嫁接是苹果(Malus pumila Mill.)无性繁殖的重要手段之一,通过嫁接能保持接穗优良的母本性状,延续品种优良特性[1];其中砧木不仅能为接穗提供物理支撑,还能增强植株的适应能力,对植株的生长发育、开始结果树龄、坐果率、产量、果实成熟期、色泽、品质、贮藏能力以及对生物和非生物胁迫的抵抗力都具有重要的影响[2]。常用的苹果砧木中M7 抗寒能力强,可在-25℃极端低温下保持70%以上存活率[3];G935 早果性和丰产性好[4];M9T337 作为矮化脱毒砧木,具有育苗简单、结果早和产量高等特点[5];新疆野苹果在较为贫瘠的山地、丘陵等土壤条件下仍能生长良好,能为嫁接品种提供稳定的生长基础[6]。然而,上述砧木在盐渍化生境中的适应性尚未明确,而新疆等核心产区因灌溉水矿化度高与蒸发强度大导致的土壤盐渍化问题日益严重。因此,系统评价这4种砧木的耐盐能力,筛选兼具优良性状与盐害耐受性的砧木材料,对实现盐渍区苹果产业的可持续发展目标具有双重意义。
已有研究发现,盐胁迫导致苹果生长减缓[7],根系和叶片发生离子外渗,严重时会导致植株死亡[8],但关于苹果砧木耐盐性相关研究主要集中在NaCl处理后某一天的生长形态、生理指标、光合作用等方面[9-10]。而在盐胁迫下,植物的反应是一个动态过程,通过动态观察,能清晰看到不同阶段的变化,如在胁迫初期,植物可能通过调节细胞膜的透性来减少盐分进入细胞;随着时间推移,会启动抗氧化防御系统来清除因盐胁迫产生的活性氧自由基[11]。鉴于此,笔者在本研究中以M7、G935、M9T337和新疆野苹果等4 种中国生产上应用较多的苹果砧木为材料,设置不同的盐分浓度,测定不同盐分浓度不同胁迫时间下苹果砧木表型与生理光合特征,探索其盐胁迫响应机制,并对4 种苹果砧木资源进行耐盐性综合评价,为建立耐盐苹果砧木苗期耐盐性筛选评价体系和耐盐苹果砧木种质资源选育提供参考。
1 材料和方法
1.1 试验材料
试验材料M7和G935均由山东泰安市亿森农业科技有限公司提供;M9T337由陕西西安市华圣现代农业集团有限公司提供;新疆野苹果由新疆霍城县清水河镇大西沟乡曾志强苗圃提供;均为一年生幼苗。其中M7,G935 和M9T337 为扦插苗,新疆野苹果为实生苗,均于2023 年3 月底定植于新疆农业大学校园中的试验基地,种植于23 cm×28 cm 的塑料花盆中,基质由泥炭土和草炭按照4∶1(V/V)配制,每盆装入等量的基质(5 kg),将花盆置于30 cm口径的托盘上,并放置于避雨棚中,定期进行浇水、除草等生长管理工作。
1.2 试验处理
在2023年7月底开始进行盐胁迫试验,NaCl溶液质量浓度分别为0、2、4、6、8、10 g·L-1,以0 g·L-1为对照组,各砧木每个梯度9 株,每3 株为1 个生物学重复。处理组用相应质量浓度的NaCl溶液浇灌,对照组用清水浇灌,为避免幼苗对盐胁迫产生应激反应,每天每盆施溶液800 mL,若底部托盘中有渗出液则浇回盆中,以避免土壤盐分的流失,3 d 后各处理均达到所设定盐浓度即开始试验。在0 d时,对所有盆栽苗进行第一次称质量,记录此时的质量为初始质量。这个质量包括花盆、基质、植株以及基质中所含的初始水分的质量,每隔4 d在同一时间称质量并补充清水,以保证盆内盐浓度不变,持续处理数次,至各砧木品种表型出现明显差异时终止试验。
1.3 试验方法
1.3.1 生长指标测定 试验开始前,测量其苗高(卷尺测量,cm)、地径(游标卡尺测量,mm),试验结束后,再次进行测量,计算苗高增量[△H,公式(1)]、地径增量[△D,公式(2)],每个指标3次重复。

1.3.2 生理指标测定 试验开始后,分别在0、5、10、15、20、25 d采样。每次均在08:00—09:00取样,摘取中上部长势好的完全展开叶片,装于冻存管内,迅速置于液氮中,贮存在-80 ℃冰箱中,用于后续相关生理指标的测定,每个指标的测定均3 次重复。叶片相对电导率采用电导仪法测定[12];丙二醛含量采用硫代巴比妥酸法测定[12];叶绿素含量采用酒精提取分光光度法测定[13];可溶性糖含量采用蒽酮比色法测定[14];可溶性蛋白含量采用考马斯亮蓝G-250法测定[14];超氧化物歧化酶活性采用四氮唑法测定[15];过氧化物酶活性采用愈创木酚法测定[15];过氧化氢酶活性采用紫外吸收法测定[15]。
1.3.3 光合参数测定 试验开始后,分别在0、5、10、15、20、25 d测量光合参数。采用德国GFS-3000便携式光合测定系统分析仪测定苗木在不同干旱胁迫条件下的光合参数[16],测定时间为09:00—11:00,选取各苹果砧木植株梢尖下数第4~5枚完全展开叶片,测定时光合有效辐射设置为1000 μmol·m-2·s-1,叶室温度为28~30 ℃。每处理测定3株,每株测定3枚叶片,取其平均值。测量参数包括净光合速率(Pn,μmol·m-2·s-1)、蒸腾速率(Tr,mmol·m-2·s-1)、气孔导度(Gs,mol·m-2·s-1)、胞间CO2浓度(Ci,μmol·mol-1)等,并计算水分利用效率(WUE=Pn/Tr)和气孔限制值(Ls=1-Ci/Ca,其中Ca为空气CO2浓度)[17]。
1.4 数据处理
试验数据采用Microsoft Excel 2016进行统计和整理,采用SPSS 26.0 软件进行单因素ANOVA 的LSD 法比较差异显著水平和主成分分析。参考赵昕玥等[18]所用方法进行主成分分析。
对测定指标进行标准化处理,用公式(3)计算NaCl胁迫处理后4种苹果砧木资源各指标的耐盐系数:
采用主成分分析法,用公式(4)、(5)计算4种材料耐盐指标的隶属函数值,用公式(6)计算4种供试材料的耐盐性综合评价值(D值)。
各主成分隶属函数值的计算公式为:
式中:U 表示隶属函数值;Xij表示第j 个苹果砧木资源第i个指标的主成分;Ximax和Ximin分别表示第i个主成分的最大值和最小值。
各主成分权重(Wi)的计算公式为:
式中:Pi表示第i个主成分的贡献率,n为选取的主成分数。
综合评价值(D)的计算公式为:
2 结果与分析
2.1 NaCl胁迫下4种苹果砧木表型的变化
通过观察苹果砧木表型变化,可以较直观地看出NaCl胁迫对植物的影响。由图1可知,干旱胁迫0~10 d 时,M7、G935、M9T337 和新疆野苹果在各NaCl处理下植株生长正常。胁迫15 d时10 g·L-1 NaCl处理下,M7、G935、M9T337和新疆野苹果的少数叶片黄化,其中M7黄化叶片最多。胁迫20 d时8 g·L-1和10 g·L-1 NaCl 处理下,各苹果砧木叶片边缘均出现不同程度的枯黄。胁迫25 d时2 g·L-1 NaCl处理下,各苹果砧木叶片较对照没有明显差异;在4~6 g·L-1 NaCl处理下,各苹果砧木叶片出现卷曲;8 g·L-1 NaCl处理下,各苹果砧木叶片出现黄化及卷曲;10 g·L-1 NaCl 处理下,M7 植株叶缘焦枯,G935 植株下部的老叶叶缘焦枯,M9T337 和新疆野苹果出现顶端叶缘焦枯的现象。

图1 NaCl 胁迫下 4 种苹果砧木幼苗的形态
Fig. 1 Morphology of four apple rootstock seedlings under NaCl stress
2.2 NaCl胁迫对4种苹果砧木生长的影响
胁迫25 d时,4种苹果砧木的苗高增量、地径增量随NaCl 浓度的增加总体呈降低趋势(表1)。G935对2 g·L-1 NaCl胁迫敏感,苗高降幅达40.00%,高于其他3 个砧木。M9T337 在4 g·L-1NaCl 胁迫下苗高增量(6.80 cm)与对照无显著差异。新疆野苹果在10 g·L-1 NaCl 胁迫下苗高降幅最小(68.66%),其他砧木降幅均超过85.00%。M9T337在4~8 g·L-1 NaCl 胁迫下地径降幅最低(14.04%~26.32%),优于降幅均超过30.00%的其他砧木。新疆野苹果在10 g·L-1 NaCl胁迫下地径增量最小(0.05 mm)、降幅最大(83.33%)。从生长指标来看,新疆野苹果的苗高增量受胁迫影响最小,M9T337 地径增量受抑制程度最轻。
表1 NaCl 处理对4 种苹果砧木生长指标的影响
Table 1 Effect of NaCl treatment on growth indexes of four apple rootstocks
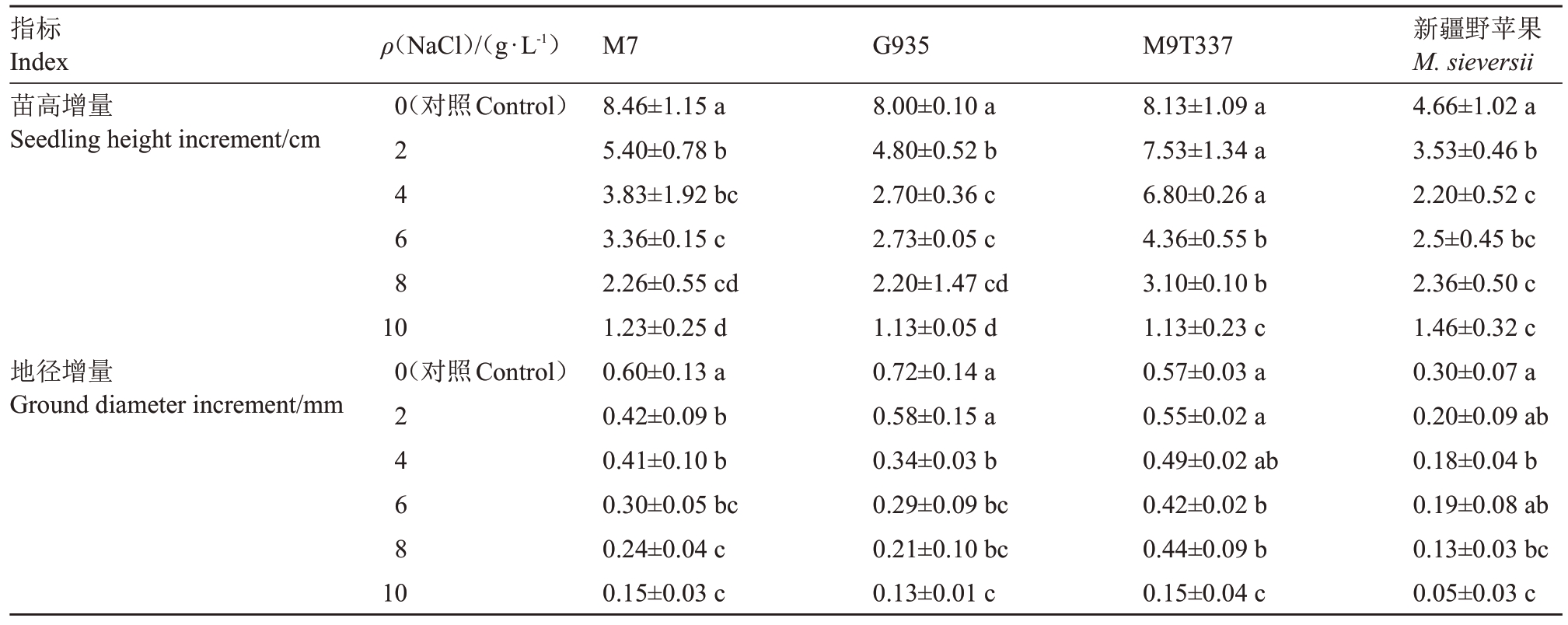
注:数据为“平均值±标准误差”。同列数据后不同小写字母表示相同砧木在不同NaCl 浓度下的(P<0.05)水平差异显著。
Note:Data are“mean±standard error”.Data in the same column followed by different small letters indicate significant differences(P<0.05)between the same rootstocks at different NaCl concentrations.
新疆野苹果M.sieversii 4.66±1.02 a 3.53±0.46 b 2.20±0.52 c 2.5±0.45 bc 2.36±0.50 c 1.46±0.32 c 0.30±0.07 a 0.20±0.09 ab 0.18±0.04 b 0.19±0.08 ab 0.13±0.03 bc 0.05±0.03 c指标Index苗高增量Seedling height increment/cm ρ(NaCl)/(g·L-1)0(对照Control)2 4 6 8地径增量Ground diameter increment/mm 10 0(对照Control)2 4 6 8 10 M7 8.46±1.15 a 5.40±0.78 b 3.83±1.92 bc 3.36±0.15 c 2.26±0.55 cd 1.23±0.25 d 0.60±0.13 a 0.42±0.09 b 0.41±0.10 b 0.30±0.05 bc 0.24±0.04 c 0.15±0.03 c G935 8.00±0.10 a 4.80±0.52 b 2.70±0.36 c 2.73±0.05 c 2.20±1.47 cd 1.13±0.05 d 0.72±0.14 a 0.58±0.15 a 0.34±0.03 b 0.29±0.09 bc 0.21±0.10 bc 0.13±0.01 c M9T337 8.13±1.09 a 7.53±1.34 a 6.80±0.26 a 4.36±0.55 b 3.10±0.10 b 1.13±0.23 c 0.57±0.03 a 0.55±0.02 a 0.49±0.02 ab 0.42±0.02 b 0.44±0.09 b 0.15±0.04 c
2.3 NaCl 胁迫下4 种苹果砧木叶片细胞膜透性的变化
2.3.1 相对电导率的变化 随着NaCl 浓度的增加和胁迫时间的延长,4 种苹果砧木相对电导率逐渐升高(图2)。胁迫至5 d 时,仅G935 相对电导率在各NaCl浓度下与对照相比无显著差异。胁迫10~20 d,各砧木在6~10 g·L-1的NaCl胁迫下的相对电导率上升速度较快。胁迫结束时,G935的相对电导率在不同NaCl处理下与其他3个砧木相比总体较低,新疆野苹果较高,在10 g·L-1的NaCl 胁迫下M7、G935、M9T337 和新疆野苹果达到最大值,分别较对照上升120.45%、98.33%、99.25%和148.71%。结果表明,在2 g·L-1的NaCl 胁迫下,各砧木的相对电导率变化范围较小,在4~10 g·L-1的NaCl胁迫下,新疆野苹果相对电导率值较其他砧木最高。
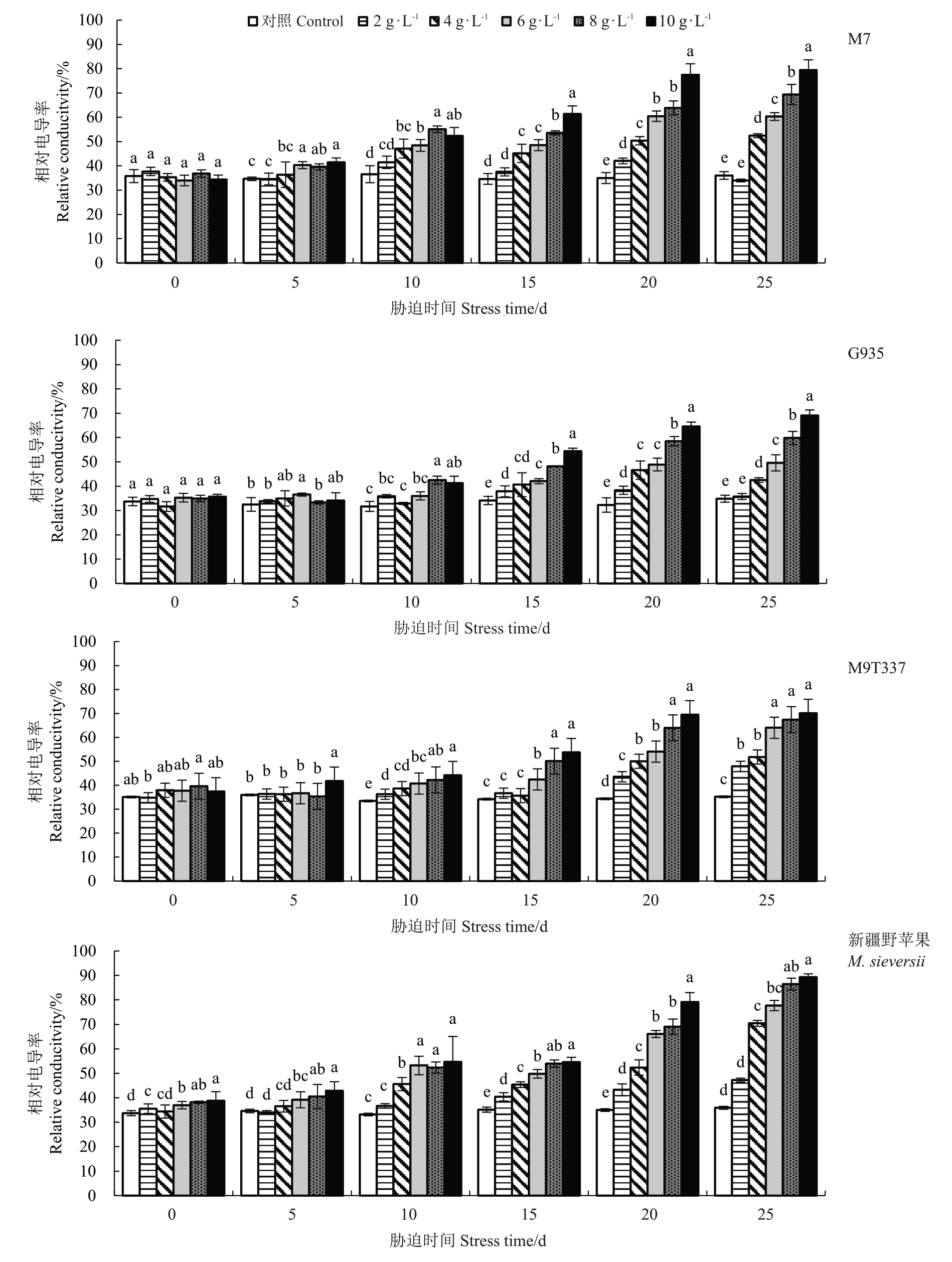
图2 NaCl 处理对苹果砧木相对电导率的影响
Fig.2 Effect of NaCl treatment on relative conductivity of apple rootstock
不同小写字母表示同一胁迫时间下不同浓度盐处理间在0.05 水平上差异显著。下同。
Different small letters indicate significant differences at the 0.05 level among different salt concentration treatments at the same stress time. The same below.
2.3.2 丙二醛含量的变化 4种苹果砧木丙二醛含量总体趋势和相对电导率一致(图3)。胁迫至5 d时,M7在各NaCl浓度下的丙二醛含量最高。胁迫至10 d 时,M9T337 在各处理下变化幅度较对照最小,仅在10 g·L-1的NaCl胁迫下与对照存在显著差异,其余砧木较对照在6~10 g·L-1胁迫下均显著增加。胁迫25 d,M7不同NaCl胁迫下丙二醛含量都高于其他3个砧木,与对照相比,G935在不同NaCl胁迫下的丙二醛含量增幅最小,增加范围在24.72%~74.51%。总体来看,盐胁迫对M7的丙二醛含量造成的影响最大。

图3 NaCl 处理对苹果砧木丙二醛含量的影响
Fig.3 Effect of NaCl treatment on malondialdehyde in apple rootstocks
2.4 NaCl 胁迫下4 种苹果砧木渗透调节物质含量的变化
2.4.1 可溶性糖含量的变化 随着胁迫时间的延长及NaCl 浓度的增加,G935 的可溶性糖含量总体呈先下降后上升趋势,M7、M9T337和新疆野苹果总体呈先上升后下降趋势(图4)。胁迫10 d,G935 和M9T337在各处理间均无显著差异;M7在8~10 g·L-1 NaCl胁迫可溶性糖含量快速上升。在胁迫15 d,与对照相比,G935 在2~6 g·L-1 NaCl 胁迫下可溶性糖含量降低,其他砧木在各NaCl浓度下均有增加。在胁迫20 d,M7在2~10 g·L-1 NaCl胁迫下可溶性糖含量最高。胁迫结束时,相同浓度下M9T337 的可溶性糖含量较其他砧木最低。结果表明,NaCl 胁迫下,M7积累了大量的可溶性糖。
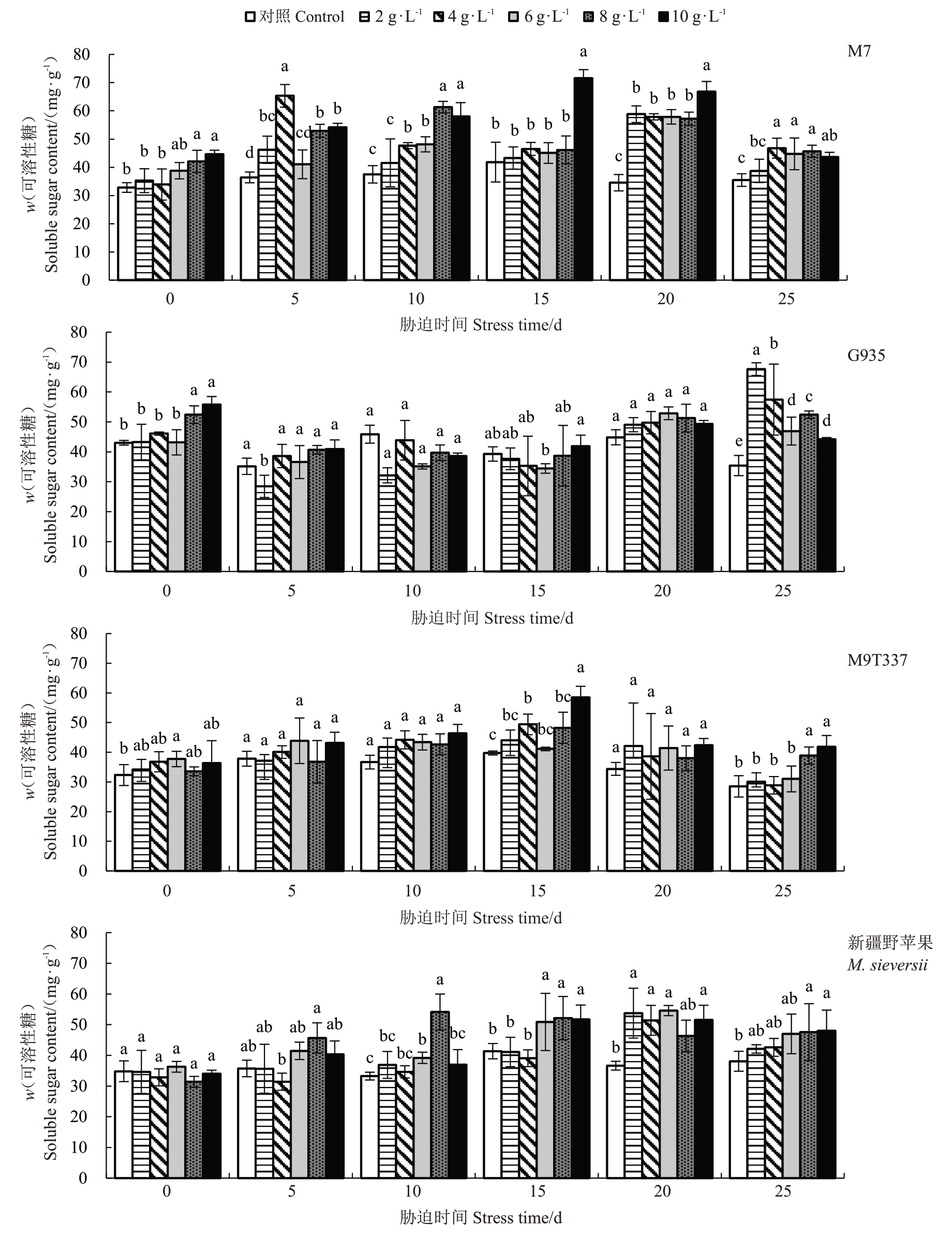
图4 NaCl 处理对苹果砧木可溶性糖含量的影响
Fig.4 Effect of NaCl treatment on soluble sugars of apple rootstocks
2.4.2 可溶性蛋白含量的变化 随着NaCl 浓度的增加和胁迫时间的延长,M7的可溶性蛋白含量总体呈先升高后降低再升高趋势,G935、M9T337和新疆野苹果总体呈先升高后降低趋势(图5)。在胁迫第5天,M7在各浓度下的可溶性蛋白含量上升幅度明显大于其他3 个砧木。在10~15 d 间,M7 可溶性蛋白含量在不同浓度下的下降速率较快,G935可溶性蛋白含量较为稳定,M9T337 仍然在各浓度下维持上升趋势,新疆野苹果仅在8~10 g·L-1的NaCl 胁迫下可溶性蛋白含量下降。在25 d 时,G935 在各Na-Cl 浓度下的可溶性蛋白含量明显高于其他3 个砧木,与对照相比最大增幅为2.43倍,其他3个砧木最大增幅均不超过1 倍。表明NaCl 胁迫下G935 有较强的诱导可溶性蛋白合成和积累的能力。
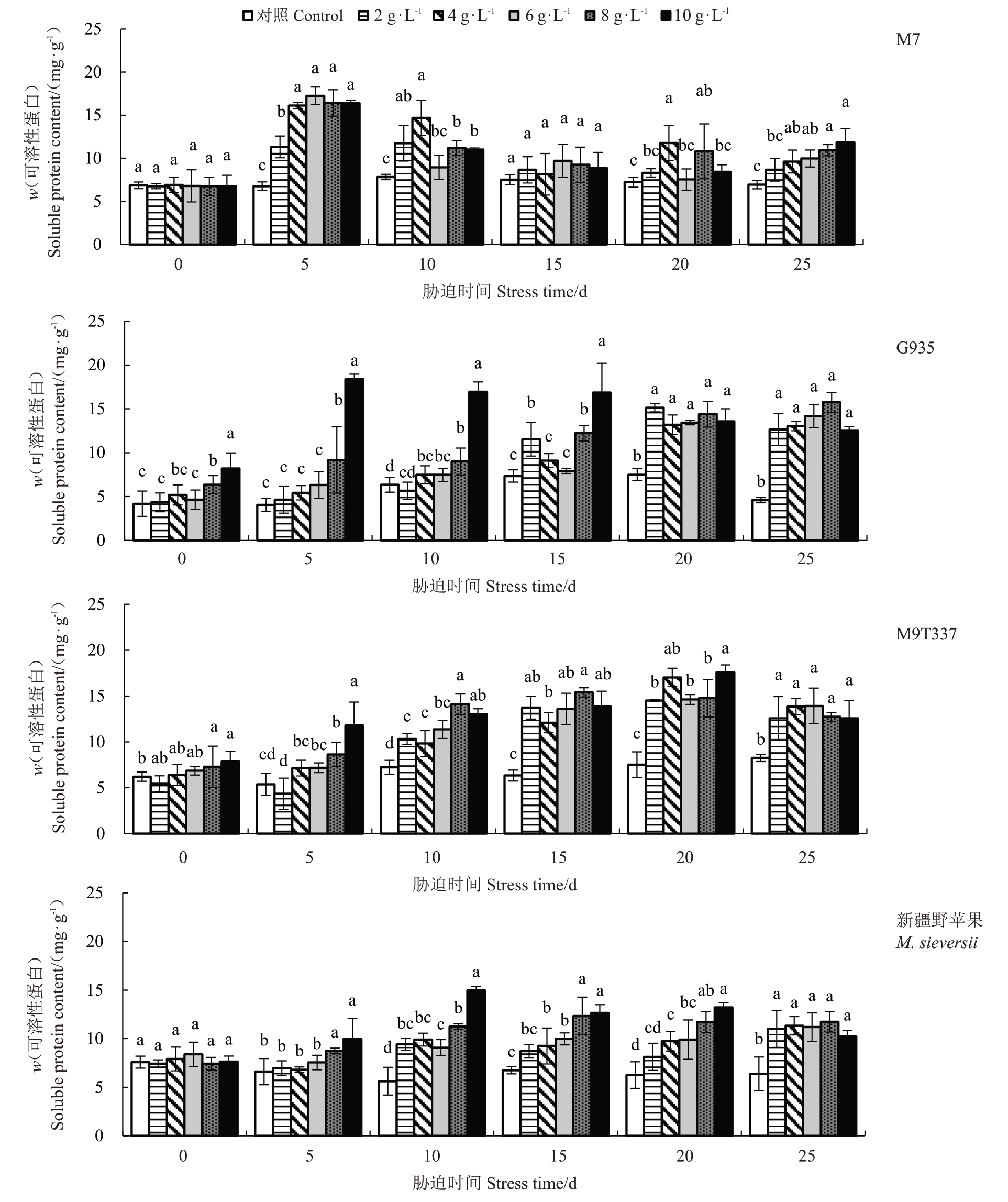
图5 NaCl 处理对苹果砧木可溶性蛋白含量的影响
Fig.5 Effect of NaCl treatment on soluble protein of apple rootstocks
2.5 NaCl 胁迫下4 种苹果砧木叶片抗氧化酶活性的变化
2.5.1 超氧化物歧化酶(SOD)活性的变化 随Na-Cl 浓度的增加和胁迫时间的延长,各苹果砧木的SOD活性总体呈先上升后下降的趋势(图6)。胁迫10 d,G935 和M9T337 的SOD 活性变化幅度较小,M7 和新疆野苹果在各NaCl 浓度下均显著高于对照,其中新疆野苹果在8 g·L-1 NaCl 胁迫下SOD 活性迅速上升较对照增幅352.86%。胁迫20 d 时,M7在不同盐浓度下与其他3 个砧木相比SOD 活性最高,在10 g·L-1 的NaCl 胁迫下SOD 活性最高值655.23 U·g-1。胁迫25 d,各砧木在不同盐胁迫下均显著高于对照,其中M9T337 的SOD 活性较低。盐胁迫下,M7和新疆野苹果的SOD活性总体较高。

图6 NaCl 处理对苹果砧木SOD 活性的影响
Fig.6 Effect of NaCl treatment on SOD activity of apple rootstocks
2.5.2 过氧化物酶(POD)活性的变化 随着NaCl浓度的增加和胁迫时间的延长,各苹果砧木的POD 活性总体呈先上升后下降的趋势(图7)。胁迫前10 d,M7 在各NaCl 胁迫下的POD 活性变化较小,G935的POD活性总体较高,M9T337和新疆野苹果在6~10 g·L-1 NaCl 胁迫下POD 活性上升较快。在胁迫20 d 时,G935 和新疆野苹果在6~10 g·L-1 NaCl 胁迫下POD 活性高于M7 和M9T337,G935 在10 g·L-1胁迫下POD活性最高(30 657.67 U·g-1),胁迫25 d时仍维持较高活性,新疆野苹果虽活性较高,但胁迫25 d 活性回落幅度较大。在胁迫结束时,4 个砧木在各NaCl 胁迫下POD 活性均显著高于对照,其中G935 的POD 活性最高。总体来说,G935 在各NaCl浓度下POD活性较高。
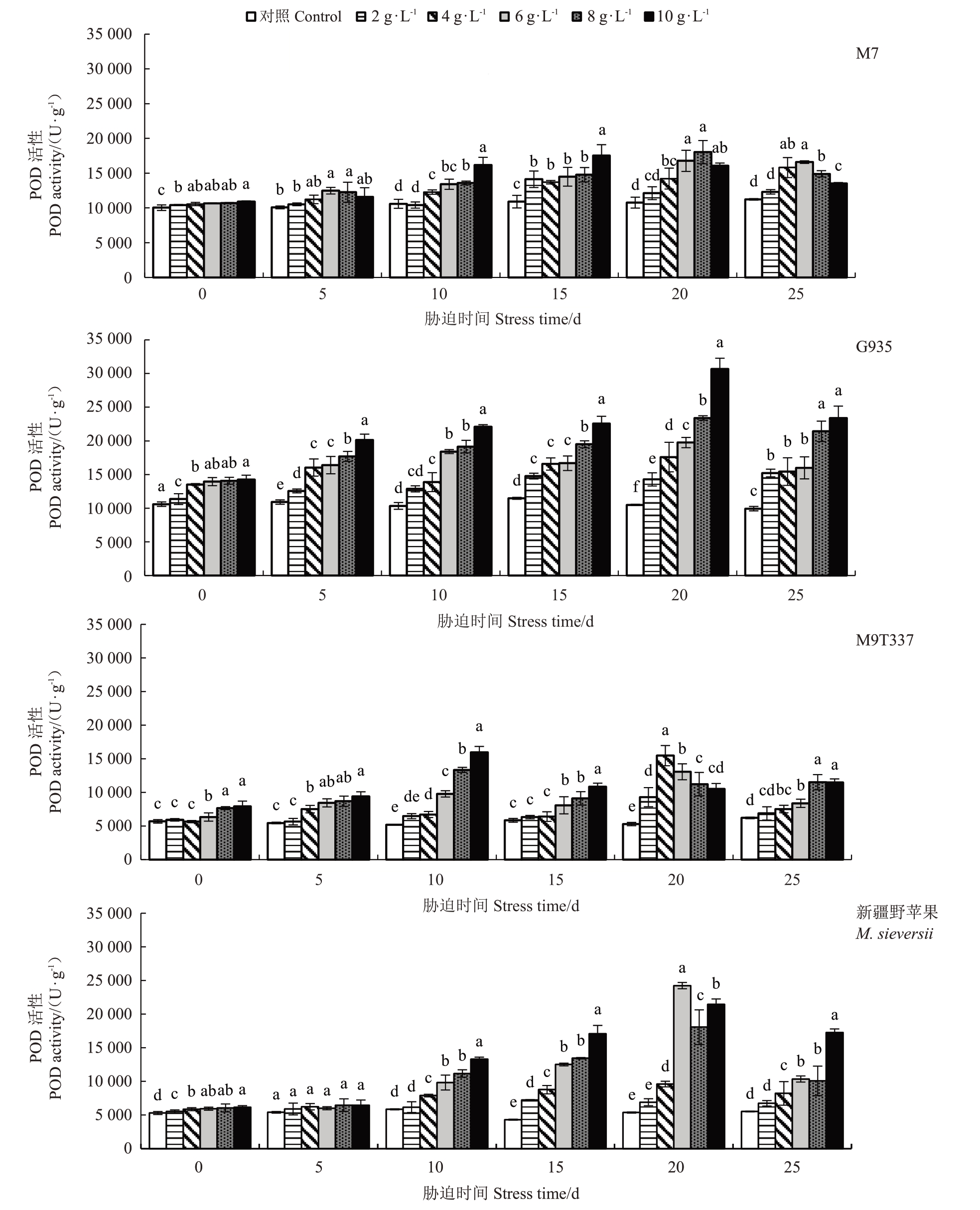
图7 NaCl 处理对苹果砧木POD 活性的影响
Fig.7 Effect of NaCl treatment on POD activity of apple rootstocks
2.5.3 过氧化氢酶(CAT)活性的变化 随着NaCl浓度的增加和胁迫时间的延长,4 种苹果砧木的CAT活性总体呈先升后降的趋势(图8)。在胁迫第10 天时,各砧木在不同NaCl 胁迫下CAT 活性均显著高于对照,与对照相比M7和G935的CAT活性增幅较大,分别在15.77%~163.51%和36.05%~142.43%;M9T337 和新疆野苹果的幅度变化均不超过90%。胁迫15 d,G935 的CAT 活性较高,在10 g·L-1的Na-Cl 胁迫下活性最高值较对照增加1.60 倍;M7 CAT活性变化范围较小(3.18%~42.18%);M9T337 和新疆野苹果CAT活性变化均不超过80%。在胁迫25 d时,与对照相比,M9T337在不同盐浓度下CAT活性变化较小,G935的变化较大。整体上看,G935在不同NaCl浓度下的CAT活性最高,M9T337和新疆野苹果不同NaCl浓度下的CAT活性变化幅度较小。
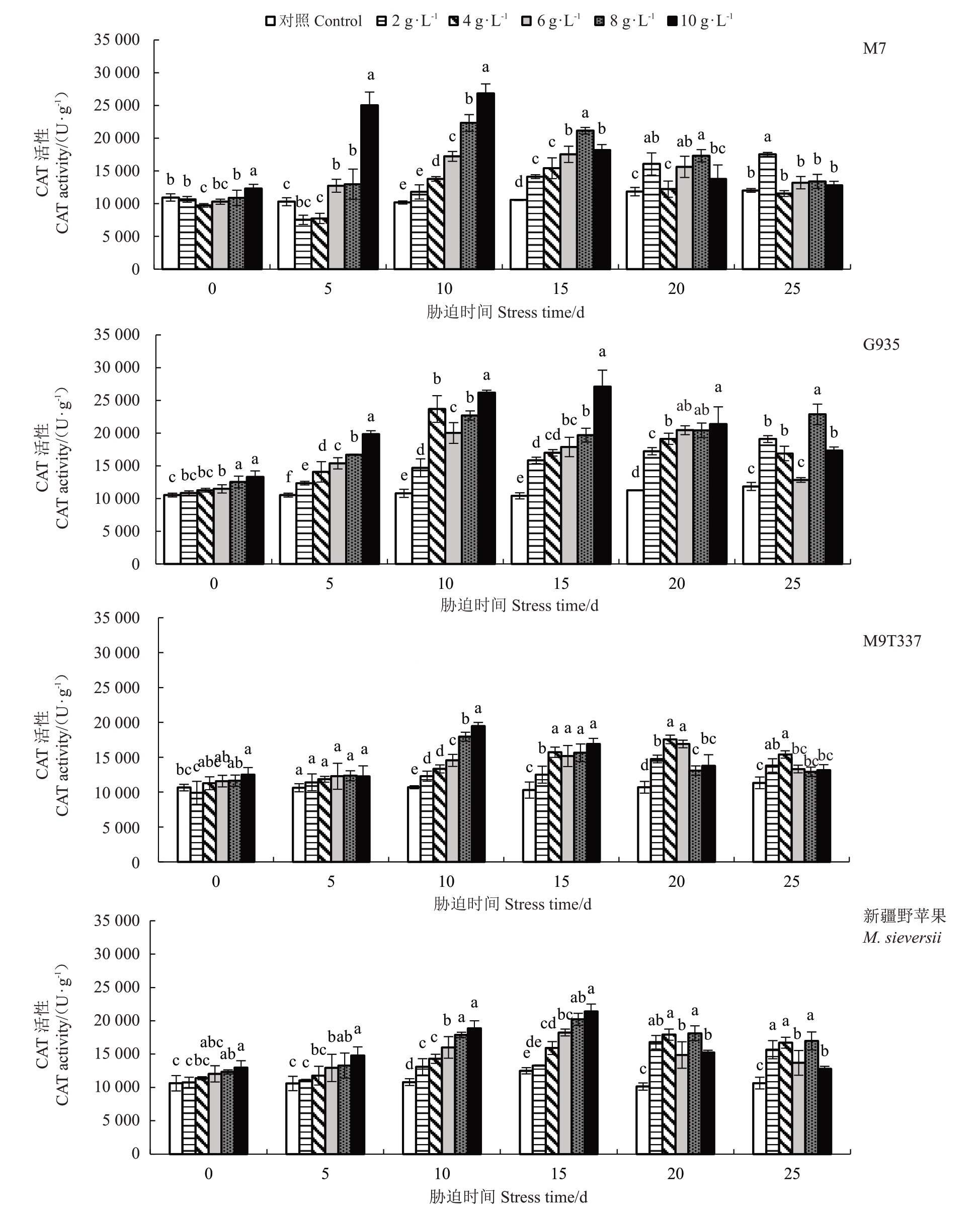
图8 NaCl 处理对苹果砧木CAT 活性的影响
Fig.8 Effect of CAT activity of apple rootstocks treated with NaCl
2.6 NaCl胁迫下4种苹果砧木叶绿素含量的变化
如图9 所示,随着盐浓度的增加和胁迫时间的延长,4 个苹果砧木叶绿素含量总体呈下降趋势。胁迫第5天,M7和新疆野苹果仅在10 g·L-1 NaCl胁迫下叶绿素含量显著低于对照(P<0.05);G935 在8~10 g·L-1 NaCl胁迫下显著低于对照;M9T337的叶绿素含量在各处理下均显著低于对照,但不同盐浓度间无显著差异。在胁迫结束时,G935的叶绿素含量较高,与对照相比降幅最小在4.06%~12.51%,其他3个砧木在8~10 g·L-1 NaCl胁迫下叶绿素含量与对照相比降幅均超过20%。总体来看,G935在胁迫结束时较其他3 个砧木叶绿素含量高,在胁迫过程中变化幅度较小。
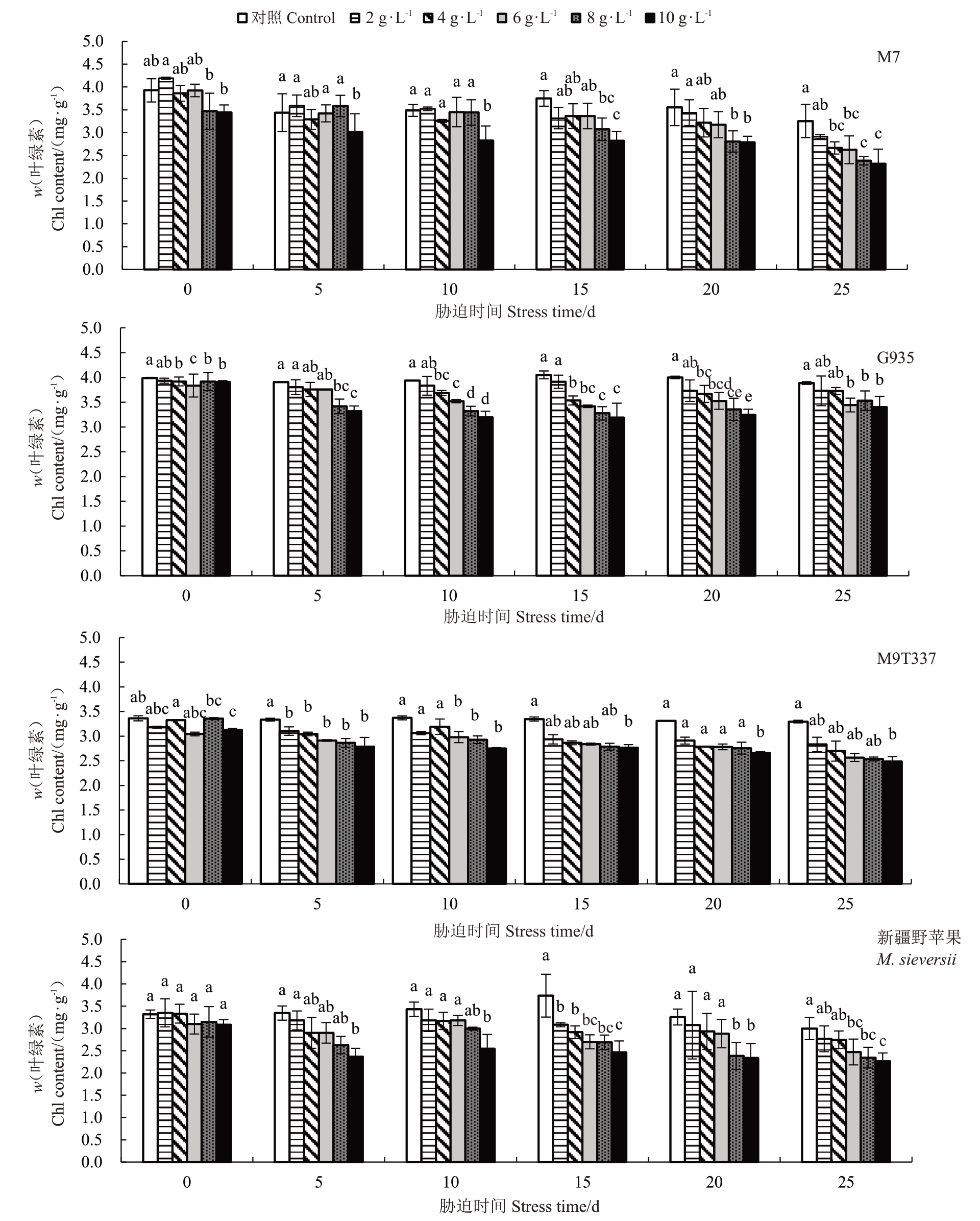
图9 NaCl 处理对苹果砧木叶绿素含量的影响
Fig.9 Effects of NaCl treatment on the chlorophyll content of walnut seedlings
2.7 光合参数
由图10可知,随着NaCl浓度的增加和胁迫时间的延长,M7、G935、M9T337和新疆野苹果的Pn总体呈下降趋势,各砧木在胁迫第10天的Pn急剧下降,随后保持在较低水平,在2~8 g·L-1 NaCl胁迫下M7的Pn总体高于其他3个砧木,但在10 g·L-1 NaCl处理下M7的Pn最低。4种苹果砧木的Ci表现出不同的变化趋势。在胁迫各时期M7、G935 和M9T337 在不同NaCl浓度下的Ci均高于对照,胁迫5~25 d时新疆野苹果在8~10 g·L-1 NaCl处理下的Ci显著低于对照。

图10 NaCl 处理对苹果砧木幼苗光合参数的影响
Fig. 10 Effeet of NaCl treatment on parameters of photosynthetic characteristies of apple rootstock seedlings
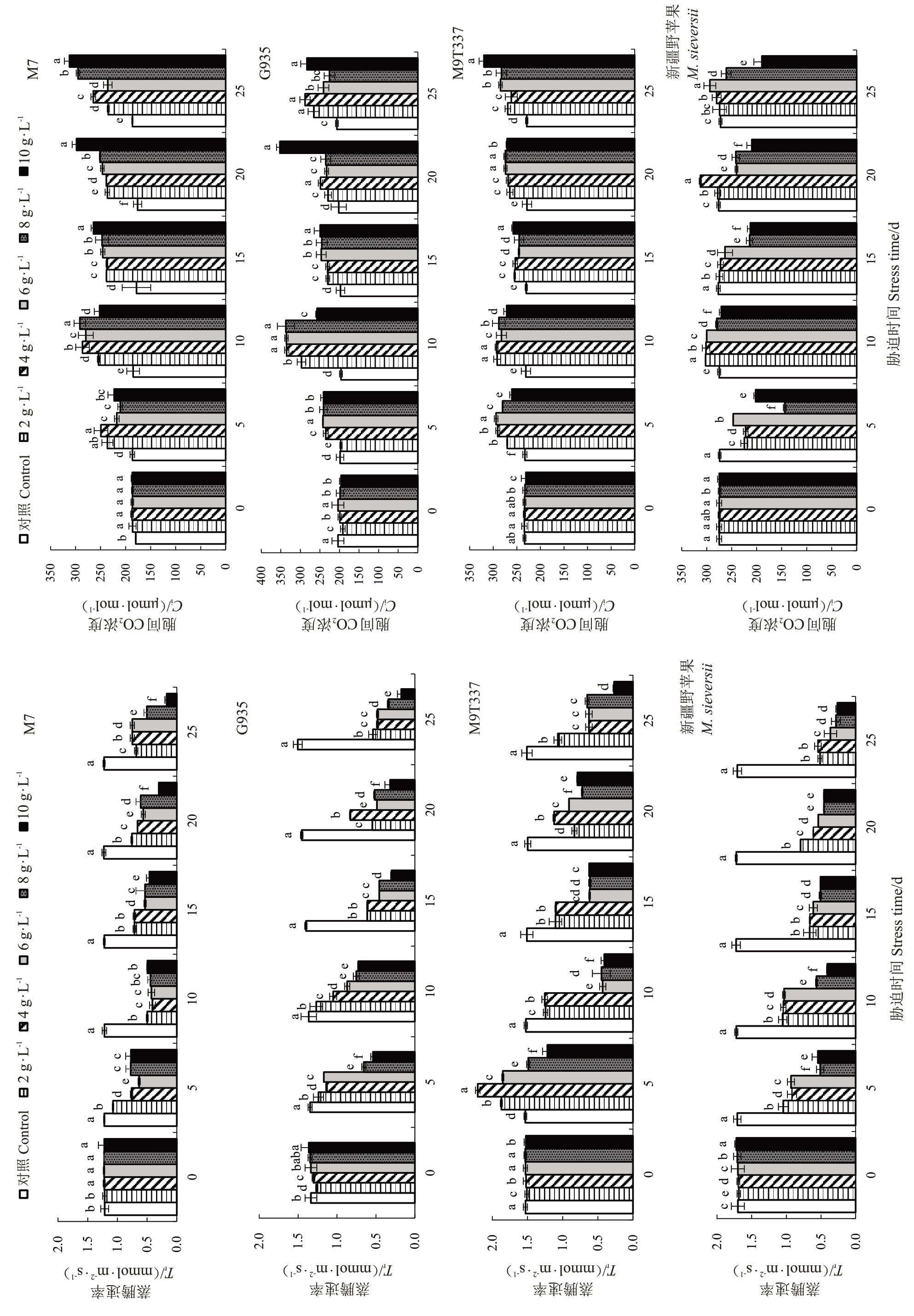
图10 (续)Fig.10(Continued)
Gs和Tr的变化趋势类似,随着NaCl浓度的增加和胁迫时间的延长,M7、G935和新疆野苹果的Gs和Tr总体呈下降趋势。M9T337 在2~6 g·L-1NaCl 处理下呈先升高后降低再升高趋势,8~10 g·L-1NaCl处理下呈逐渐降低趋势。在胁迫结束时,不同NaCl浓度下M7的Gs较对照降幅最小为17.11%~83.03%,G935较对照降幅最大为72.15%~91.30%。M7 总体上较对照的Gs、Tr和Pn的变化幅度相对较小,表现较好。
随着NaCl 浓度的增加和胁迫时间的延长,M7和新疆野苹果的WUE 总体呈先上升后下降趋势,G935 和M9T337 WUE 的变化趋势为逐渐下降(图11)。10 g·L-1 NaCl胁迫结束时,M7、G935、M9T337和新疆野苹果的WUE 相对于对照下降55.65%、50.83%、72.64%和29.64%。随着NaCl 浓度的增加和胁迫时间的延长,对4 种苹果砧木的Ls均产生了较大影响,M7、G935和新疆野苹果的Ls总体呈先下降后上升再下降趋势,M9T337呈先升高后下降再升高趋势,在胁迫5~25 d时,新疆野苹果在8~10 g·L-1 NaCl 胁迫下的Ls显著高于对照,维持在较高水平,M9T337的Ls较其他砧木变化范围较小。

图11 NaCl处理对苹果砧木水分利用效率及气孔限制值的影响
Fig. 11 Effeet of'NaCl treatment on water use efficiency and stomatal limitation value in apple rootstocks
2.8 耐盐性综合评价
对4 种苹果砧木耐盐指标进行主成分分析(表2),前3 个特征值累计贡献率达85.744%,表明这3个综合指标可以覆盖原始数据所蕴含的大部分信息,故而将原来的14种单项指标转化为3个相互独立的综合指标。根据主成分分析结果,第1主成分的贡献率为43.321%,叶绿素含量和蒸腾速率的特征向量的绝对值高于其他性状,表明第1主成分主要由叶绿素含量和蒸腾速率构成。第2 主成分贡献率为26.365%,主要由水分利用效率和SOD活性组成。第3主成分贡献率分别为16.057%,主要为胞间二氧化碳浓度。因此,初步可考虑使用上述5个相对指标作为鉴定苹果砧木苗期抗旱性的参考指标。
表2 单项指标在主成分中的特征向量
Table 2 Eigenvectors of 10 single indexes in each principal component
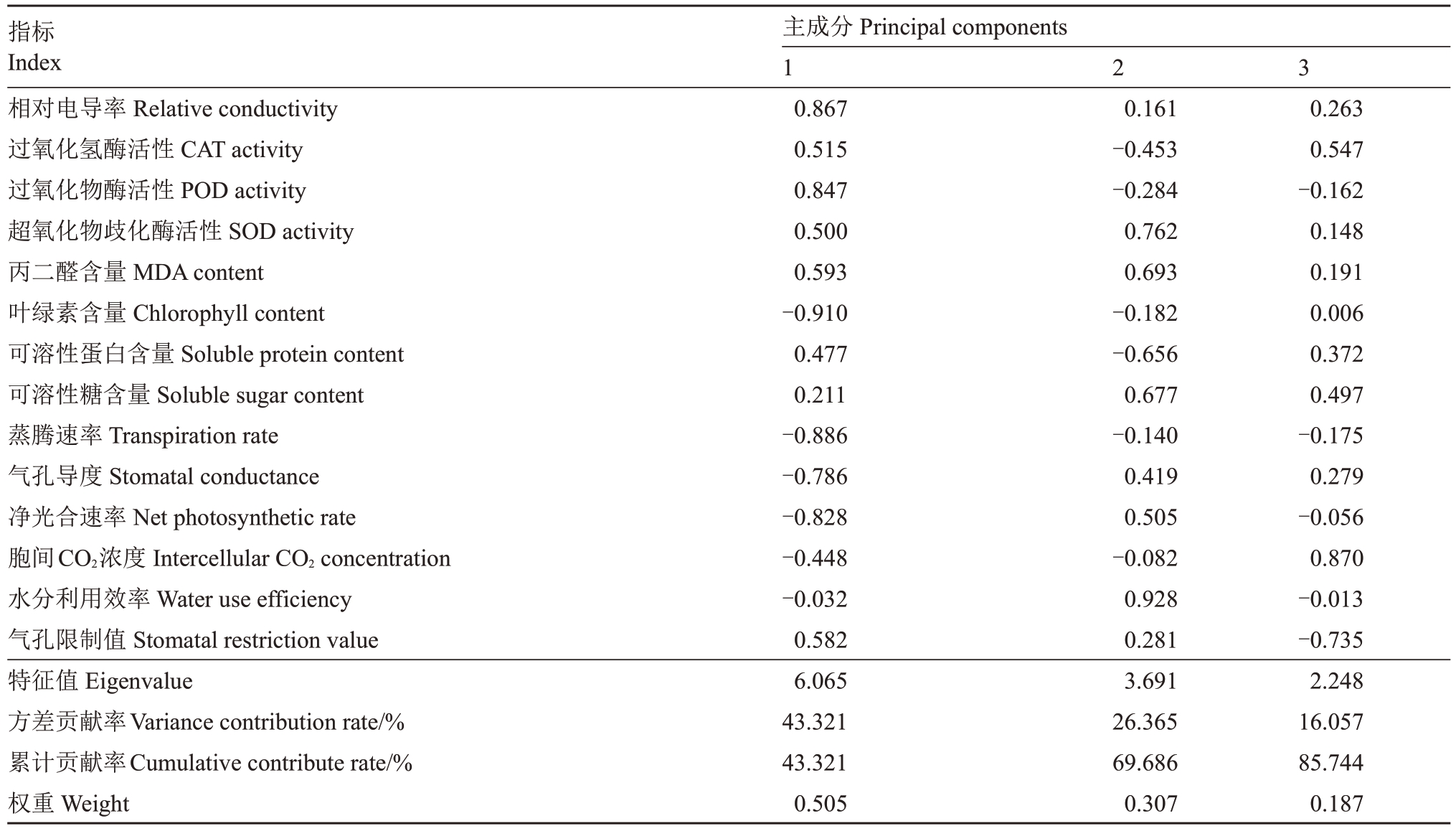
指标Index相对电导率Relative conductivity过氧化氢酶活性CAT activity过氧化物酶活性POD activity超氧化物歧化酶活性SOD activity丙二醛含量MDA content叶绿素含量Chlorophyll content可溶性蛋白含量Soluble protein content可溶性糖含量Soluble sugar content蒸腾速率Transpiration rate气孔导度Stomatal conductance净光合速率Net photosynthetic rate胞间CO2浓度Intercellular CO2 concentration水分利用效率Water use efficiency气孔限制值Stomatal restriction value特征值Eigenvalue方差贡献率Variance contribution rate/%累计贡献率Cumulative contribute rate/%权重Weight主成分Principal components 1 0.867 0.515 0.847 0.500 0.593-0.910 0.477 0.211-0.886-0.786-0.828-0.448-0.032 0.582 6.065 43.321 43.321 0.505 2 3 0.161-0.453-0.284 0.762 0.693-0.182-0.656 0.677-0.140 0.419 0.505-0.082 0.928 0.281 3.691 26.365 69.686 0.307 0.263 0.547-0.162 0.148 0.191 0.006 0.372 0.497-0.175 0.279-0.056 0.870-0.013-0.735 2.248 16.057 85.744 0.187
根据筛选出的5个相对指标计算后分别得到隶属函数值和D值。根据D值对苹果砧木苗期抗旱性进行综合评价。某一品种D 值越大,表示该品种耐盐能力越强,反之耐盐能力越弱。4 种苹果砧木的耐盐能力进行排序为M7>新疆野苹果>G935>M9T337(表3)。
表3 4 种苹果砧木耐盐能力综合评价
Table 3 Comprehensive evaluation of salt tolerance of four apple rootstocks
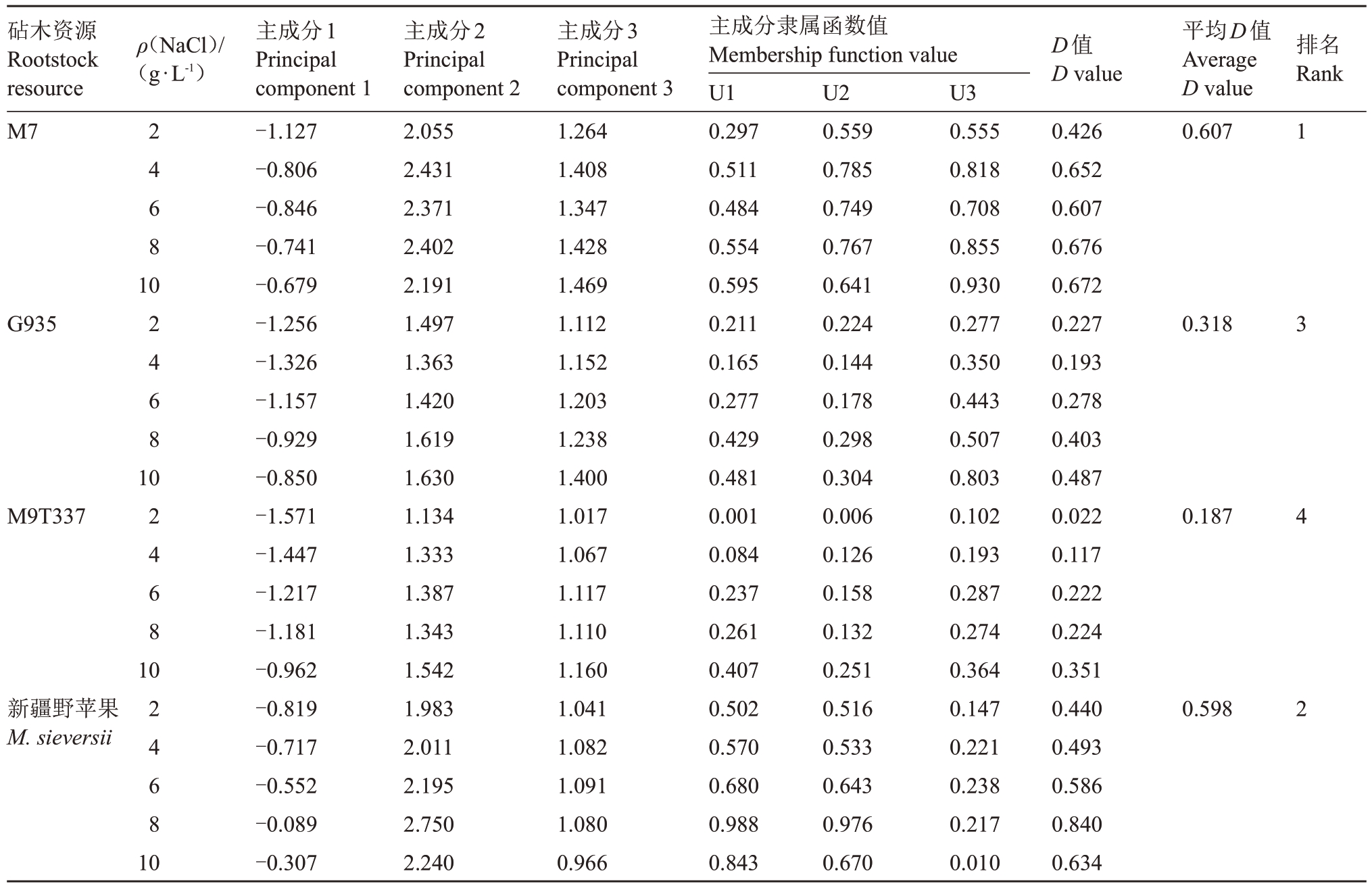
砧木资源Rootstock resource M7 ρ(NaCl)/(g·L-1)D值D value排名Rank 2 4 6 8平均D值Average D value 0.607 1 10 G935 2 4 6 8 0.318 3 10 M9T337 2 4 6 8 0.187 4 10新疆野苹果M.sieversii 2 4 6 8 0.598 2 10主成分1 Principal component 1-1.127-0.806-0.846-0.741-0.679-1.256-1.326-1.157-0.929-0.850-1.571-1.447-1.217-1.181-0.962-0.819-0.717-0.552-0.089-0.307主成分2 Principal component 2 2.055 2.431 2.371 2.402 2.191 1.497 1.363 1.420 1.619 1.630 1.134 1.333 1.387 1.343 1.542 1.983 2.011 2.195 2.750 2.240主成分3 Principal component 3 1.264 1.408 1.347 1.428 1.469 1.112 1.152 1.203 1.238 1.400 1.017 1.067 1.117 1.110 1.160 1.041 1.082 1.091 1.080 0.966主成分隶属函数值Membership function value U1 0.297 0.511 0.484 0.554 0.595 0.211 0.165 0.277 0.429 0.481 0.001 0.084 0.237 0.261 0.407 0.502 0.570 0.680 0.988 0.843 U2 0.559 0.785 0.749 0.767 0.641 0.224 0.144 0.178 0.298 0.304 0.006 0.126 0.158 0.132 0.251 0.516 0.533 0.643 0.976 0.670 U3 0.555 0.818 0.708 0.855 0.930 0.277 0.350 0.443 0.507 0.803 0.102 0.193 0.287 0.274 0.364 0.147 0.221 0.238 0.217 0.010 0.426 0.652 0.607 0.676 0.672 0.227 0.193 0.278 0.403 0.487 0.022 0.117 0.222 0.224 0.351 0.440 0.493 0.586 0.840 0.634
3 讨 论
植株的表型特征是植物对盐胁迫的综合体现,也是判断植物耐盐性直观的指标[19]。本研究结果表明,随着NaCl浓度的增加和胁迫时间的延长,4种苹果砧木资源的苗高增量、地径增量总体呈现减少的趋势,这与以往研究在植物遭受到盐胁迫时,其株高和地径随着盐浓度的增加而逐渐受到抑制的结果一致[20-21]。另外,随着胁迫程度的加深,幼苗均表现出不同程度的盐害症状,如叶片发黄、焦枯、卷曲等,其中以在8~10 g·L-1 NaCl 胁迫下,砧木受胁迫影响最严重,可能是高盐环境使土壤溶液的水势降低,远远低于植物细胞内的水势,导致幼苗根系吸水困难,细胞内的水分不断流失,出现叶片卷曲现象,高浓度的Cl-和Na+破坏细胞膜的结构和功能,使细胞内的物质泄漏,导致细胞死亡,进而使叶片出现发黄焦枯的现象。
盐胁迫条件下细胞膜损伤程度可由相对电导率大小反映[22]。笔者在本研究中发现,随着盐浓度的增加和胁迫时间的延长,4 种苹果砧木资源的相对电导率和MDA 含量总体呈现上升的趋势。说明在不同盐浓度胁迫下,4 种苹果砧木资源的质膜均受到伤害,并且随着盐浓度的增加和胁迫时间的延长受到伤害的程度逐渐加深。此外,新疆野苹果的相对电导率和MDA 含量相比于其他3 个苹果砧木较高,在胁迫结束时较对照增幅最大,表明其细胞膜损伤最严重。
可溶性糖和可溶性蛋白是促进植物适应逆境的重要渗透调节物质[22],在本研究中,在不同NaCl浓度胁迫下,随着胁迫时间的增加,最终使4种苹果砧木体内可溶性蛋白和可溶性糖含量显著高于对照,这与对盐胁迫下榉树[23]、酸樱桃[24]、罗汉松[25]的研究结果一致。通过积累可溶性糖,植物可以平衡细胞内外的水势差,减少细胞失水,从而提高植物在盐胁迫下的生存能力[26],在不同NaCl浓度胁迫下M7可溶性糖含量较其他3个砧木最高,意味着M7具有较好的抗逆性。
有关抗氧化物酶的研究主要涉及SOD、POD、CAT 等,它们协同作用共同抵抗盐分胁迫诱导的氧化伤害[27-28]。本研究结果表明,随着盐浓度的增加和胁迫时间的延长,4 种苹果砧木的SOD、POD、CAT活性总体呈现先升高后降低的趋势。这与盐胁迫下栓皮栎[29]、白蜡[30]、黑果枸杞[31]等的研究一致。表明在不同盐浓度胁迫下,为了清除过量的活性氧,减轻活性氧对细胞膜的伤害,4 种苹果砧木的抗氧化能力均得到了增强,但在长时间的盐胁迫下,超过了幼苗的耐受范围,清除过氧化物自由基的能力下降,引起植物自身代谢失衡,酶活性降低。另外,4种苹果砧木中,M9T337抗氧化物酶活性总体较低,说明在盐胁迫下M9T337清除过氧化物自由基的能力较弱。
在本研究中,在不同盐浓度胁迫下,4种苹果砧木资源的叶绿素含量均呈降低趋势,盐浓度越高叶绿素含量下降幅度越大。可能是当细胞失水时,植物为了减少水分散失,会关闭气孔,进而影响光合作用[32],光合作用的减弱会导致植物减少叶绿素的合成,甚至加速叶绿素的分解,使叶绿素含量降低[33],Dichala 等[34]也表明不同植物叶绿素在盐胁迫下呈降低趋势。相比于其他3 个苹果砧木,在不同盐浓度下,G935 的叶绿素含量较其对照降幅最小,且含量也最高,表明G935在盐胁迫下具有较强的保持叶绿素相对稳定的能力。
盐胁迫下诱导气孔关闭是植物减轻或避免盐伤害的一种生理机制,关闭气孔来避免水分通过蒸腾流失,这会导致Tr下降[35]。本研究结果表明,Gs和Tr的变化趋势类似,表明主要是因为气孔限制,气孔导度下降通常会减少二氧化碳进入叶片的量,但5~25 d时新疆野苹果在8~10g·L-1 NaCl胁迫下气孔导度的下降,并不使其胞间CO2浓度下降,反而上升,这可能是叶肉细胞的生理功能受损,非气孔限制逐渐增强[36]。光合作用受到抑制的主要因素不同,由气孔限制和非气孔限制共同作用,从而导致光合效率的下降[37],并且气孔和非气孔限制之间随着盐浓度和胁迫时间的变化而发生动态变化[38],其中,M7的Gs、Tr和Pn的变化幅度相对较小,表现较好。本研究结果表明,在不同浓度NaCl 胁迫结束时各砧木的WUE 均低于对照,表明盐胁迫对各砧木细胞造成损伤,其中G935较对照下降程度最大,说明受胁迫影响最大。
植物的耐盐性受多方面因素影响,是一个复杂的综合性状[39]。单一指标难以评价物种自身耐盐性强弱,而选择较多指标又容易造成试验成本昂贵和指标间的信息重叠[40]。主成分分析法已经被广泛应用于植物耐盐性典型指标筛选[41]。笔者在本研究中通过14种指标进行主成分分析,结果显示筛选出叶绿素含量、蒸腾速率、水分利用效率、SOD和胞间二氧化碳浓度5个指标作为苹果砧木耐盐性评价指标,其耐盐能力排序为M7>新疆野苹果>G935>M9T337。
4 结 论
不同苹果砧木在不同浓度盐胁迫下形态差异大,耐盐性亦不同。随着盐浓度的增加和胁迫时间的延长,幼苗的Gs、Tr和Pn显著降低,Ci较对照增加,REC和MDA含量持续增加,SOD、POD和CAT活性先上升后下降,盐胁迫最终使4 种苹果砧木体内可溶性蛋白和可溶性糖含量显著高于对照。结合主成分分析筛选出叶绿素含量、蒸腾速率、水分利用效率、SOD 和胞间二氧化碳浓度5 个指标为综合评价关键指标。结果表明在不同浓度盐胁迫下4种苹果砧木的耐盐能力综合排序为M7>新疆野苹果>G935>M9T337。
[1] ZHANG B Q,WU P T,ZHAO X N,WANG Y B,GAO X D,CAO X C.A drought hazard assessment index based on the VICPDSI model and its application on the Loess Plateau,China[J].Theoretical and Applied Climatology,2013,114(1):125-138.
[2] 张林森,胥生荣,张永旺,胡景江,刘富庭,李雪薇,韩明玉,马锋旺.干旱胁迫下不同中间砧嫁接苹果苗的导水特性[J].园艺学报,2013,40(11):2137-2143.ZHANG Linsen,XU Shengrong,ZHANG Yongwang,HU Jingjiang,LIU Futing,LI Xuewei,HAN Mingyu,MA Fengwang. Hydraulics characteristic of fuji apple grafted on different dwarf interstocks under drought stress[J].Acta Horticulturae Sinica,2013,40(11):2137-2143.
[3] 王来平,薛晓敏,董放,聂佩显,王金政.不同砧穗组合对富士苹果生产与果实品质的影响[J].果树学报,2022,39(9):1607-1618.WANG Laiping,XUE Xiaomin,DONG Fang,NIE Peixian,WANG Jinzheng. Effects of different rootstock-scion combinations on tree development,photosynthetic production,yield and quality of Fuji apple[J]. Journal of Fruit Science,2022,39(9):1607-1618.
[4] 李海燕,耿达立,牛春东,李翠英,管清美.苹果砧木富平楸子和G935 根系抗旱性评估[J].西北农林科技大学学报(自然科学版),2018,46(5):118-124.LI Haiyan,GENG Dali,NIU Chundong,LI Cuiying,GUAN Qingmei. Drought resistance of root system of apple rootstocks Malus prunifolia and G935[J]. Journal of Northwest A& F University(Natural Science Edition),2018,46(5):118-124.
[5] 赵文哲,刘晓,杜桂英,邓文鹏,宋新卫,肖伟,李玲.干旱胁迫及复水对M9T337 苹果砧木苗生理特性的影响[J].山东农业科学,2022,54(4):49-54.ZHAO Wenzhe,LIU Xiao,DU Guiying,DENG Wenpeng,SONG Xinwei,XIAO Wei,LI Ling. Effects of drought stress and rewatering on physiological characteristics of apple rootstock M9T337[J].Shandong Agricultural Sciences,2022,54(4):49-54.
[6] 张博,刘立强,秦伟,乌仁其米格.不同抗寒性新疆野苹果的转录组比较分析[J/OL].分子植物育种,2023:1-17.(2023-03-14).https://kns.cnki.net/kcms/detail/46.1068.s.20230313.0902.002.html.ZHANG Bo,LIU Liqiang,QIN Wei,WU Renqimige.Comparative transcriptome analysis of Malus sieversii with different cold resistance[J/OL].Molecular Plant Breeding,2023:1-17.(2023-03-14).https://kns.cnki.net/kcms/detail/46.1068.s.20230313.0902.002.html.
[7] 刘佳琪.中国苹果主产省苹果生产高质量发展水平测度及时空演变分析[D].杨凌:西北农林科技大学,2023.LIU Jiaqi. Measurement and spatiotemporal evolution analysis of high-quality development level of apple production in major apple producing provinces in China[D]. Yangling:Northwest A&F University,2023.
[8] 杜中军,翟衡,罗新书,潘志勇,程述汉.苹果砧木耐盐性鉴定及其指标判定[J].果树学报,2002,19(1):4-7.DU Zhongjun,ZHAI Heng,LUO Xinshu,PAN Zhiyong,CHENG Shuhan. Salt-tolerance identification on apple rootstocks[J].Journal of Fruit Science,2002,19(1):4-7.
[9] 杜中军,翟衡,潘志勇,解秀芹.盐胁迫下苹果砧木光合能力及光合色素的变化[J].果树学报,2001,18(4):200-203.DU Zhongjun,ZHAI Heng,PAN Zhiyong,XIE Xiuqin. Change of photosynthetic capability and pigment content of apple rootstocks under salt-stress[J]. Journal of Fruit Science,2001,18(4):200-203.
[10] 胡爱双,张小栋,王文成,李凯超,孙宇.盐胁迫对不同耐盐性八棱海棠株系生理特性的影响[J].果树学报,2021,38(3):335-343.HU Aishuang,ZHANG Xiaodong,WANG Wencheng,LI Kaichao,SUN Yu.Effects of salt stress on physiological characteristics of two strains of Malus micromalus Mak. with different salt tolerance[J].Journal of Fruit Science,2021,38(3):335-343.
[11] 韩淑敏,杨立科,布和,徐欢,周静宇,徐燕.重金属镉及盐胁迫对白榆幼苗生理特性的影响[J].森林工程,2022,38(6):19-26.HAN Shumin,YANG Like,BU He,XU Huan,ZHOU Jingyu,XU Yan.Effects of heavy metal Cd and salt stress on physiological characteristics of Ulmus pumila seedlings[J]. Forest Engineering,2022,38(6):19-26.
[12] 高俊凤. 植物生理学实验指导[M]. 北京:高等教育出版社,2006:203-204.GAO Junfeng. Experimental guidance for plant physiology[M].Beijing:Higher Education Press,2006:203-204.
[13] 严衍禄,刘心生.叶绿素测定方法的研究[J].北京农业大学学报,1982(2):53-67.YAN Yanlu,LIU Xinsheng. Studies on determination chlorophylls a and b[J].Journal of China Agricultural University,1982(2):53-67.
[14] 肖望. 植物生理学实验指导[M]. 广州:中山大学出版社,2020:45-46.XIAO Wang. Experimental guidance of plant physiology[M].Guangzhou:Sun Yat-sen University Press,2020:45-46.
[15] 王晶英.植物生理生化实验技术与原理[M].哈尔滨:东北林业大学出版社,2003:78-79.WANG Jingying. Experi mental techniques and principles of plant physiology and bio chemistry[M]. Harbin:Northeast Forestry University,2003:78-79.
[16] 吴芳,师尚礼,张立媛,张翠梅.俄罗斯百脉根种质材料的光合特性研究[J].草原与草坪,2017,37(5):21-29.WU Fang,SHI Shangli,ZHANG Liyuan,ZHANG Cuimei.Study on photosynthetic characteristics of introduced russia Lotus corniculatus materials[J].Grassland and Turf,2017,37(5):21-29.
[17] 赵文哲,刘晓,姜珊,陈敏,王旭旭,刘金宝,谭梅,王宇,李玲,高东升,陈修德,肖伟.干旱过程中‘M9T337’苹果砧木苗光合特性及MdCP2 与MdGLK1 互作分析[J]. 植物生理学报,2021,57(6):1337-1348.ZHAO Wenzhe,LIU Xiao,JIANG Shan,CHEN Min,WANG Xuxu,LIU Jinbao,TAN Mei,WANG Yu,LI Ling,GAO Dongsheng,CHEN Xiude,XIAO Wei. Photosynthetic characteristics of‘M9T337’apple rootstock seedlings and interaction between MdCP2 and MdGLK1 during drought stress[J]. Plant Physiology Journal,2021,57(6):1337-1348.
[18] 赵昕玥,王晓敏,赵云雅,郭彦英,宗蕾,程国新,郭猛,高艳明,李建设.不同地区小茴香苗期耐盐性综合评价及耐盐指标筛选[J].西北农林科技大学学报(自然科学版),2024,52(7):125-135.ZHAO Xinyue,WANG Xiaomin,ZHAO Yunya,GUO Yanying,ZONG Lei,CHENG Guoxin,GUO Meng,GAO Yanming,LI Jianshe. Comprehensive evaluation of salt tolerance of fennel at seedling stage in different regions and screening of salt-tolerant indexes[J]. Journal of Northwest A& F University (Natural Science Edition),2024,52(7):125-135.
[19] BHUSAL N,LEE M S,HAN A R,HAN A,KIM H S.Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters[J].Forest Ecology and Management,2020,465:118099.
[20] 杨文翔.盐胁迫对三个海棠砧木品种生理及生长的影响[D].南京:南京林业大学,2011.YANG Wenxiang.Effects of salt stress on the growth and physiological properties of three hitom stocks varieties[D]. Nanjing:Nanjing Forestry University,2011.
[21] LIU Z X,ZHANG H X,YANG X Y,WEI H R. Effects of soil salinity on growth,ion relations,and compatible solute accumulation of two sumac species:Rhus glabra and Rhus trilobata[J].Communications in Soil Science and Plant Analysis,2013,44(21):3187-3204.
[22] 赵昊阳,朱俊杰.植物激素对盐胁迫的响应、适应及调控机制研究进展[J/OL].分子植物育种,2023:1-22.(2023-07-24).https://kns.cnki.net/kcms/detail/46.1068.S.20230724.1431.005.html.ZHAO Haoyang,ZHU Junjie. Review of progress of response,adaptation and regulatory mechanisms of plant hormones in responses to salt stress[J/OL]. Molecular Plant Breeding,2023:1-22. (2023-07-24). https://kns.cnki.net/kcms/detail/46.1068.S.20230724.1431.005.html.
[23] ZHONG Y P,QI X J,CHEN J Y,LI Z,BAI D F,WEI C G,FANG J B. Growth and physiological responses of four kiwifruit genotypes to salt stress and resistance evaluation[J]. Journal of Integrative Agriculture,2019,18(1):83-95.
[24] PAPADAKIS I E,VENETI G,CHATZISSAVVIDIS C,THERIOS I.Physiological and growth responses of sour cherry(Prunus cerasus L.) plants subjected to short-term salinity stress[J].Acta Botanica Croatica,2018,77(2):197-202.
[25] 黄有军,王正加,郑炳松,黄胜利,吴家森,黄坚钦.盐胁迫下罗汉松生长和生理变化的研究[J].中南林业科技大学学报,2007,27(5):58-61.HUANG Youjun,WANG Zhengjia,ZHENG Bingsong,HUANG Shengli,WU Jiasen,HUANG Jianqin. Growth and physiological changes of Podocarpus macrophyllus under salt stress[J]. Journal of Central South University of Forestry &Technology,2007,27(5):58-61.
[26] ZHANG X Y,LI S H,TANG T,LIU Y D,MOBEEN TAHIR M,WANG C,MENG Z Q,NIU J X,YANG W W,MA J J,ZHANG D. Comparison of morphological,physiological,and related-gene expression responses to saline-alkali stress in eight apple rootstock genotypes[J]. Scientia Horticulturae,2022,306:111455.
[27] FENG X H,HUSSAIN T,GUO K,AN P,LIU X J. Physiological,morphological and anatomical responses of Hibiscus moscheutos to non-uniform salinity stress[J].Environmental and Experimental Botany,2021,182:104301.
[28] ZHU S P,NONG J F,LUO G T,LI Q P,WANG F S,JIANG D,ZHAO X C.Varied tolerance and different responses of five Citrus rootstocks to acid stress by principle component analysis and orthogonal analysis[J]. Scientia Horticulturae,2021,278:109853.
[29] 李志萍,张文辉,崔豫川.NaCl 和Na2CO3 胁迫对栓皮栎种子萌发及幼苗生长的影响[J].生态学报,2015,35(3):742-751.LI Zhiping,ZHANG Wenhui,CUI Yuchuan. Effects of NaCl and Na2CO3 stresses on seed germination and seedling growth of Quercus variabilis[J]. Acta Ecologica Sinica,2015,35(3):742-751.
[30] 闫文华,吴德军,燕丽萍,王因花,任飞,燕琳,刘婕,于林霖.盐胁迫下白蜡无性系苗期的耐盐性综合评价[J].北京林业大学学报,2019,41(11):44-53.YAN Wenhua,WU Dejun,YAN Liping,WANG Yinhua,REN Fei,YAN Lin,LIU Jie,YU Linlin. Comprehensive evaluation of salt tolerance of clones of Fraxinus in spp.seedling stage under salt stress[J]. Journal of Beijing Forestry University,2019,41(11):44-53.
[31] 杨万鹏.NaCl 胁迫对黑果枸杞形态特征及生理特性的影响[D].兰州:甘肃农业大学,2019.YANG Wanpeng. Effect of NaCl stress on morphological and physiological characteristics of Lycium ruthenicum Murr.[D].Lanzhou:Gansu Agricultural University,2019.
[32] BUKHAT S,MANZOOR H,ATHAR H U R,ZAFAR Z U,AZEEM F,RASUL S. Salicylic acid induced photosynthetic adaptability of Raphanus sativus to salt stress is associated with antioxidant capacity[J]. Journal of Plant Growth Regulation,2020,39(2):809-822.
[33] KARIMI S,KARAMI H,VAHDATI K,MOKHTASSI-BIDGOLI A. Antioxidative responses to short-term salinity stress induce drought tolerance in walnut[J]. Scientia Horticulturae,2020,267:109322.
[34] DICHALA O,THERIOS I,PAPADOPOULOS A,CHATZISTATHIS T,CHATZISAVVIDIS C,ANTONOPOULOU C. Effects of varying concentrations of different salts on mineral composition of leaves and roots of three pomegranate(Punica granatum L.)cultivars[J].Scientia Horticulturae,2021,275:109718.
[35] SARABI B,FRESNEAU C,GHADERI N,BOLANDNAZAR S,STREB P,BADECK F W,CITERNE S,TANGAMA M,DAVID A,GHASHGHAIE J. Stomatal and non-stomatal limitations are responsible in down-regulation of photosynthesis in melon plants grown under the saline condition:Application of carbon isotope discrimination as a reliable proxy[J].Plant Physiology and Biochemistry,2019,141:1-19.
[36] REN G X,SHI Y. The effects of drought stress on the photosynthetic parameters and dry leaf yield of Stevia rebaudina Bertoni[J]. Advanced Materials Research,2012,518/519/520/521/522/523:4786-4789.
[37] SPERLING O,PERRY A,BEN-GAL A,YERMIYAHU U,HOCHBERG U. Potassium deficiency reduces grapevine transpiration through decreased leaf area and stomatal conductance[J].Plant Physiology and Biochemistry,2024,208:108534.
[38] ASAYESH Z M,ARZANI K,MOKHTASSI-BIDGOLI A,ABDOLLAHI H. Gas exchanges and physiological responses differ among‘Pyrodwarf’clonal and‘Dargazi’seedling pear (Pyrus communis L.) rootstocks in response to drought stress[J]. Journal of Soil Science and Plant Nutrition,2023,23(4):6469-6484.
[39] MU D Y,DING C. Developing a salinity tolerance indicator for tree varieties at challenging sites and urban forests based on inferences of physiological responses:An example of Ulmus pumila[J].Trees,2022,36(2):593-607.
[40] 姬新颖,唐佳莉,李敖,郑旭,王红霞,张俊佩.盐胁迫下不同基因型核桃实生幼苗生长及生理响应[J]. 林业科学,2024,60(2):65-77.JI Xinying,TANG Jiali,LI Ao,ZHENG Xu,WANG Hongxia,ZHANG Junpei. Growth and physiological responses of walnut seedlings with different genotypes under salt stress[J]. Scientia Silvae Sinicae,2024,60(2):65-77.
[41] 李瑞强,王玉祥,孙玉兰,张磊,陈爱萍.盐胁迫对5 份无芒雀麦苗期生长和生理生化的影响及综合性评价[J].草业学报,2023,32(1):99-111.LI Ruiqiang,WANG Yuxiang,SUN Yulan,ZHANG Lei,CHEN Aiping.Effects of salt stress on the growth,physiology,and biochemistry of five Bromus inermis varieties[J]. Acta Prataculturae Sinica,2023,32(1):99-111.