土壤盐渍化是全球普遍存在的严重环境难题,对植物的生长和生产具有不利影响[1]。近年来,由于人类不恰当的灌溉方法以及极端天气状况等因素,盐碱地的面积持续增加[2]。目前,中国已有超过1亿hm2的耕地遭受盐渍化[3]。盐碱胁迫会干扰植物的一系列形态、生理、生化和分子过程[4]。例如,盐碱胁迫会导致植物气孔关闭和非气孔限制,从而使光合作用能力受到抑制[5]。此外,盐碱胁迫还会诱导植物体内的活性氧(ROS)的大量积累,如过氧化氢(H2O2)和超氧自由基(O2-·),从而对植物造成严重的氧化损伤。同时,盐碱胁迫会对植物产生离子毒性,提高钠离子与钾离子的比例,减少植物对钾、磷和钙等其他必需营养元素的吸收[6]。盐碱胁迫造成的营养失衡会导致作物生长受阻,进而影响农业产出和经济效益[7]。因此,探索增强植物对盐碱环境耐受性的方法对提升盐碱地的农业利用效率至关重要。
油菜素内酯(BRs)是一类广泛存在于高等植物中的多羟基甾醇类激素,已广泛用于农业生产。BRs 调控植物的多种过程,如种子萌发、细胞伸长、光形态建成、导管分化和根系发育[8]。此外,BRs 还参与植物对多种非生物胁迫的响应,如干旱、高温、低温、盐碱胁迫、重金属胁迫和养分缺乏[9]。例如,吴杨等[10]研究表明,外源2,4-表油菜素内酯(EBR)能够通过提高抗氧化酶活性、脯氨酸及叶绿素含量,维护细胞结构的完整,从而增强大豆幼苗的耐盐碱能力。马媛媛等[11]研究表明,外源EBR能够减轻盐碱胁迫对芸豆幼苗造成的膜脂过氧化伤害及对光合作用的非气孔限制,从而增强耐盐碱能力。郑晓东等[12]研究还表明,外源EBR 还可以通过调控生长素、赤霉素(GA)和二氢玉米素的合成以及有机酸的分泌来提高平邑甜茶幼苗的耐盐碱性。
植物内源激素和有机酸在调节生长发育和逆境响应中扮演着至关重要的角色,其含量变化能够反映植物在面临逆境胁迫时所采取的适应性行为[13]。内源激素,如吲哚乙酸(IAA)、脱落酸(ABA)、GA和玉米素(ZR)是植物体内信号传递的关键分子,它们通过影响细胞分裂、分化和逆境信号转导,调控植物对环境的适应性[14]。琥珀酸、苹果酸、酒石酸和柠檬酸等有机酸不仅参与营养物质的吸收和运输,还参与渗透压和离子平衡的调节,对维持细胞内环境稳定具有重要作用[15]。Wang等[16]研究表明,盐碱胁迫下燕麦幼苗叶片的GA3 和IAA 含量显著下降,而ABA含量显著升高。胡妮等[17]研究表明,盐胁迫下苋菜地上部甲酸和酒石酸含量显著降低,而苹果酸和草酸含量显著升高。郑晓东等[11]研究也表明,盐碱胁迫下,平邑甜茶幼苗的柠檬酸和苹果酸含量均显著升高。因此,深入探讨油菜素内酯是否能够通过调节内源激素和有机酸代谢来增强平欧杂种榛的耐盐碱性,对提高其在盐碱土壤中的生产力具有重要的理论和实践意义。
平欧杂种榛(Corylus heterophylla × C. avellana)是一种抗旱、耐瘠薄的树种,其果实不仅具有果大、壳薄、味佳等特点,还含有丰富的不饱和脂肪酸、蛋白质、氨基酸、维生素C、维生素E、抗氧化酶和酚类化合物等营养成分。因平欧杂种榛不仅有助于降低胆固醇、增强心血管健康,还具有抗炎、抗氧化和抗癌的多重益处,其已成为一种营养价值高且对健康有益的食品,深受广大消费者的喜爱[18]。近年来,榛子作为优势经济作物,在甘肃、陕西、新疆和内蒙古等中国西北地区被大面积栽培。然而,西北地区的盐碱土壤对榛子产业的发展带来了一定的挑战。由于该地区降水稀少、气候干旱,土壤盐渍化现象较为严重。盐碱土壤的改良和治理需要投入大量的资金和劳动力,榛子产业的生产成本大量增加[19]。西北地区由于干旱荒漠气候和不当的农业灌溉方式,土壤盐碱化现象越来越严重。这种盐渍化不仅限制了当地农业的发展,也对生态环境造成了影响。研究表明,盐碱胁迫可显著影响平欧杂种榛幼苗叶片的内源激素和有机酸代谢[20]。鉴于此,笔者在本研究中旨在探讨外源油菜素内酯如何通过调节内源激素、活性氧、离子和有机酸代谢,增强平欧杂种榛对盐碱胁迫的耐受性。这不仅对提高该地区平欧杂种榛的生产力具有重要意义,也对生物修复和生态保护具有理论指导价值。
1 材料和方法
1.1 植物材料与处理
本研究以平欧杂种榛优良品种达维的1年生实生苗为试验材料。试验于2024 年5 月开始,在甘肃省林业科学研究院五星坪科研基地温室大棚内进行。2024年5月15日选取180株长势一致且均匀的苗木,移栽至装有混合土壤(花园土、珍珠岩和腐殖土按2∶1∶1体积比混合)的营养钵(13 cm×9 cm)中,统一置于苗圃进行栽培管理。移栽2 周后,进行试验处理。试验共设4个处理:(1)正常栽培管理对照(CK);(2)盐碱胁迫处理(SA,200 mmol·L-1 NaCl∶Na2CO3=1∶1);(3)盐碱胁迫结合0.2 μmol·L-1 24-表油菜素内酯(EBR)处理(SE);(4)盐碱胁迫结合0.2 μmol·L-1 24-表油菜素内酯(EBR)+24 μmol·L-1芸薹素唑(BRZ)(SEB),其中BRZ 为油菜素内酯合成抑制剂,0.2 μmol·L-1 EBR 由试验前期筛选得出。每组选择15 株幼苗作为1 次重复,共进行3 次生物学重复。试验期间,每隔3 d浇灌1次盐碱溶液,共浇灌3 次,每株200 mL。盐碱胁迫后,对照和SA 处理均喷施蒸馏水,SE处理仅喷施0.2 μmol·L-1 EBR,而SEB 处理同时喷施0.2 μmol·L-1 EBR 和24 μmol·L-1 BRZ,直至叶片滴水为止。于18:00 以后进行喷施,每隔2 d 喷施1 次,共喷施5 次。此外,所有处理每隔5 d 浇灌500 mL 的Hoagland 营养液,以保证植株仅受到盐碱胁迫。其中,每株幼苗的托盘渗液又重新浇回到塑料盆中,以防止养分的流失。胁迫处理后第30 天开始表型拍照以及采样,叶片均用液氮速冻后存于-80 ℃低温冰箱,用于后续相关指标测定。
1.2 表型观察
随机选择每个处理的1株平欧杂种榛幼苗分别置于以黑布为背景的环境中拍照进行表型观察。
1.3 叶片光合气体交换参数的测定
使用光合仪(Li-6400,LI-COR公司,美国)在晴天09:00—11:00 期间随机选取每个处理中上部的3枚叶片进行净光合速率(Pn)、气孔导度(Gs)、蒸腾速率(Tr)和胞间CO2浓度(Ci)测定[21]。
1.4 生理指标测定
在盐碱胁迫第30天时,随机采取每个处理幼苗的中上部叶片,用蒸馏水清洗擦拭干净,去除叶脉后磨碎,用于测定生理指标。叶绿素a(Chl a)、叶绿素b(Chl b)含量均用丙酮萃取法[22]测定;可溶性糖(WSS)、可溶性蛋白(SP)、脯氨酸(Pro)含量分别采用3,5二硝基水杨酸法[23]、考马斯亮蓝法[24]和酸性茚三酮法[25]测定;超氧化物歧化酶(SOD)、过氧化物酶(POD)和过氧化氢酶(CAT)活性分别采用氮蓝四唑光化还原法[26]、愈创木酚法[27]和紫外吸收法[28]测定;相对电导率(REC)和丙二醛(MDA)含量分别采用电导仪法[29]和硫代巴比妥酸法[30]测定;过氧化氢(H2O2)含量、超氧阴离子产生速率(O2-·)均采用试剂盒法(索莱宝生物科技有限公司,北京)测定。内源激素(ZT、IAA、GA和ABA)含量采用高酶联免疫吸附法(ELISA,上海酶联生物科技有限公司,上海)测定。有机酸(酒石酸、琥珀酸、柠檬酸和苹果酸)含量采用高效液相色谱法[31]测定。
1.5 Na+和K+含量的测定
叶片干燥并研磨,准确称取0.5 g 叶片烘干样品,用H2SO4-H2O2进行消煮,然后使用火焰光度计测定Na+和K+的含量,具体参考Kamble等[32]的方法。
1.6 统计分析
使用SPSS 22.0 软件进行统计分析。采用单因素方差分析和Duncan 多重比较检验(p<0.05)评估平均值之间的差异。数值表示为3 次重复的平均值±标准差。
2 结果与分析
2.1 外源EBR 对盐碱胁迫下平欧杂种榛幼苗生长表型的影响
经过30 d的盐碱胁迫处理后,盐碱胁迫(SA)处理的平欧杂种榛幼苗出现明显的叶片边缘黄化以及生长缓慢现象(图1)。SE处理的平欧杂种榛幼苗受到的生长抑制明显减弱,仅叶尖存在黄化现象,总体长势明显优于SA处理。相反,在盐碱胁迫基础上同时喷施0.2 μmol·L-1EBR和24 μmol·L-1 BRZ后,SEB处理的平欧杂种榛幼苗受到的盐碱损伤未得到改善,长势明显差于SE处理。

图1 外源EBR 对盐碱胁迫下平欧杂种榛生长表型的影响
Fig.1 Effects of exogenous EBR on growth phenotype of C.heterophylla×C.heterophylla under saline-alkali stress
2.2 外源EBR对盐碱胁迫下平欧杂种榛幼苗叶片光合气体交换参数的影响
如图2 所示,盐碱胁迫(SA)处理的平欧杂种榛叶片的Pn、Gs和Tr均较对照处理显著下降,降幅分别为43.76%、53.85%和60.81%,而叶片的Ci较对照显著升高,升幅为45.47%;SE处理的平欧杂种榛幼苗叶片的Pn、Gs和Tr均较SA 处理显著升高,升幅分别为36.61%、33.33%和54.10%,而叶片的Ci较SA处理下降了21.12%。此外,SEB处理的平欧杂种榛幼苗叶片Gs和Ci与SA处理无显著差异,而叶片的Pn和Tr均显著高于SA 处理,分别为SA 处理的1.10 倍和1.28倍。
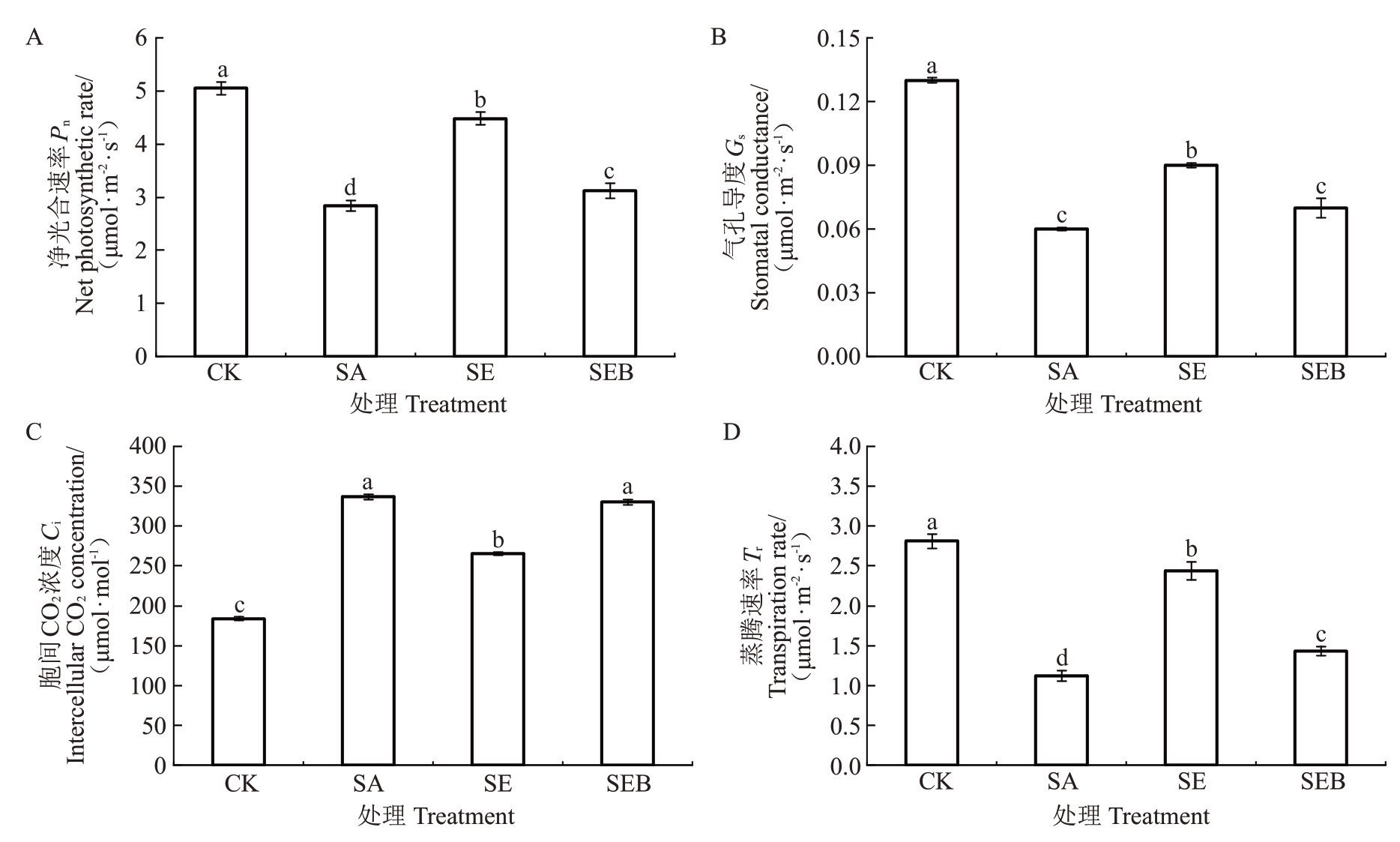
图2 外源EBR 对盐碱胁迫下平欧杂种榛幼苗叶片光合气体交换参数的影响。Fig.2 Effects of exogenous EBR on photosynthetic gas exchange parameters in leaves of C.heterophylla×C.avellana seedlings under saline-alkali stress
不同小写字母表示处理间差异显著(p<0.05)。下同。
Different small letters indicated significant differences between treatments(p<0.05).The same below.
2.3 外源EBR对盐碱胁迫下平欧杂种榛幼苗叶片光合色素含量的影响
如图3 所示,盐碱胁迫30 d 后,SA 处理的平欧杂种榛幼苗叶片的Chl a、Chl b和Chl a+b含量均较对照处理显著下降,分别为对照处理的36.32%、46.03%和38.55%;SE 处理的平欧杂种榛幼苗叶片Chl a、Chl b和Chl a+b含量均较SA处理得到不同程度的上升,其中Chl b 的升幅最大,为41.38%;此外,SEB处理的平欧杂种榛幼苗叶片Chl a、Chl b和Chl a+b含量相较SA处理均未发生显著变化。
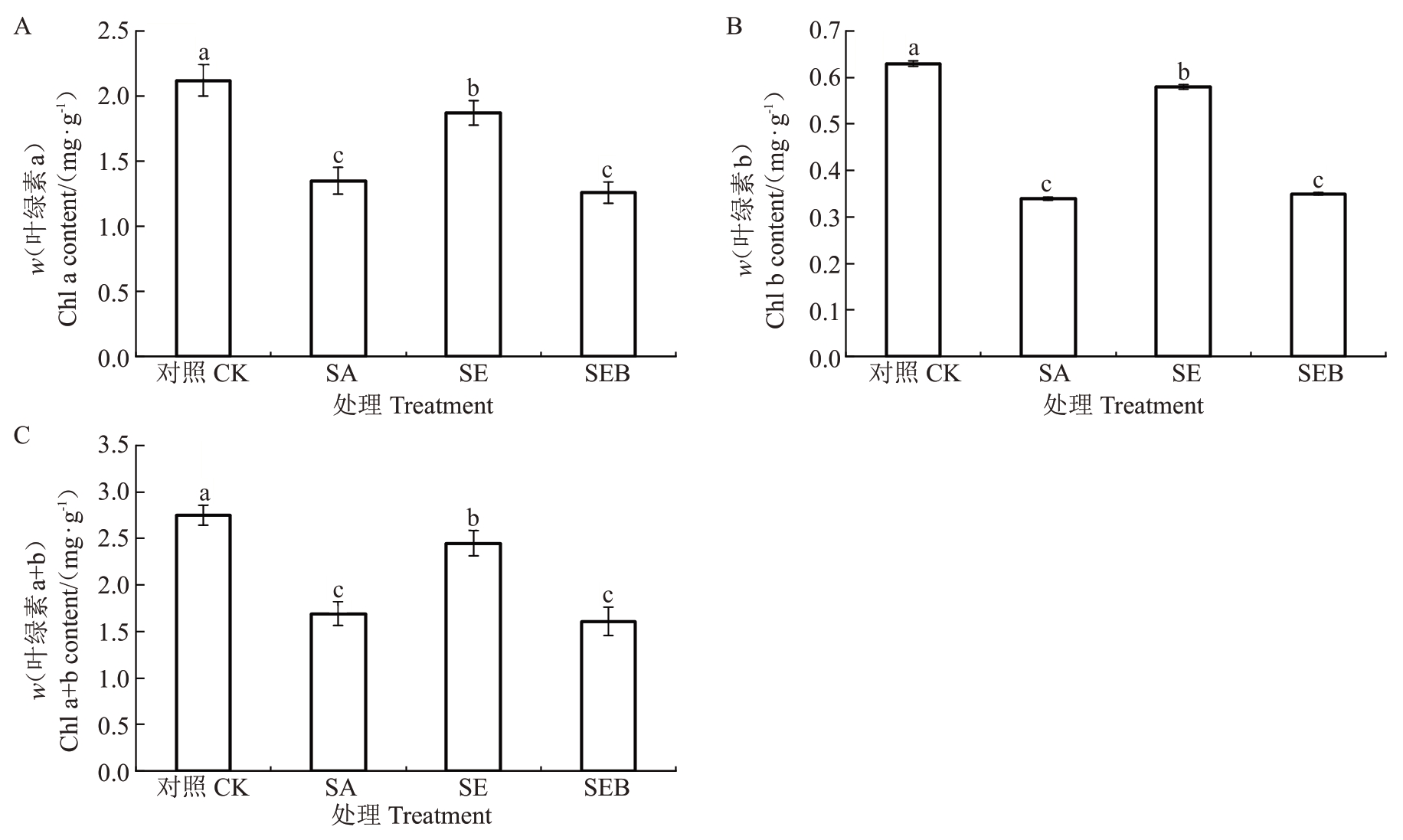
图3 外源EBR 对盐碱胁迫下平欧杂种榛幼苗叶片光合色素含量的影响
Fig.3 Effects of exogenous EBR on photosynthetic pigment content in leaves of C.heterophylla×C.avellana seedlings under saline-alkali stress
2.4 外源EBR对盐碱胁迫下平欧杂种榛幼苗叶片抗氧化酶活性和活性氧含量的影响
由图4-A、B、C 可知,盐碱胁迫30 d 后,SA 处理的平欧杂种榛幼苗叶片的抗氧化酶(SOD、POD 和CAT)活性均较对照显著升高,分别为对照的3.29倍、2.37 倍和1.50 倍;SE 处理的叶片SOD、POD 和CAT活性均较SA 处理进一步升高,分别较SA 处理升高36.83%、38.03%和51.52%。而在SA 处理的基础上同时施用0.2 μmol·L-1EBR 和24 μmol·L-1 BRZ 后,SEB处理的叶片SOD、POD和CAT活性较SE处理的升幅显著降低,分别仅为33.78%、32.94%和42.88%。
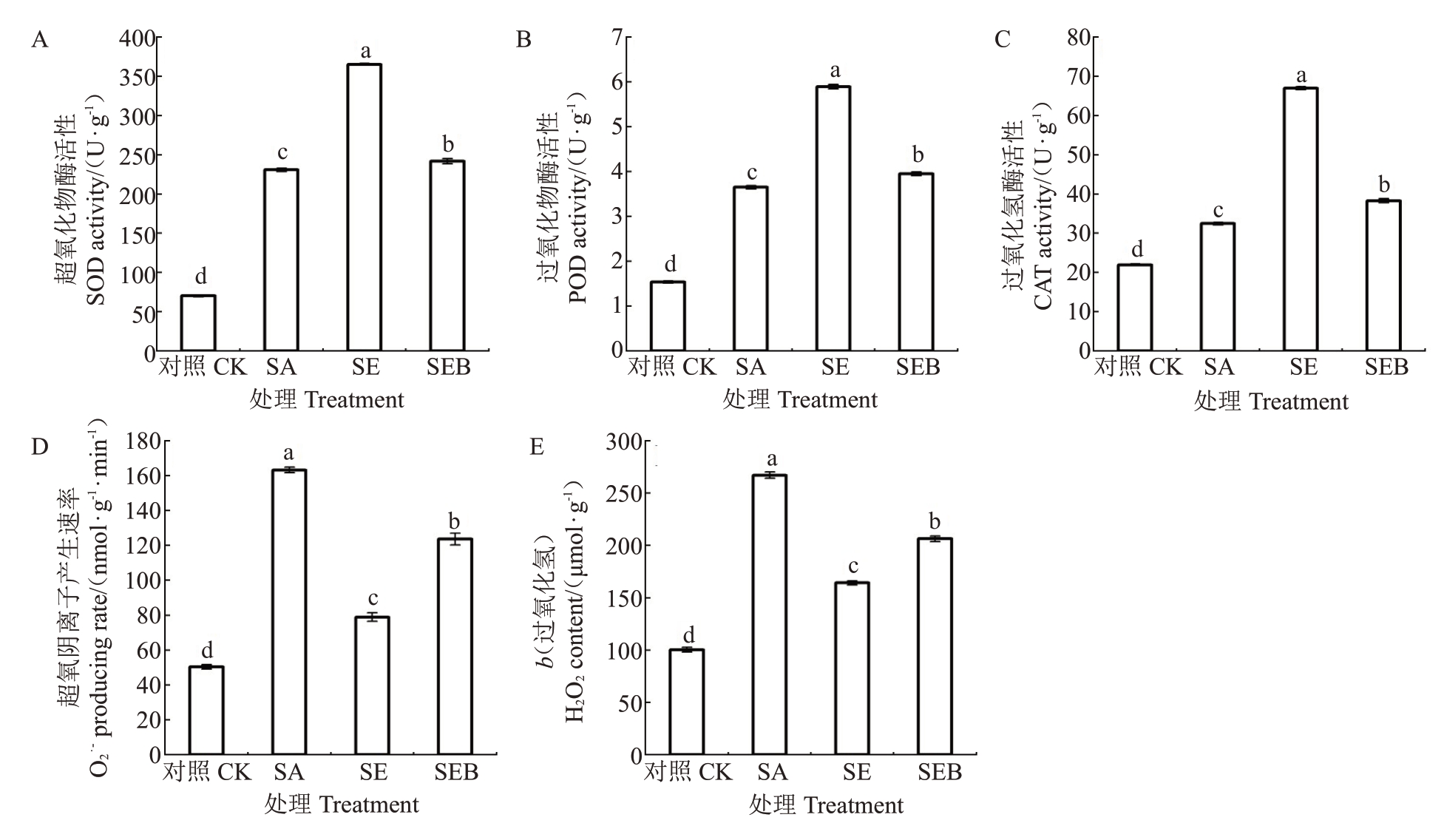
图4 外源EBR 对盐碱胁迫下平欧杂种榛幼苗叶片抗氧化酶活性和活性氧含量的影响
Fig.4 Effects of exogenous EBR on antioxidant enzyme activity and reactive oxygen species content in leaves of C.heterophylla×C.heterophylla seedlings under saline-alkali stress
如图4-D、E所示,SA处理的平欧杂种榛幼苗叶片的H2O2含量和O2-·产生速率均较对照显著升高,分别为对照的2.66 倍和3.24 倍。SE 处理的叶片H2O2含量和O2-·产生速率均较SA处理显著下降,降幅分别为38.52%和51.66%;值得注意的是,SEB 处理的叶片H2O2含量和O2-·产生速率显著高于SE 处理,升幅分别为56.73%和25.63%。
2.5 外源EBR 对盐碱胁迫下平欧杂种榛幼苗叶片渗透调节物质含量的影响
如图5所示,盐碱胁迫30 d后,SA处理的平欧杂种榛幼苗叶片的WSS、SP和Pro含量均较对照有不同程度的升高,升幅分别为61.38%、70.23%和29.43%;SE 处理的叶片WSS、SP 和Pro 含量较SA 处理进一步升高,分别是SA 处理的1.63 倍、1.57 倍和1.29 倍;而SEB 处理的叶片WSS、SP 和Pro 含量较SE 处理显著下降,降幅分别为36.02%、31.66%和40.27%。
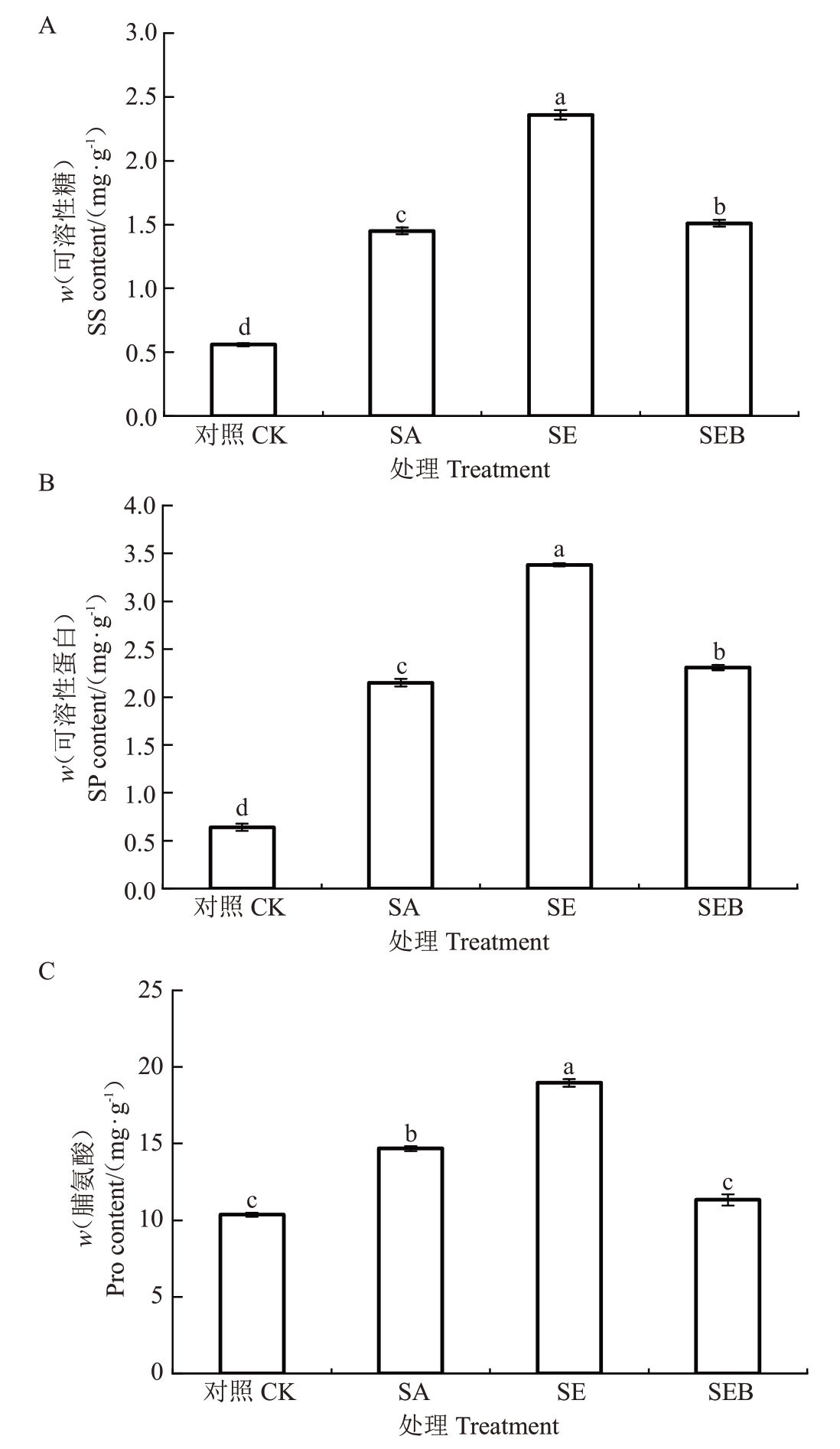
图5 外源EBR 对盐碱胁迫下平欧杂种榛幼苗叶片渗透调节物质含量的影响
Fig.5 Effects of exogenous EBR on the content of osmotic adjustment substances in leaves of C.heterophylla×C.heterophylla seedlings under saline-alkali stress
2.6 外源EBR对盐碱胁迫下平欧杂种榛幼苗叶片REC和MDA含量的影响
如图6 所示,盐碱胁迫30 d 后,SA 处理的平欧杂种榛幼苗叶片的REC和MDA含量均较对照处理显著升高,分别为对照的2.05 倍和1.98 倍;而SE 处理的REC 和MDA 含量均较SA 处理呈现不同程度的下降,降幅分别为37.70%和28.63%。值得注意的是,SEB处理的叶片REC和MDA含量较SE处理显著升高,升幅分别为28.78%和16.08%。
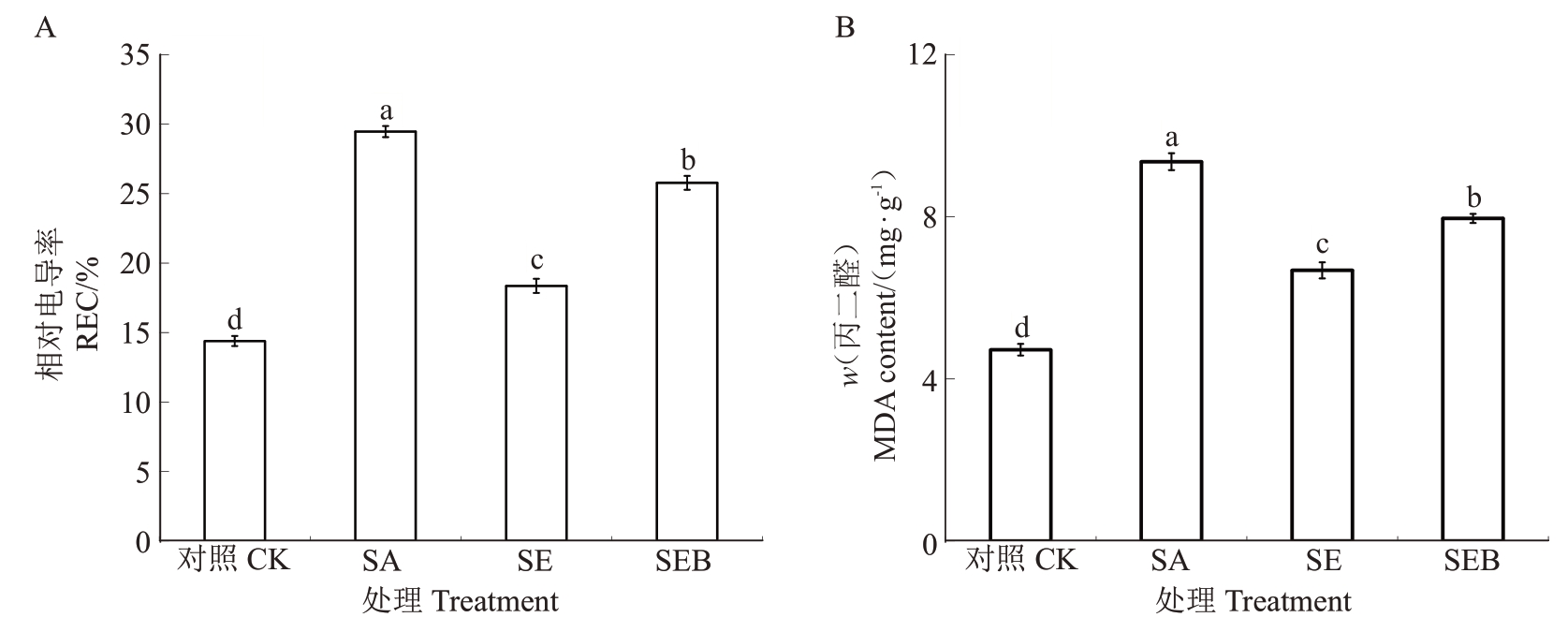
图6 外源EBR 对盐碱胁迫下平欧杂种榛幼苗叶片REC 和MDA 含量的影响
Fig.6 Effects of exogenous EBR on REC and MDA contents in leaves of C.heterophylla×C.avellana seedlings under salinealkali stress
2.7 外源EBR 对盐碱胁迫下平欧杂种榛幼苗叶片Na+和K+含量的影响
如图7 所示,盐碱胁迫30 d 后,SA 处理的平欧杂种榛幼苗叶片的Na+含量和Na+/K+值均显著高于对照,分别为对照的1.69 倍和3.49 倍,而叶片的K+含量显著低于对照处理,仅为51.64%;SE处理的叶片Na+含量和Na+/K+较SA 处理显著下降,降幅分别为20.80%、56.88%,而叶片的K+含量较SA处理显著升高,升幅为45.65%。与SE 处理相比,SEB 处理的叶片Na+和Na+/K+显著升高,升幅为23.48%和83.84%,而叶片K+含量显著降低,降幅为32.78%。
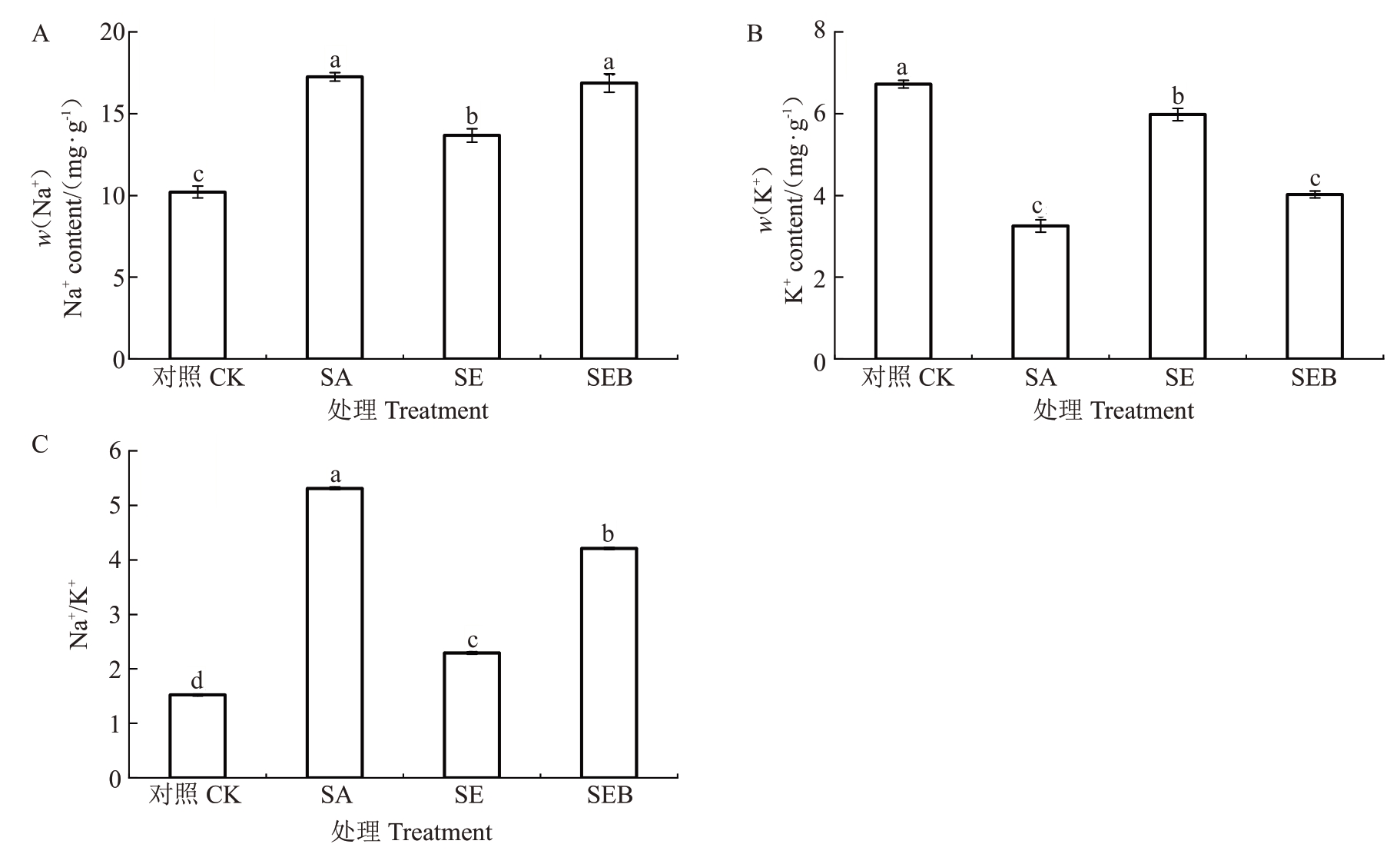
图7 外源EBR 对盐碱胁迫下平欧杂种榛幼苗叶片Na+和K+含量的影响
Fig.7 Effects of exogenous EBR on Na+and K+contents in leaves of C.heterophylla×C.avellana seedlings under salinealkali stress
2.8 外源EBR对盐碱胁迫下平欧杂种榛幼苗叶片有机酸组分含量的影响
如图8 所示,与对照处理相比,SA 处理的平欧杂种榛幼苗叶片的琥珀酸、柠檬酸、酒石酸和苹果酸含量均不同幅度的下降,降幅分别为29.27%、81.28%、52.56%和69.14%;SE 处理的叶片琥珀酸、柠檬酸、酒石酸和苹果酸含量均较SA 处理显著升高,升幅分别为21.74%、76.75%、47.15%和63.77%。值得注意的是,SEB处理的叶片琥珀酸含量与SA处理无显著差异,而柠檬酸、酒石酸和苹果酸含量显著高于SA 处理,同时也显著低于SE 处理,分别为SE的45.39%、26.02%和44.93%。
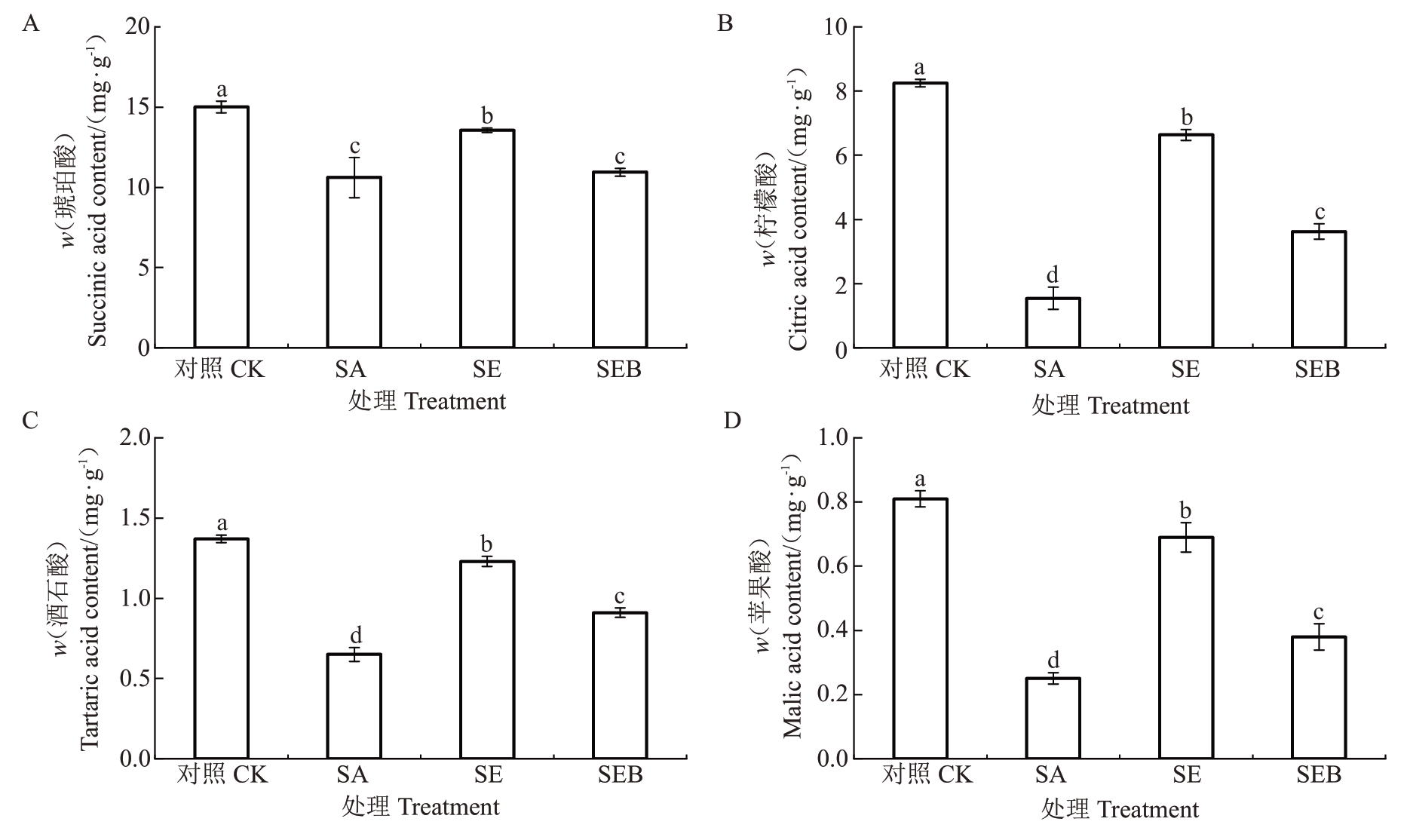
图8 外源EBR 对盐碱胁迫下平欧杂种榛幼苗叶片有机酸组分含量的影响
Fig.8 Effects of exogenous EBR on the content of organic acid components in leaves of C.heterophylla×C.heterophylla seedlings under saline-alkali stress
2.9 外源EBR 对盐碱胁迫下平欧杂种榛幼苗叶片内源激素含量的影响
如图9 所示,与对照处理相比,SA 处理的平欧杂种榛幼苗叶片的ABA 含量显著升高,升幅为44.93%,而叶片的GA、IAA和ZR含量显著降低,降幅分别为30.90%、33.55%和26.40%;SE处理的叶片ABA含量较SA处理显著下降,降幅为20.58%,而叶片的GA、IAA和ZR含量呈相反的变化趋势,分别较SA处理升高21.58%、38.40%、21.14%。值得注意的是,SEB 处理的叶片ABA、GA 和IAA 含量与SA 处理无显著差异,但叶片的ZR 含量显著低于SA 处理,较SA处理下降11.04%。
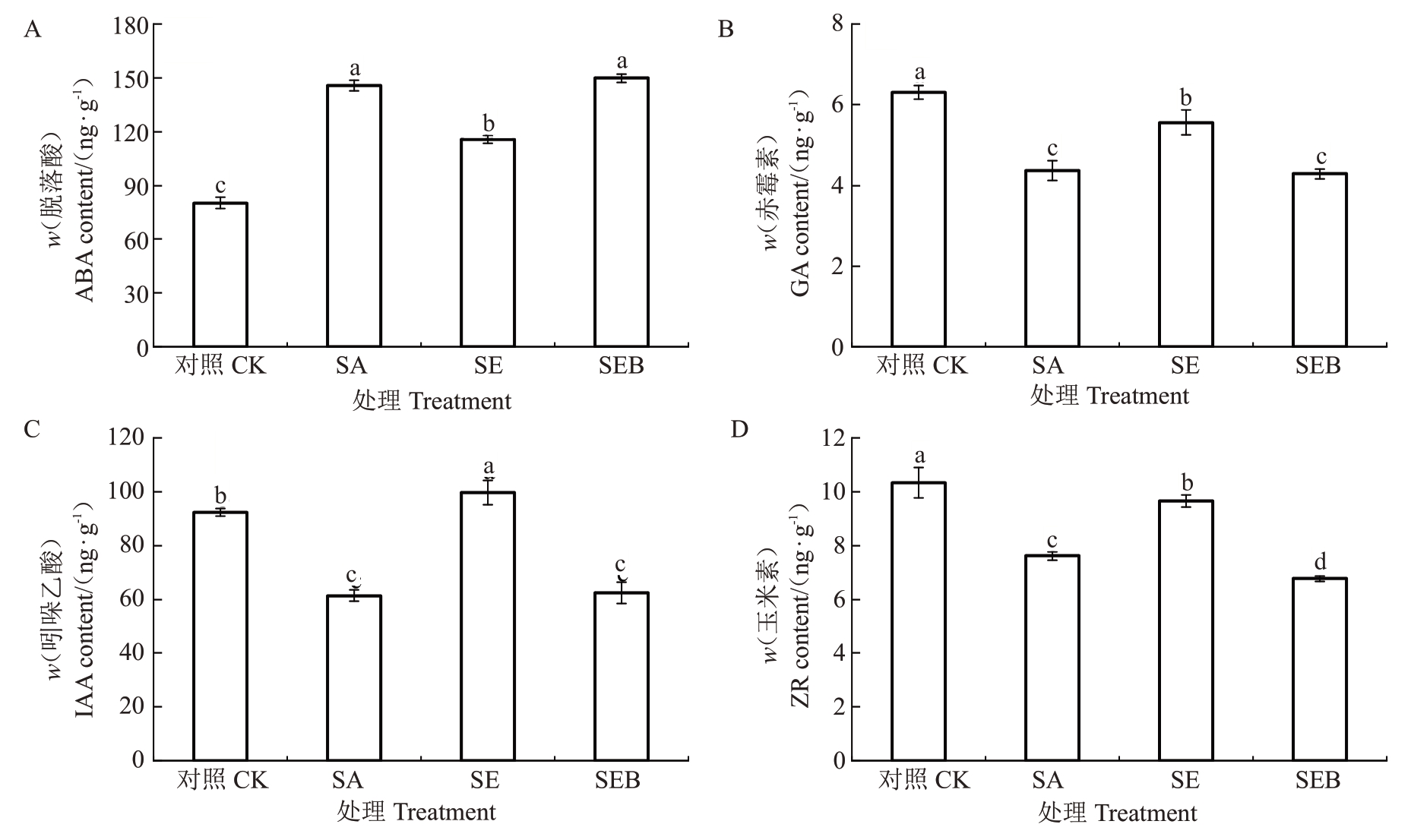
图9 外源EBR 对盐碱胁迫下平欧杂种榛幼苗叶片内源激素含量的影响
Fig.9 Effects of exogenous EBR on endogenous hormone content in leaves of C.heterophylla×C.heterophylla seedlings under saline-alkali stress
2.10 盐碱胁迫下外源EBR 对平欧杂种榛幼苗生理效应的综合评价
2.10.1 相关性分析 由图10 可知,平欧杂种榛幼苗叶片的Pn与Tr、Chl b、琥珀酸和苹果酸呈极显著正相关(p<0.01),与Chl b、Chl a+b、GA、ZR、柠檬酸和酒石酸呈显著正相关(p<0.05),与REC呈极显著负相关(p<0.01),与Ci、O2-·、H2O2、MDA、Na+、Na+/K+和ABA呈显著负相关(p<0.05)。

图10 盐碱胁迫下外源EBR 对平欧杂种榛幼苗各项生理指标的相关性分析
Fig.10 Correlation analysis of exogenous EBR on physiological indexes of C.heterophylla×C.avellana seedlings under saline-alkali stress
2.10.2 主成分分析 为全面评估外源EBR对盐碱胁迫下平欧杂种榛幼苗的生理调控特性,对主要的28 个生理指标进行了主成分分析(PCA)。总共提取到两个特征值大于1 的主成分,分别为21.525 和5.801,两者的方差贡献率分别为76.874%和20.718%,累计方差贡献率达到97.592%,满足分析要求。PC1 综合了Pn、Gs、Ci、Tr、Chl a、Chl b、Chl a+b、O2-·、H2O2、REC、MDA、Na+、K+、Na+/K+、ABA、GA、IAA、ZR、琥珀酸、柠檬酸、酒石酸和苹果酸。PC2综合了SOD、POD、CAT、WSS、SP和Pro(表1)。
表1 主成分分析及方差解释
Table 1 Principal component analysis and variance interpretation
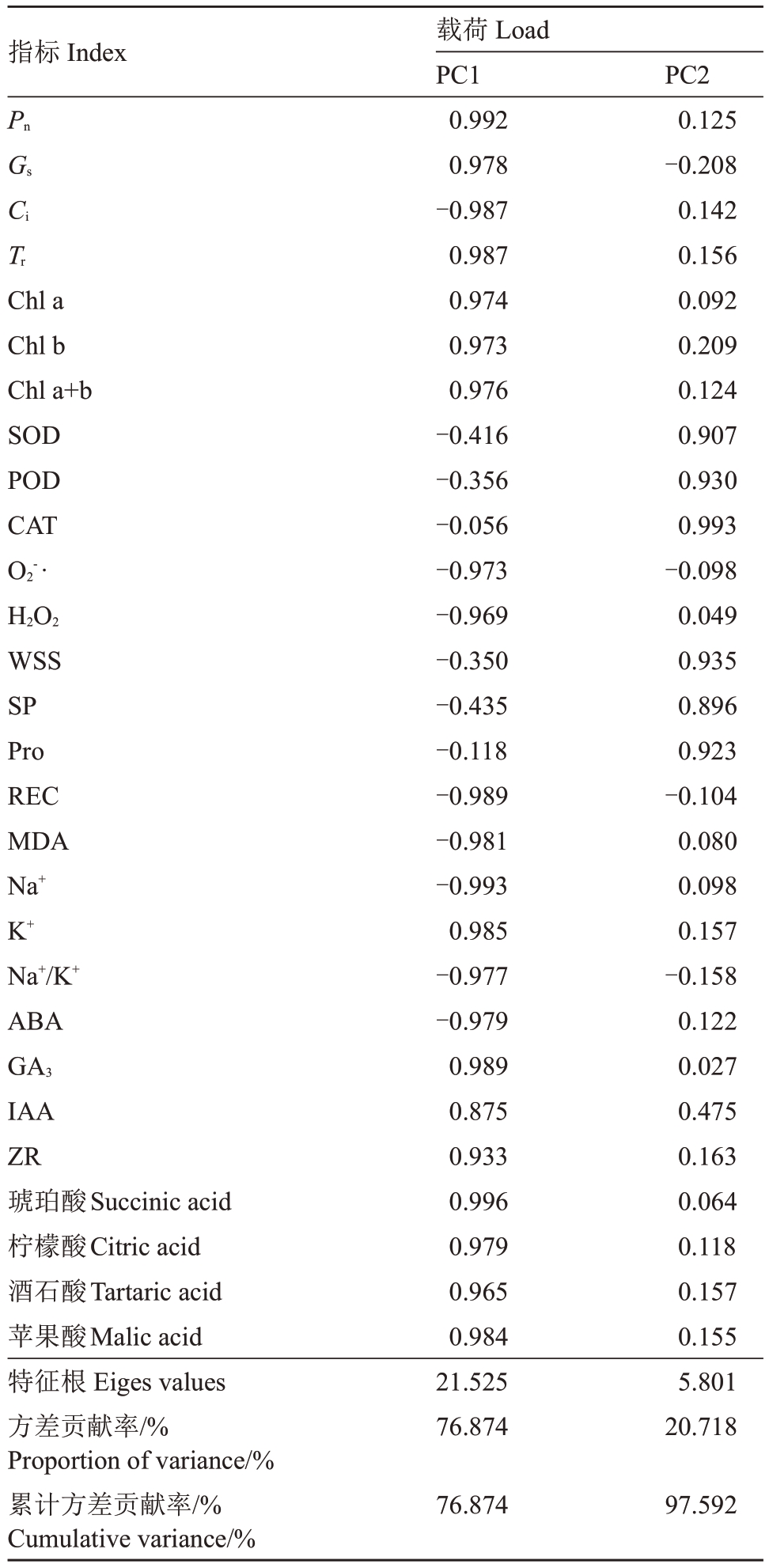
注:PC1、PC2 分别代表主成分1 和主成分2。下同。
Note:PC1 and PC2 represent principal component 1 and principal component 2,respectively.The same below.
指标Index PnGs Ci Tr Chl a Chl b Chl a+b SOD POD CAT O2-·H2O2 WSS SP Pro REC MDA Na+K+Na+/K+ABA GA3 IAA ZR琥珀酸Succinic acid柠檬酸Citric acid酒石酸Tartaric acid苹果酸Malic acid特征根Eiges values方差贡献率/%Proportion of variance/%累计方差贡献率/%Cumulative variance/%载荷Load PC1 0.992 0.978-0.987 0.987 0.974 0.973 0.976-0.416-0.356-0.056-0.973-0.969-0.350-0.435-0.118-0.989-0.981-0.993 0.985-0.977-0.979 0.989 0.875 0.933 0.996 0.979 0.965 0.984 21.525 76.874 PC2 0.125-0.208 0.142 0.156 0.092 0.209 0.124 0.907 0.930 0.993-0.098 0.049 0.935 0.896 0.923-0.104 0.080 0.098 0.157-0.158 0.122 0.027 0.475 0.163 0.064 0.118 0.157 0.155 5.801 20.718 76.87497.592
综合得分(F)是通过将每个主成分的得分与相应的方差贡献率的乘积相加来计算的。即:F=F1×76.874%+F2×20.718%。如表2 所示,各处理的综合得分分别为0.762 99(对照)、-0.799 23(SA)、0.601 22(SE)和-0.564 89(SEB)。由此证明了外源EBR 能够显著减轻盐碱胁迫对平欧杂种榛幼苗造成的伤害。
表2 盐碱胁迫下外源EBR 对平欧杂种榛幼苗生理效应的综合得分及排名
Table 2 Comprehensive score and ranking of physiological effects of exogenous EBR on Corylus heterophylla×Corylus avellana seedlings under saline-alkali stress
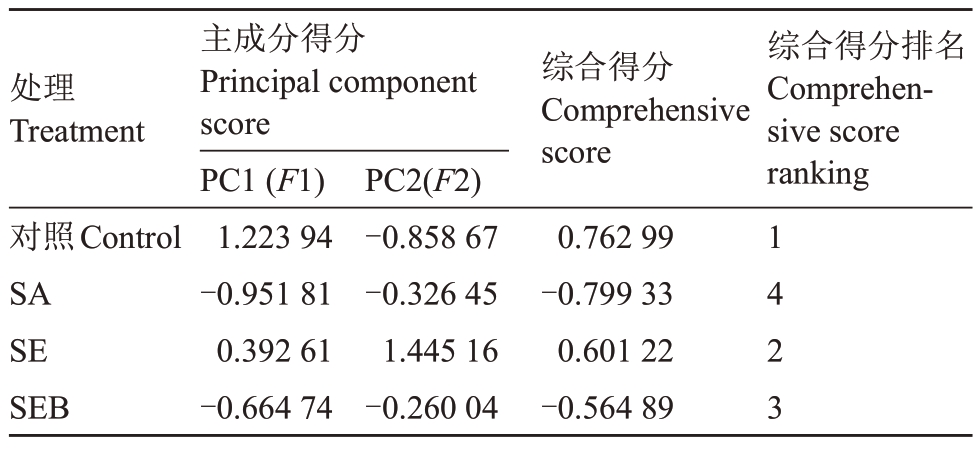
处理Treatment综合得分Comprehensive score综合得分排名Comprehensive score ranking对照Control SA SE SEB主成分得分Principal component score PC1(F1)1.223 94-0.951 81 0.392 61-0.664 74 PC2(F2)-0.858 67-0.326 45 1.445 16-0.260 04 0.762 99-0.799 33 0.601 22-0.564 89 1423
3 讨 论
盐碱胁迫是影响植物生长和发育的重要因素之一,它通过干扰植物的水分和离子平衡,导致植物生长受阻和生产力下降[33]。EBR 作为一种植物激素,已被证实在调节植物对多种胁迫的响应中发挥重要作用,包括干旱[34]、低温[35]、高温[36]胁迫和重金属[37]胁迫。本研究旨在探究外源EBR 对盐碱胁迫下平欧杂种榛幼苗氧化损伤、内源激素及有机酸代谢的影响。
光合作用不仅是植物能量和有机物合成的源泉,而且在调控植物对环境变化的适应性、气孔运动、光保护、碳氮平衡、信号转导以及昼夜节律等方面发挥着关键作用[38-40]。研究表明,盐碱胁迫会导致植物体内叶绿素合成受阻或叶绿素降解加速,进而影响光合色素的捕光能力[41]。此外,盐碱胁迫还会导致叶绿素a与叶绿素b的比例发生变化,影响光合作用中光能的分配和利用[41]。在光合气体交换参数方面,盐碱胁迫会降低Pn,减少Gs,改变Ci,以及影响Tr[42],这些变化共同导致光合作用效率下降,进而影响植物的生长和生产力。本研究结果表明,盐碱胁迫显著降低了平欧杂种榛幼苗的Chl a、Chl b 和Chl a+b含量,而外源EBR处理显著缓解了这一现象。这与前人在大豆[10]和芸豆[11]中的研究结果一致,这可能是外源EBR 能够改善植物对微量元素如铁、锰、铜和锌的吸收和运输,从而促进了叶绿素合成[43]。此外,EBR还可能通过保护光系统Ⅱ(PSⅡ)和调节非气孔因素,提高光合效率。然而,当EBR与BRZ(油菜素内酯合成抑制剂)共同施用时,叶绿素含量未显著改善,这可能是BRZ 抑制了内源EBR 的合成,从而减弱了EBR的正面效应,导致植物无法有效响应外源EBR的调节作用,进而无法缓解盐碱胁迫对光合作用的负面影响[44]。本研究结果也表明,与盐碱胁迫处理相比,EBR 显著提高了Pn、Gs和Tr,同时降低了Ci,进一步证实了EBR通过调节非气孔因素增强光合作用能力。这一发现与吴杨等[10]和马媛媛等[11]的研究结果相符,表明EBR在不同物种中具有类似的缓解盐碱胁迫的作用。
在植物面临逆境胁迫时,其内源性抗氧化防御机制会被激活,以中和积累过量的活性氧(ROS),主要包括H2O2和O2-·。这一过程对保护细胞结构的完整性、防止膜系统和蛋白质的损伤,乃至避免细胞死亡至关重要。抗氧化酶,如POD、CAT和SOD,在消除活性氧自由基和维持活性氧代谢平衡中扮演着关键角色[45-47]。本研究中,外源EBR的单独施用显著增强了平欧杂种榛幼苗叶片的SOD、POD 和CAT 活性,同时降低了H2O2含量和O2-·产生速率。这一结果与侯汶君等[48]在湖南稷子中的研究一致,这可能是外源EBR能够激活植物的抗氧化防御系统,提高抗氧化酶活性,从而增强植物清除ROS的能力。而外源EBR和BRZ同时施用后,抗氧化酶活性的提升受到抑制,H2O2含量和O2-·的积累增加,表明BRZ可能通过干扰植物响应外源EBR的信号转导途径,减弱了植物对EBR的响应,导致抗氧化酶系统的活性下降。由于抗氧化酶活性的降低,植物清除ROS的能力减弱,导致H2O2和O2-·的积累增加,从而加剧了氧化损伤[49]。MDA是膜脂过氧化作用的最终产物,是膜系统受害的重要标志之一[50]。REC的大小反映了质膜透性的大小,也是细胞膜损伤的一个指标[51]。本研究结果还发现,相较于SA处理,外源EBR显著降低了MDA 含量和REC,进一步证实了其对细胞膜的保护作用。这一发现与Gill等[49]的研究相符,表明EBR通过减少膜脂过氧化程度和维持膜完整性,增强了植物的抗逆性。值得注意的是,在盐碱胁迫处理的基础上同时施用外源EBR 和BRZ 后,叶片MDA 含量和REC 相较于SA 处理的下降幅度显著降低,表明外源BRZ可能通过干扰外源EBR的信号转导途径或者影响EBR 的生物活性,减弱了外源EBR对细胞和细胞器膜的保护作用,导致细胞膜损伤和脂质过氧化的程度没有有效降低。
渗透调节物质(WSS、SP、Pro)能帮助植物在干旱、盐分等逆境条件下维持细胞的膨压和水分平衡,同时还参与信号传递和响应,激活抗氧化防御系统,调节激素水平,从而提高植物的抗逆性[52-53]。在本研究中,外源EBR显著提高了盐碱胁迫下平欧杂种榛幼苗的WSS、SP和Pro含量,表明外源EBR能够调节植物激素的平衡并增强细胞膜的稳定性,从而使平欧杂种榛幼苗免受盐诱导的膜损伤影响,维持植物细胞膜结构的稳定。然而,当EBR与BRZ共同施用时,WSS、SP 和Pro 的积累显著减少,这可能是外源BRZ 阻碍了平欧杂种榛幼苗响应外源EBR 的信号转导,改变了与渗透调节和抗氧化防御相关的基因表达,进而抑制了体内WSS、SP和Pro的合成和积累[11]。
盐碱胁迫会造成植物体内离子失衡,从而对植物造成离子毒害现象[54]。在本研究中,外源EBR 显著降低了盐碱胁迫下平欧杂种榛幼苗叶片中的Na+含量,同时提高了K+含量,表明EBR 通过调节离子吸收和运输,有效缓解了盐碱胁迫引起的离子失衡。这一结果与高倩等[55]的研究一致,表明EBR 可能通过调控植物体内的HKT、LCT1、SLS 等K+吸收蛋白及吸收通道,减少Na+的吸收并促进对K+的吸收,从而维持离子平衡。然而,在SA 处理的基础上同时施用外源EBR和BRZ后,叶片的Na+和K+含量与SA 处理无显著差异,这可能是外源BRZ 通过抑制EBR的合成,减弱了EBR对离子吸收和运输的正向调节作用。这一发现进一步支持了EBR 在维持离子稳态中的关键作用,同时也揭示了BRZ对其效应的抑制作用。
有机酸可以通过促进养分吸收、改善根际环境、发挥化感作用以及参与信号转导等多重机制,对植物的生长发育和环境适应能力产生积极影响,是植物健康生长的关键因素[56-57]。研究表明,盐碱胁迫可以显著促进植物体内草酸、苹果酸、柠檬酸、琥珀酸、抗坏血酸等有机酸的积累[58-59]。本研究显示,盐碱胁迫显著降低了平欧杂种榛幼苗叶片中苹果酸、柠檬酸、琥珀酸及酒石酸含量。这可能是植株通过渗透调节、离子区隔化、抗氧化防御等生理机制协同作用的结果。这些机制通过调控根系分泌物组成、维持细胞离子稳态、减轻氧化损伤以及调节根际pH 值,最终提升了幼苗对盐碱逆境的适应能力[60-61]。而外源EBR 处理显著提高了盐碱胁迫下平欧杂种榛幼苗叶片的苹果酸、柠檬酸、琥珀酸和酒石酸含量,这可能是因为EBR 可以增强三羧酸循环(TCA 循环)的活性、诱导有机酸合成酶活性的提升和增强细胞膜的稳定性等途径促进苹果酸、柠檬酸等有机酸的生成[62],Wang等[57]的研究与这一结果一致。但外源EBR和BRZ联合施用后,叶片的苹果酸、柠檬酸、琥珀酸和酒石酸含量的提升受到抑制,这可能是外源BRZ通过抑制EBR的生物合成、信号转导或靶标酶活性,以及调节相反的代谢途径或基因表达,来平衡或逆转EBR促进有机酸合成的效应[63]。此外,外源BRZ 还可能直接影响参与有机酸合成途径的关键酶活性,或通过改变细胞功能和离子平衡来间接影响有机酸的合成速率,从而在联合施用下导致有机酸的整体合成减少[64]。
植物响应胁迫的过程并不是简单的依赖某种单一的植物激素,往往会存在不同激素之间的交互作用[65]。本研究中,相较于SA处理,外源EBR显著提高了平欧杂种榛幼苗叶片的IAA、GA3和ZR 含量,同时显著降低ABA 含量,表明外源EBR 可以通过调节IAA、GA3、ZR和ABA等激素的平衡,从而显著增强了平欧杂种榛幼苗对盐碱胁迫的耐受性,这一结果与郑晓东等[12]在平邑甜茶中的研究一致。但外源EBR 和BRZ 同时处理时,叶片的IAA、GA3 和ABA含量与SA处理无显著差异,而ZR含量显著降低,表明外源BRZ的加入抑制了EBR对激素的调节作用,干扰了植物激素正常合成或信号转导过程。
植物对盐碱胁迫的响应是一个极其复杂的生理生化过程,单一指标不足以全面评价其植物的抗盐碱性[66]。为了全面评估外源EBR 对盐碱胁迫下平欧杂种榛幼苗的调控作用,本研究采用主成分分析法对4个处理组的28个指标进行了综合评价。使用SPSS 22.0软件进行数据分析,结果显示两个主成分(PC1 和PC2)的贡献率分别为76.874%和20.718%,累计贡献率为97.592%。主成分分析表明,平欧杂种榛幼苗的Pn、苹果酸、酒石酸、柠檬酸、琥珀酸、GA3、ABA、REC 和WSS 等指标对外源EBR 缓解盐碱胁迫的效果敏感,适合作为筛选指标。基于这些分析,确定了0.2 μmol·L-1 EBR 可以有效缓解盐碱胁迫对平欧杂种榛幼苗的危害。
4 结 论
通过系统地分析外源EBR 对盐碱胁迫下平欧杂种榛幼苗生长及生理生化特性的影响,发现外源EBR在多个方面较SA处理均表现出显著的促进作用。EBR 不仅提高了幼苗的光合能力和叶绿素含量,促进了能量的转化和物质的积累,还显著增强了幼苗的抗氧化能力,减轻了盐碱胁迫下的氧化损伤。同时,EBR通过调节渗透调节物质的积累和离子平衡,帮助幼苗更好地适应盐碱环境。此外,EBR还促进了有机酸的合成和内源激素的平衡,进一步增强了幼苗的抗逆性。综上所述,外源EBR在盐碱地农业中具有广阔的应用前景,可为提高盐碱地作物产量和品质提供新的思路和方法。
[1] FANG S M,HOU X,LIANG X L. Response mechanisms of plants under saline-alkali stress[J]. Frontiers in Plant Science,2021,12:667458.
[2] WANG J,ZHANG Y X,YAN X R,GUO J P. Physiological and transcriptomic analyses of yellow horn (Xanthoceras sorbifolia)provide important insights into salt and saline-alkali stress tolerance[J].PLoS One,2020,15(12):e0244365.
[3] ZHANG K Y,CHANG L,LI G H,LI Y F.Advances and future research in ecological stoichiometry under saline-alkali stress[J].Environmental Science and Pollution Research,2023,30(3):5475-5486.
[4] ALI A,MAGGIO A,BRESSAN R A,YUN D J. Role and functional differences of HKT1-type transporters in plants under salt stress[J]. International Journal of Molecular Sciences,2019,20(5):1059.
[5] LI N,ZHANG Z H,GAO S,LV Y,CHEN Z J,CAO B L,XU K. Different responses of two Chinese cabbage (Brassica rapa L.ssp.pekinensis)cultivars in photosynthetic characteristics and chloroplast ultrastructure to salt and alkali stress[J]. Planta,2021,254(5):102.
[6] LIU P,WU X L,GONG B B,LÜ G Y,LI J R,GAO H B.Review of the mechanisms by which transcription factors and exogenous substances regulate ROS metabolism under abiotic stress[J].Antioxidants,2022,11(11):2106.
[7] CHEN Q,XIE H S,WEI G Y,GUO X R,ZHANG J,LU X Y,TANG Z H.Metabolic differences of two constructive species in saline-alkali grassland in China[J].BMC Plant Biology,2022,22(1):53.
[8] CHAUDHURI A,HALDER K,ABDIN M Z,MAJEE M,DATTA A. Abiotic stress tolerance in plants:Brassinosteroids navigate competently[J]. International Journal of Molecular Sciences,2022,23(23):14577.
[9] LI S M,ZHENG H X,LIN L,WANG F,SUI N.Roles of brassinosteroids in plant growth and abiotic stress response[J]. Plant Growth Regulation,2021,93(1):29-38.
[10] 吴杨,高慧纯,张必弦,张海玲,王全伟,刘鑫磊,栾晓燕,马岩松.24-表油菜素内酯对盐碱胁迫下大豆生育、生理及细胞超微结构的影响[J].中国农业科学,2017,50(5):811-821.WU Yang,GAO Huichun,ZHANG Bixian,ZHANG Hailing,WANG Quanwei,LIU Xinlei,LUAN Xiaoyan,MA Yansong.Effects of 24-brassinolide on the fertility,physiological characteristics and cell ultra-structure of soybean under saline-alkali stress[J].Scientia Agricultura Sinica,2017,50(5):811-821.
[11] 马媛媛,王智,曹金萍,罗新锐,王玉萍.2,4-表油菜素内酯对盐碱胁迫下芸豆幼苗生长及生理特性的影响[J].西北植物学报,2024,44(8):1181-1189.MA Yuanyuan,WANG Zhi,CAO Jinping,LUO Xinrui,WANG Yuping.Effects of 2,4-epibrassinolide on the growth and physiological characteristics of Phaseolus vulgaris seedlings under saline and alkaline stresses[J].Acta Botanica Boreali-Occidentalia Sinica,2024,44(8):1181-1189.
[12] 郑晓东,袭祥利,李玉琪,孙志娟,马长青,韩明三,李少旋,田义轲,王彩虹.油菜素内酯对盐碱胁迫下平邑甜茶幼苗生长的影响及调控机理研究[J]. 园艺学报,2022,49(7):1401-1414.ZHENG Xiaodong,XI Xiangli,LI Yuqi,SUN Zhijuan,MA Changqing,HAN Mingsan,LI Shaoxuan,TIAN Yike,WANG Caihong.Effects and regulating mechanism of exogenous brassinosteroids on the growth of Malus hupehensis under saline-alkali stress[J].Acta Horticulturae Sinica,2022,49(7):1401-1414.
[13] 李媛媛,王多一,高静,张岗,杜弢,郭柳,宋忠兴,唐志书,王楠.不同种类大黄萌发及幼苗内源激素与非结构性碳水化合物对温度与渗透胁迫交互的响应[J]. 生态学杂志,2024,43(7):2033-2045.LI Yuanyuan,WANG Duoyi,GAO Jing,ZHANG Gang,DU Tao,GUO Liu,SONG Zhongxing,TANG Zhishu,WANG Nan.Responses of seed germination,seedling endogenous hormones and non-structural carbohydrates of different Rheum species to the interaction of temperature and osmotic stresses[J]. Chinese Journal of Ecology,2024,43(7):2033-2045.
[14] PODLEŠÁKOVÁ K,UGENA L,SPÍCHAL L,DOLEŽAL K,DE DIEGO N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway[J].New Biotechnology,2019,48:53-65.
[15] 麻莹,张洪嘉,库都斯·阿布都沙拉木,耿淑娟,薛傲然,赵翰韦,菅柏涵.盐碱胁迫对盐地碱蓬生长、有机酸等溶质积累及其生理功能的影响[J].草地学报,2021,29(9):1934-1940.MA Ying,ZHANG Hongjia,Kudusi · Abudushalamu,GENG Shujuan,XUE Aoran,ZHAO Hanwei,JIAN Baihan. Effects of salt and alkali stresses on the growth,accumulation of organic acids and other solutes and their physiological functions of Suaeda salsa[J].Acta Agrestia Sinica,2021,29(9):1934-1940.
[16] WANG Q,LIANG X T,XIANG D B,XU W W,WANG C L,ZHAN C,REN C Z,WEI L M,ZHANG S Q,ZHANG L,WANG J Y,GUO L C.The physiological mechanism of melatonin enhancing the tolerance of oat seedlings under saline-alkali stress[J].Agronomy,2023,13(9):2343.
[17] 胡妮,陈柯罕,李取生,徐智敏,郭世鸿,余丹萍,罗涛.盐胁迫下苋菜品种有机酸变化对Cd 累积和耐盐性的影响[J].农业环境科学学报,2016,35(5):858-864.HU Ni,CHEN Kehan,LI Qusheng,XU Zhimin,GUO Shihong,YU Danping,LUO Tao. Effects of salinity-inducted organic acid variation on Cd accumulation and salinity tolerance of edible amaranth[J]. Journal of Agro-Environment Science,2016,35(5):858-864.
[18] 罗达,史彦江,宋锋惠,马合木提·阿不来提,宋子君,凌锦霞,左琛.平欧杂种榛果实经济性状与综合评价[J].东北林业大学学报,2020,48(5):45-49.LUO Da,SHI Yanjiang,SONG Fenghui,Mahmut·Ablat,SONG Zijun,LING Jinxia,ZUO Chen. Evaluation of fruit economic traits of Corylus heterophylla×C.avellane[J].Journal of Northeast Forestry University,2020,48(5):45-49.
[19] 罗达,宋锋惠,卢明艳,史彦江.盐胁迫对平欧杂种榛根系生理生化特性的影响[J].东北林业大学学报,2024,52(4):29-33.LUO Da,SONG Fenghui,LU Mingyan,SHI Yanjiang. Effects of salt stress on the physiological and biochemical characteristics of Ping’ou hybrid hazelnut root systems[J]. Journal of Northeast Forestry University,2024,52(4):29-33.
[20] 张丽.平欧杂种榛抗盐碱生理机制研究及其耐盐性评价[D].北京:中国林业科学研究院,2015.ZHANG Li. Physiological mechanisms of salt-alkaline tolerance and evaluation on salt tolerance of Ping’ou hybrid hazelnut(Corylus heterophylla Fisch.×Corylus avellana L.)[D].Beijing:Chinese Academy of Forestry,2015.
[21] 张德,张瑞,张夏燚,吴玉霞,赵婷,张仲兴,王双成,王延秀.不同抗盐性苹果砧木对盐胁迫的生理响应[J].干旱地区农业研究,2021,39(4):86-94.ZHANG De,ZHANG Rui,ZHANG Xiayi,WU Yuxia,ZHAO Ting,ZHANG Zhongxing,WANG Shuangcheng,WANG Yanxiu. Physiological response of different salt-tolerant apple rootstocks to salt stress[J].Agricultural Research in the Arid Areas,2021,39(4):86-94.
[22] ARNON D I.Copper enzymes in isolated chloroplasts.Polyphenoloxidase in beta vulgaris[J]. Plant Physiology,1949,24(1):1-15.
[23] ICHSAN C N,ANDANI R,BASYAH B,ZAKARIA S,EFENDI E.The relationship between relative water content of leaves,soluble sugars,accumulation of dry matter,and yield components of rice (Oryza sativa L.) under water-stress condition during the generative stage[J]. International Journal on Advanced Science,Engineering and Information Technology,2022,12(3):899.
[24] TULUN Ş,BILGIN M.Enhanced soluble protein and biochemical methane potential of apple biowaste by different pretreatment[J].Earth Systems and Environment,2018,2(1):85-94.
[25] GUO R,SHI L X,YANG Y F.Germination,growth,osmotic adjustment and ionic balance of wheat in response to saline and alkaline stresses[J].Soil Science and Plant Nutrition,2009,55(5):667-679.
[26] KAWAGUCHI S,KITANO T,ITO K,MINAKATA A. Sodium ion activity and electrical conductivity of poly (maleic acid)and poly (isobutylene-alt-maleic acid) in aqueous salt-free solution[J].Macromolecules,1991,24(23):6335-6339.
[27] 山雨思,代欢欢,何潇,辛正琦,吴能表.外源茉莉酸甲酯和水杨酸对盐胁迫下颠茄生理特性和次生代谢的影响[J].植物生理学报,2019,55(9):1335-1346.SHAN Yusi,DAI Huanhuan,HE Xiao,XIN Zhengqi,WU Nengbiao. Effects of exogenous methyl jasmonate and salicylic acid on physiological characteristics and secondary metabolism of Atropa belladonna under NaCl stress[J].Plant Physiology Journal,2019,55(9):1335-1346.
[28] 邹琦.植物生理学实验指导[M].北京:高等教育出版社,2003.ZOU Qi. Instruction of plant physiology experiment[M]. Beijing:Higher Education Press,2003.
[29] YUAN F,LIANG X,LI Y,YIN S S,WANG B S.Methyl jasmonate improves tolerance to high salt stress in the recretohalophyte Limonium bicolor[J]. Functional Plant Biology,2018,46(1):82-92.
[30] 赵世杰,许长成,邹琦,孟庆伟.植物组织中丙二醛测定方法的改进[J].植物生理学通讯,1994,30(3):207-210.ZHAO Shijie,XU Changcheng,ZOU Qi,MENG Qingwei. Improvement of malondialdehyde determination method in plant tissues[J]. Plant Physiology Communications,1994,30(3):207-210.
[31] KAMBLE D,CHAVAN P,JONDHALE V. Study of potassium and sodium content of mahad-raigad tertiary soil by flame photometry[J]. Asian Journal of Research in Chemistry,2021,14(6):417-420.
[32] 刘玉莲,车飞,王海,陈佰鸿,陈年来.苹果果实中糖、酸和花青苷的组分及含量特征分析[J]. 西北林学院学报,2016,31(6):236-242.LIU Yulian,CHE Fei,WANG Hai,CHEN Baihong,CHEN Nianlai. Characteristics of the components and contents of soluble sugars,organic acids and anthocyanins in apple fruit[J].Journal of Northwest Forestry University,2016,31(6):236-242.
[33] ANWAR A,BAI L Q,MIAO L,LIU Y M,LI S Z,YU X C,LI Y S. 24-epibrassinolide ameliorates endogenous hormone levels to enhance low-temperature stress tolerance in cucumber seedlings[J]. International Journal of Molecular Sciences,2018,19(9):2497.
[34] 缐旭林,张德,张仲兴,王双成,高彦龙,王延秀.2,4-表油菜素内酯对干旱胁迫下垂丝海棠生理特性的影响[J].干旱地区农业研究,2022,40(3):37-45.XIAN Xulin,ZHANG De,ZHANG Zhongxing,WANG Shuangcheng,GAO Yanlong,WANG Yanxiu. Effects of 2,4-Epibrassinolide on physiological characteristics of Malus halliana under drought stress[J].Agricultural Research in the Arid Areas,2022,40(3):37-45.
[35] 张军保,陈宇姝,田诗,于梦迪,王雪松,曹佳昂,邵庆一,杨森,金忠民,刘丽杰.油菜素内酯对低温胁迫下冬小麦Rab15-like基因表达的影响[J].东北农业大学学报,2024,55(5):28-34.ZHANG Junbao,CHEN Yushu,TIAN Shi,YU Mengdi,WANG Xuesong,CAO Jia’ang,SHAO Qingyi,YANG Sen,JIN Zhongmin,LIU Lijie. Effects of brassinosteroid on Rab15-like gene expression in winter wheat under low temperature stress[J].Journal of Northeast Agricultural University,2024,55(5):28-34.
[36] 代红军,魏强,贺琰,汪月宁,王振平.油菜素内酯对高温胁迫下葡萄花色苷合成及果实品质的影响[J].园艺学报,2023,50(8):1711-1722.DAI Hongjun,WEI Qiang,HE Yan,WANG Yuening,WANG Zhenping. Effects of brassinolide treatment on anthocyanin synthesis and quality in grape under high temperature stress[J].Acta Horticulturae Sinica,2023,50(8):1711-1722.
[37] 闫雷,孙小贺,李威,曲娟娟,孟庆尧,张钰莹,武志民,苏捷.外源2,4-表油菜素内酯对镉胁迫下黄瓜幼苗生长及光合生理特性的影响[J].东北农业大学学报,2022,53(6):10-19.YAN Lei,SUN Xiaohe,LI Wei,QU Juanjuan,MENG Qingyao,ZHANG Yuying,WU Zhimin,SU Jie.Effects of exogenous 2,4-epibrassinolide on growth and photosynthetic physiological characteristics of cucumber seedlings under cadmium stress[J]. Journal of Northeast Agricultural University,2022,53(6):10-19.
[38] JI D L,LI Q X,GUO Y J,AN W J,MANAVSKI N,MEURER J,CHI W.NADP+supply adjusts the synthesis of photosystem I in Arabidopsis chloroplasts[J]. Plant Physiology,2022,189(4):2128-2143.
[39] 黄纯倩.植物光合途径的研究进展[J].植物学研究,2024,13(3):315-321.HUANG Chunqian. Research progress of plant photosynthetic pathways[J].Botanical Research,2024,13(3):315-321.
[40] CHOI H W.From the photosynthesis to hormone biosynthesis in plants[J].Plant Pathology Journal,2024,40(2):99-105.
[41] 赵海新.碱胁迫对水稻叶绿素及叶片脯氨酸和可溶性糖含量的影响[J].作物杂志,2020(1):98-102.ZHAO Haixin. Effects of alkali stress on chlorophyll and the contents of proline and soluble sugar in rice[J]. Crops,2020(1):98-102.
[42] WANG J G,TIAN T,WANG H J,CUI J,SHI X Y,SONG J H,LI T S,LI W D,ZHONG M T,ZHANG W X.Improving the estimation accuracy of rapeseed leaf photosynthetic characteristics under salinity stress using continuous wavelet transform and successive projections algorithm[J]. Frontiers in Plant Science,2023,14:1284172.
[43] 寇江涛,康文娟,苗阳阳,师尚礼.外源EBR 对NaCl 胁迫下紫花苜蓿幼苗微量元素吸收及叶绿素荧光动力学参数的影响[J].中国生态农业学报,2016,24(3):345-355.KOU Jiangtao,KANG Wenjuan,MIAO Yangyang,SHI Shangli.Effect of exogenous 2,4-epibrassinolide on trace element absorption and chlorophyll fluorescence of Medicago sativa L.seedlings under NaCl stress[J]. Chinese Journal of Eco-Agriculture,2016,24(3):345-355.
[44] BAJGUZ A,ORCZYK W,GOŁĘBIEWSKA A,CHMUR M,PIOTROWSKA- NICZYPORUK A. Occurrence of brassinosteroids and influence of 24-epibrassinolide with brassinazole on their content in the leaves and roots of Hordeum vulgare L.cv.Golden Promise[J].Planta,2019,249(1):123-137.
[45] HUALPA-RAMIREZ E,CARRASCO-LOZANO E C,MADRID-ESPINOZA J,TEJOS R,RUIZ-LARA S,STANGE C,NORAMBUENA L. Stress salinity in plants:New strategies to cope with in the foreseeable scenario[J]. Plant Physiology and Biochemistry,2024,208:108507.
[46] 崔凯.盐碱胁迫条件下羊草抗氧化酶系统对高压芒刺静电场的响应[D].长春:东北师范大学,2006.CUI Kai. The reaction of enzymatic antioxidant system of Leymus chinensisto high-voltage prick electrostatic field under saline-alkali stress[D]. Changchun:Northeast Normal University,2006.
[47] PARVEEN N,KANDHOL N,SHARMA S,SINGH V P,CHAUHAN D K,LUDWIG-MÜLLER J,CORPAS F J,TRIPATHI D K.Auxin crosstalk with reactive oxygen and nitrogen species in plant development and abiotic stress[J]. Plant & Cell Physiology,2023,63(12):1814-1825.
[48] 侯汶君,麻冬梅,张玲,杭嘉慧.叶面喷施表油菜素内酯对湖南稷子耐盐性的调控作用[J].西北植物学报,2024,44(4):517-528.HOU Wenjun,MA Dongmei,ZHANG Ling,HANG Jiahui.Modulation of salt tolerance in Echinochloa frumentacea by foliar spraying of Epibrassinolide[J]. Acta Botanica Boreali-Occidentalia Sinica,2024,44(4):517-528.
[49] GILL S S,TUTEJA N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants[J]. Plant Physiology and Biochemistry,2010,48(12):909-930.
[50] 任雪,杨尧,杜兴翠,赖齐贤,郭巧会,章四庆.阔叶麦冬对低温变化的生理响应[J].浙江农业科学,2012,53(2):180-183.REN Xue,YANG Yao,DU Xingcui,LAI Qixian,GUO Qiaohui,ZHANG Siqing. Physiological response of liriope muscari to low temperature changes[J]. Journal of Zhejiang Agricultural Sciences,2012,53(2):180-183.
[51] 蒋倩,张瑞,李翔,高静雅,王宁,张朝铖,蒋凯.水分胁迫下4 种观赏草的生理特性[J].草业科学,2019,36(12):3024-3032.JIANG Qian,ZHANG Rui,LI Xiang,GAO Jingya,WANG Ning,ZHANG Chaocheng,JIANG Kai. Morphological and physiological responses of four ornamental grasses under drought stress[J].Pratacultural Science,2019,36(12):3024-3032.
[52] 刘铎,丛日春,党宏忠,李庆梅,刘德玺,杨庆山.柳树幼苗渗透调节物质对中、碱性钠盐响应的差异性[J].生态环境学报,2014,23(9):1531-1535.LIU Duo,CONG Richun,DANG Hongzhong,LI Qingmei,LIU Dexi,YANG Qingshan. Comparative effects of salt and alkali stresses on plant physiology of willow[J].Ecology and Environmental Sciences,2014,23(9):1531-1535.
[53] 代宇佳,罗晓峰,周文冠,陈锋,帅海威,杨文钰,舒凯.生物和非生物逆境胁迫下的植物系统信号[J]. 植物学报,2019,54(2):255-264.DAI Yujia,LUO Xiaofeng,ZHOU Wenguan,CHEN Feng,SHUAI Haiwei,YANG Wenyu,SHU Kai. Plant systemic signaling under biotic and abiotic stresses conditions[J]. Chinese Bulletin of Botany,2019,54(2):255-264.
[54] GONG Z Z,XIONG L M,SHI H Z,YANG S H,HERRERAESTRELLA L R,XU G H,CHAO D Y,LI J R,WANG P Y,QIN F,LI J,DING Y L,SHI Y T,WANG Y,YANG Y Q,GUO Y,ZHU J K. Plant abiotic stress response and nutrient use efficiency[J].Science China(Life Sciences),2020,63(5):635-674.
[55] 高倩,冯棣,刘杰,张敬敏,韩其晟.外源物缓解植物盐分胁迫的作用机理及其分类[J].植物营养与肥料学报,2021,27(11):2030-2044.GAO Qian,FENG Di,LIU Jie,ZHANG Jingmin,HAN Qisheng.Main mechanisms and classification of exogenous substances alleviating plant salt stress[J]. Journal of Plant Nutrition and Fertilizers,2021,27(11):2030-2044.
[56] PANCHAL P,MILLER A J,GIRI J. Organic acids:Versatile stress-response roles in plants[J]. Journal of Experimental Botany,2021,72(11):4038-4052.
[57] WANG J F,SHEN Q R. Roles of organic acid metabolism in plant adaptation to nutrient deficiency and aluminum toxicity stress[J]. The Journal of Applied Ecology,2006,17(11):2210-2216.
[58] 麻莹,王晓苹,姜海波,石德成.盐碱胁迫下碱地肤体内的有机酸积累及其草酸代谢特点[J].草业学报,2017,26(7):158-165.MA Ying,WANG Xiaoping,JIANG Haibo,SHI Decheng.Characteristics of organic acids accumulation and oxalate metabolism in Kochia sieversiana under salt and alkali stresses[J].Acta Prataculturae Sinica,2017,26(7):158-165.
[59] 吕家强,李长有,杨春武,胡锐.天然盐碱土壤对虎尾草茎叶有机酸积累影响及胁迫因子分析[J].草业学报,2015,24(4):95-103.LÜ Jiaqiang,LI Changyou,YANG Chunwu,HU Rui. Effect of natural saline soil on organic acid accumulation in the stem and leaf of Chloris virgata and analysis of stress factors[J]. Acta Prataculturae Sinica,2015,24(4):95-103.
[60] 邹春雷.甜菜适应碱性盐胁迫的生理机制及其转录组分析[D].哈尔滨:东北农业大学,2019.ZOU Chunlei. Physiological mechanism and transcriptom analysis of sugar beet (Beta vulgaris L.) in adaption to alkali-salt stress[D].Harbin:Northeast Agricultural University,2019.
[61] 罗达,吴正保,史彦江,宋锋惠.盐胁迫对3 种平欧杂种榛幼苗叶片解剖结构及离子吸收、运输与分配的影响[J].生态学报,2022,42(5):1876-1888.LUO Da,WU Zhengbao,SHI Yanjiang,SONG Fenghui. Effects of salt stress on leaf anatomical structure and ion absorption,transportation and distribution of three Ping’ou hybrid hazelnut seedlings[J].Acta Ecologica Sinica,2022,42(5):1876-1888.
[62] WAADT R,SELLER C A,HSU P K,TAKAHASHI Y,MUNEMASA S,SCHROEDER J I. Plant hormone regulation of abiotic stress responses[J]. Nature Reviews Molecular Cell Biology,2022,23(10):680-694.
[63] LIU L,CHEN G Q,LI S D,GU Y,LU L L,QANMBER G,MENDU V,LIU Z,LI F G,YANG Z R.A brassinosteroid transcriptional regulatory network participates in regulating fiber elongation in cotton[J].Plant Physiology,2023,191(3):1985-2000.
[64] GUO R,YANG Z Z,LI F,YAN C R,ZHONG X L,LIU Q,XIA X,LI H R,ZHAO L. Comparative metabolic responses and adaptive strategies of wheat(Triticum aestivum)to salt and alkali stress[J].BMC Plant Biology,2015,15:170.
[65] 赵怀玉,林鸿宣.植物响应盐碱胁迫的分子机制[J].土壤与作物,2020,9(2):103-113.ZHAO Huaiyu,LIN Hongxuan. Molecular mechanism of plants in responses to salt and alkali stress[J]. Soils and Crops,2020,9(2):103-113.
[66] 王绮玉,刘欢,仁增旺堆,王敬龙,罗建民,黄颖.PEG 渗透胁迫下藏沙蒿种质资源萌发特性及抗旱性评价[J].草原与草坪,2024,44(2):215-225.WANG Qiyu,LIU Huan,REN Zengwangdui,WANG Jinglong,LUO Jianmin,HUANG Ying.Evaluation of germination characteristics and drought resistance of Artemisia wellbyi germplasm under PEG osmotic stress[J]. Grassland and Turf,2024,44(2):215-225.