盐碱胁迫是影响植物生长和农业生产的重要环境因子之一,据联合国粮食及农业组织统计,全球盐渍土壤面积超过8.33亿hm2,占地球陆地面积的8.7%[1-2]。当今中国拥有约0.33亿hm2可利用的盐碱地资源,其中黄河三角洲盐碱地具有广阔的利用前景,但其生态环境脆弱、土壤盐分高、水文条件差等因素严重制约了该地区的农业生产和生态环境建设[3-4]。因此,对黄河三角洲地区盐碱地资源进行综合改良和利用,提高其农业生产能力,有助于推动当地农业可持续发展。
添加外源改良剂可以改善盐碱地土壤的理化性质,降低土壤pH和含盐量,促进盐碱地植物的生长[5]。利用耐碱嗜盐型灰绿曲霉菌微生物复配而成的水制剂,是一种生物改良剂,能够通过分泌有机酸、调节离子平衡、增强抗氧化酶活性等方式,有效缓解盐碱胁迫对植物的损伤[6]。目前,微生物菌剂对盐碱地作物促生情况的相关研究日渐增多。在苏打盐碱地增施微生物菌肥,提高了大豆幼苗的株高、根长、根瘤数、单株叶面积,增强了大豆幼苗对盐碱胁迫的耐受能力,缓解了盐碱胁迫给大豆幼苗带来的损害[7]。刘晶等[8]研究表明添加枯草芽孢杆菌菌肥有效减轻了格尔木地区的土壤盐碱化程度,促进了小黑麦幼苗的生长;叶静等[9]发现施用巨大芽孢杆菌与胶冻芽孢杆菌混合菌剂的玉米产量超出常规施肥的24.83%,说明微生物菌肥能够提升盐碱地条件下玉米的产量。
杜梨(Pyrus betulifolia Bunge)是蔷薇科梨属落叶乔木,具有耐盐碱、耐寒、耐旱等特性,是中国北方梨树栽培的主要砧木[10-11]。在盐碱地栽培梨树是充分利用盐碱地资源发展高效农业的有效途径之一。因此,探索盐碱地条件下杜梨砧木的生长发育规律具有重要现实意义。然而,关于杜梨耐盐碱性的研究多聚焦于盐碱胁迫对其生长的影响以及杜梨自身的适应机制,鲜有研究报道微生物菌剂等生物改良剂在盐碱胁迫条件下对杜梨幼苗的促生作用。笔者通过模拟黄河三角洲地区盐碱地土壤的主要盐碱成分组成,对杜梨幼苗进行混合盐碱胁迫处理,同时浇灌耐碱嗜盐型微生物水制剂,通过测定不同处理下杜梨的生理生化及叶绿素荧光参数,探究微生物水制剂对杜梨盐碱胁迫的缓解作用,以期为滨海盐碱地土壤改良及杜梨耐盐碱栽培提供一定的技术支撑。
1 材料和方法
1.1 材料
以取自山东省果树研究所天平湖试验基地的杜梨半同胞家系种子为试材,对种子进行催芽处理,待胚根露白即可播种。播种后放入光照培养箱中管理,并在系统条件下400 μmol·m-2·s -1光合光子通量密度(PPFD),温度25~28 ℃/23~25 ℃(昼/夜)和相对湿度60%~80%/50%~60%(昼/夜)进行培养。待植株长出6~8枚真叶后,选择长势一致的幼苗,移入内径7 cm、深度7 cm的营养钵中,每盆移栽1株。每盆基质(以蛭石、营养土体积比1∶1配制,经121 ℃高压灭菌2 h)为180 g。
1.2 试验设计
根据前人对黄河三角洲地区盐碱地土壤各盐碱成分组成的测定结果,山东省东营市盐碱地土壤盐分组成为70% NaCl、24.5% Na2SO4、4.6% NaHCO3[12]。按照此组分模拟黄河三角洲地区天然重度盐碱地条件,当地土壤主要养分含量如表1所示[13-14]。试验设置3个处理:清水对照(CK)、盐碱胁迫处理(SA,仅浇灌6‰盐碱溶液,pH=8.3)、耐碱嗜盐微生物水制剂+盐碱胁迫处理(HSA,浇灌200倍耐碱嗜盐微生物水制剂+6‰盐碱溶液混合液),每个处理15株幼苗。SA处理和HSA处理首次均浇灌3‰盐碱溶液(pH=7.8)50 mL·株-1,2 d之后浇灌等量的6‰盐碱溶液(pH=8.3),避免发生盐激反应。盐碱胁迫3 d后,SA处理继续浇灌6‰盐碱溶液100 mL·株-1,HSA处理同步浇灌等量的200倍微生物水制剂+6‰盐碱溶液的混合液,对照组浇灌等量清水,各处理的施用间隔时间为5 d。浇灌时注意要确保相应溶液完全渗透基质,并将渗透到托盘中的溶液重新倒回各营养钵中。处理4周后取样,样品一部分进行烘干处理,用于生物量的测定;另一部分保留鲜样,用于叶绿素含量及抗氧化酶活性的测定。耐碱嗜盐微生物水制剂购自沈阳九利肥业股份有限公司。
表1 黄河三角洲地区重度盐碱地土壤主要养分含量[13-14]
Table 1 Main nutrient content in the soil of heavily saline-alkaline soil in the Yellow River Delta[13-14]
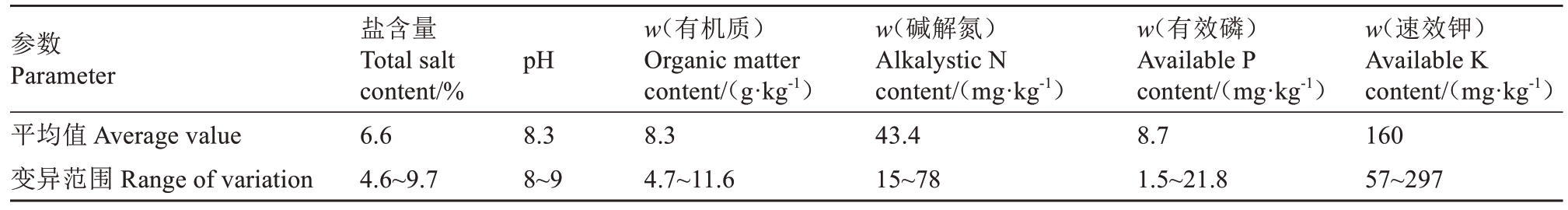
参数Parameter平均值 Average value变异范围 Range of variation盐含量Total salt content/%6.6 4.6~9.7 pH 8.3 8~9 w(有机质)Organic matter content/(g·kg-1)8.3 4.7~11.6 w(碱解氮)Alkalystic N content/(mg·kg-1)43.4 15~78 w(有效磷)Available P content/(mg·kg-1)8.7 1.5~21.8 w(速效钾)Available K content/(mg·kg-1)160 57~297
1.3 测定方法
1.3.1 生物指标的测定 分别采集杜梨幼苗的叶片和根,于105 ℃烘箱中杀青30 min,随后在80 ℃下烘干至恒质量,使用电子天平称量幼苗的叶片干质量和根干质量。在试验开始和结束时测量幼苗叶片数量,分别记作L0、L1,并按以下公式计算叶片绝对生长量(△L)。
1.3.2 叶绿素含量的测定 叶绿素相对含量使用SPAD叶绿素仪测定,结果以SPAD值表示。叶绿素含量的测定采用修改的乙醇浸提法[15]。称取叶片0.2 g,加20 mL 95%的乙醇,封口,暗处理24~36 h,至叶片完全脱色发白。利用紫外分光光度计(日立U-3900),以95%乙醇为空白对照,分别于波长665 nm、649 nm、470 nm下测定吸光度,计算叶绿素a、叶绿素b和总叶绿素含量。
1.3.3 叶绿素荧光参数的测定 采用Pocket PEA便携式植物效率分析仪(Hansatech,UK)测定叶绿素荧光参数。将待测叶片在黑暗中静置30 min,使叶片达到暗适应状态,经饱和红光(3000 μmol·m-2·s-1)激发后,记录1 s内叶片快速叶绿素荧光诱导动力学(OJIP)曲线,获取PSⅡ最大光化学效率(Fv/Fm)、光合性能指数(performance index on absorption basis,PIABS)和潜在光化学活性(Fv/Fo)。JIP-test参数的计算参考李鹏民等[16]和van Heerden等[17]的方法,相关参数及其具体含义见表2。参照苏晓琼等[18]的方法计算相对可变荧光(△Vt)。
表2 JIP测定分析所用的参数和公式
Table 2 Parameters and formulae used in JIP-test analysis

参数和公式Parameters and formulas Fo Fm FJ FI PIABS Ft Fv/Fm=(Fm-Fo)/Fm Fv/Fo=(Fm-Fo)/Fo Vt=(Ft-Fo)/(Fm-Fo)参数含义Explanation of the parameters暗适应后的最小荧光强度Minimum fluorescence intensity after dark adaptation暗适应后的最大荧光强度Maximum fluorescence intensity after dark adaptation J点处(2 ms)的荧光强度Fluorescence intensity at point J (2 ms)I点处(30 ms)的荧光强度Fluorescence intensity at point I (30 ms)以吸收光能为基础的性能指数Performance index on absorption basis任意时间点荧光强度Fluorescence intensity at any time point最大光化学效率Maximum photochemical efficiency潜在光化学活性Potential photochemical activity任意时间点相对可变荧光强度Relative variable fluorescence intensity at any time point
1.3.4 抗氧化酶活性的测定 采集幼苗新鲜叶片(避开主叶脉)或根系0.5 g,加入1 mL磷酸缓冲液(0.05 mol·L-1,pH=7.8),冰浴研磨。研磨后加入1 mL缓冲液,倒入离心管中,再加入2 mL缓冲液清洗研钵,倒入离心管中,确保完全转移。4 ℃下静置10 min后,在10 500 r·min-1条件下低温离心20 min,取上清液冷藏保存。超氧化物歧化酶(superoxide dismutase,SOD)活性采用氮蓝四唑光化还原法[19]测定。过氧化物酶(peroxidase,POD)活性采用愈创木酚比色法[20]测定。过氧化氢酶(catalase,CAT)活性采用李小方等[21]的比色法测定。
1.4 数据处理
采用 Microsoft Excel 2016软件处理数据,使用GraphPad Prism 10软件制作图表。运用SPSS26.0数据处理系统软件,采用单因素方差分析(One-way ANOVA)和Duncan多重比较法(差异显著性P<0.05)对数据进行统计分析。
2 结果与分析
2.1 微生物水制剂对盐碱胁迫下杜梨幼苗生长的影响
图1为不同处理下杜梨幼苗的生长状况。对照组杜梨幼苗的地上、地下部分生长状况良好(图1-A);SA处理的幼苗叶片自尖端开始出现卷曲、褪绿、焦枯症状,根量少、颜色呈现褐色(图1-B);HSA处理的幼苗叶片出现轻度卷曲,根量多、发褐程度低(图1-C)。综上,对照组杜梨幼苗生长状态最好,其次是HSA处理,SA处理最差。

图1 微生物水制剂对盐碱胁迫下杜梨生长状况的影响Fig. 1 Effects of microbial liquid reagent on the growth of Pyrus betulaefolia Bunge seedlings under saline-alkali stress
如图2所示,与对照相比,SA处理下的叶片绝对生长量、叶干质量、根干质量分别下降了77.27%、12.57%、26.71%;而HSA处理分别下降了70.45%、6.29%、21.12%,下降幅度均低于SA处理;较SA处理分别提高了30%、7.2%和7.63%。
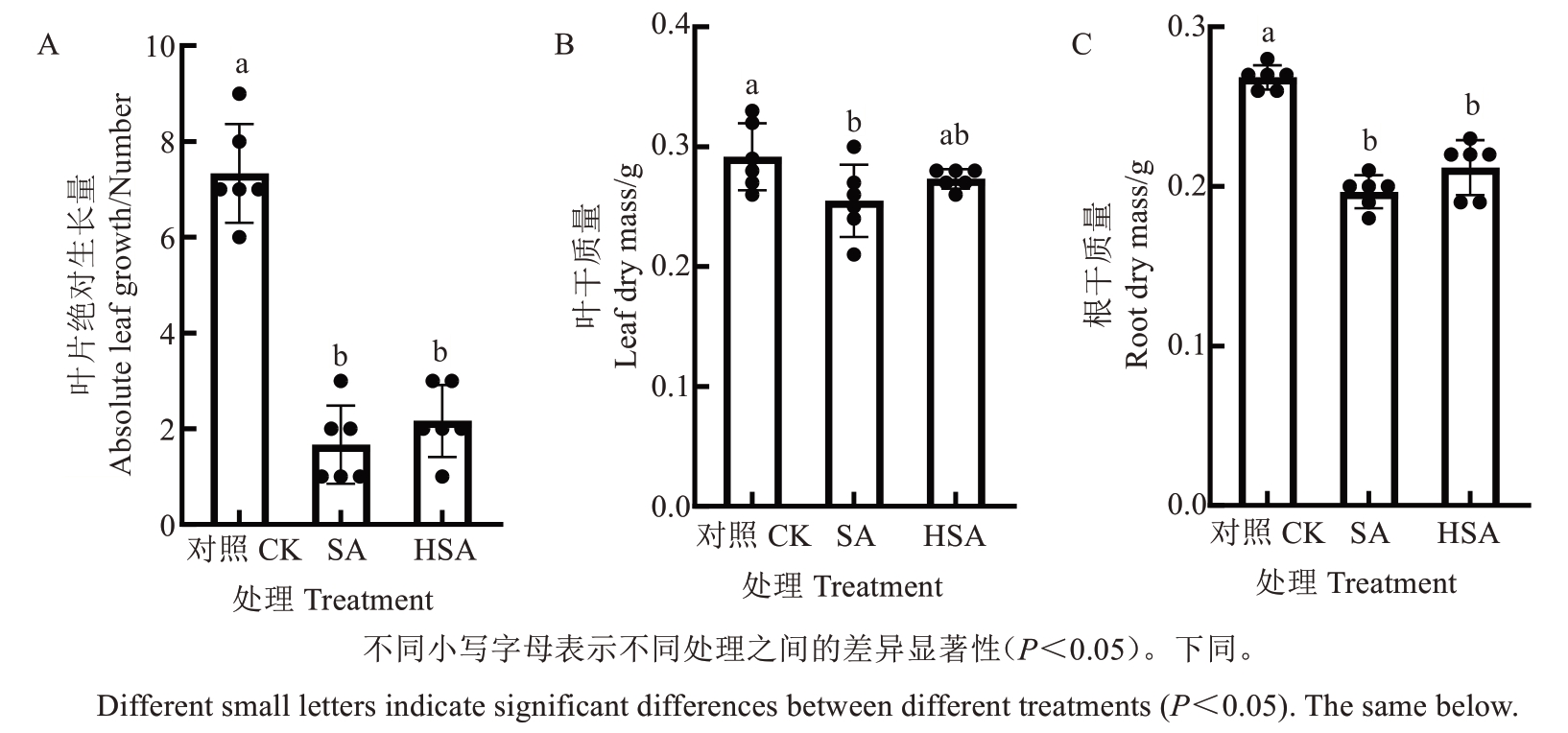
图2 微生物水制剂对盐碱胁迫下杜梨叶片绝对生长量、叶干质量和根系干质量的影响Fig. 2 Effects of microbial liquid reagent on leaf increment, leaf and root dry mass of Pyrus betulaefolia Bunge seedlings under saline-alkali stress
2.2 微生物水制剂对盐碱胁迫下杜梨幼苗叶片叶绿素指标的影响
2.2.1 叶绿素含量 图3是盐碱胁迫下杜梨幼苗施加微生物水制剂后叶绿素含量的变化情况。相较于对照,SA和HSA处理下杜梨幼苗叶片的叶绿素含量显著降低。与SA处理相比,HSA处理的SPAD值显著提升,升高了23.42%(图3-A)。与对照相比,SA和HSA处理的叶绿素a、叶绿素b和总叶绿素含量均呈现降低趋势,且差异显著。与SA处理相比,HAS处理的叶绿素a、叶绿素b和总叶绿素含量分别升高了23.21%、100.35%、44.58%(图3-B~D),表明施加微生物水制剂后缓解了盐碱胁迫条件下杜梨幼苗叶片的叶绿素降解。
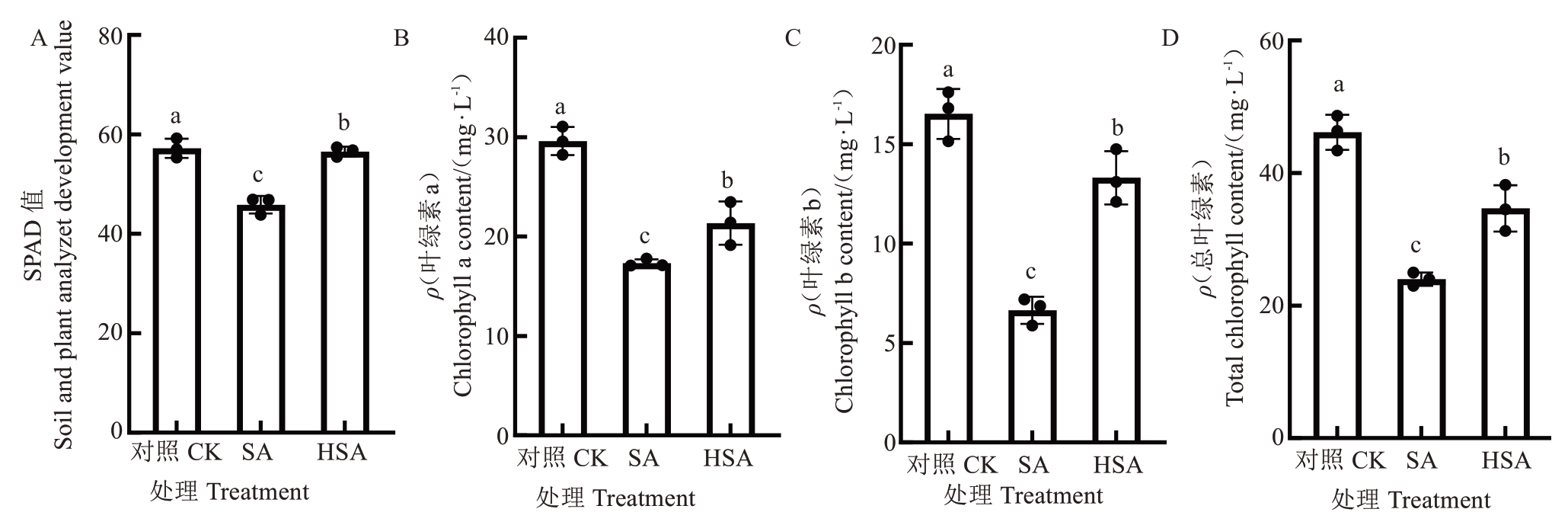
图3 微生物液体调节剂对盐碱胁迫下杜梨叶片叶绿素含量的影响
Fig. 3 Effects of microbial liquid reagent on chlorophyll content in leaves of Pyrus betulaefolia Bunge seedlings under saline-alkali stress
2.2.2 叶绿素荧光参数 如图4可知,与对照相比,盐碱胁迫下(SA处理)杜梨幼苗叶片的叶绿素荧光参数Fv/Fm、Fv/Fo和PIABS值均显著降低,分别下降了14.41%、39.02%、76.63%。而施加微生物水制剂后,杜梨幼苗叶片的Fv/Fm、Fv/Fo和PIABS值较SA处理均有所回升,其中Fv/Fm和Fv/Fo达到显著差异水平,分别升高了2.34%和12.43%。
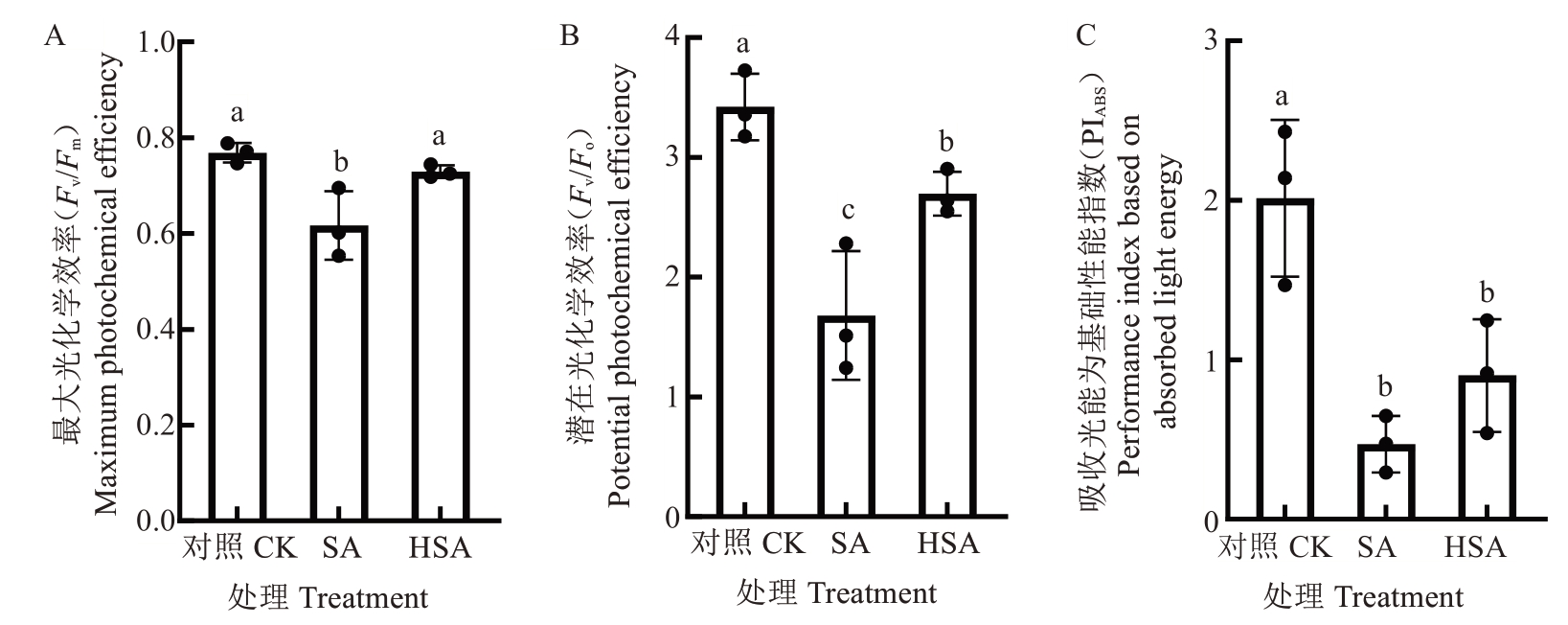
图4 微生物水制剂对盐碱胁迫下杜梨叶片荧光参数的影响
Fig. 4 Effects of microbial liquid reagent on leaf chlorophyll fluorescence parameters of Pyrus betulaefolia Bunge seedlings under saline-alkali stress
2.2.3 叶绿素荧光诱导动力学曲线 快速叶绿素荧光诱导动力学曲线(OJIP)通过监测PSⅡ光化学反应过程,可精确解析其供体侧、受体侧及反应中心电子变化情况。如图5-A所示,盐碱处理使OJIP曲线变形为OKJIP,即在O相(初始荧光)至J相(QA-第1次最大积累点)之间出现显著的K点。与对照相比,SA处理显著提高了K相和J相的荧光强度,分别升高了106.19%和66.27%;而施加微生物水制剂后,相较于SA处理,HSA处理下K相和J相的荧光强度显著降低,分别降低了42.43%、24.18%(图5-A,C)。进一步对OJIP曲线进行标准化处理后(图5-B),与对照相比,SA与HSA处理的ΔVK、ΔVJ升高,而HSA处理显著低于SA处理(图5-D)。以上结果说明HSA处理明显减缓了盐碱胁迫引起的叶绿素荧光值的变化。
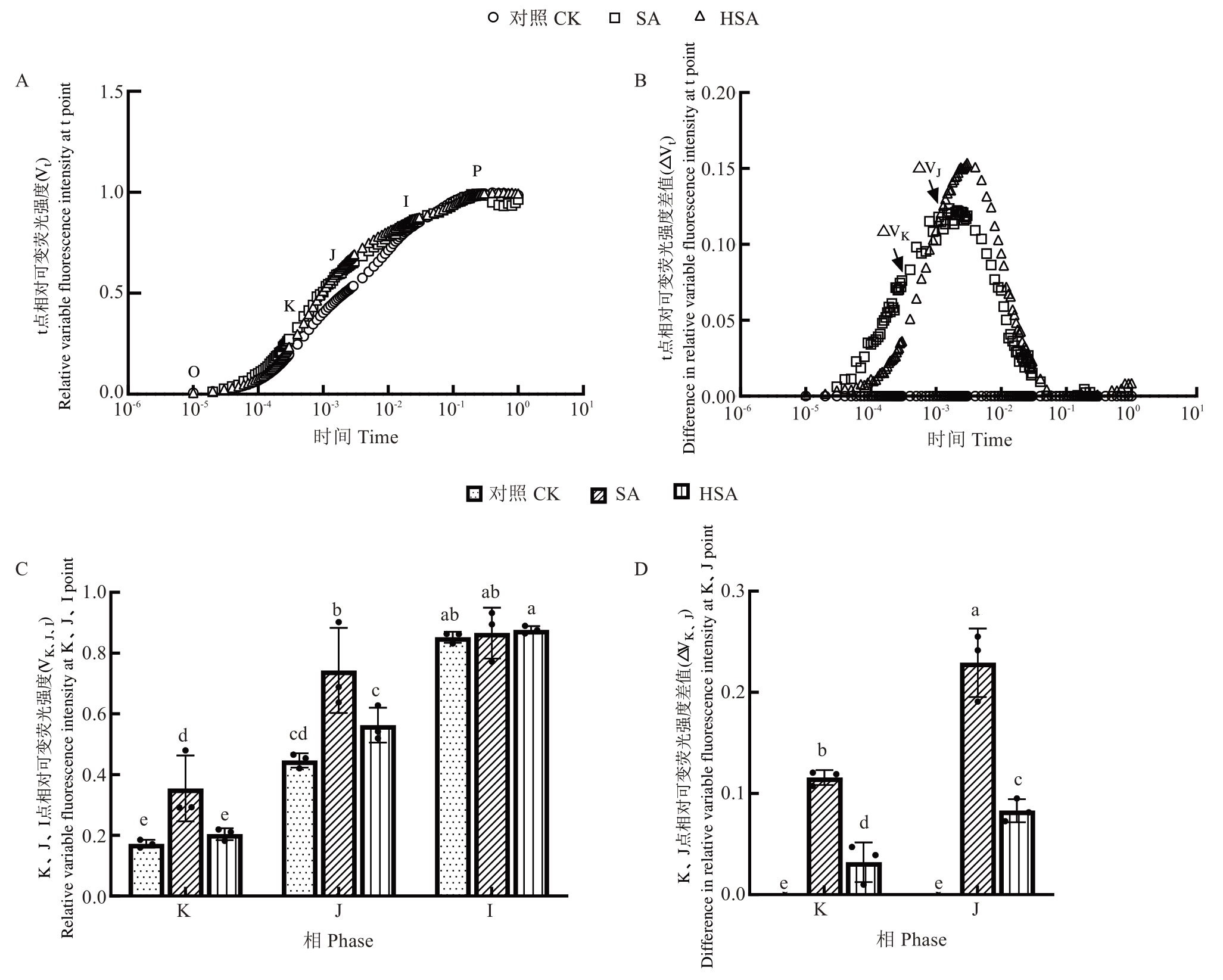
图5 微生物水制剂对盐碱胁迫下杜梨叶片OJIP曲线(A、C)和ΔVt(B、D)的影响
Fig. 5 Effects of microbial liquid reagent on OJIP curve (A, C) and ΔVt (B, D) of Pyrus betulaefolia Bunge seedlings leaves under saline-alkali stress
2.3 微生物水制剂对盐碱胁迫下杜梨幼苗抗氧化酶活性的影响
植物抗氧化酶系统在植物生长发育和抵御逆境胁迫过程中发挥着重要作用。如图6所示,盐碱胁迫下杜梨叶片和根系抗氧化酶(SOD、POD、CAT)活性发生了改变。与对照相比,盐碱胁迫(SA处理)显著提高了杜梨幼苗叶片的SOD活性,升高了29.50%;而降低了POD和CAT活性,其中CAT活性显著下降,降低了59.67%。施用微生物水制剂后(HAS处理),叶片中SOD和POD活性增强:SOD活性较对照和SA处理分别提高59.53%、23.19%;POD活性分别升高了15.05%、78.33%;而CAT活性则进一步降低。
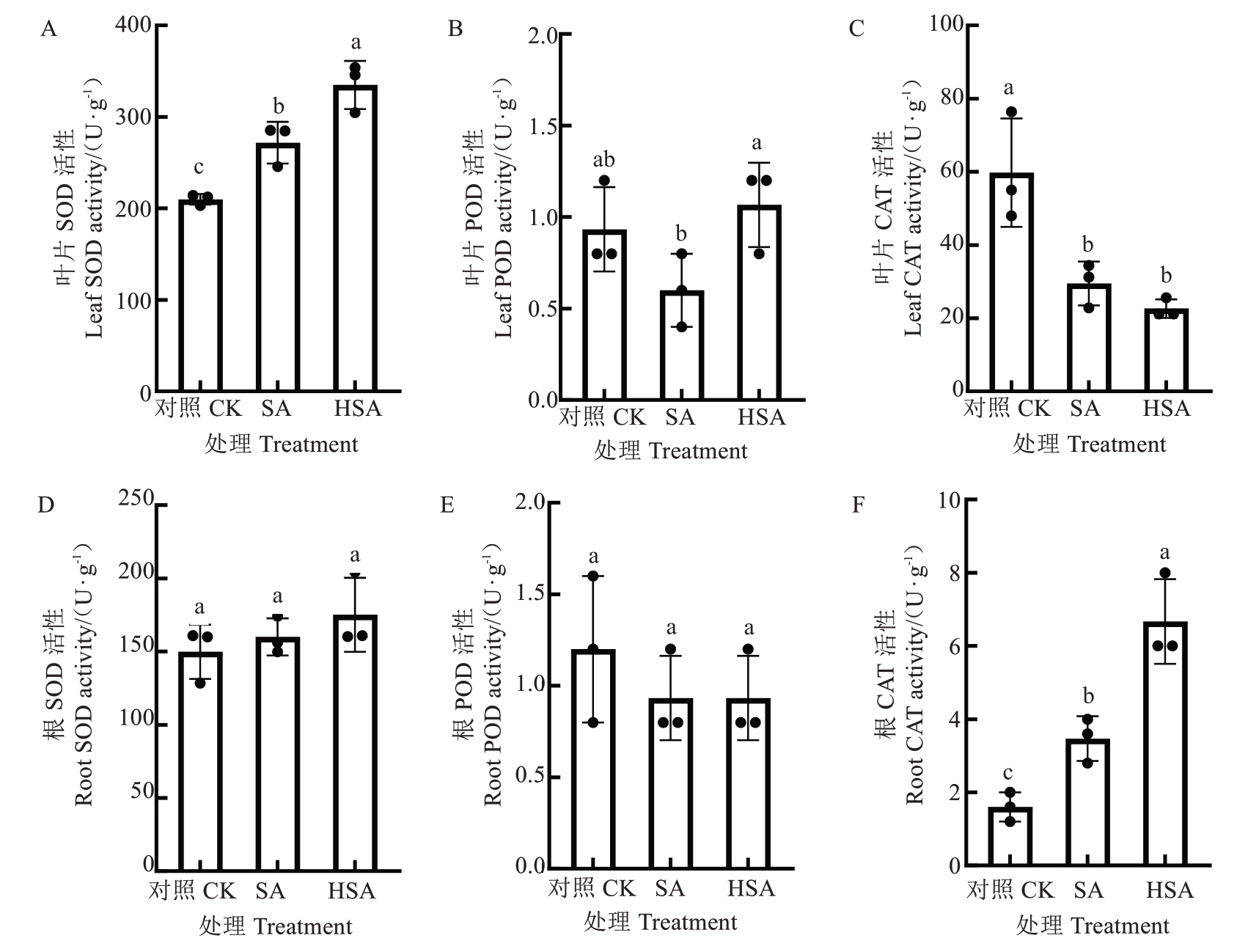
图6 微生物水制剂对盐碱胁迫下杜梨叶片和根系抗氧化酶活性的影响
Fig. 6 Effects of microbial liquid regulator on antioxidant enzyme activities in leaves and roots of Pyrus betulaefolia Bunge seedlings under saline-alkali stress
与对照相比,SA和HAS处理的杜梨幼苗根系SOD和CAT活性均增高,其中CAT活性均达到显著差异水平,分别升高了116.67%、337.50%(图6-D和6-F);而根中POD活性呈降低趋势,但无显著差异(图6-E)。盐碱胁迫条件下,施用微生物水制剂提高了根系SOD和CAT活性,其中CAT活性达到显著差异水平。
3 讨 论
盐碱胁迫会对植物产生离子毒害、渗透胁迫、营养失衡等负面影响,导致生物量积累减少,进而抑制植物生长发育,严重时导致植株死亡[22-23]。在混合盐碱胁迫处理下,甜菜幼苗的干质量和鲜质量均受到显著抑制[24]。朱晨晨等[25]研究表明盐碱胁迫导致中苜一号紫花苜蓿幼苗的株高和干质量均呈现不同程度的降低。霍宏亮等[26]在杜梨对盐碱胁迫的生理响应研究中发现,盐碱胁迫抑制了杜梨幼苗的生长,导致叶片枯萎、扭曲,直至死亡。本研究结果表明,盐碱胁迫导致杜梨幼苗的干质量和叶片绝对生长量明显减少,叶片褪绿焦枯,根系褐化,与前人研究结果一致。施用耐碱嗜盐微生物水制剂后,杜梨幼苗的生长状况得到改善,叶片干质量、根干质量和叶片绝对生长量分别较盐碱胁迫处理升高了7.2%、7.63%和30%,表明微生物水制剂在一定程度上促进了盐碱胁迫条件下杜梨幼苗的生长,这与施用微生物菌剂对盐碱地向日葵[27]和小黑麦[8]促生效果的结论一致。
光合作用是对盐碱胁迫最为敏感的生理过程之一,盐碱胁迫通过影响叶片生长及光合作用来抑制植物的光合能力[28]。叶绿素是植物叶片进行光合作用的关键色素,其含量变化直接反映光合能力。Sun等[29]研究发现随着盐碱胁迫程度的加剧,紫斑牡丹叶片呈现明显的卷曲失绿,总叶绿素含量明显下降。笔者的试验结果表明,盐碱胁迫显著降低了杜梨幼苗叶片的SPAD值和叶绿素a、叶绿素b和总叶绿素含量,导致叶片褪绿焦枯;而增施微生物水制剂后则明显提高了这些参数,有效缓解了叶绿素降解,提升了盐碱胁迫下杜梨幼苗叶片的光合能力。这与王国丽等[27]、李星志等[30]和刘晶等[8]的研究结果一致。
叶绿素荧光参数Fv/Fm、Fv/Fo和PIABS能够反映光抑制程度和光合机构的整体状态[31]。李旭新等[32]研究发现0.3% NaCl处理显著降低了Fv/Fm和PIABS值,表明盐胁迫损害光合机构,抑制了光合作用的正常进行。在本研究结果中,盐碱胁迫条件下杜梨幼苗叶片Fv/Fm、Fv/Fo和PIABS值大幅度降低,而增施耐碱嗜盐微生物水制剂则有效缓解了这些指标的降低,表明盐碱胁迫处理抑制了叶片的光化学效率和光合性能,耐碱嗜盐微生物水制剂对盐碱胁迫下杜梨幼苗叶片光合机构和光合性能起到了较好的保护作用,减轻了盐碱胁迫造成的光抑制程度。
OJIP曲线是解析PSⅡ光化学反应损伤的重要工具,其特征相的变化可精准揭示盐碱胁迫对光合机构的影响机制。在本研究结果中,盐碱胁迫导致杜梨幼苗叶片OJIP曲线从典型的OJIP型畸变为OKJIP型,且K相荧光强度及曲线标准化后的ΔVK均显著高于对照。有研究指出K点的出现和ΔVK值的升高表明PSⅡ供体侧放氧复合体(OEC)受到损伤[28,32]。因此,笔者的研究结果表明盐碱胁迫破坏了杜梨幼苗叶片PSⅡ供体侧OEC。从标准化后的ΔVK分析,HAS处理的K相荧光强度和ΔVK值均明显低于SA处理,表明施用耐碱嗜盐微生物水制剂有效减轻了OEC的损伤程度。此外,SA处理的叶片J相荧光强度和ΔVJ显著升高,反映了PSⅡ受体侧QA-的瞬时积累量增加,表明盐碱胁迫下PSⅡ受体侧QA-到QB-的电子传递过程受阻。这与干旱胁迫下辣椒叶片由受体侧电子传递抑制导致J相荧光强度和ΔVJ升高的机制一致[28]。而HAS处理的J相荧光强度和ΔVJ均显著低于SA组,表明微生物水制剂通过改善PSⅡ受体侧的电子传递效率,减少了QA-的过度积累,进而维持了光合机构的功能完整性。这一结果与上述叶绿素含量及荧光参数的改善趋势一致,进一步证明增施耐碱嗜盐微生物水制剂,通过保护光合机构的结构与功能,提升了杜梨幼苗对盐碱胁迫的适应性。
植物抗氧化酶系统(SOD、POD、CAT)是抵御盐碱胁迫诱导的氧化损伤的核心防线,其协同作用对维持细胞内活性氧平衡至关重要[33]。本研究结果中,盐碱胁迫显著提高了杜梨幼苗叶片的SOD活性,但降低了POD和CAT活性,这一结果与逆境条件下抗氧化酶的响应规律相一致,即逆境条件下SOD优先被激活以清除活性氧,而重度胁迫下POD与CAT可能因底物过载或酶蛋白结构损伤导致活性下降[26]。值得注意的是,SA处理的根系SOD和CAT活性均升高,可能与根系直接接触盐碱环境、优先启动抗氧化防御功能有关。施用耐碱嗜盐微生物水制剂后,除叶片CAT活性外,叶片和根中其他酶活性均进一步增强。其中,叶片SOD和POD、根CAT均达到显著差异水平。这与微生物菌肥通过提高盐碱地大豆[7]和小黑麦[8]的POD、SOD和CAT活性来缓解盐碱胁迫造成氧化损伤的结果一致。
此外,在本试验条件下,增施耐碱嗜盐微生物水制剂后,杜梨幼苗的生长状况及叶绿素荧光等指标完全恢复至正常生长水平,但部分指标与盐碱胁迫处理相比尚未达到显著差异水平,这可能与微生物水制剂的施用浓度或者处理时间不足有关。今后需进一步开展剂量梯度试验,筛选最优的施用浓度,并结合转录组、蛋白组等多组学技术,深入解析耐碱嗜盐微生物水制剂调控盐碱胁迫下杜梨幼苗生长的分子机制,为其田间应用提供更坚实的理论依据。
4 结 论
在盐碱胁迫条件下,施用200倍耐碱嗜盐微生物水制剂,可显著提高杜梨幼苗叶片的叶绿素含量,恢复Fv/Fm和PIABS等关键叶绿素荧光参数,不仅有效降低PSⅡ供体侧OEC的损伤程度,还能优化受体侧电子传递效率,进而增强了叶片的光合作用性能。同时,还能减轻盐碱胁迫造成的氧化损伤,最终促进幼苗生长状况的改善。
[1] 联合国粮农组织. 世界盐渍土壤分布图发布[J] . 中国农业综合开发,2021(10):64.Food and Agriculture Organization of the United Nations,FAO.World distribution map of saline soils released[J] . Agricultural Comprehensive Development in China,2021(10):64.
[2] LIU X Y,SHANG C Y,DUAN P Y,YANG J Y,WANG J B,SUI D,CHEN G,LI X J,LI G B,HU S S,HU X H. The Sl-WRKY42-SlMYC2 module synergistically enhances tomato saline-alkali tolerance by activating the jasmonic acid signaling and spermidine biosynthesis pathway[J] . Journal of Integrative Plant Biology,2025,67(5):1254-1273.
[3] 陈梦. 黄河三角洲盐碱地土壤质量评价与障碍因素分析:以黄三角农高区为例[D] . 烟台:鲁东大学,2023:2-3.CHEN Meng. Evaluation of soil quality and analysis of obstacle factors in saline-alkali land in the Yellow River Delta:A Case Study of the Agricultural High-tech Industry Demonstration Area of the Yellow River[D] . Yantai:Ludong University,2023:2-3.
[4] 王娜娜,刘宏元,李英,王艳君,董红云,张燕,张锡金,高洁,梁守真,李新华. 黄河三角洲湿地生态系统服务价值评估[J] . 山东农业科学,2022,54(2):153-158.WANG Nana,LIU Hongyuan,LI Ying,WANG Yanjun,DONG Hongyun,ZHANG Yan,ZHANG Xijin,GAO Jie,LIANG Shouzhen,LI Xinhua. Value evaluation of wetland ecosystem services in the Yellow River Delta Wetland[J] . Shandong Agricultural Sciences,2022,54(2):153-158.
[5] LEI S H,JIA X X,ZHAO C L,SHAO M G. A review of salinealkali soil improvements in China:Efforts and their impacts on soil properties[J] . Agricultural Water Management,2025,317:109617.
[6] 李欣. 微生物菌剂对盐碱胁迫下玉米幼苗的促生效果研究[D] .泰安:山东农业大学,2022:44-45.LI Xin. Growth-promoting effect of microbial inoculants on maize seedlings under saline-alkali stress[D] . Taian:Shandong Agricultural University,2022:44-45.
[7] 滕迁莹,崔明元,姜海英,吴楠,刘玉兰. 微生物菌肥增施方式对盐碱地大豆幼苗生长发育及产量的影响[J/OL] . 大豆科学,2025:1-22. (2025-04-23). https://link.cnki.net/urlid/23.1227.S.20250423.1358.006.TENG Qianying,CUI Mingyuan,JIANG Haiying,WU Nan,LIU Yulan. Effects of microbial fertilizers application methods on the growth,development of soybean seedlings and its yield in saline-alkaline soil[J/OL] . Soybean Science,2025:1-22. (2025-04-23). https://link.cnki.net/urlid/23.1227.S.20250423.1358.006.
[8] 刘晶,张树灿,鲍海娟,麻心玥,杜明川,纪金兰,王伟. 微生物菌肥对盐碱胁迫下小黑麦幼苗促生效果的影响[J] . 分子植物育种,2024,22(13):4316-4323.LIU Jing,ZHANG Shucan,BAO Haijuan,MA Xinyue,DU Mingchuan,JI Jinlan,WANG Wei. Effects of microbial fertilizer on growth-promoting effect of triticale seedlings under salinealkali stress[J] . Molecular Plant Breeding,2024,22(13):4316-4323.
[9] 叶静,陈影,屈爽,赵文超. 不同微生物菌肥对滨海盐渍土土壤质量及玉米产量的影响[J] . 环境科学,2024,45(7):4279-4292.YE Jing,CHEN Ying,QU Shuang,ZHAO Wenchao. Effects of different microbial fertilizers on soil quality and maize yield in coastal saline soil[J] . Environmental Science,2024,45(7):4279-4292.
[10] 朱文碧. 八棱海棠(Malus robusta Rehd.)和杜梨(Pyrus betu‐laefolia Bge.)PuNHA基因遗传转化研究[D] . 重庆:西南大学,2008.Zhu Wenbi. Transform Gene PuNHA into Malus robusta Rehd.and Pyrus betulaefolia Bge.[D] . Chongqing:Southwest University,2008.
[11] 冀明辉,李龙飞,高丽娟,张海娥,徐金涛,郝宝锋. 梨砧木研究进展[J] . 河北农业科学,2022,26(1):76-80.JI Minghui,LI Longfei,GAO Lijuan,ZHANG Hai’e,XU Jintao,HAO Baofeng. Research progress of pear rootstock[J] . Journal of Hebei Agricultural Sciences,2022,26(1):76-80.
[12] 龚远博. 杜梨人工林土壤微生物群落结构与菌根化杜梨苗耐盐碱胁迫机制研究[D] . 泰安:山东农业大学,2022.GONG Yuanbo. Study on soil microbial community structure of Pyrus betulifolia plantation and the mechanism of salt and alkali tolerance of arbuscular mycorrhizal Pyrus betulifolia seedlings[D] . Taian:Shandong Agricultural University,2022.
[13] 李庆梅,侯龙鱼,刘艳,马风云. 黄河三角洲盐碱地不同利用方式土壤理化性质[J] . 中国生态农业学报,2009,17(6):1132-1136.LI Qingmei,HOU Longyu,LIU Yan,MA Fengyun. Properties of saline-alkaline soil under different land use types in Yellow River Delta[J] . Chinese Journal of Eco-Agriculture,2009,17(6):1132-1136.
[14] 董合忠,辛承松,李维江,唐薇,张冬梅,罗振. 山东滨海盐渍棉田盐分和养分特征及对棉花出苗的影响[J] . 棉花学报,2009,21(4):290-295.DONG Hezhong,XIN Chengsong,LI Weijiang,TANG Wei,ZHANG Dongmei,LUO Zhen. Characteristics of salinity and fertility in coastal saline cotton fields in Shandong and their effects on cotton emergence[J] . Cotton Science,2009,21(4):290-295.
[15] 张志良,瞿伟菁. 植物生理学实验指导[M] . 3版. 北京:高等教育出版社,2003:62-63.ZHANG Zhiliang,QU Weijing. The experimental guide for plant physiology[M] . 3rd ed. Beijing:Higher Education Press,2003:62-63.
[16] 李鹏民,高辉远,STRASSER R J. 快速叶绿素荧光诱导动力学分析在光合作用研究中的应用[J] . 植物生理与分子生物学学报,2005,31(6):559-566.LI Pengmin,GAO Huiyuan,STRASSER R J. Application of the fast chlorophyll fluorescence induction dynamics analysis in photosynthesis study[J] . Acta Photophysiologica Sinica,2005,31(6):559-566.
[17] VAN HEERDEN P D R,STRASSER R J,KRÜGER G H J. Reduction of dark chilling stress in N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics[J] . Physiologia Plantarum,2004,121(2):239-249.
[18] 苏晓琼,王美月,束胜,孙锦,郭世荣. 外源亚精胺对高温胁迫下番茄幼苗快速叶绿素荧光诱导动力学特性的影响[J] . 园艺学报,2013,40(12):2409-2418.SU Xiaoqiong,WANG Meiyue,SHU Sheng,SUN Jin,GUO Shirong. Effects of exogenous spd on the fast chlorophyll fluorescence induction dynamics in tomato seedlings under high temperature stress[J] . Acta Horticulturae Sinica,2013,40(12):2409-2418.
[19] 王学奎,黄见良. 植物生理生化实验原理与技术[M] . 3版. 北京:高等教育出版社,2015:216-217.WANG Xuekui,HUANG Jianliang. Principles and techniques of plant physiological biochemical experiment[M] . 3rd ed. Beijing:Higher Education Press,2015:216-217.
[20] 李合生. 植物生理生化实验原理和技术[M] . 北京:高等教育出版社,2000:164-169.LI Hesheng. Principles and techniques of plant physiological biochemical experiment[M] . Beijing:Higher Education Press,2000:164-169.
[21] 李小方,张志良. 植物生理学实验指导[M] . 5版. 北京:高等教育出版社,2016:86-89..LI Xiaofang,ZHANG Zhiliang. Experimental guidance of plant physiology[M] . 5th ed. Beijing:Higher Education Press,2016:86-89..
[22] 张华新,刘正祥,刘秋芳. 盐胁迫下树种幼苗生长及其耐盐性[J] . 生态学报,2009,29(5):2263-2271.ZHANG Huaxin,LIU Zhengxiang,LIU Qiufang. Seedling growth and salt tolerance of tree species under NaCl stress[J] .Acta Ecologica Sinica,2009,29(5):2263-2271.
[23] SHANG C Y,LIU X Y,CHEN G,ZHENG H,KHAN A,LI G B,HU X H. SlWRKY80-mediated jasmonic acid pathway positively regulates tomato resistance to saline-alkali stress by enhancing spermidine content and stabilizing Na+/K+ homeostasis[J] .Horticulture Research,2024,11(3):uhae028.
[24] 黄春燕,苏文斌,郭晓霞,李智,菅彩媛,田露,樊福义,任霄云,宫前恒,张强. 15个甜菜品种对盐碱胁迫的生理响应及耐盐碱性评价[J] . 北方农业学报,2020,48(4):1-9.HUANG Chunyan,SU Wenbin,GUO Xiaoxia,LI Zhi,JIAN Caiyuan,TIAN Lu,FAN Fuyi,REN Xiaoyun,GONG Qianheng,ZHANG Qiang. Physiological responses and saline-alkali tolerant evaluation of 15 sugar beet varieties to saline-alkali stress[J] . Journal of Northern Agriculture,2020,48(4):1-9.
[25] 朱晨晨,史昆,何沁坤,迪力穆拉提·阿卜杜外力,刘亚玲,王赞. 中苜一号紫花苜蓿幼苗对混合盐碱胁迫的响应[J] . 中国草地学报,2024,46(8):1-9.ZHU Chenchen,SHI Kun,HE Qinkun,Dilmurat Abduwali,LIU Yaling,WANG Zan. Response of ‘Zhongmu No. 1’ alfalfa seedlings to mixed saline-alkali stress[J] . Chinese Journal of Grassland,2024,46(8):1-9.
[26] 霍宏亮,王超,杨祥,曹玉芬,田路明,董星光,张莹,齐丹,徐家玉,刘超. 杜梨对盐碱胁迫的生理响应及耐盐碱性评价[J] . 植物遗传资源学报,2022,23(2):480-492.HUO Hongliang,WANG Chao,YANG Xiang,CAO Yufen,TIAN Luming,DONG Xingguang,ZHANG Ying,QI Dan,XU Jiayu,LIU Chao. Physiological response and saline-alkali tolerance evaluation of Pyrus betulifolia resources under saline-alkali stress[J] . Journal of Plant Genetic Resources,2022,23(2):480-492.
[27] 王国丽,张晓丽,张晓霞,常芳弟,刘娜,逄焕成,师文娟,张建丽,李玉义. 施用功能微生物菌剂对重度盐碱地向日葵生长及土壤微生物的影响[J] . 中国土壤与肥料,2021(5):133-139.WANG Guoli,ZHANG Xiaoli,ZHANG Xiaoxia,CHANG Fangdi,LIU Na,PANG Huancheng,SHI Wenjuan,ZHANG Jianli,LI Yuyi. Effects of applying functional microbial agents on sunflower growth and soil microorganism in severe saline alkali soil[J] . Soil and Fertilizer Sciences in China,2021(5):133-139.
[28] 胡文海,闫小红,李晓红,曹灶桂. 24-表油菜素内酯对干旱胁迫下辣椒叶片快速叶绿素荧光诱导动力学曲线的影响[J] . 植物研究,2021,41(1):53-59.HU Wenhai,YAN Xiaohong,LI Xiaohong,CAO Zaogui. Effects of 24-epibrassinolide on the chlorophyll fluorescence transient in leaves of pepper under drought stress[J] . Bulletin of Botanical Research,2021,41(1):53-59.
[29] SUN Y,FENG X H,LI Y X,LV J,CHENG D D,LU Y Z,YU C F,GAO D M. Integrated transcriptomics and metabolomics analysis revealed the molecular mechanism of Paeonia rockii to saline-alkali stress tolerance[J] . Scientia Horticulturae,2025,340:113912.
[30] 李星志,王虹,赵丹,石运幸,刘爱新. 小麦内生耐盐菌株YN1的分离鉴定及其生物学特性研究[J] . 山东农业科学,2019,51(5):63-68.LI Xingzhi,WANG Hong,ZHAO Dan,SHI Yunxing,LIU Aixin. Study of isolation,identification and biological characteristics of wheat endophytic salt-tolerant strain YN1[J] . Shandong Agricultural Sciences,2019,51(5):63-68.
[31] 卢盼玲,杜旋,王颖,张红梅,田守波,王楠,刘娜. 碱性盐胁迫对节瓜幼苗叶绿素荧光诱导动力学的影响[J/OL] . 分子植物育种,2023:1-8. (2023-10-17). https://link.cnki.net/urlid/46.1068.S.20231016.1123.014.LU Panling,DU Xuan,WANG Ying,ZHANG Hongmei,TIAN Shoubo,WANG Nan,LIU Na. Effects of salt-alkaline stress on chlorophyll fluorescence characteristics in leaves of chieh-qua seedlings[J/OL] . Molecular Plant Breeding,2023:1-8. (2023-10-17). https://link.cnki.net/urlid/46.1068.S.20231016.1123.014
[32] 李旭新,刘炳响,郭智涛,常越霞,贺磊,陈芳,路丙社. NaCl胁迫下黄连木叶片光合特性及快速叶绿素荧光诱导动力学曲线的变化[J] . 应用生态学报,2013,24(9):2479-2484.LI Xuxin,LIU Bingxiang,GUO Zhitao,CHANG Yuexia,HE Lei,CHEN Fang,LU Bingshe. Effects of NaCl stress on photosynthesis characteristics and fast chlorophyll fluorescence induction dynamics of Pistacia chinensis leaves[J] . Chinese Journal of Applied Ecology,2013,24(9):2479-2484.
[33] ZHANG L S,SUN Y G,JI J Q,ZHAO W D,GUO W L,LI J Q,BAI Y,WANG D,YAN Z,GUO C H. Flavonol synthase gene MsFLS13 regulates saline-alkali stress tolerance in alfalfa[J] .The Crop Journal,2023,11(4):1218-1229.