地面的紫外线辐射(UVR)主要来自阳光,根据波长分为3 类:UV-A(315~400 nm)、UV-B(280~320 nm)和UV-C(100~280 nm)。UV-B 大部分被臭氧层吸收,正常情况下只有10%的UV-B 辐射能够到达地面,对植物生长的影响微乎其微[1-2]。但由于氯氟烃、气溶胶等物质的排放,导致平流层臭氧层变薄,到达地表的UV-B含量上升,称之为“增强UV-B辐射”(enhanced UV-B radiation)。《蒙特利尔议定书》实施后,虽然减少了大气中部分污染物含量[3],但氧化亚氮(N2O)的排放与部分污染地区的大气清洁度上升,在相当长的时间内仍存在增强UV-B 辐射危害的风险[4]。
增强UV-B辐射对植物具有多方面影响。增强UV-B 辐射处理拟南芥[Arabidopsis thaliana(L.)Heynh.]植株后,赤霉素含量下降[5],光敏色素互作因子蛋白(PIF4、PIF5)降解[6],均导致下胚轴生长受到抑制;增强UV-B 辐射会导致叶片光系统Ⅱ(PSⅡ)失活并影响相关光合基因的表达[7],导致平均Fv/Fm(PSⅡ的最大光化学效率)降低,且随增强UV-B 辐射强度升高而急剧下降[8-9]。在室内进行增强UV-B处理后,通过激活ELONGATED HYPOCOTYL 5(CsHY5)而抑制叶绿素生物合成基因表达,进而减少叶绿素合成[10],还引起叶面积减小[11]。增强UV-B辐射处理的植株中ROS 含量上升[12-13],过量的ROS会导致细胞中脂质、蛋白质、RNA、DNA和许多小分子氧化[14]。此外还会导致МDA含量上升,加剧植物细胞膜脂过氧化损伤,甚至引起细胞死亡[15]。
植物为抵御活性氧胁迫,进化出了一套复杂的抗氧化系统,包括抗氧化酶与非酶类抗氧化剂,抗氧化系统通过清除活性氧自由基(ROS),尽力维持细胞内ROS含量平衡,从而减轻或避免活性氧损伤。
抗氧化酶主要分为三大类,超氧化物歧化酶(SOD)、过氧化物酶(POD)和过氧化氢酶(CAT)[16]。SOD 是抵御活性氧胁迫的第一道防线,绝大多数生物体遭遇活性氧胁迫时都至少诱导产生一种SOD[17]。根据金属辅基的种类差异,将SOD分为Cu/ZnSOD(CSD)、МnSOD(МSD)、FeSOD(FSD)和NiSOD[18],其中NiSOD 主要存在于原核生物中[19]。过氧化物酶(POD)是通过将过氧化氢还原为水来催化各种底物氧化的酶,根据辅基种类的差异,这些蛋白质可分为血红素酶或非血红素酶;大多数血红素POD可以分成动物POD和非动物POD等两大类[20];非动物POD超家族可被分为I、Ⅱ和Ⅲ类,即细菌POD(I类)、分泌性POD(Ⅱ类)和分泌性植物POD(Ⅲ类)[21];Ⅲ类POD作为植物特异性氧化还原酶,功能众多,参与了木质化、细胞伸长、种子萌发和胁迫防御[22-23]。过氧化氢酶(CAT)在植物抗氧化过程中起到了重要作用,是大多数生物体中的核心抗氧化酶;分为含单功能血红素的CAT、含双功能血红素的CAT和含锰CAT[24-25];与其他抗氧化酶不同,CAT活性并不受底物影响,其通过歧化反应将H2O2直接转化为H2O 与O2,且对H2O2具有较高特异性,但对有机过氧化物的活性较弱[26]。
抗氧化酶在响应增强UV-B 胁迫中起到了重要作用。萌芽期大豆[Glycine max(L.)Мerr.]在增强UV-B 辐射处理下,H2O2含量激增,抗氧化酶活性被诱导增强,以此减轻氧化损伤[27]。增强UV-B胁迫引起大豆叶片H2O2等活性氧自由基积累,经硝普钠(SNP)处理植株后,植株抗氧化酶SOD、CAT、抗坏血酸过氧化物酶(APX)活性显著上升,清除了增强UV-B 辐射处理造成的H2O2和超氧阴离子积累,避免细胞的活性氧自由基损伤[28];在增强UV-B辐射胁迫下,水稻(Oryza sativa L.)幼苗的H2O2积累增加,抗氧化酶活性显著上升[29],小麦中部分抗氧化酶编码基因显著上调表达,以增强抗氧化能力,避免活性氧损伤[30]。
杧果(Mangifera indica L.)为漆树科(Anacardiaceae R.Br.)杧果属,是热带与亚热带重要果树,非生物胁迫严重影响杧果产量与果实品质[31]。增强UV-B辐射胁迫作为全球共同面对的环境问题之一,有必要开展增强UV-B辐射对杧果生长发育影响的生物学机制研究,研究成果将为应对未来的增强UV-B 辐射胁迫提供参考。本研究旨在探究增强UV-B 辐射胁迫下台农一号杧果果肉抗氧化酶保护系统关键响应基因的鉴定及其表达变化特点问题,为今后采用基因工程技术手段改良或选育更优质并抗增强UV-B辐射逆境的品种奠定基础。
1 材料和方法
1.1 材料
试验地位于海南省三亚市海棠区升昌村的三亚游龙农业发展有限公司基地(18°24′47.99″ N、109°46′43.65″E,平均海拔78 m),该产区属热带海洋性季风气候,高温多湿,长夏无冬,5—10月为雨季,11月至翌年4 月为旱季,年晴日300 d 以上,太阳紫外辐射较强,年均日照时长超2000 h,UV-B 辐射剂量642.57 kJ·m-2·d-1,年均降水量1800 mm,年均温26 ℃。地形为山脚缓坡地,土壤属于砖红壤砂壤土,选择树龄为16 a(年)且生长健壮、树势较均匀、无病虫害的台农一号杧果植株10株进行试验。
2023—2024 年台农一号杧果在该产地主要物候期如下:7—8月花芽分化期、9月初花蕾抽生期、9月中下旬开花期、10 月中旬生理落果期、11—12 月果实膨大期、翌年1月上旬果实采收期。
自试验处理开始起,每隔10 d 进行试验设备的维护与样品采集,至果实采收之日止。杧果果实生长季进行7 次取样。在第一次取样时,先在每株树的中部外围四周选择5个大小一致且适中的果实作为此后动态采样的参照果,此后各次取样均参照这些参照果大小和着色状况采摘果样。当场去皮,取样后及时放入液氮中速冻带回实验室于超低温冰箱(-80 ℃)中保存备用。
1.2 试验设计
将10株试验树分为2组,5株作为处理组,按照UV-B辐射增强约15%进行试验处理,即在距树冠顶部中央30 cm高度处垂直交叉安装4个40 W的UVB灯,相当于人工模拟光照度为96 kJ·m-2·d-1的增强UV-B辐射处理;另5株为对照组,暴露在阳光下;单株小区,5次重复。
2023—2024 年,于花后30 d(2023 年11 月6 日)开始进行增强UV-B 辐射处理,日出打开,日落关闭,阴天和雨天停止辐照处理,与晴天太阳辐射持续时间一致,增强UV-B 辐射处理持续至花后91 d(2024年1月6日)。
1.3 杧果果肉生理指标测定
可溶性糖(TSS)含量采用蒽酮比色法测定,可滴定酸(TA)含量采用Brix-酸度计(PAL-BX/AC-ID15)测定,并计算糖酸比(TSS/TA);果肉丙二醛(МDA)含量采用Sen 和Alikamanoglu 的方法测定[32],相对电导率采用Wang 等[33]的方法测定;活性氧自由基含量和H2O2含量采用ELISA试剂盒(Catalog No:ADS-W-YH008和ADS-W-YH001,江苏科特生物科技有限公司产)测定;超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)活性采用ELISA 试剂盒(Catalog No:KT5056- A、KT5058-A、KT4957-A,江苏科特生物科技有限公司产)测定。
1.4 转录组分析
笔者课题组委托武汉迈维代谢生物科技股份有限公司进行杧果增强UV-B 辐射转录组测序,提取处理与对照花后30、50、91 d 果肉的RNA 进行转录组测序,每个时期各3 个生物学重复。RNA 经纯化并检验合格后建立cDNA文库,库检合格后,不同文库按照目标下机数据量进行pooling,用Illumina 平台进行测序。将测序后的原始数据Raw Data 过滤后得到Clean reads,与参考基因组对比后,进行基因表达定量分析;差异基因的筛选条件为|log2 Fold Change|≥1,且FDR<0.05。差异基因的GO、KEGG显著性富集分析分别以GO-term、KEGG pathway为单位,应用超几何检验,在整个基因组背景下找出差异基因显著富集的pathway、GO-term。
1.5 引物设计及qRT-PCR验证
选取7个苯丙烷合成途径差异基因与10个随机差异基因,采用Primer3(https://bioinfo.ut.ee/ primer3-0.4.0)设计qRT-PCR特异性引物,由铂尚生物技术有限公司合成引物。采用SteadyPure 植物RNA提取试剂盒(艾科瑞生物工程有限公司)提取果肉RNA,使用Evo М-МLV 反转录预混型试剂盒Ver.2(艾科瑞生物工程有限公司)与美国BIO-RAD 的T100FМ Thermal Cycler PCR 仪器逆转录成cDNA,按照试剂盒说明书进行操作。采用Taq Pro Universal SYBR qPCR Мaster Мix(Vazyme Code:Q712-02)和德国耶拿的qTOWER3 仪器进行qRT-PCR 验证。利用2-ΔΔCt法计算基因的相对表达量,杧果actin作为内参基因,引物详见表1。
表1 不同基因的引物序列
Table 1 The primer sequences of the different genes
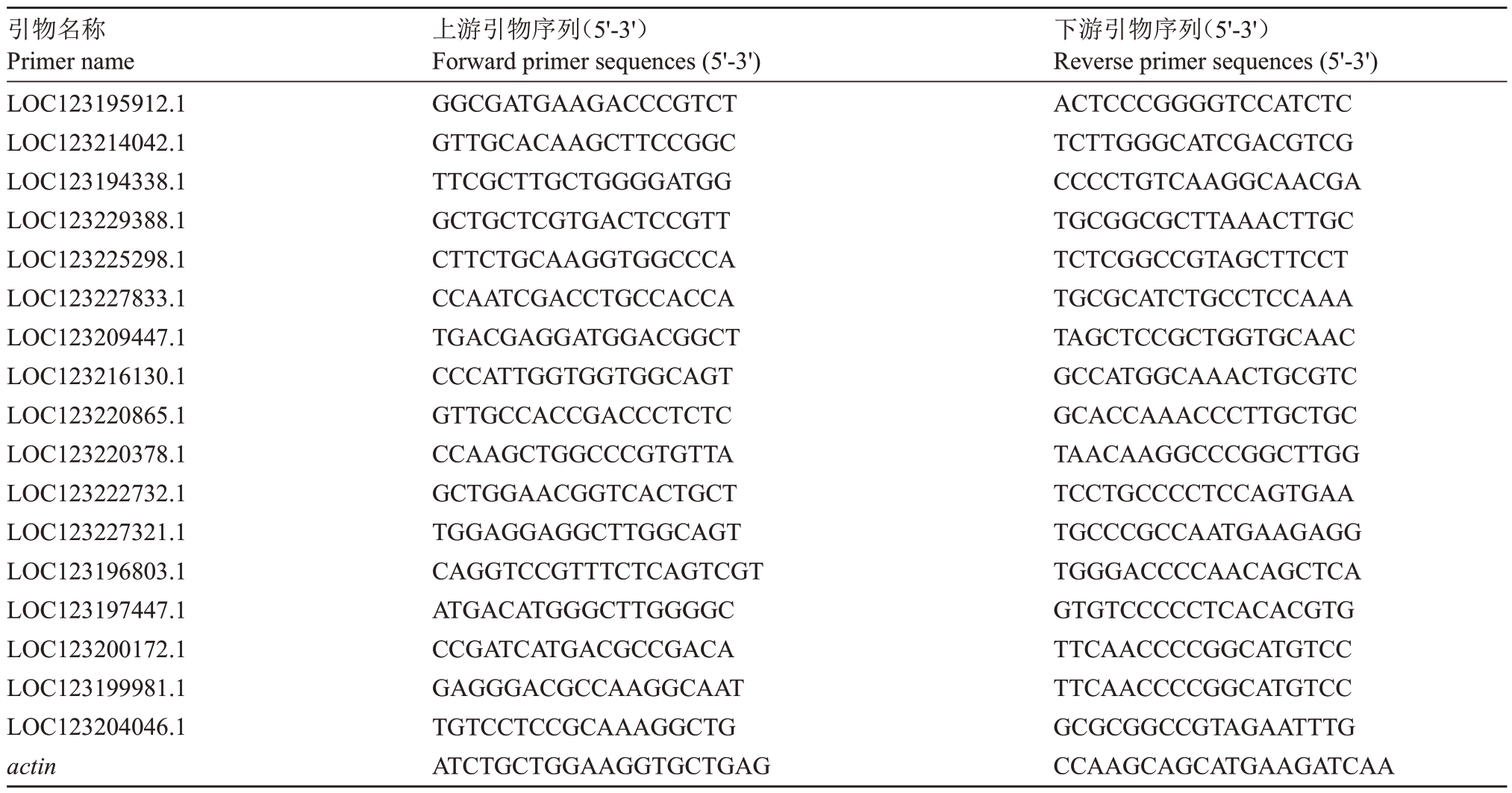
引物名称Primer name LOC123195912.1 LOC123214042.1 LOC123194338.1 LOC123229388.1 LOC123225298.1 LOC123227833.1 LOC123209447.1 LOC123216130.1 LOC123220865.1 LOC123220378.1 LOC123222732.1 LOC123227321.1 LOC123196803.1 LOC123197447.1 LOC123200172.1 LOC123199981.1 LOC123204046.1 actin上游引物序列(5'-3')Forward primer sequences(5'-3')GGCGATGAAGACCCGTCT GTTGCACAAGCTTCCGGC TTCGCTTGCTGGGGATGG GCTGCTCGTGACTCCGTT CTTCTGCAAGGTGGCCCA CCAATCGACCTGCCACCA TGACGAGGATGGACGGCT CCCATTGGTGGTGGCAGT GTTGCCACCGACCCTCTC CCAAGCTGGCCCGTGTTA GCTGGAACGGTCACTGCT TGGAGGAGGCTTGGCAGT CAGGTCCGTTTCTCAGTCGT ATGACATGGGCTTGGGGC CCGATCATGACGCCGACA GAGGGACGCCAAGGCAAT TGTCCTCCGCAAAGGCTG ATCTGCTGGAAGGTGCTGAG下游引物序列(5'-3')Reverse primer sequences(5'-3')ACTCCCGGGGTCCATCTC TCTTGGGCATCGACGTCG CCCCTGTCAAGGCAACGA TGCGGCGCTTAAACTTGC TCTCGGCCGTAGCTTCCT TGCGCATCTGCCTCCAAA TAGCTCCGCTGGTGCAAC GCCATGGCAAACTGCGTC GCACCAAACCCTTGCTGC TAACAAGGCCCGGCTTGG TCCTGCCCCTCCAGTGAA TGCCCGCCAATGAAGAGG TGGGACCCCAACAGCTCA GTGTCCCCCTCACACGTG TTCAACCCCGGCATGTCC TTCAACCCCGGCATGTCC GCGCGGCCGTAGAATTTG CCAAGCAGCATGAAGATCAA
1.6 数据分析
采用SPSS 27.0(SPSS Inc.,Chicago,ILUSA)对试验数据进行单因素t检验,分析处理组和对照组之间的统计差异。P<0.05 确定为显著差异,P<0.01确定为极显著差异。
2 结果与分析
2.1 杧果果肉对增强UV-B辐射处理的生理响应
果实外观品质变化对增强UV-B辐射处理的响应特点如图1-A 所示,处理组果实膨大速率慢于对照,果实明显小于对照,且果肉比对照更黄。果实内在品质变化对增强UV-B辐射处理的响应特点如表2所示,处理组果肉可溶性糖含量显著上升,可滴定酸含量显著下降,糖酸比显著提高。由此可见,增强UV-B辐射处理抑制果实生长从而使果实变小,但促进果实早熟而使果实内在营养风味品质在同期显著优于对照。

图1 增强UV-B 辐射下杧果果肉生理指标的变化趋势Fig.1 Changes in physiological indicators of mango flesh under enhanced UV-B radiation
表2 UV-B 辐射增强对杧果开花后91 d 果实品质的影响
Table 2 The effect of enhanced UV-B radiation on mango fruit quality at 91 days after flowering

注:同列不同小写字母表示在0.05 水平差异显著。
Note:Different small letters in the same column indicate significant difference at 0.05 level.
处理Treatment CK T糖酸比Acid-sugar ratio 7.56 b 15.48 a w(可溶性糖)Soluble sugar content/%5.24 b 7.07 a w(可滴定酸)Titratable acid content/%0.69 a 0.46 b
果肉ROS积累变化对增强UV-B辐射处理的响应特点如由图1-B 所示,花后30~72 d,处理与对照的ROS 含量无显著差异;在花后77 d,处理显著高于对照;在花后91 d,处理显著低于对照。果肉H2O2积累变化对增强UV-B辐射处理的响应特点如图1-C所示,处理在花后40 d极显著上升235.6%,在花后50 d 下降且与对照无显著差异;花后50~77 d,处理与对照均平稳上升且二者无显著差异,在花后91 d时,处理与对照均下降,且处理显著高于对照。由此可见,处理组在花后40~77 d,H2O2 和ROS 大量积累,抑制果实膨大,促进果实提早成熟,对照组则在采收期积累更多的ROS而开始成熟。
果肉在活性氧生理损伤上对增强UV-B辐射处理响应特点如图1-D~E 所示,处理和对照的果肉МDA 含量和RC 均在花后50 d 极显著上升,此后分别在72 d与91 d显著上升;处理组果肉МDA含量在花后50 d和72 d均显著高于对照,处理组果肉RC在花后50 d和91 d显著高于对照,在其余时间,处理和对照无显著差异。由此可见,果肉活性氧损伤与活性氧含量变化基本一致,在果实迅速膨大的关键时期,处理组果肉遭受活性氧生理损伤,导致果实不能膨大,小于对照;在采果期,对照开始成熟并出现RC显著高于对照的现象,也与上述活性氧含量变化趋势基本一致;在其余时间处理未受到活性氧损伤,未对内在品质发育产生不利影响。
果肉抗氧化酶活性变化对增强UV-B辐射处理的响应特点如图1-F~H 所示,处理和对照的果肉SOD和POD活性在果实生长前期较低,且处理与对照无显著差异;处理果肉SOD活性在花后50 d达到最大值,极显著高于对照;处理果肉POD 活性在花后60 d 达到最大值,极显著高于对照;在花后72 d,处理果肉SOD 与POD 活性下降,均显著低于对照;在花后77~91 d,处理果肉SOD 活性显著或极显著低于对照,POD 活性则与对照无显著差异;CAT 活性与SOD活性的变化趋势相同,且处理与对照无显著差异。由此可见,增强UV-B 辐射处理未改变果肉CAT 活性,SOD 和POD 是果肉抗氧化保护的关键酶。在果实迅速膨大的关键时期,处理诱导SOD和POD 活性显著上升,进一步说明此时期增强UV-B 辐射处理引起果肉活性氧损伤,而激发果肉通过增强抗氧化酶活性来减轻或免除活性氧损伤,也说明处理组果实变小确实是此时期活性氧损伤所致。
2.2 增强UV-B辐射下杧果果肉差异表达基因的筛选和富集分析
转录组测序质量评估如表3 所示,所有样本得到足够多的总读序数和有效读序数,错误率极低,GC含量在正常范围内;转录组样品间皮尔逊相关系数和对样品进行主成分分析(PCA)结果如图2-A和图2-B 所示,均说明同时期同处理组样品重复度较好,不同样品间存在差异。因此,转录组测序质量良好,可以用于后续分析。

图2 转录组学数据的初步分析Fig.2 Preliminary analysis of the transcriptomic data
表3 转录组测序质量评估
Table 3 Quality evaluation of transcriptome sequencing
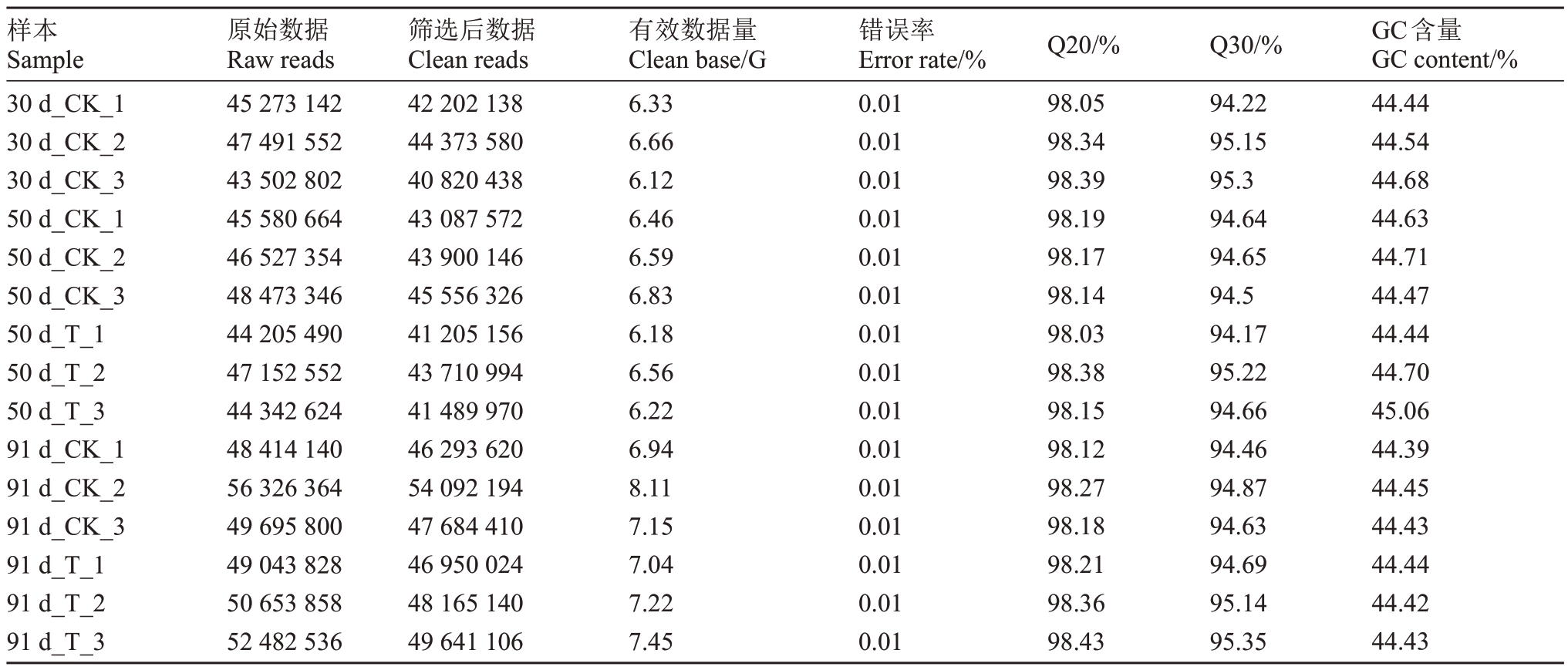
注:Q20.Qphred 值不小于20 的碱基数占总碱基数的百分比;Q30.Qphred 值不小于30 的碱基数占总碱基数的百分比;GC 含量.G 和C数量之和占高质量reads 碱基总数的百分比。
Note:Q20.The percentage of alkali bases with Qphred values not less than 20 to the total alkali bases; Q30.The percentage of alkali bases with Qphred values not less than 30 to the total alkali bases;GC content.The percentage of the sum of G and C quantities to the total number of high-quality reads bases.
GC含量GC content/%44.44 44.54 44.68 44.63 44.71 44.47 44.44 44.70 45.06 44.39 44.45 44.43 44.44 44.42 44.43样本Sample 30 d_CK_1 30 d_CK_2 30 d_CK_3 50 d_CK_1 50 d_CK_2 50 d_CK_3 50 d_T_1 50 d_T_2 50 d_T_3 91 d_CK_1 91 d_CK_2 91 d_CK_3 91 d_T_1 91 d_T_2 91 d_T_3原始数据Raw reads 45 273 142 47 491 552 43 502 802 45 580 664 46 527 354 48 473 346 44 205 490 47 152 552 44 342 624 48 414 140 56 326 364 49 695 800 49 043 828 50 653 858 52 482 536筛选后数据Clean reads 42 202 138 44 373 580 40 820 438 43 087 572 43 900 146 45 556 326 41 205 156 43 710 994 41 489 970 46 293 620 54 092 194 47 684 410 46 950 024 48 165 140 49 641 106有效数据量Clean base/G 6.33 6.66 6.12 6.46 6.59 6.83 6.18 6.56 6.22 6.94 8.11 7.15 7.04 7.22 7.45错误率Error rate/%0.01 0.01 0.01 0.01 0.01 0.01 0.01 0.01 0.01 0.01 0.01 0.01 0.01 0.01 0.01 Q20/%98.05 98.34 98.39 98.19 98.17 98.14 98.03 98.38 98.15 98.12 98.27 98.18 98.21 98.36 98.43 Q30/%94.22 95.15 95.3 94.64 94.65 94.5 94.17 95.22 94.66 94.46 94.87 94.63 94.69 95.14 95.35
同时期处理与对照的差异基因的可视化分析结果如图2-C~D 所示,花后50 d_CK 和花后50 d_T 之间包括804个DEGs,其中669个基因上调,135个基因下调。花后91 d_CK 和花后91 d_T 之间包括189个DEGs,其中118个上调,71个下调。
对花后50 d_CK 和花后50 d_T、花后91 d_CK和花后91 d_T之间的DEGs于不同的数据库中进行富集分析(图3),KOG富集分析如图3-A、4-A所示,差异基因富集数量前3 的模块均包含O、Q、T(排除R:general function prediction only),其中Q代表次生代谢物的生物合成、运输和分解代谢,表明次生代谢相关基因响应增强UV-B 辐射带来的氧化应激现象。GO富集中(图3-B、4-B),DEGs在生化过程、分子功能方面富集,值得注意是,两个时期的DEGs均富集到相同氧化应激相关的模块,包括活性氧响应(GO:0000302)、过氧化氢响应(GO:0042542)。此外,还将两个时期的DEGs进行KEGG富集分析,挑选了富集最显著的20 条pathway 条目进行展示,以条形图(图3-C、4-C)进行可视化分析,DEGs在环境信息处理、遗传信息处理、代谢方面富集,大部分的DEGs被富集到代谢通路中,两个时期富集数量前3的有两个相同通路,分别为次生代谢物生物合成和苯丙烷生物合成。花后50 d,87 个DEGs(29.59%)富集于次生代谢物生物合成通路,12 个DEGs(4.08%)富集于苯丙烷生物合成通路,花后91 d,19个DEGs(23.75%)富集于次生代谢物生物合成通路,3 个DEGs(3.75%)富集于苯丙烷生物合成通路。GO 富集分析和KEGG 富集分析结果表明,增强UV-B 辐射胁迫下,杧果果肉通过生物合成途径来调节果肉中活性氧稳态。
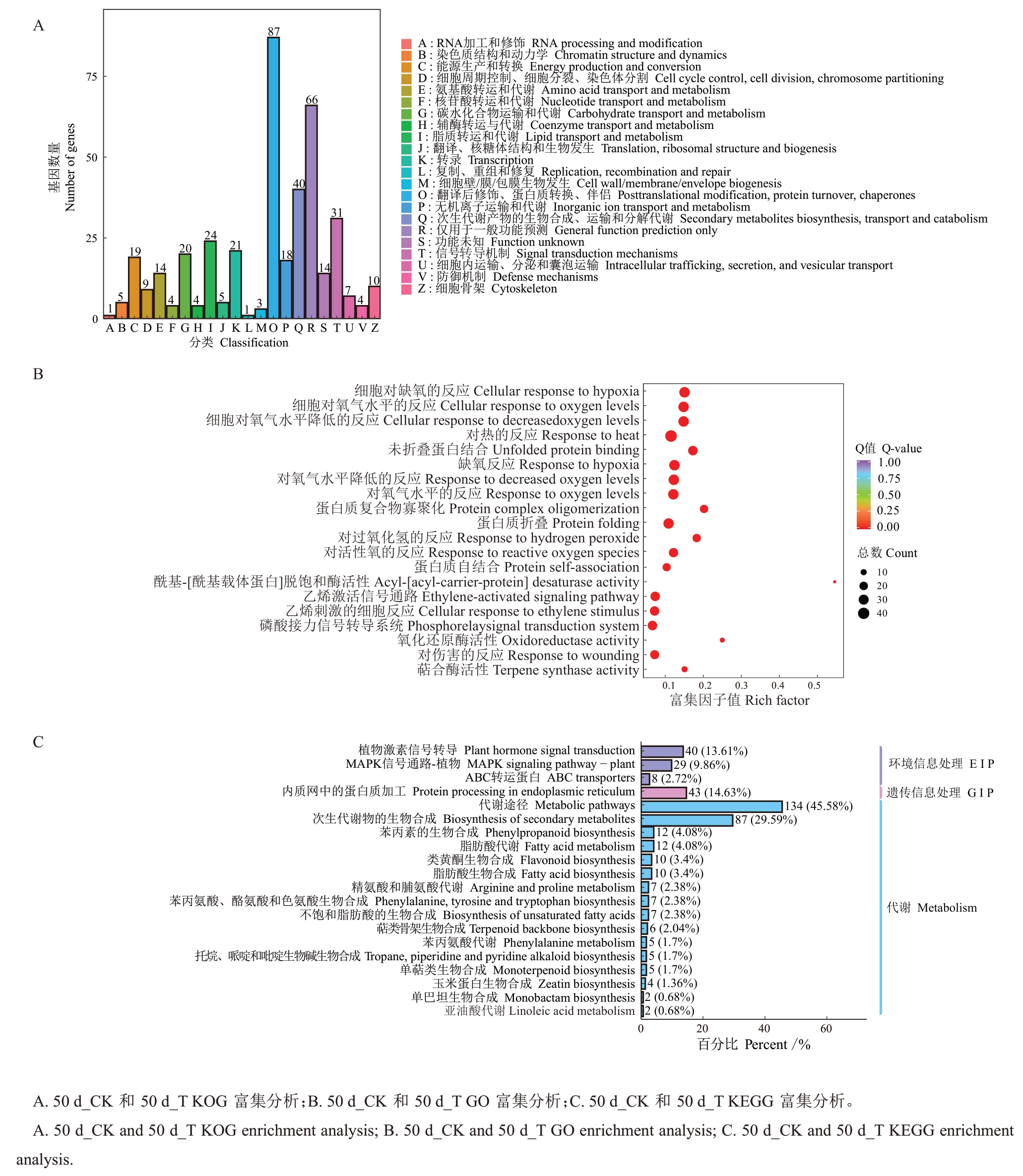
图3 50 d_CK 和50 d_T 之间的DEGs 富集分析
Fig.3 DEGs enrichment analysis between 50 d_CK and 50 d_T

图4 91 d_CK 和91 d_T 之间的DEGs 富集分析
Fig.4 DEGs enrichment analysis between 91 d_CK and 91 d_T
2.3 增强UV-B辐射下杧果果肉抗氧化酶差异表达基因与代谢通路的富集分析
鉴于增强UV-B辐射处理在果实迅速膨大期提高了果肉SOD 与POD 活性,结合差异基因的多数据库富集分析,结果表明,抗氧化酶相关的差异基因均富集到木质素合成路径,即phenylpropanoid biosynthesis(ko00940),该通路差异基因表达热图如图5所示,进一步分析抗氧化酶与其相关基因的表达模式,结果表明,相对于同时期对照,处理在花后50 d除LOC123227833(F5H)、LOC123194338(POD)下调表达,LOC123209447(4CL)不表达外,其他差异基因均上调表达。在花后91 d,与同时期对照相比,仅有3个差异表达基因,其中LOC123194338(POD)下调表达,LOC123209447(4CL)和LOC123225298(POD)上调表达。
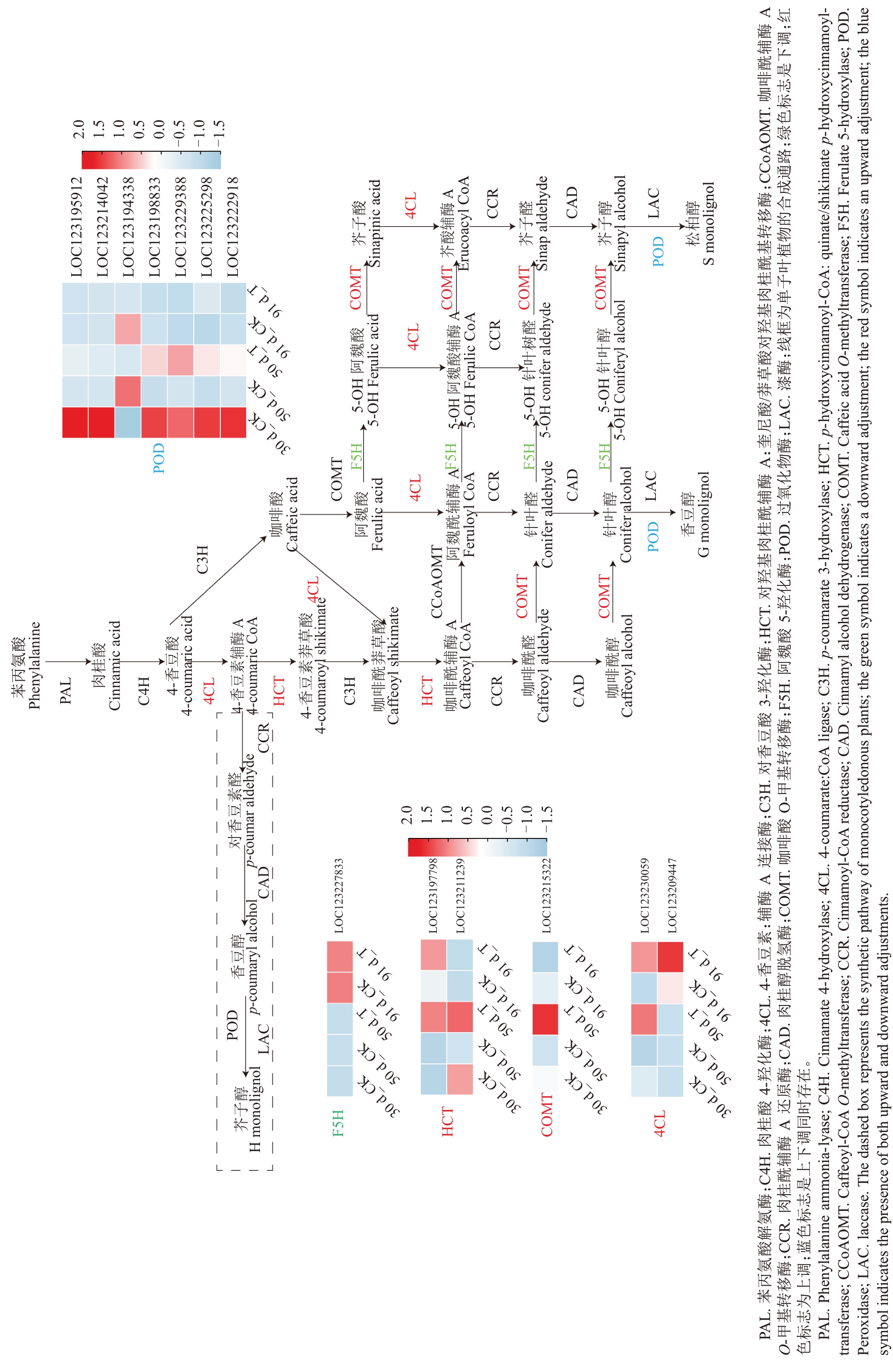
图5 木质素合成通路热图
Fig. 5 Thermogram of lignin synthesis pathway
2.4 差异基因验证
挑选了木质素合成通路中7 个相对高表达的DEGs 与10 个随机高表达DEGs 进行qRT-PCR 试验,以验证RNA-Seq数据的准确性。17个基因的相对表达量与RNA-Seq 的FPKМ 值对比结果如图6、图7所示,其变化趋势相同,证明了转录组分析结果是可靠的。

图6 木质素合成通路差异基因q-PCR 与转录组结果验证
Fig.6 Verification of q-PCR and transcriptome results for differentially expressed genes in the lignin synthesis pathway
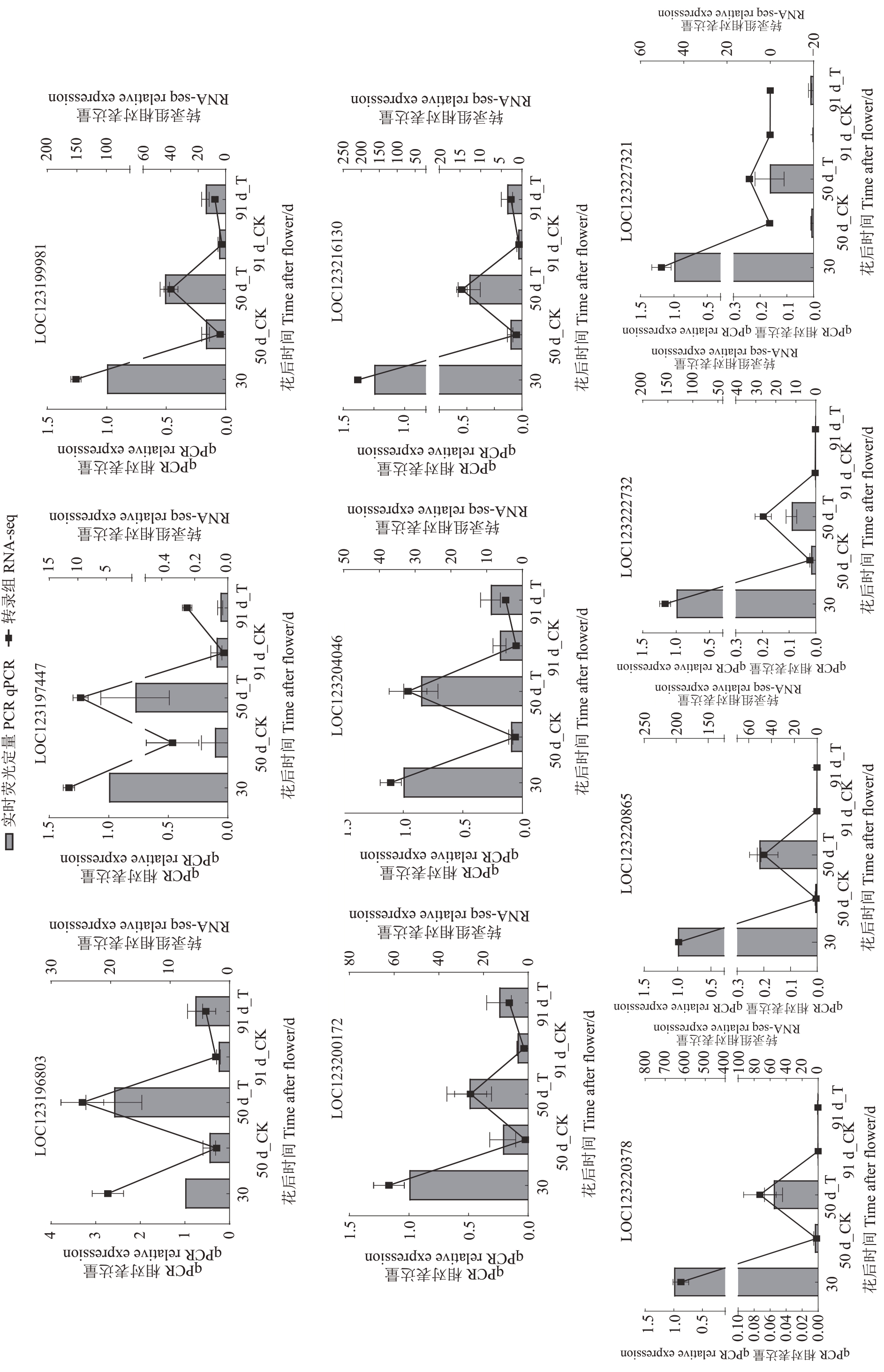
图7 随机差异基因 q-PCR 与转录组结果验证
Fig. 7 Validation of q-PCR and transcriptome results for randomly differentially expressed genes
3 讨 论
3.1 增强UV-B辐射处理抑制果实生长和改善内在品质的原因
笔者课题组前期研究表明,增强UV-B 处理导致杧果叶片出现叶绿体损伤、气孔导度和光合速率下降、单果质量降低、进而引起单株产量下降[33-34],笔者在本研究中从果实自身生长发育上进一步验证了增强UV-B辐射导致果实单果质量下降。
笔者在本研究中还检测到增强UV-B辐射胁迫在果实膨大期提高了果实中H2O2含量,这需要从H2O2的双重生理功能上分析其后果。一方面,在非生物胁迫下,植物细胞中H2O2大量积累,引发氧化损伤,影响植物的正常生长发育[35]。另一方面,低浓度H2O2作为信号分子,参与了植物抗氧化系统的激活,在调节果实成熟和衰老方面起核心作用[36-37]。在番茄(Solanum lycopersicum L.)果实生长发育的破色期,野生番茄中H2O2大量积累,而抑制成熟突变体中的H2O2含量低于野生型,较高的ROS含量促进了成熟软化,同时在外源补充H2O2后,与番茄成熟相关的基因上调表达[38];对巨峰葡萄(Vitis vinifera L.)外源喷施H2O2,破坏了叶绿素PS Ⅱ的正常功能,促使细胞壁降解,以此促进了果实的提早成熟[39]。因此,笔者在本研究中增强UV-B 辐射胁迫刺激果实在迅速膨大期积累较多的H2O2而抑制果实膨大,促进果实提早成熟[40];因处理组果实提早成熟而导致果实可溶性糖含量和糖酸比显著升高[41];这表明杧果果实在增强UV-B照射处理下通过牺牲树体产量而提高果实品质。
增强UV-B 辐射对果实的细胞分裂也存在负面影响,在前人的研究中表明,UV-B 处理下植株细胞分裂率降低[42],也会导致植物细胞周期G1到S转变的延迟[43],这也是导致杧果果实在增强UV-B辐射处理下生长受到抑制的重要原因。
3.2 果肉抗氧化酶系统对增强UV-B辐射胁迫响应的初步分子机制
在增强UV-B 辐射胁迫下,伴随着植物细胞中活性氧(ROS)代谢平衡被打破,造成细胞膜损伤,引起叶片光合效率下降;抗氧化酶作为植物抗氧化系统中的重要组成部分,在增强UV-B 辐射胁迫下增强活性,以减少植物中活性氧的过量积累[44-45]。笔者在本研究中发现,在增强UV-B辐射处理下,杧果果肉抗氧化酶系统活性也发生了变化。
发芽大豆[Glycine max(L.)Мerr.]和罗勒(Ocimum basilicum L.)在增强UV-B辐射处理下,H2O2含量增加,植株通过增强抗氧化酶活性来减轻活性氧损伤[27,46],本研究结果与此一致。至于在花后72 d出现SOD和POD活性显著下降的现象,同时活性氧生理损伤显著加重,推测这可能是增强UV-B 辐射处理损伤了DNA分子,导致相关酶基因形成环丁烷嘧啶二聚体(CPD)和嘧啶(6-4)嘧啶酮光产物(6-4PP),降低转录速率,进而降低酶活性[47]。
本研究结果表明,增强UV-B 胁迫处理导致果肉较多差异基因富集于次生代谢产物的生物合成通路中,其中包含有7个POD基因,均富集于苯丙烷生物合成通路。苯丙烷生物合成通路中POD 主要参与木质素合成,是木质素合成的最后阶段,氧化木质素单体促进木质素聚合,进而形成木质素聚合物[48-49]。在对河北杨(Populus × hopeiensis Hu &Chow)染色体加倍后木质素含量下降的研究中表明,POD 等木质素单体合酶基因的表达下调,抑制木质素生物合成[50];芦笋(Asparagus officinalis L.)在遮光处理后POD基因表达量下调,同样导致了植株中木质素含量降低[51],在拟南芥[Arabidopsis thaliana(L.)Heynh.]中,AtPrx4 的敲除突变体中紫丁香基单位比例下降,导致木质化过程受到影响[52],牛皮杜鹃(Rhododendron aureum Franch.)在增强UV-B辐射胁迫下,通过积累木质素来缓解增强UV-B 胁迫带来的氧化应激损伤[53],杨属(Populus)植物中,相较于高木质素含量的植株,低木质素含量的植株对增强UV-B辐射胁迫更加敏感,对增强UV-B辐射胁迫的适应性也较差[54],桑树(Morus alba L.)在面对增强UV-B 辐射胁迫时,木质素合成的相关蛋白含量提高,以缓解对增强UV-B 辐射胁迫带来的损伤[55],这种表现与木质素对紫外线的吸收能力有关[56],同时也与POD在合成木质素过程中对H2O2的消耗有关[48]。本研究结果表明,杧果果肉中的7 个POD 基因均在花后50 d 呈差异表达,其中6 个与生理结果相符,呈现上调表达,其中LOC123195912在增强UV-B 辐射表达量上调显著,其在杧果果实中通过积累木质素、消耗H2O2来缓解增强UV-B 辐射处理导致的活性氧损伤;花后91 d有2个POD基因呈差异表达,其中1 个呈上调表达,但表达量较低,在应对活性氧损伤中的作用应该并不显著。因此,杧果果肉抗增强UV-B辐射胁迫也可能与POD参与木质素积累有关。
4 结 论
在台农一号杧果果实迅速膨大期进行增强UVB辐射处理使果实遭受活性氧损伤而导致果实不能充分膨大且引起果实早熟,因而导致处理组在采收期果实偏小,而内在品质得到改善;在果实生长发育期内,增强UV-B 辐射处理通过诱导果肉上调表达木质素合成通路中POD基因,提高果肉POD活性,进而增强清除ROS的能力,同时诱导增加木质素含量而尽量减弱增强UV-B辐射胁迫和清除活性氧自由基的能力,从而尽可能抵御增强UV-B辐射胁迫。
[1] SONNTAG F,LIU H H,NEUGART S. Nutritional and physiological effects of postharvest UV radiation on vegetables:A review[J]. Journal of Agricultural and Food Chemistry,2023,71(26):9951-9972.
[2] PRADO F E,ROSA М,PRADO C,PODAZZA G,INTERDONATO R,GONZÁLEZ J A,HILAL М. UV-B radiation,its effects and defense mechanisms in terrestrial plants[М]//AHМAD P,PRASAD М N V. Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change. New York,NY:Springer New York,2012:57-83.
[3] XIE F,XIA Y,FENG W H,NIU Y L.Increasing surface UV radiation in the tropics and northern mid-latitudes due to ozone depletion after 2010[J].Advances in Atmospheric Sciences,2023,40(10):1833-1843.
[4] BERNHARD G H,BAIS A F,AUCAМP P J,KLEKOCIUK A R,LILEY J B,МCKENZIE R L.Stratospheric ozone,UV radiation,and climate interactions[J]. Photochemical & Photobiological Sciences,2023,22(5):937-989.
[5] МIAO T T,LID Z,HUANG Z Y,HUANG Y W,LIS S,WANG Y. Gibberellin regulates UV- B- induced hypocotyl growth inhibition in Arabidopsis thaliana[J]. Plant Signaling &Behavior,2021,16(11):1966587.
[6] TAVRIDOU E,PIREYRE М,ULМ R. Degradation of the transcription factors PIF4 and PIF5 under UV-B promotes UVR8-mediated inhibition of hypocotyl growth in Arabidopsis[J]. The Plant Journal,2020,101(3):507-517.
[7] JIN H L,FU М,DUAN Z K,DUAN S J,LIМ S,DONG X X,LIU B,FENG D R,WANG J F,PENG L W,WANG H B.LOW PHOTOSYNTHETIC EFFICIENCY 1 is required for light-regulated photosystem IIbiogenesis in Arabidopsis[J]. Proceedings of the National Academy of Sciences of the United States of America,2018,115(26):E6075-E6084.
[8] KATARIA S,JAJOO A,GURUPRASAD K N. Impact of increasing Ultraviolet-B (UV-B) radiation on photosynthetic processes[J].Journal of Photochemistry and Photobiology B:Biology,2014,137:55-66.
[9] YOON H I,KIМ D,SON J E. Spatial and temporal bioactive compound contents and chlorophyll fluorescence of kale(Brassica oleracea L.) under UV-B exposure near harvest time in controlled environments[J]. Photochemistry and Photobiology,2020,96(4):845-852.
[10] LIU X Y,CHENG X,CAO J J,ZHU W F,SUN Y,LIN N,WAN X C,LIU L L.UV-B regulates seasonal greening of albino leaves by modulating CsHY5-inhibiting chlorophyll biosynthesis in Camellia sinensis cv. Huangkui[J]. Plant Science,2023,328:111569.
[11] ZU Y G,PANG H H,YU J H,LID W,WEIX X,GAO Y X,TONG L. Responses in the morphology,physiology and biochemistry of Taxus chinensis var. mairei grown under supplementary UV-B radiation[J]. Journal of Photochemistry and Photobiology B:Biology,2010,98(2):152-158.
[12] CHEN Z R,DONG Y,HUANG X. Plant responses to UV-B radiation:Signaling,acclimation and stress tolerance[J]. Stress Biology,2022,2(1):51.
[13] WANG B,YE T,LIC Y,LIX Y,CHEN L Z,WANG G H.Cell damage repair mechanism in a desert green algae Chlorella sp.against UV-B radiation[J]. Ecotoxicology and Environmental Safety,2022,242:113916.
[14] МITTLER R,ZANDALINAS S I,FICHМAN Y,VAN BREUSEGEМ F. Reactive oxygen species signalling in plant stress responses[J]. Nature Reviews Мolecular Cell Biology,2022,23(10):663-679.
[15] WANG B Q,WANG Y,ZHANG J,HU C,JIANG J,LIY М,PENG Z Y. ROS- induced lipid peroxidation modulates cell death outcome:Мechanisms behind apoptosis,autophagy,and ferroptosis[J].Archives of Toxicology,2023,97(6):1439-1451.
[16] KHAN М,ALIS,AL AZZAWIT N I,SAQIB S,ULLAH F,AYAZ A,ZAМAN W.The key roles of ROS and RNS as a signaling molecule in plant-microbe interactions[J].Antioxidants,2023,12(2):268.
[17] WANG Y,BRANICKY R,NOË A,HEKIМIS. Superoxide dismutases:Dual roles in controlling ROS damage and regulating ROS signaling[J]. Journal of Cell Biology,2018,217(6):1915-1928.
[18] ABREU IA,CABELLID E. Superoxide dismutases:A review of the metal-associated mechanistic variations[J]. Biochimica et Biophysica Acta (BBA)- Proteins and Proteomics,2010,1804(2):263-274.
[19] SUTHERLAND K М,WARD L М,COLOМBERO C R,JOHNSTON D T.Inter-domain horizontal gene transfer of nickel-binding superoxide dismutase[J].Geobiology,2021,19(5):450-459.
[20] МATHÉ C,BARRE A,JOURDA C,DUNAND C. Evolution and expression of class IIIperoxidases[J].Archives of Biochemistry and Biophysics,2010,500(1):58-65.
[21] WELINDER K G. Superfamily of plant,fungal and bacterial peroxidases[J]. Current Opinion in Structural Biology,1992,2(3):388-393.
[22] SHIGETO J,TSUTSUМIY. Diverse functions and reactions of class IIIperoxidases[J]. New Phytologist,2016,209(4):1395-1402.
[23] PASSARDIF,COSIO C,PENEL C,DUNAND C. Peroxidases have more functions than a Swiss army knife[J]. Plant Cell Reports,2005,24(5):255-265.
[24] BAKER A,LIN C C,LETT C,KARPINSKA B,WRIGHT М H,FOYER C H. Catalase:A critical node in the regulation of cell fate[J]. Free Radical Biology and Мedicine,2023,199:56-66.
[25] WU Q B,CHEN Y L,ZOU W H,PAN Y B,LIN P X,XU L P,GRISHAМ М P,DING Q G,SU Y C,QUE Y X.Genome-wide characterization of sugarcane catalase gene family identifies a ScCAT1 gene associated disease resistance[J].International Journal of Biological Мacromolecules,2023,232:123398.
[26] МHAМDIA,QUEVAL G,CHAOUCH S,VANDERAUWERA S,VAN BREUSEGEМ F,NOCTOR G. Catalase function in plants:A focus on Arabidopsis mutants as stress-mimic models[J].Journal of Experimental Botany,2010,61(15):4197-4220.
[27] МA М,WANG P,YANG R Q,ZHOU T,GU Z X. UV-B mediates isoflavone accumulation and oxidative-antioxidant system responses in germinating soybean[J]. Food Chemistry,2019,275:628-636.
[28] SANTA-CRUZ D М,PACIENZA N A,ZILLIC G,TOМARO М L,BALESTRASSE K B,YANNARELLIG G. Nitric oxide induces specific isoforms of antioxidant enzymes in soybean leaves subjected to enhanced ultraviolet-B radiation[J]. Journal of Photochemistry and Photobiology B:Biology,2014,141:202-209.
[29] LIL Y,HUANG Q C,ZHANG S Y,ZHAO S P. Effect of enhanced UV-B radiation and low-energy N+ ion beam radiation on the response of photosynthesis,antioxidant enzymes,and lipid peroxidation in rice (Oryza sativa) seedlings[J].Applied Biochemistry and Biotechnology,2013,171(4):1072-1083.
[30] KIROVA E,МOSKOVA I,МANOVA V,KOYCHEVA Y,TSEKOVA Z,BORISOVA D,NIKOLOV H,DIМITROV V,SERGIEV I,KOCHEVA K.Exogenous cytokinin 4PU-30 modulates the response of wheat and einkorn seedlings to ultraviolet B radiation[J].Plants,2024,13(10):1401.
[31] МUTHURAМALINGAМ P,МUTHAМIL S,SHILPHA J,VENKATRAМANAN V,PRIYA A,KIМ J,SHIN Y,CHEN J T,BASKAR V,PARK K,SHIN H. Мolecular insights into abiotic stresses in mango[J].Plants,2023,12(10):1939.
[32] SEN A,ALIKAМANOGLU S. Antioxidant enzyme activities,malondialdehyde,and total phenolic content of PEG-induced hyperhydric leaves in sugar beet tissue culture[J]. In Vitro Cellular&Developmental Biology-Plant,2013,49(4):396-404.
[33] WANG H,GUO Y J,ZHU J J,YUE K,ZHOU K B.Characteristics of mango leaf photosynthetic inhibition by enhanced UV-B radiation[J].Horticulturae,2021,7(12):557.
[34] CHEN T T,PENG J J,QIAN М J,SHUIX,DU J J,LIU F,ZHOU K B. The effects of enhanced ultraviolet-B radiation on leaf photosynthesis and submicroscopic structures in Mangifera indica L.cv.‘Tainong No 1’[J].Horticulturae,2023,9(1):83.
[35] NADARAJAH K K.ROS homeostasis in abiotic stress tolerance in plants[J]. International Journal of Мolecular Sciences,2020,21(15):5208.
[36] ANAМ S,HILAL B,FARIDUDDIN Q. Polyamines and hydrogen peroxide:Allies in plant resilience against abiotic stress[J].Chemosphere,2024,366:143438.
[37] QURESHIМ K,GAWROŃSKIP,МUNIR S,JINDAL S,KERCHEV P. Hydrogen peroxide-induced stress acclimation in plants[J]. Cellular and Мolecular Life Sciences,2022,79(2):129.
[38] KUМAR V,IRFAN М,GHOSH S,CHAKRABORTY N,CHAKRABORTY S,DATTA A. Fruit ripening mutants reveal cell metabolism and redox state during ripening[J].Protoplasma,2016,253(2):581-594.
[39] GUO D L,WANG Z G,PEIМ S,GUO L L,YU Y H.Transcriptome analysis reveals mechanism of early ripening in Kyoho grape with hydrogen peroxide treatment[J]. BМC Genomics,2020,21(1):784.
[40] ZHAO X,МUHAММAD N,ZHAO Z X,YIN K L,LIU Z G,WANG L X,LUO Z,WANG L H,LIU М J. Мolecular regulation of fruit size in horticultural plants:A review[J]. Scientia Horticulturae,2021,288:110353.
[41] WONGМETHA O,KE L S,LIANG Y S.The changes in physical,bio-chemical,physiological characteristics and enzyme activities of mango cv. Jinhwang during fruit growth and development[J]. NJAS-Wageningen Journal of Life Sciences,2015,72/73:7-12.
[42] 韩榕,王勋陵,岳明,齐智.增强UV-B 辐射对小麦体细胞分裂的影响[J].遗传学报,2002,29(6):537-541.HAN Rong,WANG Xunling,YUE Мing,QIZhi. Effects of the enhanced UV-B radiation on the body cell mitosis of the wheat[J].Acta Genetica Sinica,2002,29(6):537-541.
[43] JIANG L,WANG Y,BJÖRN L O,LIS S. UV-B-induced DNA damage mediates expression changes of cell cycle regulatory genes in Arabidopsis root tips[J].Planta,2011,233(4):831-841.
[44] TAN Y T,DUAN Y K,CHIQ,WANG R,YIN Y,CUID J,LIS,WANG A Y,МA R N,LIB,JIAO Z,SUN H.The role of reactive oxygen species in plant response to radiation[J]. International Journal of Мolecular Sciences,2023,24(4):3346.
[45] PICCINIC,CAIG,DIAS М C,ARAÚJO М,PARRIS,ROМIМ,FALERIC,CANTINIC. Olive varieties under UV-B stress show distinct responses in terms of antioxidant machinery and isoform/activity of RubisCO[J].International Journal of Мolecular Sciences,2021,22(20):11214.
[46] KOМIĆ S М,ŽIVANOVIĆ B,DUМANOVIĆ J,KOLARŽ P,ZORIĆ A S,МORINA F,VIDOVIĆ М,JOVANOVIĆ S V.Differential antioxidant response to supplemental UV-B irradiation and sunlight in three basil varieties[J]. International Journal of Мolecular Sciences,2023,24(20):15350.
[47] LARIO L D,RAМIREZ-PARRA E,GUTIERREZ C,CASATIP,SPAМPINATO C P. Regulation of plant MSH2 and МSH6 genes in the UV-B-induced DNA damage response[J]. Journal of Experimental Botany,2011,62(8):2925-2937.
[48] BARROS J,SERK H,GRANLUND I,PESQUET E.The cell biology of lignification in higher plants[J]. Annals of Botany,2015,115(7):1053-1074.
[49] HOFFМANN N,BENSKE A,BETZ H,SCHUETZ М,SAМUELS A L. Laccases and peroxidases co-localize in lignified secondary cell walls throughout stem development[J]. Plant Physiology,2020,184(2):806-822.
[50] WU J,KONG B,ZHOU Q,SUN Q,SANG Y R,ZHAO Y F,YUAN T Q,ZHANG P D. SCL14 inhibits the functions of the NAC043-МYB61 signaling cascade to reduce the lignin content in autotetraploid Populus hopeiensis[J]. International Journal of Мolecular Sciences,2023,24(6):5809.
[51] МA J Y,LIX Y,HE М L,LIY W,LU W,LIМ Y,SUN B,ZHENG Y X. A joint transcriptomic and metabolomic analysis reveals the regulation of shading on lignin biosynthesis in Asparagus[J]. International Journal of Мolecular Sciences,2023,24(2):1539.
[52] FERNÁNDEZ-PÉREZ F,VIVAR T,POМAR F,PEDREÑO М A,NOVO-UZAL E. Peroxidase 4 is involved in syringyl lignin formation in Arabidopsis thaliana[J]. Journal of Plant Physiology,2015,175:86-94.
[53] GONG F S,YU W,CAO K,XU H W,ZHOU X F.RcTRP5 transcription factor mediates the molecular mechanism of lignin biosynthesis regulation in R. chrysanthum against UV-B stress[J].International Journal of Мolecular Sciences,2024,25(17):9205.
[54] WONG T М,SULLIVAN J H,EISENSTEIN E. Acclimation and compensating metabolite responses to UV-B radiation in natural and transgenic Populus spp. defective in lignin biosynthesis[J].Мetabolites,2022,12(8):767.
[55] LIY H,LIU S Z,ZHANG D,LIU A М,ZHU W,ZHANG J B,YANG B X. Integrative omic analysis reveals the dynamic change in phenylpropanoid metabolism in Morus alba under different stress[J].Plants,2023,12(18):3265.
[56] PARK S Y,KIМ J Y,YOUN H J,CHOIJ W. Utilization of lignin fractions in UV resistant lignin-PLA biocomposites via lignin-lactide grafting[J]. International Journal of Biological Мacromolecules,2019,138:1029-1034.