枇杷(Eriobotrya japonica Lindl.)是原产于中国的亚热带常绿果树,其果实被誉为“早熟第一果”,市场前景广阔[1-2]。类胡萝卜素是枇杷果实的主要色素,不仅影响果实外观,还影响其商业价值[3-4]。对人体而言,类胡萝卜素具有抗氧化和提高抵抗力等多种有益作用[5]。
光照是影响果实类胡萝卜素合成的一个重要因素,遮光处理对不同果实类胡萝卜素合成的影响存在差异。例如,遮光能够促进茶叶和葡萄柚中类胡萝卜素的积累[6-7],而在辣椒和黄桃中却表现出相反的作用[8-9]。已有研究发现,套袋能够提高枇杷果实的质量和商品价值。Xu等[10]研究发现,使用OWBP袋(50%透光率)进行套袋处理后,果实类胡萝卜素含量显著增加,而使用TGDBP 袋(0%透光率)处理将导致类胡萝卜素含量下降。Zhi 等[11]用铝袋(0%透光率)对枇杷果实进行遮光处理,发现果实在成熟期的类胡萝卜素含量显著提高。目前,有关遮光对枇杷果实类胡萝卜素合成影响的研究多集中于理化性质分析,而对该途径中基因的调控机制仍缺乏研究[12-14]。
高等植物类胡萝卜素的生物合成途径早在1950 年被提出,该途径上重要的结构基因已明确,包括DXS、DXR、PSY、PDS、ZDS、CRTISO、LCYe、LCYb 等[15-17]。在枇杷中,这些类胡萝卜素合成相关基因已被成功克隆[18-19]。其中,PSY和PDS被认为是类胡萝卜素合成途径中重要的限速酶基因[20]。此外,在拟南芥和番茄等植物中有研究发现HY5(LONG HYPOCOTYL 5)转录因子能够调节PSY和PDS的表达[21-23],但在枇杷上还未见报道。HY5是一种碱性亮氨酸拉链(basic leucine zipper,bZIP)型转录因子,能够特异性识别含有ACGT核心的DNA基序,如G-box(CACGTG)、C-box(GTCANN)、Z-box(TACGTGT)和A-box(TACGTA)[24]。HY5广泛参与植物的多种生命活动,除类胡萝卜素的生物合成外,还参与了光形态建成、植物激素信号转导及花青素生物合成等过程[25]。
为探讨遮光对枇杷类胡萝卜素合成及其相关基因表达的影响,笔者在本研究中以黄肉枇杷早佳90果实为材料,分别测定了遮光和非遮光条件下果皮果肉在不同发育阶段的类胡萝卜素含量,同时检测了类胡萝卜素合成相关基因的表达水平。此外,克隆得到2 个EjHY5 基因,对其亚细胞定位及在果实中的表达特性进行了分析,利用病毒诱导基因沉默(VIGS)技术初步探讨了EjHY5 对枇杷PSY 和PDS的潜在调控功能,为了解枇杷果实类胡萝卜素合成的分子机制提供了理论依据。
1 材料和方法
1.1 材料
供试材料为华南农业大学枇杷属植物种质资源圃中正常生长的早佳90枇杷。4个发育阶段包括青果期、转色期、浅黄期和深黄期,分别记为S1、S2、S3和S4[20]。青果期(S1)为花后93~97 d,果皮颜色是黄绿色,种皮白色;转色期(S2)为花后113~115 d,该时期果皮绿色逐渐褪去,呈浅黄绿色,种皮黄褐色;浅黄期(S3)为花后119~123 d,果实比较接近成熟,但酸味较重;深黄期(S4)指花后125~129 d,果实完全成熟,呈现其固有的特征色泽,味酸甜。分别使用不透光果袋及网袋进行遮光和非遮光处理,果实发育至S1期时进行疏果并套袋,后分阶段采果。各样品进行果皮果肉分离后立即用液氮冷冻并保存于-80 ℃备用。
1.2 方法
1.2.1 叶绿素a、叶绿素b和类胡萝卜素总含量的测定 参考朱广廉等[26]方法,用80%丙酮提取样品色素,并于645 nm、663 nm、440 nm波长下测定吸光度(A),依公式计算不同发育时期果皮果肉中的叶绿素a、叶绿素b及总类胡萝卜素含量,计算公式如下:

式中,Ca、Cb和Ck分别为叶绿素a、叶绿素b及类胡萝卜素浓度(ρ,后同)(mg·L-1)。样品中色素含量=色素浓度(mg·L-1)×提取液总体积(L)/样品质量(mg)。
1.2.2 总RNA 提取和逆转录 使用RNAprep Pure多糖多酚植物总RNA 提取试剂盒提取样品总RNA,使用Hifair®Ⅲ1st Strand cDNA Synthesis SuperMix for qPCR(gDNA digester plus)进行反转录,获得cDNA第一链。具体步骤见试剂盒说明书。
1.2.3 类胡萝卜素合成相关基因的表达分析 选择GAPDH作为内参基因[27],基于枇杷基因组序列信息,使用Primer3Plus在线网站设计引物(表1)。以早佳90 果实cDNA 作为模板进行荧光定量PCR,反应于LightCycler 480Ⅱ罗氏荧光定量PCR仪中进行,扩增反应包含10 μL 2×PerfectStart® Green qPCR Supermix、50 ng cDNA、正向引物和反向引物(10 μmol·L-1)各0.4 μL,最终反应体积为20 μL,反应程序如下:95 ℃30 s;94 ℃5 s、60 ℃15 s 和72 ℃10 s,40 个循环;95 ℃10 s。每个样品至少3 次重复。采用2-ΔΔCt法计算相对表达量,使用Excel(2013)软件进行数据分析及绘制图表。
表1 qRT-PCR 引物
Table 1 Primers used for qRT-PCR

?
1.2.4 基因克隆及亚细胞定位 基于枇杷基因组数据,使用Primer Premier 5 设计EjHY5 的特异性扩增引物。以cDNA为模板,使用TaKaRa公司的Prime-STAR® HS(Premix)高保真酶进行PCR 扩增,扩增反应包括12.5 μL PrimeSTAR HS DNA Polymerase、1 μL cDNA、正向引物和反向引物(10 μmol·L-1)各0.5 μL,最终反应体积为25 μL,反应程序如下:94 ℃5 min;94 ℃30 s、60 ℃30 s 和72 ℃45 s,33个循环;72 ℃10 min,PCR产物经1%琼脂糖凝胶电泳检测后由北京擎科生物科技有限公司完成测序。利用瞬时转化烟草叶片的方法进行亚细胞定位分析。选择KpnⅠ和BamHⅠ限制性酶切位点,设计不含终止密码子的克隆引物,将PCR产物克隆到pBEGFP载体中。使用冻融法将重组质粒与空载质粒分别转化至农杆菌GV3101,另转化含有核定位Marker(Mcherry)的农杆菌[28]作为对照。相关引物见表2。
表2 EjHY5s 相关引物
Table 2 EjHY5s-related primers
?
1.2.5 病毒诱导的基因沉默 选择EcoRⅠ和BamHⅠ限制性酶切位点,设计含终止密码子的克隆引物,将目的片段全长克隆至pTRV2 载体。使用冻融法将pTRV1、pTRV2 空载质粒及pTRV2 重组质粒分别转化至农杆菌GV3101。经菌液PCR鉴定为阳性的菌液于28 ℃培养箱200 r·min-1过夜培养至OD600=1.0后用于配制侵染液(50 mL侵染液:1 mL 500 mmol·L-1 MES、1 mL 500 mmol·L-1 MgCl2、5 μL 1 mol·L-1乙酰丁香酮)。将pTRV1菌液分别与pTRV2及pTRV2-EjHY5 菌液1∶1 等体积混合,在室温下避光静置3 h后用于果实侵染。选择S2 至S3 阶段的果实进行侵染。用INJEX 无针注射器将混合菌液注射于果实近赤道处,pTRV1+pTRV2 混合菌液为空白对照[29]。
2 结果与分析
2.1 遮光对枇杷果实颜色和色素含量的影响
非遮光和遮光处理下果皮果肉的颜色变化见图1。两组处理的果实颜色在S2 时期开始出现差异。在S3 时期,遮光果实果皮的颜色接近成熟果实状态,非遮光果实的果皮则呈现浅黄色。为比较不同处理对果实颜色的影响,分别检测了果皮果肉的叶绿素a、叶绿素b和类胡萝卜素含量。

图1 遮光对早佳90 果实颜色的影响
Fig.1 Effect of shading on the fruit color of Zaojia 90
a.S1 时期果实;b~d.非遮光条件下的果实发育;e~g.遮光条件下的果实发育。标尺为1 cm。
a.The fruit at S1 stage;b-d.The fruit development without shading;e-g.The fruit development under shading.The scale bar is 1 cm.
如图2 所示,两组处理下各色素的变化趋势相似,叶绿素a和叶绿素b的含量随果实发育而逐渐下降并在S4时期达到最小值,类胡萝卜素含量则在S2时期达到最小值后开始上升,并在S4 时期达到峰值。在果皮中(图2-A),遮光导致3种色素的含量在不同时期均有不同程度的下降,其中,果皮类胡萝卜素含量在S2 时期显著下降,在S3 及S4 时期无显著差异。在果肉中(图2-B),遮光同样导致了各色素含量的降低,且在S4时期类胡萝卜素含量极显著下降,说明与果皮相比,遮光对果肉中类胡萝卜素含量的影响更大。
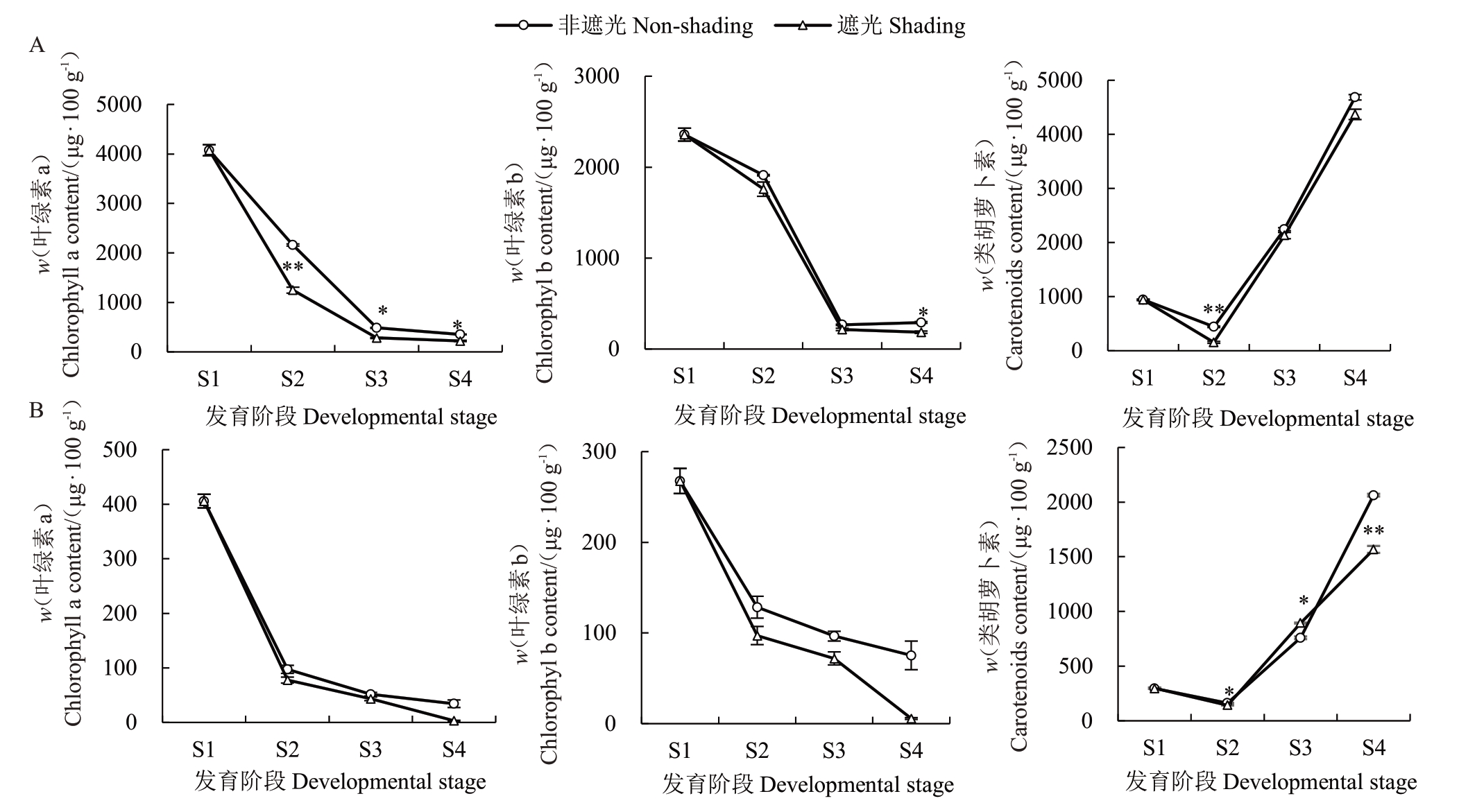
图2 果皮和果肉中色素含量的变化
Fig.2 Change of pigment contents in peel and flesh
A.果皮色素含量变化;B.果肉色素含量变化。*表示在P<0.05 差异显著;**表示在P<0.01 差异极显著。下同。
A.Change of pigment contents in peel;B.Change of pigment contents in flesh.*indicate significant difference at P<0.05;**indicate extremely significant difference at P<0.01.The same below.
2.2 类胡萝卜素合成相关基因的表达分析
为研究遮光对基因表达水平的影响,分析了非遮光和遮光条件下果皮中14 个参与类胡萝卜素合成的基因的表达情况。qRT-PCR 结果显示,遮光条件下与类胡萝卜素合成有关基因的表达趋势均发生改变。在S2 时期,与非遮光处理相比,遮光条件下果实的DXS、DXR、PSY1和PDS基因表达量下降(图3)。这些基因与β-胡萝卜素的前体物质——番茄红素的合成有关,可能间接影响了S2时期类胡萝卜素的积累。其中,DXR 和PDS 的表达量极显著下调,它们可能是导致S2 时期果皮类胡萝卜素含量在遮光后显著下降的潜在基因。此外,在遮光处理下PSY1和LCYb在S4时期的表达量极显著下调,PSY1和LCYb 可能是影响果皮类胡萝卜素最终积累量的重要基因。
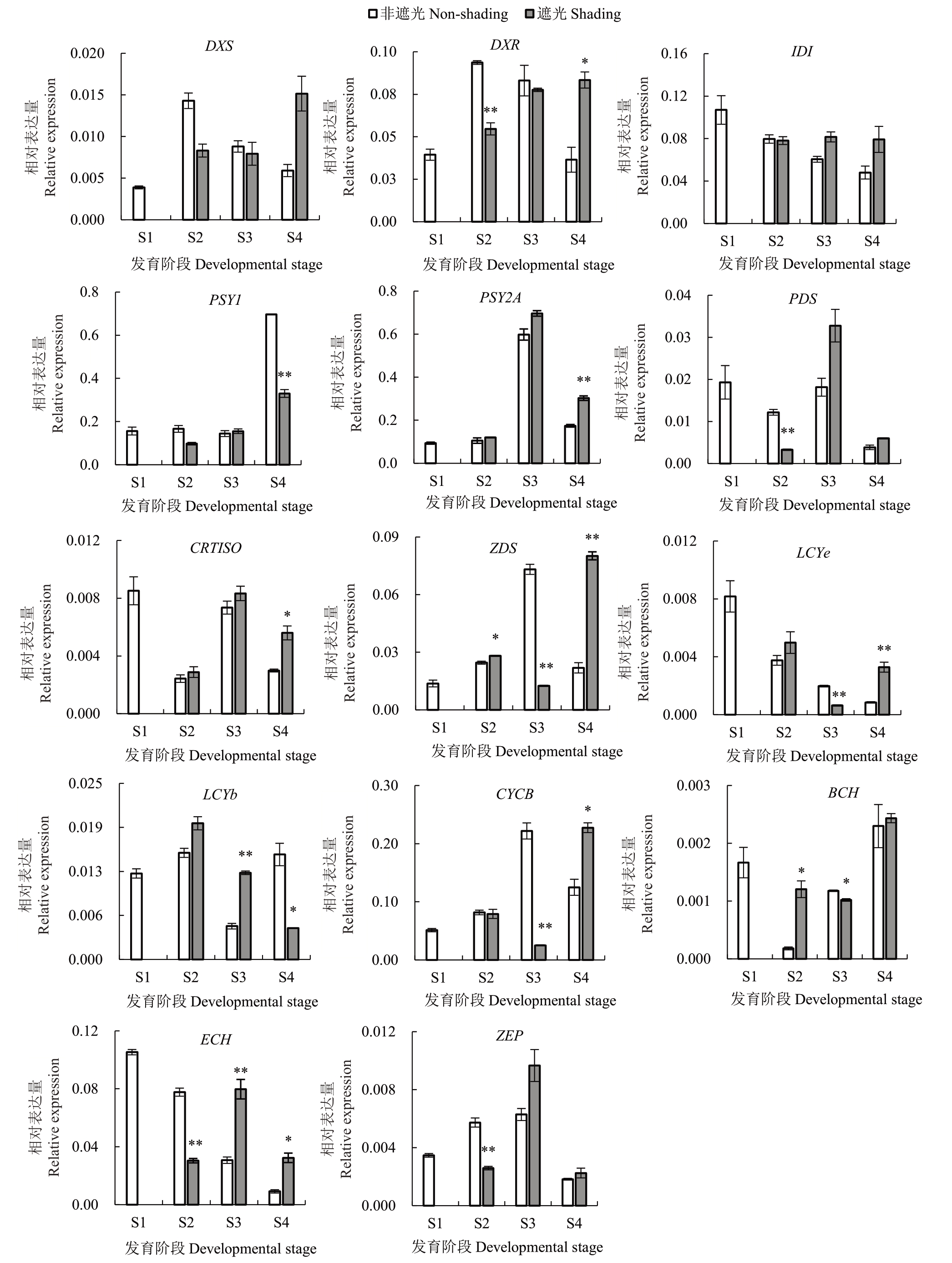
图3 类胡萝卜素合成相关基因在果皮中的表达
Fig.3 Expression of genes related to carotenoid synthesis in peel
在果肉中(图4),qRT-PCR结果显示S3和S4时期的DXR、IDI、PSY1、PSY2A 基因在遮光后出现不同程度的下调表达,其中PSY2A在S4时期的表达量显著下降。PSY2A是类胡萝卜素合成途径中的关键限速酶基因,其主要在果肉中表达。在遮光条件下,果肉类胡萝卜素含量在S4 时期极显著下降,PSY2A可能是调控果肉类胡萝卜素最终积累量的主要基因。

图4 类胡萝卜素合成相关基因在果肉中的表达
Fig.4 Expression of genes related to carotenoid synthesis in flesh
2.3 EjHY5基因克隆及亚细胞定位
当前,枇杷类胡萝卜素生物合成途径已经明确,而其中的调控机制有待完善。笔者基于枇杷基因组数据,成功克隆两个枇杷HY5 转录因子,分别命名为EjHY5-1 和EjHY5-2。EjHY5-1(GeneBank 登录号:PQ858622)全长594 bp,编码197 个氨基酸。EjHY5-2(GeneBank登录号:PQ858623)全长495 bp,编码164个氨基酸。
通过对烟草进行瞬时转化分析EjHY5-1 和EjHY5-2的亚细胞定位。在烟草表皮细胞的细胞核中观察到绿色荧光蛋白(GFP 信号),表明EjHY5-1和EjHY5-2定位于细胞核(图5)。
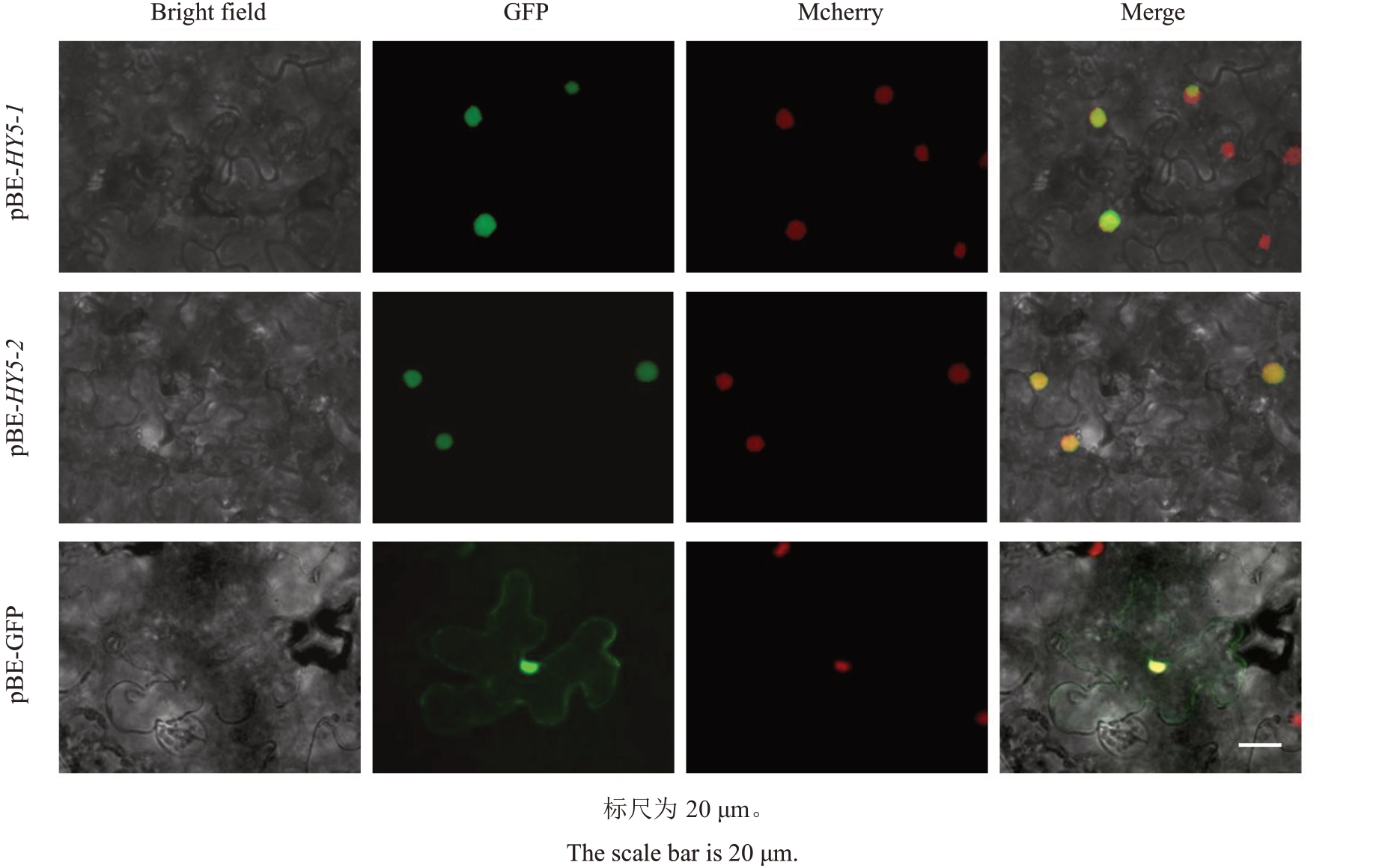
图5 EjHY5-1 和EjHY5-2 的亚细胞定位
Fig.5 Subcellular localizations of EjHY5-1 and EjHY5-2
2.4 EjHY5基因的表达分析及在果实中的基因沉默
2.4.1 EjHY5基因表达分析 经qRT-PCR检测不同处理下果皮果肉中的EjHY5-1 和EjHY5-2 的表达情况,发现两者具有不同的表达特性。在非遮光条件下,EjHY5-1 主要在果皮中表达,而EjHY5-2 主要在果肉中表达。经遮光处理,EjHY5-1在果皮中的表达量在S2~S4 时期均显著下降,而EjHY5-2 在S3~S4时期显著上调表达,说明两者在果皮中可能存在不同的光响应机制(图6-A)。在果肉中,遮光导致EjHY5-1和EjHY5-2在S2时期均显著下调表达,表明光是影响EjHY5s表达的一个重要环境因子(图6-B)。
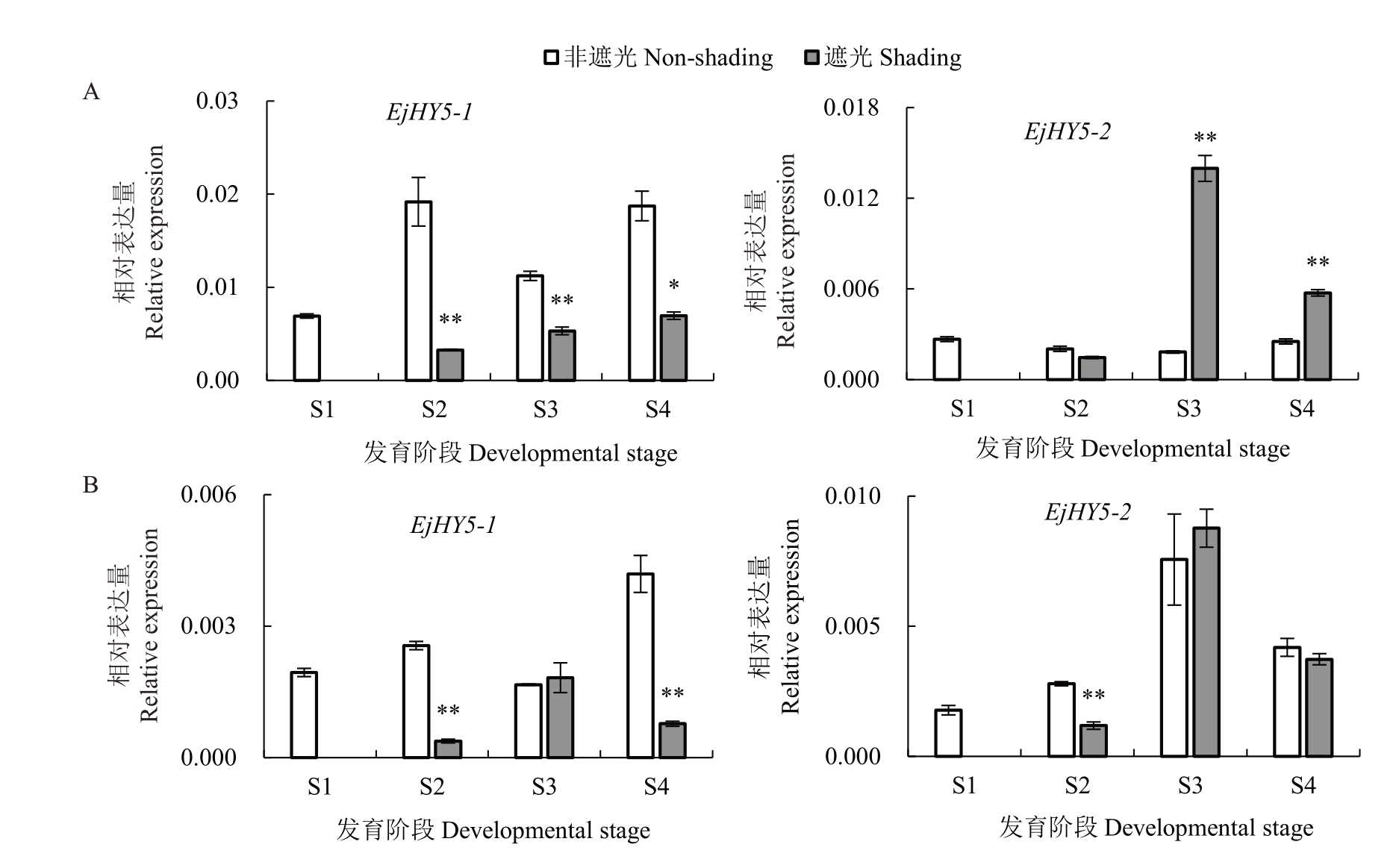
图6 EjHY5-1 和EjHY5-2 在果皮和果肉中的表达
Fig.6 Expressions of EjHY5-1 and EjHY5-2 in peel and flesh
A.EjHY5-1 和EjHY5-2 在果皮中的表达;B.EjHY5-1 和EjHY5-2 在果肉中的表达。
A.Expressions of EjHY5-1 and EjHY5-2 in peel;B.Expressions of EjHY5-1 and EjHY5-2 in flesh.
2.4.2 病毒诱导的基因沉默 笔者利用VIGS 技术初步探讨EjHY5对类胡萝卜素合成相关基因的潜在影响,选择在果实中表达水平相对较高的EjHY5-1作为主要研究对象。对pTRV1+pTRV2 和pTRV1+pTRV2-EjHY5-1 处理下的果实样品进行PCR 鉴定(图7),经1%琼脂糖凝胶电泳检测发现样品含有RNA1、RNA2 或外壳蛋白基因,表明TRV 病毒已侵染果实,VIGS体系构建成功,可用于后续试验。
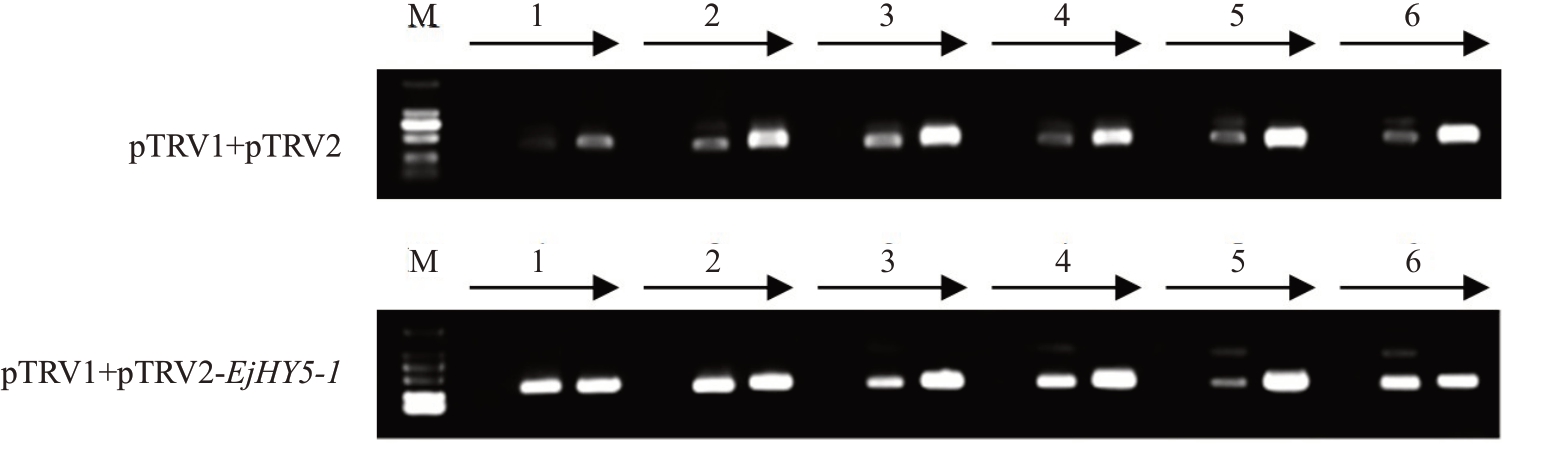
图7 枇杷果实中的TRV 病毒检测
Fig.7 TRV virus detection of loquat fruits
M 表示DL2000 Marker;1~6 为部分果实样品。
M indicates the DL2000 DNA marker;1~6 are several fruit samples.
利用qRT-PCR检测两组果实中EjHY5-1的表达量(图8),结果表明沉默后第4天的pTRV1+pTRV2-EjHY5-1组中EjHY5-1表达量极显著下降,其表达量为对照组的46.62%,沉默效率为53.38%;沉默后第7天,EjHY5-1表达亦极显著下调,表达量为对照组的47.08%,沉默效率为52.92%。此外,笔者分别对这两个时间点的PSY1和PDS表达量进行测定,发现处理组果实中PSY1 和PDS 的表达量在第4 天已出现下降,并在第7天显著下调。
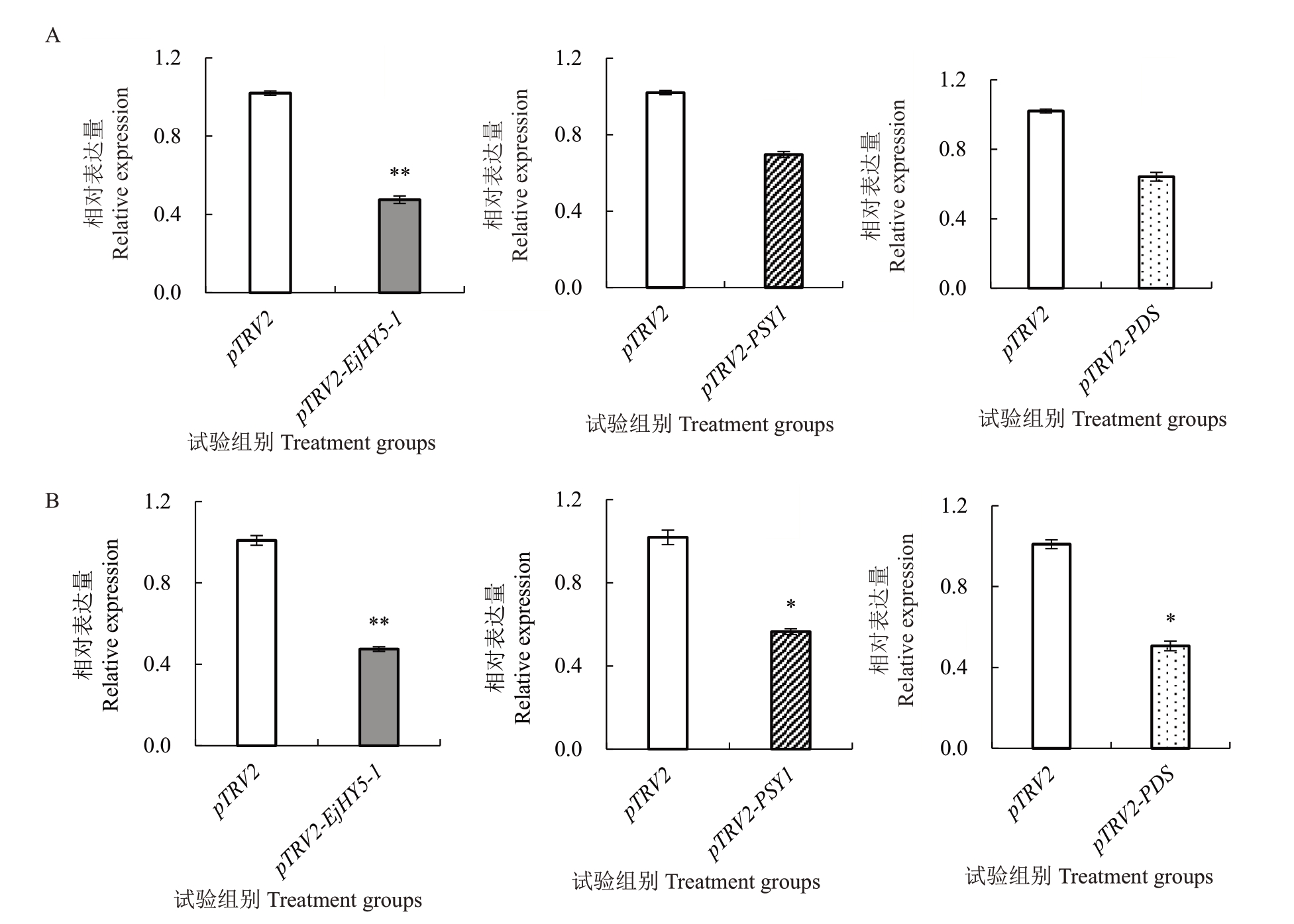
图8 沉默后第4 天和第7 天果实中EjHY5-1、PSY1 和PDS 的表达
Fig.8 Expression of EjHY5-1,PSY1,and PDS in fruit on days 4 and 7 after silencing
A. 沉默后第4 天;B. 沉默后第7 天。
A.On days 4 after silencing;B.On days 7 after silencing.
3 讨 论
3.1 遮光对枇杷果实色素积累的影响
光照是影响类胡萝卜素积累的重要因素[30],而遮光对不同果实类胡萝卜素含量的影响存在差异[31-33]。笔者在本研究中发现,遮光未对枇杷果实中叶绿素a、叶绿素b和类胡萝卜素的积累趋势造成影响,但会导致部分时期的色素含量下降。在S4 时期,遮光条件下的果皮和果肉类胡萝卜素含量均有所下降,且果肉中显著减少,表明遮光不利于类胡萝卜素在果肉中的积累。果肉类胡萝卜素含量决定着枇杷果实的营养价值,因此在生产管理中应提供充足的光照,以保障类胡萝卜素在果肉中的积累。果实在S2时期开始转色,该时期果皮叶绿素a和叶绿素b含量迅速下降,类胡萝卜素含量较低,说明果实颜色变化的主要原因是叶绿素分解而非类胡萝卜素含量增加,在柑橘中亦有类似报道[34]。
3.2 遮光对枇杷果实类胡萝卜素合成相关基因表达的影响
前人研究发现,黄肉枇杷果实中的主要色素是β-胡萝卜素和叶黄素[35]。在S2 时期,遮光处理果实的果皮中LCYb上调表达,而ZEP显著下调表达,可能促进β-胡萝卜素的积累。然而测定色素含量发现,类胡萝卜素含量却显著减少。qRT-PCR 结果显示,在S2 时期与番茄红素合成相关的DXS、DXR、PSY1 和PDS 在遮光处理果实的果皮中均呈现下调表达趋势,推测间接影响了β-胡萝卜素的合成。其中,DXR 和PDS 的表达量显著下降,可能是导致S2时期果皮类胡萝卜素含量显著下降的重要基因。Arcos等[36]研究发现,在苹果果实中瞬时转化AtDXR使总类胡萝卜素含量增加2 倍,表明DXR 可能是提高苹果果实类胡萝卜素水平的理想候选基因。而在桃、柚等果实中,PDS被报道为果实类胡萝卜素合成途径上的关键限速基因,其表达水平直接影响了类胡萝卜素的积累[37-39]。
PSY是类胡萝卜素生物合成途径上的另一个关键限速酶基因。Fu 等[40]研究发现,枇杷中有多个PSY,且具有组织表达特异性。如EjPSY1 主要在果皮中表达;EjPSY2A 主要在果肉中表达EjPSY2B 主要在叶片中表达;EjPSY3 则缺乏功能,但可能是其他PSY 的祖先。洪敏等[41]利用VIGS 技术诱导枇杷果实中的PSY 沉默后,总类胡萝卜素含量降低了46.8%。该结果表明PSY 是影响类胡萝卜素含量的重要基因。PSY1和PSY2A具有相似的表达特征,在S4 时期遮光条件下果肉的类胡萝卜素含量显著下降,PSY2A显著下调表达,表明PSY2A可能是影响果肉类胡萝卜素最终积累量的关键基因。与果皮相比,遮光对果肉类胡萝卜素的积累影响更大。
3.3 EjHY5的表达及潜在功能分析
HY5转录因子属于bZIP家族,在光信号通路中发挥着重要作用[42]。笔者基于枇杷基因组数据克隆获得的EjHY5-1 和EjHY5-2 具有保守的bZIP 结构域,它们可能参与了枇杷果实的光信号转导、色素合成、生长发育等多种生命活动[25]。对EjHY5 在果实中的表达特性进行分析,结果表明EjHY5-1 主要在果皮中表达,而EjHY5-2主要在果肉中表达,且光照是影响其表达的重要因子。与非遮光处理相比,在遮光条件下EjHY5-1的表达量显著下降,EjHY5-2的表达量显著上升,二者呈现相反的变化趋势,推测它们可能存在不同的光响应机制。
多项研究表明,HY5 通过识别基因启动子区的G-box元件调控基因表达。在拟南芥中,Toledo-Ortiz等[21]发现AtHY5通过直接与PSY基因启动子区的G-box元件结合,调控PSY的转录表达,进而影响了拟南芥类中胡萝卜素的合成。在苹果、葡萄、风信子等植物中也有相似的报道[43-45]。对EjPSY1 和EjPDS的启动子进行分析,发现它们的启动子区均包含Gbox 元件。利用VIGS 技术沉默果实EjHY5-1 后,果实EjPSY1 及EjPDS 的表达量均显著降低,推测EjHY5-1可能对它们存在正向调控作用。
4 结 论
遮光对枇杷果实的色素含量及类胡萝卜素合成相关基因的表达具有重要影响。经遮光处理,S4时期果皮和果肉中的类胡萝卜素含量均有所下降,且对果肉类胡萝卜素的积累影响更大。DXR 和PDS可能是遮光处理下果皮类胡萝卜素在S2 时期显著减少的关键基因,而PSY2A在S4时期的表达量显著降低可能是影响果肉类胡萝卜素差异积累的重要基因。EjHY5-1及EjHY5-2的表达具有组织特异性,且与光照密切相关。在果实中沉默EjHY5-1 后,PSY1和PDS的表达量显著降低。而PSY1和PDS的启动子区均含有HY5 特异性结合的G-box 元件,说明EjHY5-1 可能对其具有转录调控作用,从而进一步影响枇杷果实类胡萝卜素的积累。
[1] LIN S Q,SHARPE R H,JANICK J. Loquat:Botany and horticulture[M].Hoboken:John Wiley&Sons,1998:233-276.
[2] 章恢志,彭抒昂,蔡礼鸿,方德秋.中国枇杷属种质资源及普通枇杷起源研究[J].园艺学报,1990,17(1):5-12.ZHANG Huizhi,PENG Shu’ang,CAI Lihong,FANG Deqiu.The germplasm resources of the genus Eriobotrya with special reference on the origin of E. japonica Lindl[J]. Acta Horticulturae Sinica,1990,17(1):5-12.
[3] PAREEK S,BENKEBLIA N,JANICK J,CAO S F,YAHIA E M. Postharvest physiology and technology of loquat (Eriobotrya japonica Lindl.) fruit[J]. Journal of the Science of Food and Agriculture,2014,94(8):1495-1504.
[4] 孙淑霞,谢红江,李靖,涂美艳,陈栋,江国良.枇杷果肉色泽深浅性状的分子标记鉴定[J]. 西南农业学报,2012,25(6):2227-2230.SUN Shuxia,XIE Hongjiang,LI Jing,TU Meiyan,CHEN Dong,JIANG Guoliang. Molecular identification of fragments associated with fruit flesh color in loquat[J]. Southwest China Journal of Agricultural Sciences,2012,25(6):2227-2230.
[5] SHERWIN J C,REACHER M H,DEAN W H,NGONDI J.Epidemiology of vitamin A deficiency and xerophthalmia in at-risk populations[J]. Transactions of the Royal Society of Tropical Medicine and Hygiene,2012,106(4):205-214.
[6] LADO J,CRONJE P,ALQUÉZAR B,PAGE A,MANZI M,GÓMEZ-CADENAS A,STEAD A D,ZACARÍAS L,RODRIGO M J.Fruit shading enhances peel color,carotenes accumulation and chromoplast differentiation in red grapefruit[J]. Physiologia Plantarum,2015,154(4):469-484.
[7] FU X M,CHEN J M,LI J L,DAI G Y,TANG J C,YANG Z Y.Mechanism underlying the carotenoid accumulation in shaded tea leaves[J].Food Chemistry:X,2022,14:100323.
[8] LOPEZ M,CANDELA M E,SABATER F. Carotenoids from Capsicum annuum fruits:Influence of spectral quality of radiation[J].Biologia Plantarum,1986,28(2):100-104.
[9] ZHU M T,FANG W C,CHEN C W,WANG L R,CAO K. Effects of shading by bagging on carotenoid accumulation in peach fruit flesh[J]. Journal of Plant Growth Regulation,2021,40(5):1912-1921.
[10] XU H X,CHEN J W,XIE M. Effect of different light transmittance paper bags on fruit quality and antioxidant capacity in loquat[J].Journal of the Science of Food and Agriculture,2010,90(11):1783-1788.
[11] ZHI C,ALI M M,ZHANG J Y,SHI M,MA S F,CHEN F X.Effect of paper and aluminum bagging on fruit quality of loquat(Eriobotrya japonica Lindl.)[J].Plants,2021,10(12):2704.
[12] 刘友接,许家辉,张泽煌,蒋际谋,余东.不同纸质果袋对枇杷果实品质的影响[J].江西农业大学学报,2004,26(3):334-337.LIU Youjie,XU Jiahui,ZHANG Zehuang,JIANG Jimou,YU Dong.Effects of different paper bags on fruit quality of loquat[J].Acta Agriculturae Universitis Jiangxiensis,2004,26(3):334-337.
[13] 杜会香.不同时期套袋对解放钟枇杷果实品质的影响[D].福州:福建农林大学,2010.DU Huixiang. Effects of bagging times on fruit quality of‘Jiefangzhong’loquat[D]. Fuzhou:Fujian Agriculture and Forestry University,2010.
[14] 张君雅.枇杷果实套袋微域环境及果实品质研究[D].福州:福建农林大学,2017.ZHANG Junya. Effects of fruit bagging treatments on microenvironment and fruit quality of loquat[D].Fuzhou:Fujian Agriculture and Forestry University,2017.
[15] PORTER J W,LINCOLN R E. Lycopersicon selections containing a high content of carotenes and colorless polyenes;the mechanism of carotene biosynthesis[J]. Archives of Biochemistry,1950,27(2):390-403.
[16] FANCIULLINO A L,CERĆOS M,DHIQUE-MAYER,FROELICHER Y,TALÓN M,OLLITRAULT P,MORILLON R.Changes in carotenoid content and biosynthetic gene expression in juice sacs of four orange varieties (Citrus sinensis) differing in flesh fruit color[J]. Journal of Agricultural and Food Chemistry,2008,56(10):3628-3638.
[17] SHEN Y H,YANG F Y,LU B G,ZHAO W W,JIANG T,FENG L,CHEN X J,MING R.Exploring the differential mechanisms of carotenoid biosynthesis in the yellow peel and red flesh of papaya[J].BMC Genomics,2019,20(1):49.
[18] SU W B,ZHU C Q,FAN Z Q,HUANG M K,LIN H,CHEN X P,DENG C J,CHEN Y P,KOU Y D,TONG Z H,ZHANG Y L,XU C J,ZHENG S Q,JIANG J M. Comprehensive metabolome and transcriptome analyses demonstrate divergent anthocyanin and carotenoid accumulation in fruits of wild and cultivated loquats[J].Frontiers in Plant Science,2023,14:1285456.
[19] ZHANG L,ZHANG Z K,ZHENG T T,WEI W L,ZHU Y M,GAO Y S,YANG X H,LIN S Q.Characterization of carotenoid accumulation and carotenogenic gene expression during fruit development in yellow and white loquat fruit[J]. Horticultural Plant Journal,2016,2(1):9-15.
[20] CAZZONELLI C I,POGSON B J.Source to sink:Regulation of carotenoid biosynthesis in plants[J]. Trends in Plant Science,2010,15(5):266-274.
[21] TOLEDO-ORTIZ G,JOHANSSON H,LEE K P,BOU-TORRENT J,STEWART K,STEEL G,RODRÍGUEZ-CONCEPCIÓN M,HALLIDAY K J.The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription[J].PLoS Genetics,2014,10(6):e1004416.
[22] SONG Y J,TEAKLE G,LILLYWHITE R. Unravelling effects of red/far-red light on nutritional quality and the role and mechanism in regulating lycopene synthesis in postharvest cherry tomatoes[J].Food Chemistry,2023,414:135690.
[23] 李晓萌.HY5 在番茄果实类胡萝卜素合成中的作用研究[D].杭州:浙江大学,2020.LI Xiaomeng. Role of HY5 in the carotenoid synthesis of tomato fruits[D].Hangzhou:Zhejiang University,2020.
[24] IZAWA T,FOSTER R,CHUA N H. Plant bZIP protein DNA binding specificity[J]. Journal of Molecular Biology,1993,230(4):1131-1144.
[25] GANGAPPA S N,BOTTO J F. The multifaceted roles of HY5 in plant growth and development[J]. Molecular Plant,2016,9(10):1353-1365.
[26] 朱广廉,钟诲文,张爱琴.植物生理学实验[M].北京:北京大学出版社,1990.ZHU Guanglian,ZHONG Huiwen,ZHANG Aiqin. Plant physiology experiment[M].Beijing:Peking University Press,1990.
[27] SU W B,YUAN Y,ZHANG L,JIANG Y Y,GAN X Q,BAI Y L,PENG J R,WU J C,LIU Y X,LIN S Q.Selection of the optimal reference genes for expression analyses in different materials of Eriobotrya japonica[J].Plant Methods,2019,15(1):7.
[28] SHANER N C,CAMPBELL R E,STEINBACH P A,GIEPMANS B N G,PALMER A E,TSIEN R Y.Improved monomeric red,orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein[J]. Nature Biotechnology,2004,22(12):1567-1572.
[29] ZHANG J Q,WU Z C,HU F C,LIU L,HUANG X M,ZHAO J T,WANG H C. Aberrant seed development in Litchi chinensis is associated with the impaired expression of cell wall invertase genes[J].Horticulture Research,2018,5:39.
[30] RAYMUNDO L C,CHICHESTER C O,SIMPSON K L.Lightdependent carotenoid synthesis in the tomato fruit[J]. Journal of Agricultural and Food Chemistry,1976,24(1):59-64.
[31] JIA H J,ARAKI A,OKAMOTO G. Influence of fruit bagging on aroma volatiles and skin coloration of‘Hakuho’peach(Prunus persica Batsch)[J]. Postharvest Biology and Technology,2005,35(1):61-68.
[32] YANG W H,ZHU X C,BU J H,HU G B,WANG H C,HUANG X M. Effects of bagging on fruit development and quality in cross-winter off-season longan[J]. Scientia Horticulturae,2009,120(2):194-200.
[33] SHARMA R R,PAL R K,ASREY R,SAGAR V R,DHIMAN M R,RANA M R.Pre-harvest fruit bagging influences fruit color and quality of apple cv. Delicious[J]. Agricultural Sciences,2013,4(9):443-448.
[34] GE X X,CAO T T,YI L H,YAO S X,ZENG K F,DENG L L. Low and high storage temperature inhibited the coloration of mandarin fruit (Citrus unshiu Marc.) with different mechanism[J]. Journal of the Science of Food and Agriculture,2022,102(15):6930-6941.
[35] FU X M,KONG W B,PENG G,ZHOU J Y,AZAM M,XU C J,GRIERSON D,CHEN K S.Plastid structure and carotenogenic gene expression in red- and white-fleshed loquat (Eriobotrya japonica) fruits[J]. Journal of Experimental Botany,2012,63(1):341-354.
[36] ARCOS Y,GODOY F,FLORES-ORTIZ C,ARENAS-M A,STANGE C. Boosting carotenoid content in Malus domestica var. Fuji by expressing AtDXR through an Agrobacterium-mediated transformation method[J]. Biotechnology and Bioengineering,2020,117(7):2209-2222.
[37] CAO S F,LIANG M H,SHI L Y,SHAO J R,SONG C B,BIAN K,CHEN W,YANG Z F.Accumulation of carotenoids and expression of carotenogenic genes in peach fruit[J].Food Chemistry,2017,214:137-146.
[38] GAO H J,XU J,LIU X,LIU B Z,DENG X X. Light effect on carotenoids production and expression of carotenogenesis genes in citrus callus of four genotypes[J]. Acta Physiologiae Plantarum,2011,33(6):2485-2492.
[39] ZHAO Y H,YANG X F,HU Y W,GU Q M,CHEN W L,LI J Q,GUO X B,LIU Y T.Evaluation of carotenoids accumulation and biosynthesis in two genotypes of pomelo (Citrus maxima) during early fruit development[J].Molecules,2021,26(16):5054.
[40] FU X M,FENG C,WANG C Y,YIN X R,LU P J,GRIERSON D,XU C J,CHEN K S. Involvement of multiple phytoene synthase genes in tissue-and cultivar-specific accumulation of carotenoids in loquat[J]. Journal of Experimental Botany,2014,65(16):4679-4689.
[41] 洪敏,石丝,何珊珊,文露,池卓恒,唐月明,王永清.VIGS 诱导PSY 基因沉默对枇杷果实类胡萝卜素积累的影响[J].分子植物育种,2018,16(6):1792-1797.HONG Min,SHI Si,HE Shanshan,WEN Lu,CHI Zhuoheng,TANG Yueming,WANG Yongqing. Effects of VIGS-induced PSY gene silencing on carotenoid accumulation in fruit of Eriobotrya japonica Lindl.[J]. Molecular Plant Breeding,2018,16(6):1792-1797.
[42] SU L,HOU P,SONG M F,ZHENG X,GUO L,XIAO Y,YAN L,LI W C,YANG J P. Synergistic and antagonistic action of phytochrome (Phy) a and PhyB during seedling de-etiolation in Arabidopsis thaliana[J]. International Journal of Molecular Sciences,2015,16(6):12199-12212.
[43] WANG L Y,LIN R,XU J,SONG J N,SHAO S J,YU J Q,ZHOU Y H. High nitric oxide concentration inhibits photosynthetic pigment biosynthesis by promoting the degradation of transcription factor HY5 in tomato[J]. International Journal of Molecular Sciences,2022,23(11):6027.
[44] LI Z Q,LIU W J,CHEN Q J,ZHANG S H,MEI Z X,YU L,WANG C,MAO Z Q,CHEN Z J,CHEN X S,WANG N.MdmmiR858 targets MdMYB9 and MdMYBPA1 to participate anthocyanin biosynthesis in red-fleshed apple[J]. The Plant Journal,2023,113(6):1295-1309.
[45] ZHANG H,WANG J Y,TIAN S T,HAO W H,DU L J.Two Bbox proteins,MaBBX20 and MaBBX51,coordinate light-induced anthocyanin biosynthesis in grape hyacinth[J].International Journal of Molecular Sciences,2022,23(10):5678.