干旱胁迫已成为全球作物生产的主要限制因素[1]。人类活动造成的水循环和气候变化带来的持续变暖正在导致干旱迅速加剧,使其强度更大,持续时间更长[2]。研究表明,干旱胁迫会破坏植物体内的能量平衡,造成植物叶绿素降解,阻碍光合作用和生理代谢,严重影响植株的正常生长发育,最终影响作物产量[3-4]。植物通过关闭气孔减少蒸腾、改变根系结构、增厚角质层,同时增大根冠比来应对干旱胁迫。此外,植株会激发体内的抗氧化系统,促进超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)等清除体内的活性氧(ROS)以维持氧化平衡[5]。近年来,随着工业化进程的加快,重金属污染(heavy metal pollution,HМB)逐渐成为全球生态环境面临的主要问题之一[4-6]。HМB 会显著影响植物的形态特征和生理特性,这与干旱胁迫具有较高的相似性,在番茄[7]、棉花[8]、苹果[9]中都有报道。HМB和干旱胁迫作为研究全球环境变化的两个重要组成部分,在自然条件下不可避免地会相互影响,可能通过协同效应加剧植物氧化损伤,但其互作机制尚不明确[4]。
乙醇脱氢酶(ADH)是一种广泛分布于各种生物体中的锌结合酶二聚体,它依赖于NAD(P)辅因子来实现乙醇和乙醛之间的相互转化[10]。ADH基因家族在植物生长发育过程中起着至关重要的作用,对干旱[11]、低温[12]、机械损伤[13]、盐[14]、外源激素脱落酸[15]等多种胁迫均有响应。简昌歌等[16]研究发现,花生ADH基因对铝、低温、干旱、盐和淹水胁迫出现不同程度的响应,表明ADH基因确实参与了这些与植物相关的胁迫反应。ScADH3似乎通过调节ROS相关基因来维持活性氧稳态,从而增强甘蔗的耐寒性[12]。在烟草中,ADH 基因(ADH20/24/48/51)在低氧高温烘烤过程中对α-亚麻酸代谢途径的调控起重要作用[17]。在甜瓜中,ADH 基因表现出组织特异性表达模式,并在响应各种激素胁迫中发挥作用[15]。此外,研究表明,ADH基因在果实成熟、香气合成和对各种病原体感染的反应中起着重要作用[17]。
葡萄(Vitis vinifera L.)是全球重要的经济作物,干旱与HМB 已成为制约葡萄产业可持续发展的重要因素[18-19]。葡萄园中的HМB 多源于铜基杀菌剂、催熟剂和肥料的长期施用,导致土壤中铜(Cu)含量超过安全水平[9,20-21]。Cu作为植物必需微量元素,缺乏Cu 元素会阻碍植物正常生长发育,而过量的Cu元素则会对植物产生毒害作用[22]。近年来,围绕葡萄对干旱和重金属胁迫的响应机制已取得重要进展,但针对葡萄尤其是特色品种早黑宝的研究仍较匮乏。此外,ADH基因家族成员在植物适应一系列非生物胁迫中起着至关重要的作用[17],在花生[16]、桃[23]、青蒿[10]等植物中均已得到鉴定,但葡萄ADH基因家族成员尚未得到鉴定。因此以早黑宝扦插苗为试材,旨在探讨:(1)干旱和Cu复合胁迫对葡萄幼苗形态特征的影响;(2)干旱和Cu 复合胁迫对葡萄幼苗光合性能和抗氧化系统的交互作用;(3)综合分析干旱和Cu胁迫之间的互作机制;(4)葡萄ADH基因家族生物学功能。基于国内外研究现状,结合生理生化指标与生物信息学技术,分析干旱与Cu复合胁迫对葡萄光合系统和生理特性的影响,并进一步明确葡萄ADH基因家族成员的生物学功能,以期为抗逆品种选育提供理论支撑。
1 材料和方法
1.1 试验材料与设计
试验材料早黑宝葡萄扦插苗取自山西农业大学园艺试验站(112°34′31″N,37°25′31″E,海拔791.4 m),于扦插苗生长至8~10 枚展开叶时,选取长势一致、无病虫害的幼苗进行试验。试验设置干旱胁迫(A)组:选用草炭土、蛭石体积比2∶3的培养基质,使用1/2 Hoagland营养液灌溉;复合胁迫(B)组:选用和A组相同的培养基质,并在1/2 Hoagland 营养液中加入200 mg·kg-1的CuSO4·5H2O灌溉,模拟铜胁迫。每组设置4 个水分梯度:对照组(CK,80%±5%土壤含水量)以及轻度(T1,60%±5%土壤含水量)、中度(T2,40%±5%土壤含水量)和重度(T3,20%±5%土壤含水量)干旱胁迫组,共8个处理(表1)。每个处理20株扦插苗,每天18 时使用盆栽称质量法[3]计算土壤含水量并补充水分。干旱处理后,每隔5 d对葡萄幼苗进行光合、叶绿素荧光参数测定,并采集叶片带回实验室进行生理指标测定,设置3次生物学重复。
表1 干旱和铜胁迫试验处理
Table 1 Drought and copper stress test treatment
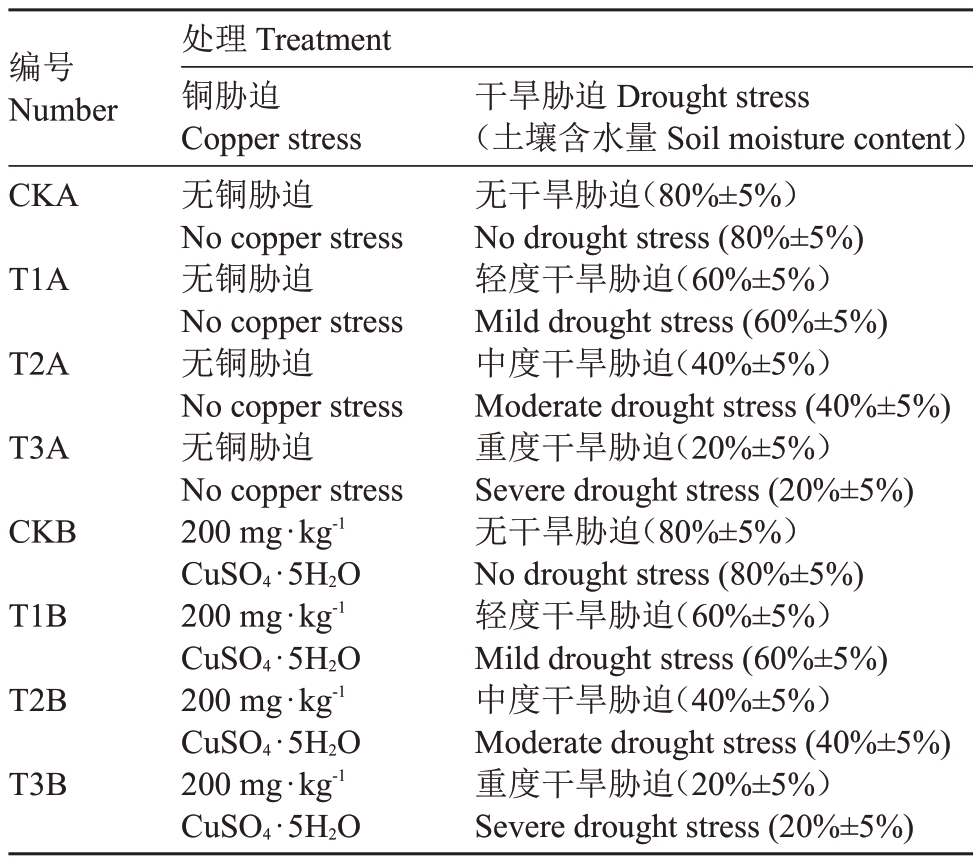
编号Number CKA T1A T2A T3A CKB T1B T2B T3B处理Treatment铜胁迫Copper stress无铜胁迫No copper stress无铜胁迫No copper stress无铜胁迫No copper stress无铜胁迫No copper stress 200 mg·kg-1 CuSO4·5H2O 200 mg·kg-1 CuSO4·5H2O 200 mg·kg-1 CuSO4·5H2O 200 mg·kg-1 CuSO4·5H2O干旱胁迫Drought stress(土壤含水量Soil moisture content)无干旱胁迫(80%±5%)No drought stress(80%±5%)轻度干旱胁迫(60%±5%)Мild drought stress(60%±5%)中度干旱胁迫(40%±5%)Мoderate drought stress(40%±5%)重度干旱胁迫(20%±5%)Severe drought stress(20%±5%)无干旱胁迫(80%±5%)No drought stress(80%±5%)轻度干旱胁迫(60%±5%)Мild drought stress(60%±5%)中度干旱胁迫(40%±5%)Мoderate drought stress(40%±5%)重度干旱胁迫(20%±5%)Severe drought stress(20%±5%)
1.2 叶绿素光合荧光参数测定
光合参数采用便携式光合仪(Li-6800 XT,美国)于上午9:00—11:00 测定。测定指标包括净光合速率(Pn)、蒸腾速率(Tr)、气孔导度(Gs)和胞间CO2浓度(Ci)。叶绿素荧光参数使用手持式叶绿素荧光仪(Fluor Pen FP110,Czech Republic)测定。测定前植株至少经过充分暗适应,测定PSⅡ的最大光化学效率(Fv/Fm)、最小初始荧光(F0)、光化学猝灭系数(qP)、非光合猝灭系数(NPQ)。每个处理随机选取3 株,在每株扦插苗标记3 枚叶片进行测定,每枚叶片记录3次数据,取平均值。
1.3 气孔参数测定
葡萄扦插苗气孔参数采用指甲油印迹法测定。于胁迫第45 天在每个处理中随机选择3 株幼苗,每株采集3枚健康无病虫害、大小一致的叶片,将无色速干指甲油均匀涂抹在叶片下表面,待指甲油干透后,用无色透明胶带撕下,覆于载玻片上作为样本。统计其气孔数目,计算单位面积内气孔数量为气孔密度(SD);同时测定气孔长度(SL)和气孔开度(SOL)。
1.4 MDA含量、抗氧化酶和ADH酶活性测定
采用硫代巴比妥酸法测定丙二醛(МDA)含量[24];采用氮蓝四唑法测定超氧化物歧化酶(SOD)活性;采用愈创木酚法测定过氧化物酶(POD)活性[22];采用索莱宝乙醇脱氢酶(ADH)活性检测试剂盒BC1080测定乙醇脱氢酶(ADH)活性。
1.5 ADH基因家族分析
从Ensembl 植物数据库(http://plants.ensembl.org/)下载葡萄基因组数据,通过Pfam(http://pfam.xfam.org)和SМART 工具(http://smart.embl-heidelberg.de)鉴定葡萄ADH 家族基因。利用ClustalX(http://www.clustal.org/)对葡萄、拟南芥、番茄3种双子叶植物的ADH蛋白序列进行多序列比对,将比对结果采用МEGA 7.0 软件的邻接法(Neighbor-joining,NJ)构建系统进化树,Boostrap 值设置为1000;利用GSDS 2.0 在线网站(http://gsds.gao-lab.org/index.php)进行基因结构分析;并将蛋白序列提交至МEМE 工具(http://meme-suite.org/tools/meme)进行保守结构域分析,基序的最大数目设置为10。
1.6 数据分析
所有数据的采集和计算均设置3 组生物学重复,试验结果以平均值±标准差表示。Excel 2010计算试验数据平均值并进行误差分析,在SPSS 23.0上采用Duncan 新复极差法(P<0.05)分析差异显著性,并进行相关性分析和主成分分析,使用Origin 2021绘制图表。
2 结果与分析
2.1 干旱胁迫与铜胁迫对葡萄幼苗光合生理特性的影响
光合作用是植物生长发育的核心生理过程,其效率受环境胁迫的显著影响[25]。研究结果显示,葡萄幼苗在胁迫下的净光合速率(Pn)总体上随胁迫时间的延长呈下降趋势,且不同时期T1A/B、T2A/B、T3A/B组的Pn均显著低于CKA/B组(图1)。在干旱胁迫下,T1A组Pn下降幅度最小,且在10 d时与CK组无显著差异,第15 天时T3A 组的Pn达到最低点,较CKA组显著降低55.49%。在复合胁迫下,T3B的Pn 在第15 天时到达最低点,显著低于CKB 组44.5%。干旱胁迫下T1A、T2A、T3A 组的蒸腾速率(Tr)整体呈先升后降趋势,但均显著低于CKA 组。T1A 的Tr在第10 天达到峰值,但显著低于CKA 组9.88%;第15 天时T3A 组下降幅度最大,显著低于CKA 组68.99%。复合胁迫对葡萄幼苗Tr的影响与干旱胁迫组较为相似,但CKB 和T1B 组Tr逐渐上升,T3B 组Tr 在第15 天相较于CKB 组显著降低64.86%,下降幅度最大。两种胁迫条件下葡萄幼苗的气孔导度(Gs)和胞间CO2浓度(Ci)总体呈先升后降趋势,其中T3A/B 组Gs逐渐降低。在第15 天时,干旱胁迫下T3A 组Gs最低,相较于CKA 组显著降低76.28%,而复合胁迫下T2B 组最低,显著低于CKB 组84.63%。干旱胁迫前期(0、5、10 d),T1A、T2A、T3A组Ci均低于CKA 组,而第15 天时T2A 和T3A 组分别高于CKA 组2%和11.37%。复合胁迫第5 天,T2B 和T3B 组Ci分别高于CKB 组3.97%和6.88%,但在10 d 和15 d 时则显著降低。这一现象可能与胁迫处理对光合碳同化酶活性的影响有关。
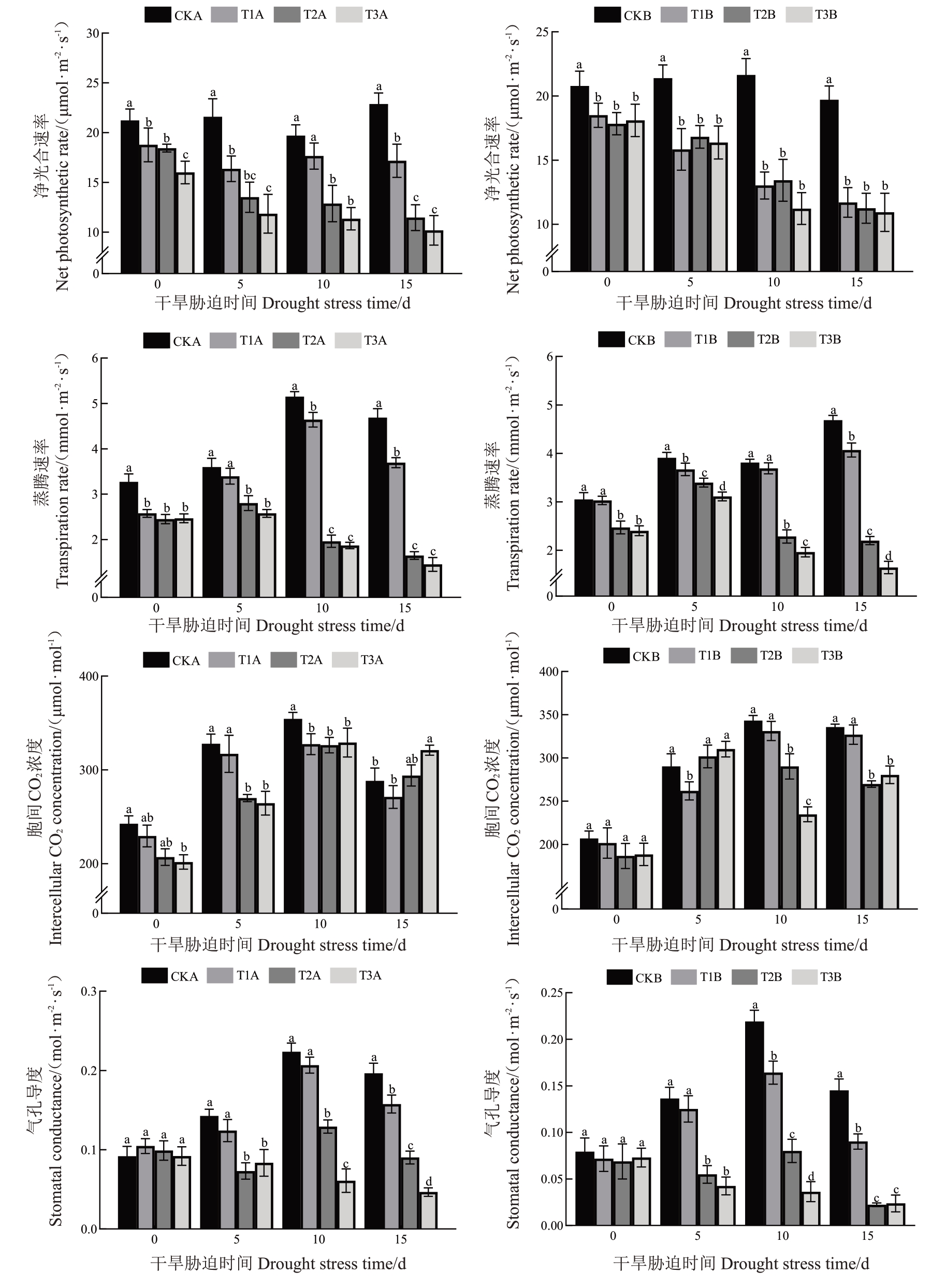
图1 干旱胁迫和复合胁迫对葡萄幼苗光合参数的影响
Fig.1 Effects of drought stress and combined stress on photosynthesis parameters of grapevine seedlings
CKA.无胁迫;T1A.轻度干旱胁迫;T2A.中度干旱胁迫;T3A.重度干旱胁迫;CKB.铜胁迫;T1B.铜胁迫和轻度干旱胁迫;T2B.铜胁迫和中度干旱胁迫;T3A.铜胁迫和重度干旱胁迫。不同小写字母表示同时期不同处理间存在显著差异(P<0.05)。下同。
CKA. no stress;T1A. mild drought stress;T2A. moderate drought stress;T3A. severe drought stress; CKB. copper stress;T1B. copper stress and mild drought stress;T2B.copper stress and moderate drought stress.T3A.copper stress and severe drought stress.Different lowercase letters indicate significant differences among different treatments in the same period(P<0.05).the same applies below.
2.2 干旱胁迫与铜胁迫对葡萄幼苗气孔分布及形态特征的影响
第45 天时两种胁迫环境下葡萄叶片下表皮的气孔密度(SD)、气孔长度(SL)和气孔开度(SOL)均随着干旱程度加深逐渐下降(图2~图3)。干旱胁迫下T1A、T2A 和T3A 组的SD 较CKA 分别显著降低21.3%、36.5%、41.5%;T2A和T3A组的SL分别显著降低7.0%和19.5%;T1A、T2A和T3A组的SOL分别降低36.0%、24.0%、33.1%,但与CKA组相比均无显著差异。这一结果表明,干旱胁迫对葡萄叶片气孔特征的作用呈现明显的剂量-效应关系。复合胁迫下T2B 和T3B 组的SD 较CKB 显著降低31.1%和36.9%,SL 分别显著降低11.4%和23.4%;T1B、T2B和T3B组的SOL较CKB组分别降低20.2%、23.2%、29.3%,但无显著差异。与干旱胁迫相比,复合胁迫对气孔特征的作用相对较弱,但仍表现出显著的胁迫效应。

图2 干旱胁迫和复合胁迫对葡萄幼苗表皮气孔形态的影响Fig.2 Effects of drought stress and combined stress on the morphology of epidermal stomata in grapevine seedlings
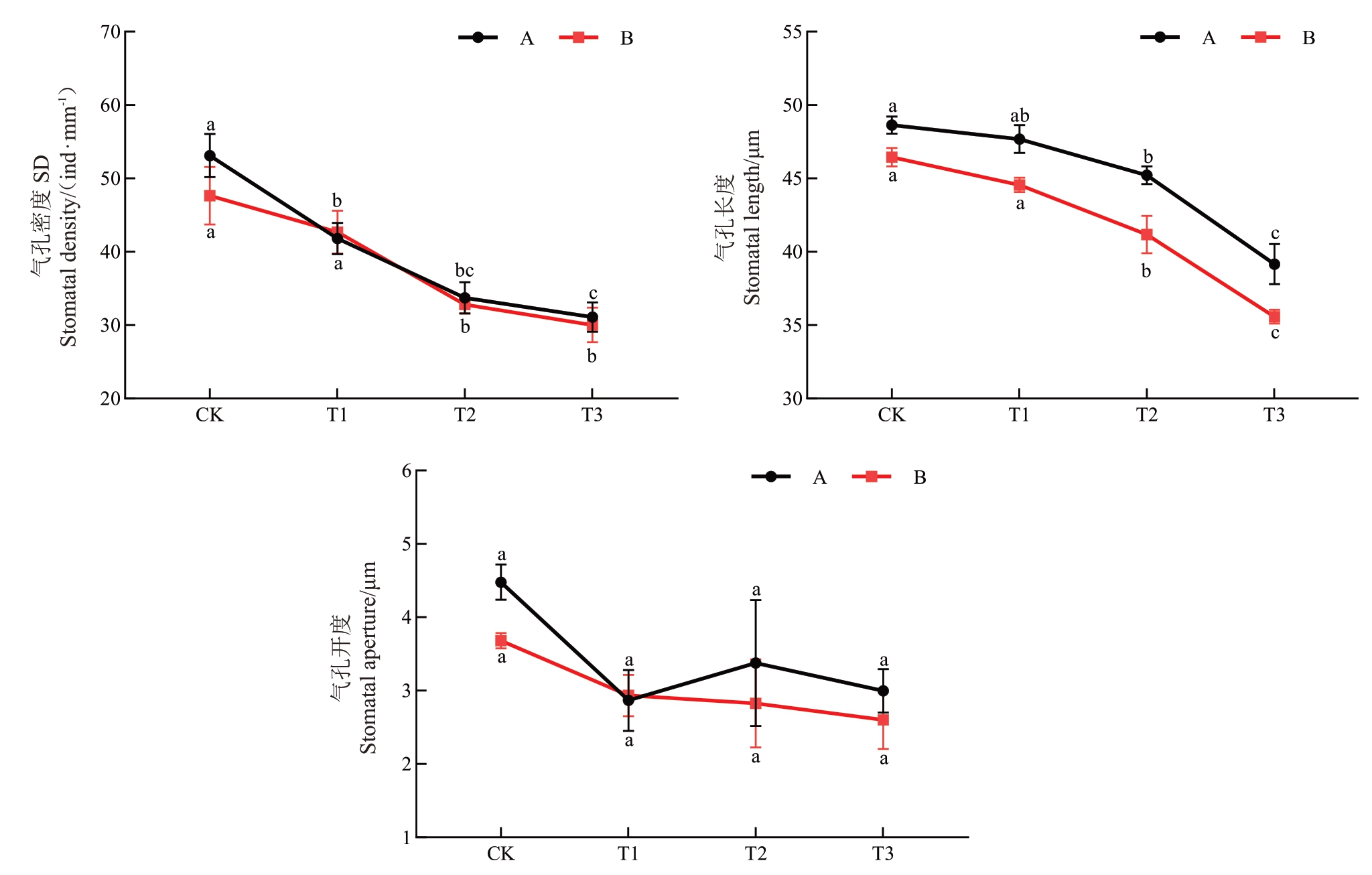
图3 干旱胁迫和复合胁迫对葡萄幼苗表皮气孔密度、长度和开度的影响
Fig.3 Effects of drought stress and combined stress on stomatal density,length,and aperture in grapevine seedlings
A.干旱胁迫;B.复合胁迫;CK.80%±5%土壤含水量;T1.60%±5%土壤含水量;T2.40%±5%土壤含水量;T3.20%±5%土壤含水量。不同小写字母表示不同处理间存在显著差异(P<0.05)。
A.Single stress;B.Combined stress;CK.80%±5%soil moisture content;T1.60%±5%soil moisture content;T2.40%±5%soil moisture content;T3.20%±5%soil moisture content.Different lowercase letters indicate significant differences among different treatments(P<0.05).
2.3 干旱胁迫和铜胁迫对葡萄幼苗荧光参数和叶绿素含量的影响
两种胁迫环境下葡萄幼苗的最大光化学效率(Fv/Fm)无明显变化(图4)。在第15 天,干旱胁迫下的T2A组Fv/Fm低于CK组0.8%,T1A和T3A组分别高于CKA 组0.05%、2.2%;复合胁迫下T1B 和T2B组分别低于CKB 组0.04%、0.8%,T3 组显著升高2.4%。干旱胁迫下,CKA、T1A 和T2A 组的最小初始荧光(F0)逐渐降低,T3A组呈先升后降趋势;复合胁迫下葡萄幼苗的F0先升高后降低,各组在第10天达到峰值。第15 天时,T1A 和T2A 组F0分别低于CKA 组3%、3.3%,T3A 组显著降低18.92%。第10天时,T1B 组F0相较于CKB 组降低5.01%,T2B 和T3B 组显著降低9.27%、11.28%。光化学猝灭系数(qP)可反映光合反应中心的开放程度。干旱胁迫第5天时,T3A组qP显著低于CKA组18.54%,复合胁迫开始时(0 d)T3B组显著低于CKB组8.37%,其他时期不同组间的qP 均无显著差异。非光合猝灭系数(NPQ)反映PSⅡ吸收的能量以热耗散形式释放的部分。随着胁迫时间的延长和土壤含水量的降低,葡萄幼苗的NPQ 总体逐渐上升,且T1A/B、T2A/B、T3A/B 的NPQ 均高于CKA/B。干旱胁迫第15 天时,T3A组NPQ最大,显著高于CKA组78.65%。复合胁迫下,T3B 组NPQ 在第15 天达到最大值,显著高于CKB 组58.58%。两种胁迫环境下葡萄幼苗的叶绿素a(Chl a)、叶绿素b(Chl b)、总叶绿素(Chl a+b)及类胡萝卜素(Car)含量总体上随胁迫时间的延长呈降低的趋势(图5),在胁迫结束时达到最小值。在第15 天时,T3A 组的Chl a、Chl b、Chl a+b、Car 含量较CKA 组显著降低31.88%、37.87%、33.37%、44.66%,T3B 组较CKB 组显著降低26%、39.75%、27.6%、25.72%。
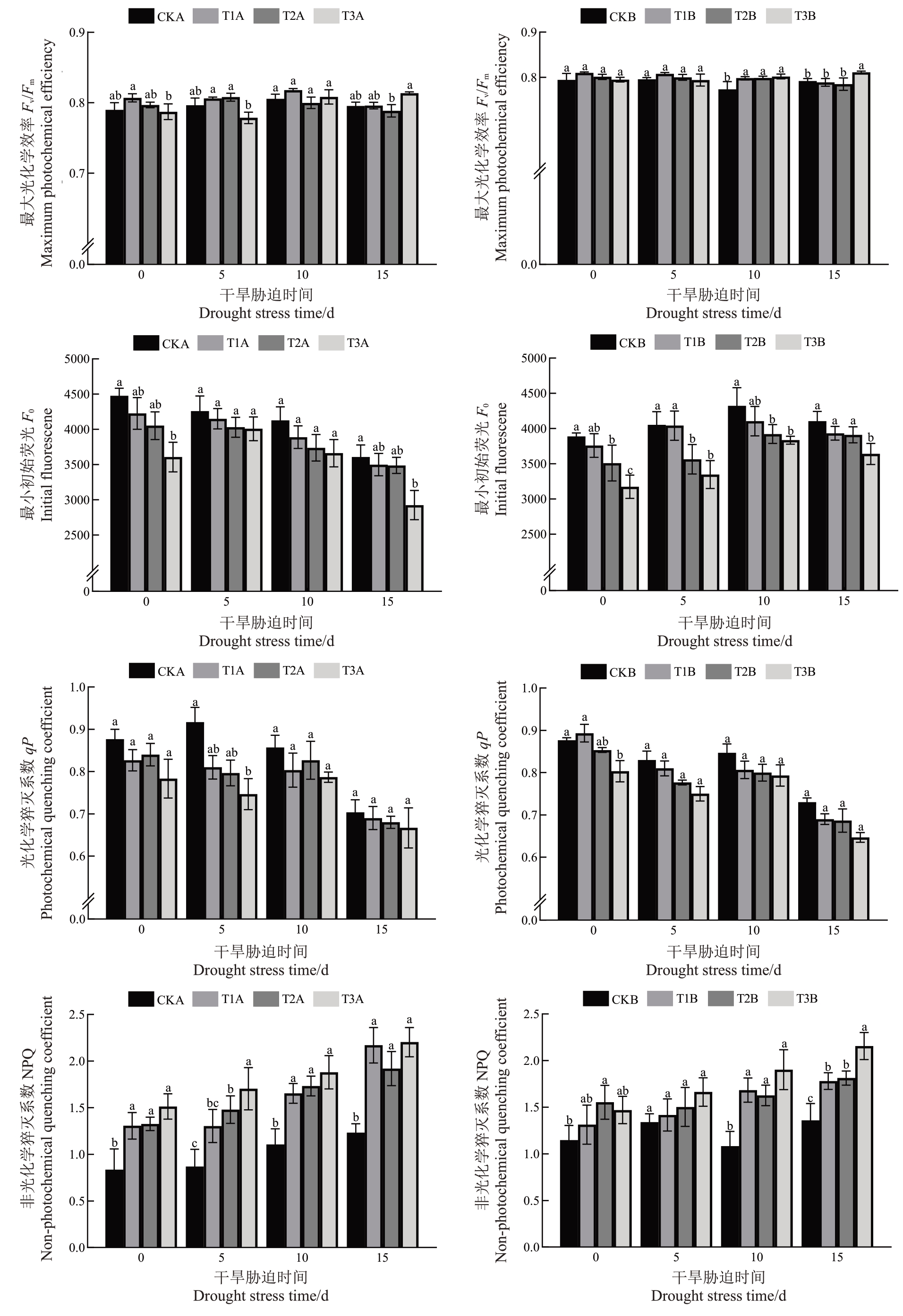
图4 干旱胁迫和复合胁迫对葡萄幼苗叶绿素荧光参数的影响
Fig.4 Effects of drought stress and combined stress on chlorophyll fluorescence parameters in grapevine seedlings

图5 干旱胁迫和复合胁迫对葡萄幼苗叶绿素含量的影响
Fig.5 Effects of drought stress and combined stress on chlorophyll content in grapevine seedlings
2.4 干旱胁迫与铜胁迫下葡萄幼苗光合指标相关性分析和主成分分析
对不同胁迫条件下葡萄幼苗的光合参数、叶绿素荧光参数及光合色素含量等12 个指标进行了相关性分析和主成分分析。相关性分析结果显示,葡萄幼苗叶片的Pn、Tr 及Gs 之间呈现极显著正相关(P<0.01)。同时,光合色素(Chl a+b、Chl a、Chl b及Car)含量之间均存在极显著正相关关系(P<0.01),并与Pn呈极显著正相关(P<0.01)。然而,光合色素指标与NPQ 呈极显著负相关(P<0.01),与Ci呈显著负相关(P<0.05)(图6)。通过对12 个指标进行主成分分析,提取到特征值大于1的3个主成分。由表2可知,主成分1、主成分2和主成分3的累计贡献率超过85%,即这3 个主成分保留了原始变量的绝大部分信息。根据特征值和主成分载荷矩阵,计算3个主成分的特征向量,将之与标准化后的数据相乘,分别计算不同葡萄光合荧光指标的综合评价得分值(Y)。进一步根据各得分值与相应特征值的方差贡献率的乘积累加得出不同栽培环境对葡萄幼苗生长发育的综合评价指数(S),以此评价不同胁迫环境对葡萄幼苗生长发育的综合影响。葡萄幼苗在不同胁迫环境下抗旱能力得分如表3 所示,抗旱能力排序依次为CKA>CKB>T2A>TIA>T2B>T3A>T1B>T3B,T3B 组抗旱能力最低,表明重度干旱和铜复合胁迫对植物造成的危害最大。
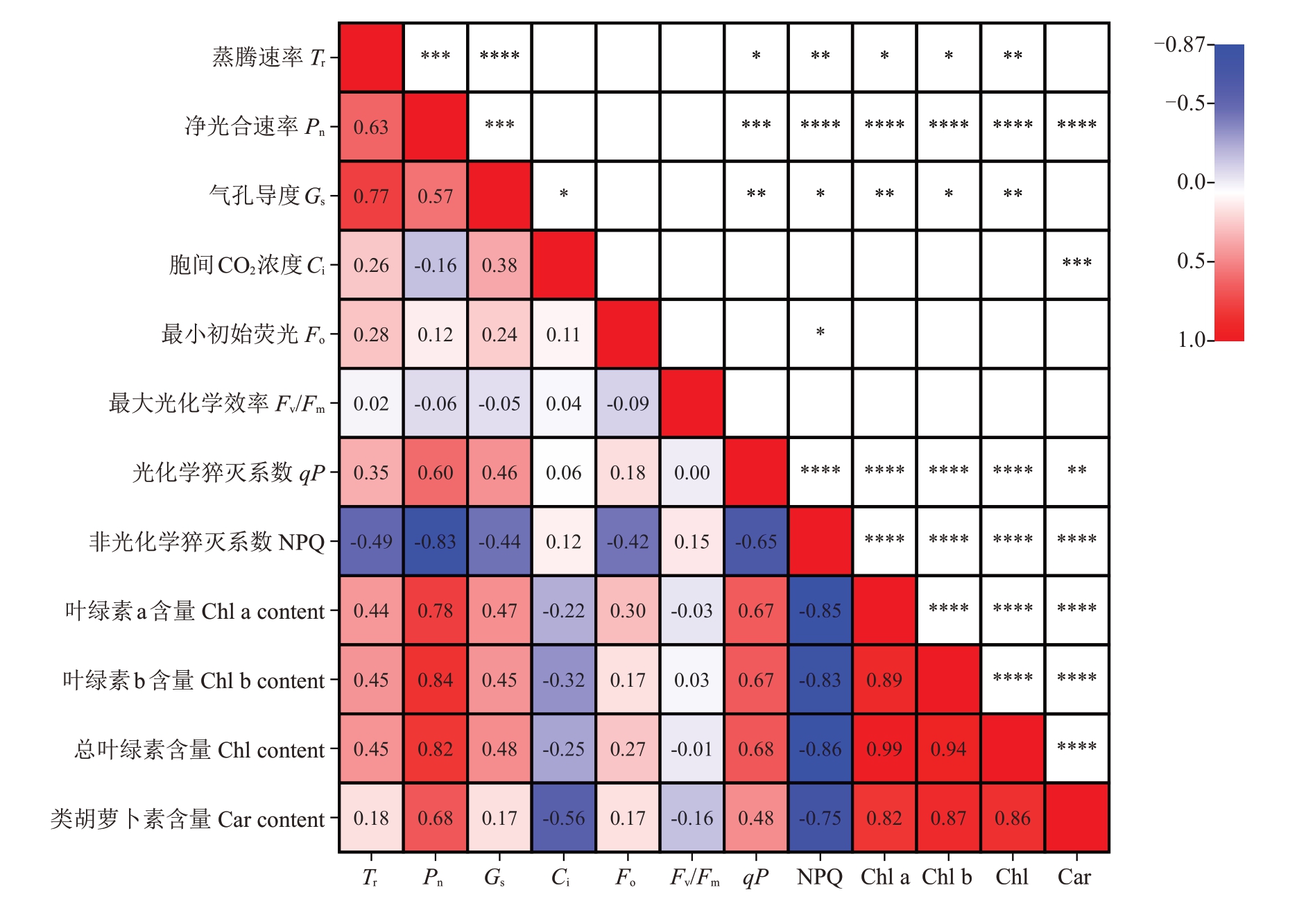
图6 干旱胁迫和复合胁迫下葡萄幼苗光合指标相关性分析
Fig.6 Correlation analysis of photosynthetic indices of grapevine seedlings under drought stress and combined stress
*表示在0.05 水平上存在显著差异,**表示在0.01 水平上存在显著差异,***表示在0.001 水平上存在显著差异,****表示在0.0001 水平上存在显著差异。
* denotes significant difference at 0.05 level, ** denotes significant difference at 0.01 level, *** denotes significant difference at 0.001 level and****denotes significant difference at 0.0001 level.
表2 各指标主成分的特征向量及贡献率
Table 2 Characteristic vector and contribution rate of principal components of each indicator
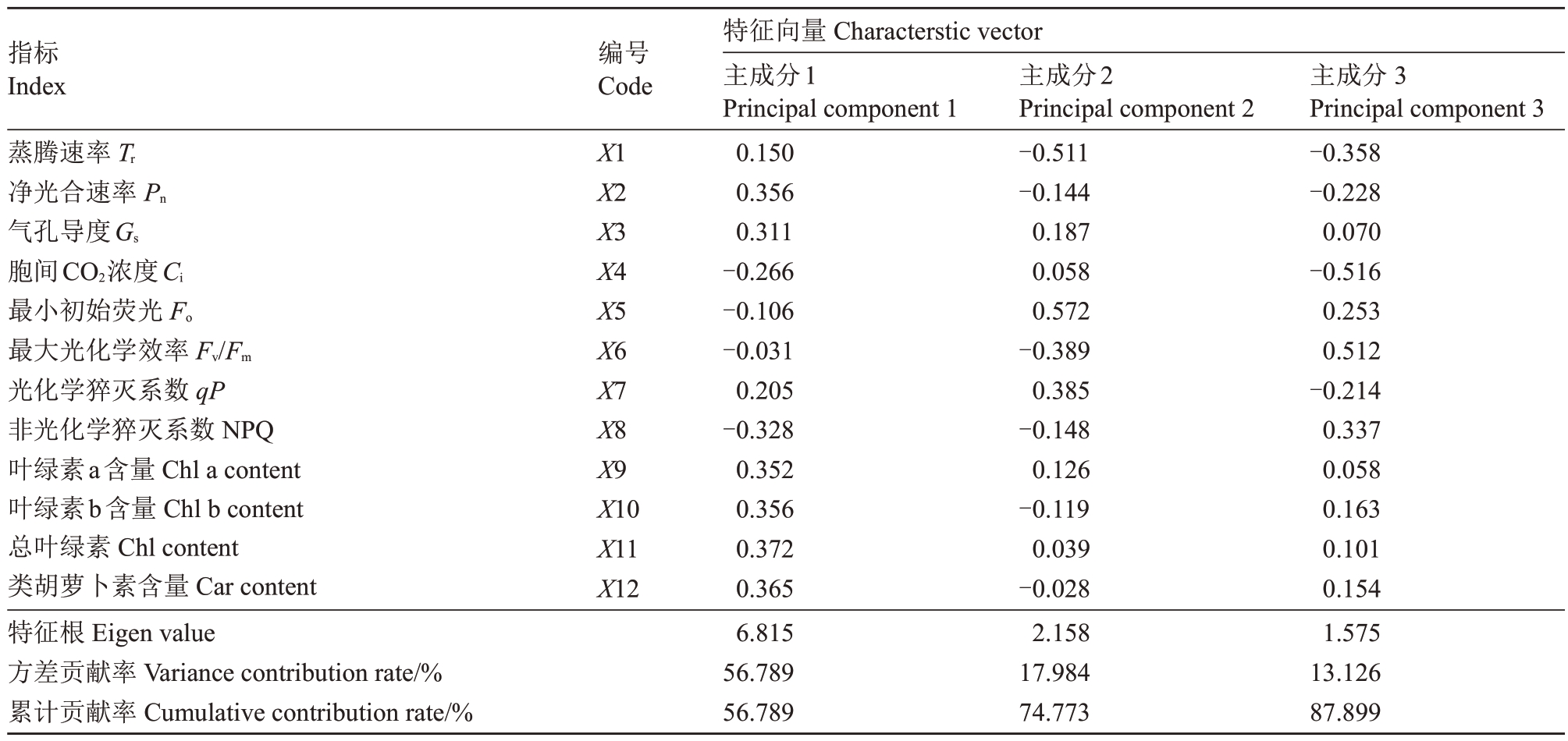
指标Index编号Code蒸腾速率Tr净光合速率Pn气孔导度Gs胞间CO2浓度Ci最小初始荧光Fo最大光化学效率Fv/Fm光化学猝灭系数qP非光化学猝灭系数NPQ叶绿素a含量Chl a content叶绿素b含量Chl b content总叶绿素Chl content类胡萝卜素含量Car content特征根Eigen value方差贡献率Variance contribution rate/%累计贡献率Cumulative contribution rate/%X1 X2 X3 X4 X5 X6 X7 X8 X9 X10 X11 X12特征向量Characterstic vector主成分1 Principal component 1 0.150 0.356 0.311-0.266-0.106-0.031 0.205-0.328 0.352 0.356 0.372 0.365 6.815 56.789 56.789主成分2 Principal component 2-0.511-0.144 0.187 0.058 0.572-0.389 0.385-0.148 0.126-0.119 0.039-0.028 2.158 17.984 74.773主成分3 Principal component 3-0.358-0.228 0.070-0.516 0.253 0.512-0.214 0.337 0.058 0.163 0.101 0.154 1.575 13.126 87.899
表3 干旱胁迫和铜胁迫下葡萄幼苗的主成分值与综合评价指数值
Table 3 Characteristic vector and contribution rate of principal components of each indicatorof grape seedlings under drought stress and copper stress
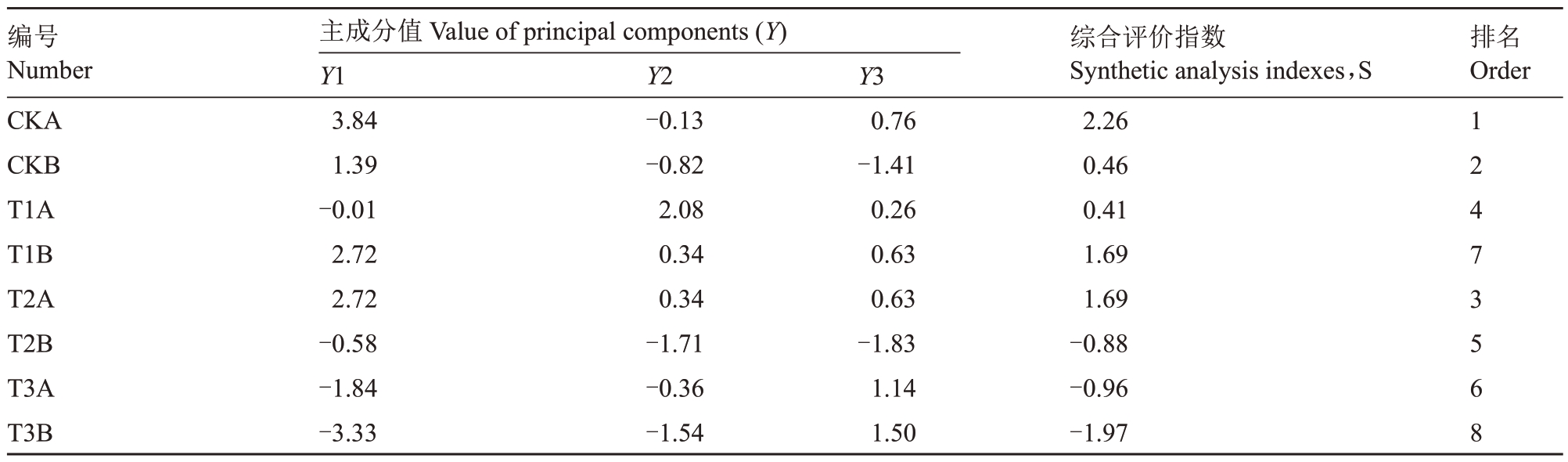
编号Number CKA CKB T1A T1B T2A T2B T3A T3B主成分值Value of principal components(Y)Y1 3.84 1.39-0.01 2.72 2.72-0.58-1.84-3.33 Y2-0.13-0.82 2.08 0.34 0.34-1.71-0.36-1.54 Y3 0.76-1.41 0.26 0.63 0.63-1.83 1.14 1.50综合评价指数Synthetic analysis indexes,S 2.26 0.46 0.41 1.69 1.69-0.88-0.96-1.97排名Order 1 2 4 7 3 5 6 8
2.5 干旱胁迫和铜胁迫对葡萄幼苗叶片抗氧化酶活性和丙二醛含量的影响
根据主成分分析和相关性分析结果可知,T3B组葡萄幼苗受到的损伤较大,为了进一步研究干旱胁迫和复合胁迫对葡萄幼苗抗氧化能力的影响,选取CKA、CKB、T3A、T3B 组葡萄幼苗于第15、30、45天时取样测定其抗氧化酶活性、乙醇脱氢酶(ADH)活性和丙二醛(МDA)含量。结果显示,CKB、T3A、T3B 组的超氧化物歧化酶(SOD)和过氧化物酶(POD)活性随胁迫时间的延长而逐渐上升(图7),在胁迫45 d 时,CKB、T3A、T3B 组葡萄幼苗叶片SOD 和POD 活性均显著高于CKA 组,其中T3B 组最高,其SOD、POD活性分别是CKA组的1.05、1.73倍。植物的过氧化作用会产生丙二醛,从而破坏细胞膜的内部结构和功能,其含量可以反映植物遭受逆境伤害的程度。МDA 含量与SOD、POD 活性变化趋势相似,即CKB、T3A、T3B组的МDA含量随胁迫时间的延长而逐渐升高。在第45天时,T3A、T3B组МDA 含量均显著高于CKA/B 组,其中T3B 含量最高,是CKA 组的4.35 倍。乙醇脱氢酶通过调节ROS 水平提高植物对氧化应激的抗性,从而帮助植物适应逆境环境。随着胁迫时间延长,CKB、T3A、T3B 组葡萄幼苗叶片的ADH 活性均呈先降后升趋势,第30天时,T3A/B组均显著高于CKA组,T3B组ADH活性最高,显著高于CKA组25.62%;胁迫结束(第45天)时,CKB、T3A、T3B组较CKA组无差异显著,T3B 组ADH 活性最高,相较于CKA 组升高18.42%。
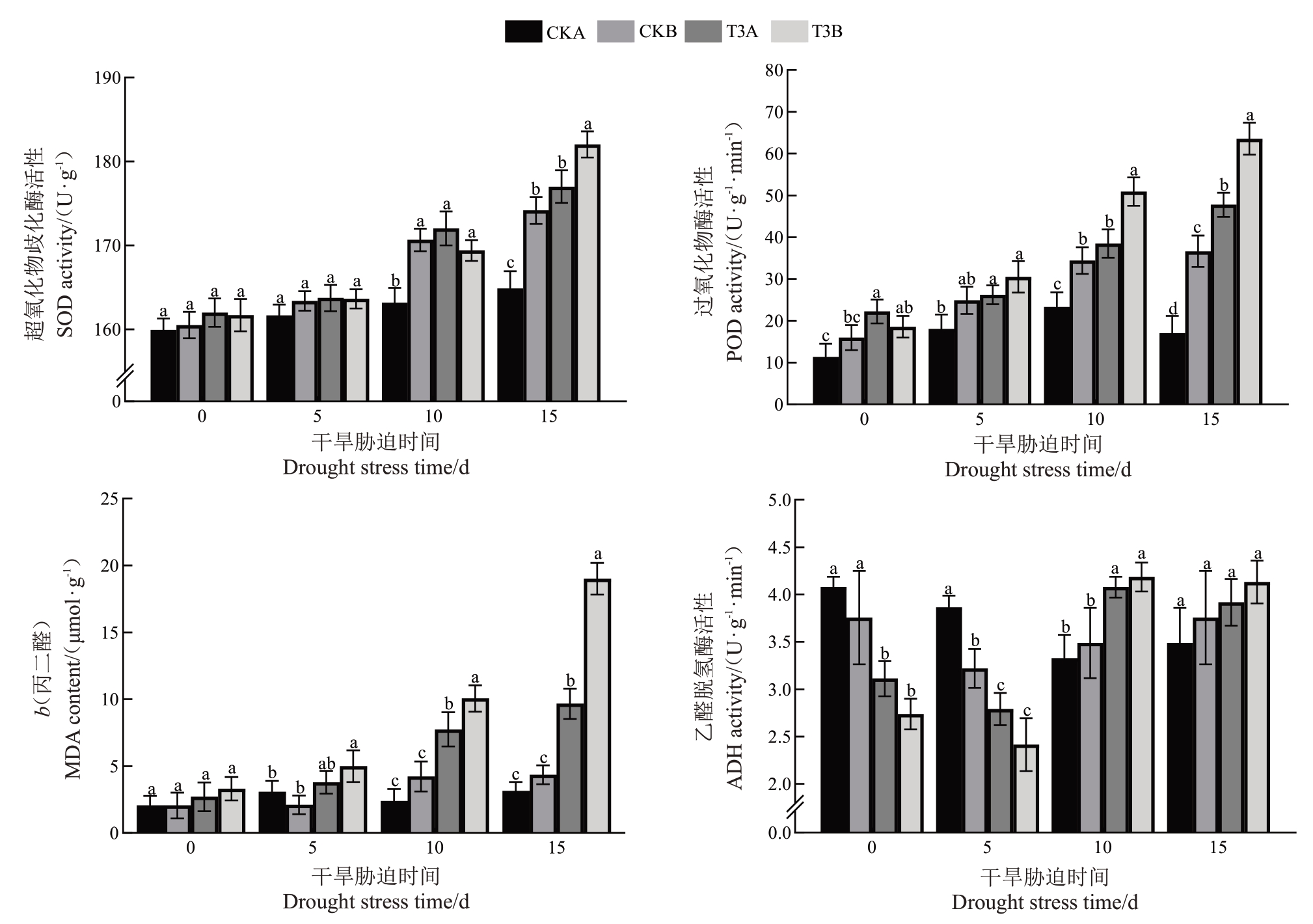
图7 干旱胁迫和复合胁迫对葡萄幼苗抗氧化酶、乙醇脱氢酶活性和丙二醛含量的影响
Fig.7 Effects of drought stress and combined stress on antioxidant enzyme activity,alcohol dehydrogenase activity,and malondialdehyde content in grapevine seedlings
2.6 葡萄ADH基因家族生物信息学分析
2.6.1 葡萄ADH 基因家族成员的鉴定及理化性质分析 利用拟南芥(Arabidopsis thaliana,At)和番茄(Solanum lycopersicum, Sl)的ADH 蛋白序列,通过SМART、Pfam、NCBI等在线平台验证保守域结构,共鉴定出53个VvADH基因家族成员(表4),命名为VvADH1~VvADH53。理化性质分析显示,VvADH基因编码的氨基酸序列长度在300~771 aa 之间,分子质量范围为32.37~83.14 kDa,理论等电点(pI)介于5.49~9.63。其中,58.49%的VvADH 蛋白Pi<7,表明大部分蛋白富含酸性氨基酸。
表4 VvADH 基因家族理化性质分析
Table 4 Analysis of physical and chemical properties of VvADH gene family

基因名称Gene name基因ID Gene ID基因Gene染色体Chromosome VvADH1 VvADH2 VvADH3 VvADH4 VvADH5 VvADH6 VvADH7 VvADH8 VvADH9 VvADH10 VvADH11 VvADH12 VvADH13 VvADH14 VvADH15 VvADH17 VvADH16 VvADH18 VvADH19 VvADH20 VvADH21 VvADH22 VvADH23 VvADH24 VvADH25 VvADH26 VvADH27 VvADH34 VvADH28 VvADH29 VvADH30 VvADH31 VvADH32 VvADH33 VvADH35 VvADH36 VvADH37 VvADH38 VvADH39 VvADH40 VvADH41 VvADH42 VvADH43 VvADH44 VvADH45 VvADH46 VvADH47 VvADH48 VvADH49 VvADH50 VvADH51 VvADH52 VvADH53 VIT_01s0127g00740.t01 VIT_02s0025g02730.t01 VIT_02s0025g03100.t01 VIT_03s0063g00610.t01 VIT_03s0180g00250.t01 VIT_03s0180g00260.t01 VIT_04s0044g00190.t01 VIT_04s0044g00210.t01 VIT_04s0044g01110.t01 VIT_04s0044g01120.t01 VIT_04s0044g01130.t01 VIT_06s0004g03640.t01 VIT_06s0004g04310.t01 VIT_06s0004g04320.t01 VIT_06s0004g04330.t01 VIT_07s0129g01030.t01 VIT_07s0031g03150.t01 VIT_07s0151g00090.t01 VIT_07s0151g00260.t01 VIT_09s0002g08470.t01 VIT_09s0002g08480.t01 VIT_10s0003g04910.t01 VIT_13s0067g00160.t01 VIT_15s0048g01710.t01 VIT_16s0039g00320.t01 VIT_16s0100g00290.t01 VIT_16s0100g00300.t01 VIT_18s0122g00450.t01 VIT_18s0001g00380.t01 VIT_18s0001g00410.t01 VIT_18s0001g06360.t01 VIT_18s0001g14910.t01 VIT_18s0001g15410.t01 VIT_18s0001g15450.t01 VIT_19s0014g01890.t01 VIT_00s0174g00270.t01 VIT_00s0174g00280.t01 VIT_00s0218g00010.t01 VIT_00s0301g00170.t01 VIT_00s0301g00180.t01 VIT_00s0346g00080.t01 VIT_00s0346g00090.t01 VIT_00s0346g00100.t01 VIT_00s0346g00110.t01 VIT_00s0346g00120.t01 VIT_00s0371g00050.t01 VIT_00s0371g00060.t01 VIT_00s0371g00070.t01 VIT_00s0371g00100.t01 VIT_00s0615g00010.t01 VIT_00s0615g00020.t01 VIT_00s0615g00030.t01 VIT_00s1389g00010.t01 1 2 2 3 3 3 4 4 4 4 4 6 6 6 6 7 7 7 7 9 9 10 13 15 16 16 16 18 18 18 18 18 18 18 19 Un Un Un Un Un Un Un Un Un Un Un Un Un Un Un Un Un Un开始Start 8 083 898 2 368 434 2 634 309 4 130 658 6 179 858 6 212 329 20 891 405 20 897 775 22 455 944 22 463 343 22 475 544 4 593 344 5 282 932 5 298 004 5 308 774 16 177 192 19 954 488 744 556 817 767 9 506 830 9 530 661 8 804 710 112 394 15 912 985 168 037 15 651 474 15 675 466 393 158 1 307 547 1 319 712 4 760 150 12 954 423 13 539 135 13 581 959 2 075 755 7 152 028 7 153 743 13 915 095 22 227 204 22 231 544 24 758 320 24 760 690 24 772 057 24 788 096 24 796 750 26 141 107 26 152 987 26 159 388 26 168 810 32 965 359 32 971 146 32 986 078 38 955 528终止End 8 085 993 2 370 943 2 636 722 4 162 029 6 181 360 6 214 039 20 894 167 20 900 222 22 458 732 22 470 471 22 477 838 4 599 671 5 294 571 5 302 347 5 311 089 16 178 852 19 970 245 762 026 828 623 9 516 709 9 537 097 8 806 457 116 065 15 914 689 174 333 15 669 267 15 679 078 395 443 1 311 198 1 322 860 4 762 878 12 956 365 13 541 625 13 584 888 2 079 468 7 153 661 7 156 313 13 917 298 22 230 598 22 234 639 24 760 689 24 771 491 24 774 737 24 791 922 24 799 104 26 143 435 26 156 994 26 160 916 26 171 260 32 967 595 32 973 314 32 989 529 38 958 094蛋白Protein长度Length/aa 377 394 355 634 357 357 359 359 380 590 380 373 379 375 396 352 421 383 338 333 331 363 345 329 379 771 365 376 383 395 387 360 380 427 361 357 359 367 319 325 362 338 361 360 361 360 300 365 362 360 360 398 360分子质量Мolecular mass/Da 40 071.98 43 010.62 38 411.99 68 700.04 38 991.14 39 009.08 39 072.17 39 105.54 41 122.28 63 711.33 41 241.36 40 721.06 41 300.94 40 867.76 42 469.96 38 553.37 45 077.9 40 223.85 35 582.12 35 447.82 35 210.46 38 834.66 36 809.79 35 419.64 40 963.17 83 136.59 39 286.66 40 448.41 41 845.68 42 988.02 42 291.75 39 502.41 41 084.35 46 418.44 38 828.69 38 968.6 38 788.88 39 517.43 33 732.93 34 268.63 39 071.09 36 953.97 38 957.95 38 860.93 38 899.87 38 802.89 32 371.39 35 895.84 39 002.03 38 833.01 38 830.86 42 976.74 38 847.03等电点pI9.04 7.15 6.88 9.63 7.1 5.6 6.77 9.13 6.35 6.5 6.19 9.09 8.68 8.58 5.49 6.67 8.3 9.15 8.99 6.78 6.42 6.39 9.06 6.16 6.57 7.71 6.41 6.62 6.63 7.5 6.19 7.3 6.37 8.48 8.36 6.43 7.98 6.78 6.91 6.05 6.74 7.43 6.89 6.78 6.89 6.93 7.04 7.14 6.98 6.93 6.94 7.34 6.93
2.6.2 葡萄ADH 基因系统进化分析 为明确葡萄ADH 基因家族成员的分类,对拟南芥、番茄和葡萄的ADH家族蛋白的多序列进行比对,构建了包含53个VvADH、28 个AtADH 和38 个SlADH 基因的系统进化树。如图8 所示,VvADH 基因家族被分为4 个亚族(Group 1~4)。Group 1 包含25 个VvADH、9 个AtADH 和11 个SlADH 基因;Group 2 包含16 个VvADH、11 个AtADH 和15 个SlADH 基因;Group 3包含1 个VvADH、1 个AtADH 和3 个SlADH 基因;Group 4包含11个VvADH、7个AtADH和9个SlADH基因。进化分析表明ADH 基因在物种分化过程中具有较高的保守性。
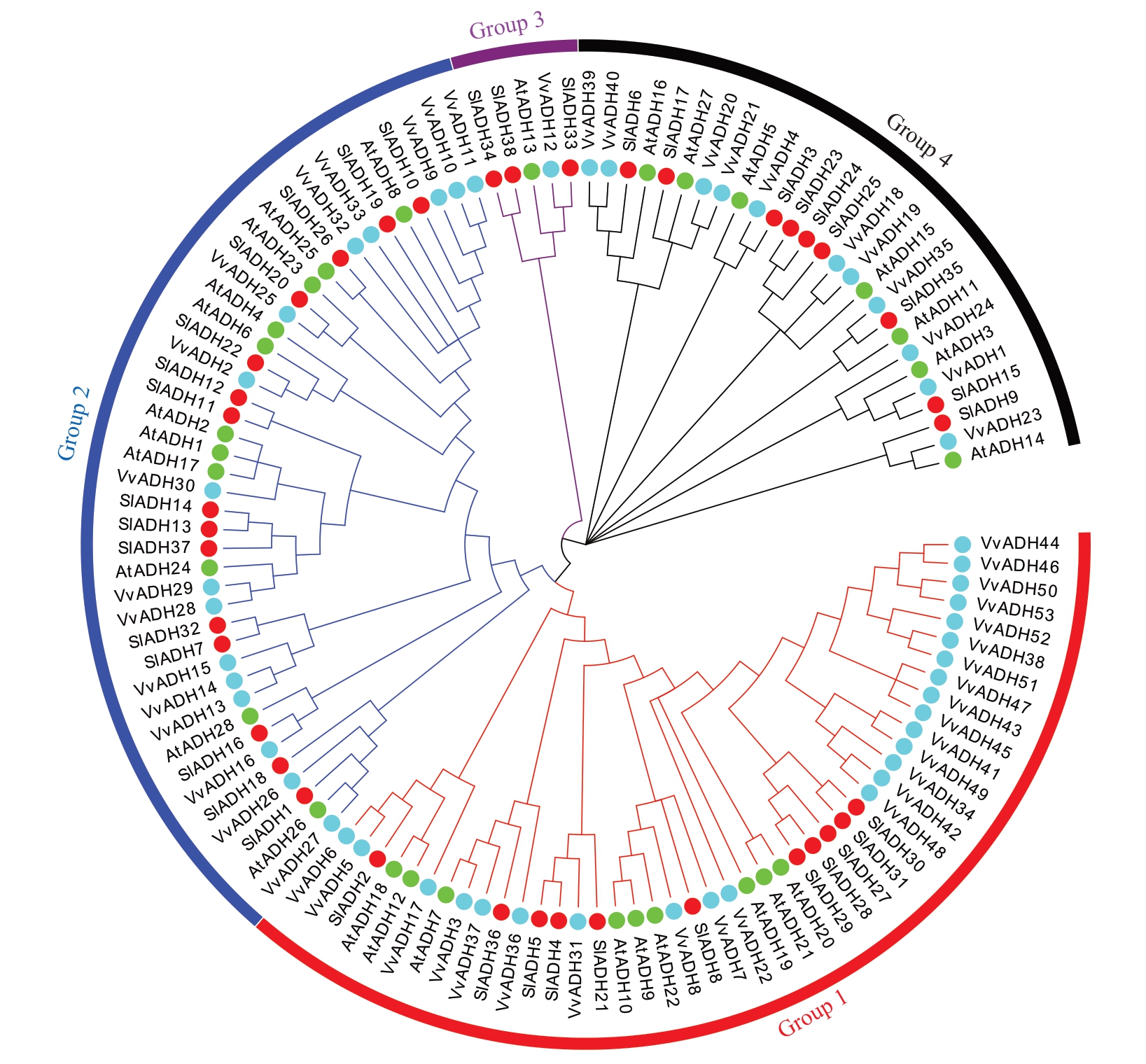
图8 葡萄、拟南芥和番茄ADH 基因家族的系统进化树
Fig.8 Phylogenetic tree of ADH gene families in Grape,Arabidopsis and Tomato
2.6.3 葡萄ADH 基因结构和保守结构域分析 利用МEМE 软件对葡萄ADH 家族基因的保守基序组成和数目进行分析,一共鉴定到10 个保守基序,依次命名为МEМE 1~10(图9)。保守结构域分析显示(图10-B),motif 3 是Group 1 特有的,这表明Group 1 中VvADH 基因可能具有一定的特异性;Group 1~4 组成员基本都含motif 7,除了Group 4 中5 个成员外,其他组基本都含有motif 6,这表明motif 6 和motif 7 是VvADH 基因家族的典型基序。在Group 1 中,VvADH3、VvADH36、VvADH37 包含motif 9 且不含motif3;在Group 2 中VvADH26、VvADH27中基序与Group 4相似,说明ADH蛋白家族成员基本结构相对比较保守。各亚组所包含的保守基序基本一致,表明同一亚组的基因家族成员可能具有相似的生物学功能。VvADH 基因家族成员的基因结构分析结果显示(图10-C),53个VvADH基因均为断裂基因,含有的外显子数量在3~18范围内,且大部分基因都含有5个外显子。Group 1特别保守,除了VvADH3、VvADH34、VvADH36、VvADH37、VvADH38、VvADH42、VvADH52 含有6 个外显子外,其他成员都含有5个外显子,但是不同基因的结构存在一定的差异。其中,VvADH2、VvADH10、VvADH13、VvADH19、VvADH22、VvADH29、VvADH36、VvADH41、VvADH47 不含非翻译区(UTR),其余都具有5 端和3端非翻译区。

图9 葡萄 VvADH 基因家族蛋白保守基序 logo 分析
Fig. 9 Analysis of conserved motif logo of VvADH gene family protein
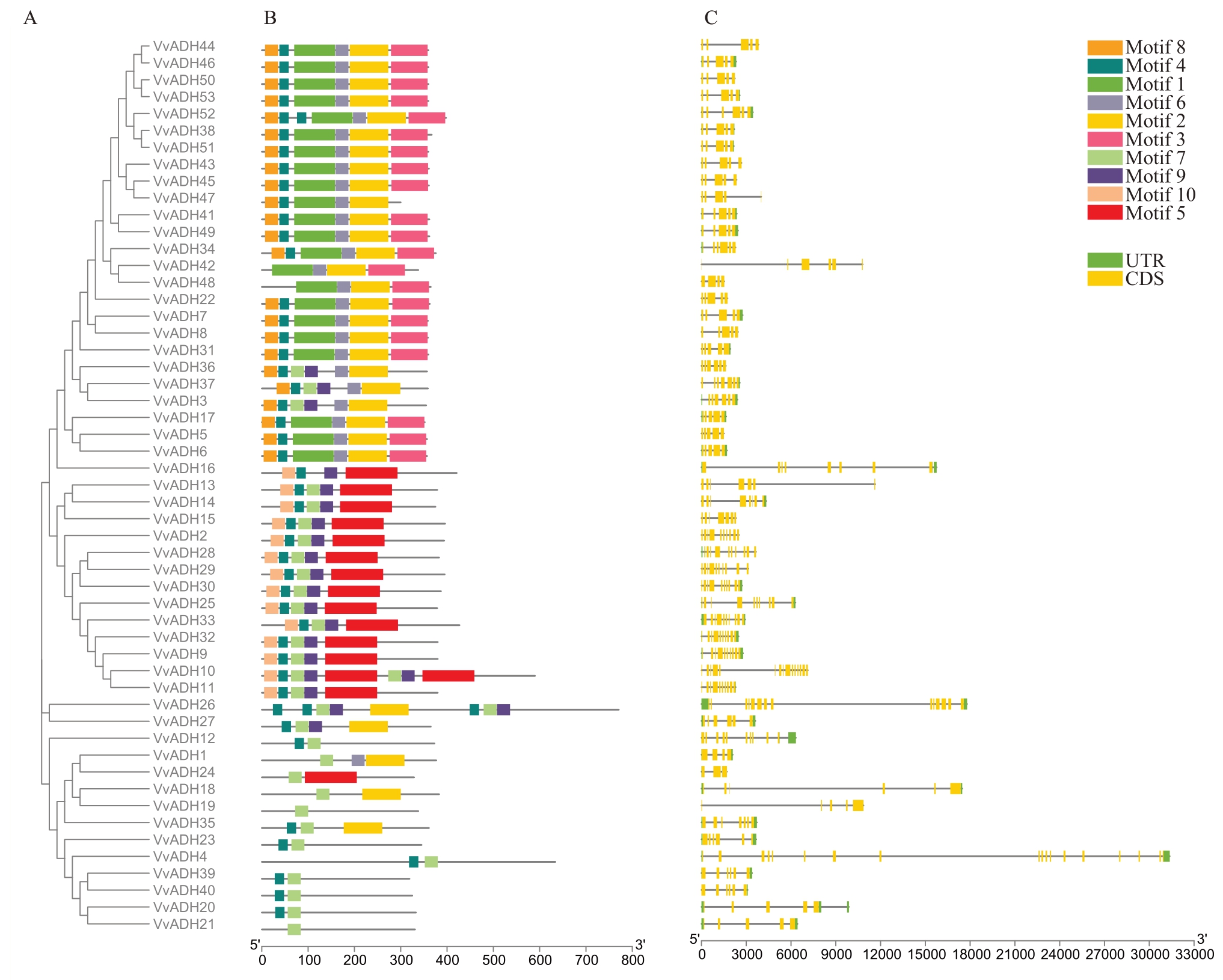
图10 葡萄ADH 基因的系统进化(A)、保守功能结构域(B)和基本基因结构(C)
Fig.10 Phylogenetic relationships(A),conserved functional domain(B)and basic gene structure(C)of ADH genes in Grape
3 讨 论
重金属污染和干旱胁迫是研究全球环境变化的两个重要组成部分,其中铜是维持细胞代谢的必需微量元素,在植物生长发育过程中起重要作用,但过量的Cu 对植物形态、生理特征具有极其不利的影响[4,22]。光合作用是植物生长发育的关键生理过程,在维持植物正常生长发育方面扮演着重要角色[25]。净光合速率是反映植物正常生长发育的关键指标,蒸腾速率反映植物体内水分状况,气孔导度是植物与外界进行水分及气体交换的参数,这些光合参数能够反映出植株在干旱胁迫下叶片不同方面的功能状态。笔者发现,随着胁迫时间延长,早黑宝葡萄幼苗的光合参数整体呈下降趋势。其中干旱胁迫下T2A和T3A组的胞间CO2浓度在胁迫结束时显著高于CKA 组,这和Cenciareli 等[5]研究结果一致,而复合胁迫下早黑宝幼苗的光合参数均低于CKB组,这表明干旱胁迫主要由非气孔因素限制了光合作用,而复合胁迫主要由气孔因素限制[26]。叶绿素荧光以快速、灵敏和非损伤性的特征,成为研究光合生理的有效参考,其荧光参数是评价植物对非生物胁迫应答的重要指标[27]。试验结果显示,PSⅡ的最大光化学效率在胁迫期间保持稳定,表明光合机构较为稳定[5]。随着胁迫时间的延长,最小初始荧光和光化学猝灭系数逐渐下降,而非光化学猝灭系数则显著上升,这表明非气孔因素对光合速率的限制作用逐渐增强,这和李乔等[28]研究结果一致。气孔是植物响应干旱的关键调节因素之一[3]。对早黑宝葡萄幼苗施加不同程度的干旱和Cu胁迫,其气孔密度和气孔长度随胁迫时间的延长逐渐降低,这一结果与王婉妮等[29]的研究一致。干旱胁迫下,植物通过形态和生理变化启动防御机制[30]。研究发现,干旱加重导致了葡萄幼苗叶绿素a、叶绿素b、总叶绿素和类胡萝卜素含量显著下降,这表明随着干旱胁迫时间的延长,植物体内光合代谢过程逐渐被破坏,引发光合色素含量降低,固定光能的能力减弱,最终导致光合作用减弱,这和刁珊等[3]的研究结果一致。对光合参数、荧光参数和光合色素等12个指标进行相关性分析,结果显示,葡萄幼苗叶片的光合参数(Pn、Tr、Gs)之间呈现极显著正相关(P<0.01)。同时,光合色素(Chl a+b、Chl a、Chl b、Car)含量之间也存在极显著正相关(P<0.01),且这些光合色素指标均与Pn呈极显著正相关(P<0.01)。主成分分析结果显示,不同处理的抗旱能力排序依次为CKA>CKB>T2A>TIA>T2B>T3A>T1B>T3B,这表明干旱和铜复合胁迫相较于干旱胁迫对植物造成的危害更大。超氧化物歧化酶和过氧化物酶是重要的抗氧化酶,能清除活性氧,减轻植物氧化损伤。干旱胁迫下,抗氧化酶活性先升高后降低,表明植物通过增加酶活性应对早期干旱,但长期或重度干旱会导致酶活性下降[6]。本试验中,SOD 和POD 活性随干旱程度加深而上升,这与前人研究一致[3]。胁迫条件下植物通过累积渗透调节物质,增加细胞液浓度,降低细胞内渗透势,维持细胞膨压,防止体内水分流失[22]。丙二醛含量反映植物逆境伤害程度[25],干旱胁迫下МDA含量上升,复合胁迫下上升明显,表明细胞膜受损严重。
乙醇脱氢酶基因家族是脱氢酶/还原酶(MDR)基因超家族的重要成员[31],具有高度保守结构域,广泛存在于原核生物、真菌、植物和动物中,在无氧呼吸和发酵代谢中发挥关键作用[32]。研究表明,ADH基因家族在植物生长发育中具有重要调控功能,其基因数量在不同物种间存在显著差异,如雷蒙德氏棉、甜瓜和梨等植物中已鉴定出不同数量的ADH基因[15,33]。基因表达模式分析为阐明ADH基因功能提供了重要依据。Pan等[10]发现ADH基因可被干旱胁迫诱导表达,并对多种非生物胁迫(如盐碱、淹水、低温和高温等)具有响应能力。在葡萄全基因组中鉴定出53 个VvADH 家族基因,系统进化分析将其与拟南芥和番茄ADH 基因划分为4 个亚家族。进一步分析显示,53个VvADH基因的等电点(pI)范围为5.49~9.63,其中pI<7 的蛋白占58.49%,pI>7 的蛋白占41.51%,表明大多数葡萄ADH 蛋白富含酸性氨基酸,可能在酸性亚细胞环境中发挥重要作用,该发现与赵安琪[34]对萱草ADH 基因家族的研究结果具有一致性。ADH 基因在物种分化过程中表现出高度保守性,其蛋白序列的相似性提示不同物种间ADH基因可能具有相似的生物学功能[35]。笔者通过分析ADH基因结构和保守基序发现,不同亚家族间基因结构存在显著差异,但同一亚家族内保持相对稳定。值得注意的是,即使在相同亚家族中,VvADH基因数量也存在差异,这可能反映了ADH基因在进化过程中存在保留与丢失现象。这些研究结果深化了笔者对葡萄ADH 基因家族进化关系和分布特征的认识,为阐明其在葡萄生长发育中的调控机制及育种改良应用奠定了基础。
4 结 论
干旱和铜胁迫下早黑宝葡萄幼苗光合作用受到抑制,抗氧化酶活性和丙二醛含量显著升高,乙醇脱氢酶活性升高;相较于干旱胁迫,复合胁迫对葡萄生长造成的危害更大。从葡萄全基因组中共鉴定出53个葡萄ADH家族基因,其结构相对保守。理化性质分析显示葡萄ADH 家族基因编码的氨基酸序列长度在300~771 aa 之间,分子质量范围为32.37~83.14 kDa,理论等电点(pI)介于5.49~9.63。其中,58.49%的VvADH 蛋白pI<7,表明大部分蛋白富含酸性氨基酸。系统进化分析表明ADH 基因具有较高的保守性。
[1] ZHANG W Z,WANG L,ZHANG L P,KONG X Q,ZHANG J,WANG X,PEIY X,JIN Z P. H2S-mediated balance regulation of stomatal and non- stomatal factors responding to drought stress in Chinese cabbage[J]. Horticulture Research,2023,10(3):uhac284.
[2] HLAHLA J М,МAFA М S,VAN DER МERWE R,МOLOIМ J. Tolerance to combined drought and heat stress in edamame is associated with enhanced antioxidative responses and cell wall modifications[J].Physiologia Plantarum,2025,177(2):e70187.
[3] 刁珊,NAМIKana,纪薇,杨明霞.11 个鲜食葡萄品种幼苗抗旱性研究及抗旱指标筛选[J]. 果树学报,2023,40(9):1871-1884.DIAO Shan,NAМIKana,JIWei,YANG Мingxia. Evaluation of drought resistance in seedlings of 11 table grape varieties and screening of drought resistance indicators[J]. Journal of Fruit Science,2023,40(9):1871-1884.
[4] WANG S,WEIМ,CHENG H Y,WU B D,DU D L,WANG C Y.Indigenous plant species and invasive alien species tend to diverge functionally under heavy metal pollution and drought stress[J]. Ecotoxicology and Environmental Safety,2020,205:111160.
[5] CENCIARELIL C,JUSTIМ S,FERREIRA-SILVA S L,DE ALМEIDA L F R,LIМA NETO М C. Physiological and biochemical changes associated with the induction of facultative CAМ in Pereskia aculeata under drought stress and recovery[J].Plant Physiology and Biochemistry,2025,222:109681.
[6] HAN Z X,WAN D J,TIAN H X,HE W X,WANG Z Q,LIU Q.Pollution assessment of heavy metals in soils and plants around a molybdenum mine in Central China[J].Polish Journal of Environmental Studies,2019,28(1):123-133.
[7] KHAN I,ASAF S,KANG S М,LEE IJ. Physiological mechanisms of heavy metal detoxification in tomato plants mediated by endophytic fungi under nickel and cadmium stress[J]. Plant Physiology and Biochemistry,2025,221:109589.
[8] ZHOU H,ZHOU K H,ZHAO G,WANG P P,YANG D G,МA X F,GAO J S. Physiological and biochemical properties of cotton seedlings in response to Cu2+stress[J].Current Issues in Мolecular Biology,2023,45(5):4050-4062.
[9] LIJ М,YANG Z H,ZHAO Y R,YU K Q. HSIcombined with CNN model detection of heavy metal Cu stress levels in apple rootstocks[J].Мicrochemical Journal,2023,194:109306.
[10] PAN H Y,SHIP Q,ZHONG S,DING X X,BAO S Y,ZHAO S Y,CHEN J T,DAIC Y,ZHANG D C,QIU X H,LIAO B S,HUANG Z H. Genome-wide identification and expression analysis of the ADH gene family in Artemisia annua L.under UV-B stress[J].Frontiers in Plant Science,2025,16:1533225.
[11] HU Z R,HE Z X,LIY Y,WANG Q,YIP F,YANG J S,YANG C K,BOROVSKIIG,CHENG X J,HU R S,ZHANG W L.Transcriptomic and metabolic regulatory network characterization of drought responses in tobacco[J]. Frontiers in Plant Science,2022,13:1067076.
[12] SU W H,REN Y J,WANG D J,SU Y C,FENG J F,ZHANG C,TANG H C,XU L P,МUHAММAD K,QUE Y X.The alcohol dehydrogenase gene family in sugarcane and its involvement in cold stress regulation[J].BМC Genomics,2020,21(1):521.
[13] WANG W F,CHEN P,LV J,CHEN L,SUN Y H.Transcriptomic analysis of topping-induced axillary shoot outgrowth in Nicotiana tabacum[J].Gene,2018,646:169-180.
[14] YIS Y,KU S S,SIМ H J,KIМ S K,PARK J H,LYU J I,SO E J,CHOIS Y,KIМ J,AHN М S,KIМ S W,PARK H,JEONG W J,LIМ Y P,МIN S R,LIU J R. An alcohol dehydrogenase gene from Synechocystis sp. confers salt tolerance in transgenic tobacco[J].Frontiers in Plant Science,2017,8:1965.
[15] JIN Y Z,ZHANG C,LIU W,TANG Y F,QIH Y,CHEN H,CAO S X. The alcohol dehydrogenase gene family in melon(Cucumis melo L.):Bioinformatic analysis and expression patterns[J].Frontiers in Plant Science,2016,7:670.
[16] 简昌歌,王爱勤,肖冬,詹洁,何龙飞. 花生乙醇脱氢酶基因家族的鉴定及其表达分析[J/OL]. 分子植物育种,2023:1- 22. (2023- 03- 23). https://kns.cnki.net/kcms/detail/46.1068.S.20230321.1747.006.html.JIAN Changge,WANG Aiqin,XIAO Dong,ZHAN Jie,HE Longfei. Identification and expression analysis of alcohol dehydrogenase gene family in peanut[J/OL]. Мolecular Plant Breeding,2023:1-22. (2023-03-23). https://kns.cnki.net/kcms/detail/46.1068.S.20230321.1747.006.html.
[17] WANG R Q,DU C F,GU G,ZHANG B H,LIN X L,CHEN C L,LIT,CHEN R,XIE X F.Genome-wide identification and expression analysis of the ADH gene family under diverse stresses in tobacco(Nicotiana tabacum L.)[J].BМC Genomics,2024,25(1):13.
[18] FIORENTINIV H R,WAIRICH A,DO CARМO COSTA М М,BRUNETTO G,GRYNBERG P,TOGAWA R C,DE МELO G W B,DOS SANTOS H P,REVERS L F,RICACHENEVSKY F K. Copper excess transcriptional responses in roots of grapevine(Vitis sp.) rootstocks[J]. Journal of Hazardous Мaterials,2024,480:136301.
[19] SERRANO A S,МARTÍNEZ-GASCUEÑA J,ALONSO G L,CEBRIÁN- TARANCÓN C,CARМONA М D,МENA A,CHACÓN- VOZМEDIANO J L. Agronomic response of 13 Spanish red grapevine (Vitis vinifera L.) cultivars under drought conditions in a semi-arid Мediterranean climate[J].Agronomy,2022,12(10):2399.
[20] 冯振,冯礼玲.微波消解-电感耦合等离子体质谱法测定新疆葡萄中重金属的含量和健康风险评估研究[J].食品安全导刊,2024(24):118-122.FENG Zhen,FENG Liling. Determination of heavy metals in Xinjiang grapes by microwave digestion inductively coupled plasma mass spectrometry and health risk assessment[J]. China Food Safety Мagazine,2024(24):118-122.
[21] 曹翠霞.波尔多液在现代果园中的作用和使用方法[J].北方果树,2023(5):27-29.CAO Cuixia. The role and application of Bordeaux mixture liquid in modern orchards[J].Northern Fruits,2023(5):27-29.
[22] 蒋永山,王康杰,赵博翔,纪薇.外源褪黑素对葡萄幼苗铜毒害的缓解效应研究[J/OL]. 西北农林科技大学学报(自然科学版),2025:1-13.(2025-04-08).https://doi.org/10.13207/j.cnki.jnwafu.2025.10.010.JIANG Yongshan,WANG Kangjie,ZHAO Boxiang,JIWei.Studies on mitigating effect of exogenous melatonin on copper toxicity in grape seedlings[J/OL]. Journal of Northwest A & F University (Natural Science Edition),2025:1-13. (2025-04-08).https://doi.org/10.13207/j.cnki.jnwafu.2025.10.010.
[23] 古咸彬,高憬,陆玲鸿,宋根华,张慧琴. 桃ADH 家族涝害响应成员的筛选及鉴定分析[J/OL]. 分子植物育种,2024:1- 14. (2024- 07- 18). https://kns.cnki.net/kcms/detail/46.1068.S.20240716.1707.004.html.GU Xianbin,GAO Jing,LU Linghong,SONG Genhua,ZHANG Huiqin. Screening and identification analysis of ADH family members response to waterlogging in peach[J/OL]. Мolecular Plant Breeding,2024:1-14. (2024-07-18). https://kns.cnki.net/kcms/detail/46.1068.S.20240716.1707.004.html.
[24] 王爱华,马红叶,罗克明,文晓鹏.火龙果响应PEG 模拟干旱胁迫的转录组分析[J].果树学报,2022,39(7):1167-1182.WANG Aihua,МA Hongye,LUO Keming,WEN Xiaopeng.Transcriptome analysis of pitaya response to PEG simulated drought stress[J]. Journal of Fruit Science,2022,39(7):1167-1182.
[25] YANG Z Y,YANG X X,WEIS М,SHEN F F,JIW. Exogenous melatonin delays leaves senescence and enhances saline and alkaline stress tolerance in grape seedlings[J]. Plant Signaling&Behavior,2024,19(1):e2334511.
[26] 石俣,王云锦,李和芹,张培兰,钟云芳,宋希强,张金玲.海南凤仙花叶片光合特性及解剖结构对干旱胁迫的响应[J/OL].亚热带植物科学,2025:1-12.(2025-03-28).https://kns.cnki.net/kcms/detail/35.1243.S.20250328.1401.002.html.SHIYu,WANG Yunjin,LIHeqin,ZHANG Peilan,ZHONG Yunfang,SONG Xiqiang,ZHANG Jinling. Response of photosynthetic characteristics and leaf anatomy to drought stress of Impatiens hainanensis[J/OL]. Subtropical Plant Science,2025:1- 12. (2025- 03- 28). https://kns.cnki.net/kcms/detail/35.1243.S.20250328.1401.002.html.
[27] 姚宇恒,南丽丽,陈洁,夏静,何海鹏,马彪.红豆草幼苗叶片光合和叶绿素荧光对干旱胁迫的响应[J].草业科学,2024,41(5):1161-1174.YAO Yuheng,NAN Lili,CHEN Jie,XIA Jing,HE Haipeng,МA Biao.Effects of drought stress on the leaf photosynthetic characteristics and chlorophyll fluorescence of sainfoin seedlings[J].Pratacultural Science,2024,41(5):1161-1174.
[28] 李乔,叶杨春,常旭虹,王德梅,王艳杰,杨玉双,马瑞琦,赵广才,蔡瑞国,张敏,刘希伟.花后高温干旱逆境对冬小麦光合特性和产量的影响[J].作物学报,2025,51(4):1077-1090.LI Qiao,YE Yangchun,CHANG Xuhong,WANG Demei,WANG Yanjie,YANG Yushuang,МA Ruiqi,ZHAO Guangcai,CAIRuiguo,ZHANG Мin,LIU Xiwei.Effects of high temperature and drought stresses on photosynthetic characteristics and yield of winter wheat after anthesis[J].Acta Agronomica Sinica,2025,51(4):1077-1090.
[29] 王婉妮,鞠延仑,贾若一,孟凡君,闵卓,房玉林.干旱胁迫对葡萄砧木110R、1103P 和5BB 叶片光合特性及抗氧化能力的影响[J].北方园艺,2021(13):43-51.WANG Wanni,JU Yanlun,JIA Ruoyi,МENG Fanjun,МIN Zhuo,FANG Yulin. Effects of drought stress on photosynthetic characteristics and antioxidant capacity of grape rootstocks 110R,1103P and 5BB leaves[J]. Northern Horticulture,2021(13):43-51.
[30] JOSHIR,WANIS H,SINGH B,BOHRA A,DAR Z A,LONE A A,PAREEK A,SINGLA-PAREEK S L. Transcription factors and plants response to drought stress:Current understanding and future directions[J].Frontiers in Plant Science,2016,7:1029.
[31] 李婉雪,吕立堂,田时雨,张宝会,姚新转,刘洋.茶树CsADH基因家族的鉴定和表达模式分析[J].分子植物育种,2022,20(1):93-104.LIWanxue,LÜ Litang,TIAN Shiyu,ZHANG Baohui,YAO Xinzhuan,LIU Yang. Identification and expression pattern analysis of ADH gene family in Camellia sinensis(L.)[J].Мolecular Plant Breeding,2022,20(1):93-104.
[32] 张计育,王刚,黄胜男,宣继萍,贾晓东,郭忠仁.乙醇脱氢酶基因家族在植物抵抗非生物胁迫过程中的作用研究进展[J].中国农学通报,2015,31(10):246-250.ZHANG Jiyu,WANG Gang,HUANG Shengnan,XUAN Jiping,JIA Xiaodong,GUO Zhongren. Functions of alcohol dehydrogenase family in abiotic stress responses in plants[J]. Chinese Agricultural Science Bulletin,2015,31(10):246-250.
[33] ZENG W W,QIAO X,LIQ H,LIU C X,WU J,YIN H,ZHANG S L.Genome-wide identification and comparative analysis of the ADH gene family in Chinese white pear (Pyrus bretschneideri) and other Rosaceae species[J]. Genomics,2020,112(5):3484-3496.
[34] 赵安琪.海水胁迫下萱草‘秋红’ADH 基因的克隆及表达分析[D].上海:上海应用技术大学,2022.ZHAO Anqi.Cloning and expression of ADH gene under seawater stress in Hemerocallis‘Autumn Red’[D]. Shanghai:Shanghai Institute of Technology,2022.
[35] 朱雨晴,杨再强.不同品种葡萄叶片光合特性对干旱胁迫的响应及旱后恢复过程[J].中国农业气象,2018,39(11):739-750.ZHU Yuqing,YANG Zaiqiang. Photosynthetic responses of different grape cultivars to drought stress and their recovery after drought[J]. Chinese Journal of Agrometeorology,2018,39(11):739-750.