渗透胁迫是植物生长发育过程中面临的主要不利环境之一,对世界农业生产造成了极大危害[1]。渗透胁迫会抑制种子萌发和根系形态建成,抑制幼苗生长,导致植物花粉败育,降低作物产量,加速植物衰老,严重时甚至导致植物死亡[2]。渗透调节是指植物通过调节细胞内溶质的含量来保持细胞渗透压的过程,是植物响应渗透胁迫的重要调节机制[3]。甘氨酸甜菜碱(glycine betaine,GB)是一种广泛存在于高等植物细胞中的季铵碱类化合物[4],被认为是在植物遭受渗透胁迫时发挥主要渗透调节作用的一种甜菜碱[5]。在高等植物中,GB的合成首先以胆碱为底物,在胆碱单加氧酶(choline monooxygenase,CMO)催化下氧化为甜菜碱醛;然后,甜菜碱醛在甜菜碱醛脱氢酶(betaine aldehyde dehydrogenase,BADH)催化下最终合成GB[6]。1989 年,Brouquisse 等[7]首次在菠菜(Spinacia oleracea)叶绿体中分离出CMO 蛋白,克隆到CMO 基因并认为CMO 属于单编码基因。CMO 蛋白仅在植物中存在,CMO 是加氧酶超家族Rieske 型加氧酶家族成员[8]。CMO是植物GB合成过程中的限速酶[9],具有Rieske型[2Fe-2S]和单核非血红素铁中心,通过催化不可逆的羟基化反应,利用分子氧和还原铁氧还蛋白(Fdred)提供的电子,将胆碱氧化成甜菜碱醛[10]。在多种非生物胁迫条件下,不同植物CMO基因的上调表达可以显著提高植物的非生物胁迫抗性[11-13]。通过基因工程过表达CMO 基因可以赋予非GB 积累植物合成积累GB的能力以增强其非生物抗性[14]。
香蕉(Musa spp.)作为重要的热带亚热带水果,是关系世界粮食安全、人类健康和地区发展的重要作物[15]。1955年,Simmonds等[16]提出香蕉由尖叶蕉(Musa acuminata;A 基因组)和长梗蕉(Musa balbisiana;B基因组)两个原始野生蕉种内或种间杂交后代进化而成,还有少数的Musa schizocarpa(S 基因组)和Musa textilis(T 基因组)。在非生物胁迫条件下,同一物种不同基因型个体间存在不同的生理反应和基因表达模式,具有特定基因型的物种可能产生各种表型,从而在适应环境的过程中得以继续生存[17]。Ravi 等[18]认为Musa balbisiana 可能是在极端天气条件下被人类驯化而成。因此,相较Musa acuminata 具有更强的抗旱能力,并通过对干旱条件下不同基因型香蕉进行表型分析发现,ABB基因型香蕉比其他基因型香蕉具有更强的抗旱性[19],在干旱胁迫条件下不同基因型香蕉与耐旱性相关的基因、蛋白均存在差异表达现象[20];Hu 等[21]通过表型和生理分析认为,ABB 基因型的粉蕉比AAA 基因型的巴西蕉更耐渗透胁迫、冷胁迫和盐胁迫;在多种胁迫联合作用下,DREB 基因在Hill banana(AAB 基因型)中的低表达使得其耐旱性和耐热性强于Grand Nain(AAA 基因型)[22]。前期研究表明,外源GB 可缓解渗透胁迫下香蕉的生理响应[23];在不同基因型香蕉品种中鉴定到分别来源于香蕉A、B 基因组的CMO 基因,其结构存在不同特征,且渗透胁迫响应能力存在差异[24]。因此,笔者拟对鉴定得到的香蕉A、B 基因组的CMO 基因功能进行验证,旨在揭示A、B基因组香蕉CMO基因的功能差异,为探索香蕉A、B基因组基因功能差异和抗逆育种提供参考。
1 材料和方法
1.1 材料
以湛江AA(AA 基因型)、巴西蕉(AAA 基因型)、广东大蕉(AAB基因型)和金粉(ABB基因型)4种不同基因型香蕉的生长健壮、长势一致、五叶一心的组培苗为材料,采集全株叶片,取样后迅速放入液氮速冻,然后于-80 ℃超低温冰箱保存备用。
本氏烟草(Nicotiana tabacum)由武汉伯远生物科技有限公司提供。将野生型本氏烟草种子和转基因烟草种子分别用超纯水或20 mmol·L-1 GB 浸泡24 h,经过75%乙醇消毒1 min 后,用无菌水冲洗3次,每次1 min,再经10%次氯酸钠溶液消毒10 min后,用无菌水冲洗5次,每次1 min。各基因型取30粒种子分别点播在含0和150 mmol·L-1甘露醇的MS培养基上,置于25 ℃、16 h光照/8 h黑暗的培养箱中培养。试验设置3次重复,于第10天统计种子萌发率。
将点播在含0 mmol·L-1甘露醇的MS 培养基上生长10 d的野生型本氏烟草和转基因烟草的幼苗移栽至含150 mmol·L-1甘露醇的MS 培养基上,置于25 ℃,16 h 光照/8 h 黑暗的培养箱中竖直培养。每组3株重复,于第10天测量主根长度。
1.2 酵母表达载体的构建及转化
利用同源重组法将在湛江AA、巴西蕉、广东大蕉和金粉中鉴定得到的CMO-A、CMO-H、CMO-B1和CMO-B2等4种CMO基因的编码序列(参考朱博为等[24]的方法)构建到pYES2-NTB 酵母表达载体(瑞源生物,南京)上。载体经BamHⅠ和EcoRⅠ限制性核酸内切酶线性化,37 ℃水浴30 min,10 ×FastDigest Green Buffer(赛默飞,上海)作为缓冲液。利用带有BamHⅠ和EcoRⅠ限制性核酸内切酶酶切位点的特异性同源臂引物(表1)扩增CMO基因编码序列,使用2×Seamless Cloning Mix(博迈德生物,北京)无缝克隆酶对CMO 基因扩增片段和pYES2-NTB 线性化载体进行连接,50 ℃,15 min。将连接产物转化DH5α大肠杆菌感受态(唯地生物,上海),提取阳性质粒。使用Yeastmaker™Yeast Transformation System 2 酵母转化试剂盒(TaKaRa,Japan),采用醋酸锂转化法进行酵母转化试验,按照说明书操作。涂布于SD-Ura(含2%葡萄糖)固体培养基(泛基诺,北京)上,倒置于28 ℃培养箱培养2~3 d。菌液PCR筛选阳性克隆。
表1 本研究所用引物
Table 1 Primers used in this study

?
1.3 酵母功能互补试验
将阳性酵母菌液以1%的体积加入到SD-Ura(含2%葡萄糖)液体培养基中摇菌至OD600=1.2~1.4,4000 r·min-1,离心5 min 去上清液;用不含碳源的SD-Ura 液体培养基清洗酵母细胞并振荡培养3 h,4000 r·min-1,离心5 min去上清液;添加SGUra(含2%半乳糖)液体培养基继续振荡培养8 h,调整OD600=0.6。按100、10-1、10-2、10-3、10-4浓度梯度各取5 μL点涂于相应的SG-Ura固体培养基上,置于28 ℃培养箱培养2~3 d(CK)。其中INVSC1酵母菌株(瑞源生物,南京)用于渗透胁迫、盐胁迫和极端生长温度筛选,ycf1菌株(瑞源生物,南京)用于重金属胁迫筛选。甘露醇模拟渗透胁迫,NaCl模拟盐胁迫,CdCl2模拟重金属胁迫,18 ℃培养箱模拟低温胁迫。
1.4 过表达载体构建及农杆菌转化
利用同源重组法将鉴定得到的4 种CMO 基因的编码序列(去除终止密码子)构建到pGFPGUSplus 过表达载体上。载体使用XbaⅠ限制性核酸内切酶线性化,37 ℃水浴30 min,10×FastDigest Green Buffer 作为缓冲液。利用带有XbaⅠ限制性核酸内切酶酶切位点的特异性同源臂引物(表1)扩增CMO 基因编码序列,将CMO 基因扩增片段和线性化载体进行连接。将连接产物转化DH5α 大肠杆菌感受态,提取阳性质粒。取1 μL质粒加入50 μL GV3101农杆菌感受态细胞(唯地生物,上海)中,充分混匀后吸取至电转杯中,电转后加入1 mL LB液体培养基,充分混匀后吸取至1.5 mL 离心管中,于摇床30 ℃、180 r·min-1振荡培养30 min,将活化好的农杆菌菌液吸取50 μL接种于YEP固体培养基上,30 ℃暗培养48 h。挑取单克隆菌落,于300 μL YEP液体培养基中,30 ℃,200 r·min-1振荡过夜培养,菌液PCR筛选阳性克隆。
1.5 烟草遗传转化及阳性植株筛选
烟草转化由武汉伯远生物科技有限公司完成。烟草种子使用75%乙醇消毒30 s,无菌水清洗1 min,再使用84 消毒液消毒3~5 min,无菌水清洗3 次,每次1 min。将消毒后的烟草种子播种于萌发培养基上,25 ℃,16 h 光照/8 h 黑暗培养4~5 周,将无菌烟草叶片用手术刀切成小块接种于预培养基上。向侵染液中加入阳性农杆菌菌液,制备OD600=0.2的农杆菌重悬液,将预培养2~3 d的烟草叶片放置于农杆菌悬浮液中侵染10~15 min,将侵染后的烟草叶片放置于滤纸上,经晾干后接种于共培养基上,暗培养48~72 h。将共培养2 d 后的叶片转入诱导培养基上诱导愈伤组织,约10 d,待其长出愈伤组织。挑选符合标准的愈伤组织,接种于潮霉素抗性的筛选培养基上,培养时间15~30 d,培养温度(23±2)℃。将二筛生长旺盛的阳性愈伤组织接种至分化培养基上,每皿4~5个愈伤,25 ℃16 h光照/8 h黑暗培养15~30 d。在分化过程中,愈伤组织如有幼苗形成,将其接种至壮苗培养基上生长7~10 d。采用CTAB法提取烟草基因组DNA,使用潮霉素特异性引物进行PCR检测。
1.6 亚细胞定位
于25 ℃、16 h 光照/8 h 黑暗、5000 lx 的培养箱培养本氏烟草,定时浇水,培养一个月后用于试验。通过激光共聚焦显微镜(Nikon C2-ER)观察烟草叶片细胞并采集图像,488 nm激发光下检测绿色荧光蛋白(Green fluorescent protein,GFP)荧光,使用640 nm激发光检测叶绿体自发荧光。
1.7 数据统计与分析
数据导入SPSS 软件(v22)进行统计学分析,采用ANOVA进行方差分析,采用Duncan’s新复极差法进行作多重比较分析,不同小写字母表示差异显著(P<0.05),采用Origin软件(v2022)绘图。
2 结果与分析
2.1 转香蕉CMO 基因酵母对渗透、低温和盐胁迫的响应
为验证A、B 基因组CMO 基因功能差异,利用INVSC1敏感突变酵母进行功能互补试验。结果显示,在SG-Ura培养基28 ℃条件(图1-A)下转化pYES2空载体的INVSC1对照酵母(CK)与重组酵母生长情况无明显差异;在添加1.0 mol·L-1甘露醇(图1-B)、18 ℃条件下(图1-C)和添加1.0 mol·L-1 Nacl(图1-D)的SG-Ura培养基上转化的4种INVSC1重组酵母生长情况均显著优于CK,但转化4种香蕉CMO的INVSC1酵母间生长情况无明显差异。表明在渗透、低温和盐胁迫下,转化4种香蕉CMO均可以提高INVSC1酵母抗性,且在INVSC1酵母中4种香蕉CMO功能无明显差异。
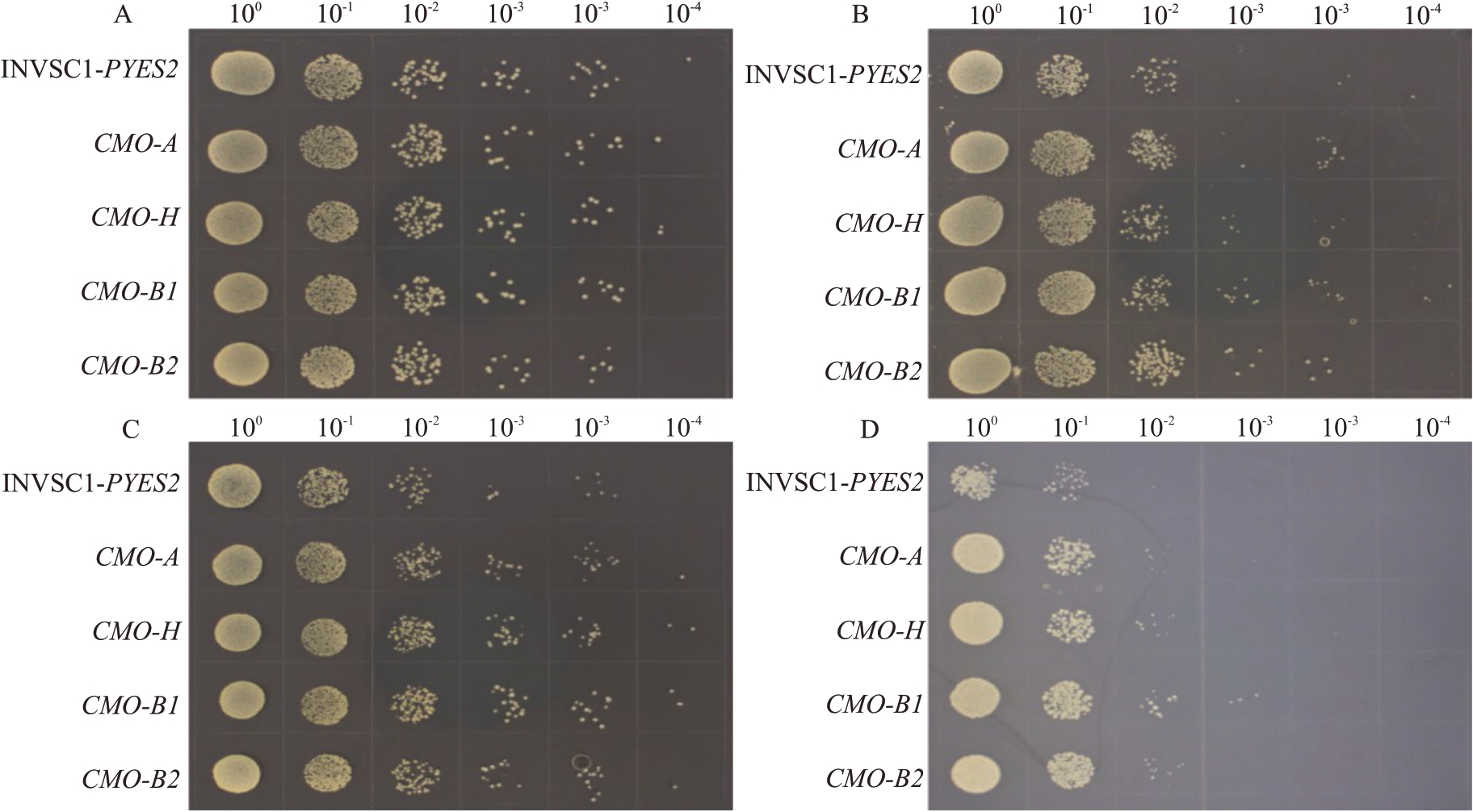
图1 转香蕉CMO 基因酵母对渗透、低温和盐胁迫的响应
Fig.1 Response of transgenic CMO gene yeast to osmotic,low-temperature,and salt stresses
生长条件:A.SG-Ura 培养基28 ℃培养(CK);B.添加1.0 mol·L-1 甘露醇的SG-Ura 培养基28 ℃培养;C.SG-Ura 培养基18 ℃培养;D.添加1.0 mol·L-1 Nacl 的SG-Ura 培养基28 ℃培养。
Growth conditions:A.SG-Ura media cultured at 28 ℃(CK);B.SG-Ura media cultured at 28 ℃with the addition of 1.0 mol·L-1 mannitol;C.SGUra media cultured at 18 ℃;D.SG-Ura media cultured at 28 ℃with the addition of 1.0 mol·L-1 NaCl.
2.2 转香蕉CMO基因酵母对重金属胁迫的响应
利用ycf1 重金属离子敏感型突变酵母进行功能互补试验。结果如图2所示:在不含CdCl2的SGUra 培养基上转化pYES2 空载体的ycf1 对照酵母(CK)与转化4种香蕉CMO 的ycf1酵母生长情况无明显差异(图2-A),在含40 mmol·L-1 CdCl2的SGUra培养基上转化CMO-A、CMO-H、CMO-B1、CMOB2 的ycf1 酵母生长情况显著优于转化pYES2 空载体的ycf1 酵母(图2-B)。表明在重金属胁迫条件下,转化4种香蕉CMO基因均可以提高ycf1酵母耐重金属胁迫能力,且转化CMO-H、CMO-B1 的ycf1酵母生长情况优于转化CMO-A、CMO-B2 的ycf1 酵母。
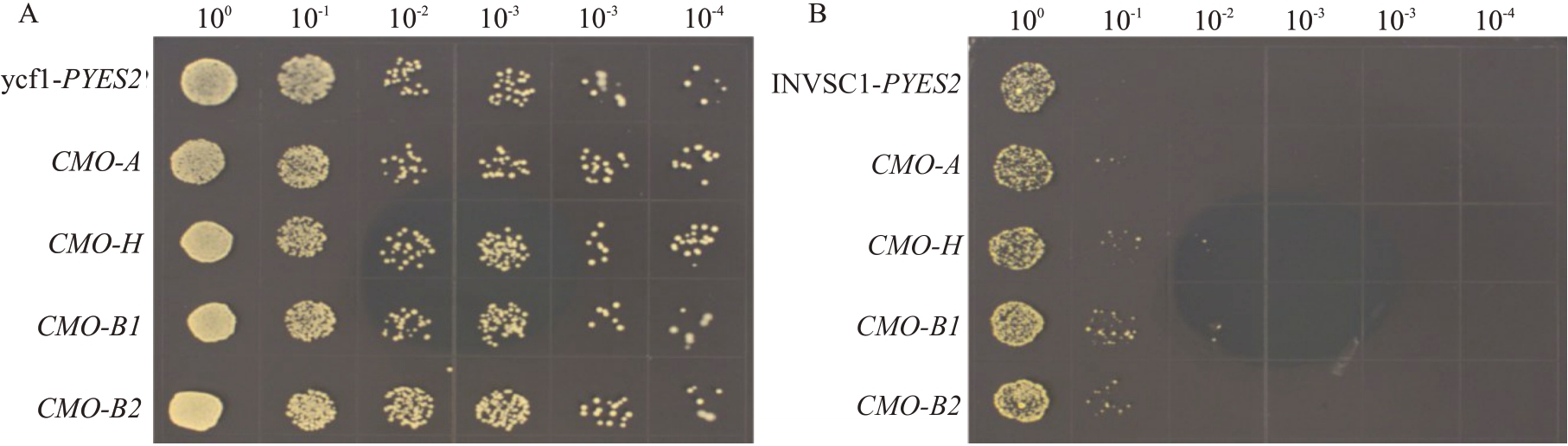
图2 转香蕉CMO 基因酵母对重金属胁迫的响应
Fig.2 Response of transgenic CMO gene yeast to heavy metal stress
生长条件:A.SG-Ura 培养基28 ℃培养(CK);B.添加40 mmol·L-1 Cdcl2 的SG-Ura 培养基28 ℃培养。
Growth conditions:A.SG-Ura media cultured at 28 ℃(CK);B.SG-Ura media with 40 mmol·L-1 Cdcl2 added,cultured at 28 ℃.
2.3 香蕉CMO转基因烟草鉴定
提取转化4种香蕉CMO基因烟草的DNA,进行PCR 鉴定。结果见图3,电泳结果显示得到501 bp目的片段,与pGFPGUSplus载体潮霉素基因片段大小一致,表明香蕉不同CMO基因已经成功转入。转化CMO-A、CMO-H、CMO-B1、CMO-B2 基因的烟草,其阳性率分别为95.45%、100%、100%、76.19%。
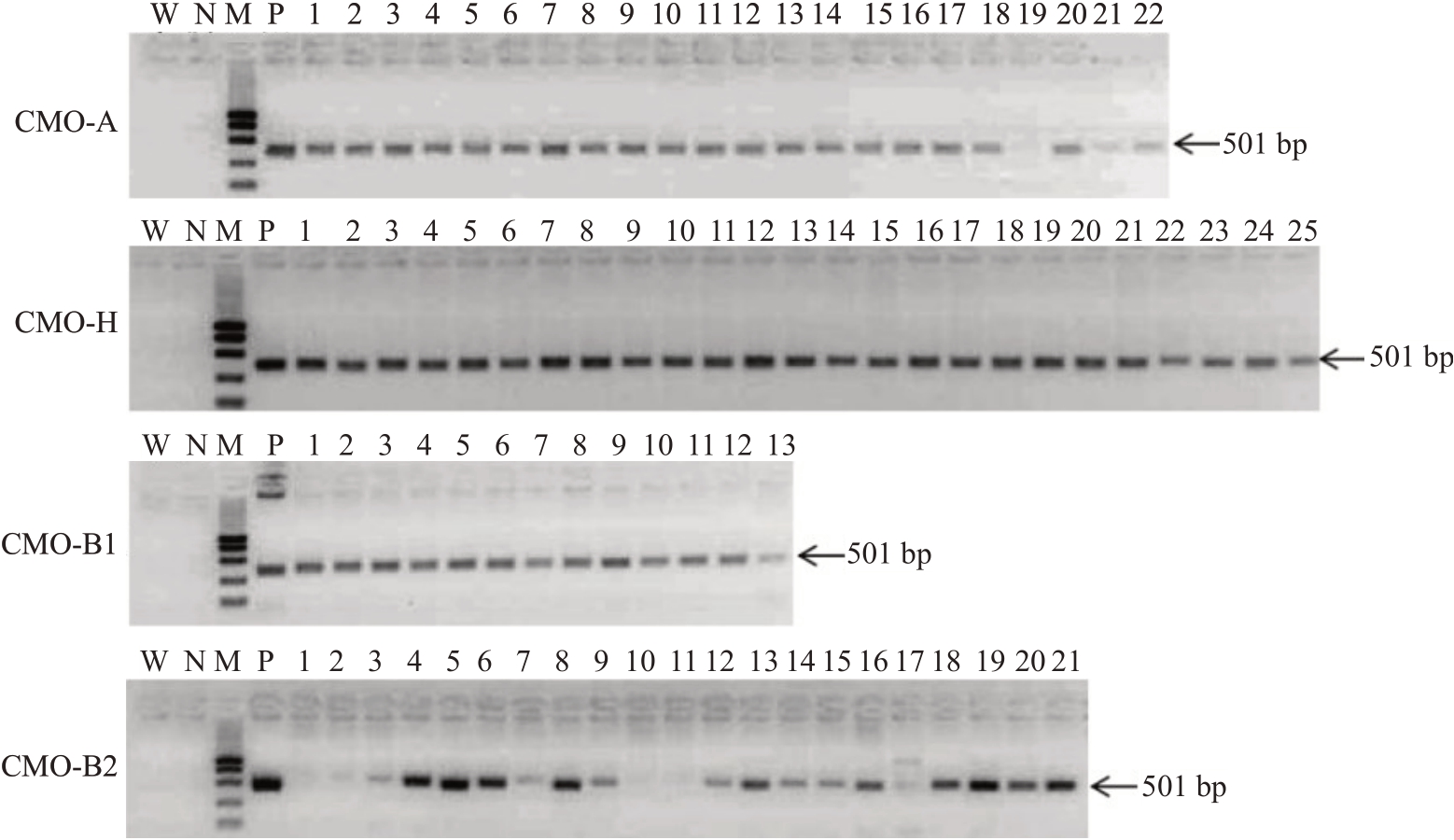
图3 香蕉CMO 转基因烟草鉴定
Fig.3 Transformation of banana CMO gene tobacco identification
泳道:W.水;N.阴性对照;M.1000 bp Marker;P.阳性对照。
Lane:W.Water;N.Negative control;M.1000 bp Marker;P.Positive control.
2.4 渗透胁迫对过表达香蕉CMO基因烟草种子萌发的影响
为探究渗透胁迫对过表达香蕉A、B 基因组CMO 基因烟草种子萌发的影响,将经超纯水或20 mmol·L-1 GB 处理后野生型烟草和过表达香蕉A、B 基因组CMO 基因烟草的种子点播于MS 与150 mmol·L-1甘露醇+MS 培养基上。由图4 可知,10 d 时,在MS 培养基上,WT 和过表达香蕉A、B 基因组CMO 基因烟草种子萌发率无显著差异;在150 mmol·L-1甘露醇+MS 培养基上,WT 和过表达香蕉A、B 基因组CMO 基因烟草种子萌发率较MS培养基上种子萌发率均显著降低,过表达CMO-H、CMO-B1和CMO-B2基因烟草种子萌发率显著高于WT。在渗透胁迫条件下,20 mmol·L-1 GB 处理后WT 和过表达香蕉A、B 基因组CMO 基因烟草种子萌发率较150 mmol·L-1甘露醇+MS培养基上种子萌发率均显著升高,分别上升了23.33%、42.22%、30%、33.33%和33.33%。
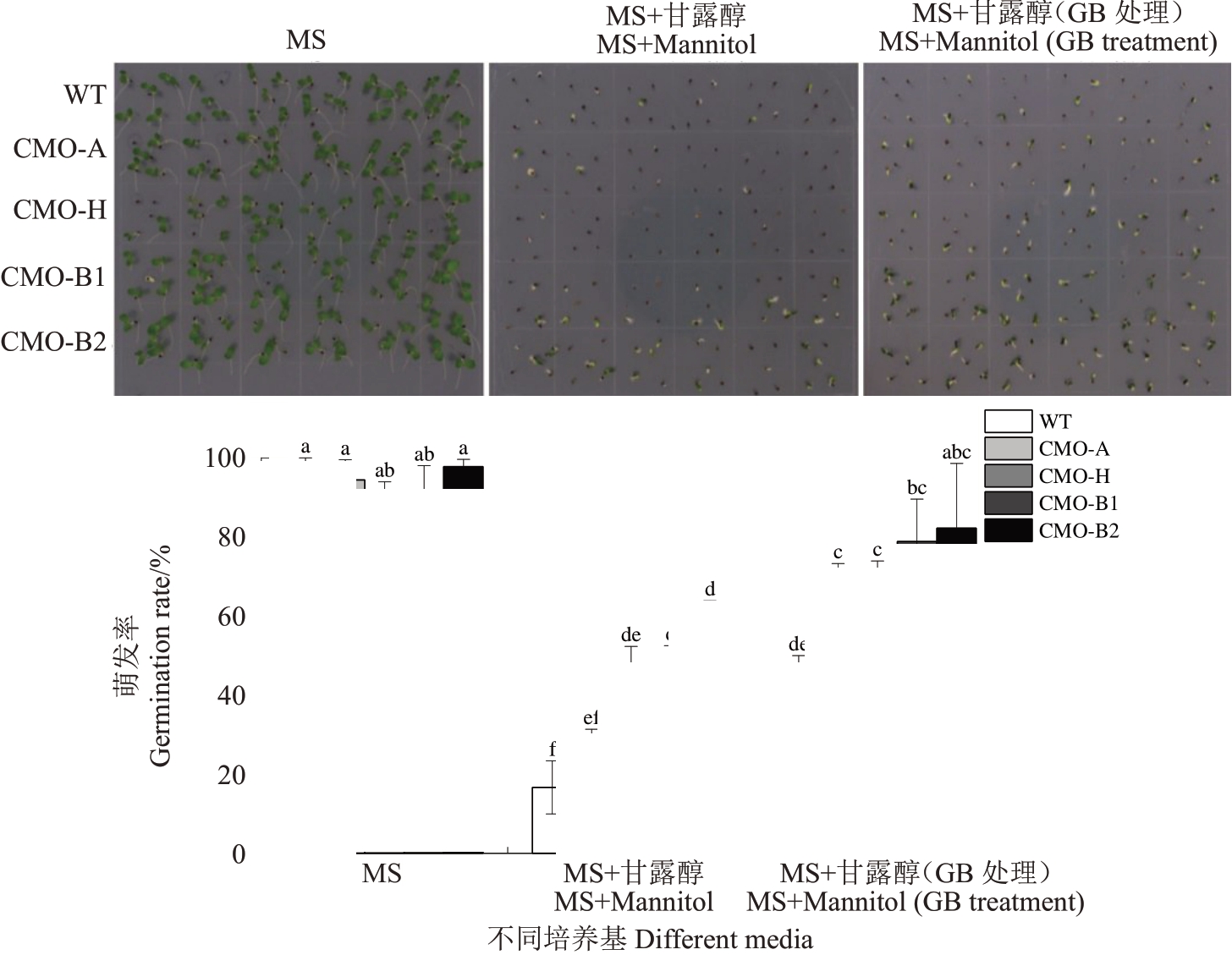
图4 渗透胁迫对过表达香蕉CMO 基因烟草种子萌发的影响
Fig.4 Effects of osmotic stress on the germination of banana CMO gene transgenic tobacco seeds
不同小写字母表示在0.05 水平差异显著。下同。
Different small letters indicate significant difference at 0.05 level.The same below.
2.5 渗透胁迫对过表达香蕉CMO基因烟草幼苗根长的影响
将萌发10 d 后的野生型烟草和过表达香蕉A、B基因组CMO基因烟草幼苗移栽至含150 mmol·L-1甘露醇的MS 培养基上竖直培养10 d,观察烟草幼苗根的生长情况。结果表明(图5),在渗透胁迫条件下,转化香蕉CMO-B1 与CMO-B2 基因烟草根长较WT 根长显著增加,转化香蕉CMO-A 与CMO-H基因烟草根长与WT 根长之间无显著差异。WT 与转化香蕉CMO-A、CMO-H、CMO-B1、CMO-B2 基因烟草的根长分别达到1.80、2.03、2.07、2.57、2.57 cm,分别是WT根长的1.13、1.15、1.43、1.43倍。
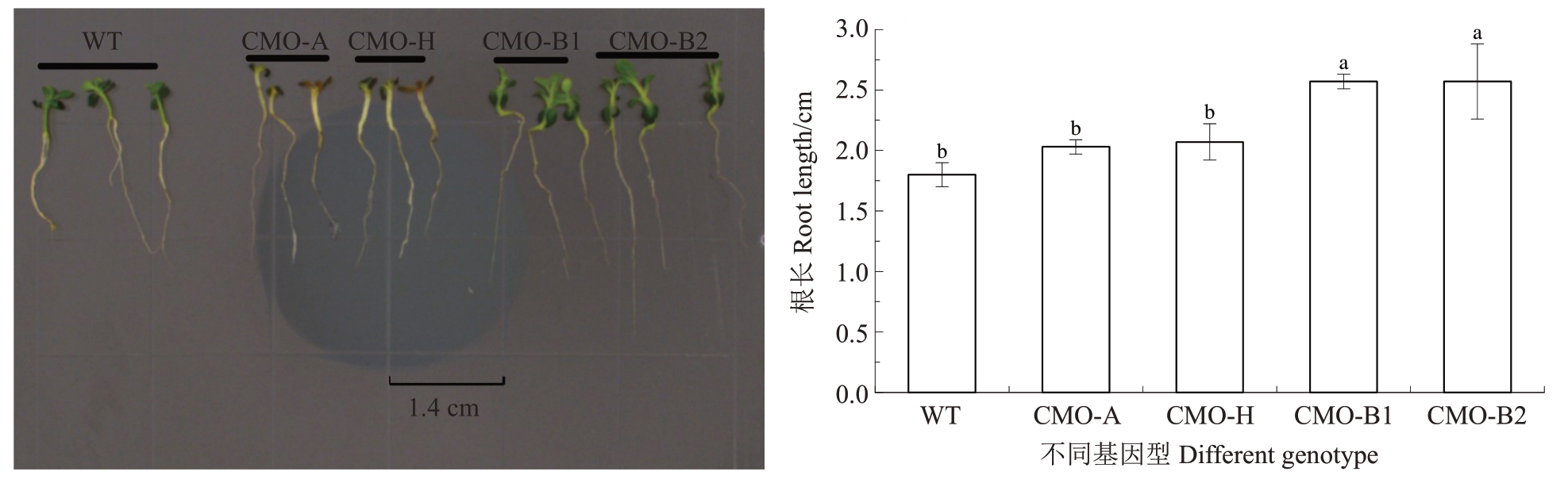
图5 渗透胁迫对过表达香蕉CMO 基因烟草幼苗根长的影响
Fig.5 Effects of osmotic stress on root length of tobacco seedlings transgenic with banana CMO genes
2.6 香蕉CMO蛋白亚细胞定位
为探究A、B 基因组香蕉CMO 蛋白表达情况,在激光共聚焦显微镜下观察香蕉A、B 基因组CMO基因烟草叶片。结果如图6 所示,在整个细胞中可以观察到对照空载体所发出的GFP 信号,4 种香蕉CMO-GFP 融合蛋白的绿色荧光均与叶绿体自发的红色荧光完全重合,表明4种香蕉CMO蛋白主要定位在叶绿体中。

图6 香蕉CMO 蛋白亚细胞定位(比例尺=25 μm)
Fig.6 Subcellular localization of banana CMO proteins(Bar=25 μm)
3 讨 论
目前,CMO 基因已经在多种高等植物中被鉴定,并发现其在植物GB 合成以及响应胁迫反应过程中起重要作用[25]。在4种不同基因型香蕉中鉴定到分别具有或兼具香蕉A、B基因组特征的CMO基因,并且发现不同基因型香蕉CMO基因对渗透胁迫具有不同程度的响应能力,推测其基因结构差异可能导致其功能存在差异。因此,为进一步探究香蕉A、B基因组与不同基因组香蕉抗逆性相关差异,对基因功能进行验证。
酵母是研究高等真核生物的一种重要模式生物,将异源基因导入酵母突变体中并促进重组蛋白在特定环境下的表达以改变转基因酵母相关性状是植物基因功能验证的一种常见方法[26]。Mitsuya等[27]将大麦CMO 重组到PYES2 载体上并导入酵母中,大量诱导大麦CMO 重组蛋白表达,获得了具有高催化活性的蛋白,说明酵母表达系统可以表达具有活性的外源CMO 蛋白。将植物GB 生物合成途径引入酵母可以有效提高其对各种非生物胁迫的耐受性。Wang等[28]将大豆BADH和CMO基因导入酵母中,发现在盐、甲醇和高温胁迫下,重组酵母中CMO 和BADH 蛋白活性较转化空载体的对照酵母显著升高并且重组酵母中GB的积累量显著高于对照,重组酵母表现出更强的胁迫抗性。过表达外源GB合成基因已被证明能显著增强植物非生物胁迫耐受性。Wu等[29]发现盐角草CMO基因可提高转基因烟草的盐胁迫抗性,而Shen 等[30]研究表明,过表达菠菜CMO 的本氏烟草通过内源GB 积累增强耐旱性。笔者利用PYES2 酵母表达系统和烟草遗传转化技术验证香蕉不同基因组的CMO基因功能,通过观察非生物胁迫条件下酵母菌落和烟草的生长状况,首次论证了香蕉B 基因组CMO(CMO-B1 和CMO-B2)在渗透和重金属胁迫下具有功能优势。这一结果与Hu等[21]关于ABB基因型香蕉高抗逆性的报道一致,表明B 基因组可能通过进化压力筛选出更高效的胁迫响应基因。本研究结果证实,在渗透胁迫下过表达香蕉B基因组CMO-B1和CMO-B2的烟草种子萌发率显著高于野生型,且外源GB的添加可协同增强其渗透胁迫抗性。该协同效应表明内源性GB合成与外源补充存在功能互补,验证了前人报道的互作机制[31]。由此推测,B 基因组CMO 可能通过双重途径增强植物胁迫响应,一是增强GB 生物合成效率,二是调控下游胁迫信号转导通路。
在高等植物中,关于CMO 蛋白定位的报道主要是在细胞的叶绿体基质中[32]和过氧化物酶体中[27]。这也被认为是GB 合成主要发生在叶绿体基质或细胞质基质[33-34]中的原因。CMO 催化底物(胆碱)的利用效率被认为是限制植物GB 合成过程的关键因素[35],由于胆碱的合成发生在细胞质中[36],一般需要通过转运体转运到叶绿体基质参与GB 合成[37]。然而并非所有高等植物都可以在自然条件下合成GB,有研究者认为部分植物叶绿体基质中缺乏功能性CMO 或无CMO 导致其不能自然积累GB,例如拟南芥[38]、烟草[39]和水稻[40]等。Gubernator等[41]认为具有Rieske 型[2Fe-2S]结构域的蛋白定位在叶绿体基质中,与本研究亚细胞定位结果一致。
4 结 论
笔者揭示香蕉B基因组CMO基因(CMO-B1和CMO-B2)在渗透胁迫响应中的功能显著优于A 基因组基因。功能试验结果表明,过表达CMO-B1 或CMO-B2 可增强酵母和烟草对渗透、盐及重金属胁迫的抗性,其转基因烟草种子萌发率提高25%以上,根长增加1.43 倍,且外源GB 协同缓解渗透损伤。亚细胞定位结果证实香蕉A、B基因组CMO均定位于叶绿体上。笔者未在香蕉中直接验证香蕉A、B基因组CMO基因的功能差异,未来需通过香蕉稳定转化体系进一步确认其功能特异性。此外,CMO与BADH的协同作用机制仍需深入解析。本研究结果为解析香蕉A、B 基因组抗逆性差异及分子育种提供了关键理论依据。
[1] SKRIVER K,MUNDY J.Gene expression in response to abscisic acid and osmotic stress[J]. The Plant Cell,1990,2(6):503-512.
[2] DA CUNHA VALENÇA D,DE MOURA S M,TRAVASSOSLINS J,ALVES-FERREIRA M,MEDICI L O,ORTIZ-SILVA B,MACRAE A,REINERT F. Physiological and molecular responses of Setaria viridis to osmotic stress[J]. Plant Physiology and Biochemistry,2020,155:114-125.
[3] HELLEBUSI J A. Osmoregulation[J]. Annual Review of Plant Physiology,1976,27:485-505.
[4] ASHRAF M,FOOLAD M R. Roles of glycine betaine and proline in improving plant abiotic stress resistance[J]. Environmental and Experimental Botany,2007,59(2):206-216.
[5] 徐保红,杨洁.甘氨酸甜菜碱与植物抗胁迫能力[J].新疆大学学报(自然科学版),2008,25(3):349-352.XU Baohong,YANG Jie. Glycine betaine and its relationships with plant stress resistance[J]. Journal of Xinjiang University(Natural Science Edition),2008,25(3):349-352.
[6] HANSON A D,WYSE R.Biosynthesis,translocation,and accumulation of betaine in sugar beet and its progenitors in relation to salinity[J].Plant Physiology,1982,70(4):1191-1198.
[7] BROUQUISSE R,WEIGEL P,RHODES D,YOCUM C F,HANSON A D. Evidence for a ferredoxin-dependent choline monooxygenase from spinach chloroplast stroma[J].Plant Physiology,1989,90(1):322-329.
[8] MITCHELL A J,WENG J K.Unleashing the synthetic power of plant oxygenases:From mechanism to application[J].Plant Physiology,2019,179(3):813-829.
[9] HIBINO T,WADITEE R,ARAKI E,ISHIKAWA H,AOKI K,TANAKA Y,TAKABE T.Functional characterization of choline monooxygenase,an enzyme for betaine synthesis in plants[J].Journal of Biological Chemistry,2002,277(44):41352-41360.
[10] CARRILLO-CAMPOS J,RIVEROS-ROSAS H,RODRÍGUEZSOTRES R,MUÑOZ-CLARES R A.Bona fide choline monoxygenases evolved in Amaranthaceae plants from oxygenases of unknown function:Evidence from phylogenetics,homology modeling and docking studies[J]. PLoS One,2018,13(9):e0204711.
[11] XU Z J,SUN M L,JIANG X F,SUN H P,DANG X M,CONG H Q,QIAO F.Glycinebetaine biosynthesis in response to osmotic stress depends on jasmonate signaling in watermelon suspension cells[J].Frontiers in Plant Science,2018,9:1469.
[12] KHAN M S,YU X,KIKUCHI A,ASAHINA M,WATANABE K N. Genetic engineering of glycine betaine biosynthesis to enhance abiotic stress tolerance in plants[J]. Plant Biotechnology,2009,26(1):125-134.
[13] RUSSELL B L,RATHINASABAPATHI B,HANSON A D. Osmotic stress induces expression of choline monooxygenase in sugar beet and amaranth[J].Plant Physiology,1998,116(2):859-865.
[14] YIM H H,VILLAREJO M R.Gene expression and osmoregulation in bacteria[M]//Cellular and Molecular Physiology of Cell Volume Regulation.Boca Raton:CRC Press,2020:335-346.
[15] HESLOP-HARRISON J S,SCHWARZACHER T. Domestication,genomics and the future for banana[J].Annals of Botany,2007,100(5):1073-1084.
[16] SIMMONDS N W,SHEPHERD K. The taxonomy and origins of the cultivated bananas[J]. Journal of the Linnean Society of London,Botany,1955,55(359):302-312.
[17] HILKER M,SCHMÜLLING T. Stress priming,memory,and signalling in plants[J]. Plant,Cell & Environment,2019,42(3):753-761.
[18] RAVI I,VAGANAN M M.Abiotic stress tolerance in banana[M].RAO N K S,SHIVASHANKARA K S,LAXMAN R H.Abiotic stress physiology of horticultural crops. India:Springer,2016:207-222.
[19] RAVI I,UMA S,VAGANAN M M,MUSTAFFA M M. Phenotyping bananas for drought resistance[J]. Frontiers in Physiology,2013,4:9.
[20] MATTOS-MOREIRA L A,FERREIRA C F,AMORIM E P,PIROVANI C P,DE ANDRADE E M,FILHO M A C,DA SILVA LEDO C A. Differentially expressed proteins associated with drought tolerance in bananas(Musa spp.)[J].Acta Physiologiae Plantarum,2018,40(3):60.
[21] HU W,DING Z H,TIE W W,YAN Y,LIU Y,WU C L,LIU J H,WANG J S,PENG M,XU B Y,JIN Z Q.Comparative physiological and transcriptomic analyses provide integrated insight into osmotic,cold,and salt stress tolerance mechanisms in banana[J].Scientific Reports,2017,7:43007.
[22] JANGALE B L,CHAUDHARI R S,AZEEZ A,SANE P V,SANE A P,KRISHNA B. Independent and combined abiotic stresses affect the physiology and expression patterns of DREB genes differently in stress-susceptible and resistant genotypes of banana[J].Physiologia Plantarum,2019,165(2):303-318.
[23] 唐露,朱博为,乔飞,李新国.外源甜菜碱对渗透胁迫下巴西蕉幼苗生长和生理的影响[J].西南林业大学学报(自然科学),2023,43(2):26-34.TANG Lu,ZHU Bowei,QIAO Fei,LI Xinguo.Effects of exogenous betaine on physiology of Brazil banana under osmotic stress[J]. Journal of Southwest Forestry University (Natural Sciences),2023,43(2):26-34.
[24] 朱博为,于佳玄,李新国,刘菊华.香蕉A、B 基因组胆碱单加氧酶基因克隆及渗透胁迫下表达特性分析[J].热带作物学报,2024,45(8):1538-1551.ZHU Bowei,YU Jiaxuan,LI Xinguo,LIU Juhua. Cloning and expression characteristics of choline monooxygenase gene in banana A and B genomes under osmotic stress[J]. Chinese Journal of Tropical Crops,2024,45(8):1538-1551.
[25] AHMAD Z,ANJUM S,AHMAD WARAICH E,AYUB M A,AHMAD T,TARIQ R M S,AHMAD R,IQBAL M A.Growth,physiology,and biochemical activities of plant responses with foliar potassium application under drought stress:A review[J].Journal of Plant Nutrition,2018,41(13):1734-1743.
[26] KARBALAEI M,REZAEE S A,FARSIANI H.Pichia pastoris:A highly successful expression system for optimal synthesis of heterologous proteins[J]. Journal of Cellular Physiology,2020,235(9):5867-5881.
[27] MITSUYA S,KUWAHARA J,OZAKI K,SAEKI E,FUJIWARA T,TAKABE T. Isolation and characterization of a novel peroxisomal choline monooxygenase in barley[J]. Planta,2011,234(6):1215-1226.
[28] WANG S H,YAO Q H,TAO J M,QIAO Y S,ZHANG Z.Co-ordinate expression of glycine betaine synthesis genes linked by the FMDV 2A region in a single open reading frame in Pichia pastoris[J]. Applied Microbiology and Biotechnology,2007,77(4):891-899.
[29] WU S,SU Q,AN L J. Isolation of choline monooxygenase(CMO) gene from Salicornia europaea and enhanced salt tolerance of transgenic tobacco with CMO genes[J]. Indian Journal of Biochemistry&Biophysics,2010,47(5):298-305.
[30] SHEN Y G,DU B X,ZHANG W K,ZHANG J S,CHEN S Y.AhCMO,regulated by stresses in Atriplex hortensis,can improve drought tolerance in transgenic tobacco[J].Theoretical and Applied Genetics,2002,105(6):815-821.
[31] 李茂富,韦建学,符良峰,李绍鹏.外源甜菜碱对低温胁迫下香蕉内源甜菜碱合成的影响[J]. 西北植物学报,2011,31(7):1400-1404.LI Maofu,WEI Jianxue,FU Liangfeng,LI Shaopeng. Effects of exogenous betaine on the endogenous betaine synthesis in banana under low temperature stress[J].Acta Botanica Boreali-Occidentalia Sinica,2011,31(7):1400-1404.
[32] RATHINASABAPATHI B,MCCUE K F,GAGE D A,HANSON A D. Metabolic engineering of glycine betaine synthesis:Plant betaine aldehyde dehydrogenases lacking typical transit peptides are targeted to tobacco chloroplasts where they confer betaine aldehyde resistance[J].Planta,1994,193(2):155-162.
[33] SAKAMOTO A,MURATA N.The role of glycine betaine in the protection of plants from stress:Clues from transgenic plants[J].Plant,Cell&Environment,2002,25(2):163-171.
[34] MANSOUR M M F,ALI E F. Glycinebetaine in saline conditions:An assessment of the current state of knowledge[J].Acta Physiologiae Plantarum,2017,39(2):56.
[35] NUCCIO M L,RUSSELL B L,NOLTE K D,RATHINASABAPATHI B,GAGE D A,HANSON A.The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing choline monooxygenase[J].The Plant Journal,1998,16(4):487-496.
[36] JARIN A,GHOSH U K,HOSSAIN M S,MAHMUD A,KHAN M A R.Glycine betaine in plant responses and tolerance to abiotic stresses[J].Discover Agriculture,2024,2(1):127.
[37] DETTMER J,URSACHE R,CAMPILHO A,MIYASHIMA S,BELEVICH I,O’REGAN S,MULLENDORE D L,YADAV S R,LANZ C,BEVERINA L,PAPAGNI A,SCHNEEBERGER K,WEIGEL D,STIERHOF Y D,MORITZ T,KNOBLAUCH M,JOKITALO E,HELARIUTTA Y. CHOLINE TRANSPORTER-LIKE1 is required for sieve plate development to mediate long-distance cell-to-cell communication[J].Nature Communications,2014,5:4276.
[38] LAI S J,LAI M C,LEE R J,CHEN Y H,YEN H E.Transgenic Arabidopsis expressing osmolyte glycine betaine synthesizing enzymes from halophilic methanogen promote tolerance to drought and salt stress[J].Plant Molecular Biology,2014,85(4):429-441.
[39] SUN H J,SUN X W,WANG H,MA X L.Advances in salt tolerance molecular mechanism in tobacco plants[J].Hereditas,2020,157(1):5.
[40] MO X,QIAN J Y,LIU P,ZENG H L,CHEN G H,WANG Y.Exogenous betaine enhances the protrusion vigor of rice seeds under heat stress by regulating plant hormone signal transduction and its interaction network[J]. Antioxidants,2022,11(9):1792.
[41] GUBERNATOR B,KRÓLICZEWSKI J,KALLAS T,SZCZEPANIAK A.Iron-sulfur cluster reconstitution of spinach chloroplast Rieske protein requires a partially prefolded apoprotein[J].Biochimica et Biophysica Acta(BBA)-Proteins and Proteomics,2006,1764(4):735-742.