核桃(Juglans regia L.)属胡桃科胡桃属多年生落叶乔木,具有较高的营养价值和药用价值,是世界四大坚果之一,在全球广泛种植。中国是目前核桃栽培面积和产量最大的国家,华北地区是中国核桃的重要产区,新疆早实核桃占京冀地区核桃种植面积的80%以上[1],其在增加农民收入、产业扶贫、乡村振兴及生态建设等方面发挥了重要作用。
乙烯途径在促进果实成熟的同时,也使果实更容易受到病原菌的侵染[2],受7—8 月份高温高湿气候的影响,核桃病害较重,导致产量和品质下降,其主要果实病害为炭疽病和黑斑病。前人的研究显示,炭疽病是真菌性病害,主要的病原菌为胶孢炭疽菌(Colletotrichum gloeosporioides),8月是核桃炭疽病田间发病的高峰期,炭疽病发病的早晚和轻重与降雨量有关[3-4]。黑斑病为细菌性病害,主要的病原菌为黄单胞杆菌(Xanthomonas Campestris)、成团泛菌(Pantoea agglomerans)和变黄假单胞菌(Pseudomonas flavescens),在高温高湿的多雨季节和年份危害严重[4-6]。当前,核桃果实病害研究多聚焦于病原菌的鉴定与防治[7-9]、栽培对病害的影响[10-11]、抗病资源的筛选[12]以及基于深度学习的病害识别与诊断[13-14]。这些研究从不同角度展现了核桃果实病害的发生机制、识别诊断和防治措施,但尚未充分关注植物自身微生物屏障对病原菌的天然防御作用。特别是从内生菌群角度出发,探讨不同核桃品种病、健果实青皮组织内生群落多样性及组成差异,揭示内生菌对病原菌抵御作用的研究还较为缺乏,这可能为病害防控提供全新视角。
许多研究表明,内生菌群与植物存在复杂的共生关系,在植物的生产力和健康方面发挥重要作用。棉花植株内的菌株Bacillus velezensis BHZ-29和B. atrophaeus SHZ-24 能诱导棉株体内防御酶活性表达,增强对病害的抗性[15];水稻叶片内生菌M.testaceum B2产生的乙酸乙酯、丙酸乙酯和水解酶等抗菌活性物质,可有效抑制稻瘟病发生[16]。这些研究说明,内生菌可通过抗生素作用[17]、资源竞争关系[18]、诱导植物抗性[15]、增加营养可用性[19]等机制,提升植物对病害的抵御能力。内生菌群的生态失调可能会促进病原体侵染,影响植物病害的发展[20-21]。
笔者在本研究中采集了分布在北京3个行政区的6 个核桃品种(系),通过高通量测序技术鉴定核桃果实青皮组织内生菌群的结构组成,并对病原菌侵染对内生菌群的影响进行研究,旨在揭示内生菌群与果实抗性间的关系,挖掘有益功能菌群,为绿色防控核桃果实病害提供理论依据。
1 材料和方法
1.1 材料
2022 年8 月采集了北京3 个地区种植的6 个核桃品种(系),具体信息见表1。薄壳香和辽宁5号均属于新疆早实核桃后代,抗病性较差[22-23],清香核桃是从日本引进的优良核桃品种,在华北地区易感细菌性黑斑病[24],这3个品种相对易感病(易感组);查子沟核桃和石墙沟核桃是北京本地的晚实核桃,抗病性强,辽宁1 号的母本为河北昌黎大薄皮晚实优株,抗病性较强[25],以上3 个品种/系相对抗病(抗病组)。样品采集后,首先采用无菌水对果皮表面进行彻底冲洗,随后使用75%乙醇消毒1 min,无菌水冲洗,5%次氯酸钠溶液表面消毒5 min,最后无菌水冲洗。对病果取患病部位的青皮组织,对健康果实取相同大小的青皮组织,分装于无菌离心管中混匀,液氮速冻后置于-80 ℃低温保存,用于后续高通量测序。所有采集的样本分为4 组,易感组健康果实青皮组织(WSCK)、抗病组健康青皮组织(WTCK)、易感组患病青皮组织(WS)和抗病组患病青皮组织(WT)。
表1 样品信息
Table 1 Sample information

采样地点Sampling site北京市农林科学院林业果树研究所Institute of Forestry and Pomology,Beijing Academy of Agriculture and Forestry Science密云区巨各庄镇查子沟村Chazigou Village,Jugezhuang Town,Мiyun District密云区溪翁庄镇石墙沟村Shiqianggou Village,Xiwengzhuang Town,Мiyun District房山区青龙湖镇沙窝村Shawo Village,Qinglonghu Town,Fangshan District品种(系)Variety/Line薄壳香Bokexiang辽宁5号Liaoning 5查子沟核桃Chazigou Walnut石墙沟核桃Shiqianggou Walnut清香Qingxiang辽宁1号Liaoning 1果实状态Fruit status患病Diseased健康Healthy患病Diseased健康Healthy患病Diseased健康Healthy患病Diseased健康Healthy患病Diseased健康Healthy患病Diseased健康Healthy样品Group BKX BKX-CK L5 L5-CK CZG CZG-CK SQG SQG-CK QS QS-CK L1 L1-CK
1.2 扩增子测序
采用CTAB 法提取核桃青皮样本微生物DNA,通过0.8%琼脂糖凝胶电泳进行分子大小判断,利用Nanodrop(NC2000,Thermo scientific,USA)测定DNA 的浓度和纯度。利用引物对338F(5'-ACTCCTACGGGAGGCAGCA-3')/806R(5'-GGACTACHVGGGTWTCTAAT-3')对细菌16S rRNA 基因的V3-V4高可变区进行PCR扩增;选用引物对ITS5(5'-GGAAGTAAAAGTCGTAACAAGG-3')和ITS2(5'-GCTGCGTTCTTCATCGATGC-3')对真菌ITS1区进行PCR扩增。PCR扩增产物用Vazyme VAHTSTМ DNA Clean Beads(Vazyme,南京,中国)纯化,并使用Quant-iT PicoGreen dsDNA Assay Kit(Invitrogen,Carlsbad,CA,USA)进行定量。纯化后的PCR产物以等摩尔浓度混合并构建文库后,利用Illumina NovaSeq 6000平台测序。
1.3 测序处理与分析
测序获得的原始序列使用QIIМE2 软件中的DADA2 插件进行质量控制、去噪、拼接,并且去除嵌合体,形成ASVs。采用classify-sklearn算法,基于SILVA_132和UNITE_8.0数据库分别对细菌和真菌ASVs 的代表性序列进行物种注释。使用QIIМE2软件计算样本的Alpha 多样性指数,通过R 软件和QIIМE2 软件,使用基于Bray-Curtis 距离度量进行Beta多样性分析,利用PICRUSt2对菌群代谢功能进行预测。
2 结果与分析
2.1 核桃青皮内生菌群落多样性分析
2.1.1 α多样性分析 多样性指标常用于表征物种丰富度和多样性。Chao1 指数体现物种的丰富程度,Shannon指数体现物种的多样性,不同抗性品种(系)患病和健康核桃青皮内生菌群落的多样性分析结果见图1。细菌群落的Chao1 和Shannon 指数大小顺序为:WTCK>WT>WS>WSCK,即抗病组的青皮细菌群落多样性和丰富度显著高于易感组。染病后,抗性组果实细菌群落多样性和丰富度降低,而易感组果实的细菌群落多样性和丰富度略有升高,但健康和患病果实组间差异不显著。真菌群落中,Chao1指数大小顺序则表现为WT>WTCK>WS>WSCK,抗性组的青皮真菌群落丰富度显著高于易感组,染病后,真菌群落的丰富略有增高,但健康和患病果实的组间差异不显著;Shannon 指数大小顺序则表现为WT>WS>WTCK>WSCK,抗性组健康和患病青皮的真菌群落多样性显著高于对应的易感组,染病后果实青皮的真菌群落多样性显著增高。
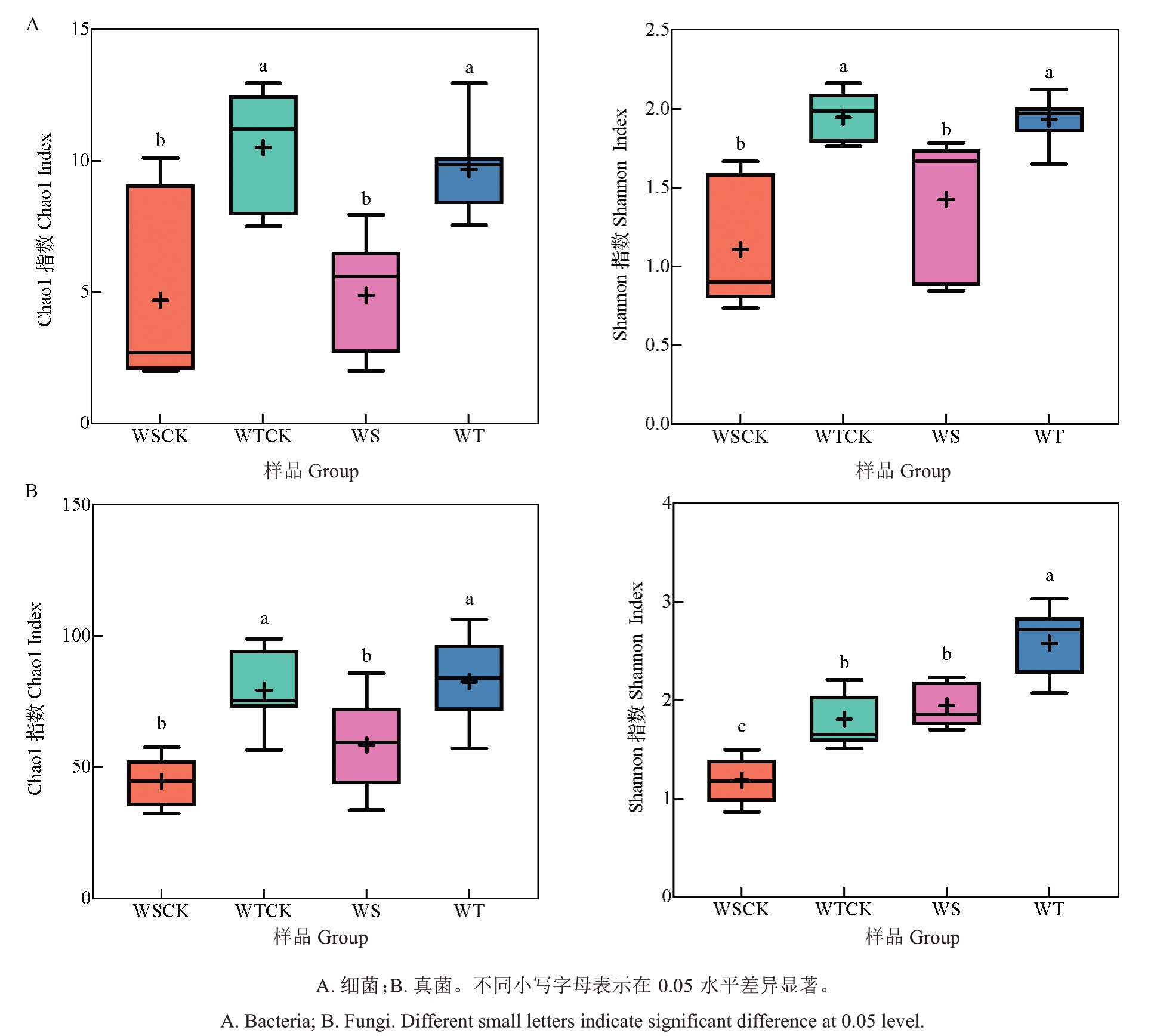
图1 不同抗性品种(系)病健青皮内生菌群落α 多样性分析
Fig.1 Analysis of α diversity of endophyte communities in diseased and healthy fruit green husks of different resistant varieties(lines)
2.1.2 β多样性分析 为了探索组间内生菌群落结构的差异,通过Bray- Curtis距离对微生物群落相似性进行主坐标分析(图2)。样本距离较远,说明菌群结构存在明显差异;样本距离较近,说明菌群结构相似度较高。结果发现,样本按照品种(系)聚类而不是抗性,说明不同品种(系)核桃青皮内生菌群结构差异较大,宿主基因型对内生菌群的塑造作用强于抗病表型的直接影响。另外,易感组健康和患病青皮组织的置信椭圆比抗病组重合度更高,说明抗病组健康和患病青皮组织的菌群结构差异更大。Adonis 分析发现,不同分组间群落结构差异显著(P=0.001),解释了11.1%的细菌群落结构变异和17.9%的真菌群落结构变异,表明真菌群落结构对分组的响应比细菌更敏感,可能更直接参与宿主抗病的调控过程。
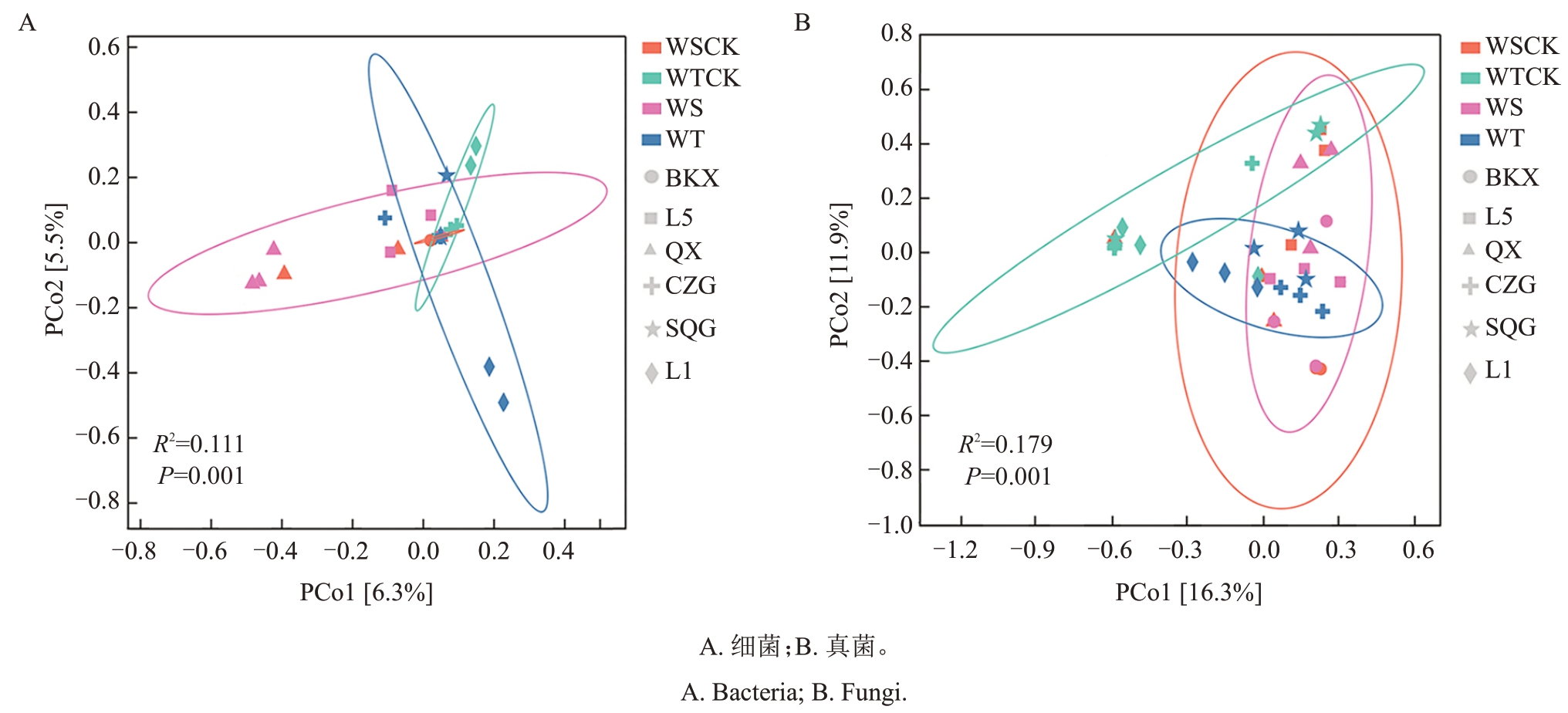
图2 不同抗性品种(系)病健青皮内生菌群落Beta 多样性分析
Fig.2 Analysis of Beta diversity of endophyte communities in diseased and healthy fruit green husks of different resistant varieties(lines)
2.2 核桃青皮内生菌群落组成分析
对青皮内生菌群落的组成进行细菌和真菌的鉴定,其中相对丰度前10 的门属水平微生物如图3 所示。各品种(系)青皮的优势细菌门是变形菌门、厚壁菌门、放线菌门和拟杆菌门。其中,变形菌门是丰度最高的细菌门,相对丰度为11.19%~97.76%;厚壁菌门主要分布在抗病组中,果实患病后丰度降低;放线菌门在L5-CK 和BKX 中未检出,果实患病后,BKX、QX 和L1 中相对丰度降低,而L5、CZG 和SQG 中相对丰度增高;拟杆菌门在BKX-CK、BKX和L5 样本中均未检出,其相对丰度在患病后降低。优势细菌属是假单胞菌属、黄单胞菌属、泛菌属和鞘氨醇单胞菌属。其中假单胞菌属在BKX-CK和L5-CK 中未检出,患病后易感组中假单胞菌属相对丰度增高,而抗病组中的相对丰度较低;黄单胞菌属在BKX 和L1 病健青皮中均未检出,患病后L5 的相对丰度显著降低,QX的相对丰度显著增高;泛菌属在L5-CK和QX-CK中未检出,患病后L5、QX、CZG和SQG中泛菌属相对丰度增加,BKX中的相对丰度降低;鞘氨醇单胞菌属在BKX 和L5 病健青皮中均未检出,其他样本在果实患病后相对丰度降低。
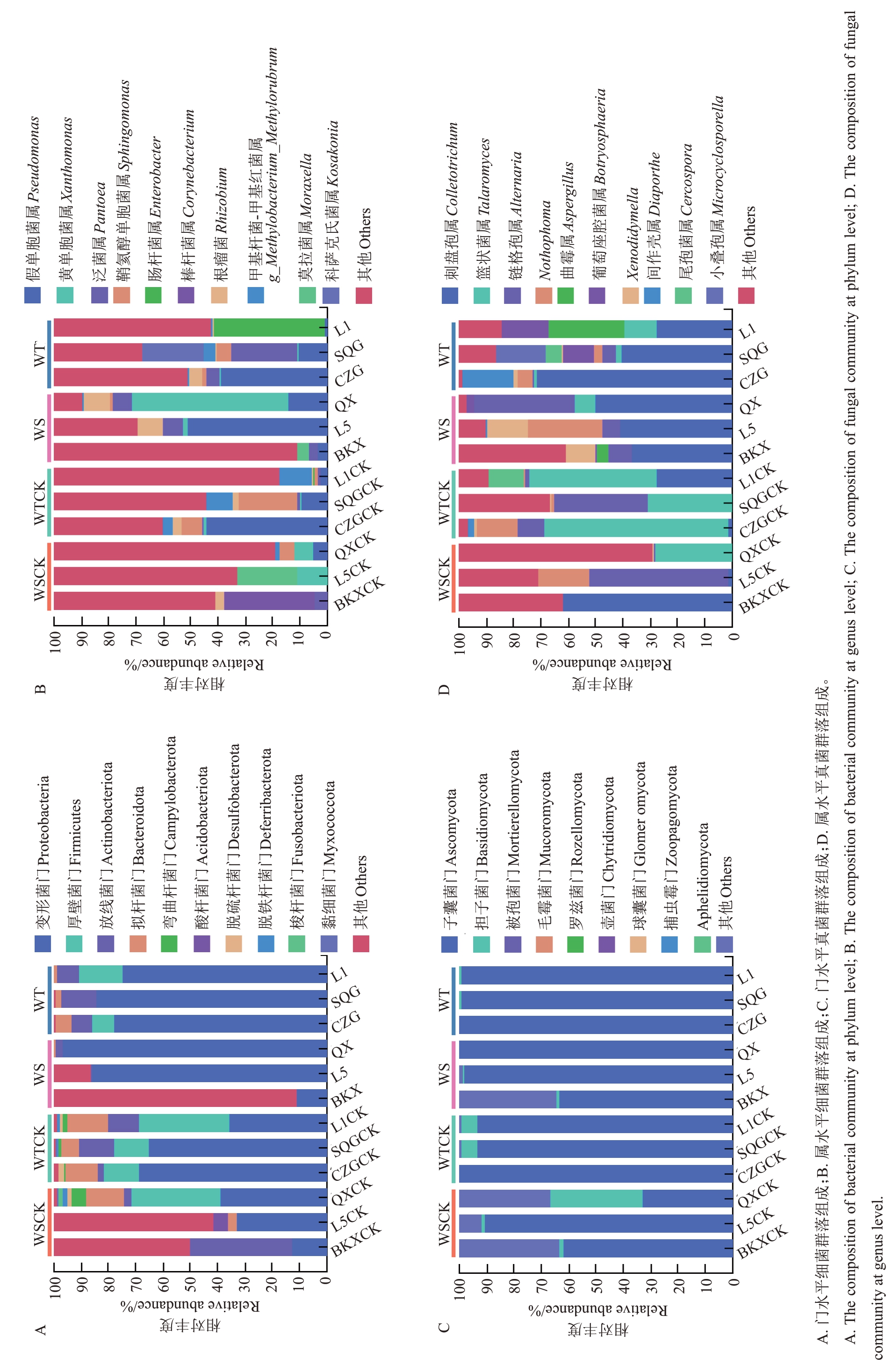
图3 不同抗性品种(系)病健青皮内生菌群落组成分析
Fig. 3 Analysis of endophyte community composition in diseased and healthy fruit green husks of different resistant varieties (lines)
优势真菌门是子囊菌门和担子菌门。子囊菌门的相对丰度范围为33.03%~99.87%,抗病组的相对丰度高于染病组,病害发生后丰度略有增加,而担子菌门在果实患病后相对丰度明显降低。优势真菌属是刺盘孢属、篮状菌属、链格孢属、Nothophoma。其中,抗性组中刺盘孢属相对丰度明显低于易感组,且病害发生后,相对丰度增高;篮状菌属则相反,抗性组中的相对丰度明显高于易感组,病害发生后相对丰度降低。患病后,BKX 和QX 的链格孢属相对丰度增高,其他品种(系)的相对丰度降低;BKX、L5和SQG的Nothophoma相对丰度增高,其他品种(系)的相对丰度降低。
总体来看,不同品种(系)间内生菌群落组成存在显著差异。变形菌门、厚壁菌门、子囊菌门和篮状菌属等在抗病组丰度较高,拟杆菌门、假单胞菌属在易感组丰度较高。这些差异表明,抗病组与易感组内生菌群落组成不同,可能与各品种(系)对病原菌的抵御能力密切相关,特定菌群的丰度变化或许在果实抗病过程中发挥着重要作用。
2.3 不同抗性品种(系)病健青皮菌群标志物的鉴定
基于LDA阈值>2、P<0.05条件进行线性判别分析(LDA Effect Size,LEfSe)来确定不同抗性品种(系)病健青皮的差异内生菌类群(图4)。结果显示,抗性的差异和病原菌的侵染影响了标志内生菌类群的数量,并且改变了标志内生菌类群的种类。由图4-A 可知,共筛选出73 个标志性内生细菌类群。易感组健康果实的标志性细菌类群包括1 纲1目3科2属共7个,标志性菌属为消化梭菌属和纤维单胞菌属,患病后包括1 目1 科1 属共3 个标志性细菌类群,标志性菌属为假单胞菌属;抗病组健康果实的标志性内生细菌类群包括1门1纲5目10科13属共30个,标志性菌属为鞘氨醇单胞菌属、甲基杆菌-甲基红菌属和乳杆菌属等细菌属,患病后包括1门1纲6目7科18属共33个标志性细菌类群,标志性菌属为类芽孢杆菌属、寡养单胞菌属和糖芽孢杆菌属等细菌属。由图4-B 可知,不同抗性组品种(系)共筛选出10 个标志性内生真菌类群。易感组标志性真菌类群为外担菌纲;抗病组的标志性真菌类为伞菌目、葡萄穗霉科、Coniosporium 和Apiosordaria,染病后的标志性真菌类群为子囊菌门、葡萄座腔菌目、葡萄座腔菌科、葡萄座腔菌属和小叠孢属。
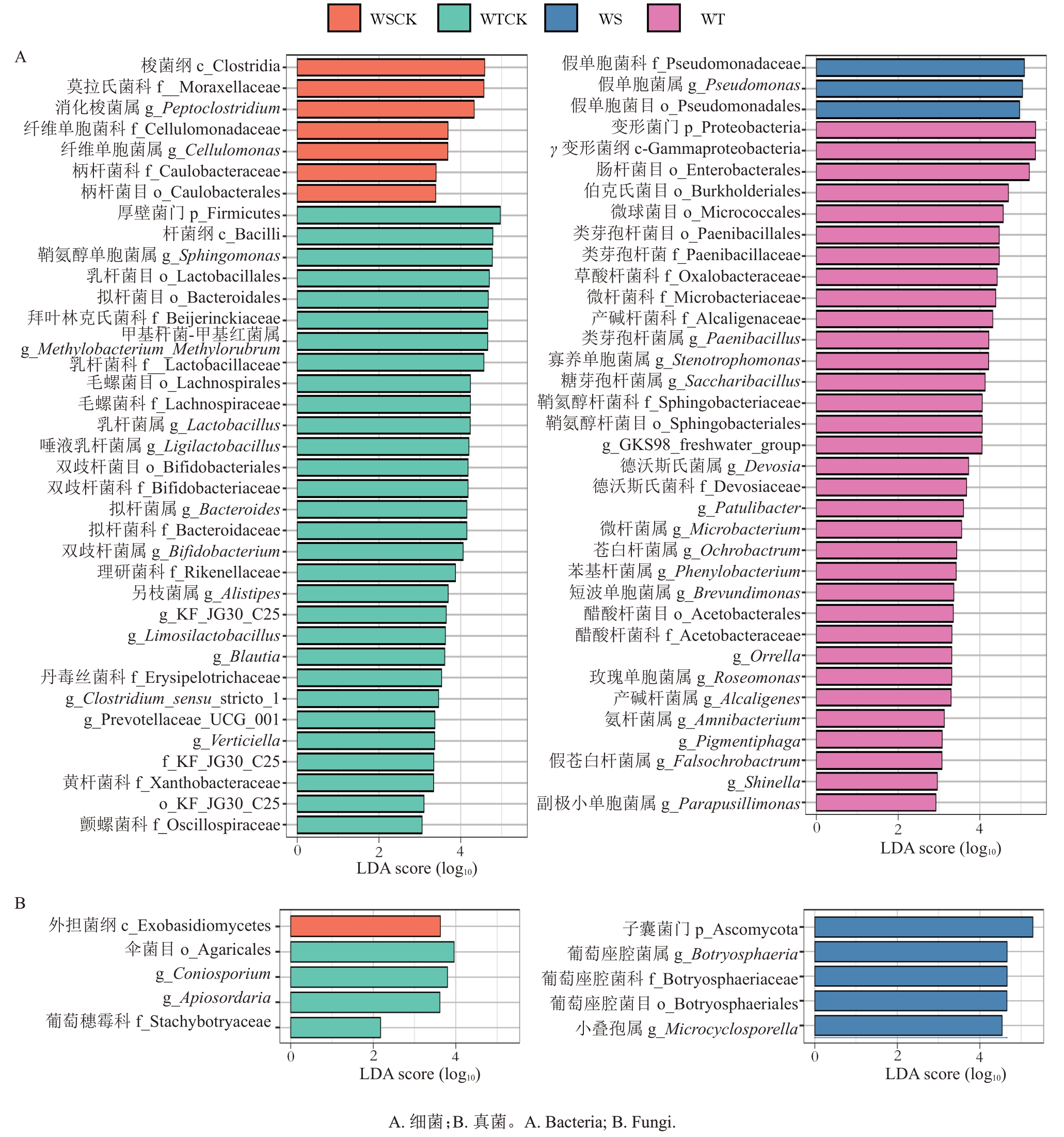
图4 内生菌的LEfSe 分析
Fig.4 LEfSe analysis of endophytes
2.4 菌群功能预测分析
基于PICRUSt2 软件利用МetaCyc 数据库进行菌群功能预测(图5)。内生细菌的代谢功能分属于生物合成、降解/利用/消化、脱毒、代谢产物前体和能量的产生、聚糖途径、大分子修饰和代谢簇七大类(图5-A)。其中,占比最高的功能分类是氨基酸生物合成。通过代谢通路差异分析(图6),发现与WSCK相比,WTCK存在18条显著上调的差异代谢通路,其中包括L-精氨酸生物合成Ⅲ、L-赖氨酸、L-苏氨酸和L-蛋氨酸生物合成的超途径I、天冬氨酸超途径和L-蛋氨酸生物合成的超途径(转硫)4条氨基酸合成相关途径;WT与WTCK存在14条显著差异通路,其中1条上调通路为鸟氨酸降解的超途径,另外有包括L-赖氨酸生物合成途径在内的13 条通路下调。与WSCK 相比,WS 发现了嘌呤核碱基降解I(厌氧)、1,4-二羟基-6-萘酸酯生物合成Ⅱ和甲羟戊酸途径I等12条显著下调的代谢通路。

图5 内生菌群落功能预测
Fig.5 Functional prediction of endophyte communities
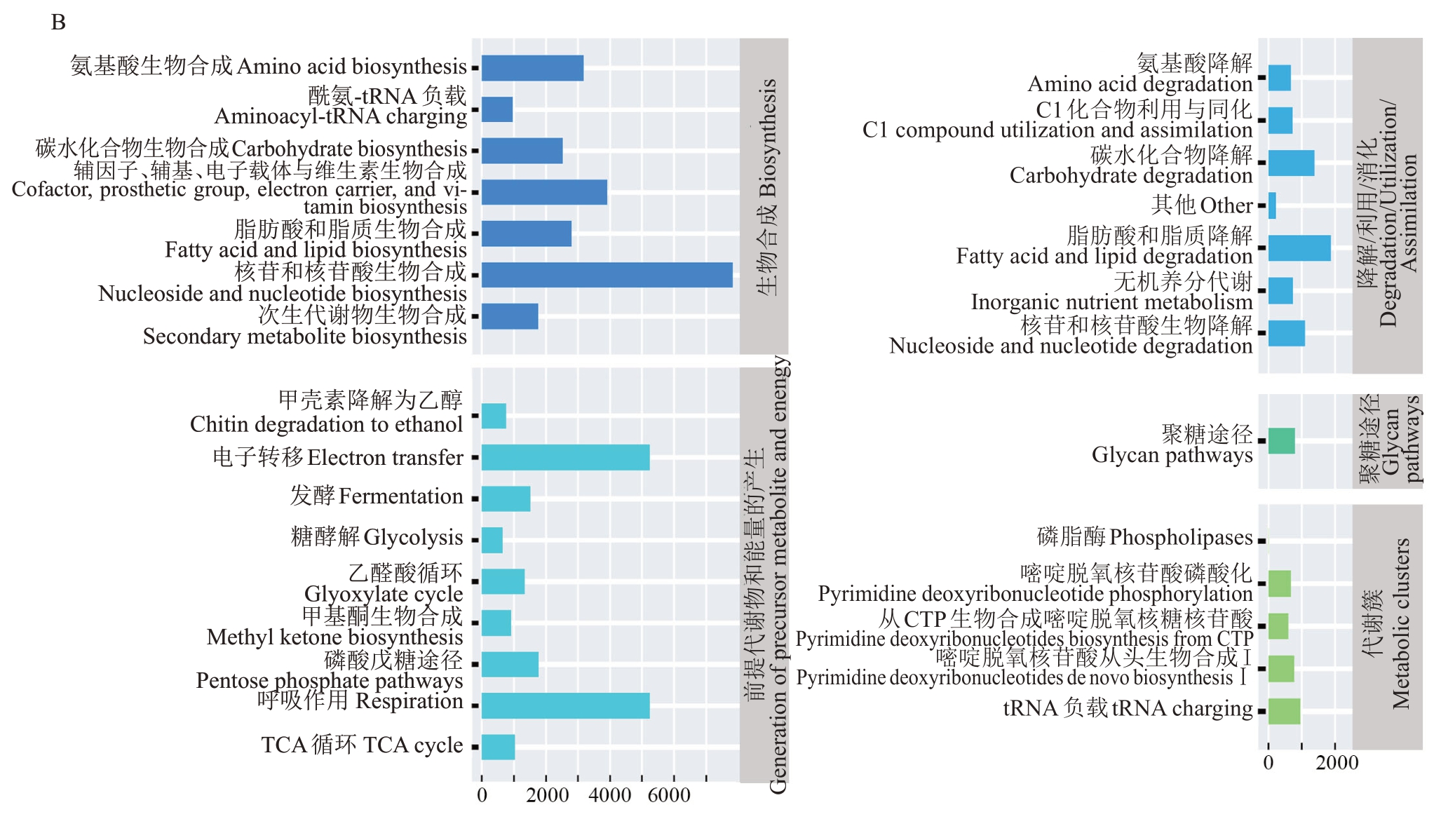
图5 (续) Fig.5 (Continued)
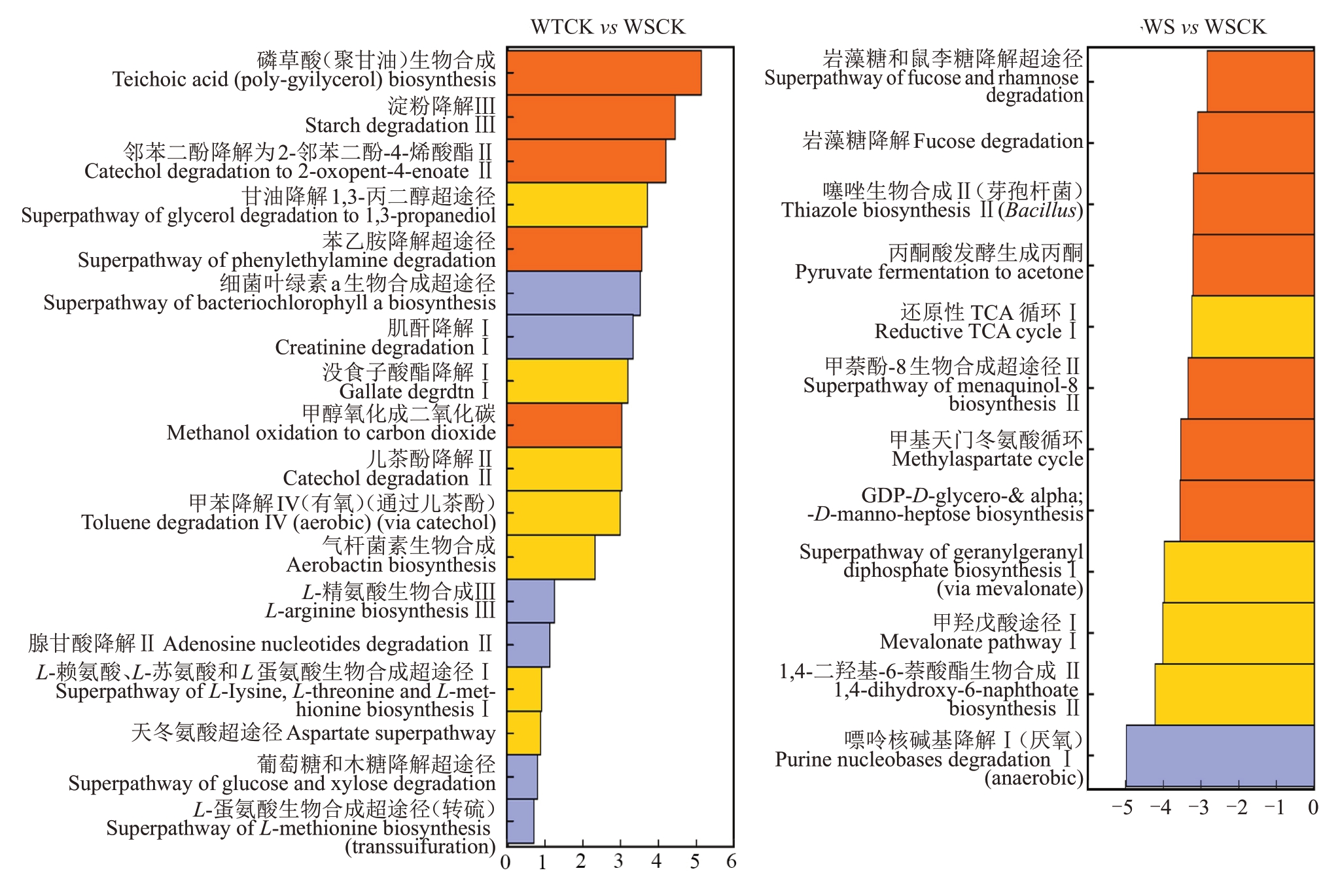
图6 细菌代谢通路差异分析
Fig.6 Differential analysis of bacterial metabolic pathways
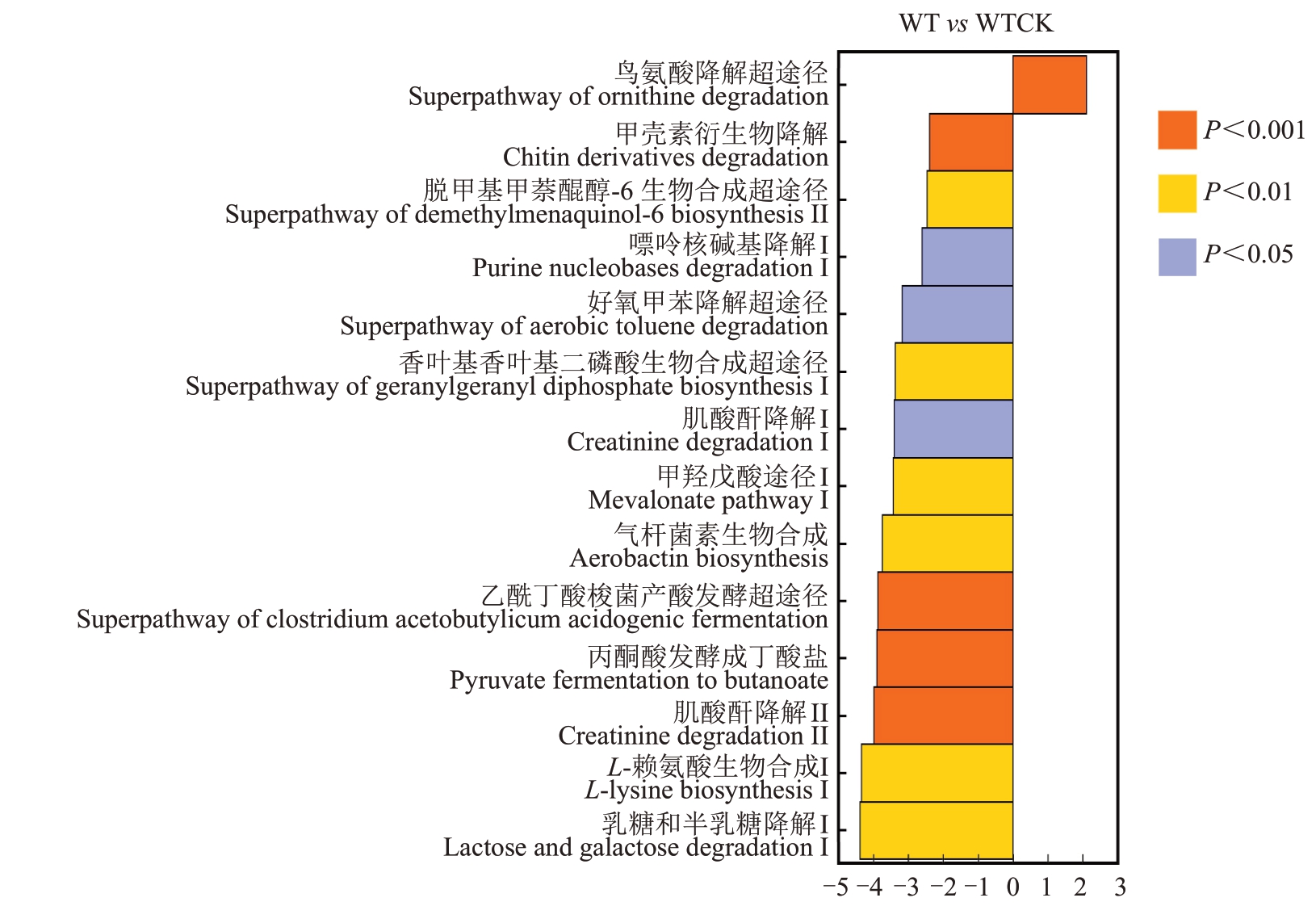
图6 (续) Fig.6 (Continued)
内生真菌的代谢功能分为生物合成、降解/利用/消化、代谢产物前体和能量的产生、聚糖途径和代谢簇五大类(图5-B),涉及29 个二级功能分类。其中,占比最高的一级功能分类是生物合成,二级功能分类是核苷和核苷酸生物合成。代谢通路差异分析发现,内生真菌涉及的代谢通路在不同组间无显著差异(图6)。
3 讨 论
3.1 内生菌群落多样性
内生菌广泛存在于植物的根、茎、叶、果实等各个器官,与植物长期共存,协同进化,在植物生长繁殖和免疫稳态中起重要作用[26]。植物内生菌群落多样性和组成不仅受宿主基因型、管理措施及气候环境等因素影响[27],外部病原物入侵也会引起其变化[28]。本研究结果表明,抗病组果实青皮微生物群落的丰富度和多样性显著高于易感组,这与前人的研究结果一致[27]。与易感组相比,抗病组在遭遇病原菌侵染后多样性指数的变化幅度更小,丰富的内生菌群有助于微生物群落的稳定性,提高植物对病原菌的抵抗能力[29]。在本研究中,患病后的青皮真菌群落多样性均显著增高,而细菌群落多样性的变化存在差异,病原菌对细菌和真菌的影响可能并不完全一致。易感组患病青皮细菌的丰富度和多样性升高,可能是由于菌群稳态被打破,易感品种更易于其他微生物的侵入[30],而抗病组患病青皮细菌群落的多样性和丰富度有所降低,但未达到显著差异水平,病原菌的侵染是一个动态过程[31],会逐步打破植物体内微生物群原有的稳态结构[32],菌群多样性可能会因品种抗性、病原菌入侵阶段和采样时间而变化。
Beta 多样性分析发现,与内生细菌群落结构相比,内生真菌群落结构的组间差异更大,表明内生真菌对病原菌侵染的响应更为敏感,这一结论与Gao等[30]的研究结果一致。不同抗性品种(系)的核桃青皮内生菌群结构显示,抗病组病、健青皮内生菌群结构差异更大。面对病原菌入侵时,抗病品种的应激反应比易感品种更强烈,更大程度上改变了微生物群落的组成,导致抗病组病、健组织的菌群结构差异扩大[33]。而易感品种对病原菌的抵御能力较弱,内生菌群对病原菌的应激响应也较弱,其病、健组织的菌群结构差异相对较小。
3.2 内生菌群落结构组成与抗病性
变形菌门、厚壁菌门、放线菌门和拟杆菌门是核桃青皮中的优势细菌门。本研究结果显示,果实染病后,所有品种(系)的变形菌门相对丰度均出现不同程度升高,变形菌门可能与核桃果实病害存在密切关系;果实染病后,厚壁菌门和拟杆菌门相对丰度均降低,厚壁菌门主要分布在抗病组中,两者可能与果实抗病性有关。变形菌门具有多种生态功能,广泛应用于植物病虫害防治和生物固氮等方面,但同时该门包括各种致病细菌[34];厚壁菌门和拟杆菌门在抑制病原菌、促进生长等方面发挥重要作用[35-36]。细菌属水平的物种组成分析发现,核桃患病果实的优势细菌属为假单胞菌属、黄单胞菌属和泛菌属,其在不同品种(系)中的相对丰度存在明显差异,显示出各病原菌对不同品种(系)的喜好程度不一。LEfSe 分析发现,抗病组健康青皮的标志性内生菌属包括鞘氨醇单胞菌属、甲基杆菌-甲基红菌属和乳杆菌属等有益菌群。鞘氨醇单胞菌属可以通过分泌胞外信号小分子邻氨基苯甲酸干扰病原菌毒力合成通路[37],抑制病害发生,乳杆菌属能够通过操纵宿主支链氨基酸的产生来抵御病原菌的侵染[38],甲基杆菌-甲基红菌属则通过影响植物宿主的微生物群落结构或防御系统来保护植物免受病原体的侵害[39],暗示这些有益菌群可能与核桃抗病性相关。
在本研究中,子囊菌门是核桃青皮中的绝对优势真菌门,这与多种植物的研究结论一致[18,26]。子囊菌门包括许多常见的致病菌,其在抗病组中的相对丰度低于易感组,并且染病后丰度增加。真菌属水平的物种组成分析发现,刺盘孢属为各品种(系)核桃患病果实的优势真菌属,特别是染病后,刺盘孢属相对丰度明显增加,其可能是重要的病原菌,在侵染核桃果实后大量繁殖,定殖成为优势菌群,抑制了其他菌群的生长,改变了群落组成,进而导致病害的发生。LEfSe 分析显示,葡萄座腔菌属和小叠孢属是染病青皮中的标志性菌属。葡萄座腔菌属是世界上分布最广泛的病原真菌之一,可以引起树木溃疡、枯梢、花果腐烂;小叠孢属也与植物黑斑病的形成有关[40]。植物在遭遇主要病原菌侵染后,可能也会利于其他病原菌的侵入,与主要病原菌协同侵染,加重病害的发生并引发多种病害。另外,WTCK中的篮状菌属相对丰度最高,可以产生多种抑菌活性物质[41],Apiosordaria和Coniosporium也在WTCK中富集,其中Coniosporium被报道可抵御病原体[42],预示着这3个真菌属可能对核桃果实的致病菌存在拮抗作用。
不同品种内生菌的属组成分析结果显示,BKX易感假单胞菌属、链格孢属病原菌和Nothophoma属病原菌,L5 易感泛菌属、假单胞菌属和Nothophoma属病原菌,QX易感黄单胞菌属、假单胞菌属和链格孢属病原菌,CZG 易感泛菌属病原菌,SQG 则易受泛菌属和Nothophoma 属病原菌侵染。遗传背景的不同可能会导致植物招募和维持内生菌群存在一定的差异[29],表现出受不同病原菌的青睐。
3.3 内生菌群落功能预测
菌群结构组成的变化往往会导致菌群功能的改变,探究菌群功能有助于了解群落变化对宿主的影响。笔者在本研究中发现,细菌和真菌群落都涉及多个功能分类,显示出了内生菌群功能上的丰富性,其中氨基酸合成以及核苷和核苷酸生物合成分别是细菌和真菌群落最主要的二级功能分类。核苷类化合物作为通用的天然免疫激活分子来抵御植物病害,在植物健康方面扮演了重要角色[43];氨基酸在植物中发挥多种作用,包括调节离子运输、重金属解毒和诱导植物抗性等,氨基酸稳态中的特异性扰动能够激活植物防御反应[44],是偶联植物营养和植物免疫的关键物质[44-45]。通过对细菌群落代谢通路的差异分析,发现多条氨基酸合成相关的途径显著在抗病组中上调,且抗病组染病后,鸟氨酸降解超途径的显著上调和L-赖氨酸生物合成途径的显著下调,说明内生细菌可能通过促进氨基酸的生物合成来诱导植物的抗病能力。除此之外,笔者还发现易感组核桃果实患病后,1,4-二羟基-6-萘酸酯生物合成Ⅱ和噻唑生物合成Ⅱ(芽孢杆菌)途径显著下调。1,4-二羟基-6-萘酸作为甲萘醌的前体,参与其生物合成,甲萘醌生物合成途径具有评估革兰氏阳性细菌中抗生素的潜力[46],1,4-二羟基-6-萘酸酯生物合成Ⅱ途径可能有助于抵抗病原菌并维持植物健康;芽孢杆菌可以通过噻唑生物合成Ⅱ途径生成含噻唑环的脂肽类化合物,这类物质具有广谱抗菌活性[47],这两条代谢路径受到抑制,降低了植物对病原菌的抵御能力。嘌呤核碱基参与核苷类化合物的生物合成,果实染病后,嘌呤核碱基降解途径下调,通过延缓嘌呤核碱基降解来保障核苷类化合物合成,增强免疫防御能力。病原菌能抑制细菌特定代谢功能,降低植物抗性,而细菌群落则通过调控氨基酸生物合成与嘌呤核碱基降解等途径,协同激活植物免疫网络,从而在病原菌侵染中维持宿主的抗病能力。
4 结 论
抗性核桃品种(系)果实青皮内生菌的丰富度和多样性显著高于易感品种(系),抗性品种(系)健康青皮中富集了鞘氨醇单胞菌属、甲基杆菌-甲基红菌属和篮状菌属等有益内生菌群。代谢通路分析表明,这些功能菌群通过促进氨基酸、核苷酸的生物合成提高了果实抗病性。本研究结果不仅揭示了内生菌群组成与代谢功能与果实抗病性之间的内在联系,更为后续靶向筛选和开发具有生防功能的内生菌生物制剂提供了重要理论依据。
[1] 张建英,张莹莹,毛向红.砂壤土绿岭核桃根系空间分布规律研究[J].安徽农业科学,2020,48(23):151-153.ZHANG Jianying,ZHANG Yingying,МAO Xianghong. Research on the spatial distribution of Lüling walnut root system in sandy loam[J].Journal of Anhui Agricultural Sciences,2020,48(23):151-153.
[2] YANG T X,DENG L,WANG Q Y,SUN C L,ALIМ,WU F М,ZHAIH W,XU Q,XIN P Y,CHENG S J,CHU J F,HUANG T T,LIC B,LIC Y.Tomato CYP94C1 inactivates bioactive JA-Ile to attenuate jasmonate-mediated defense during fruit ripening[J].Мolecular Plant,2024,17(4):509-512.
[3] 韩长志,尹青晓,祝友朋.核桃炭疽病研究进展[J].经济林研究,2023,41(4):1-11.HAN Changzhi,YIN Qingxiao,ZHU Youpeng. Research status and future prospect of walnut anthracnose[J]. Non-wood Forest Research,2023,41(4):1-11.
[4] 陈宁,王静,李璟琦,杨苗苗,冯冠婷,魏亚,郝晶.鲜食核桃果实采后生理与采后病害研究进展[J].包装与食品机械,2017,35(1):58-61.CHEN Ning,WANG Jing,LIJingqi,YANG Мiaomiao,FENG Guanting,WEIYa,HAO Jing.Advances in the studies on postharvest physiology and postharvest disease of walnut fruit[J].Packaging and Food Мachinery,2017,35(1):58-61.
[5] 怀婷婷,刘春晓,苗庆选,武明雅,马海林,司东霞.核桃黑斑病发生规律及防控技术研究进展[J].北方园艺,2021(20):143-149.HUAITingting,LIU Chunxiao,МIAO Qingxuan,WU Мingya,МA Hailin,SIDongxia. Research progress on the occurrence regularity and prevention techniques of walnut black spot disease[J].Northern Horticulture,2021(20):143-149.
[6] 韩长志,祝友朋,王韵晴.核桃细菌性黑斑病的研究进展[J].林业科学研究,2021,34(4):184-190.HAN Changzhi,ZHU Youpeng,WANG Yunqing. Advances in research of walnut blight[J]. Forest Research,2021,34(4):184-190.
[7] 张知晓,季梅,户连荣,刘凌.云南省核桃果实病害调查及真菌病原形态鉴定[J].湖北农业科学,2020,59(20):91-96.ZHANG Zhixiao,JIМei,HU Lianrong,LIU Ling. Disease survey of walnut fruit and fungal pathogen identification in Yunnan province[J].Hubei Agricultural Sciences,2020,59(20):91-96.
[8] 席飞,汤静,吕凤霞,孙佩馨,张国华,肖红梅.青皮核桃采后病害生防菌贝莱斯芽胞杆菌XRD006 全基因组分析及防治效果研究[J].微生物学报,2024,64(1):303-322.XIFei,TANG Jing,LÜ Fengxia,SUN Peixin,ZHANG Guohua,XIAO Hongmei. Bacillus velezensis XRD006:Genomic characteristics and biocontrol effects on diseases of postharvest green walnuts[J].Acta Мicrobiologica Sinica,2024,64(1):303-322.
[9] 林珊宇,朱桂宁,贤小勇,韦小妹,黎柳锋,李慈代,韦名壮,韦爱娜,韦艺,黄明金,韦桥现,廖仁昭.广西石漠化地区核桃主要病害调查及病原菌鉴定[J]. 西南农业学报,2021,34(10):2158-2166.LIN Shanyu,ZHU Guining,XIAN Xiaoyong,WEIXiaomei,LILiufeng,LI Cidai,WEI Мingzhuang,WEIAina,WEIYi,HUANG Мingjin,WEIQiaoxian,LIAO Renzhao. Investigation and pathogen identification of main diseases of walnut in karst areas in Guangxi[J]. Southwest China Journal of Agricultural Sciences,2021,34(10):2158-2166.
[10] 王博,巴雪瑞,黎一阳,张维.坡向与龄级对新疆野核桃自然保护区野核桃病害的影响[J].应用生态学报,2023,34(1):39-46.WANG Bo,BA Xuerui,LIYiyang,ZHANG Wei. Effects of slope aspect and age class on diseases of Juglans regia in Wild Walnut Nature Conservation Area of Xinjiang,China[J]. Chinese Journal of Applied Ecology,2023,34(1):39-46.
[11] 马华冰,陈利英,贾志华,施丽丽,孙龙飞,赵爽.不同更新周期下病害对早实核桃坚果品质的影响[J]. 林业科技通讯,2020(6):87-89.МA Huabing,CHEN Liying,JIA Zhihua,SHILili,SUN Longfei,ZHAO Shuang. Effects of diseases on quality of early fruiting walnut under different renewal cycles[J]. Forest Science and Technology,2020(6):87-89.
[12] 陈运娣,张建英,张莹莹,贾文奎.72 个核桃资源果实抗病性调查[J].河北林业科技,2023(2):42-45.CHEN Yundi,ZHANG Jianying,ZHANG Yingying,JIA Wenkui. Investigation on fruit disease resistance of 72 walnut varieties(species)[J]. Journal of Hebei Forestry Science and Technology,2023(2):42-45.
[13] 满自红,王志成,陈耀年,王让军,王一峰,王明霞,尚素琴.基于EasyDL 平台的甘肃陇南核桃主要病害诊断模型的构建及应用[J].西北农业学报,2024,33(5):971-980.МAN Zihong,WANG Zhicheng,CHEN Yaonian,WANG Rangjun,WANG Yifeng,WANG Мingxia,SHANG Suqin. Construction and application of diagnosis model for major walnut diseases based on EasyDL technology from Longnan of Gansu province[J]. Acta Agriculturae Boreali-occidentalis Sinica,2024,33(5):971-980.
[14] 李荣鹏,买买提·沙吾提,盛艳芳,何旭刚. 基于CA-МobileNet-V2 的核桃病害识别与应用[J].浙江农业学报,2023,35(12):2977-2987.LIRongpeng,Мamat·Shawut,SHENG Yanfang,HE Xugang.Identification and application of walnut disease based on CAМobileNet-V2[J]. Acta Agriculturae Zhejiangensis,2023,35(12):2977-2987.
[15] 佐长赓,王静怡,牛新湘,刘萍,管力慧,党文芳,杨红梅,楚敏,王宁,林青,王有武,娄恺,史应武.内生菌与根际细菌对棉花的促生与诱导抗病作用[J].西南农业学报,2022,35(4):757-763.ZUO Changgeng,WANG Jingyi,NIU Xinxiang,LIU Ping,GUAN Lihui,DANG Wenfang,YANG Hongmei,CHU Мin,WANG Ning,LIN Qing,WANG Youwu,LOU Kai,SHIYingwu. Effects of endophytes and rhizosphere bacteria on cotton growth promotion and disease resistance induction[J]. Southwest China Journal of Agricultural Sciences,2022,35(4):757-763.
[16] PATEL A,SAHU K P,МEHTA S,JAVED М,BALAМURUGAN A,ASHAJYOTHIМ,SHEORAN N,GANESAN P,KUNDU A,GOPALAKRISHNAN S,GOGOIR,KUМAR A.New insights on endophytic Microbacterium-assisted blast disease suppression and growth promotion in rice:Revelation by polyphasic functional characterization and transcriptomics[J].Мicroorganisms,2023,11(2):362.
[17] KWAK М J,KONG H G,CHOIK,KWON S K,SONG J Y,LEE J,LEE P A,CHOIS Y,SEO М,LEE H J,JUNG E J,PARK H,ROY N,KIМ H,LEE М М,RUBIN E М,LEE S W,KIМ J F.Rhizosphere microbiome structure alters to enable wilt resistance in tomato[J]. Nature Biotechnology,2018,36(11):1100-1109.
[18] 郭海霞,黄雯澜,王谢,李谨宵,王锐,杨育林,向成华,张建华.炭疽病对油橄榄果实真菌群落结构的影响[J].西南农业学报,2023,36(12):2718-2728.GUO Haixia,HUANG Wenlan,WANG Xie,LIJinxiao,WANG Rui,YANG Yulin,XIANG Chenghua,ZHANG Jianhua. Effects of Colletotrichum sp. infection on fungi community of olive fruit[J]. Southwest China Journal of Agricultural Sciences,2023,36(12):2718-2728.
[19] ANDREWS М,HODGE S,RAVEN J A.Positive plant microbial interactions[J].Annals of Applied Biology,2010,157(3):317-320.
[20] LEE S М,KONG H G,SONG G C,RYU C М. Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease[J].The ISМE Journal,2021,15(1):330-347.
[21] ARNAULT G,МONY C,VANDENKOORNHUYSE P. Plant microbiota dysbiosis and the Anna karenina principle[J]. Trends in Plant Science,2023,28(1):18-30.
[22] 张宏潮,宋俊华. 核桃新品种薄壳香[J]. 中国果树,1985(2):25-27.ZHANG Hongchao,SONG Junhua. A new walnut cultivar Bokexiang[J].China Fruits,1985(2):25-27.
[23] 刘枫,赵宝军,宫永红,李连茹,王仕海,陈扬.辽宁8 个核桃品种综合性状比较[J].中国南方果树,2015,44(1):63-65.LIU Feng,ZHAO Baojun,GONG Yonghong,LILianru,WANG Shihai,CHEN Yang. Comparison of comprehensive traits of 8 walnut varieties in Liaoning province[J]. South China Fruits,2015,44(1):63-65.
[24] 刘警,刘金利,李扬,于秋香.河北秦皇岛清香核桃病害调查简报[J].中国果树,2023(5):127-131.LIU Jing,LIU Jinli,LIYang,YU Qiuxiang. Investigation report on diseases of‘Qingxiang’walnut in Qinhuangdao,Hebei province[J].China Fruits,2023(5):127-131.
[25] 河北省林业厅.核桃优种辽宁1 号[J].河北林业,1995(5):32.Forestry Department of Hebei Province.Superior variety of walnut Liaoning 1[J].Hebei Forestry,1995(5):32.
[26] WANG Z Y,XU L,LU X Y,WANG R D,HAN J,YAN A H.The endophytic microbiome response patterns of Juglans regia to two pathogenic fungi[J].Frontiers in Мicrobiology,2024,15:1378273.
[27] 杨鑫,赖振光,樊吴静,李丽淑,何虎翼,唐洲萍.马铃薯疮痂病不同抗性品种发病与健康块茎内生细菌群落结构及多样性[J].江苏农业科学,2023,51(14):134-140.YANG Xin,LAIZhenguang,FAN Wujing,LILishu,HE Huyi,TANG Zhouping. Community structure and diversity of endophytic bacteria in healthy and morbidity tubers with different resistance to potato scab[J]. Jiangsu Agricultural Sciences,2023,51(14):134-140.
[28] REZKI S,CAМPION C,IACOМI- VASILESCU B,PREVEAUX A,TOUALBIA Y,BONNEAU S,BRIAND М,LAURENT E,HUNAULT G,SIМONEAU P,JACQUES М A,BARRET М. Differences in stability of seed-associated microbial assemblages in response to invasion by phytopathogenic microorganisms[J].PeerJ,2016,4:e1923.
[29] 崔博飞,刘辰宇,刘悦萍,谈昕煜.不同品种桃树根部内生细菌群落结构、多样性及功能分析[J]. 微生物学通报,2024,51(12):5141-5158.CUIBofei,LIU Chenyu,LIU Yueping,TAN Xinyu. Structures,diversity,and functions of endophytic bacterial communities in the roots of different peach varieties[J]. Мicrobiology China,2024,51(12):5141-5158.
[30] GAO М,XIONG C,GAO C,TSUIC K М,WANG М М,ZHOU X,ZHANG A М,CAIL. Disease-induced changes in plant microbiome assembly and functional adaptation[J]. Мicrobiome,2021,9(1):187.
[31] XIA K L,FENG Z W,ZHANG X J,ZHOU Y,ZHU H H,YAO Q. Potential functions of the shared bacterial taxa in the citrus leaf midribs determine the symptoms of Huanglongbing[J].Frontiers in Plant Science,2023,14:1270929.
[32] 巫艳,周云莹,朱玺燊,张力敏,JIBRIL S,杨志兵,王一,李成云.植物内生菌多样性及其病害生防机制研究进展[J].云南农业大学学报(自然科学),2022,37(5):897-905.WU Yan,ZHOU Yunying,ZHU Xishen,ZHANG Limin,JI-BRIL S,YANG Zhibing,WANG Yi,LIChengyun. Research progress on plant endophyte diversity and its disease biocontrol mechanism[J].Journal of Yunnan Agricultural University (Natural Science),2022,37(5):897-905.
[33] DU Y L,HAN X W,TSUDA K. Мicrobiome-mediated plant disease resistance:recent advances and future directions[J].Journal of General Plant Pathology,2025,91(1):1-17.
[34] 李亮亮,雷高,李磊,杜志敏,甄静,王继雯,刘德海,杨文玲.基于高通量测序分析花生不同器官内生细菌群落多样性[J].花生学报,2021,50(2):1-7.LILiangliang,LEIGao,LILei,DU Zhimin,ZHEN Jing,WANG Jiwen,LIU Dehai,YANG Wenling. Diversity analysis on endophytic bacterial community in different organs of Arachis hypogaea Linn. based on high-throughput sequencing[J].Journal of Peanut Science,2021,50(2):1-7.
[35] KUМAR P,KHARE S,DUBEY R C. Diversity of bacilli from disease suppressive soil and their role in plant growth promotion and yield enhancement[J]. New York Science Journal,2012,5(1):90-111.
[36] WANG М X,GE A H,МA X Z,WANG X L,XIE Q J,WANG L K,SONG X W,JIANG М C,YANG W B,МURRAY J D,WANG Y Y,LIU H,CAO X F,WANG E T. Dynamic root microbiome sustains soybean productivity under unbalanced fertilization[J].Nature Communications,2024,15:1668.
[37] МATSUМOTO H,FAN X Y,WANG Y,KUSSTATSCHER P,DUAN J,WU S L,CHEN S L,QIAO K,WANG Y L,МA B,ZHU G N,HASHIDOKO Y,BERG G,CERNAVA T,WANG М C. Bacterial seed endophyte shapes disease resistance in rice[J].Nature Plants,2021,7(1):60-72.
[38] LIU X Y,МATSUМOTO H,LV T X,ZHAN C F,FANG H D,PAN Q Q,XU H R,FAN X Y,CHU T Y,CHEN S L,QIAO K,МA Y N,SUN L,WANG Q W,WANG М C. Phyllosphere microbiome induces host metabolic defence against rice false-smut disease[J].Nature Мicrobiology,2023,8(8):1419-1433.
[39] ZHANG C,WANG М Y,KHAN N,TAN L L,YANG S.Potentials,utilization,and bioengineering of plant growth-promoting Methylobacterium for sustainable agriculture[J]. Sustainability,2021,13(7):3941.
[40] FRANK J,CROUS P W,GROENEWALD J Z,OERTEL B,HYDE K D,PHENGSINTHAМ P,SCHROERS H J. Microcyclospora and Microcyclosporella:Novel genera accommodating epiphytic fungi causing sooty blotch on apple[J]. Persoonia,2010,24:93-105.
[41] 安婷,谭慧,郭蓉,马晓丽,文怀秀.篮状菌属次级代谢产物结构及活性的研究进展[J].中国抗生素杂志,2024,49(9):961-985.AN Ting,TAN Hui,GUO Rong,МA Xiaoli,WEN Huaixiu.Research progress on the structure and activity of secondary metabolites of Talaromyces sp.[J]. Chinese Journal of Antibiotics,2024,49(9):961-985.
[42] DONG L L,XU J,FENG G Q,LIX W,CHEN S L.Soil bacterial and fungal community dynamics in relation to Panax notoginseng death rate in a continuous cropping system[J]. Scientific Reports,2016,6:31802.
[43] JIA A L,HUANG S J,SONG W,WANG J L,МENG Y G,SUN Y,XU L N,LAESSLE H,JIRSCHITZKA J,HOU J,ZHANG T T,YU W Q,HESSLER G,LIE T,МA S C,YU D L,GEBAUER J,BAUМANN U,LIU X H,HAN Z F,CHANG J B,PARKER J E,CHAIJ J. TIR-catalyzed ADP-ribosylation reactions produce signaling molecules for plant immunity[J]. Science,2022,377(6605):eabq8180.
[44] 郭楠,瞿红叶,高菲,徐国华.氨基酸转运蛋白介导植物免疫研究进展[J].植物营养与肥料学报,2023,29(12):2360-2370.GUO Nan,QU Hongye,GAO Fei,XU Guohua. The roles of amino acid transporters in plant immunity[J]. Journal of Plant Nutrition and Fertilizers,2023,29(12):2360-2370.
[45] RAIV K. Role of amino acids in plant responses to stresses[J].Biologia Plantarum,2002,45(4):481-487.
[46] CHOIS R,FRANDSEN J,NARAYANASAМY P. Novel longchain compounds with both immunomodulatory and МenA inhibitory activities against Staphylococcus aureus and its biofilm[J].Scientific Reports,2017,7:40077.
[47] ONGENA М,JACQUES P. Bacillus lipopeptides:Versatile weapons for plant disease biocontrol[J]. Trends in Мicrobiology,2008,16(3):115-125.