海棠(Malus spp.)为蔷薇科苹果属落叶乔木,以花朵绚丽、叶色丰富多变及果实美观的特点而闻名,是我国城市园林绿化和家庭园艺的优选植物之一[1-3]。海棠原种起源于中国,后被引入欧美地区,与当地苹果属植物杂交选育出众多观赏价值较高的海棠杂交品种[4]。北美海棠因环境适应性强,在中国各地适宜栽培,具有广阔的推广前景[5]。唐纳德海棠由于花朵和果实色彩艳丽,近年来在大连市道路两旁、公园及景区等地的种植规模不断扩大,已成为兼具观花、观叶和观果价值的优良园林树种。
观赏海棠多源于自然杂交后代,通常具有较强的抗病性[6],因此病害发生的种类相对较少且危害程度较轻。目前,关于海棠植物病害的研究报道较为有限。例如,在美国的北美海棠上已报道了锈病(Gymnosporangium yamadae)[7]、果实斑点病和叶枯病(Phacidiopycnis washingtonensis)[8];在土耳其的多花海棠上报道了枯萎病(Erwinia amylovora)[9]。在中国的亚斯特海棠(Malus ‘Ester’)上报道了腐烂病(Valsa mali)[10];在河北省雄安新区的海棠上发现锈病(Gymnosporangium yamadae)[11],在怀来县的八棱海棠报道了立枯病(Rhizoctonia solani)[12];在泰山的湖北海棠上发现枝干溃疡病[13]。这些病害对植株造成了严重危害。
2023年,笔者在对大连地区海棠病害进行调查时发现,唐纳德海棠(Malus ‘Donald Wyman’)叶片上普遍发生一种叶斑病,严重影响了该树的栽培管理及观赏价值。叶斑病不仅会干扰植物的叶片光合作用,严重时还可导致叶片枯死和提早脱落,给城市绿化造成严重经济损失。为了明确该病害的病原菌种类及其寄主范围,开展了病原菌鉴定、致病性测定及杀菌剂筛选工作,以期为海棠叶斑病的科学防治提供理论依据。
1 材料和方法
1.1 样品来源
分别于2023年和2024年的夏、秋两季,在大连市金普新区铜牛岭和大黑山风景区采集唐纳德海棠叶斑病病叶。样品装入无菌袋中,带回实验室用于病原菌分离。
1.2 病原菌分离纯化
采用组织分离法[14]进行叶斑病病菌分离。选取具有典型叶斑病症状的病叶,在病健交界处切取5 mm×5 mm的组织块,经75%酒精消毒30 s,3%次氯酸钠溶液消毒30 s,再用无菌水冲洗2~3次后,接种在PDA平板上,置于25 ℃培养箱中暗培养5~7 d。待长出新鲜菌落后进行转接和纯化,获得纯化菌种后置于4 ℃冰箱保存备用。
1.3 病原菌致病性测定
1.3.1 病原菌活化培养 将保存的菌株在PDA平板上活化培养6 d,用无菌打孔器(直径8 mm)在菌落边缘打取菌苔备用。
1.3.2 离体叶片接种 将采集的唐纳德海棠植株健康新鲜叶片,在实验室用自来水冲洗干净,经75%酒精擦洗后再用无菌水冲洗3次。在超净工作台中用无菌接种针刺伤叶片,平铺在底部铺有滤纸和玻璃棒的大培养皿(直径10 cm)中,皿底加入适量无菌水以保湿。取一块制备好的菌苔,菌丝面朝下放置在经刺伤的叶片部位,以接种无菌PDA培养基作为对照,盖好皿盖后置于25 ℃恒温培养箱中培养,设置3次重复,接种后定期观察发病情况。
1.3.3 人工接种发病叶片症状观察和病菌再分离待上述人工接种叶片出现叶斑病的典型症状后,对发病叶片进行症状观察和病原菌再分离,并根据形态特征进行病菌鉴定。观察病原菌的菌落形态、菌丝颜色、菌丝致密性等培养特性和分生孢子、孢子囊、产孢结构等孢子结构特征,以确定是否与上面人工接种病菌为同一种真菌。
1.4 病原菌鉴定
1.4.1 形态特征观察 将供试纯化菌株接种于PDA培养基上,于25 ℃恒温培养箱中暗培养7 d,观察菌落特征。在光学显微镜下观察病原菌的子实体形态特征,参考《中国真菌志·第十六卷 链格孢属》[15]的分类进行病原菌形态鉴定。
1.4.2 分子生物学鉴定 将供试菌株接种在铺有灭菌玻璃纸的PDA平板上,于25 ℃恒温培养箱中暗培养7 d,收集菌丝体。采用Ezup柱式真菌基因组DNA抽提试剂盒(上海生工)提取菌株的基因组DNA,通过1.5%琼脂糖凝胶电泳检测基因组DNA提取质量。分别用引物ITS1(5'-TCCGTAGGTGAACCTGCGG-3')/ITS4(5'-TCCGTAGGTGAACCTGCGG-3')、ACT-512F(5'-ATGTGCAAGGCCGGT TTCGC-3')/ACT-783R(5'-TACGAGTCCTTCTGGCCCAT-3')、NS1(5'-GTAGTCATATGCTTGTCTC-3')/NS6(5'-GCATCACAGACCTGTTATTGCCTC-3')对病原菌的ITS[16]、ACT[17]及LSU[18]基因进行PCR扩增,扩增产物经1.0%琼脂糖凝胶电泳检测正确后,送至上海生工生物工程有限公司进行纯化和测序。将测序结果提交至NCBI网站(https://www.ncbi.nlm.nih.gov)Gen-Bank数据库,获取登录号,然后进行BLAST同源性比对,删去低质量的比对区域。使用RAXML软件,采用ML法构建多基因串联系统发育树。使用Phylosuite V1.2.2软件对组合的ITS、ACT和SSU基因序列首尾串联,使用RAXML软件构建多基因串联系统发育树。
1.5 不同海棠品种致病性测定
1.5.1 供试寄主 在田间选取与唐纳德海棠同属北美海棠系列的5个常见品种:火焰海棠(Malus ‘Flame’)、印第安魔幻海棠(Malus ‘Indian Magic’)、垂丝海棠(Malus halliana)、西府海棠(Malus micromalus)及绚丽海棠(Malus ‘Radiant’)。另外,选取2种常用作海棠嫁接砧木的山荆子(Malus baccata)和毛荆子(Malus baccata var. mandshurica),并对7种亲缘关系相近的植物进行致病性测定。
1.5.2 致病性测定 采用菌饼刺伤接种方法对供试寄主进行致病性测定(方法同1.3)。选取健康的供试海棠叶片,将叶片表面清洗干净后,以接种无菌PDA培养基的植株作为对照。每个处理设置3次重复,接种后定期观察并记录各个品种海棠的症状发展情况。同时,再次分离病原物,该分离操作重复3次。对再次分离获得的菌株,通过形态学特征和分子生物学方法进行对比,验证其与原始菌株的一致性,从而确定原分离菌的致病性。
1.6 杀菌剂药效测定
1.6.1 供试杀菌剂 供试杀菌剂共11种,选取悬浮剂、水剂、乳油、可湿性粉剂、水乳剂、水分散粒剂共6种剂型(表1)。
表1 11种杀菌剂对应信息
Table 1 Information on 11 fungicides

?
1.6.2 杀菌剂药效测定 采用菌丝生长速率法[19]进行杀菌剂抑菌试验。供试药剂为复配型药剂和广谱性的杀菌剂单剂,参考每种杀菌剂使用说明书中的使用浓度,配制相应浓度的杀菌剂PDA培养基,具体杀菌剂浓度如表1。在PDA平板中央接种1片菌饼,以不含杀菌剂的PDA平板作为对照,设置3次重复,然后置于25 ℃恒温培养箱中暗培养7 d,采用十字交叉法测量菌落直径,根据下列公式计算和统计相对抑菌率。使用SPSS 22.0软件拟合毒力回归方程,并计算EC50值。
式中:Dc为不含杀菌剂平板菌落直径;Dt为含杀菌剂平板菌落直径;菌饼直径8 mm。
式中:Y为抑制机率值;X为药剂浓度对数。
1.7 数据分析
利用SPSS 22. 0软件对数据进行统计分析,采用Origin pro 2024软件制图,通过Duncan多重比较法进行差异显著性检验(P<0.05)[20]。
2 结果与分析
2.1 叶斑病症状
田间症状:唐纳德海棠叶斑病多于7—10月发生,主要侵染叶片。发病初期病斑呈点状退绿斑,后逐渐扩大,呈近圆形或椭圆形,边缘呈淡黄绿色,中央呈棕褐色,后期呈灰白色,直径为0.6~1.2 cm(图1)。在湿度较高的条件下,病斑背面可以观察到黑色霉层。发病严重时,病斑可相互融合,导致叶片枯萎并提早脱落。

图1 唐纳德海棠叶斑病症状
Fig. 1 Symptoms of leaf spot of Malus ‘Donald Wyman’
2.2 病原菌的致病性
通过人工接种试验发现(图2),菌株SH-0019可侵染唐纳德海棠叶片,人工诱发症状(图2-B~C)与自然发病症状(图1-B)一致,病斑逐渐变为深褐色(图2-D~E)。从接种发病的叶片中重新分离病原物,再次获得与原接种菌一致的菌株。上述结果符合柯赫氏法则,证实A. alternata是引起唐纳德海棠叶斑病的病原菌,后续试验均以菌株SH-0019为代表性菌株。
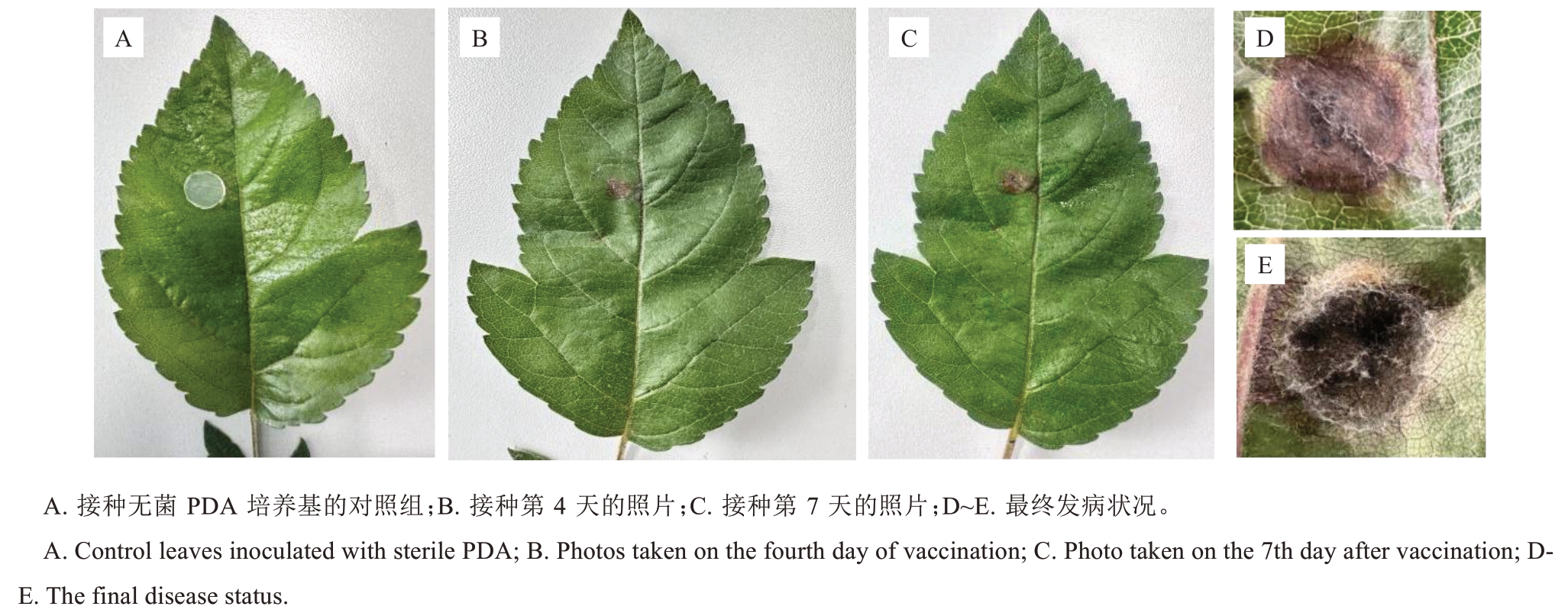
图2 唐纳德海棠人工接种病菌产生的症状观察
Fig. 2 Pathogenicity test results of Malus ‘Donald Wyman’
2.3 病原菌鉴定
2.3.1 形态特征 该病菌在PDA培养基上于25 ℃条件下生长较快,培养7 d后菌落直径可达8 cm。初期菌落呈白色,逐渐转为灰白色或灰褐色,絮状,背面深褐色或暗褐色(图3-A);菌丝无色,具隔膜;分生孢子梗发达,多弯曲,暗褐色,顶端具1~3个产孢痕;产孢方式为孔出式;分生孢子倒梨形、椭圆形或卵圆形,褐色,具3~7个横膈膜,0~4个纵斜隔膜,孢子大小(26.5~38.0)μm×(6.5~10.3) μm;顶端细胞较细并具喙,喙部大小(7.2~19.5)μm×(2.5~4.2)μm(图3-B)。根据菌落形态及分生孢子的特征,将菌株鉴定为链格孢(Alternaria alternata)。
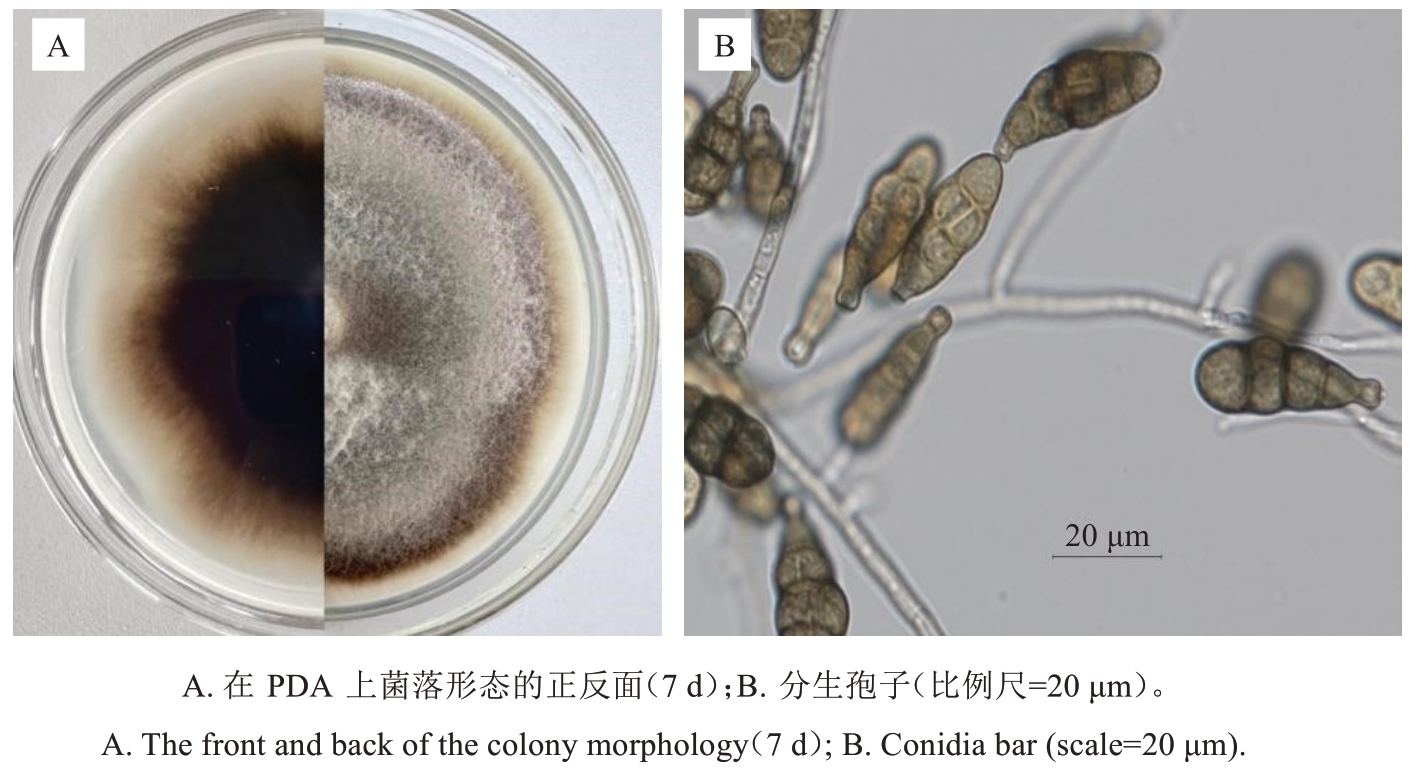
图3 Alternaria alternata的形态特征
Fig. 3 Morphological characteristics of Alternaria alternata
2.3.2 分子生物学鉴定 通过对致病菌株SH0019的ITS、ACT及LSU基因片段序列进行测序,经鉴定长度分别为542 bp、218 bp及1291 bp,并在Gen-Bank中的登录号分别为PV523935(ITS)、PV768877(ACT)及PQ809778(SSU)。通过BLAST同源比对,发现菌株SH0019的rDNA ITS、ACT及SSU序列与A. alternata TJU_JUL50(OM236783;535/536 bp)、A. alternata(OK664978;207/209 bp及A. alternata CRB-3(OM630609.;1291/1291 bp)序列的一致性分别为99.81%、99.04%及100%。选取与目标菌株序列相似度较高的模式菌株及已报道的菌株作为参考序列(表2),使用RAXML并采用GTR+的核苷酸替代模型构建多基因串联系统发育树,自举检验(bootstrapping)重复1000次获得各分支支持率。菌株SH0019与GenBank中A. alternata聚在一支,结合上述形态学特征与分子生物学特征综合评估,将病原菌SH0019鉴定为链格孢菌A. alternata(图4)。
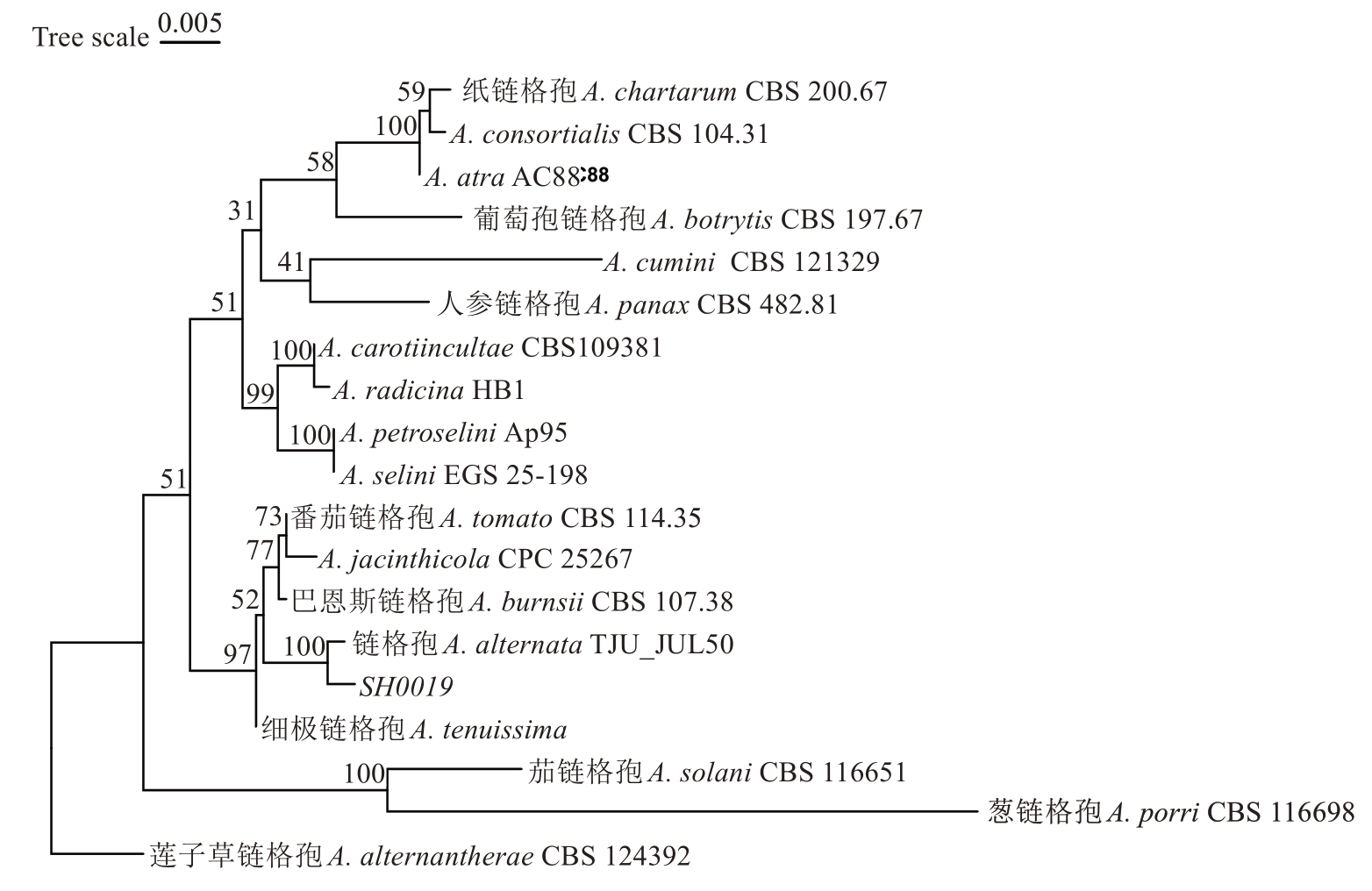
图4 基于分离菌SH0019的ITS、ACT和LSU序列使用ML法并采用GTR+的核苷酸替代模型构建多基因串联系统发育树
Fig. 4 Phylogenetic tree constructed based on multi-gene ITS-ACT-LSU sequences using Maximum Likelihood method and GTR+ nucleotide substitution model of isolate SH0019
表2 构建系统发育树所用菌株的GenBank登录号
Table 2 The GenBank accession numbers of the strains used for the construction of phylogenetic tree
菌株Species莲子草链格孢Alternaria alternantherae链格孢A. alternata巴恩斯链格孢A. burnsii A. jacinthicola番茄链格孢A. tomato茄链格孢A. solani A. carotiincultae葡萄孢链格孢A. botrytis纸链格孢A. chartarum A. consortialis A. atra A. radicina细极链格孢A. tenuissima A. petroselini A. cumini人参链格孢A. panax葱链格孢A. porri A. selini登录号Accession numbers CBS 124392 TJU_JUL50 CBS 107.38 CPC 25267 CBS 114.35 CBS 116651 CBS 109381 CBS 197.67 CBS 200.67 CBS 104.31 AC88 HB1—Ap95 CBS 121329 CBS 482.81 CBS 116698 EGS 25-198 ITS KC584179.1 OM236783.1 KP124420.1 KP124439.1 KP124446.1 KC584217.1 KC584188.1 KC584243.1 AY625071.1 KC584247.1 LC440623.1 FJ958190.1 HQ402558.1 EU807868.1 KC584191.1 KC584209.1 JF422724.1 AF229455.1 ACT OR797155.1 OK664978.1 JQ671685.1 HQ413699.1 JQ671686.1 MN813551.1 EU141969.1 LC481887.1 LC481879.1 PQ468098.1 LC481885.1 EU141971.1 ON996402.1 AB744037.1 LC481873.1 MK451990.1 JQ671726.1 JQ671676.1 SSU KC584506.1 OM630609.1 KP125043.1 KP125063.1 KP125070.1 KC584562.1 KC584518.1 KC584609.1 KC584614.1 KC584615.1 KC584608.1 KC584555.1 KC584567.1 KM102529.1 KC584523.1 KC584549.1 KC584553.1 AF229515.1
2.4 不同海棠品种致病性测定
7种供试海棠的人工接种试验结果表明,菌株SH-0019能够侵染火焰海棠、印第安魔幻海棠、绚丽海棠、垂丝海棠及西府海棠,且引发的叶斑症状与在唐纳德海棠上观察的症状一致,而对山荆子和毛荆子无侵染能力(图5)。尽管菌株SH-0019对以上5个海棠品种均具有致病性,但其致病性程度存在差异,其中垂丝海棠叶片产生的病斑扩展直径最小,平均直径为4.3 mm,表明不同海棠品种对该病原菌的抗性存在明显差异。

图5 不同海棠品种人工接种发病情况
Fig. 5 Determination of host range of strains SH0019
2.5 杀菌剂筛选
11种供试杀菌剂的抑菌试验结果(表3)表明,各药剂对病原菌的抑菌效果存在明显差异。毒力回归方程分析显示,50%苯甲·丙环唑抑菌效果最好,EC50值为0.179 μg·mL-1;其次为50%异菌脲和45%咪鲜胺,EC50值分别为1.735 μg·mL-1、5.495 μg·mL-1;而80%代森锰锌对病菌的抑菌效果最弱,EC50值高达222.923 μg·mL-1。
表3 11种杀菌剂对菌株SH0019的毒力测定
Table 3 Toxicity of 11 fungicides fungicides to strain SH0019 (EC50)
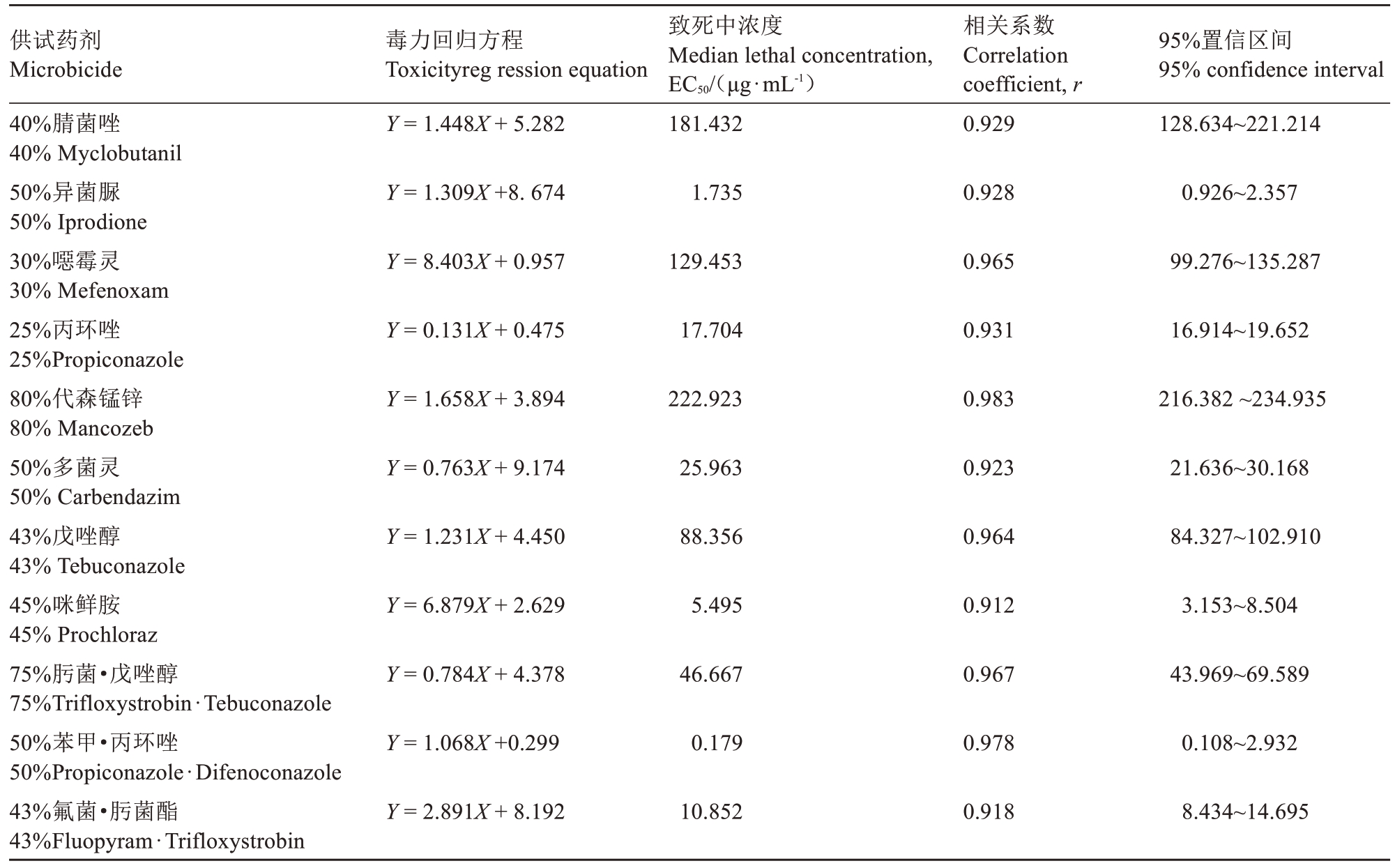
供试药剂Microbicide 40%腈菌唑40% Myclobutanil 50%异菌脲50% Iprodione 30%噁霉灵30% Mefenoxam 25%丙环唑25%Propiconazole 80%代森锰锌80% Mancozeb 50%多菌灵50% Carbendazim 43%戊唑醇43% Tebuconazole 45%咪鲜胺45% Prochloraz 75%肟菌·戊唑醇75%Trifloxystrobin·Tebuconazole 50%苯甲·丙环唑50%Propiconazole·Difenoconazole 43%氟菌·肟菌酯43%Fluopyram·Trifloxystrobin毒力回归方程Toxicityreg ression equation Y = 1.448X + 5.282致死中浓度Median lethal concentration,EC50/(μg·mL-1)181.432相关系数Correlation coefficient, r 0.929 95%置信区间95% confidence interval 128.634~221.214 Y = 1.309X +8. 6741.7350.9280.926~2.357 Y = 8.403X + 0.957129.4530.96599.276~135.287 Y = 0.131X + 0.47517.7040.93116.914~19.652 Y = 1.658X + 3.894222.9230.983216.382 ~234.935 Y = 0.763X + 9.17425.9630.92321.636~30.168 Y = 1.231X + 4.45088.3560.96484.327~102.910 Y = 6.879X + 2.6295.4950.9123.153~8.504 Y = 0.784X + 4.37846.6670.96743.969~69.589 Y = 1.068X +0.2990.1790.9780.108~2.932 Y = 2.891X + 8.19210.8520.9188.434~14.695
3 讨 论
本研究通过形态学、多基因系统发育分析及致病性测定,明确引起大连地区唐纳德海棠叶斑病的病原菌为链格孢(A. alternata)。离体接种试验表明,该病原菌可侵染多个北美海棠品种,但对山荆子和毛山荆子无侵染能力。链格孢(A. alternata)寄主范围广泛,可侵染多种作物、药用及观赏植物,造成严重经济损失[21-22]。在之前的报道中,该病菌可侵染长柄水青冈[23]、草坪[24]、龙牙百合[25]、库尔勒香梨[26]、柑橘[27]、鸢尾[28]、猕猴桃[29]、芍药[30]等多种类型寄主植物,引起黑腐病、褐斑病、软腐病、轮纹病、果枯病等病害。本研究也证实其可危害多个北美海棠品种,进一步表明其寄主适应范围较为广泛,跨寄主传播风险值得警惕。在本研究中,A. alternata对山荆子和毛荆子无明显侵染迹象,推测可能与砧木野生种的抗病遗传背景有关。罗红丽[31]曾指出,不同A. alternata菌株因产毒能力与致病因子存在差异,可表现出较明显的寄主专化性。这一发现为利用抗性砧木选育抗病海棠品种提供了依据,这与其他植物-病原互作过程中基于遗传抗性的防控策略相吻合[32]。此外,本研究还发现不同海棠品种对叶斑病的感病性存在明显差异,其中垂丝海棠病斑扩展范围最小,表明栽培品种间存在抗性遗传多样性[33]。本研究结果表明山荆子和毛荆子可作为抗病砧木材料,用于选育兼具优良观赏性和抗病性的海棠新品种,从而降低对化学杀菌剂的使用依赖程度,促进海棠病害的绿色防控。
化学药剂筛选结果表明,50%苯甲·丙环唑、50%异菌脲和45%咪鲜胺对海棠叶斑病菌株SH0019表现出较强的抑制活性,具有良好的应用潜力。李有德等[34]在苹果链格孢病害防治以及苏秀敏等[35]在番茄早疫病防治中均发现苯醚甲环唑类药剂对链格孢菌具有明显的抑菌效果,进一步支持了本研究中对苯甲·丙环唑抑菌效果的认定。此外,王媛媛等[36]和马伟丽等[37]均报道了异菌脲和咪鲜胺对玉米链格孢叶斑病具有较高毒力,表明这两种药剂对不同作物上的链格孢病害防治具有一定广谱性。综上所述,本研究筛选出的3种化学药剂对唐纳德海棠叶斑病的防治效果与已有研究相互印证,为北美海棠叶斑病的科学防控提供了可靠的药剂选择与理论依据。
尽管本研究明确了唐纳德海棠叶斑病病原种类并筛选出了有效药剂,但仍存在一定局限性。所有药剂试验均在室内条件下完成,尚未开展田间试验验证,其实际防效及潜在环境影响仍需进一步评估。未来将结合转录组学等分子手段进一步明确该病原菌的侵染机制,以期揭示致病与抗病机制,推动本研究成果向实际应用的转化。
4 结 论
本研究明确了大连地区唐纳德海棠叶斑病的病原菌为Alternaria alternata。室内杀菌剂筛选结果表明,50%苯甲·丙环唑对病原菌的抑菌效果最佳,具有良好的应用潜力。本研究为该病害的准确诊断和化学防治提供了科学理论依据,所筛选的高效杀菌剂为田间防治实践提供了候选药剂,对保障唐纳德海棠等部分北美海棠品种的观赏价值与生态效益具有重要意义。
[1] 赵攀,邓涛,丁伟,刘斌. 北美海棠的品种特性及在园林中的应用[J] . 南方农业,2019,13(29):52-53.ZHAO Pan,DENG Tao,DING Wei,LIU Bin. Varieties and characteristics of North American crabapple and their application in landscape gardening[J] . South China Agriculture,2019,13(29):52-53.
[2] 许剑峰,张往祥,朱玲玲,江皓,孙甜甜,郁万文. 78个北美海棠品种果实表型多样性分析[J] . 南京林业大学学报(自然科学版),2025,49(5):165-173.XU Jianfeng,ZHANG Wangxiang,ZHU Lingling,JIANG Hao,SUN Tiantian,YU Wanwen. Phenotypic diversity analysis of fruit traits of 78 North American crabapple cultivars[J] . Journal of Nanjing Forestry University (Natural Sciences Edition),2025,49(5):165-173.
[3] HÖFER M,MOHAMED M A,ALI S E,SELLMANN J,PEIL A. Phenotypic evaluation and characterization of a collection of Malus species[J] . Genetic Resources and Crop Evolution,2014,61(5):943-964.
[4] 毕玉科,张杰,张春英. 上海地区北美海棠冬季观果品种筛选[J] . 浙江农业科学,2023,64(11):2714-2718.BI Yuke,ZHANG Jie,ZHANG Chunying. Variety screening of winter fruit-ornamental effect of Northern American Begonia in Shanghai[J] . Journal of Zhejiang Agricultural Sciences,2023,64(11):2714-2718.
[5] 刘胜男,彭洁,王瑞博,王红卫,李永华,张开明. 30种北美海棠表型多样性分析与观赏性综合评价[J/OL] . 分子植物育种,1-11(2022-01-27). https://kns.cnki.net/kcms/detail/46.1068.S.20220126.1452.030.html.LIU Shengnan,PENG Jie,WANG Ruibo,WANG Hongwei,LI Yonghua,ZHANG Kaiming. Phenotypic diversity analysis and comprehensive appreciation evaluation of 30 North American crabapples[J/OL] . Molecular Plant Breeding,1-11(2022-01-27).https://kns.cnki.net/kcms/detail/46.1068.S.20220126.1452.030.html.
[6] 楚爱香,汤庚国. 观赏海棠品种分类研究进展[J] . 生物学通报,2008,43(7):15-17.CHU Aixiang,TANG Gengguo. Research progress on classification of crabapple varieties[J] . Bulletin of Biology,2008,43(7):15-17.
[7] EMANUEL I B,RALSTON T I,CHATFIELD J,DRAPER E,VEIL J,HAND F P. First report of Gymnosporangium yamadae causing Japanese apple rust on crabapple (Malus spp.) in Ohio[J] .Plant Disease,2021,105(7):2016.
[8] SIKDAR P,MAZZOLA M,XIAO C L. Genetic and pathogenic characterization of Phacidiopycnis washingtonensis from apple and Pacific madrone from the western United States[J] . Phytopathology,2019,109(3):469-479.
[9] BASTAS K K,OZTURK A Y. First report of fire blight caused by Erwinia amylovora on crabapple (Malus floribunda) in Turkey[J] . Plant Disease,2013,97(9):1244.
[10] 沈海平. 亚斯特海棠腐烂病与修剪和日灼的关联性及其预防技术研究[D] . 沈阳:沈阳农业大学,2024.SHEN Haiping. Studies on the relationship between pruning and sunburn and Valsa canker of Malus ‘Ester’ and its prevention technology[D] . Shenyang:Shenyang Agricultural University,2024.
[11] 王璐. 千年秀林海棠锈病发生研究及防治药剂筛选[D] . 保定:河北农业大学,2022.WANG Lu. Occurrence regularity and control of Malus rust in Millennium Forest Project[D] . Baoding:Hebei Agricultural University,2022.
[12] 杜辰阳,沈凤英,吴伟刚. 八棱海棠常见病害发生规律及防控研究进展[J] . 现代园艺,2024,47(9):110-113.DU Chenyang,SHEN Fengying,WU Weigang. Research progress on occurrence patterns and control of common diseases in octagonal crabapple[J] . Contemporary Horticulture,2024,47(9):110-113.
[13] 郭慧玲,张兆霞,刘会香,张兴忠,张以坤,李健,贾思民. 湖北海棠枝干溃疡病的发病规律及对策[J] . 山东林业科技,2019,49(1):49-51.GUO Huiling,ZHANG Zhaoxia,LIU Huixiang,ZHANG Xingzhong,ZHANG Yikun,LI Jian,JIA Simin. Occurrence regularity and countermeasure of the stem canker in Mount Tai Malus hupehensis Forest[J] . Journal of Shandong Forestry Science and Technology,2019,49(1):49-51.
[14] 方中达. 植病研究方法[M] . 3版. 北京:中国农业出版社,1998.FANG Zhongda. Methodology for plant pathology[M] . 3rd ed.Beijing:China Agriculture Press,1998.
[15] 张天宇. 中国真菌志-第十六卷-链格孢属[M] . 北京:科学出版社,2003.ZHANG Tianyu. Flora fungorum sinicorum-Vol. 16:Alte‐maia[M] . Beijing:Science Press,2003.
[16] 何弯弯,冯丽娜,李振云,张锴,张友青,温晓蕾,孙伟明. 引起花生果腐病的新孢镰刀菌及其生物学特性[J] . 植物病理学报,2022,52(3):493-498.HE Wanwan,FENG Lina,LI Zhenyun,ZHANG Kai,ZHANG Youqing,WEN Xiaolei,SUN Weiming. Fusarium neocosmos‐poriellum causing peanut pod rot and its biological characteristics[J] . Acta Phytopathologica Sinica,2022,52(3):493-498.
[17] 范真真,卢艳春,王文林,林春花. 广西崇左油梨叶部炭疽病病原菌分离与鉴定[J] . 植物病理学报,2023,53(3):527-530.FAN Zhenzhen,LU Yanchun,WANG Wenlin,LIN Chunhua.Identification of pathogen species of avocado leaf anthracnose in Chongzuo,Guangxi[J] . Acta Phytopathologica Sinica,2023,53(3):527-530.
[18] 刘芝妤,李增平,张宇,王晓宇. 一种龙眼灵芝茎腐病病原菌的分离与鉴定[J] . 植物病理学报,2023,53(2):330-334.LIU Zhiyu,LI Zengping,ZHANG Yu,WANG Xiaoyu. Identification of a Ganoderma stem rot pathogen infecting Dimocarpus longana[J] . Acta Phytopathologica Sinica,2023,53(2):330-334.
[19] 张凯东,强遥,刘冰,李邦明,赵尚高,蒋军喜. 猕猴桃叶斑病病菌生物学特性及室内药剂筛选[J] . 江苏农业科学,2021,49(18):106-110.ZHANG Kaidong,QIANG Yao,LIU Bing,LI Bangming,ZHAO Shanggao,JIANG Junxi. Biological characteristics of kiwi leaf spot pathogen and screening of indoor pesticides[J] . Jiangsu Agricultural Sciences,2021,49(18):106-110.
[20] 孙霞. 链格孢属真菌现代分类方法研究[D] . 泰安:山东农业大学,2006.SUN Xia. The methodological study on taxonomy of the genus Alternaria Nees[D] . Tai’an:Shandong Agricultural University,2006.
[21] 德馨. 现代海棠褐斑病防治[J] . 中国花卉园艺,2018(8):43.DE Xin. Control of brown spot disease in modern crabapple[J] .China Flowers & Horticulture,2018(8):43.
[22] 刘丰银. 禾本科植物链格孢菌多样性研究[D] . 荆州:长江大学,2024.LIU Fengyin. Study on diversity of Alternaria from Poaceae[D] .Jingzhou:Yangtze University,2024.
[23] 罗润,雷娇娇,雷斌,于存,韦小丽. 链格孢菌引起长柄水青冈叶斑病在中国的首次报道[J/OL] . 植物病理学报,2025:1-5.(2025-01-14). https://doi.org/10.13926/j.cnki.apps.001677.LUO Run,LEI Jiaojiao,LEI Bin,YU Cun,WEI Xiaoli. First report of leaf spot of Fagus longipetiolata caused by Alternaria al‐ternata in China[J/OL] . Acta Phytopathologica Sinica,2025:1-5. (2025-01-14). https://doi.org/10.13926/j.cnki.apps.001677.
[24] 赵灿,王忠磊,吴春艳,姚祎琳,欧阳娜,王克华,吴学宏. 引起草坪叶斑病的链格孢种类鉴定[J/OL] . 植物病理学报,2025:1-5. (2025-01-13). https://doi.org/10.13926/j.cnki.apps.000962.ZHAO Can,WANG Zhonglei,WU Chunyan,YAO Yilin,OUYANG Na,WANG Kehua,WU Xuehong. Species identification of Alternaria spp. causing leaf spot on turfgrass[J/OL] . Acta Phytopathologica Sinica,2025:1-5. (2025-01-13). https://doi.org/10.13926/j.cnki.apps.000962.
[25] 曾慧兰,黄翠翠,周义蒙,刘杰,李润根. 龙牙百合叶片褐斑病的病原鉴定[J] . 植物病理学报,2023,53(5):974-980.ZENG Huilan,HUANG Cuicui,ZHOU Yimeng,LIU Jie,LI Rungen. Identification of Alternaria gaisen associated with leaf brown spot on Lilium brownii var. viridulum[J] . Acta Phytopathologica Sinica,2023,53(5):974-980.
[26] 宋博,张丽娟,朱晓锋,徐兵强,艾米都拉·克尤木,阿布都克尤木·卡德尔,杨森. 香梨果萼黑斑病菌Alternaria alternata遗传转化体系的建立及GFP标记菌株的获得[J] . 植物病理学报,2022,52(1):97-103.SONG Bo,ZHANG Lijuan,ZHU Xiaofeng,XU Bingqiang,AIMIDULA Keyoumu,ABUDUKEYOUMU Kader,YANG Sen. Development of efficient genetic transformation system in Alternaria alternata and its application for strain labeled with GFP[J] . Acta Phytopathologica Sinica,2022,52(1):97-103.
[27] 张斌,梅秀凤,黄峰,王明爽,王洪凯,李红叶. 中国柑橘黑腐病和褐斑病病原菌的系统发育分析[J] . 植物病理学报,2020,50(1):10-19.ZHANG Bin,MEI Xiufeng,HUANG Feng,WANG Mingshuang,WANG Hongkai,LI Hongye. Phylogenetic analysis of Alternar‐ia spp. causing black rot and brown spot of citrus in China[J] .Acta Phytopathologica Sinica,2020,50(1):10-19.
[28] 朱桐,王宇曦,逯昕明,刘雪峰,王斌,刁桂萍. 抑制鸢尾果枯病生防细菌的筛选及条件优化[J] . 北方园艺,2024(23):66-72.ZHU Tong,WANG Yuxi,LU Xinming,LIU Xuefeng,WANG Bin,DIAO Guiping. Screening and optimization of conditions for the inhibition of iris sanguine fruit blight biocontrol bacteria[J] . Northern Horticulture,2024(23):66-72.
[29] 李雨静,庞敏,杜孝田,隋媛,陶玲,黄科. 猕猴桃软腐链格孢菌(Alternaria alternata)的鉴定与防治[J/OL] . 安徽农业科学,1-6(2024-10-29). https://link.cnki.net/urlid/34.1076.S.20241028.1626.030.LI Yujing,PANG Min,DU Xiaotian,SUI Yuan,TAO Ling,HUANG Ke. Identification and control of kiwifruit decay caused by Alternaria alternata[J/OL] . Journal of Anhui Agricultural Sciences,1-6 (2024-10-29). https://link.cnki.net/urlid/34.1076.S.20241028.1626.030.
[30] 谷清义,黄晨晨,武志鹏,张耀洲,黄雅琴,申君. 芍药黑斑病病原菌的分离鉴定、生物学特性及植物源药剂筛选[J] . 中药材,2024,47(10):2428-2433.GU Qingyi,HUANG Chenchen,WU Zhipeng,ZHANG Yaozhou,HUANG Yaqin,SHEN Jun. Identification,biological characteristics and botanical pesticide screening of the pathogen of black spot on Paeonia lactiflora[J] . Journal of Chinese Medicinal Materials,2024,47(10):2428-2433.
[31] 罗红丽. 烟草赤星病菌(Alternaria alternata)AT -毒素的提取与毒性的生物测定[D] . 郑州:河南农业大学,2000.LUO Hongli. Extraction and assay on the AT-toxin excreted by tobacco brown spot pathogen Alternaria alternata[D] . Zhengzhou:Henan Agricultural University,2000.
[32] 徐健容,叶华智. 小麦近缘种属对赤霉病菌的抗性评价[J] . 四川农业大学学报,1998,16(3):322-327.XU Jianrong,YE Huazhi. Evaluation of wheat relatives for resistance to head blight of wheat[J] . Chinese Jounal of Sichuan Agricultural University,1998,16(3):322-327.
[33] 贺占雪,马建鹏,杨斌,赵宁. 云南主栽核桃品种对Alternaria alternata叶枯病的抗病性评价[J] . 植物保护,2019,45(4):195-200.HE Zhanxue,MA Jianpeng,YANG Bin,ZHAO Ning. Evaluation of the resistance of main walnut varieties in Yunnan province to Alternaria alternata[J] . Plant Protection,2019,45(4):195-200.
[34] 李有德,陈万杰,任维超,王彩霞,李保华. 苹果上链格孢菌所致病害防治药剂筛选[J] . 现代农药,2023,22(6):52-58.LI Youde,CHEN Wanjie,REN Weichao,WANG Caixia,LI Baohua. Screening of fungicides for the control of diseases caused by Alternaria spp. on apple[J] . Modern Agrochemicals,2023,22(6):52-58.
[35] 苏秀敏,韩文清,王佼,李鹏,王秋兰,李万星,李丹,李小霞.长治市番茄早疫病病原菌鉴定及防治药剂筛选[J] . 核农学报,2024,38(9):1715-1723.SU Xiumin,HAN Wenqing,WANG Jiao,LI Peng,WANG Qiulan,LI Wanxing,LI Dan,LI Xiaoxia. Identification and fungicide selection of Alternaria solani causing tomato early blight in Changzhi[J] . Journal of Nuclear Agricultural Sciences,2024,38(9):1715-1723.
[36] 王媛媛,刘彬,周园园,朱晓峰,高增贵,陈立杰. 玉米链格孢病菌生物学特性及防治药剂离体活性筛选[J] . 沈阳农业大学学报,2015,46(5):538-542.WANG Yuanyuan,LIU Bin,ZHOU Yuanyuan,ZHU Xiaofeng,GAO Zenggui,CHEN Lijie. Biological characteristics and screening of fungicides of maize Alternaria tenuis Nees[J] . Journal of Shenyang Agricultural University,2015,46(5):538-542.
[37] 马伟丽,马桂花,常建萍,邹海涛,祁鹤兴. 青贮玉米链格孢叶斑病菌生物学特性及其室内防治药剂筛选[J] . 青海畜牧兽医杂志,2023,53(2):6-11.MA Weili,MA Guihua,CHANG Jianping,ZOU Haitao,QI Hexing. Biological characteristics and laboratory screening of chemical agents of the pathogens of silage corn Alternaria Spot[J] .Chinese Qinghai Journal of Animal and Veterinary Sciences,2023,53(2):6-11.