琯溪蜜柚[Guanxi honey pomelo(Citrus grandis)]是福建省重要的柑橘属经济作物,平和县种植面积达48 333.33 hm2,为中国琯溪蜜柚第一大种植县。作为中国名特优柚类品种,蜜柚产业对福建省平和县的经济发展起着至关重要的作用[1-2]。琯溪蜜柚炭疽病是由刺盘孢属(Colletotrichum spp.)真菌引起的一种重要病害,在平和县琯溪蜜柚种植区普遍发生,病重果园发病率超过60%,主要危害叶片、枝梢、花及果实[3]。叶片受害通常发生在叶缘、叶尖或主脉,产生褐色病斑,病部枯死后多呈“V”字形,最后枯萎脱落;枝梢受害一般从叶柄、基部腋芽处开始产生病斑,随后发展至枝条,枝条自上而下枯死;染病花蕾呈褐色腐烂状,导致落花;果实受害初期出现暗绿色油渍状不规则病斑,幼果易脱落,成熟果变褐腐烂并导致落果,还可在贮藏期发生,引起果实腐烂[4]。
据国内外报道,引起柑橘类作物炭疽病的病原种类有胶孢炭疽菌复合种、尖孢炭疽菌复合种、平头炭疽菌复合种、博宁炭疽菌复合种、长真孢炭疽菌复合种、C.orchidearum复合种、C.magnum复合种和热带生炭疽菌(C.tropicicola)单系种等[5-7]。其中,胶孢炭疽菌复合种下的胶孢炭疽菌(C.gloeosporioides)是目前报道最多的种,在世界柑橘产区均可发生,为柑橘炭疽病的主要病原菌,对柑橘产业危害最大[8-10]。
刺盘孢属真菌种类繁多,具有多个复合种群,复合群下有多个单系种,种间形态差异微小,单独依据形态学特征对刺盘孢属真菌进行分类鉴定存在较大的困难[11-12];刺盘孢属真菌的ITS 序列保守性过强,对于多数近缘种构建的系统发育树在关键进化节点支持率低,ITS 在该属的系统分类研究中同样存在一些局限性[13]。近年来,以形态特征为基础,结合致病性测定与多基因联合聚类分析的多相法已被广泛应用于柑橘炭疽病病原菌的分类研究[14-15]。Wang等[7]采用形态特征观察结合多基因系统发育分析和致病性测定,将华盛顿脐橙炭疽病病原菌鉴定为胶孢炭疽菌(C.gloeosporioides)、果生炭疽菌(C.fructicola)、澳大利亚炭疽菌(C.australianum)、喀斯特炭疽菌(C.karstii)和可可炭疽菌(C.theobromicola)。
笔者课题组前期研究发现,引起琯溪蜜柚炭疽病的病原菌为胶孢炭疽菌(C.gloeosporioides)[3],但目前尚未见关于该病的病原种类多样性及致病力分析方面的研究报道。为明确琯溪蜜柚炭疽病的病原种类多样性,笔者在本研究中采集琯溪蜜柚炭疽病典型病样进行刺盘孢属真菌分离、形态特征观察及多基因系统发育分析,并对其进行致病性测定研究。研究结果可为深入研究琯溪蜜柚炭疽病的病原菌致病机制以及制定行之有效的防治措施提供理论依据。
1 材料和方法
1.1 材料
2019 年至2021 年,在福建省平和县13 个乡镇的琯溪蜜柚炭疽病发生严重的果园进行系统调查,观察蜜柚炭疽病在新梢抽发期及果实膨大期的田间症状特点。采集具有典型症状的发病样品,将不同发病症状的样品分别用塑封袋密封并于当天带回实验室及时分离。
1.2 方法
1.2.1 菌株分离纯化 在新鲜病样的病健交界处切取4 mm×4 mm大小的组织块,依次用70%乙醇表面消毒30 s,0.1%升汞消毒60 s,无菌水漂洗3次,于无菌滤纸上晾干后,置于PDA 平板上,28 ℃恒温黑暗培养,待长出菌落后,挑取菌落边缘单菌丝于新的PDA 平板上进行纯化。将纯化后的菌株分别置于PDA斜面上4 ℃保存和滤纸片上-20 ℃保存备用。
1.2.2 培养性状和形态特征观察 挑取各菌株的菌丝接种至PDA 平板中央,28 ℃黑暗培养,每天记录观察菌落的生长情况并测量菌落直径。待菌株长满平板时将其表面划伤诱导产孢,用接种针挑取分生孢子于滴有无菌水的载玻片上,在光学显微镜下观察分生孢子的形态特征。取60 μL分生孢子悬浮液滴于无菌凹槽载玻片上,置于25 ℃黑暗培养12 h,在光学显微镜下观察附着胞形态特征,同时计算附着胞形成率。附着胞形成率/%=附着胞形成孢子数/调查总孢子数×100。
1.2.3 多基因系统发育分析 刮取PDA 平板上28 ℃培养7 d的菌丝,用SK8259试剂盒提取供试菌株基因组DNA,用于PCR 扩增的模板。采用引物ITS1/ITS4[16]、ACT-512F/ACT-783R[17]、Bt2a/Bt2b[18]、GDF/GDR[19]和GSF1/GSR2[20]分别扩增菌株的内转录间隔区(ITS)序列及β-微管蛋白(TUB2)、肌动蛋白(ACT)、3-磷酸甘油醛脱氢酶(GAPDH)和谷氨酞胺合成酶(GS)基因的部分序列,具体引物信息见表1。PCR 反应体系和扩增程序参考前人的研究方法[16-20]。将PCR 扩增产物在1%的琼脂糖凝胶上电泳,在紫外灯下,切取目的片段,使用凝胶纯化试剂盒(AXYGEN)纯化回收DNA片段。所有引物由生工生物工程(上海)股份有限公司合成,并完成测序。
表1 基因引物名称及序列
Table 1 Gene primer name and sequence
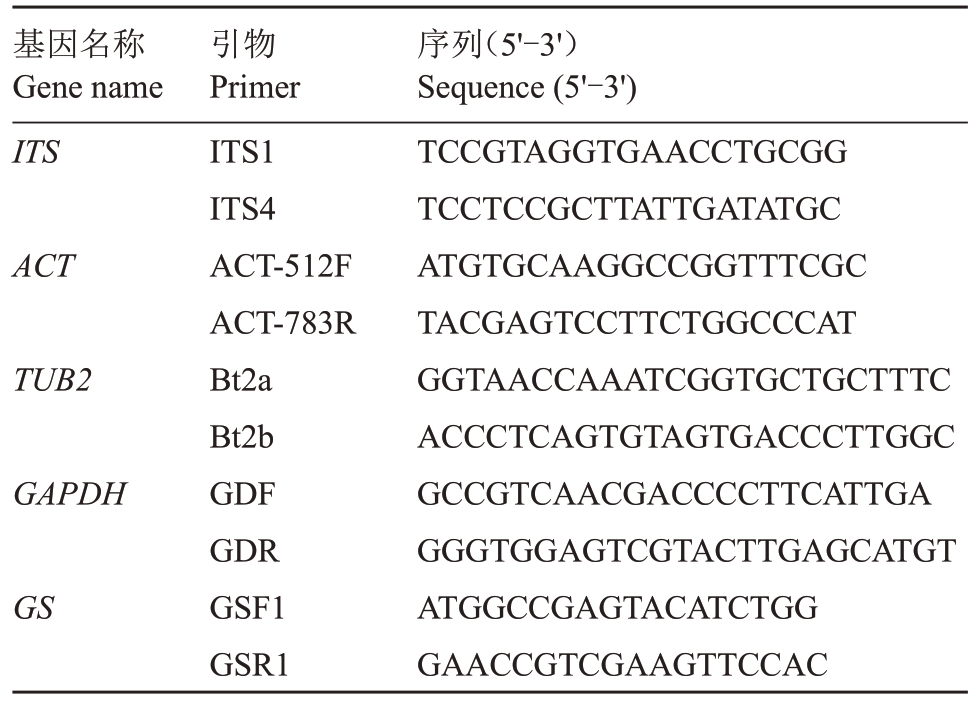
基因名称Gene name ITS ACT TUB2 GAPDH GS引物Primer ITS1 ITS4 ACT-512F ACT-783R Bt2a Bt2b GDF GDR GSF1 GSR1序列(5'-3')Sequence(5'-3')TCCGTAGGTGAACCTGCGG TCCTCCGCTTATTGATATGC ATGTGCAAGGCCGGTTTCGC TACGAGTCCTTCTGGCCCAT GGTAACCAAATCGGTGCTGCTTTC ACCCTCAGTGTAGTGACCCTTGGC GCCGTCAACGACCCCTTCATTGA GGGTGGAGTCGTACTTGAGCATGT ATGGCCGAGTACATCTGG GAACCGTCGAAGTTCCAC
在GenBank(https://www.ncbi.nlm.nih.gov/)数据库使用BLAST搜索比对,下载与本研究中相似性较高的刺盘孢属真菌序列作为参考(具体菌株编号及序列登录号见表2)。使用DNAMAN9.0 软件对本研究中得到的基因序列与GenBank中下载的刺盘孢属真菌序列进行多重比对和分析,必要时用MEGA 7.0对序列进行手工校正。采用软件PhyloSuite v1.2.2 将所有比对好的ITS、ACT、TUB2、GAPDH和GS 基因序列首尾串联。采用最大似然法(Maximum Likelihood,ML),使用MEGA 7.0构建系统发育树,用自展检验法以1000次重复计算分支支持率。
表2 本研究用于系统发育分析的菌株序列信息
Table 2 List of isolates of Colletotrichum species used for phylogenetic analyses in this study

注:本研究所获得菌株加粗表示。
Note:The isolates obtained in this study are expressed in bold.
菌种名Species name分离物编号Isolate No.寄主Host来源Origin基因序列登录号GenBank accession number ITSACTTUB2GAPDHGS C.gloeosporioides C.fructicola C.karstii CG1 ZH3 C-47 JFMYTJ34 ZH6 HNLD-10 YCH32 AHMYTJ2 NSMYTJ4 WZMYTJ7 BZMYTJ23 GQMYTJ26 LXMYTJ29 SGMYTJ35 XZMYTJ37 WFMYTJ40 WFMYTJ48 GQMYTJ55 CLMYTJ59 HUNTJLYC8 OCAC4 GZAAS5.09501 DXMYTJ5 AHMYTJ11 DXMYTJ13 CLMYTJ15 GQMYTJ18 BZMYTJ20 XXMYTJ21 AHMYTJ22 SGMYTJ25 WZMYTJ28 GQMYTJ32 WFMYTJ33 WZMYTJ34 BZMYTJ36 NSMYTJ38 NSMYTJ41 WZMYTJ42 XZMYTJ43 JFMYTJ44 GQMYTJ45 XZMYTJ46 CLMYTJ47 WZMYTJ49 Punica granatum/Capsicum annuum Guanximiyou pomelo Cyclocaryay paliurus Strawberry Jasminum nudiflorum Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Camellia oleifera Small cardamom Citrus sinensis Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Albania China India China China China China China China China China China China China China China China China China China India China China China China China China China China China China China China China China China China China China China China China China China China MT300326 MT476850 MG282160 PQ624726 MT476840 MK629873 MT626035 PQ624695 PQ624697 PQ624700 PQ624714 PQ624717 PQ624720 PQ624727 PQ624729 PQ624732 PQ624740 PQ624747 PQ624750 MF615464 KJ813595 JQ247629 PQ624698 PQ624704 PQ624705 PQ624707 PQ624710 PQ624711 PQ624712 PQ624713 PQ624716 PQ624719 PQ624723 PQ624724 PQ624725 PQ624728 PQ624730 PQ624733 PQ624734 PQ624735 PQ624736 PQ624737 PQ624738 PQ624739 PQ624741 MT332146 MT500918 MG729649 PQ603050 MT500908 MK675237 MT741778 PQ603019 PQ603021 PQ603024 PQ603038 PQ603041 PQ603044 PQ603051 PQ603053 PQ603056 PQ603064 PQ603071 PQ603074 MF615469 KJ813445 JQ247653 PQ603022 PQ603028 PQ603029 PQ603031 PQ603034 PQ603035 PQ603036 PQ603037 PQ603040 PQ603043 PQ603047 PQ603048 PQ603049 PQ603052 PQ603054 PQ603057 PQ603058 PQ603059 PQ603060 PQ603061 PQ603062 PQ603063 PQ603065 MT332145 MT501094 MG383569 PQ616674 MT501084 MK681417 MT683674 PQ616643 PQ616645 PQ616648 PQ616662 PQ616665 PQ616668 PQ616675 PQ616677 PQ616680 PQ616688 PQ616695 PQ616698 MF615484 KJ813470 JQ247641 PQ616646 PQ616652 PQ616653 PQ616655 PQ616658 PQ616659 PQ616660 PQ616661 PQ616664 PQ616667 PQ616671 PQ616672 PQ616673 PQ616676 PQ616678 PQ616681 PQ616682 PQ616683 PQ616684 PQ616685 PQ616686 PQ616687 PQ616689 MT332147 MT501050 MG729659 PQ616558 MT501040 MK675259 MT741781 PQ616527 PQ616529 PQ616532 PQ616546 PQ616549 PQ616552 PQ616559 PQ616561 PQ616564 PQ616572 PQ616579 PQ616582 MF615474 KJ813545 JQ247605 PQ616530 PQ616536 PQ616537 PQ616539 PQ616542 PQ616543 PQ616544 PQ616545 PQ616548 PQ616551 PQ616555 PQ616556 PQ616557 PQ616560 PQ616562 PQ616565 PQ616566 PQ616567 PQ616568 PQ616569 PQ616570 PQ616571 PQ616573 MT332150 MW344714 MG729657 PQ616616 MW344704 MK681395 PQ046875 PQ616585 PQ616587 PQ616590 PQ616604 PQ616607 PQ616610 PQ616617 PQ616619 PQ616622 PQ616630 PQ616637 PQ616640 MF615479 KJ813570 JQ247618 PQ616588 PQ616594 PQ616595 PQ616597 PQ616600 PQ616601 PQ616602 PQ616603 PQ616606 PQ616609 PQ616613 PQ616614 PQ616615 PQ616618 PQ616620 PQ616623 PQ616624 PQ616625 PQ616626 PQ616627 PQ616628 PQ616629 PQ616631
表2 (续) Table 2 (Continued)

菌种名Species name分离物编号Isolate No.寄主Host来源Origin基因序列登录号GenBank accession number ITSACTTUB2GAPDHGS C.brevisporum C.cliviicola C.truncatum PQ616632 PQ616633 PQ616634 PQ616635 PQ616638 PQ616639 PQ616641 KU319455 MN737615 JQ247611 PQ616584 PQ616589 PQ616591 PQ616596 PQ616598 PQ616599 PQ616611 PQ616612 PQ616621 MK585943 MG729673 PQ616586 PQ616592 PQ616593 PQ616608 PQ616636 MK681396 MG703489 KJ486112 PQ616605 C.plurivorum C.dematium C.okinawense C.acutatum Curvularia lunata XZMYTJ50 LXMYTJ51 SGMYTJ52 JFMYTJ53 BZMYTJ56 BZMYTJ58 LXMYTJ60 YYGXZ07 JXHTC19 GZAAS5.09545 XXMYTJ1 AHMYTJ6 LXMYTJ8 WFMYTJ14 SGMYTJ16 XZMYTJ17 XXMYTJ30 SGMYTJ31 XXMYTJ39 S37 C-77 DXMYTJ3 AHMYTJ9 JFMYTJ10 WFMYTJ27 XXMYTJ54 BJ-3 C-3 OOC72 GQMYTJ24 LFN0016 GX018 JZB330314 PC7 PP3 19301A 43380 A112 Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Pepper Dalbergia odorifera Citrus medica Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Morus alba Capsicum annuum Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Guanximiyou pomelo Clausena lansium Capsicum annuum Allium cepa(onion)Guanximiyou pomelo Soybean Diospyros kaki Prunus avium Carica papaya Papaya Olea europaea cv.Galega Vulgar Prunus dulcis(almond)Saccharum sp.China China China China China China China China China China China China China China China China China China China China India China China China China China China India India China Brasil China China China China Portugal Australia Brazil PQ624742 PQ624743 PQ624744 PQ624745 PQ624748 PQ624749 PQ624751 KU319458 MF993572 JQ247623 PQ624694 PQ624699 PQ624701 PQ624706 PQ624708 PQ624709 PQ624721 PQ624722 PQ624731 KY986892 MG282172 PQ624696 PQ624702 PQ624703 PQ624718 PQ624746 MK629874 MG204564 KJ486149 PQ624715 MK142673 MN092338 OL378294 OQ642143 MK649935 PP508294 MT254972 MT683262 PQ603066 PQ603067 PQ603068 PQ603069 PQ603072 PQ603073 PQ603075 KU319457 MG515612 JQ247647 PQ603018 PQ603023 PQ603025 PQ603030 PQ603032 PQ603033 PQ603045 PQ603046 PQ603055 KY986904 MG729670 PQ603020 PQ603026 PQ603027 PQ603042 PQ603070 MK675238 MG703483 KJ485890 PQ603039 KT696277 MN092324 OL471275 OQ723039 MK790071 PP506870 MT305716 MT757139 PQ616690 PQ616691 PQ616692 PQ616693 PQ616696 PQ616697 PQ616699 KU319453 MG515615 JQ247635 PQ616642 PQ616647 PQ616649 PQ616654 PQ616656 PQ616657 PQ616669 PQ616670 PQ616679 MF033886 MG383581 PQ616644 PQ616650 PQ616651 PQ616666 PQ616694 MK681418 MG204619 KJ485927 PQ616663 MK188482 MN092341 OL471279 OQ723040 MK790073 PP506921 MT270256 MT820144 PQ616574 PQ616575 PQ616576 PQ616577 PQ616580 PQ616581 PQ616583 KU319456 MN737614 JQ247599 PQ616526 PQ616531 PQ616533 PQ616538 PQ616540 PQ616541 PQ616553 PQ616554 PQ616563 KY986898 MG729674 PQ616528 PQ616534 PQ616535 PQ616550 PQ616578 MK675260 MG703491 KJ486075 PQ616547 MK139901 MN092335 OL471273 OQ723038 MK790069 PP506775 MT305691 MW091454--------
1.2.4 致病性测定及致病力评估 根据柯赫氏法则将分离获得的刺盘孢属真菌进行致病性测定,采用孢子悬浮液刺伤接种离体叶片和枝条、活体叶片。以健康无伤、成熟度好、大小相近的琯溪蜜柚嫩叶和枝条以及健康幼苗为接种材料,洗去表面灰尘后用75%乙醇表面消毒,无菌水冲洗3次,自然风干。用无菌昆虫针(Φ=0.5 mm)刺伤嫩叶和枝条表面(深度约1 mm),向伤口表面喷洒孢子悬浮液(浓度为1×106 孢子·mL-1),每个菌株接种叶片9枚,枝条9个,3次重复,以接种无菌水为对照。待悬浮液自然风干后将离体叶片和枝条置于塑料盒中,放入25 ℃、12 h/12 h光暗交替、相对湿度90%的人工气候箱保湿培养,蜜柚苗接种后置于温室大棚。期间观察并记录发病情况,发病后从病斑上再分离、纯化菌株。采用十字交叉法测量病斑直径,根据不同分离株的病斑大小,评估致病力。
1.2.5 病原菌菌丝生长速率、附着胞形成率与致病性相关性分析 利用Excel对菌丝生长速率、附着胞形成率与致病力相关性进行分析,0<|r|≤0.3,无相关性;0.3<|r|<0.8,弱相关性;|r|≥0.8,强相关性。
2 结果与分析
2.1 刺盘孢属真菌分离纯化
从平和县13 个乡镇(小溪镇、山格镇、文峰镇、南胜镇、坂仔镇、安厚镇、大溪镇、霞寨镇、五寨乡、九峰镇、芦溪镇、国强乡、长乐乡)的18个蜜柚果园和3个蜜柚苗圃中共采集256份具有炭疽病典型症状的样品(叶片165份、果实48份和枝梢43份)。通过组织分离共获得367 株分离物,经对培养性状和形态特征观察后初步分析鉴定,共有350 株刺盘孢属真菌。根据分离菌株的形态特征和ITS序列分析主要有5 类复合种,其中220 株为胶孢炭疽菌复合种、76株为博宁炭疽菌复合种、29株为C.magnum复合种、15 株为C.orchidearum 复合种,10 株为平头炭疽菌复合种,其分离频率分别为62.86%、21.71%、8.29%、4.29%和2.86%。
2.2 刺盘孢属真菌形态特征鉴定
胶胞炭疽菌复合种:其中有128 株在PDA 培养基上的培养性状、分生孢子均一样。代表菌株JFMYTJ34菌落为深灰色,气生菌丝发达(图1-A1),背面明显可见扇形的角边区,中间橘黄色,边缘深灰色(图1-B1)。培养10 d 左右可产生砖红色分生孢子堆,分生孢子单胞,无色,圆柱形,两端钝圆,有1~2个油球,大小为(12.0~18.8)μm×(4.0~6.2)μm(图1-C1);附着胞单生或散生,深褐色,近圆形或不规则形,边缘整齐,大小为(8.0~16.5)μm×(4.0~8.5)μm(图1-D1)。另外92株在PDA培养基上的培养性状存在差异,菌落颜色为灰白色至灰色等12种不同的培养特征。代表菌株AHMYTJ2 在PDA 培养基上生长迅速,菌落灰白色,边缘整齐光滑,气生菌丝发达、绒毛状(图1-A2),背面浅灰色至浅橄榄灰色(图1-B2)。分生孢子堆橙红色,分生孢子光滑,无色,有油球,单胞,圆柱状、棍棒状,两端钝圆,基部偶有平截,大小为(13.0~17.0)μm×(4.0~6.0)μm(图1-C2);附着胞单生或散生,浅褐色,椭圆形、棒状或不规则状,边缘完整,大小为(4.0~17.0)μm×(3.0~8.0)μm(图1-D2)。
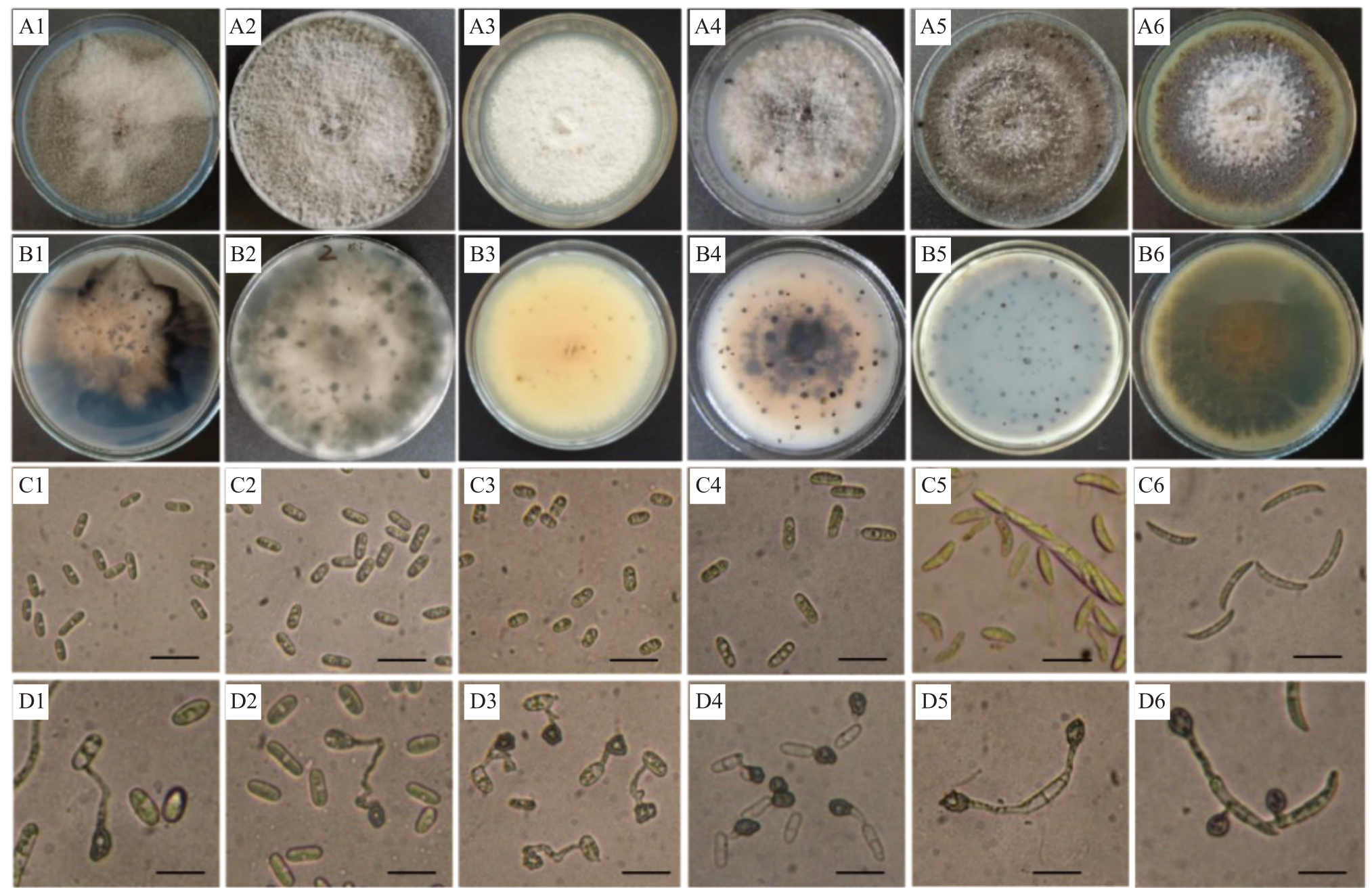
图1 刺盘孢属真菌代表菌株的培养性状及形态特征
Fig.1 Colony and morphological characteristics of representative strains belonging to six Colletotrichum spp.
A1~A6.代表菌株在PDA 培养基上培养10 d 的正面菌落状态;B1~B6.代表菌株在PDA 培养基上培养10 d 的反面菌落状态;C1~C6.代表菌株的分生孢子;D1~D6.代表菌株的附着胞;1.JFMYTJ34;2.AHMYTJ2;3.BZMYTJ20;4.XXMYTJ1;5.DXMYTJ3;6.GQMYTJ24。标尺=20 μm。
A1-A6.Front views of 10 d PDA culture of representative strains;B1-B6.Back views of 10 d PDA culture of representative strains;C1-C6.Conidia of representative strains; D1-D6. Appressorium of representative strains; 1. JFMYTJ34; 2. AHMYTJ2; 3. BZMYTJ20; 4. XXMYTJ1; 5. DXMYTJ3;6.GQMYTJ24.Scale=20 μm.
博宁炭疽菌复合种:76株菌的培养性状差异较大,有30种不同的培养性状。代表菌株BZMYTJ20在PDA 培养基上生长较慢,菌落乳白色,气生菌丝稀疏,绒毛状或卷毛状(图1-A3),背面浅褐色(图1-B3)。分生孢子堆橙色,分生孢子透明,无隔膜,圆柱状,顶部钝圆,基部脐状突起,大小为(12.0~17.0)μm×(5.5~7.5)μm(图1-C3);附着胞单生或散生,浅棕色至深褐色,形状多样,多数为不规则型,边缘完整,大小为(8.6~11.0)μm×(6.0~8.5)μm(图1-D3)。
C. magnum 复合种:各菌株间培养性状存在较大差异,有9 种不同的培养特征。代表菌株XXMYTJ1在PDA培养基上菌落灰白色,气生菌丝发达(图1-A4),背面橘红色伴有黑色小点(图1-B4)。分生孢子橙红色,圆柱形,两端钝圆,大小为(13.0~19.0)μm×(4.0~5.5)μm(图1-C4);附着胞单生,棕色到深棕色,椭圆形、棒状或不规则形,边缘完整,大小为(7.0~15.5)μm×(5.0~8.0)μm(图1-D4)。
C.orchidearum复合种:各菌株间培养性状差异较大,有5种不同的培养特征。代表菌株DXMYTJ3在PDA培养基上生长较快,菌落颜色灰褐色至墨绿色,中间形成环状轮纹圈,气生菌丝发达(图1-A5),背面深灰色至灰色(图1-B5)。分生孢子无色,单胞,椭圆形、弧形,两端钝圆,中间偶有缢缩,大小为(13.0~19.0)μm×(4.0~7.0)μm(图1-C5);附着胞浅褐色至褐色,椭圆形或棒状,边缘偶有凸起,大小为(9.0~15.0)μm×(3.0~13.0)μm(图1-D5)。
平头炭疽菌复合种:10株在PDA培养基上的培养性状、分生孢子均一样。代表菌株GQMYTJ24在PDA培养基上生长较慢,菌落卡其色至橙黄色,气生菌丝不发达(图1-A6),背面杏黄色至桔黄色(图1-B6)。分生孢子镰刀型,光滑,无色,单胞,两端尖,中间有1个油球,大小为(20.0~27.0)μm×(2.0~4.0)μm(图1-C6);附着胞浅褐色,椭圆形或卵圆形,边缘完整,大小为(7.0~18.0)μm×(5.0~11.0)μm(图1-D6)。
2.3 刺盘孢属真菌多基因系统发育分析
从胶孢炭疽菌复合种、博宁炭疽菌复合种、C.magnum 复合种、C.orchidearum 复合种和平头炭疽菌复合种中各选取13株、30株、9株、5株和1株培养性状有较大差异的代表菌株共58 株刺盘孢属真菌进行多基因系统发育分析。分别提取DNA 作为模板,扩增其ITS、ACT、TUB2、GAPDH和GS序列并测序,得到大小分别为500、250、500、300和1000 bp左右的特异性片段。将5 个基因按顺序首尾相连(ITS-ACT-TUB2-GAPDH-GS),以菌株Curvularia lunata 为外类群,采用MEGA 7.0 构建系统发育树。结果表明(图2),58 株刺盘孢属真菌在系统发育树上聚类为6 个不同的分支,其中菌株JFMYTJ34 与胶孢炭疽菌(C.gloeosporioides)单独聚类为一个小分支,GQMYTJ26 等12 株与果生炭疽菌(C.fructicola)聚类为一个分支;BZMYTJ20 等30 株与喀斯特炭疽菌(C.karstii)聚类为一个大分支;XXMYTJ1等9 个菌株聚类为短孢炭疽菌(C. brevisporum),AHMYTJ9 等5 株聚类为兰花炭疽菌(C.cliviicola),菌株GQMYTJ24 与平头炭疽菌(C.truncatum)聚类为一个分支。结合培养性状和分生孢子形态特征结果,从平和县琯溪蜜柚炭疽病样品中分离获得的刺盘孢属真菌分属于6 个种,分别为胶孢炭疽菌(C.gloeosporioides)、果生炭疽菌(C.fructicola)、喀斯特炭疽菌(C.karstii)、短孢炭疽菌(C.brevisporum)、兰花炭疽菌(C. cliviicola)和平头炭疽菌(C. truncatum)。
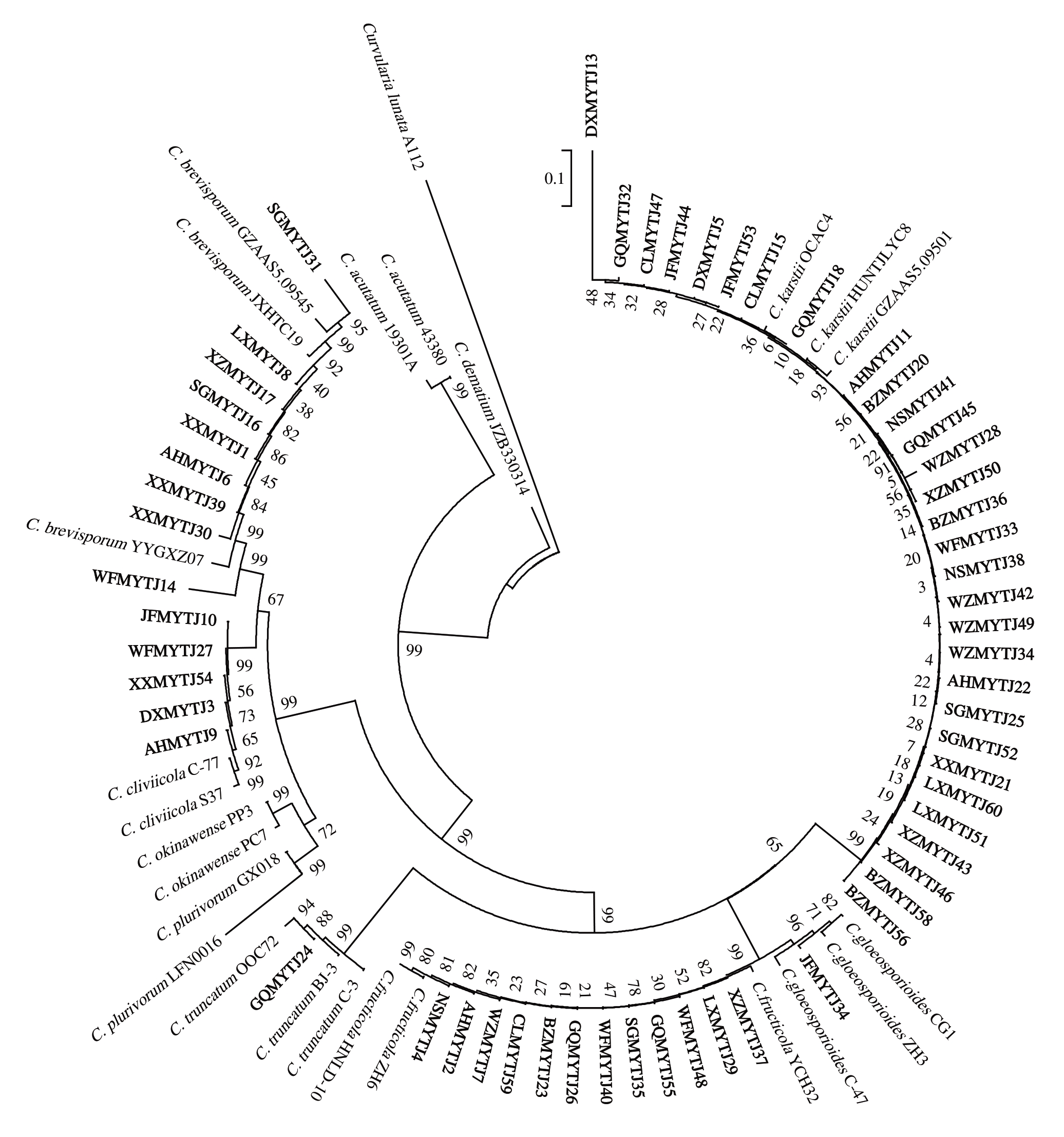
图2 58 株刺盘孢属真菌基于ITS、ACT、TUB2、GADPH、GS 序列的多基因系统发育树
Fig.2 Phylogenetic tree of 58 Colletotrichum species based on the combined ITS-ACT-TUB2-GAPDH-GS
本研究获得的菌株加粗表示。
The isolates obtained in this study are expressed in bold.
2.4 致病性测定
选取培养性状有较大差异的58 株刺盘孢属真菌菌株(同多基因系统发育分析),采用孢子悬浮液刺伤接种离体的叶片和枝条。致病性测定结果表明,不同菌株对健康蜜柚叶片和枝条的致病情况不同(表3),其中胶孢炭疽菌(C. gloeosporioides)JFMYTJ34及12株果生炭疽菌(C.fructicola)接种后病原菌迅速侵入有伤口的蜜柚组织,7 d后达到发病高峰期,叶片病斑呈浅灰褐色至深褐色、近圆形,略微凹陷(图3-K~M),枝条病斑呈淡褐色至深褐色,椭圆形至梭形,稍微下陷,病健交界分明(图3-B~D),症状表现与田间植株症状一致。9株喀斯特炭疽菌(C. karstii)接种3 d 后开始出现水渍症状病斑,9 d后达到发病高峰期,叶片病斑呈灰白色椭圆形,稍凸起(图3-N~O),枝条病斑呈浅褐色稍下陷(图3-E~F)。9 株短孢炭疽菌(C.brevisporum)接种3 d 后开始出现青褐色水渍小斑,随后病斑迅速扩大,9 d后叶片病斑可穿透背面使叶片变软(图3-P~Q),枝条病斑逐渐发展至整个枝条并使其腐烂(图3-G~H)。平头炭疽菌(C.truncatum)GQMYTJ24接种5 d 后开始出现青色水渍小斑,病斑逐渐扩大,向下凹陷,10 d后枝条上的病斑都出现干枯症状(图3-I,R)。21 株喀斯特炭疽菌(C. karstii)、5 株兰花炭疽菌(C.cliviicola)及对照不发病。
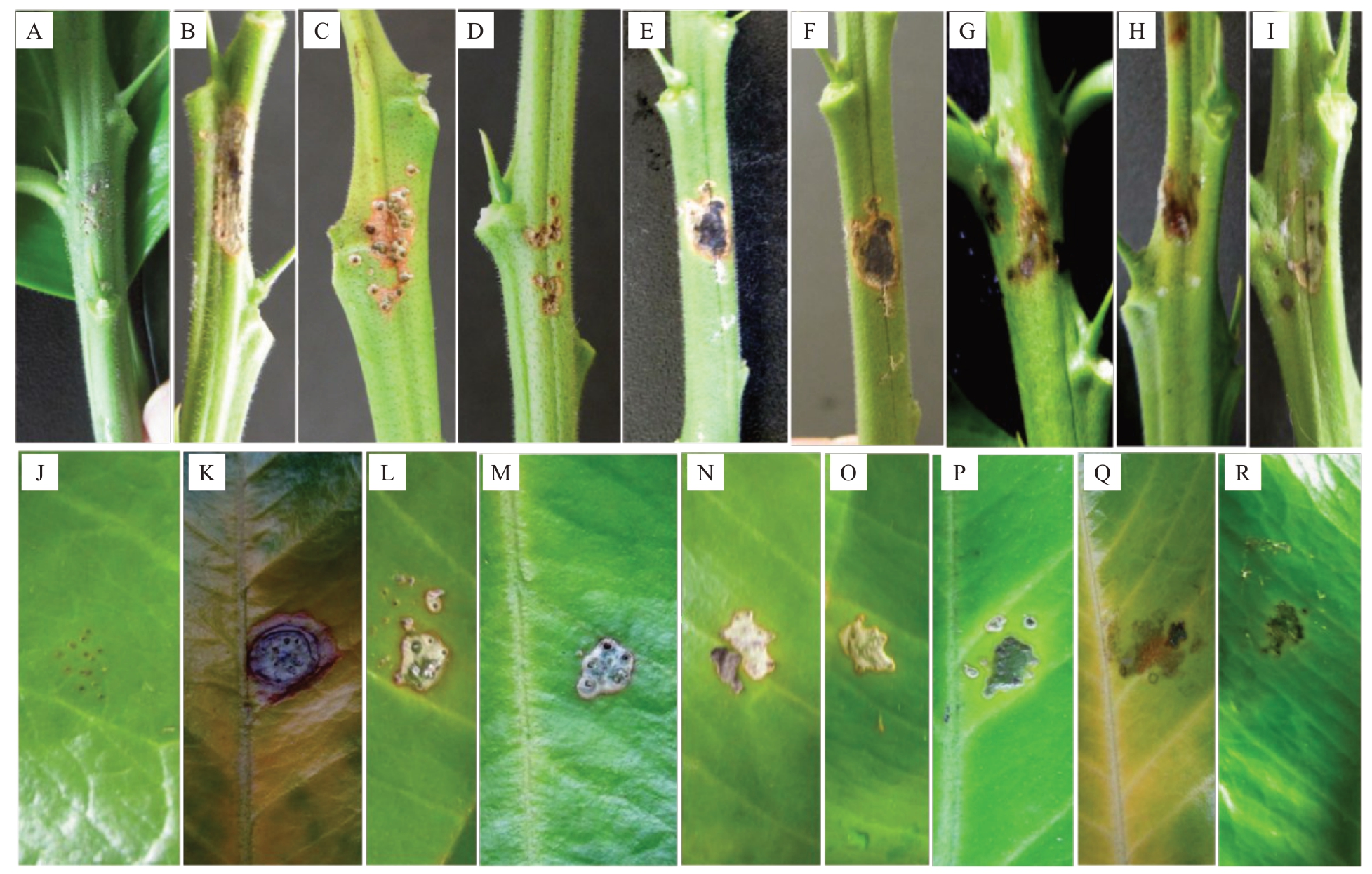
图3 5 种刺盘孢属真菌代表菌株接种离体琯溪蜜柚枝条和叶片产生的症状
Fig.3 Symptoms of Guanximiyou pomelo twigs and leaves inoculated with five Colletotrichum spp.
A、J.接种清水的对照;B、K.接种胶胞炭疽菌JFMYTJ34 的枝条和叶片;C、L.接种果生炭疽菌AHMYTJ2 的枝条和叶片;D、M.接种果生炭疽菌NSMYTJ4 的枝条和叶片;E、N.接种喀斯特炭疽菌BZMYTJ20 的枝条和叶片;F、O.接种喀斯特炭疽菌WZMYTJ34 的枝条和叶片;G、P.接种短孢炭疽菌XXMYJ1 的枝条和叶片;H、Q.接种短孢炭疽菌WFMYTJ14 的枝条和叶片;I、R.接种平头炭疽菌GQMYTJ24 的枝条和叶片。
A,J.Controls inoculated with water;B,K.Inoculated twigs and leaves with C.gloeosporioides JFMYTJ34;C,L.Inoculated twigs and leaves with C. fructicola AHMYTJ2; D, M. Inoculated twigs and leaves with C. fructicola NSMYTJ4; E, N. Inoculated twigs and leaves with C. karstii BZMYTJ20;F,O.Inoculated twigs and leaves with C.karstii WZMYTJ34;G,P.Inoculated twigs and leaves with C.brevisporum XXMYJ1;H,Q.Inoculated twigs and leaves with C.brevisporum WFMYTJ14;I,R.Inoculated twigs and leaves with C.truncatum GQMYTJ24.
表3 琯溪蜜柚炭疽病病原菌菌丝生长速率、附着胞形成率及叶片病斑长度
Table 3 Mycelial growth rate and appressorium formation rate of pathogens,lesion lengths of Guanximiyou pomelo leaves
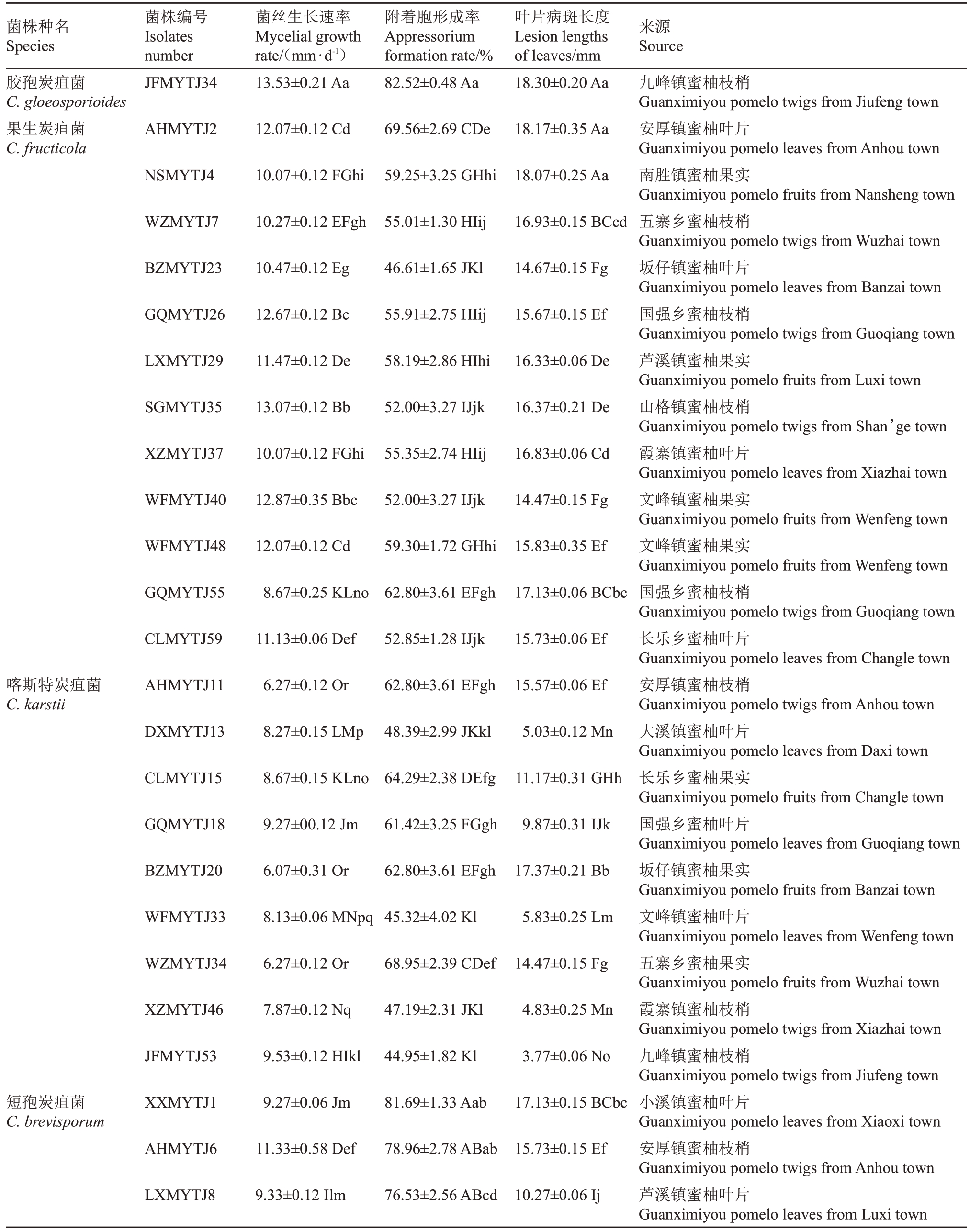
注:同列不同大写字母表示不同菌株在0.01 水平差异极显著,不同小写字母表示不同菌株在0.05 水平差异显著。
Note:Different capital letters in the same column indicate extremely significant differences among different strains at the 0.01 level, Different small letters indicate significant differences at the 0.05 level.
菌株种名Species菌株编号Isolates number菌丝生长速率Mycelial growth rate/(mm·d-1)附着胞形成率Appressorium formation rate/%叶片病斑长度Lesion lengths of leaves/mm来源Source胶孢炭疽菌C.gloeosporioides果生炭疽菌C.fructicola JFMYTJ34 13.53±0.21 Aa 82.52±0.48 Aa 18.30±0.20 Aa AHMYTJ2 12.07±0.12 Cd 69.56±2.69 CDe 18.17±0.35 Aa NSMYTJ4 10.07±0.12 FGhi 59.25±3.25 GHhi 18.07±0.25 Aa WZMYTJ7 10.27±0.12 EFgh 55.01±1.30 HIij 16.93±0.15 BCcd BZMYTJ23 10.47±0.12 Eg 46.61±1.65 JKl 14.67±0.15 Fg GQMYTJ26 12.67±0.12 Bc 55.91±2.75 HIij 15.67±0.15 Ef LXMYTJ29 11.47±0.12 De 58.19±2.86 HIhi 16.33±0.06 De SGMYTJ35 13.07±0.12 Bb 52.00±3.27 IJjk 16.37±0.21 De XZMYTJ37 10.07±0.12 FGhi 55.35±2.74 HIij 16.83±0.06 Cd WFMYTJ40 12.87±0.35 Bbc 52.00±3.27 IJjk 14.47±0.15 Fg WFMYTJ48 12.07±0.12 Cd 59.30±1.72 GHhi 15.83±0.35 Ef GQMYTJ55 8.67±0.25 KLno 62.80±3.61 EFgh 17.13±0.06 BCbc CLMYTJ59 11.13±0.06 Def 52.85±1.28 IJjk 15.73±0.06 Ef喀斯特炭疽菌C.karstii AHMYTJ11 6.27±0.12 Or 62.80±3.61 EFgh 15.57±0.06 Ef DXMYTJ13 8.27±0.15 LMp 48.39±2.99 JKkl 5.03±0.12 Mn CLMYTJ15 8.67±0.15 KLno 64.29±2.38 DEfg 11.17±0.31 GHh GQMYTJ18 9.27±00.12 Jm 61.42±3.25 FGgh 9.87±0.31 IJk BZMYTJ20 6.07±0.31 Or 62.80±3.61 EFgh 17.37±0.21 Bb WFMYTJ33 8.13±0.06 MNpq 45.32±4.02 Kl 5.83±0.25 Lm WZMYTJ34 6.27±0.12 Or 68.95±2.39 CDef 14.47±0.15 Fg XZMYTJ46 7.87±0.12 Nq 47.19±2.31 JKl 4.83±0.25 Mn JFMYTJ53 9.53±0.12 HIkl 44.95±1.82 Kl 3.77±0.06 No短孢炭疽菌C.brevisporum XXMYTJ1 9.27±0.06 Jm 81.69±1.33 Aab 17.13±0.15 BCbc AHMYTJ6 11.33±0.58 Def 78.96±2.78 ABab 15.73±0.15 Ef LXMYTJ8 9.33±0.12 Ilm 76.53±2.56 ABcd 10.27±0.06 Ij九峰镇蜜柚枝梢Guanximiyou pomelo twigs from Jiufeng town安厚镇蜜柚叶片Guanximiyou pomelo leaves from Anhou town南胜镇蜜柚果实Guanximiyou pomelo fruits from Nansheng town五寨乡蜜柚枝梢Guanximiyou pomelo twigs from Wuzhai town坂仔镇蜜柚叶片Guanximiyou pomelo leaves from Banzai town国强乡蜜柚枝梢Guanximiyou pomelo twigs from Guoqiang town芦溪镇蜜柚果实Guanximiyou pomelo fruits from Luxi town山格镇蜜柚枝梢Guanximiyou pomelo twigs from Shan’ge town霞寨镇蜜柚叶片Guanximiyou pomelo leaves from Xiazhai town文峰镇蜜柚果实Guanximiyou pomelo fruits from Wenfeng town文峰镇蜜柚果实Guanximiyou pomelo fruits from Wenfeng town国强乡蜜柚枝梢Guanximiyou pomelo twigs from Guoqiang town长乐乡蜜柚叶片Guanximiyou pomelo leaves from Changle town安厚镇蜜柚枝梢Guanximiyou pomelo twigs from Anhou town大溪镇蜜柚叶片Guanximiyou pomelo leaves from Daxi town长乐乡蜜柚果实Guanximiyou pomelo fruits from Changle town国强乡蜜柚叶片Guanximiyou pomelo leaves from Guoqiang town坂仔镇蜜柚果实Guanximiyou pomelo fruits from Banzai town文峰镇蜜柚叶片Guanximiyou pomelo leaves from Wenfeng town五寨乡蜜柚果实Guanximiyou pomelo fruits from Wuzhai town霞寨镇蜜柚枝梢Guanximiyou pomelo twigs from Xiazhai town九峰镇蜜柚枝梢Guanximiyou pomelo twigs from Jiufeng town小溪镇蜜柚叶片Guanximiyou pomelo leaves from Xiaoxi town安厚镇蜜柚枝梢Guanximiyou pomelo twigs from Anhou town芦溪镇蜜柚叶片Guanximiyou pomelo leaves from Luxi town
表3 (续) Table 3 (Continued)
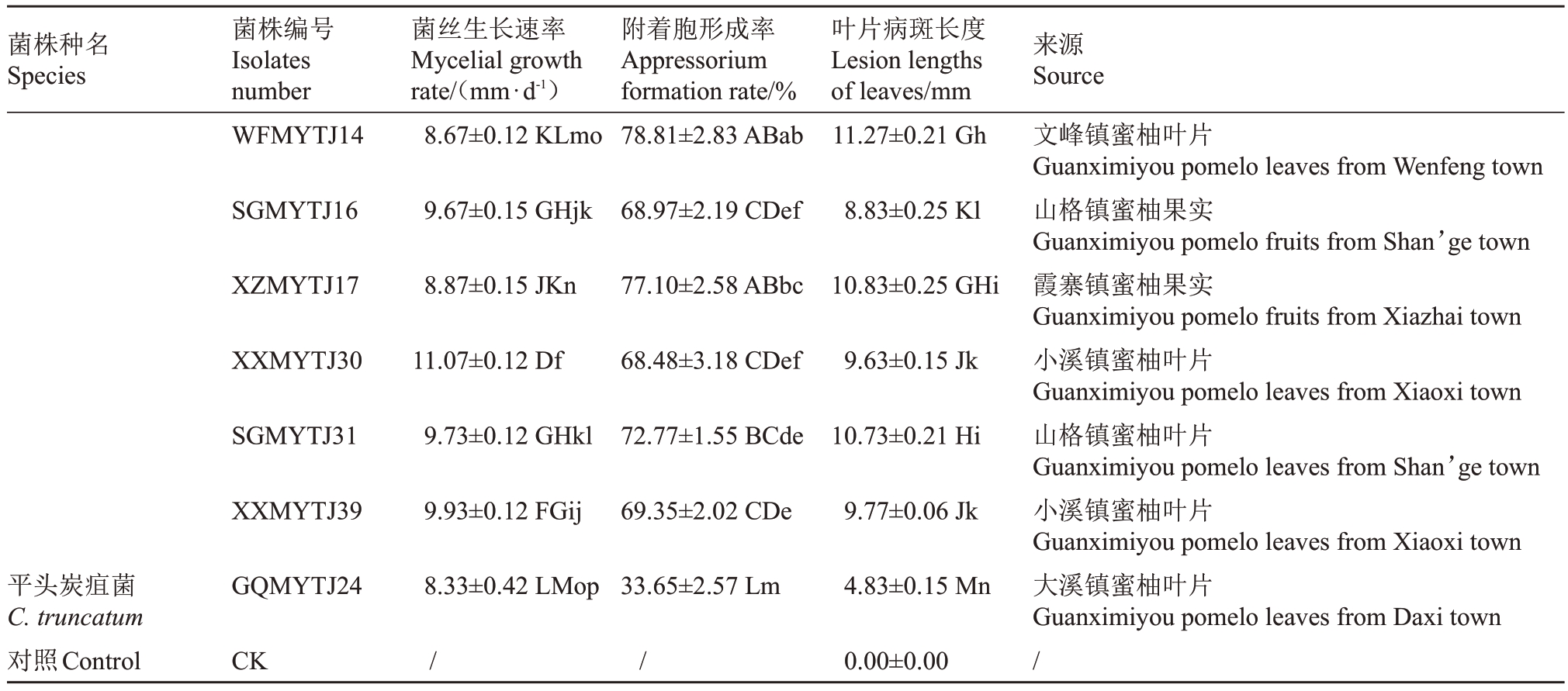
菌株种名Species菌株编号Isolates number菌丝生长速率Mycelial growth rate/(mm·d-1)附着胞形成率Appressorium formation rate/%叶片病斑长度Lesion lengths of leaves/mm来源Source WFMYTJ14 8.67±0.12 KLmo 78.81±2.83 ABab 11.27±0.21 Gh SGMYTJ16 9.67±0.15 GHjk 68.97±2.19 CDef 8.83±0.25 Kl XZMYTJ17 8.87±0.15 JKn 77.10±2.58 ABbc 10.83±0.25 GHi XXMYTJ30 11.07±0.12 Df 68.48±3.18 CDef 9.63±0.15 Jk SGMYTJ31 9.73±0.12 GHkl 72.77±1.55 BCde 10.73±0.21 Hi XXMYTJ39 9.93±0.12 FGij 69.35±2.02 CDe 9.77±0.06 Jk平头炭疽菌C.truncatum对照Control GQMYTJ24 8.33±0.42 LMop 33.65±2.57 Lm 4.83±0.15 Mn CK //0.00±0.00文峰镇蜜柚叶片Guanximiyou pomelo leaves from Wenfeng town山格镇蜜柚果实Guanximiyou pomelo fruits from Shan’ge town霞寨镇蜜柚果实Guanximiyou pomelo fruits from Xiazhai town小溪镇蜜柚叶片Guanximiyou pomelo leaves from Xiaoxi town山格镇蜜柚叶片Guanximiyou pomelo leaves from Shan’ge town小溪镇蜜柚叶片Guanximiyou pomelo leaves from Xiaoxi town大溪镇蜜柚叶片Guanximiyou pomelo leaves from Daxi town/
从5种致病的刺盘孢属真菌中各选取一株致病性最强的代表菌株进行健康蜜柚盆栽幼苗叶片有伤接种,结果表明,这些代表菌株均能使叶片产生干枯的病斑,发病率为100%。接种3 d 后叶片开始出现轻微症状,与田间发病初期症状一致,7 d 后症状加重,叶片病斑面积扩大,不同种的刺盘孢属真菌产生的病斑大小差异明显,与田间发病中、后期症状一致(图4)。
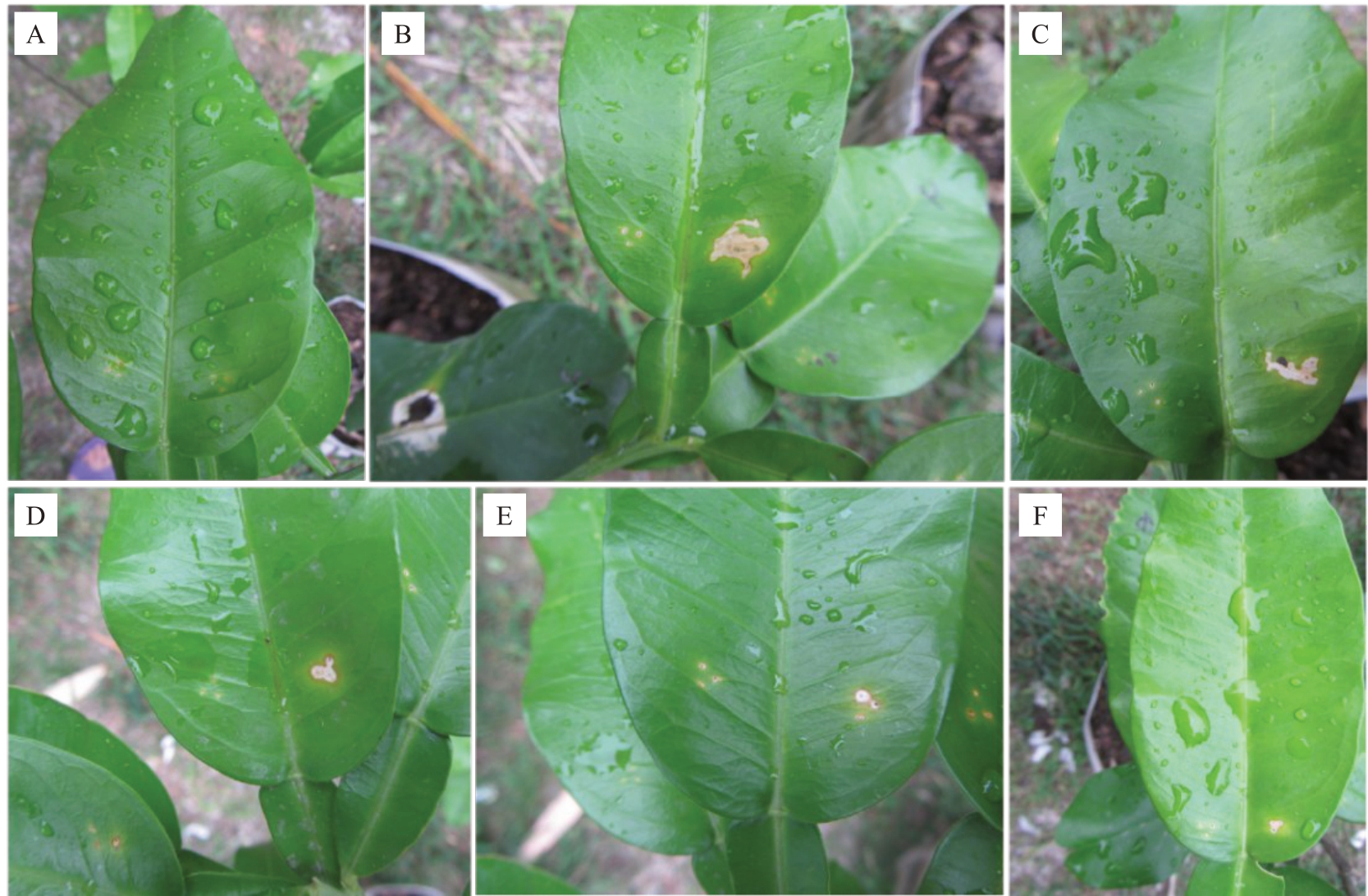
图4 5 种刺盘孢属真菌代表菌株接种盆栽琯溪蜜柚叶片产生的症状
Fig.4 Symptoms of Guanximiyou pomelo leaves in vivo after inoculated with representative isolates of five Colletotrichum spp.
A.接种清水的对照;B.胶胞炭疽菌JFMYTJ34;C.果生炭疽菌AHMYTJ2;D.喀斯特炭疽菌BZMYTJ20;E.短孢炭疽菌XXMYJ1;F.平头炭疽菌GQMYTJ24。
A. Controls inoculated with water; B. C. gloeosporioides JFMYTJ34; C. C. fructicola AHMYTJ2; D. C. karstii BZMYTJ20; E. C. brevisporum XXMYJ1;F.C.truncatum GQMYTJ24.
对以上离体接种和盆栽接种发病的组织取样进行病原菌再分离,得到的病原菌经形态学和分子生物学鉴定,结果与接种的病原菌菌株完全一致。结果表明,胶孢炭疽菌(C.gloeosporioides)、果生炭疽菌(C.fructicola)、喀斯特炭疽菌(C.karstii)、短孢炭疽菌(C.brevisporum)和平头炭疽菌(C.truncatum)5种刺盘孢属真菌均是引起福建琯溪蜜柚炭疽病的病原菌。
2.5 病原菌菌丝生长速率、附着胞形成率及致病力分析
5种致病的刺盘孢属真菌菌丝生长速率在6.07~13.53 mm·d-1之间(表3),其中胶孢炭疽菌(C.gloeo-sporioides)的平均生长速率最快,为13.53 mm·d-1;果生炭疽菌(C. fructicola)次之,平均生长速率为11.28 mm·d-1;短孢炭疽菌(C. brevisporum)和平头炭疽菌(C. truncatum)居中,平均生长速率分别为9.76 mm·d-1和8.33 mm·d-1;喀斯特炭疽菌(C.karstii)的平均生长速率最慢,平均生长速率为7.81 mm·d-1。5 种刺盘孢属真菌的附着胞形成率在33.65%~82.52%之间(表3),其中胶孢炭疽菌(C.gloeosporioides)最高,为82.52%;其次是短孢炭疽菌(C.brevisporum),平均附着胞形成率为74.74%;果生炭疽菌(C. fructicola)和喀斯特炭疽菌(C. karstii)居中,分别为56.57%和56.23%;平头炭疽菌(C. truncatum)最低,为33.65%。
调查分析5种致病的刺盘孢属真菌接种琯溪蜜柚叶片10 d 后的病害严重程度及病斑长度(表3)。结果表明,不同种的刺盘孢属真菌致病力存在明显差异,胶孢炭疽菌(C. gloeosporioides)的致病力最强,接种叶片的平均病斑长度为18.30 mm;其次是果生炭疽菌(C.fructicola),平均病斑长度为16.35 mm;短孢炭疽菌(C.brevisporum)的致病力居中,平均病斑长度为11.58 mm;平头炭疽菌(C.truncatum)的致病力较弱,平均病斑长度为4.83 mm。喀斯特炭疽菌(C. karstii)不同菌株之间的致病力存在较大差异,菌株BZMYTJ20 接种叶片的平均病斑长度为17.37 mm,菌株JFMYTJ53 接种叶片的平均病斑长度仅为3.77 mm,而DXMYTJ5、XXMYTJ21 和BZMYTJ56等菌株接种叶片后不发病。
2.6 病原菌菌丝生长速率、附着胞形成率与致病力相关性分析
对5种致病的刺盘孢属真菌菌丝生长速率与致病力进行相关性分析(图5),结果表明,其相关系数r为0.373 3,0.3<|r|<0.8,表明菌丝生长速率与致病力之间呈弱相关性。对5种致病的刺盘孢属真菌附着胞形成率与致病力进行相关性分析(图6),结果表明,其相关系数r 为0.364 1,0.3<|r|<0.8,表明附着胞形成率与致病力之间呈弱相关性。
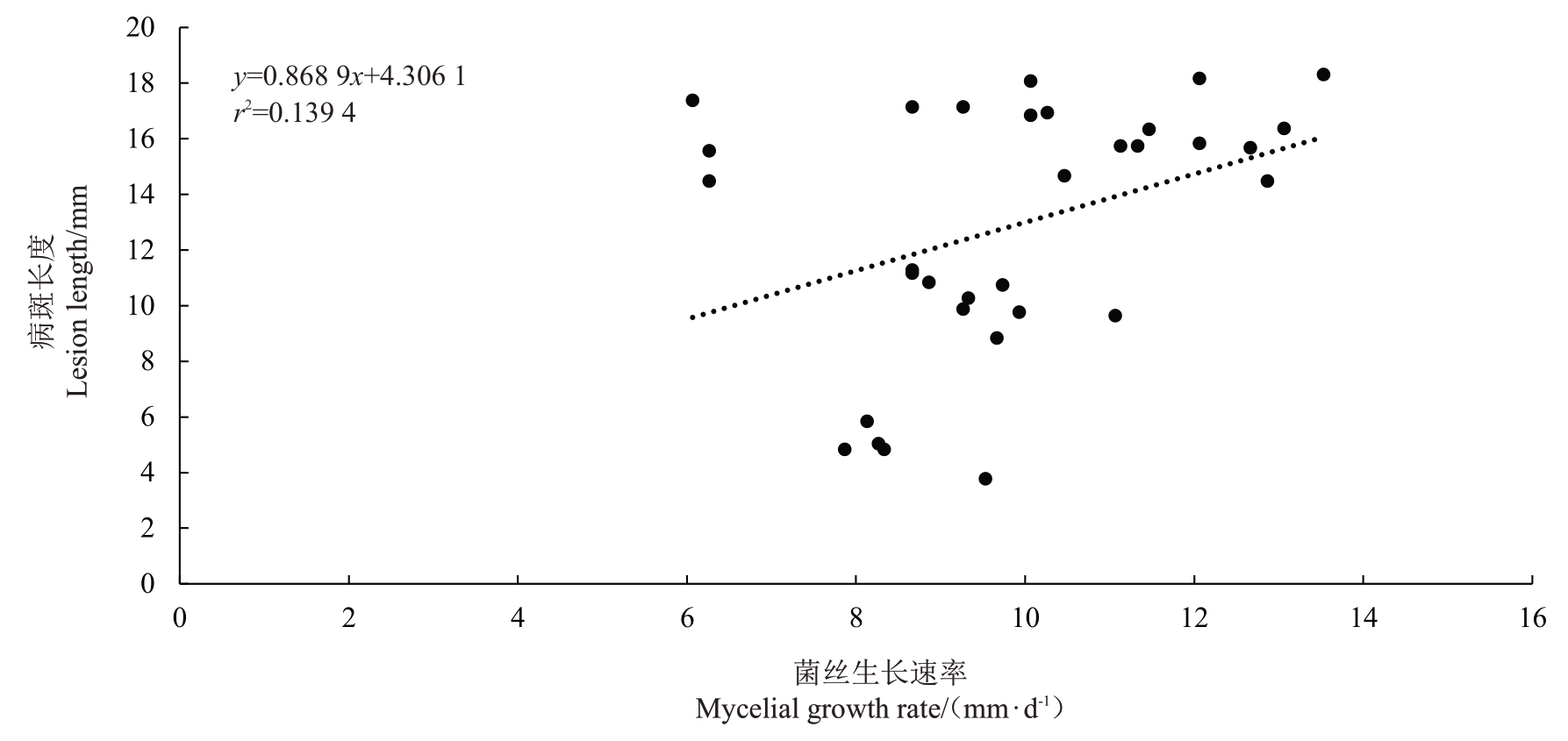
图5 5 种刺盘孢属真菌菌株菌丝生长速率与致病力相关性分析
Fig.5 Linear analysis of the relationship between pathogenicity and mycelial growth rate of five Colletotrichum spp.
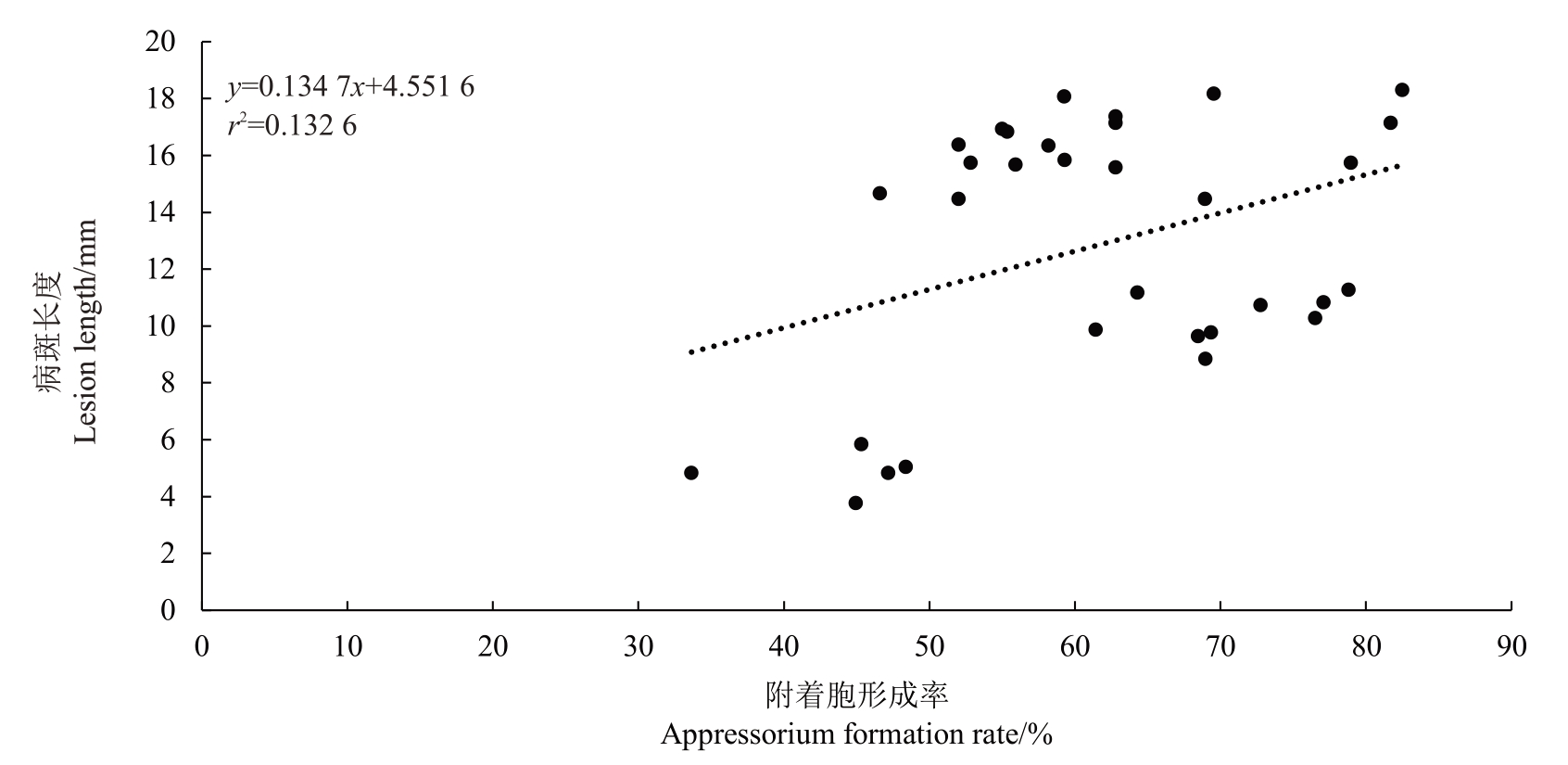
图6 5 种刺盘孢属真菌菌株附着胞形成率与致病力相关性分析
Fig.6 Linear analysis of the relationship between pathogenicity and appressorium formation rate of five Colletotrichum spp.
3 讨 论
炭疽病是琯溪蜜柚的主要病害之一,可在整个生育期造成严重危害,极大地影响了琯溪蜜柚产业的健康发展。刺盘孢属真菌作为柑橘炭疽病的主要病原菌,种类繁多[21],据报道,国内外关于柑橘刺盘孢属真菌的记录有100 余种,其中超过10 种是柑橘炭疽病的病原菌[22]。中国2017 年报道琯溪蜜柚炭疽病病原菌为胶孢炭疽菌(C.gloeosporioides)[3],之后一直未见其他刺盘孢属真菌侵染琯溪蜜柚的报道。笔者课题组于2019 年在福建省平和县琯溪蜜柚种植区发现该病的田间症状具有多样性,通过形态学特征观察和多基因系统发育分析,将琯溪蜜柚相关刺盘孢属真菌鉴定为5 类刺盘孢复合种下的6种单系种,揭示了琯溪蜜柚刺盘孢属真菌种类的多样性。Peng等[23]在云南和贵州柑橘主产区分离到的柑橘相关刺盘孢属真菌,鉴定为胶胞炭疽菌复合种下的胶胞炭疽菌(C.gloeosporioides)和果生炭疽菌(C.fructicola)、博宁炭疽菌复合种下的博宁炭疽菌(C.boninense)和喀斯特炭疽菌(C.karstii)、C.magnum 复合种下的短孢炭疽菌(C.brevisporum)、尖孢炭疽菌复合种的西蒙氏炭疽菌(C.simmondsii)和C.murrayae,与本研究结果不尽相同。
柑橘炭疽病可由多种刺盘孢属真菌复合侵染,国内已报道的柑橘炭疽病病原种类有胶孢炭疽菌(C.gloeosporioides)、果生炭疽菌(C.fructicola)、博宁炭疽菌(C.boninense)、喀斯特炭疽菌(C.karstii)、短孢炭疽菌(C.brevisporum)、平头炭疽菌(C.truncatum)、热带生炭疽菌(C.tropicicola)、暹罗炭疽菌(C.siamense)、普洛柏炭疽菌(C.plurivorum)和江西炭疽菌(C. jiangxiense)等,其中胶孢炭疽菌(C.gloeosporioides)是主要的病原菌[24-25]。国外已报道的柑橘炭疽病病原种类有胶孢炭疽菌(C.gloeosporioides)、果生炭疽菌(C.fructicola)、澳大利亚孢炭疽菌(C. australianum)、喀斯特炭疽菌(C. karstii)、尖孢炭疽菌(C.acutatum)、荷花炭疽菌(C.nymphaeae)、C. catinaense、C. limonicola、C. helleniense 和可可炭疽菌(C.theobromicola)等[21,26]。Huang等[5]从福建琯溪蜜柚无症状的叶片中分离获得胶孢炭疽菌(C.gloeosporioides)和喀斯特炭疽菌(C.karstii),然而未对这些菌株进行致病性测定,尚不确定其是否对琯溪蜜柚致病。本研究结果发现有5种刺盘孢属真菌可引起琯溪蜜柚炭疽病,与国内外研究结果相一致,证实琯溪蜜柚炭疽病病原种类具有多样性。笔者在本研究中通过盆栽、田间活体接种试验,表明不同的病原在田间所造成的症状存在明显差异,琯溪蜜柚炭疽病不同的田间症状与病原种类的多样性息息相关,因此,进行准确的病原种类鉴定可为该病的田间症状诊断和有效防控提供重要的信息,促进蜜柚产业健康发展。
形态学观察结果表明,5 种琯溪蜜柚炭疽病病原菌在PDA培养基上的培养性状存在差异,菌落生长速度快慢不一,不同菌株之间附着胞产生速度及形成率差异也较大。胶胞炭疽菌(C. gloeosporioides)培养性状稳定,各个菌株培养性状基本一致,菌丝生长速率最快,而喀斯特炭疽菌(C.karstii)培养性状极其不稳定,分离的菌株中培养性状各异,菌丝生长速率最慢;短孢炭疽菌(C.brevisporum)产生附着胞的速度最快且形成率最高,平头炭疽菌(C.truncatum)产生附着胞的速度最慢且形成率最低。琯溪蜜柚炭疽病菌的形态特征存在较大差异,表明该菌具有丰富的生理生化特性,其在侵染寄主时会引起致病力的差异,菌株的培养性状、菌丝生长速率和附着胞产生速度及形成率可能对刺盘孢属真菌的致病力起重要作用[27-28]。本研究致病力测定结果发现,5种病原菌的致病力存在较大差异,不同种刺盘孢属真菌及同种的不同菌株对琯溪蜜柚存在明显的致病力分化现象,胶胞炭疽菌(C.gloeosporioides)致病力最强,喀斯特炭疽菌(C.karstii)的平均致病力最弱,与Guarnaccia 等[21]对欧洲柑橘炭疽病病原菌致病力测定的结果一致。然而Mayorquin 等[29]对美国加利福尼亚小柑橘炭疽病的病原菌进行致病力研究发现,喀斯特炭疽菌(C.karstii)是该病的主要致病菌,其致病力明显强于胶胞炭疽菌(C.gloeosporioides),这进一步证实了不同产区柑橘炭疽病病原菌的致病力存在较大差异。笔者在本研究中还发现喀斯特炭疽菌(C. karstii)不同菌株之间的致病力存在较大差异,有的菌株致病力较强,有的较弱,有的不致病,Mario 等[30]研究表明,3 株不同的喀斯特炭疽菌(C.karstii)分离株对甜橙叶片同样表现出明显的致病力差异,其中1株的致病力显著高于其他2株,但并未发现不致病的菌株,可能与选取的代表菌株数量较少有关。对5种病原菌的菌丝生长速率和致病力进行相关性分析,发现二者有一定程度的正相关关系,表明病原菌在侵染寄主的过程中菌丝生长速率越快,致病力可能越大,危害性越强。5种病原菌的附着胞形成率和致病力相关性分析证实二者呈弱相关性,表明病原菌在侵染寄主的过程中附着胞形成率与致病力相关。致病力差异还与其他多种因素相关,刺盘孢属真菌的果胶裂解酶活性及致病相关基因如MAP激酶基因Cgl-SLT2、漆酶基因Lac1等对其致病力起着重要的调控作用[31-32]。因此,琯溪蜜柚炭疽病病原菌致病力差异的分子机制还有待进行深入全面的研究。
刺盘孢属真菌寄主范围广泛,同一种刺盘孢属真菌可同时侵染多种植物,造成严重的病害。来源于意大利柑橘的胶孢炭疽菌(C.gloeosporioides)可以侵染柑橘、杧果、辣椒、草莓、番石榴和木瓜等作物,引起炭疽病[14,33];Prihastuti 等[34]发现果生炭疽菌(C.fructicola)可以引起咖啡发生炭疽病,随后其他研究表明该菌也可以侵染柑橘、李、桃、梨和猕猴桃等植物,发生炭疽病[35];短孢炭疽菌(C.brevisporum)最初来源于彩叶凤梨炭疽病病叶,同时该菌可以侵染柑橘、南瓜、辣椒等经济作物,引起炭疽病[36-37];喀斯特炭疽菌(C.karstii)可引起柑橘、山茶、咖啡、番茄等作物发生炭疽病病害[38];平头炭疽菌(C.truncatum)是大豆、柑橘、番木瓜等作物炭疽病的病原菌[39-40]。因此,有必要进一步对上述琯溪蜜柚炭疽病刺盘孢属真菌进行其他作物的致病性测定,明确来源于琯溪蜜柚的刺盘孢属真菌是否会侵染其他作物,以便在果园管理中防范交互感染。
4 结 论
引起福建省平和县琯溪蜜柚炭疽病的病原菌有胶孢炭疽菌(C. gloeosporioides)、果生炭疽菌(C.fructicola)、喀斯特炭疽菌(C. karstii)、短孢炭疽菌(C.brevisporum)和平头炭疽菌(C.truncatum),其中胶孢炭疽菌(C. gloeosporioides)为优势病原菌,果生炭疽菌(C.fructicola)、喀斯特炭疽菌(C.karstii)、短孢炭疽菌(C.brevisporum)和平头炭疽菌(C.truncatum)是琯溪蜜柚炭疽病的新病原。5种琯溪蜜柚炭疽病病原菌的菌丝生长速率及附着胞形成率与致病力均呈弱相关性。
[1] 赖宝春,姚锦爱.蜜柚间座壳黑点病菌(Diaporthe citri)LAMP 可视化检测技术的建立[J].福建农业学报,2022,37(11):1470-1475.LAI Baochun,YAO Jin’ai. Establishment of a LAMP assay for rapid detecting Diaporthe citri on pomelo[J]. Fujian Journal of Agricultural Sciences,2022,37(11):1470-1475.
[2] 陶晶霞,王玉雯,李晓娜,张利军,张建翔,张华,廖文强,姜玉英,吴良泉,李延,郭九信.琯溪蜜柚地上部新生器官生物量和钙镁养分累积特征[J].果树学报,2024,41(1):101-112.TAO Jingxia,WANG Yuwen,LI Xiaona,ZHANG Lijun,ZHANG Jianxiang,ZHANG Hua,LIAO Wenqiang,JIANG Yuying,WU Liangquan,LI Yan,GUO Jiuxin.Accumulation dynamics of biomass,Ca and Mg nutrient in the aboveground newborn organs of Guanximiyou pomelo tree[J]. Journal of Fruit Science,2024,41(1):101-112.
[3] 赖宝春,姚锦爱.福建蜜柚炭疽病菌的生物学特性及高效防治药剂筛选[J].福建农业学报,2022,37(6):789-793.LAI Baochun,YAO Jin’ai.Biological characteristics of Colletotrichum gloeosporioides and fungicides for disease control on honey pomelo in Fujian[J]. Fujian Journal of Agricultural Sciences,2022,37(6):789-793.
[4] 赖宝春,吴顺章,郑春明,王家瑞.琯溪蜜柚炭疽病病原鉴定[J].果树学报,2017,34(9):1178-1184.LAI Baochun,WU Shunzhang,ZHENG Chunming,WANG Jiarui.Identification of pathogen causing Guanxi honey pomelo anthracnose in Pinghe county of Fujian province[J]. Journal of Fruit Science,2017,34(9):1178-1184.
[5] HUANG F,CHEN G Q,HOU X,FU Y S,CAI L,HYDE K D,LI H Y. Colletotrichum species associated with cultivated citrus in China[J].Fungal Diversity,2013,61(1):61-74.
[6] 刘欢欢.四川省柑橘炭疽病病原鉴定和遗传多样性分析[D].雅安:四川农业大学,2020.LIU Huanhuan. Colletotrichum species and genetic diversity of cultivated citrus in Sichuan province[D]. Ya’an:Sichuan Agricultural University,2020.
[7] WANG W X,DE SILVA D D,MOSLEMI A,EDWARDS J,ADES P K,CROUS P W,TAYLOR P W J. Colletotrichum species causing anthracnose of citrus in Australia[J].Journal of Fungi,2021,7(1):47.
[8] LIMA W G,SPÓSITO M B,AMORIM L,GONÇALVES F P,DE FILHO P A M. Colletotrichum gloeosporioides,a new causal agent of citrus post-bloom fruit drop[J]. European Journal of Plant Pathology,2011,131(1):157-165.
[9] AIELLO D,CARRIERI R,GUARNACCIA V,VITALE A,LAHOZ E,POLIZZI G. Characterization and pathogenicity of Colletotrichum gloeosporioides and C. karstii causing preharvest disease on citrus sinensis in Italy[J].Journal of Phytopathology,2015,163(3):168-177.
[10] RAMOS A P,TALHINHAS P,SREENIVASAPRASAD S,OLIVEIRA H. Characterization of Colletotrichum gloeosporioides,as the main causal agent of Citrus anthracnose,and C.karstii as species preferentially associated with lemon twig dieback in Portugal[J].Phytoparasitica,2016,44(4):549-561.
[11] DAMM U,SATO T,ALIZADEH A,GROENEWALD J Z,CROUS P W. The Colletotrichum dracaenophilum,C. magnum and C.orchidearum species complexes[J].Studies in Mycology,2019,92:1-46.
[12] 李少卡,赵亚,王祥和,胡福初,陈哲,范鸿雁.海南荔枝炭疽病病原菌鉴定及遗传多样性分析[J].农业生物技术学报,2021,29(4):673-687.LI Shaoka,ZHAO Ya,WANG Xianghe,HU Fuchu,CHEN Zhe,FAN Hongyan. Identification and genetic diversity analysis of Litchi chinensis Colletotrichum spp.in Hainan[J].Journal of Agricultural Biotechnology,2021,29(4):673-687.
[13] MORIWAKI J,TSUKIBOSHI T,SATO T.Grouping of Colletotrichum species in Japan based on rDNA sequences[J].Journal of General Plant Pathology,2002,68(4):307-320.
[14] WEIR B S,JOHNSTON P R,DAMM U. The Colletotrichum gloeosporioides species complex[J].Studies in Mycology,2012,73:115-180.
[15] DAMM U,CANNON P F,WOUDENBERG J H C,CROUS P W. The Colletotrichum acutatum species complex[J]. Studies in Mycology,2012,73:37-113.
[16] WHITE T J,BRUNS T,LEE S,TAYLOR J.Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics[M]//INNIS M A,GELFAND D H,SNINSKY J J,WHITE T J. PCR Protocols:A Guide to Methods and Applications.Amsterdam:Academic Press,1990:315-322.
[17] CARBONE I,KOHN L M.A method for designing primer sets for speciation studies in filamentous ascomycetes[J].Mycologia,1999,91(3):553-556.
[18] GLASS N L,DONALDSON G C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes[J]. Applied and Environmental Microbiology,1995,61(4):1323-1330.
[19] GUERBER J C,LIU B,CORRELL J C,JOHNSTON P R.Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns,mtDNAand intron RFLPs,and mating compatibility[J].Mycologia,2003,95(5):872-895.
[20] STEPHENSON S A,GREEN J R,MANNERS J M,MACLEAN D J. Cloning and characterisation of glutamine synthetase from Colletotrichum gloeosporioides and demonstration of elevated expression during pathogenesis on Stylosanthes guianensis[J].Current Genetics,1997,31(5):447-454.
[21] GUARNACCIA V,GROENEWALD J Z,POLIZZI G,CROUS P W. High species diversity in Colletotrichum associated with citrus diseases in Europe[J].Persoonia,2017,39:32-50.
[22] 田宇曦,闵勇,陈凌,刘晓艳.柑橘炭疽病分类鉴定和防治研究进展[J].中南农业科技,2023(12):230-232.TIAN Yuxi,MIN Yong,CHEN Ling,LIU Xiaoyan. Research progress on classification,identification,and control of citrus anthracnose[J]. South-Central Agricultural Science and Technology,2023(12):230-232.
[23] PENG LJ,YANGYL,KEVIN D H,BAHKALIAH,LIU ZY.Colletotrichum species on citrus leaves in Guizhou and Yunnan provinces,China[J].Cryptogamie,Mycologie,2012,33(3):267-283.
[24] CHENG B P,HUANG Y H,PENG A T,LING J F,SONG X B,CHEN X.First report of leaf and fruit spot of Citrus reticulata Blanco cv. Nian Ju caused by Colletotrichum truncatum in China[J].Plant Disease,2014,98(3):422.
[25] 谢桃明.四川主栽柑橘品种叶片潜伏炭疽菌研究[D].雅安:四川农业大学,2019.XIE Taoming.Study on the latent Colletotrichum in the main citrus leaves in Sichuan province[D]. Ya’an:Sichuan Agricultural University,2019.
[26] SHIVAS R G,TAN Y P,EDWARDS J,DINH Q,MAXWELL A,ANDJIC V,LIBERATO J R,ANDERSON C,BEASLEY D R,BRANSGROVE K,COATES L M,COWAN K,DANIEL R,DEAN J R,LOMAVATU M F,MERCADO-ESCUETA D,MITCHELL R W,THANGAVEL R,TRAN-NGUYEN L T T,WEIR B S. Colletotrichum species in Australia[J]. Australasian Plant Pathology,2016,45(5):447-464.
[27] 王葵娣,王文华,郑服丛.炭疽菌附着胞的研究进展[J].中国农学通报,2007,23(1):265-270.WANG Kuidi,WANG Wenhua,ZHENG Fucong. Advance research on appressorium of Colletotrichum[J]. Chinese Agricultural Science Bulletin,2007,23(1):265-270.
[28] 李菲菲,龙超安.柑橘炭疽病菌的分离、鉴定及在果实上的潜伏侵染特性[J].果树学报,2015,32(1):108-114.LI Feifei,LONG Chao’an.Isolation,identification and latent infection characteristics of citrus anthracnose pathogens[J]. Journal of Fruit Science,2015,32(1):108-114.
[29] MAYORQUIN J S,NOURI M T,PEACOCK B B,TROUILLAS F P,DOUHAN G W,KALLSEN C,ESKALEN A. Identification,pathogenicity,and spore trapping of Colletotrichum karstii associated with twig and shoot dieback in California[J].Plant Disease,2019,103(7):1464-1473.
[30] RIOLO M,ALOI F,PANE A,CARA M,CACCIOLA S O.Twig and shoot dieback of citrus,a new disease caused by Colletotrichum species[J].Cells,2021,10(2):449.
[31] 韦运谢,刘晓妹,张贺,张欣,漆艳香,谢艺贤,陆英,曹申文,蒲金基. 杧果炭疽病菌漆酶基因lac1 的克隆与序列特征分析[J].果树学报,2013,30(2):202-206.WEI Yunxie,LIU Xiaomei,ZHANG He,ZHANG Xin,QI Yanxiang,XIE Yixian,LU Ying,CAO Shenwen,PU Jinji.Cloning and sequence analysis of laccase gene lac1 from Colletotrichum gloeosporioides on mango[J]. Journal of Fruit Science,2013,30(2):202-206.
[32] YONGHY,BAKARFDA,ILLIASRM,MAHADINM,MURAD A M A.Cgl-SLT2 is required for appressorium formation,sporulation and pathogenicity in Colletotrichum gloeosporioides[J].Brazilian Journal of Microbiology,2014,44(4):1241-1250.
[33] SANDERS G M,KORSTEN L. Comparison of cross inoculation potential of South African avocado and mango isolates of Colletotrichum gloeosporioides[J]. Microbiological Research,2003,158(2):143-150.
[34] PRIHASTUTI H,CAI L,CHEN H,MCKENZIE E H C,HYDE K D. Characterization of Colletotrichum species associated with coffee berries in Northern Thailand[J]. Fungal Diversity,2009,39:89-109.
[35] 亓政良,徐芳菲,王先洪,傅敏,王利平,洪霓,王国平.福建李叶斑病病原菌种类鉴定及致病性研究[J].果树学报,2023,40(11):2423-2434.QI Zhengliang,XU Fangfei,WANG Xianhong,FU Min,WANG Liping,HONG Ni,WANG Guoping. Identification and pathogenicity of pathogenic species of plum leaf spot disease in Fujian[J].Journal of Fruit Science,2023,40(11):2423-2434.
[36] 张琳,彭琳,邵郅伟,付长然,高洁,刘丽萍.南瓜炭疽病菌Colletotrichum brevisporum 生物学特性及药剂防治[J].植物保护,2021,47(4):59-65.ZHANG Lin,PENG Lin,SHAO Zhiwei,FU Changran,GAO Jie,LIU Liping. Biological characteristics and indoor fungicide screening of Colletotrichum brevisporum causing pumpkin anthracnose[J].Plant Protection,2021,47(4):59-65.
[37] DE ALMEIDA L B,MATOS K S,ASSIS L A G,HANADA R E,DA SILVA G F. First report of anthracnose of Capsicum chinense in Brazil caused by Colletotrichum brevisporum[J]. Plant Disease,2017,101(6):1035.
[38] DAMM U,CANNON P F,WOUDENBERG J H C,JOHNSTON P R,WEIR B S,TAN Y P,SHIVAS R G,CROUS P W.The Colletotrichum boninense species complex[J]. Studies in Mycology,2012,73:1-36.
[39] RAMOS A M,TADIC L F,CINTO I,CARMONA M,GALLY M. Molecular characterization of Colletotrichum species causing soybean anthracnose in Argentina[J]. Mycotaxon,2013,123(1):457-465.
[40] 张玉杰,孙文秀,唐利华,黄穗萍,莫贱友,郭堂勋,陈小林,黄辉晔,李其利.广西番木瓜炭疽病的病原菌鉴定[J].植物病理学报,2023,53(3):518-521.ZHANG Yujie,SUN Wenxiu,TANG Lihua,HUANG Suiping,MO Jianyou,GUO Tangxun,CHEN Xiaolin,HUANG Huiye,LI Qili. Identification of the pathogen of Papaya anthracnose in Guangxi[J].Acta Phytopathologica Sinica,2023,53(3):518-521.