石榴(Punica granatum L.)具有经济效益、营养价值与文化内涵[1-3],其果实对保持人类健康和预防疾病有重要作用[4-5]。石榴在中国的栽培面积较大,形成了以四川、云南、河南、安徽、山东、陕西和新疆为代表的七大主产区[6]。
土壤是果树生长发育的基础,其理化性质和养分含量对果实品质和产量具有重要的影响[7]。施肥能够显著改善土壤养分供给状况,是提升单位面积果树产量的关键措施[8]。然而,长期过度依赖化肥,不仅难以持续提升果树产量,还可能引发土壤退化和环境污染等问题,制约果树产业的健康发展[9]。
在石榴生长发育过程中,季节性干旱和土壤沙化会影响果实的产量和品质[10]。过度施肥会导致土壤板结、养分流失,易形成裂果和脓包果[11]。改善土壤水肥供应状况对提高石榴的产量与品质至关重要。充足的水肥条件不仅可促进苗木根系的发育、增强其吸收养分和水分的能力,还可有效提高苗木的抗逆性,使其在干旱、寒冷等逆境下表现出更强的适应能力。不同于传统化学肥料,微生物菌肥是一种以微生物为主要活性成分的特殊生物制剂,包含大量有益的微生物菌种[12],能够加速土壤中氮、磷等元素的分解,提高植物对养分的吸收利用率[13],调节土壤微生物群落结构并增加微生物数量[14-15],抑制土传病害的发生。此外,菌肥中的有益微生物在代谢过程中会产生防御酶和抗生素,从而增强果树的抗病性和耐逆性[16]。作为新型肥料,微生物菌肥通过活化有机质、提高土壤酶活性等方式改善土壤质量,促进园艺作物的生长[17],提高果实品质[18]。例如,固氮菌可提高石榴叶片的氮素含量,促进叶绿素的合成[19];溶磷酶能将土壤中的磷元素转化为易于植物吸收的形式,从而影响植物的生长发育。
微生物菌肥对不同果树的生长发育及抗逆性均有显著作用。例如,施用微生物菌肥可提高油桃植株株高并降低冠幅,增加苹果叶片叶绿素含量、促进幼苗光合作用以及增强其对碱胁迫的耐受性[20-21]。此外,微生物菌肥还能通过调节果实发育过程,实现果树增产提质,如促进葡萄果实的增糖、着色及提前成熟,增加苹果单果质量和提高百香果产量[22-24]。目前,关于微生物菌肥在石榴上的应用研究较为有限,且主要集中在成龄石榴树的应用效果方面。研究发现竹纤维高分子菌肥可以促进突尼斯软籽石榴的植株生长,优化果形和粒质量,并提高果实可溶性固形物和总糖含量[25]。然而,关于微生物菌肥对石榴幼苗早期生长发育影响的研究却鲜有报道,不同菌肥对石榴幼苗生长的影响机制也尚不明确。目前,市面上微生物菌肥种类繁多,尽管这些菌肥在诸多作物上已表现出良好的应用前景,但适宜石榴幼苗生长发育的菌肥仍需进一步筛选和确定。基于此,笔者在本研究中设计了5 种不同的微生物菌肥处理,包括单一功能菌、复合功能菌等类型,系统评价不同菌肥对石榴幼苗生长量和光合特性等方面的影响,以期筛选出有助于石榴幼苗生长发育的菌肥,为石榴的绿色生产和科学施肥提供理论依据和实践指导。
1 材料和方法
1.1 试验地点和材料
试验于2023年3—12月在国家园艺种质资源库(郑州)进行。试验地属于北温带大陆性季风气候,四季分明,年平均气温14.4 ℃,年平均降水量640.9 mm,无霜期220 d,年日照时间约2400 h。
选取上年10月扦插的华冠石榴苗为试验材料,选取大小和根系基本相同的幼苗栽植在直径30 cm、高30 cm 的营养袋中,采用栽培基质为标准泥炭(GREEN TERRA),吸水性65%,粒径规格0~20 mm,有机质含量(w,后同)50%~80%,肥料含量0.8 kg·m-3,与果园沙质土混合体积比为3∶1,每个营养袋约含13.5 kg 栽培基质,栽培基质pH 值为5.81,按照常规露地栽培管理。
供试微生物菌肥分别为:活力源(国光园林科技有限公司),有效活菌数枯草芽孢杆菌、侧孢短芽孢杆菌≥0.2 亿·g-1;哈茨木霉菌(山东君德生物科技有限公司),有效活菌数≥10.0 亿·g-1;微生物菌剂(粉状)(荥阳西里多作物保护有限公司),有效活菌数枯草芽孢杆菌≥5.0亿·g-1;微生物菌剂(颗粒)(郑州中科化工产品有限公司),有效活菌数地衣芽孢杆菌、胶冻样类芽孢杆菌≥1.0 亿·g-1;土壤活化剂(史丹利农业集团股份有限公司),有效活菌数≥3.0亿·g-1。
1.2 试验设计
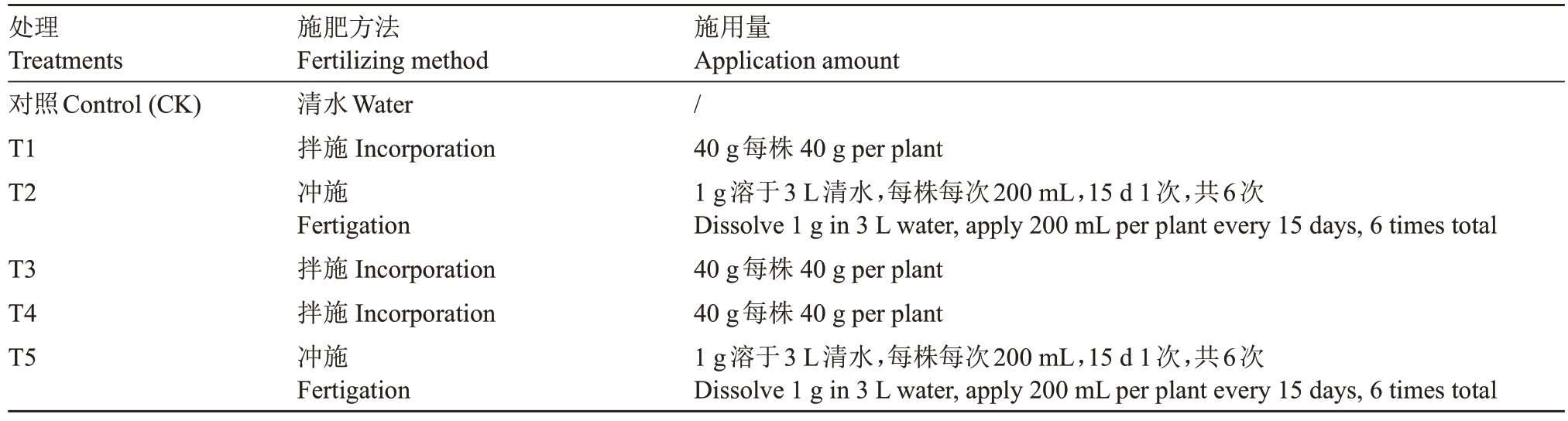
试验设6 个处理(表1),每个处理10 株石榴幼苗,以清水为对照(CK),设置5种微生物菌肥处理,分别为国光活力源(T1)、君德哈茨木霉菌(T2)、西里多微生物菌剂(粉状)(T3)、中科微生物菌剂(颗粒)(T4)、史丹利土壤活化剂(T5)。菌肥按照生产厂家推荐方法施用,冲施处理时,对照与其他处理用等量清水浇灌。
表1 不同微生物菌肥处理方法
Table 1 Different microbial fertilizer treatment methods
?
1.3 测定指标及方法
1.3.1 石榴生长量测定 使用卷尺测定石榴幼苗从苗木基部到生长点的高度,即为株高,使用游标卡尺测定石榴幼苗地径粗度,同时统计新生枝条数目。使用万分天平测定每个处理的植株鲜质量,每个处理设置3个生物学重复。
1.3.2 叶绿素及类胡萝卜素含量测定 采集石榴幼苗中上部东、西、南、北4 个不同生长方向健康完整的成熟叶片,每个植株取8枚叶片,3次重复,共测定24 枚叶片,测定方法参考郭雁君等[26]的报道。将叶片擦净后去除叶脉并切碎,称取0.2 g 于试管中,加入10 mL 95%乙醇,避光提取直至叶片变白。采用Spectra Max i3x 多功能酶标仪(Molecular Devices公司)分别测定在波长470、649、665 nm 下吸光度值,并应用Arnon 公式[27]计算叶片叶绿素a、叶绿素b、类胡萝卜素及叶绿素总量,每个处理设置3 次生物学重复。
1.3.3 光合参数的测定 选取完全展开的叶片,采用CIRAS-3 便携式光合作用测定系统(PP-SYSTEMS 公司)测定净光合速率(net photosynthetic rate,Pn)、蒸腾速率(transpiration rate,Tr)、气孔导度(stomatal conductance,Gs)、细胞间CO2 浓度(intercellular CO2 concentration,Ci)、水分利用效率(water use efficiency,WUE)等光合参数。在典型晴天分别于8:00、10:00、14:00、16:00 进行测定。每片处理设置3次生物学重复,2次技术重复。
1.3.4 土壤酸碱度测定 土壤pH值的测定参考《中华人民共和国国家环境保护标准HJ 962—2018》,采用电位法(水土体积比2.5∶1),土壤质量为风干干质量。
1.3.5 数据处理 试验数据使用Excel 2016进行整理和计算,使用SPSS 26 进行方差分析和多重比较分析。
2 结果与分析
2.1 不同菌肥处理对石榴幼苗生长状况的影响
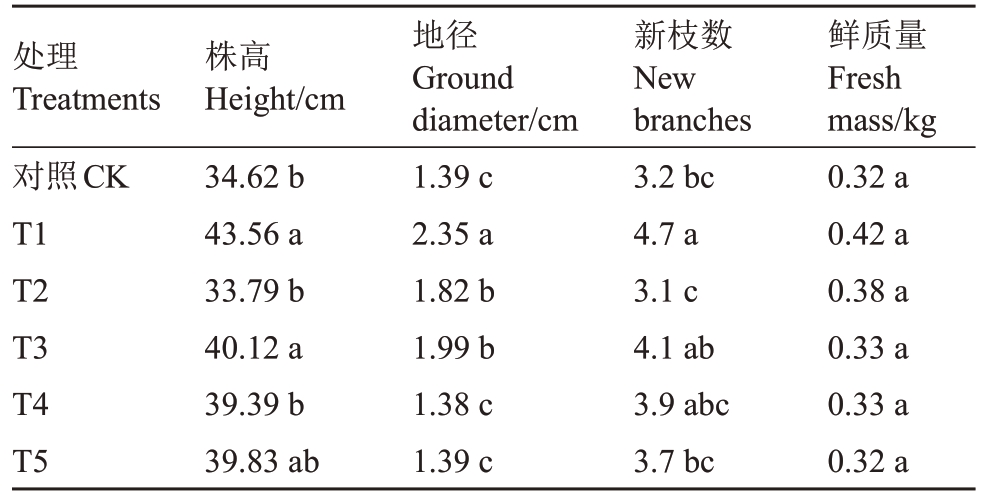
为了研究不同菌肥处理对石榴幼苗生长状况的影响,分别测定了幼苗的株高、地径和新枝数目以及幼苗鲜质量。结果表明,幼苗株高从大到小依次为T1>T3>T5>T4>CK>T2,在0.05 水平上T1、T3处理显著高于CK,T1、T3 处理分别比CK 高出25.82%、15.89%。地径从大到小依次为T1>T3>T2>T5=CK>T4,在0.05 水平上T1、T2、T3 处理均显著高于CK,T1、T2、T3 分别比CK 高出69.06%、30.94%和43.17%,T2 和T3 处理之间没有显著差异。新枝数目从大到小依次为T1>T3>T4>T5>CK>T2,在0.05 水平上T1 处理显著高于CK,T1 比CK高出46.88%。石榴幼苗鲜质量从高到低分别为T1>T2>T3=T4>T5=CK,在0.05 水平上各处理与CK均无显著差异。具体如表2所示。
表2 不同菌肥处理对石榴幼苗生长状况的影响
Table 2 Effects of different treatments on growth status of pomegranate seedlings
注:同一列不同小写字母表示在0.05 水平差异显著。下同。
Note:Different small letters in the same column indicate significant differences at the 0.05 level.The same below.
?
2.2 不同菌肥处理对石榴叶片叶绿素含量的影响
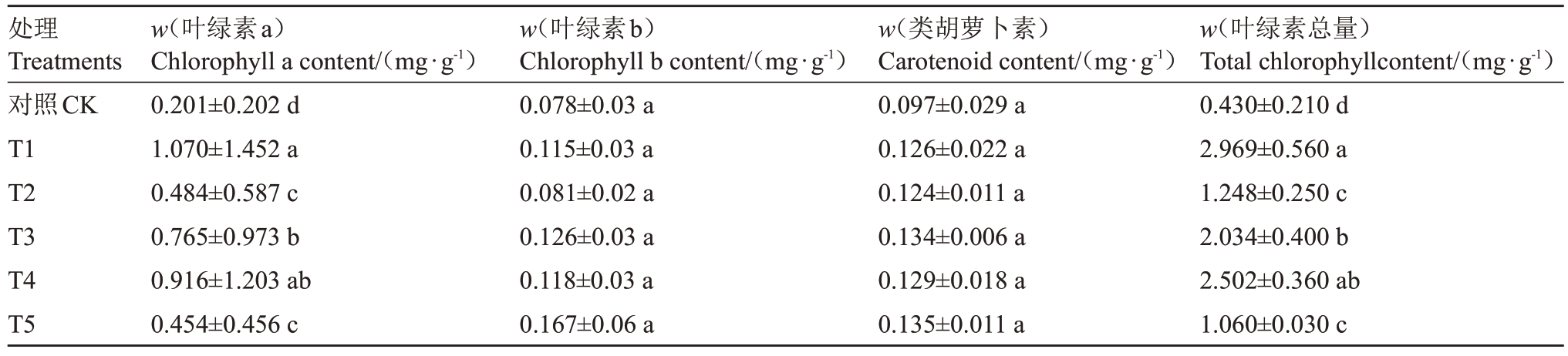
叶绿素是植物光合作用最重要的物质,不同菌肥处理下石榴叶片叶绿素含量影响石榴幼苗的生长。结果表明不同菌肥处理对叶片中的叶绿素含量影响显著。各处理叶片叶绿素a、叶绿素b、类胡萝卜素及叶绿素总量相对CK均有所上升。幼苗叶绿素a 含量从大到小依次为T1>T4>T3>T2>T5>CK,在0.05 水平上各个处理均显著高于CK,其中T4 和T1、T3 处理之间没有显著差异,T2 和T5 之间差异不显著。幼苗叶绿素总量从大到小依次为T1>T4>T3>T2>T5>CK,在0.05 水平上各处理均显著高于CK,其中T4和T1、T3处理之间没有显著差异,T2和T5之间差异不显著。具体如表3所示。
表3 不同菌肥处理下石榴叶片叶绿素含量
Table 3 Effects of different treatments on chlorophyll content of pomegranate seedlings leaves
?
2.3 不同菌肥处理对石榴叶片光合特性的影响
2.3.1 不同菌肥处理对叶片净光合速率(Pn)和气孔导度(Gs)日变化的影响 菌肥处理改变了石榴叶片净光合速率的日变化模式,T2、T3、T4、T5 推迟了峰值出现时间并影响了特定时段的光合速率,其中,T2在08:00显著降低光合速率,而T3在16:00显著提高光合速率。具体表现为:各处理和CK均在16:00达到最低值。CK 和T1 的叶片净光合速率在08:00达到最高值,随后持续下降,而T2、T4和T5在08:00后逐步上升,10:00达到峰值后缓慢下降,在14:00到16:00 呈现加速下降的趋势;T3 的叶片净光合速率在08:00 到10:00 下降,10:00 到14:00 回升,在14:00达到峰值后下降。与CK相比,T2在08:00叶片净光合速率显著降低,T3 在16:00 叶片净光合速率显著增加,其他各处理与CK均无显著差异(图1)。
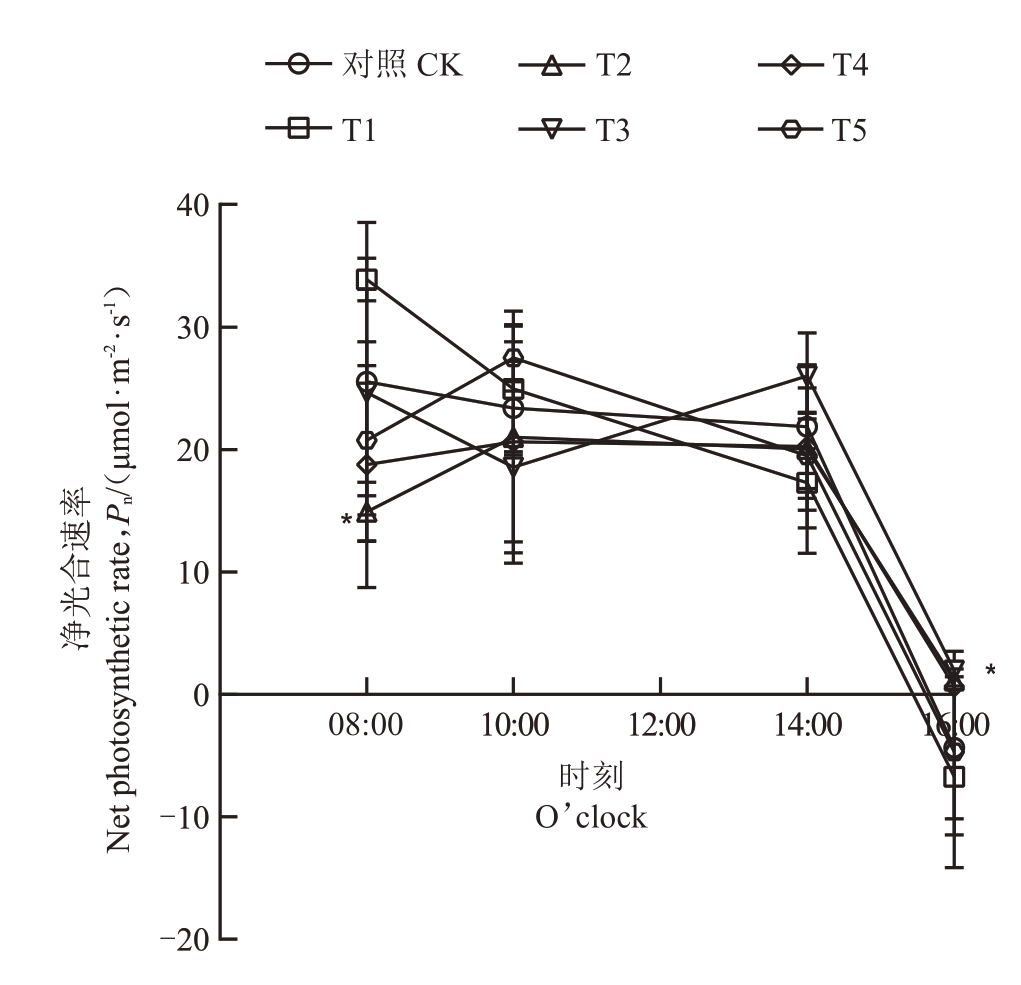
图1 石榴叶片净光合速率日变化
Fig.1 Daily variation of net photosynthetic rate of pomegranate seedlings leaves
菌肥处理改变了石榴叶片气孔导度的日变化趋势,部分处理(T1、T3)的峰值时间推迟至14:00,且T1、T4、T5在早晨显著提高气孔导度。具体表现为:CK、T2、T4 和T5 叶片气孔导度在08:00 开始增加,10:00 达到峰值后开始下降,16:00 达到最低值;T1叶片气孔导度在08:00 后呈上升趋势,10:00 达到峰值后逐渐下降,在14:00达到最低值;T3叶片气孔导度在08:00开始持续上升,在14:00达到峰值后下降,16:00 达到最低值。与CK 比,T1、T4、T5 在08:00 叶片气孔导度显著增加,T1 在10:00 叶片气孔导度显著增加外,其他各处理与CK均无显著差异(图2)。
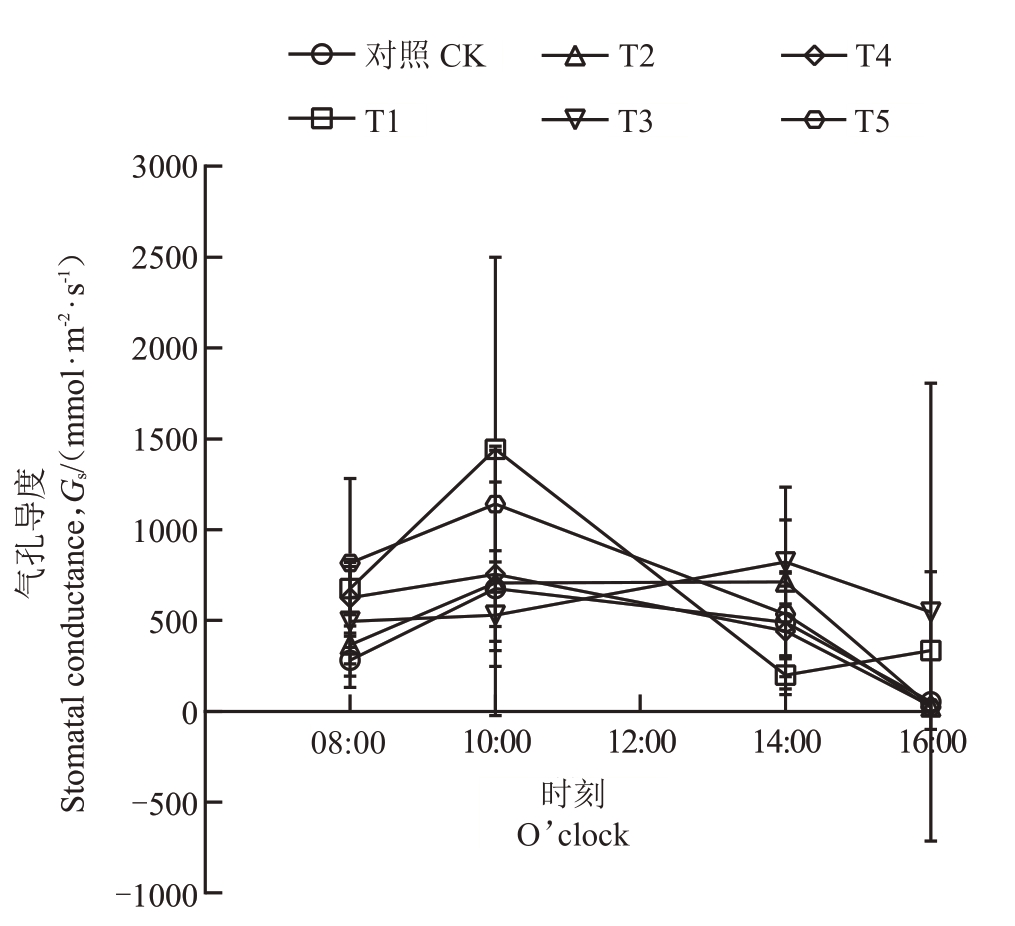
图2 石榴叶片气孔导度的日变化
Fig.2 Daily variation of stomatal conductance of pomegranate seedlings leaves
2.3.2 不同菌肥处理对石榴叶片蒸腾速率(Tr)和细胞间CO2浓度(Ci)日变化的影响 菌肥处理显著改变了石榴叶片蒸腾速率的日变化模式,T2、T3 的峰值推迟至14:00,且T1、T3、T4、T5 在08:00 显著提高蒸腾速率,而T1 在14:00 显著降低蒸腾速率。具体表现为:在菌肥处理和对照处理下,CK、T4 和T5 的叶片蒸腾速率在08:00 后逐步上升,在10:00 达到峰值后下降,16:00 最低;T1 的叶片蒸腾速率在08:00开始上升,10:00达到峰值后下降,在14:00达到最低值;T2和T3的叶片蒸腾速率在08:00后持续上升,在14:00达到峰值后下降。与CK比,T1、T3、T4、T5在8:00 叶片蒸腾速率显著升高,T1 在14:00 叶片蒸腾速率显著降低,T1在16:00叶片蒸腾速率显著升高,其他各处理与CK均无显著差异(图3)。菌肥处理显著改变了石榴叶片胞间CO2浓度的日变化规律,T1、T2、T5呈现双峰波动模式,而T3则持续下降。具体表现为:CK 和T4 胞间CO2浓度在08:00最高,随后逐渐下降,在14:00到达最低值后回升;T1 胞间CO2浓度在08:00 到10:00 上升,10:00 到14:00 下降,14:00 达到最低值后上升,在16:00 达到峰值;T2 和T5 的胞间CO2浓度在08:00 开始下降,10:00 达到最低值后逐渐上升,T2 在08:00 最高,T5在16:00 达到峰值;T3 胞间CO2浓度整体呈下降趋势,在08:00最高,16:00最低。与CK相比,T3在08:00胞间CO2浓度显著增加,T1在10:00胞间CO2浓度显著降低,T1 在14:00 胞间CO2 浓度显著增加,T2、T3、T4 在16:00胞间CO2浓度显著降低,其他各处理与CK均无显著差异(图4)。
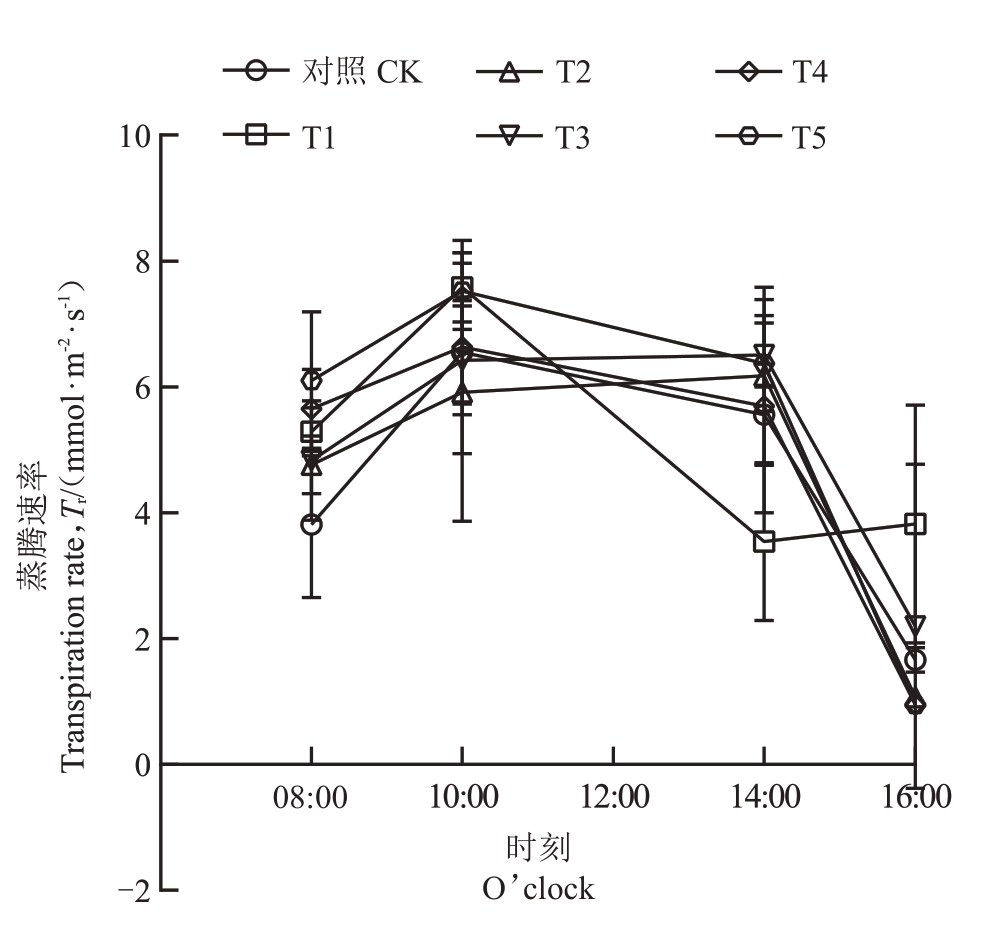
图3 石榴叶片蒸腾速率日变化
Fig.3 Daily variation of transpiration rate of pomegranate seedlings leaves
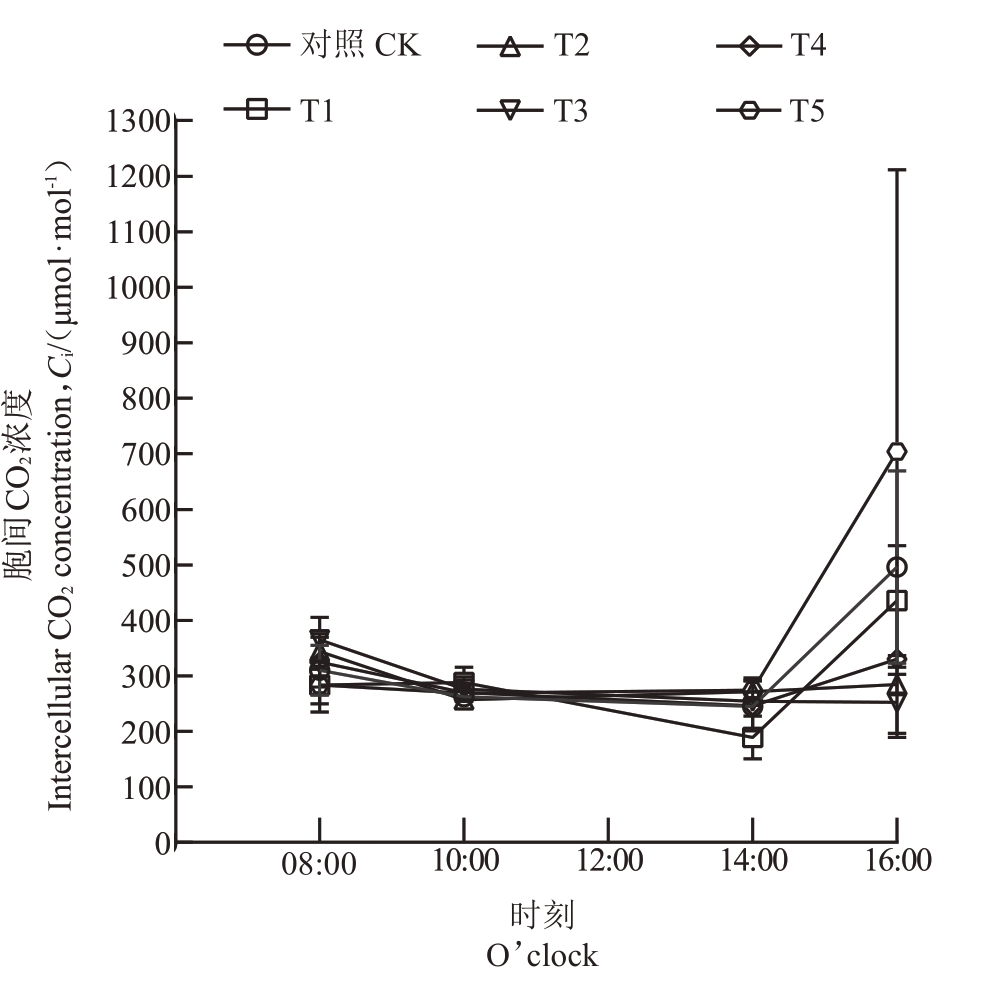
图4 石榴叶片胞间CO2浓度日变化
Fig.4 Daily variation of intercellular CO2 concentration of pomegranate seedlings leaves
2.3.3 不同菌肥处理对水分利用效率(WUE)日变化的影响 菌肥处理显著改变了石榴叶片水分利用效率的日变化模式,部分处理T2、T5 使峰值提前至10:00,且T2、T4、T5在08:00显著降低水分利用效率,而T1在14:00显著提高效率。具体表现为:CK、T1、T3和T4的叶片水分利用效率在08:00最高,之后逐渐下降,在10:00到14:00逐渐回升,在14:00达到次高值后再度下降,16:00 达到最低值。T2 和T5 的叶片水分利用效率在08:00 后持续上升,在10:00 达到峰值后逐渐下降,16:00最低。与CK相比,T2、T4、T5在08:00叶片水分利用效率显著降低,T1在14:00叶片水分利用效率显著升高,T2、T5在14:00叶片水分利用效率显著降低,T2、T3在16:00叶片水分利用效率显著升高,其他各处理与CK均无显著差异(图5)。
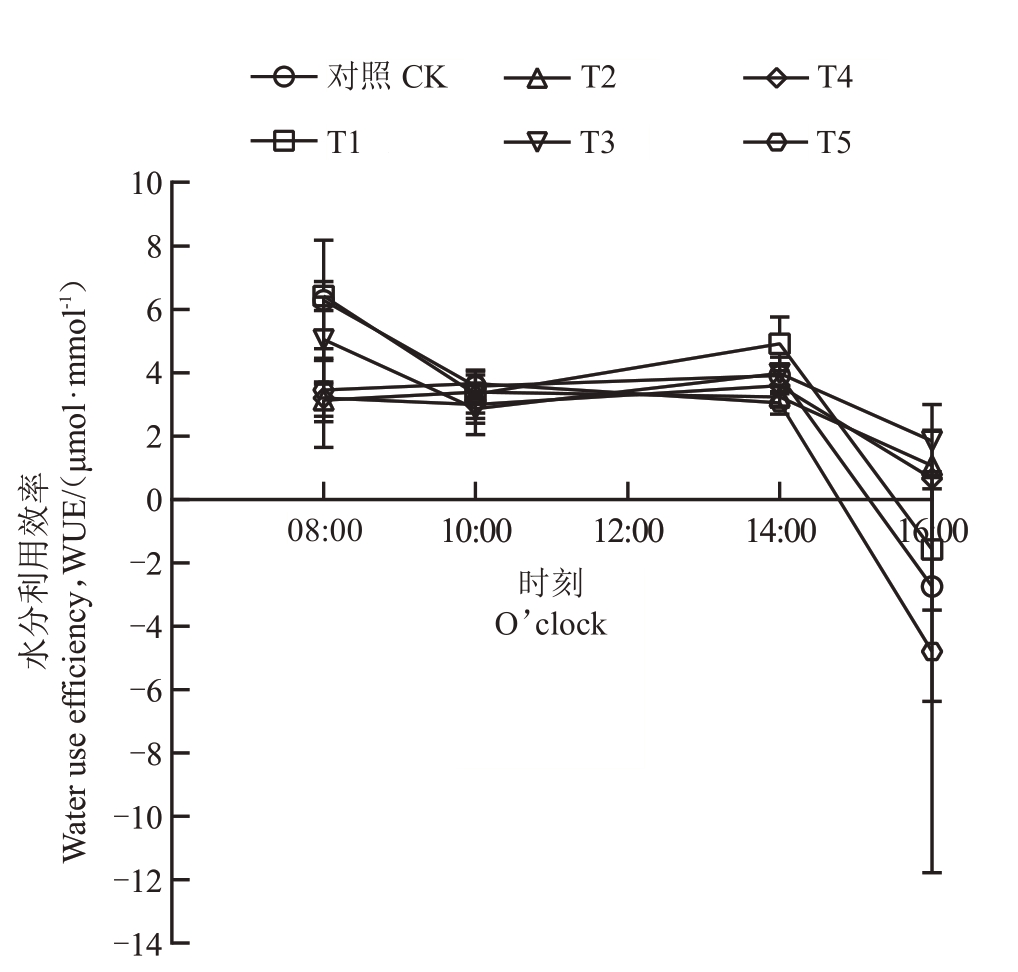
图5 石榴叶片水分利用效率日变化
Fig.5 Daily variation of water use efficiency of pomegranate seedlings leaves
2.3.4 各测定参数的相关性分析 石榴叶片光合参数间存在不同的相关性(表4)。净光合速率与气孔导度、蒸腾速率、水分利用效率之间呈极显著正相关,与胞间CO2浓度呈极显著负相关。气孔导度与蒸腾速率、水分利用效率之间呈极显著正相关。蒸腾速率与胞间CO2浓度呈极显著负相关,与水分利用效率呈极显著正相关。胞间CO2浓度与水分利用效率呈极显著负相关。
表4 光合参数间的相关性分析
Table 4 Correlation analysis between photosynthetic parameters
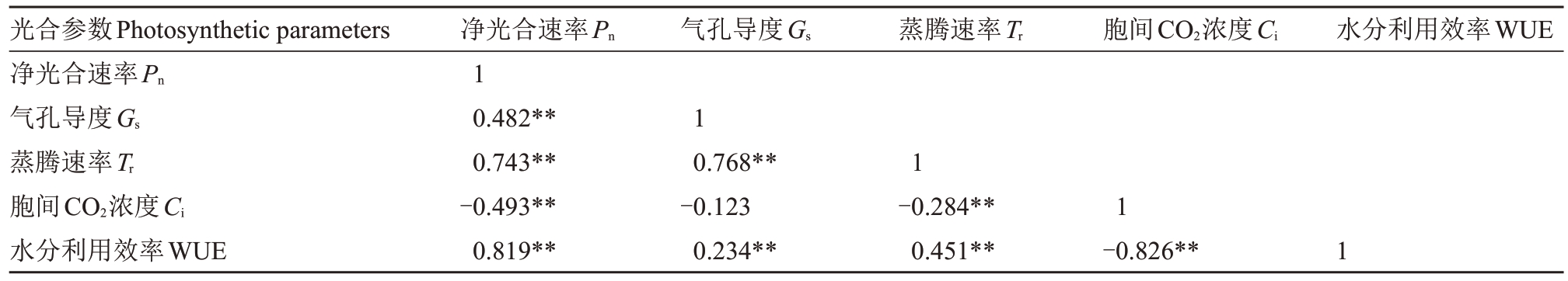
注:**在0.01 水平显著相关。
Note:**means the correlation is significant at the 0.01 level.
?
2.4 不同菌肥处理对土壤酸碱度的影响
为了研究不同菌肥处理对土壤酸碱度的影响,分别测定不同处理下栽培基质土壤pH。与栽培基质初始pH相比(5.81),落叶后对照和各处理的土壤pH 均有所升高。施用微生物菌肥后降低了土壤pH,从高到低依次为CK>T4>T3>T2>T5>T1,但在0.05水平上各处理与CK均无显著差异(图6)。
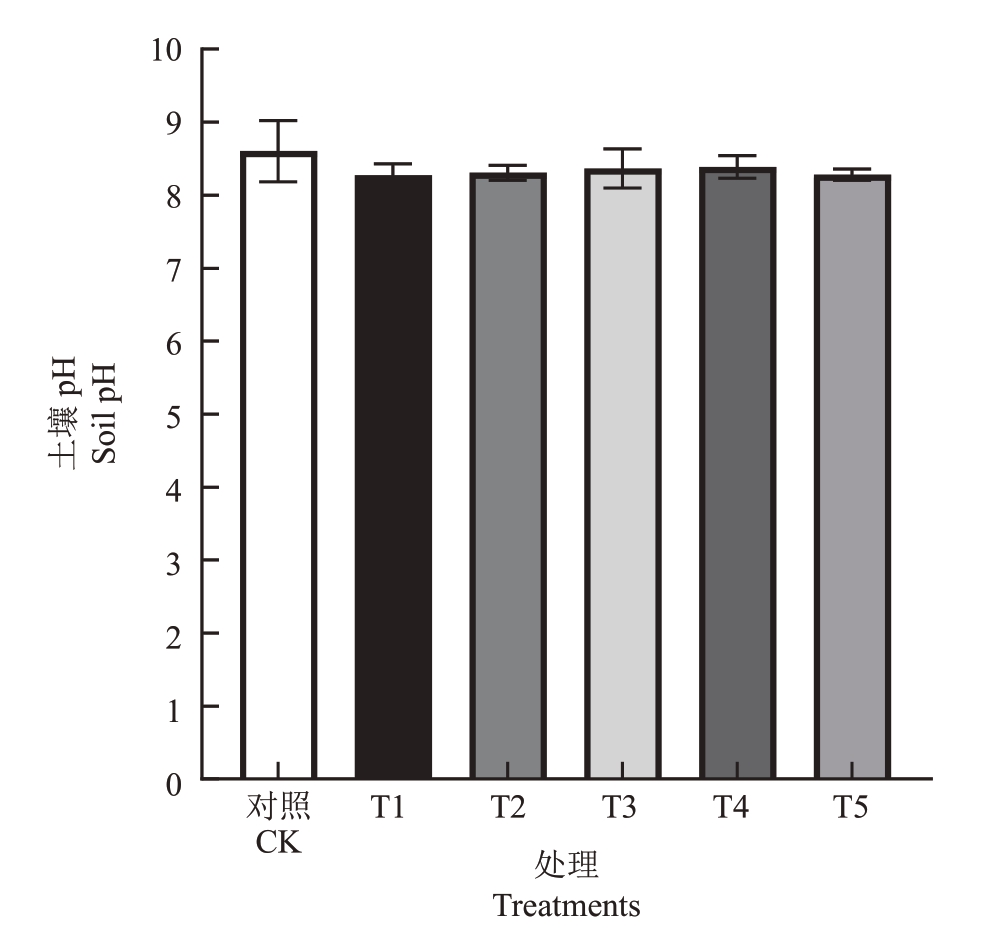
图6 不同菌肥处理下土壤pH
Fig.6 Effects of different treatments on soil pH
3 讨 论
与单一过量施用化学肥料相比,施用微生物菌肥能够改善土壤团粒结构,提升土壤养分含量并调节微生物群落[28],从而促进果树生长发育,提高苹果和梨等果树产量和果实品质[29-31]。
微生物菌剂的效果不仅与活菌数密切相关,还受菌株特性、活性及其在特定环境中的适应性等因素影响。尽管高活菌数通常被认为与菌剂效果呈正相关,但研究表明菌剂的生物有效性同样关键。例如,活力源的枯草芽孢杆菌有效活菌数≥0.2 亿·g-1,而哈茨木霉菌可达10.0亿·g-1,这表明菌剂的效率并非单纯依赖于活菌数,而是与其在特定环境中发挥的功能密切相关。
本试验中,5 种微生物菌剂均能够促进石榴幼苗的生长,但作用机制存在差异。其中,拌施的活力源菌肥效果最佳,处理后幼苗的株高、地径、新枝数分别比对照组高出25.82%、69.06%、46.88%,叶绿素a含量与叶绿素总含量均显著高于对照组,石榴幼苗叶片气孔导度、蒸腾速率和水分利用效率与对照组相比显著增加,胞间CO2浓度则显著降低。活力源富含枯草芽孢杆菌和侧孢短芽孢杆菌,枯草芽孢杆菌可以通过诱导植物合成吲哚乙酸等激素,并通过解磷作用促进黄瓜幼苗的生长和光合作用,增加叶绿素含量[32],产生脂肽类生物表面活性剂,抑制植物病原菌活性[33],在石榴中其能抑制枯萎病菌,从而减少幼苗病害的发生[34-36],同时其分泌物能够使土壤中的矿物金属离子溶出,降低土壤盐碱度,对受盐碱胁迫的植物具有促生作用[37];侧孢短芽孢杆菌能够增加根际土壤中的细菌数量,与真菌竞争营养和生存空间,并通过有益微生物产生抗菌物质,抑制有害真菌的生存。此外,两种芽孢杆菌在营养物质和氧气的竞争中都具有较强的优势,能够促进玉米和柑橘对N、P、K等营养元素的吸收[38-40]。已有研究表明在水稻中与单一菌种菌剂相比,多菌种菌剂的使用效果更好,这可能与不同菌种之间功能互补、相互协同所带来的综合效应有关[41]。因此,推测活力源处理促进石榴幼苗生长,可能与上述两种芽孢杆菌的联合作用密切相关。相比之下,哈茨木霉菌侧重于病害控制,通过分泌抗菌物质和与根系共生关系增强幼苗的抗病性[42];粉状枯草芽孢杆菌制剂在快速定植和促生物质分泌方面效果明显,有助于根系发育;颗粒状地衣芽孢杆菌和胶冻样类芽孢杆菌制剂,主要通过固氮、解磷、解钾提高土壤肥力[43-45];土壤活化剂则改善土壤结构,提升保水保肥能力。
活力源效果较好可能是因为其菌种组合和高效成分具有协同作用,有利于土壤环境优化和石榴幼苗的快速、健康生长。此外本试验中尽管由于雨水冲刷等环境因素,落叶后各处理组土壤pH值均较初始栽培基质有所升高,但菌肥处理组土壤pH值在数值上普遍低于对照组,表明菌肥可能通过微生物活动或有机酸的分泌对土壤酸碱度产生影响。
4 结 论
与对照组相比,施用微生物菌肥可显著促进石榴幼苗的生长发育,其中施用活力源后,石榴幼苗植株的整体生长和发育状态最好。活力源微生物菌肥主要通过提高叶绿素的含量,增加气孔导度,提高水分和胞间CO2的利用效率,促进石榴叶片光合作用,最终表现为石榴幼苗株高、地径、新枝数的显著增加。此外,施用微生物菌肥一定程度上可降低土壤pH值,具有缓解土壤碱含量过高的作用。本研究所得结论可为实际生产提供参考。
[1] ALALAWI S,ALBALAWI F,RAMJI D P.The role of punicalagin and its metabolites in atherosclerosis and risk factors associated with the disease[J]. International Journal of Molecular Sciences,2023,24(10):8476.
[2] ALAMI M,BOUMEZOUGH K,KHALIL A,RAMCHOUN M,BOULBAROUD S,FULOP T,MORVARIDZADEH M,BERROUGUI H.The modulatory bioeffects of pomegranate(Punica granatum L.) polyphenols on metabolic disorders:Understanding their preventive role against metabolic syndrome[J]. Nutrients,2023,15(23):4879.
[3] PARVEEN S,BATOOL A,SHAFIQ N,RASHID M,SULTAN A,WONDMIE G F,BIN JARDAN Y A,BROGI S,BOURHIA M. Developmental landscape of computational techniques to explore the potential phytochemicals from Punica granatum peels for their antioxidant activity in Alzheimer’s disease[J].Frontiers in Molecular Biosciences,2023,10:1252178.
[4] MARINÉ-CASADÓ R,TEICHENNÉ J,TOBAJAS Y,CAIMARI A,VILLAR A,ZANGARA A,MULÀ A,DEL BAS J M.Pomegranate natural extract Pomanox® positively modulates skin health-related parameters in normal and UV-induced photoaging conditions in Hs68 human fibroblast cells[J]. International Journal of Food Sciences and Nutrition,2023,74(1):51-63.
[5] EL HOSRY L,BOU-MITRI C,BOU DARGHAM M,ABOU JAOUDEH M,FARHAT A,EL HAYEK J,BOU MOSLEH J M,BOU-MAROUN E. Phytochemical composition,biological activities and antioxidant potential of pomegranate fruit,juice and molasses:A review[J].Food Bioscience,2023,55:103034.
[6] 侯乐峰,罗华,毕润霞,郝兆祥,谭伟,张立华.我国石榴育种四十年回顾与展望[J].北方园艺,2022(24):139-147.HOU Lefeng,LUO Hua,BI Runxia,HAO Zhaoxiang,TAN Wei,ZHANG Lihua. Review and prospect of pomegranate breeding in China in the past 40 years[J].Northern Horticulture,2022(24):139-147.
[7] 张博文,郭素娟.不同土壤类型条件下板栗品质评价与研究[J].果树学报,2023,40(9):1904-1914.ZHANG Bowen,GUO Sujuan. Evaluation and research on chestnut quality under different soil conditions[J]. Journal of Fruit Science,2023,40(9):1904-1914.
[8] 徐永杰,姜德志,李莉,王瑞文,王代全,王晓飞,王其竹.不同类型有机肥对核桃园土壤、生长结果特性和坚果品质的影响[J].果树学报,2025,42(1):141-150.XU Yongjie,JIANG Dezhi,LI Li,WANG Ruiwen,WANG Daiquan,WANG Xiaofei,WANG Qizhu. Effects of different types of organic fertilizers on soil,growth and fruiting characteristics,and nut quality in walnut orchards[J]. Journal of Fruit Science,2025,42(1):141-150.
[9] 于会丽,谢宁,徐国益,邵微,徐变变,乔宪生,司鹏.减量化肥配施海藻复合物对葡萄产量、品质和养分吸收的影响[J].果树学报,2022,39(4):584-592.YU Huili,XIE Ning,XU Guoyi,SHAO Wei,XU Bianbian,QIAO Xiansheng,SI Peng. Effects of chemical fertilizer reduction combined with seaweed complex application on yield,fruit quality and nutrient absorption of grape[J]. Journal of Fruit Science,2022,39(4):584-592.
[10] NASRABADI M,RAMEZANIAN A,ESHGHI S,SARKHOSH A.Chilling and heat requirement of pomegranate(Punica granatum L.)trees grown under sustained deficit irrigation[J].Scientia Horticulturae,2020,263:109117.
[11] 吴婉莉,刘鲜艳,姚刚,刘皓.中医农业制剂对石榴产量品质与园地土壤的影响[J].西北园艺,2023(12):76-78.WU Wanli,LIU Xianyan,YAO Gang,LIU Hao.Effects of traditional Chinese medicine agricultural preparations on pomegranate yield,quality,and garden soil[J]. Northwest Horticulture,2023(12):76-78.
[12] LIU J,LI H,YUAN Z Y,FENG J J,CHEN S H,SUN G Z,WEI Z H,HU T T. Effects of microbial fertilizer and irrigation amount on growth,physiology and water use efficiency of tomato in greenhouse[J].Scientia Horticulturae,2024,323:112553.
[13] 姜霞,赵俊禧,石盼盼,王小萱,杜晨晖,张朔生,詹海仙.不同菌肥对党参生理及其根际土壤环境的影响[J].中国实验方剂学杂志,2025,31(13):241-251.JIANG Xia,ZHAO Junxi,SHI Panpan,WANG Xiaoxuan,DU Chenhui,ZHANG Shuosheng,ZHAN Haixian.Effects of different microbial fertilizers on physiology and rhizosphere soil environment of Codonopsis pilosula[J]. Chinese Journal of Experimental Traditional Medical Formulae,2025,31(13):241-251.
[14] 许泽华,马军,李百云,郭鑫年,邢润东,周涛.不同用量有机肥和生物菌肥配施对设施番茄土壤性能和品质的影响[J].中国瓜菜,2025,38(3):131-137.XU Zehua,MA Jun,LI Baiyun,GUO Xinnian,XING Rundong,ZHOU Tao. Effects of different amounts of organic fertilizer and biofungal fertilizer on soil properties and quality of tomato in facility[J]. China Cucurbits and Vegetables,2025,38(3):131-137.
[15] 叶勇,吴康云,肖玖军,邓廷飞,牟玉梅,张力,涂德辉,邢丹.不同生物菌肥对山地辣椒产量与品质的影响[J].中国瓜菜,2022,35(11):50-55.YE Yong,WU Kangyun,XIAO Jiujun,DENG Tingfei,MOU Yumei,ZHANG Li,TU Dehui,XING Dan.Effects of biological fertilizers on yield and quality of mountain peppers[J]. China Cucurbits and Vegetables,2022,35(11):50-55.
[16] 姜宇,王娟,刘力勇,谭巍,王志伟,冯一新,王琳,李金龙.化肥与微生物菌肥配施对番茄生长、产量和品质的影响[J].中国蔬菜,2024(8):103-108.JIANG Yu,WANG Juan,LIU Liyong,TAN Wei,WANG Zhiwei,FENG Yixin,WANG Lin,LI Jinlong. The effects of combined application of chemical fertilizers and microbial fertilizers on tomato growth,yield,and quality[J]. China Vegetables,2024(8):103-108.
[17] 段迪瀚,刘情宇,荣梦瑶,李元杰,毕世权,张少斌.微生物菌肥的特点及其作用机制研究进展[J].农业技术与装备,2022(8):98-99.DUAN Dihan,LIU Qingyu,RONG Mengyao,LI Yuanjie,BI Shiquan,ZHANG Shaobin. Research progress on the characteristics and mechanism of microbial fertilizer[J]. Agricultural Technology&Equipment,2022(8):98-99.
[18] 武杞蔓,张金梅,李玥莹,张颖.有益微生物菌肥对农作物的作用机制研究进展[J].生物技术通报,2021,37(5):221-230.WU Qiman,ZHANG Jinmei,LI Yueying,ZHANG Ying. Recent advances on the mechanism of beneficial microbial fertilizers in crops[J].Biotechnology Bulletin,2021,37(5):221-230.
[19] 张佼,屈锋,朱玉尧,杨甲甲,胡晓辉.增施有机肥和微生物菌剂对春季杨凌设施番茄产量和品质的影响[J].西北农业学报,2019,28(5):767-773.ZHANG Jiao,QU Feng,ZHU Yuyao,YANG Jiajia,HU Xiaohui. Effects of more organic fertilizer and microbial agents on yield and quality of spring greenhouse tomato in Yangling[J].Acta Agriculturae Boreali-occidentalis Sinica,2019,28(5):767-773.
[20] 王鹏,韩娟,国淑梅,牛贞福,王清伟.土壤微生物菌剂对大棚油桃植株特性的影响研究[J].东北农业科学,2019,44(2):52-56.WANG Peng,HAN Juan,GUO Shumei,NIU Zhenfu,WANG Qingwei. Effects of soil microbial inoculants on plant characteristics of nectarine in greenhouse[J].Journal of Northeast Agricultural Sciences,2019,44(2):52-56.
[21] 左晓婷,王超鹏,张杭,孙玉芳.微生物菌肥对碱胁迫下新疆野苹果幼苗生理特性的影响[J]. 果树资源学报,2025,6(1):14-20.ZUO Xiaoting,WANG Chaopeng,ZHANG Hang,SUN Yufang.Effects of microbial fertilizer on physiological characteristics of Malus sieversii under alkali stress[J].Journal of Fruit Resources,2025,6(1):14-20.
[22] 车建美,赖恭梯,李思雨,郭奥琳,陈冰星,陈杏,刘波,赖呈纯.复合微生物菌剂对葡萄生长、品质及根际土壤环境的影响[J].生物技术通报,2024,40(8):264-274.CHE Jianmei,LAI Gongti,LI Siyu,GUO Aolin,CHEN Bingxing,CHEN Xing,LIU Bo,LAI Chengchun.Effects of compound microbial agent on the growth,quality and rhizosphere environment of grape[J].Biotechnology Bulletin,2024,40(8):264-274.
[23] 李百云,许泽华,郭鑫年,周涛,贾永华.不同微生物菌剂对土壤肥力及苹果果实品质的影响[J].果树资源学报,2024,5(6):55-60.LI Baiyun,XU Zehua,GUO Xinnian,ZHOU Tao,JIA Yonghua.Effects of different microbial agents on soil fertility and apple fruit quality in orchards[J]. Journal of Fruit Resources,2024,5(6):55-60.
[24] 张小英,李嘉昱,王叶,张子雄,滕尧,陈彩霞,龙秀琴.微生物菌剂对百香果生长发育及果实产量品质的影响[J].江苏农业科学,2024,52(13):155-160.ZHANG Xiaoying,LI Jiayu,WANG Ye,ZHANG Zixiong,TENG Yao,CHEN Caixia,LONG Xiuqin. Influences of microbial agents on growth,fruit yield and quality of Passiflora edulis[J].Jiangsu Agricultural Sciences,2024,52(13):155-160.
[25] 于嘉欣,龙文聪,肖析蒙,成思轩,杨壮,何明珠,杨瑶君.竹纤维高分子菌肥对软籽石榴产量及品质的影响[J].中国土壤与肥料,2022(3):143-147.YU Jiaxin,LONG Wencong,XIAO Ximeng,CHENG Sixuan,YANG Zhuang,HE Mingzhu,YANG Yaojun. Effect of bamboo fiber macromolecule fertilizer on the yield and quality of soft seed pomegranate[J].Soil and Fertilizer Sciences in China,2022(3):143-147.
[26] 郭雁君,吉前华,杜鹏飞,黄火金,许冰玲.低温胁迫对2 种主要砧穗组合砂糖橘幼树抗寒性的影响[J].果树学报,2022,39(5):784-799.GUO Yanjun,JI Qianhua,DU Pengfei,HUANG Huojin,XU Bingling. Effect of two main rootstock species on cold resistance of Shatangju mandarin saplings under low temperature stress[J].Journal of Fruit Science,2022,39(5):784-799.
[27] ARNON D I.Copper enzymes in isolated chloroplasts.Polyphenoloxidase in beta vulgaris[J]. Plant Physiology,1949,24(1):1-15.
[28] 樊建霞,吴琼,游龙,覃贵勇.不同微生物菌肥对连作番茄品质、土壤性质及微生物活性的影响[J].江苏农业科学,2024,52(1):197-204.FAN Jianxia,WU Qiong,YOU Long,QIN Guiyong. Influences of different bio-fertilizer on quality,soil properties and microbial activities of continuous cropping tomatoes[J].Jiangsu Agricultural Sciences,2024,52(1):197-204.
[29] 王晓艳.不同菌肥对油茶叶内源激素及氮磷钾含量和林下土壤理化性质的影响[J].江苏林业科技,2021,48(5):28-32.WANG Xiaoyan. Effects of different bacterial fertilizers on soil properties,leaf endogenous hormones and N,P,K contents in Camellia oleifera Abel[J].Journal of Jiangsu Forestry Science&Technology,2021,48(5):28-32.
[30] 杜倩,李琳,刘铁男,梁素钰.复合菌肥对盐渍土土壤微生物多样性的影响[J].中国农学通报,2022,38(2):38-43.DU Qian,LI Lin,LIU Tienan,LIANG Suyu. Effects of compound microbial fertilizer on soil microbial diversity in saline soil[J]. Chinese Agricultural Science Bulletin,2022,38(2):38-43.
[31] 杨瑞,赵国康,张树武,徐秉良,石海春.木霉T6 生物菌肥对苹果砧木M9T337 幼苗生长的促进作用[J]. 中国果树,2022(5):34-38.YANG Rui,ZHAO Guokang,ZHANG Shuwu,XU Bingliang,SHI Haichun.Effect of Trichoderma longibrachiatum T6 biofertilizer on the growth promotion of apple rootstock M9T337 seedlings[J].China Fruits,2022(5):34-38.
[32] 王程成,张琦,段黄金,张驰,闫敏,吉爽秋,穆凯代斯罕·伊萨克.生物菌肥对‘新梨7 号’树体营养及土壤养分的影响[J].塔里木大学学报,2022,34(1):64-70.WANG Chengcheng,ZHANG Qi,DUAN Huangjin,ZHANG Chi,YAN Min,JI Shuangqiu,Mukaidaisi·Yisake. Effects of bio-bacterial fertilizer on tree nutritional and soil quality of‘Xinli No. 7’[J]. Journal of Tarim University,2022,34(1):64-70.
[33] 谷清义,王妍佳,李梦楠,李蒙,刘松虎,申君.枯草芽孢杆菌BSCY-1 对黄瓜种子萌发和幼苗生长特性的影响[J].北方园艺,2024(10):17-23.GU Qingyi,WANG Yanjia,LI Mengnan,LI Meng,LIU Songhu,SHEN Jun.Effects of a Bacillus subtilis BSCY-1 on the seed germination and seedling growth and physiological characteristics of cucumber[J].Northern Horticulture,2024(10):17-23.
[34] MNIF I,HAMMAMI I,ALI TRIKI M,AZABOU M C,ELLOUZE-CHAABOUNI S,GHRIBI D. Antifungal efficiency of a lipopeptide biosurfactant derived from Bacillus subtilis SPB1 versus the phytopathogenic fungus,Fusarium solani[J].Environmental Science and Pollution Research,2015,22(22):18137-18147.
[35] MNIF I,GRAU-CAMPISTANY A,CORONEL-LEÓN J,HAMMAMI I,ALI TRIKI M,MANRESA A,GHRIBI D. Purification and identification of Bacillus subtilis SPB1 lipopeptide biosurfactant exhibiting antifungal activity against Rhizoctonia bataticola and Rhizoctonia solani[J]. Environmental Science and Pollution Research,2016,23(7):6690-6699.
[36] 周银丽,韦福翠,李彩红,杨伟,胡先奇.石榴枯萎病菌拮抗菌株1 的分离鉴定及定植能力研究[C]//中国园艺学会石榴分会.第四届中国石榴博览会暨第十届全国石榴生产与科研研讨会.中国石榴研究进展(四).北京:中国林业出版社,2022:227-233.ZHOU Yinli,WEI Fuchui,LI Caihong,YANG Wei,HU Xianqi.Isolation and ldentification of pomegranate wilt pathogen antagonism Bacillus strain 1 and its colonization ability studies[C]//Pomegranate Branch of Chinese Society for Horticultural Science. The 4th China Pomegranate Expo and the 10th National Pomegranate Production and Research Seminar. The research Progress of Pomegranate (Ⅳ). Beijing:China Forestry Publishing House,2022:227-233.
[37] CHOWDHURY S P,HARTMANN A,GAO X W,BORRISS R.Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42:A review[J]. Frontiers in Microbiology,2015,6:780.
[38] 叶玢妤,俞跃,何沛益,谢启晓,杨瑞滢,吴嘉睿,刘怡琳,林子然,赖威宇,许凌祎,刘鹏,洪华嫦.丛枝菌根真菌-枯草芽孢杆菌组合体系对盐碱土壤微生态的修复作用[J].中国环境科学,2025,45(1):440-449.YE Fenyu,YU Yue,HE Peiyi,XIE Qixiao,YANG Ruiying,WU Jiarui,LIU Yilin,LIN Ziran,LAI Weiyu,XU Lingyi,LIU Peng,HONG Huachang. Remediation of saline-alkali soil microecology by a combined system of mycorrhizal fungus-Bacillus subtilis[J]. China Environmental Science,2025,45(1):440-449.
[39] 李婧,揣峻峰,王磊,张东旭,肖艳,徐志文.枯草芽孢杆菌发酵上清液对玉米植株生长和养分吸收的影响[J].安徽农业科学,2019,47(10):138-140.LI Jing,CHUAI Junfeng,WANG Lei,ZHANG Dongxu,XIAO Yan,XU Zhiwen.Effects of rushing liquid supernatant in the fermentation of Bacillus subtilis on growth and nutrients uptake of maize[J]. Journal of Anhui Agricultural Sciences,2019,47(10):138-140.
[40] 刘文欢,邱芳颖,王娅,陈朗,马岩岩,吕强,易时来,谢让金,郑永强.枯草芽孢杆菌液态肥对柑橘养分吸收和果实品质的影响[J].园艺学报,2022,49(3):509-518.LIU Wenhuan,QIU Fangying,WANG Ya,CHEN Lang,MA Yanyan,LÜ Qiang,YI Shilai,XIE Rangjin,ZHENG Yongqiang.Effects of liquid microbial fertilizer application of Bacillus subtilis on nutrient absorption and fruit quality of citrus[J].Acta Horticulturae Sinica,2022,49(3):509-518.
[41] 陈慧君.微生物肥料菌种应用与效果分析[D].北京:中国农业科学院,2013.CHEN Huijun.Application of functional species and effect evaluation in microbal fertilizers [D]. Beijing:Chinese Academy of Agricultural Sciences,2013
[42] 常峻嘉,盖佳鑫,陶刚,莫转龙海.哈茨木霉菌对烟草的促生及其黑胫病的诱导抗性评价[J].中国农业科技导报,2024,26(10):168-176.CHANG Junjia,GAI Jiaxin,TAO Gang,MO Zhuanlonghai.Evaluation of the growth-promoting effect of Trichoderma harzianum on tobacco and its induced resistance to black shank disease[J]. Journal of Agricultural Science and Technology,2024,26(10):168-176.
[43] 程凯凯,王秀艳,郭周倩,邢明振,张云鸽.白菜软腐病病原菌鉴定及地衣芽孢杆菌XW02 对其的抑制作用[J].河北农业科学,2024,28(4):49-53.CHENG Kaikai,WANG Xiuyan,GUO Zhouqian,XING Mingzhen,ZHANG Yunge. Identification of soft rot disease pathogen of Chinese cabbage and inhibition effect of Bacillus licheniformis XW02[J]. Journal of Hebei Agricultural Sciences,2024,28(4):49-53.
[44] 娄梦奇,王晓帆,李明蔚,聂丽萍,廖美德.胶冻样类芽孢杆菌对水稻纹枯病病害的防治及田间测产研究[J].安徽农业科学,2023,51(16):140-142.LOU Mengqi,WANG Xiaofan,LI Mingwei,NIE Liping,LIAO Meide. Control of jelly-like Bacillus on rice sheath blight and field yield measurement of rice[J].Journal of Anhui Agricultural Sciences,2023,51(16):140-142.
[45] 张杰,武才女,刘燕燕,陈鑫,高芳芳,马亚君,荣文俊,王保平,田丰庆.增施胶冻样类芽孢杆菌对烟叶产量和品质的影响[J].山西师范大学学报(自然科学版),2022,36(3):68-76.ZHANG Jie,WU Cainü,LIU Yanyan,CHEN Xin,GAO Fangfang,MA Yajun,RONG Wenjun,WANG Baoping,TIAN Fengqing. Effect of increasing Paenibacillus mucilaginosus on yield and quality of tobacco[J]. Journal of Shanxi Normal University(Natural Science Edition),2022,36(3):68-76.