蓝莓(Blueberry)为杜鹃花科(Ericaceae)越橘属(Vaccinium L.)植物,原产于北美洲[1]。蓝莓保健价值高[2-3],但目前国内的主栽品种如优瑞卡(Eureka)、莱格西(Legacy)等均为从国外引进[4]。选育具有市场竞争力强的本土蓝莓良种对中国蓝莓产业发展具有重要意义。随着现代分子生物学的发展,通过基因编辑技术可针对性地推动蓝莓种质改良与创新,加快育种进程,分子育种结合杂交育种成为主要的育种方法[5]。
高效稳定的蓝莓遗传转化体系是发掘蓝莓关键基因、研究蓝莓优良性状、进行蓝莓精确育种的重要研究手段[6]。农杆菌介导的遗传转化是应用最广的植物遗传转化方法之一,其转化原理为携带Ti质粒的根癌农杆菌或携带Ri 质粒的发根农杆菌可以在侵染植物后将质粒中的T-DNA 片段整合到植物基因组中,从而诱导得到转基因植株[7-8]。在农杆菌介导的遗传转化体系中,菌液浓度是影响转化效率的主要因素之一,不同种类植物耐受的菌液浓度不同,菌液浓度过低则转化效率低,浓度过高时易损害植物细胞[9]。现有的蓝莓遗传转化方法主要是以叶片为外植体的根癌农杆菌转化法[10-12],操作相对繁琐耗时,也需要相应的组织培养体系,且不定芽转化率较低。Mei等[13]提出的以茎段等器官为外植体的注射转化法(RAPID)是通过直接将菌液注入植株相应部位,可实现马铃薯、甘薯等植物的快速转化,同时提出表面活性剂是注射法转化的重要变量。与根癌农杆菌转化法相比,发根农杆菌转化法具有操作简单、成本低等优点,也可以诱导转化单细胞产生独立的毛状根,避免出现嵌合体问题。柑橘[14]、苹果[15]、闽楠[16]等植物均已建立了毛状根转化体系,也有研究实现了完整植株的转化[17],但发根农杆菌在蓝莓的遗传转化中少有应用。上述研究为蓝莓茎段的体外快速转化提供了应用实例。
南高丛蓝莓品种蓝美1 号(Vaccinium corymbosum‘Lanmei 1’)是中国国家级林木良种(编号:国R-ETS-VC-006-2018),遗传背景复杂[18-19],具有耐热性强[20]、氮素利用效率高[21]、适应性强、丰产性好[22]、果实花青素含量高[23]等优点,其基因资源的开发利用对中国蓝莓种质创新具有重要意义。在本研究中,笔者以蓝美1号的绿枝茎段作为外植体,采用注射法,通过不同菌液浓度和添加剂的组合对最佳转化条件进行筛选,以期建立由发根农杆菌K599介导的蓝莓遗传转化体系,为后续蓝莓的基因功能研究和高效良种选育提供一定的技术支持。
1 材料和方法
1.1 材料
1.1.1 植物材料 试验材料来源于江苏省中国科学院植物研究所蓝莓试验苗圃的蓝美1 号地栽植株。选取当年生的直径3~5 mm、半木质化的绿枝,去除顶端幼嫩茎段,修剪成长为6~8 cm、带2 个以上腋芽、去除叶片的插条作为外植体,保湿待用。
1.1.2 农杆菌的获得 发根农杆菌(Agrobacterium rhizogenes)菌株为K599,带有表达卡那霉素(Kanamycin)抗性基因、潮霉素(Hygromycin)抗性基因以及绿色荧光蛋白报告基因(Green Fluorescent Protein,GFP)的pMDC83 质粒。该甘油菌受赠于南京农业大学农学院。
1.2 农杆菌活化与侵染液制备
从-20 ℃冰箱中取出发根农杆菌K599 甘油菌液,在含有50 mg·L-1卡那霉素的LB 固体培养基上划线活化,28 ℃暗培养1~2 d。挑取单菌落加入含有50 mg·L-1卡那霉素的LB 液体培养基中,28 ℃、120 r·min-1过夜培养。以每100 mL培养基加入1 mL菌液的比例复摇菌液,28 ℃、120 r·min-1过夜培养至菌液颜色为果粒橙色。将菌液6500 r·min-1、10 min离心后倒去上清液,沉淀菌体加入含AS(乙酰丁香酮,Acetosyringone)的重悬液(WPM+100 μmol·L-1 AS,pH 5.3)吸打混匀,调整OD600值至1.0,黑暗条件下28 ℃、120 r·min-1活化2 h后进行注射。
1.3 蓝莓茎段的遗传转化
1.3.1 不同菌液浓度对转化效率的影响 根据前期预试验结果,蓝美1 号叶盘法转化的最适菌液浓度为OD600值0.5左右。本试验选用的蓝莓绿枝木质化程度较高,推测其适用更高的菌液浓度,因此设置K599 侵染液的菌液浓度(OD600值)梯度为0.5、1.0、1.5。
使用1 mL 无菌注射器将侵染液注入插条两端和腋芽芽点,具体操作如下:将针头斜向刺入芽点基部或距离切口0.3~0.6 cm 的茎段,保持针头浅插并且长度约1 mm的针尖已完全没入植株的状态,缓慢匀速地向每个注射点注射0.2~0.3 mL侵染液。注射时一手以镊子固定茎段,另一手注射的同时轻压针管防止脱落,注射结束后沿针口方向抽出针头。每个处理接种26个外植体(图1)。
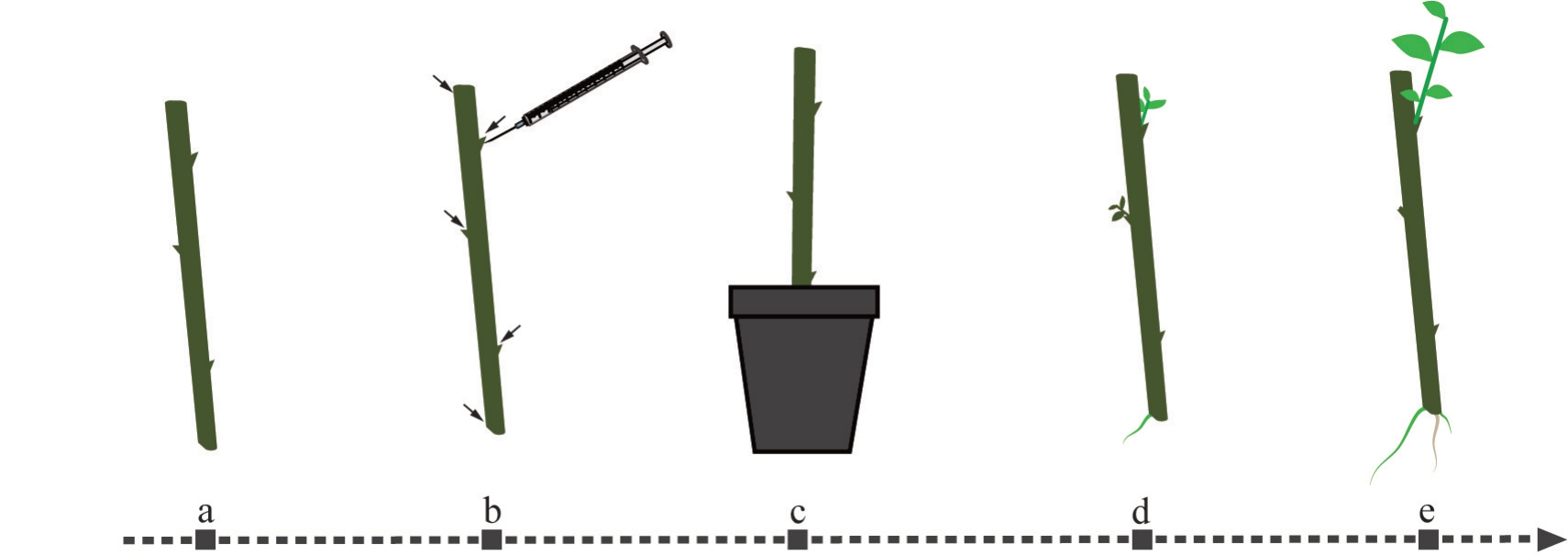
图1 注射法转化的简要流程图
Fig.1 Brief flowchart of transformation for the injection method
a.插条制备;b.侵染;c.扦插;d.筛选GFP 阳性植株;e.取材鉴定。d-e 图中亮绿色部分表示GFP 阳性新生组织。
a.Cutting preparation;b.Infection;c.Cuttage;d.Screening of GFP-positive plants;e.Sampling for identification.In figures d-e,the bright green areas indicate newly formed GFP-positive tissues.
1.3.2 不同添加剂组合对转化效率的影响 K599侵染液OD600值为1.0,设置添加AS与表面活性剂S L-77(Surfactant L-77)的组合试验。重悬液中分别添加:①100 μmol·L-1 AS,不添加S L-77;②200 μmol·L-1 AS,不添加S L-77;③100 μmol·L-1 AS,添加0.05%S L-77;④200 μmol·L-1 AS,添加0.05%S L-77;共4个处理。使用1 mL 无菌注射器将侵染液注入插条两端和腋芽芽点,具体操作同1.3.1,每个处理接种40个外植体。
1.3.3 蓝莓茎段的扦插培养 插条经侵染后,底部浸泡5000 mg·L-1 IBA溶液9~10 s,扦插在基质土中暗培养4 d后转为光照培养,育苗盘全程盖有保湿透明的塑料罩。暗培养后的第一周每日喷施1000 倍多菌灵溶液防霉,生根前对茎段定期喷水保湿,在25 ℃、16 h 光照/8 h 黑暗的条件下培养。培养45 d左右插条生根发芽后进行荧光筛选。
1.3.4 转基因植株的鉴定 为鉴定蓝莓遗传转化株系的有效性,使用体视荧光显微镜(Zeiss SteREO Discovery.V8,蔡司,德国)在488 nm 蓝光下观察侵染植株的荧光表达情况,以野生型植株的自发荧光作为阴性对照,筛选出表达GFP信号的新生侧芽和根系并拍照。为鉴定目的基因是否整合到蓝莓基因组中,进一步提取拟阳性扦插苗的侧枝茎叶,在液氮冷冻条件下研磨成粉,使用植物基因组DNA提取试剂盒(FastClean Plant Genomic DNA Kit,康为世纪CWBIO,CW05715)提取DNA,具体步骤参照试剂盒说明书。设计引物Kgfp-F:5'-GAAGTTCGAGGGCGACA-3',Kgfp-R:5'-CGTTGGGGTCTTTGCTTA-3',扩增条带长度302 bp,引物由安徽通用生物股份有限公司合成。使用高保真酶(康为世纪CWBIO,CW2969)通过PCR 克隆GFP 基因片段,反应体系(20 μL)为2×Super Pfx Master Mix(Dye)10.0 μL、Forward Primer 0.8 μL、Reverse Primer 0.8 μL、ddH2O 7.4 μL、DNA 1.0 μL;采用二步法反应程序,具体设定为:98 ℃3 min,(98 ℃10 s,66 ℃30 s;35个循环),72 ℃1 min。反应结束后,通过1%琼脂糖凝胶电泳检测PCR 产物,有目的条带的即为阳性,另外使用上海勤翔凝胶成像系统(Gene Sens 2100,勤翔,中国)拍照。
1.4 数据统计与分析
使用Excel 统计和计算外植体的存活率与GFP芽诱导率,根据GFP芽诱导率与PCR检测结果计算转化率。计算公式如下:存活率/%=存活外植体数量/外植体总数量×100;诱导率/%=产生GFP芽的外植体数量/外植体总数量×100;转化率=PCR 结果阳性的DNA样品数量/提取的DNA样品总数量×诱导率。使用Adobe Illustrator 2023作图。
2 结果与分析
2.1 不同菌液浓度对蓝莓转化效率的影响
通过注射法转化蓝美1 号绿枝茎段,筛选转化最适的K599菌液浓度(OD600值)。结果(表1)表明:当菌液OD600值从0.5 到1.0 时,外植体存活率提高;当菌液OD600值从1.0 到1.5 时,外植体存活率相同,均为96.15%。随着菌液浓度的提高,外植体新芽的GFP 芽诱导率先升后降。因此,菌液OD600值为1.0时侵染效果最佳,外植体存活率为96.15%,GFP 芽诱导率为42.31%。
表1 菌液浓度对蓝美1 号茎段转化效率的影响
Table 1 Effects of bacterial solution concentration on transformation efficiency of Lanmei 1 stems
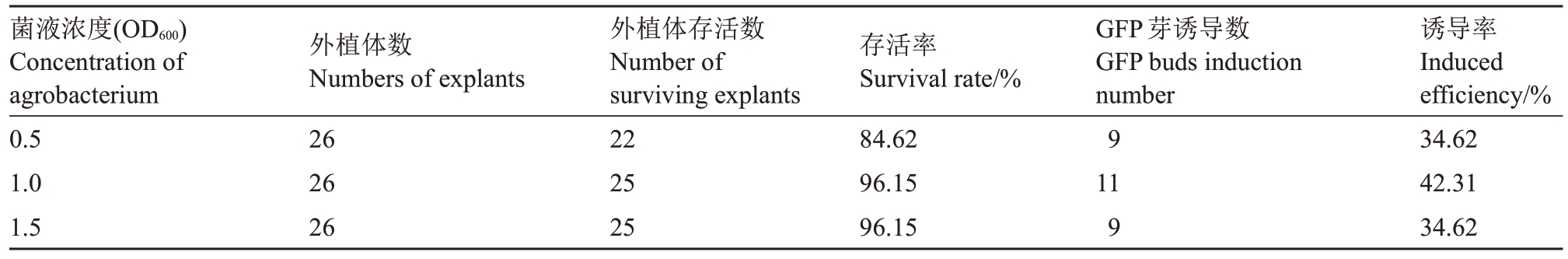
菌液浓度(OD600)Concentration of agrobacterium 0.5 1.0 1.5外植体数Numbers of explants 26 26 26外植体存活数Number of surviving explants 22 25 25存活率Survival rate/%84.62 96.15 96.15 GFP芽诱导数GFP buds induction number 9 11 9诱导率Induced efficiency/%34.62 42.31 34.62
2.2 不同添加剂组合对蓝莓转化效率的影响
通过注射法转化蓝美1号绿枝茎段,在K599农杆菌的重悬液中进行添加AS或S L-77。结果(表2)表明:重悬液中添加AS或S L-77均会降低外植体存活率,同时均能提高GFP芽诱导率。在重悬液中同时添加200 μmol·L-1 AS与0.05%S L-77时侵染效果最佳,外植体存活率为80.00%,GFP 芽诱导率为42.50%。
表2 添加剂组合对蓝美1 号茎段转化效率的影响
Table 2 Effects of additive combination on transformation efficiency of Lanmei 1 stems
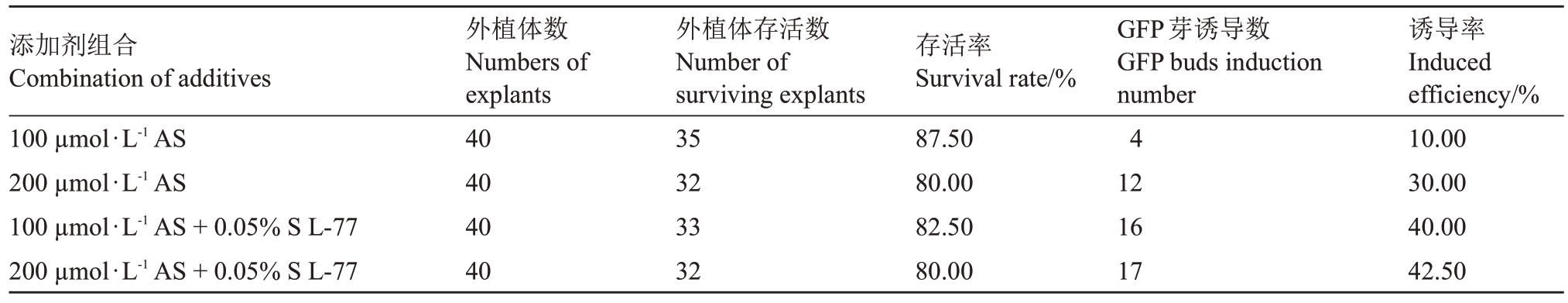
添加剂组合Combination of additives 100 μmol·L-1 AS 200 μmol·L-1 AS 100 μmol·L-1 AS+0.05%S L-77 200 μmol·L-1 AS+0.05%S L-77外植体数Numbers of explants 40 40 40 40外植体存活数Number of surviving explants 35 32 33 32存活率Survival rate/%87.50 80.00 82.50 80.00 GFP芽诱导数GFP buds induction number 4 12 16 17诱导率Induced efficiency/%10.00 30.00 40.00 42.50
2.3 转基因植株的筛选与鉴定
对蓝美1 号遗传转化株系进行荧光检测(激发波长488 nm),观察到侧芽或毛状根表达为绿色荧光信号的株系(图2),对以上株系进行筛选和统计。为鉴定目的基因是否整合到蓝莓基因组中,随机抽选20 株拟阳性植株的GFP 芽提取DNA,通过PCR 克隆GFP 基因片段进行验证(图2-G),结果测得14株转化植株,假阳性率为30%。结合遗传转化株系的最高GFP芽诱导率为42.50%,注射法侵染蓝美1号绿枝茎段的转化率最高可达29.75%。

图2 转基因植株的筛选与鉴定
Fig.2 Screening and identification of transgenic plants
A-F 为体视荧光显微镜下拍摄的荧光照片(激发光波长488 nm),a-f 为对应A-F 的明场照片。A. 野生型植株叶片;B. 野生型植株根系;C.未表达GFP 的阴性叶片;D. 未表达GFP 的阴性毛状根;E. 表达GFP 的阳性叶片;F. 表达GFP 的阳性毛状根。标尺=1000 μm。G. 转化株系的分子鉴定:M 为DNA Marker DL2000,1 为空白对照,2 为野生型植株的阴性对照,3 为农杆菌液的阳性对照,4-10 为转化植株DNA 的PCR 产物。
A-F are fluorescence photos taken by a stereo fluorescence microscope(excitation wavelength 488 nm),and a-f are bright field photos corresponding to A-F.A.wild-type plant leaves;B.wild-type plant roots;C.GFP-negative control leaves;D.GFP-negative control hairy roots;E.GFP-positive leaves;F.GFP-positive hairy roots.Bar=1000 μm.G.Molecular identification of transgenic lines:M represents DNA Marker DL2000,1 represents blank control,2 represents wild-type plant negative control,3 represents Agrobacterium suspension positive control,4-10 represent PCR products of transgenic plant DNA.
3 讨 论
蓝莓遗传转化体系主要以叶片为外植体,且基于组织培养技术进行,操作相对繁琐耗时。本研究直接将发根农杆菌菌液注射到蓝莓茎段中进行侵染,最高转化率为29.75%,不仅优于已发表蓝莓叶盘法遗传转化体系的不定芽转化率[10-12],并且更加简单、快速、经济。同时,注射法也可以用于转化未建立组培快繁体系的蓝莓品种,适用范围更广泛。
发根农杆菌能够诱导植物伤口产生大量毛状根[24],但本研究中经注射侵染的蓝莓茎段的芽点及上端切口处未见毛状根的发生,这可能说明发根农杆菌对毛状根的诱导与其侵入的植株部位相关。菌液浓度是影响植物遗传转化效率的关键因素,越橘属植物遗传转化体系采用的菌液OD600值多在0.4~0.8[11,25],并且外植体多取自组培植株。考虑到蓝莓绿枝木质化程度较高,且菌斑涂抹法这类农杆菌浓度高、侵染时间短的侵染方式已在苹果[15]、豌豆等[26]植株的转化上获得较好效果,本研究选择菌液OD600值0.5~1.5 的浓度范围进行筛选试验。结果显示,OD600值为1.0 时外植体的转化效率最高,表明外植体耐受的菌液浓度与自身生理状态相关。本研究中外植体取自相对粗壮的地栽植株绿枝,这可能导致最适菌液浓度可以相应增加。农杆菌对茎段的诱导效果随菌液浓度的增加先升后降[9],笔者发现农杆菌菌液经重悬活化制成侵染液后,OD600值为1.5 的侵染液底部出现少量白色死亡菌团,表明农杆菌活性在该菌液浓度下受到抑制,这可能导致菌液OD600值1.0~1.5间转化效率降低。Mei等[13]在“RAPID法”的研究中发现化学添加剂AS 与表面活性剂S L-77的组合可以显著提高甘薯茎段的转化效率。AS 可诱发农杆菌质粒DNA 上Vir 区基因的活化和表达,促使农杆菌的T-DNA向宿主细胞核转移,被广泛用于植物的遗传转化[27];表面活性剂能显著降低液体-固体间的表面张力,增强菌液对外植体的浸润效果,在拟南芥的浸花转化法上早有应用[28]。本研究将该添加剂组合用于蓝莓茎段的遗传转化,结果发现当重悬液含100 μmol·L-1 AS 时,再添加0.05%S L-77后诱导率提高了30%,转化效率得到明显提高,与Mei等[13]的结果一致;同时,当重悬液含200 μmol·L-1 AS时,添加0.05%S L-77 后诱导率仅提高了12.5%,这可能说明表面活性剂的效果与该添加剂组合的配比相关。两轮注射法侵染试验存在2 个月时差,在同一侵染条件下,前者的GFP 芽诱导率为42.31%,而后者为10.00%,表明外植体的生理状态对转化效率影响较大。在南京地区,南高丛蓝莓植株的枝条生长在春季夏初和秋季有两次高峰期,可选在一年内的5月下旬至9月下旬取半木质化绿枝进行侵染试验,探究最佳取材期,进一步优化蓝莓遗传转化体系[1,29]。
在构建蓝莓遗传转化体系的试验中,笔者分别选用了蓝美1 号地栽植株绿枝和组培苗茎段(数据未发表)作为外植体进行转化试验。与本研究相比,蓝美1 号组培苗茎段在进行注射侵染后存活率极低,可能1 mL注射器针头造成的创伤对组培苗茎段影响较大,外植体易萎蔫死亡,从而导致转化试验的失败;同时,组培苗茎段能依据切-浸-芽转化体系[17]实现转化,说明同种蓝莓茎段在不同生理状态适用的遗传转化条件不尽相同。
4 结 论
本研究以蓝美1号绿枝茎段作为外植体构建了简单快速的发根农杆菌K599 介导的蓝莓遗传转化体系,获得了转化植株。本研究所得遗传转化体系的转化方法为:选取直径3~5 mm 的当年生半木质化绿枝,去顶并剪成6~8 cm、带2个以上芽点、去除叶片的插条,使用菌液OD600为1.0、添加200 μmol·L-1 AS与0.05%S L-77的K599侵染液注射插条芽点及两端切口进行侵染,暗培养4 d。最快可在45 d获得阳性转化植株,最高转化率为29.75%。
[1] 顾姻,贺善安.蓝浆果与蔓越桔[M].北京:中国农业出版社,2001:1-9.GU Yin,HE Shanan. Blueberry and cranberry[M]. Beijing:China Agriculture Press,2001:1-9.
[2] 潘美华,程哲灏. 蓝莓的营养成分及其保健功能的研究进展[J].食品安全导刊,2022(22):107-109.PAN Meihua,CHENG Zhehao. Research progress on nutritional components and health function of blueberry[J]. China Food Safety Magazine,2022(22):107-109.
[3] DILEK B,MELTEM Ö. Quercetin suppresses cell proliferation using the apoptosis pathways in MCF-7 and MDA-MB-231 human breast carcinoma cells in monolayer and spheroid model cultures[J]. South African Journal of Botany,2023,162:259-270.
[4] 刘庆忠,崔冬冬,朱东姿.世界及中国蓝莓产业现状[J].落叶果树,2024,56(4):1-7.LIU Qingzhong,CUI Dongdong,ZHU Dongzi. Current situation of blueberry industry in the world and China[J]. Deciduous Fruits,2024,56(4):1-7.
[5] 公旭彤,杜乾慧,刘桂婷,卢雅妮,李雨彤,宋清秋,吕梓茜,王楠,张文基,王贺新,赵丽娜,刘国玲,徐国辉.近30 年世界蓝莓新品种资源及育种趋势分析[J].植物遗传资源学报,2025,26(2):218-236.GONG Xutong,DU Qianhui,LIU Guiting,LU Yani,LI Yutong,SONG Qingqiu,LÜ Zixi,WANG Nan,ZHANG Wenji,WANG Hexin,ZHAO Lina,LIU Guoling,XU Guohui.Analysis of new blueberry varieties and breeding trends in the world in recent 30 years[J]. Journal of Plant Genetic Resources,2025,26(2):218-236.
[6] SU W B,XU M Y,RADANI Y,YANG L M.Technological development and application of plant genetic transformation[J].International Journal of Molecular Sciences,2023,24(13):10646.
[7] PĂCURAR D I,THORDAL-CHRISTENSEN H,PĂCURAR M L,PAMFIL D,BOTEZ C,BELLINI C. Agrobacterium tumefaciens:From crown gall tumors to genetic transformation[J].Physiological and Molecular Plant Pathology,2011,76(2):76-81.
[8] 王燕燕,王丹,崔馨文,于放.植物毛状根应用的研究[J].食品与发酵工业,2023,49(21):293-302.WANG Yanyan,WANG Dan,CUI Xinwen,YU Fang.Advances on the application of hairy roots in plants[J].Food and Fermentation Industries,2023,49(21):293-302.
[9] 孙华军,李国瑞,陈永胜,黄凤兰,李跃,邢超,赵永,陈晓凤,包长春,张智勇.农杆菌介导的植物遗传转化影响因素研究进展[J].安徽农业科学,2015,43(24):26-27.SUN Huajun,LI Guorui,CHEN Yongsheng,HUANG Fenglan,LI Yue,XING Chao,ZHAO Yong,CHEN Xiaofeng,BAO Changchun,ZHANG Zhiyong. Research progress on influencing factors of Agrobacterium-mediated plant genetic transformation[J].Journal of Anhui Agricultural Sciences,2015,43(24):26-27.
[10] SONG G Q,SINK K C. Agrobacterium tumefaciens-mediated transformation of blueberry (Vaccinium corymbosum L.) [J].Plant Cell Reports,2004,23(7):475-484.
[11] 刘嘉欣.农杆菌介导的不同蓝莓品种遗传转化体系的建立[D].北京:北京林业大学,2018.LIU Jiaxin. Agrobacterium-mediated genetic transformation of different blueberry varieties[D]. Beijing:Beijing Forestry University,2018.
[12] 覃雪晶.几个蓝莓品种离体叶片再生及遗传转化体系的优化[D].北京:北京林业大学,2020.QIN Xuejing. Optimization of leaves regeneration and genetic transformation system of several blueberry varieties[D].Beijing:Beijing Forestry University,2020.
[13] MEI G G,CHEN A,WANG Y R,LI S Q,WU M Y,HU Y L,LIU X,HOU X L. A simple and efficient in planta transformation method based on the active regeneration capacity of plants[J].Plant Communications,2024,5(4):100822.
[14] XIAO Y X,DUTT M,MA H J,XIAO C,TONG Z,WANG Z Q,HE X J,SUN Z H,QIU W M.Establishment of an efficient root mediated genetic transformation method for gene function verification in Citrus[J].Scientia Horticulturae,2023,321:112298.
[15] 周雪婷,武凯凯,崔蓝芳,张坤玺,白团辉,史江莉,焦健,王苗苗,刘昱,赵玉洁,万然,郝鹏博,郑先波.苹果发根农杆菌介导转化体系构建与优化[J/OL]. 果树学报,2024:1-14. (2024-03-14).https://doi.org/10.13925/j.cnki.gsxb.20240046.ZHOU Xueting,WU Kaikai,CUI Lanfang,ZHANG Kunxi,BAI Tuanhui,SHI Jiangli,JIAO Jian,WANG Miaomiao,LIU Yu,ZHAO Yujie,WAN Ran,HAO Pengbo,ZHENG Xianbo.Construction and optimization of transformation system mediated by Agrobacterium rhizogenes in apple[J/OL].Journal of Fruit Science,2024:1-14. (2024-03-14). https://doi.org/10.13925/j.cnki.gsxb.20240046.
[16] 吴梦洁,洪家都,李芳燕,周生财,林二培,程龙军.发根农杆菌介导的闽楠遗传转化体系构建与优化[J].核农学报,2023,37(8):1516-1522.WU Mengjie,HONG Jiadu,LI Fangyan,ZHOU Shengcai,LIN Erpei,CHENG Longjun. Construction and optimization of genetic transformation system mediated by Agrobacterium rhizogenes in Phoebe bournei[J].Journal of Nuclear Agricultural Sciences,2023,37(8):1516-1522.
[17] CAO X S,XIE H T,SONG M L,LU J H,MA P,HUANG B Y,WANG M G,TIAN Y F,CHEN F,PENG J,LANG Z B,LI G F,ZHU J K.Cut-dip-budding delivery system enables genetic modifications in plants without tissue culture[J]. The Innovation,2023,4(1):100345.
[18] 於虹,曾其龙,杨曙方,陈华江,贺善安.中国蓝莓产业中品种资源的选用取向与创新[J]. 浙江农业科学,2018,59(6):924-927.YU Hong,ZENG Qilong,YANG Shufang,CHEN Huajiang,HE Shan’an.Study on germplasm resources selection and innovation of blueberry industries in China[J]. Journal of Zhejiang Agricultural Sciences,2018,59(6):924-927.
[19] 王亮,韦继光,葛春峰,於虹,姜燕琴,杨曙方,曾其龙,田亮亮.基于SSR 标记分析蓝莓品种‘蓝美1 号’自由授粉子代遗传多样性及群体遗传结构[J].植物资源与环境学报,2022,31(3):35-43.WANG Liang,WEI Jiguang,GE Chunfeng,YU Hong,JIANG Yanqin,YANG Shufang,ZENG Qilong,TIAN Liangliang.Analysis on genetic diversity and population genetic structure of open-pollinated progenies of Vaccinium corymbosum‘Lanmei 1’based on SSR marker[J]. Journal of Plant Resources and Environment,2022,31(3):35-43.
[20] 韦继光,曾其龙,姜燕琴,蒋佳峰,田亮亮,刘梦溪,於虹.南高丛蓝莓品种蓝美1 号的光合特性研究[J].中国果树,2022(1):62-67.WEI Jiguang,ZENG Qilong,JIANG Yanqin,JIANG Jiafeng,TIAN Liangliang,LIU Mengxi,YU Hong. Photosynthetic characteristics of Vaccinium corymbosum cv.‘Lanmei 1’[J]. China Fruits,2022(1):62-67.
[21] 宋佳蓉,刘梦溪,葛春峰,张鹏程,赵刚,於虹,曾其龙.不同蓝莓品种幼苗氮素利用效率和适宜施氮水平分析[J].植物资源与环境学报,2024,33(3):58-68.SONG Jiarong,LIU Mengxi,GE Chunfeng,ZHANG Pengcheng,ZHAO Gang,YU Hong,ZENG Qilong. Analyses on nitrogen use efficiency and appropriate nitrogen application level for seedlings of different blueberry (Vaccinium spp.) cultivars[J].Journal of Plant Resources and Environment,2024,33(3):58-68.
[22] 蓝美.鲜食与加工兼用型南高丛蓝莓新品种蓝美1 号[J].农村百事通,2016(3):25.LAN Mei. A new southern highbush blueberry variety‘Lanmei 1’for fresh food and processing[J]. Nongcun Baishitong,2016(3):25.
[23] 郑嘉伟,王兰娇,李大婧,柴智,张晓晓,黄午阳.蓝莓花青素生物合成相关基因表达与分子进化分析[J].食品科学,2022,43(2):184-191.ZHENG Jiawei,WANG Lanjiao,LI Dajing,CHAI Zhi,ZHANG Xiaoxiao,HUANG Wuyang. Expression and molecular evolution of genes related to anthocyanin biosynthesis in blueberries[J].Food Science,2022,43(2):184-191.
[24] 张思佳,吴凡颖,李小姗,常明明,刘悦萍,杨海清.桃毛根转化体系的建立与优化[J].北京农学院学报,2024,39(3):63-68.ZHANG Sijia,WU Fanying,LI Xiaoshan,CHANG Mingming,LIU Yueping,YANG Haiqing. Establishment and optimization of peach hairy root transformation system[J]. Journal of Beijing University of Agriculture,2024,39(3):63-68.
[25] 李晨,吴清楠,汪晴晴,张凌云,覃雪晶.乌饭树再生及遗传转化体系的建立[J].农业科学,2024(5):523-534.LI Chen,WU Qingnan,WANG Qingqing,ZHANG Lingyun,QIN Xuejing. The establishment of regeneration and genetic transformation system for Vaccinium bracteatum Thunb.[J].Hans Journal of Agricultural Sciences,2024(5):523-534.
[26] 冯志娟,刘娜,卜远鹏,张古文,王斌,龚亚明.发根农杆菌介导的菜用豌豆遗传转化体系构建[J]. 园艺学报,2024,51(10):2439-2448.FENG Zhijuan,LIU Na,BU Yuanpeng,ZHANG Guwen,WANG Bin,GONG Yaming. Establishment of Agrobacterium rhizogenes-mediated genetic transformation system in vegetable pea[J].Acta Horticulturae Sinica,2024,51(10):2439-2448.
[27] 邓艺,曾炳山,赵思东,刘英,裘珍飞,李湘阳,王曙.乙酰丁香酮在农杆菌介导的遗传转化中的作用机制及应用[J].安徽农业科学,2010,38(5):2229-2232.DENG Yi,ZENG Bingshan,ZHAO Sidong,LIU Ying,QIU Zhenfei,LI Xiangyang,WANG Shu. Mechanism and application of acetosyringone in Agrobacterium-mediated transformation[J]. Journal of Anhui Agricultural Sciences,2010,38(5):2229-2232.
[28] VERMA S S,CHINNUSAMY V,BANSA K C.A simplified floral dip method for transformation of Brassica napus and B.carinata[J].Journal of Plant Biochemistry and Biotechnology,2008,17(2):197-200.
[29] 杨海燕,吴文龙,闾连飞,赵慧芳,李维林.3 个南高丛蓝莓品种在南京的表现及栽培要点[J].南方园艺,2021,32(5):45-47.YANG Haiyan,WU Wenlong,LÜ Lianfei,ZHAO Huifang,LI Weilin.The performance and cultivation points of three southern highbush blueberry varieties in Nanjing[J]. Southern Horticulture,2021,32(5):45-47.