番木瓜(Carica papaya)属于番木瓜科番木瓜属,是一种多年生草本果树,加工型大瓜可用于提取木瓜蛋白酶,做木瓜酱菜,成熟的番木瓜果肉富含各类维生素和营养元素,具有较高的商业价值,是一种极具发展潜力的热带水果[1-2]。近年来,在广西南宁由茄腐镰刀菌(Fusarium solani)侵染导致的番木瓜枯萎病发生严重,病情发展迅速。该病症状为茎基部最先开始腐烂,最后整个植株倒伏死亡,严重制约番木瓜产业的发展[3]。目前,生产上防治番木瓜枯萎病的方法是施用化学农药,但此防治措施不环保且不利于生态环境的可持续发展,因此使用分子生物学手段及高通量测序技术研究土壤微生物多样性,寻找对病原菌有拮抗作用的有益菌,开发潜在的微生物具有重要意义。土壤中真菌、细菌的群落构成差异会影响微生物的功能多样性以及病害的发生[4-7]。杨俊誉等[8]借助高通量测序技术,针对感染白粉病以及健康状态下的草莓植株,对根际原核生物群落开展研究,结果显示,两者的群落种类大致相仿,然而相对丰度却存在极显著差异。邓晓[9]应用16S rRNA基因克隆文库技术,针对香蕉枯萎病发病样地和健康样地的土壤细菌遗传多样性进行了深入剖析与比较,发现患病样地中植株的根际以及非根际土壤细菌遗传多样性相较于健康样地的水平更低。肖蓉等[10]对患草莓炭疽病根际土壤细菌群落进行了高通量测序,发现两种生境下细菌的群落结构存在差异,且健康土壤中的有益微生物群体数量要比患病土壤高。马凤娟等[11]经研究指出,生防菌XP1 在香蕉枯萎病的综合防治体系中,展现出极为突出的效能。通过对香蕉根际微生态系统的精准调控,显著提升了根际土壤细菌群落中具有促生、抗病等功能的有益菌种群的相对丰度,从根本上优化了根际土壤微生态结构,有力保障了香蕉植株的健康生长。目前,针对番木瓜患枯萎病植株根际土壤微生物群落结构以及多样性的相关研究未有公开报道。笔者在本研究中探讨番木瓜患枯萎病对根际土壤细菌和真菌多样性的影响,从微生物学角度挖掘对番木瓜枯萎病具有抑制作用的菌群,以期为揭示番木瓜枯萎病发病机制及利用微生物生态学调控土壤环境奠定基础,并为寻找有效的番木瓜枯萎病生防菌提供理论依据。
1 材料和方法
1.1 土壤样品采集
于番木瓜枯萎病高发的4—5月期间,在广西壮族自治区南宁市番木瓜种质资源保护广西创新基地开展研究,材料选取田间严重患枯萎病的番木瓜品种穗中红植株以及健康植株。从健康植株根际采集的土壤样品标记为“ HS”,而从患病植株根际采集的土壤样品标记为“ DS”。在发病区域,运用五点取样法选取植株,每个取样点选取1 株发病植株,把5 株发病植株的根际土壤混合均匀,作为一个样本,并标记为一个重复。按照同样方式,共采集3个重复,分别标记为“ DS1”、“ DS2”和“ DS3”。对于健康植株根际土壤,采用相同方法采集,3 个重复依次标记为“ HS1”、“ HS2”和“ HS3”。采集样本将整棵植株连同根系从土里挖出,用抖根法将附在根际的土壤收集进无菌袋中,放入冰盒后,带回实验室过筛处理,采集所得的全部样品均放置于-80 ℃环境中备用。
1.2 土壤微生物总DNA提取
使用E.Z.N.A.® Soil DNA Kit 试剂盒(美国OMEGA 公司)提取根际土壤微生物DNA,按说明书操作,并通过琼脂糖凝胶电泳和紫外分光光度计检测DNA质量与浓度。
1.3 高通量测序
针对真菌ITS1~ITS2 区域,设计引物fITS7 和ITS4[12],针对细菌16S rDNA 的V3~V4 区域,设计引物338F和806R[13]。引物添加测序接头和Barcode序列后,使用Phusion® High-Fidelity PCR Master Mix进行PCR 扩增。扩增条件:98 ℃预变性30 s,98 ℃变性10 s,54 ℃复性30 s,72 ℃延伸45 s,35个循环,最后72 ℃延伸10 min。PCR产物经2%琼脂糖凝胶电泳检测,使用AMPure XT beads 回收目标片段。文库经Qubit定量和NaOH变性后,在MiSeq测序仪上进行2×300 bp 双端测序。测序与分析由广西联川生物信息科技有限公司完成。
1.4 数据处理与统计分析
采用FLASH[14]拼接双端序列,采用Vsearch[15](v2.3.4,Vsearch)过滤嵌合体。通过Vsearch算法将相似性>97%的clean tags 聚类为OTU[16]。物种注释基于Blast比对RDP[17]、Unite和NT-16S数据库,参数设置为RDP 置信度0.8,Blast 最小identity 90%,最小query覆盖度80%,最大evalue 1e-5。选取门和属水平丰度前20 的物种绘制柱状图,并进行Alpha多样性分析、Beta多样性分析、LeFSe分析。
2 结果与分析
2.1 番木瓜根际土壤真菌种群多样性和群落结构分析
2.1.1 样品测序结果分析 运用Vsearch 算法,将所有样品均一化后绘制成韦恩图,更直观地看出HS和DS 中的OTUs 数量(图1)。HS 中包含872 个OTUs,DS 中共包含854 个OTUs。HS 和DS 有613个共有OUTs,HS特有的OTUs有259个,DS中特有的OTUs 有241 个,这表明HS 中的真菌种类多于DS。OTUs丰度曲线随着测序数据量的增加逐渐趋于平缓(图2),表明测序数据量较为合理,继续增加数据对新物种(OTUs)的发现贡献有限。
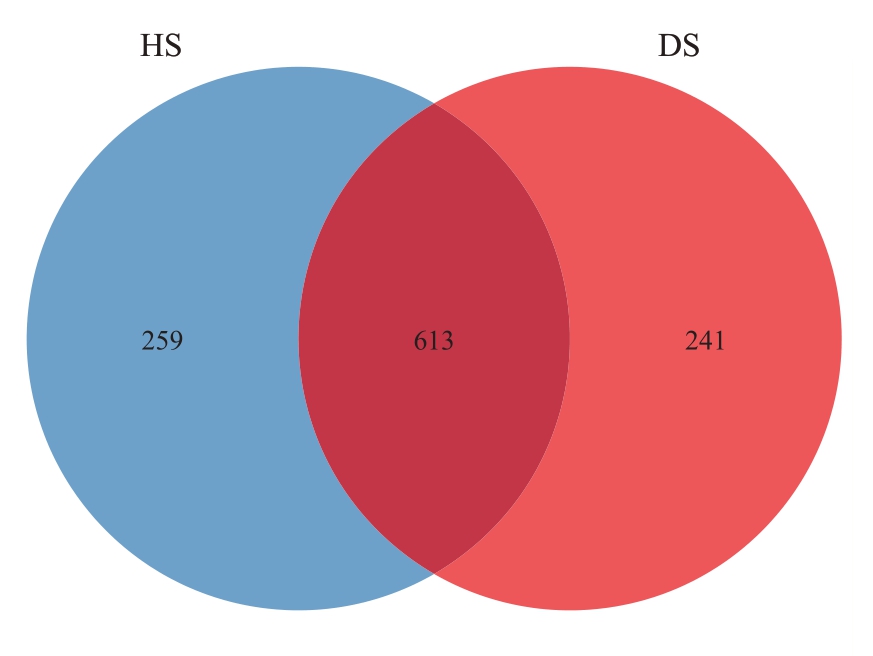
图1 番木瓜根际土壤样品真菌OTUs 韦恩图
Fig.1 Venn diagram release curve of fungal OTUs in papaya rhizosphere soil samples
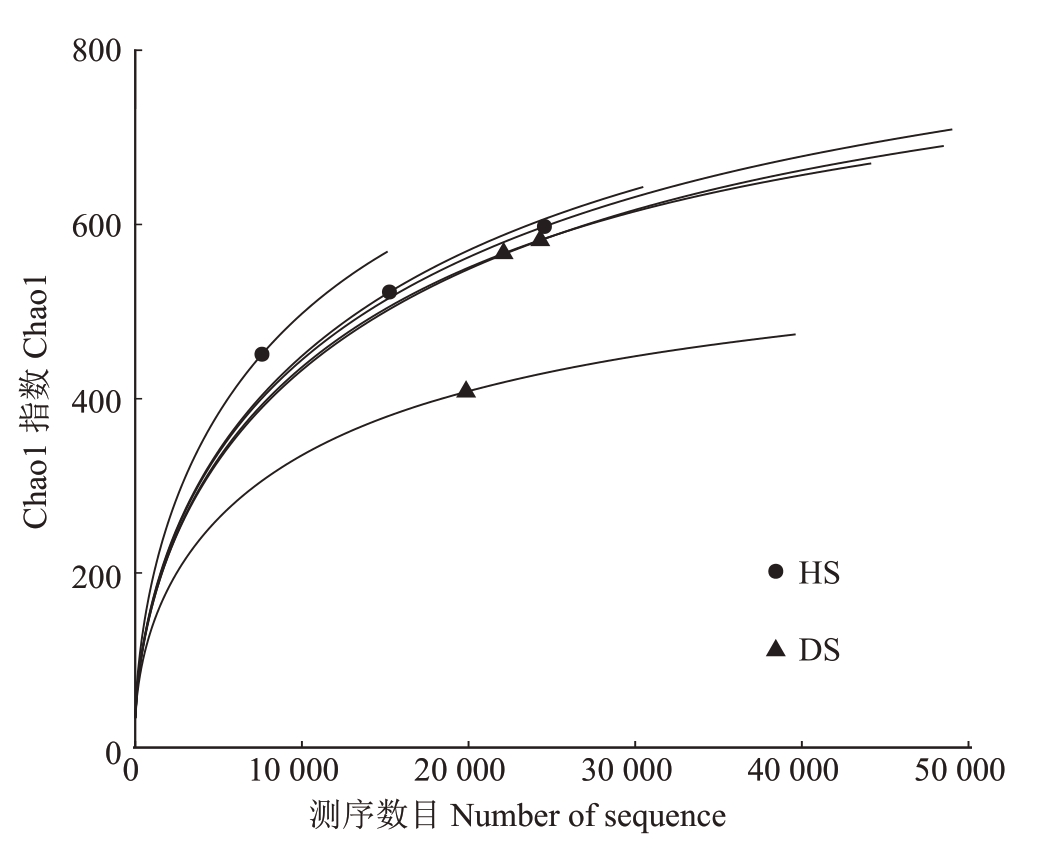
图2 番木瓜根际土壤样品真菌OTUs 丰度稀释曲线图
Fig.2 Dilution curve of fungal OTUs abundance in papaya rhizosphere soil samples
2.1.2 根际土壤真菌α 多样性分析 所有样品的Goods_coverage指数均达到99%(表1),说明测序结果能够充分反映番木瓜根际土壤真菌的多样性。HS 组的根际土壤真菌的Observed_specie、Chao1 指数分别为513.00、711.55,DS 中对应的指数分别为424.33、555.84,相比HS样品减少了20.90%、28.01%,说明HS 的真菌群落物种数目多。HS 真菌的Shannon、Simpson指数分别为5.13、0.92,DS 相对应的指数分别为4.39、0.82,相比HS的指数减少了16.86%、12.20%,说明HS 的真菌群落物种丰度以及均匀度高于DS。综上,HS的真菌群落多样性高于DS。
表1 番木瓜根际土壤真菌微生物丰富度和多样性
Table 1 Fungal community richness and diversity in the rhizosphere soil of papaya
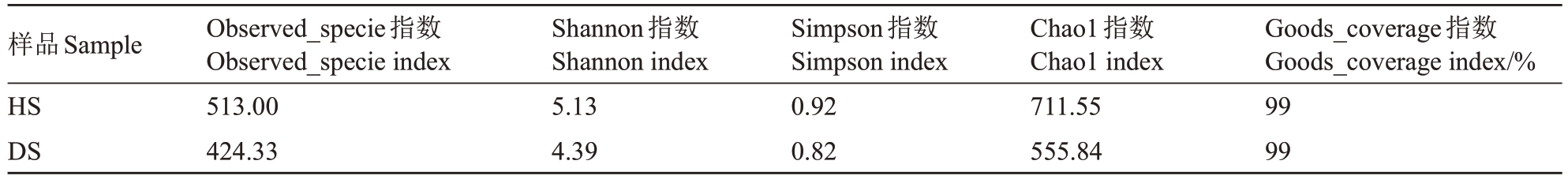
?
2.1.3 根际土壤真菌β 多样性分析 通过Weighted_UniFrac 进行PCoA 分析观察样品组之间的群落结构差异(图3),在真菌群落结构中,PCo1 为64.9%,PCo2 为22.6%,HS 组与DS 组的细菌群落结构存在差异,说明枯萎病病原菌侵染改变了番木瓜真菌微生物群落结构。
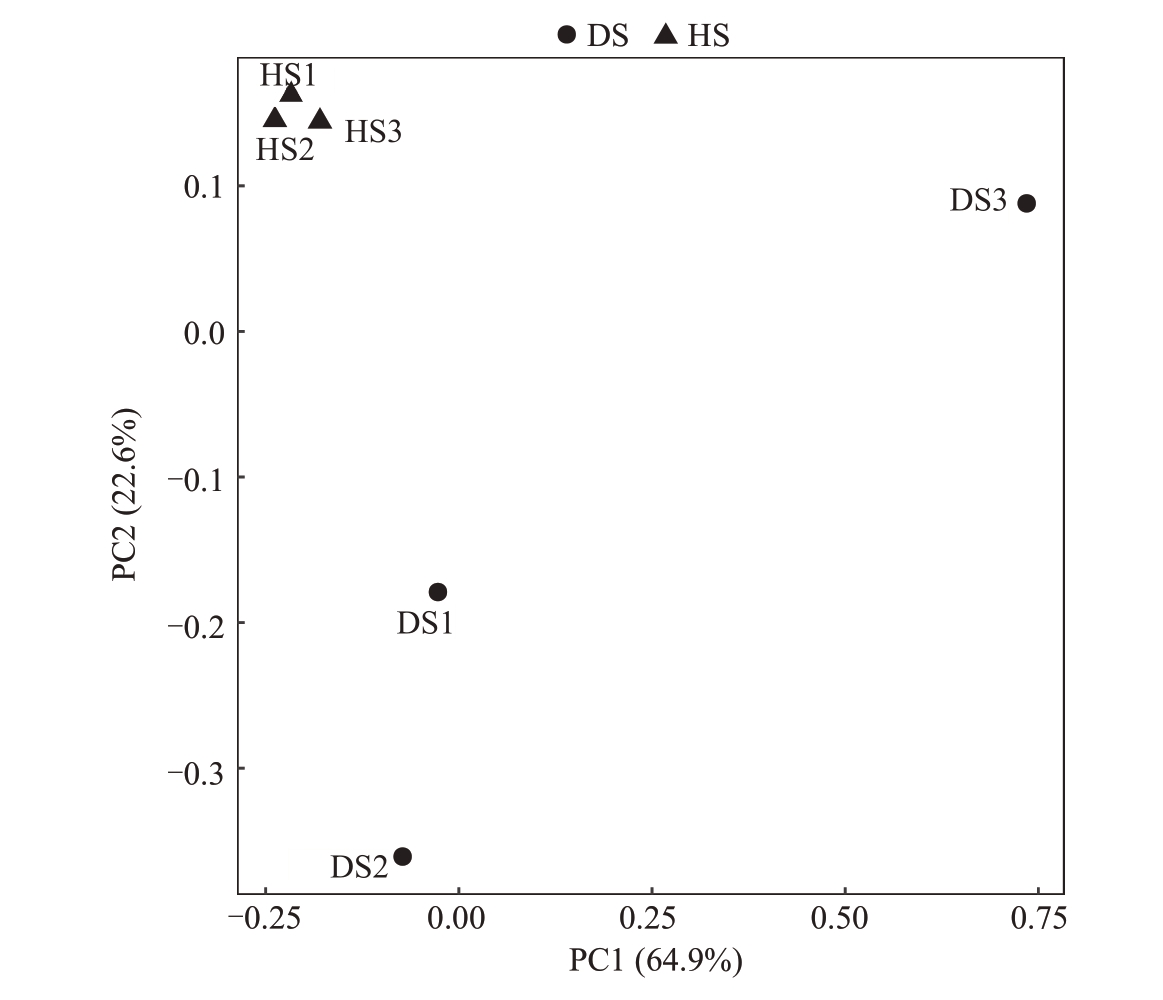
图3 番木瓜根际土壤样品真菌PCoA 2D 图
Fig.3 2D image of fungal PCoA in papaya rhizosphere soil samples
2.1.4 真菌门水平的注释及相对丰度 在真菌门的水平上共注释出6 个门,包括子囊菌门(Ascomycota)、担子菌门(Basidiomycota)、Fungi_unclassified、接合菌门(Zygomycota)、球囊菌门(Glomeromycota)、壶菌门(Chytridiomycota)(图4)。HS和DS的最优势门均为子囊菌门,HS中各个门的相对丰度分别为:子囊菌门63.16%、担子菌门33.23%、未分类真菌门1.98%、接合菌门1.06%、球囊菌门0.09%、壶菌门0.48%。DS 中各个门的相对丰度分别为:子囊菌门50.08%、担子菌门45.66%、未分类真菌门2.63%、接合菌门0.99%、球囊菌门0.59%、壶菌门0.04%。HS中的子囊菌门、接合菌门、壶菌门的相对丰度比DS高,分别是DS中对应门的1.26倍、1.12倍、12.00倍。但是DS中的担子菌门、Fungi_unclassified、球囊菌门比HS高,分别是HS中对应门的1.37倍、1.33倍、6.56倍。
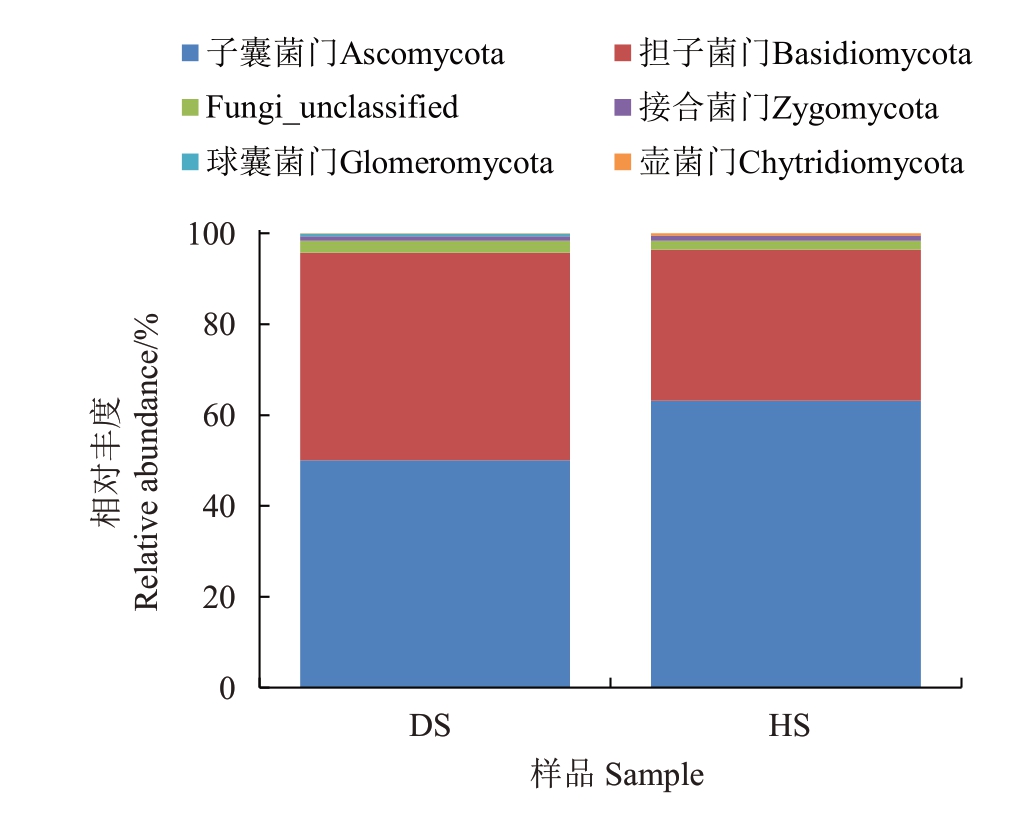
图4 两组土壤真菌在门水平上的群落组成
Fig.4 Community composition of soil fungi at the phylum level in two groups of soil samples
2.1.5 真菌属水平的注释及相对丰度 相对丰度排名前20 的真菌属(图5),在HS 中Agaricomycetes_unclassified的相对丰度为21.34%,为优势属,在DS中的相对丰度为0.01%,相差2134倍。假裸囊菌属(Pseudogymnoascus)在HS中排名第二,相对丰度为19.34%,在DS中的相对丰度为8.10%。梭孢壳属(Thielavia)的相对丰度在HS中排名第三,相对丰度为8.30%,而在DS 中的相对丰度为15.29%。HS 中根金藻属(Rhizopycnis)的相对丰度为5.43%,在DS中的相对丰度为0.61%。DS 中的优势属为锥盖伞属(Conocybe),相对丰度为18.51%,在HS中的相对丰度为0.24%,两者相差77.13 倍。DS 中的梭孢壳属(Thielavia)的相对丰度为15.29%,排名第二。弹球菌属(Sphaerobolus)在DS 中的相对丰度排名第三,为10.77%,在HS 中的相对丰度为1.63%。隐球菌属(Cryptococcus)在DS 中的相对丰度为7.06%,在HS 中的相对丰度为0.76%。其中Agaricomycetes_unclassified、假裸囊菌属(Pseudogymnoascus)、根金藻属(Rhizopycnis)、粗糙孔菌属(Trechispora)、Eurotiomycetes_unclassified、小戴卫霉属(Davidiella)、Basidiomycota_unclassified、腐质霉属(Humicola)在HS中的相对丰度均高于DS。隐球菌属(Cryptococcus)、白环蘑属(Leucoagaricus)、裸伞属(Gymnopilus)在DS中的相对丰度均高于HS。
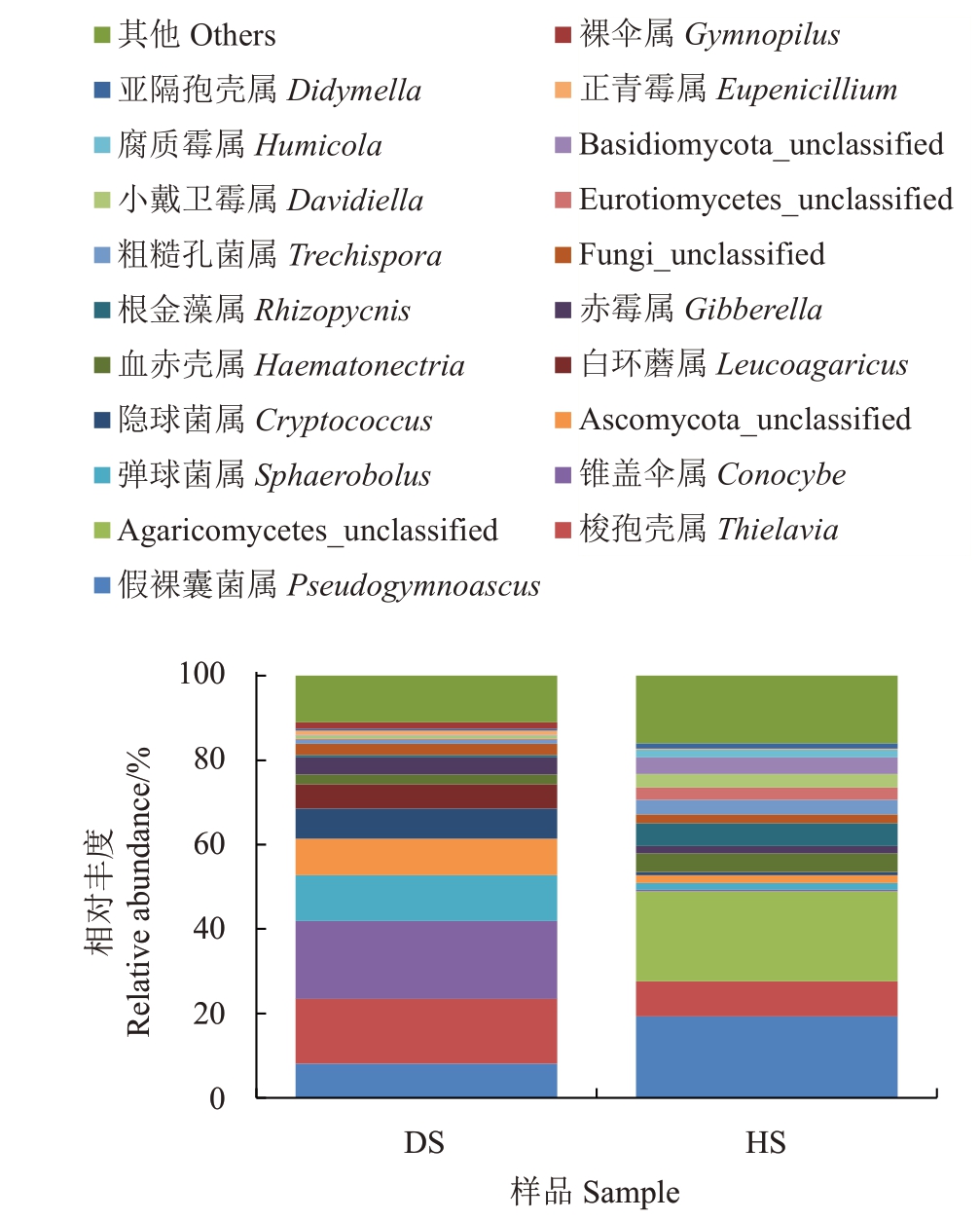
图5 两组土壤真菌在属水平上的群落组成
Fig.5 Community composition of fungi at the genus level in two groups of soil samples
2.1.6 真菌微生物差异物种分析 利用LefSe(LDA Effect Size)对患枯萎病番木瓜真菌微生物群落菌属的差异物种进行分析(图6),HS 中主要差异物种为Agaricomycetes_unclassified、假裸囊菌属(Pseudogymnoascus)、根金藻属(Rhizopycnis)等,DS中主要差异物种为隐球菌属(Cryptococcus)、白环蘑属(Leucoagaricus)等。
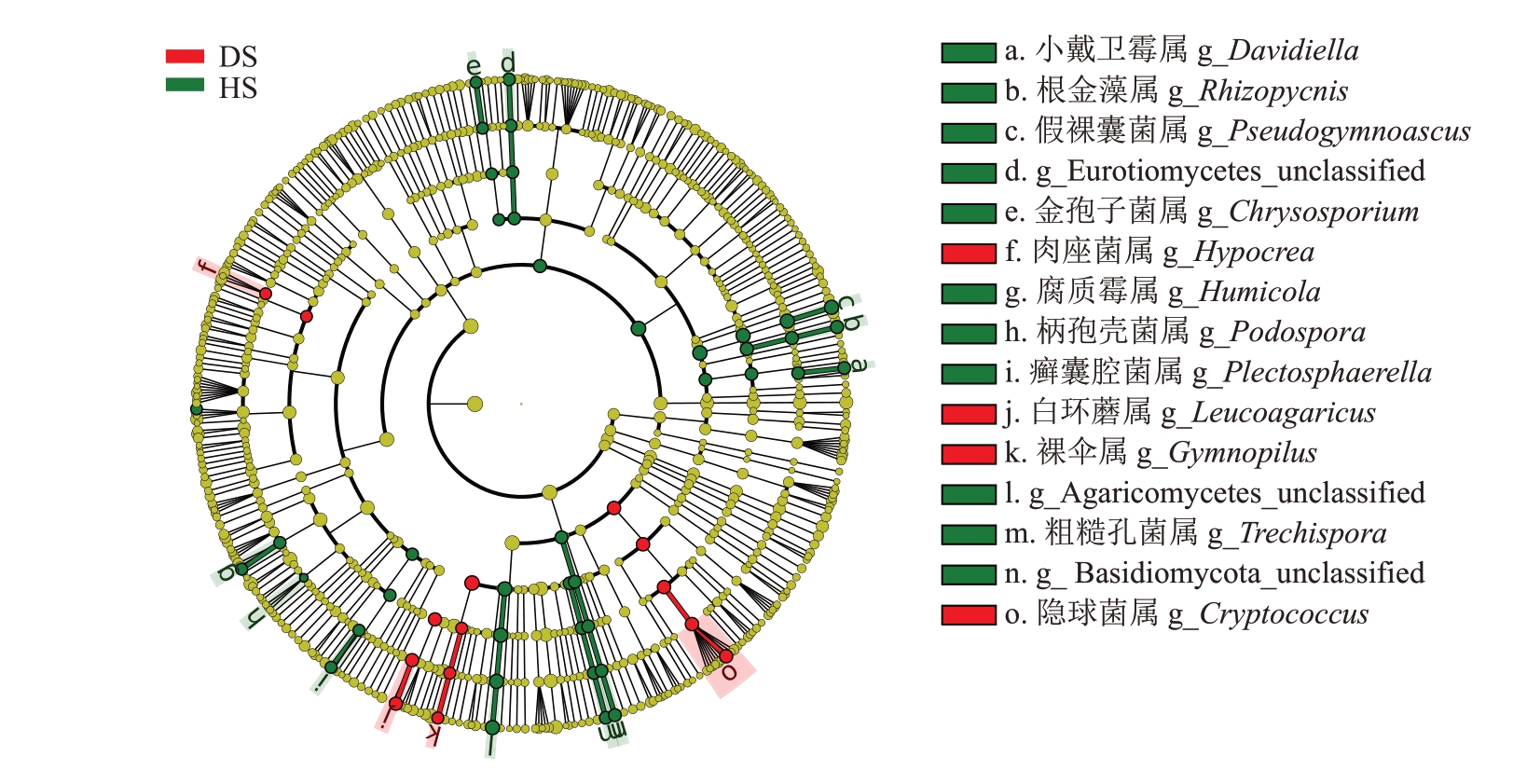
图6 两组土壤真菌菌属差异物种LefSe 分析
Fig.6 LefSe analysis of differential species of fungal genera between the two groups of soil samples
2.2 番木瓜根际土壤细菌种群多样性和群落结构分析
2.2.1 样品测序结果分析 采用Vsearch 算法,将所有样品均一化后绘制成韦恩图(图7),更直观地看出HS 和DS 中的OTUs 数量。HS 中包含7203 个OTUs,DS 中包含7976 个OTUs。HS 和DS 有5431个共有OUTs,HS 特有的OTUs 有1772 个,DS 中特有的OTUs有2545个,说明DS中的细菌种类要多于HS。随着测序规模的扩大,曲线逐渐趋于平稳(图8),这表明测序数据量已接近合理范围,继续增加数据仅能发现少量新物种(OTUs)。
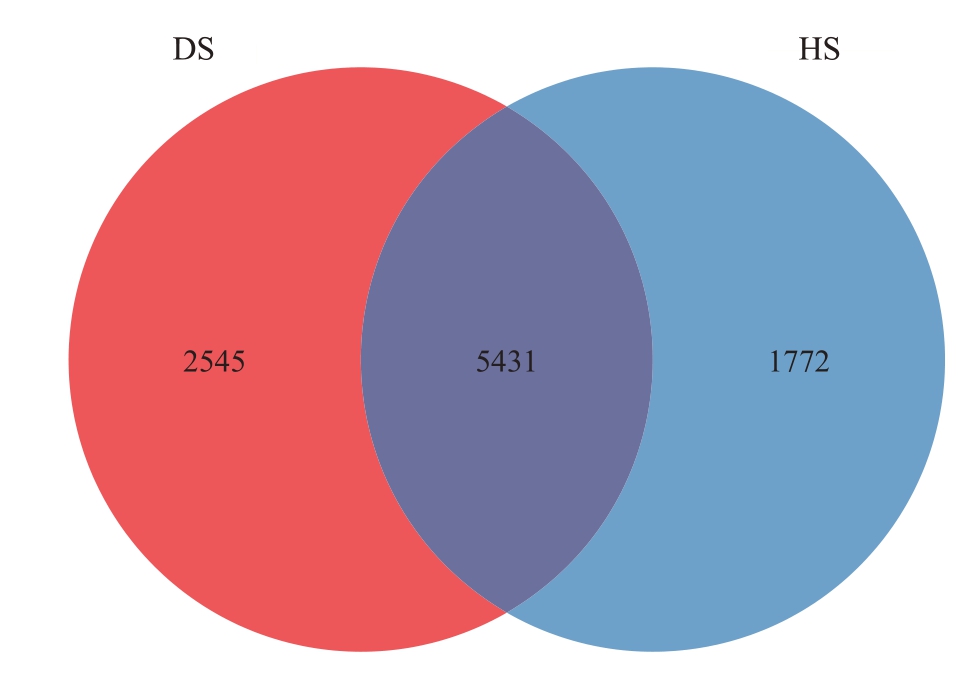
图7 番木瓜根际土壤样品细菌OTUs 韦恩图
Fig.7 Venn diagram of bacteria OTUs in papaya rhizosphere soil samples
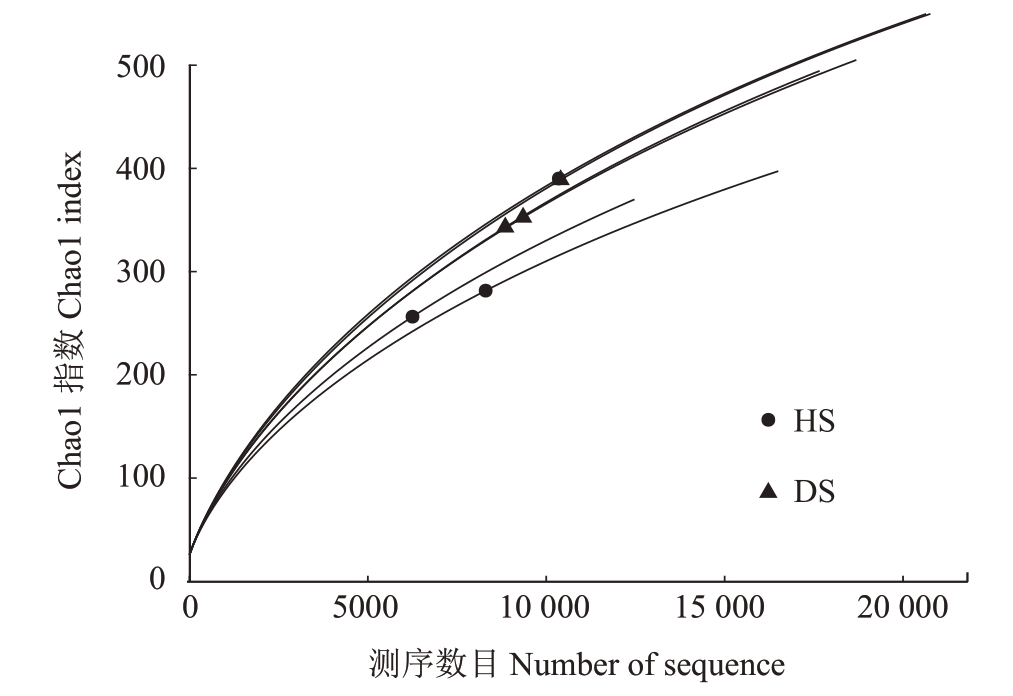
图8 番木瓜根际土壤样品细菌OTUs 丰度稀释曲线图
Fig.8 Dilution curve of bacterial OTUs abundance in papaya rhizosphere soil samples
2.2.2 根际土壤细菌α多样性分析 如表4所示,所有样品的Goods_coverage 指数达到83%,说明测序结果能够较为全面地反映番木瓜根际土壤细菌的多样性。HS 组细菌的Observed_specie、Chao1 指数分别为3 552.00、6 467.18,DS 中对应的指数分别为3 895.00、7 160.43,相比健康植株样品增加了8.86%、9.68%,说明DS的细菌群落物种数目相对较高。HS 中细菌的Shannon、Simpson 指数分别为10.49、1.00,DS 相对应的指数分别为10.70、1.00,Shannon 指数相比HS 增加了1.96%。由此可以看出,DS 的细菌群落物种丰度以及均匀度略高于HS。但是Simpson 指数达到了1.00,说明两个组中细菌种类无限多,丰富度最高,均匀度最高。综上,DS的细菌群落多样性高于HS。
表4 番木瓜根际土壤细菌丰富度和多样性
Table 4 Diversity and richness of bacterial communities in the rhizosphere soil of papaya
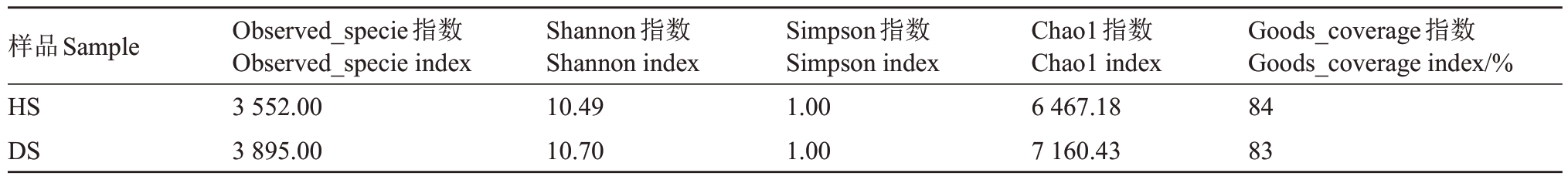
?
2.2.3 根际土壤细菌β 多样性分析 通过Weighted_UniFrac 进行PCoA 分析观察样品组之间的群落结构差异(图9),在细菌群落结构中,PCo1 为63.8%,PCo2 为14.8%,HS 组与DS 组的细菌群落结构存在差异,说明枯萎病病原菌侵染改变了番木瓜细菌微生物群落结构。
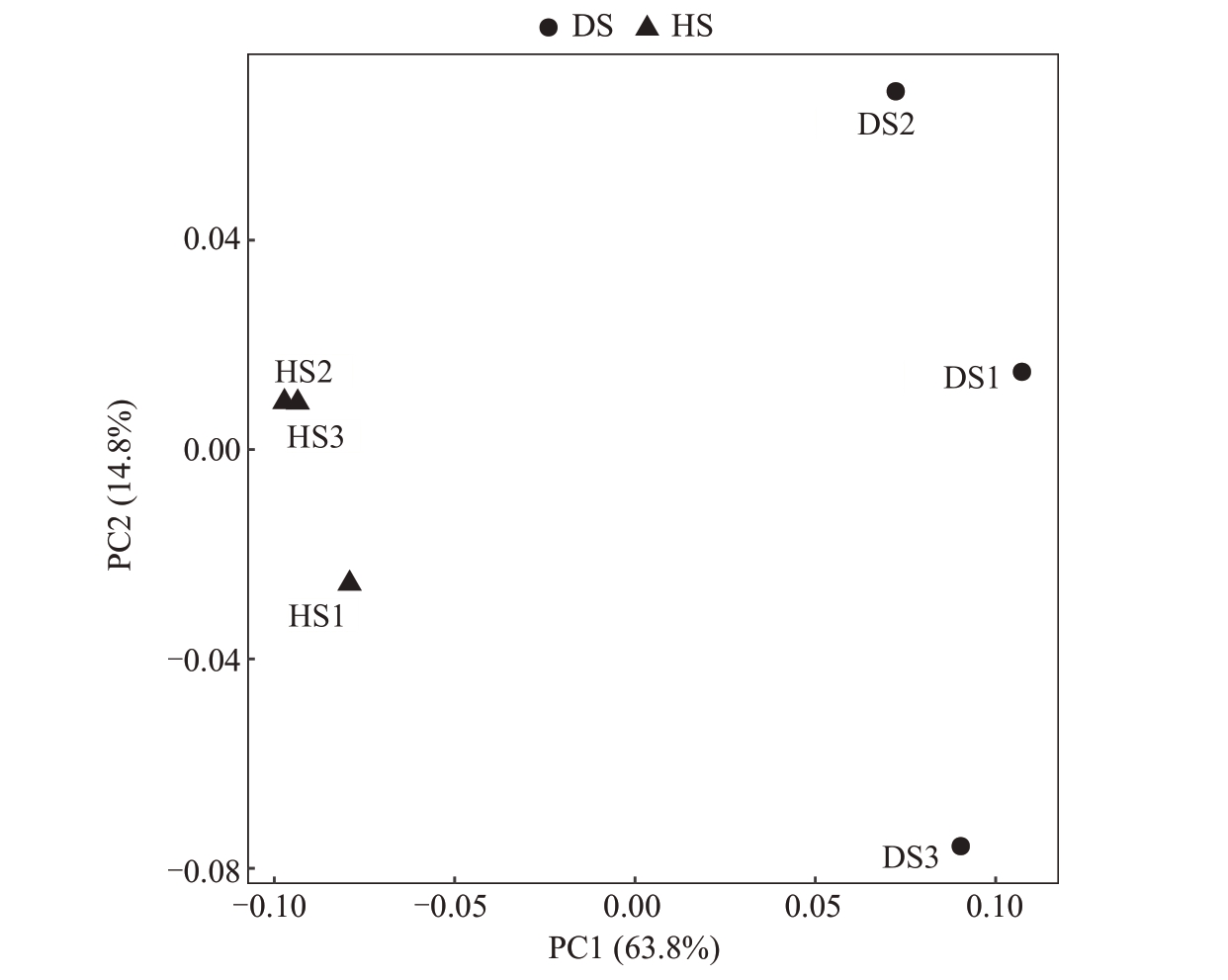
图9 番木瓜根际土壤样品细菌PCoA 2D 图
Fig.9 2D image of bacterial PCoA in papaya rhizosphere soil samples
2.2.4 细菌门水平的注释及相对丰度 在细菌门的水平上共注释出21 个门。HS 和DS 在门分类水平上相对丰度Top20 的群落组成(图10),HS 和DS 的优势门均为变形菌门(Proteobacteria),DS中的相对丰度为42.68%,HS 中的相对丰度为37.98%。相对丰度排名第二的门为放线菌门(Actinobacteria),在HS中的相对丰度为28.03%,在DS中的相对丰度为24.44%。相对丰度排名第三的门为酸杆菌门(Acidobacteria),在HS 中的相对丰度为13.46%,在DS中的相对丰度为10.51%。其余注释出的门相对丰度在10%以下,在两个组中的相对丰度相当。以上结果表明患病样本DS 中匿杆菌门(Latescibacteria)、酸杆菌门(Acidobacteria)、放线菌门(Actinobacteria)、拟杆菌门(Bacteroidetes)、硝化螺旋菌门(Nitrospirae)均低于健康样本HS,而栖热菌门(Deinococcus-Thermus)、变形菌门(Proteobacteria)、Candidatus_Saccharibacteria、绿弯菌门(Chloroflexi)、厚壁菌门(Firmicutes)、Parcubacteriacandidate_division_WPS-2均高于健康样本HS。
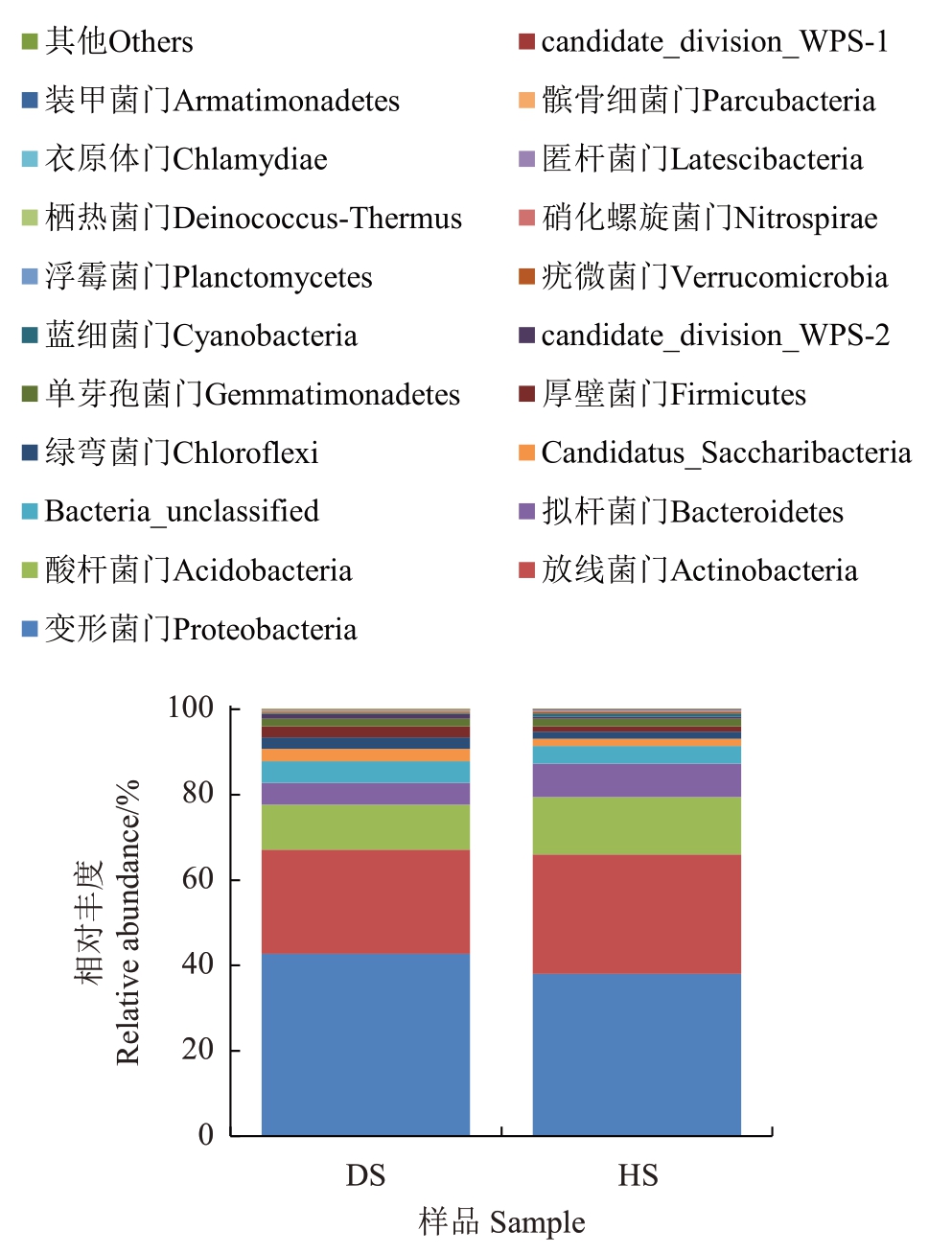
图10 两组土壤细菌在门水平上的群落组成
Fig.10 Community composition of soil bacteria at the phylum level in two groups of soil samples
2.2.5 细菌属水平的注释及相对丰度 属水平上共注释出601个属,列出了相对丰度排名前20的属(图11),其他属归类为Others。HS 中Others 的相对丰度为50.89%,在DS中的相对丰度为49.79%,两者相差不大。除了Others外,在HS中相对丰度排名第一的属为Actinomycetales_unclassified,相对丰度为7.52%,在DS中相对丰度为6.54%。DS中除了Others 外,相对丰度排名第一的属为朱氏杆菌属(Chujaibacter),相对丰度为7.00%,该属在HS 中的相对丰度为4.33%。相对丰度排名前20 的属中,Actinomycetales_unclassified 放线菌目未分纲、Gp3、Gaiella、Gp1、Thermomonosporaceae_unclassified 高温单胞菌科未分属、黄杆菌属(Flavobacterium)在DS 样本中的相对丰度要低于HS;而朱氏杆菌属(Chujaibacter)、Bacteria_unclassified、Saccharibacteria_genera_incertae_sedis、Acidimicrobiales_unclassified 酸微菌目未分科、硫杆菌属(Thiobacillus)、黄色类固醇杆菌属(Steroidobacter)的相对丰度要高于HS。
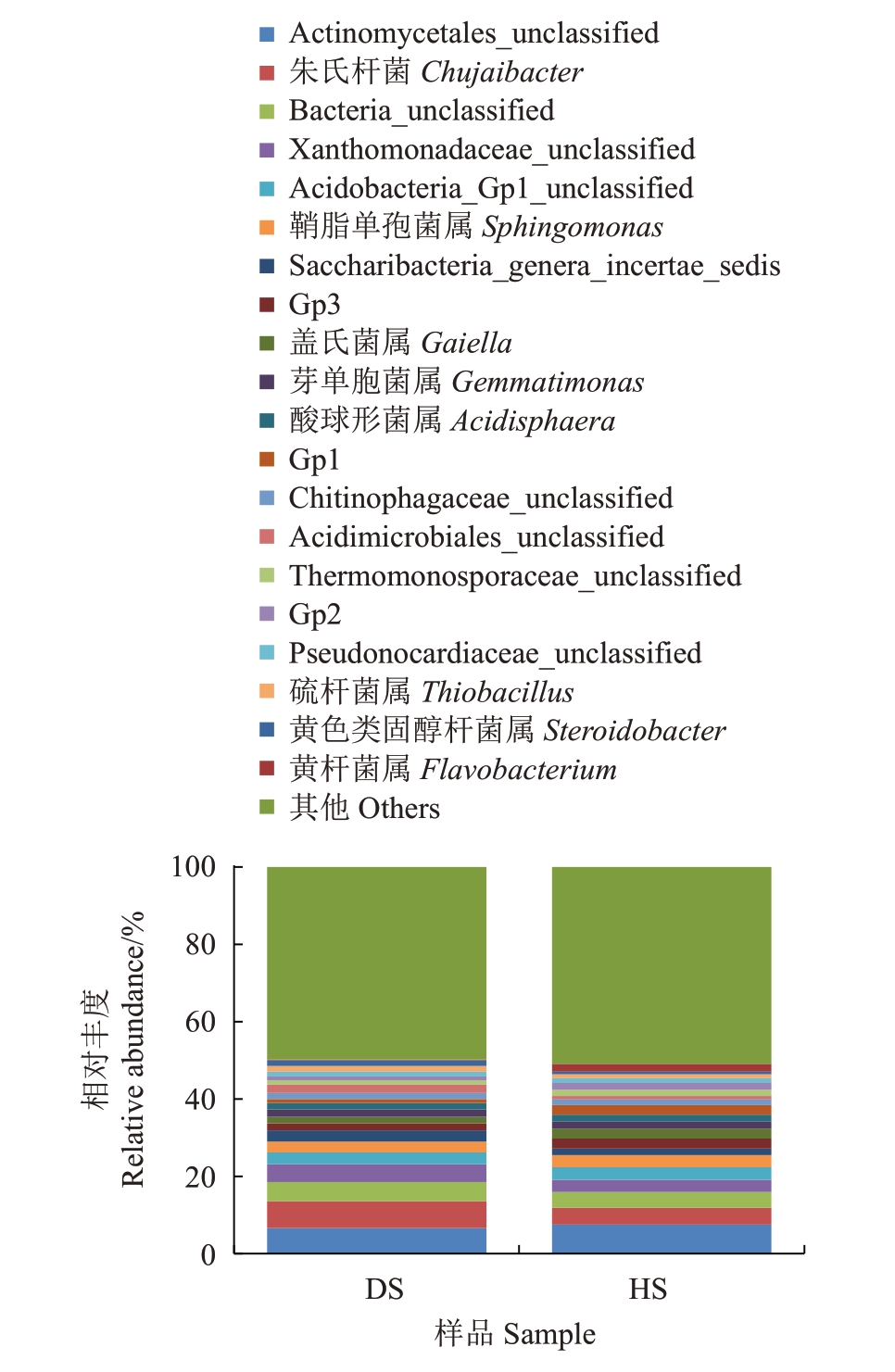
图11 两组土壤细菌在属水平上的群落组成
Fig.11 Community composition of two groups of soil bacteria at the genus level
2.2.6 细菌微生物差异物种分析 利用LefSe(LDA Effect Size)对患枯萎病番木瓜细菌微生物群落菌属的差异物种进行分析(图12),HS中主要差异物种为Actinomycetales_unclassified、黄杆菌属(Flavobacterium)、Gp1等,DS中主要差异物种为朱氏杆菌属(Chujaibacter)、黄色类固醇杆菌属(Steroidobacter)等。
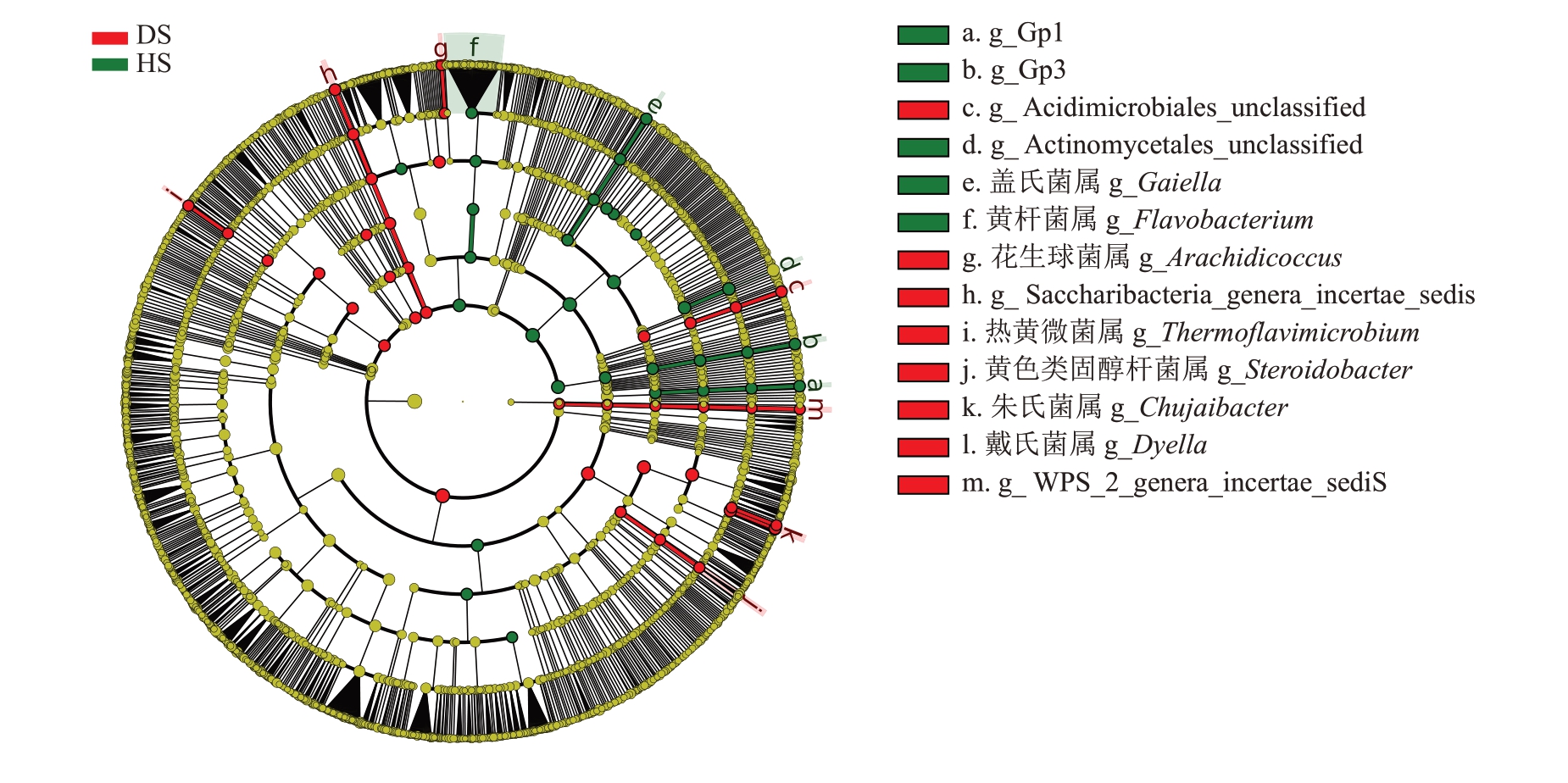
图12 两组土壤细菌菌属差异物种LefSe 分析
Fig.12 LefSe analysis of differential species of soil bacterial genera of between two groups of soil samples
3 讨 论
3.1 番木瓜患枯萎病对植株根际土壤真菌群落组成和丰度的影响
本研究结果表明,子囊菌门在两组样品真菌门类中均为优势门,其在HS 组中的相对丰度高于DS组。担子菌门在两个组中的相对丰度同样较高,HS组中占比33.23%,DS组中则更高,达45.66%。子囊菌门是当下已知真菌里规模最为庞大的一个门,其已知种类数量超过了64 000多种[18]。该门微生物可通过腐生、寄生等方式获取营养,其中部分寄生性子囊菌会导致植物病害,症状表现为枝枯、叶斑、根腐、茎腐和果(穗)腐等[19]。担子菌包括有益和有害的种类,有害种类会导致许多农作物的病害,如黑粉菌和锈菌;而有益种类中,部分具备分解纤维素与木质素的能力,而且其中一部分还能与植物构建共生关系,进而形成菌根,有助于植株更好地利用和吸收养分[20]。在本研究中,由于番木瓜患枯萎病,首先是生长点出现萎蔫、坏死现象,担子菌门在患病植株中的相对丰度比健康植株更高,推测可能是参与了植株的分解过程,最后导致整株枯死。其余4 个真菌门的相对丰度较低,接合菌门部分种类能够与植物形成菌根,少数种类可寄生植物、人和动物体上并引发病害[19]。此外,还有一个未分类的真菌门,其中是否存在潜在的拮抗菌类群,还有待进一步探索。
在番木瓜根际土壤真菌群落结构的属水平上,HS 组中Agaricomycetes_unclassified 呈现高度富集状态,其分类地位为伞菌纲担子菌门未分类科,可能其在抵御番木瓜枯萎病方面具有显著作用,与番木瓜的健康状态密切相关,具体菌种还有待进一步研究确定。假裸囊菌属(Pseudogymnoascus)、粗糙孔菌属(Trechispora)等菌属在HS 中的相对丰度均高于DS。研究发现,假裸囊菌具有抵抗极端环境以及进行特殊生理代谢的能力[21],也有研究表明,粗糙孔菌属极可能具备生物防治的功能[22]。在患病土壤中,锥盖伞属(Conocybe)高度富集,弹球菌属(Sphaerobolus)、隐球菌属(Cryptococcus)、梭孢壳属(Thielavia)等在DS中的相对丰度均高于HS。锥盖伞属(Conocybe)对土壤中的纤维素具有较强的分解活性[20]。有研究指出,在香榧根腐病中,隐球菌属(Cryptococcus)在病株土壤中丰度增加,可能是香榧根腐病的致病菌[23],在番木瓜枯萎病中,锥盖伞属(Conocybe)、孢壳属(Thielavia)等这些真菌属,在侵染植株导致枯萎病发生的过程中可能起到关键作用。
3.2 番木瓜患枯萎病对植株根际土壤细菌群落组成和丰度的影响
研究结果表明,在门水平上,HS与DS中细菌的优势门均为变形菌门(Proteobacteria),其中,放线菌门、酸杆菌门在HS 中的相对丰度较高,变形菌门在DS 中的相对丰度较高。酸杆菌门细菌具备合成多种生物活性物质(如抗生素、抗真菌及抗病毒化合物)的遗传基础,在植物生长发育中扮演着重要角色[24]。据报道,放线菌能够抑制病原菌微生物的生长、提高植物抗病能力并促进植物生长[25]。研究表明,在健康植株上酸杆菌门和放线菌门呈现富集状态[26],与本研究结果类似,这表明这两种菌门在番木瓜健康生长中发挥着重要作用,番木瓜患病后,根际土壤中这两种门类的细菌可抵御病原体的侵害。有研究指出,变形菌门中多为病原菌,在有病害的样品中相对丰度较高[27],与本研究结果一致。
本研究结果表明,Actinomycetales_unclassified(7.52%)和朱氏杆菌属(Chujaibacter)(7.00%)分别是HS 与DS 中相对丰度排名第一的属。黄杆菌属(Flavobacterium)的相对丰度在HS 中较高,黄杆菌属(Flavobacterium)在病原细菌侵染植株时可抑制其发展[28]。朱氏杆菌属(Chujaibacter)、黄色类固醇杆菌属(Steroidobacter)等的相对丰度在DS中较高,有研究认为朱氏杆菌属(Chujaibacter)为有益菌[29-30],可能是番木瓜患病后,会吸引有益菌属来抵御病原菌侵害,增强植株抵抗力,因此番木瓜枯萎病的根际土壤中朱氏杆菌属(Chujaibacter)的数量增多。
4 结 论
番木瓜枯萎病导致根际土壤细菌的多样性提高,真菌的丰富度下降,进而使番木瓜植株根际细菌、真菌群落的结构发生变化。在微生物群落中,部分有益菌发挥优势来抵御番木瓜枯萎病病原菌,同时也有致病菌参与到患病植株的致病过程,最后导致植株死亡。这些微生物的变化对番木瓜枯萎病以及健康植株生长产生的影响还需要通过对菌种分离纯化及鉴定培养进行研究。本研究结果为揭示番木瓜枯萎病的发生机制及寻找有效的番木瓜枯萎病生防菌提供了理论依据。
[1] 秦溱.番木瓜的应用价值与开发利用研究进展[J].食品工业,2017,38(1):234-237.QIN Qin. Research progress on application value and development of Papaya[J].The Food Industry,2017,38(1):234-237.
[2] 郭文场,丁向清,周淑荣,包秀芳,刘佳贺,尚建勋.番木瓜的种植和开发利用[J].特种经济动植物,2012,15(9):43-46.GUO Wenchang,DING Xiangqing,ZHOU Shurong,BAO Xiufang,LIU Jiahe,SHANG Jianxun. Cultivation and development utilization of Papaya[J]. Special Economic Animal and Plant,2012,15(9):43-46.
[3] 冯岩,陈健,罗金棠,夏恭澍,杜洪忠.中国番木瓜枯萎病病原菌的首次鉴定[J].植物检疫,2011,25(1):55-59.FENG Yan,CHEN Jian,LUO Jintang,XIA Gongshu,DU Hongzhong.First identification on pathogen of Papaya wilt disease in China[J].Plant Quarantine,2011,25(1):55-59.
[4] 杨珍,戴传超,王兴祥,李孝刚.作物土传真菌病害发生的根际微生物机制研究进展[J].土壤学报,2019,56(1):12-22.YANG Zhen,DAI Chuanchao,WANG Xingxiang,LI Xiaogang. Advance in research on rhizosphere microbial mechanisms of crop soil-borne fungal diseases[J].Acta Pedologica Sinica,2019,56(1):12-22.
[5] HARTMANN M,FREY B,MAYER J,MÄDER P,WIDMER F.Distinct soil microbial diversity under long-term organic and conventional farming[J]. The ISME Journal,2015,9(5):1177-1194.
[6] LARKIN R P. Soil health paradigms and implications for disease management[J]. Annual Review of Phytopathology,2015,53:199-221.
[7] XIONG W,ZHAO Q Y,ZHAO J,XUN W B,LI R,ZHANG R F,WU H S,SHEN Q R.Different continuous cropping spans significantly affect microbial community membership and structure in a vanilla-grown soil as revealed by deep pyrosequencing[J].Microbial Ecology,2015,70(1):209-218.
[8] 杨俊誉,魏世杰,苏代发,张振荣,陈杉艳,罗志伟,沈雪梅,赖泳红,JAMIL A,童江云,崔晓龙.温室中患白粉病与健康草莓植株根际原核生物群落的比较研究[J].西南农业学报,2020,33(1):85-91.YANG Junyu,WEI Shijie,SU Daifa,ZHANG Zhenrong,CHEN Shanyan,LUO Zhiwei,SHEN Xuemei,LAI Yonghong,JAMIL A,TONG Jiangyun,CUI Xiaolong. Study on prokaryotic communities in rhizospheres of powdery mildew-infected and non-infected strawberry in greenhouse by high-through sequencing technology[J]. Southwest China Journal of Agricultural Sciences,2020,33(1):85-91.
[9] 邓晓.香蕉枯萎病区土壤微生物多样性研究[D].海口:海南大学,2012.DENG Xiao. Study on soil microbial diversity in wards infected by banana Fusarium wilt[D].Haikou:Hainan University,2012.
[10] 肖蓉,曹秋芬,聂园军,张春芬,邓舒,孙海峰,李倩.基于高通量测序患炭疽病草莓根际与健康草莓根际细菌群落的比较研究[J].中国农学通报,2017,33(11):14-20.XIAO Rong,CAO Qiufen,NIE Yuanjun,ZHANG Chunfen,DENG Shu,SUN Haifeng,LI Qian.A comparative study on rhizosphere soil bacterial communities of healthy strawberry and infected strawberry with anthracnose by high-throughput sequencing[J]. Chinese Agricultural Science Bulletin,2017,33(11):14-20.
[11] 马凤娟,孙杰,徐培智,解开治,李夏,顾文杰,卢钰升,徐如玉.生防菌XP1 对香蕉枯萎病防效及土壤细菌群落多样性的影响[J].南方农业学报,2019,50(9):1981-1989.MA Fengjuan,SUN Jie,XU Peizhi,XIE Kaizhi,LI Xia,GU Wenjie,LU Yusheng,XU Ruyu. Effects of biocontrol bacteria XP1 on banana Fusarium wilt and diversity of bacterial community in soil[J]. Journal of Southern Agriculture,2019,50(9):1981-1989.
[12] KARLSSON I,FRIBERG H,STEINBERG C,PERSSON P.Fungicide effects on fungal community composition in the wheat phyllosphere[J].PLoS One,2014,9(11):e111786.
[13] FADROSH D W,MA B,GAJER P,SENGAMALAY N,OTT S,BROTMAN R M,RAVEL J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform[J].Microbiome,2014,2(1):6.
[14] MAGOČ T,SALZBERG S L. FLASH:Fast length adjustment of short reads to improve genome assemblies[J].Bioinformatics,2011,27(21):2957-2963.
[15] ROGNES T,FLOURI T,NICHOLS B,QUINCE C,MAHÉ F.VSEARCH:A versatile open source tool for metagenomics[J].PeerJ,2016,4:e2584.
[16] REN L L,ZHANG R B,RAO J,XIAO Y,ZHANG Z,YANG B,CAO D P,ZHONG H,NING P,SHANG Y,LI M K,GAO Z C,WANG J W.Transcriptionally active lung microbiome and its association with bacterial biomass and host inflammatory status[J].mSystems,2018,3(5):e00199-18.
[17] MEYER F,PAARMANN D,D’SOUZA M,OLSON R,GLASS E M,KUBAL M,PACZIAN T,RODRIGUEZ A,STEVENS R,WILKE A,WILKENING J,EDWARDS R A. The metagenomics RAST server- a public resource for the automatic phylogenetic and functional analysis of metagenomes[J].BMC Bioinformatics,2008,9:386.
[18] FREY S D,KNORR M,PARRENT J L,SIMPSON R T.Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests[J]. Forest Ecology and Management,2004,196(1):159-171.
[19] 谢联辉.普通植物病理学[M].北京:科学出版社,2006.XIE Lianhui. General plant pathology[M]. Beijing:Science Press,2006.
[20] 胡骞予,赵娅红,吕怡颖,邓晨宵,肜磊,卢超,余磊,戴利利,齐颖,高鹏华,蔡宪杰,闫鼎,黄飞燕,韩天华.生物炭不同施用量对烟草根际土壤真菌群落结构的影响[J].西南农业学报,2023,36(10):2254-2260.HU Qianyu,ZHAO Yahong,LÜ Yiying,DENG Chenxiao,RONG Lei,LU Chao,YU Lei,DAI Lili,QI Ying,GAO Penghua,CAI Xianjie,YAN Ding,HUANG Feiyan,HAN Tianhua.Effect of different application of biochar application on fungal community structure in tobacco rhizosphere soil[J]. Southwest China Journal of Agricultural Sciences,2023,36(10):2254-2260.
[21] 吴振强,戴瑞卿,赖宝春,林明辉,王家瑞.健康与感染枯萎病辣椒植株根际土壤真菌群落结构与多样性[J].现代农业科技,2020(7):184-187.WU Zhenqiang,DAI Ruiqing,LAI Baochun,LIN Minghui,WANG Jiarui. Fungus community structure and diversity of rhizosphere soil of healthy and Fusarium wilt chilli plants[J].Modern Agricultural Science and Technology,2020(7):184-187.
[22] 陈海生,陈韬略,蔡林生,李振宇,方昉,金思远.猕猴桃根腐病根际土壤酶活性及真菌群落组成研究[J].中国南方果树,2024,53(2):144-151.CHEN Haisheng,CHEN Taolue,CAI Linsheng,LI Zhenyu,FANG Fang,JIN Siyuan. Research on soil enzyme activity and fungal community structure in rhizosphere soil of Actinidia chinensis Planch.with root rot[J].South China Fruits,2024,53(2):144-151.
[23] 冯雨星.香榧根腐病株根区土壤微生物群落特征研究[D].杭州:浙江农林大学,2019.FENG Yuxing.Characterization of microbial communities in the root zone soil of Torreya grandis cv.Merrillii suffering from rootrot disease[D].Hangzhou:Zhejiang A&F University,2019.
[24] KALAM S,BASU A,AHMAD I,SAYYED R Z,EL-ENSHASY H A,DAILIN D J,SURIANI N L. Recent understanding of soil acidobacteria and their ecological significance:A critical review[J].Frontiers in Microbiology,2020,11:580024.
[25] 严佳成,李艳如,陈让让,吴洁,蒋继宏.植物内生放线菌研究进展[J].生物资源,2020,42(1):9-21.YAN Jiacheng,LI Yanru,CHEN Rangrang,WU Jie,JIANG Jihong. Advances on plant endophytic actinomycetes[J]. Biotic Resources,2020,42(1):9-21.
[26] 张仁军,陈雅琼,张洁梅,姚正平,吴金虎,侯正学,殷红慧,徐天养,欧阳进,王亮,陈穗云.健康与根结线虫病烟田根际土壤微生物群落对比分析[J].中国农学通报,2021,37(26):124-132.ZHANG Renjun,CHEN Yaqiong,ZHANG Jiemei,YAO Zhengping,WU Jinhu,HOU Zhengxue,YIN Honghui,XU Tianyang,OUYANG Jin,WANG Liang,CHEN Suiyun.Comparative analysis of microbial communities in rhizosphere soil of healthy and root-knot nematodes-infected tobacco fields[J].Chinese Agricultural Science Bulletin,2021,37(26):124-132.
[27] 刘浩浩,钟彩虹,刘巍,李黎,黄丽丽.猕猴桃溃疡病不同发病程度下花部微生物群落结构和多样性分析[J]. 果树学报,2024,41(11):2323-2334.LIU Haohao,ZHONG Caihong,LIU Wei,LI Li,HUANG Lili.Microbial community structure and diversity of kiwifruit flowers infected by different degrees of bacterial canker[J]. Journal of Fruit Science,2024,41(11):2323-2334.
[28] 李想,殷全玉.健康和易感青枯病烟田土壤细菌群落结构特征及其标志菌[J].中国烟草科学,2024,45(3):68-76.LI Xiang,YIN Quanyu. Soil bacterial community structure and its signature bacteria in healthy and susceptible to tobacco bacterial wilt soils[J].Chinese Tobacco Science,2024,45(3):68-76.
[29] 番华彩,魏薇,曾莉,徐胜涛,李舒,郭志祥,郑泗军.香蕉枯萎病和健康植株根际土壤细菌群落结构差异对比分析[J].西南农业学报,2021,34(9):1885-1891.FAN Huacai,WEI Wei,ZENG Li,XU Shengtao,LI Shu,GUO Zhixiang,ZHENG Sijun. Comparative analysis on difference of bacterial community structure in rhizosphere soil between banana Fusarium wilt and healthy plants[J].Southwest China Journal of Agricultural Sciences,2021,34(9):1885-1891.
[30] 余高,赵仕龙,孙约兵,罗有亮,陈芬.覆盖材料对云贵高原柑橘园土壤理化性质和细菌群落特征以及柑橘品质的影响[J].生态学报,2024,44(8):3408-3422.YU Gao,ZHAO Shilong,SUN Yuebing,LUO Youliang,CHEN Fen.Effects of mulching materials on soil physio-chemical properties,bacterial community characteristics,and citrus quality in orange orchards on the Yunnan-Guizhou Plateau[J].Acta Ecologica Sinica,2024,44(8):3408-3422.