质地作为衡量果实品质的一个重要因素,不仅影响果实口感和消费者选择,而且对后期果实贮藏和运输有较大影响,决定果树产业的发展。引起果实质地变化的外部因素主要有温度、机械伤害、光照等,内部因素主要有基因、酶、转录因子、激素等。果实的质地变化通常表现为软化、硬化、糊状、粉状和脆性等,软化是成熟新鲜果实最显著且不可逆转的特征之一,也有一些果实如番木瓜、枇杷和梨等在收获后质地硬化[1]。果实组织的渗透状态和细胞壁结构重塑可能是质地变化的主要原因,质地变化过程涉及多种细胞壁降解酶、修饰酶的活性变化[2]。桃果实采后成熟过程中多聚半乳糖醛酸酶(PG)活性升高,且PG活性越高,软化越快[3];杧果的软化过程中伴随着细胞壁果胶酶(PE)活性的持续升高,说明PE在控制杧果软化过程中起主要作用[4]。木聚糖酶基因与草莓果实软化相关[5],其在木瓜成熟和软化过程中起主要作用[6]。植物激素乙烯抑制猕猴桃Ade-miR164 及其前体miRNA(Ade-MIR164b)的表达,且转录因子AdNAC6、AdNAC7(均为miR164的预测靶标)表达量升高,AdNAC6 和AdNAC7 蛋白作为转录激活因子,并与乙烯合成酶基因的启动子结合[7];ABA 则通过调控乙烯合成酶和信号蛋白基因(ACS1、ACO1、ETR2、ERF2)的表达,进而影响杧果、桃果实成熟与软化进程[4,8]。果实质地变化受多基因协同调控,分别沉默细胞壁修饰基因如SlPG2a、SlPME2、SlTBG4、SlCEL2、SlEXP1,番茄果实质地只有轻微或无变化[9];而同时沉默SlPG2a、Sl-EXP1基因,其果实在整个成熟期显著变硬[10]。
果实质地与木质素含量之间存在较强的关联性。对于大多数果实来说,木质素含量会随着果实成熟软化逐渐降低,胁迫状态下则会导致木质素含量升高。光氧化胁迫与高温协同作用,刺激苹果果实引发多种防御机制,导致木质素含量升高、组织的生理生化和形态改变,形成高硬度果肉[11]。木质素生物合成涉及一系列限速酶、关键酶,包括PAL、COMT、4CL、CAD、CCR和POD等,CCR是木质素单体形成的关键酶,Ⅲ类POD在木质素的聚合化过程中起着重要作用[12]。转录因子通过调控木质素合成重要基因的功能,促进或抑制木质素合成,改变果实质地[13]。杨树ERF139 抑制维管束射线形成和加快木质素积累,次生细胞壁合成、盐和干旱胁迫响应基因是其潜在靶基因[14]。PbrMYB4通过结合启动子区域的AC-I元件激活Pbr4CL1基因表达,显著提高梨果实木质素含量且木质部和木纤维细胞壁增厚,沉默该基因则降低木质素含量[15]。葡萄VibZIP14转录因子与VlCOMT基因启动子区域的G-box结合,直接激活该基因表达,参与木质素合成[16]。
桑葚作为一种呼吸跃变型水果,在成熟后期,果实质地发生剧烈变化,表现为口感软化且伴随着果色、黄酮类物质含量的改变。截至目前,关于桑葚的研究大多数聚焦于成分分析、发酵、采后贮藏等方面,未见其质地、木质素变化及影响二者的分子机制报道。结合其他果实研究成果,推测木质素参与桑葚成熟过程中的质地变化,较多木质素不利于桑葚口感的形成。高通量测序具有成本低、数据量大等优点,有助于揭示果实质地变化的潜在分子机制。转录组测序结果表明,不同品种西瓜间的质地差异可能主要与果胶、纤维素、半纤维素有关,另外激素、转录因子、过氧化物酶等相关基因也可能参与质地变化进程[17]。基于转录组的加权基因共表达网络分析发现,PAL、HCT、4CL2、C4H 等11 个基因是柚子果实汁胞粒化过程中最显著的差异表达基因[18];不同质地的刺梨与柑橘果实转录组测序数据均鉴定到木质素合成通路基因[19-20]。笔者在本研究中以3个不同发育阶段的桑葚为试验材料,通过测定果实的质地变化及木质素含量,结合转录组测序鉴定的差异表达基因,分析桑葚果实质地变化的生理与潜在的分子机制,为解析桑葚成熟过程中的质地软化机制提供参考。
1 材料和方法
1.1 材料
果桑品种为安葚,桑树种植于承德医学院蚕桑科技园。2018 年6—7 月收集桑葚,分别于开花后20 d(绿果期,Mulberry Green Fruits,MGF)、35 d(红果期,Mulberry Red Fruits,MRF)、45 d(黑果期,八至九成熟,Mulberry Black Fruits,MBF)采集大小一致、无病虫害果实(图1),每个时期取3 个生物学重复,每个重复采集30 个果实,清理表面后迅速投入液氮中,于-80 ℃保存。转录组测序工作由北京康普森生物公司完成。

图1 桑葚果实不同成熟时期
Fig.1 The different development phases of mulberry fruits
1.2 果实质地及木质素含量的测定
果实质地特性指标(硬度、黏附性、内聚性、弹性、胶黏性、咀嚼性)用装有直径5 mm探头的质构仪(TMS-PRO,美国FTC 公司)测定[21];木质素采用浓硫酸法测定[22]。
1.3 总RNA提取、文库制备及转录组测序
使用RNAprep Pure Plant Kit(天根,北京)提取桑葚的总RNA,同时利用Nano Drop 和Agilent 2 100评估其纯度、浓度和完整性。提取总RNA后,用带有Oligo(dT)的磁珠富集真核生物mRNA,随后使用PrimeScriptTM ⅡFirst cDNA 合成试剂盒(TaKa-Ra,美国)合成第一链cDNA,并使用随机引物合成第二条cDNA 链,然后经过QiaQuick PCR 试剂盒纯化之后做末端修复并连接测序接头,在Illumina HiSeq 2500 上测序。对获得的数据经过质控过滤,得到clean reads,将clean reads从头组装成Unigene。
1.4 桑葚转录组木质素合成相关基因的表达分析
使用RSEM(v.1.3.0)软件将基因表达归一化为RPKM 值,采用DEseq(v1.20.0)软件以log2(Fold-Change)|≥1 & Padj≤0.05 为标准,对Unigene 表达进行差异分析,筛选出显著差异表达的基因(DEGs)。通过对差异基因KEGG 代谢通路途径的分析,筛选与木质素合成代谢途径相关的基因,利用TBtools软件绘制热图。
1.5 qRT-PCR验证
选择木质素合成通路中的10 个基因(C4H2、C4H7、PAL1、PAL3、4CL2、POD2、POD6、POD14、CCR1、CCoAOMT2),以桑树Ribosomal protein L 15为内参基因,使用Primer Premier5.0软件设计特异性引物(表1)。每个反应体系为10 μL,包含SYBR Green Master Mix 5 μL、正、反向引物各0.2 μL,cDNA 0.5 μL,ddH2O 4.1 μL。扩增反应用荧光定量PCR 仪(伯乐CFX96,美国)进行,扩增条件如下:95 ℃1 min,95 ℃10 s,50 ℃30 s,循环40次,熔解曲线采用仪器默认程序收集。每个样品3次生物学重复,3 次技术重复,使用2-ΔΔCt方法进行相对定量计算。差异显著性分析使用IBM SPSS 23.0软件(P<0.05)。
表1 用于qRT-PCR 验证的引物序列
Table 1 Primer sequences of qRT-PCR
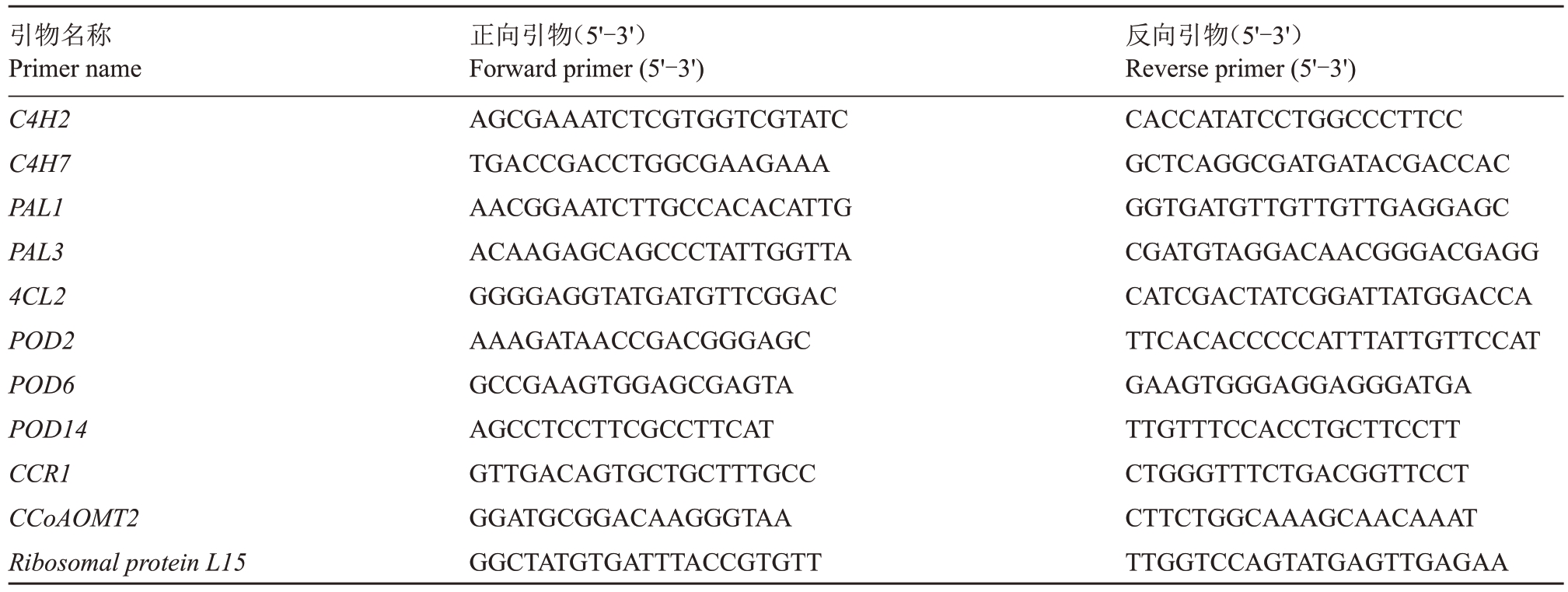
引物名称Primer name C4H2 C4H7 PAL1 PAL3 4CL2 POD2 POD6 POD14 CCR1 CCoAOMT2 Ribosomal protein L15正向引物(5'-3')Forward primer(5'-3')AGCGAAATCTCGTGGTCGTATC TGACCGACCTGGCGAAGAAA AACGGAATCTTGCCACACATTG ACAAGAGCAGCCCTATTGGTTA GGGGAGGTATGATGTTCGGAC AAAGATAACCGACGGGAGC GCCGAAGTGGAGCGAGTA AGCCTCCTTCGCCTTCAT GTTGACAGTGCTGCTTTGCC GGATGCGGACAAGGGTAA GGCTATGTGATTTACCGTGTT反向引物(5'-3')Reverse primer(5'-3')CACCATATCCTGGCCCTTCC GCTCAGGCGATGATACGACCAC GGTGATGTTGTTGTTGAGGAGC CGATGTAGGACAACGGGACGAGG CATCGACTATCGGATTATGGACCA TTCACACCCCCATTTATTGTTCCAT GAAGTGGGAGGAGGGATGA TTGTTTCCACCTGCTTCCTT CTGGGTTTCTGACGGTTCCT CTTCTGGCAAAGCAACAAAT TTGGTCCAGTATGAGTTGAGAA
1.6 木质素与果实质地相关性分析
冗余分析(RDA)使用CANOCO 5.0 软件完成。
2 结果与分析
2.1 不同成熟期桑葚木质素含量及质地的变化
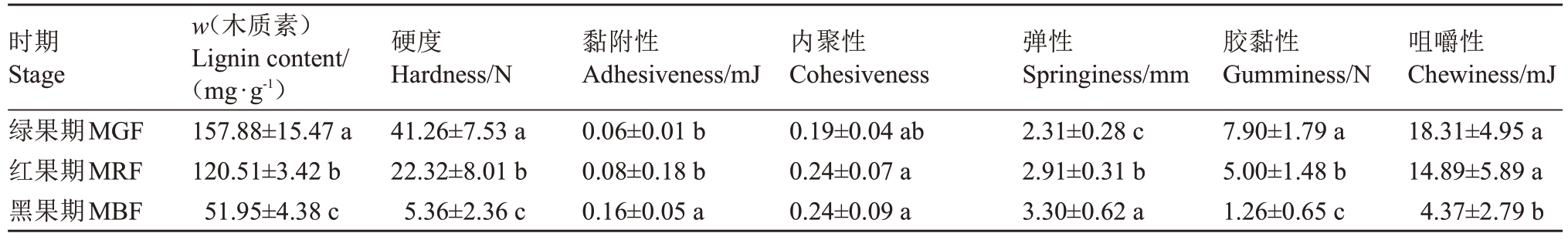
对绿果期(MGF)、红果期(MRF)和黑果期(MBF)3 个时期桑葚的木质素含量及质地进行比较。结果(表2)表明,桑葚木质素含量从绿果期至黑果期显著降低,其中绿果期的木质素含量是黑果期的3 倍。桑葚硬度随着果实成熟呈逐渐下降趋势,各发育时期差异显著,绿果期硬度分别为红果期和黑果期的1.85倍和7.70倍。桑葚弹性随果实成熟度增加逐渐升高,各发育时期之间差异显著。胶黏性和咀嚼性均随着果实成熟而逐渐下降,变化规律与果实木质素含量和硬度趋势一致,其中果实咀嚼性从绿果期到红果期无显著差异,分别是黑果期的4.19 倍和3.41 倍,均与黑果期差异显著。果实的黏附性在绿果期和红果期无显著差异,其他发育时期之间差异显著。果实的内聚性随着果实的成熟没有显著变化。
表2 不同成熟期桑葚果实质地特征参数比较
Table 2 Comparison of texture parameters detected from mulberry during different development phases
注:不同小写字母表示差异显著(P<0.05)。Note:Different small letters significant difference at P<0.05.
时期Stage绿果期MGF红果期MRF黑果期MBF w(木质素)Lignin content/(mg·g-1)157.88±15.47 a 120.51±3.42 b 51.95±4.38 c咀嚼性Chewiness/mJ 18.31±4.95 a 14.89±5.89 a 4.37±2.79 b硬度Hardness/N 41.26±7.53 a 22.32±8.01 b 5.36±2.36 c黏附性Adhesiveness/mJ 0.06±0.01 b 0.08±0.18 b 0.16±0.05 a内聚性Cohesiveness 0.19±0.04 ab 0.24±0.07 a 0.24±0.09 a弹性Springiness/mm 2.31±0.28 c 2.91±0.31 b 3.30±0.62 a胶黏性Gumminess/N 7.90±1.79 a 5.00±1.48 b 1.26±0.65 c
2.2 桑葚转录组测序数据分析
对3个不同时期桑葚样品进行高通量转录组测序(表3),共获得73.39 Gb clean date,每个样品平均clean date 为8.16 Gb,各样品Q20 碱基百分比在97.61%~97.91%之间(>97%),Q30 碱基百分比在93.60%~94.13%之间(>93%),GC 含量在45.63%~46.41%(>45%),测序错误率均在0.02%左右,表明整体测序过滤质量良好,可用于后续的转录组分析。总计获得51 895 个Unigenes,总长度约为60 533 758 bp,N50 长度为1885 bp,序列平均长度为1166 bp,Unigenes长度在300~400 bp区间的数量最多,为10 609 个,占比20.44%(图2),生成的转录组数据可以满足后续试验要求。
表3 9 个样本转录组数据质控
Table 3 Summary of 9 samples sequencing data quality
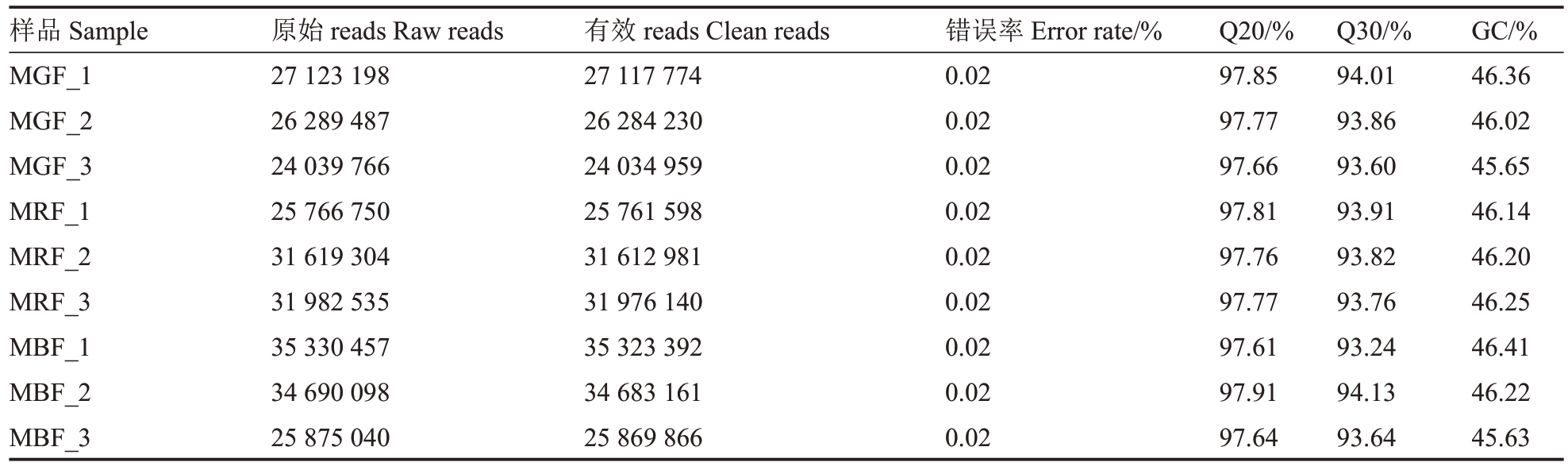
样品Sample MGF_1 MGF_2 MGF_3 MRF_1 MRF_2 MRF_3 MBF_1 MBF_2 MBF_3原始reads Raw reads 27 123 198 26 289 487 24 039 766 25 766 750 31 619 304 31 982 535 35 330 457 34 690 098 25 875 040有效reads Clean reads 27 117 774 26 284 230 24 034 959 25 761 598 31 612 981 31 976 140 35 323 392 34 683 161 25 869 866错误率Error rate/%0.02 0.02 0.02 0.02 0.02 0.02 0.02 0.02 0.02 Q20/%97.85 97.77 97.66 97.81 97.76 97.77 97.61 97.91 97.64 Q30/%94.01 93.86 93.60 93.91 93.82 93.76 93.24 94.13 93.64 GC/%46.36 46.02 45.65 46.14 46.20 46.25 46.41 46.22 45.63
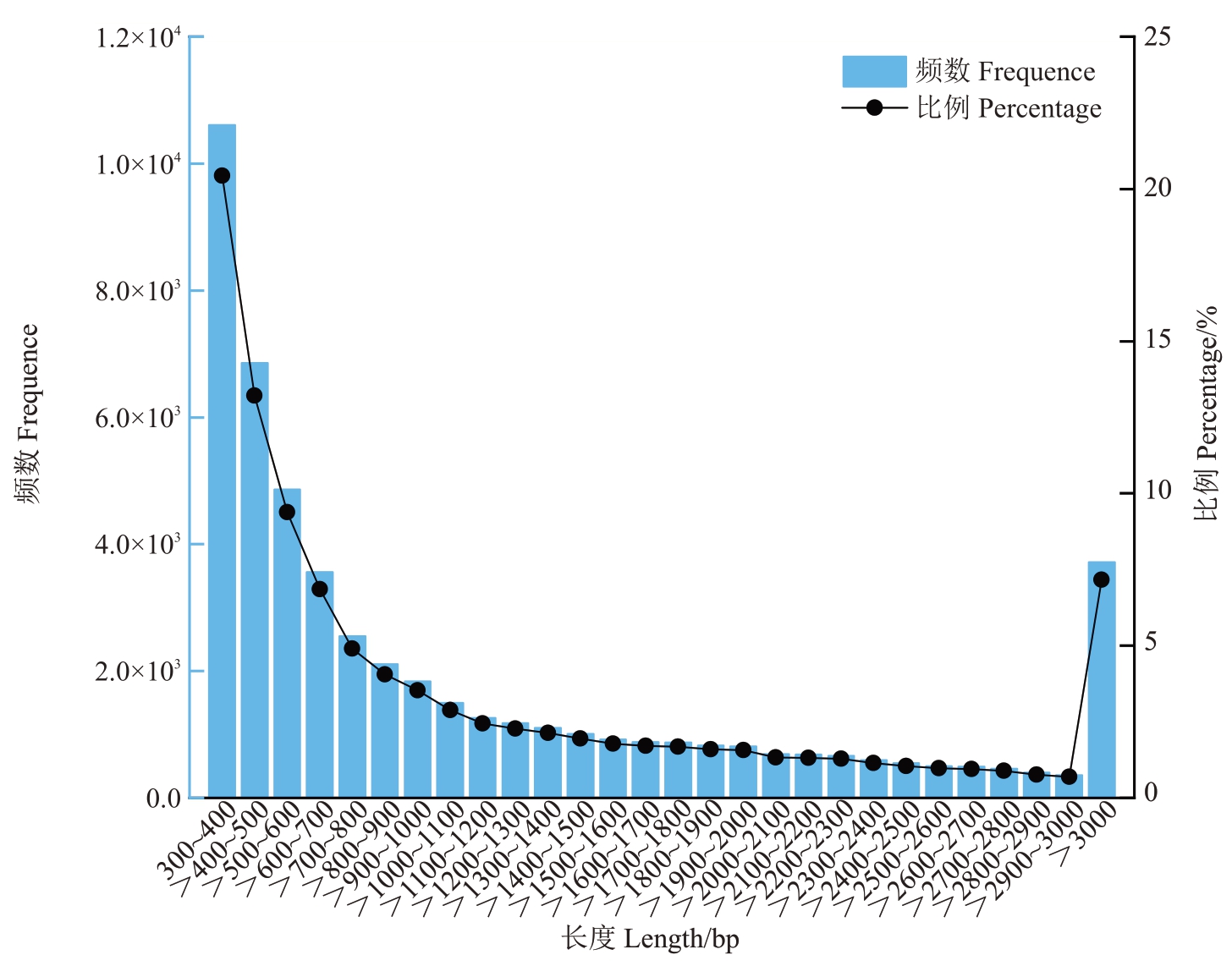
图2 Unigene 长度分布
Fig.2 Length distribution of Unigene
2.3 不同成熟期桑葚差异表达基因分析与功能注释
3 个发育时期桑葚共表达的Unigene 有36 385个,绿果期和黑果期特有表达Unigene均为5343个,而红果期特有表达Unigene 为3189 个(图3-A)。采用DESeq 2 软件以|log2(FoldChange)| ≥1 & FDR≤0.05 为筛选条件对不同发育时期基因Unigene 表达进行差异分析,筛选差异表达基因(DEGs)。结果表明,3 个比较组共有10 207 个DEGs,红果期相较于绿果期,黑果期相较于绿果期,黑果期相较于红果期,共有656 个共表达的DEGs(图3-B)。通过比较绿果期和红果期DEGs,共获得8572 个DEGs,其中上调基因有4353 个、下调基因4219 个;比较绿果期和黑果期的DEGs,共获得1900 个DEGs,其中上调基因有1140个、下调基因760个;比较红果期和黑果期的DEGs,共获得6716 个DEGs,其中上下调基因分别有3599 个、3117 个。3 个比较组中上调DEGs数目均多于下调DEGs数目,且DEGs最多的是绿果期和红果期的比较组(图3-B~D)。
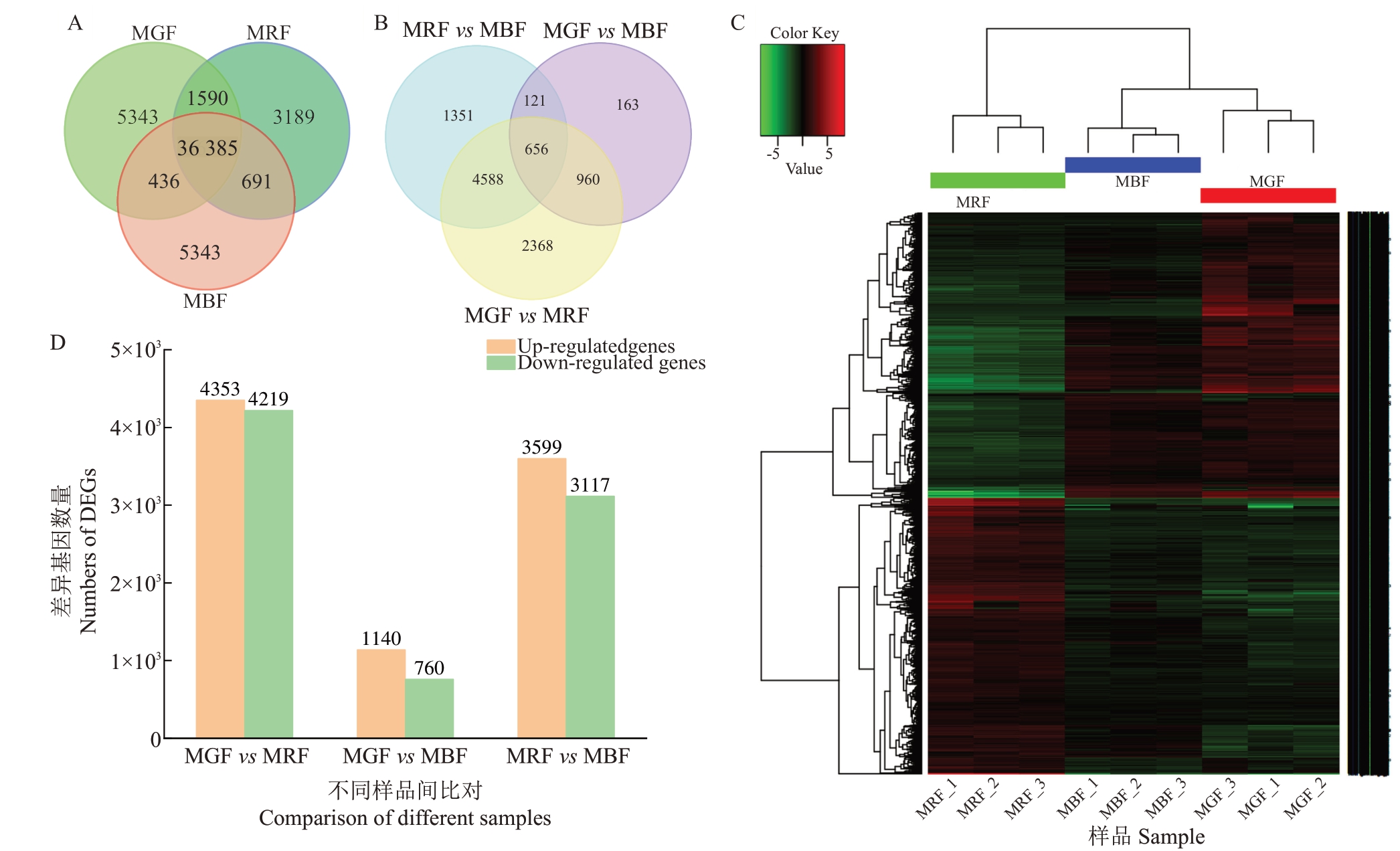
图3 韦恩图及DEGs 表达模式
Fig.3 Venn diagram and expression pattern of DEG
A.不同发育阶段Unigene 维恩图;B.不同发育阶段DEGs 维恩图;C.DEGs 的表达模式;D.DEGs 统计图。
A.Venn diagram of Unigene detected from different samples; B.Venn diagram of DEGs among different development stages; C. Expression pattern of DEGs;D.DEGs statistics.
2.4 与木质素合成相关基因的表达分析
通过对3 个发育时期桑葚中与木质素相关的DEGs进行筛选,共鉴定到45个DEGs(图4),并对其FPKM值绘制热图分析。45个DEGs可以聚类为3种表达模式,2个候选DEGs[TRINITY_DN45720_c2_g6(POD15)、TRINITY_DN37868_c0_g1(POD12)]表达呈逐渐上调趋势,即桑葚成熟过程中2个基因的FPKM 值逐渐升高。19个候选DEGs 表现为在红果期高表达,在绿果期和黑果期低表达,FPKM值总体表现为先上调后下调的趋势。24个候选DEGs在绿果期高表达,在红果期和黑果期表现为低表达,整个发育时期表现为下调趋势,即桑葚转色期时低表达,与桑葚果实发育过程中3个时期的木质素含量和质地指标变化趋势相一致。
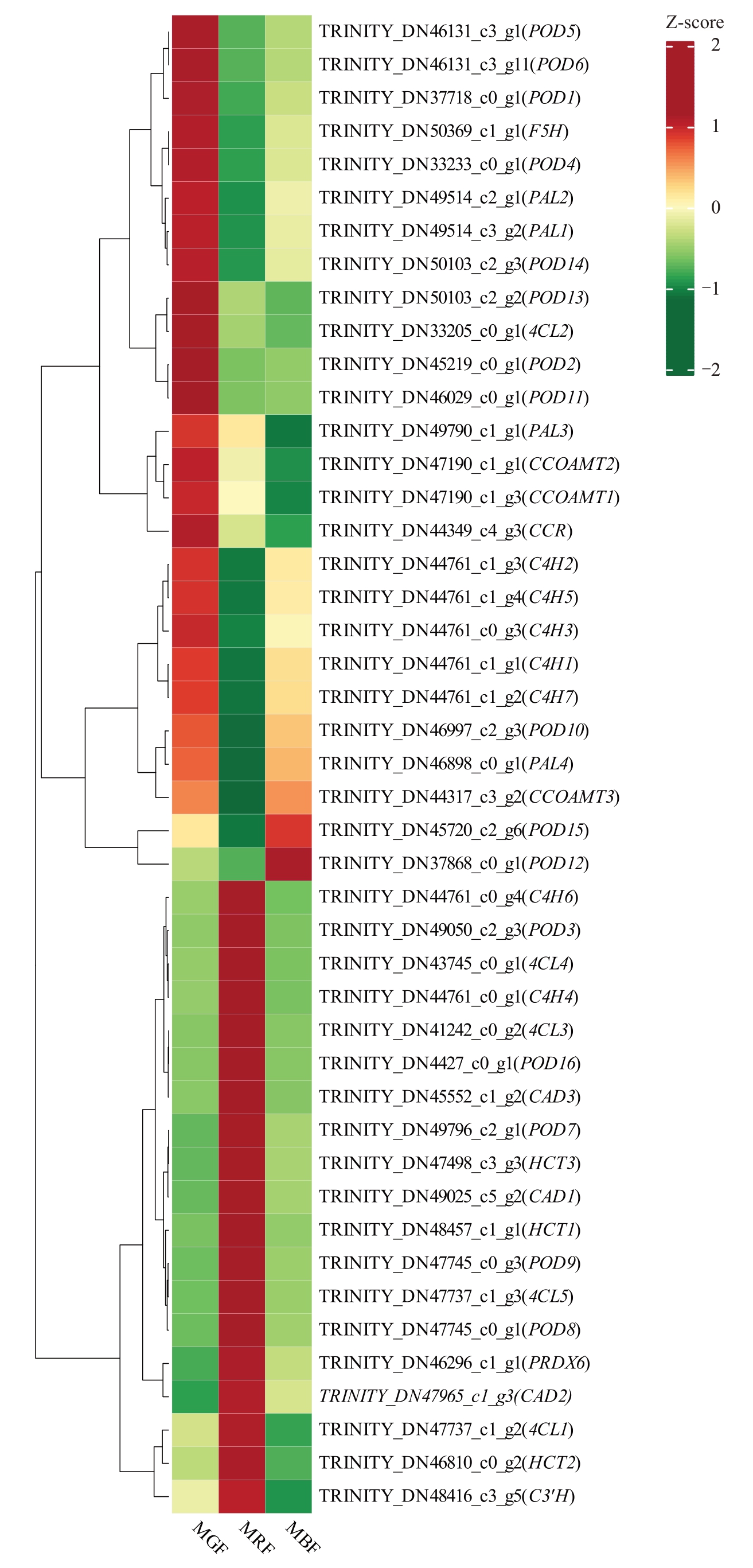
图4 桑葚木质素合成相关差异基因的聚类热图
Fig.4 Clustering and heatmap of DEGs related to lignin biosynthesis in mulberry
对苯丙烷代谢通路分析发现(图5),上游木质素合成通路中鉴定出4 个苯丙氨酸解氨酶(PAL1、PAL2、PAL3、PAL4)DEGs、5 个肉桂酸-4-羟化酶(C4H1、C4H2、C4H3、C4H5、C4H7)DEGs、1 个4-香豆酸辅酶-A-连接酶(4CL2)DEGs、1 个阿魏-5-羟化酶(F5H)DEGs、3 个咖啡酰辅酶A 3-O-甲基转移酶(CCoAOMT1、CCoAOMT2、CCoAOMT3)DEGs、1 个肉桂酰辅酶A 还原酶(CCR)DEGs,共计15 个DEGs,且均显著下调,与木质素含量和质地品质指标变化趋势相一致。下游代谢物木质素(lignin)、紫丁香基木质素(syringyl lignin)、对羟基苯酚木质素(p-Hydroxyphenyl lignin)、愈创木酚木质素(Guaiacyl lignin)和5-羟基愈创木酚木质素(5-Hydroxyguaiacyl lignin)等合成通路中鉴定到16个DEGs,其中9 个过氧化氢酶(POD1、POD2、POD4、POD5、POD6、POD10、POD11、POD13、POD14)DEGs 在绿果期高表达,在转色期低表达,并且和木质素含量和质地品质指标变化趋势相一致。综合上述结果,筛选出上下游木质素合成通路中24个DEGs(PAL1、PAL2、PAL3、PAL4、C4H1、C4H2、C4H3、C4H5、C4H7、4CL2、F5H、CCoAOMT1、CCoAOMT2、CCoAOMT3、CCR、POD1、POD2、POD4、POD5、POD6、POD10、POD11、POD13、POD14),推测其可能是桑葚成熟过程中参与果实软化进程的重要基因。
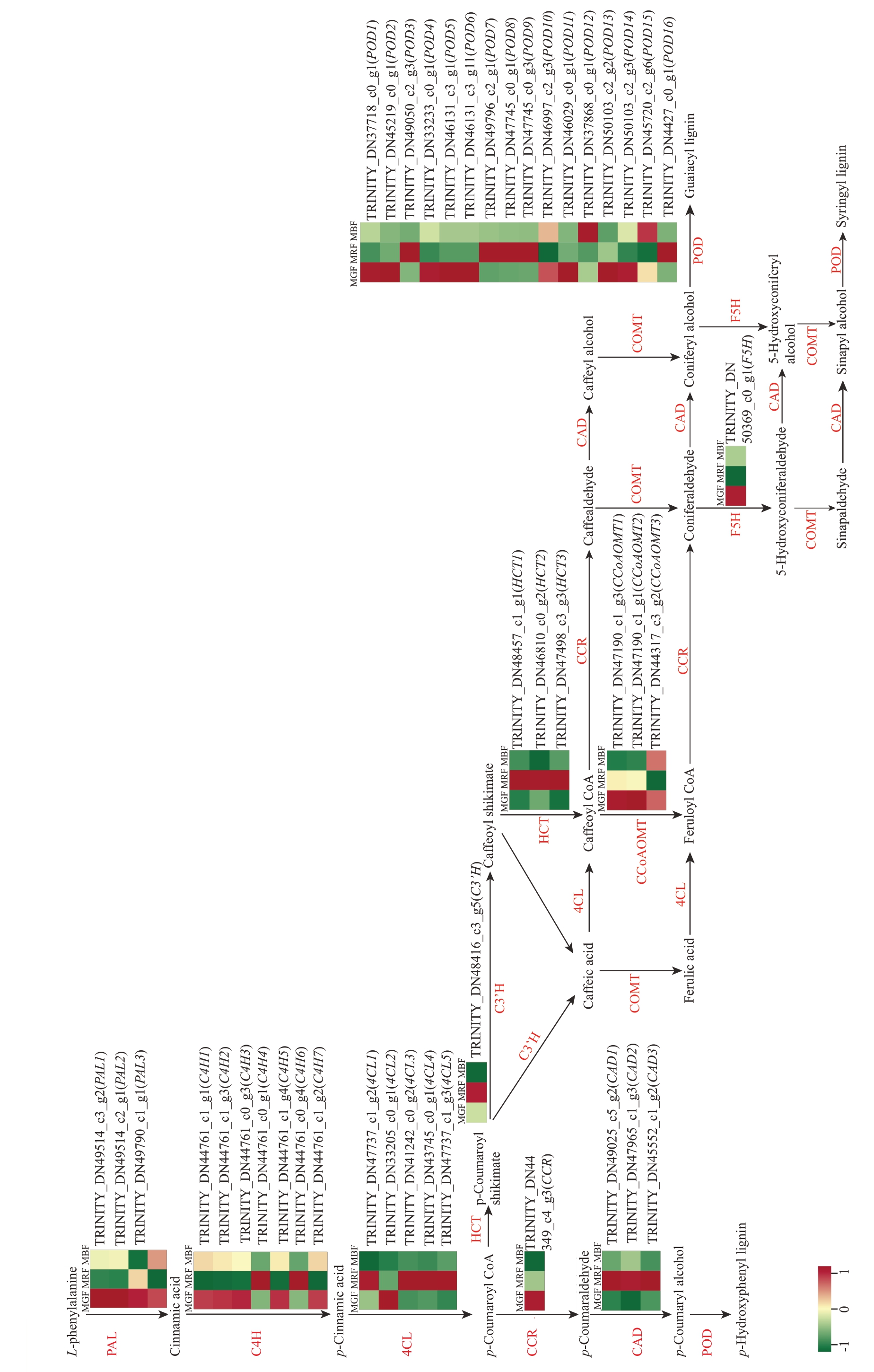
图5 木质素生物合成途径中的差异表达基因
Fig. 5 DEGs obtained in lignin biosynthesis pathway
2.5 差异表达基因的qRT-PCR验证
从木质素上下游合成通路中的24个DEGs中随机选择10 个木质素合成途径的关键DEGs 进行qRT-PCR 试验验证(图6)。随着桑葚果实的成熟,C4H7、CCR、PAL1、PAL3、POD2、POD14、CCoAOMT2等7个基因的荧光定量检测结果与转录组数据一致且均下调表达,特别是绿果期表达量均高于红果期,推测这7个基因可能负调控桑葚成熟软化进程。虽然4CL2、C4H2、POD6 等基因的qRT-PCR 检测表达量与转录组数据的变化倍数存在部分差异,但是基因表达水平的趋势是一致的,说明转录组分析可靠。

图6 qRT-PCR 验证10 个差异基因的表达情况
Fig.6 Expression levels of 10 DEGs were determined by qRT-PCR
2.6 果实质地变软的关键因素分析
通过RDA 分析木质素与6 种果实质地特性的关系,结果(图7-A)表明,木质素与果实咀嚼性呈现极显著的正相关性,其次为胶黏性和硬度,其中木质素与三者的相关性基本一致;而木质素与果实的黏附性、弹性和内聚性呈负相关。通过RDA分别分析了木质素和果实质地特性与木质素合成代谢相关基因相关性,结果(图7-B、C)表明,CCR对木质素的生成影响最大,其次为CCoAOMT1、CCoAOMT2、PAL3、C3H 和4CL2 等,POD12 和CAD2 等基因对木质素的生成无显著关联。4CL2 对果实硬度和胶黏性变化影响最大,其次为POD13、CCoAOMT1、CCoAOMT2、PAL3、C3H。CCoAOMT1 对果实的咀嚼性影响最大,其次为CCoAOMT2、PAL3、C3H。POD12 与果实硬度、胶黏性、咀嚼性无显著关联。CAD2对果实弹性、粘附性和内聚性变化影响最大,其次为CAD1、POD12、POD15。RDA分析结果与前述中基因表达模式趋势一致,证明了试验结果的可靠性。
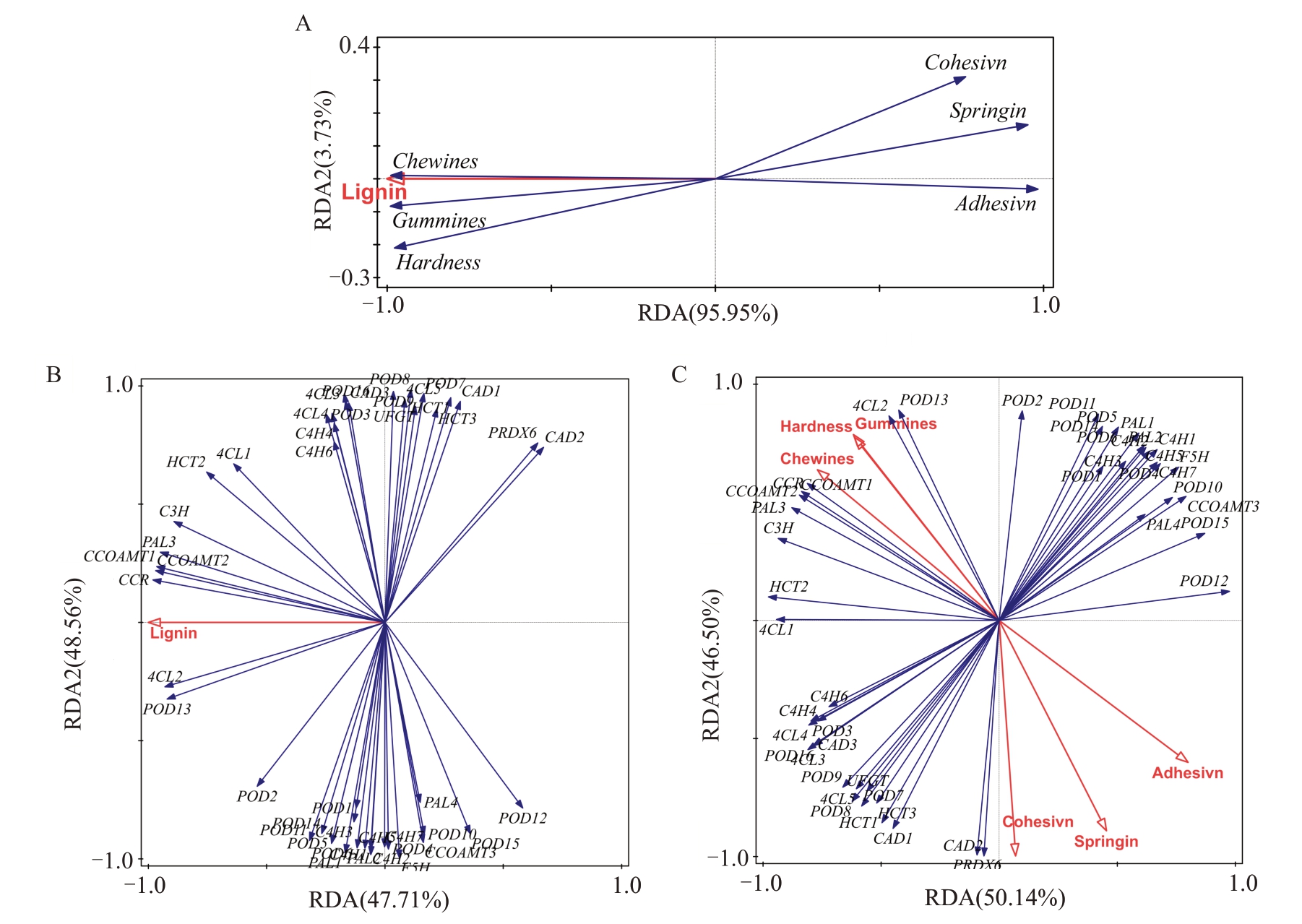
图7 RDA 分析影响质地的主要因子
Fig.7 The main factors that affected texture was identified by RDA
A.木质素含量与果实质地特性相关性;B.木质素含量与木质素合成代谢相关基因相关性;C.果实质地特性与木质素合成代谢相关基因相关性;横纵坐标分别表示在整体解释量中的重要值。
A.The correlation between lignin content and texture quality; B. Lignin content and genes related to lignin synthetic metabolism; C. Fruit texture quality and genes related to lignin synthetic metabolism.The horizontal and vertical coordinates represent the importance value in the overall interpretation volume.
3 讨 论
桑葚果实在发育前期表现为口感硬、味道差等特点,成熟期口感硬度则显著降低且风味浓郁。果实质地的评价通常采用感官评价、仪器测定两种方式。感官评价比较主观,能真正反映人对果实质地的感觉信息,仪器测量可量化、客观、数据重现性较好,理想情况下,感官评价与仪器测量相结合是识别和评价果实质地的最佳方法。围绕果实质地形成及影响因素,已有大量的报道,这一过程涉及多种物质的转化、多个基因的表达与沉默等,其生理与分子机制较复杂。高通量测序技术的特点为解析果实质地形成的分子机制提供了新的途径和视角。
木质素是细胞壁的重要组成成分之一,为细胞壁提供刚性支撑,是引起果实质地变化的主要因素之一[23-24]。Wang 等[25]研究发现,随着甜瓜木质素含量增加,其愈伤组织的硬度随之增大,而内聚性和弹性随之减小,这与本研究中桑葚木质素含量与果实硬度呈正相关、与果实内聚性和弹性呈负相关的结果相一致,推测桑葚的成熟软化与木质素含量降低存在关联性。木质素的合成主要是通过苯丙烷途径,其中PAL、C4H、4CL、CCR、POD、CCoAOMT 等基因是木质素合成途径关键调控基因,且这些基因表达量与木质素合成量呈正相关[26-27]。笔者在本研究中鉴定获得木质素合成通路中的45 个DEGs,其中24个DEGs的表达量与木质素合成含量变化趋势一致,C4H7、CCR、PAL1、PAL3、POD2、POD14、CCoAOMT2等7个基因可能协作调控桑葚成熟软化进程。PAL 是木质素生物合成途径关键限速基因,Korth等[28]研究表明,在烟草中过表达PAL基因,PAL酶活性和木质素含量均显著升高;Cai等[27]在低温储藏枇杷试验中发现,PAL 表达量与木质素含量呈正相关。本研究结果表明,PAL1、PAL2、PAL3、PAL4等4 个基因PAL 表达量与木质素含量呈正相关,其中PAL1 对木质素的合成及硬度软化影响最大且呈正相关,推测PAL 可能是参与调控木质素合成的重要基因。C4H是一类细胞色素P450基因,主要功能为调控细胞结构。Sewalt 等[29]研究发现,在烟草中抑制C4H基因表达,其细胞构成发生变化且木质素含量出现下降,C4H 表达量与木质素含量呈正相关。桑葚C4H1、C4H2、C4H3、C4H5、C4H7等5个基因表达量与木质素含量呈正相关,表明C4H基因对桑葚软化过程发挥重要作用。CCR、CCoAOMT 对木质素生成也有较大影响。CCR是木质素生物合成单信号通路中的第一个固定酶,研究发现在转基因杨树[30]、玉米[31]中CCR的活性降低,木质素含量显著下降。桑葚CCR 基因在木质素的生成中表现为正相关,且随着桑葚的成熟,CCR 表达量随之降低。CCoAOMT基因在木质素合成及组分构成中起到重要的调控作用,在亚麻植物研究中发现,抑制CCoAOMT基因的表达,木质素含量显著降低,同时植株形态表现为畸形[32]。桑葚转录组数据中共筛选到3 个CCoAOMT 差异表达基因,CCoAOMT1 和CCoAOMT2与木质素的合成呈正相关。这些结果对桑葚的质地评价具有参考意义,为优质遗传资源的有效选择和精确定位育种方法的建立提供了依据。
4 结 论
通过质地、木质素测定分析,发现桑葚果实成熟过程中的硬度等质地指标在不同的发育时期呈现不同特征,即硬度、胶黏性和咀嚼性逐渐下降,弹性逐渐升高,黏附性在成熟期显著变化,内聚性无显著变化;木质素含量逐渐降低。转录组测序数据中共鉴定到45 个木质素合成相关的差异表达基因。21 个差异基因表达量与木质素合成含量变化趋势一致且均下调表达。RDA 分析表明不同的基因与质地指标、木质素合成等的相关性不一致,说明桑葚质地形成、木质素合成是由多因素、多基因参与调控的生长发育进程,本研究结论为后续开展相关的分子机制探讨奠定了理论基础。
[1] CONTADOR L,SHINYA P,INFANTE R. Texture phenotyping in fresh fleshy fruit[J].Scientia Horticulturae,2015,193:40-46.
[2] SHI Y N,LI B J,SU G Q,ZHANG M X,GRIERSON D,CHEN K S. Transcriptional regulation of fleshy fruit texture[J].Journal of Integrative Plant Biology,2022,64(9):1649-1672.
[3] QIAN M,ZHANG Y K,YAN X Y,HAN M Y,LI J J,LI F,LI F R,ZHANG D,ZHAO C P. Identification and expression analysis of polygalacturonase family members during peach fruit softening[J]. International Journal of Molecular Sciences,2016,17(11):1933.
[4] ZAHARAH S S,SINGH Z,SYMONS G M,REID J B. Mode of action of abscisic acid in triggering ethylene biosynthesis and softening during ripening in mango fruit[J].Postharvest Biology&Technology,2013,75:37-44.
[5] HIRSCH M,LANGER S E,MARINA M,ROSLI H G,CIVELLO P M,MARTÍNEZ G A,VILLARREAL N M. Expression profiling of endo-xylanases during ripening of strawberry cultivars with contrasting softening rates. Influence of postharvest and hormonal treatments[J]. Journal of the Science of Food and Agriculture,2021,101(9):3676-3684.
[6] SHEN Y H,LU B G,FENG L,YANG F Y,GENG J J,MING R,CHEN X J.Isolation of ripening-related genes from ethylene/1-MCP treated papaya through RNA-seq[J]. BMC Genomics,2017,18(1):671.
[7] WANG W Q,WANG J,WU Y Y,LI D W,ALLAN A C,YIN X R. Genome-wide analysis of coding and non-coding RNA reveals a conserved miR164-NAC regulatory pathway for fruit ripening[J].New Phytologist,2020,225(4):1618-1634.
[8] LUO H,DAI S J,REN J,ZHANG C X,DING Y,LI Z,SUN Y F,JI K,WANG Y P,LI Q,CHEN P,DUAN C R,WANG Y,LENG P.The role of ABA in the maturation and postharvest life of a nonclimacteric sweet cherry fruit[J]. Journal of Plant Growth Regulation,2014,33(2):373-383.
[9] WANG D D,SAMSULRIZAL N H,YAN C,ALLCOCK N S,CRAIGON J,BLANCO-ULATE B,ORTEGA-SALAZAR I,MARCUS S E,BAGHERI H M,PEREZ FONS L,FRASER P D,FOSTER T,FRAY R,KNOX J P,SEYMOUR G B. Characterization of CRISPR mutants targeting genes modulating pectin degradation in ripening tomato[J]. Plant Physiology,2019,179(2):544-557.
[10] POWELL A L T,KALAMAKI M S,KURIEN P A,GURRIERI S,BENNETT A B. Simultaneous transgenic suppression of LePG and LeExp1 influences fruit texture and juice viscosity in a fresh market tomato variety[J]. Journal of Agricultural and Food Chemistry,2003,51(25):7450-7455.
[11] TORRES C A,AZOCAR C,RAMOS P,PÉREZ-DÍAZ R,SEPULVEDA G,MOYA-LEÓN M A. Photooxidative stress activates a complex multigenic response integrating the phenylpropanoid pathway and ethylene,leading to lignin accumulation in apple (Malus domestica Borkh.) fruit[J]. Horticulture Research,2020,7:22.
[12] WANG Y,ZHANG X F,YANG S L,YUAN Y B. Lignin involvement in programmed changes in peach-fruit texture indicated by metabolite and transcriptome analyses[J]. Journal of Agricultural and Food Chemistry,2018,66(48):12627-12640.
[13] ZHAO X W,WANG Q,WANG D,GUO W,HU M X,LIU Y L,ZHOU G K,CHAI G H,ZHAO S T,LU M Z.PagERF81 regulates lignin biosynthesis and xylem cell differentiation in poplar[J].Journal of Integrative Plant Biology,2023,65(5):1134-1146.
[14] WESSELS B,SEYFFERTH C,ESCAMEZ S,VAIN T,ANTOS K,VAHALA J,DELHOMME N,KANGASJÄRVI J,EDER M,FELTEN J,TUOMINEN H. An AP2/ERF transcription factor ERF139 coordinates xylem cell expansion and secondary cell wall deposition[J].New Phytologist,2019,224(4):1585-1599.
[15] LIU D L,XUE Y S,WANG R Z,SONG B B,XUE C,SHAN Y F,XUE Z L,WU J. PbrMYB4,a R2R3-MYB protein,regulates pear stone cell lignification through activation of lignin biosynthesis genes[J]. Horticultural Plant Journal,2025,11(1):105-122.
[16] YU P,LI S Q,SUN Y D,MENG X X,SHI Q F,ZHAO X C,YU Y H. Transcription factor VlbZIP14 inhibits postharvest grape berry abscission by directly activating VlCOMT and promoting lignin biosynthesis[J].International Journal of Molecular Sciences,2024,25(17):9479.
[17] GAO Y,GUO Y,SU Z Y,YU Y,ZHU Z C,GAO P,WANG X Z.Transcriptome analysis of genes related to fruit texture in watermelon[J].Scientia Horticulturae,2020,262:109075.
[18] LI X T,HUANG H T,RIZWAN H M,WANG N Y,JIANG J Y,SHE W Q,ZHENG G H,PAN H L,GUO Z X,PAN D M,PAN T F. Transcriptome analysis reveals candidate lignin- related genes and transcription factors during fruit development in pomelo(Citrus maxima)[J].Genes,2022,13(5):845.
[19] LU M,MA W T,LIU Y Q,AN H M,LUDLOW R A.Transcriptome analysis reveals candidate lignin-related genes and transcription factors in Rosa roxburghii during fruit ripening[J].Plant Molecular Biology Reporter,2020,38(2):331-342.
[20] WANG X,LIN L J,TANG Y,XIA H,ZHANG X C,YUE M L,QIU X,XU K,WANG Z H. Transcriptomic insights into citrus segment membrane’s cell wall components relating to fruit sensory texture[J].BMC Genomics,2018,19(1):280.
[21] 王彬彬,李娜,贾漫丽,陈秀灵,范伟,夏爱华,高玉军,李季生.质构仪检测桑葚质地品质的方法研究[J].果树学报,2021,38(11):2014-2020.WANG Binbin,LI Na,JIA Manli,CHEN Xiuling,FAN Wei,XIA Aihua,GAO Yujun,LI Jisheng. Measuring texture quality of mulberry fruit using a texture analyser[J].Journal of Fruit Science,2021,38(11):2014-2020.
[22] 王广龙.胡萝卜肉质根发育过程中激素和品质的变化规律研究[D].南京:南京农业大学,2016.WANG Guanglong. Change patterns of hormones and quality characters during taproot growth and development in carrot[D].Nanjing:Nanjing Agricultural University,2016.
[23] ZHAO Q,DIXON R A.Transcriptional networks for lignin biosynthesis:More complex than we thought [J]. Trends in Plant Science,2011,16(4):227-233.
[24] COSGROVE D J. Plant expansins:Diversity and interactions with plant cell walls[J].Current Opinion in Plant Biology,2015,25:162-172.
[25] WANG B,LI Z C,HAN Z H,XUE S L,BI Y,PRUSKY D.Effects of nitric oxide treatment on lignin biosynthesis and texture properties at wound sites of muskmelons[J]. Food Chemistry,2021,362:130193.
[26] LI X,ZANG C,GE H,ZHANG J,GRIERSON D,YIN X R,CHEN K S.Involvement of PAL,C4H,and 4CL in chilling injury-induced flesh lignification of loquat fruit[J]. HortScience,2017,52(1):127-131.
[27] CAI C,LI X,CHEN K S.Acetylsalicylic acid alleviates chilling injury of postharvest loquat(Eriobotrya japonica Lindl.)fruit[J].European Food Research and Technology,2006,223(4):533-539.
[28] KORTH K L,BLOUNT J W,CHEN F,RASMUSSEN S,LAMB C,DIXON R A. Changes in phenylpropanoid metabolites associated with homology-dependent silencing of phenylalanine ammonia-lyase and its somatic reversion in tobacco[J].Physiologia Plantarum,2001,111(2):137-143.
[29] SEWALT V J H,NI W,BLOUNT J W,JUNG H G,MASOUD S A,HOWLES P A,LAMB C,DIXON R A. Reduced lignin content and altered lignin composition in transgenic tobacco downregulated in expression of L-phenylalanine ammonia-lyase or cinnamate 4-hydroxylase[J].Plant Physiology,1997,115(1):41-50.
[30] LEPLÉ J C,DAUWE R,MORREEL K,STORME V,LAPIERRE C,POLLET B,NAUMANN A,KANG K Y,KIM H,RUEL K,LEFÈBVRE A,JOSELEAU J P,GRIMA-PETTENATI J,DE RYCKE R,ANDERSSON-GUNNERÅS S,ERBAN A,FEHRLE I,PETIT-CONIL M,KOPKA J,POLLE A,MESSENS E,SUNDBERG B,MANSFIELD S D,RALPH J,PILATE G,BOERJAN W. Downregulation of cinnamoyl-coenzyme A reductase in poplar:Multiple-level phenotyping reveals effects on cell wall polymer metabolism and structure[J]. The Plant Cell,2007,19(11):3669-3691.
[31] TAMASLOUKHT B,LAM S J W Q,MARTINEZ Y,TOZO K,BARBIER O,JOURDA C,JAUNEAU A,BORDERIES G,BALZERGUE S,RENOU J P,HUGUET S,MARTINANT J P,TATOUT C,LAPIERRE C,BARRIÈRE Y,GOFFNER D,PICHON M. Characterization of a cinnamoyl-CoA reductase 1(CCR1) mutant in maize:Effects on lignification,fibre development,and global gene expression[J]. Journal of Experimental Botany,2011,62(11):3837-3848.
[32] DAY A,NEUTELINGS G,NOLIN F,GREC S,HABRANT A,CRÔNIER D,MAHER B,ROLANDO C,DAVID H,CHABBERT B,HAWKINS S.Caffeoyl coenzyme A O-methyltransferase down-regulation is associated with modifications in lignin and cell-wall architecture in flax secondary xylem[J].Plant Physiology and Biochemistry,2009,47(1):9-19.