杂交杏李(Prunus salicina×P.armeniaca)是通过李和杏种间杂交而选育的新型果树,果实外观(如果实大小)和品质(如含糖量)显著优于传统杏、李品种,被誉为“21世纪水果新骄子”[1]。目前,我国主栽的杂交杏李品种,如味帝、恐龙蛋等,均为从国外引进品种,栽培面积约2 万hm2,其中近一半分布在新疆地区[2]。新疆地处我国西北,属于气候偏冷的经济林栽培边缘地区,冬季极端低温持续时间长,冻害频繁[3]。近年来,新疆气温屡创新低,杂交杏李遭受大面积冻害,造成了严重的经济损失。低温已成为制约杂交杏李生长发育最主要的环境因素之一。因此,鉴定现有杂交杏李品种(系)的抗寒性,筛选抗寒种质,对培育具有自主知识产权的抗寒杏李优良品种具有重要意义。
抗寒亲本的选择是培育抗寒优良品种的关键。植物在低温胁迫下,细胞膜是受损的原初部位,相对电导率(REC)能够准确反映细胞膜的受损程度[4-5]。目前,通过测定REC并拟合Logistic方程计算半致死温度(LT50)以评价果树抗寒性的方法已广泛应用于葡萄[6]、鲜食枣[7]、百香果[8]以及扁桃[9]等经济林果的研究中。此外,脯氨酸(Pro)、可溶性糖(SS)等渗透调节物质含量,及超氧化物歧化酶(SOD)、过氧化物酶(POD)等抗氧化酶活性,也在一定程度上反映了植株的抗寒水平[10-12]。然而,植物的抗寒能力受多因素调控,单一指标评价存在局限性。隶属函数法通过综合多项指标进行评价,结果更为可靠[13-15]。王季姣等[16]同时结合LT50和隶属函数法对75份酿酒葡萄进行抗寒性评价,成功筛选出抗寒能力最强的种质。
近年来,低温响应研究大多集中在模式植物及主要的粮食作物和经济作物中,而杂交杏李作为新树种,其抗寒性研究报道较少[17]。笔者所在团队是我国杂交杏李的最初引进单位,经过十余年的栽培育种,已选育出丰富的杏李优系。笔者以6 个主栽杏李品种和4 个杏李优系为材料,通过测定REC 和LT50进行抗寒性鉴定,并探索不同抗寒性种质对低温胁迫的生理响应,结合隶属函数法综合评价其抗寒性,旨在为杂交杏李抗寒育种提供抗性材料,并为抗寒杏李的推广应用提供依据。
1 材料和方法
1.1 材料
笔者以6个主栽杂交杏李品种(风味玫瑰、风味皇后、味帝、恐龙蛋、味厚和味王)及4 个杏李优系(XL7、XL8、XL9 和XL10)为试验材料,所有材料均保存于中国林业科学研究院经济林研究所杏李种质资源圃(河南原阳)。于2023年12月中下旬,分别剪取10 份种质粗度一致、生长健康(无病虫害和机械损伤)的一年生休眠枝条各30 根,然后立即用湿润的报纸包裹,迅速带回实验室备用。
1.2 低温处理
将所有休眠枝条清洗干净并修剪为30 cm左右的枝段(去除顶端和基部),参照郭艳等[18]对板栗枝条的处理方法进行低温胁迫试验。将每个基因型的枝条分为5 份,每份6 根,用保鲜膜包裹后分别放置于-10、-15、-20、-25和-30 ℃低温下处理12 h,然后以2 ℃·h-1的速度升温至0 ℃,取出枝条于室温解冻1 h。低温处理后,部分枝条用于测定相对电导率,剩余枝条经液氮速冻后保存于-80 ℃冰箱,用于后续生理指标的测定。
1.3 相对电导率及低温半致死温度的测定
参照文献[19]进行相对电导率的测定,具体步骤如下:首先将低温处理过的枝条剪成1~2 mm 的薄片(避开芽眼),混合均匀称取2 g,放入装有20 mL蒸馏水的50 mL离心管中,置于摇床(200 r·min-1)浸提2 h,使用Mettler Toledo S470-K电导率仪(梅特勒托利多科技有限公司,中国上海)测定初始电导率R1,然后将离心管沸水浴30 min后,放入摇床200 r·min-1振摇30 min,冷却至室温后测定终电导率值R2。相对电导率计算公式为:REC/%=R1/R2×100。
利用Logistic回归方程y=k/(1+ae-bx)拟合相对电导率与温度的关系,计算半致死温度。其中y 为枝条相对电导率,x 为处理温度,k 为饱和值,a、b 为方程参数[20]。
1.4 生理生化指标的测定
将-80 ℃保存的枝条研磨成粉,用于测定以下生理指标:可溶性糖含量的测定采用蒽酮法[20];脯氨酸含量的测定采用茚三酮比色法[21];丙二醛含量的测定采用硫代巴比妥酸法[22];超氧化物歧化酶活性、过氧化物酶活性、超氧阴离子(O2-·)含量和过氧化氢含量均采用商业化试剂盒(苏州梦犀生物医药科技有限公司,中国)测定。
1.5 隶属函数分析
参照杨凤翔等[23]的方法,采用隶属函数法综合评价样品抗寒性。与抗寒性呈正相关的指标(Pro、SS含量和SOD、POD活性)的隶属度计算公式为:U(Xij)=(Xij-Xmin)/(Xmax-Xmin)。与抗寒性呈负相关的指标(H2O2、O2·-和MDA)的隶属度计算公式为:U(Xij)=1-(Xij-Xmin)/(Xmax-Xmin)。其中:Xij表示指标测定值;Xmax、Xmin为所有参试材料中指标的最大值和最小值。
1.6 数据分析
所有试验均设置3 个生物学重复,采用Microsoft Excel 2016 整理数据,采用IBM SPSS Statistics 22.0 进行方差分析(LSD 法,p<0.05),采用Origin-Pro 2021绘图。
2 结果与分析
2.1 低温胁迫对杂交杏李休眠枝条相对电导率的影响及LT50的确定
如图1所示,10个杂交杏李种质休眠枝条的相对电导率(REC)均随着温度降低逐渐上升。在-10 ℃至-20 ℃范围内,所有基因型的REC均未超过50%,表明在此温度区间内,低温造成的细胞膜损伤是可逆的。然而,当温度降至-25 ℃时,味帝、恐龙蛋和XL10 的REC 值已超过50%,表明其细胞膜系统遭受不可逆损伤。在-30 ℃处理下,味王(49.05%)、XL9(51.82%)和XL8(59.12%)的REC值相对较低,而其余7 个基因型的REC 值均超过60%,其中味厚的REC 值最高(67.56%)。这一结果表明,味王和XL9 在极端低温条件下表现出更强的耐受性,其抗寒能力高于其他基因型。
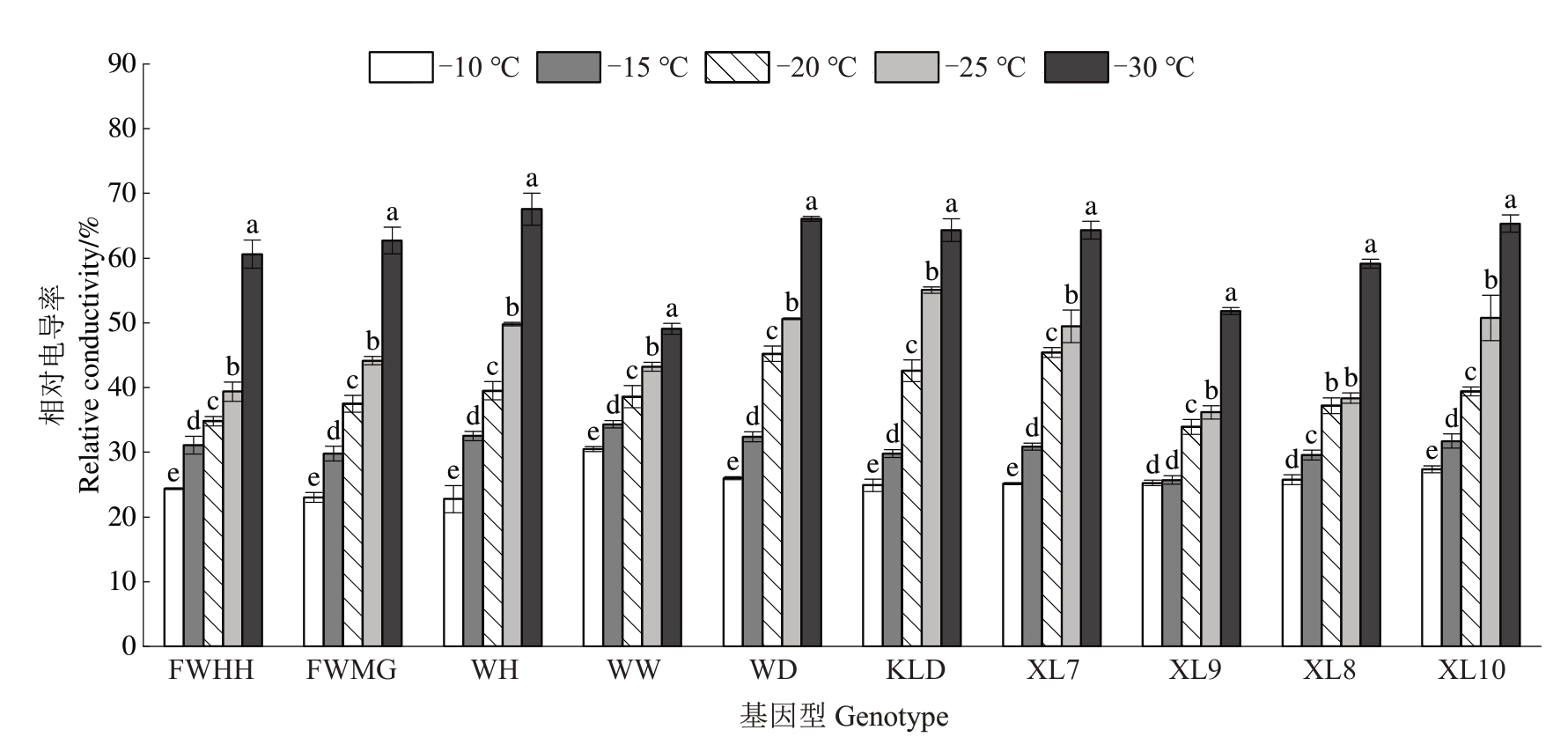
图1 低温处理下杂交杏李枝条的相对电导率变化
Fig.1 The changes in the relative conductivity of branches of Prunus salicina×P.armeniaca under low temperature treatments
FWHH、FWMG、WH、WW、WD 和KLD 分别代表风味皇后、风味玫瑰、味厚、味王、味帝和恐龙蛋。不同小写字母表示同一份基因型的不同处理在0.05 水平差异显著。下同。
FWHH,FWMG,WH,WW,WD and KLD represent Fengweihuanghou,Fengweimeigui,Weihou,Weiwang,Weidi and Konglongdan,respectively.Different small letters indicate significant difference among different treatments of the same genotype at 0.05 level.The same below.
Logistic 回归分析发现,10 个基因型的半致死温度(LT50)范围为-23.02~-31.67 ℃,拟合度均高于0.82(表1)。它们的抗寒性划分为3个等级:高度抗寒型(LT50<-30 ℃,包括味王和XL9)、中度抗寒型(-30 ℃≤LT50≤-25 ℃,包括风味皇后、风味玫瑰和XL8)及低温敏感型(LT50>-25 ℃,包括味厚、味帝、恐龙蛋、XL7和XL10)。
表1 10 个杂交杏李基因型的枝条半致死温度
Table 1 LT50 in shoots of ten Prunus salicina×P.armeniaca genotypes
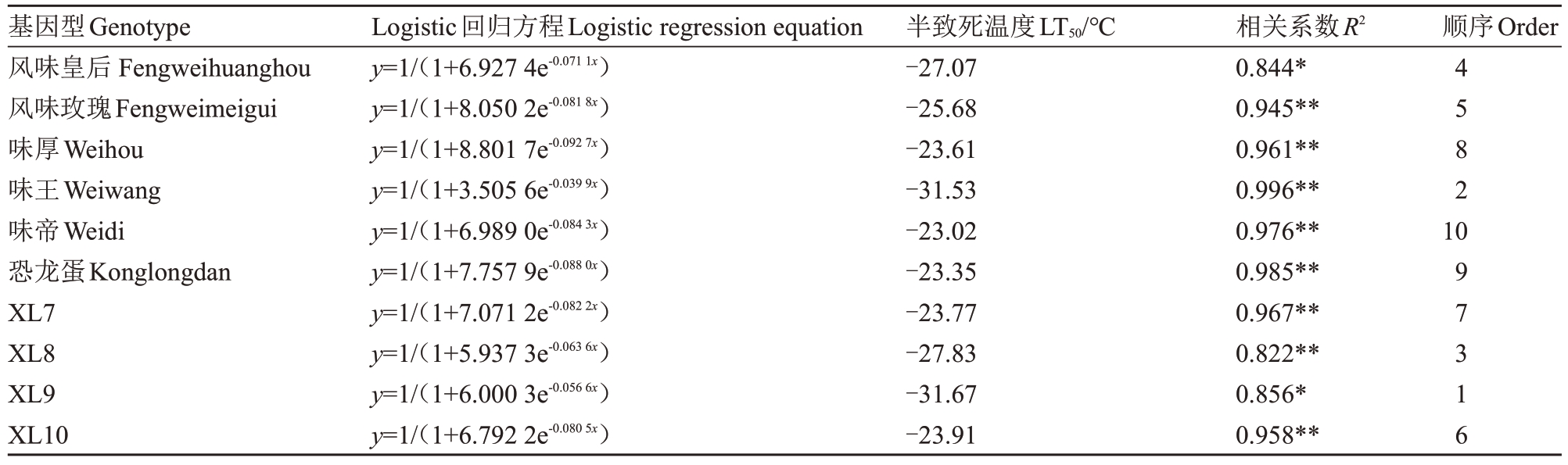
注:*和**分别表示在0.05水平和0.01水平上显著相关。
Note:*and**represent significant correlations at the 0.05 and 0.01 levels,respectively.
基因型Genotype风味皇后Fengweihuanghou风味玫瑰Fengweimeigui味厚Weihou味王Weiwang味帝Weidi恐龙蛋Konglongdan XL7 XL8 XL9 XL10 Logistic回归方程Logistic regression equation y=1/(1+6.927 4e-0.0711x)y=1/(1+8.050 2e-0.0818x)y=1/(1+8.801 7e-0.0927x)y=1/(1+3.505 6e-0.0399x)y=1/(1+6.989 0e-0.0843x)y=1/(1+7.757 9e-0.0880x)y=1/(1+7.071 2e-0.0822x)y=1/(1+5.937 3e-0.0636x)y=1/(1+6.000 3e-0.0566x)y=1/(1+6.792 2e-0.0805x)半致死温度LT50/℃-27.07-25.68-23.61-31.53-23.02-23.35-23.77-27.83-31.67-23.91相关系数R2 0.844*0.945**0.961**0.996**0.976**0.985**0.967**0.822**0.856*0.958**顺序Order 4582 10 97316
2.2 低温胁迫对杂交杏李休眠枝条活性氧含量的影响
选择2 个抗寒基因型(味王和XL9)及2 个敏感基因型(味帝和恐龙蛋)进一步探索低温生理响应机制。如图2-A 所示,在低温胁迫下,4 个基因型的超氧阴离子(O2-·)含量整体上呈先上升后下降的趋势。味王和XL9 的O2-·含量分别在-25 ℃和-20 ℃达到峰值。然而,-30 ℃处理与-10 ℃处理无显著差异。恐龙蛋的O2-·含量在-30 ℃和-10 ℃处理下无显著差异,但是在-15 ℃处理下比-10 ℃显著提高了142.79%。味帝的O2-·含量在-25 ℃达到峰值,-30 ℃处理较-10 ℃处理显著提高了33.42%。
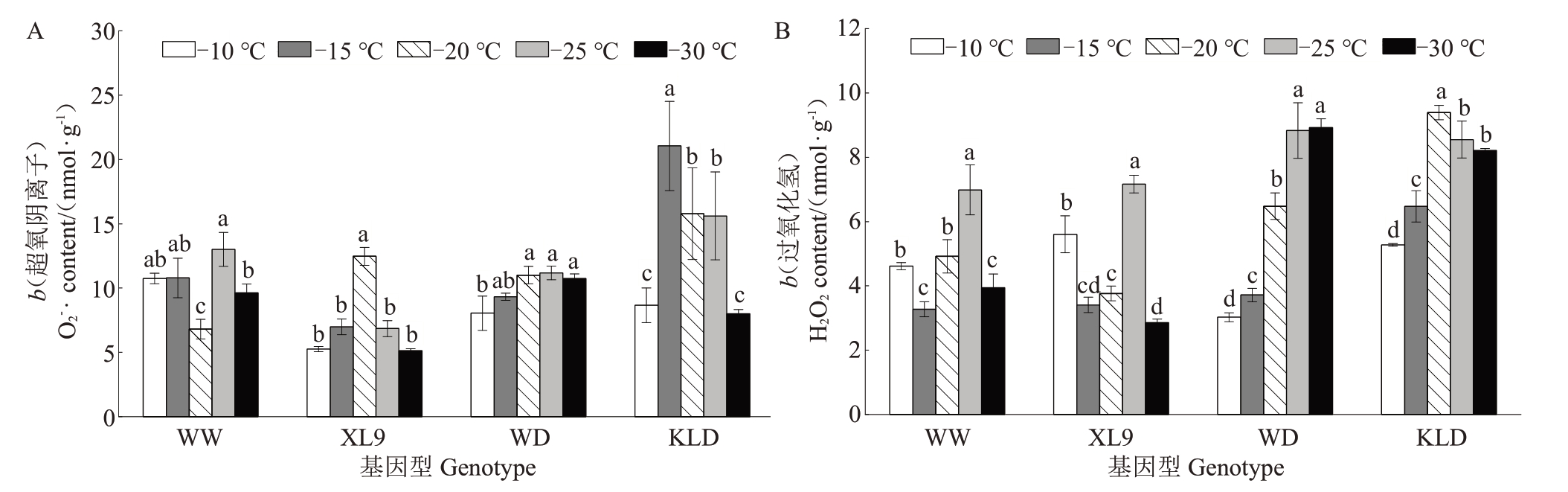
图2 低温处理下不同抗性杂交杏李枝条超氧阴离子(A)和过氧化氢(B)含量的变化
Fig.2 The changes in the O2·-(A)and H2O2(B)of branches of Prunus salicina×P.armeniaca with different resistance under low temperature treatments
如图2-B 所示,味王和XL9 的H2O2含量呈先下降后上升再下降的变化趋势,整体处于较低水平;恐龙蛋的H2O2含量在-20 ℃处理下达到峰值,较-10 ℃处理显著提高了78.18%;味帝的H2O2含量随温度的降低持续上升,-30 ℃处理较-10 ℃处理显著提高了195.36%。综上,味王和XL9 在低温胁迫下能够使活性氧含量保持在相对稳定的水平,其抗寒能力优于味帝和恐龙蛋。
2.3 低温胁迫对杂交杏李休眠枝条丙二醛含量的影响
如图3 所示,味王和XL9 的MDA 含量随温度降低呈先上升后下降的趋势,分别在-25 ℃和-20 ℃处理下达到峰值,较-10 ℃处理分别显著提高了18.35%和28.60%。在-30 ℃处理下,它们的MDA含量低于-10 ℃。味帝和恐龙蛋的MDA含量随温度降低持续上升,-30 ℃低温处理较-10 ℃处理分别显著提高了117.52%和36.78%。这表明低温胁迫下,味帝和恐龙蛋的细胞膜损伤更为严重。
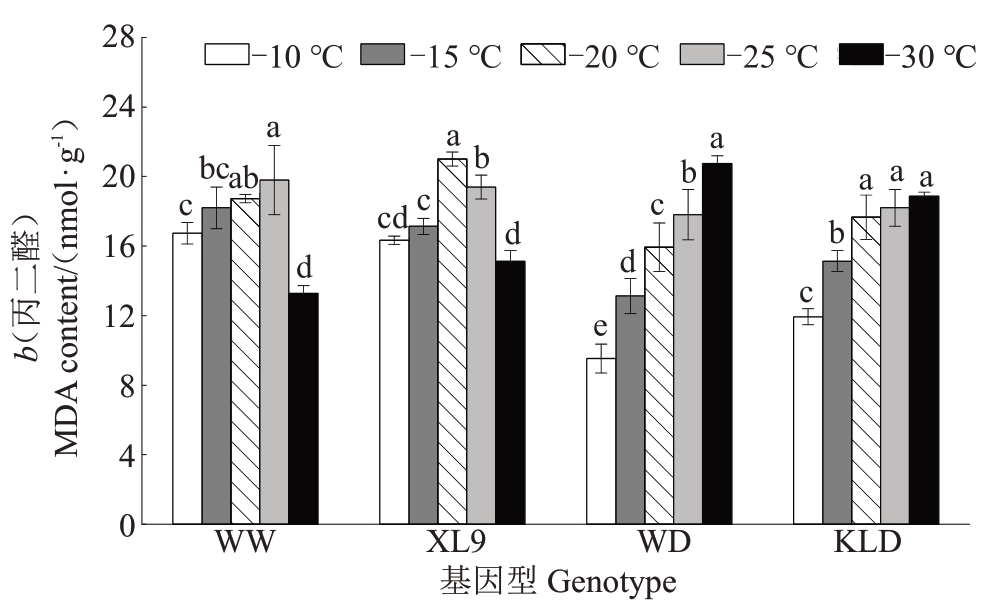
图3 低温处理下不同抗性杂交杏李枝条丙二醛含量的变化
Fig.3 The changes in MDA content of branches of Prunus salicina×P.armeniaca with different resistance under low temperature treatments
2.4 低温胁迫对杂交杏李休眠枝条抗氧化酶活性的影响
如图4-A 所示,低温处理下,味王、XL9 和恐龙蛋的SOD 活性整体上呈升-降-升趋势,而味帝的SOD 活性呈降-升-降趋势。在-30 ℃处理下,味王和XL9 的SOD 活性较-10 ℃处理分别显著提高了80.54%和38.46%,而味帝和恐龙蛋的SOD 活性低于-10 ℃处理。
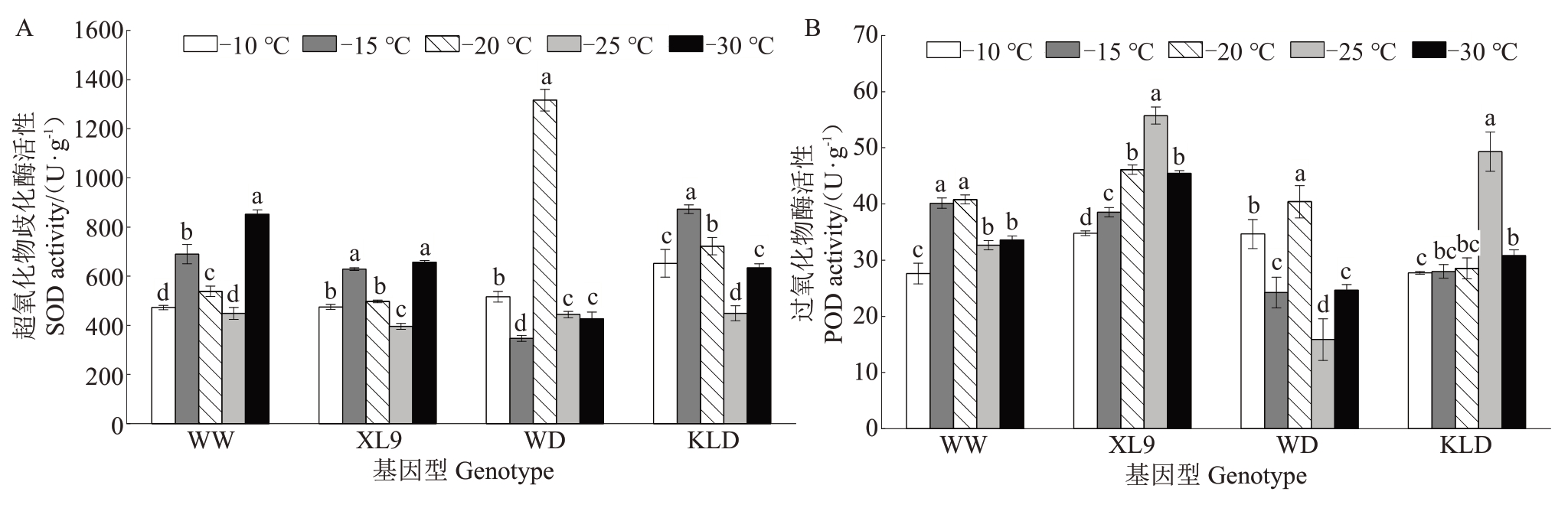
图4 低温处理下不同抗性杂交杏李枝条的SOD(A)和POD(B)活性变化
Fig.4 The changes in the SOD(A)and POD(B)activities of branches of Prunus salicina×P.armeniaca with different resistance under low temperature treatments
如图4-B 所示,低温胁迫下,味王、XL9 和恐龙蛋的POD 活性整体上呈先上升后下降的趋势,其中,XL9 和恐龙蛋在-25 ℃处理下达到最高,分别较-10 ℃处理显著提高了60.14%和77.89%;味王在-20 ℃处理下达到峰值,较-10 ℃处理显著提高了47.83%。味帝的POD 活性波动较大,在-30 ℃处理下,味王和XL9的POD活性高于味帝和恐龙蛋。这表明味王和XL9 在低温胁迫下拥有更强的抗氧化能力,其抗寒性高于味帝和恐龙蛋。
2.5 低温胁迫对杂交杏李休眠枝条渗透调节物质含量的影响
如图5-A 所示,味王和XL9 的可溶性糖含量随温度降低呈先上升后下降的趋势,分别在-20 ℃和-25 ℃达到峰值,较-10 ℃处理分别显著提高了17.98%和22.00%,而味帝和恐龙蛋的可溶性糖含量随温度降低整体上呈下降趋势,与-10 ℃处理相比,-30 ℃处理分别显著降低了36.27%和44.24%。
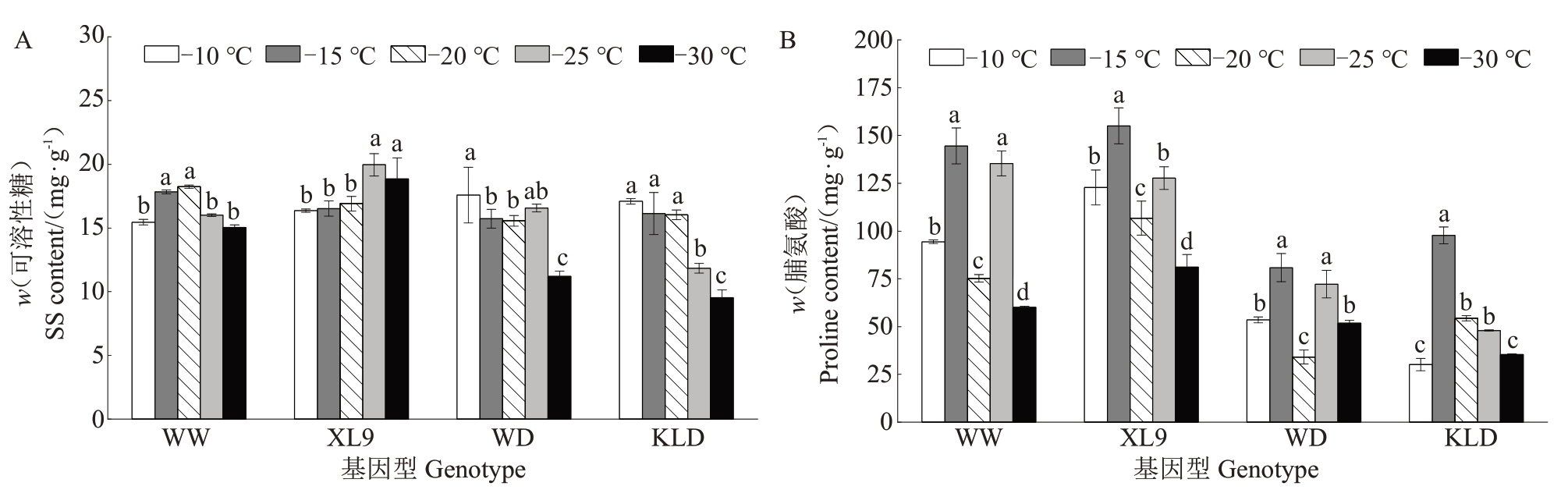
图5 低温处理下不同抗性杂交杏李枝条可溶性糖(A)和脯氨酸(B)含量的变化
Fig.5 The changes in the soluble sugar(A)and proline(B)contents of branches of Prunus salicina×P.armeniaca with different resistance under low temperature treatments
如图5-B 所示,低温处理下,味王、XL9 和味帝的脯氨酸含量呈升-降-升-降的趋势,恐龙蛋的脯氨酸含量呈先上升后下降的趋势。整体来看,低温处理下,味王和XL9 的脯氨酸含量要高于味帝和恐龙蛋。这表明,味王和XL9 在低温胁迫下能够维持自身渗透调节能力,其抗寒性优于味帝和恐龙蛋。
2.6 4个杂交杏李基因型抗寒性综合评价
通过隶属函数法计算4 个杂交杏李基因型的平均隶属值(表2),由高到低依次为XL9、味王、恐龙蛋和味帝。味王和XL9 的平均隶属值高于味帝和恐龙蛋,表明其抗寒性更强,与LT50鉴定结果一致。
表2 4 个杏李品种枝条抗寒隶属函数值及综合评价
Table 2 The membership function values and comprehensive evaluation of the cold resistance of four Prunus salicina×P.armeniaca branches
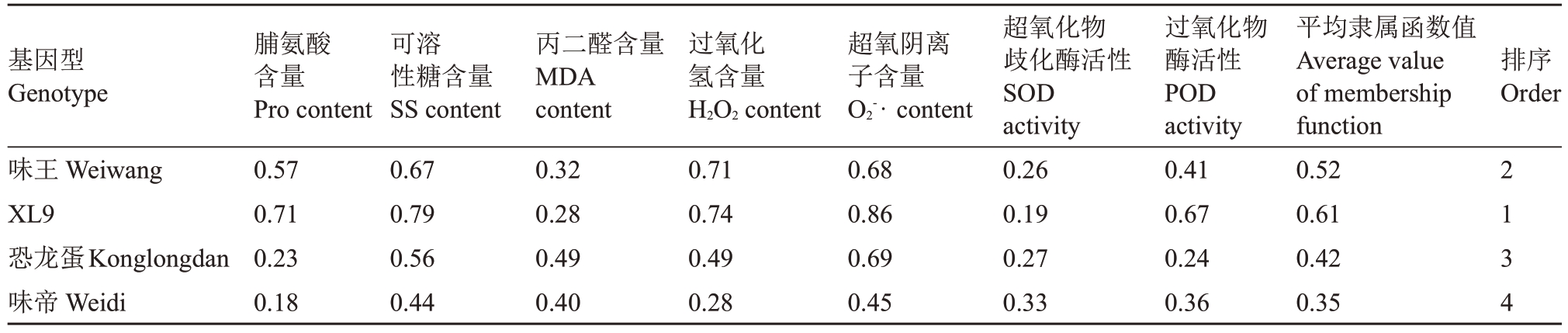
基因型Genotype排序Order味王Weiwang XL9恐龙蛋Konglongdan味帝Weidi脯氨酸含量Pro content 0.57 0.71 0.23 0.18可溶性糖含量SS content 0.67 0.79 0.56 0.44丙二醛含量MDA content 0.32 0.28 0.49 0.40过氧化氢含量H2O2 content 0.71 0.74 0.49 0.28超氧阴离子含量O2-· content 0.68 0.86 0.69 0.45超氧化物歧化酶活性SOD activity 0.26 0.19 0.27 0.33过氧化物酶活性POD activity 0.41 0.67 0.24 0.36平均隶属函数值Average value of membership function 0.52 0.61 0.42 0.35 2134
3 讨 论
3.1 杂交杏李基因型的抗寒性鉴定
细胞膜是植物抵御低温的首要屏障,其稳定性直接影响抗寒能力[24]。相对电导率(REC)是评估细胞膜损伤的重要指标,REC 越小,表明植物细胞膜受到的损伤越小[25]。笔者通过测定REC结合Logistic 方程计算半致死温度(LT50),发现杂交杏李的LT50 在-23.02~-31.67 ℃之间,抗寒性强弱顺序为XL9>味王>XL8>风味皇后>风味玫瑰>XL10>XL7>味厚>恐龙蛋>味帝。其中,味王和XL9的表现突出(LT50<-30 ℃),抗寒性显著强于新疆主栽品种恐龙蛋(LT50=-23.35 ℃)和味帝(LT50=-23.02 ℃)。这一结果与新疆产区恐龙蛋和味帝频繁发生冻害的现象相吻合,而味王和XL9可作为推广应用的候选种质及抗寒育种的优选亲本。
3.2 不同抗性杂交杏李对低温胁迫的生理响应机制
在长期进化过程中,植物已形成了一套系统且复杂的响应机制以应对低温胁迫。活性氧(ROS)是植物自身重要的信号分子,低温胁迫诱导的ROS积累是细胞损伤的主要因素[26-28]。超氧化物歧化酶(SOD)和过氧化物酶(POD)是植物抗氧化系统的主要屏障。SOD 能催化O2-·生成H2O2,H2O2 可由POD 分解[29-30]。本研究揭示抗寒杏李基因型(味王和XL9)通过动态调控SOD 和POD 活性来实现ROS稳态;在-30 ℃处理下,其SOD活性较-10 ℃处理显著提高80.54%(味王)和38.46%(XL9),POD活性峰值较-10 ℃处理显著提高47.83%(味王)和60.14%(XL9)。这种“先激活后维持”的酶活性调节模式,有效将H2O2和O2-·含量控制在较低水平,与低温敏感型品种的持续氧化损伤形成鲜明对比。值得注意的是,味帝在-30 ℃时H2O2积累量是-10 ℃处理的2.95 倍,且伴随MDA 含量激增117.52%,表明其抗氧化系统在极端低温下完全崩溃。这表明,抗寒资源在一定的低温处理条件下,会通过提高自身的抗氧化酶活性,从而减少低温对植物的氧化伤害,这与Cansev等[31]在油橄榄中的研究结果一致。
可溶性糖含量提高可以增加细胞渗透浓度,低温胁迫下,其含量的增加能够提高细胞的保水性,从而增强植物的抗寒能力[32];脯氨酸能够促进蛋白质水合作用,产生疏水骨架蛋白,进而起到保护细胞的作用[33];这两者是植物应对渗透胁迫的重要策略[34-35]。笔者发现抗寒基因型呈现“阈值响应”特征:味王和XL9分别在-20 ℃和-25 ℃时的SS含量分别较-10 ℃处理显著提高17.98%和22.00%,而敏感基因型恐龙蛋的SS含量随温度降低持续下降。脯氨酸代谢路径的差异尤为显著-抗寒基因型通过“波动积累”模式维持渗透平衡,而味帝的Pro含量在-30 ℃时较峰值下降42.70%,揭示其渗透调节能力随胁迫加剧而丧失。这种代谢可塑性差异可能是决定抗寒等级的关键生理基础。
隶属函数法是综合评价植物抗寒性的有效方法,平均隶属值越大,抗寒性越强[36]。研究者常常同时利用LT50和隶属函数进行抗性分析,评价结果更为准确可靠。孙世航[37]利用LT50和隶属函数法对9个猕猴桃基因型的抗寒性进行评价,结果表明,这两种评价的结果基本一致。在本研究中,隶属函数法整合7 项生理指标的综合评价结果与LT50高度一致,证实了两种方法的互补性。值得注意的是,XL9在隶属度评分中(0.61)略高于味王(0.52),提示除细胞膜稳定性外,持续性的渗透调节能力可能贡献额外抗性。这为后续抗寒育种提供了多维度筛选指标:在LT50初筛基础上,可结合SOD活性增幅、SS积累速率等参数建立分级评价标准。
4 结 论
10 个杏李种质的REC 均随温度下降而上升,LT50在-23.02~-31.67 ℃之间,其抗寒性划分为3 个等级:高度抗寒型(LT50<-30 ℃,味王和XL9)、中度抗寒型(-30 ℃≤LT50≤-25 ℃,风味皇后、风味玫瑰和XL8)和低温敏感型(LT50>-25 ℃,味厚、味帝、恐龙蛋、XL7 和XL10)。通过隶属函数综合评价进一步证明了LT50评价结果的准确性,味王和XL9 的抗寒性强于其他基因型,可作为杂交杏李抗寒育种的优良亲本,且具备在新疆等寒冷地区推广的潜力。
[1] 李泰山,韩卫娟,杜改改,刁松锋,冯延芝,杨绍彬,岳华峰,李芳东,傅建敏. 杏李不同品种果实香气成分分析[J]. 林业科学,2017,53(9):123-132.LI Taishan,HAN Weijuan,DU Gaigai,DIAO Songfeng,FENG Yanzhi,YANG Shaobin,YUE Huafeng,LI Fangdong,FU Jianmin.Volatile characteristics of different Prunus domestica × armeniaca cultivars evaluated by HS-SPME with GC-MS[J]. Scientia Silvae Sinicae,2017,53(9):123-132.
[2] 杨红丽.不同杏李品种抗寒生理研究[D].乌鲁木齐:新疆农业大学,2014.YANG Hongli. Study on the physiological characteristics of cold-resistance in different Prunus domestica[D].Urumqi:Xinjiang Agricultural University,2014.
[3] LI H,WANG X F,CHEN S J,HOU P. Dynamic analysis and evaluation of Xinjiang forest resources:Based on RS and GIS[J].Journal of Geographical Sciences,2005,15(3):346-352.
[4] 王文举,张亚红,牛锦凤,王振平.电导法测定鲜食葡萄的抗寒性[J].果树学报,2007,24(1):34-37.WANG Wenju,ZHANG Yahong,NIU Jinfeng,WANG Zhenping.Study on cold tolerance of table grape cultivars by measuring the conductivity[J]. Journal of Fruit Science,2007,24(1):34-37.
[5] 李文明,辛建攀,魏驰宇,田如男.植物抗寒性研究进展[J].江苏农业科学,2017,45(12):6-11.LI Wenming,XIN Jianpan,WEI Chiyu,TIAN Runan. Research progress of plant cold resistance[J].Jiangsu Agricultural Sciences,2017,45(12):6-11.
[6] 李凯. 7 个鲜食葡萄品种抗寒性评价[D]. 石河子:石河子大学,2015.LI Kai. Evaluation on cold resistance of seven table grape cultivars[D].Shihezi:Shihezi University,2015.
[7] 高拖弟. 6 个不同鲜食枣品种抗寒性研究[D]. 陕西:榆林学院,2023.GAO Tuodi. The study on cold resistance of six different freshenble jujube varieties[D].Shaanxi:Yulin University,2023.
[8] 吴凤婵.百香果砧木抗寒抗旱性评价及筛选[D].贵阳:贵州大学,2022.WU Fengchan.Evaluation and screening of cold and drought resistance of passion fruit rootstock[D].Guiyang:Guizhou University,2022.
[9] 欧欢. 不同扁桃品种抗寒性研究[D]. 阿拉尔:塔里木大学,2019.OU Huan. Study on cold resistance of different almond cultivars[D].Aler:Tarim University,2019.
[10] 张博,刘立强,秦伟,乌仁其米格.新疆野苹果抗寒生理生化机制研究[J].经济林研究,2021,39(4):60-68.ZHANG Bo,LIU Liqiang,QIN Wei,Wurenqimige. Study on physiological and biochemical mechanism of cold resistance of Malus sieversii[J]. Non-wood Forest Research,2021,39(4):60-68.
[11] 高京草,王慧霞,李西选.可溶性蛋白、丙二醛含量与枣树枝条抗寒性的关系研究[J].北方园艺,2010(23):18-20.GAO Jingcao,WANG Huixia,LI Xixuan. Relationship between soluble protein,MDA,and jujube tree cold hardiness[J]. Northern Horticulture,2010(23):18-20.
[12] 王佳.低温胁迫对不同杏品种抗性指标的影响[J].山西林业科技,2014,43(2):20-22.WANG Jia.Influence of low temperature stress on resistance index of different apricot varieties[J].Shanxi Forestry Science and Technology,2014,43(2):20-22.
[13] 张淑文,梁森苗,朱婷婷,任海英,郑锡良,戚行江.不同杨梅品种的耐低温能力比较[J]. 浙江农业学报,2020,32(10):1772-1779.ZHANG Shuwen,LIANG Senmiao,ZHU Tingting,REN Haiying,ZHENG Xiliang,QI Xingjiang.Cold tolerance of different Chinese bayberry varieties[J]. Acta Agriculturae Zhejiangensis,2020,32(10):1772-1779.
[14] 杨复康,杨燕君,宋永宏,李静江,吕振兵.不同杏品种抗寒性及生理指标[J].北方园艺,2021(3):27-32.YANG Fukang,YANG Yanjun,SONG Yonghong,LI Jingjiang,LÜ Zhenbing. Cold resistance and physiological indexes of different apricot cultivars[J].Northern Horticulture,2021(3):27-32.
[15] 何伟,艾军,范书田,杨义明,王振兴,赵滢,乔永在,张亚凤,李晓燕. 葡萄品种及砧木抗寒性评价方法研究[J]. 果树学报,2015,32(6):1135-1142.HE Wei,AI Jun,FAN Shutian,YANG Yiming,WANG Zhenxing,ZHAO Ying,QIAO Yongzai,ZHANG Yafeng,LI Xiaoyan.Study on evaluation method for cold resistance of grape cultivars and rootstock[J]. Journal of Fruit Science,2015,32(6):1135-1142.
[16] 王季姣,王世伟,潘越,李亚兰,李树德.新疆天山北麓产区酿酒葡萄种质抗寒性鉴定及综合评价[J]. 果树学报,2024,41(10):1933-1946.WANG Jijiao,WANG Shiwei,PAN Yue,LI Yalan,LI Shude.Identification and comprehensive evaluation of cold resistance of wine grape germplasms in Northern Tianshan Region,Xinjiang[J].Journal of Fruit Science,2024,41(10):1933-1946.
[17] 杨红丽,李建贵,徐业勇,王明.新疆阿克苏地区6 个杏李品种抗寒性研究[J].新疆农业科学,2014,51(10):1782-1786.YANG Hongli,LI Jiangui,XU Yeyong,WANG Ming. Study on the cold resistance of six varieties of Prunus domestica planted in Aksu,Xinjiang[D]. Xinjiang Agricultural Sciences,2014,51(10):1782-1786.
[18] 郭燕,张树航,李颖,张馨方,韩斌,王广鹏,杨阳.我国几个板栗品种抗寒性综合评价[J].中国农业大学学报,2019,24(4):52-63.GUO Yan,ZHANG Shuhang,LI Ying,ZHANG Xinfang,HAN Bin,WANG Guangpeng,YANG Yang. Comprehensive evaluation on the cold resistance of several main Chinese chestnut cultivars[J]. Journal of China Agricultural University,2019,24(4):52-63.
[19] 焦其庆,冯立娟,尹燕雷,崔洪涛.石榴冻害及抗寒评价研究进展[J].植物生理学报,2019,55(4):425-432.JIAO Qiqing,FENG Lijuan,YIN Yanlei,CUI Hongtao. Research progress on evaluation of freezing injury and cold resistance of pomegranate[J].Plant Physiology Journal,2019,55(4):425-432.
[20] 刘敏,靳娟,阿布都卡尤木·阿依麦提,樊丁宇,郝庆,杨磊,赵晓梅,耿文娟.新疆3 个鲜食枣品种的抗寒性评价[J].新疆农业科学,2023,60(4):916-924.LIU Min,JIN Juan,Abudoukayoumu·Ayimaiti,FAN Dingyu,HAO Qing,YANG Lei,ZHAO Xiaomei,GENG Wenjuan.Evaluation of cold resistance of three fresh edible jujube cultivars in Xinjiang[J]. Xinjiang Agricultural Sciences,2023,60(4):916-924.
[21] 王贺,刘国成,吕德国,赵德英,秦嗣军.‘寒富’苹果与亲本系品种抗寒生理指标的比较研究[J]. 北方园艺,2007(10):32-34.WANG He,LIU Guocheng,LÜ Deguo,ZHAO Deying,QIN Sijun.Studies on the physiological index related to cold resistance in‘Hanfu’and its parents plant[J]. Northern Horticulture,2007(10):32-34.
[22] 李俊才,刘成,王家珍,蔡忠民,沙守峰.洋梨枝条的低温半致死温度[J].果树学报,2007,24(4):529-532.LI Juncai,LIU Cheng,WANG Jiazhen,CAI Zhongmin,SHA Shoufeng. Study on the semi-lethal temperatures for European pear cultivars[J].Journal of Fruit Science,2007,24(4):529-532.
[23] 杨凤翔,金芳,颜霞.不同草莓品种抗寒性综合评价[J].果树学报,2010,27(3):368-372.YANG Fengxiang,JIN Fang,YAN Xia. Comprehensive evaluation of different strawberry varieties’tolerance to coldness[J].Journal of Fruit Science,2010,27(3):368-372.
[24] 杨梅.15 个果桑品种抗寒性研究[D].杨凌:西北农林科技大学,2012.YANG Mei.Study on cold-hardiness mechanism of mulberry[D].Yangling:Northwest A&F University,2012.
[25] 井俊丽,刘铭潇,高美娜,徐继忠,张学英,周莎莎.不同苹果中间砧枝条在越冬期间的生理特性变化及抗寒性比较[J].河北农业大学学报,2022,45(4):25-31.JING Junli,LIU Mingxiao,GAO Meina,XU Jizhong,ZHANG Xueying,ZHOU Shasha.Comparison of physiological characteristics and cold resistance of branches of different apple interstocks during overwintering[J]. Journal of Hebei Agricultural University,2022,45(4):25-31.
[26] YANG W,LIU X D,CHI X J,WU C G,LI Y Z,SONG L L,LIU X M,WANG Y F,WANG F W,ZHANG C,LIU Y,ZONG J M,LI H Y. Dwarf apple MbDREB1 enhances plant tolerance to low temperature,drought,and salt stress via both ABA-dependent and ABA-independent pathways[J]. Planta,2011,233(2):219-229.
[27] DING Y L,YANG S H.Surviving and thriving:How plants perceive and respond to temperature stress[J]. Developmental Cell,2022,57(8):947-958.
[28] 张旭,朱珍珍,孙鲁龙,李凤龙,韦德闯,朱佳顺,樊良栋,赵政阳.陇东地区不同矮化中间砧对‘长富2 号’苹果抗寒性的影响[J].果树学报,2020,37(7):985-996.ZHANG Xu,ZHU Zhenzhen,SUN Lulong,LI Fenglong,WEI Dechuang,ZHU Jiashun,FAN Liangdong,ZHAO Zhengyang.Effects of different dwarfing interstocks on cold resistance of‘Changfu 2’apple in Longdong Area[J]. Journal of Fruit Science,2020,37(7):985-996.
[29] CHO U H,PARK J O.Mercury-induced oxidative stress in tomato seedlings[J].Plant Science,2000,156(1):1-9.
[30] SHAH K,NAHAKPAM S. Heat exposure alters the expression of SOD,POD,APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars[J].Plant Physiology and Biochemistry,2012,57:106-113.
[31] CANSEV A,GULEN H,ERIS A. Cold-hardiness of olive (Olea europaea L.) cultivars in cold-acclimated and non-acclimated stages:Seasonal alteration of antioxidative enzymes and dehydrin-like proteins[J].The Journal of Agricultural Science,2009,147(1):51-61.
[32] 王红平,董铁,刘兴禄,尹晓宁,孙文泰,牛军强,马明.5 个苹果砧木品种枝条的低温半致死温度及耐寒性评价[J].果树学报,2020,37(4):495-501.WANG Hongping,DONG Tie,LIU Xinglu,YIN Xiaoning,SUN Wentai,NIU Junqiang,MA Ming.A study on the cold resistance and the semi-lethal temperatures for branches of five apple rootstock cultivars[J]. Journal of Fruit Science,2020,37(4):495-501.
[33] 陈仁伟,张晓煜,杨豫,李芳红,冯蕊,李红英,王静,苏雨弦,丁永平.‘赤霞珠’酿酒葡萄根颈和不同根系部位抗寒性比较[J].生态学杂志,2021,40(9):2754-2762.CHEN Renwei,ZHANG Xiaoyu,YANG Yu,LI Fanghong,FENG Rui,LI Hongying,WANG Jing,SU Yuxian,DING Yongping. Comparison of cold resistance in root collar and different parts of root of Cabernet Sauvignon[J].Chinese Journal of Ecology,2021,40(9):2754-2762.
[34] 韩立群,马凯,丁军伟,闫鹏,梅闯,王继勋.低温处理下新疆野生核桃的生理响应及抗寒性评价[J]. 西北林学院学报,2019,34(5):98-101.HAN Liqun,MA Kai,DING Junwei,YAN Peng,MEI Chuang,WANG Jixun. Physiological response and evaluation of cold resistance of Xinjiang wild walnut under low temperature stress[J].Journal of Northwest Forestry University,2019,34(5):98-101.
[35] CAMPOS P S,QUARTIN V N,RAMALHO J C,NUNES M A.Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp.plants[J].Journal of Plant Physiology,2003,160(3):283-292.
[36] 范宗民,孙军利,赵宝龙,刘怀锋,于坤,章智钧,刘晶晶.不同砧木‘赤霞珠’葡萄枝条抗寒性比较[J]. 果树学报,2020,37(2):215-225.FAN Zongmin,SUN Junli,ZHAO Baolong,LIU Huaifeng,YU Kun,ZHANG Zhijun,LIU Jingjing. Evaluation of cold resistance of one-year shoots from‘Cabernet Sauvignon’grape vine grafted on different rootstocks[J]. Journal of Fruit Science,2020,37(2):215-225.
[37] 孙世航.猕猴桃抗寒性评价体系的建立与应用[D].北京:中国农业科学院,2018.SUN Shihang.Establishment and application of evaluation method of freezing tolerance in Actinidia[D].Beijing:Chinese Academy of Agricultural Sciences,2018.