色泽是影响果实市场价值和消费者购买意愿的标准之一[1]。果树和蔬菜等园艺作物中的类胡萝卜素、花青素和叶绿素通常是影响着色的三大色素,其呈色主要由类胡萝卜素或花青素的积累以及叶绿素降解的比例决定[2-4]。近年来,环境因素(如光、温度、植物激素)通过调控转录因子以及表观遗传修饰对色素代谢的分子机制逐渐被揭示。然而,当前研究多聚焦于单一色素的代谢过程,对跨通路调控节点的整合分析还不充分。此外,三类色素间的竞争性合成或协同积累机制也缺乏系统性的探讨。因此,笔者在本文中围绕近年来果实着色过程中三类色素(类胡萝卜素、花青素和叶绿素)的分子调控机制研究展开综述,结合多组学以及基因编辑技术,旨在发现跨通路代谢的关键调控因子(如MYB、bHLH、NAC)。这将为使用分子标记辅助育种进一步筛选色素代谢关键基因提供理论依据。同时,定向增强色素积累也可提高果实的营养品质、贮藏性和抗逆性,对优质品种选育和果实产量提质增效具有重要意义。
1 类胡萝卜素
类胡萝卜素(Carotenoid)作为脂溶性萜类化合物,通过异戊二烯骨架构建C40或C30结构[5],通常由其多烯链共轭双键赋予果实黄、橙、红、紫等色泽[6]。除呈色功能外,类胡萝卜素还兼具光保护、抗氧化以及为植物激素(如ABA)的合成提供前体等作用[7],同时参与光吸收过程,是果实呈色的重要化合物类型之一[8]。高等植物体内类胡萝卜素主要通过MEP(甲基赤藓糖醇)途径合成[9],影响类胡萝卜素合成的关键酶有八氢番茄红素合成酶(PSY)、八氢番茄红素脱饱和酶(PDS)、ζ-胡萝卜素脱饱和酶(ZDS)、ζ-胡萝卜素异构酶(ZISO)、胡萝卜素顺式-反式异构酶(CRTISO)、β-羟化酶(BCH)、玉米黄质环氧化酶(ZEP)、β-胡萝卜素羟化酶(CHYB)、β-环化酶(LCYb)、番茄红素ε-环化酶(LCYe)、ε-羟化酶(ECH)和β-羟化酶(BCH)等[10-12]。
1.1 环境因素
果实中类胡萝卜素的合成代谢途径是一个复杂的、多方面控制的过程。已有的研究结果表明,环境因素(如水、温度、矿质元素、外源植物激素等)能够通过调节代谢途径中相关酶的基因表达来控制果实中类胡萝卜素的含量。在遮光的柑橘果实中,类胡萝卜素生物合成的关键基因PSY、PDS、ZDS1、LCY2a、LCY2b和CHX的表达显著下调,但并未影响类胡萝卜素前体途径(MEP)基因(DXS、HDR1、GGPPS1)以及分解代谢基因(CCD4b1)的表达[13],这表明光照信号对类胡萝卜素代谢的调控具有路径特异性。温度则对果实中类胡萝卜素的积累具有双重调控特性:在柑橘果实生长过程中,土壤低温影响根系对氮素的吸收,氮素供应的减少会促进类胡萝卜素合成相关基因(如PSY)的表达,从而促进果实着色[14];在5 ℃低温条件下贮藏柑橘时,虽然类胡萝卜素合成相关基因CcPSY2、CcCHYB和CcZEP的表达上调,但低温同样导致叶绿素循环相关基因的上调,叶绿素显著积累导致类胡萝卜素的颜色无法呈现,果皮无法着色[15]。因此在生产和贮藏过程中,需采取针对性的控温技术以促进果树果实类胡萝卜素的积累,进而提高果实品质和市场价值。
植物激素对促进果实着色起着重要作用。近年来,外源激素应用对内源激素平衡及类胡萝卜素合成相关酶基因表达影响的研究不断深入,进一步完善了植物激素调控类胡萝卜素合成途径的理论框架。Li等[16]研究发现外源施加乙烯(ETH)的蓝莓果实中,ETH 负调控VcPSY、VcPDS、VcZ-ISO、VcZDS、VcCHYE/LUT1 和VcCHYB/LUT5 合成,却显著提高了类胡萝卜素裂解基因(VcCCD1)、ABA 生物合成的关键基因(VcNCED1)表达量,使得ABA 在蓝莓中得到积累并促进果实成熟。而外源ABA 诱导的CsERF110-CsERF53模块则促进了柑橘果皮类胡萝卜素的合成[17],这表明乙烯和ABA之间存在复杂的互作网络来调控类胡萝卜素的代谢途径。此外,外源茉莉酸甲酯(MeJA)通过激活柑橘中的CsMPK6-CsMYC2 信号模块,抑制CsMYC2 激活CsCCD4b、CsPSY、CsLCYb、CsBCH等基因的启动子表达,从而抑制类胡萝卜素的积累[18]。目前,植物激素调控类胡萝卜素合成的分子机制逐渐清晰,但外源激素与内源激素协同调控的作用机制仍需完善,未来研究应聚焦于外源激素对内源激素信号通路关键基因的调控,阐明其如何间接激活类胡萝卜素合成酶基因的转录,从而完善激素互作的理论框架。
1.2 转录因子
转录因子对类胡萝卜素的合成调控复杂且多样,目前在果实中鉴定出MADS-box、NAC、MYB、bHLH 和bZIP 等参与类胡萝卜素的合成,表1 列出主要转录因子类型、调控的关键基因以及具体调控路径[19-36]。MADS转录因子通常在柑橘、桃的果皮中促进类胡萝卜素合成基因(如PSY)的表达,并且在柑橘中特异性激活CsSGR(叶绿素降解基因)启动子,稳定叶绿素和类胡萝卜素的动态平衡[19-20]。NAC转录因子则作为杧果、番木瓜中类胡萝卜素合成的正调控因子,影响关键合成基因PDS的转录活性[22-23]。此外NAC还能与乙烯转录因子CpEIN3a启动子中的NAC 结合位点(NABS)结合协同激活番木瓜CpPDS2/4的表达[24]。MYB、bHLH等转录因子同样能够影响类胡萝卜素合成途径中相关酶的基因表达[26-29],MYB24 则能够在葡萄果皮花青素缺乏的条件下被光照激活,并与类胡萝卜素代谢基因(CRTISO2)的启动子结合,调控花青素和类胡萝卜素的竞争性合成[26]。其他转录因子如WRKY、SBPbox 等蛋白家族的转录机制也逐渐被阐明[31-33]。
表1 调控果实类胡萝卜素合成的转录因子及具体路径
Table 1 Transcription factors and specific pathways regulating carotenoid synthesis in fruits

转录因子Transcription factor物种Species名称Name调控路径Regulatory pathway参考文献Reference MADS-box转录因子MADS-box transcription factor柑橘Citrus CsMADS3[19]桃[20]Prunus persica PpMADS2,PpMADS3榴莲Durio zibethinus L.DzAGL6-1[21]NAC转录因子NAC transcription factor杧果Mangifera indica L.NAC6[22]番木瓜Carica papaya L.CpNAC1[23]CpNAC2[24]金橘Fortunella crassifolia Swingle FcrNAC22[25]MYB转录因子MYB transcription factor葡萄Vitis vinifera MYB24[26]木瓜Papaya CpMYB1,CpMYB2[27]柑橘Citrus reticulata CrMYB33促进CsPSY1、CsLCYb2、CsSGR(叶绿素降解基因)表达,正调控维持二者动态平衡。CsMADS3 promotes the expression of CsPSY1, CsLCYb2, and CsSGR(chlorophyll degradation genes)and maintain their dynamic balance.二者互作增强PpPSY和PpCHYB启动子的转录活性,正调控类胡萝卜素合成。The two interact to enhance the transcriptional activity of the PpPSY and PpCHYB promoters and positively regulate carotenoid synthesis.与CArG元件相互作用来激活DzPSY的启动子活性,促进类胡萝卜素积累。DzAGL6-1 interacts with CArG elements to activate the promoter activity of DzPSY and promote carotenoid accumulation.激活PSY、PDS、ZDS、CRTISO、BCH 和VDE 的表达,上调类胡萝卜素含量。NAC6 activates of PSY,PDS,ZDS,CRTISO,BCH,VDE and up-regulated carotenoid content.CpNAC1 结合CpPDS2/4 启动子中的NAC 结合位点(NABS)基序,促进类胡萝卜素合成。CpNAC1 binds to the NAC binding site (nabs) motif in the CpPDS2/4 promoter and promote carotenoid accumulation.CpNAC2 与乙烯转录因子CpEIN3a 互作,激活CpPDS2/4、CpLCYe、CpCHYb,正调控类胡萝卜素积累。CpNAC2 interacts with the ethylene transcription factor CpEIN3a, activating CpPDS2/4,CpLCYe,CpCHYb,and promote carotenoid accumulation.红光特异性激活FcrNAC22,随后FcrLCYB1、FcrBCH2c 表达上升,促进类胡萝卜素合成。Red light specifically activates FcrNAC22, the expression of FcrLCYB1,FcrBCH2c increased,and promote carotenoid accumulation.在光照和辐射刺激色斑的情况下,MYB24被激活并结合CRTISO2,正调控类胡萝卜素积累。In the presence of light and radiation-stimulated pigmentation,MYB24 is activated and binds to CRTISO2, positively regulating carotenoid accumulation.CpMYB1、CpMYB2 抑制类胡萝卜素合成基因的表达,负调控类胡萝卜素合成。CpMYB1 and CpMYB2 inhibit the expression of carotenoid synthesis genes,and negatively regulate carotenoid synthesis.CrMYB33 激活CrLCYb2、CrBCH2 转录活性,同时抑制叶绿素降解酶CrCLH,正向调控类胡萝卜素和叶绿素合成。CrMYB33 activates the transcriptional activity of CrLCYb2 and CrBCH2,and participating in the regulation of chlorophyll degradation.[28]
表1 (续) Table 1 (Continued)

转录因子Transcription factor物种Species名称Name调控路径Regulatory pathway参考文献Reference bHLH转录因子bHLH transcription factor柑橘Citrus spp.CsTT8[29]bZIP转录因子bZIP transcription factor柑橘Citrus spp.CsbZIP44[30]WRKY转录因子WRKY transcription factor桃PpWRKY4[31]P.persica枇杷Eriobotrya japonica Lindl.EjWRKY6[32]SBP-box 转录因子SBP-box transcription factor枇杷Eriobotrya japonica Lindl.EjSBP01 EjSBP09[33]AP2/ERF转录因子AP2/ERFtranscription factor苹果Malus domestica MdAP2-34[34]柑橘Citrus sinensis L.Osbeck CsERF061[35]ABI转录因子ABI transcription factor苹果M.domestica Borkh.MdABI5激活CsPDS、CsLCYE、CsZEP 和CsNCED2 表达,促进类胡萝卜素合成。CsTT8 activates CsPDS, CsLCYE, CsZEP and CsNCED2 expression,and promote carotenoid accumulation.参与CsHB5-CsbZIP44 转录模块,激活类胡萝卜素合成基因CsDXR、CsGGPPs、CsBCH1和CsNCED2表达,促进类胡萝卜素积累。CsbZIP44 participates in the CsHB5-CsbZIP44 transcription module and activate of carotenoid synthesis genes CsDXR, CsGGPPs, CsBCH1,CsDXR,CsGGPPs,CsBCH1 and CsNCED2 expression,promotes carotenoid accumulation.抑制类胡萝卜素降解基因PpCCD4表达,正调控类胡萝卜素合成。Inhibition of carotenoid degradation gene PpCCD4 expression positively regulates carotenoid synthesis.喷施ABA 促进EjWRKY6 表达,诱导类胡萝卜素合成相关基因EjPSY1、EjLCYB、EjBCH2转录。ABA spraying promoted EjWRKY6 expression and induced the transcription of carotenoid synthesis- related genes EjPSY1, EjLCYB, and EjBCH2.黄色果肉EjSBP01、EjSBP09 表达上调,正调控类胡萝卜素合成和积累。Yellow pulp EjSBP01 and EjSBP09 expression was up-regulated and was regulating carotenoid synthesis and accumulation.增强MdPSY2-1的转录活性,类胡萝卜素得到积累。MdAP2-34 promotes the transcriptional activity of MdPSY2-1 and accumulate carotenoids.激活PSY1、PDS、CRTISO、LCYb1、BCH、ZEP、NCED3、CCD1 和CCD4的启动子,促进类胡萝卜素合成。CsERF061 activates the promoters of PSY1, PDS, CRTISO, LCYb1,BCH,ZEP,NCED3,CCD1 and CCD4 to promote carotenoid synthesis.MdABI5-MdMYBS1 级联调控类胡萝卜素的积累,激活MdPSY2-1、MdLCYb等合成基因的表达。MdABI5-MdMYBS1 cascade regulates the accumulation of carotenoids,and activates the expression of synthetic genes such as MdPSY2-1 and MdLCYb.[36]
激素信号转导途径的相关转录因子参与果实内源激素的合成以及类胡萝卜素的代谢。乙烯响应因子(AP2/ERF)是最主要的转录因子,能够直接调控合成途径基因的表达,也可与MADS-box、MYB 等转录因子互作间接调控。在苹果和柑橘中MdAP2-34、CsERF061 结合PSY、LCYb、CRTISO 等启动子[34-35],也可在木瓜中CpMADS4 与CpERF9 相互作用抑制PDS 和LCYe 的表达从而减少类胡萝卜素的积累[37]。除了AP2/ERF外,生长素响应因子SlARF、油菜素内酯响应因子SlBZR等都在调控番茄果实的类胡萝卜素合成[38-39],但对于果树果实来说其他激素信号转导的转录因子仍缺少深入研究。
1.3 表观修饰
DNA甲基化是表观遗传中一种主要的机制,类胡萝卜素代谢相关基因的DNA 甲基化变化趋势也为完善果实着色的分子调控网络提供新的理论支撑。甜樱桃着色期间类胡萝卜素降解相关基因的5’UTR 区域存在DNA 低甲基化[40]。目前研究利用改变DNA甲基化的化学抑制剂揭示了DNA甲基化在调节类胡萝卜素生成中的作用途径[41]。5-氮杂胞苷(5-aza)是一种DNA 甲基化抑制剂,应用5-aza 可激活CpCCD1 等分解基因的转录活性,诱导柑橘果实中类胡萝卜素降解[42-43]。RNA修饰也参与对类胡萝卜素合成的调控,miRNAs、lncRNAs 能够靶向调节类胡萝卜素生物合成的相关TFs 和关键酶基因。黄桃果皮中PpMYB9与类胡萝卜素合成相关,mdmmiR858则通过靶向剪切抑制PpMYB9 基因的表达,为miRNA 参与类胡萝卜素合成提供理论基础[44]。甜瓜lncRNAs的靶基因则直接富集于NCED1、PSY1等合成基因,促进类胡萝卜素的积累[45]。
2 花青素
花青素(Anthocyanidin),又称花色素,是属于植物类黄酮化合物中的一种水溶性糖苷,广泛存在于果实、叶片和花瓣中,赋予植物器官红色、橙色、紫色、蓝色或黑色[46]。该色素分子具有类黄酮物质所特有的“C6-C3-C6 三环”碳骨架结构,其化学结构为3,5,7-羟基-2-苯基苯并吡喃,在自然界中主要呈现6种基本形态,包括天竺葵素(Pelargonidin)、矢车菊素(Cyanidin)、芍药花素(Peonidin)、飞燕草素(Delphinidin)、矮牵牛素(Petunidin)和锦葵素(Malvidin)[47]。在不同pH下花青素呈现不同颜色,当植物细胞液泡pH<7 时呈红色,酸性越强则颜色越红,pH在7~8时呈紫色,pH在8~11范围时为蓝色,碱性越强则为蓝黑色[48]。糖基化是提高花青素稳定性并改变花青素颜色的重要修饰手段,通常发生在A 环的糖基化程度越高,花青素越偏向紫色,其中C-5位点糖基化使花青素颜色向红紫色偏移[49]。在金属离子螯合作用中,Mg2+、Fe3+分别与飞燕草素、矢车菊素结合形成蓝色[50],Cu2+、Fe2+则能够提高花青素色泽[51]。此外花青素通过共价键与酚类、氨基酸、有机酸和生物碱等其他小分子或次生代谢产物结合有助于花青素色泽的稳定形成[52]。作为植物应对生物与非生物胁迫的重要化合物,花青素生物合成的分子调控机制主要围绕结构基因的编码功能及转录因子的调控网络展开。核心结构基因编码花青素生物合成途径中的关键酶,如苯丙氨酸解氨酶(PAL)、查尔酮合成酶(CHS)、查尔酮异构酶(CHI)、黄酮3-羟化酶(F3H)、二氢黄酮醇4-还原酶(DFR)、花青素合成酶(ANS)、糖基转移酶(UFGT)等[53]。转录调控层面则以MYB-bHLH-WD40(MBW)复合体为代表,通过顺式作用元件与结构基因启动子互作实现多层级调控。近年来,花青素的抗氧化、抗衰老、预防心脑血管疾病等医疗价值被广泛关注,因此明确花青素合成的代谢途径以及分子机制对高效合成花青素至关重要。
2.1 转录因子
研究表明,MYB、bHLH、WD 蛋白家族成员及其形成的MYB-bHLH-WD40(MBW)复合物是花青素生物合成的核心转录调控模块[54]。这些转录因子通过特异性识别结构基因的启动子区域并特异性结合,动态调节靶基因的转录活性,从而调控花青素的生物合成。
在果树果实中,MYB家族的调控花青素合成的功能具有显著多样性。红肉苹果是目前遗传育种研究的热点,新疆1 号红肉苹果中MYB10 通过直接激活花青素生物合成的基因ANS、UFGT,显著提升果实花青素的含量[55]。而Wang等[56]发现,野生红肉苹果具有较高的黄酮类化合物含量。进一步研究发现,苹果果实中的MYB12 能够与bHLH3/33 形成异源二聚体,并特异性结合无色花青素还原酶(LAR)基因启动子,促进LAR基因表达,从而驱动原花青素合成。根据苹果和拟南芥的R2R3-MYB蛋白序列,在梨基因组中共鉴定出184个R2R3-MYB转录因子候选基因[57],梨果实中PyMYB10 的转录水平与花青素合成的结构基因(PyANS、PyDFR)表达呈正相关[58];蓝莓中已鉴定出437 条具有SANT 结构域的MYB 序列,共88 个VcMYBs 在果皮中更高表达[59]。VcMYB1 激活AtPAL、AtCHS 和AtDFR 等花青素结构基因的表达,从而使花青素积累[60],而VmMYBYPA1.1 的敲除显著抑制CHS、DFR 和ANS 启动子的活性,果实花青素含量下降[61-62]。
bHLH 作为碱性螺旋-环-螺旋(basic helix-loophelix,bHLH)结构域转录因子,具有调控植物生长发育、参与植物抗逆性、次生代谢等作用[63],在果树果实中,bHLH 通常与MYBs 家族协同调控花青素合成途径中的关键基因。Li等[64]研究表明bHLH转录因子的N 端存在MYB 相互作用区(IR),能够与R2R3-MYB蛋白相互作用,早酥梨果皮中PbbHLH2与PbMYB9/10形成复合物,协同激活类黄酮合成途径基因的转录起始。在苹果中MdbHLH162 破坏花青素激活的MdMYB1-MdbHLH3/33复合物的形成,并削弱MdDFR、MdUF3GT表达,同时整合GA和JA信号,负调控花青素的生物合成[65]。通过转录组分析,Wang 等[66]在红心猕猴桃中发现,AcMYB123-AcbHLH42 复合体通过激活AcANS 和AcF3GT 基因的表达,促进果实内果皮红色表型形成。
WD 蛋白作为MBW 复合物的核心组分,能够增强MBW 调控网络的稳定性。Liu 等[67]从猕猴桃中鉴定出AcMYBF110-AcbHLH4-AcWDR1 和Ac-MYBF110-AcbHLH5-AcWDR1 两种复合体,通过调控AcbHLH1 和AcWDR1 的转录活性来间接影响花青素代谢。草莓FaMYB5主导的FaMYB5-FaEGL3-FaLWD1-like复合物通过直接激活F3’H、AHA10和LAR基因来促进花青素和原花青素的积累[68]。无花果的WD蛋白FcTTG1与FcMYB114、FcMYB123和FcbHLH42 蛋白形成互作网络,精细调控花青素的合成[69]。
近年研究发现,除了MBW 复合体的多层级调控外,其他转录因子家族也被逐渐发掘参与花青素合成代谢。不同于MBW 转录因子间的互作机制,其他转录因子则更多通过与MYB、bHLH协同调控花青素生物合成的关键酶基因表达,从而影响花青素的积累或降解(表2)[70-85]。bZIP 家族成员HY5 已被鉴定为拟南芥、苹果、血橙和番茄花青素生物合成的正调控因子,蓝莓中VcbZIP55 与VcMYB1 启动子上的G-BOX 基序结合,激活VcMYB1 的表达,从而促进花青素的合成[70]。NAC 转录因子是跨通路代谢的重要节点,能够调控多种色素的合成,蓝莓Vc-NAC072 可与MYB 转录因子AtPAP1 互作并激活花青素合成基因的表达[76],苹果MdNAC52 也能通过MdMYB9 和MdMYB11 协同提高MdDFR、MdANS、MdUFGT 的转录活性[77]。而葡萄的VvNAC17 则能促进花青素的合成,从而提高植物的抗干旱胁迫能力[86],为转录因子互作的多层级调控花青素的合成网络提供新思路。WRKY 基因家族除了能与MYB、bHLH相互作用外[79],还能够在光照条件下与HY5 转录因子协同促进苹果果实中花青素的合成[73]。此外,乙烯信号途径的相关转录因子ERF 也能与不同MYB 转录因子互作,通过不同的作用途径对花青素合成进行正向或反向调控[81-82]。ARF、BZR转录因子则更多抑制花青素合成基因的表达,从而负调控花青素和积累[83-85]。
表2 其他调控花青素合成的转录因子及作用机制
Table 2 Other transcription factors regulating anthocyanin synthesis and mechanisms of action

转录因子Transcription factor bZIP转录因子bZIP transcription factor物种Species蓝莓Vaccinium corymbosum甜樱桃Vitis vinifera L.名称Name VcbZIP55参考文献Reference[70]PavbZIP6[71][72]HY5转录因子HY5 transcription factor李子Prunus salicina桃P.persica PsbZIP1 PsbZIP10 PpHY5[73]砂梨Pyrus pyrifolia PyHY5[74]苹果M.domestica MdHY5[75]NAC转录因子NAC transcription factor蓝莓Vaccinium corymbosum苹果M.domestica VcNAC072[76]MdNAC52[77]苹果M.domestica Borkh.MdNAC1[78]WRKY转录因子WRKY transcription factor红梨Pyrus L.PyWRKY26[79]苹果M.domestica Borkh.MdWRKY72[80]ERF转录因子ERF transcription factor桑葚Morus alba L.梨Pyrus spp.ERF5[81]PpERF105[82]ARF转录因子ARF transcription factor苹果M.domestica MdARF2[83]草莓Fragaria×ananassa苹果M.domestica FveARF2[84]BZR转录因子BZR transcription factor MdBEH2.2调控路径Regulatory pathway激活VcMYB1的表达,正调控花青素的合成。VcbZIP55 activates the expression of VcMYB1 and promote the synthesis of anthocyanins.增强DFR、ANS、UFGT等合成基因的表达,正调控花青素合成。PavbZIP6 enhances the expression of synthetic genes such as DFR,ANS,and UFGT and positively regulates anthocyanin synthesis.二者与PsUFGT 启动子结合,正调控花青素积累。Both bind to the PsUFGT promoter to promote anthocyanin accumulation.激活花青素合成的结构基因PpCHS1、PpCHS2、PpDFR1以及PpMYB10.1的表达,正调控花青素合成。The structural genes PpCHS1, PpCHS2, PpDFR1, and PpMYB10.1, which activate anthocyanin synthesis,are regulating anthocyanin synthesis.参与PyMYB10和PyWD40共同调控的花青素合成途径,激活CHS、ANS、UFGT表达,促进花青素积累。PyHY5 participates in the anthocyanin synthesis pathway co-regulated by Py-MYB10 and PyWD40,activations of CHS, ANS, and UFGT expression for anthocyanin accumulation.与MdBBX22 蛋白协同激活MdMYB10 和MdCHS,促进花青素的生物合成。MdHY5 interactes with MdBBX22 protein to activate MdMYB10 and MdCHS to promote anthocyanin biosynthesis.与MYB转录因子AtPAP1互作并激活花青素合成基因的表达。VcNAC072 with MYB transcription factor AtPAP1 and activation of anthocyanin synthesis gene expression.通过MdMYB9和MdMYB11协同激活花青素合成的结构基因LAR、ANR的表达,正调控花青素合成。MdNAC52 interactes with MdMYB9 and MdMYB11 and activate structural genes LAR,ANR and positive regulation of anthocyanin synthesis.与MdbZIP23互作并激活MdMYB10、MdUFGT基因的表达,促进花青素积累。MdNAC1 interactes with MdbZIP23 and activates the expression of Md-MYB10,MdUFGT genes to promote anthocyanin accumulation.与PybHLH3、PyMYB114 互作并激活花青素合成基因PyDFR、PyANS 和PyUFGT的表达,正调控花青素合成。PyWRKY26 interactes with PybHLH3、PyMYB114 and activates the expression of anthocyanin synthesis genes PyDFR, PyANS and PyUFGT, which positively regulate anthocyanin synthesis光照诱导下,与MdHY5、MdMYB1 共同激活MdANS、MdDFR、MdUFGT 的表达,正调控花青素积累。Light-induced co-activation of MdANS, MdDFR, and MdUFGT expression with MdHY5 and MdMYB1 positively regulated anthocyanin accumulation与MYBA和F3H 基因互作促进花青素合成。ERF5 interactes with MYBA and F3H genes promotes anthocyanin synthesis.与PpMYB140互作并抑制花青素合成基因的表达,负调控花青素合成。PpERF105 interactes with PpMYB140 and inhibit of anthocyanin synthesis gene expression,and negative regulation of anthocyanin synthesis.抑制花青素合成的相关结构基因MdDFR、MdCHS和MdUFGT表达,抑制花青素积累。MdARF2 inhibitions of anthocyanin accumulation by suppressing the expression of structural genes related to anthocyanin synthesis, MdDFR, MdCHS and MdUFGT.抑制花青素合成的相关结构基因FaCHS等的表达,负调控花青素合成。FveARF2 inhibitions of the expression of structural genes related to anthocyanin synthesis,such as FaCHS,negatively regulates anthocyanin synthesis通过MdBEH2.2-MdMYB60 复合物抑制ANS、ANR、FLS和ANR的转录,负调控花青素积累。MdBEH2.2 negatively regulates anthocyanin accumulation by repressing the transcription of ANS, ANR, FLS, ANR through the MdBEH2.2-MdMYB60 complex.[85]
2.2 环境因素
近年来,环境因素对果树果实花青素合成的分子调控机制研究取得了显著进展。其核心调控路径在于通过调控MBW复合物的动态形成从而激活花青素代谢通路。研究表明环境信号可双向调控花青素生物合成通路的关键基因[87],花青素的稳定性低[88],环境因子和内源激素协同调控花青素合成-降解的动态平衡,目前已知的主要调控因子包括光、温度、糖、水分和外源植物激素等。
在光照条件下,光信号通常激活HY5(bZIP 家族成员),通过与MYB、bHLH 等形成复合物从而促进果实花青素的合成。光信号通路通过FvHY5-FvbHLH9 异源二聚体来调控草莓果实的花青素合成[89]。Xing 等[90]在苹果中研究发现,MdMPK6 激酶介导的MdHY5 蛋白磷酸化可显著提高MdMYB1、MdCHI 和MdUFGT 基因的表达水平。HY5 转录因子还能够与B-BOX(BBX)共同在光信号传导通路中调控花青素的生物合成。苹果的MdBBX22 与MdHY5 相互作用并增强MdHY5 与其靶基因MdCHS启动子的结合能力,从而促进UV-B 诱导的花青素积累[75]。在蓝光的特异性激活条件下,草莓建立FaCRY1-FaCOP1-FaHY5信号模块从而显著上调CHS、ANS等结构基因的表达,同时FaHY5在单独作用下必须依靠FaBBX22蛋白相互作用,协同调控花青素的生物合成[91]。此外光照不仅可以单一影响花青素合成,在温度的作用下,二者可以协同调控花青素的积累,Huang等[92]在血橙中发现的CsRuby1基因启动子区域,同时包含光响应G-box 元件、LTRE(低温响应元件)和MYC结合位点,在光照和低温的同时诱导下,血橙中的CsRuby1基因被显著激活,提高花青素的积累效率。低温也能通过影响MYB、bHLH等复合物从而促进花青素的合成。海棠果实在低温胁迫下ABA 显著在果皮积累,诱导Mp-MYB11-MpbHLH79 复合物的形成,激活花青素合成关键基因MpCHS的表达[93]。黄酮、类黄酮化合物的积累被证实可以改善植物对干旱胁迫的适应性[94],ABA 信号通路、MYB/bHLH 转录因子的信号级联调控发挥着重要作用。对蓝莓在干旱胁迫下的代谢组和转录组分析发现,黄酮类代谢物显著积累,ABA 信号通路中的ABF、MYBs、bHLHs 和黄酮生物合成基因的调控网络能够调节干旱诱导的蓝莓叶片中黄酮代谢物的积累[95]。
喷施外源植物激素作为调控花青素代谢的关键技术方法,在果树栽培中能够显著促进花青素的积累。各种内源激素的信号通路受体与MBW复合物之间的互作网络是目前研究的热点,外源激素如何影响或改变调控网络的形成仍是未来的研究方向,主要包括ETH、ABA、IAA 和JA 等植物激素。转录因子互作网络中MYB 基因家族能够整合多激素信号。ABA 通过MdABI5-MdMYB1-MdbHLH3 三元复合体激活靶基因MdDFR、MdUF3GT 的表达[96]。在蓝莓中首次鉴定出6 个SnRK2 家族成员(VcSnRK2.1~6),其中VcSnRK2.3 的表达与果实成熟和ABA 信号通路呈正相关,ABA 诱导的VcSnRK2.3(蔗糖非发酵-1-相关蛋白激酶2)能与VcMYB1相互作用,促进花青素的生物合成[97]。内源JA信号通路蛋白MdJAZ1 抑制MdTRB1-MdMYB9 途径对花青素的合成,负向调节MeJA诱导的花青素和PA的积累[98]。此外外源NAA处理后MdIAA121-His蛋白的迅速降解可释放与之结合的MdARF13,随后MdARF13与MdMYB10形成复合体协同抑制靶基因的表达,负调控花青素的生物合成[99]。乙烯信号通路响应因子AP2/ERFs 也在不同激素处理条件下调控花青素的合成:梨果实中ETH通过PpERF9(ERF转录因子)构建双重抑制机制,一方面PpERF9 与PpMYB114的启动子结合直接抑制其表达,另一方面形成一个PpERF9-PpRAP2.4-PpMYB114 的调控回路,从而抑制梨中花青素的生物合成[100];Li等[101]发现同时用ETH和NAA处理苹果果实后,MdARF5-1抑制正调控因子MdERF3的表达来负向调控花青素合成,而MdIAA29可通过竞争结合减弱抑制作用。
2.3 表观修饰
DNA 甲基化水平的下降能有效促进果实中花青素的合成。苹果的MdROS1通过降低花青素相关基因CHS、CHI、F3’H、ANS、UFGT和MYB10的启动子甲基化水平从而促进花青素的积累[102]。桃果实在16 ℃低温贮藏下,PpF3H、PpANS 等基因的甲基化水平显著降低,同时用5-aza 处理桃果肉,显著诱导花青素积累[103]。长链非编码RNA(lncRNA)作为表观遗传的调控网络的核心部分,在果实花青素积累中发挥着重要作用,Ma 等[104]在苹果中构建了一个MdWRKY1-MdLNC499-MdERF109的转录级联,光信号转录因子MdWRKY1 通过激活lncRNA MdLNC499的转录活性,进而诱导MdERF109表达,最终MdERF109蛋白诱导苹果着色前期花青素相关基因的表达。而在草莓果实中的lncRNA FRILAIR作为一种非规范的靶模拟物,能够结合miR397分子并且促进一种漆酶-11样蛋白LAC11a的转录,进而使花青素在果实成熟过程中沉淀着色[105]。近年来研究发现,miRNAs-MYB协同调控花青素的生物合成[106],Zhang 等[107]在梨果实中发现一个PyPIF5-PymiR156a-PySPL9-PyMYB114/10 模块,光照下调光敏色素因子PIF5 并释放miR156a,随后PySPL9被miR156a 切割降解,进而抑制PySPL9-Py-MYB114/10异源聚体的形成,重新激活花青素的生物合成[108]。此外,组蛋白修饰作用在果实中同样调控花青素的合成。苹果中MdSnRK1.1 与MdJAZ18相互作用并磷酸化,以促进其26S 蛋白酶体介导的降解并释放MdbHLH3,MdbHLH3 与MdMYB1/9、MdTTG1 形成MBW 复合物,从而激活MdDFR、MdANS、MdANR 和MdUF3GT 的表达,并促进花青素和原花青素(PA)的生物合成[109]。同时苹果的MdBT2通过泛素化-蛋白酶体途径调控MdTCP46的稳定性,形成动态的“MdBT2-MdTCP46-MdMYB1”调控模块,在高光强刺激下MdBT2 表达受抑制,增强MdTCP46 与MdMYB1 的协同作用并激活花青素合成基因,促进果实着色[110]。
3 叶绿素
叶绿素(Chlorophylls,CHIs)是一种广泛存在于植物、藻类和某些细菌中的四吡咯化合物[111-112],CHIs经过修饰后主要有5 种类型,分别为叶绿素a、叶绿素b、叶绿素c(c1、c2、c3)、叶绿素d 和叶绿素f[113]。叶绿素在光合作用过程中起核心作用,吸收光能并驱动电子传递链最终产生ATP、NADPH 等化学能[113],同时叶绿素的抗氧化活性、抗炎症、抗癌以及抗肥胖等医疗保健作用也逐渐被关注[114]。值得注意的是,叶绿素代谢网络与类胡萝卜素、花青素等代谢途径存在复杂的交互作用,三类色素共同调控果实色泽的形成[115-116],因此更需进一步探究和完善叶绿素代谢途径涉及的分子调控机制。现阶段,针对果实叶绿素代谢的分子调控研究,更多集中于叶绿素合成或降解途径关键基因的转录活性。合成途径关键酶包括谷氨酰-tRNA 合成酶(GluRS)、原叶绿素酸酯氧化还原酶(POR)、脱植基叶绿素a 加氧酶(CAO)和叶绿素合酶(CHLG),降解途径则由脱镁叶绿酸a 加氧酶(PAO)、脱镁螯合酶(SGR)、脱镁叶绿素脱镁叶绿酸水解酶(PPH)、红色叶绿素分解产物还原酶(RCCR)、叶绿素b 还原酶(NYC、NOL)等协同调控[117-118]。
3.1 转录因子
果实叶绿素代谢的分子机制离不开转录因子的精细调控,已有较多的转录因子被发现能够在植物中影响叶绿素的代谢过程。但对于果树果实来说,已经验证并具有明确调控叶绿素代谢功能的调控因子仍然较少,目前研究的主要核心包括GOLDEN2-LIKE(GLK)、MYB、NAC、bHLH和APRR等转录因子家族(表3)[28,115,119-134]。
表3 调控叶绿素合成的主要转录因子及作用机制
Table 3 Main transcription factors and mechanisms regulating chlorophyll synthesis
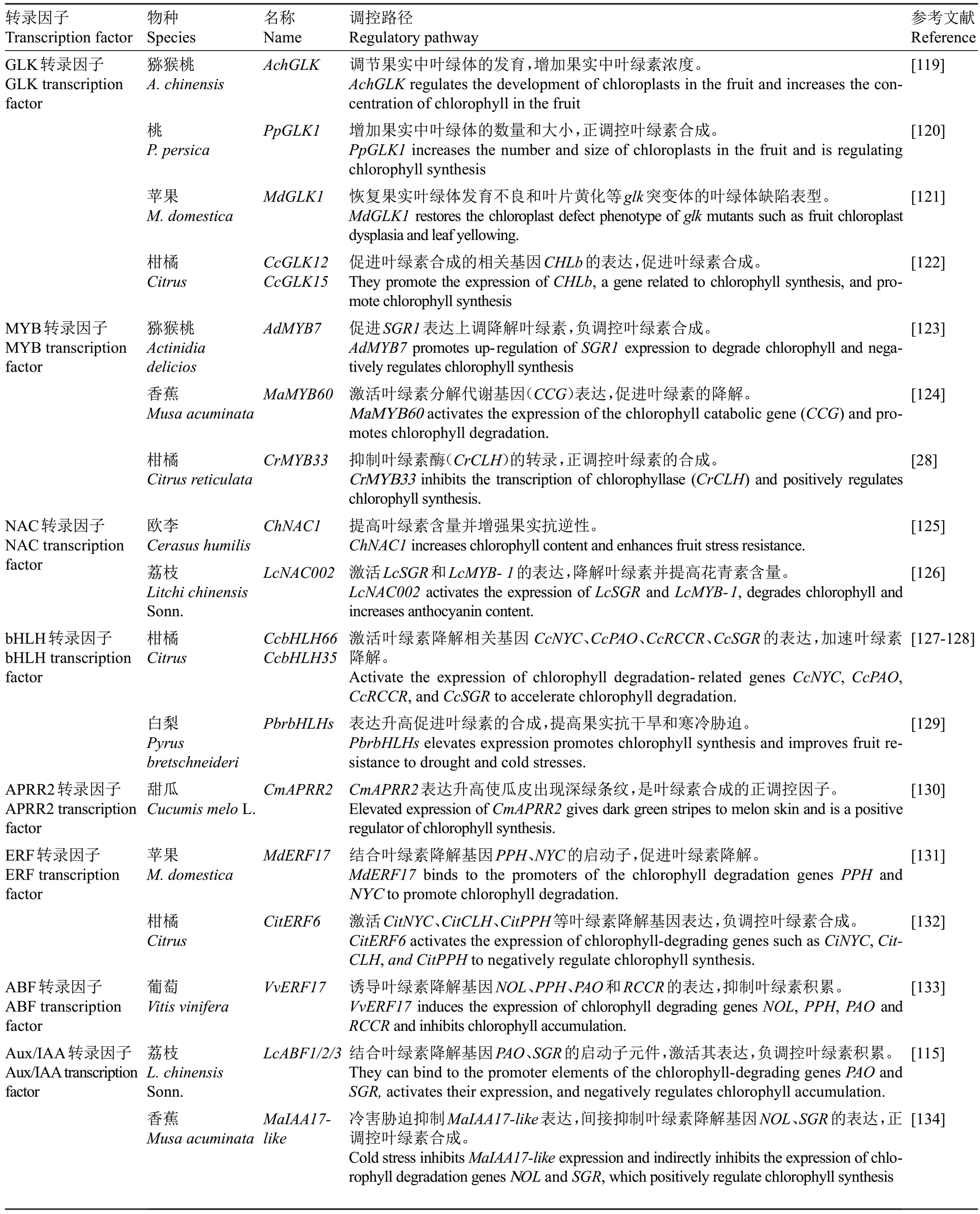
转录因子Transcription factor GLK转录因子GLK transcription factor物种Species猕猴桃A.chinensis名称Name AchGLK参考文献Reference[119]桃PpGLK1[120]P.persica苹果M.domestica MdGLK1[121]柑橘Citrus CcGLK12 CcGLK15[122]MYB转录因子MYB transcription factor猕猴桃Actinidia delicios香蕉Musa acuminata AdMYB7[123]MaMYB60[124]柑橘Citrus reticulata CrMYB33[28]NAC转录因子NAC transcription factor ChNAC1[125]欧李Cerasus humilis荔枝Litchi chinensis Sonn.柑橘Citrus LcNAC002[126]bHLH转录因子bHLH transcription factor CcbHLH66 CcbHLH35[127-128]白梨Pyrus bretschneideri甜瓜Cucumis melo L.PbrbHLHs [129]APRR2转录因子APRR2 transcription factor ERF转录因子ERF transcription factor CmAPRR2[130]苹果M.domestica MdERF17[131]柑橘Citrus CitERF6[132]ABF转录因子ABF transcription factor Aux/IAA转录因子Aux/IAAtranscription factor葡萄Vitis vinifera VvERF17[133]荔枝L.chinensis Sonn.香蕉Musa acuminata LcABF1/2/3[115]MaIAA17-like调控路径Regulatory pathway调节果实中叶绿体的发育,增加果实中叶绿素浓度。AchGLK regulates the development of chloroplasts in the fruit and increases the concentration of chlorophyll in the fruit增加果实中叶绿体的数量和大小,正调控叶绿素合成。PpGLK1 increases the number and size of chloroplasts in the fruit and is regulating chlorophyll synthesis恢复果实叶绿体发育不良和叶片黄化等glk突变体的叶绿体缺陷表型。MdGLK1 restores the chloroplast defect phenotype of glk mutants such as fruit chloroplast dysplasia and leaf yellowing.促进叶绿素合成的相关基因CHLb的表达,促进叶绿素合成。They promote the expression of CHLb, a gene related to chlorophyll synthesis, and promote chlorophyll synthesis促进SGR1表达上调降解叶绿素,负调控叶绿素合成。AdMYB7 promotes up-regulation of SGR1 expression to degrade chlorophyll and negatively regulates chlorophyll synthesis激活叶绿素分解代谢基因(CCG)表达,促进叶绿素的降解。MaMYB60 activates the expression of the chlorophyll catabolic gene(CCG)and promotes chlorophyll degradation.抑制叶绿素酶(CrCLH)的转录,正调控叶绿素的合成。CrMYB33 inhibits the transcription of chlorophyllase (CrCLH) and positively regulates chlorophyll synthesis.提高叶绿素含量并增强果实抗逆性。ChNAC1 increases chlorophyll content and enhances fruit stress resistance.激活LcSGR和LcMYB-1的表达,降解叶绿素并提高花青素含量。LcNAC002 activates the expression of LcSGR and LcMYB-1,degrades chlorophyll and increases anthocyanin content.激活叶绿素降解相关基因CcNYC、CcPAO、CcRCCR、CcSGR的表达,加速叶绿素降解。Activate the expression of chlorophyll degradation-related genes CcNYC, CcPAO,CcRCCR,and CcSGR to accelerate chlorophyll degradation.表达升高促进叶绿素的合成,提高果实抗干旱和寒冷胁迫。PbrbHLHs elevates expression promotes chlorophyll synthesis and improves fruit resistance to drought and cold stresses.CmAPRR2表达升高使瓜皮出现深绿条纹,是叶绿素合成的正调控因子。Elevated expression of CmAPRR2 gives dark green stripes to melon skin and is a positive regulator of chlorophyll synthesis.结合叶绿素降解基因PPH、NYC的启动子,促进叶绿素降解。MdERF17 binds to the promoters of the chlorophyll degradation genes PPH and NYC to promote chlorophyll degradation.激活CitNYC、CitCLH、CitPPH等叶绿素降解基因表达,负调控叶绿素合成。CitERF6 activates the expression of chlorophyll-degrading genes such as CiNYC,Cit-CLH,and CitPPH to negatively regulate chlorophyll synthesis.诱导叶绿素降解基因NOL、PPH、PAO和RCCR的表达,抑制叶绿素积累。VvERF17 induces the expression of chlorophyll degrading genes NOL, PPH, PAO and RCCR and inhibits chlorophyll accumulation.结合叶绿素降解基因PAO、SGR的启动子元件,激活其表达,负调控叶绿素积累。They can bind to the promoter elements of the chlorophyll-degrading genes PAO and SGR,activates their expression,and negatively regulates chlorophyll accumulation.冷害胁迫抑制MaIAA17-like表达,间接抑制叶绿素降解基因NOL、SGR的表达,正调控叶绿素合成。Cold stress inhibits MaIAA17-like expression and indirectly inhibits the expression of chlorophyll degradation genes NOL and SGR,which positively regulate chlorophyll synthesis[134]
GLK 被认为是叶绿素生物合成的主调控因子[135],猕猴桃的AchGLK 在番茄中异源表达使叶绿素得到积累,过表达AchGLK 的果实叶绿体大小和类囊体颗粒堆叠厚度都显著增加[119]。桃果实中利用VIGS病毒沉默PpGLK1的表达,其靶基因PpPORA、PpCHLH 等转录水平下降[120]。An 等[121]发现异位表达MdGLK1则能恢复拟南芥glk1glk2双突变体的叶绿体缺陷表型,如叶绿体发育不良和叶片黄化等,并提高HEMA1、GUN4、CHLH 和CAO 的表达水平,促进叶绿素的合成。Frangedakis等[135]发现在拟南芥glk 突变体中仍有叶绿素残留,并寻找到MYB转录因子与GLK 共同调控叶绿体发育和叶绿素合成酶基因表达的证据。在果树果实中,尽管研究已发现较多MYB 参与叶绿素代谢,但其与GLK 协同作用的调控路径仍需深入研究。猕猴桃的AdMYB7促进SGR1 表达上调并降解叶绿素[123],香蕉Ma-MYB60在高温胁迫下被MaBAH1 E3连接酶介导的泛素化修饰并降解,减弱MaMYB60 对叶绿素分解代谢基因的激活,这也为环境因子通过翻译后修饰MYB-TFs提供了新证据[124]。NAC在调控果实成熟过程中发挥着关键作用,在果树果实中NAC的研究较多集中于类胡萝卜素和花青素合成代谢[136],对叶绿素的代谢调控有待进一步探讨。但欧李果实在干旱胁迫时过表达ChNAC1能促进叶绿素积累并提高抗逆性,帮助果实在胁迫环境下更好成熟[125]。荔枝中的LcNAC002 显著激活LcSGR 和LcMYB-1 的表达,降解叶绿素并提高花青素含量,促进荔枝果实的着色[126]。bHLH转录因子在柑橘果皮中的呈色机制表现出多重作用,通过与叶绿素降解相关基因(CcNYC、CcPAO、CcRCCR和CcSGR)以及类胡萝卜素合成关键基因(CcPSY1、CcBCH2、CcNCED5)的结合激活[127-128],形成两类色素的代谢平衡。此外bHLH的功能也在白梨等中得到验证[129]。不同于通过转录组分析方式挖掘果实中与叶绿素代谢有关的转录因子,Oren等[130]利用双亲/多亲群体设计和深度测序,发现甜瓜的多等位基因CmAPRR2 是叶绿素合成的正调控因子,这为研究叶绿素代谢途径中的分子作用机制提供了新思路。
激素信号转导相关转录因子通过调控叶绿素代谢相关基因的表达,在叶绿素代谢中同样重要,从而影响果实的着色和成熟。乙烯响应因子(AP2/ERFs)帮助果实去绿和软化,MdERF17基因编码区丝氨酸(Ser)重复数量影响ERF17蛋白的转录活性以及与叶绿素降解基因(MdPPH)的结合能力,是影响苹果果皮退绿的重要遗传因素[131]。同时葡萄、柑橘中也存在相应的ERFs激活叶绿素降解基因如PPH、PAO和RCCR 等,完成果实在成熟和采后中的脱绿过程[132-133]。内源ABA 通常在叶绿素降解开始迅速增加,三种ABA响应因子(ABF1/2/3)是荔枝着色的重要转录调控因子,其识别LcPAO和LcSGR启动子区域的ABA 响应元件并激活LcMYB1,从而促进叶绿素降解和花青素合成[115]。IAA响应因子(ARFs)也在果实的叶绿素代谢中有所报道,Chen等[134]通过分析香蕉中MaIAA17-like在正常和低温储藏时的转录水平,发现冷害会抑制MaIAA17-like的表达,间接影响MaIAA17-like 对MaNOL 和MaSGR1 的激活表达,这也表明冷储会延缓香蕉果实的脱绿和成熟。转录组分析揭示香蕉成熟过程中乙烯、ABA和IAA激素信号响应因子的表达趋势,发现在香蕉脱绿时MaERFs和MaARF19-like表达上调,相应促进叶绿素降解相关基因MaSGR1、MaPPH1的表达,但MaABI5-like却显著下调[137]。细胞分裂素氧化酶(CKX)则抑制MaSGR1的表达,延缓叶绿素的分解[138]。乙烯和ABA信号通路的相关转录因子能够在果实脱绿过程中靶向激活叶绿素降解的关键基因(SGR、PAO),从而促进叶绿素的降解,而IAA、CK则拮抗乙烯和ABA的协同作用,抑制降解基因的表达从而延缓果实的脱绿。
3.2 环境因素
果树果实的叶绿素代谢离不开栽培环境的改良和完善,但目前只有较少的研究以分子机制为落点探究环境因素对叶绿素代谢的影响,现有的研究主要聚焦光环境、金属离子元素和外源植物激素等。
光照是植物进行光合作用不可或缺的外界条件,不同光质影响果实颜色转变的途径存在差异。采后柑橘经蓝光LED 处理可激活内源乙烯合成通路的关键基因(CitACS1、CitACO)以及信号转导元件(CitETR1、CitEIN2、CitEIL1和CitERF2)等基因的表达间接促进叶绿素降解[139],而LED白光处理贮藏早酥梨则表现出CAO酶基因的高表达,抑制果实内乙烯生成和叶绿素降解[140]。除了光质,光信号转录因子的调控对叶绿素代谢也尤为重要,在番茄、马铃薯等园艺作物上HY5、PIFs 等光响应因子的作用机制逐渐清晰[141-142],但果树果实中光信号转导与叶绿素代谢的分子互作网络仍需探究。
叶绿素合成的核心途径之一为原卟啉Ⅸ(protoporphyrin Ⅸ,Proto Ⅸ)的合成,镁、铁螯合酶将镁、铁离子送入Proto Ⅸ中推动叶绿素的合成[143],金属离子的稳态调控对叶绿素代谢同样重要。Song等[144]研究了缺铁条件时葡萄Fe2+转运蛋白VvIRT的基因表达趋势,发现IRT1缺失突变体的叶绿素含量显著低于野生型。而桃树喷施MgCl2可诱导Mg2+转运蛋白PpMGT家族基因表达上调,加快植物体对外源Mg2+的吸收利用,进而影响叶绿素的生物合成[145]。这些研究表明了离子转运蛋白在叶绿素代谢途径中发挥的关键作用。
外源激素处理通过调控转录因子网络进而动态平衡叶绿素的合成或降解,同时帮助果实更快地着色和成熟。乙烯是促进果实褪绿和成熟过程中使用较为广泛的外源激素,不但直接影响着叶绿素降解基因的表达,还对ERFs 和某些转录因子起调控作用。早熟柑橘往往有果皮和果肉无法同时成熟的特性[146],因此外源乙烯处理使柑橘果皮褪绿和着色十分必要。已有研究证实CcbHLH35、CitERF13 都被显著诱导并结合叶绿素降解基因NYC1、PAO、RCCR和PPH,协同介导乙烯诱导的叶绿素降解[128,147]。此外,柠檬、苹果等果实经乙烯处理后促进PPH、NYC等基因表达,加速叶绿素的降解[148-149]。除了乙烯,ABA同样是促进果实成熟的主要外源激素,单独施用ABA能促进叶绿素的降解[150]。利用ABA作为对照,外源NAA 虽能降低叶绿素的含量,但其对叶绿素降解基因的转录激活水平低于ABA[151]。而外源细胞分裂素(CPPU)对比ABA则表现出直接对SGR表达的抑制作用,从而缓解叶绿素的降解[152]。此外,其他激素如GA、MeJA 等也参与叶绿素代谢相关基因的表达[153-154],形成复杂的激素互作网络。
3.3 表观遗传
叶绿素代谢的表观遗传调控机制研究近年来取得重要突破,特别是DNA甲基化方面,DNA甲基化修饰与基因表达之间的相互作用是动态且复杂的,调控效果取决于具体的基因和表观遗传的环境。在菠萝组织培养过程中,低DNA甲基化水平导致叶绿素降解途径相关基因高度表达同时引起叶绿体的发育异常[155],柑橘和草莓低水平的DNA甲基化也会引起叶绿素合成基因的表达下降,导致无法着色[43,156]。长链非编码RNA 在果实成熟过程中是重要的调节因子。蓝莓miR156a-SPL12模块能动态调控叶绿素代谢相关基因(CAB、CLH2、NYC1)的表达[157]。桃果实在UV-B 的处理下miR171c 表达显著下调,解除其对SCL的抑制,从而间接促进叶绿素的合成[158]。组蛋白修饰的泛素化作用能够调控叶绿素的生物合成:高温诱导香蕉中MaNIP1 蛋白表达从而泛素化降解MaNYC1,抑制香蕉的褪绿过程[159];乙烯则促进苹果E3 泛素连接酶MdPUB24 表达上调,后者泛素化MdBEL7 并解除其对叶绿素降解基因(MdCLH、MdPPH2和MdRCCR2)的抑制[160]。
4 展 望
类胡萝卜素、花青素与叶绿素代谢的动态平衡受环境、激素与转录网络协同调控。目前对于叶绿素与类胡萝卜素在果实成熟过程中积累的时序性、花青素和类胡萝卜素的竞争性合成以及三者间的协同或拮抗效应仍缺乏系统和深入的解析。当前研究焦点逐渐从单一的色素代谢转向三类色素的协同或拮抗机制,并逐渐发掘更多调控三类色素代谢的关键基因节点(如MYB、bHLH、NAC)。多组学联合分析(如代谢组、转录组、蛋白质组)在揭示三类色素代谢的分子调控途径中至关重要。转录组分析筛选三类色素代谢途径的关键基因及其表达水平,鉴定核心转录因子并明确其在跨通路中的整合功能,代谢组动态分析色素代谢物(如类黄酮代谢物、叶绿素a/b 以及β-胡萝卜素)的积累趋势,将代谢物水平与核心转录因子进行关联分析,明确关键基因的具体调控功能。而蛋白质组学则聚焦翻译后修饰对酶活性的动态调控,在蛋白层面明确核心转录因子的修饰对色素代谢的调控模式。核心转录因子通过完善的基因编辑手段(如CRISPR/Cas9),进行敲除或过表达,定向调节目标色素的积累或降解,协调三类色素的代谢平衡(图1)。
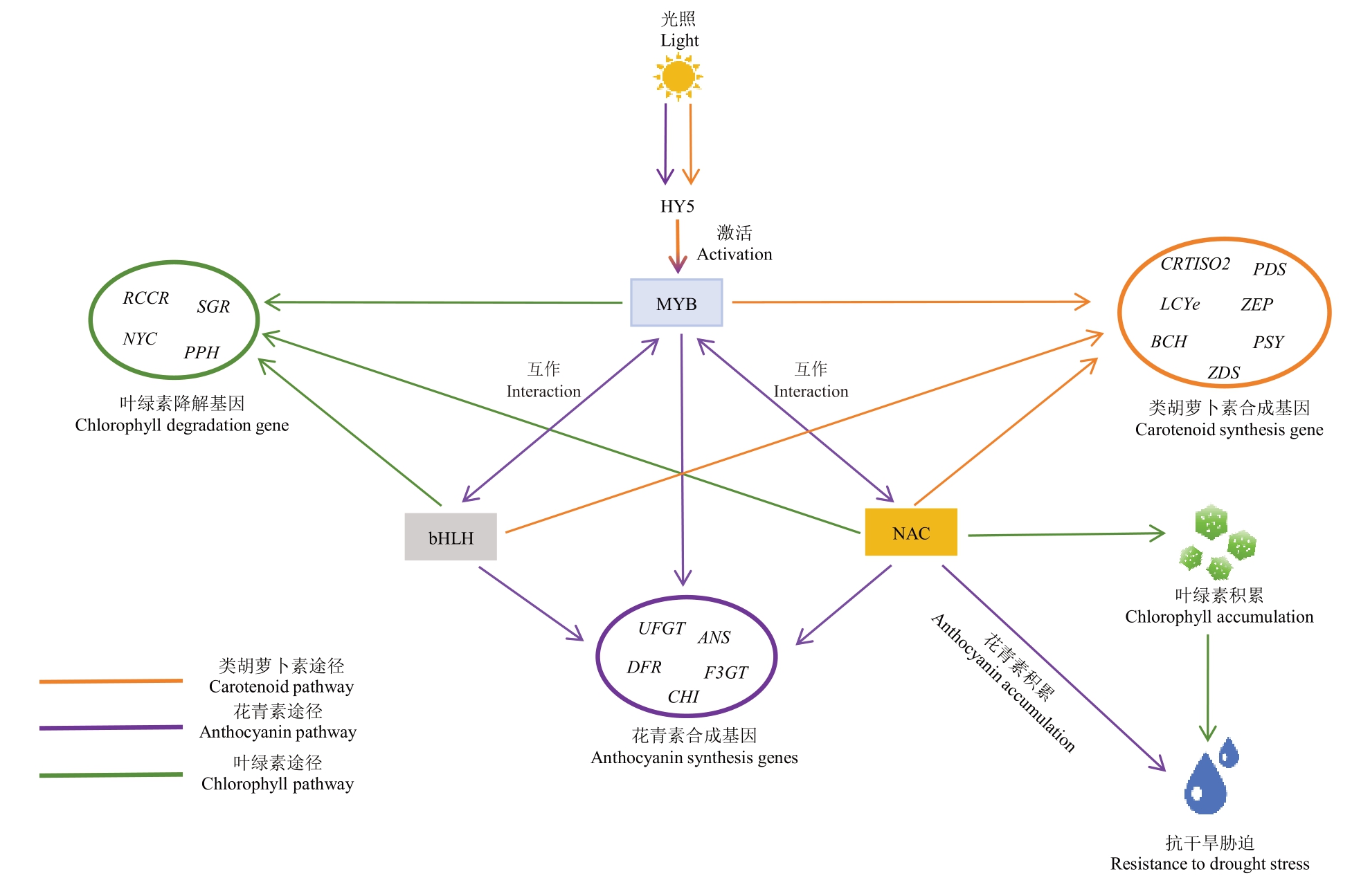
图1 跨通路代谢关键转录因子调控网络
Fig.1 Cross pathway metabolic key transcription factor regulatory network
图中箭头表示促进作用。该图根据梁敏华等[22]、Zhang 等[26]、Sun 等[29]、An 等[65]、Sun 等[77]、Jin 等[86]、Xing 等[90]、Wei 等[124]、Wang 等[125]、Zou等[126]、Wang 等[127]的研究绘制。
The arrows in the figure indicate facilitation.The figure is drawn according to the results of Liang Minhua et al.[22],Zhang et al.[26],Sun et al.[29],An et al.[65],Sun et al.[77],Jin et al.[86],Xing et al.[90],Wei et al.[124],Wang et al.[125],Zou et al.[126],and Wang et al[127].
目前,表观遗传的研究方向也逐渐与环境胁迫和激素信号进行关联。但表观遗传与激素信号的互作机制尚不完善,例如表观遗传如何通过激活激素信号传导途径的相关转录因子从而影响果实呈色仍需进一步研究。此外,环境胁迫(如低温、干旱)如何通过表观遗传手段调控色素代谢的分子机制,是提高果树果实抗逆性的重要方向。
本文整合了三类色素代谢的分子调控框架,提出了跨通路色素代谢的关键节点,通过系统梳理环境-基因-色素代谢的调控途径,为设施栽培定向调控果实色素积累提供理论依据。在设施栽培中建立光质、温度与激素协同互作模式,最大程度实现果实色素积累,提高市场竞争力和经济收益。同时整合表观遗传修饰涉及的分子调控机制,合理利用外源激素或小分子抑制剂(如甲基化抑制剂5-AZ),实现对果实着色的精准控制。
果实的色泽不仅是外观指标,也与营养性(如三类色素的抗氧化性等医用价值)、贮藏性(叶绿素降解与果实软化)以及抗逆性(花青素响应胁迫)密切相关。果实着色的分子调控正从单一途径向多维度的调控网络迈进,未来需探索果实色泽调控网络与果实综合品质的关联,通过深化理论研究并整合新型技术手段,实现果实色泽的精准设计,为顺应市场需求高效化、高品质培育优良果树品种。
[1] MUHAMMAD N,LUO Z,YANG M,LIU Z G,LIU M J. The underlying molecular mechanisms of external factors influencing fruit coloration in fruit trees[J].Scientia Horticulturae,2023,309:111615.
[2] MUHAMMAD N,LIU Z G,WANG L X,YANG M S,LIU M J.The underlying molecular mechanisms of hormonal regulation of fruit color in fruit-bearing plants[J].Plant Molecular Biology,2024,114(5):104.
[3] 刁卫楠,朱红菊,刘文革.蔬菜作物中类胡萝卜素研究进展[J].中国瓜菜,2021,34(1):1-8.DIAO Weinan,ZHU Hongju,LIU Wenge. Research progress on carotenoids in vegetable crops[J]. China Cucurbits and Vegetables,2021,34(1):1-8.
[4] 胡紫蔚,张蕾琛,陈英子,朱勇,章豪,黄芸萍.西瓜果实类胡萝卜素组分含量与瓤色相关性分析[J].中国瓜菜,2025,38(1):31-38.HU Ziwei,ZHANG Leichen,CHEN Yingzi,ZHU Yong,ZHANG Hao,HUANG Yunping. Correlation analysis between watermelon flesh color with carotenoid compositions and content[J].China Cucurbits and Vegetables,2025,38(1):31-38.
[5] 陆晨飞,高月霞,黄河,戴思兰.植物类胡萝卜素代谢及调控研究进展[J].园艺学报,2022,49(12):2559-2578.LU Chenfei,GAO Yuexia,HUANG He,DAI Silan. Carotenoid metabolism and regulation in plants[J].Acta Horticulturae Sinica,2022,49(12):2559-2578.
[6] MAOKA T. Carotenoids as natural functional pigments[J]. Journal of Natural Medicines,2020,74(1):1-16.
[7] SUN T H,RAO S,ZHOU X S,LI L. Plant carotenoids:Recent advances and future perspectives[J]. Molecular Horticulture,2022,2(1):3.
[8] 何静娟,范燕萍.观赏植物花色相关的类胡萝卜素组成及代谢调控研究进展[J].园艺学报,2022,49(5):1162-1172.HE Jingjuan,FAN Yanping. Progress in composition and metabolic regulation of carotenoids related to floral color[J]. Acta Horticulturae Sinica,2022,49(5):1162-1172.
[9] 樊宝莲,王晓云.转录因子调控植物类胡萝卜素合成途径的研究进展[J].分子植物育种,2021,19(13):4401-4408.FAN Baolian,WANG Xiaoyun. Research progress of transcription factors regulating carotenoid synthesis pathway in plant[J].Molecular Plant Breeding,2021,19(13):4401-4408.
[10] ZHOU W Q,ZHAO S R,XU M,NIU Y Y,NASIER M,FAN G Q,QUAN S W,ZHANG S K,WANG Y T,LIAO K.Identification of key genes controlling carotenoid metabolism during apricot fruit development by integrating metabolic phenotypes and gene expression profiles[J]. Journal of Agricultural and Food Chemistry,2021,69(32):9472-9483.
[11] LEVIN G. Promoting carotenoid biosynthesis in the NUD(X23)[J].The Plant Cell,2024,36(5):1588-1589.
[12] KARNIEL U,BERKE N A,MANN V,HIRSCHBERG J. Perturbations in the carotenoid biosynthesis pathway in tomato fruit reactivate the leaf-specific phytoene synthase 2[J]. Frontiers in Plant Science,2022,13:844748.
[13] LADO J,ALÓS E,MANZI M,CRONJE P J R,GÓMEZ-CADENAS A,RODRIGO M J,ZACARÍAS L. Light regulation of carotenoid biosynthesis in the peel of mandarin and sweet orange fruits[J].Frontiers in Plant Science,2019,10:1288.
[14] MESEJO C,LOZANO-OMEÑACA A,MARTÍNEZ-FUENTES A,REIG C,GAMBETTA G,MARZAL A,MARTÍNEZALCÁNTARA B,GRAVINA A,AGUSTÍ M.Soil-to-fruit nitrogen flux mediates the onset of fruit-nitrogen remobilization and color change in citrus[J].Environmental and Experimental Botany,2022,204:105088.
[15] GE X X,CAO T T,YI L H,YAO S X,ZENG K F,DENG L L.Low and high storage temperature inhibited the coloration of mandarin fruit (Citrus unshiu Marc.) with different mechanism[J].Journal of the Science of Food and Agriculture,2022,102(15):6930-6941.
[16] LI X B,ZHANG D D,PAN X H,KAKAR K U,NAWAZ Z.Regulation of carotenoid metabolism and ABA biosynthesis during blueberry fruit ripening[J].Plant Physiology and Biochemistry,2024,206:108232.
[17] SUN Q,HE Z C,FENG D,WEI R R,ZHANG Y Z,YE J L,CHAI L J,XU J,CHENG Y J,XU Q,DENG X X.The abscisic acid-responsive transcriptional regulatory module CsERF110-CsERF53 orchestrates citrus fruit coloration[J]. Plant Communications,2024,5(11):101065.
[18] YUE P T,JIANG Z H,SUN Q,WEI R R,YIN Y Z,XIE Z Z,LARKIN R M,YE J L,CHAI L J,DENG X X. Jasmonate activates a CsMPK6-CsMYC2 module that regulates the expression of β-citraurin biosynthetic genes and fruit coloration in orange(Citrus sinensis)[J].The Plant Cell,2023,35(4):1167-1185.
[19] ZHU K J,CHEN H Y,MEI X H,LU S W,XIE H P,LIU J W,CHAI L J,XU Q,WURTZEL E T,YE J L,DENG X X. Transcription factor CsMADS3 coordinately regulates chlorophyll and carotenoid pools in Citrus hesperidium[J].Plant Physiology,2023,193(1):519-536.
[20] 肖翔,周储江,金舒婉,施丽愉,杨震峰,曹士锋,陈伟.PpMADS2 与PpMADS3 协同调控黄肉桃果实类胡萝卜素积累机制的研究[J].园艺学报,2023,50(6):1173-1186.XIAO Xiang,ZHOU Chujiang,JIN Shuwan,SHI Liyu,YANG Zhenfeng,CAO Shifeng,CHEN Wei. Mechanism of PpMADS2 and PpMADS3 synergistically regulating carotenoids accumulation in peach fruit[J]. Acta Horticulturae Sinica,2023,50(6):1173-1186.
[21] TANTISUWANICHKUL K,KOMAKI S,WATANABE M,TOHGE T,SIRIKANTARAMAS S. Unveiling the regulatory role of DzAGL6-1 in carotenoid biosynthesis during durian (Durio zibethinus) fruit development[J]. Plant Cell Reports,2024,43(9):217.
[22] 梁敏华,梁瑞进,杨震峰,邓鸿铃.NAC6 转录因子在芒果果实采后类胡萝卜素代谢过程中的表达及影响[J]. 食品科学,2024,45(16):77-87.LIANG Minhua,LIANG Ruijin,YANG Zhenfeng,DENG Hongling.Expression of NAC6 transcription factor and its impact on carotenoid metabolism in postharvest mango fruit[J]. Food Science,2024,45(16):77-87.
[23] FU C C,HAN Y C,FAN Z Q,CHEN J Y,CHEN W X,LU W J,KUANG J F. The papaya transcription factor CpNAC1 modulates carotenoid biosynthesis through activating phytoene desaturase genes CpPDS2/4 during fruit ripening[J]. Journal of Agricultural and Food Chemistry,2016,64(27):5454-5463.
[24] FU C C,HAN Y C,KUANG J F,CHEN J Y,LU W J. Papaya CpEIN3a and CpNAC2 co-operatively regulate carotenoid biosynthesis-related genes CpPDS2/4,CpLCY-e and CpCHY-b during fruit Ripening[J]. Plant and Cell Physiology,2017,58(12):2155-2165.
[25] GONG J L,ZENG Y L,MENG Q N,GUAN Y J,LI C Y,YANG H B,ZHANG Y Z,AMPOMAH-DWAMENA C,LIU P,CHEN C W,DENG X X,CHENG Y J,WANG P W. Red lightinduced kumquat fruit coloration is attributable to increased carotenoid metabolism regulated by FcrNAC22[J]. Journal of Experimental Botany,2021,72(18):6274-6290.
[26] ZHANG C,DAI Z W,FERRIER T,ORDUÑA L,SANTIAGO A,PERIS A,WONG D C J,KAPPEL C,SAVOI S,LOYOLA R,AMATO A,KOZAK B,LI M M,LIANG A K,CARRASCO D,MEYER- REGUEIRO C,ESPINOZA C,HILBERT G,FIGUEROA-BALDERAS R,CANTU D,ARROYO-GARCIA R,ARCE-JOHNSON P,CLAUDEL P,ERRANDONEA D,RODRÍGUEZ-CONCEPCIÓN M,DUCHÊNE E,HUANG S C,CASTELLARIN S D,TORNIELLI G B,BARRIEU F,MATUS J T. MYB24 orchestrates terpene and flavonol metabolism as light responses to anthocyanin depletion in variegated grape berries[J].The Plant Cell,2023,35(12):4238-4265.
[27] FU C C,CHEN H J,GAO H Y,LU Y,HAN C,HAN Y C.Two papaya MYB proteins function in fruit ripening by regulating some genes involved in cell-wall degradation and carotenoid biosynthesis[J].Journal of the Science of Food and Agriculture,2020,100(12):4442-4448.
[28] TIAN S L,YANG Y Y,FANG B,UDDIN S,LIU X G. The CrMYB33 transcription factor positively coordinate the regulation of both carotenoid accumulation and chlorophyll degradation in the peel of citrus fruit[J].Plant Physiology and Biochemistry,2024,209:108540.
[29] SUN Q,HE Z C,WEI R R,YIN Y Z,YE J L,CHAI L J,XIE Z Z,GUO W W,XU J,CHENG Y J,XU Q,DENG X X. Transcription factor CsTT8 promotes fruit coloration by positively regulating the methylerythritol 4-phosphate pathway and carotenoid biosynthesis pathway in citrus (Citrus spp.)[J]. Horticulture Research,2023,10(11):uhad199.
[30] SUN Q,HE Z C,WEI R R,ZHANG Y,YE J L,CHAI L J,XIE Z Z,GUO W W,XU J,CHENG Y J,XU Q,DENG X X. The transcriptional regulatory module CsHB5-CsbZIP44 positively regulates abscisic acid-mediated carotenoid biosynthesis in citrus (Citrus spp.)[J]. Plant Biotechnology Journal,2024,22(3):722-737.
[31] 宋聪豪,姜超,金自清,张海朋,王小贝,侯楠,程钧,王伟,郑先波,冯建灿,连晓东,谭彬.PpWRKY4 通过影响PpCCD4 表达调控桃果实类胡萝卜素积累[J]. 果树学报,2024,41(8):1504-1512.SONG Conghao,JIANG Chao,JIN Ziqing,ZHANG Haipeng,WANG Xiaobei,HOU Nan,CHENG Jun,WANG Wei,ZHENG Xianbo,FENG Jiancan,LIAN Xiaodong,TAN Bin. PpWRKY4 regulates carotenoids accumulation in peach fruit by affecting the expression of PpCCD4[J].Journal of Fruit Science,2024,41(8):1504-1512.
[32] YU Y,BAO Z Y,ZHOU Q H,WU W,CHEN W,YANG Z F,WANG L,LI X W,CAO S F,SHI L Y. EjWRKY6 is involved in the ABA-induced carotenoid biosynthesis in loquat fruit during ripening[J].Foods,2024,13(17):2829.
[33] SONG H Y,ZHAO K,JIANG G L,SUN S X,LI J,TU M Y,WANG L L,XIE H J,CHEN D. Genome-wide identification and expression analysis of the SBP-box gene family in loquat fruit development[J].Genes,2024,15(1):23.
[34] DANG Q Y,SHA H Y,NIE J Y,WANG Y Z,YUAN Y B,JIA D J. An apple (Malus domestica) AP2/ERF transcription factor modulates carotenoid accumulation[J]. Horticulture Research,2021,8(1):223.
[35] ZHU K J,SUN Q,CHEN H Y,MEI X H,LU S W,YE J L,CHAI L J,XU Q,DENG X X.Ethylene activation of carotenoid biosynthesis by a novel transcription factor CsERF061[J]. Journal of Experimental Botany,2021,72(8):3137-3154.
[36] JIA D J,LI Y C,JIA K,HUANG B C,DANG Q Y,WANG H M,WANG X Y,LI C Y,ZHANG Y G,NIE J Y,YUAN Y B.Abscisic acid activates transcription factor module MdABI5-Md-MYBS1 during carotenoid- derived apple fruit coloration[J].Plant Physiology,2024,195(3):2053-2072.
[37] FU C C,HAN Y C.CpMADS4 and CpERF9 jointly regulate carotenoid synthesis related genes during papaya fruit ripening[J].Food Research International,2025,203:115864.
[38] HAO Y W,HU G J,BREITEL D,LIU M C,MILA I,FRASSE P,FU Y Y,AHARONI A,BOUZAYEN M,ZOUINE M.Auxin response factor SlARF2 is an essential component of the regulatory mechanism controlling fruit ripening in tomato[J].PLoS Genetics,2015,11(12):e1005649.
[39] LIU L H,JIA C G,ZHANG M,CHEN D L,CHEN S X,GUO R F,GUO D P,WANG Q M. Ectopic expression of a BZR1-1D transcription factor in brassinosteroid signalling enhances carotenoid accumulation and fruit quality attributes in tomato[J].Plant Biotechnology Journal,2014,12(1):105-115.
[40] KUHN N,ARELLANO M,PONCE C,HODAR C,CORREA F,MULTARI S,MARTENS S,CARRERA E,DONOSO J M,MEISEL L A. RNA-seq and WGBS analyses during fruit ripening and in response to ABA in sweet cherry (Prunus avium) reveal genetic and epigenetic modulation of auxin and cytokinin genes[J].Journal of Plant Growth Regulation,2025,44(3):1165-1187.
[41] ANWAR S,BRENYA E,ALAGOZ Y,CAZZONELLI C I. Epigenetic control of carotenogenesis during plant development[J].Critical Reviews in Plant Sciences,2021,40(1):23-48.
[42] XU J D,WANG X,CAO H B,XU H D,XU Q,DENG X X.Dynamic changes in methylome and transcriptome patterns in response to methyltransferase inhibitor 5-azacytidine treatment in citrus[J].DNA Research,2017,24(5):509-522.
[43] HUANG H,LIU R E,NIU Q F,TANG K,ZHANG B,ZHANG H,CHEN K S,ZHU J K,LANG Z B. Global increase in DNA methylation during orange fruit development and ripening[J].Proceedings of the National Academy of Sciences of the United States of America,2019,116(4):1430-1436.
[44] ZHENG J R,YANG X Y,YE J B,SU D X,WANG L N,LIAO Y L,ZHANG W W,WANG Q J,CHEN Q W,XU F. Multiomics analysis provides new insights into the regulatory mechanism of carotenoid biosynthesis in yellow peach peel[J].Molecular Horticulture,2023,3(1):23.
[45] TIAN Y Y,BAI S,DANG Z H,HAO J F,ZHANG J,HASI A.Genome-wide identification and characterization of long noncoding RNAs involved in fruit ripening and the climacteric in Cucumis melo[J].BMC Plant Biology,2019,19(1):369.
[46] SUN L P,HUO J T,LIU J Y,YU J Y,ZHOU J L,SUN C D,WANG Y,LENG F. Anthocyanins distribution,transcriptional regulation,epigenetic and post- translational modification in fruits[J].Food Chemistry,2023,411:135540.
[47] 王硕,郑秀文,王琪,冯慧智,刘冠,张萌萌.果树中花青素合成及其分子调控机制研究进展[J/OL]. 分子植物育种,2022:1-10. (2022- 07- 29). https://kns.cnki.net/kcms/detail/46.1068.S.20220728.1710.014.html.WANG Shuo,ZHENG Xiuwen,WANG Qi,FENG Huizhi,LIU Guan,ZHANG Mengmeng. Research progress on anthocyanin synthesis and its molecular regulation mechanism in fruit trees[J/OL].Molecular Plant Breeding,2022:1-10.(2022-07-29).https://kns.cnki.net/kcms/detail/46.1068.S.20220728.1710.014.html.
[48] 乔廷廷,郭玲.花青素来源、结构特性和生理功能的研究进展[J].中成药,2019,41(2):388-392.QIAO Tingting,GUO Ling. Research progress on the source,structural properties and physiological functions of anthocyanins[J]. Chinese Traditional Patent Medicine,2019,41(2):388-392.
[49] 张丽,范维娟,郑臻颖,杨俊,康乐,王红霞.花青素糖基化、甲基化和酰基化修饰的研究现状[J]. 分子植物育种,2023,21(7):2378-2387.ZHANG Li,FAN Weijuan,ZHENG Zhenying,YANG Jun,KANG Le,WANG Hongxia. Research progress of anthocyanin glycosylation,methylation and acylation modification in plants[J].Molecular Plant Breeding,2023,21(7):2378-2387.
[50] 朱晓路,陈文博,王玉玲,贾国超.食品中的花青素及其与金属离子的相互作用[J].食品工业,2022,43(9):249-253.ZHU Xiaolu,CHEN Wenbo,WANG Yuling,JIA Guochao.Anthocyanins in food and the interaction with metal ions[J]. The Food Industry,2022,43(9):249-253.
[51] 崔海鹏,郭健龙,王大全,杨剑婷.花青素加工稳定性及其研究进展[J].食品与发酵工业,2024,50(13):388-397.CUI Haipeng,GUO Jianlong,WANG Daquan,YANG Jianting.Stability of anthocyanins during processing and research progress[J]. Food and Fermentation Industries,2024,50(13):388-397.
[52] WANG J D,ZHAO Y Q,SUN B,YANG Y T,WANG S P,FENG Z R,LI J Y. The structure of anthocyanins and the copigmentation by common micromolecular copigments:A review[J].Food Research International,2024,176:113837.
[53] GAO H N,JIANG H,CUI J Y,YOU C X,LI Y Y.Review:The effects of hormones and environmental factors on anthocyanin biosynthesis in apple[J].Plant Science,2021,312:111024.
[54] GIL-MUÑOZ F,SÁNCHEZ-NAVARRO J A,BESADA C,SALVADOR A,BADENES M L,NAVAL M D M,RÍOS G.MBW complexes impinge on anthocyanidin reductase gene regulation for proanthocyanidin biosynthesis in persimmon fruit[J].Scientific Reports,2020,10:3543.
[55] 孙晓红.红肉苹果MYB10 及其启动子对花青苷合成相关基因的调控作用[D].长沙:湖南农业大学,2017.SUN Xiaohong. Regulation of related genes involved in anthocyanin biosynthesis by the MYB10 and its promoter in red flesh apple[D].Changsha:Hunan Agricultural University,2017.
[56] WANG N,XU H F,JIANG S H,ZHANG Z Y,LU N L,QIU H R,QU C Z,WANG Y C,WU S J,CHEN X S. MYB12 and MYB22 play essential roles in proanthocyanidin and flavonol synthesis in red-fleshed apple (Malus sieversii f. niedzwetzkyana)[J].The Plant Journal,2017,90(2):276-292.
[57] FENG S Q,XU Y C,YANG L,SUN S S,WANG D Y,CHEN X S. Genome-wide identification and characterization of R2R3-MYB transcription factors in pear[J]. Scientia Horticulturae,2015,197:176-182.
[58] FENG S Q,WANG Y L,YANG S,XU Y T,CHEN X S.Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10[J].Planta,2010,232(1):245-255.
[59] WANG H Y,ZHAI L L,WANG S W,ZHENG B T,HU H L,LI X Y,BIAN S M.Identification of R2R3-MYB family in blueberry and its potential involvement of anthocyanin biosynthesis in fruits[J].BMC Genomics,2023,24(1):505.
[60] TANG Q,CHI F M,LIU H D,ZHANG H J,SONG Y. Singlemolecule real-time and illumina sequencing to analyze transcriptional regulation of flavonoid synthesis in blueberry[J].Frontiers in Plant Science,2021,12:754325.
[61] KARPPINEN K,LAFFERTY D J,ALBERT N W,MIKKOLA N,MCGHIE T,ALLAN A C,AFZAL B M,HÄGGMAN H,ESPLEY R V,JAAKOLA L.MYBA and MYBPA transcription factors co-regulate anthocyanin biosynthesis in blue-coloured berries[J].New Phytologist,2021,232(3):1350-1367.
[62] GÜNTHER C S,DARE A P,MCGHIE T K,DENG C,LAFFERTY D J,PLUNKETT B J,GRIERSON E R P,TURNER J L,JAAKOLA L,ALBERT N W,ESPLEY R V. Spatiotemporal modulation of flavonoid metabolism in blueberries[J]. Frontiers in Plant Science,2020,11:545.
[63] 安昌,陆琳,沈梦千,陈盛圳,叶康卓,秦源,郑平.植物bHLH基因家族研究进展及在药用植物中的应用前景[J].生物技术通报,2023,39(10):1-16.AN Chang,LU Lin,SHEN Mengqian,CHEN Shengzhen,YE Kangzhuo,QIN Yuan,ZHENG Ping. Research progress of bHLH gene family in plants and its application prospects in medical plants[J].Biotechnology Bulletin,2023,39(10):1-16.
[64] LI X Y,XIANG F X,HAN W,QIE B Q,ZHAI R,YANG C Q,WANG Z G,XU L F.The MIR-domain of PbbHLH2 is involved in regulation of the anthocyanin biosynthetic pathway in“Red Zaosu”(Pyrus bretschneideri Rehd.) pear fruit[J]. International Journal of Molecular Sciences,2021,22(6):3026.
[65] AN J P,XU R R,WANG X N,ZHANG X W,YOU C X,HAN Y P. MdbHLH162 connects the gibberellin and jasmonic acid signals to regulate anthocyanin biosynthesis in apple[J]. Journal of Integrative Plant Biology,2024,66(2):265-284.
[66] WANG L H,TANG W,HU Y W,ZHANG Y B,SUN J Q,GUO X H,LU H,YANG Y,FANG C B,NIU X L,YUE J Y,FEI Z J,LIU Y S.A MYB/bHLH complex regulates tissue-specific anthocyanin biosynthesis in the inner pericarp of red-centered kiwifruit Actinidia chinensis cv. Hongyang[J]. The Plant Journal,2019,99(2):359-378.
[67] LIU Y F,MA K X,QI Y W,LV G W,REN X L,LIU Z D,MA F W.Transcriptional regulation of anthocyanin synthesis by MYBbHLH-WDR complexes in kiwifruit (Actinidia chinensis)[J].Journal of Agricultural and Food Chemistry,2021,69(12):3677-3691.
[68] YUE M L,JIANG L Y,ZHANG N T,ZHANG L X,LIU Y Q,LIN Y X,ZHANG Y T,LUO Y,ZHANG Y,WANG Y,LI M Y,WANG X R,CHEN Q,TANG H R. Regulation of flavonoids in strawberry fruits by FaMYB5/FaMYB10 dominated MYBbHLH-WD40 ternary complexes[J]. Frontiers in Plant Science,2023,14:1145670.
[69] FAN Z Y,ZHAI Y L,WANG Y,ZHANG L,SONG M Y,FLAISHMAN M A,MA H Q. Genome-wide analysis of anthocyanin biosynthesis regulatory WD40 gene FcTTG1 and related family in Ficus carica L.[J]. Frontiers in Plant Science,2022,13:948084.
[70] TANG Q,WANG X,MA S R,FAN S T,CHI F M,SONG Y.Molecular mechanism of abscisic acid signaling response factor VcbZIP55 to promote anthocyanin biosynthesis in blueberry(Vaccinium corymbosum)[J]. Plant Physiology and Biochemistry,2024,210:108611.
[71] GAI S L,DU B Y,XIAO Y Q,ZHANG X,TURUPU M,YAO Q S,WANG X Y,YAN Y Z,LI T H. bZIP transcription factor PavbZIP6 regulates anthocyanin accumulation by increasing abscisic acid in sweet cherry[J].International Journal of Molecular Sciences,2024,25(18):10207.
[72] SHEN S L,HU X L,CHENG J,LOU L C,HUAN C,ZHENG X L. PsbZIP1 and PsbZIP10 induce anthocyanin synthesis in plums(Prunus salicina cv.Taoxingli)via PsUFGT by methyl salicylate treatment during postharvest[J]. Postharvest Biology and Technology,2023,203:112396.
[73] ZHAO Y,MIN T,CHEN M J,WANG H X,ZHU C Q,JIN R,ALLAN A C,KUI L W,XU C J. The photomorphogenic transcription factor PpHY5 regulates anthocyanin accumulation in response to UVA and UVB irradiation[J]. Frontiers in Plant Science,2021,11:603178.
[74] WANG Y Y,ZHANG X D,ZHAO Y R,YANG J,HE Y Y,LI G C,MA W R,HUANG X L,SU J. Transcription factor PyHY5 binds to the promoters of PyWD40 and PyMYB10 and regulates its expression in red pear‘Yunhongli No. 1’[J]. Plant Physiology and Biochemistry,2020,154:665-674.
[75] AN J P,WANG X F,ZHANG X W,BI S Q,YOU C X,HAO Y J. MdBBX22 regulates UV-B-induced anthocyanin biosynthesis through regulating the function of MdHY5 and is targeted by MdBT2 for 26S proteasome-mediated degradation[J].Plant Biotechnology Journal,2019,17(12):2231-2233.
[76] 宋杨,刘红弟,王海波,张红军,刘凤之.越橘VcNAC072 克隆及其促进花青素积累的功能分析[J].中国农业科学,2019,52(3):503-511.SONG Yang,LIU Hongdi,WANG Haibo,ZHANG Hongjun,LIU Fengzhi. Molecular cloning and functional characterization of VcNAC072 reveals its involvement in anthocyanin accumulation in blueberry[J]. Scientia Agricultura Sinica,2019,52(3):503-511.
[77] SUN Q G,JIANG S H,ZHANG T L,XU H F,FANG H C,ZHANG J,SU M Y,WANG Y C,ZHANG Z Y,WANG N,CHEN X S. Apple NAC transcription factor MdNAC52 regulates biosynthesis of anthocyanin and proanthocyanidin through MdMYB9 and MdMYB11[J].Plant Science,2019,289:110286.
[78] LIU W J,MEI Z X,YU L,GU T T,LI Z Q,ZOU Q,ZHANG S H,FANG H C,WANG Y C,ZHANG Z Y,CHEN X S,WANG N. The ABA-induced NAC transcription factor MdNAC1 interacts with a bZIP-type transcription factor to promote anthocyanin synthesis in red-fleshed apples[J]. Horticulture Research,2023,10(5):uhad049.
[79] LI C,WU J,HU K D,WEI S W,SUN H Y,HU L Y,HAN Z,YAO G F,ZHANG H.PyWRKY26 and PybHLH3 cotargeted the PyMYB114 promoter to regulate anthocyanin biosynthesis and transport in red-skinned pears[J]. Horticulture Research,2020,7:37.
[80] HU J F,FANG H C,WANG J,YUE X X,SU M Y,MAO Z L,ZOU Q,JIANG H Y,GUO Z W,YU L,FENG T,LU L,PENG Z G,ZHANG Z Y,WANG N,CHEN X S. Ultraviolet B-induced MdWRKY72 expression promotes anthocyanin synthesis in apple[J].Plant Science,2020,292:110377.
[81] MO R L,HAN G M,ZHU Z X,ESSEMINE J,DONG Z X,LI Y,DENG W,QU M N,ZHANG C,YU C. The ethylene response factor ERF5 regulates anthocyanin biosynthesis in‘Zijin’mulberry fruits by interacting with MYBA and F3H genes[J]. International Journal of Molecular Sciences,2022,23(14):7615.
[82] NI J B,PREMATHILAKE A T,GAO Y H,YU W J,TAO R Y,TENG Y W,BAI S L.Ethylene-activated PpERF105 induces the expression of the repressor-type R2R3-MYB gene PpMYB140 to inhibit anthocyanin biosynthesis in red pear fruit[J]. The Plant Journal,2021,105(1):167-181.
[83] WANG C K,HAN P L,ZHAO Y W,YU J Q,YOU C X,HU D G,HAO Y J. Genome-wide analysis of auxin response factor(ARF) genes and functional identification of MdARF2 reveals the involvement in the regulation of anthocyanin accumulation in apple[J]. New Zealand Journal of Crop and Horticultural Science,2021,49(2/3):78-91.
[84] YI S N,MAO J X,ZHANG X Y,LI X M,ZHANG Z H,LI H.FveARF2 negatively regulates fruit ripening and quality in strawberry[J].Frontiers in Plant Science,2022,13:1023739.
[85] WANG Y C,MAO Z L,JIANG H Y,ZHANG Z Y,WANG N,CHEN X S.Brassinolide inhibits flavonoid biosynthesis and redflesh coloration via the MdBEH2.2-MdMYB60 complex in apple[J].Journal of Experimental Botany,2021,72(18):6382-6399.
[86] JIN Z L,WANG W N,NAN Q,LIU J W,JU Y L,FANG Y L.VvNAC17,a grape NAC transcription factor,regulates plant response to drought-tolerance and anthocyanin synthesis[J]. Plant Physiology and Biochemistry,2025,219:109379.
[87] 胡可,韩科厅,戴思兰.环境因子调控植物花青素苷合成及呈色的机理[J].植物学报,2010,45(3):307-317.HU Ke,HAN Keting,DAI Silan.Regulation of plant anthocyanin synthesis and pigmentation by environmental factors[J]. Chinese Bulletin of Botany,2010,45(3):307-317.
[88] ENARU B,DREȚCANU G,POP T D,STǍNILǍ A,DIACONEASA Z. Anthocyanins:Factors affecting their stability and degradation[J].Antioxidants,2021,10(12):1967.
[89] LI Y,XU P B,CHEN G Q,WU J,LIU Z C,LIAN H L. Fvb-HLH9 functions as a positive regulator of anthocyanin biosynthesis by forming a HY5-bHLH9 transcription complex in strawberry fruits[J].Plant&Cell Physiology,2020,61(4):826-837.
[90] XING Y F,SUN W J,SUN Y Y,LI J L,ZHANG J,WU T,SONG T T,YAO Y C,TIAN J.MPK6-mediated HY5 phosphorylation regulates light-induced anthocyanin accumulation in apple fruit[J].Plant Biotechnology Journal,2023,21(2):283-301.
[91] LIU Y Q,TANG L,WANG Y P,ZHANG L X,XU S Q,WANG X,HE W,ZHANG Y T,LIN Y X,WANG Y,LI M Y,WANG X R,ZHANG Y,LUO Y,CHEN Q,TANG H R.The blue light signal transduction module FaCRY1-FaCOP1-FaHY5 regulates anthocyanin accumulation in cultivated strawberry[J]. Frontiers in Plant Science,2023,14:1144273.
[92] HUANG D,YUAN Y,TANG Z Z,HUANG Y,KANG C Y,DENG X X,XU Q. Retrotransposon promoter of Ruby1 controls both light- and cold-induced accumulation of anthocyanins in blood orange[J]. Plant,Cell & Environment,2019,42(11):3092-3104.
[93] CHEN M K,LIANG X L,YANG Y,WANG Y,WEI J,DUAN Y,PENG H X,DUAN Y,HUANG Y X,ZOU W T,LI H H.MpYABBY2 promotes ABA accumulation and influences Mp-MYB11/MpbHLH79 complex to regulate anthocyanin biosynthesis in Malus‘Pinkspire’fruits under cold stress[J]. Postharvest Biology and Technology,2025,222:113341.
[94] NAING A H,KIM C K.Abiotic stress-induced anthocyanins in plants:Their role in tolerance to abiotic stresses[J]. Physiologia Plantarum,2021,172(3):1711-1723.
[95] FENG X H,BAI S N,ZHOU L X,SONG Y,JIA S J,GUO Q X,ZHANG C Y.Integrated analysis of transcriptome and metabolome provides insights into flavonoid biosynthesis of blueberry leaves in response to drought stress[J]. International Journal of Molecular Sciences,2024,25(20):11135.
[96] AN J P,ZHANG X W,LIU Y J,WANG X F,YOU C X,HAO Y J. ABI5 regulates ABA-induced anthocyanin biosynthesis by modulating the MYB1-bHLH3 complex in apple[J]. Journal of Experimental Botany,2021,72(4):1460-1472.
[97] WANG X,TANG Q,CHI F M,LIU H D,ZHANG H J,SONG Y.Sucrose non-fermenting1-related protein kinase VcSnRK2.3 promotes anthocyanin biosynthesis in association with VcMYB1 in blueberry[J].Frontiers in Plant Science,2023,14:1018874.
[98] AN J P,XU R R,LIU X,ZHANG J C,WANG X F,YOU C X,HAO Y J. Jasmonate induces biosynthesis of anthocyanin and proanthocyanidin in apple by mediating the JAZ1-TRB1-MYB9 complex[J].The Plant Journal,2021,106(5):1414-1430.
[99] WANG Y C,WANG N,XU H F,JIANG S H,FANG H C,SU M Y,ZHANG Z Y,ZHANG T L,CHEN X S.Auxin regulates anthocyanin biosynthesis through the Aux/IAA-ARF signaling pathway in apple[J].Horticulture Research,2018,5:59.
[100] NI J B,WANG S M,YU W J,LIAO Y F,PAN C,ZHANG M M,TAO R Y,WEI J,GAO Y H,WANG D S,BAI S L,TENG Y W. The ethylene-responsive transcription factor PpERF9 represses PpRAP2.4 and PpMYB114 via histone deacetylation to inhibit anthocyanin biosynthesis in pear[J]. The Plant Cell,2023,35(6):2271-2292.
[101] LI H L,LIU Z Y,WANG X N,HAN Y P,YOU C X,AN J P.E3 ubiquitin ligases SINA4 and SINA11 regulate anthocyanin biosynthesis by targeting the IAA29-ARF5-1-ERF3 module in apple[J].Plant,Cell&Environment,2023,46(12):3902-3918.
[102] YU L J,SUN Y Y,ZHANG X,CHEN M C,WU T,ZHANG J,XING Y F,TIAN J,YAO Y C. ROS1 promotes low temperature-induced anthocyanin accumulation in apple by demethylating the promoter of anthocyanin-associated genes[J]. Horticulture Research,2022,9:uhac007.
[103] ZHU Y C,ZHANG B,ALLAN A C,KUI L W,ZHAO Y,WANG K,CHEN K S,XU C J.DNA demethylation is involved in the regulation of temperature-dependent anthocyanin accumulation in peach[J].The Plant Journal,2020,102(5):965-976.
[104] MA H Y,YANG T,LI Y,ZHANG J,WU T,SONG T T,YAO Y C,TIAN J. The long noncoding RNA MdLNC499 bridges Md-WRKY1 and MdERF109 function to regulate early-stage lightinduced anthocyanin accumulation in apple fruit[J]. The Plant Cell,2021,33(10):3309-3330.
[105] TANG Y J,QU Z P,LEI J J,HE R Q,ADELSON D L,ZHU Y L,YANG Z B,WANG D. The long noncoding RNA FRILAIR regulates strawberry fruit ripening by functioning as a noncanonical target mimic[J].PLoS Genetics,2021,17(3):e1009461.
[106] ZHAO Y,SUN J L,CHERONO S,AN J P,ALLAN A C,HAN Y P.Colorful hues:Insight into the mechanisms of anthocyanin pigmentation in fruit[J].Plant Physiology,2023,192(3):1718-1732.
[107] ZHANG B,YANG H J,QU D,ZHU Z Z,YANG Y Z,ZHAO Z Y. The MdBBX22-miR858-MdMYB9/11/12 module regulates proanthocyanidin biosynthesis in apple peel[J].Plant Biotechnology Journal,2022,20(9):1683-1700.
[108] LIU H N,SHU Q,KUI L W,ALLAN A C,ESPLEY R V,SU J,PEI M S,WU J. The PyPIF5-PymiR156a-PySPL9-PyMYB114/MYB10 module regulates light-induced anthocyanin biosynthesis in red pear[J].Molecular Horticulture,2021,1(1):14.
[109] LIU X J,AN X H,LIU X,HU D G,WANG X F,YOU C X,HAO Y J. MdSnRK1.1 interacts with MdJAZ18 to regulate sucrose-induced anthocyanin and proanthocyanidin accumulation in apple[J].Journal of Experimental Botany,2017,68(11):2977-2990.
[110] AN J P,LIU Y J,ZHANG X W,BI S Q,WANG X F,YOU C X,HAO Y J.Dynamic regulation of anthocyanin biosynthesis at different light intensities by the BT2-TCP46-MYB1 module in apple[J].Journal of Experimental Botany,2020,71(10):3094-3109.
[111] BJÖRN L O,PAPAGEORGIOU G C,BLANKENSHIP R E,GOVINDJEE.A viewpoint:Why chlorophyll a[J].Photosynthesis Research,2009,99(2):85-98.
[112] SUN D N,WU S L,LI X H,GE B S,ZHOU C X,YAN X J,RUAN R,CHENG P F. The structure,functions and potential medicinal effects of chlorophylls derived from microalgae[J]. Marine Drugs,2024,22(2):65.
[113] PROCTOR M S,SUTHERLAND G A,CANNIFFE D P,HITCHCOCK A. The terminal enzymes of (bacterio) chlorophyll biosynthesis[J]. Royal Society Open Science,2022,9(5):211903.
[114] MARTINS T,BARROS A N,ROSA E,ANTUNES L. Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food:A comprehensive review[J]. Molecules,2023,28(14):5344.
[115] HU B,LAI B,WANG D,LI J Q,CHEN L H,QIN Y Q,WANG H C,QIN Y H,HU G B,ZHAO J T. Three LcABFs are involved in the regulation of chlorophyll degradation and anthocyanin biosynthesis during fruit ripening in Litchi chinensis[J].Plant and Cell Physiology,2019,60(2):448-461.
[116] LU Y X,SHEN X C,LI Y C,XU Y N,CHEN Y H,CHEN Y S,HU X L,LI X L,SUN X P,GONG J L. Regulation of chlorophyll and carotenoid metabolism in citrus fruit[J]. Horticultural Plant Journal,2025,11(3):951-962.
[117] 张巧丽,陈笛,宋艳萍,朱鸿亮,罗云波,曲桂芹.番茄果实叶绿素代谢转录调控网络研究进展[J].园艺学报,2023,50(9):2031-2047.ZHANG Qiaoli,CHEN Di,SONG Yanping,ZHU Hongliang,LUO Yunbo,QU Guiqin. Review on transcriptional regulation of chlorophyll metabolism network in tomato fruits[J].Acta Horticulturae Sinica,2023,50(9):2031-2047.
[118] 王伟,徐跃进,万正杰.黄瓜叶绿素降解关键酶基因PPH 和PAO cDNA 片段的克隆与表达初步分析[J].园艺学报,2011,38(6):1104-1110.WANG Wei,XU Yuejin,WAN Zhengjie. Cloning and expression analysis of key genes PPH and PAO for chlorophyll degradation in cucumber[J]. Acta Horticulturae Sinica,2011,38(6):1104-1110.
[119] LI G W,CHEN D Y,TANG X F,LIU Y S.Heterologous expression of kiwifruit (Actinidia chinensis) GOLDEN2-LIKE homolog elevates chloroplast level and nutritional quality in tomato(Solanum lycopersicum)[J].Planta,2018,247(6):1351-1362.
[120] CHEN M,LIU X,JIANG S H,WEN B B,YANG C,XIAO W,FU X L,LI D M,CHEN X D,GAO D S,LI L. Transcriptomic and functional analyses reveal that PpGLK1 regulates chloroplast development in peach (Prunus persica)[J]. Frontiers in Plant Science,2018,9:34.
[121] AN X H,TIAN Y,CHEN Y H,LI E M,LI M,CHENG C G.Functional identification of apple MdGLK1 which regulates chlorophyll biosynthesis in Arabidopsis[J]. Journal of Plant Growth Regulation,2019,38(3):778-787.
[122] XIONG B,CHEN H Z,MA Q Q,YAO J F,WANG J L,WU W J,LIAO L,WANG X,ZHANG M F,HE S Y,HE J X,SUN G C,WANG Z H. Genome-wide analysis of the GLK gene family and its expression at different leaf ages in the Citrus cultivar Kanpei[J].Plants,2024,13(7):936.
[123] AMPOMAH-DWAMENA C,THRIMAWITHANA A H,DEJNOPRAT S,LEWIS D,ESPLEY R V,ALLAN A C. A kiwifruit(Actinidia deliciosa)R2R3-MYB transcription factor modulates chlorophyll and carotenoid accumulation[J]. New Phytologist,2019,221(1):309-325.
[124] WEI W,YANG Y Y,LAKSHMANAN P,KUANG J F,LU W J,PANG X Q,CHEN J Y,SHAN W. Proteasomal degradation of MaMYB60 mediated by the E3 ligase MaBAH1 causes high temperature-induced repression of chlorophyll catabolism and green ripening in banana[J].The Plant Cell,2023,35(5):1408-1428.
[125] WANG F,WANG J W,SUN L J,SONG X S. The molecular cloning and functional characterization of ChNAC1,a NAC transcription factor in Cerasus humilis[J]. Plant Growth Regulation,2019,89(3):331-343.
[126] ZOU S C,ZHUO M G,ABBAS F,HU G B,WANG H C,HUANG X M.Transcription factor LcNAC002 coregulates chlorophyll degradation and anthocyanin biosynthesis in litchi[J].Plant Physiology,2023,192(3):1913-1927.
[127] WANG H L,LIU Q,DENG S F,CHEN J L,HAN J,ZHU R,ZENG K F,DENG L L. Transcription factor CcbHLH66 regulates mandarin fruit coloration via modulating the expression of chlorophyll degradation related genes CcRCCR and CcNYC[J].Postharvest Biology and Technology,2024,218:113188.
[128] LIU Q,DENG S F,LIU L,WANG H L,YUAN L Y,YAO S X,ZENG K F,DENG L L.The chlorophyll and carotenoid metabolism in postharvest mandarin fruit peels is co-regulated by transcription factor CcbHLH35[J].Postharvest Biology and Technology,2024,216:113030.
[129] DONG H Z,CHEN Q M,DAI Y Q,HU W J,ZHANG S L,HUANG X S. Genome-wide identification of PbrbHLH family genes,and expression analysis in response to drought and cold stresses in pear (Pyrus bretschneideri)[J]. BMC Plant Biology,2021,21(1):86.
[130] OREN E,TZURI G,VEXLER L,DAFNA A,MEIR A,FAIGENBOIM A,KENIGSWALD M,PORTNOY V,SCHAFFER A A,LEVI A,BUCKLER E S,KATZIR N,BURGER J,TADMOR Y,GUR A.The multi-allelic APRR2 gene is associated with fruit pigment accumulation in melon and watermelon[J].Journal of Experimental Botany,2019,70(15):3781-3794.
[131] HAN Z Y,HU Y N,LV Y D,ROSE J K C,SUN Y Q,SHEN F,WANG Y,ZHANG X Z,XU X F,WU T,HAN Z H. Natural variation underlies differences in ETHYLENE RESPONSE FACTOR17 activity in fruit peel degreening[J]. Plant Physiology,2018,176(3):2292-2304.
[132] LI S J,XIE X L,LIU S C,CHEN K S,YIN X R.Auto-and mutual-regulation between two CitERFs contribute to ethylene-induced citrus fruit degreening[J]. Food Chemistry,2019,299:125163.
[133] LU S W,ZHANG M W,ZHUGE Y X,FU W H,OUYANG Q X,WANG W R,REN Y H,PEI D,FANG J G.VvERF17 mediates chlorophyll degradation by transcriptional activation of chlorophyll catabolic genes in grape berry skin[J]. Environmental and Experimental Botany,2022,193:104678.
[134] CHEN H C,SONG Z Y,WANG L H,LAI X H,CHEN W X,LI X P,ZHU X Y.Auxin-responsive protein MaIAA17-like modulates fruit ripening and ripening disorders induced by cold stress in‘Fenjiao’banana[J]. International Journal of Biological Macromolecules,2023,247:125750.
[135] FRANGEDAKIS E,YELINA N E,BILLAKURTHI K,HUA L,SCHREIER T,DICKINSON P J,TOMASELLI M,HASELOFF J,HIBBERD J M. MYB-related transcription factors control chloroplast biogenesis[J].Cell,2024,187(18):4859-4876.e22.
[136] 卓茂根,王惠聪.NAC 转录因子在果实成熟中的调控作用[J].果树学报,2023,40(7):1455-1470.ZHUO Maogen,WANG Huicong. The roles of NAC transcription factors in regulating fruit ripening[J]. Journal of Fruit Science,2023,40(7):1455-1470.
[137] SONG Z Y,QIN J J,YAO Y L,LAI X H,ZHENG W,CHEN W X,ZHU X Y,LI X P.A transcriptomic analysis unravels key factors in the regulation of stay-green disorder in peel of banana fruit (Fenjiao) caused by treatment with 1-MCP[J]. Postharvest Biology and Technology,2020,168:111290.
[138] HUANG H,HE W D.Application of exogenous cytokinin regulates cytokinin oxidase and antioxidant activity to maintain chlorophyll pigment during ripening of banana fruit[J].Food Bioscience,2023,55:102998.
[139] YUAN Z Y,DENG L L,YIN B F,YAO S X,ZENG K F. Effects of blue LED light irradiation on pigment metabolism of ethephon-degreened mandarin fruit[J]. Postharvest Biology and Technology,2017,134:45-54.
[140] MI H B,ZHOU X,YANG J,CHEN J X,LIU B. LED white light treatment delays postharvest senescence of‘Zaosu’pear fruit with inhibited chlorophyll degradation[J]. Horticulturae,2024,10(1):32.
[141] ZHANG C L,WU Y J,LIU X R,ZHANG J Y,LI X,LIN L,YIN R H. Pivotal roles of ELONGATED HYPOCOTYL5 in regulation of plant development and fruit metabolism in tomato[J].Plant Physiology,2022,189(2):527-540.
[142] HAN Y W,YANG J W,ZHANG N,GONG Y T,LIU M,QIAO R,JIAO X H,ZHU F J,LI X X,SI H J. Genome-wide identification of phytochrome-interacting factor (PIF) gene family in potatoes and functional characterization of StPIF3 in regulating shade-avoidance syndrome[J].Agronomy,2024,14(4):873.
[143] STENBAEK A,JENSEN P E. Redox regulation of chlorophyll biosynthesis[J].Phytochemistry,2010,71(8/9):853-859.
[144] SONG Z Z,WANG X,LI M Y,NING Y Z,SHI S P,YANG G R,ZHANG H X,TANG M L,PENG B. Isolation,heterologous expression,and functional determination of an iron regulated transporter (IRT) gene involved in Fe2+ transport and tolerance to Fe2+ deficiency in Vitis vinifera[J]. Plant Cell,Tissue and Organ Culture,2024,156(2):65.
[145] 周平,颜少宾,郭瑞,金光.桃镁离子转运蛋白MGT 基因家族鉴定与表达分析[J].园艺学报,2024,51(3):463-478.ZHOU Ping,YAN Shaobin,GUO Rui,JIN Guang. Identification and expressional analysis of MGT gene family in Prunus persica[J].Acta Horticulturae Sinica,2024,51(3):463-478.
[146] SDIRI S,NAVARRO P,MONTERDE A,BENABDA J,SALVADOR A. New degreening treatments to improve the quality of citrus fruit combining different periods with and without ethylene exposure[J]. Postharvest Biology and Technology,2012,63(1):25-32.
[147] YIN X R,XIE X L,XIA X J,YU J Q,FERGUSON I B,GIOVANNONI J J,CHEN K S. Involvement of an ethylene response factor in chlorophyll degradation during citrus fruit degreening[J].The Plant Journal,2016,86(5):403-412.
[148] MITALO O W,OTSUKI T,OKADA R,OBITSU S,MASUDA K,HOJO Y,MATSUURA T,MORI I C,ABE D,ASICHE W O,AKAGI T,KUBO Y,USHIJIMA K.Low temperature modulates natural peel degreening in lemon fruit independently of endogenous ethylene[J].Journal of Experimental Botany,2020,71(16):4778-4796.
[149] FERNÁNDEZ-CANCELO P,GINÉ-BORDONABA J,TEIXIDÓ N,ALAMAR M C.An insight into the hormonal interplay regulating pigment changes and colour development in the peel of‘Granny Smith’,‘OPAL®’and‘Royal Gala’apples[J].Journal of Plant Growth Regulation,2025,44(3):1116-1132.
[150] SINGH S P,SAINI M K,SINGH J,PONGENER A,SIDHU G S.Preharvest application of abscisic acid promotes anthocyanins accumulation in pericarp of litchi fruit without adversely affecting postharvest quality[J]. Postharvest Biology and Technology,2014,96:14-22.
[151] MA G,ZHANG L C,KUDAKA R,INABA H,FURUYA T,KITAMURA M,KITAYA Y,YAMAMOTO R,YAHATA M,MATSUMOTO H,KATO M. Exogenous application of ABA and NAA alleviates the delayed coloring caused by puffing inhibitor in citrus fruit[J].Cells,2021,10(2):308.
[152] HU B,LI J Q,WANG D,WANG H C,QIN Y H,HU G B,ZHAO J T. Transcriptome profiling of Litchi chinensis pericarp in response to exogenous cytokinins and abscisic acid[J]. Plant Growth Regulation,2018,84(3):437-450.
[153] QIU X,XU Y H,XIONG B,DAI L,HUANG S J,DONG T T,SUN G C,LIAO L,DENG Q X,WANG X,ZHU J,WANG Z H. Effects of exogenous methyl jasmonate on the synthesis of endogenous jasmonates and the regulation of photosynthesis in citrus[J].Physiologia Plantarum,2020,170(3):398-414.
[154] KEAWMANEE N,MA G,ZHANG L C,YAHATA M,MURAKAMI K,YAMAMOTO M,KOJIMA N,KATO M. Exogenous gibberellin induced regreening through the regulation of chlorophyll and carotenoid metabolism in Valencia oranges[J].Plant Physiology and Biochemistry,2022,173:14-24.
[155] LIU Y H,PRIYADARSHANI S V G N,CHI M R,YAN M K,MOHAMMADI M A,ZHANG M,ZHOU Q,WANG L L,LUO T T,WAI M H,WANG X M,CAI H Y,WANG H F,QIN Y.Epigenetic modification mechanisms of chloroplasts mutants in pineapple leaves during somatic regeneration[J]. Horticultural Plant Journal,2023,9(3):509-522.
[156] CHENG J F,NIU Q F,ZHANG B,CHEN K S,YANG R H,ZHU J K,ZHANG Y J,LANG Z B. Downregulation of RdDM during strawberry fruit ripening[J]. Genome Biology,2018,19(1):212.
[157] LI S G,ZHANG J Y,ZHANG L Q,FANG X P,LUO J,AN H S,ZHANG X Y. Genome-wide identification and comprehensive analysis reveal potential roles of long non-coding RNAs in fruit development of southern highbush blueberry (Vaccinium corymbosum L.)[J].Frontiers in Plant Science,2022,13:1078085.
[158] LI S X,SHAO Z R,FU X L,XIAO W,LI L,CHEN M,SUN M Y,LI D M,GAO D S. Identification and characterization of Prunus persica miRNAs in response to UVB radiation in greenhouse through high-throughput sequencing[J]. BMC Genomics,2017,18(1):938.
[159] LUO Q,WEI W,YANG Y Y,WU C J,CHEN J Y,LU W J,KUANG J F,SHAN W.E3 ligase MaNIP1 degradation of NONYELLOW COLORING1 at high temperature inhibits banana degreening[J].Plant Physiology,2023,192(3):1969-1981.
[160] WEI Y,JIN J T,XU Y X,LIU W T,YANG G X,BU H D,LI T,WANG A D.Ethylene-activated MdPUB24 mediates ubiquitination of MdBEL7 to promote chlorophyll degradation in apple fruit[J].The Plant Journal,2021,108(1):169-182.