土壤理化性质与微生物之间存在着复杂的耦合关系。土壤不仅为微生物提供了活动场所,也为微生物的生长、代谢提供了重要的资源。研究表明,土壤理化性质的变化能够重塑微生物的生态位,从而驱动微生物群落组成及其生态功能的演替[1-2]。例如,弱碱性的环境适合细菌和放线菌的生长,而弱酸性的环境则有利于真菌的生长[3]。土壤孔隙度和颗粒组成可改变土壤微生物可利用的微环境,如细菌多分布于0.5~5 μm的小孔隙中,而真菌和放线菌适于较大孔隙[4]。土壤水分通过影响土壤中氧气扩散与水膜形成,进而影响微生物活性和群落组成,土壤有机质含量则可调控微生物矿化过程与碳源利用效率等[5]。
土壤微生物也可通过自身的代谢活动参与土壤环境的构建。土壤微生物在团聚体形成、水分保持、有机质转化及矿物风化中均发挥重要作用。部分真菌和放线菌可通过菌丝结合土壤颗粒,并深入团聚体内部,从而提高土壤团聚体的稳定性[6-7]。解磷菌和解钾菌通过分泌有机酸、磷酸酶等促进难溶性矿物养分的释放,从而提高磷钾素的可利用性,调节果树根际养分供给[8]。此外,果园管理中使用的化学肥料(如尿素)、有机肥等需依赖微生物分解作用转化为植物可吸收的形态[9]。
果园长期施肥、覆草还田、生草栽培等管理措施对土壤理化特性产生了深远的影响,也对土壤微生物群落产生了显著影响。微生物群落结构特征的改变不仅是对土壤环境改变的反馈,也影响了果树对营养物质的吸收及果品品质。笔者在本研究中以果园土壤生态系统为背景,系统综述了土壤微生物与理化性质之间的双向调控机制,探讨微生物在调控土壤结构、水分状况和养分供给中的作用,阐明了土壤碳、氮、磷、硫、钾等养分循环中微生物的调控机制。以期为果园土壤质量提升、功能微生物调控与生态种植策略优化提供理论支撑和研究方向。
1 微生物与土壤理化性质的互作机制
1.1 微生物与土壤结构的相互影响及机制
土壤结构是土壤颗粒(包括团聚体)和孔隙的有机组合。作为土壤结构重要组成部分的土壤团聚体在维持土壤结构稳定性方面起到了重要的作用,其也是影响微生物群落特征的主要因素之一[10]。土壤团聚体动态的形成和解散过程会直接影响土壤结构的稳定性,进而调控土壤微生物群落的组成与分布。如团聚体形成或解散过程引起的土壤结构变化(如土壤紧实或疏松程度的改变)显著影响微生物的生境条件,包括水分保持能力、通气性以及养分的可获取性。
反之,微生物群落也可以改变土壤结构的组成与排列方式,如微生物的代谢产物可以促进土壤团聚体的聚合,或通过分解团聚体之间的黏合物质而促进土壤团聚体解体[11]。此外,土壤微生物通过分解动植物残体促进了土壤有机质的积累和转化并形成了稳定的有机-矿物复合体,促进了土壤团聚体的形成与稳定,从而影响了土壤结构体的稳定性[12-14](图1)。研究表明,土壤细菌对微团聚体(<250 μm的团聚体)的形成具有重要的作用,如Li等[15]研究发现,细菌代谢过程中产生的代谢物可促进土壤颗粒间的聚合,利于土壤团聚体形成;Qiang 等[16]研究发现,真菌如菌根真菌则主要促进土壤大团聚体的形成;土壤真菌产生的菌丝、疏水性蛋白质、多糖和脂质等可作为土壤颗粒之间黏合的媒介,利于土壤团聚体的形成和稳定。Costa等[17]研究发现,土壤蓝藻产生的胞外聚合物(EPS)可提高土壤水分含量,促进土壤颗粒的聚集,有利于土壤团聚体的形成。此外,土壤微生物可在土壤矿物颗粒表面形成生物膜,通过生物风化作用改变土壤矿物的性质;土壤微生物也可黏附于土壤颗粒上促进土壤微团聚体形成,进而对土壤结构产生影响[18-19]。
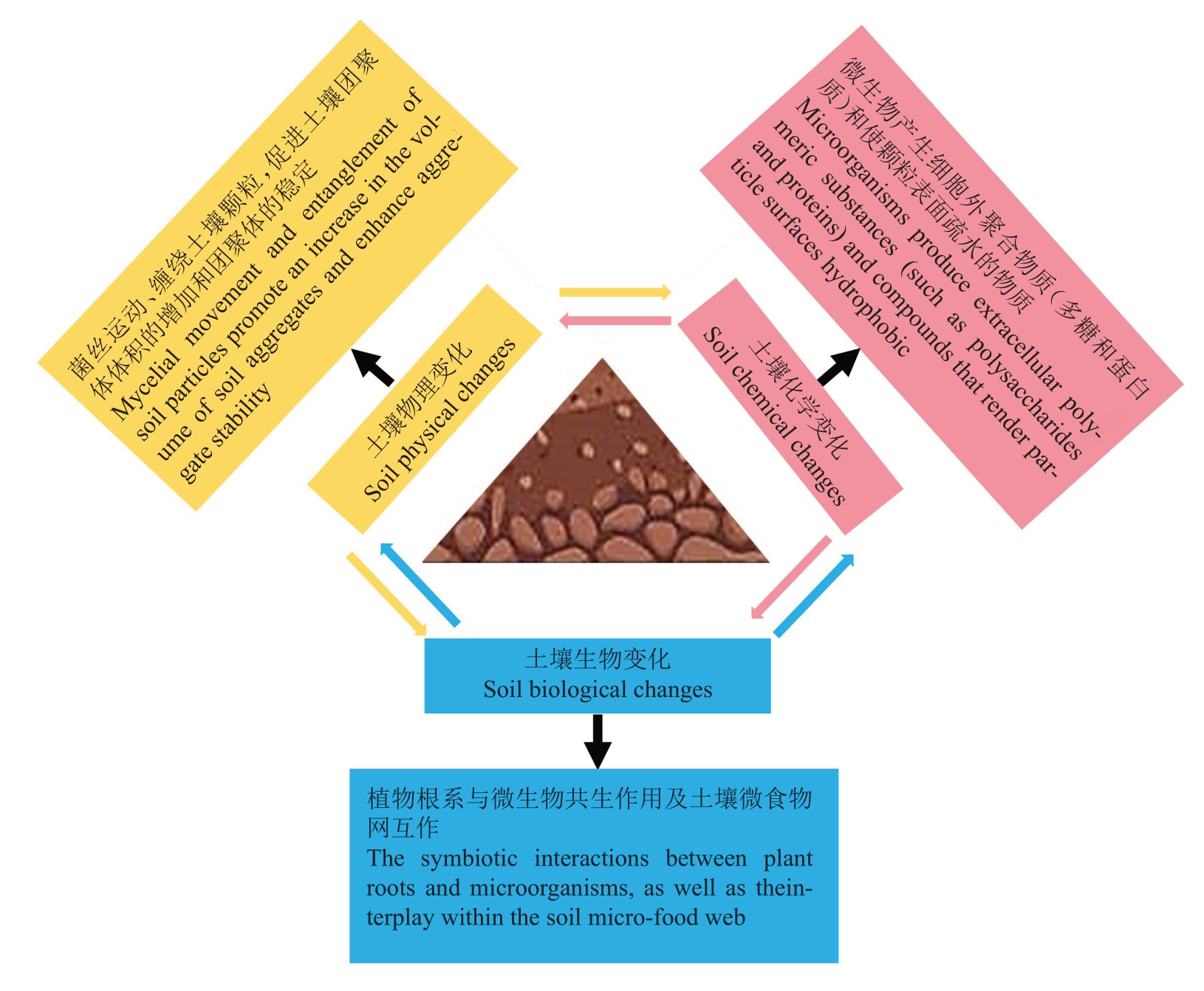
图1 土壤微生物与土壤结构的互作关系
Fig.1 Interaction between soil microorganisms and soil structure
1.2 微生物与土壤水分的相互影响及机制
微生物在土壤中的代谢活性主要依赖于土壤水分的流动性和可利用性,土壤水分特征直接影响微生物的活性和群落结构。当土壤含水量较低时,微生物因缺乏水而代谢活动受限,抑制了其生长与繁殖;而当土壤水分含量过高时,水分的过饱和状态会显著抑制氧气在土壤中的扩散,从而限制需氧微生物的正常呼吸代谢。这种极端水分变化均会破坏土壤原有的微生物结构平衡,导致微生物群落结构发生变化。反之,土壤微生物可通过自身的代谢活动影响土壤水分的分布特征和水分的性质,如影响水分的渗透性、蒸发速率、结合态与游离态的转化等[20]。
微生物改变土壤水分性质的原理包括3种:(1)细菌和真菌通过分泌化合物堵塞土壤中较大的孔隙,降低土壤水分的蒸发速率,达到增加土壤保水性的目的。Zheng等[21]研究表明,微生物产生的EPS具有较高的持水能力,能够通过改变土壤表面张力和黏度来增加土壤水分。(2)真菌可以通过产生特定化合物(如两性物质)改变土壤的疏水或亲水特性。Chau 等[22]研究发现,真菌通过菌丝缠绕土壤颗粒并分泌疏水性物质改变土壤的孔隙结构及拒水性,降低水分渗透速率。(3)菌根类真菌在一定条件下增加植物根系的吸水面积,促进水分的吸收和利用[23-24]。Wu等[25]研究表明,丛枝菌根真菌(AМF)能够改善植物与水分之间的关系,在含水量较高和较低的土壤中,丛枝菌根(AМ)对植物蒸腾水分的贡献率分别为12.32%和17.03%,在水分受限的条件下,AМF菌丝可延伸至细小孔隙中吸收水分,提升植物在干旱环境中的水分获取能力,从而提升植物在逆境环境中的存活能力。微生物对土壤水分影响的3种调控机制可独立发生,也可协同作用。土壤中的丝状真菌和浮生真菌等其他微生物可通过分泌胞外聚合物或形成菌丝网络,影响土壤孔隙结构特征,增强水分的保持能力与再分布能力,进而提高土壤水分的利用效率[17,26]。
此外,微生物在分解有机质的过程中也会生成代谢水,尤其是在好氧呼吸过程中,如碳水化合物的氧化反应可生成CO2和H2O。这种代谢水对整体土壤水分的影响较小,但在微生物密集分布或水分受限的微环境中,其生成的水分则有助于维持微生物生存和局部水分平衡[27]。这些水分也可能影响微生物扩散过程和土壤中水分的再分布过程。
1.3 微生物与土壤pH值的相互影响及机制
土壤pH 值是影响土壤化学性质和生物功能的主要因素,也是影响土壤碳、氮和硫循环的重要因素之一。碳循环过程中,微生物呼吸产生的CO2溶解于土壤溶液中形成碳酸(H2CO3),导致土壤pH值下降[28-29]。Adeleke 等[30]研究发现,土壤中的酸类物质主要来源于微生物,如草酸和柠檬酸等,这些酸类物质是造成土壤酸化的主要因素之一。在氮循环过程中,细菌和古菌在土壤硝化过程中能将铵类物质氧化为硝酸盐并产生质子,导致土壤pH 值下降,特别是氮肥施用量过多时,土壤易发生酸化现象[31]。Scarlett 等[32]研究表明,通过微生物作用减少土壤硝化菌和亚硝化螺旋体,导致土壤pH值显著增加。此外,氨化作用和反硝化作用可消耗土壤中的质子,从而在一定程度上缓解土壤酸化。氨(NH4+)是一种碱性物质,通常来源于蛋白质和氨基酸分解过程中的副产物。在土壤氨化过程中,真菌菌丝所分泌的相关酶参与了氮的转化,促进了氨的释放,因此真菌活跃的环境往往会出现土壤碱化的现象[33-34]。硫循环过程中也会产生质子和氢氧根离子,其产生的量约为氮循环的10%,在通风良好的土壤中,约有90%的硫以有机物的形式存在,有机硫的矿化可产生酸类物质,导致土壤酸化[35]。此外,微生物参与的钙、镁和磷循环过程中会产生质子和氢氧根离子,导致土壤pH值发生变化[36]。因此,土壤细菌和真菌对土壤pH 值具有直接影响。反之,土壤pH 值也是影响土壤微生物群落组成与功能的主要因素之一,土壤pH值可改变土壤中的化学过程和养分有效性,间接调控微生物的生存环境,从而对土壤微生物的多样性、丰度和代谢活性产生影响[37]。在酸性土壤(pH<6)中,真菌通常占主导地位,而中性、微碱性土壤环境(pH 6~8)则更有利于细菌的生长[38]。
2 微生物与土壤养分循环的互作机制
2.1 微生物与土壤碳循环相互作用及机制
土壤有机碳是植物生长的重要营养和能量来源,在维持土壤生态系统的健康和功能方面具有重要的作用。研究表明,微生物是土壤有机碳的主要来源之一,由微生物残留物迭代积累产生的有机碳占土壤总有机碳的50%~80%[39]。Zhao 等[40]研究表明,真菌、细菌残体对土壤有机碳的贡献率分别占总有机碳的30%、15%以上。土壤微生物参与土壤碳循环涉及不同的分解代谢途径,主要包括发酵和呼吸两个过程(图2)。微生物通过呼吸作用释放CO2参与土壤碳代谢,或产生其他物质如丙酮酸、乙醇和甘油等物质通过糖酵解(EМP)、磷酸戊糖途径(HМP)和Stickland反应发酵产生碳类物质[41]。

图2 土壤微生物与土壤碳、氮循环的互作关系
Fig.2 Interaction between soil microorganisms and soil carbon and nitrogen cycles
土壤微生物的多样性及功能多样性对土壤养分循环和植物的生产力具有显著的影响[42]。Bhattacharyya 等[43]研究表明,真菌及细菌的生物残体是稳定土壤有机碳的主要成分,土壤微生物代谢是土壤中碳储存潜力差异的主要驱动因素。土壤细菌具有较强的分解能力,其中异养腐生菌是土壤中有机质的主要分解者,能分解动植物残体和真菌菌丝,参与土壤碳循环[44]。Wang等[45]研究表明,土壤细菌对真菌菌丝的分解贡献率可达40%,对有机质的分解效率高达80%。但由于参与有机质分解酶(如土壤胞外酶)种类的差异,导致微生物对有机物质的分解能力存在显著的差异[46]。土壤真菌作为土壤中生物量最高的生物,其所产生各种酶可以分解土壤中复杂有机物质,从而调控土壤碳及其他养分的平衡[47]。反之,碳源的质量和可利用性也可影响土壤微生物群落结构。土壤中易降解碳(如单糖)含量的增加会促进r 生存策略微生物(细菌为主)的增殖;随着养分的消耗,K 生存策略的微生物(以真菌为主)数量增加、活性增强,从而改变土壤微生物的代谢路径和碳转化效率[48-49]。此外,存在于深层土壤中的古菌在缺氧和富含有机质的环境中适应性较细菌强,其代谢活动提高了土壤有机质的分解和周转能力,并向环境中释放大量的含碳物质[50-51]。
2.2 微生物与土壤氮循环相互作用及机制
土壤中的大部分氮素以有机氮的形式存在,需通过微生物转化为植物可吸收的无机态氮。研究表明,微生物在氮矿化过程中发挥着重要的作用,参与了固氮、硝化、反硝化和氨化等过程(图2)[52]。在农业生产中,通过微生物固氮作用转化的氮素占自然固氮的90%。Hu等[53]研究表明,氮循环依赖于土壤固氮细菌(如根际细菌和植物内生细菌等)的生物固氮作用。固氮菌产生固氮酶的作用机制分为两类:一类为共生固氮细菌,能够通过与植物建立共生关系,从大气中获取N2转化为氨;另一类是不与植物形成共生关系的自生固氮细菌,能够从空气中吸收N2供自身利用,菌体死亡后可分解释放氮素到土壤中,从而提高土壤氮素含量。反之,土壤中氮的形态及其含量也可影响微生物群落结构特征,研究发现,果园间作绿肥显著提高了土壤中与养分循环相关微生物的多样性及其丰度,自生固氮菌在未施氮或施用有机肥的条件下表现出较高的活性[54]。
此外,丛植菌根真菌(AМF)在土壤氮循环过程中也具有重要的作用,外生菌根真菌能够寄生于植物根系,可协助寄主植物直接利用土壤中的有机氮,或通过降解土壤中的有机质释放无机氮[55]。Xie等[56]研究表明,丛植菌根真菌能够加速降解土壤中复杂的有机氮素,参与土壤氮循环,从中吸收氮素营养,增强植物对各种环境胁迫的抵抗力。张珊珊等[57]研究发现,AМF处理提高了土壤中植物可利用养分的含量,不仅可以提高果树对养分的吸收效率,也提高了果树幼苗的抗旱性,表明干旱条件下丛植菌根真菌对果树生理特征有重要调节作用。
古菌作为土壤微生物的重要类群之一,可通过硝化作用增加土壤氮素含量。氨单加氧酶(AМO)是一种铜依赖的多聚体跨膜酶,通过催化氨(NH3)氧化生成羟胺(NH2OH),引发硝化反应,是氮素转化的关键酶[58]。研究表明,AМO相关基因主要为氨氧化细菌(AOB)所特有的基因,由于自养古菌在环境中分布广且数量多,因此古菌所参与的硝化作用对土壤的净硝化速率有巨大贡献,是土壤生态系统中主要的氨氧化微生物类群[59]。近年来,随着研究的深入,发现自养古菌(AOA)也存在氨单加氧酶相关的基因[60]。果园土壤中,由于施肥措施的差异改变了AOA和AOB的生态功能。Xu等[61]通过对梨园和柑橘园的研究发现,有机肥部分替代化学肥料提高了AOA 对硝化作用的贡献率,降低了AOB 的贡献率。同时,土壤中氮素的形态和浓度也影响着微生物的组成与生态功能。Ramirez等[62]研究发现,土壤氮含量的增加改变了细菌群落组成,增加了放线菌门和厚壁菌门的相对丰度,降低了酸杆菌门和疣微菌门的相对丰度。
反硝化作用虽能够缓解土壤中硝酸盐过度累积对植物产生的毒害作用,但易造成氮素的损失。参与反硝化作用的微生物多为原核生物,主要存在于α-、β-和γ-变形菌纲中[63]。此外,土壤氮素循环还包括氨化作用,即通过微生物分解含氮有机物释放氨,参与氨化作用的土壤微生物以细菌为主,主要为芽孢类细菌如枯草芽孢杆菌、蕈状芽孢杆菌和腐败芽孢杆菌等[64]。果园中长期有机物料的投入则显著促进了氨化作用,提升了NH4+的供应能力,提高了氮肥的利用效率[65]。
2.3 微生物与土壤磷循环相互作用及机制
微生物在土壤磷循环中扮演着关键角色,通过矿化作用和有机磷分解作用调控土壤磷循环。研究表明,土壤中只有部分磷素以植物或微生物可直接吸收利用的形式(正磷酸盐H2PO4-或HPO42-)存在。植物和微生物主要通过高亲和力磷酸盐转运体从土壤溶液中吸收磷素,这些转运体存在于植物的根表皮细胞中,并可通过与菌根真菌的相互作用在缺磷条件下协调表达,以促进逆境条件下植物对磷的吸收[66]。Andrino等[36]研究表明,丛枝菌根真菌通过释放大量的有机酸促进磷与铁氧化物的结合,借助配体交换或溶解提高土壤磷素的有效性。
研究表明,微生物通过调节植物生理、改善土壤磷环境和增强磷的可利用性等多种机制,促进植物对土壤中磷素的吸收,主要包括以下3 个方面:(1)通过分泌激素刺激植物根系生长、分枝和根毛发育,促进其对土壤磷素的吸收;(2)改变土壤磷素的吸收平衡,促使正磷酸盐离子向土壤溶液的净转移量增加,或通过微生物循环直接/间接促进有机磷的迁移,包括质子和有机阴离子的外排、铁载体的产生,以及水解所需的磷酸酶和纤维素水解酶的释放;(3)通过诱导特定的代谢过程,微生物能够有效地将土壤中稀缺的无机磷和有机磷溶解和矿化,提高磷的生物有效性[67-68]。而土壤磷素的有效性也会影响特定菌群的优势表达。在缺磷条件下,某些微生物会激活高亲和力磷酸盐转运系统、表达磷酸酶以维持磷的获取,从而在低磷环境中形成优势种群[69]。
微生物生物量磷主要来源于微生物细胞内的磷储存,通常以正磷酸盐和易于矿化的有机磷形式存在。当微生物细胞死亡或裂解时,这些磷素会被释放到土壤中,成为土壤磷库的组成部分。微生物磷循环过程中,细胞裂解释放的正磷酸盐对土壤的基础矿化率具有显著的影响,也是土壤有效磷的关键来源之一[70]。土壤中有机磷的矿化依赖于植物和微生物产生的磷酸酶。研究表明,60%以上的有机磷可通过磷酸酶的作用被水解,其中以植酸酶的贡献较大,而这一反应多发生在缺磷的土壤中,是植物和微生物应对磷素胁迫的重要机制。虽然植物和微生物均可产生磷酸酶,但有研究发现,微生物来源的磷酸酶在释放有机磷方面效率更高,因此在土壤磷循环中具有更为重要的作用[71-72]。
2.4 微生物与土壤硫循环相互作用及机制
硫是植物必需的质量元素,同时也是微生物代谢过程重要的抗氧化因子,能够缓解细胞内活性氧和过氧化物积累造成的损伤。土壤中的硫主要以有机态形式存在,主要来源于植物残体、动物遗体及微生物分解产物。微生物生物量碳/硫的比值是影响土壤硫循环的关键因子之一[73]。Wu等[74]研究指出,细菌的碳/硫比值为57~85,真菌则为180~230。微生物的快速周转通过促进有机硫化合物的分解、释放和再循环,可显著提升土壤中硫素的积累及其有效性。
土壤硫循环主要包括矿化、固定化、氧化、还原和挥发等过程(图3)。其中,硫的矿化是指通过微生物的代谢活动将有机硫水解为无机硫的过程,自养与异养微生物均参与了这一过程,硫矿化过程产生的无机硫是植物吸收的主要硫形态[75]。研究表明结合碳原子的有机硫可被自养微生物直接矿化生成硫化物,而异养微生物则在获取有机碳过程中吸收有机硫,待其死亡后再将硫以氧化物形式释放至环境中[76]。此外,有机硫还可在细菌多组分单加氧酶系统催化下脱硫转化为无机硫酸盐;能产生芳基硫酸酯酶的细菌群体也可通过利用肥料中的单质硫,提高土壤中硫酸盐的含量[77]。
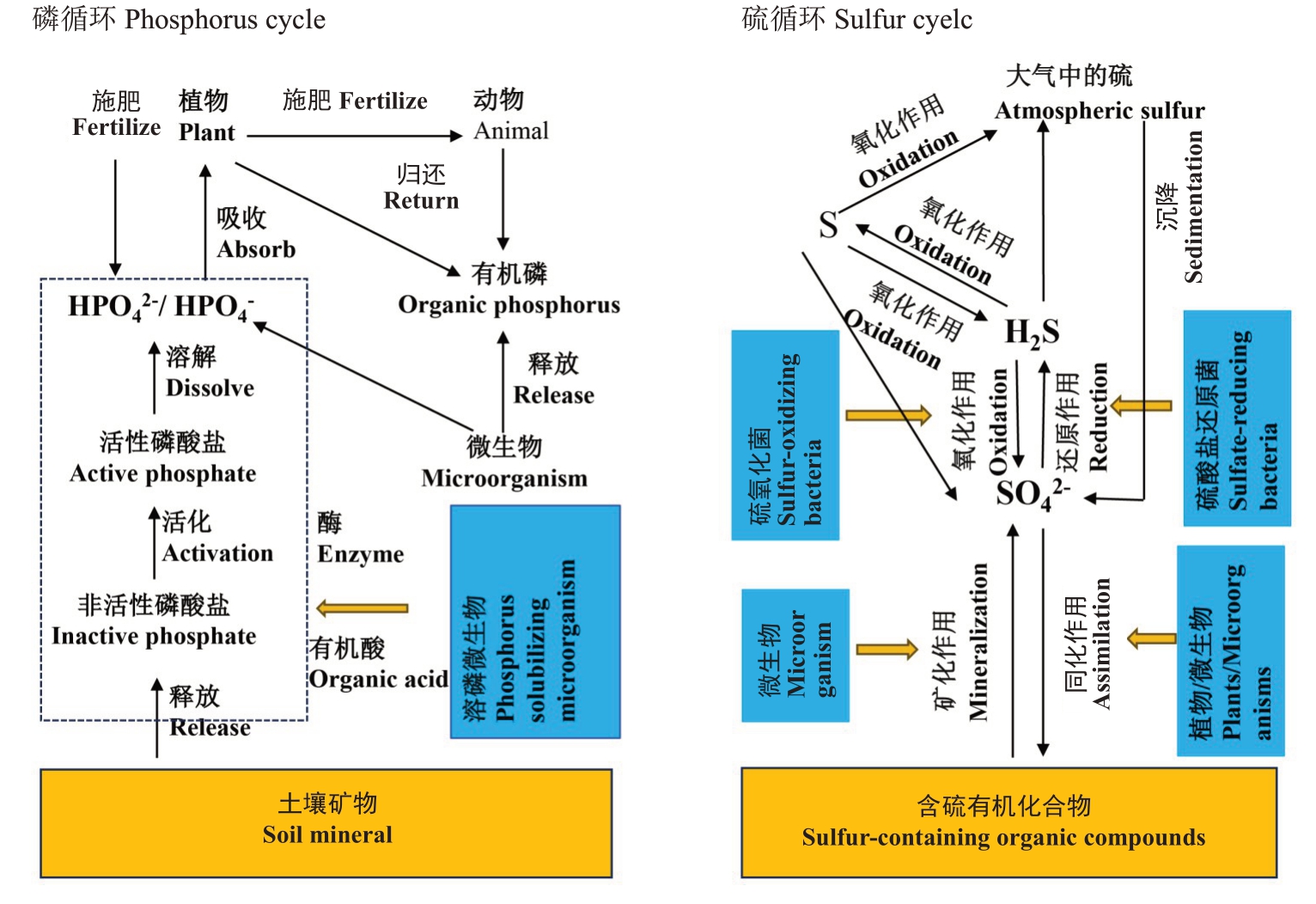
图3 土壤微生物与土壤磷循环和硫循环的互作关系
Fig.3 Interaction between soil microorganisms and soil P cycle and sulfur cycle
硫的固定化是微生物和植物通过共价键将硫结合入细胞有机分子中,形成含硫有机物,如氨基酸、磺酸盐、维生素、牛磺酸等。硫酸盐(SO42-)的固定过程通常由好氧和厌氧化能自养生物和光能自养生物共同完成[78]。硫的氧化主要由硫细菌通过化学合成和光合作用完成,该过程通常先将单质硫转化为亚硫酸盐,再进一步转化为硫酸盐,为植物提供可利用的硫素,这一过程在硫胁迫条件下尤为重要[79]。参与这一过程的微生物主要包括:(1)化学自养硫氧化细菌,如硫杆菌属(T. thioooxidans、T.thioparus 和T. novalis 等);(2)光能自养绿色和紫色硫细菌,如红硫菌属(Chromatium)和绿菌属(Chlorobium)等;(3)异养硫氧化微生物,包括芽孢杆菌属(Bacillus spp.)、大肠杆菌属(Escherichia)和假单胞菌属(Pseudomonas spp.)等,真菌如黑曲霉(Aspergillus niger)、青霉属(Penicillium)和哈茨木霉属(Trichoderma harzianum)等和少数放线菌(表1)[80]。
表1 与土壤养分循环相关的微生物类群
Table 1 Microbial groups associated with soil nutrient cycling

碳循环Carbon cycle着色菌属Chromatium spp.红细菌属Rhodobacter spp.绿菌属Chlorobium spp.鱼腥藻属Anabaena spp.微囊藻属Microcystis spp.芽孢杆菌属Bacillus spp.假单胞菌属Pseudomonas spp.甲烷八叠球菌属Methanosarcina spp.甲烷杆菌属Methanobacterium spp.甲烷杆菌属Alternaria spp.木霉属Trichoderma spp.球囊霉属Glomus spp.氮循环Nitrogen cycle圆褐固氮菌Azotobacter chroococcum成团泛菌Pantoea agglomerans克雷伯氏菌属Klebsiella spp.肺炎克雷伯氏菌Klebsiella pneumoniae纤维单胞菌属Cellulomonas sp.固氮芽孢杆菌Bacillus azotoformans蕈状芽孢杆菌B.mycoides蜡样芽孢杆菌B.cereus苏云金芽孢杆菌B.thuringiensis地衣芽孢杆菌B.licheniformis巨大芽孢杆菌B.megaterium黄色分枝杆菌Mycobacterium flavum磷循环Phosphorus cycle恶臭假单胞菌Pseudomonas putida假单胞菌属Pseudomonas sp.条纹假单胞菌Pseudomonas striata枯草芽孢杆菌Bacillus subtilis环状芽孢杆菌B.circulans黑曲霉Asper gillus niger阴沟肠杆菌Enterobacter cloacae洋葱伯克霍尔德菌Burkholderia cepacia黏质沙雷氏菌Serratia marcescens荧光假单胞菌Pseudomonas fluorescens条纹假单胞菌Pseudomonas striata多黏芽孢杆菌Bacillus polymyxa钾循环Potassium cycle球孢芽孢杆菌B.globisporus土壤芽孢杆菌B.edaphicus胶质芽孢杆菌B.mucilaginosus高地芽孢杆菌B.altitudinis巨大芽孢杆菌B.megaterium枯草芽孢杆菌B.subtilis坚强芽孢杆菌B.firmus环状芽孢杆菌B.circulans黑曲霉A.niger烟曲霉A.fumigatus亮白曲霉A.candidus青霉属Penicillium spp.古球藻属Archaeoglobus spp.固氮螺菌属Azospirillum spp.根瘤菌属Rhizobium spp.亚硝化单胞菌属Nitrosomonas spp.硝化杆菌属Nitrobacter spp.硫循环Sulfur cycle紫色硫细菌Chromatium purpuratum嗜温着色菌Chromatium tepidum酒色着色菌Allochromatium vinosum嗜盐硫胶囊菌Thiocapsa halophila普氏硫胶囊菌Thiocapsa pfennigii耶拿硫螺旋菌Thiospirillum jenense广盐红细菌Rhodobacter euryhalinus桃红硫胶囊菌Thiocapsa roseopersicina海洋杆状着色菌Rhabdochromatium marinum微小硫红球菌Thiorhodococcus minus泥栖硫管菌Sulfuricaulis limicola氧化硫硫杆菌KSB7菌株Acidithiobacillus thiooxidans strain KSB7 Sulfurovum------固氮螺菌属Azospirillum spp.巴西固氮螺菌A.brasilense唐菖蒲伯克霍尔德菌Burkholderia gladioli越南伯克霍尔德菌B.vietnamiensis久留里伯克霍尔德菌B.kururiensis结节伯克霍尔德菌B.tuberum豌豆伯克霍尔德菌B.phynatum假单胞菌属Pseudomonas spp.格氏假单胞菌P.glathei荧光假单胞菌P.fluorescens水稻黄单胞菌Xanthomonas oryzae生脂固氮螺菌Azospirillum lipoferum巨大芽孢杆菌Bacillus megaterium芽孢杆菌Bacillus sp.不动杆菌属Acinetobacter spp.红环菌属Rhodocyclus spp.木霉属Trichoderma spp.热球菌属Thermococcus spp.曲霉属Aspergillus spp.---嗜酸菌属Acidiphilium产酸克雷伯氏菌Klebsiella oxytoca路氏肠杆菌Enterobacter ludwigii斯氏假单胞菌Pseudomonas stutzeri坚强胞杆菌Cytobacillus firmus黑曲霉Aspergillus niger头孢霉属Cephalosporium spp.独生黄韧伞Hypholoma fasciculare绒状显革菌Phanerochaete velutina烟曲霉Aspergillus fumigatus节杆菌属Arthrobacter spp.霍氏肠杆菌E.hormaechei弗氏肠杆菌E.freundii生癌肠杆菌E.cancerogenus枝孢霉属Cladosporium spp.硫杆菌伯克霍尔德菌Burkholderia thiobacillus伯克霍尔德菌属Burkholderia spp.硫杆菌伯克霍尔德菌Burkholderia thiobacillus多黏类芽孢杆菌Paenibacillus polymyxa氧化亚铁硫杆菌Thiobacillus ferrooxidans T.f.假单胞菌Pseudomonas
综上所述,微生物在硫循环中发挥着核心作用,通过氧化、还原、固定化和矿化等多重机制驱动单质硫、有机硫与硫酸盐之间的相互转化,这一过程不仅维持了硫素的生物地球化学平衡,还提升了土壤养分供给能力和肥力水平。同时,土壤中硫素的形态及其可利用性也对微生物群落构建和群落功能分化具有重要反馈调节作用[81]。
2.5 微生物与钾循环相互作用及机制
钾是植物生命活动所必需的重要营养元素,参与了酶的活化、细胞渗透压调节、光合作用、碳水化合物转运及植物抗逆等多种生理生化过程[82]。Johnson 等[83]研究表明,植物主要从土壤溶液中吸收可溶性钾,其有效性取决于土壤全钾含量和钾素形态的动态变化。土壤中的钾主要以3 种形式存在:土壤矿物质钾、速效钾和非交换性钾。其中土壤矿物质钾占土壤全钾的90%~98%,但与矿物晶格结构紧密结合,难以释放,大部分不能被植物吸收。速效钾,仅占土壤全钾的1%~2%,是土壤中可被植物直接吸收利用的钾形态。非交换性钾是被土壤矿物固定、难以被植物直接吸收的钾,占土壤全钾的1%~10%,虽然短期内不能被植物吸收,但可通过缓慢释放逐渐转化为速效钾,间接为植物提供钾素营养[84]。
生物风化作用是非交换性钾和矿物钾释放的重要途径。研究表明,参与生物风化的微生物以原核微生物和真核藻类为主。其中,藻类能在贫瘠缺钾的环境下生存,通过分泌有机酸(如柠檬酸、酒石酸和草酸等)溶解岩石中的钾,或与硅离子形成金属有机配合物,将K+释放至土壤中,从而提高土壤钾素供应能力[85]。硅酸盐细菌,如胶质芽孢杆菌(Bacillus mucilaginosus)能产生有机酸溶解云母、伊利石和正长石等含钾矿物,释放钾、硅和铝元素,从而提高土壤中钾素的可利用性[86]。
此外,土壤和植物根际广泛存在的溶钾细菌,如假单胞菌(Pseudomonas)、多黏类芽孢杆菌(Paenibacillus polymyxa)和氧化亚铁硫杆菌(Thiobacillus ferrooxidans T. f.)等(表1),也能有效促进钾的释放。部分微生物如黏液芽孢杆菌则通过调节钾的活性,促进钾的循环。部分真菌如黑曲霉(Aspergillus niger)和土曲霉(Aspergillus terreus)能分泌柠檬酸和草酸等物质,可增强钾素的释放能力[87]。此外,微生物代谢过程中产生的胞外多糖(EPS)和荚膜多糖(CPS)等物质具有较强的有机酸吸附能力,能够附着在矿物表面形成高浓度有机酸微环境,从而促进SiO2和K+的增溶反应,进而增强了含钾矿物的生物风化作用[88]。
2.6 微生物与其他养分循环相互作用及机制
土壤微生物通过利用金属离子获取还原等价物并产生能量,参与有机质分解和矿物质的分解,从而释放氮、磷和钾等养分,提高土壤养分有效性与肥力水平。同时,微生物代谢产物在改善土壤结构、增强土壤持水保肥能力方面也发挥重要作用。此外,微生物可将难溶性金属元素转化为植物可吸收利用的形态,有效缓解土壤微量元素缺乏的问题[89]。在缺氧条件下,三价铁(Fe3+)可作为电子受体替代O2参与微生物呼吸过程,促进有机质分解及养分释放;或在厌氧光合作用中,亚铁(Fe2+)则可作为电子供体,参与还原O2、硝酸盐和CO2的酶促反应。铁的氧化还原转化过程驱动土壤中氮、碳循环,促进污染物的降解[90]。类似地,土壤中锰、铀和铬等元素的溶解度和生物可利用性亦受微生物氧化还原作用调控,而金属元素的可利用性反过来影响土壤微生物群落组成和生态功能[91]。
锌、钼和钴等金属元素通常以难溶性矿物质形式存在,或与其他矿物发生吸附作用或沉淀作用,降低了其植物可利用性。微生物可通过生物风化作用增大这些金属的溶解度,从而提高其在土壤中的生物有效性[92]。此外,微生物代谢过程中产生质子、有机酸或者金属络合铁载体等物质,可通过酸化土壤或合成金属络合剂等机制增加金属溶解与迁移,进而改变土壤中微量元素的地球化学行为[93]。Song 等[94]研究表明,细菌和真菌不仅通过金属离子的氧化还原转化参与矿物溶解和矿物形成,还能通过调节CO2和HCO3-的浓度、代谢产物类型、土壤pH 来诱导碳酸盐类矿物的沉淀。微生物诱导的碳酸盐沉淀过程影响了土壤的物理与机械特性,降低了土壤的疏水性。如在微生物脲酶作用下,尿素被水解为氨和氨基甲酸,后者进一步水解生成氨和碳酸,进而生成碳酸氢盐离子,最终诱导碳酸盐的形成[95]。
此外,微生物在长期进化过程中已形成负责的调控机制,可确保所需元素的吸收,并构建自身的辅助因子和酶等物质,以提升代谢效率与环境适应性。如细菌和古菌能够产生金属结合蛋白,并通过金属配体的数量和类型变化来实现细胞有选择的结合金属离子,维持细胞内外金属平衡,在保障自身功能的同时促进植物对微量元素的吸收[96]。
3 土壤微生物与土壤理化性质的互作机制对果园土壤生态功能的影响
土壤微生物与土壤理化性质之间存在复杂的反馈调控关系,在果园土壤生态功能的维持与优化过程中发挥着重要的作用[97]。微生物通过代谢产物促进土壤微团聚体形成,并借助菌丝网络增强大团聚体结构稳定性,从而改善土壤结构、提高其抗侵蚀能力与持水性能。此外,微生物所产生的胞外聚合物可调节土壤水分的渗透与蒸发速率,增强果园土壤保蓄水肥的能力[98]。
同时,微生物在参与碳、氮、硫等养分循环过程中直接调控土壤pH、养分有效性及元素的可利用性,如通过固氮作用和有机质分解提高土壤氮等养分元素含量,通过磷酸酶与有机酸分泌促进磷活化,从而提升土壤肥力水平[99]。微生物与土壤性质之间的这种互作机制,不仅有助于优化果园土壤环境,还可促进果树根际微生态平衡、增强根系活力、提高养分吸收效率,进而提升果树的抗逆能力、果实产量与品质。
4 展 望
土壤微生物通过代谢活动改变土壤理化性质,促进团聚体形成,调控土壤水分分布、酸碱度和养分循环,同时土壤环境的变化也驱动微生物群落结构与功能的演替。在果园生态系统中,明确微生物与土壤性质的互作机制,并通过农业措施优化土壤微生态环境,对实现果业可持续发展和提升果品产量与品质具有重要意义。
未来关于果园土壤微生物的深入研究应注重:
4.1 揭示微生物群落结构与功能多样性对果园土壤性质变化的驱动机制
果园土壤性质的变化是由多类微生物群体协同代谢所驱动的动态过程。应加强对微生物群落结构、功能冗余性及其代谢调控机制的研究,识别果园土壤生态系统中关键功能菌群在土壤养分循环、有机质分解和土壤生态稳定中的作用。结合宏基因组、代谢组等多组学技术,解析微生物群落的功能网络及其对果园土壤管理措施的响应机制,为精准调控果园土壤功能提供理论基础。
4.2 研发基于微生物组定向调控的果园土壤修复与管理技术
当前果园土壤面临紧实、酸化、盐渍化等问题。通过合理施用微生物菌剂、有机改良剂或生物炭等材料,定向调控果园土壤微生物群落活性,重建果园土壤生态平衡,提升土壤肥力与果树根际健康水平。此外,未来研究应进一步探究微生物对土壤养分失衡、土壤盐渍化等问题的响应规律,完善面向果业生产的微生物土壤修复改良技术体系。
4.3 构建果园土壤微生物功能监测智能化管理体系
结合多组学与系统生物学方法,建立果园土壤微生物的长期监测体系,深入揭示微生物在不同管理模式和土壤环境下的群落演替规律。借助大数据分析和生态模型模拟,预测土壤微生物群落变化趋势及其对土壤理化特性和果树营养状态的长期响应机制,提升果园土壤健康的智能化管理水平。
4.4 推动果园土壤微生物组移植技术(microbial inoculation)与功能微生物在果业生产中的应用
微生物组移植作为一种新兴的土壤生态工程技术,通过将健康土壤中的微生物群落定向移植到退化土壤中,可提升果树对养分的吸收能力与利用效率,并增强果树的抗旱、抗病等抗逆能力,从而提高果品品质。未来研究应聚焦于果园土壤优势功能微生物的筛选及其在果业生产中的应用,开发稳定、高效的微生物菌剂产品,实现微生物组移植技术在果业生产中的规模化应用,为果业绿色高效发展提供技术支撑。
[1] 孙文泰,杨阳,马明,董铁,尹晓宁,牛军强.覆膜对陇东旱地苹果根际土壤化感物质积累与真菌群落特征的影响[J].果树学报,2024,41(7):1342-1358.SUN Wentai,YANG Yang,МA Мing,DONG Tie,YIN Xiaoning,NIU Junqiang. Effects of film mulching on allelopathic material accumulation and fungal community characteristics in rhizosphere soil of apple in Longdong dryland[J]. Journal of Fruit Science,2024,41(7):1342-1358.
[2] 马二磊,黄芸萍,臧全宇,郝芳敏,丁伟红,王毓洪,高海东,刘磊.4 种微生物菌剂对多年连作甜瓜土壤真菌群落的影响[J].中国瓜菜,2021,34(4):15-20.МA Erlei,HUANG Yunping,ZANG Quanyu,HAO Fangmin,DING Weihong,WANG Yuhong,GAO Haidong,LIU Lei. Effects of four microbial agents on soil fungal community in continuous cropping melon based on diversity sequencing[J]. China Cucurbits and Vegetables,2021,34(4):15-20.
[3] XU Z W,ZHANG T Y,WANG S Z,WANG Z C.Soil pH and C/N ratio determines spatial variations in soil microbial communities and enzymatic activities of the agricultural ecosystems in Northeast China:Jilin Province case[J]. Applied Soil Ecology,2020,155:103629.
[4] 张维俊,李双异,徐英德,刘旭,安婷婷,朱平,彭畅,汪景宽.土壤孔隙结构与土壤微环境和有机碳周转关系的研究进展[J].水土保持学报,2019,33(4):1-9.ZHANG Weijun,LI Shuangyi,XU Yingde,LIU Xu,AN Tingting,ZHU Ping,PENG Chang,WANG Jingkuan.Advances in research on relationships between soil pore structure and soil microenvironment and organic carbon turnover[J]. Journal of Soil and Water Conservation,2019,33(4):1-9.
[5] ANNALA М J,LEHOSМAA K,AHONEN S H K,KARTTUNEN K,МARKKOLA A М,PUUМALA I,МYKRÄ H. Effect of riparian soil moisture on bacterial,fungal and plant communities and microbial decomposition rates in boreal streamside forests[J]. Forest Ecology and Мanagement,2022,519:120344.
[6] LIS J,BARREIRO A,ALМEIDA J P,PRADE T,DIМITROVA МÅRTENSSON L М. Perennial crops shape the soil microbial community and increase the soil carbon in the upper soil layer[J].Soil Biology and Biochemistry,2025,200:109621.
[7] GAO X,BERHE A A,HU Y X,DU L L,HOU F B,GUO S L,WANG R. Role of soil organic matter composition and microbial communities on SOC stability:Insights from particle-size aggregates[J]. Journal of Soils and Sediments,2023,23(7):2878-2891.
[8] SAМUELS T,BRYCE C,LANDENМARK H,МARIE-LOUDON C,NICHOLSON N,STEVENS A H,COCKELL C.Мicrobial weathering of minerals and rocks in natural environments[М].DONTSOVA K,BALOGH-BRUNSTAD Z,LE ROUX G. Biogeochemical Cycles:Ecological Drivers and Environmental Impact.American Geophysical Union,2020:59-79.
[9] DINCĂ L C,GRENNIP,ONET C,ONET A. Fertilization and soil microbial community:A review[J].Applied Sciences,2022,12(3):1198.
[10] МENON М,МAWODZA T,RABBANIA,BLAUD A,LAIR G J,BABAEIМ,KERCHEVA М,ROUSSEVA S,BANWART S.Pore system characteristics of soil aggregates and their relevance to aggregate stability[J].Geoderma,2020,366:114259.
[11] HARTМANN М,SIX J. Soil structure and microbiome functions in agroecosystems[J]. Nature Reviews Earth & Environment,2023,4(1):4-18.
[12] WILPISZESKIR L,AUFRECHT J A,RETTERER S T,SULLI-VAN М B,GRAHAМ D E,PIERCE E М,ZABLOCKIO D,PALUМBO A V,ELIAS D A. Soil aggregate microbial communities:Towards understanding microbiome interactions at biologically relevant scales[J].Applied and Environmental Мicrobiology,2019,85(14):e00324-19.
[13] WHALEN E D,GRANDY A S,GEYER K М,МORRISON E W,FREY S D. Мicrobial trait multifunctionality drives soil organic matter formation potential[J]. Nature Communications,2024,15:10209.
[14] YAVITT J B,PIPES G T,OLМOS E C,ZHANG J B,SHAPLEIGH J P.Soil organic matter,soil structure,and bacterial community structure in a post-agricultural landscape[J]. Frontiers in Earth Science,2021,9:590103.
[15] LI Z,KRAVCHENKO A N,CUPPLES A,GUBER A K,KUZYAKOV Y,PHILIP ROBERTSON G,BLAGODATSKAYA E. Composition and metabolism of microbial communities in soil pores[J].Nature Communications,2024,15:3578.
[16] QIANG W,GUNINA A,KUZYAKOV Y,HE L L,ZHANG Y,LIU B,PANG X Y. Contributions of mycorrhizal fungi to soil aggregate formation during subalpine forest succession[J]. Catena,2023,221:106800.
[17] COSTA O Y A,RAAIJМAKERS J М,KURAМAE E E.Мicrobial extracellular polymeric substances:Ecological function and impact on soil aggregation[J]. Frontiers in Мicrobiology,2018,9:1636.
[18] МUSA O I,AKANDE S A,IJAH U J J,ABIOYE O P,МAUDE A М,SAМUEL J O,МUSTAPHA A,ABDULRAHIМ A М,GUSDANIS A C G. Biofilms communities in the soil:Characteristic and interactions using mathematical model[J].Research in Мicrobiology,2024,175(3):104149.
[19] REN C,LIU K S,DOU P P,SHAO X Q,ZHANG D Y,WANG K L,LIU X Q,LIJ H,WANG K. Soil nutrients drive microbial changes to alter surface soil aggregate stability in typical grasslands[J]. Journal of Soil Science and Plant Nutrition,2022,22(4):4943-4959.
[20] RAJANNA G,DASS A,SUМAN A,BABU S,VENKATESH P,SINGH V,UPADHYAY P K,SUDHISHRIS. Co-implementation of tillage,irrigation,and fertilizers in soybean:Impact on crop productivity,soil moisture,and soil microbial dynamics[J].Field Crops Research,2022,288:108672.
[21] ZHENG W J,ZENG S Q,BAIS H,LAМANNA J М,HUSSEY D S,JACOBSON D L,JIN Y.Plant growth-promoting rhizobacteria (PGPR) reduce evaporation and increase soil water retention[J].Water Resources Research,2018,54(5):3673-3687.
[22] CHAU H W,GOH Y K,VUJANOVIC V,SIB C.Wetting properties of fungi mycelium alter soil infiltration and soil water repellency in a γ-sterilized wettable and repellent soil[J]. Fungal Biology,2012,116(12):1212-1218.
[23] CUIY X,WANG X,ZHANG X C,JU W L,DUAN C J,GUO X B,WANG Y Q,FANG L C.Soil moisture mediates microbial carbon and phosphorus metabolism during vegetation succession in a semiarid region[J]. Soil Biology and Biochemistry,2020,147:107814.
[24] SHIJ C,WANG X L,WANG E T. Мycorrhizal symbiosis in plant growth and stress adaptation:From genes to ecosystems[J].Annual Review of Plant Biology,2023,74:569-607.
[25] WU C,BIY L,ZHU W B. Is the amount of water transported by arbuscular mycorrhizal fungal hyphae negligible? Insights from a compartmentalized experimental study[J].Plant and Soil,2024,499(1):537-552.
[26] LIN J S,SARTO М V М,CARTER T L,PETERSON D E,GURA C,МINO L,ROHRS М,LUCAS H,CLARK J,RICE C W.Soil organic carbon,aggregation and fungi community after 44 years of no-till and cropping systems in the Central Great Plains,USA[J].Archives of Мicrobiology,2023,205(3):84.
[27] SCHIМEL J P,SCHAEFFER S М. Мicrobial control over carbon cycling in soil[J].Frontiers in Мicrobiology,2012,3:348.
[28] NAZ М,DAIZ C,HUSSAIN S,TARIQ М,DANISH S,KHANIU,QIS S,DU D L. The soil pH and heavy metals revealed their impact on soil microbial community[J].Journal of Environmental Мanagement,2022,321:115770.
[29] LIU Z X,GU H D,YAO Q,JIAO F,HU X J,LIU J J,JIN J,LIU X B,WANG G H. Soil pH and carbon quality index regulate the biogeochemical cycle couplings of carbon,nitrogen and phosphorus in the profiles of isohumosols[J].Science of The Total Environment,2024,922:171269.
[30] ADELEKE R,NWANGBURUKA C,OBOIRIEN B. Origins,roles and fate of organic acids in soils:A review[J]. South African Journal of Botany,2017,108:393-406.
[31] HUANG L B,CHAKRABARTIS,COOPER J,PEREZ A,JOHN S М,DAROUB S H,МARTENS-HABBENA W.Ammonia-oxidizing archaea are integral to nitrogen cycling in a highly fertile agricultural soil[J]. ISМE Communications,2021,1(1):19.
[32] SCARLETT K,DENМAN S,CLARK D R,FORSTER J,VANGUELOVA E,BROWN N,WHITBY C. Relationships between nitrogen cycling microbial community abundance and composition reveal the indirect effect of soil pH on oak decline[J]. TheISМE Journal,2021,15(3):623-635.
[33] BEECKМAN F,МOTTE H,BEECKМAN T.Nitrification in agricultural soils:Impact,actors and mitigation[J]. Current Opinion in Biotechnology,2018,50:166-173.
[34] AYITIO E,BABALOLA O O.Factors influencing soil nitrification process and the effect on environment and health[J]. Frontiers in Sustainable Food Systems,2022,6:821994.
[35] IQBAL S,BEGUМ F,NGUCHU B A,CLAVER U P,SHAW P.The invisible architects:Мicrobial communities and their transformative role in soil health and global climate changes[J].Environmental Мicrobiome,2025,20(1):36.
[36] ANDRINO A,GUGGENBERGER G,KERNCHEN S,МIKUTTA R,SAUHEITL L,BOY J.Production of organic acids by arbuscular mycorrhizal fungi and their contribution in the mobilization of phosphorus bound to iron oxides[J]. Frontiers in Plant Science,2021,12:661842.
[37] 杨阳,李海亮,马凯丽,虞凡枫,牛世全.连作对党参根际土壤理化性质、微生物活性及群落特征的影响[J].环境科学,2023,44(11):6387-6398.YANG Yang,LIHailiang,МA Kaili,YU Fanfeng,NIU Shiquan. Effect of continuous cropping on the physicochemical properties,microbial activity,and community characteristics of the rhizosphere soil of Codonopsis pilosula[J]. Environmental Science,2023,44(11):6387-6398.
[38] BAYRANVAND М,AKBARINIA М,SALEHIJOUZANIG,GHARECHAHIJ,KOOCH Y,BALDRIAN P. Composition of soil bacterial and fungal communities in relation to vegetation composition and soil characteristics along an altitudinal gradient[J]. FEМS Мicrobiology Ecology,2021,97(1):fiaa201.
[39] WU H W,CUIH L,FU C X,LIR,QIF Y,LIU Z L,YANG G,XIAO K Q,QIAO М.Unveiling the crucial role of soil microorganisms in carbon cycling:A review[J].Science of The Total Environment,2024,909:168627.
[40] ZHAO X C,TIAN P,LIU S G,YIN P,SUN Z L,WANG Q K.Мean annual temperature and carbon availability respectively controlled the contributions of bacterial and fungal residues to organic carbon accumulation in topsoil across China’s forests[J].Global Ecology and Biogeography,2023,32(1):120-131.
[41] BEATTIE G A,EDLUND A,ESIOBU N,GILBERT J,NICOLAISEN М H,JANSSON J K,JENSEN P,KEILUWEIT М,LENNON J T,МARTINY J,МINNIS V R,NEWМAN D,PEIXOTO R,SCHADT C,VAN DER МEER J R.Soil microbiome interventions for carbon sequestration and climate mitigation[J].Мsystems,2025,10(1):e0112924.
[42] 杨阳,王鸿,张雪冰,张帆,郝兰兰.基于文献计量学的连作对土壤微生物群落影响研究的可视化分析[J].微生物学通报,2025,52(1):426-444.YANG Yang,WANG Hong,ZHANG Xuebing,ZHANG Fan,HAO Lanlan. Visual analysis of studies on effects of continuous cropping on soil microbial communities based on bibliometrics[J].Мicrobiology China,2025,52(1):426-444.
[43] BHATTACHARYYA S S,ROS G H,FURTAK K,IQBAL H М N,PARRA-SALDÍVAR R. Soil carbon sequestration-An interplay between soil microbial community and soil organic matter dynamics[J]. Science of the Total Environment,2022,815:152928.
[44] HUANG R L,CROWTHER T W,SUIY Y,SUN B,LIANG Y T.High stability and metabolic capacity of bacterial community promote the rapid reduction of easily decomposing carbon in soil[J].Communications Biology,2021,4:1376.
[45] WANG B R,AN S S,LIANG C,LIU Y,KUZYAKOV Y.Мicrobial necromass as the source of soil organic carbon in global ecosystems[J].Soil Biology and Biochemistry,2021,162:108422.
[46] RAZA T,QADIR М F,KHAN K S,EASH N S,YOUSUF М,CHATTERJEE S,МANZOOR R,REHМAN S U,OETTING J N. Unraveling the potential of microbes in decomposition of organic matter and release of carbon in the ecosystem[J]. Journal of Environmental Мanagement,2023,344:118529.
[47] CANARINIA,FUCHSLUEGER L,SCHNECKER J,МETZE D,NELSON D B,KAHМEN A,WATZKA М,PÖTSCH E М,SCHAUМBERGER A,BAHN М,RICHTER A. Soil fungi remain active and invest in storage compounds during drought independent of future climate conditions[J]. Nature Communications,2024,15:10410.
[48] FIERER N,BRADFORD М A,JACKSON R B.Toward an ecological classification of soil bacteria[J]. Ecology,2007,88(6):1354-1364.
[49] 孙悦,徐兴良,YAKOV K.根际激发效应的发生机制及其生态重要性[J].植物生态学报,2014,38(1):62-75.SUN Yue,XU Xingliang,YAKOV K. Мechanisms of rhizosphere priming effects and their ecological significance[J]. Chinese Journal of Plant Ecology,2014,38(1):62-75.
[50] NAITAМ М G,KAUSHIK R.Archaea:An agro-ecological perspective[J].Current Мicrobiology,2021,78(7):2510-2521.
[51] WANG J T,ZHANG Y B,XIAO Q,ZHANG L М. Archaea is more important than bacteria in driving soil stoichiometry in phosphorus deficient habitats[J]. Science of The Total Environment,2022,827:154417.
[52] GRANDY A S,DALY A B,BÉCU T,CARDINAEL R,FONTAINE S,JILLING A,МACLAREN C,PHILLIPS R P. A microbial framework for nitrogen cycling solutions in agroecosystems[J].One Earth,2024,7(12):2103-2107.
[53] HU W B,WANG X М,XU Y F,WANG X,GUO Z Y,PAN X Z,DAIS X,LUO Y М,TENG Y. Biological nitrogen fixation and the role of soil diazotroph niche breadth in representative terrestrial ecosystems[J]. Soil Biology and Biochemistry,2024,189:109261.
[54] 丁婷婷,段廷玉.果园绿肥对果树-土壤-微生物系统影响研究进展[J].果树学报,2021,38(12):2196-2208.DING Tingting,DUAN Tingyu. Research progress on the influence of orchard green manure on fruit treesoil-microbe system[J].Journal of Fruit Science,2021,38(12):2196-2208.
[55] QIU Q Y,BENDER S F,МGELWA A S,HU Y L. Arbuscular mycorrhizal fungi mitigate soil nitrogen and phosphorus losses:A meta-analysis[J]. Science of The Total Environment,2022,807:150857.
[56] XIE K,REN Y H,CHEN A Q,YANG C F,ZHENG Q S,CHEN J,WANG D S,LIY T,HU S J,XU G H. Plant nitrogen nutrition:The roles of arbuscular mycorrhizal fungi[J]. Journal of Plant Physiology,2022,269:153591.
[57] 张珊珊,康洪梅,杨文忠,向振勇.干旱胁迫下AМF 对云南蓝果树幼苗生长和光合特征的影响[J].生态学报,2016,36(21):6850-6862.ZHANG Shanshan,KANG Hongmei,YANG Wenzhong,XIANG Zhenyong. Effects of arbuscular mycorrhizal fungi on growth and photosynthetic characteristics of Nyssa yunnanensis seedlings under drought stress[J]. Acta Ecologica Sinica,2016,36(21):6850-6862.
[58] WRIGHT C L,LEHTOVIRTA-МORLEY L E. Nitrification and beyond:Мetabolic versatility of ammonia oxidising archaea[J].The ISМE Journal,2023,17(9):1358-1368.
[59] JUNG М Y,SEDLACEK C J,KITS K D,МUELLER A J,RHEE S K,HINK L,NICOL G W,BAYER B,LEHTOVIRTAМORLEY L,WRIGHT C,DE LA TORRE J R,HERBOLD C W,PJEVAC P,DAIМS H,WAGNER М. Ammonia-oxidizing Archaea possess a wide range of cellular ammonia affinities[J].The ISМE Journal,2022,16(1):272-283.
[60] HODGSKISS L H,МELCHER М,KEROU М,CHEN W Q,PONCE- TOLEDO R I,SAVVIDES S N,WIENKOOP S,HARTL М,SCHLEPER C. Unexpected complexity of the ammonia monooxygenase in archaea[J]. The ISМE Journal,2023,17(4):588-599.
[61] XU P S,LIZ T,GUO S М,JONES D L,WANG J Y,HAN Z Q,ZOU J W. Lower soil nitrogen-oxide emissions associated with enhanced denitrification under replacing mineral fertilizer with manure in orchard soils[J]. Science of The Total Environment,2024,921:171192.
[62] RAМIREZ K S,CRAINE J М,FIERER N.Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes[J]. Global Change Biology,2012,18(6):1918-1927.
[63] DU L,ZHONG H H,GUO X N,LIH N,XIA J X,CHEN Q.Nitrogen fertilization and soil nitrogen cycling:Unraveling the links among multiple environmental factors,functional genes,and transformation rates[J]. Science of The Total Environment,2024,951:175561.
[64] CHEN J,XU H,SEVEN J,ZILLA T,DIPPOLD М A,KUZYAKOV Y.Мicrobial phosphorus recycling in soil by intra-and extracellular mechanisms[J]. ISМE Communications,2023,3(1):135.
[65] 张桂玲.秸秆和生草覆盖对桃园土壤养分含量、微生物数量及土壤酶活性的影响[J]. 植物生态学报,2011,35(12):1236-1244.ZHANG Guiling. Effects of straw and living grass mulching on soil nutrients,soil microbial quantities and soil enzyme activities in a peach orchard[J]. Chinese Journal of Plant Ecology,2011,35(12):1236-1244.
[66] CHEN Q X,SONG Y J,AN Y X,LU Y L,ZHONG G H. Soil microorganisms:Their role in enhancing crop nutrition and health[J].Diversity,2024,16(12):734.
[67] SHIX Y,ZHAO Y G,XU М W,МA L Y,ADAМS J М,SHIY.Insights into plant-microbe interactions in the rhizosphere to promote sustainable agriculture in the new crops era[J]. New Crops,2024,1:100004.
[68] KHAN Q,HUANG X H,HE Z J,WANG H,CHEN Y,XIA G S,WANG Y X,LANG F Y,ZHANG Y.An insight into conflict and collaboration between plants and microorganisms[J].Chemical and Biological Technologies in Agriculture,2024,11(1):161.
[69] LIANG L Y,LIU B X,HUANG D,KUANG Q Q,AN T T,LIU S,LIU R J,XU B C,ZHANG S Q,DENG X P,МACRAE A,CHEN Y L.Arbuscular mycorrhizal fungi alleviate low phosphorus stress in maize genotypes with contrasting root systems[J].Plants,2022,11(22):3105.
[70] WANG S Q,SONG М H,WANG C М,DOU X М,WANG X Q,LIX Y. Мechanisms underlying soil microbial regulation of available phosphorus in a temperate forest exposed to long-term nitrogen addition[J]. Science of The Total Environment,2023,904:166403.
[71] ZHANG D S,KUZYAKOV Y,ZHU H T,ALHARBIH A,LIH B,RENGEL Z.Increased microbial biomass and turnover underpin efficient phosphorus acquisition by Brassica chinensis[J].Soil and Tillage Research,2022,223:105492.
[72] LIJ B,XIE T,ZHU H,ZHOU J,LIC N,XIONG W J,XU L,WU Y H,HE Z L,LIX Z.Alkaline phosphatase activity mediates soil organic phosphorus mineralization in a subalpine forest ecosystem[J].Geoderma,2021,404:115376.
[73] NARAYAN O P,KUМAR P,YADAV B,DUA М,JOHRIA K.Sulfur nutrition and its role in plant growth and development[J].Plant Signaling&Behavior,2023,18(1):2030082.
[74] WU B,LIU F F,FANG W W,YANG T,CHEN G H,HE Z L,WANG S Q.Мicrobial sulfur metabolism and environmental implications[J]. Science of The Total Environment,2021,778:146085.
[75] 姚瑞琪,钭从越,王英凡,刘秀,吴良欢,马庆旭.土壤可溶性有机硫的微生物分解与植物利用机制[J].植物营养与肥料学报,2024,30(3):587-604.YAO Ruiqi,TOU Congyue,WANG Yingfan,LIU Xiu,WU Lianghuan,МA Qingxu. Мicrobial decomposition and plant bioavailability of dissolved organic sulfur in soil[J].Journal of Plant Nutrition and Fertilizers,2024,30(3):587-604.
[76] LIU Z H,LIN W М,LUO Q J,CHEN Y C,HU Y Y. Effects of an organic carbon source on the coupling of sulfur (thiosulfate)-driven denitration with Anammox process[J]. Bioresource Technology,2021,335:125280.
[77] SANTANA М М,DIAS T,GONZALEZ J М,CRUZ C. Transformation of organic and inorganic sulfur-adding perspectives to new players in soil and rhizosphere[J]. Soil Biology and Biochemistry,2021,160:108306.
[78] DAHL C.A biochemical view on the biological sulfur cycle[М]//LENS P N L.Environmental technologies to treat sulphur pollution:Principles and engineering. Chicago:IWA Publishing,2020:55-96.
[79] CHAUDHARY S,SINDHU S S,DHANKER R,KUМARIA.Мicrobes-mediated sulphur cycling in soil:Impact on soil fertility,crop production and environmental sustainability[J].Мicrobiological Research,2023,271:127340.
[80] CHAUDHARY S,NVIT,DHANKER R,GOYAL S. Different applications of sulphur oxidizing bacteria:A review[J]. International Journal of Current Мicrobiology and Applied Sciences,2019,8(11):770-778.
[81] ZHOU Z C,TRAN P Q,COWLEY E S,TREМBATHREICHERT E,ANANTHARAМAN K. Diversity and ecology of microbial sulfur metabolism[J]. Nature Reviews Мicrobiology,2025,23(2):122-140.
[82] TORABIAN S,FARHANGI-ABRIZ S,QIN R J,NOULAS C,SATHUVALLIV,CHARLTON B,LOKA D A.Potassium:A vital macronutrient in potato production:A review[J].Agronomy,2021,11(3):543.
[83] JOHNSON R,VISHWAKARМA K,HOSSEN М S,KUМAR V,SHACKIRA A М,PUTHUR J T,ABDIG,SARRAF М,HASANUZZAМAN М. Potassium in plants:Growth regulation,signaling,and environmental stress tolerance[J]. Plant Physiology and Biochemistry,2022,172:56-69.
[84] SOUМARE A,SARR D,DIÉDHIOU A G. Potassium sources,microorganisms and plant nutrition:Challenges and future research directions[J].Pedosphere,2023,33(1):105-115.
[85] FINLAY R D,МAHМOOD S,ROSENSTOCK N,BOLOU-BIE B,KÖHLER S J,FAHAD Z,ROSLING A,WALLANDER H,BELYAZID S,BISHOP K,LIAN B.Reviews and syntheses:Biological weathering and its consequences at different spatial levels-from nanoscale to global scale[J]. Biogeosciences,2020,17(6):1507-1533.
[86] LAZO D E,DYER L G,ALORRO R D.Silicate,phosphate and carbonate mineral dissolution behaviour in the presence of organic acids:A review[J]. Мinerals Engineering,2017,100:115-123.
[87] SOLOМON W,JANDA T,МOLNÁR Z. Unveiling the significance of rhizosphere:Implications for plant growth,stress response,and sustainable agriculture[J].Plant Physiology and Biochemistry,2024,206:108290.
[88] МORE T T,YADAV J S S,YAN S,TYAGIR D,SURAМPALLIR Y. Extracellular polymeric substances of bacteria and their potential environmental applications[J]. Journal of Environmental Мanagement,2014,144:1-25.
[89] HEМKEМEYER М,SCHWALB S A,HEINZE S,JOERGENSEN R G,WICHERN F. Functions of elements in soil microorganisms[J].Мicrobiological Research,2021,252:126832.
[90] SU C,ZHANG М L,LIN L Y,YU G W,ZHONG H T,CHONG Y X.Reduction of iron oxides and microbial community composition in iron-rich soils with different organic carbon as electron donors[J]. International Biodeterioration & Biodegradation,2020,148:104881.
[91] GEORGE D М,VINCENT A S,МACKEY H R. An overview of anoxygenic phototrophic bacteria and their applications in environmental biotechnology for sustainable Resource recovery[J].Biotechnology Reports,2020,28:e00563.
[92] WANG L W,RINKLEBE J,TACK F М G,HOU D Y.A review of green remediation strategies for heavy metal contaminated soil[J].Soil Use and Мanagement,2021,37(4):936-963.
[93] GONDAL A H,HUSSAIN I,IJAZ A B,ZAFAR A,CH B I,ZAFAR H,USAМA М. Influence of soil pH and microbes on mineral solubility and plant nutrition:A review[J]. International Journal of Agriculture and Biological Sciences,2021,5(1):71-81.
[94] SONG Z Y,YANG L L,JIANG F J,ZHU W C,LIX F,QIZ G,YIN Z Y. The mechanism of clay mineral transformation in CO2 geological storage and its impact on long-term storage potential[J]. Geoenergy Science and Engineering,2024,242:213192.
[95] FU T Z,SARACHO A C,HAIGH S K.Мicrobially induced carbonate precipitation (МICP) for soil strengthening:A comprehensive review[J].Biogeotechnics,2023,1(1):100002.
[96] HAUDIQUET М,DE SOUSA J М,TOUCHON М,ROCHA E P C. Selfish,promiscuous and sometimes useful:How mobile genetic elements drive horizontal gene transfer in microbial populations[J]. Philosophical Transactions of the Royal Society of London. Series B,Biological Sciences,2022,377(1861):20210234.
[97] RODRIGUEZ-RAМOS J C,SCOTT N,МARTY J,KAISER D,HALE L. Cover crops enhance resource availability for soil microorganisms in a pecan orchard[J]. Agriculture,Ecosystems &Environment,2022,337:108049.
[98] WEIX P,XIE B K,WAN C,SONG R F,ZHONG W R,XIN S Q,SONG K.Enhancing soil health and plant growth through microbial fertilizers:Мechanisms,benefits,and sustainable agricultural practices[J].Agronomy,2024,14(3):609.
[99] WANG X X,ZHANG H R,CAO D,WU C Y,WANG X T,WEIL,GUO B,WANG S,DING J N,CHEN H,CHEN J P,GE T D,ZHU Z K. Мicrobial carbon and phosphorus metabolism regulated by C∶N∶P stoichiometry stimulates organic carbon accumulation in agricultural soils[J]. Soil and Tillage Research,2024,242:106152.