桃[Prunus persica(L.)Batsch]是蔷薇科李属核果类落叶果树,原产于中国西部,是中国重要的经济树种之一。桃蚜(Myzus persicae Sulzer)危害是造成桃树减产和桃果实品质降低的一个重要因素,不仅是直接取食造成危害,还间接导致光合效率下降以及蚜虫取食造成病毒传播等,对桃农造成的损失更大[1]。自20世纪90年代以来,国内外研究机构鉴定了多份抗性种质资源,包括法国农科院鉴定了3 份抗桃蚜种质,分别是Rubra、Weeping flower peach(后文简写为WFP)和山桃P1908[2-4];王力荣等[5]2001年对国内419 份资源进行抗桃蚜鉴定,发现山桃(P.davidiana)和寿星桃(P.persica var.densa)类抗桃蚜性较强,筛选出了帚形山桃、红寿星、粉寿星等抗桃蚜种质。但目前产业中抗性品种严重匮乏,抗性种质中帚形山桃和山桃P1908 为野生近缘种,Rubira主要作为砧木,而WFP(P.persica var.weeping)和寿星桃类主要作为观赏桃品种,在鲜食桃中直接利用较为困难。目前主要报道的抗桃蚜普通桃只有毛桃种质2013-04-20R[6]和栽培桃来源的09 南3-30[7]等,无法满足桃抗桃蚜育种的需求。在生产中,桃蚜主要利用农药进行防治,不仅影响桃产业绿色健康发展,而且污染环境。因此,挖掘利用抗性种质资源,开发抗性育种分子标记,培育抗桃蚜桃品种是解决桃蚜危害的根本途径。
目前已挖掘到了多个抗桃蚜位点。1994 年,Monet等[4]鉴定出WFP的抗桃蚜性状是由Rm1单基因显性控制的,并由Pascal 等[8]在2017 年将Rm1 定位到1 号染色体底部;Sauge 等[9]推测Rubira 抗桃蚜性由Rm2单基因显性控制,Lambert等[10-11]将Rm2也定位到了1 号染色体末端,随后又将Rm2 定位区间进一步缩小至SNP_ICA131 130和SNP_ICA126 668之间,包含6 个共分离的SNP。牛良等[12]发现寿星桃抗性基因Rm3,该基因也为单基因显性控制,张南南等[13]在2017 年对Rm3 基因进行了定位,Pan 等[14]确定了Rm3 区间内的抗桃蚜候选基因NLR1。山桃的抗桃蚜性被认为是多基因控制的数量性状,Sauge等[15]发现控制山桃P1908抗性的8个QTL位点分布在7个连锁群上,并确定了主效位点位于3号染色体上分子标记AG106附近。王君秀等[16-17]利用BSA和关联分析,将山桃抗桃蚜主效QTL 定位到3 号染色体上950 kb 的区间,并通过以山桃的两个单倍型基因组作为参考基因组,进一步缩小了山桃的抗桃蚜区间,结合转录组分析,确定了山桃抗桃蚜的主效基因PdaWRKY4。
王新卫等[18]基于山桃抗桃蚜主效QTL qGPAR-3-1 紧密连锁的InDel 位点,开发了山桃InDel24 分子标记,该标记对高抗(HR)、抗(R)和中抗(MR)3种不同等级的抗性鉴定准确率在95%以上,同时,王新卫等[6]根据多年田间调查,发现了一个抗桃蚜的毛桃种质2013-04-20R,并根据该毛桃种质抗桃蚜性状紧密连锁的InDel 位点开发了Pp-InDel-23分子标记(后简称InDel23)。潘磊等[19]在进行Rm3定位的过程中,发现了一个与其紧密连锁的20 bp的特异性插入片段,从而开发了P62 分子标记,并对该分子标记进行了通用性验证,结果表明,该标记不仅在寿星桃类抗桃蚜分离群体中适用,在栽培种毛桃和野生近缘种中均能进行抗桃蚜性状区分。此外,潘磊等[7]发现了一种栽培种来源的抗桃蚜新种质09 南3-30,该材料对桃蚜表现为强烈的趋避型抗性,随后将该种质抗性位点定位在了3 号染色体上的5.44 Mb 候选区段内,并由此开发出QMR分子标记[20]。
为验证抗桃蚜分子标记的准确性,促进分子标记在育种中的应用,笔者在本研究中对4 个杂交群体及1 个实生群体进行了抗桃蚜表型评价,结合群体表型及育成抗桃蚜品种对4个不同来源的抗桃蚜分子标记进行了有效性验证,确认了抗蚜观赏桃品种的抗性来源,并在抗桃蚜群体中选出了12个抗桃蚜且农艺性状优良的种质,为后期抗桃蚜品种的选育提供了支撑。
1 材料和方法
1.1 材料
以育成的8 个抗桃蚜观赏桃品种、一个抗桃蚜栽培桃品种以及4 个杂交群体和1 个实生群体及其亲本为试验材料。其中,满天红为[2-7(白凤×红寿星)]自然实生,白花山碧桃为(山桃×碧桃)自然杂交,南一区西29-13为[(橡皮油桃姊妹系99-5-69×帚形山桃)F1]自然实生。所有试验材料均来自中国农业科学院郑州果树研究所新乡育种基地及国家葡萄桃种质资源圃(郑州),材料信息如表1。
表1 试验材料信息
Table 1 Test material information
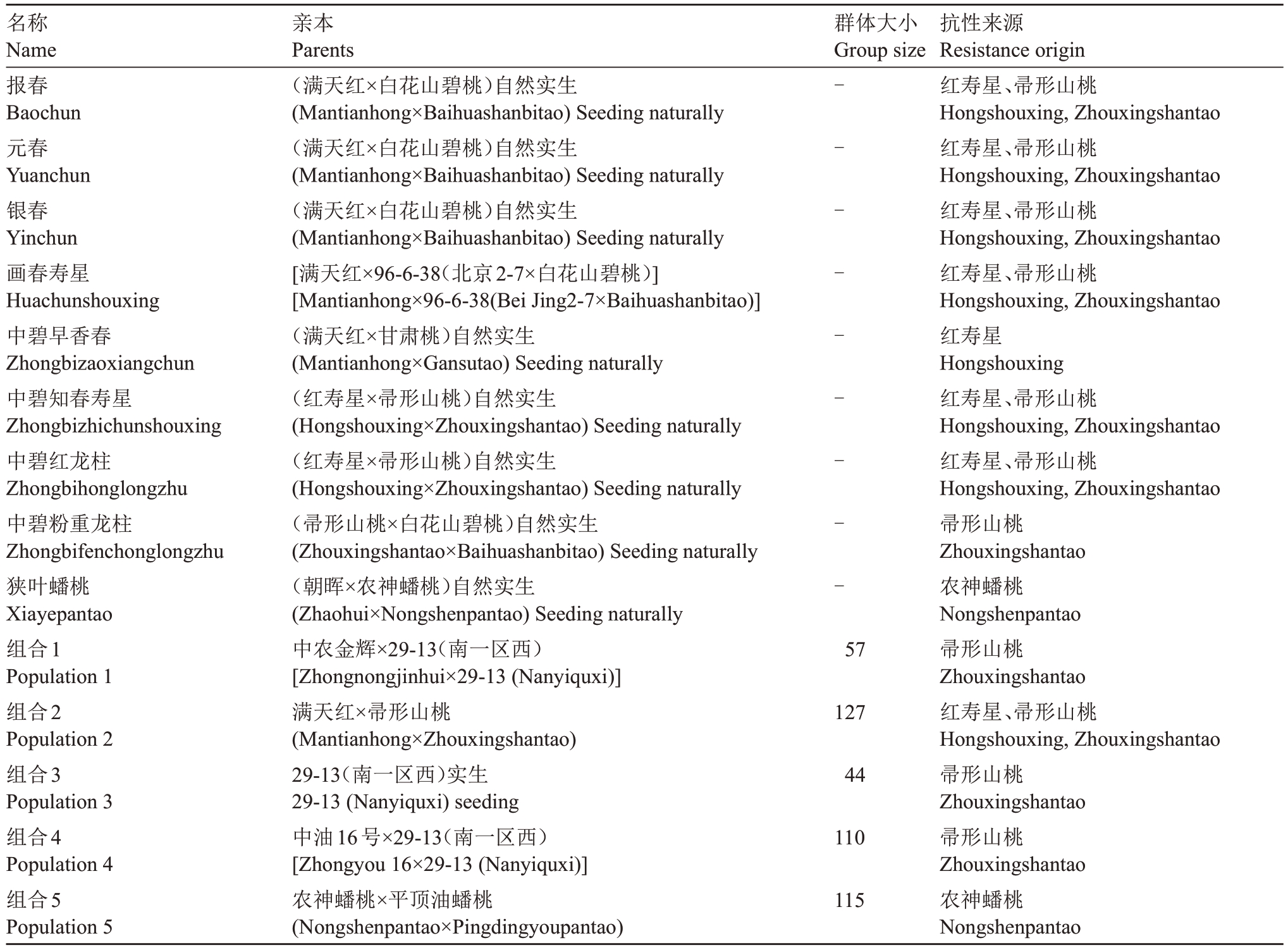
名称Name报春Baochun元春Yuanchun银春Yinchun画春寿星Huachunshouxing中碧早香春Zhongbizaoxiangchun中碧知春寿星Zhongbizhichunshouxing中碧红龙柱Zhongbihonglongzhu中碧粉重龙柱Zhongbifenchonglongzhu狭叶蟠桃Xiayepantao组合1 Population 1组合2 Population 2组合3 Population 3组合4 Population 4组合5 Population 5亲本Parents(满天红×白花山碧桃)自然实生(Mantianhong×Baihuashanbitao)Seeding naturally(满天红×白花山碧桃)自然实生(Mantianhong×Baihuashanbitao)Seeding naturally(满天红×白花山碧桃)自然实生(Mantianhong×Baihuashanbitao)Seeding naturally[满天红×96-6-38(北京2-7×白花山碧桃)][Mantianhong×96-6-38(Bei Jing2-7×Baihuashanbitao)](满天红×甘肃桃)自然实生(Mantianhong×Gansutao)Seeding naturally(红寿星×帚形山桃)自然实生(Hongshouxing×Zhouxingshantao)Seeding naturally(红寿星×帚形山桃)自然实生(Hongshouxing×Zhouxingshantao)Seeding naturally(帚形山桃×白花山碧桃)自然实生(Zhouxingshantao×Baihuashanbitao)Seeding naturally(朝晖×农神蟠桃)自然实生(Zhaohui×Nongshenpantao)Seeding naturally中农金辉×29-13(南一区西)[Zhongnongjinhui×29-13(Nanyiquxi)]满天红×帚形山桃(Mantianhong×Zhouxingshantao)29-13(南一区西)实生29-13(Nanyiquxi)seeding中油16号×29-13(南一区西)[Zhongyou 16×29-13(Nanyiquxi)]农神蟠桃×平顶油蟠桃(Nongshenpantao×Pingdingyoupantao)群体大小Group size---------57 127 44 110 115抗性来源Resistance origin红寿星、帚形山桃Hongshouxing,Zhouxingshantao红寿星、帚形山桃Hongshouxing,Zhouxingshantao红寿星、帚形山桃Hongshouxing,Zhouxingshantao红寿星、帚形山桃Hongshouxing,Zhouxingshantao红寿星Hongshouxing红寿星、帚形山桃Hongshouxing,Zhouxingshantao红寿星、帚形山桃Hongshouxing,Zhouxingshantao帚形山桃Zhouxingshantao农神蟠桃Nongshenpantao帚形山桃Zhouxingshantao红寿星、帚形山桃Hongshouxing,Zhouxingshantao帚形山桃Zhouxingshantao帚形山桃Zhouxingshantao农神蟠桃Nongshenpantao
1.2 方法
1.2.1 农艺性状评价 为筛选优异抗性资源,对试验材料进行基本农艺性状评价。对目标群体成熟期进行初步确定,在八至九成熟时进行采样,选取树冠中部外围6个果实,每株均在相近位置选取,测量其果实质量、可溶性固形物含量,并对其风味进行评价,具体方法及评价标准参考王力荣等[21]编著的《桃种质资源描述规范和数据标准》。
1.2.2 抗/感桃蚜表型鉴定 在桃蚜盛发期对未经农药处理自然感蚜的目标群体进行表型鉴定,选取靠上部位的嫩梢进行观察,根据嫩梢上桃蚜的有无、卷曲程度对每个单株进行表型鉴定,具体方法及评价标准参考王力荣等[21]编著的《桃种质资源描述规范和数据标准》。
1.2.3 基因组DNA提取 每份材料取适量嫩叶,分别装入2.0 mL 的离心管中,加入钢珠,在液氮中研磨后用CTAB 试剂盒(艾德莱生物公司)进行基因组DNA 提取,提取后用NanoDrop1000 spectrophotometer(Themo Scientific)紫外分光光度计对DNA浓度和纯度进行测定,然后用无菌水将DNA 稀释到20 ng·μL-1保存至-20 ℃备用。
1.2.4 分子标记检测 利用前人开发的4个分子标记InDel24[17]、P62[19]、InDel23[6]、QMR[20]对杂交后代群体单株进行检测,引物由北京普乐海生物科技有限公司合成,引物序列见表2,4 个标记的PCR 扩增体系均为20 μL,包括2×Mix混合液10 μL(南京诺唯赞生物科技股份有限公司,南京),模板DNA 1 μL(20 ng·μL-1),上、下游引物各1 μL(10 μmol·μL-1),ddH2O 7 μL;反应程序为:95 ℃预变性5 min,95 ℃变性1 min,58 ℃退火1 min,72 ℃延伸10 s,35个循环;72 ℃延伸5 min。反应产物用6%聚丙烯酰胺凝胶电泳进行检测。
表2 聚丙烯酰胺凝胶电泳所用引物序列信息
Table 2 Information on primers used for polyacrylamide gel electrophoresis
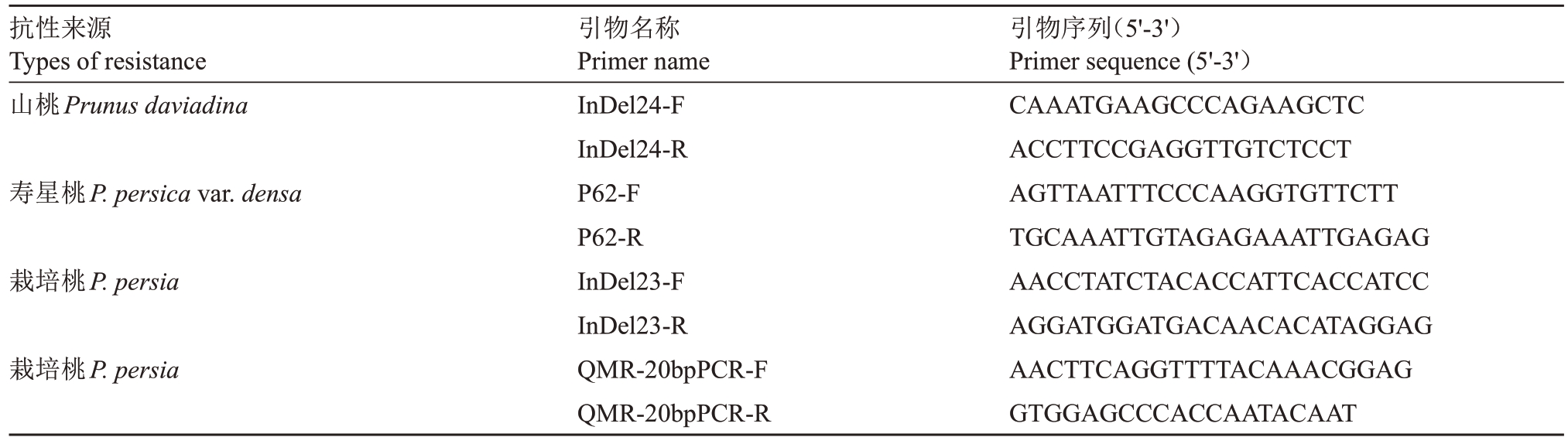
抗性来源Types of resistance山桃Prunus daviadina寿星桃P.persica var.densa栽培桃P.persia栽培桃P.persia引物名称Primer name InDel24-F InDel24-R P62-F P62-R InDel23-F InDel23-R QMR-20bpPCR-F QMR-20bpPCR-R引物序列(5'-3')Primer sequence(5'-3')CAAATGAAGCCCAGAAGCTC ACCTTCCGAGGTTGTCTCCT AGTTAATTTCCCAAGGTGTTCTT TGCAAATTGTAGAGAAATTGAGAG AACCTATCTACACCATTCACCATCC AGGATGGATGACAACACATAGGAG AACTTCAGGTTTTACAAACGGAG GTGGAGCCCACCAATACAAT
1.3 数据分析
使用Microsoft Excel 2019进行数据处理。群体抗桃蚜率/%=抗桃蚜植株数量/群体总株数×100,分子标记准确率/%=表型与分子标记一致的株数/群体总株数×100。
2 结果与分析
2.1 表型鉴定结果
抗桃蚜植株嫩叶舒展,未见蚜虫或者有零星几只,未对植株造成任何影响,而不抗桃蚜的植株嫩叶甚至整个枝头叶片均卷曲,严重影响植株生长,如图1。杂交群体田间调查结果见表3,组合1、组合3、组合4所有单株均为抗桃蚜植株,抗桃蚜等级为高抗;组合2 抗桃蚜率为99.21%,126 株抗桃蚜植株均为高抗,1 株感蚜植株为高感;组合5 抗桃蚜率为63.48%,其中抗桃蚜植株与感蚜植株分别为73株和42 株(χc2=8.37>χ20.05=3.84,p<0.05),不符合孟德尔遗传1∶1 的分离规律。杂交组合的亲本中,南一区西29-13、满天红、帚形山桃、农神蟠桃为抗蚜亲本,而中农金辉、中油16 号、平顶油蟠桃为感蚜亲本。报春、元春、银春、满天红、画春寿星、中碧早香春、中碧知春寿星、中碧红龙柱、中碧粉重龙珠、红寿星、白花山碧桃、狭叶蟠桃均为抗蚜种质。

图1 抗桃蚜叶片(左)与感桃蚜叶片(右)对比
Fig.1 Comparison between leaves resistant to green peach aphid(left)and leaves susceptible to green peach aphid(right)
表3 桃蚜田间自然发生情况调查
Table 3 Investigation of the natural occurrence of peach aphid in the field
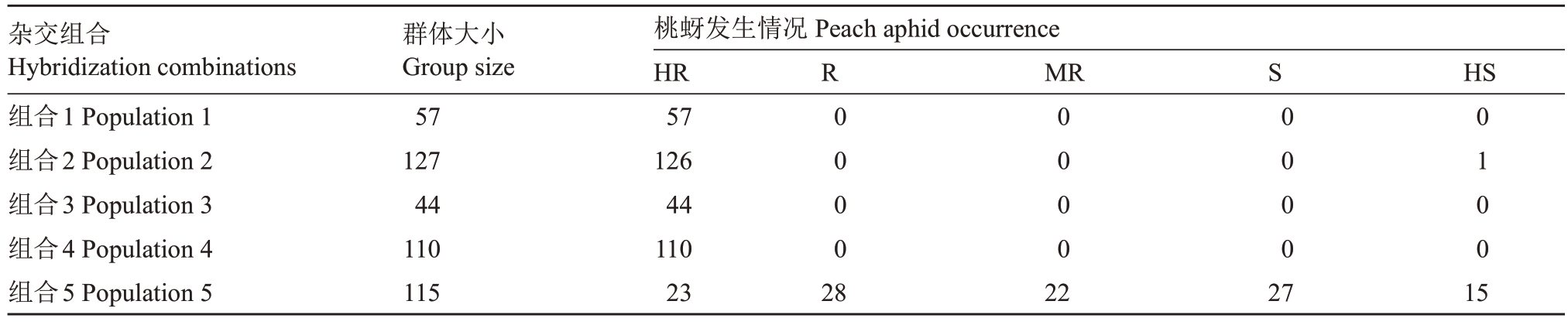
注:表中HR、R、MR 为高抗、抗和中抗,抗桃蚜程度递减,S、HS 为感蚜和高感蚜,感蚜程度递增。
Note:In the Table,HR,R and MR represent high resistance,aphid resistance,and moderate resistance,with the degree of resistance decreasing;S and HS represent aphid susceptible and highly aphid susceptible,with the degree of aphid susceptible increasing.
杂交组合Hybridization combinations组合1 Population 1组合2 Population 2组合3 Population 3组合4 Population 4组合5 Population 5群体大小Group size 57 127 44 110 115桃蚜发生情况Peach aphid occurrence HR 57 126 44 110 23 R0000 MR HS 0000 S0000 0100 15 282227
2.2 分子标记InDel24的检测结果
育成品种中,报春、元春、银春、画春寿星及亲本中白花山碧桃、帚形山桃、南一区西29-13具有山桃来源的抗桃蚜性,其中,南一区西29-13 为纯合抗性,如图2。利用InDel 分子标记InDel24 对帚形山桃后代进行基因型检测。结果显示,在组合1(图3)、组合2、组合3(图4)和组合4中分子标记的准确率分别达到了100%、99.21%、100%和100%。其中,在组合1、组合2 和组合4 中,聚丙烯酰胺凝胶电泳均能扩增出两条清晰的条带,将抗性条带记为A,感蚜条带记为a,则在这3个杂交组合里所有的植株的抗桃蚜基因型均为Aa。在组合3中,36株单株仅有一条抗桃蚜条带,基因型为AA,为纯合抗性,其余8株基因型为Aa,为杂合抗性。结合其亲本情况,组合1和组合4均为母本感桃蚜父本抗桃蚜杂交组合,父本在该位点为纯合抗性,故后代均为杂合抗桃蚜植株,组合2母本具有寿星桃来源的抗性,但认为其不具备山桃来源抗性,因此后代在该位点均为杂合抗性。组合3 为纯合抗性植株实生后代,推测全部为纯合抗性,可能由于非严格的自花授粉,出现了个别单株为杂合抗性的情况。
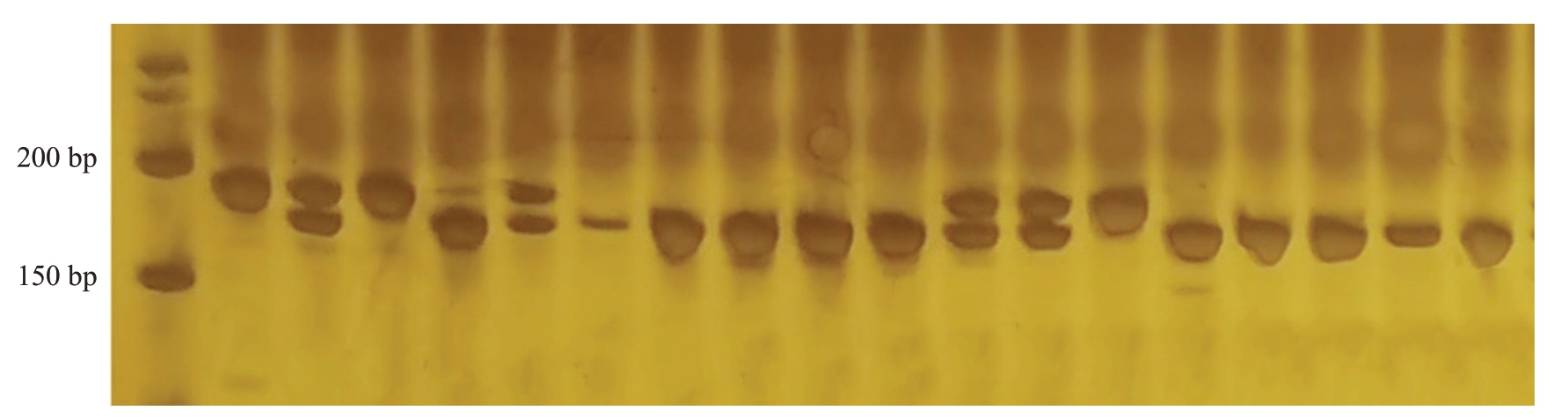
图2 分子标记InDel24 在部分品种及组合亲本中的条带Fig.2 Bands of molecular marker InDel24 in some varieties and combined parents
从左到右不同泳道分别为Marker、报春、元春、银春、满天红、画春寿星、中碧早香春、中碧知春寿星、中碧红龙柱、中碧粉重龙珠、红寿星、白花山碧桃、帚型山桃、29-13(南一区西)、中农金辉、中油16 号、农神蟠桃、平顶油蟠桃、狭叶蟠桃。图5、7、9 与此相同。
From left to right, the lanes are Marker, Baochun,Yuanchun,Yinchun, Mantianhong, Huachunshouxing, Zhongbizaoxiangchun, Zhongbizhichunshouxing,Zhongbihonglongzhu,Zhongbifenchonglongzhu,Hongshouxing,Baihuashanbitao,Zhouxingshantao,29-13(Nanyiquxi),Zhongnongjinhui,Zhongyou 16,Nongshenpantao,Pingdingyoupantao,Xiayepantao.Figures 5,7 and 9 are the same.
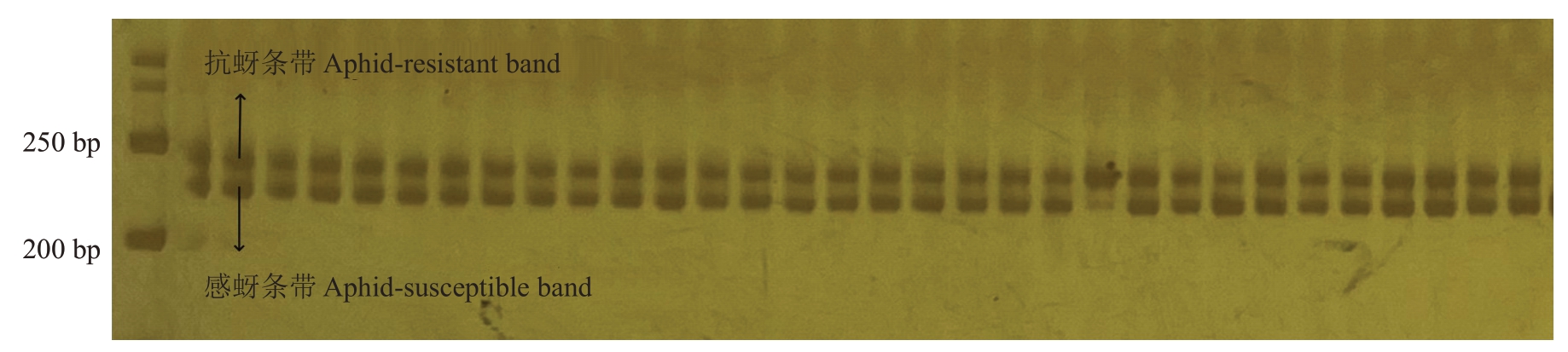
图3 分子标记InDel24 在组合1 中的部分条带
Fig.3 The molecular marker InDel24 is partially banded in combination 1
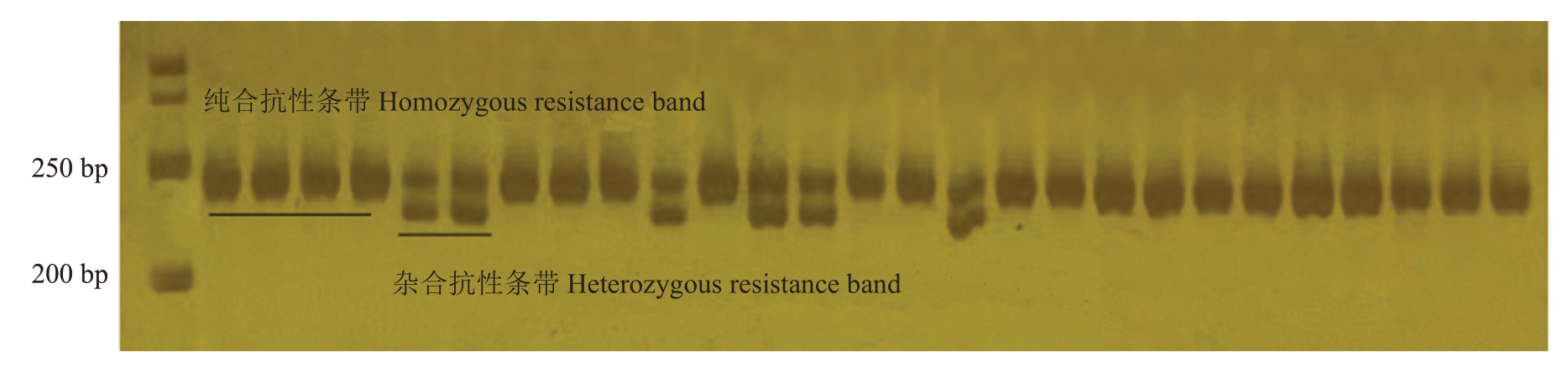
图4 分子标记InDel24 在组合3 中的部分条带
Fig.4 The molecular marker InDel24 is partially banded in combination 3
2.3 分子标记InDel23的检测结果
分子标记Indel23 对部分育成品种及杂交群体亲本进行检验(图5),发现育成品种中元春、银春、满天红、画春寿星、中碧早香春、中碧知春寿星、中碧红龙柱、中碧粉重龙柱均含有抗性条带且与表型相符合,其中,元春、中碧早香春、中碧红龙柱为纯合抗性。结合亲本关系及扩增结果来看,Indel23可能可以用来区分满天红的抗性,如图6,推测可能扩增的是同源基因,于是用Indel23鉴定组合2中的96份材料,有26 份未扩增出有效条带,70 份为抗性杂合条带,分子标记准确率为72.92%。由于分子标记Indel23 来自毛桃,因此分子标记InDel23 鉴定组合5,所有单株均扩增出了约100 bp 的条带,无法区分抗感单株,此分子标记不适用于该群体及其后代的抗桃蚜植株鉴定。
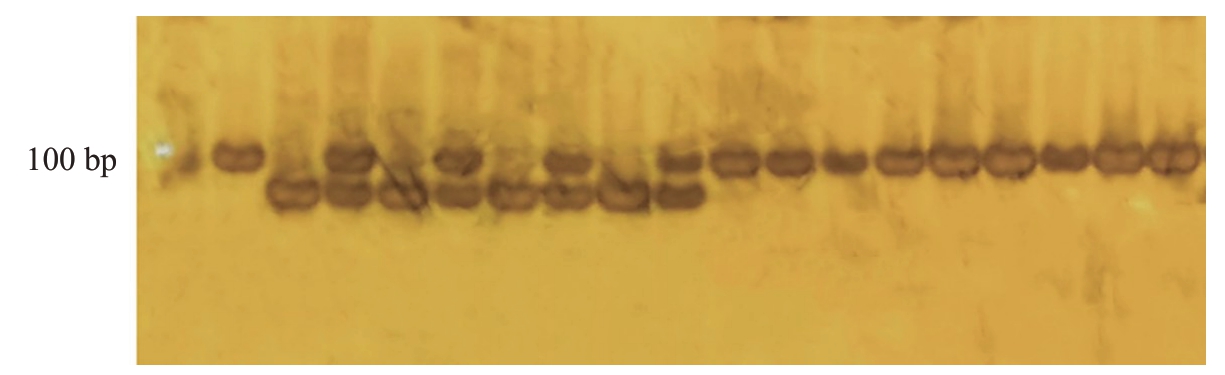
图5 分子标记InDel23 在部分品种及组合亲本中的条带
Fig.5 Bands of molecular marker InDel23 in some varieties and combined parents
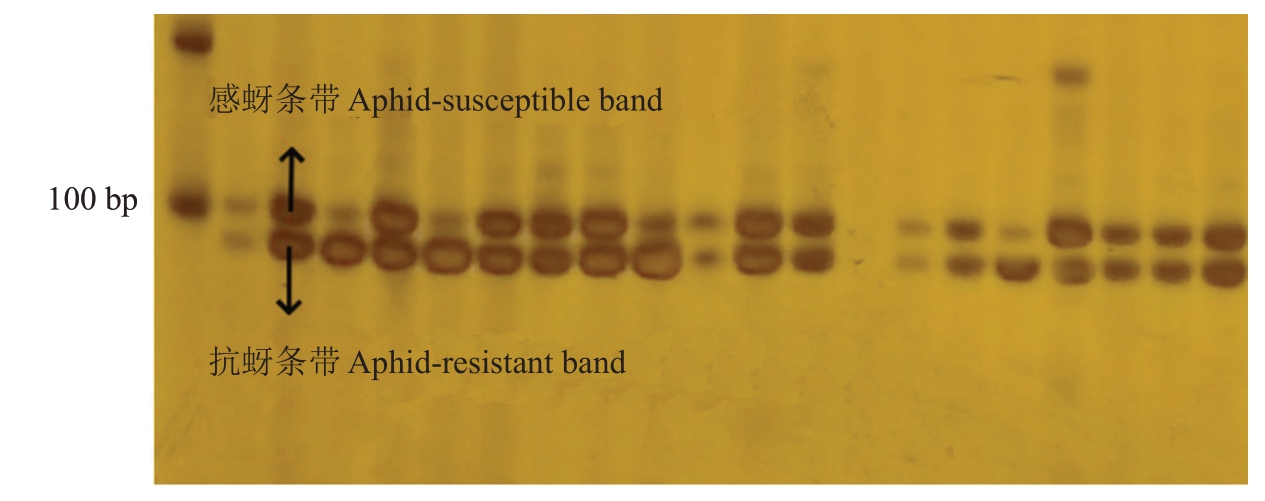
图6 分子标记InDel23 在组合2 中的部分条带
Fig.6 The molecular marker InDel23 is partially banded in combination 2
2.4 分子标记P62的检测结果
分子标记P62检测育成品种结果显示(图7),育成品种中,元春、银春、画春寿星、中碧早香春、中碧知春寿星、中碧红龙柱、中碧粉红龙柱具有寿星桃来源的抗性;亲本中,除红寿星外,白花山碧桃、帚形山桃、南一区西29-13 也能扩增出抗性条带,其中,中碧早香春、中碧知春寿星、中碧红龙柱、中碧粉重龙柱为纯合抗性。用该分子标记检测组合1、组合2(图8)和组合3,结果显示,P62 在组合1 和组合3 中均无法有效区分抗桃蚜植株。对于组合2,分子标记鉴定准确率为99.21%,所有植株均为杂合抗性,满天红杂交群体后代可用该分子标记进行区分。该分子标记鉴定组合5,所有单株均扩增出了约200 bp的条带,无法区分抗感单株,此分子标记不适用于该栽培桃群体及其后代的抗桃蚜植株鉴定。
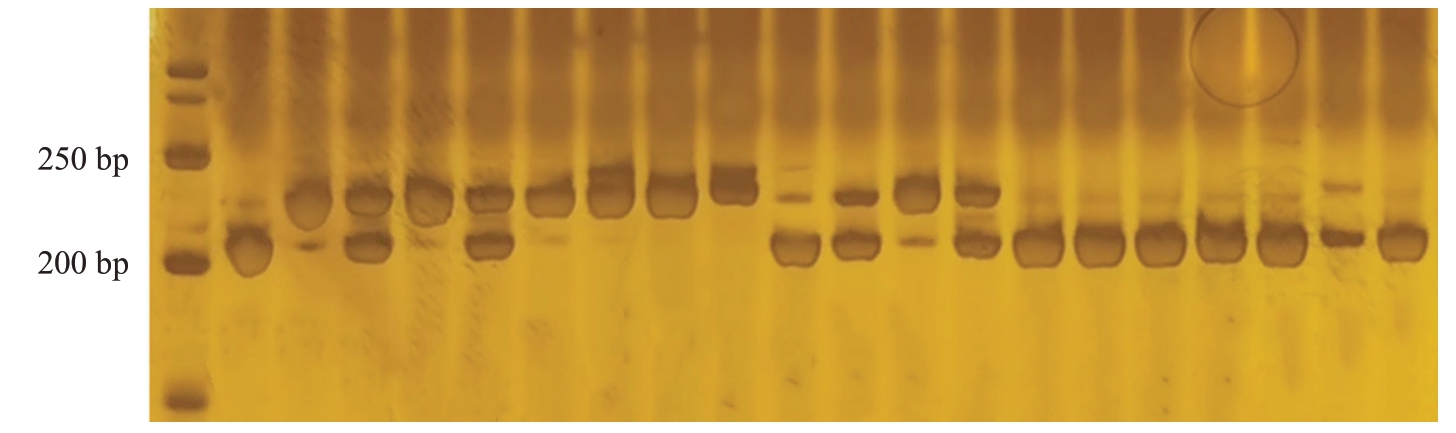
图7 分子标记P62 在部分品种及组合亲本中的条带
Fig.7 Bands of molecular marker P62 in some varieties and combined parents
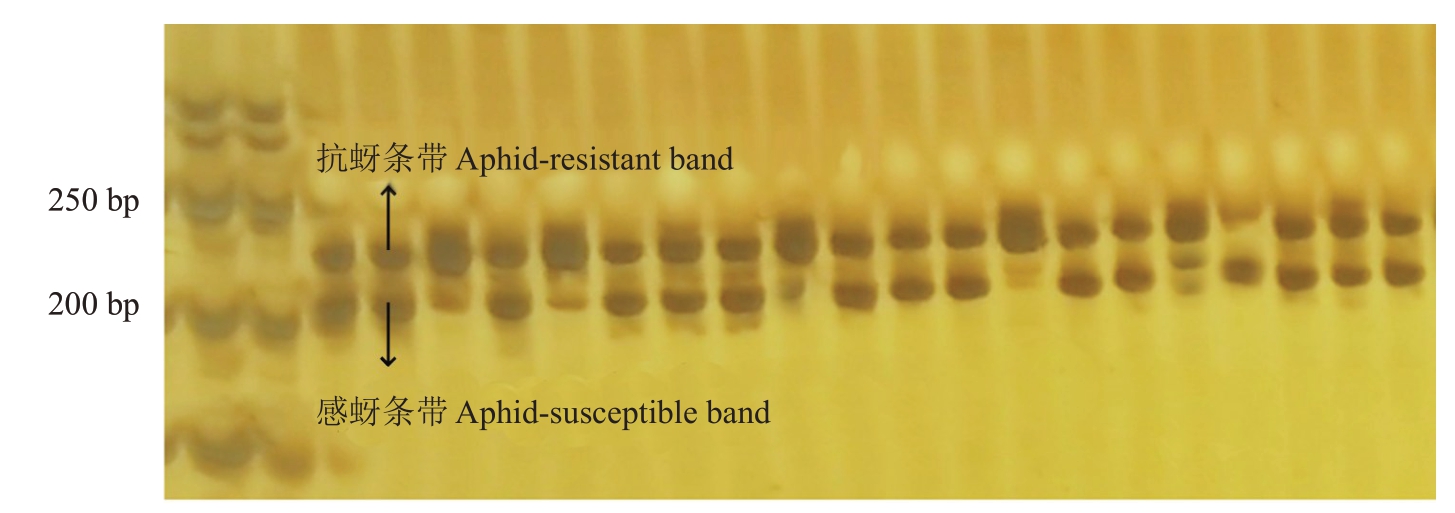
图8 分子标记P62 在组合2 中的部分条带
Fig.8 Molecular marker P62 in a partial band in combination 2
2.5 分子标记QMR的检测结果
分子标记QMR鉴定结果显示(图9),育成品种中,中碧知春寿星和狭叶蟠桃为抗性植株,且均为杂合抗性。鉴定组合5的结果显示(图10),在115份材料中,分子标记鉴定结果与表型相符的有72份,准确率为62.61%。在表型与基因型相符的72份材料中,有6株为纯合感桃蚜植株,66株为杂合抗桃蚜植株。
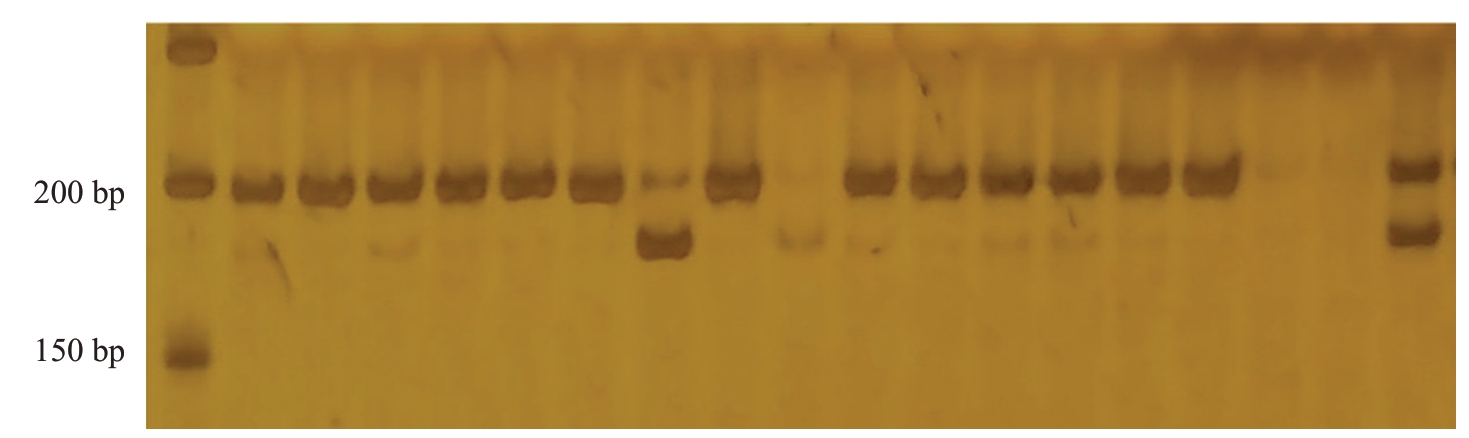
图9 分子标记QMR 在部分品种及组合亲本中的条带
Fig.9 Bands of molecular marker QMR in some varieties and combined parents
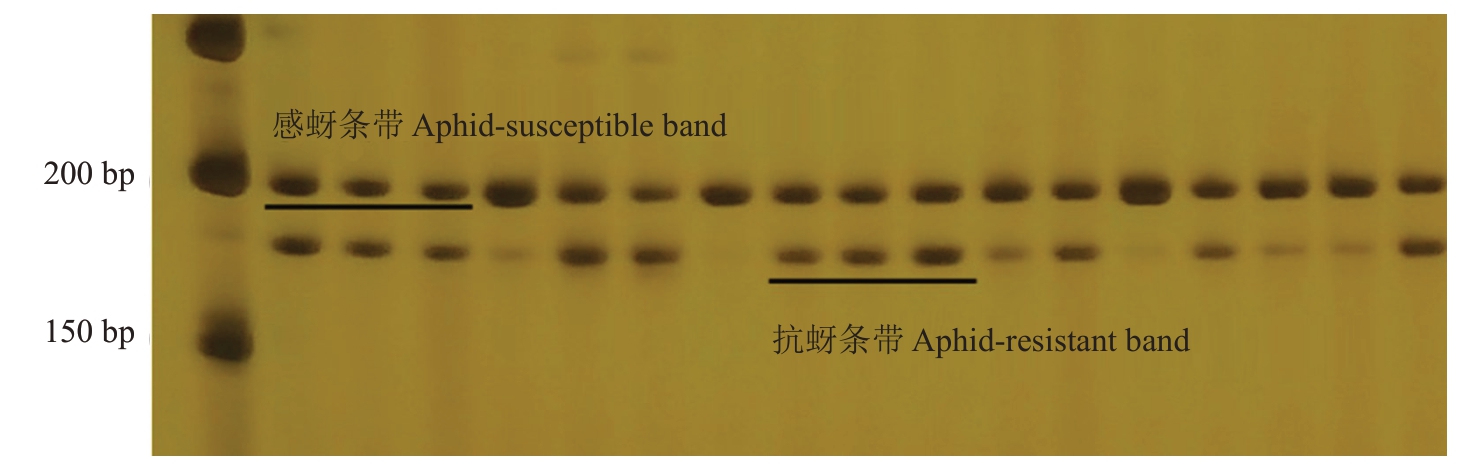
图10 分子标记QMR 在组合5 中的部分条带
Fig.10 Molecular marker QMR in a partial band in combination 5
2.6 抗桃蚜优株筛选
结合抗桃蚜表型鉴定及分子标记验证结果,从抗桃蚜单株中根据果实质量、可溶性固形物含量以及风味出发,在组合1中筛选出了12个抗桃蚜优株,见表5、图11。在所挑选的优株中,以小果型为主(50 g<平均单果质量≤100 g),其中,ZCC-1707-95等4 株平均单果质量达到了中果型水平(100 g<平均单果质量≤150 g);可溶性固形物含量有8株达到了极高[可溶性固形物含量(w)≥14%]的标准,其余4 株也均达到了高(12%≤可溶性固形物含量<14%)标准。
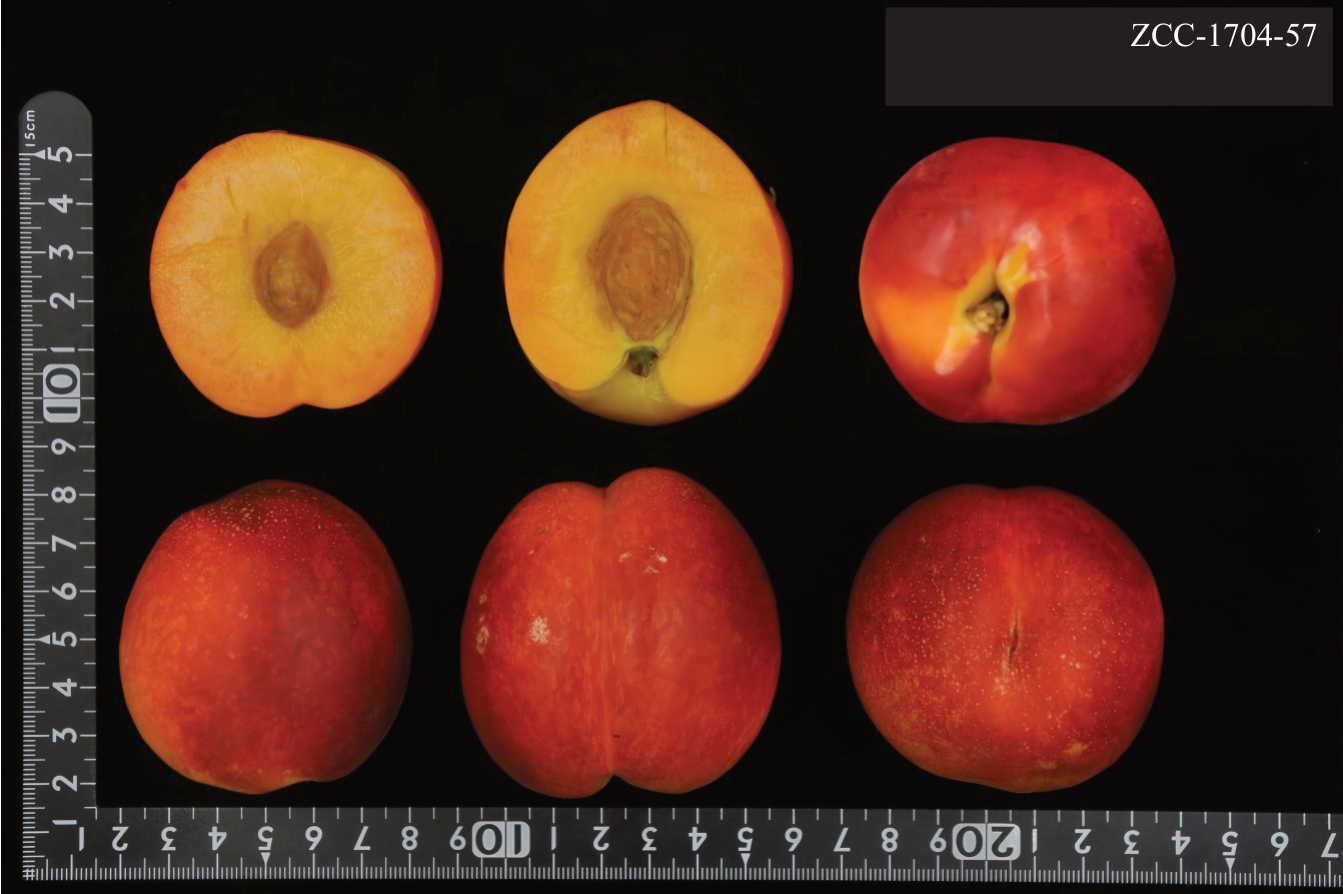
图11 ZCC-1704-57 标准照
Fig.11 Standard photo of ZCC-1704-57
表5 农艺性状评价
Table 5 Evaluation of agronomic traits
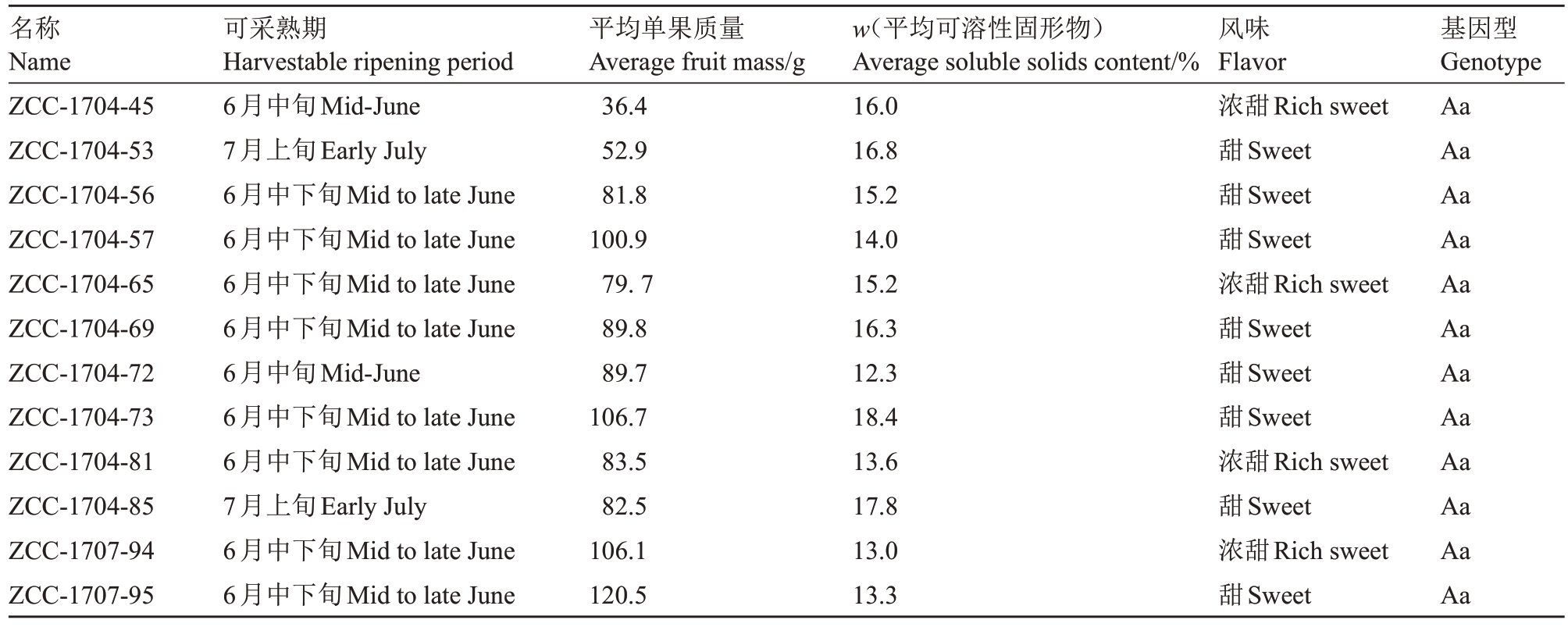
名称Name ZCC-1704-45 ZCC-1704-53 ZCC-1704-56 ZCC-1704-57 ZCC-1704-65 ZCC-1704-69 ZCC-1704-72 ZCC-1704-73 ZCC-1704-81 ZCC-1704-85 ZCC-1707-94 ZCC-1707-95可采熟期Harvestable ripening period 6月中旬Mid-June 7月上旬Early July 6月中下旬Mid to late June 6月中下旬Mid to late June 6月中下旬Mid to late June 6月中下旬Mid to late June 6月中旬Mid-June 6月中下旬Mid to late June 6月中下旬Mid to late June 7月上旬Early July 6月中下旬Mid to late June 6月中下旬Mid to late June平均单果质量Average fruit mass/g 36.4 52.9 81.8 100.9 79.7 89.8 89.7 106.7 83.5 82.5 106.1 120.5 w(平均可溶性固形物)Average soluble solids content/%16.0 16.8 15.2 14.0 15.2 16.3 12.3 18.4 13.6 17.8 13.0 13.3风味Flavor浓甜Rich sweet甜Sweet甜Sweet甜Sweet浓甜Rich sweet甜Sweet甜Sweet甜Sweet浓甜Rich sweet甜Sweet浓甜Rich sweet甜Sweet基因型Genotype Aa Aa Aa Aa Aa Aa Aa Aa Aa Aa Aa Aa
3 讨 论
桃对桃蚜的抗性主要有3 种,一是以山桃类为代表的抗生性,二是以寿星桃类为代表的趋避性,三是在栽培桃里发现的与寿星桃抗性相似但无过敏反应的趋避性抗性。文中选用的4个分子标记来自以上3 种抗性。Indel24 来源于山桃抗性[17],在山桃杂交后代中进行验证时准确率高,可直接用于新品种选育。王力荣等[22]推测白花山碧桃为山桃与普通桃、碧桃的杂交后代。利用山桃标记对白花山碧桃及其后代进行验证,其中报春、元春、银春、画春寿星均能扩增出抗性条带,验证了白花山碧桃抗性来源于帚形山桃。InDel23 分子标记来源于栽培桃种质2013-04-20R,且标记仅在该种质的群体中进行了验证,需要对通用性进行进一步检验[6],文中检测该标记在其他栽培桃中适用性的同时,发现该标记还可以对满天红的后代进行抗桃蚜性状的区分,扩增片段是否为同源基因有待进一步验证。P62分子标记能够有效地区分寿星桃群体,同时,潘磊等[19]发现在桃属近缘种中,P62也能进行抗桃蚜性状的区分,并在后续研究中表明Rm3 基因引物在野生近缘种中扩增到的基因只是其同源基因而非Rm3基因本身,笔者在本研究中用该标记鉴定帚形山桃、南一区西29-13及其杂交后代时发现,在帚形山桃和南一区西29-13中能够扩增到该分子标记,但对其杂交群体进行鉴定时,发现准确性较差。QMR分子标记开发过程中利用的抗桃蚜亲本的抗性来源于从美国引进的品种农神蟠桃[20],笔者课题组在农神蟠桃的杂交后代中发现了抗桃蚜性极强的狭叶蟠桃,在调查过程中发现以农神蟠桃为母本的杂交群体(组合5)在田间表现出了抗桃蚜性,并出现了表型分离,该分离比不符孟德尔遗传规律,推测其抗桃蚜基因可能不是单基因显性遗传。QMR 分子标记在鉴别农神蟠桃后代单株狭叶蟠桃时结果准确,而在农神蟠桃×平顶油蟠桃杂交群体中准确率仅有62.61%,因此,适用于该类型的抗桃蚜标记有待进一步开发。
8 个育成品种中,除中碧早香春仅有寿星桃来源抗性外,其余7 个品种的亲本均为两种不同来源的抗性植株,分别将山桃来源的抗/感基因型记为A/a,寿星桃来源的抗/感基因型记为B/b,在鉴定过程中发现,元春、银春、画春寿星同时可被山桃和寿星桃来源的抗桃蚜标记中检测,基因型分别为AaBb、AABb、AaBb;而报春只能在山桃来源的抗桃蚜标记检测出抗性条带,基因型为AAbb;中碧早香春、中碧知春寿星、中碧红龙柱、中碧粉重龙柱仅能在寿星桃来源的抗桃蚜标记检测出抗性条带,基因型均为aaBB。推测单抗品种在杂交过程中亲本基因型为杂合,后代出现了分离导致该来源的抗性基因丢失。
在上述的遗传机制中,山桃对桃蚜的抗性是多基因控制的数量性状,抗桃蚜机制相对于单基因(Rm1、Rm2、Rm3)控制的抗性而言较为复杂,抗性效果较为持久[23],正是这一复杂性,为杂交过程中优株的筛选创造了更多的可能性。山桃除了在抗桃蚜上有优异表现外,在抗寒方面也胜于普通桃,Cao等[24]研究发现,山桃抗性基因数目在进化过程中显著增加,揭示了山桃综合抗性强的分子机制。虽然山桃综合抗性强,但是农艺性状差,不易利用,因此需要进行多代杂交选育,结合其复杂的抗性机制,选育出的抗桃蚜植株可能具有丰富的表型差异,符合多样性育种目标[25]。综上所述,在主要的抗桃蚜类型中对已开发的分子标记进行适用性验证,具有较强的实用意义。
4 结 论
笔者在本研究中明确了8个育成品种的抗性来源,进一步确认了分子标记InDel24在帚形山桃杂交群体中的高准确率,P62 分子标记可以用于准确鉴别寿星桃及其杂交后代的抗桃蚜性状,而对于栽培桃中的农神蟠桃群体,InDel23 和P62 无法对其鉴别,QMR标记准确率不高,仍然需要进一步研究。
[1] PASCAL T,PFEIFFER F,KERVELLA J,LACROZE J P,SAUGE M H,WEBER W E. Inheritance of green peach aphid resistance in the peach cultivar‘Rubira’[J]. Plant Breeding,2002,121(5):459-461.
[2] SAUGE M H,KERVELLA J,PASCAL T. Settling behaviour and reproductive potential of the green peach aphid Myzus persicae on peach varieties and a related wild Prunus[J]. Entomologia Experimentalis et Applicata,1998,89(3):233-242.
[3] MASSONIÉ G,MAISON P,MONET R,GRASSELLY C.Résistance au puceron vert du pêcher,Myzus persicae Sulzer(Homoptera Aphididae)chez Prunus persica(L.)Batsch et d’autres espèces de Prunus[J].Agronomie,1982,2(1):63-70.
[4] MONET R,MASSONIÉ G.Déterminisme génétique de la résistance au puceron vert(Myzus persicae)chez le pêcher.Résultats complémentaires[J].Agronomie,1994,14(3):177-182.
[5] 王力荣,朱更瑞,方伟超,左覃元,韩立新.桃种质资源对桃蚜的抗性评价[J].果树学报,2001,18(3):145-147.WANG Lirong,ZHU Gengrui,FANG Weichao,ZUO Qinyuan,HAN Lixin.Study on the resistance to peach aphid(Myzus persicae Sulzer) of peach germplasm[J]. Journal of Fruit Science,2001,18(3):145-147.
[6] 王新卫,王力荣,曹珂,陈昌文,方伟超,朱更瑞,薛梅真.一个与毛桃种质抗绿色桃蚜性状紧密连锁的InDel 标记及其应用:CN201711111942.7[P].2021-11-30.WANG Xinwei,WANG Lirong,CAO Ke,CHEN Changwen,FANG Weichao,ZHU Gengrui,XUE Meizhen.An InDel marker closely linked to the resistance trait of green peach aphid in hairy peach germplasm and its application:CN201711111942.7[P].2021-11-30.
[7] 潘磊,闫乐乐,鲁振华,曾文芳,崔国朝,牛良,王志强.一类桃树桃蚜抗性新种质09 南3-30[J].果树学报,2021,38(6):895-900.PAN Lei,YAN Lele,LU Zhenhua,ZENG Wenfang,CUI Guochao,NIU Liang,WANG Zhiqiang. 09N3-30,a new peach germplasm with green peach aphid resistance[J]. Journal of Fruit Science,2021,38(6):895-900.
[8] PASCAL T,ABERLENC R,CONFOLENT C,HOERTER M,LECERF E,TUÉRO C,LAMBERT P. Mapping of new resistance (Vr2,Rm1) and ornamental (Di2,pl) Mendelian trait loci in peach[J].Euphytica,2017,213(6):132.
[9] SAUGE M H,MUS F,LACROZE J P,PASCAL T,KERVELLA J,POËSSEL J L. Genotypic variation in induced resistance and induced susceptibility in the peach-Myzus persicae aphid system[J].Oikos,2006,113(2):305-313.
[10] LAMBERT P,PASCAL T. Mapping Rm2 gene conferring resistance to the green peach aphid (Myzus persicae Sulzer) in the peach cultivar“Rubira®”[J].Tree Genetics&Genomes,2011,7(5):1057-1068.
[11] LAMBERT P,CAMPOY J A,PACHECO I,MAUROUX J B,DA SILVA LINGE C,MICHELETTI D,BASSI D,ROSSINI L,DIRLEWANGER E,PASCAL T,TROGGIO M,ARANZANA M J,PATOCCHI A,ARÚS P. Identifying SNP markers tightly associated with six major genes in peach [Prunus persica (L.)Batsch] using a high-density SNP array with an objective of marker-assisted selection(MAS)[J].Tree Genetics&Genomes,2016,12(6):121.
[12] 牛良,鲁振华,曾文芳,崔国朝,潘磊,徐强,李国怀,王志强.‘粉寿星’对桃绿蚜抗性的遗传分析[J]. 果树学报,2016,33(5):578-584.NIU Liang,LU Zhenhua,ZENG Wenfang,CUI Guochao,PAN Lei,XU Qiang,LI Guohuai,WANG Zhiqiang.Inheritance analysis of resistance to green peach aphids (Myzus persicae Sulzer)for peach cultivar‘Fen Shouxing’(Prunus persica var.densa)[J].Journal of Fruit Science,2016,33(5):578-584.
[13] 张南南,鲁振华,崔国朝,潘磊,曾文芳,牛良,王志强. 基于SNP 标记桃抗蚜性状的基因定位[J].中国农业科学,2017,50(23):4613-4621.ZHANG Nannan,LU Zhenhua,CUI Guochao,PAN Lei,ZENG Wenfang,NIU Liang,WANG Zhiqiang.Gene mapping of aphidresistant for peach using SNP markers[J]. Scientia Agricultura Sinica,2017,50(23):4613-4621.
[14] PAN L,LU Z H,YAN L L,ZENG W F,SHEN Z J,YU M L,BU L L,CUI G C,NIU L,WANG Z Q.NLR1 is a strong candidate for the Rm3 dominant green peach aphid (Myzus persicae)resistance trait in peach[J]. Journal of Experimental Botany,2022,73(5):1357-1369.
[15] SAUGE M H,LAMBERT P,PASCAL T. Co-localisation of host plant resistance QTLs affecting the performance and feeding behaviour of the aphid Myzus persicae in the peach tree[J].Heredity,2011,108(3):292-301.
[16] 王君秀.山桃抗蚜基因的发掘及抗性机制解析[D].武汉:华中农业大学,2023.WANG Junxiu. Identification of genes and resistance mechanism of Prunus davidiana against aphid[D]. Wuhan:Huazhong Agricultural University,2019.
[17] WANG J X,LI Y,WANG X W,CAO K,CHEN C W,WU J L,FANG W C,ZHU G R,CHEN X J,GUO D D,WANG J,ZHAO Y L,FAN J Q,LIU S N,LI W Q,BIE H L,XU Q,WANG L R. Haplotype-resolved genome of a heterozygous wild peach reveals the PdaWRKY4-PdaCYP716A1 module mediates resistance to aphids by regulating betulin biosynthesis[J].Journal of Integrative Plant Biology,2024,66(12):2716-2735.
[18] 王新卫,白翠营,王力荣,朱更瑞,方伟超,曹珂,陈昌文,李勇,郭健,丁体玉,关利平,张倩.一组与山桃抗蚜主效QTL qGPAR-3-1 紧密连锁的InDel 位点及其应用:CN201610804468.5[P].2020-10-09.WANG Xinwei,BAI Cuiying,WANG Lirong,ZHU Gengrui,FANG Weichao,CAO Ke,CHEN Changwen,LI Yong,GUO Jian,DING Tiyu,GUAN Liping,ZHANG Qian.A group of InDel loci closely linked to the main QTL qGPAR-3-1 of mountain peach anti-aphid and their applications:CN201610804468.5[P].2020-10-09.
[19] 潘磊,牛良,樊美丽,王志强,鲁振华,曾文芳,崔国朝.与桃抗蚜性状紧密连锁的分子标记,用于检测桃抗蚜性状的引物、试剂盒、方法及其应用:CN201910266708.4[P].2022-12-13.PAN Lei,NIU Liang,FAN Meili,WANG Zhiqiang,LU Zhenhua,ZENG Wenfang,CUI Guochao. Molecular markers closely linked to peach aphid resistance traits,primers,kits,methods and applications for detecting peach aphid resistance traits:CN201910266708.4[P].2022-12-13.
[20] 潘磊,王志强,牛良,鲁振华,曾文芳,崔国朝,闫乐乐.与栽培种来源抗桃绿蚜性状紧密连锁的分子标记、引物、应用及品种选育方法:CN202011231906.6[P].2022-08-19.PAN Lei,WANG Zhiqiang,NIU Liang,LU Zhenhua,ZENG Wenfang,CUI Guochao,YAN Lele. Molecular markers,primers,applications and variety breeding methods closely linked to the resistance traits of peach green aphids from cultivated varieties:CN202011231906.6[P].2022-08-19.
[21] 王力荣,朱更瑞.桃种质资源描述规范和数据标准[M].北京:中国农业出版社,2005.WANG Lirong,ZHU Gengrui. Descriptors and data standard for peach(Prunus persica L.)[M].Beijing:China Agriculture Press,2005.
[22] 王力荣,朱更瑞,方伟超.中国桃遗传资源[M].北京:中国农业出版社,2012.WANG Lirong,ZHU Gengrui,FANG Weichao. Peach genetic resource in China[M].Beijing:China Agriculture Press,2012.
[23] PALLOIX A,AYME V,MOURY B. Durability of plant major resistance genes to pathogens depends on the genetic background,experimental evidence and consequences for breeding strategies[J].New Phytologist,2009,183(1):190-199.
[24] CAO K,PENG Z,ZHAO X,LI Y,LIU K Z,ARUS P,FANG W C,CHEN C W,WANG X W,WU J L,FEI Z J,WANG L R.Chromosome-level genome assemblies of four wild peach species provide insights into genome evolution and genetic basis of stress resistance[J].BMC Biology,2022,20(1):139.
[25] 王力荣. 中国桃品种改良历史回顾与展望[J]. 果树学报,2021,38(12):2178-2195.WANGLirong.HistoryandprospectofpeachbreedinginChina[J].Journal of Fruit Science,2021,38(12):2178-2195.