绿原酸(chlorogenic acid,CGA)作为一种重要的次生代谢物质广泛存在于植物中,因其较强的生物活性和药理作用,在植物的生长发育、抵御生物和非生物胁迫等方面发挥着重大的作用。绿原酸是咖啡酸和奎宁酸形成的酯,是植物通过苯丙烷途径合成的羟基肉桂酸[1],化学名3-O-咖啡酰奎酸,其分子式为C16H18O9,系统命名为1,3,4,5-四羟基环己烷羧酸3-(3,4-二羟基肉桂酸酯)。绿原酸也是一种对植物病原真菌有活性的杀菌剂[2]。研究表明,外源施加绿原酸对果蔬的病害防治和果实品质保持有积极作用。樱桃番茄果实采后外源施加绿原酸可以明显抑制病害镰刀菌腐烂病菌(Fusarium fujikuroi)分生孢子萌发、芽管伸长、细胞活力和菌丝生长,显著抑制腐烂病的发生[3]。外源绿原酸可以有效保护桃果实在储存期间免受扩展青霉(Penicillium expansum)的感染[4]。绿原酸对桃果实灰霉病也具有防治效果,可以抑制真菌孢子萌发、芽管伸长、细胞活力和菌丝穿透,有效降低桃果实上灰霉病菌(Botrytis cinerea)在25 ℃下贮藏的病斑直径和孢子产量,降低桃的自然腐烂率,延缓果实的成熟和衰老,保持桃果实的贮藏品质[5]。绿原酸还可以通过影响采后油桃防御相关蛋白的变化并减少ROS的产生,来减少油桃成熟过程中的氧化损伤,提高其抗氧化能力,同时也可通过降低乙烯产量,减少丙二醛(MDA)的积累,延迟果实软化,维持桃果实的采后品质[6-7]。在猕猴桃中,绿原酸可通过诱导线粒体氧化应激有效抑制间座壳菌(Diaporthe sp.)的侵染,显著减少果实腐烂[8]。同样,在烟草(Nicotiana tobacum)中施加绿原酸能显著减轻烟草疫霉(Phytophthora nicotianae)的生长,减少烟草黑胫病造成的损失,且抑制作用随着绿原酸施加浓度的递增而增强[9]。此外,绿原酸的积累也有助于提高植物的抗寒性、抗旱性、耐盐性以及抵御强光照射或紫外辐射的能力[10-11]。由此可见,绿原酸在植物的生长发育、抗病、抗虫以及抵御外界胁迫环境中扮演着重要的角色。
桃(Prunus persica L.)为蔷薇科果树,桃花提取物具有强大的抗氧化和酪氨酸酶抑制活性[12-14]。有研究利用HPLC-PDA 方法在不同开花阶段的桃花中发现6 种酚类化合物,绿原酸作为主要成分占其总量的62.08%~71.09%,桃花抗氧化能力与总酚含量和总黄酮含量以及绿原酸、肉桂酸和山奈酚-3-O-半乳糖苷含量之间存在显著相关性,而酪氨酸酶抑制活性与总酚、总黄酮、绿原酸、槲皮素-3-O-鼠李糖苷、山奈酚-3-O-半乳糖苷和肉桂酸的相关性较低。桃花的抗氧化活性比酪氨酸酶抑制活性更依赖于酚类化合物[15]。进一步研究桃花中筛选出的25 种化合物,发现其中包括8种酚酸和17种黄酮类化合物,并且不同栽培条件下桃花定量成分中绿原酸的浓度均为最高,表明桃花绿原酸含量与外界环境之间没有相关性[16]。有研究发现,乙醇回流可以浸提绿原酸,其方法操作简单,成本低,但其选择性差,杂质成分多,后续纯化困难[17]。蒸汽爆破辅助超声法提取金银花绿原酸可以缩短提取时间,提高提取效率,但其设备成本高,可能有效成分会被破坏[18]。笔者在本研究中采用甲醇辅助超声法提取桃花中的绿原酸,区别于常见单一的乙醇回流浸提,也不同于蒸汽爆破辅助超声这种侧重物理作用的提取方式,该技术将甲醇作为辅助试剂与超声法相结合,甲醇具有独特的溶解性能,与超声的空化、机械等作用协同,开辟了一种新的提取路径。
桃褐腐病主要引起果实腐烂,也可危害花、叶及枝条等,已给桃产业带来严重经济损失[19-20]。有研究表明,植物天然提取物对桃褐腐病菌丝体生长有抑制效果[21-23]。基于以上需求,本试验通过建立和优化一套利用超声辅助甲醇浸提桃花中绿原酸的方法,获得桃花绿原酸浸提液,并利用该提取液体外处理桃褐腐病的主要致病菌-美澳型核果链核盘菌(Monilinia fructicola),测定其抑菌效果,以期为桃花价值的开发利用和褐腐病菌的防治提供数据支撑。
1 材料和方法
1.1 试验材料
1.1.1 供试材料 桃花采摘于河北省秦皇岛市昌黎县石桥营基地(119.23° E,39.73° N)。选择不同花色的桃品系,R-2(红花)、P-3(粉花)和W-7(白花)如图1所示。取新鲜桃花花瓣,置于烘箱42 ℃烘干至恒质量,将桃花花瓣粉碎,得到桃花粉末,密封保存备用。

图1 不同品系桃花样品
Fig.1 Samples of peach blossoms of different strains
1.1.2 培养基及供试药剂 马铃薯葡萄糖琼脂(PDA)培养基:马铃薯200 g,D-葡萄糖20 g,琼脂粉20 g,蒸馏水1000 mL。绿原酸标准品(纯度≥98%,北京谱析标准技术有限公司);甲醇(色谱纯,北京蓝弋生物有限公司)以及甲酸(色谱纯,北京蓝弋生物有限公司)。
1.1.3 供试的病原菌 桃褐腐病菌(M. fructicola)由江西农业大学保存并提供。
1.2 试验方法
1.2.1 桃花浸提液的制备方法 确定料液比。以75%甲醇为溶剂,按照桃花粉末与溶剂质量比为1∶10、1∶20和1∶30,将混合物于4 ℃浸提过夜,然后用超声波处理2 次,每次60 min,超声频率20 kHz,超声功率200 W,超声温度40 ℃,以10 000 r·min-1离心10 min,取上清液;将第二次超声处理和第一次超声处理所得上清液合并,经0.22 μm滤膜抽滤后,采用HPLC测定样品中绿原酸的含量,比较并确定最佳料液比。根据确定的最佳料液比,在3种桃花色粉末中加入不同浓度(φ,后同)的甲醇(60%、70%和80%),按照上述操作确定浸提溶剂甲醇的最佳浓度。
1.2.2 高效液相色谱测定桃花浸提液中绿原酸的含量 采用高效液相色谱法对桃花浸提液进行成分分析。高效液相色谱仪分析的色谱条件为:使用以十八烷基硅烷键合硅胶为填充剂的色谱柱,以0.1%甲酸水溶液为流动相A,以甲醇为流动相B 进行梯度洗脱;流速为1 mL·min-1;检测波长为280 nm;进样量20 μL;梯度洗脱条件为:0~10 min,流动相A的浓度变化为70%~55%,流动相B 的浓度变化为30%~45%;10~20 min,流动相A的浓度变化为55%~49%,流动相B的浓度变化为45%~51%;20~35 min,流动相A 的浓度变化为49%~20%,流动相B 的浓度变化为51%~80%;35~36 min,流动相A的浓度变化为20%~0,流动相B的浓度变化为80%~100%。
1.2.3 桃花绿原酸浸提液对桃褐腐病菌菌丝生长的抑制试验 笔者在本研究中从第20 天的桃褐腐病菌菌株中取直径7 mm的果孢菌塞备用。PDA培养基经高压蒸汽灭菌处理后,自然冷却至适宜温度,向其中加入不同浓度(ρ,后同)桃花浸提液或者绿原酸标准品(0.2、0.3 或0.4 mg·mL-1),混匀,随后制备平板,以PDA为对照。将7 mm菌塞置于平板的中心,25 ℃培养,观察处理不同时间(0、1、3、5 d)桃褐腐病菌的生长情况,每个处理设3 次重复。使用ImageJ测量并记录桃褐腐病菌菌落直径。菌丝生长抑制率(Y)/%=[(对照组菌落直径-菌塞直径)-(处理组菌落直径-菌塞直径)]/(对照组菌落直径-菌塞直径)×100。
2 结果与分析
2.1 不同品系桃花的盛花期
为了研究不同颜色桃花浸提液中绿原酸的含量差别,对花色不同的桃品系的盛花期进行了调查,并确定最佳取样时间。通过对白色(W-7)、粉色(P-3)、红色(R-2)3个颜色桃花的盛花期进行连续观测(表1),分别在3个桃品系的盛花期采集桃花花瓣样品,用于后续浸提物的制备。
表1 不同品系桃花的盛花期调查(2023 年)
Table 1 Flowering period of different peach strains(In 2023)
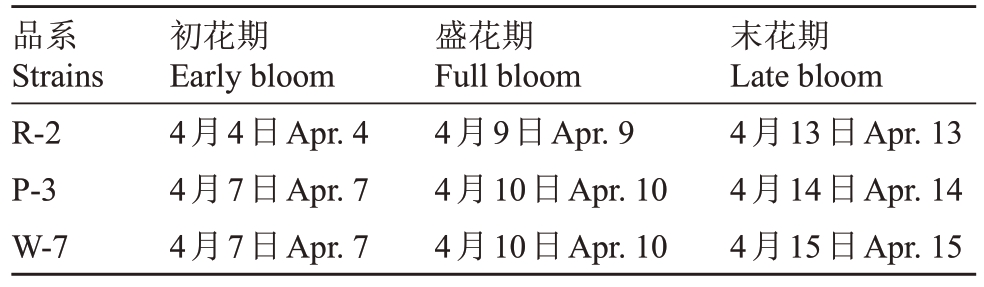
品系Strains R-2 P-3 W-7初花期Early bloom 4月4日Apr.4 4月7日Apr.7 4月7日Apr.7盛花期Full bloom 4月9日Apr.9 4月10日Apr.10 4月10日Apr.10末花期Late bloom 4月13日Apr.13 4月14日Apr.14 4月15日Apr.15
2.2 不同品系桃花绿原酸提取条件的确定
采用超声波辅助甲醇浸提法提取桃花中的绿原酸,HPLC测定R-2、P-3和W-7品系桃花浸提液中绿原酸的含量,确定从3种桃花色中提取绿原酸的最优条件均为料液质量比1∶10,浸提溶液为80%甲醇(图2)。
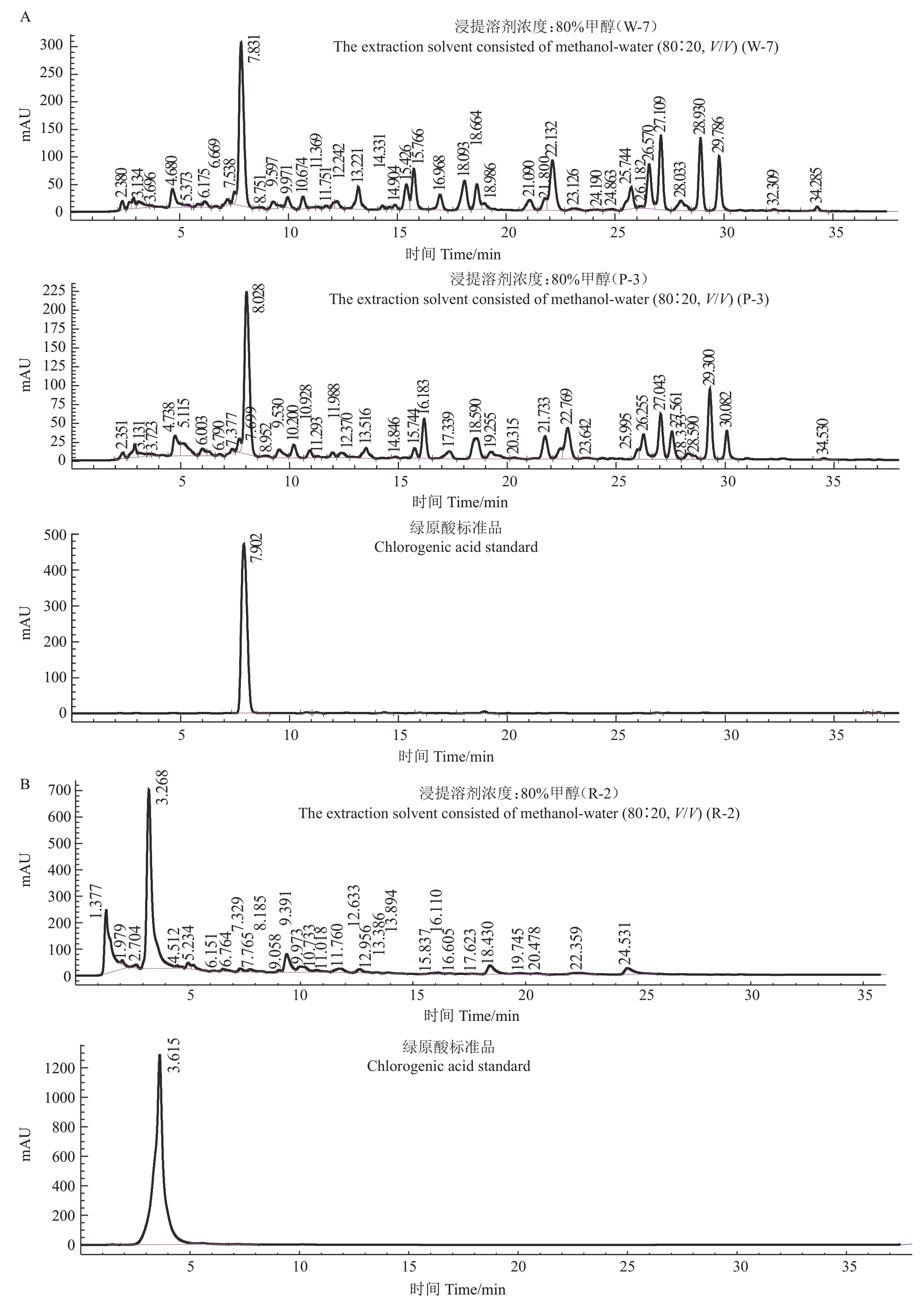
图2 HPLC 测定桃花浸提液中的化学成分
Fig.2 HPLC determination of chemical compounds in the extract of peach blossoms
在最优条件下,红色桃花(R-2)、粉色桃花(P-3)及白色桃花(W-7)中绿原酸含量(ρ,后同)分别为0.440、0.324 和0.417 mg·mL-1(图3),3 个不同花色桃品系绿原酸含量无明显差别。
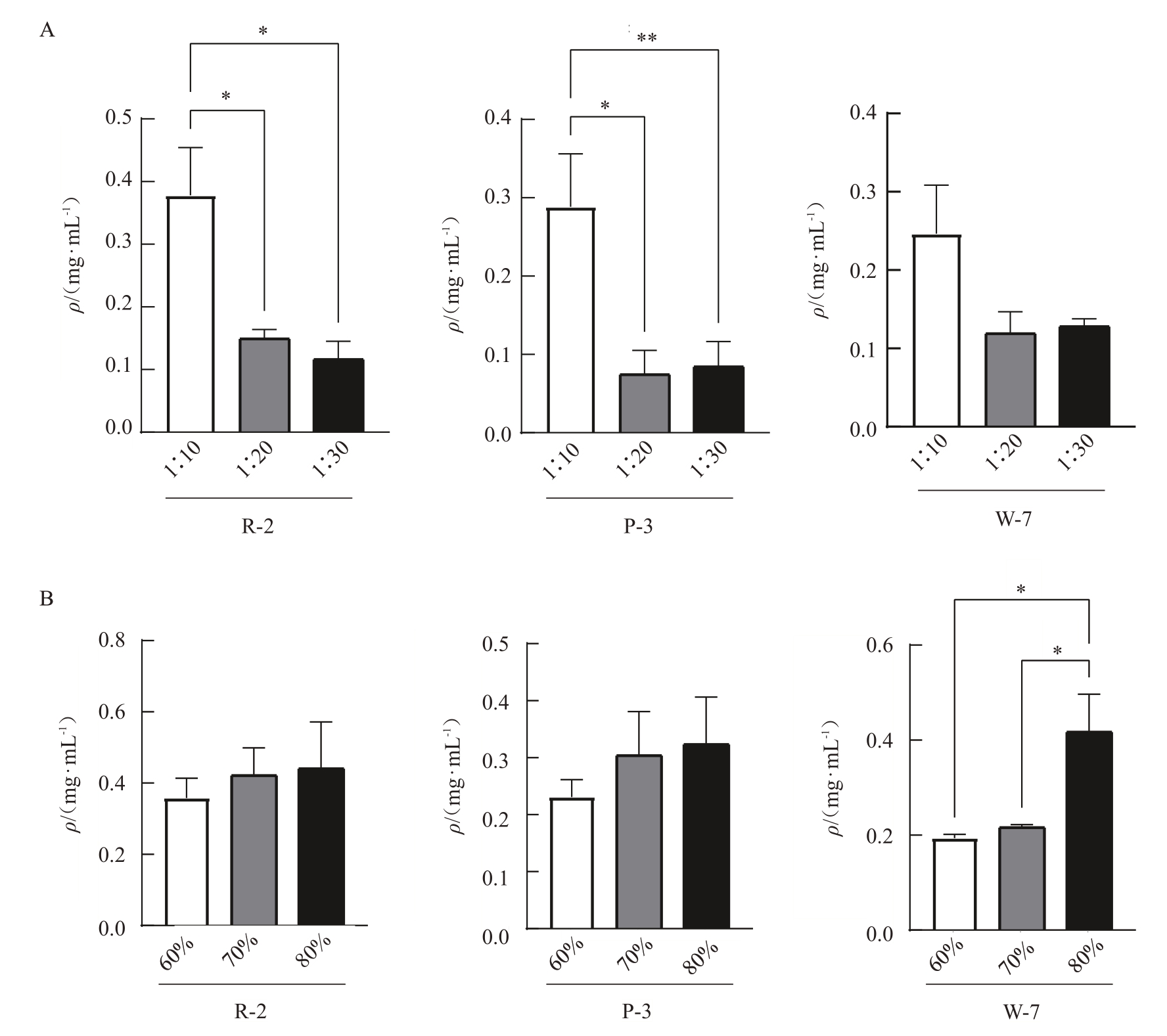
图3 HPLC 测定不同品系桃花绿原酸的提取效果
Fig.3 HPLC determination of chlorogenic acid extraction efficiency of three peach blossom
A. 不同料液比绿原酸提取含量;B. 不同甲醇浓度绿原酸提取含量。**p<0.01,*p<0.05。
A.Extracted content of chlorogenic acid at different material-liquid ratio;B.Extracted content of chlorogenic acid at different methanol concentrations.**p<0.01,*p<0.05.
2.3 桃褐腐病菌的形态
桃褐腐病菌及其对桃愈伤组织侵染表型如图4所示,褐腐病菌白色菌丝体能够稳固地黏附在愈伤组织的表面,并且在局部区域桃愈伤组织开始出现变色现象,由原本正常的颜色逐渐变为褐色或暗褐色。组织质地也发生了变化,从原本相对紧实变得松软、水渍状。由此可见,桃褐腐病菌对愈伤组织具有明显的侵染能力,在愈伤组织表面保持良好的径向生长能力。
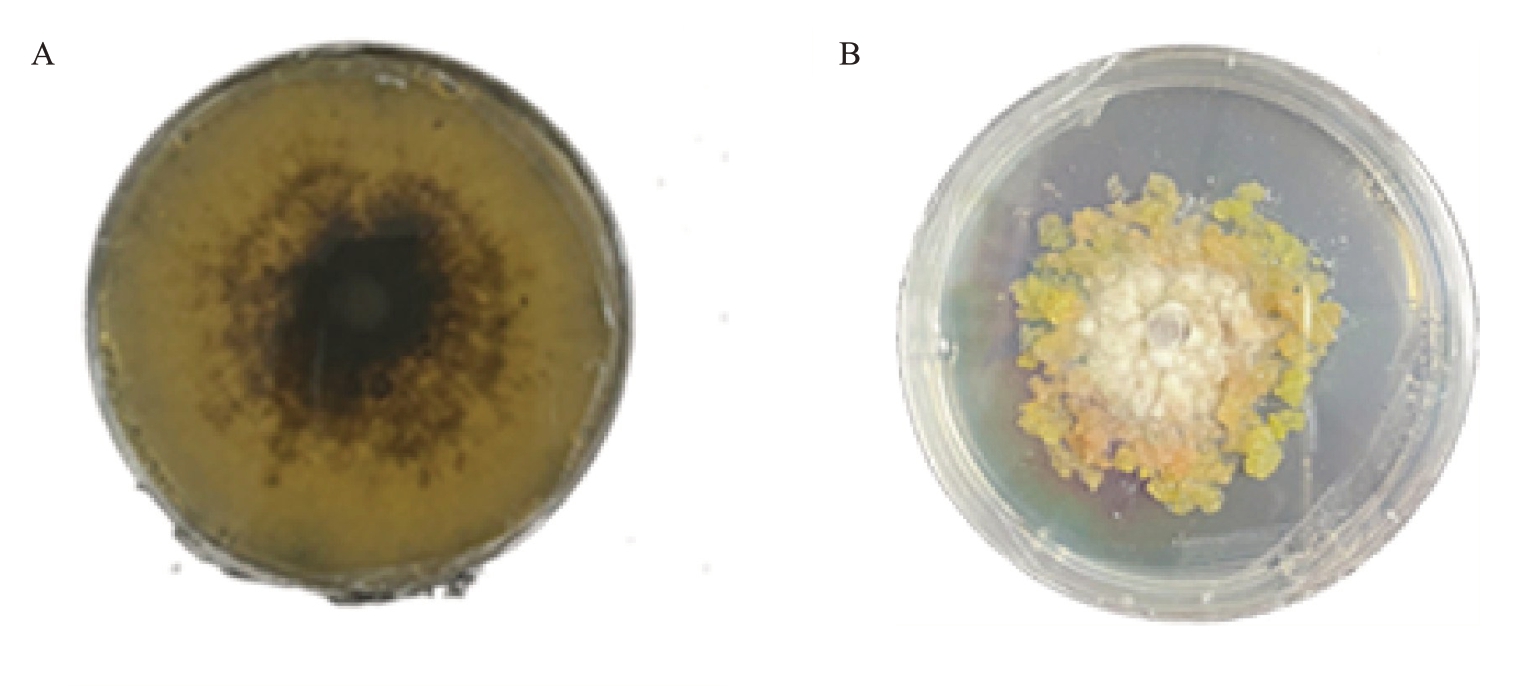
图4 桃褐腐病菌的形态及侵染效果
Fig.4 Morphology and infection effect of M.fructicola
A.桃褐腐病菌落形态;B. 桃褐腐病菌侵染桃愈伤组织。
A.The colony morphology of peach brown rot pathogen M.fructicola;B.The diagram of M.fructicola infecting peach callus tissue.
2.4 桃花绿原酸浸提液对桃褐腐病菌的抑制效果
采用不同浓度(0、0.2、0.3 或0.4 mg·mL-1)的绿原酸标准品及桃花绿原酸浸提液处理桃褐腐病菌,不同时间(0、1、3、5 d)对桃褐腐病菌的生长以及抑菌情况见图5,同时通过测定菌落直径计算抑菌率,采用统计学方法对不同处理组间的抑菌效果进行比较分析。对照组中,0.2 mg·mL-1和0.4 mg·mL-1绿原酸标准品对桃褐腐病菌均有抑制效果。试验组不同浓度桃花绿原酸浸提液对桃褐腐病菌均显示出抑制作用。当浓度为0.3 或0.4 mg·mL-1时,绿原酸标准品和桃花绿原酸浸提液抑制效果均为最佳,第5 天时抑菌率分别为67.21%(绿原酸标准品)、21.38%(W-7)、21.47%(P-3)以及34.65%(R-2)(表2)。桃花浸提液对目标病原菌生长具有显著的抑制作用,且抑菌效果具有时间依赖性和浓度依赖性。
表2 绿原酸及桃花浸提液对桃褐腐病菌抑制效果
Table 2 The inhibition effects of chlorogenic acid and peach blossom extracts against peach brown rot pathogen
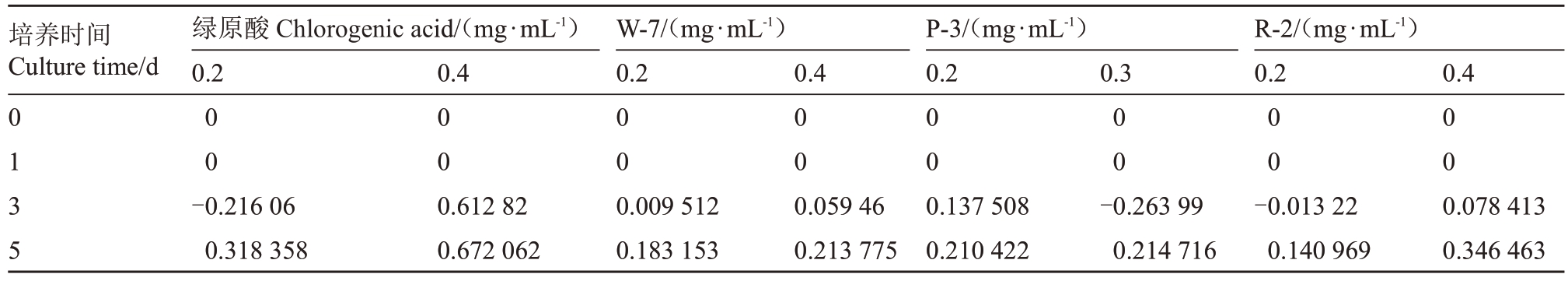
培养时间Culture time/d绿原酸Chlorogenic acid/(mg·mL-1)0.2 0.4 W-7/(mg·mL-1)0.2 0.4 P-3/(mg·mL-1)0.2 0.3 R-2/(mg·mL-1)0.2 0.4 0 1 3 5 0 0-0.216 06 0.318 358 0 0 0.612 82 0.672 062 0 0 0.009 512 0.183 153 0 0 0.059 46 0.213 775 0 0 0.137 508 0.210 422 0 0-0.263 99 0.214 716 0 0-0.013 22 0.140 969 0 0 0.078 413 0.346 463
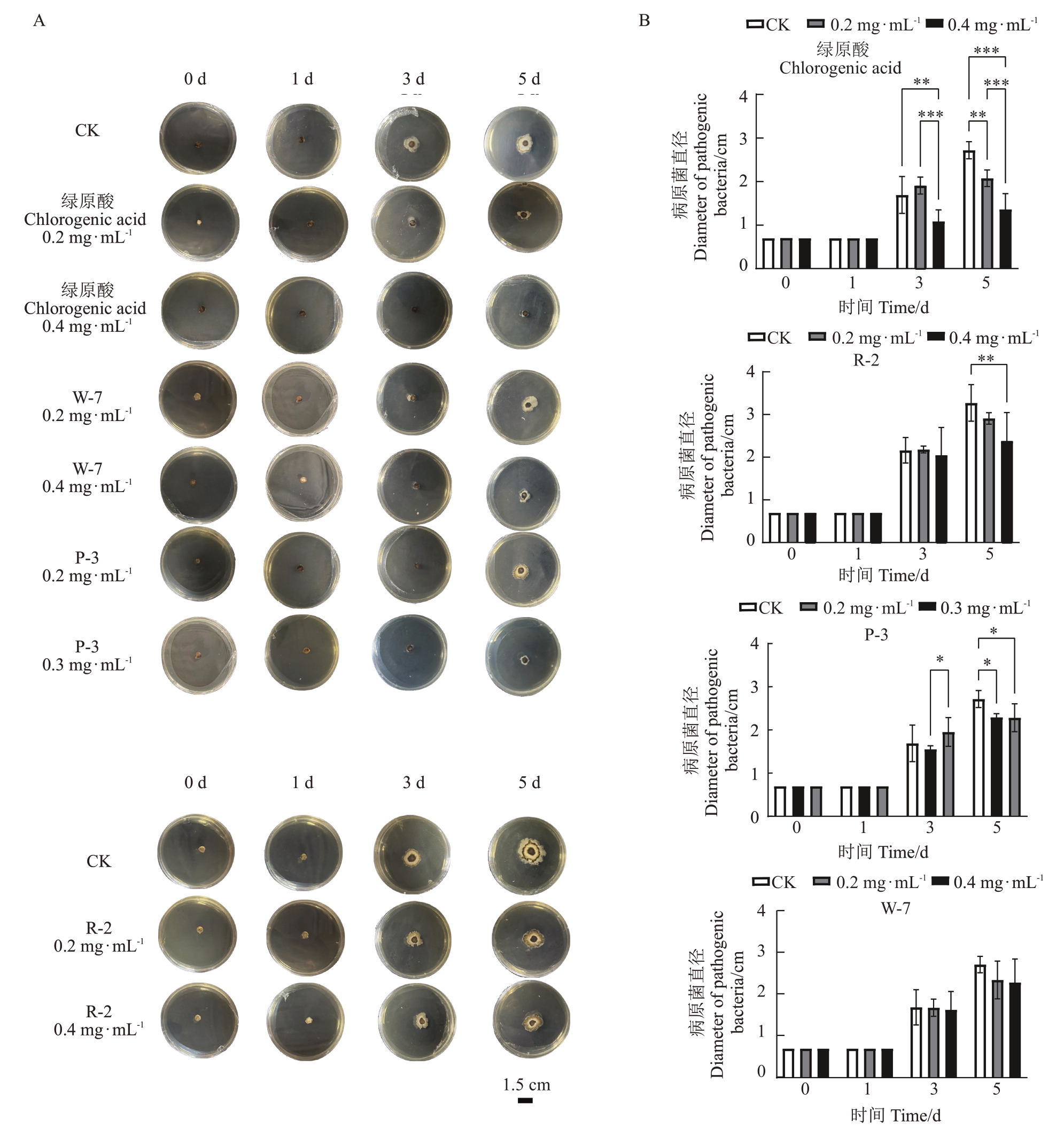
图5 绿原酸及桃花浸提液对褐腐病菌的抑制效果
Fig.5 The inhibition of chlorogenic acid and peach blossom extracts against peach brown rot pathogen
A.不同浓度绿原酸及桃花浸提液对桃褐腐病菌的抑制效果(标尺:1.5 cm);B.不同浓度绿原酸及桃花浸提液对桃褐腐病菌的抑制效果比较。***p<0.001,**p<0.01,*p<0.05。
A.Inhibition effect of different concentrations of chlorogenic acid and peach blossom extract on peach brown rot pathogen(Scale bar:1.5 cm);B.Comparison of the inhibition effect of chlorogenic acid and peach blossom extracts at different concentrations on peach brown rot pathogen.***p<0.001,**p<0.01,*p<0.05.
2.5 桃花绿原酸浸提工艺及对桃褐腐病菌的抑制流程
笔者在本研究中通过系统化的试验流程,探究不同品种桃花花瓣中活性物质的提取方法及其对桃褐腐病菌的抑制效果。首先,在样品制备阶段,采集不同品种的桃花,取花瓣并在42 ℃烘箱中烘干。经研磨成粉末,并进行精确称质量。在浸提阶段,通过单因素试验确定最佳料液比,并确定80%的甲醇作为浸提溶剂,结合超声波辅助提取技术,以提高提取效率。浸提过程在4 ℃条件下过夜进行,以确保活性物质的充分释放。随后,通过离心分离上清液,抽滤处理后采用HPLC进行成分及含量分析,以定量测定目标活性物质绿原酸。最后,在浸提物质应用效果评估阶段,将提取物以不同浓度(0.2、0.3或0.4 mg·mL-1)加入PDA培养基中,观察其对桃褐腐病菌生长的抑制效果。试验在25 ℃培养箱中进行,分别于0、1、3、5 d 观测病菌的生长情况,测定菌落直径,计算菌丝生长抑制率,以评估提取物的抑菌效果(图6)。
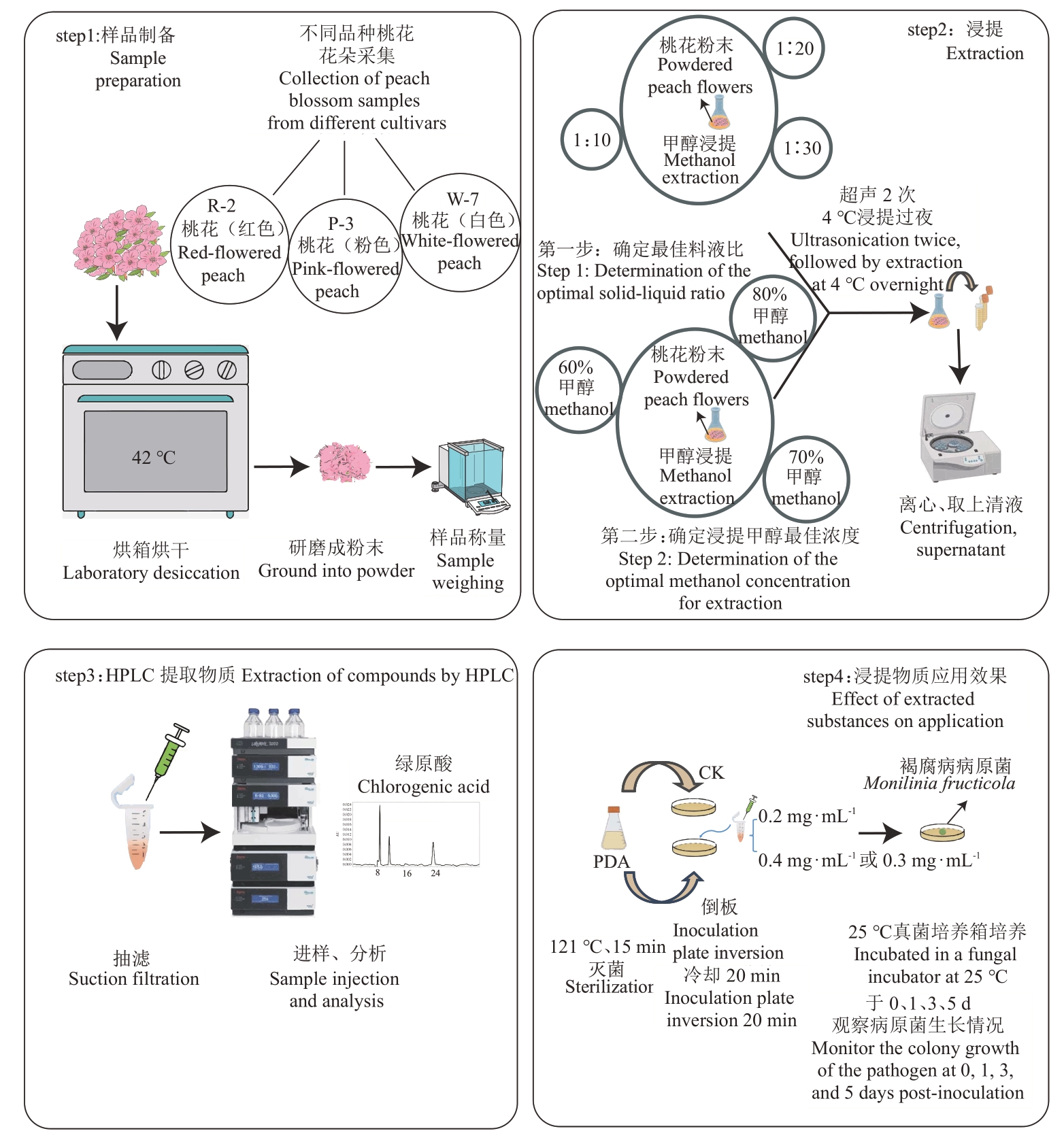
图6 不同品系桃花浸提液的提取工艺及应用效果模式
Fig.6 Extraction process and application effect mode of peach blossom extracts of different strains
3 讨 论
绿原酸属于一种酚类次生代谢产物,是植物体在有氧呼吸过程中经莽草酸途径合成的一种苯丙素类物质,存在于大多蔷薇科果树不同组织中。绿原酸的提取和研究也成为天然提取物研究热点。绿原酸水溶性好,易溶于热水、乙醇及丙酮等极性溶剂,在植物中提取剂大多采用水、醇、酮类等极性较强的溶剂。绿原酸的提取方法主要有浸提法、乙醇回流法、超声波提取法、超滤法、树脂吸附法等提取法[24-25]。桃是蔷薇科重要的经济果树,桃花作为重要的生殖器官,含有丰富的绿原酸[26]。其提取方法主要采用超声辅助乙醇方法[27],以甲醇作为浸提溶剂提取桃花绿原酸的研究尚未见报道。本试验建立和优化了一套超声波辅助甲醇浸提桃花绿原酸的方法,综合现有的试验数据,利用单因素法确定了桃花中提取绿原酸的最优条件均为料液比1∶10,浸提溶液为80%的甲醇。利用此提取方法,得到的桃花中绿原酸浓度分别为0.44、0.324 和0.417 mg·mL-1,从提取效率和节约成本上来说具有重要意义。
绿原酸对多种生物体表现出抗菌活性,包括细菌和真菌[2,28-29]。对于不同细菌,绿原酸的作用机制是主要包括抑制细菌生物膜形成、增加细胞膜通透性和破坏细胞完整性、扰乱细胞代谢及调控信号转导通路等[28]。而对于真菌而言,绿原酸可通过抑制孢子萌发、芽管伸长、菌丝生长,降低细胞活力和菌丝体渗透性以及干扰真菌细胞完整性来抑制病原真菌的生长[2,25,30]。桃褐腐病主要引起果实腐烂,也可危害花、叶及枝条等,作为一种在中国广泛分布的病害,具有发病症状严重、传染力强的特点,给桃产业带来巨大的经济损失[19-20]。有研究表明,植物天然提取物花椒、薄荷和大蒜提取物对桃褐腐病菌丝体生长有抑制效果[21-23]。绿原酸对该病致病菌的作用尚未见研究。本试验中采用不同浓度的绿原酸及桃花绿原酸浸提液处理桃褐腐病菌(M.fructicola),系统评估了绿原酸的抑菌效果。抑菌效果具有时间依赖性和浓度依赖性,当浓度为0.4 mg·mL-1时,标准品和桃花绿原酸浸提液抑制效果为最佳。有研究发现,当绿原酸浓度为15 mg·mL-1时,真菌(Sclerotinia sclerotiorum、Fusarium solani、Verticillium dahliae、Botrytis cinerea和Cercospora sojina)的孢子萌发均被完全抑制,对部分菌丝的抑制率超过96%,具体情况因真菌种类而异[2]。笔者在本研究中使用0.2~0.4 mg·mL-1浸提液处理桃褐腐病菌,使用的绿原酸浓度低于上述研究,其对桃褐腐病菌的生长也具有显著的抑制作用,但强度受到限制。
4 结 论
本试验建立了一套超声辅助甲醇浸提提取桃花中绿原酸的方法,采用超声波浸提功率200 W,超声温度40 ℃,浸提时间60 min,在料液质量比1∶10,浸提溶剂为80%甲醇溶液条件下,浸提2 次效果最佳。将桃花绿原酸浸提液处理桃褐腐病菌后病菌生长受到抑制,具有较好的抑制效果。
[1] LALLEMAND L A,ZUBIETA C,LEE S G,WANG Y C,ACAJJAOUI S,TIMMINS J,MCSWEENEY S,JEZ J M,MCCARTHY J G,MCCARTHY A A. A structural basis for the biosynthesis of the major chlorogenic acids found in coffee[J]. Plant Physiology,2012,160(1):249-260.
[2] MARTÍNEZ G,REGENTE M,JACOBI S,DEL RIO M,PINEDO M,DE LA CANAL L. Chlorogenic acid is a fungicide active against phytopathogenic fungi[J]. Pesticide Biochemistry and Physiology,2017,140:30-35.
[3] KAI K,WANG R,BI W L,MA Z T,SHI W,YE Y W,ZHANG D F. Chlorogenic acid induces ROS-dependent apoptosis in Fusarium fujikuroi and decreases the postharvest rot of cherry tomato[J]. World Journal of Microbiology and Biotechnology,2021,37(6):93.
[4] JIAO W X,LI X X,WANG X M,CAO J K,JIANG W B.Chlorogenic acid induces resistance against Penicillium expansum in peach fruit by activating the salicylic acid signaling pathway[J].Food Chemistry,2018,260:274-282.
[5] DAI B E,WANG Y X,ZHOU H J,WANG L F,ZHOU L,MAO J X,ZHANG S Y,SHEN S L,ZHENG X L,HUAN C. Control efficiency and potential mechanisms of chlorogenic acid against postharvest gray mold caused by Botrytis cinerea on peach fruit[J].Postharvest Biology and Technology,2024,218:113134.
[6] XI Y,JIAO W X,CAO J K,JIANG W B.Effects of chlorogenic acid on capacity of free radicals scavenging and proteomic changes in postharvest fruit of nectarine[J]. PLoS One,2017,12(8):e0182494.
[7] XI Y,FAN X G,ZHAO H D,LI X H,CAO J K,JIANG W B.Postharvest fruit quality and antioxidants of nectarine fruit as influenced by chlorogenic acid[J]. LWT-Food Science and Technology,2017,75:537-544.
[8] ZHANG D F,BI W L,KAI K,YE Y W,LIU J.Effect of chlorogenic acid on controlling kiwifruit postharvest decay caused by Diaporthe sp.[J]. LWT-Food Science and Technology,2020,132:109805.
[9] 张豫丹,马晓寒,李俊领,许自成,贾玮,石秋环.绿原酸对烟草疫霉的抑制作用及对烟草黑胫病的防治效果研究[J].作物杂志,2022(2):230-236.ZHANG Yudan,MA Xiaohan,LI Junling,XU Zicheng,JIA Wei,SHI Qiuhuan.Inhibitory effect of chlorogenic acid on Phytophthora nicotiana and its control effect on tobacco black shank disease[J].Crops,2022(2):230-236.
[10] 马志桃.绿原酸对草莓采后灰霉病的抑制作用机理研究[D].合肥:合肥工业大学,2022.MA Zhitao. Inhibitory mechanism of chlorogenic acid on postharst Botrytis cinerea of strawberry[D]. Hefei:Hefei University of Technology,2022.
[11] 辛邵南.滩涂适生菊芋绿原酸合成途径相关基因响应高温与盐胁迫的机理分析[D].南京:南京农业大学,2017.XIN Shaonan.Analysis of mechanism of relative gene responses of high temperature and salt stresses to chlorogenic acid synthesis pathway in Jerusalem artichoke growth in tidal flats[D].Nanjing:Nanjing Agricultural University,2017.
[12] LI C M,WANG M H.Antioxidant activity of peach blossom extracts[J]. Journal of the Korean Society for Applied Biological Chemistry,2011,54(1):46-53.
[13] 刘杰超,张巧莲,焦中高,张春岭,吕真真,刘慧,王思新.桃花提取物对酪氨酸酶的抑制作用及其动力学分析[J].果树学报,2014,31(5):836-841.LIU Jiechao,ZHANG Qiaolian,JIAO Zhonggao,ZHANG Chunling,LÜ Zhenzhen,LIU Hui,WANG Sixin. Inhibitory effect of peach flower extract on tyrosinase and its kinetics analysis[J].Journal of Fruit Science,2014,31(5):836-841.
[14] 刘杰超,张春岭,吕真真,刘慧,焦中高.桃花中总酚和总黄酮的提取及抗氧化活性研究[J].食品安全质量检测学报,2013,4(6):1750-1756.LIU Jiechao,ZHANG Chunling,LÜ Zhenzhen,LIU Hui,JIAO Zhonggao. Extraction of total phenolics and flavanoids from peach blossoms and their antioxidant activity[J]. Journal of Food Safety&Quality,2013,4(6):1750-1756.
[15] LIU J C,JIAO Z G,YANG W B,ZHANG C L,LIU H,LÜ Z Z.Ⅴariation in phenolics,flavanoids,antioxidant and tyrosinase inhibitory activity of peach blossoms at different developmental stages[J].Molecules,2015,20(11):20460-20472.
[16] FUY,SUNRQ,YANGJF,WANGLL,ZHAOP,CHENSQ.Characterization and quantification of phenolic constituents in peach blossom by UPLC-LTQ-Orbitrap-MS and UPLC-DAD[J].Natural Product Communications,2020,15(1):1934578X19884437.
[17] 刘丹赤,杨建华,李鹏.牛蒡叶中绿原酸提取工艺研究[J].江苏农业科学,2009,37(4):305-306.LIU Danchi,YANG Jianhua,LI Peng. Study on extraction technology of chlorogenic acid from burdock leaves[J]. Jiangsu Agricultural Sciences,2009,37(4):305-306.
[18] 王丽,胡付侠,张明,李晓桐,王崇队,范祺,马超.蒸汽爆破辅助超声提取金银花绿原酸及其抗氧化、抗炎活性研究[J].食品科技,2025,50(3):225-234.WANG Li,HU Fuxia,ZHANG Ming,LI Xiaotong,WANG Chongdui,FAN Qi,MA Chao. Extraction of chlorogenic acid from Lonicera japonica Thunb. by steam explosion assisted ultrasonography and its antioxidant and anti-inflammatory activities[J].Food Science and Technology,2025,50(3):225-234.
[19] 薛璐,李勇,方伟超,杨英军,王力荣.150 份桃种质资源果实褐腐病抗性评价[J].植物遗传资源学报,2025,26(1):148-156.XUE Lu,LI Yong,FANG Weichao,YANG Yingjun,WANG Lirong. Evaluation of resistant to brown rot in peach fruits for 150 peach germplasm resources[J].Journal of Plant Genetic Resources,2025,26(1):148-156.
[20] 纪兆林,谈彬,朱薇,董京萍,朱峰,徐敬友,童蕴慧.我国不同产区桃褐腐病病原鉴定与分析[J]. 微生物学通报,2019,46(4):869-878.JI Zhaolin,TAN Bin,ZHU Wei,DONG Jingping,ZHU Feng,XU Jingyou,TONG Yunhui. Identification and analysis of the peach brown rot pathogens from different peach-growing areas in China[J].Microbiology China,2019,46(4):869-878.
[21] 莫熙礼,赵同贵,邓伟,吴彤林,杨云彩,赵应婉.花椒提取物对桃褐腐病菌的抑制作用及酶活性的影响[J]. 北方园艺,2014(20):129-132.MO Xili,ZHAO Tonggui,DENG Wei,WU Tonglin,YANG Yuncai,ZHAO Yingwan. Effect of extract of Zanthoxylum bungeanum on pathogen inhibition and activities of antioxidative enzyme of Monilinia fructicolain in peach[J]. Northern Horticulture,2014(20):129-132.
[22] 莫熙礼,赵同贵,邓伟,吴彤林,杨云彩,赵应婉,李本华.植物提取物对桃褐腐病菌的抑制作用[J].江苏农业科学,2015,43(6):124-126.MO Xili,ZHAO Tonggui,DENG Wei,WU Tonglin,YANG Yuncai,ZHAO Yingwan,LI Benhua. Inhibitory effect of plant extracts on Monilinia fructicola in peach[J].Jiangsu Agricultural Sciences,2015,43(6):124-126.
[23] 张瑶. 茶叶提取物对桃采后软腐病菌的抑菌活性及作用机制[D].武汉:华中农业大学,2013.ZHANG Yao.Antifungal activity of tea extract against Rhizopus nigricans on harvested peaches and the mechanism[D]. Wuhan:Huazhong Agricultural University,2013.
[24] YUE Y Y,HUANG Q W,FU Y,CHANG J.A quick selection of natural deep eutectic solvents for the extraction of chlorogenic acid from herba artemisiae scopariae[J]. RSC Advances,2020,10(39):23403-23409.
[25] MANGIAPELO L,BLASI F,IANNI F,BAROLA C,GALARINI R,ABUALZULOF G W,SARDELLA R,ⅤOLPI C,COSSIGNANI L. Optimization of ultrasound-assisted extraction of chlorogenic acid from potato sprout waste and enhancement of the in vitro total antioxidant capacity[J].Antioxidants,2023,12(2):348.
[26] SU Z W,JIA H R,SUN M,CAI Z X,SHEN Z J,ZHAO B T,LI J Y,MA R J,YU M L,YAN J.Integrative analysis of the metabolome and transcriptome reveals the molecular mechanism of chlorogenic acid synthesis in peach fruit[J]. Frontiers in Nutrition,2022,9:961626.
[27] ZENG G M,RAN Y J,HUANG X,LI Y,ZHANG M L,DING H,MA Y G,MA H S,JIN L B,SUN D.Optimization of ultrasonic-assisted extraction of chlorogenic acid from tobacco waste[J].International Journal of Environmental Research and Public Health,2022,19(3):1555.
[28] 李阳昱,李庆蓉,陈孝红,薛丽,和平安,吕梅,杨旭.绿原酸抗菌作用及机制的研究进展[J].中国抗生素杂志,2024,49(2):141-150.LI Yangyu,LI Qingrong,CHEN Xiaohong,XUE Li,HE Ping’an,LÜ Mei,YANG Xu.Advances in research on the antibacterial effects and mechanism of chlorogenic acid[J]. Chinese Journal of Antibiotics,2024,49(2):141-150.
[29] LOU Z X,WANG H X,ZHU S,MA C Y,WANG Z P.Antibacterial activity and mechanism of action of chlorogenic acid[J].Journal of Food Science,2011,76(6):M398-M403.
[30] ZHANG D F,MA Z T,KAI K,HU T T,BI W L,YANG Y Y,SHI W,WANG Z S,YE Y W. Chlorogenic acid induces endoplasmic reticulum stress in Botrytis cinerea and inhibits gray mold on strawberry[J].Scientia Horticulturae,2023,318:112091.