西瓜作为全球重要的经济作物,2023年全球种植面积超过304万hm2[1]。在其栽培过程中常受到高温胁迫的限制,不仅抑制西瓜的生长发育,还会显著降低其产量和品质[2]。同时连作障碍与土传病害对产业影响严重,导致减产30%~50%,严重时甚至绝收[3]。传统化学防治引发生态污染,而抗病育种周期长(8~10年)。因此,嫁接技术成为综合防控最有效的绿色手段[4],近年来被广泛应用于改善作物的抗病性,耐低温、耐旱等耐逆性和抗连作栽培等方面[5-7]。
西瓜苗期是生理代谢的敏感期,高温会触发一系列连锁反应,包括叶片厚度、栅栏组织厚度、海绵组织厚度和组织紧密度变化,叶绿体膜稳定性受破坏和PSⅡ反应中心失活等光合系统的损伤,Fv/Fm参数下降,净光合速率、蒸腾速率和气孔导度降低,发生氧化应激、膜脂过氧化、渗透调节失衡及信号通路调控异常,最终抑制幼苗生长、降低存活率,且不同西瓜品种在耐高温方面存在一定差异[8-9]。嫁接技术能够缓解高温胁迫对西瓜生长的抑制,提高光合效率,并增强抗氧化酶活性,从而促进植物的生物量积累和提升整体耐热性[10]。此外,不同砧木对嫁接西瓜的光合特性也有显著影响。研究发现,南瓜砧木嫁接的西瓜在高温条件下表现出更高的叶绿素含量、净光合速率(Pn)和叶绿素荧光参数(如Fv/Fm),表明嫁接可能通过改善光合系统来提高西瓜的高温耐性[11-12]。在叶片颜色方面,嫁接西瓜表现出与自根苗不同的变化趋势。例如,葫芦砧木嫁接的西瓜在高温胁迫下叶片颜色更加鲜绿,而自根苗则出现褪绿现象[13-14]。叶绿素含量是衡量植物光合能力的重要指标。研究显示,在嫁接瓜类中叶绿素含量通常高于自根苗,这可能是由于嫁接技术提高了根系的吸收能力和叶片的光合效率[15-16]。过氧化物酶(POD)作为植物抗氧化系统的重要组成部分,其活性在嫁接西瓜中高于自根苗,这表明嫁接可通过增强抗氧化酶活性来减轻高温引起的氧化损伤[17-18]。此外,嫁接还能够降低叶片中丙二醛(MDA)含量,进一步证明了嫁接技术对高温胁迫的缓解作用[13-14,19]。
嫁接西瓜的主要砧木类型有南瓜、葫芦和野生西瓜。其中,野生西瓜砧木可提高西瓜果实番茄红素等营养物质含量,近年来已成为西瓜嫁接砧木的重要应用类型之一[20]。野生西瓜砧木嫁接成活率显著高于南瓜砧木,同时果实可溶性糖含量、番茄红素含量显著高于南瓜砧木,且无南瓜砧木常见的异味问题[21-23]。因此,在生产中成为高品质精品西瓜嫁接栽培的首要选择。本研究以西瓜自根苗为对照,对自根苗和野生西瓜砧木嫁接的西瓜苗进行高温处理,围绕光合荧光参数、叶片颜色、叶绿素含量及过氧化物酶活性等关键指标展开深入探讨,为优化西瓜的高温耐性提供理论依据和技术支持。
1 材料和方法
1.1 试验材料
所用材料为野生西瓜类型砧木品种野壮1 号(YZ1),西瓜品种早佳(ZJ)。其中野壮1 号种子由宁波市丰登种业科技有限公司提供,早佳种子由新疆昌农种业有限责任公司提供。
1.2 试验方法
试验在宁波市特色园艺作物品质调控与抗性育种重点实验室进行。选择饱满一致的砧木种子进行温汤浸种、催芽。待种子露白后播种于填充有育苗基质的50 孔穴盘中,置于人工气候箱内暗培养,温度为26 ℃,湿度为90%。顶土后,培养条件调整为光周期16 h 光照/8 h 黑暗,光照度约为25 000 lx,昼夜温度为24 ℃/16 ℃,昼夜湿度为80%/85%。接穗种子浸种、催芽,待其露白后播种于填充有育苗基质的方盘中,置于人工气候箱内暗培养,温度为26 ℃,湿度为90%。顶土后,培养条件调整为光周期12 h光照/12 h 黑暗,光照度约8000 lx,昼夜温度26 ℃/20 ℃,昼夜湿度80%/85%。以野壮1 号为砧木,早佳为接穗。待砧木真叶展开、接穗生长至子叶直立但未展开时,采用顶插接法嫁接。嫁接后,穴盘浸水至基质水分饱和,穴盘上加盖育苗盖,顶部透气孔密闭,置于培养箱内。前3 d 无光照,温度28 ℃,湿度95%;第4~7 天透气孔少量打开,光周期12 h 光照/12 h 黑暗,光照度8000 lx,昼夜温度26 ℃/20 ℃,湿度90%;第8~10天,透气孔全部打开,光周期16 h光照/8 h黑暗,光照度20 000 lx,昼夜温度24 ℃/16 ℃,昼夜湿度85%/90%;第11天及之后,去掉育苗盖,光周期16 h 光照/8 h 黑暗,光照20 000 lx,昼夜温度24 ℃/16 ℃,昼夜湿度80%/85%。
嫁接成活后,进行高温处理。试验设置嫁接苗高温(YZ1/ZJ-T)、自根苗高温(ZJ-T)、嫁接苗常温(YZ1/ZJ-CK)和自根苗常温(ZJ-CK)4个处理,均设3次重复,每次重复25株。处理组设置为光周期16 h光照/8 h 黑暗,光照度25 000 lx,温度42 ℃,昼夜湿度80%/85%。对照组设置为光周期16 h光照/8 h黑暗,光照度25 000 lx,昼夜温度24 ℃/16 ℃,昼夜湿度80%/85%。
1.3 测定项目与方法
高温处理5 d后测定接穗下胚轴粗度和长度;利用SPAD 502 plus 叶绿素仪测定接穗子叶和第一片真叶的相对叶绿素含量(SPAD 值);利用LS171 色差仪测定接穗子叶和第一片真叶的叶片颜色,L*(亮度)、a*(红-绿)、b*(黄-蓝)值。其中ΔL*(处理组L*-对照组L*)为正,表明处理组颜色比对照组浅(偏白),ΔL*为负,表明处理组颜色比对照组深(偏黑);Δa*(处理组a*-对照组a*)为正,表明处理组颜色比对照组红(偏红),Δa*为负,表明处理组颜色比对照组绿(偏绿);Δb*(处理组b*-对照组b*)为正,表明处理组颜色比对照组黄(偏黄),Δb*为负,表明处理组颜色比对照组蓝(偏蓝)。
利用Junior-PAM 调制叶绿素荧光仪测定光合相关参数。植株暗处理20 min后,利用Fo′模式对接穗第一片真叶测定初始荧光Fo,最大荧光Fm,光合电子传递速率ETR(μmol·m-2·s-1),光化学淬灭系数qP,非光化学猝灭系数NPQ,光保护的重要指标PSⅡ调节性能量耗散的量子产额Y(NPQ),光损伤的重要指标PSⅡ非调节性能量耗散的量子产额Y(NO),以及通过公式计算的PSⅡ最大光化学效率即原初光能转化效率Fv/Fm,PSⅡ实际光化学效率Y(Ⅱ)=(Fm′-F′)/Fm′,PSⅡ潜在光化学效率Fv/Fo=(Fm-Fo)/Fo。
叶绿素a、叶绿素b及总叶绿素含量使用叶绿素含量测定试剂盒(分光法)测定;可溶性糖含量使用可溶性糖含量试剂盒(分光法)测定;丙二醛含量使用丙二醛含量测试试剂盒(分光法)测定;过氧化物酶活性使用过氧化物酶试剂盒(分光法)测定;过氧化氢酶活性使用过氧化氢酶(CAT)试剂盒(分光法)测定。测定方法均按照试剂盒说明书进行。所用试剂盒均来自苏州格锐思生物科技有限公司。
1.4 数据分析
利用WPS office 进行方差分析,并通过在线数据分析平台OmicShare tools,利用方差分析LSD 多重比较的方法进行组间差异分析。
2 结果与分析
2.1 高温对嫁接和自根西瓜苗期农艺及生理性状的影响
高温及嫁接均可显著提高接穗下胚轴粗度,但在嫁接苗和自根苗中,高温对下胚轴长度影响不显著(表1)。高温对自根苗子叶SPAD值影响不显著,对嫁接苗的影响显著。对于真叶,高温对自根苗及嫁接苗的影响均显著,但嫁接苗高温处理下与自根苗对照无显著差异。
表1 高温下嫁接和自根西瓜苗期农艺及生理性状表现
Table 1 Performance of agronomic and physiological traits in grafted and self rooted watermelon seedlings under high temperature
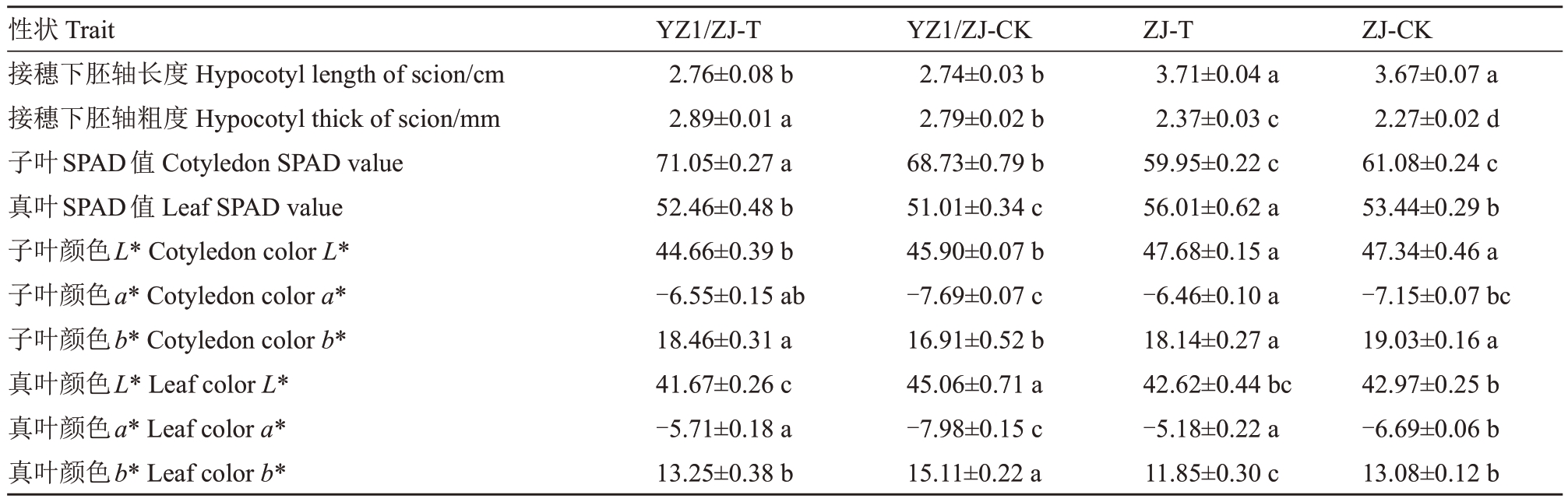
注:不同字母代表差异显著(P<0.05)。下同。
Note:Different letters represent significant differences(P<0.05).The same below.
?
高温对子叶颜色L*影响则不显著,可导致真叶颜色变深(表1)。嫁接降低了高温及常温的子叶和真叶颜色a*,但仅真叶达显著水平。高温导致嫁接苗及自根苗子叶和真叶颜色a*均显著升高。高温导致自根苗子叶和真叶颜色b*降低(19.03 vs 18.14和13.08 vs 11.85),但嫁接苗高温下子叶和真叶颜色b*分别表现为显著上升和降低。另外,高温及嫁接对真叶的影响总体均大于子叶。
2.2 高温对嫁接和自根西瓜苗期光合荧光参数的影响
高温胁迫显著降低了光系统Ⅱ的实际量子效率Y(Ⅱ)。嫁接苗在高温下Y(Ⅱ)为0.34,居于自根苗高温(0.24)和常温(0.50)之间(表2)。电子传递速率结果显示,嫁接高温处理的ETR(27.36)显著高于自根苗高温处理(19.11),但较各自对照降低约32.4%和52.5%。qP值反映PSⅡ反应中心的开放程度。嫁接苗高温处理的qP(0.64)显著高于自根苗(0.47)。NPQ 值显示自根苗高温处理(0.55)显著高于嫁接苗(0.47)。值得注意的是,对照组NPQ 值显著高于高温处理。Y(NO)反映PSⅡ的不可逆损伤程度。自根苗高温处理的Y(NO)最高(0.50),显著高于嫁接苗(0.43),这与Fv/Fm和Fv/Fo的结果一致。
表2 高温下嫁接和自根西瓜苗期光合荧光参数表现
Table 2 Performance of photosynthetic fluorescence parameters in grafted and self rooted watermelon seedlings under high temperature
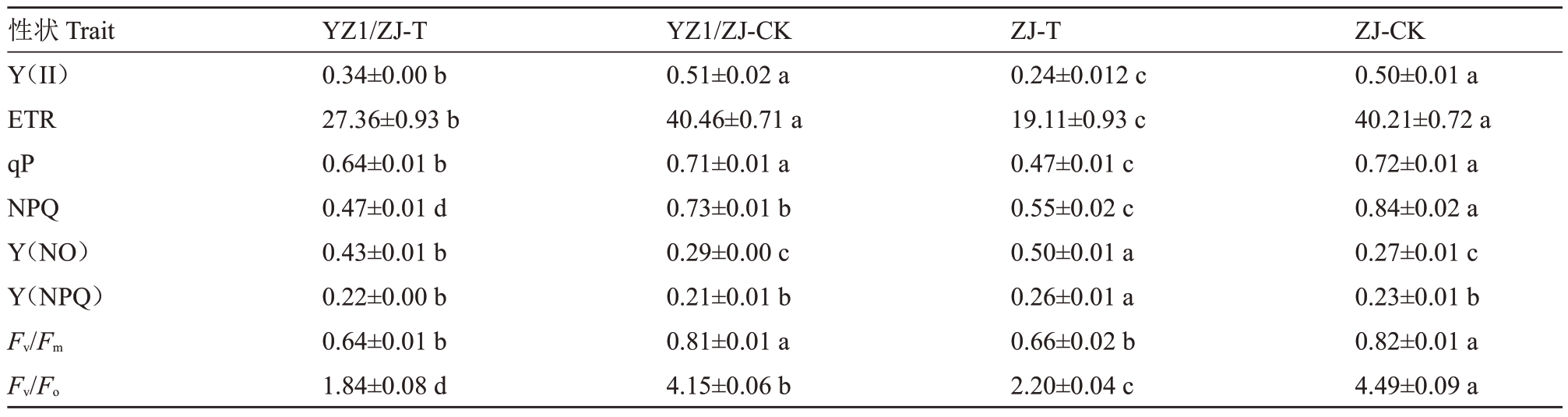
?
2.3 高温对嫁接和自根西瓜苗期逆境相关生理指标的影响
常温下嫁接对西瓜叶片叶绿素a含量、叶绿素b含量和总叶绿素含量影响均不显著(表3)。但高温下嫁接苗叶绿素b 及总叶绿素含量显著高于自根苗,且高温导致叶绿素a 降低的变幅在嫁接苗中更小,导致叶绿素b 和总叶绿素含量在嫁接苗中则为上升。
表3 高温下嫁接和自根西瓜苗期逆境相关物质含量
Table 3 Adversity related substances content in grafted and self rooted watermelon seedlings under high temperature
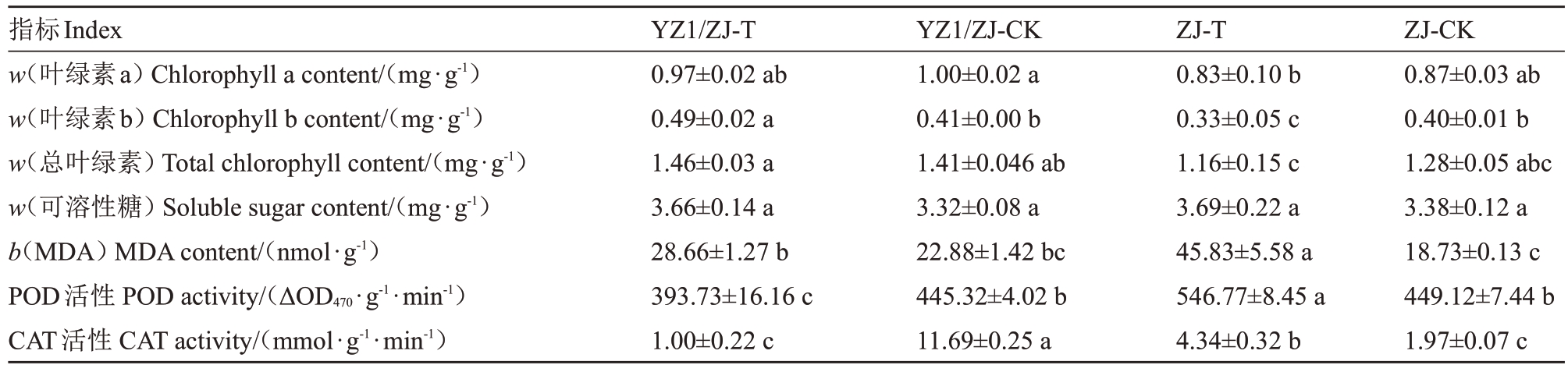
?
可溶性糖含量在各组间无显著差异(表3)。高温导致自根苗和嫁接苗MDA 含量均升高,但自根苗升高显著,且自根苗高温组MDA 含量最高(45.83 nmol·g-1)。高温导致自根苗POD活性升高,且高温组活性最高(546.77 ΔOD470·g-1·min-1);导致嫁接苗POD 活性降低,且高温组最高(393.73 ΔOD470·g-1·min-1)。对于CAT活性,与POD 活性一样,高温导致自根苗显著升高,嫁接苗显著降低。
3 讨 论
3.1 嫁接通过协调形态与色素代谢增强高温适应性
嫁接苗下胚轴粗度在高温下显著增加,且显著高于自根苗高温和常温处理,表现出更显著的形态适应性。这一现象可能与高温通过改变相关基因表达,促进生长素合成,进而促进下胚轴增粗,强化机械支撑,从而提高高温抗性[24-25]。值得注意的是,高温对下胚轴长度的促进作用在嫁接/自根苗间无显著差异,表明嫁接主要调控径向生长而非纵向延伸。
在色素代谢层面,嫁接苗子叶SPAD 值显著高于自根苗,且叶色参数L*值降低、a*值增幅较小。与茶树的研究结果类似,表明嫁接可减轻高温引起的叶绿素降解和叶片失绿现象[26-28]。这源于两个关键机制:(1)叶绿素稳定性增强:嫁接苗高温下总叶绿素含量较自根苗高25.9%,与SPAD 值呈正相关[29]。高叶绿素含量维持了光捕获能力,为光合电子传递提供基础。(2)捕光复合体调控:茶树研究显示,黄化叶片中CAB 基因家族(如CAB1)表达下调导致叶绿素a/b 结合蛋白减少,引发L*值升高和a*值异常[27]。嫁接可能通过稳定CAB 基因表达,避免高温下的色素紊乱。
3.2 光合机构稳定性:荧光参数揭示的耐热核心机制
高温破坏光系统稳定性,但嫁接可提高接穗的叶绿素含量、光合速率,保持电子传递系统稳定,维持了更高效的光能转化效率[30-31]。嫁接苗Y(Ⅱ)较自根苗高,且qP 值显著高于自根苗,与前人研究结果一致。这归因于三重保护机制:(1)电子传递链稳定性:嫁接苗ETR 降幅显著低于自根苗,表明其光合电子传递系统受高温抑制程度较轻。耐热水稻品种同样在高温下表现出ETR 稳定性,与Rubisco 活化酶活性呈正相关[32]。(2)热耗散精准调控:自根苗NPQ值高于嫁接苗,反映其通过增强非光化学淬灭耗散过剩光能,但代价是光能利用效率下降[33-34]。嫁接苗的较低NPQ与更高qP结合,实现光保护与光化学效率的平衡。(3)PSⅡ损伤防控:自根苗Y(NO)显著高于嫁接苗,证实其PSⅡ核心遭受更严重光损伤[35]。Fv/Fo值在嫁接苗中异常降低,可能与PSⅡ天线重组相关:PsbS蛋白缺失的拟南芥仍能通过增强ΔpH 诱导能量耗散,但响应速度延迟[36]。嫁接可能通过调控类囊体膜流动性优化该过程。同时嫁接可引发P700 等光合相关RNA 在砧穗间的跨物种移动,但砧穗间的亲缘关系对RNA 转移具有显著影响[37-38],这可能是本砧嫁接对高温耐性的影响与异砧嫁接有所不同的原因。
高温下嫁接苗的光合保护策略见图1。
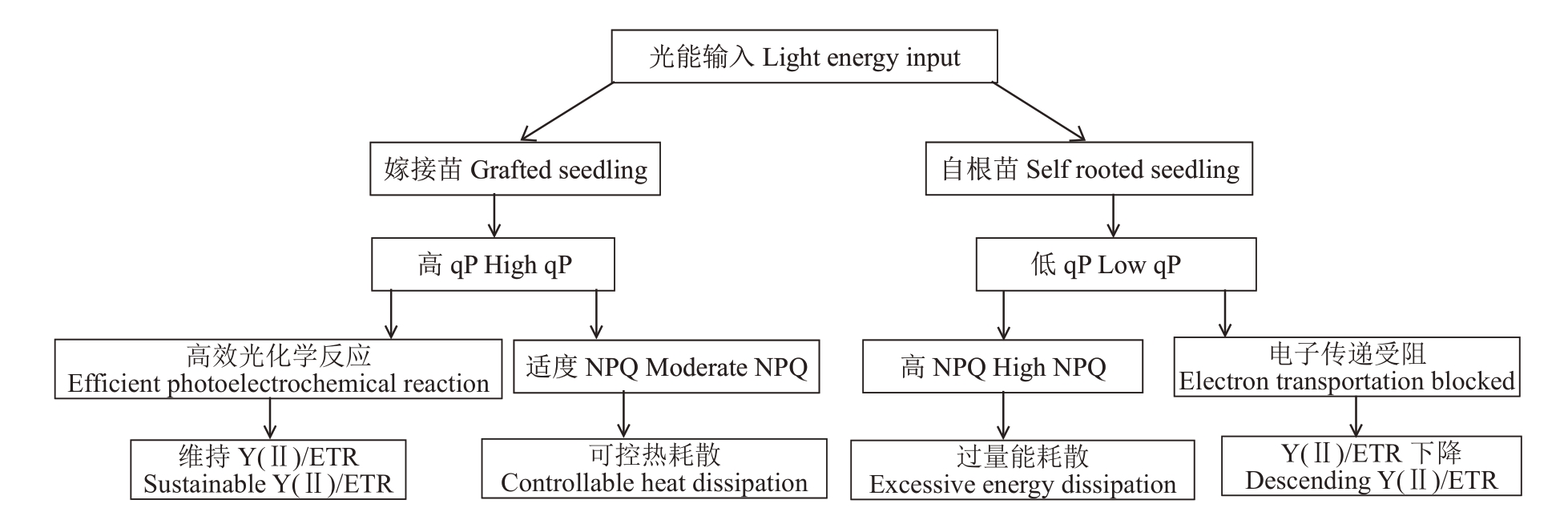
图1 高温下嫁接苗光合保护策略
Fig.1 Photosynthetic protection strategies of grafted seedlings under high temperature
3.3 膜系统保护与氧化应激平衡
高温可导致植株叶绿素降解,膜脂过氧化,MDA 含量增加,POD 活性升高[39-40],但嫁接可显著缓解高温诱导的膜脂过氧化[41-42]。与前人研究结果一致,笔者在本研究中发现自根苗MDA 含量在高温下激增,而嫁接苗中增加并不显著,这种差异可能源于两类协同机制。一是膜脂过氧化抑制:叶绿素降解与MDA 积累呈显著负相关[40]。嫁接苗的高叶绿素含量直接减少活性氧(ROS)爆发,降低膜系统损伤风险。二是抗氧化酶动态调节:自根苗POD活性在高温下显著升高,反映其处于氧化应激状态;而嫁接苗POD活性较低,表明其基础抗氧化能力足以清除ROS[42]。
3.4 嫁接耐热性的生理整合效应
本砧嫁接(西瓜/西瓜)相较于异砧(如南瓜砧木)可能具有独特优势:(1)物质运输优化:南瓜砧木虽提高西瓜壮苗指数,但可能导致糖代谢紊乱[24]。本研究中嫁接苗可溶性糖含量无显著变化,暗示其碳分配更协调。(2)信号传导保真性:同种砧穗间内源激素(如ABA)和逆境信号分子(如H2O2)的传导效率更高。外源ABA 可增强NPQ 和rETR[31],本砧嫁接可能通过类似通路协同光保护来提高抗逆性。番茄盐胁迫试验显示,BR通路在非本砧中的激活程度高于本砧,暗示其对逆境的响应存在遗传距离依赖性[43],进一步表明本砧嫁接可能在逆境胁迫方面具有优势。(3)昼夜节律整合:拟南芥中ELF3 通过液-液相分离感知温度,调控PIF4 介导的下胚轴生长[44]。嫁接可能通过维持生物钟基因稳态,避免高温导致的节律紊乱。
3.5 局限性
尽管笔者对本砧嫁接在室内条件下影响西瓜苗期高温耐性的生理机制进行了一定的解析,但处理的温度和时间还不够全面。后续为了更全面地了解本砧嫁接对西瓜高温耐性的影响机制,还需要在不同温度和不同处理时长下,针对更多的表型及生理生化和分子表现进行研究。另外,伸蔓期、开花期等更多发育阶段的高温胁迫影响尚需完善,并进行不同发育阶段影响机制的联合分析。
4 结 论
笔者在本研究中通过对本砧嫁接的西瓜及西瓜自根苗在苗期高温处理后的农艺性状、叶片颜色、SPAD值、光合荧光参数、叶绿素含量和可溶性糖含量等逆境相关物质的分析,得出以下结论:(1)高温胁迫下,嫁接苗较自根苗的下胚轴粗度显著增加,通过强化机械支撑来增强抗逆性,表现出更显著的形态适应性;(2)嫁接苗子叶SPAD 值显著高于自根苗,维持更高的叶绿素含量,减轻了高温引起的叶绿素降解和叶片失绿现象;(3)嫁接苗ETR 降幅显著低于自根苗,Y(NPQ)和Y(NO)稳定性高于自根苗;(4)嫁接抑制了高温导致的MDA含量和POD活性的升高,减轻了氧化应激,维持膜稳定性。总体上,本砧嫁接通过三重互作机制以提升西瓜苗期高温耐性:(1)形态:强化下胚轴稳定性,维持叶绿体色素稳态;(2)光合:优化光化学效率与热耗散平衡,减少不可逆光损伤;(3)细胞:抑制膜脂过氧化并协调抗氧化酶活性,应激响应更高效。可将本砧在提高抗病性及低温耐性的前提下,应用于易受高温胁迫的地区,进行高品质西瓜栽培生产,以提升西瓜产业的稳定性。本研究结果在一定程度上明确了本砧嫁接对西瓜高温耐性的影响机制,为后续野生西瓜耐热基因的挖掘及进一步西瓜本砧育种和本砧嫁接栽培提供了理论参考。
[1] FAO. Global Horticulture Production Report[R]. Rome:FAO Press,2025
[2] 张力.西瓜耐热性指标鉴定及材料筛选研究[D].南宁:广西大学,2014.ZHANG Li. Studies on indexes for identification of heat tolerance of watermelon and selection of heat tolerance materials[D].Nanning:Guangxi University,2014.
[3] 郑秀革.西瓜枯萎病的预防[J].农民致富之友,2017(1):46.ZHENG Xiuge. Prevention of watermelon wilt disease[J].Friends of Wealthy Farmers,2017(1):46.
[4] 王毓洪,高天一,张华峰,黄芸萍,严蕾艳,邢乃林,王迎儿,应泉盛.设施西甜瓜连作障碍综合防控技术[J].中国蔬菜,2018(11):81-83.WANG Yuhong,GAO Tianyi,ZHANG Huafeng,HUANG Yunping,YAN Leiyan,XING Nailin,WANG Yinger,YING Quansheng. Comprehensive prevention and control technology for continuous cropping obstacles of melon in facilities[J]. China Vegetables,2018(11):81-83.
[5] 王彦刚,孙德祥,严文倩,申太荣,韩道杰.不同砧木嫁接对西瓜生长及其品质、产量的影响[J].宁夏农林科技,2020,61(2):4-6.WANG Yangang,SUN Dexiang,YAN Wenqian,SHEN Tairong,HAN Daojie. Effects of different rootstocks on plant growth,fruit quality and yield of grafted watermelon[J].Ningxia Journal of Agriculture and Forestry Science and Technology,2020,61(2):4-6.
[6] 刘调平.双根南瓜嫁接西瓜设施育苗技术试验报告[J].农业科技与信息,2020,17(3):35.LIU Diaoping. Experimental report on facility seedling cultivation technology of double root pumpkin grafted watermelon[J].Agricultural Science-Technology and Information,2020,17(3):35.
[7] 贺学强,余立云,赵小红,何春花,杨帆.宁夏中部干旱带早春嫁接西瓜“一窝两膜”栽培模式[J].北方园艺,2024(19):151-154.HE Xueqiang,YU Liyun,ZHAO Xiaohong,HE Chunhua,YANG Fan. Early spring grafted watermelon “ One nest,two films”cultivation model in the arid zone of central Ningxia[J].Northern Horticulture,2024(19):151-154.
[8] 张力,李桂芬,李文信,覃斯华,黄金艳,何毅,洪日新.高温胁迫对不同西瓜幼苗生长和生理特性的影响及综合评价[J].中国瓜菜,2015,28(4):13-17.ZHANG Li,LI Guifen,LI Wenxin,QIN Sihua,HUANG Jinyan,HE Yi,HONG Rixin. Effect of high temperature stress on the growth and physiological property of different watermelon seedlings and their comprehensive assessment[J]. China Cucurbits and Vegetables,2015,28(4):13-17.
[9] 张梁葛,石文昕,李爱,张卫华,林萍,薛佳.不同耐热型西瓜幼苗对高温胁迫的生理响应[J].华北农学报,2024,39(5):117-127.ZHANG Liangge,SHI Wenxin,LI Ai,ZHANG Weihua,LIN Ping,XUE Jia. Physiological response of different heat-tolerant watermelon seedlings to high temperature stress[J]. Acta Agriculturae Boreali-Sinica,2024,39(5):117-127.
[10] TAO M Q,JAHAN M S,HOU K,SHU S,WANG Y,SUN J,GUO S R.Bitter melon(Momordica charantia L.)rootstock improves the heat tolerance of cucumber by regulating photosynthetic and antioxidant defense pathways[J]. Plants,2020,9(6):692.
[11] 张红梅,王平,金海军,丁小涛,余纪柱.高温对不同砧木黄瓜嫁接苗生长、光合和叶绿素荧光特性的影响[J].上海农业学报,2016,32(5):40-45.ZHANG Hongmei,WANG Ping,JIN Haijun,DING Xiaotao,YU Jizhu. Effects of high temperature on the growth,photosynthesis and chlorophyll fluorescence characteristics of grafted cucumber with different rootstocks[J]. Acta Agriculturae Shanghai,2016,32(5):40-45.
[12] 郝婷.不同砧木对嫁接黄瓜缓解高温胁迫的生理机制研究[D].南京:南京农业大学,2014.HAO Ting. Study on physiological mechanism of different rootstocks relieving heat stress of grafted cucumber[D]. Nanjing:Nanjing Agricultural University,2014.
[13] 邢乃林,张蕾琛,黄芸萍,王毓洪.砧穗互作对嫁接西瓜高温耐性的影响[J].浙江农业科学,2021,62(10):1988-1990.XING Nailin,ZHANG Leichen,HUANG Yunping,WANG Yuhong. Effect of rootstock and scion interaction on high temperature tolerance of grafted watermelon[J]. Journal of Zhejiang Agricultural Sciences,2021,62(10):1988-1990.
[14] 赵依杰,陈清西,吴宇芬,林强.砧木对小型嫁接西瓜生理生化的影响[J].中国农学通报,2008,24(6):319-323.ZHAO Yijie,CHEN Qingxi,WU Yufen,LIN Qiang. Effect of rootstocks on physiology and biochemistry of small grafted watermelon[J].Chinese Agricultural Science Bulletin,2008,24(6):319-323.
[15] 侯树安. CmHY5/CmoHY5-CmoNRT2.1 调控嫁接薄皮甜瓜幼苗硝态氮吸收的分子机制[D].沈阳:沈阳农业大学,2022.HOU Shu’an. Molecular mechanism of CmHY5/CmoHY5-CmoNRT2.1 regulating nitrate uptake in grafted oriental melon seedlings[D]. Shenyang:Shenyang Agricultural University,2022.
[16] HUANG Y,JIAO Y Y,NAWAZ M A,CHEN C,LIU L,LU Z,KONG Q S,CHENG F,BIE Z L. Improving magnesium uptake,photosynthesis and antioxidant enzyme activities of watermelon by grafting onto pumpkin rootstock under low magnesium[J].Plant&Soil,2016,409(1):229-246.
[17] 刘成静,王崇启,焦自高,张志忠,王艳艳,董玉梅,肖守华.高温胁迫下西瓜嫁接苗耐热性和保护酶活性的研究[J].长江蔬菜,2009(4):50-53.LIU Chengjing,WANG Chongqi,JIAO Zigao,ZHANG Zhizhong,WANG Yanyan,DONG Yumei,XIAO Shouhua. Study on heat resistance and protective enzyme of grafted watermelon seedlings under high temperature stress[J]. Journal of Changjiang Vegetables,2009(4):50-53.
[18] 任丽华,陈晖,高秋美,孟庆峰.不同砧木嫁接西瓜叶片抗氧化酶活性研究[J].山东农业科学,2013,45(8):77-78.REN Lihua,CHEN Hui,GAO Qiumei,MENG Qingfeng. Study on antioxidant enzyme activity of watermelon leaves grafted with different rootstocks[J]. Shandong Agricultural Sciences,2013,45(8):77-78.
[19] 仪泽会,毛丽萍,赵婧.嫁接对复合盐碱胁迫下青椒幼苗生长、抗氧化能力及渗透调节能力的影响[J].植物生理学报,2020,56(9):1943-1954.YI Zehui,MAO Liping,ZHAO Jing. Effects of grafting on growth,antioxidant capacity and osmotic adjustment capacity of green pepper seedlings under mixed salt-alkali stress[J]. Plant Physiology Journal,2020,56(9):1943-1954.
[20] 邢乃林,严蕾艳,王毓洪,黄芸萍.不同类型砧木嫁接对西瓜果实品质和镉含量的影响[J].江西农业学报,2022,34(8):17-21.XING Nailin,YAN Leiyan,WANG Yuhong,HUANG Yunping.Effects of different types of rootstocks on fruit quality and cadmium content of grafted watermelon[J]. Acta Agriculturae Jiangxi,2022,34(8):17-21.
[21] 高军红,廖华俊.嫁接对西瓜果品品质的影响[J].中国瓜菜,2006,19(5):12-14.GAO Junhong,LIAO Huajun. Effect of grafting on watermelon quality[J].China Cucurbits and Vegetables,2006,19(5):12-14.
[22] 孟文慧.不同砧木对西瓜若干重要性状的影响[D].杨凌:西北农林科技大学,2008.MENG Wenhui.Effects of different rootstocks on several important characters in Citrullus lanatus[D].Yangling:Northwest A&F University,2008.
[23] 赵利强.不同种类砧木嫁接对西瓜生长发育和果实品质的影响[D].武汉:华中农业大学,2014.ZHAO Liqiang. Effects of different rootstocks on the growth and quality of grafted watermelon[D]. Wuhan:Huazhong Agricultural University,2014.
[24] 任慧转,周海姣,尧甜,潘文博,丁明.不同砧木对西瓜断根嫁接苗生长发育的影响[J].中国瓜菜,2024,37(7):107-110.REN Huizhuan,ZHOU Haijiao,YAO Tian,PAN Wenbo,DING Ming.Effects of different rootstocks on the growth and development of root-cutting grafted watermelon seedlings[J]. China Cucurbits and Vegetables,2024,37(7):107-110.
[25] SUN J Q,QI L L,LI Y N,CHU J F,LI C Y.PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth[J].PLoS Genetics,2012,8(3):e1002594.
[26] 范延艮.‘黄金芽’茶树不同色泽新梢多组学比较及生理特性研究[D].泰安:山东农业大学,2019.FAN Yangen. Multiomics comparison and physiological characteristics of different colour shoots of Camellia sinensis var.huangjinya[D].Taian:Shandong Agricultural University,2019.
[27] 钟思彤,张亚真,游小妹,陈志辉,孔祥瑞,林郑和,伍慧妮,金珊,陈常颂.茶树叶片黄化变异相关的CAB 基因家族鉴定及关键基因挖掘[J].茶叶科学,2024,44(2):175-192.ZHONG Sitong,ZHANG Yazhen,YOU Xiaomei,CHEN Zhihui,KONG Xiangrui,LIN Zhenghe,WU Huini,JIN Shan,CHEN Changsong.Identification of CAB gene family and excavation of key genes related to leaf yellowing variationin tea plants (Camellia sinensis)[J]. Journal of Tea Science,2024,44(2):175-192.
[28] YANG L,XIA L C,ZENG Y,HAN Q Q,ZHANG S. Grafting enhances plants drought resistance:Current understanding,mechanisms,and future perspectives[J]. Frontiers in Plant Science,2022,13:1015317.
[29] 郝学明,王响铃,宋柏权,王孝纯,王秋红,周建朝.甜菜叶片SPAD 值和光合色素的相关性研究[J].农学学报,2019,9(10):65-70.HAO Xueming,WANG Xiangling,SONG Baiquan,WANG Xiaochun,WANG Qiuhong,ZHOU Jianchao.Correlation analysis of SPAD value and photosynthetic pigment in sugarbeet leaves[J].Journal of Agriculture,2019,9(10):65-70.
[30] 宋丽莉,赵华强,朱小倩,董根西,谢戎.高温胁迫对水稻光合作用和叶绿素荧光特性的影响[J]. 安徽农业科学,2011,39(22):13348-13353.SONG Lili,ZHAO Huaqiang,ZHU Xiaoqian,DONG Genxi,XIE Rong. Effect of high temperature stress on photosynthesis and chlorophyll fluorescence of rice[J]. Journal of Anhui Agricultural Sciences,2011,39(22):13348-13353.
[31] 隆春艳,古洪辉,汪正香,蒋雄,杨翠芹,秦耀国.外源脱落酸对高温胁迫下菠菜光合与叶绿素荧光参数的影响[J].四川农业大学学报,2017,35(1):24-30.LONG Chunyan,GU Honghui,WANG Zhengxiang,JIANG Xiong,YANG Cuiqin,QIN Yaoguo. Effects of exogenous abscisic acid on the photosynthesis and chlorophy Ⅱfluorescence parameters of spinach under high temperature stress[J]. Journal of Sichuan Agricultural University,2017,35(1):24-30.
[32] 王松.耐/感水稻品种光合作用对极端高温的反应[D].荆州:长江大学,2022.WANG Song. Response of photosynthesis to extreme high temperature in rice genotypes with contrasting heat tolerance[D].Jingzhou:Yangtze University,2022.
[33] GILMOREAM.Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves[J].Physiologia Plantarum,1997,99(1):197-209.
[34] JOHNSON M P,RUBAN A V. Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced ΔpH[J]. Journal of Biological Chemistry,2011,286(22):19973-19981.
[35] LIU J,LU Y,HUA W,LAST R L.A new light on photosystem II maintenance in oxygenic photosynthesis[J]. Frontiers in Plant Science,2019,10:975.
[36] JOHNSON M P,RUBAN A V.Arabidopsis plants lacking PsbS protein possess photoprotective energy dissipation[J]. The Plant Journal,2010,61(2):283-289.
[37] RUBIO B,STAMMITTI L,COOKSON S J,TEYSSIER E,GALLUSCI P. Small RNA populations reflect the complex dialogue established between heterograft partners in grapevine[J].Horticulture Research,2022,9:uhab067.
[38] NING K,ZHOU W X,CAI X Q,YAN L Y,MA Y C,XIE A,WANG Y H,XU P. Rootstock–scion exchanging mRNAs participate in watermelon fruit quality improvement[J]. International Journal of Molecular Sciences,2025,26(11):5121.
[39] 马德华,庞金安,霍振荣,李淑菊.高温对黄瓜幼苗膜脂过氧化作用的影响[J].西北植物学报,2000,20(1):141-144.MA Dehua,PANG Jin’an,HUO Zhenrong,LI Shuju. Effect of high temperature on membrane lipid peroxidation in leaf of cucumber seedling[J].Acta Botanica Boreali-Occidentalia Sinica,2000,20(1):141-144.
[40] 曹慧,韩振海,许雪峰.水分胁迫下苹果属植物叶片叶绿素降解的膜脂过氧化损伤作用[J]. 中国农业科学,2003,36(10):1191-1195.CAO Hui,HAN Zhenhai,XU Xuefeng. Membrane lipid peroxidation damage effect of chlorophyll degradation in Malus seedlings under water stress[J]. Scientia Agricultura Sinica,2003,36(10):1191-1195.
[41] 翁锦周,林江波,林加耕,张梅坤,陈永快,曾日秋,吴水金.盐胁迫对桉树幼苗的生长及叶绿素含量的影响[J].热带作物学报,2007,28(4):15-20.WENG Jinzhou,LIN Jiangbo,LIN Jiageng,ZHANG Meikun,CHEN Yongkuai,ZENG Riqiu,WU Shuijin.Effect of salt stress on the growth and the content of chlorophyll in seedling leaves of eucalyptus[J]. Chinese Journal of Tropical Crops,2007,28(4):15-20.
[42] 李能芳,荀琳,郭学君,周庆阳.嫁接对茄子植株过氧化物酶同工酶的影响[J].西南园艺,1999(4):24-25.LI Nengfang,XUN Lin,GUO Xuejun,ZHOU Qingyang. Effect of grafting on peroxidase isoenzyme of egg plant[J]. Southwest Horticulture,1999(4):24-25.
[43] WU X L,YUAN D,BIAN X Y,HUO R X,LÜ G Y,GONG B B,LI J R,LIU S C,GAO H B.Transcriptome analysis showed that tomato-rootstock enhanced salt tolerance of grafted seedlings was accompanied by multiple metabolic processes and gene differences[J].Frontiers in Plant Science,2023,14:1167145.
[44] LAOSUNTISUK K,DOHERTY C J. The intersection between circadian and heat-responsive regulatory networks controls plant responses to increasing temperatures[J]. Biochemical Society Transactions,2022,50(3):1151-1165.