猕猴桃(Actinidia chinensis)是猕猴桃属落叶果树,其果实营养丰富、风味独特,被誉为“水果之王”,原产于中国,现已有多个国家广泛种植[1]。随着猕猴桃种植面积的日益扩大、气候环境的变化和猕猴桃品种的更替,猕猴桃软腐病的发生日趋严重,病果率达到50%[2]。猕猴桃软腐病是一种真菌病害,在果实成熟期即可染病,通常在贮藏期危害严重,导致果实明显凹陷变软,快速腐烂的同时伴随发酵的酸臭味,直接导致果实不能食用[3]。目前,国内外关于猕猴桃软腐病病原菌的报道有多种,包括Botryosphaeria dothidea、Diaporthe ambigua、D. eres、D. actinidiae、Phoma ex‐igua、Colletotrichum fioriniae、Botrytis cinerea、Peni‐cillium expansum、Pestalotiopsis microspora和Alter‐naria alternata等[4-14],其中B. dothidea和Diaporthe sp.为中国猕猴桃软腐病病原菌的主要优势类群[15-16]。
关于防治猕猴桃软腐病的报道不多,主要是室内毒力试验和田间防治试验。毒力试验表明,咪鲜胺和苯醚甲环唑等药剂对猕猴桃软腐病病原菌有较好的抑制作用[17-18];莫飞旭等[19]田间试验表明,生长期使用四霉素和戊唑醇复配剂能够较好地预防采后猕猴桃软腐病;邓蕾等[20]研究表明,代森铵、琥胶肥酸铜、铜大师和春雷·王铜是防治猕猴桃果实腐烂病的高效药剂;谢文静等[2]研究表明,在生长期使用肟菌酯·戊唑醇和唑醚·氟酰胺防治猕猴桃软腐病的效果较好,贮藏期使用咪鲜胺浸果处理防效较好。室内毒力试验和田间试验防治靶标的病原菌多是B.dothidea,田间防治试验的病果未分离鉴定。鉴于猕猴桃软腐病的病原菌种类不单一,不同地区和品种的防治效果可能有所不同,应加强研究。
通过开展杀菌剂对猕猴桃软腐病的田间防治试验,并采集试验地的病果分离病原菌,对其进行形态学和分子鉴定,旨在明确该地区猕猴桃软腐病的病原菌种类,以及不同杀菌剂对该病的防治效果,以期为猕猴桃软腐病的正确诊断和防治提供理论基础和科学依据。
1 材料和方法
1.1 材料
供试植物:中猕2号猕猴桃。
供试药剂:具体名称、剂型、施药浓度及生产厂家见表1。
表1 供试杀菌剂
Table 1 The fungicides used in this study
注:SC. 悬浮剂;EC. 乳油;WG . 水分散粒剂;WP. 可湿性粉剂;WP. 可湿性粉剂;EW. 水乳剂。下同。
Note:SC. Suspension concentrate; EC. Emulsifiable concentrate; WG. Water dispersible granule; WP. Water power; EW. Emulsion in water; AS.Aqueous solutions. The same below.
药剂Fungicide 25%吡唑醚菌酯 Pyraclostrobin SC 75%肟菌·戊唑醇Trifloxystrobin·Tebuconazole WG 3%中生菌素Zhongshengmycin WP 50% 咯菌腈 Fludioxonil WP 10%苯醚甲环唑Difenoconazole WG 1×1010 CFU·g-1枯草芽孢杆菌Bacillus subtilis WP 3%中生菌素 Zhongshengmycin WP+50%咯菌腈 Fludioxonil WP 25%丙环唑 Propiconazole EW 80%多菌灵Carbendazim WP 40%氟硅唑Flusilazole EC 25%咪鲜胺Prochloraz EC 25%嘧菌酯Azoxystrobin SC 43%戊唑醇Tebuconazole SC+5%氨基寡糖素Oligosaccharins AS 43%戊唑醇Tebuconazole SC 47%春雷·王铜Kasugamycin·Copper oxychloride WG 50%异菌脲Iprodione WG 86.2%氧化亚铜Cuprous oxide WG生产厂家Manufacturer江苏三山农药有限公司Jiangsu Sanshan Pesticide Co., Ltd.拜耳农药有限公司Bayer [China] Co., Ltd.陕西标正作物科学有限公司Shaanxi Biaozheng Crop Technology Co., Ltd.瑞士先正达作物保护有限公司 Swiss Syngenta Crop Protection Co., Ltd.天津市汉邦植物保护有限责任公司Tianjin Highpoint Plant Protection Co., Ltd.青岛星牌作物科学有限公司Qingdao Star Agrochemicals Co., Ltd.陕西标正作物科学有限公司Shaanxi Biaozheng Crop Technology Co., Ltd.瑞士先正达作物保护有限公司 Swiss Syngenta Crop Protection Co., Ltd.陕西标正作物科学有限公司Shaanxi Biaozheng Crop Technology Co., Ltd.陕西标正作物科学有限公司Shaanxi Biaozheng Crop Technology Co., Ltd.江西巴菲特化工有限公司Jiangxi Buffett Chemical Co., Ltd.天津市汉邦植物保护有限责任公司Tianjin Highpoint Plant Protection Co., Ltd.先正达农药有限公司 Syngenta Crop Protection Co., Ltd.济南一农化工有限公司Jinan Yirong Chemical Industry Co., Ltd.江西正邦作物保护股份有限公司Jiangxi Zhengbang Crop Protection Co., Ltd..济南一农化工有限公司Jinan Yirong Chemical Industry Co., Ltd.一帆生物科技集团有限公司Yifan Biotechnology Group Co., Ltd.江西禾益化工股份有限公司Jiangxi Heyi Chemical Co., Ltd.挪威劳道克斯公司Laudox Norway AS用药量(倍)Dosage (multiple)2000 5000 800 2000 4000 1000 800+2000 1000 1000 6000 1000 1000 4000+4000 4000 800 1000 800
1.2 方法
1.2.1 菌株的分离 采用组织分离法分离病原菌[21],病果采自河南省新乡市中国农业科学院试验基地。取病果的病健交界处组织数块,置于0.5%的次氯酸钠溶液中消毒60 s,然后移至PDA平板培养基上,于25 ℃下培养5 d。选取代表性菌落挑取单菌丝尖端,得到纯菌株进行培养,保存在PDA斜面,放置于4 ℃冰箱备用。
1.2.2 菌株的形态特征 病原菌形态特征:将菌株接种在PDA平板上,于25 ℃光照培养箱中培养3~7 d,观察菌落颜色、形状和质地。产生孢子后,观察分生孢子的颜色、大小和形态特征等。
1.2.3 致病性测定 离体果实接种:选取大小相近的健康猕猴桃果实,用无菌水清洗2次,在超净工作台上晾干后待用。将菌株接种在PDA培养基上,25 ℃下培养6 d。使用5 mm直径打孔器制备菌饼。每个果实用昆虫针刺伤1个伤口,在伤口处接种1个菌饼。每个分离物接种10个果实,3次重复,以接种空白PDA培养基的果实作为对照。接种后置于保鲜盒中,25 ℃保存,定期观察并记录发病情况。
1.2.4 分子鉴定 将菌株接种到铺有玻璃纸的PDA平板上,25 ℃恒温培养箱中培养5 d,刮取菌丝。按照真菌基因组DNA快速提取试剂盒说明书提取DNA。采用引物对ITS1/ITS4、Bt2a/Bt2b和EF1-728F/EF1-986R分别对提取的DNA进行PCR扩增[22-24],扩增产物用1%琼脂糖凝胶电泳检测,送至河南尚亚生物技术有限公司测序。通过MEGA11.0软件构建邻接系统发育树,自展(bootstrap)循环抽样检测1000次。
1.2.5 田间防治试验 田间防治试验于2023年和2024年在河南省新乡市中国农业科学院试验基地5年生猕猴桃园进行。供试品种为中猕2号,易感染猕猴桃软腐病。按照表1中的药剂和浓度进行猕猴桃软腐病的田间防治试验。2023年分别在6月8日、7月14日、8月4日、8月25日和9月15日进行药剂喷施处理,在果实成熟期10月16日采收果实。2024年分别在5月30日、6月28日、7月29日、8月28日和9月12日进行药剂喷施处理,在果实成熟期10月12日采收果实。试验设置保护行,采用随机区组排列,以喷施清水为空白对照,共14个处理,每个处理3次重复,每小区3株树。在果实采收期采果,2023年每小区随机采收100个果,每个处理共计300个果,置于室温贮存;2024年每小区随机采收200个果(100个混合装,100个单独分装),每个处理共计600个果,置于室温贮存。在贮藏15 d和30 d时,调查软腐病病果数,按下列公式(1)、(2)计算各处理的病果率和田间防效[21]。

1.3 数据分析
采用Excel和DPS对试验数据进行统计分析,应用Duncan氏新复极差法进行差异显著性检验。
2 结果与分析
2.1 病害症状
果实自然发病的主要症状表现为果皮上形成略微凹陷的病斑。随着病情加重,病斑内部果肉出现乳化、软腐的症状,不同病原菌引起的症状在外观上没有明显区别(图1)。

图1 猕猴桃软腐病症状
Fig. 1 Symptoms of kiwifruit soft rot
2.2 病原菌分离和形态特征
从82份样品中,分离得到79株分离物。依据菌株的形态特征,可将这些分离物划分为2类(图2)。Ⅰ类包括44株分离物,分离频率为53.66%。分离物在PDA培养基上培养,菌落呈白色茸毛状,菌落背面子实体着生位置颜色较深呈黑点状,产生α型和β型分生孢子。α型分生孢子,单孢,无色,纺锤形至椭圆形,大小(5.6~8.7)μm×(2.4~3.1)μm;β型分生孢子,单孢,无色,弯线形,大小(18.7~33.6)μm×(1.1 ~2.1)μm。依据形态特征,初步判断Ⅰ类分离菌株为间座壳属(Diaporthe sp.)。Ⅱ类包括35株分离物,分离频率为42.68%,分离物在PDA培养基上培养,菌落初期为白色,后期颜色逐渐加深变为灰褐色,菌丝发达,菌落背面为黑褐色。分生孢子,单胞,无色,近梭形,大小(17.3~27.6)μm×(5.3~9.1)μm。依据形态特征,初步判断Ⅱ类分离物为葡萄座腔菌属(Bot‐ryosphaeria sp.)。
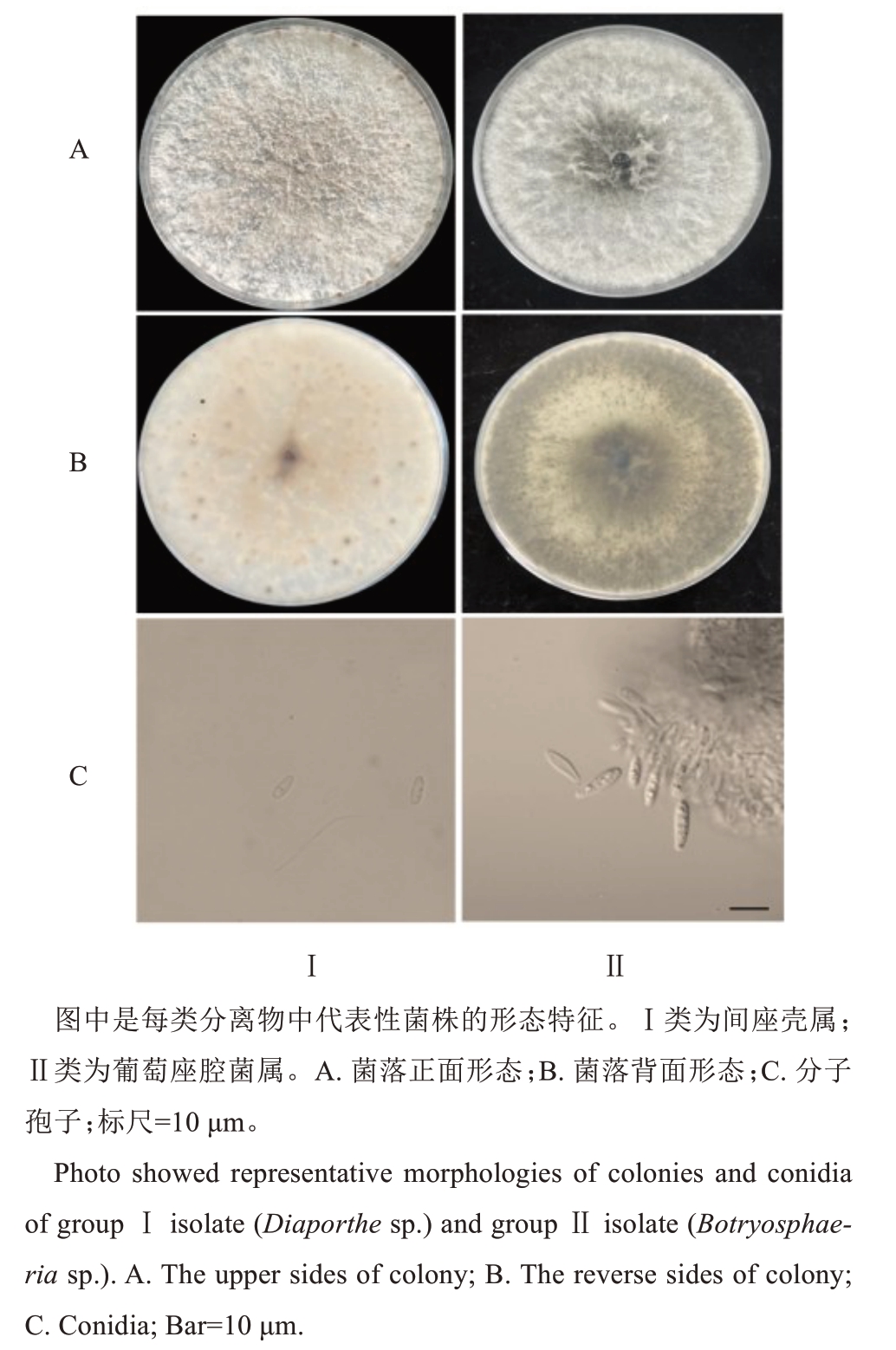
图2 分离菌株的形态特征
Fig. 2 Morphology of fungi isolates
2.3 猕猴桃软腐病病原菌的分子鉴定
基于多基因位点分析,菌株MF-1和MF-3鉴定为猕猴桃软腐病的致病菌。序列比对结果显示,在ITS区域,菌株MF-1与D. eres(NFIF-1-1)的相似性为100%,MF-3与B. dothidea(BDCYP1)的相似性为100%;在EF-1α基因位点,MF-1与D. eres(SL-MHB-9)的相似性为99.70%,MF-3与B. dothidea(XG-221)的相似性为99.26%;在β-tubulin(TUB)基因位点,MF-1与D. eres(LNCY6-1)的相似性为100%,MF-3与B. dothidea(JZB310205)的相似性为100%。系统发育分析显示(图3),基于ITS、EF-1α和TUB基因串联构建的系统发育树中,菌株MF-1与D. eres参考菌株聚为一支(bootstrap=100%),MF-3与B. do‐thidea参考菌株聚为一支(bootstrap=100%)。结合形态学特征,最终确定引起猕猴桃软腐病的病原菌为D. eres和B. dothidea。
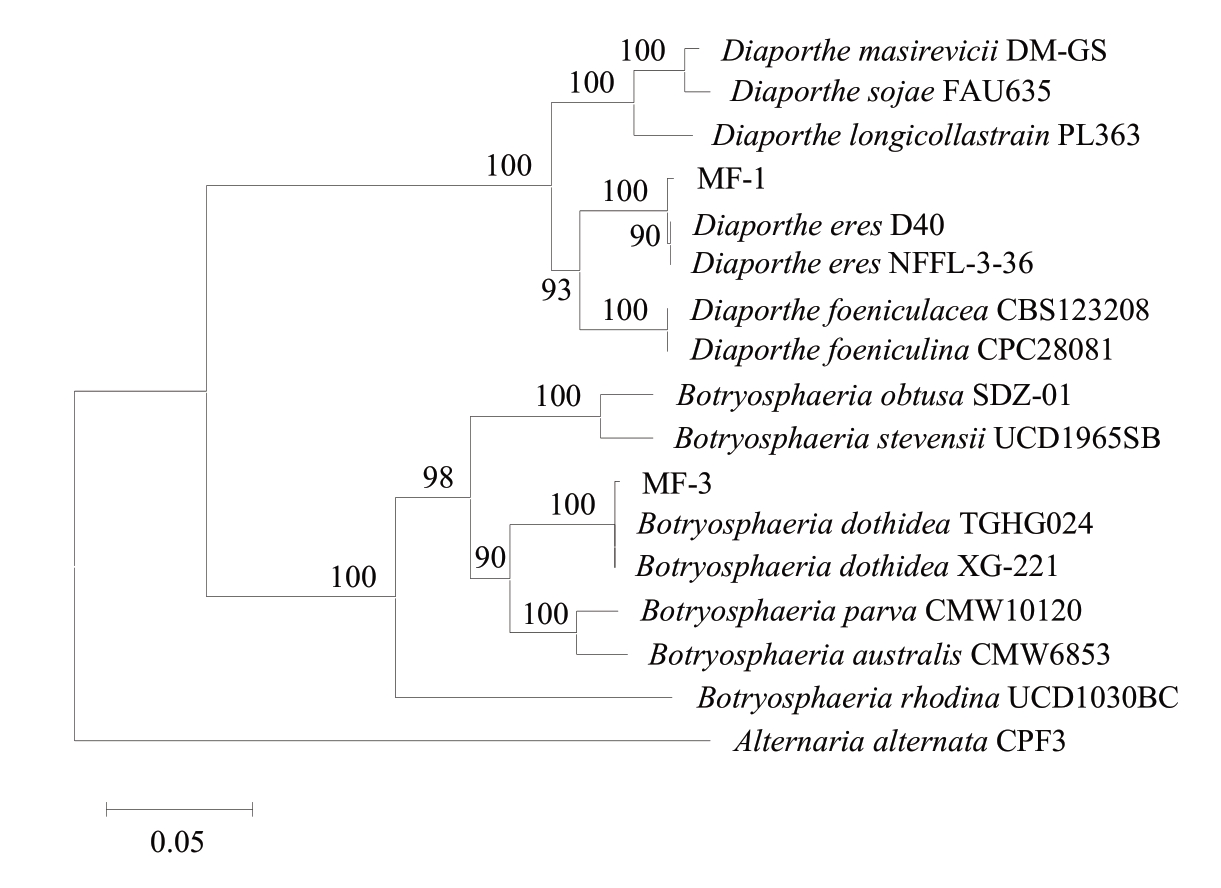
图3 基于ITS、EF-lα和TUB基因序列构建NJ系统发育树
Fig. 3 The neighbor-joining tree based on combined ITS, EF-lα and TUB sequences
2.4 病原菌的致病性
通过有伤接种法,对分离获得的菌株进行回接试验。接种分离物的猕猴桃果实在接种后7 d形成与自然发病相似的明显症状,而对照组不发病(图4)。D. eres菌株致病力较强,病斑平均直径为28.05 mm;B. dothidea菌株致病力较弱,病斑平均直径为18.71 mm。对接种后发病的果实再次进行分离鉴定,可以得到与接种分离物一致的菌株,证明D. eres和B. dothidea是引起猕猴桃软腐病的病原菌。

图4 分离菌株对猕猴桃的致病性测定Fig. 4 Pathogenicity of isolates to kiwifruits
2.5 杀菌剂对猕猴桃软腐病的田间防效
2023年田间试验调查结果表明(表2),贮藏15 d的防效在85%以上的药剂有5种,分别是10%苯醚甲环唑水分散粒剂、75%肟菌·戊唑醇水分散粒剂、25%吡唑醚菌酯悬浮剂、40%氟硅唑乳油和43%戊唑醇悬浮剂,防效分别为94.56%、93.17%、91.79%、87.24%和85.85%,防效差异不显著;贮藏30 d后,这5种药剂的防效分别为85.43%、77.47%、75.34%、80.18%和78.97%,除25%吡唑醚菌酯悬浮剂外,其他药剂间防效差异不显著。25%咪鲜胺乳油、25%丙环唑乳油、80%多菌灵可湿性粉剂、100亿CFU·g-1枯草芽孢杆菌可湿性粉剂和25%嘧菌酯悬浮剂贮藏15 d的防效分别为82.63%、80.41%、77.06%、72.90%和74.61%;贮藏30 d的防效分别为68.81%、54.42%、67.56%、61.55%和56.87%。50%咯菌腈可湿性粉剂的防效最差,贮藏15 d和30 d的防效均在50%以下;其次是3%中生菌素可湿性粉剂,贮藏15 d和贮藏30 d的防效分别为68.98%和52.02%;但3%中生菌素可湿性粉剂和25%咯菌腈悬浮剂混合使用能够提高防效,贮藏15 d和贮藏30 d的防效分别为83.44%和64.98%。
表2 杀菌剂对猕猴桃软腐病的田间防效(2023)
Table 2 Control effects of fungicides on kiwifruit soft rot in the field (2023)

注:不同小写字母表示同列间存在显著差异(P<0.05)。下同。
Note:Different small letters indicate significant difference in the same column (P<0.05). The same below.
药剂 Fungicide 10%苯醚甲环唑 Difenoconazole WG 75%肟菌·戊唑醇 Trifloxystrobin·Tebuconazole WG 25%吡唑醚菌酯 Pyraclostrobin SC 40%氟硅唑Flusilazole EC 43%戊唑醇Tebuconazole SC 3%中生菌素 Zhongshengmycin WP+50%咯菌腈 Fludioxonil WP 25%咪鲜胺 Prochloraz EC 25%丙环唑 Propiconazole EW 80%多菌灵Carbendazim WP 1×1010 CFU·g-1枯草芽孢杆菌Bacillus subtilis WP 25%嘧菌酯Azoxystrobin SC 3%中生菌素Zhongshengmycin WP 50% 咯菌腈 Fludioxonil WP清水对照CK贮藏15 d Store 15 d病果率Diseased fruit rate/%1.00±0.58 i 1.33±0.28 fg 1.67±0.33 fg 2.67±0.53 efg 3.00±0.58 defg 3.33±0.33 defg 3.67±0.45 cdefg 4.00±0.58 cdef 4.67±0.41 cde 5.33±0.88 cde 5.67±0.36 cd 6.33±0.67 c 10.67±1.20 b 21.00±2.08 a平均防效Average control efficacy/%94.56±3.43 a 93.17±2.47 ab 91.79±2.08 ab 87.24±2.87 abc 85.85±2.03 abc 83.44±3.50 bcd 82.63±2.08 bcd 80.41±3.55 cd 77.06±3.68 cde 72.90±2.19 de 74.61±3.31 de 68.98±5.41 e 48.77±5.10 f贮藏30 d Store 30 d病果率Diseased fruit rate/%5.67±1.24 g 8.67±0.92 hi 9.33±0.88 gh 7.67±1.03 hi 8.00±0.38 hi 13.33±0.76 ef 12.00±1.15 fg 17.33±0.35 cd 12.33±0.33 fg 14.67±1.33 def 16.33±0.67 cde 18.33±1.20 c 23.67±0.66 b 38.33±2.40 a平均防效Average control efficacy/%85.43±2.25 a 77.47±1.30 ab 75.34±3.39 bc 80.18±2.03 ab 78.97±1.26 ab 64.98±3.03 de 68.81±1.31 cd 54.42±3.00 fg 67.56±2.28 cd 61.55±3.88 def 56.87±4.19 efg 52.02±3.07 g 37.99±2.19 h
2024年田间试验结果表明(表3~表4),分装贮藏的病果率普遍稍低于不分装贮藏,防效普遍稍高于不分装贮藏。该结果与2023年的相似,10%苯醚甲环唑水分散粒剂、75%肟菌·戊唑醇水分散粒剂、25%吡唑醚菌酯悬浮剂、40%氟硅唑乳油和43%戊唑醇悬浮剂的防效较高,不分装贮藏15 d的防效均在84%以上,贮藏30 d的防效均在74%以上;分装贮藏15 d的防效均在87%以上,贮藏30 d的防效均在78%以上。47%春雷·王铜可湿性粉剂也表现出良好的防效,无论是否分装其贮藏15 d的防效均在78%以上,贮藏30 d的防效均在74%以上。100亿CFU·g-1枯草芽孢杆菌可湿性粉剂不分装贮藏15 d和30 d的防效分别是68.98%和66.53%,分装贮藏15 d和30 d的防效分别是75.23%和70.44%;50%异菌脲可湿性粉剂不分装贮藏15 d和30 d的防效分别是52.94%和45.62%,分装贮藏15 d和30 d的防效分别是58.92%和46.41%;100亿CFU·g-1枯草芽孢杆菌可湿性粉剂和50%异菌脲可湿性粉剂混用不分装贮藏15 d和30 d的防效分别是68.61%和64.43%,分装贮藏15 d和30 d的防效分别是71.04%和68.55%,两者混用并未表现出明显的增效作用;43%戊唑醇悬浮剂和5%氨基寡糖素AS混用也未表现出明显的增效作用。
表3 杀菌剂对猕猴桃软腐病的田间防效 (2024)
Table 3 Control effects of fungicides on kiwifruit soft rot in the field(2024)
药剂 Fungicides 10%苯醚甲环唑 Difenoconazole WG 40%氟硅唑Flusilazole EC 43%戊唑醇Tebuconazole SC 75%肟菌·戊唑醇Trifloxystrobin·Tebuconazole WG 43%戊唑醇Tebuconazole SC+5%氨基寡糖素Oligosaccharins AS 25%吡唑醚菌酯 Pyraclostrobin SC 47%春雷·王铜Kasugamycin·Copper oxychloride WG 25%咪鲜胺 Prochloraz EC 1×1010 CFU·g-1枯草芽孢杆菌Bacillus subtilis WP 1×1010 CFU·g-1枯草芽孢杆菌Bacillus subtilis WP+50%异菌脲Iprodione WG 50%异菌脲Iprodione WG 86.2%氧化亚铜Cuprous oxide WG清水对照CK贮藏15 d Store 15 d病果率Diseased fruit rate/%2.33±2.65 e 2.67±0.67 e 3.00±1.45 e 3.33±0.88 e 3.67±1.20 e 4.33±0.88 de 5.33±0.88 cde 5.67±0.67 cde 7.67±0.58 cd 8.33±0.67 c平均防效Average control efficacy/%91.00±2.07 a 89.72±2.39 a 88.44±2.06 a 87.23±1.79 a 85.78±2.74 a 84.03±3.81 a 80.33±1.54 ab 78.88±2.14 bc 68.98±5.41 e 68.61±3.11 c贮藏30 d Store 30 d病果率Diseased fruit rate/%7.67±1.67 d 8.33±0.33 d 8.00±2.03 d 7.33±3.84 d 8.67±2.40 d 10.67±1.53 cd 9.67±1.67 cd 12.67±1.45 cd 14.33±0.58 c 14.67±0.88 c平均防效Average control efficacy/%81.25±3.02 a 79.98±2.69 a 80.60±2.90 a 82.31±4.15 a 79.64±2.85 a 74.52±3.62 abc 77.48±2.53 ab 69.67±1.75 bc 66.53±2.85 bc 64.43±2.38 c 12.33±0.33 b 13.33±0.33 b 27.00±0.33 a 52.94±9.08 d 50.32±3.88 d 22.67±0.88 b 24.00±0.88 b 42.33±1.20 a 45.62±7.06 d 42.88±6.27 d
表4 杀菌剂对猕猴桃软腐病的田间防效(分装储藏)(2024)
Table 4 Control effects of fungicides on kiwifruit soft rot in the field (Subpackage store) (2024)
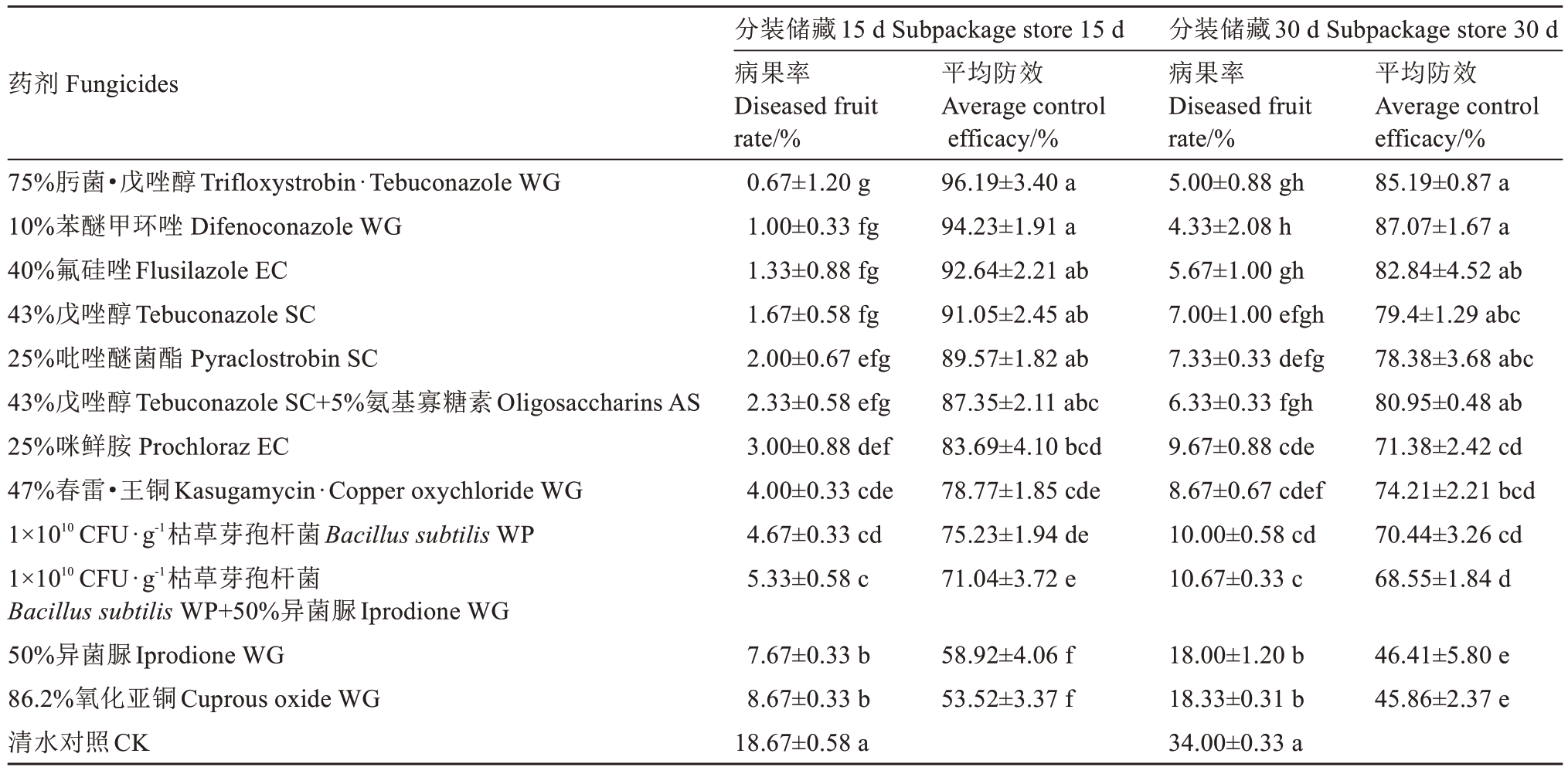
药剂 Fungicides 平均防效Average control efficacy/%75%肟菌·戊唑醇Trifloxystrobin·Tebuconazole WG 10%苯醚甲环唑 Difenoconazole WG 40%氟硅唑Flusilazole EC 43%戊唑醇Tebuconazole SC 25%吡唑醚菌酯 Pyraclostrobin SC 43%戊唑醇Tebuconazole SC+5%氨基寡糖素Oligosaccharins AS 25%咪鲜胺 Prochloraz EC 47%春雷·王铜Kasugamycin·Copper oxychloride WG 1×1010 CFU·g-1枯草芽孢杆菌Bacillus subtilis WP 1×1010 CFU·g-1枯草芽孢杆菌Bacillus subtilis WP+50%异菌脲Iprodione WG 50%异菌脲Iprodione WG 86.2%氧化亚铜Cuprous oxide WG清水对照CK分装储藏15 d Subpackage store 15 d病果率Diseased fruit rate/%0.67±1.20 g 1.00±0.33 fg 1.33±0.88 fg 1.67±0.58 fg 2.00±0.67 efg 2.33±0.58 efg 3.00±0.88 def 4.00±0.33 cde 4.67±0.33 cd 5.33±0.58 c 96.19±3.40 a 94.23±1.91 a 92.64±2.21 ab 91.05±2.45 ab 89.57±1.82 ab 87.35±2.11 abc 83.69±4.10 bcd 78.77±1.85 cde 75.23±1.94 de 71.04±3.72 e分装储藏30 d Subpackage store 30 d病果率Diseased fruit rate/%5.00±0.88 gh 4.33±2.08 h 5.67±1.00 gh 7.00±1.00 efgh 7.33±0.33 defg 6.33±0.33 fgh 9.67±0.88 cde 8.67±0.67 cdef 10.00±0.58 cd 10.67±0.33 c平均防效Average control efficacy/%85.19±0.87 a 87.07±1.67 a 82.84±4.52 ab 79.4±1.29 abc 78.38±3.68 abc 80.95±0.48 ab 71.38±2.42 cd 74.21±2.21 bcd 70.44±3.26 cd 68.55±1.84 d 7.67±0.33 b 8.67±0.33 b 18.67±0.58 a 58.92±4.06 f 53.52±3.37 f 18.00±1.20 b 18.33±0.31 b 34.00±0.33 a 46.41±5.80 e 45.86±2.37 e
3 讨 论
研究表明,引起猕猴桃软腐病的病原菌种类较多,如D. ambigua、D. eres、D. actinidiae、B. dothidea和A. alternata等[7,12,15,25-26]。笔者通过症状观察、形态学鉴定以及系统发育树分析,将引起猕猴桃软腐病的2种病原菌鉴定为D. eres和B. dothidea,分离频率分别为53.66%和42.68%。D. eres分离比例高于B. dothidea,这与Li等[16]的研究结果一致,但与Zhou等[27]报道的B. dothidea是猕猴桃软腐病优势病原菌的结果不一致。这可能是因为病样采集地点和品种的不同,表明不同地区和品种的病原菌种类会有所变化,应根据当地具体情况合理优化调整用药种类。本文病样采集和田间防治试验是同一地点的同一品种,可能对制定相应的猕猴桃软腐病防控策略更具指导意义。
猕猴桃软腐病潜伏侵染危害重,严重影响猕猴桃产业的发展,目前仍以化学防治为主要措施。然而,中国目前尚未登记用于防治猕猴桃软腐病的杀菌剂(http://www.chinapesticide.org.cn)。乱用、滥用情况普遍存在,用药缺乏科学的指导,在贮藏15 d和30 d时防治效果不理想。连续2年的田间试验结果表明,10%苯醚甲环唑水分散粒剂、75%肟菌·戊唑醇水分散粒剂、25%吡唑醚菌酯悬浮剂、40%氟硅唑乳油和43%戊唑醇悬浮剂5种杀菌剂对猕猴桃软腐病均表现出良好稳定的防治效果,这一结果与三唑类、甲氧基丙烯酸酯类杀菌剂具有广谱、高效的特性吻合[28]。尽管药剂的防治效果随贮藏时间延长而下降,但其防效仍能保持在74%以上,表明药剂具有较好的持效性。然而,长期贮藏过程中防效的衰减也表明后续研究需要关注农药残留与食品安全问题。笔者在本研究中发现,75%肟菌·戊唑醇水分散粒剂不分装情况下最高防效为93.17%,与谢文静等[2]报道的75%肟菌酯·戊唑醇水分散粒剂田间防效为92.30%的结果一致。40%氟硅唑乳油不分装情况下最高防效为87.24%,与谢文静等[2]报道的400 g·L-1氟硅唑乳油防治效果为32.84%的结果不一致。43%戊唑醇悬浮剂和5%氨基寡糖素AS混用没有表现出明显的增效作用,与何立楠等[29]报道的戊唑醇与氨基寡糖素复配对猕猴桃软腐病有增效结果有所不同。这些差异可能是因为文献报道的猕猴桃软腐病的病原菌为B. dothidea,本文中猕猴桃软腐病的病原菌为D. eres和B. dothidea,且D. eres分离比例高于B. dothidea;此外,试验品种和试验地点等因素不同也可能造成结果不一致。
此外,100亿CFU·g-1枯草芽孢杆菌可湿性粉剂和50%异菌脲可湿性粉剂混用并未表现出明显的增效作用,无论是否分装其贮藏30 d的防效均在70%以下,这与刘淑娟等[30]报道的生防芽孢杆菌与扑海因混配对番茄早疫病菌具有增效作用的结果不一致,可能是因为笔者在本研究中防治的是猕猴桃软腐病,与前人研究的防治靶标不同,还需重复试验。值得注意的是,分装贮藏的防效高于不分装贮藏,这可能是由于分装贮藏减少了果实间机械损伤和病原菌接触传播的风险[31],同时分装贮藏能够通过物理隔离作用降低病原菌再侵染概率。
4 结 论
猕猴桃软腐病病原菌为D. eres和B. dothidea,10%苯醚甲环唑水分散粒剂、75%肟菌·戊唑醇水分散粒剂、25%吡唑醚菌酯悬浮剂、40%氟硅唑乳油和43%戊唑醇悬浮剂对猕猴桃软腐病具有良好的田间防治效果,分装贮藏的防效普遍稍高于不分装贮藏。
[1] 齐秀娟,郭丹丹,王然,钟云鹏,方金豹. 我国猕猴桃产业发展现状及对策建议[J] . 果树学报,2020,37(5):754-763.QI Xiujuan,GUO Dandan,WANG Ran,ZHONG Yunpeng,FANG Jinbao. Development status and suggestions on Chinese kiwifruit industry[J] . Journal of Fruit Science,2020,37(5):754-763.
[2] 谢文静,朱宇航,徐菁,李治菲,姚凯凯,吴翠平,陈华保,马苗苗,龚国淑. 不同杀菌剂对猕猴桃软腐病菌(Botryosphaeria dothidea)的防治效果评价[J] . 果树学报,2024,41(11):2335-2346.XIE Wenjing,ZHU Yuhang,XU Jing,LI Zhifei,YAO Kaikai,WU Cuiping,CHEN Huabao,MA Miaomiao,GONG Guoshu.Control efficacy of different fungicides against kiwifruit soft rot caused by Botryosphaeria dothidea[J] . Journal of Fruit Science,2024,41(11):2335-2346.
[3] 左盼盼,付苏,彭丽桃,范刚,杨书珍,李杰. 猕猴桃采后软腐病病原菌鉴定及香芹酚对其控制效果[J] . 华中农业大学学报,2020,39(6):15-22.ZUO Panpan,FU Su,PENG Litao,FAN Gang,YANG Shuzhen,LI Jie. Identification of soft rot pathogens in postharvest kiwifruit and its control effect by carvacrol[J] . Journal of Huazhong Agricultural University,2020,39(6):15-22.
[4] PENNYCOOK S R. Fungal fruit rots of Actinidia deliciosa (kiwifruit)[J] . New Zealand Journal of Experimental Agriculture,1985,13(4):289-299.
[5] OPGENORTH D C. Storage rot of California-grown kiwifruit[J] .Plant Disease,1983,67(4):382.
[6] MANNING M A,MEIER X,OLSEN T L,JOHNSTON P R.Fungi associated with fruit rots of Actinidia chinensis ‘Hort16A’in New Zealand[J] . New Zealand Journal of Crop and Horticultural Science,2003,31(4):315-324.
[7] AUGER J,PÉREZ I,ESTERIO M. Diaporthe ambigua associated with post-harvest fruit rot of kiwifruit in Chile[J] . Plant Disease,2013,97(6):843.
[8] LUONGO L,SANTORI A,RICCIONI L,BELISARIO A. Pho‐mopsis sp. associated with postharvest fruit rot of kiwifruit in Italy[J] . Journal of Plant Pathology,2011,93(1):205-209.
[9] POTI T,KISAKI G,ARITA K,AKIMITSU K. Identification and characterization of Colletotrichum species causing kiwifruit anthracnose in Kagawa Prefecture,Japan[J] . Journal of General Plant Pathology,2023,89(2):84-90.
[10] SUI Y,LIAO Q H,LENG J S,CHEN Z. Eco-friendly biocontrol strategies for management of postharvest fungal decays in kiwifruit:A review[J] . International Journal of Food Microbiology,2025,432:111106.
[11] WANG L,HOU H,ZHOU Z Q,TU H T,YUAN H B. Identification and detection of Botryosphaeria dothidea from kiwifruit(Actinidia chinensis) in China[J] . Plants,2021,10(2):401.
[12] LI L,PAN H,LIU W,CHEN M Y,ZHONG C H. First report of Diaporthe actinidiae causing stem end rot of kiwifruit during post-harvest in China[J] . Plant Disease,2017,101(6):1054.
[13] LI L,PAN H,CHEN M Y,ZHONG C H. First report of Pestalo‐tiopsis microspora causing postharvest rot of kiwifruit in Hubei province,China[J] . Plant Disease,2016,100(10):2161.
[14] LI L,PAN H,LIU W,CHEN M Y,ZHONG C H. First report of Alternaria alternata causing postharvest rot of kiwifruit in China[J] . Plant Disease,2017,101(6):1046.
[15] 封露. 我国主产区猕猴桃采后腐烂病原菌多样性[D] . 武汉:华中农业大学,2021.FENG Lu. Pathogen diversity of postharvest rot of kiwifruit at the major cultivation areas of China[D] . Wuhan:Huazhong Agricultural University,2021.
[16] LI L,PAN H,CHEN M Y,ZHANG S J,ZHONG C H. Isolation and identification of pathogenic fungi causing postharvest fruit rot of kiwifruit (Actinidia chinensis) in China[J] . Journal of Phytopathology,2017,165(11/12):782-790.
[17] 胡容平,石军,林立金,叶慧丽,姚琳,周游. 四川猕猴桃软腐病防治初步研究[J] . 西南农业学报,2017,30(2):366-370.HU Rongping,SHI Jun,LIN Lijin,YE Huili,YAO Lin,ZHOU You. Preliminary study on control efficiency against soft rot disease of kiwifruit in Sichuan[J] . Southwest China Journal of Agricultural Sciences,2017,30(2):366-370.
[18] 吴文能,张起,雷霁卿,黄亚欣,陈晨蕰,王瑞,曹森. ‘贵长’猕猴桃软腐病病原菌分离鉴定及抑菌药剂筛选[J] . 北方园艺,2018(16):47-54.WU Wenneng,ZHANG Qi,LEI Jiqing,HUANG Yaxin,CHEN Chenwen,WANG Rui,CAO Sen. Identification and pharmaceutical screening of kiwifruit soft rot disease on ‘Guichang’ gooseberry[J] . Northern Horticulture,2018(16):47-54.
[19] 莫飞旭,石金巧,潘东妹,黄亚欣,吴素芳,尹显慧,龙友华. 四霉素与戊唑醇复配对猕猴桃软腐病的防控效果[J] . 中国植保导刊,2019,39(2):71-74.MO Feixu,SHI Jinqiao,PAN Dongmei,HUANG Yaxin,WU Sufang,YIN Xianhui,LONG Youhua. Controlling efficiency of tetramycin and tebuconazole mixture against kiwifruit soft rot disease[J] . China Plant Protection,2019,39(2):71-74.
[20] 邓蕾,潘慧,张莹华,钟彩虹,胡秋舲,谢义福,周鹏,雷靖,李黎. 猕猴桃果实腐烂病高效防治药剂的田间验证[J] . 中国南方果树,2020,49(4):127-129.DENG Lei,PAN Hui,ZHANG Yinghua,ZHONG Caihong,HU Qiuling,XIE Yifu,ZHOU Peng,LEI Jing,LI Li. Field validation of an effective control agent for kiwifruit fruit rot[J] . South China Fruits,2020,49(4):127-129.
[21] WANG L,TU H T,HOU H,ZHOU Z Q,YUAN H B,LUO C X,GU Q S. Occurrence and detection of carbendazim resistance in Botryosphaeria dothidea from apple orchards in China[J] .Plant Disease,2022,106(1):207-214.
[22] WHITE T J,BRUNS T,LEE S,TAYLOR J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics[M] //INNIS M A,GELFAND D H,SNINSKY J J,WHITE T J. PCR protocols:A guide to methods and applications. Amsterdam:Elsevier,Academic Press,1990:315-322.
[23] GLASS N L,DONALDSON G C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes[J] . Applied and Environmental Microbiology,1995,61(4):1323-1330.
[24] CARBONE I,KOHN L M. A method for designing primer sets for speciation studies in filamentous ascomycetes[J] . Mycologia,1999,91(3):553-556.
[25] REN Y L,WANG T,TANG J,JIANG Y J,HUANG Y X,ZHANG C P,PENG J,WANG J,WANG S S,WANG J. Identification of the pathogens and laboratory bioactivity determination of the rot disease of kiwifruit (Actinidia spp.)[J] . Journal of Chemistry,2022,2022(1):2293297.
[26] LEE J G,LEE D H,PARK S Y,HUR J S,KOH Y J. First report of Diaporthe actinidiae,the causal organism of stem-end rot of kiwifruit in Korea[J] . The Plant Pathology Journal,2001,17(2):110-113.
[27] ZHOU Y,GONG G S,CUI Y L,ZHANG D X,CHANG X L,HU R P,LIU N,SUN X F. Identification of Botryosphaeriaceae species causing kiwifruit rot in Sichuan province,China[J] .Plant Disease,2015,99(5):699-708.
[28] 严明,柏亚罗. 甲氧基丙烯酸酯类等四大类杀菌剂市场概况及前景展望[J] . 现代农药,2016,15(6):1-8.YAN Ming,BAI Yaluo. Market overview and prospect outlook on four fungicide sectors including methoxyacrylates[J] . Modern Agrochemicals,2016,15(6):1-8.
[29] 何立楠,石金巧,张荣全,罗桂洪,袁腾,樊荣,尹显慧,吴小毛,龙友华. 戊唑醇与氨基寡糖素复配对猕猴桃软腐病的防控效果[J] . 世界农药,2021,43(10):25-29.HE Linan,SHI Jinqiao,ZHANG Rongquan,LUO Guihong,YUAN Teng,FAN Rong,YIN Xianhui,WU Xiaomao,LONG Youhua. The control effects of the binary mixture of tebuconazole and oligosaccharides on kiwifruit soft rot Botryosphaeria dothidea[J] . World Pesticide,2021,43(10):25-29.
[30] 刘淑娟,陈秀蓉,杨成德,薛莉. 生防芽孢杆菌与扑海因混配对番茄早疫病菌的抑制作用[J] . 甘肃农业大学学报,2007,42(1):49-53.LIU Shujuan,CHEN Xiurong,YANG Chengde,XUE Li. Inhibiting effect of the mixture of iprodione and Bacillus subtilis on Alternaria solani[J] . Journal of Gansu Agricultural University,2007,42(1):49-53.
[31] ROMANAZZI G,SANZANI S M,BI Y,TIAN S P,GUTIÉRREZ MARTÍNEZ P,ALKAN N. Induced resistance to control postharvest decay of fruit and vegetables[J] . Postharvest Biology and Technology,2016,122:82-94.