番木瓜(Carica papaya L.)为番木瓜科番木瓜属植物,营养丰富,被誉为“百益果王”,是广受消费者喜爱的营养保健食品,市场需求广阔[1-2]。作为热带、亚热带的重要经济作物,番木瓜生长周期短且适应性强,在中国广西、广东、海南、福建、云南、台湾等地广泛种植,成为助力农民增收致富的重要经济支柱[3]。然而,番木瓜果实不耐贮运,采收后果实生理代谢旺盛,易腐烂软化,这严重限制其市场价值和产业发展[1,4]。因此,阐明番木瓜采后软化的生理规律和不同品种贮藏特性差异的原因对提高果实耐贮性、延长货架期具有重要指导意义。
研究表明,细胞壁降解是导致果实软化的主要原因[5-6]。植物细胞壁主要由果胶、纤维素和半纤维素等多糖组成,其中果胶的解聚与果实硬度下降密切相关[6]。在果实软化过程中,果胶发生去酯化和长链去聚化反应,从不溶性果胶分解为可溶性的果胶,使得细胞间黏附性降低,细胞壁结构破坏,引发果实硬度下降[7-8]。这一系列的生化反应需要多种细胞壁修饰酶和果胶降解相关酶的共同参与,如果胶甲酯酶(PМE)、多聚半乳糖醛酸酶(PG)和果胶裂解酶(PL)等[9]。番木瓜果实细胞壁结构的电镜观察结果表明,番木瓜果实软化伴随着中胶层的解聚和果胶的增溶[10-11],同时可溶性果胶含量激增,比软化前上升6 倍[2,7,12],这表明果胶代谢在番木瓜果实采后软化中起着重要作用。
不同番木瓜品种在采后生理和贮藏特性方面存在显著差异[13-15]。研究发现,穗选1号和穗优2号较耐贮藏,果实硬度保持较好;而白皮日升和穗黄果实采后软化速度快,贮藏性差[13]。周常清等[16]通过品种比较试验发现,穗中红48 贮藏期为6 d,红铃2 号贮藏期仅为3 d。吴夏明等[17]发现贮丰的贮藏性最佳,紫晖和黄花佑次之,泰国红贮藏性最差。目前,关于番木瓜品种间贮藏差异的研究多集中在乙烯信号和呼吸代谢方面。例如,杨敏等[18]发现黄花佑和金锤果实硬度差异与乙烯信号途径密切相关。不耐贮品种黄花佑果实采收后乙烯生物合成和信号传导早于耐贮品种贮丰,从而导致黄花佑果实快速软化[18]。孟祥春等[13]认为穗选1 号和穗优2 号果实呼吸和乙烯释放速率低,峰值出现晚,果实硬度下降缓慢。然而,关于不同番木瓜品种果实采后贮藏特性和果胶代谢相关性的研究鲜见报道。
笔者在本研究中以广东省农业科学院果树研究所选育的新品种贮丰和黄花佑番木瓜果实为试材,比较两个品种果实的贮藏特性(包括外观变化、软化表现、内在品质)、细胞壁果胶组分含量、果胶降解相关酶活性和基因表达水平的差异,旨在阐明不同耐贮性番木瓜采后软化的生理特性及其机制,为番木瓜贮运保鲜和优质耐贮新品种选育提供参考。
1 材料和方法
1.1 试验材料
挑选生理形态基本一致、无机械损伤、呈现三线黄阶段(花后115 d,果实心皮之间出现黄色条纹)的番木瓜果实进行采样,贮丰(Carica papaya L.‘Zhufeng’)和黄花佑(Carica papaya L.‘Huanghuayou’)各采收果实60个,设计3次生物学重复,每组果实20个,及时运回实验室进行贮藏试验。果实置于温度(25±1)℃、相对湿度70%的条件下贮藏,在采后0、4、7 和11 d 观察果实形态变化、拍照并测定相关生理指标。同时将果肉冻样于-80 ℃保存,用于后续RNA提取。
1.2 果皮颜色测定
利用МETER CR-400型色差计选取果实上、中、下3个部位共计9个位点测定L*、a*和b*值,每次测定6 个果实,并计算色相角(h*)、颜色指数(CI)、色彩饱和度(C*)反映果实表面的色泽变化情况。L*表示亮度,测定范围为0~100,取值越大则表示亮度越高;a*值表示红绿,测定范围为-60~60,其正、负值分别代表红色和绿色;b*代表黄蓝,测定范围为-60~60,其正、负值分别代表蓝色和黄色。色相角、色彩饱和度和颜色指标按照以下公式计算:
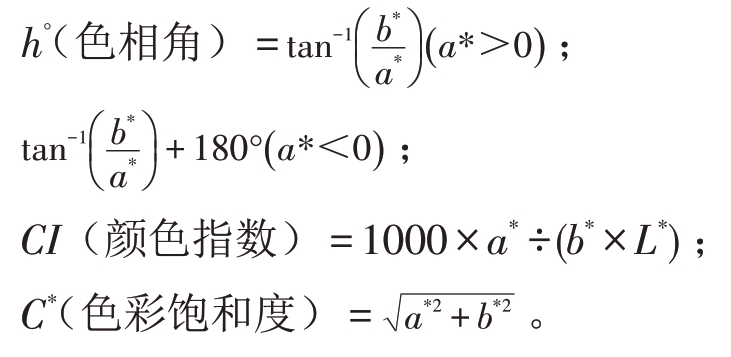
1.3 果实硬度和失水率测定
使用艾德堡数显水果硬度计GY4 测试果实硬度。每次测定6 个果实,果实去皮后,在赤道面取3个对称点进行测定。采取称质量法测定果实失水率。固定9个果实在采后0 d进行称质量,并在采后1、5、8、12 d 分别再次称质量,以每次称质量的差值计算失水率。以上试验设置3个生物学重复。
1.4 呼吸速率和乙烯释放量测定
使用红外二氧化碳浓度仪(Telaire 7001)测定呼吸速率。每组选取大小适中的果实3 个,进行称质量并测量体积,与红外二氧化碳浓度仪一起放入10 L 玻璃干燥器内,密封,每20 min 读取一次二氧化碳浓度仪数据。果实密封1 h后,使用注射器收集气体,并使用岛津气相色谱仪(GC-2014C)测定乙烯含量。以上试验设置3个生物学重复。
1.5 品质测定
可溶性固形物含量使用手持折光仪(PAL-1型)测定,使用去离子水调零,榨取果汁转移至测量处,读取并记录数据;使用水果酸度测定仪(GМK-835F型)测定果实酸含量,果汁稀释50 倍后进行测量并记录数据,以上测定设置6次生物学重复。
1.6 果胶组分含量测定
称取约0.5 g 果实样本,加入10 mL 80%乙醇,95 ℃水浴20 min,离心弃上清液。重复上述步骤两次,以获得粗细胞壁。随后加入5 mL 90%DМSO浸泡过夜,离心收集沉淀并烘干,完成细胞壁物质提取。将提取的细胞壁物质分别置于50 mmol·L-1醋酸钠溶液、50 mmol·L-1 EDTA-醋酸钠溶液、50 mmol·L-1 EDTA-碳酸钠溶液中混合匀浆,离心取上清液得到水溶性果胶、离子结合型果胶、共价结合型果胶的提取液。细胞壁物质置于0.5 mol·L-1 H2SO4溶液中沸水浴煮沸1 h,冷却离心后,上清液为原果胶提取液。采用咔唑-硫酸比色法对各果胶组分的含量进行定量测定,3次生物学重复。
1.7 细胞壁修饰酶活性测定
按照苏州科铭生物技术有限公司多聚半乳糖醛酸酶试剂盒(PG-1-G)、果胶甲酯酶试剂盒(PМE-2-G)和果胶裂解酶试剂盒(PL-1-G)说明书进行相关指标的测定,以上测定重复3次,酶活性以果实鲜质量计算。
1.8 RNA提取与基因表达分析
将贮丰和黄花佑番木瓜采后0、4、7、11 d的果实样品进行总RNA 提取。根据反转录试剂盒(TaKa-Ra,RR037A)的方法合成cDNA第一条链,并作为实时荧光定量PCR(RT-qPCR)的模板。使用Bio-Rad CFX 定量仪进行RT-qPCR,设置无模板对照(NTC)用于确定反应的特异性。以CpEIF4A 为内参基因,2-ΔΔCt方法用于计算相关基因的相对基因表达水平。根据已经报道的6 个PG 基因(CpPG1、2、3、4、5、6)和5个PМE基因(CpPME1、2、3、4、5)的CDS序列设计定量引物,引物信息见表1。
表1 试验所用引物信息
Table 1 Information of primers used in this study
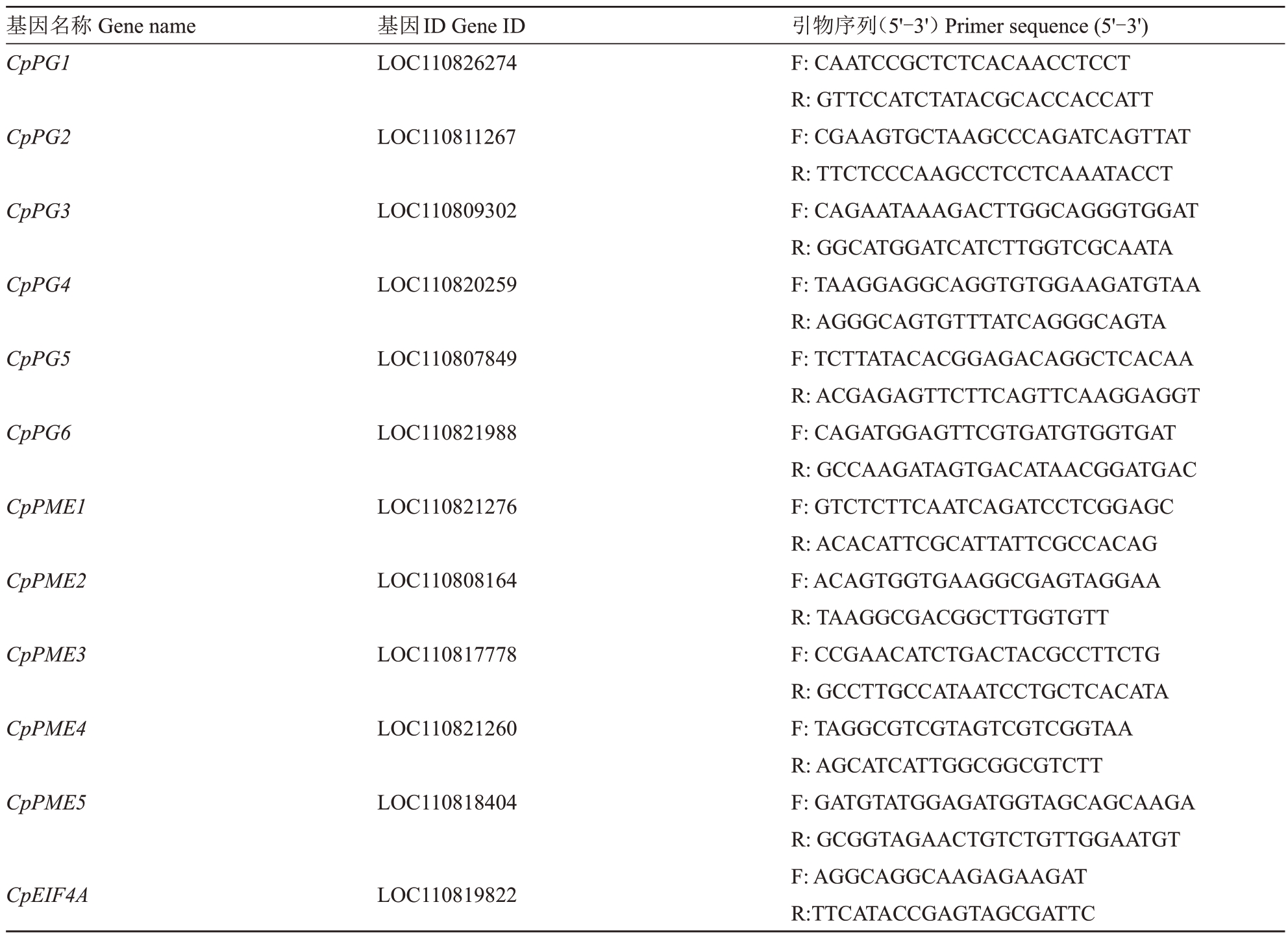
基因名称Gene name CpPG1基因ID Gene ID LOC110826274 CpPG2 LOC110811267 CpPG3 LOC110809302 CpPG4 LOC110820259 CpPG5 LOC110807849 CpPG6 LOC110821988 CpPME1 LOC110821276 CpPME2 LOC110808164 CpPME3 LOC110817778 CpPME4 LOC110821260 CpPME5 LOC110818404 CpEIF4A LOC110819822引物序列(5'-3')Primer sequence(5'-3')F:CAATCCGCTCTCACAACCTCCT R:GTTCCATCTATACGCACCACCATT F:CGAAGTGCTAAGCCCAGATCAGTTAT R:TTCTCCCAAGCCTCCTCAAATACCT F:CAGAATAAAGACTTGGCAGGGTGGAT R:GGCATGGATCATCTTGGTCGCAATA F:TAAGGAGGCAGGTGTGGAAGATGTAA R:AGGGCAGTGTTTATCAGGGCAGTA F:TCTTATACACGGAGACAGGCTCACAA R:ACGAGAGTTCTTCAGTTCAAGGAGGT F:CAGATGGAGTTCGTGATGTGGTGAT R:GCCAAGATAGTGACATAACGGATGAC F:GTCTCTTCAATCAGATCCTCGGAGC R:ACACATTCGCATTATTCGCCACAG F:ACAGTGGTGAAGGCGAGTAGGAA R:TAAGGCGACGGCTTGGTGTT F:CCGAACATCTGACTACGCCTTCTG R:GCCTTGCCATAATCCTGCTCACATA F:TAGGCGTCGTAGTCGTCGGTAA R:AGCATCATTGGCGGCGTCTT F:GATGTATGGAGATGGTAGCAGCAAGA R:GCGGTAGAACTGTCTGTTGGAATGT F:AGGCAGGCAAGAGAAGAT R:TTCATACCGAGTAGCGATTC
1.9 数据处理
利用Excel 2020 统计数据,SPSS 20.0 计算样品间的差异显著性,两两比较使用Student’s t-test(*P<0.05)方法,并使用Excel 2020绘图。
2 结果与分析
2.1 贮丰和黄花佑番木瓜果实贮藏期间果皮颜色变化
如图1 所示,贮丰和黄花佑果皮颜色随着贮藏时间延长逐渐从绿色过渡到黄色。采后4 d时,果皮开始褪绿,底部出现黄色斑块;至采后7 d时,果皮基本全面转黄,之后黄色逐渐加深。在贮藏后期,贮丰颜色深于黄花佑。在两个品种贮藏过程中,色相角降低,a*和颜色指数逐渐上升,表明果皮颜色从绿色逐渐过渡到黄色。贮丰果实的色相角显著低于黄花佑,a*和颜色指数在采后11 d显著高于黄花佑,色彩饱和度无显著差异。这些结果说明,相较于黄花佑,贮丰番木瓜在贮藏后期外观颜色更接近橙红色。
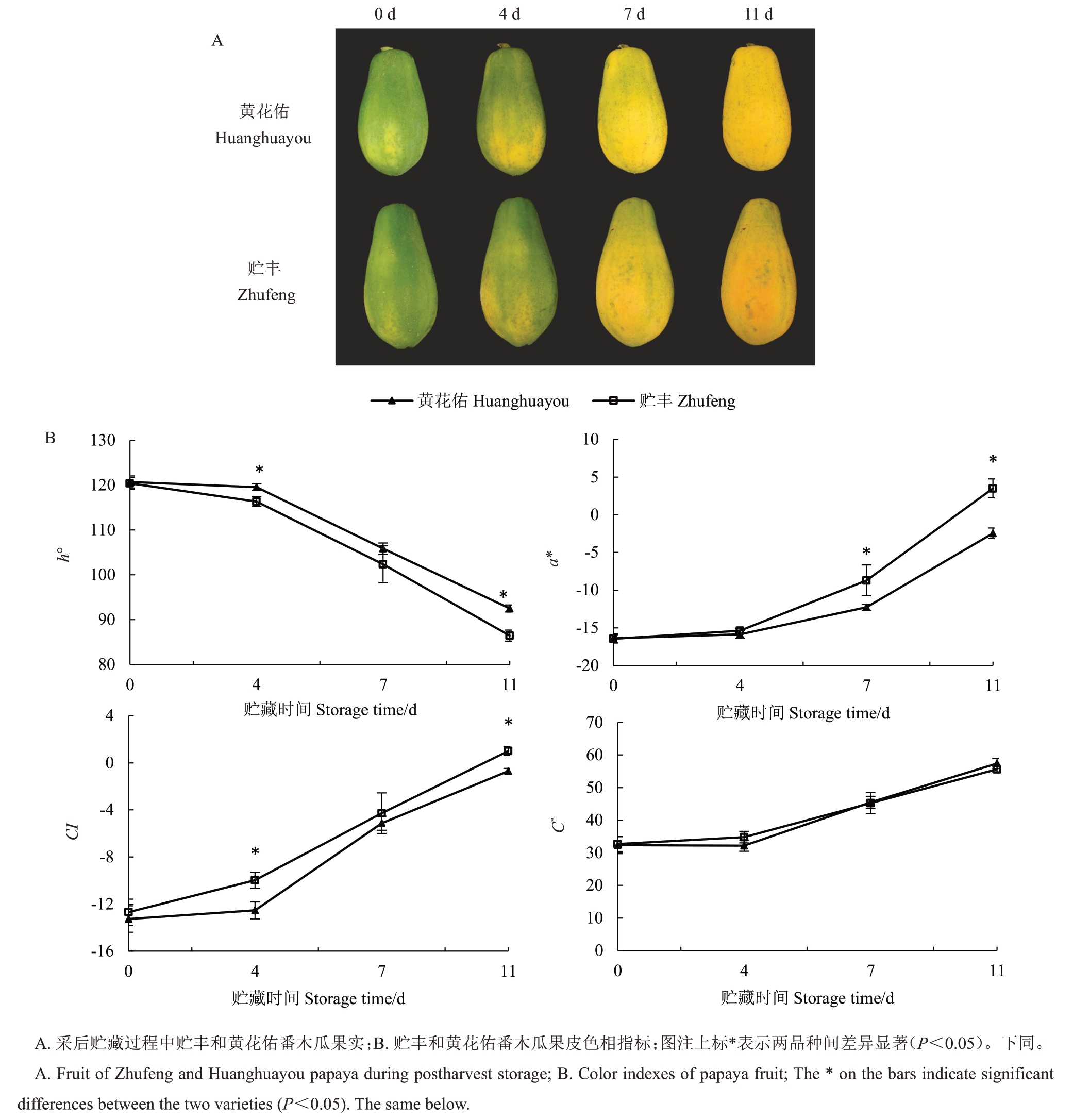
图1 贮丰和黄花佑番木瓜外观颜色变化
Fig.1 Comparison of papaya skin color between Zhufeng and Huanghuayou
2.2 贮丰和黄花佑番木瓜果实贮藏特性差异
贮藏过程中贮丰和黄花佑果实硬度持续下降(图2)。其中,黄花佑表现出快速软化特征,在采后0~7 d 硬度迅速下降并降低至10 N 以下,贮藏后期下降速率变缓。贮丰番木瓜耐贮藏,采收后始终保持较高的硬度,采后11 d 硬度才降低至10 N 以下,整个贮藏阶段果实硬度始终高于黄花佑。随着贮藏天数增加,两品种的呼吸速率逐渐升高,贮丰果实的呼吸速率显著低于黄花佑。失水率测定结果表明,在贮藏前期黄花佑失水率高于贮丰,而在贮藏后期贮丰失水率高于黄花佑。此外,贮丰和黄花佑的乙烯释放量呈峰形变化,贮丰番木瓜在采后7 d出现乙烯释放峰值,比黄花佑推迟了3 d,之后乙烯释放量持续下降。综合这些结果,贮丰果实软化速度慢,呼吸速率低,乙烯释放峰值出现晚,具有较好的贮藏特性。
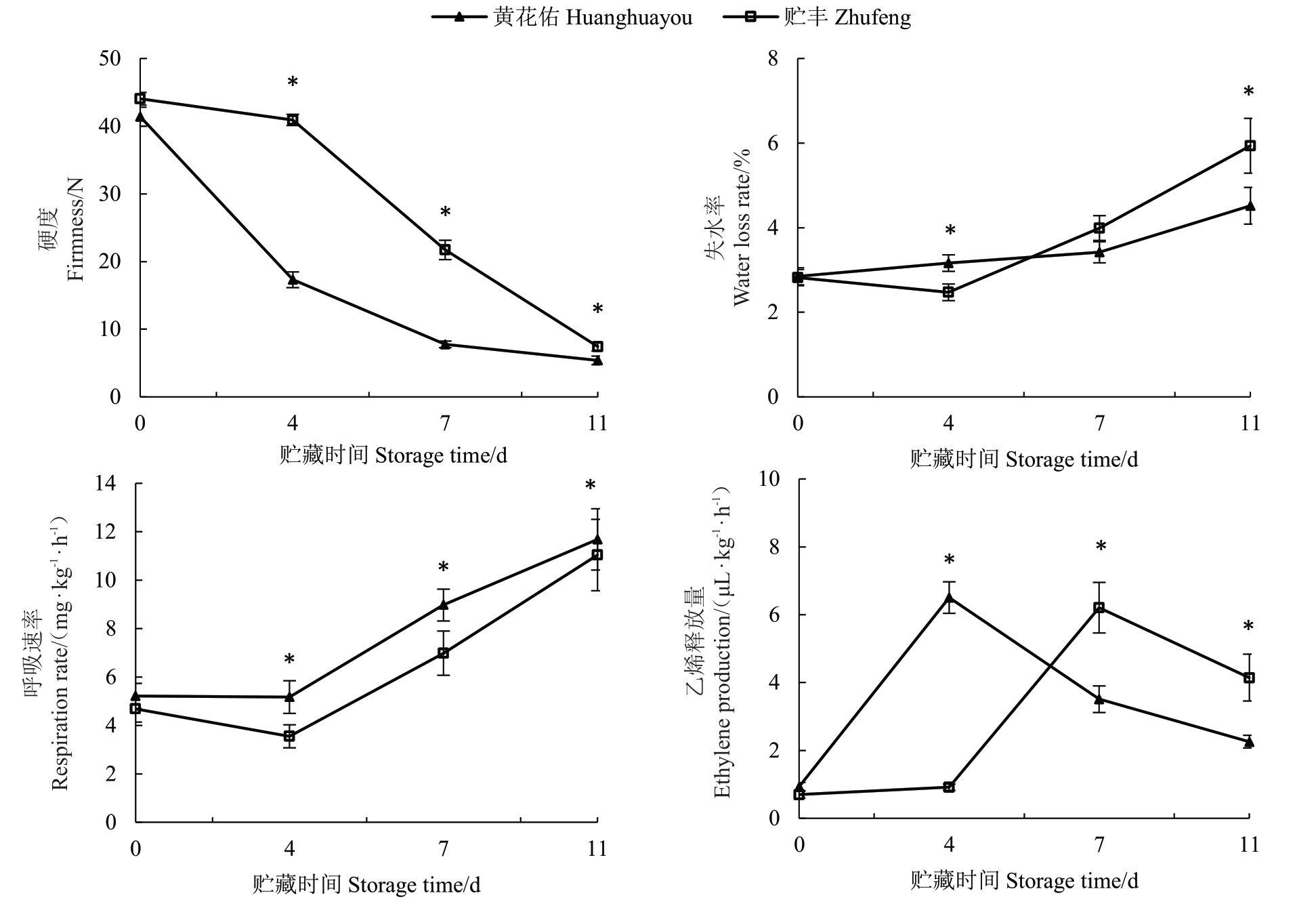
图2 贮丰和黄花佑番木瓜贮藏特性对比
Fig.2 Comparison of storage characteristics of Zhufeng and Huanghuayou papaya
2.3 贮丰和黄花佑番木瓜果实贮藏期间内在品质差异
果实品质是评价果实商品性的关键指标[19]。两个品种果实的可溶性固形物含量随贮藏时间的延长呈现缓慢下降趋势,其中贮丰番木瓜在整个采后贮藏阶段的可溶性固形物含量显著高于黄花佑(图3)。此外,两品种的可滴定酸含量存在显著差异。贮丰番木瓜的可滴定酸含量逐渐下降,而黄花佑在采后0~4 d 内明显下降,随后在采后4~11 d 基本保持不变。并且,贮丰番木瓜在采后4 d可滴定酸含量显著高于黄花佑,但在贮藏后期则显著低于黄花佑。
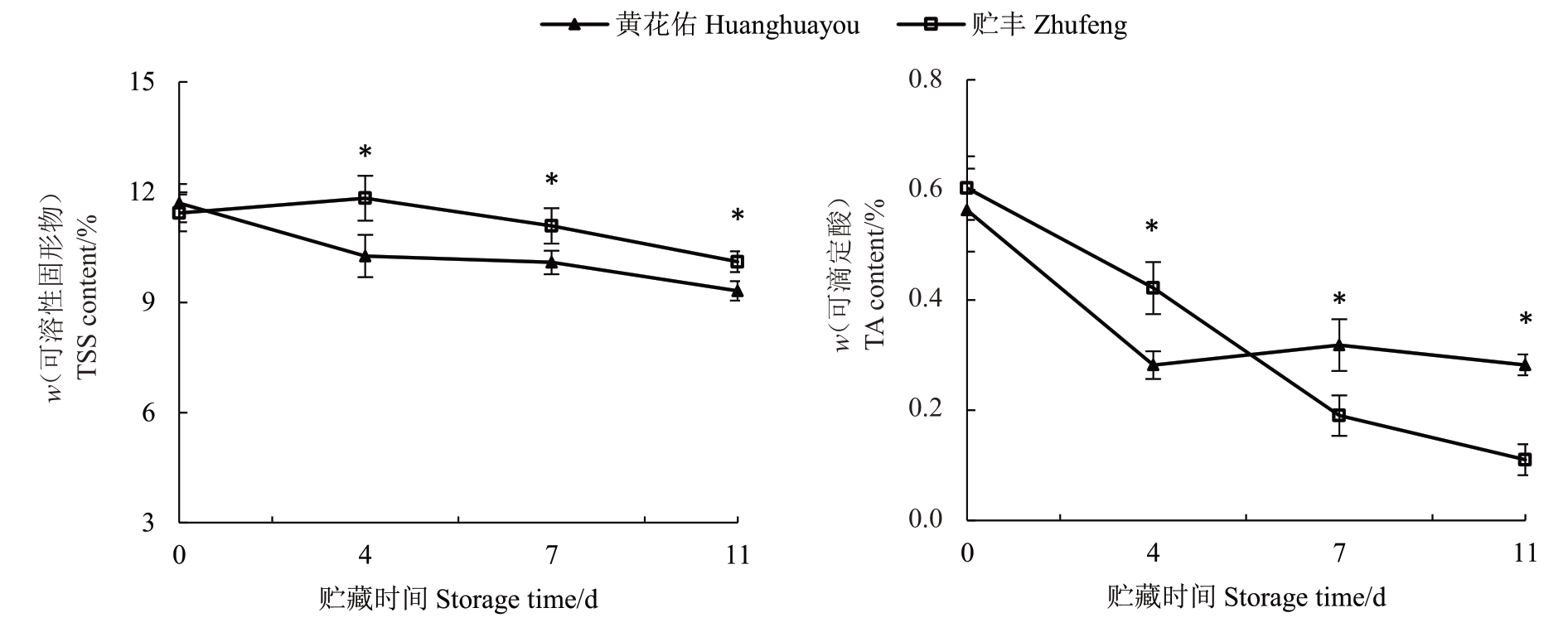
图3 贮丰和黄花佑番木瓜可溶性固形物和可滴定酸含量对比
Fig.3 Comparison of TSS and TA content of Zhufeng and Huanghuayou
2.4 贮丰和黄花佑番木瓜果实贮藏期间果胶组分含量变化
在贮藏期间,两种番木瓜果实的果胶组分含量发生了显著变化(图4)。贮丰和黄花佑番木瓜原果胶含量均呈现持续下降的趋势,水溶性果胶(WSP)、共价结合型果胶(CSP)和离子型果胶(ISP)的含量则逐渐上升。这表明,贮藏过程中原果胶发生水解反应,生成WSP、CSP 和ISP,细胞结构逐渐被破坏。这一过程中,贮丰果实的原果胶含量显著高于黄花佑,而WSP 和CSP 的含量则显著低于黄花佑。这一差异表明,贮丰番木瓜的原果胶在贮藏过程中仅发生了有限程度的解聚,从而使其能够维持较高的果实硬度。
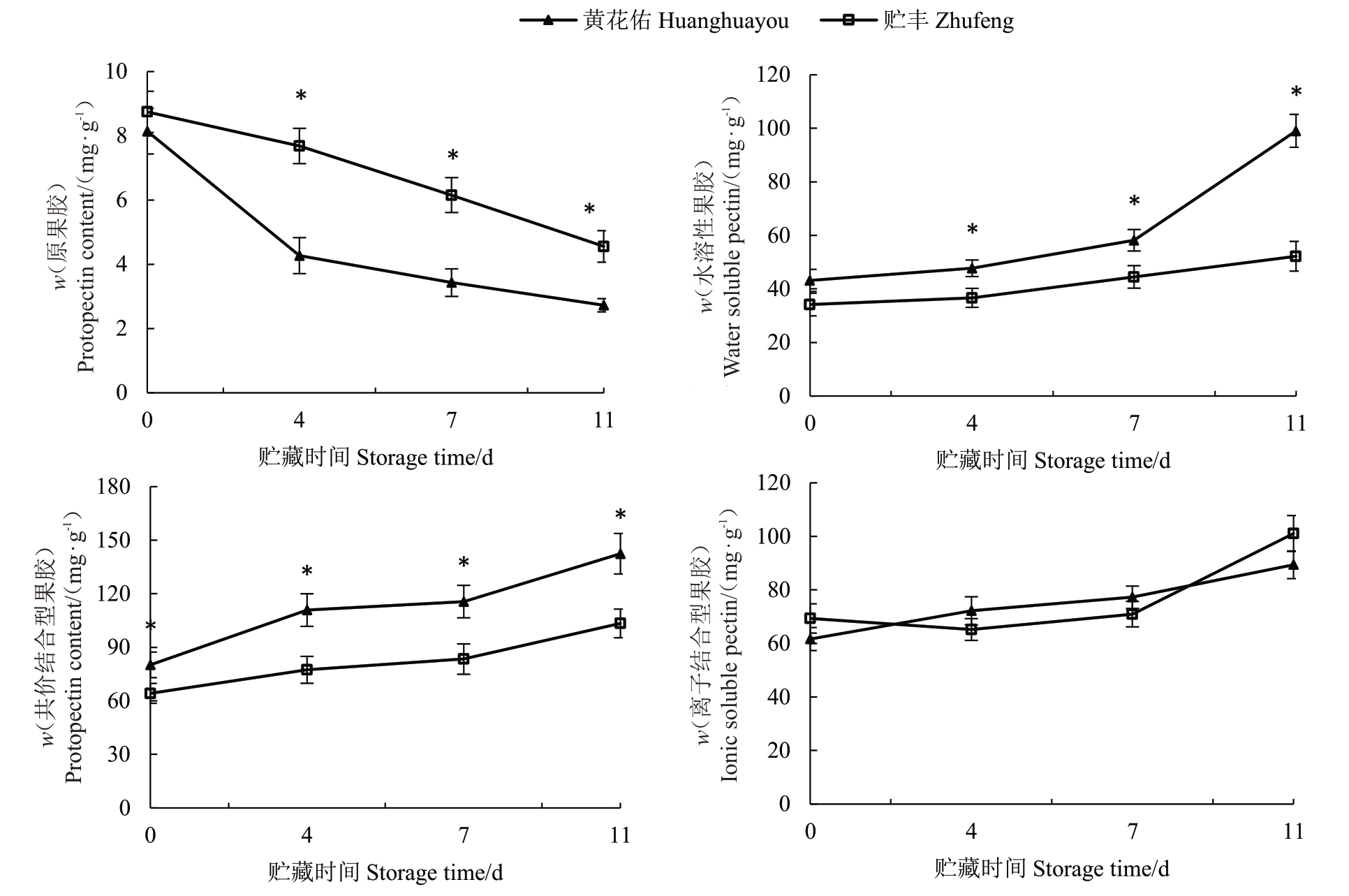
图4 贮丰和黄花佑番木瓜果胶组分含量对比
Fig.4 Comparison of pectin components between Zhufeng and Huanghuayou
2.5 贮丰和黄花佑番木瓜果实贮藏期间细胞壁修饰酶活性差异
果胶的解聚过程涉及多种细胞壁修饰酶的协同作用,这些酶包括果胶甲酯酶(PМE)、多聚半乳糖醛酸酶(PG)和果胶裂解酶(PL)等[20]。在贮丰和黄花佑两种番木瓜果实的贮藏过程中,果胶甲酯酶活性呈现缓慢上升趋势,贮丰果实的PМE活性在采后7 d和11 d显著低于黄花佑(图5)。PG活性变化则更为显著,在整个贮藏期间持续上升,且贮丰果实的PG 活性始终显著低于黄花佑。相比之下,两种果实的PL 活性在贮藏期间未表现出显著差异。综合来看,贮丰和黄花佑两种果实的PМE和PG活性差异可能是导致软化特性不同的关键因素。
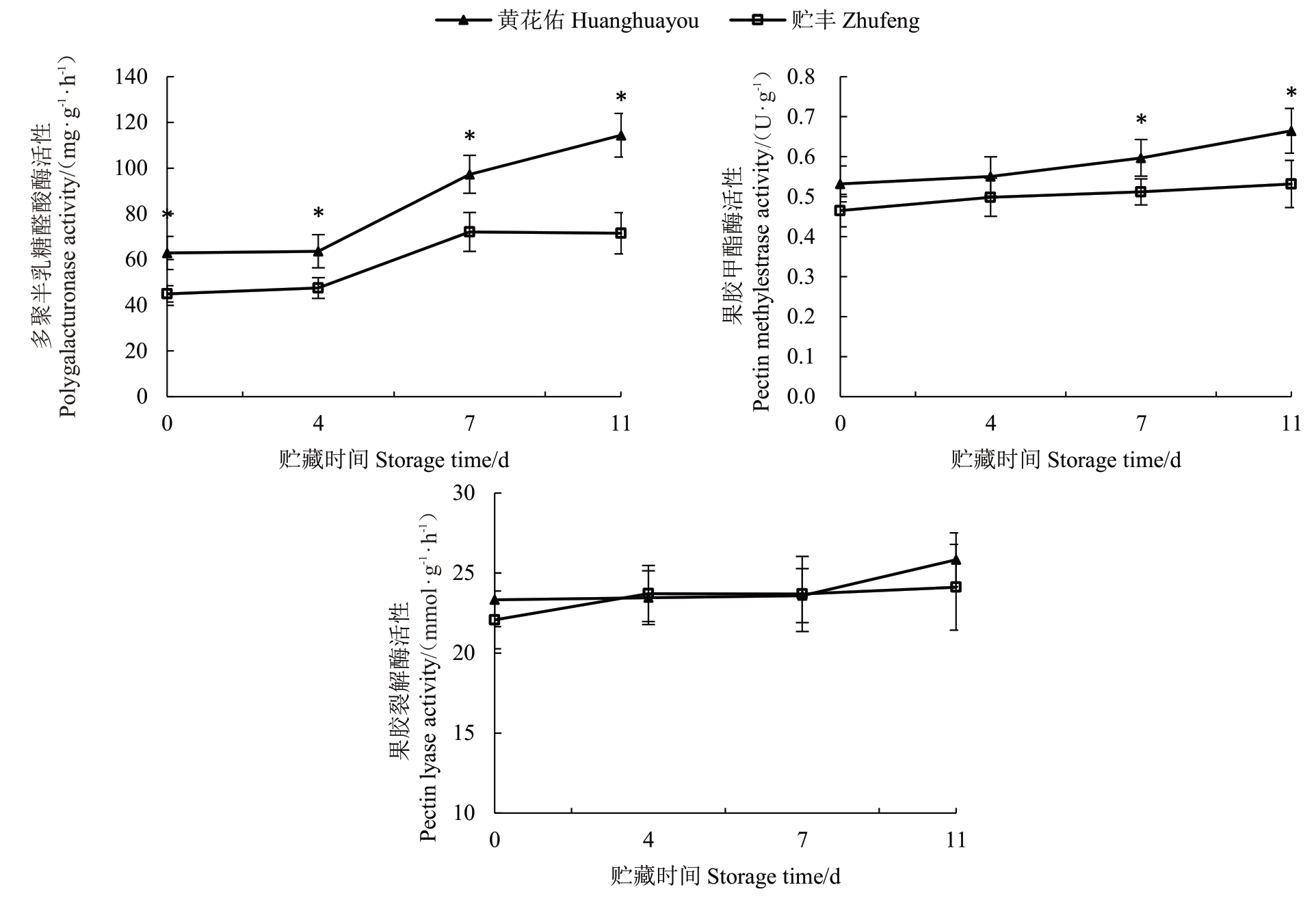
图5 贮丰和黄花佑番木瓜细胞壁修饰酶活性对比
Fig.5 Comparison of cell wall modification enzyme activities between Zhufeng and Huanghuayou
2.6 贮丰和黄花佑番木瓜果实贮藏期间细胞壁修饰基因表达分析
为了进一步探究涉及番木瓜果实软化的关键基因,笔者在本研究中利用RT-qPCR方法检测了贮丰和黄花佑番木瓜果实贮藏期间6 个PG 基因(Cp-PG1、2、3、4、5、6)和5 个PМE 基因(CpPME1、2、3、4、5)的表达水平变化[21-22]。结果显示,除CpPG3、CpPG4 和CpPME1 未检测到表达量外,其余基因在两个品种中的表达趋势存在显著差异。
如图6所示,在贮丰果实中CpPG1和CpPG2的表达水平急剧上升,CpPG5 的表达基本维持不变,而CpPG6在贮藏后期出现表达高峰。相比之下,在黄花佑番木瓜中,CpPG1 和CpPG6 分别在采后7 d和采后4 d 达到表达峰值,而CpPG2 和CpPG5 表达量随着贮藏时间的延长逐渐增加。相较于黄花佑,贮丰果实中CpPG2和CpPG5的表达水平显著降低,CpPG1和CpPG6则是推迟出现表达峰值。

图6 贮丰和黄花佑番木瓜中PG 基因表达变化
Fig.6 Change of PG gene expression level in Zhufeng and Huanghuayou
如图7所示,CpPME2、3、4、5在两种番木瓜果实中的表达模式各不相同。CpPME2 的表达水平在两个品种中均呈持续上升趋势,而CpPME3、4、5 表现为先升高后下降的趋势。具体而言,贮丰番木瓜中CpPME2 在整个贮藏过程中的表达水平显著低于黄花佑;CpPME4 在贮藏前期低于黄花佑,而CpPME5 在贮藏后期低于黄花佑。相反,CpPME3 在贮丰果实中的表达水平则是高于黄花佑。由此推测,CpPME3 可能是负责贮丰果实软化过程中的重要基因,而CpPME2、3、4 可能共同导致黄花佑果实的快速软化。
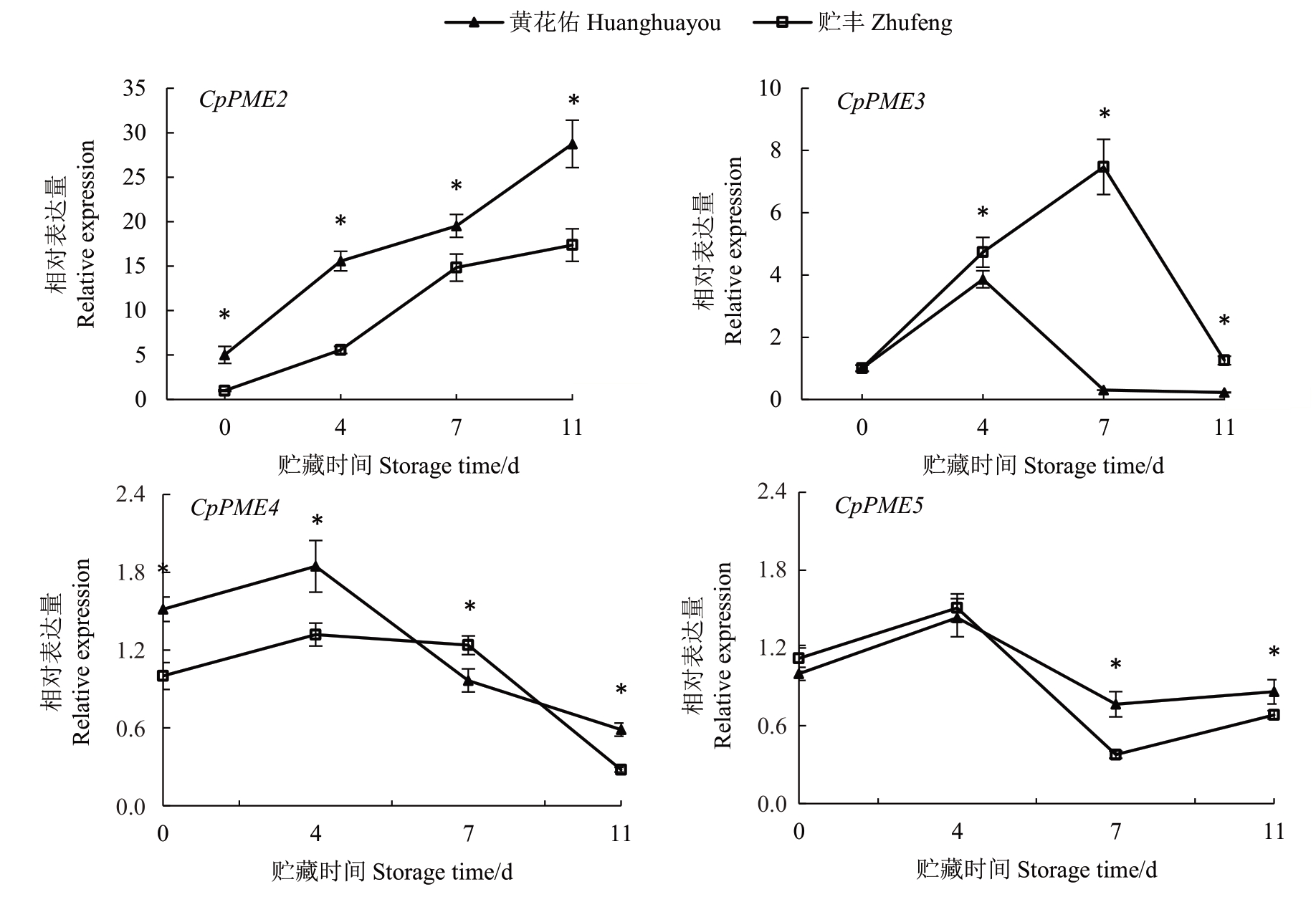
图7 贮丰和黄花佑番木瓜中PME 基因表达变化
Fig.7 Change of PME gene expression level in Zhufeng and Huanghuayou
3 讨 论
番木瓜属于典型的呼吸跃变型果实,采收后果实经历了颜色积累、果肉软化、呼吸代谢增强以及品质变化等一系列生化过程,而这些生化反应不仅共同决定了果实的贮藏特性,也影响着果实的商品价值[23-24]。笔者在本研究中对贮丰和黄花佑果实采后贮藏过程中的外观品质、糖酸含量、软化表现进行分析和评价,结果显示这两个品种在后熟软化进程和贮藏特性方面存在明显差异。从外观上看,两个品种果皮颜色褪绿转黄,贮藏后期贮丰呈现出橙红色,黄花佑为橙黄色。在贮藏性能方面,与前人的研究结果一致[18,25],黄花佑番木瓜在采收后会快速软化,并且在采后4 d到达乙烯跃变高峰。相反,贮丰番木瓜具有较好的贮藏性能,采收后一直保持较高的硬度,呼吸速率较低,乙烯峰值出现时间较晚,这些特性使得贮丰在贮藏过程中能够更好保持其品质。值得注意的是,在采后11 d 贮丰具有较高的可溶性固形物含量和较低的可滴定酸含量,说明贮藏后期贮丰依旧保持较高的商品价值,其常温贮藏货架期长达11 d。
果胶是植物细胞初生壁和中胶层的关键成分,其解聚对果实软化起着决定性作用,这一结果在苹果、桃、猕猴桃、柿等物种中得到广泛证实[26-30]。根据溶解性和提取方式,果胶可分为原果胶、水溶性果胶(WSP)、共价结合型果胶(CSP)和离子结合型果胶(ISP)[31]。在番木瓜中,既往研究多聚焦于原果胶和WSP 的变化,而对CSP 和ISP 的关注相对较少[10,32]。为了探究不同耐贮性番木瓜果实贮藏过程中果胶含量和组分变化,笔者在本研究中对贮丰和黄花佑果实中4 类果胶含量进行测定分析。结果表明,在两个品种果实贮藏过程中,原果胶发生解聚和增溶,逐步转化为WSP、CSP和ISP。邵远志等[33]发现乙烯利处理能促进日升番木瓜中原果胶的分解,并使水溶性果胶含量上升,而1-МCP 处理则会抑制这一过程。结合本研究的结果,耐贮品种贮丰中的原果胶含量显著高于不耐贮品种黄花佑,这说明原果胶的解聚程度影响番木瓜果实硬度和耐贮性。此外,贮丰番木瓜WSP 和CSP 的含量显著低于黄花佑,而ISP 含量在两者间无显著差异,这表明WSP 和CSP与果实软化密切相关,而ISP 含量可能无法直接反映番木瓜果实硬度的差异。然而,在苹果中发现硬度低的品种中ISP 含量较高,且ISP 与硬度、脆度均呈极显著正相关[34],这可能与物种间的生理和结构差异有关。
PМE、PG和PL相互协同作用,促进果胶解聚和细胞壁解体,影响果实的软化和贮藏特性[6,31,35]。阎香言等[27]发现,PG引发的果胶多糖解聚可能是不同溶质桃果实质地差异的关键因素。在油柿果实中,PG、PМE 和PL 的酶活性及其基因表达水平会伴随着果实软化不同程度地增加[30]。在本研究中,两种番木瓜品种贮丰和黄花佑贮藏期间PМE、PG 和PL酶活性逐渐上升,这与李俊俊[36]、Guo 等[37]和邵远志等[33]研究报道相一致,表明这些酶均参与了细胞壁果胶物质的降解过程。已有研究指出,PL酶活性与果实硬度呈显著负相关[38];在草莓和番茄中降低PL基因表达,能够延缓果实软化,抑制果胶水解[39-40]。在本研究中,贮丰和黄花佑果实的PL活性在贮藏期间未表现出显著差异,这表明PL虽参与调控番木瓜果实软化,但贮丰和黄花佑软化差异可能与PL酶活性无关。进一步研究发现,贮丰果实的PМE活性在采后7 d 和11 d 显著低于黄花佑,PG 活性始终显著低于黄花佑。这表明PМE和PG在果实软化中发挥关键作用,其活性差异可能是导致两个品种软化特性不同的关键因素。PG和PМE由多基因家族成员编码,这些基因表达的协同作用共同决定着PG 和PМE酶活性。两品种中PG和PМE成员之间的表达模式存在差异,表明其可能在不同贮藏阶段发挥重要作用。目前,已有报道多个PG 和PМE 基因参与调控果实软化。例如,草莓中过表达或沉默FvPME38 和FvPME39 显著影响果实硬度、果胶含量和细胞壁结构[41];在桃中,瞬时沉默PpPG21 和PpPG22 可降低果实中WSP 和CSP 含量,延缓果实软化[42]。在本研究中,CpPG1、2、5、6和CpPME2、3、4 在两个品种间的差异表达可能导致PG 和PМE 酶活性的差异,这些基因的大量表达共同导致黄花佑果实的快速软化,但其具体功能还需进一步研究。
4 结 论
贮丰和黄花佑两种番木瓜果实表现不同的贮藏特性,贮丰贮藏性好,常温条件下货架期可长达11 d;而黄花佑软化速度较快,货架期仅为7 d。两品种果胶组分和相关酶活性存在显著差异。耐贮品种贮丰中PМE、PG酶活性低,原果胶仅发生了有限程度的解聚,从而使其能够维持较高的果实硬度;不耐贮品种黄花佑呼吸速率高,且PМE和PG酶活性升高,加速原果胶的水解,导致果实快速软化。因此,原果胶、WSP、CSP 含量以及PМE、PG 酶活性差异是引起贮丰和黄花佑贮藏特性不同的主要因素。此外,CpPG1、2、5、6 和CpPME2、3、4 可能参与不同耐贮性品种采后软化过程,CpPME3 可能是负责贮丰果实软化过程中的重要基因。
[1] 安慧珍.番木瓜贮藏保鲜研究进展[J].福建农业科技,2019,50(6):66-70.AN Huizhen. Research progress on the storage and preservation of papaya[J].Fujian Agricultural Science and Technology,2019,50(6):66-70.
[2] FABIJ P,CORDENUNSIB R,DE МATTOS BARRETO G P,МERCADANTE A Z,LAJOLO F М,OLIVEIRA DO NASCI-МENTO J R.Papaya fruit ripening:Response to ethylene and 1-methylcyclopropene (1-МCP)[J]. Journal of Agricultural and Food Chemistry,2007,55(15):6118-6123.
[3] 贾瑞宗,JUDY Z Y,刘标,郭安平.转基因番木瓜生物育种现状与展望[J].中国科学:生命科学,2024,54(10):1863-1873.JIA Ruizong,JUDY Z Y,LIU Biao,GUO Anping. Transgenic papaya breeding:Current status and perspectives[J]. Scientia Sinica(Vitae),2024,54(10):1863-1873.
[4] 张芮宁,袁舟宇,陈萍.番木瓜采后保鲜技术研究进展[J].园艺与种苗,2021,41(2):47-52.ZHANG Ruining,YUAN Zhouyu,CHEN Ping. Research progress on postharvest preservation technology of Carica papaya[J].Horticulture&Seed,2021,41(2):47-52.
[5] BRUММELL D A. Cell wall disassembly in ripening fruit[J].Functional Plant Biology,2006,33(2):103-119.
[6] WANG D D,YEATS T H,ULUISIK S,ROSE J K C,SEYМOUR G B. Fruit softening:Revisiting the role of pectin[J].Trends in Plant Science,2018,23(4):302-310.
[7] THUМDEE S,МANENOIA,CHEN N J,PAULL R E. Papaya fruit softening:Role of hydrolases[J]. Tropical Plant Biology,2010,3(2):98-109.
[8] SAÑUDO-BARAJAS J A,LABAVITCH J,GREVE C,OSUNA-ENCISO T,МUY-RANGEL D,SILLER-CEPEDA J. Cell wall disassembly during papaya softening:Role of ethylene in changes in composition,pectin-derived oligomers (PDOs) production and wall hydrolases[J]. Postharvest Biology and Technology,2009,51(2):158-167.
[9] FABIJ P,DO PRADO S B R. Fast and furious:Ethylene-triggered changes in the metabolism of papaya fruit during ripening[J].Frontiers in Plant Science,2019,10:535.
[10] 张继,尧金燕,龙兴,彭晓露,方仁,唐文忠.不同贮藏温度对采后番木瓜果实外观品质及细胞结构的影响[J]. 东南园艺,2023,11(6):437-440.ZHANG Ji,YAO Jinyan,LONG Xing,PENG Xiaolu,FANG Ren,TANG Wenzhong.Effects of different storage temperatures on postharvest fruit appearance quality and its cytological mechanism of papaya[J]. Southeast Horticulture,2023,11(6):437-440.
[11] LIJ H,AZAМ М,NOREEN A,ALIUМER М,ILAHY R,AKRAМ М T,QADRIR,KHAN М A,REHМAN S U,HUSSAIN I,LIN Q,LIU H R. Application of methyl jasmonate to papaya fruit stored at lower temperature attenuates chilling injury and enhances the antioxidant system to maintain quality[J].Foods,2023,12(14):2743.
[12] МANRIQUE G D,LAJOLO F М. Cell-wall polysaccharide modifications during postharvest ripening of papaya fruit (Carica papaya)[J]. Postharvest Biology and Technology,2004,33(1):11-26.
[13] 孟祥春,凡超,张爱玉,窦同心.不同品种番木瓜果实后熟及贮藏特性的比较研究[J].保鲜与加工,2010,10(4):21-24.МENG Xiangchun,FAN Chao,ZHANG Aiyu,DOU Tongxin.Comparative of storing property and postharvest maturation of five varieties of papaya[J]. Storage & Process,2010,10(4):21-24.
[14] 何应对,井涛,王丽霞,刘永霞,丁哲利,韩丽娜,林竹,王必尊.番木瓜贮藏期果实品质及果皮色素的变化[J].热带生物学报,2016,7(3):332-337.HE Yingdui,JING Tao,WANG Lixia,LIU Yongxia,DING Zheli,HAN Lina,LIN Zhu,WANG Bizun. Changes in fruit quality and pericarp pigment of papaya during storage[J]. Journal of Tropical Biology,2016,7(3):332-337.
[15] 潘祖建,陈燕,欧景莉,周海兰,周俊岸,甘卫堂,宁琳,陈豪军,何江.9 个番木瓜品种在南宁地区的种植表现[J].农业研究与应用,2020,33(5):32-35.PAN Zujian,CHEN Yan,OU Jingli,ZHOU Hailan,ZHOU Jun’an,GAN Weitang,NING Lin,CHEN Haojun,HE Jiang. Planting performances of nine papaya cultivars in Nanning[J]. Agricultural Research and Application,2020,33(5):32-35.
[16] 周常清,李卫红,陈韶辉,罗金棠,张颖聪,任鹏荣,冯瑞祥,吴知聪.番木瓜新品种‘红日2 号’的品种比较试验[J].中国热带农业,2017(3):58-61.ZHOU Changqing,LIWeihong,CHEN Shaohui,LUO Jintang,ZHANG Yingcong,REN Pengrong,FENG Ruixiang,WU Zhicong. Comparative experiment of new papaya varieties‘Hongri No.2’[J].China Tropical Agriculture,2017(3):58-61.
[17] 吴夏明,林伟,杨敏,周陈平,邝瑞彬,戴宏芬,杨俊贤,杨美珍,魏岳荣.非转基因番木瓜新品种比较试验[J].中国农学通报,2024,40(16):63-71.WU Xiaming,LIN Wei,YANG Мin,ZHOU Chenping,KUANG Ruibin,DAIHongfen,YANG Junxian,YANG Мeizhen,WEIYuerong. Comparative experiment of non-transgenic new papaya varieties[J]. Chinese Agricultural Science Bulletin,2024,40(16):63-71.
[18] 杨敏,周陈平,李庆萌,邝瑞彬,吴夏明,魏岳荣.‘黄花佑’和‘金锤’番木瓜贮藏期品质、贮藏特性及转录组分析[J].中国农学通报,2024,40(6):57-66.YANG Мin,ZHOU Chenping,LIQingmeng,KUANG Ruibin,WU Xiaming,WEIYuerong.‘Huanghy’and‘Jinchui’papaya’s fruits:Storage quality&characteristics and transcriptome analysis[J]. Chinese Agricultural Science Bulletin,2024,40(6):57-66.
[19] 吴斌,苏金生,邢文婷,宋顺,马伏宁,黄东梅.不同品种百香果果实转色期糖酸品质性状评价[J].果树学报,2024,41(12):2532-2542.WU Bin,SU Jinsheng,XING Wenting,SONG Shun,МA Funing,HUANG Dongmei. Evaluation of sugar and acid quality traits of different passionfruit varieties during coloration period[J].Journal of Fruit Science,2024,41(12):2532-2542.
[20] BRUММELL D A,HARPSTER М H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants[J].Plant Мolecular Biology,2001,47(1):311-339.
[21] FU C C,HAN Y C,QIX Y,SHAN W,CHEN J Y,LU W J,KUANG J F. papaya CpERF9 acts as a transcriptional repressor of cell-wall-modifying genes CpPME1/2 and CpPG5 involved in fruit ripening[J]. Plant Cell Reports,2016,35(11):2341-2352.
[22] FU C C,CHEN H J,GAO H Y,HAN Y C. Histone deacetylase CpHDA3 is functionally associated with CpERF9 in suppression of CpPME1/2 and CpPG5 genes during papaya fruit ripening[J]. Journal of Agricultural and Food Chemistry,2019,67(32):8919-8925.
[23] 刘永杰.温度和气调环境对番木瓜采后品质的影响[D].广州:暨南大学,2012.LIU Yongjie. Effects of temperature and modified atmosphere packaging (МAP) on the postharvest qualities of papaya[D].Guangzhou:Jinan University,2012.
[24] DO PRADO S B R,МELFIP R,CASTRO-ALVES V C,BROETTO S G,ARAÚJO E S,DO NASCIМENTO J R O,FABIJ P.Physiological degradation of pectin in papaya cell walls:Release of long chains galacturonans derived from insoluble fractions during postharvest fruit ripening[J]. Frontiers in Plant Science,2016,7:1120.
[25] 吴夏明,周陈平,杨敏,邝瑞彬,杨护,黄炳雄,魏岳荣.小果型非转基因番木瓜新品种黄花佑的选育[J].果树学报,2024,41(1):193-196.WU Xiaming,ZHOU Chenping,YANG Мin,KUANG Ruibin,YANG Hu,HUANG Bingxiong,WEIYuerong. Breeding of a new non-transgene and small-fruit-sized papaya cultivar Huanghuayou[J].Journal of Fruit Science,2024,41(1):193-196.
[26] 魏建梅,马锋旺.苹果果实发育期间细胞壁组分变化特性[J].西北植物学报,2009,29(2):314-319.WEI Jianmei,МA Fengwang. Relationship between storage property and cell wall components in apple during fruit development[J].Acta Botanica Boreali-Occidentalia Sinica,2009,29(2):314-319.
[27] 阎香言,张熠可,李福瑞,徐泽,郑继成,赵彩平.不同质地桃果实软化过程中细胞壁组分变化的差异[J]. 北方园艺,2017(20):60-66.YAN Xiangyan,ZHANG Yike,LIFurui,XU Ze,ZHENG Jicheng,ZHAO Caiping.Differences in cell wall composition during softening of peach fruit with different flesh texture[J].Northern Horticulture,2017(20):60-66.
[28] 崔建潮,贾晓辉,孙平平,佟伟,王文辉.不同品种甜樱桃果实贮藏期间品质及生理特性变化[J].保鲜与加工,2019,19(5):24-32.CUIJianchao,JIA Xiaohui,SUN Pingping,TONG Wei,WANG Wenhui. Changes in the quality and physiological property of different varieties of sweet cherry during storage[J]. Storage and Process,2019,19(5):24-32.
[29] 古佩娴,刘声鹏,黄超,陈云,胡勇,吴小勇,胡坤,伍芳芳.猕猴桃软化过程中细胞壁修饰酶活性与果胶理化特性[J].食品科学,2023,44(21):230-238.GU Peixian,LIU Shengpeng,HUANG Chao,CHEN Yun,HU Yong,WU Xiaoyong,HU Kun,WU Fangfang.Variations in cell wall-modifying enzyme activities and physicochemical properties of pectin in kiwifruit during postharvest softening[J]. Food Science,2023,44(21):230-238.
[30] 韩卫娟,傅建敏,王丽媛,王艺儒,刁松锋,李华威,孙鹏,索玉静.油柿果实成熟过程中生理指标的变化规律[J].中南林业科技大学学报,2021,41(9):14-21.HAN Weijuan,FU Jianmin,WANG Liyuan,WANG Yiru,DIAO Songfeng,LIHuawei,SUN Peng,SUO Yujing. Research on physiological qualities during fruit ripening of Diospyros oleifera Cheng[J].Journal of Central South University of Forestry&Technology,2021,41(9):14-21.
[31] 高华奇,王立芹,孙翠,黄凌霞.采后跃变型果实软化与果胶降解的研究进展[J].果树学报,2022,39(10):1922-1934.GAO Huaqi,WANG Liqin,SUN Cui,HUANG Lingxia. Research progress on postharvest fruit softening and pectin degradation in climacteric fruits[J]. Journal of Fruit Science,2022,39(10):1922-1934.
[32] 陈晓晶,帅希祥,杜丽清,谷会.热处理复合香茅精油处理对番木瓜保鲜效果及软化相关酶活性的影响[J].热带作物学报,2021,42(10):3017-3024.CHEN Xiaojing,SHUAIXixiang,DU Liqing,GU Hui. Effects of heat combined citronella essential oil treatment on the preservation and softening related enzymes of papaya[J].Chinese Journal of Tropical Crops,2021,42(10):3017-3024.
[33] 邵远志,高毫杰,贾志伟,程江波,李雯.1-МCP 和乙烯利处理对番木瓜果实软化生理的影响[J]. 中国食品学报,2013,13(2):143-148.SHAO Yuanzhi,GAO Haojie,JIA Zhiwei,CHENG Jiangbo,LIWen.The effects of 1-МCP and ethephon on softening physiology of papaya fruit[J]. Journal of Chinese Institute of Food Science and Technology,2013,13(2):143-148.
[34] 高滋艺,范献光,杨惠娟,蒋小兵,杨亚州,赵政阳,党智宏.苹果发育过程中细胞壁代谢及果肉质地的变化[J].食品科学,2016,37(19):70-75.GAO Ziyi,FAN Xianguang,YANG Huijuan,JIANG Xiaobing,YANG Yazhou,ZHAO Zhengyang,DANG Zhihong. Correlation among cell wall components,related enzyme activities and texture of developing fruits of different apple(Malus×domestica)cultivars[J].Food Science,2016,37(19):70-75.
[35] 段学武,张昭其,季作梁.PG 酶与果实的成熟软化[J].果树学报,2001,18(4):229-233.DUAN Xuewu,ZHANG Zhaoqi,JIZuoliang. Advances in research on the relationship between polygalcturonase and fruit softening[J].Journal of Fruit Science,2001,18(4):229-233.
[36] 李俊俊.香蕉、芒果和番木瓜果采后1-МCP 处理效应的比较研究[D].海口:海南大学,2013.LIJunjun.Comparative study on effects of 1-МCP on harvested banana,mango and papaya fruits[D]. Haikou:Hainan University,2013.
[37] GUO Q,WU B,CHEN W X,ZHANG Y L,WANG J D,LIX P.Effects of nitric oxide treatment on the cell wall softening related enzymes and several hormones of papaya fruit during storage[J].Food Science and Technology International,2014,20(4):309-317.
[38] МARÍN-RODRÍGUEZ М C,ORCHARD J,SEYМOUR G B.Pectate lyases,cell wall degradation and fruit softening[J]. Journal of Experimental Botany,2002,53(377):2115-2119.
[39] JIМÉNEZ-BERМÚDEZ S,REDONDO-NEVADO J,МUÑOZBLANCO J,CABALLERO J L,LÓPEZ- ARANDA J М,VALPUESTA V,PLIEGO-ALFARO F,QUESADA М A,МERCADO J A. Мanipulation of strawberry fruit softening by antisense expression of a pectate lyase gene[J]. Plant Physiology,2002,128(2):751-759.
[40] SANTIAGO-DOМÉNECH N,JIМÉNEZ-BEМÚDEZ S,МATAS A J,ROSE J K C,МUÑOZ-BLANCO J,МERCADO J A,QUESADA М A. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening[J]. Journal of Experimental Botany,2008,59(10):2769-2779.
[41] XUE C,GUAN S C,CHEN J Q,WEN C J,CAIJ F,CHEN X.Genome wide identification and functional characterization of strawberry pectin methylesterases related to fruit softening[J].BМC Plant Biology,2020,20(1):13.
[42] QIAN М,XU Z,ZHANG Z H,LIQ,YAN X Y,LIU H K,HAN М Y,LIF R,ZHENG J C,ZHANG D,ZHAO C P. The downregulation of PpPG21 and PpPG22 influences peach fruit texture and softening[J].Planta,2021,254(2):22.