土壤盐渍化是全球性挑战,现已成为农业最常见的非生物胁迫之一。长时间盐害导致细胞渗透压和氧化胁迫,打破营养平衡,严重阻碍葡萄的生长发育,影响产量和品质[1]。据统计,2050年,全球约50%可耕地盐渍化[2]。新疆是中国最大盐渍土区域,占中国灌溉面积的32%以上,同时拥有最大的葡萄种植面积和最高的市场份额。而葡萄对盐胁迫敏感或中度敏感[3]。因此,研究盐胁迫响应代谢途径关键基因及其调控机制,对培育改良耐盐葡萄新品种至关重要。
植物为了感知和应对外界盐胁迫进化出复杂的防御机制,包括代谢、生理和分子调控等过程。目前,关于植物应对盐胁迫所触发的信号转导路径,已有诸多研究报道,常见的包括:盐超敏(salt overly sensitive,SOS)信号传导路径[4]、钙依赖性蛋白激酶(calcium-dependent protein kinase,CDPK)级联反应路径[5-6]、脱落酸(abscisic acid,ABA)信号传导通路[7]、磷脂信号通路以及丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)级联反应路径等[8],以上多种信号转导路径相互交织,通过形成复杂的网络结构,协同调控植物的耐盐生理响应过程。其中,ROS信号转导通路又包括单线态氧(1O2)、超氧自由基(O2)、过氧化氢(H2O2)和羟基自由基(·OH)[9]。H2O2在细胞间隙中通过腺嘌呤二核苷酸磷酸(NADPH)氧化酶催化产生,而RBOH是合成该蛋白质的关键基因[10],在非生物胁迫(盐、寒冷、高光、热、重金属)中具有重要生物学功能[11-13]。例如,在水稻中,NaCl处理可显著上调OsRBOHA 的表达,敲除该基因会降低水稻对盐胁迫的耐受性,表现为抑制根系中NADPH氧化酶活性和H2O2含量的增加,进而抑制根系生长;与之相反,过表达OsRBOHA则能提高水稻耐盐性[14]。在番茄中,盐胁迫下RBOH转录因子可激活H2O2介导的信号通路,诱导番茄幼苗产生解毒机制如激活抗氧化系统及ABA生物合成和信号转录因子,提高其耐盐性[15]。此外,在烟草中,NtBHLH123转录因子可通过与NtRBO‐HE启动子上的E-box基序结合来激活其表达,进而增强烟草对盐胁迫的耐受性,且该调控依赖于NtB‐HLH123-NtRBOHE信号转导途径[16]。这些研究从多个角度揭示了RBOH基因参与植物耐盐调控的复杂网络。在植物中,首个被鉴定的水稻RBOHA为哺乳动物gp91phox同源物[17]。研究发现,葡萄中有7个RBOH基因(VvRBOHA-J)具有组织表达特异性,且不同家族基因响应干旱、盐、白粉病、水杨酸和脱落酸的规律存在差异。其中,VvRBOHA的表达水平在盐胁迫和干旱处理后显著升高[18]。此外,多种转录因子(MYB、MYC、WRKY、bHLH等)也参与ROS介导的非生物胁迫调控[19]。如VvMYB108A直接结合ACS1启动子并激活其转录,促进乙烯合成和提高耐盐性[20]。WRKY(TaWRKY)和RAV(TaRAV)蛋白特异性结合TaeIF5A1启动子的W-box激活其表达[21]。
笔者课题组前期通过对葡萄耐盐功能分析,发现H2O2参与调控通路,其中VvRBOHA在盐胁迫下显著表达,而关于VvRBOHA基因耐盐作用机制与调控网络尚不清晰。因此本试验中构建了盐胁迫下葡萄cDNA文库,并利用该文库筛选与其可能互作的蛋白,以探究其参与调控葡萄盐胁迫的作用分子机制。
1 材料和方法
1.1 植物材料和试验设计
试验于2022年在石河子大学进行,选用6年生的巨峰葡萄(Vitis labrusca ‘Kyoho’)自根树进行盐处理。当浆果达到早期变色阶段(7月6日)时,在根系周围(10 cm)灌溉50 L 150 mmol·L-1 NaCl溶液。试验采用随机区组设计,设置3次独立重复,每次使用5株葡萄,每株树保留10个垂直果枝。处理12 h后,随机采集根、茎、叶和果实样品,并立即用液氮冷冻。
1.2 方法
1.2.1 酵母cDNA文库构建 采用Trizol试剂盒(Invitrogen,美国)提取葡萄总RNA,再使用Beaver-Beads™寡聚(dT)mRNA midi试剂盒(Beaver,中国)分离mRNA。利用SMART cDNA文库构建试剂盒(TaKaRa,日本)合成双链cDNA。将cDNA与三框attB1重组连接,进行分级和收集,再使用Clontech CHROMA SPIN™和TE-1000柱试剂盒(TaKaRa,日本)去除小片段。采用Gateway技术构建酵母文库:首先将cDNA克隆到pDONR222载体中,并与BP Clonase®Ⅱ酶(Invitrogen)孵育16~20 h,转化至大肠杆菌DH10B感受态细胞。通过LR重组反应构建二级酵母文库,将一级文库质粒克隆到pGADT7-DEST载体中,并与LR Clonase®Ⅱ酶(Invitrogen)孵育16~20 h,转化至大肠杆菌DH10B感受态细胞。然后将二级文库细菌溶液加入甘油保存,使用高纯度质粒提取试剂盒(TIANGEN,中国)提取质粒。
1.2.2 鉴定文库滴度 取10 μL二级文库细菌溶液,用SOC培养基稀释10 000倍。取100 μL稀释液涂布在100 μg·mL-1氨苄青霉素的LB培养基上,在37 ℃培养12~14 h。从长出菌落的固体培养基上随机挑选24个克隆,使用T7/3' AD引物进行PCR 鉴定。将二级文库载体转化至酵母Y187感受态细胞,并进行10-2、10-4、10-5、10-6梯度稀释,涂布于SD/-Leu固体培基上,在30 ℃培养3~5 d,计算转化效率和存储容量。根据提供的公式(1)和(2)使用血细胞计数器计量细胞密度[22]。

1.2.3 转染诱饵载体的菌株自我激活鉴定 克隆VvRBOH基因的5'上游高转录活性区域(-1070~0 bp),经EcoRⅠ和SacⅡ(TaKaRa)酶切后,连接到pHIS2质粒中,构建诱饵载体pHIS2-proVvRBOH启动子。重组载体序列鉴定后,将pHIS2-proVvRBOH启动子诱饵载体(约100 ng)和pGADT7猎物载体(约100 ng)共转入酵母菌Y187细胞,检测自激活和毒性。转化后的酵母接种于50mL SD/-Trp液体培养基,在30 ℃、200 r·min-1条件下培养24 h。通过紫外分光光度法测定培养基OD600值,检测诱饵载体是否对细胞有毒性。随后,将转化的酵母菌株涂布在SD/-Leu/-Trp(DDO)、SD/-Leu/-Trp/-His(TDO)以及含不同3-AT浓度(0、10、20、30、40、50、75、100、110、120、130和140 mmol·L-1)的SD/-Leu/-Trp/-His的培养基上,在30 ℃培养3~5 d,检测自激活。
1.2.4 阳性互作蛋白筛选 将25 μL酵母文库载体转入携带pHIS2-proVvRBOH诱饵载体的Y187酵母菌细胞中。将转化后的酵母细胞涂布在DDO培养基上,检测文库转化效率。同时,将转化细胞接种在120 mmol·L-1 3-AT的TDO筛选培养基上,在30 ℃培养3~5 d。当有直径1~2 mm的互作克隆出现时,收集菌落进行NGS筛选和测序。测序结果通过NCBI数据库的BLAST进行比对,鉴定与proVvR‐BOHA存在相互作用的候选蛋白。
1.2.5 NGS测序 提取阳性酵母克隆mRNA,用pGADT7载体通用引物PCR扩增猎物cDNA,FastQC检测碱基质量,Trimmomatic去除接头、低质量碱基(Q<20)及短读长(<50 bp),筛除含N比例>5%的reads。根据载体通用引物修剪非猎物序列,保留插入片段区域提取特异性序列。使用Bowtie2/BWA将reads比对到筛库文库参考序列(如猎物 cDNA 文库数据库),统计各猎物基因的reads数[23]。使用基因本体论注释(gene ontology,GO;http://geneontology.org/)和京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG;https://www.kegg.jp/)数据库对筛选基因进行富集分析和注释。
1.2.6 酵母回转验证 选取8个候选蛋白,将其克隆至pGADT7载体。将pGADT7-候选蛋白和pHIS2-proVvRBOHA共转染Y187 Gold酵母菌,涂布在DDO缺陷培养基上。在30 ℃培养3~5 d后,观察菌落生长情况。选取生长良好的菌落,制备1倍、10倍、100倍稀释液,涂布在120 mmol L-1 3-AT的TDO筛选培养基上,再次在30 ℃培养3~5 d。通过观察DDO和TDO筛选培养基的菌落生长情况,验证这8个候选蛋白与pHIS2-proVvRBOHA是否存在相互作用。
2 结果与分析
2.1 盐诱导酵母杂交cDNA文库的构建与鉴定
巨峰葡萄植株经过150 mmol·L-1 NaCl处理12 h后,收集并混合根、茎、叶片和果实,提取总RNA。结果显示,总RNA A260/A280比值在1.8~2.2之间,并具有完整的28S和18S rRNA条带(图1-A)。使用3对引物通过LD-PCR扩增得到均匀分散的ds cDNA条带(图1-B)。标准化分析显示,除了少量低于500 bp的片段外,ds cDNA长度分布与未标准化的样品相似(图1-C),证明标准化过程成功,满足了cDNA文库构建的要求。
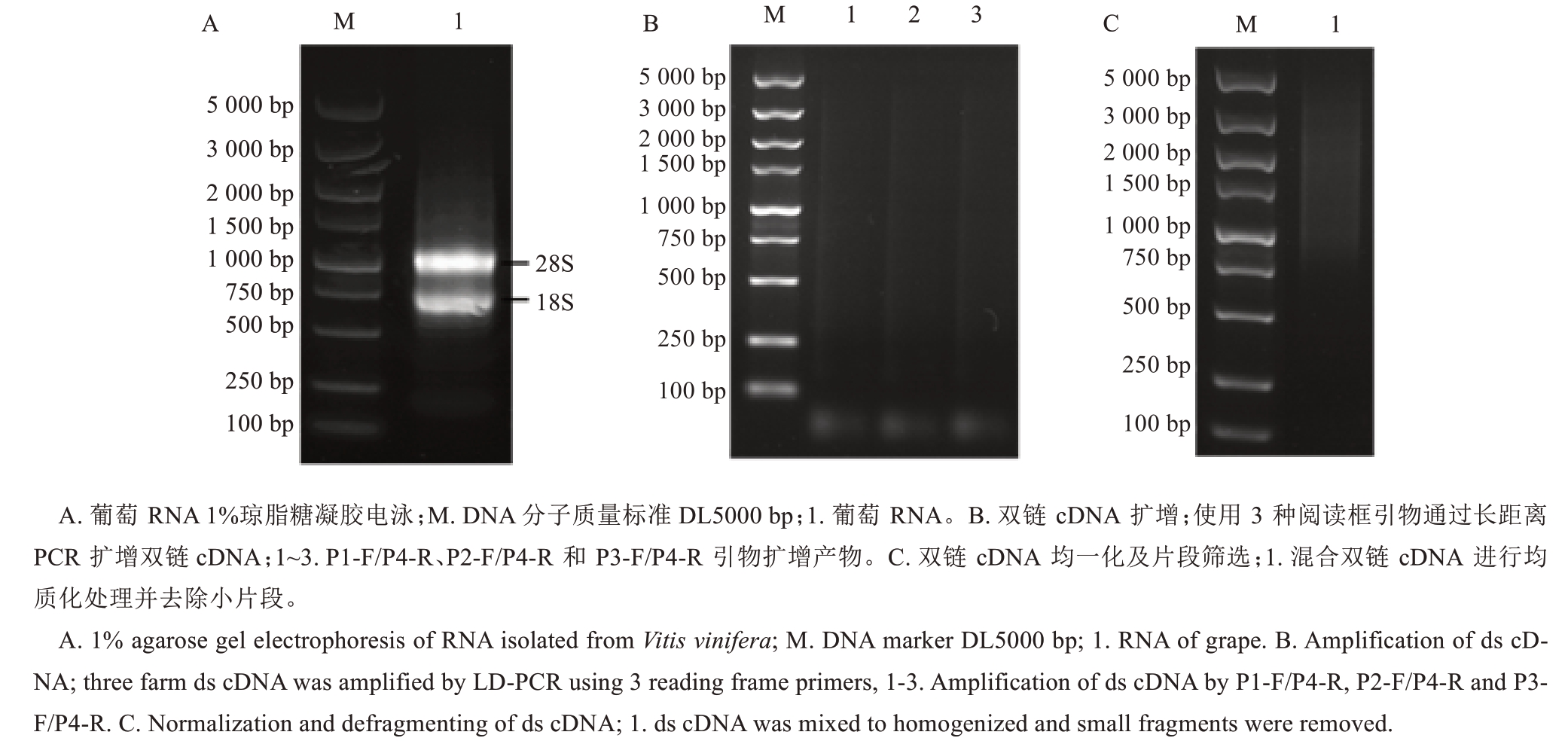
图1 葡萄RNA提取鉴定、LD-PCR扩增ds cDNA以及均一化的琼脂糖凝胶电泳
Fig. 1 Agarose gel electrophoresis of grape RNA extraction and identification, ds cDNA amplified by LD-PCR,and normalization
纯化的双链cDNA与pGADT7载体连接后,转化至大肠杆菌DH5α感受态细胞中。文库容量检测结果显示,单个平板上获得约3100个克隆(图2-A),文库滴度为3.10×108 CFU·mL-1。从3个培养板中随机挑选24个克隆,鉴定其文库插入片段。结果(图2-B)显示,所有扩增产物均有500~2000 bp的条带,重组率为100%。
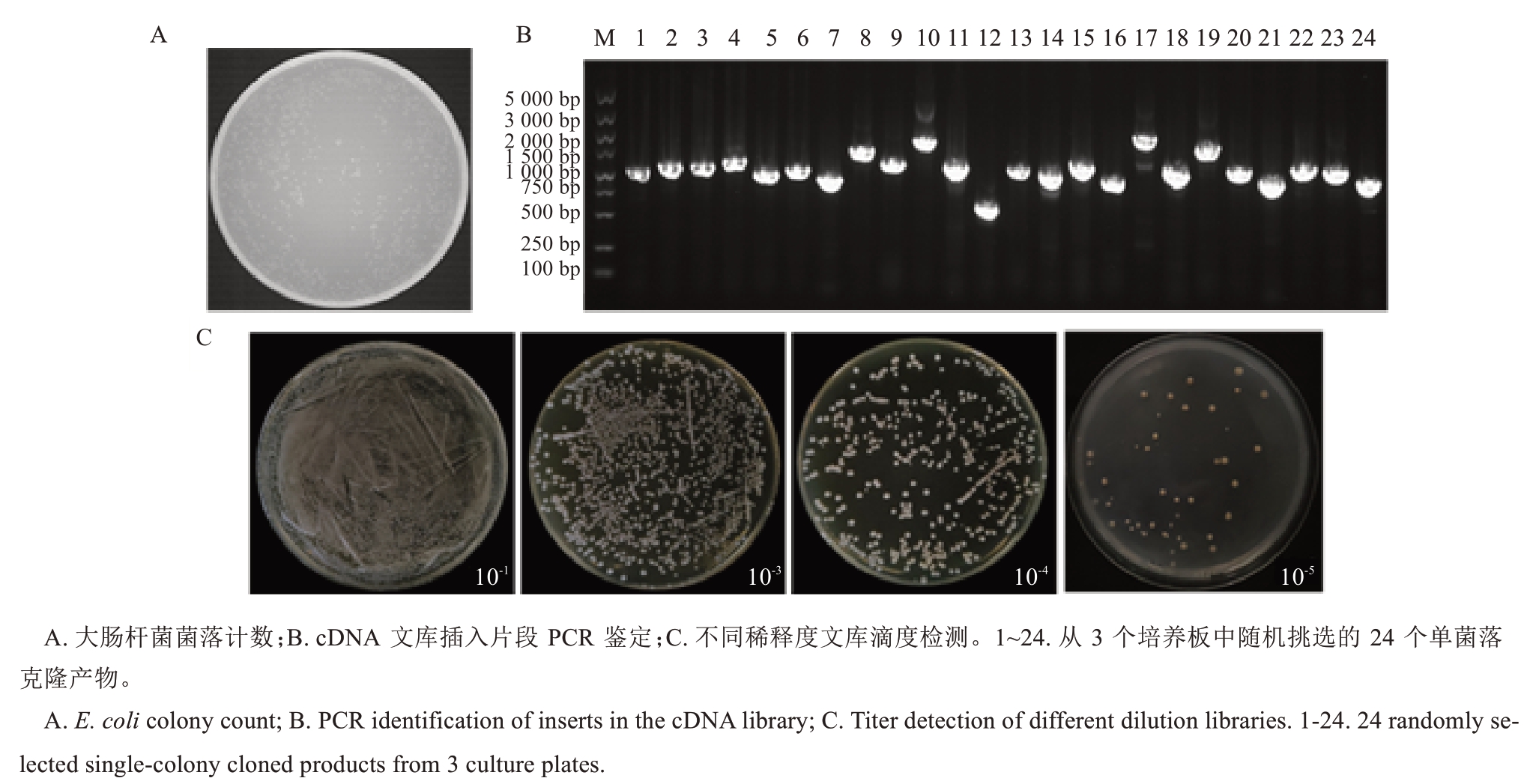
图2 盐诱导葡萄酵母cDNA文库的构建与鉴定分析Fig. 2 Construction and identification analysis of a salt-induced cDNA library from grape yeast
将文库载体转染到Y187菌株中获得酵母文库,在含有0.9% NaCl的YPDA平板上培养稀释至10-1、10-3、10-4和10-5的酵母细胞。并取100 μL稀释的酵母涂布在100 mL SD/-Leu缺陷培养基上(图2-C),倒置培养3~5 d后,用血细胞计数板计数,文库滴度为2.8×107 CFU·mL-1,cDNA插入细胞密度为2.8×107 CFU·mL-1×50 mL=1.4×109 CFU·μg-1。成功构建了具有大容量和合适插入片段的酵母杂交文库。
2.2 VvRBOHA启动子的诱饵报告菌株的构建和自激活检测
基于本课题组前期试验,初步筛选出盐响应基因VvRBOHA。因此,克隆VvRBOHA启动子(proV‐vRBOHA),连接线性化pHIS2载体并进行1%琼脂糖凝胶电泳验证(图3-A)。利用在线分析工具Plant-CARE,发现proVvRBOHA中存在响应脱落酸、茉莉酸甲酯、水杨酸、乙烯、盐胁迫相关的顺式作用元件,以及与多个转录因子结合的位点如MYB转录因子结合的MYB、WRKYs转录因子结合的W-box等位点(图3-B)。
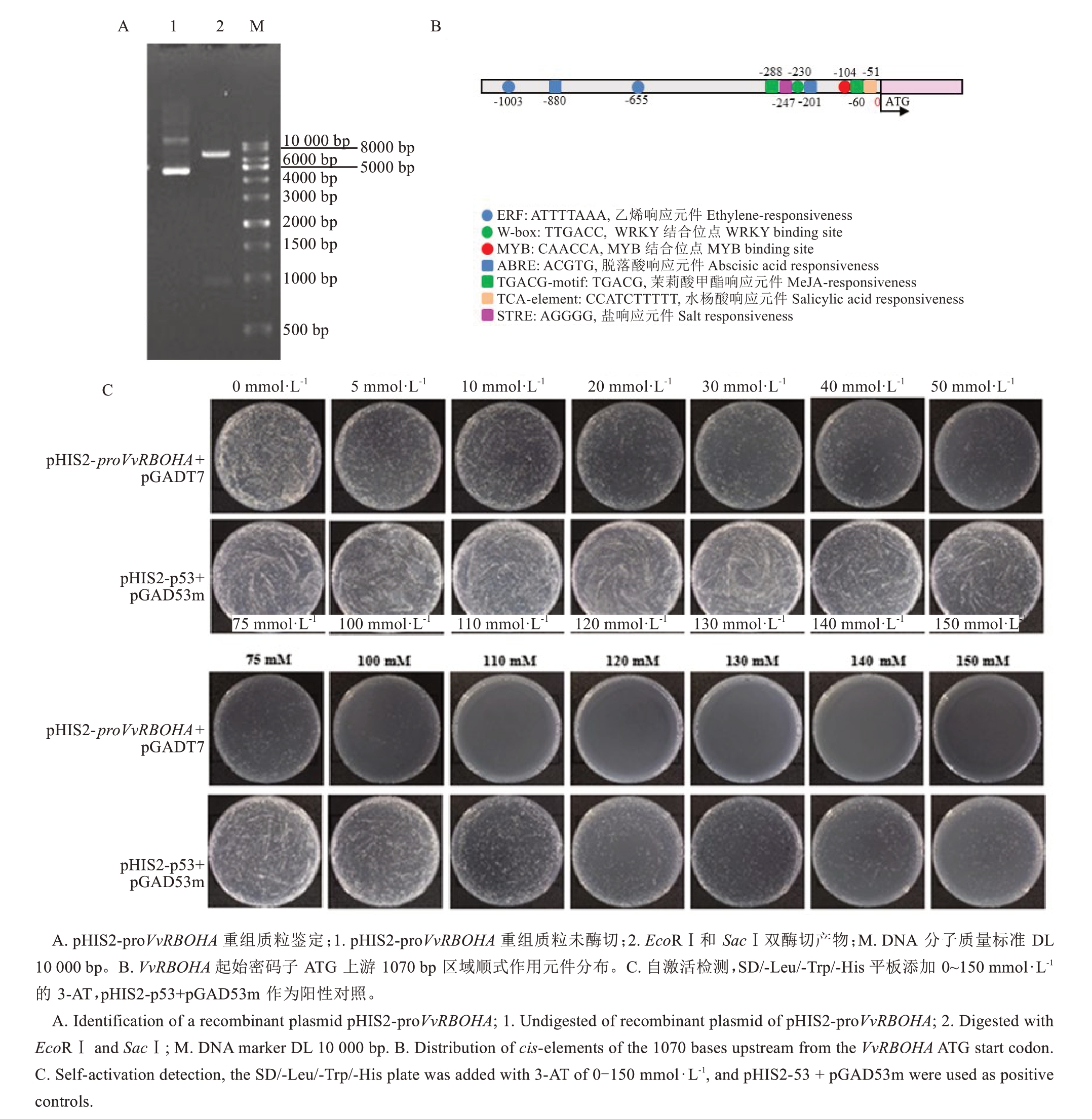
图3 pHIS2-proVvRBOHA 重组质粒鉴定、启动子顺式作用元件分析及自激活检测
Fig. 3 Identification of recombinant bacteria, analysis of the cis-element of pHIS2-proVvRBOHA and self-activation detection
酵母单杂交筛库前,对pHIS2-proVvRBOHA重组载体在酵母中的毒性和自激活进行检测。结果表明,重组载体成功导入酵母宿主细胞,并于含有pGADT7猎物载体的SD/-Leu/-Trp平板上生长,表明该重组载体无毒性。然而,在SD/-Leu/-Trp/-His平板上,重组载体展现出自激活His报告基因的能力。而添加120 mmol·L-1的3-AT后,该自激活现象得以完全抑制(图3-C)。这些结果表明,在后续Y1H文库筛选中,需要使用120 mmol·L-1 3-AT作为抑制自激活的筛选浓度。因此,该诱饵载体在酵母中无毒性,但存在自激活现象,可通过优化筛选条件加以解决,为后续的文库筛选做好准备。
2.3 文库筛选和候选蛋白的鉴定
将携带pHIS2-proVvRBOHA诱饵载体的酵母菌株培养24 h,作为受体菌株。随后,将此受体菌株与葡萄cDNA文库共转染,收集转染了两种质粒的酵母细胞。用0.9% NaCl悬浮细胞,并在SD/-Trp/-Leu/-His+120 mmol·L-1 3AT选择平板上培养3~7 d。随机选择阳性克隆,提取酵母载体后进行PCR检测(图4)。
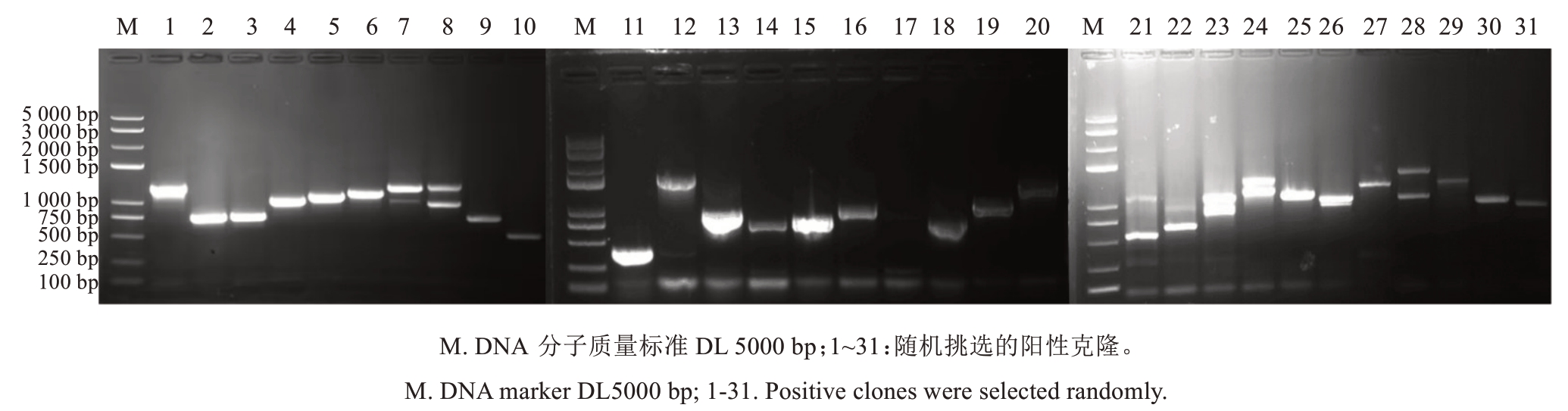
图4 筛选获得的阳性载体插入片段PCR检测
Fig. 4 PCR detection of insert size of positive vector screened out
PCR扩增结果显示,绝大多数插入片段大小集中在500~1500 bp范围内,少数为2条带。将所有从筛选平板上获得的阳性菌株进行NGS筛选。结果显示,成功筛选出1514个阳性基因,包括278个转录因子(TF)、404个激酶(Kinase),以及832个其他基因(图5-A)。

图5 候选蛋白的GO和KEGG通路分析
Fig. 5 GO and KEGG pathway analysis of the candidate proteins
GO功能注释结果显示从Y1H试验中筛选得到的候选相互作用蛋白主要富集在三个过程:生物过程427个基因、细胞组分94个基因、分子功能61个基因(图5-B)。KEGG通路注释结果显示,这些候选蛋白主要参与富集的生物学途径是蛋白激酶、转录因子、外泌体途径、植物-病原体互作和植物激素信号转导等(图5-C)。
挑选Reads读数大于100的转录因子和激酶,利用NCBI在线工具进行同源序列比对分析,发现多种响应逆境胁迫信号的蛋白质(表1)。
表1 候选蛋白(reads > 100)比对分析
Table 1 BLAST analysis of candidate proteins (reads > 100)
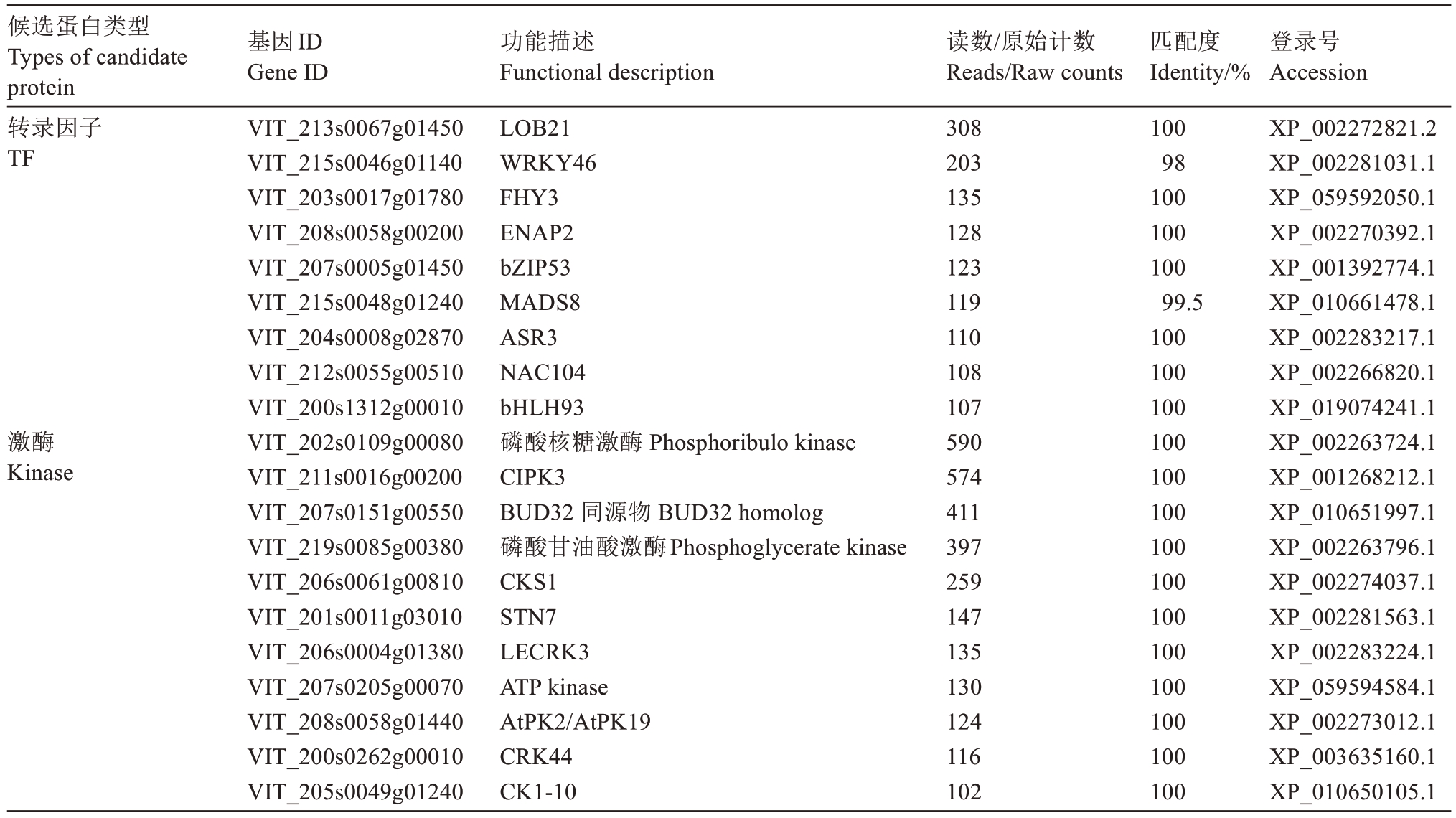
Biological processprotein modification processGO:00362161.67E-111.531042 Molecular functioncatalytic activity, acting on a pGO:01400933.44E-151.655158 Molecular functiontransferase activity, transferrinGO:001677402.650358 Biological processnucleobase-containing compGO:00346561.68E-121.577089 Molecular functionkinase activityGO:001630503.049649 Biological processcell communicationGO:00071536.77E-121.566428 Cellular componentplasma membraneGO:00058848.28E-041.221446 Biological processobsolete regulation of cellularGO:20001109.33E-151.683537 Biological processregulation of nucleobase-conGO:00192164.37E-111.544408候选蛋白类型Types of candidate protein转录因子TF激酶Kinase基因ID Gene ID VIT_213s0067g01450 VIT_215s0046g01140 VIT_203s0017g01780 VIT_208s0058g00200 VIT_207s0005g01450 VIT_215s0048g01240 VIT_204s0008g02870 VIT_212s0055g00510 VIT_200s1312g00010 VIT_202s0109g00080 VIT_211s0016g00200 VIT_207s0151g00550 VIT_219s0085g00380 VIT_206s0061g00810 VIT_201s0011g03010 VIT_206s0004g01380 VIT_207s0205g00070 VIT_208s0058g01440 VIT_200s0262g00010 VIT_205s0049g01240功能描述Functional description LOB21 WRKY46 FHY3 ENAP2 bZIP53 MADS8 ASR3 NAC104 bHLH93磷酸核糖激酶 Phosphoribulo kinase CIPK3 BUD32 同源物 BUD32 homolog磷酸甘油酸激酶Phosphoglycerate kinase CKS1 STN7 LECRK3 ATP kinase AtPK2/AtPK19 CRK44 CK1-10 Biological processregulation of macromolecule GO:001055 Biological processregulation of gene expressionGO:001046 Biological processphosphate-containing compoGO:000679 Biological processphosphorus metabolic procesGO:000679 Biological processorganic cyclic compound biosGO:190136 Biological processheterocycle biosynthetic procGO:001813 Biological processregulation of nitrogen compoGO:005117 Biological processregulation of primary metaboGO:008009 Biological processRNA metabolic processGO:001607 Biological processresponse to organic substanceGO:001003 Biological processaromatic compound biosynthGO:001943 Biological processphosphorylationGO:001631 Biological processmacromolecule modificationGO:004341 Cellular componentcell peripheryGO:007194 Biological processprotein modification processGO:003621 Molecular functioncatalytic activity, acting on a pGO:014009 Molecular functiontransferase activity, transferrinGO:001677 Biological processnucleobase-containing compGO:003465 Molecular functionkinase activityGO:001630 Biological processcell communicationGO:000715 Cellular componentplasma membraneGO:000588读数/原始计数Reads/Raw counts 308 203 135 128 123 119 110 108 107 590 574 411 397 259 147 135 130 124 116 102匹配度Identity/%100 98 100 100 100 99.5 100 100 100 100 100 100 100 100 100 100 100 100 100 100登录号Accession XP_002272821.2 XP_002281031.1 XP_059592050.1 XP_002270392.1 XP_001392774.1 XP_010661478.1 XP_002283217.1 XP_002266820.1 XP_019074241.1 XP_002263724.1 XP_001268212.1 XP_010651997.1 XP_002263796.1 XP_002274037.1 XP_002281563.1 XP_002283224.1 XP_059594584.1 XP_002273012.1 XP_003635160.1 XP_010650105.1
2.4 候选蛋白回转验证
为了验证proVvRBOHA Y1H筛选效率,从278个候选转录因子中,克隆了8个包含完整开放阅读框(ORF)的基因,并构建到pGADT7载体中。将这8个pGADT7-ORF重组载体分别与pHIS2-proVvR‐BOH共转化酵母,结果在TDO培养基上都能正常生长(图6)。随着稀释比例的增大,菌落数量逐渐减少,说明proVvRBOHA与这8个候选蛋白存在相互作用。以上结果表明,利用proVvRBOHA进行Y1H筛库效率为100%,并初步确认了8个候选蛋白与VvRBOHA启动子存在相互作用关系。
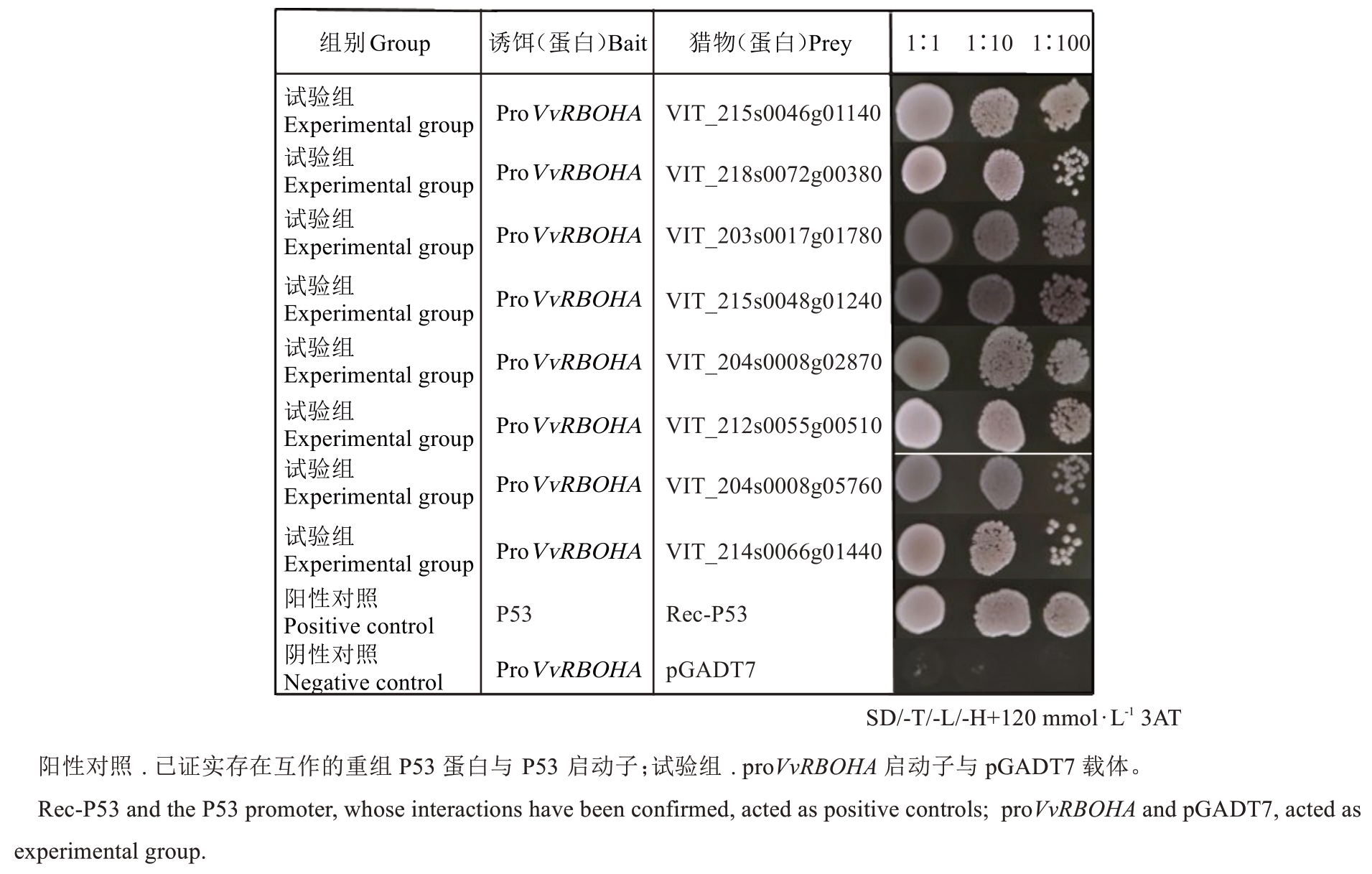
图6 酵母单杂交候选蛋白回转验证Fig. 6 Retest of yeast one-hybrid candidate proteins
3 讨 论
分子间的相互作用对阐明细胞内信号转导机制非常重要,而构建酵母文库(如酵母单杂交文库、双杂交文库)是研究基因功能、分析蛋白质互作等领域的一种非常有效的途径[24],且已应用到各种植物基础研究领域[25-27]。其质量可从库容量、冗余率和插入片段大小等方面评估[28]。一般情况下,构建高质量的植物酵母文库原始浓度在107~108 CFU·mL-1,如番茄[29]、水稻[30]、葡萄[31]。在本研究中,建立了基于盐胁迫下葡萄的高质量cDNA文库,原始库浓度为7.76×107 CFU·mL-1,随机选择的单克隆插入片段为500~1500 bp,重组率为100%,表明该文库具有完整性。
用于验证DNA-蛋白质之间相互作用的技术一般包括酵母单杂交系统(Y1H)、凝胶迁移试验(EMSA)和染色质免疫共沉淀(ChIP)。其中,Y1H技术是一种有效且便利的实验方法,可用于在酵母中识别转录因子与顺式作用元件的特异性结合。Y1H技术从Gateway克隆系统中开发和应用,但只适用于被运输到细胞核的蛋白质[32]。使用Y1H进行候选蛋白质筛选,对发现新基因和研究蛋白质相互作用调控网络至关重要[33]。另外,传统筛库方法(如菌落PCR、测序)通常需逐个挑选克隆,仅能分析数十至数百个阳性克隆,难以覆盖文库的完整多样性。而NGS可一次性对数百万级克隆进行测序,理论上能覆盖酵母文库的全部基因(如酵母cDNA文库含约1.5×106个克隆),同时通过计算阳性克隆的测序读数,量化不同调控蛋白与启动子的互作频率或结合强度避免因样本量不足导致的遗漏。例如,在柑橘溃疡病研究中,利用Y1H结合NGS筛选,发现了新型转录因子CsDOF5.8与CsPrx25启动子互作,而该因子在传统候选基因筛选中常被忽略[34]。同样,本文利用Y1H和NGS筛选技术共得到读数高于100的1516个候选互作靶蛋白,其中转录因子有278个,激酶相关蛋白达405个,为解析葡萄耐盐性提供候选蛋白库。此外,构建互作蛋白的基因GO注释、KEGG,预测了多个调控蛋白的结构域(如bHLH、MYB)。
从葡萄和沉香树的基因组中分别检索到7个和14个RBOH基因,这些假定的RBOH蛋白含有保守的结构域,表明它们在遭受各种胁迫时可能参与压力诱导的ROS生成。例如,葡萄RBOHA基因启动子区域富含各种应激反应元件,包括水杨酸、盐胁迫应激反应、多种激素(脱落酸、茉莉酸甲酯、乙烯)元件以及2种转录因子(MYBs和WRKYs)的结合位点。从278个候选转录因子中随机挑选8个候选蛋白,并通过回转验证试验确认了它们与VvRBOH启动子存在相互作用,此结果证实了该文库质量及筛选效率均较好。
转录因子在调节多种植物过程的信号网络中扮演重要角色[35-36]。多个研究发现,转录因子可以发挥双重功能,既可以作为抑制因子,也可以作为激活因子,参与调控各种关键的植物生理过程。如Fc‐MYB3通过与FcRBOH启动子结合,正调节无花果响应γ射线辐射胁迫造成的ROS积累[37];WRKY转录因子BnaWSR1ca直接结合到RBOH的启动子区域,控制油菜细胞死亡和叶片衰老[38];而BdWRKY19是ROS生成的负调节因子,可降低对短柄草锈菌的抗性[39]。本文酵母单杂交试验筛选出多个转录因子,如WRKY、NAC、bZIP和bHLH等,以及多个激酶,暗示了RBOH基因在耐盐中可能受到多个转录因子与激酶的共同调控。例如,WRKY转录因子蛋白上的D基序能够被激酶MAPK磷酸化形成蛋白复合体,从而调节其分子功能拟南芥AtWRKY22和AtWRKY29基因参与MAPK磷酸化反应,最终导致AtWRKY22和AtWRKY29基因表达量上调,寄主抗性增强[40]。激酶ox1通过磷酸化RBOH,促进ROS爆发,从而进一步增强拟南芥MAPK的持续激活[41]。因此,RBOH基因可能通过磷酸化本身或通过与转录因子结合调控ROS信号通路响应葡萄包括盐胁迫在内的各种逆境。
4 结 论
本文成功构建了具有大容量和合适插入片段的酵母杂交文库,利用该文库进行酵母单杂交筛选候选蛋白效率较高,并成功鉴定出与VvRBOH启动子存在相互作用的多个候选转录因子和激酶,为深入探索ROS信号与候选转录因子途径之间的相互作用以及盐胁迫途径中的调控机制提供了新的思路。
[1] LU X,MA L,ZHANG C C,YAN H K,BAO J Y,GONG M S,WANG W H,LI S,MA S Y,CHEN B H. Grapevine (Vitis vinif‐era) responses to salt stress and alkali stress:Transcriptional and metabolic profiling[J] . BMC Plant Biology,2022,22(1):528.
[2] ALI AAZAMI M,MEHRABANI L V,HASHEMI T,HASSANPOURAGHDAM M B,RASOULI F. Soil-based nano-graphene oxide and foliar selenium and nano-Fe influence physiological responses of ‘Sultana’ grape under salinity[J] . Scientific Reports,2022,12:4234.
[3] ZHAO M X,LI J J,SHI X N,MALIK M S,QUAN Y,GUO D H,WANG L,WANG S P. Effects of exogenous plant regulators on growth and development of ‘Kyoho’ grape under salt alkali stress[J] . Frontiers in Plant Science,2023,14:1274684.
[4] ALI A,PETROV V,YUN D J,GECHEV T. Revisiting plant salt tolerance:Novel components of the SOS pathway[J] .Trends in Plant Science,2023,28(9):1060-1069.
[5] WANG L X,LIU Z G,HAN S K,LIU P,SADEGHNEZHAD E,LIU M J. Growth or survival:What is the role of calmodulinlike proteins in plant?[J] . International Journal of Biological Macromolecules,2023,242:124733.
[6] BIN L H,XU Z L,CHU Y Q,YAN Y,NIE X J,SONG W N.Genome-wide analysis of calcium-dependent protein kinase(CDPK) family and functional characterization of TaCDPK25-U in response to drought stress in wheat[J] . Environmental and Experimental Botany,2023,209:105277.
[7] XU T,ZHANG M,CHEN T C,GONG L L,HU L L,YANG J,SI H X,WU Y Y. Identification of ABA signaling pathway genes and their differential regulation in response to suboptimal light stress in grape (Vitis vinifera L.)[J] . Horticulturae,2023,9(7):789.
[8] DE ZELICOURT A,COLCOMBET J,HIRT H. The role of MAPK modules and ABA during abiotic stress signaling[J] .Trends in Plant Science,2016,21(8):677-685.
[9] ZHANG Y M,WANG Y,WEN W X,SHI Z R,GU Q S,AHAMMED G J,CAO K,SHAH JAHAN M,SHU S,WANG J,SUN J,GUO S R. Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber[J] . Autophagy,2021,17(10):2876-2890.
[10] ZHANG Y L,ZHANG Y W,LUO L,LU C Y,KONG W W,CHENG L B,XU X Y,LIU J H. Genome wide identification of Respiratory burst oxidase homolog (Rboh) genes in Citrus sinen‐sis and functional analysis of CsRbohD in cold tolerance[J] . International Journal of Molecular Sciences,2022,23(2):648.
[11] PARDO-HERNÁNDEZ M,LÓPEZ-DELACALLE M,MARTÍGUILLEN J M,MARTÍNEZ-LORENTE S E,RIVERO R M.ROS and NO phytomelatonin-induced signaling mechanisms under metal toxicity in plants:A review[J] . Antioxidants,2021,10(5):775.
[12] HASANUZZAMAN M,BHUYAN M H M B,ZULFIQAR F,RAZA A,MOHSIN S M,AL MAHMUD J,FUJITA M,FOTOPOULOS V. Reactive oxygen species and antioxidant defense in plants under abiotic stress:Revisiting the crucial role of a universal defense regulator[J] . Antioxidants,2020,9(8):681.
[13] LIU F,LIU Q,WU J H,WANG Z Q,GENG Y J,LI J,ZHANG Y,LI S. Arabidopsis calcineurin B-like-interacting protein kinase 8 and its functional homolog in tomato negatively regulates ABA-mediated stomatal movement and drought tolerance[J] .Plant,Cell & Environment,2024,47(7):2394-2407.
[14] WANG Q W,NI L,CUI Z Z,JIANG J J,CHEN C,JIANG M Y.The NADPH oxidase OsRbohA increases salt tolerance by modulating K+ homeostasis in rice[J] . The Crop Journal,2022,10(6):1611-1622.
[15] MARDANI-KORRANI F,AMOOAGHAIE R,AHADI A,GHANADIAN M. RBOH-dependent signaling is involved in He-Ne laser-induced salt tolerance and production of rosmarinic acid and carnosol in Salvia officinalis[J] . BMC Plant Biology,2024,24(1):798.
[16] WALHOUT A J M,TEMPLE G F,BRASCH M A,HARTLEY J L,LORSON M A,VAN DEN HEUVEL S,VIDAL M. GATEWAY recombinational cloning:Application to the cloning of large numbers of open reading frames or ORFeomes[J] . Methods in Enzymology,2000,328:575-592.
[17] WU B Y,LI P,HONG X F,XU C H,WANG R,LIANG Y. The receptor-like cytosolic kinase RIPK activates NADP-malic enzyme 2 to generate NADPH for fueling ROS production[J] . Molecular Plant,2022,15(5):887-903.
[18] CHENG C X,XU X Z,GAO M,LI J,GUO C L,SONG J Y,WANG X P. Genome-wide analysis of respiratory burst oxidase homologs in grape (Vitis vinifera L.)[J] . International Journal of Molecular Sciences,2013,14(12):24169-24186.
[19] WEI W,YANG Y Y,LAKSHMANAN P,KUANG J F,LU W J,PANG X Q,CHEN J Y,SHAN W. Proteasomal degradation of MaMYB60 mediated by the E3 ligase MaBAH1 causes high temperature-induced repression of chlorophyll catabolism and green ripening in banana[J] . The Plant Cell,2023,35(5):1408-1428.
[20] XU L L,XIANG G Q,SUN Q H,NI Y,JIN Z X,GAO S W,YAO Y X. Melatonin enhances salt tolerance by promoting MYB108A-mediated ethylene biosynthesis in grapevines[J] .Horticulture Research,2019,6:114.
[21] WANG L Q,XU C X,WANG C,WANG Y C. Characterization of a eukaryotic translation initiation factor 5A homolog from Tamarix androssowii involved in plant abiotic stress tolerance[J] . BMC Plant Biology,2012,12:118.
[22] 孙熹微,周丽霞,李静,杨耀东. 香水椰子cDNA酵母文库的构建及BADH2基因启动子互作蛋白的筛选鉴定[J/OL] . 分子植物育种,2023:1-16. (2023-11-15). https://kns.cnki.net/kcms/detail/46.1068.S.20231115.1458.024.html.SUN Xiwei,ZHOU Lixia,LI Jing,YANG Yaodong. Construction of Yeast cDNA library of aromatic coconut and identification of B4DH2 gene promoter interacting proteins[J/OL] . Molecular Plant Breeding,2023:1-16. (2023-11-15). https://kns.cnki.net/kcms/detail/46.1068.S.20231115.1458.024.html.
[23] LANGMEAD B,SALZBERG S L. Fast gapped-read alignment with bowtie 2[J] . Nature Methods,2012,9(4):357-359.
[24] FANG Z,ZHANG K,LI J,MA J,YE C X. Construction of a membrane yeast two-hybrid library and screening of MsPYR1-like interacting proteins in Malus sieversii[J] . Molecular Biotechnology,2025,67(6):2319-2338.
[25] XIA Y,YE Q H,LAI Z X,WANG H Q,CHUNG J P. The role of ethylene regulator MaACO2 interacting proteins in fruit ripening using nuclear yeast cDNA technology in banana[J] . Scientia Horticulturae,2025,339:113888.
[26] DENG H R,TIAN H,YANG L Y,OU S Y,WANG H,GENG G D,ZHANG S Q. Construction of a yeast hybrid library and identification of proteins regulating CaABI3/VP1-1 expression in Capsicum annuum var. conoides[J] . Phyton-International Journal of Experimental Botany,2024,93(12):3273-3291.
[27] CAO Y W,BI M M,YANG P P,SONG M,HE G R,WANG J,YANG Y,XU L F,MING J. Construction of yeast one-hybrid library and screening of transcription factors regulating LhMYBSPLATTER expression in Asiatic hybrid lilies (Lilium spp.)[J] .BMC Plant Biology,2021,21(1):563.
[28] XU Y Q,ZHOU J J,LIU Q Q,LI K P,ZHOU Y. Construction and characterization of a high-quality cDNA library of Cymbidi‐um faberi suitable for yeast one- and two-hybrid assays[J] .BMC Biotechnology,2020,20(1):4.
[29] GARCÍA-RÍOS M,FUJITA T,LAROSA P C,LOCY R D,CLITHERO J M,BRESSAN R A,CSONKA L N. Cloning of a polycistronic cDNA from tomato encoding gamma-glutamyl kinase and gamma-glutamyl phosphate reductase[J] . Proceedings of the National Academy of Sciences of the United States of America,1997,94(15):8249-8254.
[30] TIAN Y,ZENG H,WU J C,HUANG J,GAO Q,TANG D Y,CAI L P,LIAO Z Y,WANG Y,LIU X M,LIN J Z. Screening DHHCs of S-acylated proteins using an OsDHHC cDNA library and bimolecular fluorescence complementation in rice[J] . The Plant Journal,2022,110(6):1763-1780.
[31] 黄桂媛,张瑛,林玲,韩佳宇,时晓芳,曹雄军,郭荣荣. 酵母单杂交文库构建及巨峰葡萄VvFT基因启动子上游调控因子筛选[J] . 南方农业学报,2020,51(12):2875-2883.HUANG Guiyuan,ZHANG Ying,LIN Ling,HAN Jiayu,SHI Xiaofang,CAO Xiongjun,GUO Rongrong. Yeast one-hybrid library construction and screening of upstream regulators of VvFT promoter in Kyoho grape[J] . Journal of Southern Agriculture,2020,51(12):2875-2883.
[32] ZHANG L L,FU X R,YE J X,CHEN S C,JIN J Y,LIU W X,ZHANG Z H,ZHOU L J,CHEN S M,FANG W M,SONG A P,CHEN F D. CmbZIP19 inhibits lateral bud elongation via the brassinolide pathway in Chrysanthemum[J] . The Plant Journal,2025,121(6):e70080.
[33] LI Y X,TIAN X C,LIU T F,SHI Y J,LI Y H,WANG H T,CUI Y L,LU S Y,GONG X Q,MAO K,LI M J,MA F W,LI C Y.MdSINA2-MdNAC104 module regulates apple alkaline resistance by affecting γ-aminobutyric acid synthesis and transport[J] .Advanced Science,2024,11(35):2400930.
[34] FU J,FAN J,ZHANG C X,FU Y Y,XIAN B H,YU Q Y,HUANG X,YANG W,CHEN S C,HE Y R,LI Q. Highthroughput screening system of citrus bacterial canker-associated transcription factors and its application to the regulation of citrus canker resistance[J] . Journal of Integrative Agriculture,2024,23(1):155-165.
[35] RUSHTON P J,SOMSSICH I E,RINGLER P,SHEN Q J.WRKY transcription factors[J] . Trends in Plant Science,2010,15(5):247-258.
[36] GAO W,XU F C,GUO D D,ZHAO J R,LIU J,GUO Y W,SINGH P K,MA X N,LONG L,BOTELLA J R,SONG C P.Calcium-dependent protein kinases in cotton:Insights into early plant responses to salt stress[J] . BMC Plant Biology,2018,18(1):15.
[37] SONG M Y,CHEN Z Y,BAHAYIDING W,LI J P,MA H Q,WANG Z R. The transcription factor FcMYB3 responds to 60Co γ-ray irradiation of axillary buds in Ficus carica L. by activating the expression of the NADPH oxidase,FcRbohD[J] . Frontiers in Plant Science,2024,15:1476126.
[38] CUI X,ZHAO P Y,LIANG W W,CHENG Q,MU B B,NIU F F,YAN J L,LIU C L,XIE H,KAV N N V,DEYHOLOS M K,JIANG Y Q,YANG B. A rapeseed WRKY transcription factor phosphorylated by CPK modulates cell death and leaf senescence by regulating the expression of ROS and SA-synthesis-related genes[J] . Journal of Agricultural and Food Chemistry,2020,68(28):7348-7359.
[39] WANG N,FAN X,HE M Y,HU Z Y,TANG C L,ZHANG S,LIN D X,GAN P F,WANG J F,HUANG X L,GAO C X,KANG Z S,WANG X J. Transcriptional repression of TaNOX10 by TaWRKY19 compromises ROS generation and enhances wheat susceptibility to stripe rust[J] . The Plant Cell,2022,34(5):1784-1803.
[40] ZHOU X,JIANG Y J,YU D Q. WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis[J] . Molecules and Cells,2011,31(4):303-313.
[41] MA M M,WANG P,CHEN R B,BAI M,HE Z Y,XIAO D,XU G Y,WU H,ZHOU J M,DOU D L,BI G Z,LIANG X X.The OXIDATIVE SIGNAL-INDUCIBLE1 kinase regulates plant immunity by linking microbial pattern-induced reactive oxygen species burst to MAP kinase activation[J] . The Plant Cell,2024,37(1):koae311.