在中国北方冷凉气候区,早春气温变化剧烈,昼夜温差大,由于土壤比热容远高于空气,导致土温上升明显滞后于气温,使苹果根系通常处于亚低温下。亚低温是指0 ℃以上但低于植株生长发育的最适温度,影响植株的正常生长发育但不会使植株致死的温度[1]。根区亚低温会导致苹果根系生理功能紊乱和生长发育滞后,对地上部的生命活动影响显著。根系是植物吸收水分和养分的主要器官,且其对低温的响应要比地上部更敏感[2-3]。大量研究已发现,根区低温能够直接影响根系发育、水分和矿质元素的吸收、抗氧化系统和碳氮代谢等生理过程,同时影响地上部光合作用,进而抑制整个植株的生长[4]。苹果根系生长最适宜的土壤温度为15~24 ℃,当土温在3~4 ℃时新根开始生长[5]。当土温为5 ℃时,根系处于亚低温胁迫下,导致其氧化还原状态、相关基因的表达、渗透物质代谢以及生长状态等多方面的改变[6]。
糖在调节植物生理代谢与生长发育中具有重要作用。研究表明,葡萄糖预处理会诱导叶片酸性转化酶(AI)、中性转化酶(NI)、可溶性总糖、葡萄糖和果糖含量增加,保护黄瓜幼苗免受脱水胁迫伤害[7]。盐胁迫下外源葡萄糖和果糖预处理可以提高小黑麦叶片磷酸果糖激酶(PFK)和己糖激酶(HK)活性,这表明外源糖可通过影响糖代谢,减轻盐胁迫对小黑麦的伤害[8]。植物中的蔗糖磷酸合成酶可以促进蔗糖合成,中性转化酶和酸性转化酶将部分蔗糖分解产生葡萄糖和果糖,蔗糖合成酶催化蔗糖可逆转化为葡萄糖和果糖。外源糖可调节植物细胞内碳水化合物代谢相关酶活性及基因表达,进而影响碳水化合物含量[9]。外源葡萄糖与果糖处理可显著提高红地球葡萄试管苗叶片蔗糖合成酶(SS)、蔗糖磷酸合成酶(SPS)等酶活性,导致蔗糖含量增加,叶片的蔗糖供应能力也随之提高,从而促进植株的生长[10]。外源蔗糖处理可显著提高大豆根系SPS、AI及NI 活性,促进植株内积累蔗糖和果糖,进而提高大豆的抗性,缓解低磷胁迫对大豆的损伤[11]。
糖类物质的增加可调节细胞渗透压、保护生物膜系统、加快糖代谢产生更多能量和ATP。此外,糖被认为是调节氧化胁迫的重要化合物[12]。外源糖处理可以通过调节抗氧化酶活性,清除过量活性氧(ROS),从而提高细胞膜的稳定性,缓解逆境胁迫[13-14]。在根区亚低温胁迫下,外源蔗糖处理能够提高山定子幼苗根系抗氧化酶(CAT、POD、SOD 和APX)活性,减少植株ROS的积累量[15]。在拟南芥中蔗糖处理可以消除长时间黑暗中的活性氧应激,提高植株的耐缺氧能力[16]。小分子可溶性糖与其代谢途径相关酶被广泛认为与氧化应激和活性氧信号转导有关,内源性糖为还原型谷胱甘肽(GSH)的产生创造还原能力,有助于清除过氧化氢(H2O2)[17]。研究发现葡萄糖和蔗糖可通过磷酸戊糖途径为抗坏血酸-谷胱甘肽(AsA-GSH)循环提供还原力,有效提高AsA和GSH的再生效率[18]。同时较高浓度的可溶性糖可以作为活性氧的清除剂。三氯蔗糖可以作为拟南芥羟自由基的清除剂,通过与羟自由基的非酶促反应清除ROS,促进细胞ROS动态平衡[19]。
山定子作为苹果常用砧木,其根系在中国北方冷凉地区早春时期常处于亚低温状态下,易遭受氧化胁迫损伤[20]。为探索不同外源糖处理在调节山定子根系响应亚低温中的生理作用,笔者在本研究中通过外源添加蔗糖、葡萄糖和果糖,研究根区亚低温下不同外源糖对山定子幼苗根系抗氧化系统及糖代谢的影响,探讨不同外源糖对根区亚低温下山定子根系生理功能的调节作用,以期为苹果根系低温适应性调控研究提供理论参考。
1 材料和方法
1.1 试验材料与处理
本试验在沈阳农业大学果树科研基地进行,试材采用山定子(Malus baccata Borkh.)实生幼苗。种子层积发芽后点播于穴盘中,待幼苗生长有7~8 片真叶时移栽到规格为16 cm×16 cm 营养钵中进行沙培培养,每隔1 d 浇灌1 次Hoagland 营养液,基于前期预试验发现,土施90 mmol·L-1的蔗糖溶剂效果最好,为保证3种糖提供相同的能量(即相同碳分子含量),葡萄糖与果糖的浓度设置为180 mmol·L-1。当山定子实生幼苗真叶长至15 片时,选取长势一致、无病虫害的健壮幼苗进行试验处理(表1)。
表1 试验设置
Table 1 Design of the experiment
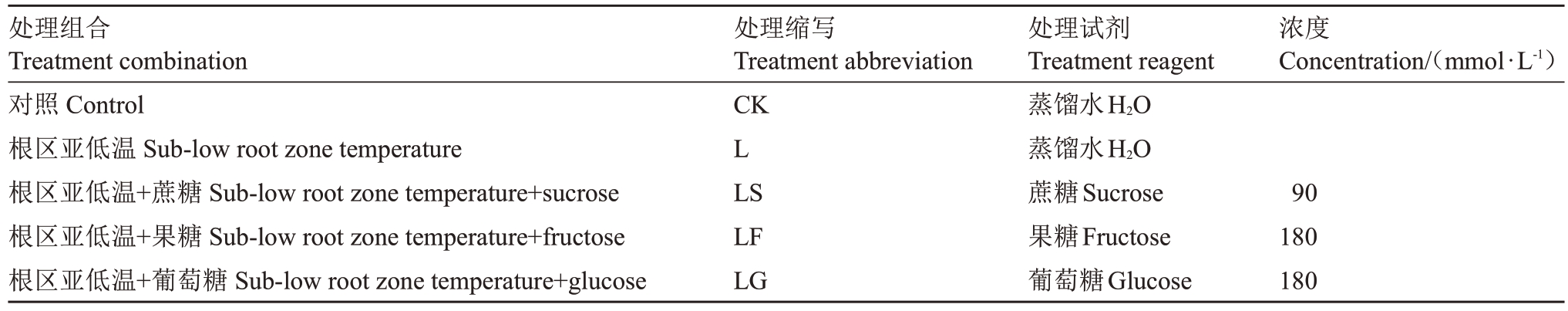
处理组合Treatment combination对照Control根区亚低温Sub-low root zone temperature根区亚低温+蔗糖Sub-low root zone temperature+sucrose根区亚低温+果糖Sub-low root zone temperature+fructose根区亚低温+葡萄糖Sub-low root zone temperature+glucose处理缩写Treatment abbreviation CK L LS LF LG处理试剂Treatment reagent蒸馏水H2O蒸馏水H2O蔗糖Sucrose果糖Fructose葡萄糖Glucose浓度Concentration/(mmol·L-1)90 180 180
1.2 葡萄糖、果糖和蔗糖含量测定
山定子根系蔗糖、葡萄糖、果糖含量采用液相色谱仪进行测定[21]。糖含量测定使用高效液相色谱仪型号为AngiLent-1260,示差折光检测器为RID检测器,进样量10 μL,柱温80 ℃,流动相超纯水。
山定子幼苗在人工气候室中进行预培养,培养温度设定为20 ℃(昼)/10 ℃(夜),光照14 h,湿度70%。预培养2 d后进行根区亚低温处理,将幼苗放进恒温低温槽中,使根部处于(5±0.2)℃条件下。
采用灌根方式进行糖处理,每钵浇灌溶液体积约为250 mL。试材预培养1 d后分别浇灌不同糖溶剂,并在24 h后放入低温槽中进行根区亚低温处理,具体参考永辉等[20]的处理方法。
处理48 h时取样,3次生物学重复,每次重复为5株。根系取样:用自来水将根系表面土壤冲洗干净,吸水纸擦干,剪取山定子幼苗根部的白色细根,用锡纸包好后于液氮中速冻,保存在-80 ℃冰箱备用。
1.3 糖代谢相关酶活性测定
蔗糖代谢关键酶酶液的提取方法与测定方法:称取0.2 g山定子幼苗根系样品,加入3 mL Hepes缓冲液,旋涡混匀后4 ℃过夜,次日于13 000 r·min-1、4 ℃下离心10 min[22]。
己糖激酶(HK)、磷酸果糖激酶(PFK)活性采用江苏酶免实业有限公司生产的ELISA检测试剂盒进行测定,测定方法参照各试剂盒的说明书。
1.4 活性氧含量、丙二醛含量及抗氧化酶活性测定
H2O2含量测定:称取0.06 g 山定子幼苗根系样品,加入2 mL的TCA溶液,于10 000 r·min-1、4 ℃下离心10 min,取1 mL上清液,加入0.1 mL 20%TiCl4溶液、0.2 mL浓氨水,于5000 r·min-1、25 ℃下离心10 min,沉淀用3 mL硫酸溶解,在A410下测定吸光值[23]。
超氧自由基(O2.-)测定方法:参照FAROOQ M A[24]的试验方法并稍作改动,称取山定子根系0.1 g,加入2 mL 50 mmol·L-1 的Kpp(pH7.8),室温振荡20 min,于1000 r·min-1、25 ℃下离心10 min,取上清液,二次离心,加入0.9 mL Kpp与0.1 mL盐酸羟胺,充分混匀,25 ℃反应20 min,然后依次加入1 mL对氨基苯磺酸与1 mL α-萘胺,25 ℃反应20 min,在A530下测定吸光值。
丙二醛含量测定采用硫代巴比妥酸比色法[25]。抗氧化酶液提取方法[26]:稍作改动,称取0.075 g样品,加入0.075 g 交联聚乙烯吡咯烷酮(pvpp)与1.5 mL 100 mmol·L-1 磷酸钾缓冲液(pH 7.8)(含0.5%聚乙二醇对异辛基苯基),漩涡混匀,4 ℃过夜,于8000 r·min-1、4 ℃下离心35 min,取上清液,即酶液。
SOD 活性用硝基蓝四唑(NBT)法测定[27];POD活性采用愈创木酚法测定[28];CAT活性的测定:抗氧化酶提取液0.1 mL,加入1 mL 50 mmol·L-1 Tirs-HCl溶液、0.2 mL 200 mmol·L-1过氧化氢溶液与1.7 mL H2O 在A240下,读3 min,每30 s 读一次数[29];APX 活性测定方法:抗氧化酶提取液0.05 mL,分别加入0.1 mL 0.5 mol·L-1 Kpp 缓冲液、0.1 mL 2 mmol·L-1 AsA 溶液、0.1 mL 过氧化氢溶液和0.65 mL H2O,在A290的条件下测定5 min吸光值[30];GR活性测定:抗氧化酶提取液0.05 mL,分别加入0.55 mL H2O、0.1 mL 1 mol·L-1 Hepes溶液、0.1 mL 5 mmol·L-1 EDTA溶液、0.1 mL 1 mmol·L-1 NADPH溶液和0.1 mL 5 mmol·L-1 GSSG 溶液,在25 ℃下反应5 min,在A340条件下测定5 min吸光值[31]。
1.5 抗氧化物含量测定
AsA、DHA、GSH 和GSSG 含量测定参考方法:称取0.2 g根系粉末加入2 mL 5%的TCA进行提取,于13 000 r·min-1、4 ℃下离心15 min,取上清液用于测定[32-33]。
1.6 数据统计与分析
使用Excel 2019软件对试验数据进行梳理分析和作图,使用SPSS Statistics 22.0 软件对数据进行差异显著性检验(p<0.05)、统计分析、单因素方差分析(ANOVA)及多重比较(Duncan法)。
2 结果与分析
2.1 外源糖对根区亚低温下山定子根系糖代谢的影响
2.1.1 外源糖对根区亚低温下山定子根系糖含量的影响 如图1 所示,与CK 相比,L 处理下根系可溶性糖、蔗糖、葡萄糖和果糖含量显著升高。与L处理相比,施用外源糖不同程度提高了可溶性糖、蔗糖、葡萄糖和果糖含量,LG处理的可溶性糖和葡萄糖含量显著高于其他两种糖处理;蔗糖含量在LF 和LS与LS和LG处理之间无显著差异;果糖含量在LF和LG 处理之间无显著差异,且LS 处理的果糖含量显著低于LF、LG处理。
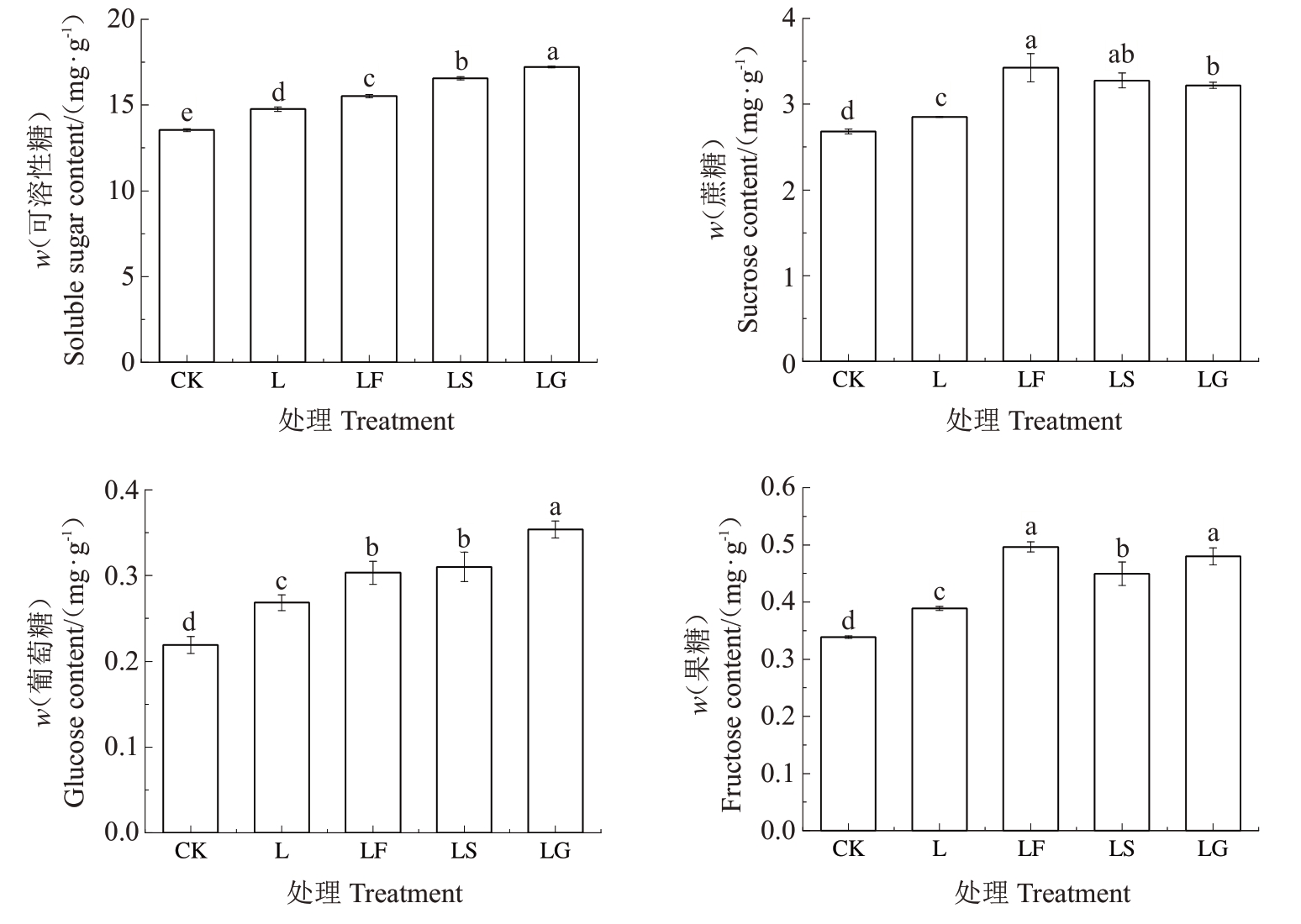
图1 外源糖对根区亚低温下根系糖组分含量的影响
Fig.1 Effects of exogenous sugars on the content of sugar components in sub-low root zone temperature
不同小写字母表示不同处理差异显著(p<0.05)。下同。
Different small letters indicate significant difference between different treatments(p<0.05).The same below.
2.1.2 外源糖对根区亚低温下根系糖代谢相关酶活性的影响 SPS催化尿苷二磷酸葡萄糖和6-磷酸果糖产生磷酸蔗糖,再发生脱磷酸反应生成蔗糖和磷酸;SS催化尿苷二磷酸葡萄糖和果糖产生蔗糖和尿苷二磷酸,反应可逆。如图2 所示,L 处理后根系SPS 活性和SS 活性(合成方向)均显著升高。与L处理相比,LF、LS、LG 处理下SPS 活性和SS 活性(合成方向)均显著提高,SPS 活性分别提高了47.51%、56.09%和51.60%,但各处理间无显著差异;SS 活性(合成方向)分别提高了22.51%、33.36%和23.26%,其中LS 处理下SS 活性显著高于LF 和LG处理。
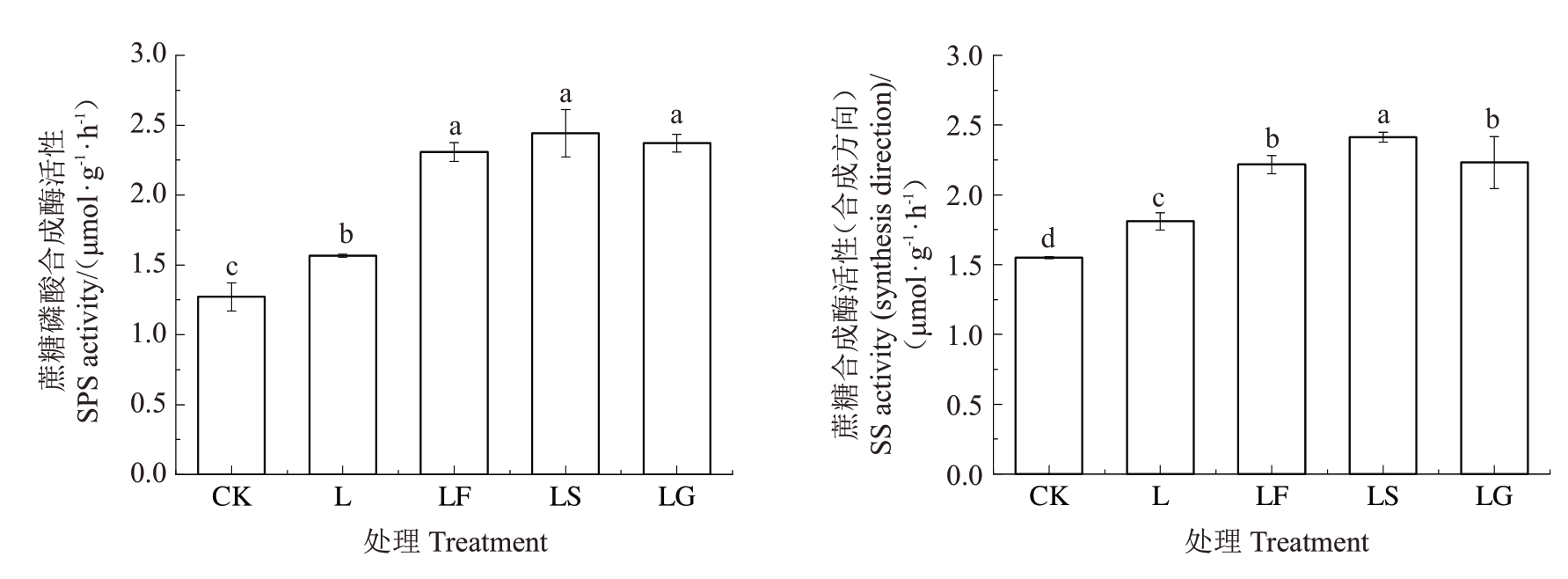
图2 外源糖对根区亚低温下根系SPS 和SS(合成方向)活性的影响
Fig.2 Effects of exogenous sugars on the SPS and SS(synthesis direction)activities of sub-low root zone temperature
酸性转化酶、中性转化酶能将蔗糖水解成葡萄糖和果糖。SS 催化蔗糖和尿苷二磷酸产生尿苷二磷酸葡萄糖和果糖,反应可逆。如图3所示,L处理后根系AI、NI和SS活性(分解方向)显著升高。与L处理相比,LF、LS和LG处理均显著提高了AI、NI和SS 活性(分解方向),其中AI 活性分别提高了33.82%、42.64%和34.94%,AI 活性在LF、LS 和LG处理之间无显著变化;NI 活性分别提高了35.52%、93.30%和38.33%,LS 处理的NI 活性显著高于LF、LG 处理;SS 活性(分解方向)分别提高了28.02%、48.16%和40.58%,SS 活性(分解方向)在LS 和LG、LF和LG处理之间无显著差异。
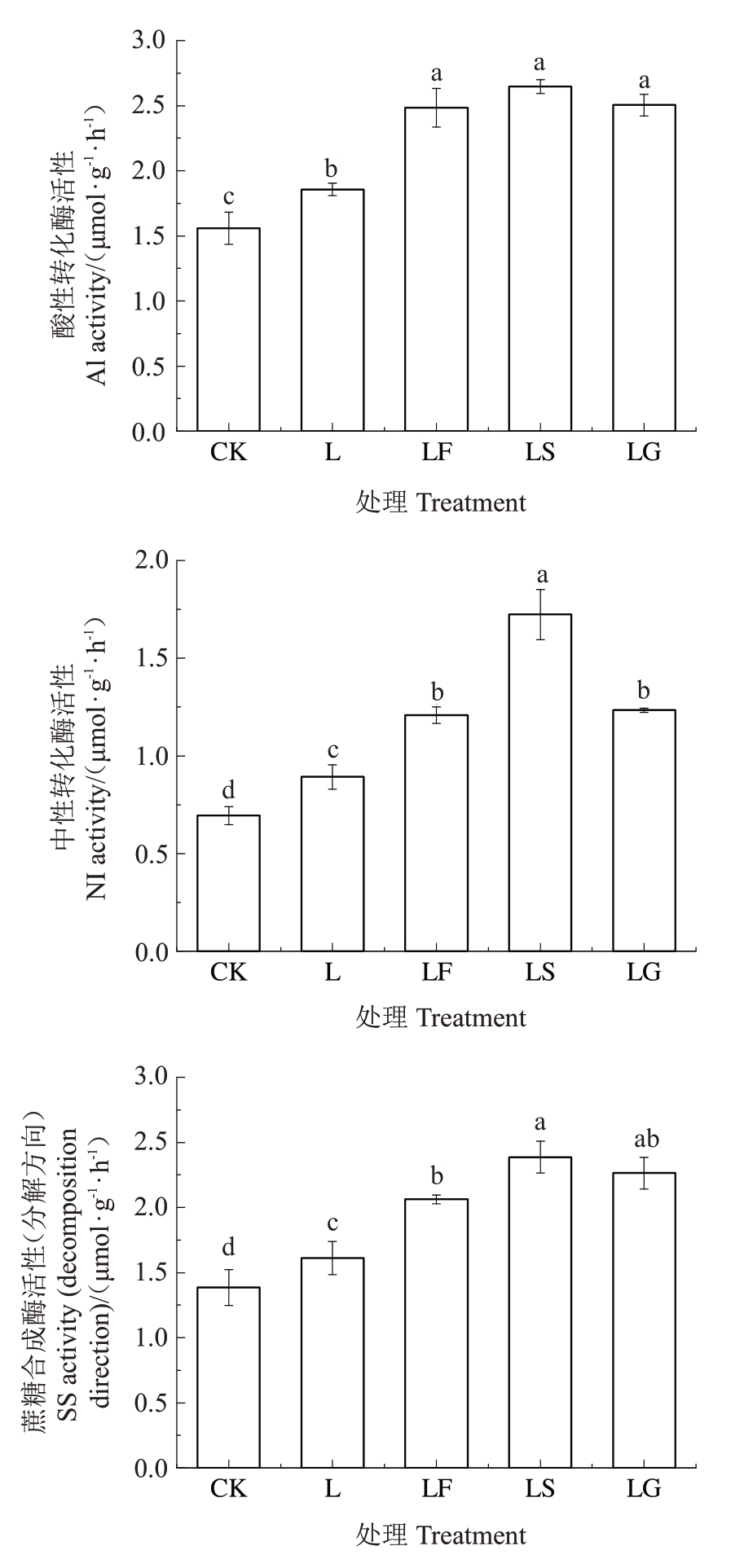
图3 外源糖对根区亚低温下根系AI、NI 和SS(分解方向)活性的影响
Fig.3 Effects of exogenous sugars on AI,NI and SS(decomposition direction)activities of sub-low root zone temperature
如图4所示,L处理后根系HK、PFK活性显著升高。与L处理相比,LF、LS和LG处理均显著提高了HK、PFK 活性,其中HK 活性分别提高了21.28%、21.36%和25.04%,且HK 活性在LF、LS 和LG 处理之间无显著变化;PFK 活性分别提高了26.71%、35.83%和17.83%,且LF与LS处理之间无显著差异。
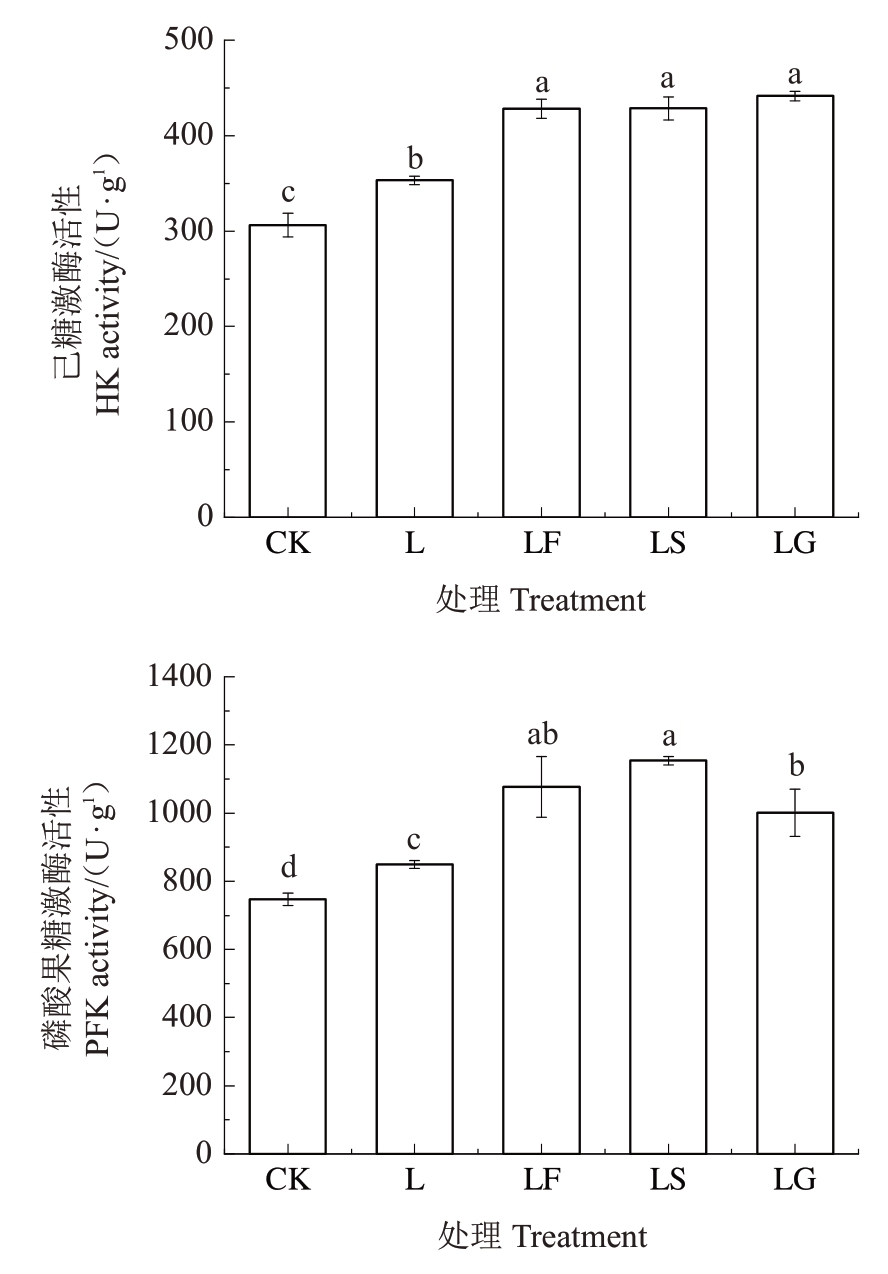
图4 外源糖对根区亚低温下根系己糖激酶和磷酸果糖激酶活性的影响
Fig.4 Effects of exogenous sugars on the activities of hexokinase and phosphofructokinase in sub-low root zone temperature
2.2 外源糖对根区亚低温下山定子根系抗氧化系统的影响
2.2.1 外源糖对根区亚低温下山定子根系丙二醛含量、相对电导率和活性氧含量的影响 如图5所示,L处理根系MDA含量与相对电导率显著提高。与L处理相比,LF、LS、LG处理MDA含量与相对电导率显著降低,MDA 含量分别降低15.12%、20.41%和16.99%,LS 处理的MDA 含量要显著低于其他两种糖处理;相对电导率分别降低了23.15%、36.05%和27.82%,其中相对电导率在LS和LG、LF和LG处理之间无显著差异。
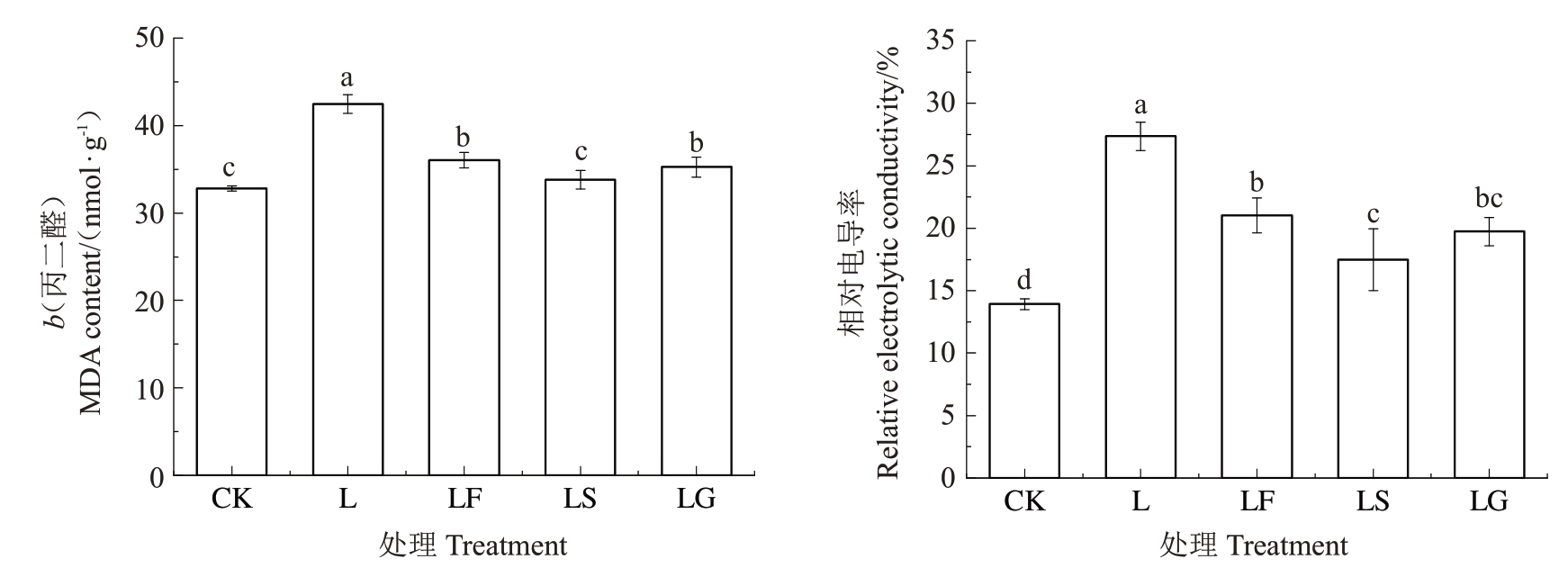
图5 外源糖对根区亚低温下根系丙二醛含量与相对电导率的影响
Fig.5 Effects of exogenous sugars on malondialdehyde content and relative conductivity in sub-low root zone temperature
如图6 所示,与CK 相比,L 处理根系O2·-、H2O2含量显著增加。与L 处理相比,LF、LS、LG 处理O2·-、H2O2 含量显著降低,O2·-含量分别降低了13.98%、22.47%和16.05%,O2·-在LS和LG、LF和LG处理之间无显著差异;H2O2 含量分别降低了10.83%、15.19%和11.96%,LS处理的H2O2含量显著低于LF、LG处理。
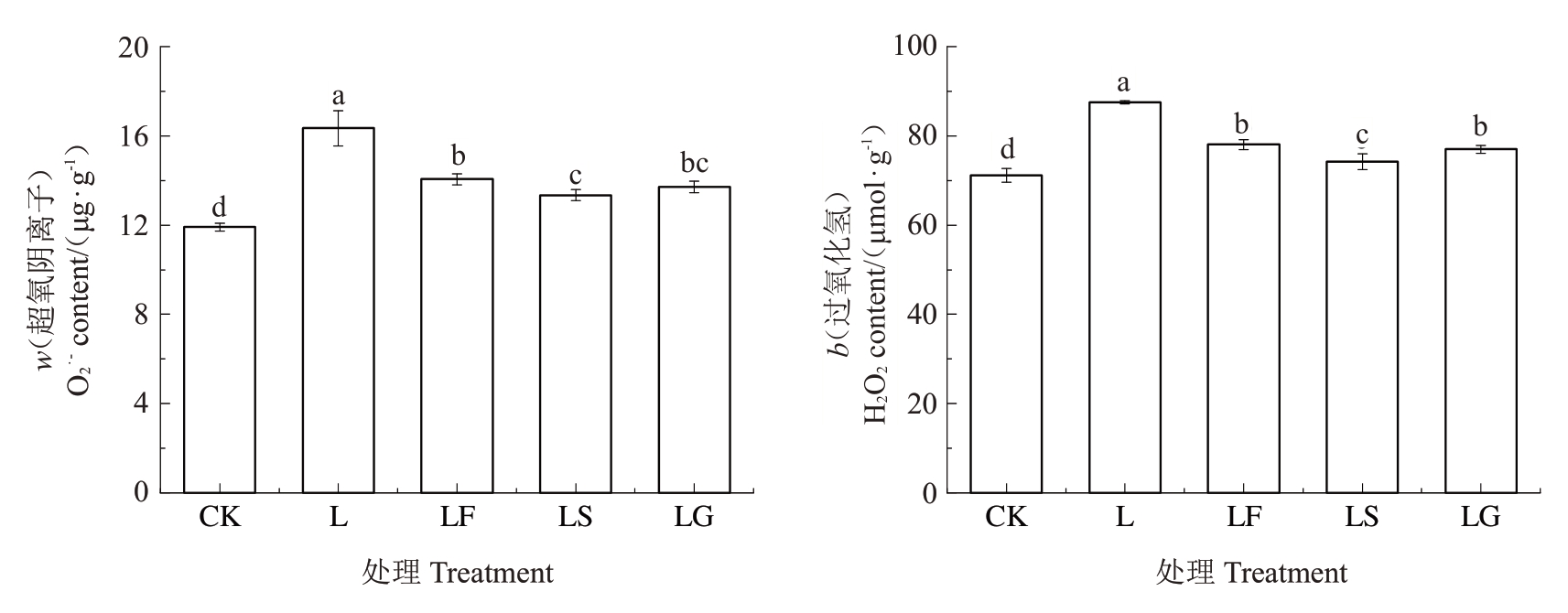
图6 外源糖对根区亚低温下根系活性氧含量的影响
Fig.6 Effects of exogenous sugars on the content of reactive oxygen species in sub-low root zone temperature
2.2.2 外源糖对根区亚低温下山定子根系抗氧化酶活性的影响 如图7 所示,L 处理诱导根系SOD、POD、CAT活性显著升高。与L 处理相比,LF、LS、LG 处理根系SOD、POD、CAT 活性显著升高,SOD活性分别升高了12.19%、23.28%和15.12%;POD活性分别升高了15.30%、28.14%和19.51%;CAT活性分别升高了26.12%、29.92%和25.57%,LS 处理的酶活性显著高于其他两种糖的处理。
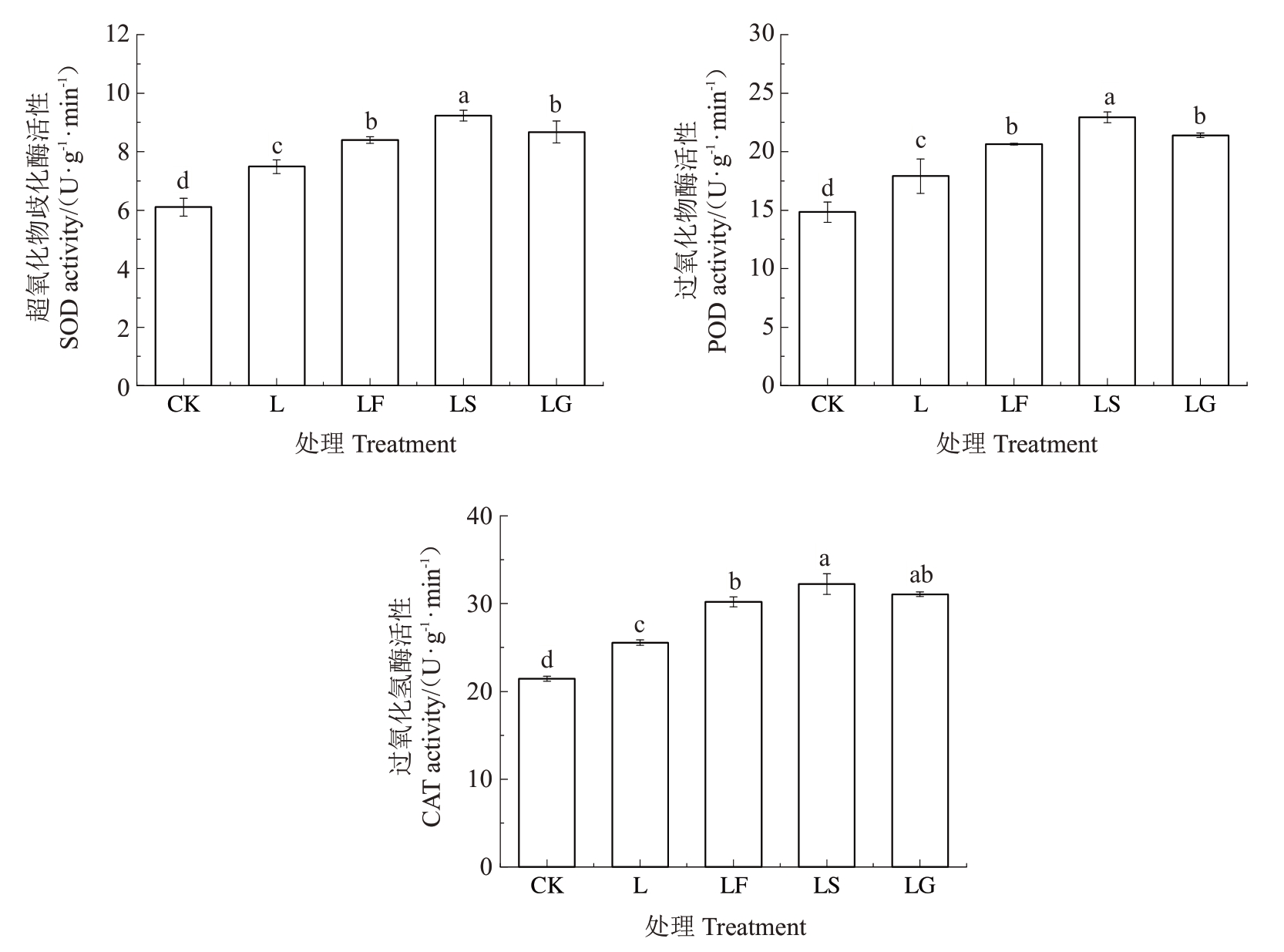
图7 外源糖对根区亚低温下根系超氧化物歧化酶、过氧化物酶和过氧化氢酶活性的影响
Fig.7 Effects of exogenous sugars on the activities of superoxide dismutase,peroxidase and catalase in sub-low root zone temperature
APX 属于过氧化物酶家族,通过催化H2O2的还原来维持细胞中的氧化还原平衡。GR 催化GSSG 还原生成GSH,维持植物体内的GSH 含量和GSH 库的氧化还原状态,并清除ROS。如图8所示,与CK 相比,L 处理后根系APX 活性无显著变化,GR 活性有所下降但变化不显著。与L 处理相比,不同糖处理后APX 和GR 活性均显著提高,APX 活性分别提高了30.50% 、44.65%和39.95%,GR 活性分别提高了37.15%、49.31%和41.92%。山定子根系APX、GR 活性从高到低依次是LS>LG>LF。
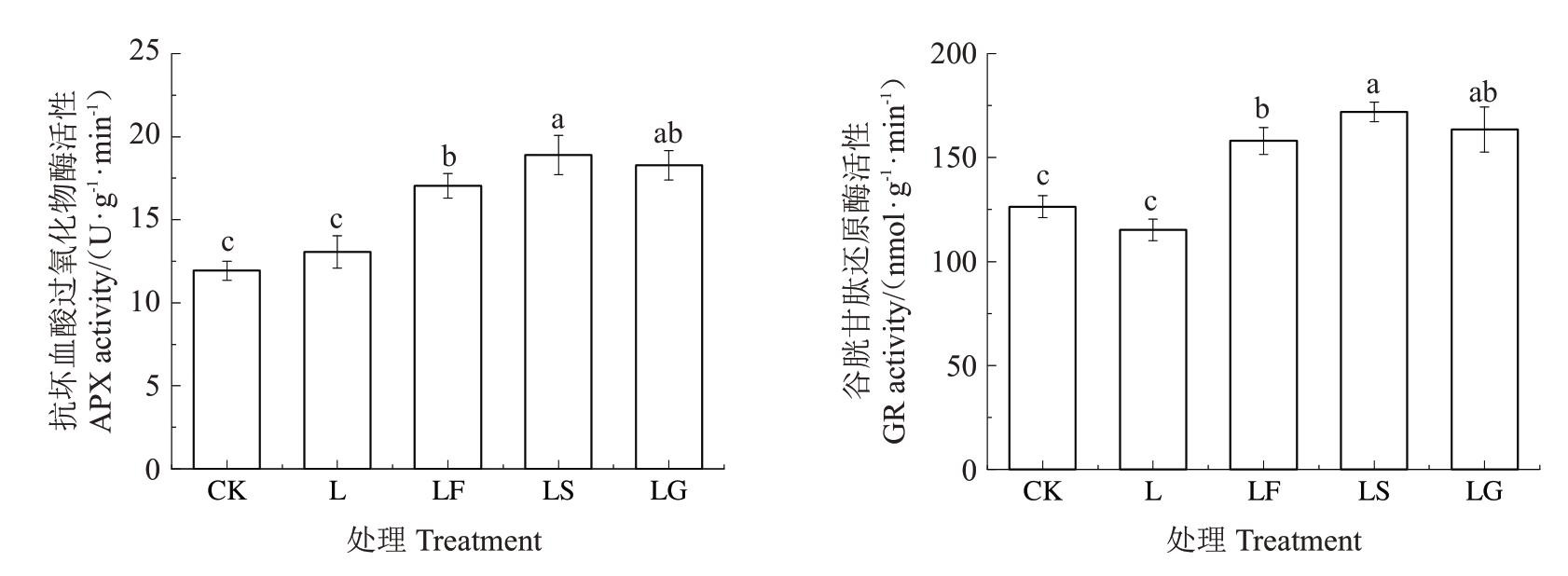
图8 外源糖对根区亚低温下根系抗坏血酸过氧化酶和谷胱甘肽还原酶活性的影响
Fig.8 Effects of exogenous sugars on the activities of ascorbate peroxidase and glutathione reductase in sub-low root zone temperature
2.2.3 外源糖对根区亚低温下山定子根系抗氧化剂含量的影响 在AsA-GSH 循环中,AsA 和GSH 也参与了ROS 解毒过程,低温胁迫严重影响了植物氧化还原平衡,导致DHA 和GSSG 的过度积累。如图9 所示,L 处理的AsA 含量无显著变化,DHA、GSH 和GSSG 含量显著升高,AsA/DHA 显著降低,GSH/GSSG 无显著变化;与L 处理相比,不同糖处理后,AsA 与DHA 含量显著增加,AsA/DHA 显著升高,且在LF、LS、LG 处理之间无显著变化;GSH含量显著升高,GSSG含量显著降低,GSH/GSSG显著升高,且LS 处理的GSH/GSSG 显著高于LF 和LG处理。
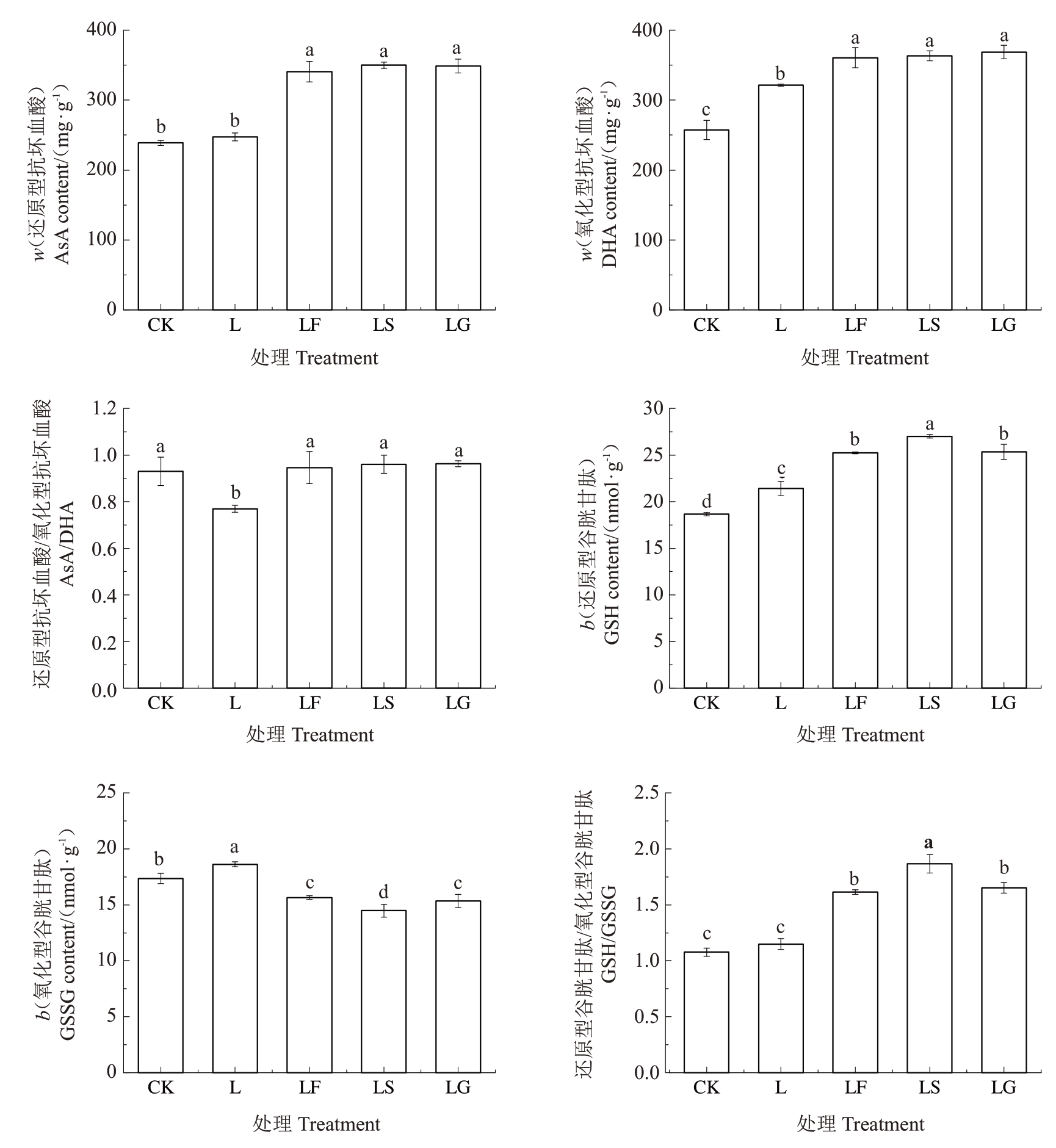
图9 外源糖对根区亚低温下山定子根系抗氧化物质含量的影响
Fig.9 Effects of exogenous sugars on the content of antioxidant substances in sub-low root zone temperature
3 讨 论
糖是光合、呼吸作用的底物,能为植物的生长发育提供碳骨架和能量,也能增强植株的抗逆性[34]。逆境胁迫会诱导植物积累不同可溶性糖(如蔗糖、葡萄糖、果糖和海藻糖)和多元醇(如水杨醇、甘露醇和山梨醇)[35]。蔗糖代谢与植物低温适应性密切相关[36]。研究表明,低温胁迫会导致麻疯树幼苗SPS和转化酶活性升高,可溶性糖含量增加[37]。蔗糖、葡萄糖和果糖等可溶性糖可作为渗透分子和信号分子,参与植物对环境胁迫的响应[38]。在本试验中,根区亚低温显著提高了根系中蔗糖代谢酶SPS、SS(合成和分解方向)和AI活性,促进根系中蔗糖、葡萄糖和果糖积累量增加,这与前人的研究结果相一致[39]。而在低温下可溶性糖的积累可提高细胞的渗透调节能力,在根系响应低温逆境时起到积极作用[40]。在本试验中,根区亚低温处理下山定子根系HK和PFK活性显著升高,这有利于将己糖磷酸化,诱导糖信号,产生更多能量,可为糖的合成代谢提供足够的还原力和能量[41]。
糖类作为安全无毒的天然物质,在生产中可应用于增强园艺作物抗低温能力、提高作物品质与产量等方面[42]。植物对不同糖及不同糖浓度所产生的响应不同。研究表明,外源葡萄糖处理会诱导叶片AI、NI活性及可溶性总糖、葡萄糖和果糖含量提高,保护黄瓜幼苗免受脱水胁迫伤害[6]。外源蔗糖显著提高了叶用莴苣中可溶性糖、蔗糖含量,提高了SS、SPS 活性,并随着生长时间的延长而逐渐提高[43]。在本试验中,根区亚低温下添加蔗糖、葡萄糖和果糖均能使山定子幼苗根系中可溶性糖含量升高,且与SS、SPS、AI和NI活性的增强相对应。同时,根系中O2·-、H2O2、MDA含量和相对电导率降低,说明外源糖处理在一定程度上可激活糖代谢酶,促进可溶性糖积累,从而降低ROS含量,缓解脂质过氧化,保护山定子幼苗免受低温胁迫损伤。
ROS的过量积累导致细胞膜脂质过氧化,表现为MDA含量的增加[44-45]。在本试验中,根区亚低温引起山定子根系O2·-和H2O2的积累与相对电导率的提高,ROS 含量升高加剧根系膜脂过氧化,表现为MDA积累,同时由于应激反应,SOD、POD和CAT活性在短期亚低温下升高,但笔者课题组前期研究发现,长时间的根区亚低温会抑制抗氧化酶活性。APX和GR作为AsA-GSH循环中重要的酶,主要通过参与AsA和GSH再生以及维持AsA-GSH循环来清除ROS[46-47]。外源糖通过诱导保护性酶活性升高成为其有效缓解逆境胁迫的重要机制之一[48]。本研究结果表明,外源添加果糖、蔗糖、葡萄糖可以提高山定子幼苗根系中SOD、POD、CAT、APX 和GR 活性,与AsA、GSH 抗氧化剂、糖组分含量,AsA/DHA和GSH/GSSG水平升高的趋势相一致。抗氧化酶活性、抗氧化剂和糖组分含量的升高导致O2·-、H2O2、MDA 含量和相对电导率降低。说明外源糖处理导致根区亚低温下山定子幼苗根系中蔗糖等含量升高,维持较高的AsA/DHA 和GSH/GSSG,使AsA 和GSH 能够在低温胁迫下AsA-GSH 循环中发挥作用,同时诱导抗氧化酶清除ROS,进而减轻根系的氧化损伤。
不同园艺作物在不同低温条件下所适宜的糖种类和糖浓度存在差异。在本研究中,外源添加蔗糖、果糖、葡萄糖均可通过促进糖代谢,积累可溶性糖等渗透物质,调节抗氧化系统,减轻根系活性氧诱导的氧化损伤,进而提高山定子幼苗对根区亚低温胁迫的耐受性。且3 种糖处理中蔗糖的处理效果最好,分析原因可能是:糖作为碳源为植物提供能量来源,蔗糖较葡萄糖能更好地调节基质(培养基)的渗透压;蔗糖也是一种重要的信号物质,参与调控植物的多种生理过程[42];研究表明,蔗糖对氧化胁迫的保护作用是由于其信号转导作用,从而完成对ROS的清除[49]。此外,蔗糖可通过转化酶或蔗糖合成酶分解成己糖进一步参与调节相关生理代谢。研究结果可为从糖调控角度改良苹果低温抗性提供理论参考,对冷凉气候区苹果生产中糖的施用具有指导意义。
4 结 论
根区亚低温处理48 h 会诱导山定子根系SS、SPS 等糖代谢关键酶活性升高,糖含量增加,SOD、POD等抗氧化酶活性和AsA、GSH等抗氧化物含量升高,同时山定子根系中ROS、MDA 含量和相对电导率升高,导致根系膜脂过氧化程度加剧。外源添加果糖、蔗糖、葡萄糖可通过调节糖代谢、抗氧化系统减轻根系氧化损伤,且外源蔗糖处理对根区亚低温胁迫下山定子幼苗的氧化损伤缓解效果要好于果糖和葡萄糖处理。
[1] ZHANG M Z,WANG L L,YE D,CHEN X,WU Z Y,LIN X J,CHEN W J,BIAN H W,HAN N,ZHU M Y. Sucrose treatment alters floral induction and development in vitro in gloxinia[J].In Vitro Cellular & Developmental Biology- Plant,2012,48(2):167-171.
[2] GHOSH D,XU J.Abiotic stress responses in plant roots:A proteomics perspective[J].Frontiers in Plant Science,2014,5:6.
[3] 赵鹏,常涛,张玉鑫.根区温度对甜瓜幼苗生长的影响[J].北方园艺,2008(12):88-90.ZHAO Peng,CHANG Tao,ZHANG Yuxin.Effects of root zone temperature on growth of muskmelon seedlings[J]. Northern Horticulture,2008(12):88-90.
[4] ANWAR A,LI Y S,HE C X,YU X C. 24-Epibrassinolide promotes NO3- and NH4+ ion flux rate and NRT1 gene expression in cucumber under suboptimal root zone temperature[J]. BMC Plant Biology,2019,19(1):225.
[5] 杨洪强,束怀瑞.苹果根系研究[M].北京:科学出版社,2007.YANG Hongqiang,SHU Huairui. Studies on apple roots[M].Beijing:Science Press,2007.
[6] JAN N,MAJEED U,ANDRABI K I,JOHN R. Cold stress modulates osmolytes and antioxidant system in Calendula officinalis[J].Acta Physiologiae Plantarum,2018,40(4):73.
[7] HUANG Y W,NIE Y X,WAN Y Y,CHEN S Y,SUN Y,WANG X J,BAI J G. Exogenous glucose regulates activities of antioxidant enzyme,soluble acid invertase and neutral invertase and alleviates dehydration stress of cucumber seedlings[J]. Scientia Horticulturae,2013,162:20-30.
[8] 李改玲.外源糖调控盐胁迫下小黑麦糖代谢及光合特性的研究[D].哈尔滨:东北农业大学,2016.LI Gailing. Exogenous sugar alleviate effects of salt stress on the sugar metabolism and photosynthetic characteristics of triticale[D].Harbin:Northeast Agricultural University,2016.
[9] HO S,CHAO Y,TONG W,YU S.Sugar coordinately and differentially regulates growth- and stress-related gene expression via a complex signal transduction network and multiple control mechanisms[J].Plant Physiology,2001,125(2):877-890.
[10] 米宝琴.外源糖对葡萄试管苗糖代谢的分子调控机理研究[D].兰州:甘肃农业大学,2016.MI Baoqin. The study of molecular regulation mechanism on exogenous sugar effect sugar metabolism of grape plantlets in vitro[D].Lanzhou:Gansu Agricultural University,2016.
[11] 孔令剑.蔗糖处理下大豆苗期根系对低磷胁迫的响应[D].沈阳:沈阳农业大学,2018.KONG Lingjian.Responses of soybean seedlings root system to low phosphorus stress under sucrose treatment[D]. Shenyang:Shenyang Agricultural University,2018.
[12] VAN DEN ENDE W,VALLURU R.Sucrose,sucrosyl oligosaccharides,and oxidative stress:Scavenging and salvaging[J].Journal of Experimental Botany,2009,60(1):9-18.
[13] BAQUE M A,ELGIRBAN A,LEE E J,PAEK K Y.Sucrose regulated enhanced induction of anthraquinone,phenolics,flavonoids biosynthesis and activities of antioxidant enzymes in adventitious root suspension cultures of Morinda citrifolia (L.)[J].Acta Physiologiae Plantarum,2012,34(2):405-415.
[14] BAI L Q,DENG H H,ZHANG X C,YU X C,LI Y S.Gibberellin is involved in inhibition of cucumber growth and nitrogen uptake at suboptimal root-zone temperatures[J]. PLoS One,2016,11(5):e0156188.
[15] ZHAO X P,LIU H Y,YONG H,LI L J,LYU D G. Effects of exogenous sucrose on root physiology and transcriptome of Malus baccata Borkh.under sub-low root-zone temperature[J].Scientia Horticulturae,2024,337:113474.
[16] ROSENWASSER S,ROT I,SOLLNER E,MEYER A J,SMITH Y,LEVIATAN N,FLUHR R,FRIEDMAN H. Organelles contribute differentially to reactive oxygen species-related events during extended darkness[J].Plant Physiology,2011,156(1):185-201.
[17] BOLOURI-MOGHADDAM M R,LE ROY K,XIANG L,ROLLAND F,VAN DEN ENDE W. Sugar signalling and antioxidant network connections in plant cells[J]. The FEBS Journal,2010,277(9):2022-2037.
[18] XIANG L,LI Y,ROLLAND F,VAN DEN ENDE W. Neutral invertase,hexokinase and mitochondrial ROS homeostasis:Emerging links between sugar metabolism,sugar signaling and ascorbate synthesis[J].Plant Signaling&Behavior,2011,6(10):1567-1573.
[19] MATROS A,PESHEV D,PEUKERT M,MOCK H P,VAN DEN ENDE W.Sugars as hydroxyl radical scavengers:Proof-ofconcept by studying the fate of sucralose in Arabidopsis[J]. The Plant Journal,2015,82(5):822-839.
[20] 永辉,王鹏,覃壮志,吕德国,李丽杰.不同外源糖对根区亚低温下山定子生长和养分吸收的影响[J].应用生态学报,2024,35(7):1825-1832.YONG Hui,WANG Peng,QIN Zhuangzhi,LÜ Deguo,LI Lijie.Effect of different exogenous sugars on the growth and nutrient uptake of Malus baccata Borkh. under sub-low root zone temperature[J]. Chinese Journal of Applied Ecology,2024,35(7):1825-1832.
[21] 陆晓晨.根域亚低温胁迫下山定子根系GABA 代谢特征及其生理效应研究[D].沈阳:沈阳农业大学,2017.LU Xiaochen. The metabolic characteristics and physiological regulation of GABA in Malus baccata Borkh. roots under sublow temperature of root-zone[D]. Shenyang:Shenyang Agricultural University,2017.
[22] 田红梅,方凌,王艳,王明霞,江海坤,严从生,张其安.越瓜果实发育过程中糖的变化与相关酶活性的关系[J].中国蔬菜,2013(8):66-70.TIAN Hongmei,FANG Ling,WANG Yan,WANG Mingxia,JIANG Haikun,YAN Congsheng,ZHANG Qi’an. Relationship between sugar content change and interrelated enzyme activity in crisp melon fruit development process[J]. China Vegetables,2013(8):66-70.
[23] LI L J,LU X C,MA H Y,LYU D G. Comparative proteomic analysis reveals the roots response to low root-zone temperature in Malus baccata[J]. Journal of Plant Research,2018,131(5):865-878.
[24] FAROOQ M A,LI L,ALI B,GILL R A,WANG J,ALI S,GILL M B,ZHOU W J.Oxidative injury and antioxidant enzymes regulation in arsenic-exposed seedlings of four Brassica napus L.cultivars[J]. Environmental Science and Pollution Research,2015,22(14):10699-10712.
[25] DU F,XU J N,LI D,WANG X Y. The identification of novel and differentially expressed apple-tree genes under low-temperature stress using high-throughput Illumina sequencing[J].Molecular Biology Reports,2015,42(3):569-580.
[26] LI L J,LU X C,MA H Y,LÜ D G.Comparative proteomic analysis reveals the roots response to low root-zone temperature in Malus baccata[J]. Journal of Plant Research,2018,131:865-878.
[27] AFZAL J,HU C,IMTIAZ M,ELYAMINE A M,RANA M S,IMRAN M,FARAG M A. Cadmium tolerance in rice cultivars associated with antioxidant enzymes activities and Fe/Zn concentrations[J]. International Journal of Environmental Science and Technology,2019,16(8):4241-4252.
[28] ZHANG S S,ZHANG H M,QIN R,JIANG W S,LIU D H.Cadmium induction of lipid peroxidation and effects on root tip cells and antioxidant enzyme activities in Vicia faba L.[J]. Ecotoxicology,2009,18(7):814-823.
[29] EMAMVERDIAN A,DING Y L,MOKHBERDORAN F,XIE Y F,ZHENG X,WANG Y J. Silicon dioxide nanoparticles improve plant growth by enhancing antioxidant enzyme capacity in bamboo (Pleioblastus pygmaeus) under lead toxicity[J]. Trees,2020,34(2):469-481.
[30] MAKSIMOVIĆ J D,ZHANG J Y,ZENG F R,ŽIVANOVIĆ B D,SHABALA L,ZHOU M X,SHABALA S. Linking oxidative and salinity stress tolerance in barley:Can root antioxidant enzyme activity be used as a measure of stress tolerance?[J].Plant and Soil,2013,365(1):141-155.
[31] WANG J L,ZENG Q,ZHU J G,LIU G,TANG H Y.Dissimilarity of ascorbate-glutathione (AsA-GSH) cycle mechanism in two rice (Oryza sativa L.) cultivars under experimental free- air ozone exposure[J]. Agriculture,Ecosystems & Environment,2013,165:39-49.
[32] YU J,CANG J,LI Y P,HUANG R,LU Q W,WANG X T,LIU L J,XU Q H,ZHANG K J.Salicylic acid-induced antioxidant protection against low temperature in cold-hardy winter wheat[J].Acta Physiologiae Plantarum,2016,38(11):261.
[33] LI L J,LU X C,MA H Y,LYU D G. Jasmonic acid regulates the ascorbate-glutathione cycle in Malus baccata Borkh. roots under low root-zone temperature[J]. Acta Physiologiae Plantarum,2017,39(8):174.
[34] 罗玉.植物中的糖代谢及其相关酶[J].文山师范高等专科学校学报,2004,17(2):155-159.LUO Yu. The sugar metabolism and the relational enzymes in plants[J]. Journal of Wenshan Teachers’College,2004,17(2):155-159.
[35] ZULFIQAR F,AKRAM N A,ASHRAF M. Osmoprotection in plants under abiotic stresses:New insights into a classical phenomenon[J].Planta,2019,251(1):3.
[36] JIANG H Y,LI W,HE B J,GAO Y H,LU J X.Sucrose metabolism in grape (Vitis vinifera L.) branches under low temperature during overwintering covered with soil[J].Plant Growth Regulation,2014,72(3):229-238.
[37] WANG H B,GONG M,XIN H,TANG L Z,DAI D Q,GAO Y,LIU C. Effects of chilling stress on the accumulation of soluble sugars and their key enzymes in Jatropha curcas seedlings[J].Physiology and Molecular Biology of Plants,2018,24(5):857-865.
[38] LI C,LIU Y,TIAN J,ZHU Y S,FAN J J.Changes in sucrose metabolism in maize varieties with different cadmium sensitivities under cadmium stress[J].PLoS One,2020,15(12):e0243835.
[39] JIANG H Y,GAO Y H,WANG W T,HE B J. Evaluation of cold resistance in grapevines via photosynthetic characteristics,carbohydrate metabolism and gene expression levels[J]. Acta Physiologiae Plantarum,2016,38(10):251.
[40] SULMON C,GOUESBET G,BINET F,MARTIN-LAURENT F,EL AMRANI A,COUÉE I. Sucrose amendment enhances phytoaccumulation of the herbicide atrazine in Arabidopsis thaliana[J].Environmental Pollution,2007,145(2):507-515.
[41] 孙永梅,刘丽杰,冯明芳,王军虹,苍晶,李速,包雨卓,王秀田.植物在低温胁迫下的糖代谢研究进展[J].东北农业大学学报,2015,46(7):95-102.SUN Yongmei,LIU Lijie,FENG Mingfang,WANG Junhong,CANG Jing,LI Su,BAO Yuzhuo,WANG Xiutian. Research progress of sugar metabolism of plants under cold stress[J].Journal of Northeast Agricultural University,2015,46(7):95-102.
[42] YOON J,CHO L H,TUN W,JEON J S,AN G. Sucrose signaling in higher plants[J].Plant Science,2021,302:110703.
[43] 常丽丽,廖宗文,陈日远,刘厚诚,宋世威,孙光闻.外源蔗糖对NO3-胁迫下叶用莴苣碳氮代谢的影响[J].北方园艺,2016(1):10-15.CHANG Lili,LIAO Zongwen,CHEN Riyuan,LIU Houcheng,SONG Shiwei,SUN Guangwen.Effect of exogenous sucrose on nitrogen and carbon metabolism of lettuce under NO3- stress[J].Northern Horticulture,2016(1):10-15.
[44] HUNG K T,CHENG D G,HSU Y T,KAO C H.Abscisic acidinduced hydrogen peroxide is required for anthocyanin accumulation in leaves of rice seedlings[J].Journal of Plant Physiology,2008,165(12):1280-1287.
[45] BHATTACHARJEE S. Heat and chilling induced disruption of redox homeostasis and its regulation by hydrogen peroxide in germinating rice seeds(Oryza sativa L.,Cultivar Ratna)[J].Physiology and Molecular Biology of Plants,2013,19(2):199-207.
[46] ZHANG Q,ZHANG J Z,CHOW W S,SUN L L,CHEN J W,CHEN Y J,PENG C L. The influence of low temperature on photosynthesis and antioxidant enzymes in sensitive banana and tolerant plantain (Musa sp.) cultivars[J]. Photosynthetica,2011,49(2):201-208.
[47] MIR A R,SIDDIQUI H,ALAM P,HAYAT S. Foliar spray of Auxin/IAA modulates photosynthesis,elemental composition,ROS localization and antioxidant machinery to promote growth of Brassica juncea[J]. Physiology and Molecular Biology of Plants,2020,26(12):2503-2520.
[48] 赵莹,杨克军,赵长江,李佐同,王玉凤,付健,郭亮,李文胜.外源糖调控玉米光合系统和活性氧代谢缓解盐胁迫[J].中国农业科学,2014,47(20):3962-3972.ZHAO Ying,YANG Kejun,ZHAO Changjiang,LI Zuotong,WANG Yufeng,FU Jian,GUO Liang,LI Wensheng.Alleviation of the adverse effects of salt stress by regulating photosynthetic system and active oxygen metabolism in maize seedlings[J].Scientia Agricultura Sinica,2014,47(20):3962-3972.
[49] RAMEL F,SULMON C,BOGARD M,COUÉE I,GOUESBET G.Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets[J].BMC Plant Biology,2009,9:28.