在全球柑橘总产量中,宽皮柑橘占据重要地位,约占世界柑橘总产量的32.09%[1]。椪柑(Citrus reticulata Blanco)作为中国长期以来主栽宽皮柑橘类型之一,因其具有果皮易剥、果肉脆嫩、风味浓郁等优良特性,在鲜食柑橘市场表现突出的商品优势。早蜜椪柑是辛女椪柑芽变而来的早熟品种,在11 月上旬成熟[2]。由于其较早的采收时间,极大地降低了与普通椪柑品种同时上市的竞争力。然而,该品种存在果皮较薄、易裂果的问题,显著影响其商业价值和食用品质。与早蜜椪柑面临相同问题的还有当前商品价值极高的品种,如阳光一号[3]、不知火[4]、甘平[5]等。因此,探究一种有效的裂果防控方法对提高产量和改善品质具有至关重要的意义。
柑橘裂果的发生与果皮发育密切相关。研究表明,正常果实果皮细胞排列紧密且有序,而易裂的果实果皮细胞则多表现为松散无序[6-7]。果皮细胞壁的强度和完整性直接影响果实的抗裂性[8-10]。易裂和耐裂品种硬度的比较也证实了易裂果实的果皮硬度明显较低[11-13]。此外,果皮厚度同样对柑橘裂果和品质有重要影响。果皮过薄容易导致采前裂果,而果皮太厚容易发生粗皮大果,这两者都会导致柑橘产量和品质的严重下降[14-15]。赤霉素(gibberellins,GA3)作为一种重要的植物激素,主要作用是加速细胞的伸长,同时对细胞的分裂也有促进作用[16]。在生产实践中,往往通过喷施外源赤霉素来促进果皮增厚从而预防裂果[17]。李永杰等[18]研究表明,10 mg·kg-1赤霉素处理显著促进甘平果皮增厚,有效抑制裂果发生,且不影响果实内在品质。邹河清等[19]也发现,在红江橙上施用赤霉素能够促进果皮增厚进而减少裂果发生。以上研究表明,喷施外源赤霉素能够作为一种有效手段,通过增厚果皮来防控裂果,间接提升柑橘产量和品质。
为探索GA3在早蜜椪柑裂果防控中的最适浓度,同时保证果皮的正常发育,笔者在早蜜椪柑果实细胞分裂期开展了系列浓度GA3处理,结果发现100 mg·L-1 GA3表现出最好的防裂果效果;在此基础上,进一步分析了该浓度下果皮厚度及细胞壁成分的动态变化规律,旨在揭示GA3调控柑橘果皮发育预防裂果的生理机制,以期为生产上裂果防控提供理论依据和实践指导。
1 材料和方法
1.1 材料
试验于2024 年在湖南省湘西土家族苗族自治州泸溪县(110°7′E,28°15′N)开展,供试材料为枳壳[Poncirus trifoliate(L.)Raf.]砧木高接的早蜜椪柑,其中辛女椪柑作为中间砧。试验地土壤类型为紫砂质土,早蜜椪柑盛花期观测记录为5月1日。
1.2 试验设计
对照(CK)为自然生长状态下的早蜜椪柑,喷施清水。处理组为50、100 和200 mg·L-1 GA3,并加入0.05%的吐温-80 作为表面活性剂,使用背负式喷雾器在分裂期喷施2次,即盛花后21和28 d(days after full bloom,DAF)。每个处理组8 株树,药液用量为16 L。为探究分裂期应用GA3对不同发育阶段果实外观形态、果皮厚度、硬度和裂果率的影响,采样时间分别设定为分裂后期(70 DAF)、膨大后期(130 DAF)和成熟期(190 DAF);为探究100 mg·L-1 GA3处理组果皮厚度和细胞壁成分的动态变化,于分裂期(30、40、50、60、70 DAF)进行采样。
1.3 样品采集
采样前,使用游标卡尺随机测量早蜜椪柑树上100个以上果实的赤道部(横径),计算平均值,以平均值上下轻微浮动作为采样标准;采样时,使用游标卡尺测定果实横径,将符合采样标准的果实采取下来;采样后,每个处理组的果实放入装有冰袋的保温箱中,当天运送至国家柑橘改良中心长沙分中心进行后续处理。
1.4 果实横径、果实纵径、单果质量、果皮厚度的测定
利用游标卡尺测量果实最大横径和最大纵径,使用电子天平测定单果质量,每个处理组至少测量40个果实。
果皮厚度的测量:在果实最大横径的位置切开,用体式荧光显微镜(徕卡,M205 FCA,新加坡)测定切面相对两点的果皮总厚度,并采用平均值作为单个果实的果皮厚度,每个处理组至少测量20个果实。
1.5 果皮硬度的测定
采用FTC TMS-Tough 专业食品物性分析仪(美国)测定果皮硬度。从每个果实上切取3 块约1 cm×1 cm 的小片进行硬度测定,并取平均值作为该果实的硬度,每个处理组至少测定10个果实。测定程序如下:测试探头为p/2,测试速度为1 mm·s-1,起始力为0.375 N,形变量设定为60%。
1.6 裂果率统计
在成熟期进行裂果情况的统计,每个处理统计3株树,裂果总数包括树上裂果数和落地裂果数,裂果率/%=裂果总数/总果数×100。
1.7 细胞壁成分测定
随机选取10 个无病虫害、一致性好的果实,用手术刀从赤道区域切取大约1/3 的总果皮,立即使用液氮速冻,随后通过研磨仪研磨成粉末后存放在-80 ℃冰箱备用。取出部分样品,置于烘箱中烘干后用于细胞壁成分的测定。总果胶、纤维素、木质素含量均使用相应试剂盒(G0717W,G0715W,G0708W,格锐思生物)测定。半纤维素含量使用半纤维素含量检测试剂盒(BC4445,Solarbio)测定。
1.8 果皮显微结构观察
将CK和100 mg·L-1GA3处理采摘下来的果实立即置于固定液中,进行石蜡切片,每个处理3个果实,具体方法参考许园园等[20]的报道。通过SlideⅤiewer软件观察切片图像,选取代表性区域(≥10个区域)进行统计,分析细胞大小和细胞层数的差异。
1.9 数据处理
采用GraphPad Prism 8 对数据进行处理和绘图,使用Adobe Photoshop 2024 处理图片,采用IBM SPSS Statistics 26软件进行差异显著性分析。
2 结果与分析
2.1 不同浓度GA3对早蜜椪柑果皮发育的影响
2.1.1 GA3对早蜜椪柑不同发育阶段果皮外观形态的影响 50、100、200 mg·L-1 GA3处理早蜜椪柑后,观察不同发育阶段(70、130和190 DAF)果实果皮粗糙程度的变化。结果显示,不同浓度GA3处理均可使70 DAF 果实果皮光滑度下降,呈现粗糙表型;而在130 DAF 和190 DAF 果实中,所有GA3处理果皮粗糙度与CK相比并无显著差异(图1)。此外,对不同浓度GA3处理早蜜椪柑果实的单果质量和横径、纵径进行了分析。结果表明,盛花后70 d和130dGA3处理与对照果实在单果质量、横径、纵径方面差异不显著,而50mg·L-1GA3处理在盛花后190 d的单果质量和纵径与对照相比显著减小(图2)。

图1 不同浓度GA3处理对早蜜椪柑不同发育阶段果皮粗糙度的影响
Fig.1 Effects of different GA3 concentrations on peel roughness of Zaomi Ponkan during fruit development
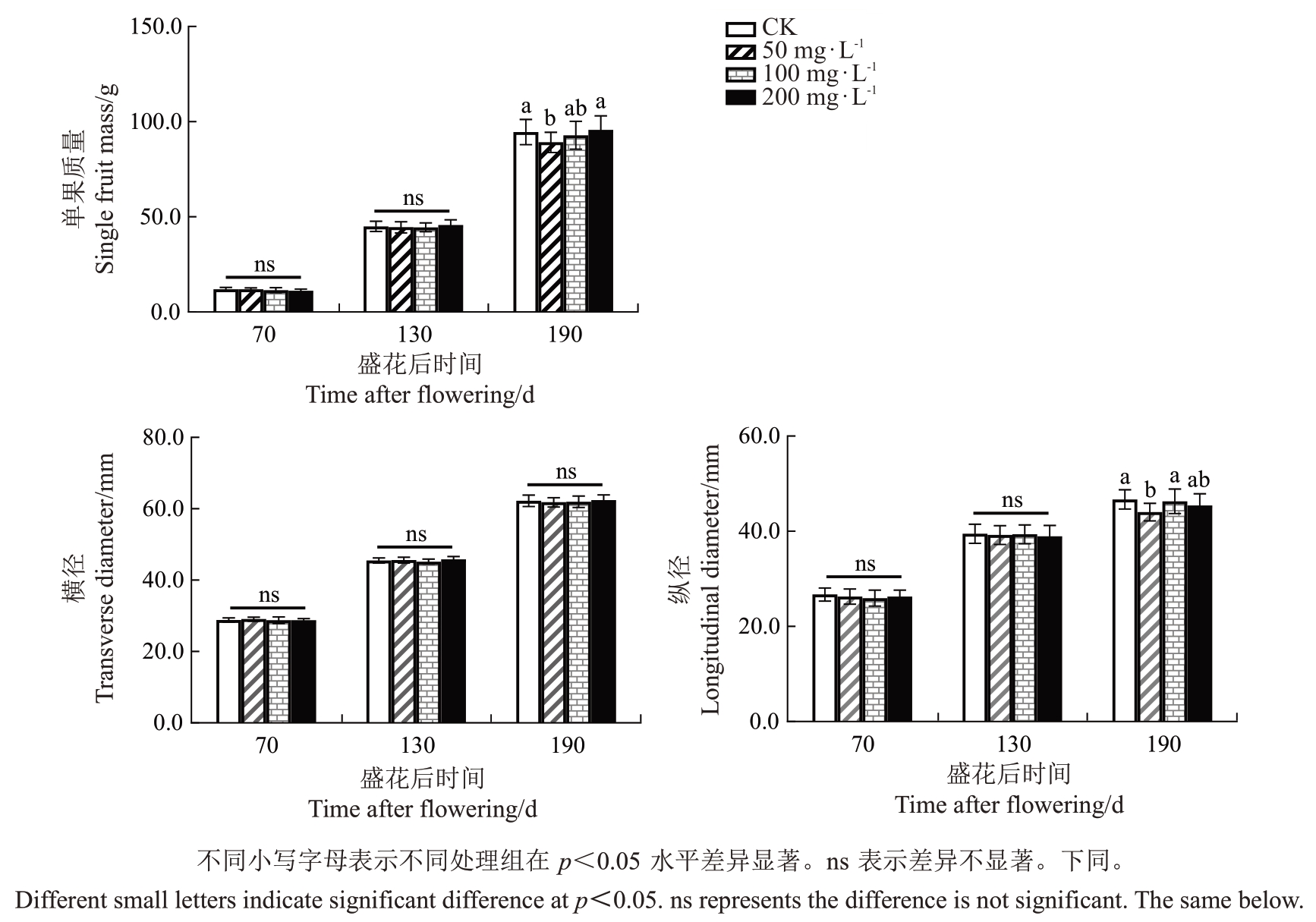
图2 不同浓度GA3处理对早蜜椪柑不同发育阶段果实单果质量、横径和纵径的影响
Fig.2 Effects of different GA3 concentrations on single fruit mass,transverse diameter and longitudinal diameter of Zaomi Ponkan during fruit development
2.1.2 GA3对早蜜椪柑不同发育阶段果皮厚度的影响 在同一发育阶段的比较中,不同浓度GA3处理的早蜜椪柑果皮厚度都呈现显著增厚。具体表现为,70 DAF经50、100和200 mg·L-1 GA3处理后果皮厚度相较于CK 分别增加了9.4%、11.4%、14.4%;在130 DAF时,这一增加比例为13.1%、9.7%、19.3%;在190 DAF时,增加比例则为9.9%、8.6%、15.6%(图3)。
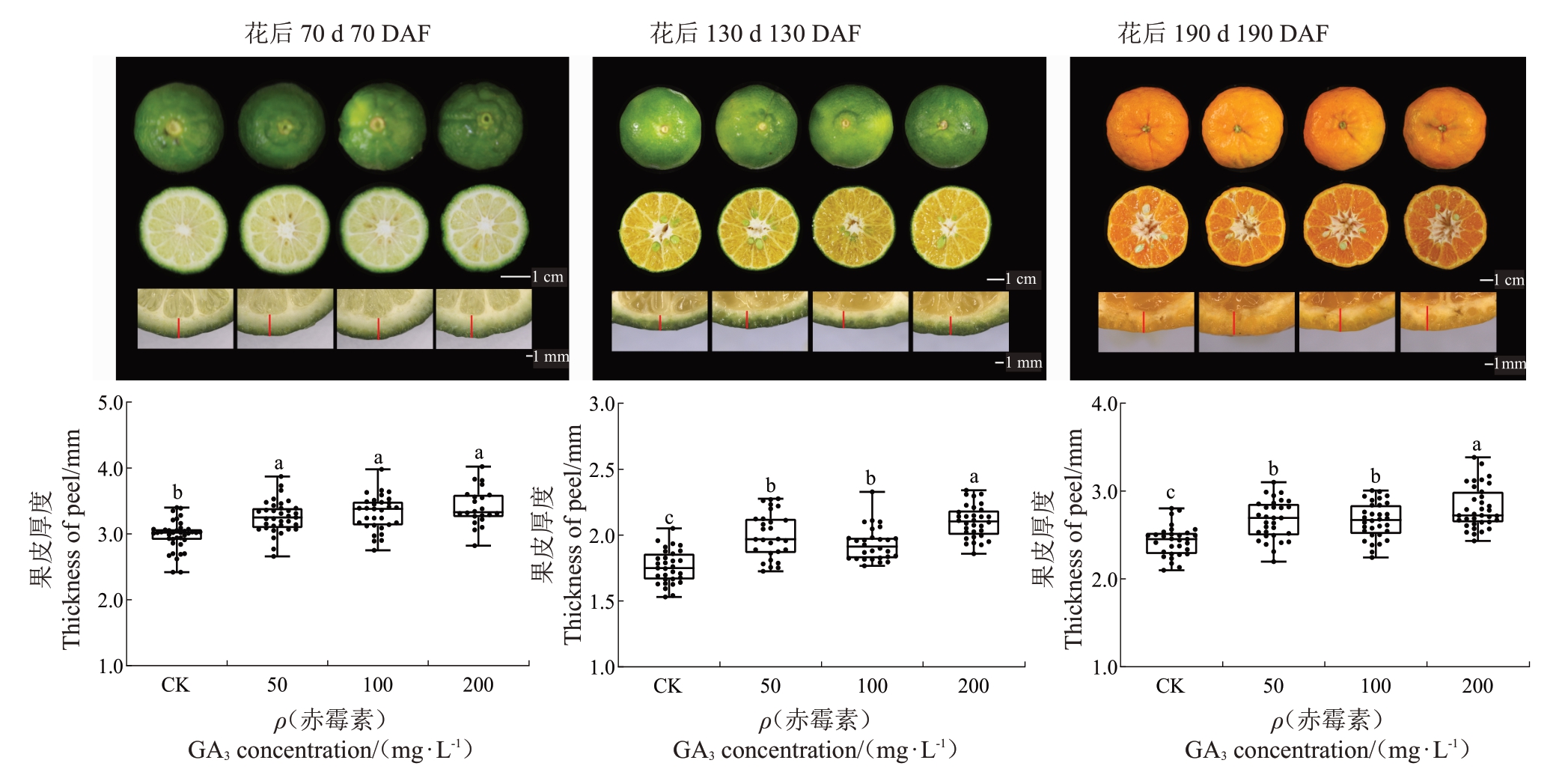
图3 不同浓度GA3处理对早蜜椪柑不同发育阶段果皮厚度的影响
Fig.3 Effects of different concentrations of GA3 treatment on the peel thickness of Zaomi Ponkan during fruit development
2.1.3 GA3对早蜜椪柑果皮硬度的影响 成熟期(190 DAF)的果皮硬度反映了果实的易剥程度和贮藏性能。数据显示,CK 组的果皮硬度为4.71 N,经50、100和200 mg·L-1 GA3处理后,果皮硬度分别增加至5.38 N、5.26 N和5.41 N。尽管所有GA3处理组的果皮硬度均稍有增加,但这些变化并不显著(图4)。
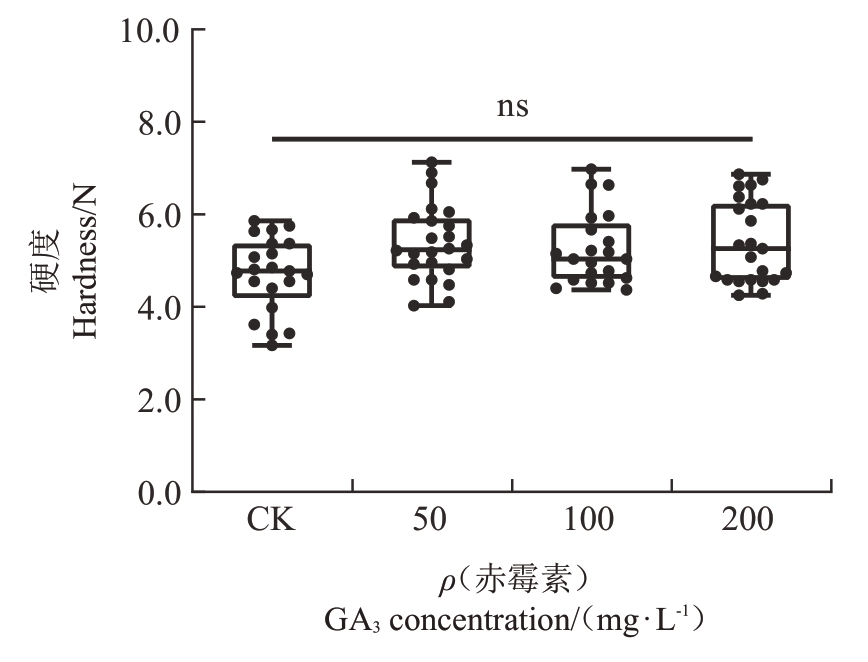
图4 不同GA3浓度对早蜜椪柑果皮硬度的影响
Fig.4 Effects of different GA3 concentrations on the peel hardness of Zaomi Ponkan
2.1.4 GA3对早蜜椪柑裂果率的影响 通过对成熟期(190 DAF)果实裂果率进行分析,以探索GA3预防早蜜椪柑裂果的最适浓度。结果显示,CK 组的裂果率约为19.91%,而经50、100和200 mg·L-1 GA3处理后,裂果率分别降低至10.72%、8.06%、8.70%。整体而言,不同浓度GA3都具有较好的防裂果效果,其中以100 mg·L-1 GA3处理最佳,但各处理间未有显著差异(表1)。
表1 不同GA3浓度对早蜜椪柑裂果率的影响
Table 1 Effects of different GA3 concentrations on the fruit cracking rate of Zaomi Ponkan
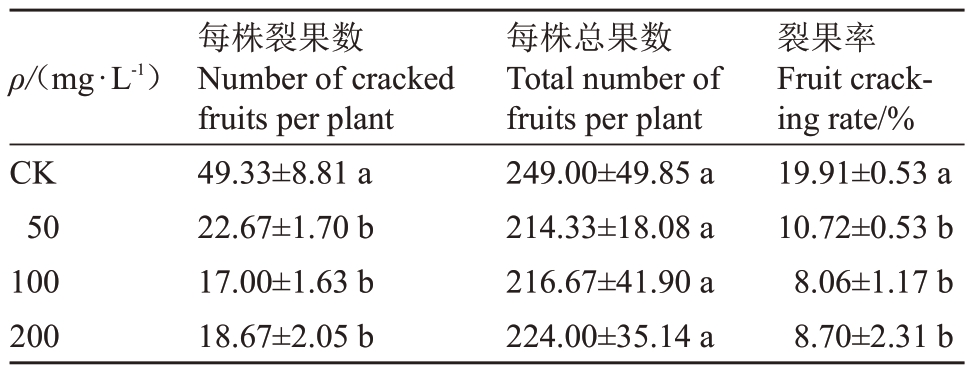
注:不同小写字母表示处理间差异显著(p<0.05)。
Note:Different small letters indicate significant difference among different treatments(p<0.05).
裂果率Fruit cracking rate/%19.91±0.53 a 10.72±0.53 b 8.06±1.17 b 8.70±2.31 b ρ/(mg·L-1)CK 50 100 200每株裂果数Number of cracked fruits per plant 49.33±8.81 a 22.67±1.70 b 17.00±1.63 b 18.67±2.05 b每株总果数Total number of fruits per plant 249.00±49.85 a 214.33±18.08 a 216.67±41.90 a 224.00±35.14 a
2.2 100 mg·L-1GA3调控早蜜椪柑细胞分裂期果皮发育的生理机制
2.2.1 GA3对早蜜椪柑果皮厚度动态发育的影响100mg·L-1GA3不仅对早蜜椪柑防裂效果最好,且在发育早期(60 DAF)果皮便已增厚,并达到顶峰,因而选择该浓度用来研究分裂期(30、40、50、60、70 DAF)果皮发育规律的生理机制。果皮厚度结果显示,CK和100 mg·L-1 GA3处理组的果皮厚度发育规律一致,表现为先增加后下降的趋势,随着果皮发育从30 DAF到60 DAF逐渐增厚并达到峰值,60 DAF之后果皮厚度急剧下降。具体来看,CK 在40、50、60、70 DAF的果皮厚度分别为3.34、3.40、3.48、2.98 mm。与CK相比,100 mg·L-1 GA3处理在相同时间点的果皮分别增厚了5.7%、10.0%、8.0%、11.4%,差异极显著,其中40 DAF是果皮厚度差异的关键时期(图5)。
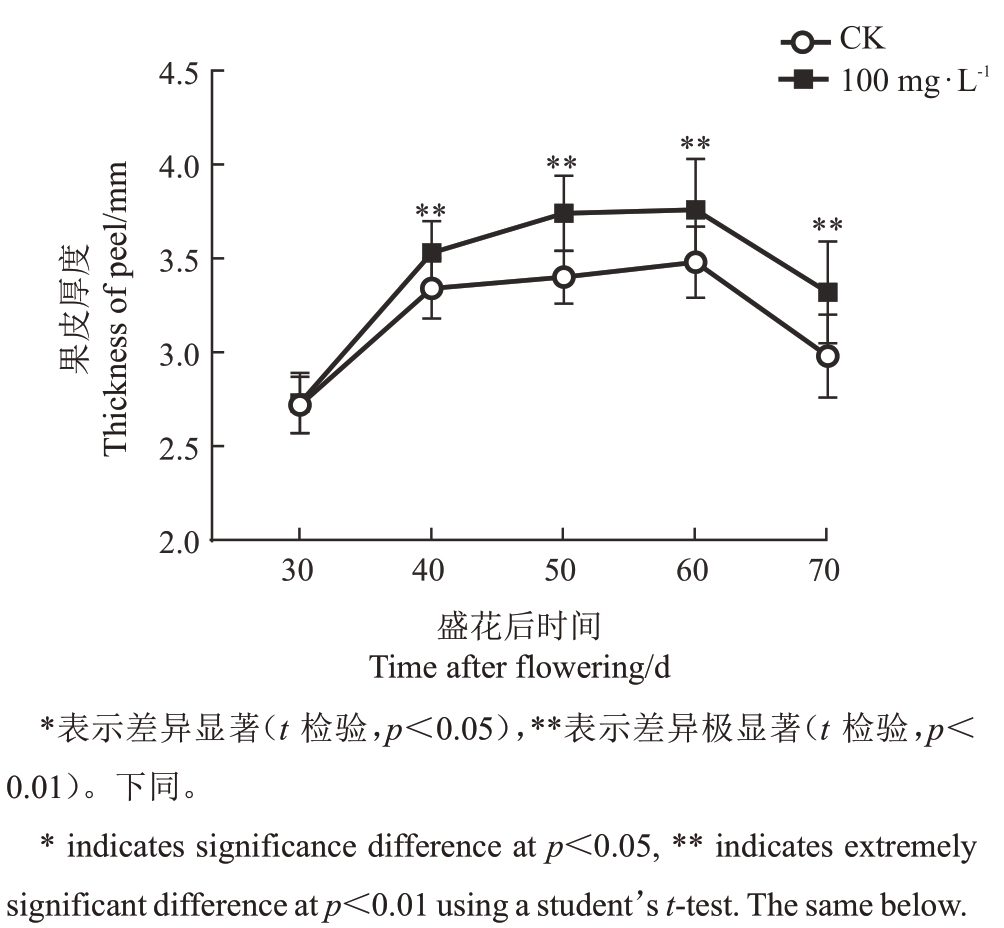
图5 100 mg·L-1 GA3处理下的早蜜椪柑果皮厚度动态变化
Fig.5 Peel thickness dynamics in Zaomi Ponkan with 100 mg·L-1 GA3 treatment
2.2.2 GA3对早蜜椪柑果皮纤维素、半纤维素、木质素、果胶含量的影响 为了探究GA3预防裂果与细胞壁成分含量之间的关系,进一步检测了30~70 DAF的果皮中纤维素、半纤维素、木质素和果胶的含量。结果显示,经100 mg·L-1 GA3处理后,半纤维素含量在30~70 DAF和木质素含量在50~70 DAF均显著高于CK,趋势明显;相比之下,虽然纤维素含量在50、60 DAF 显著低于CK,果胶含量在70 DAF显著高于CK,但整体差异不大(图6)。这些结果表明,GA3预防裂果可能与果皮半纤维素和木质素含量的提高有关。
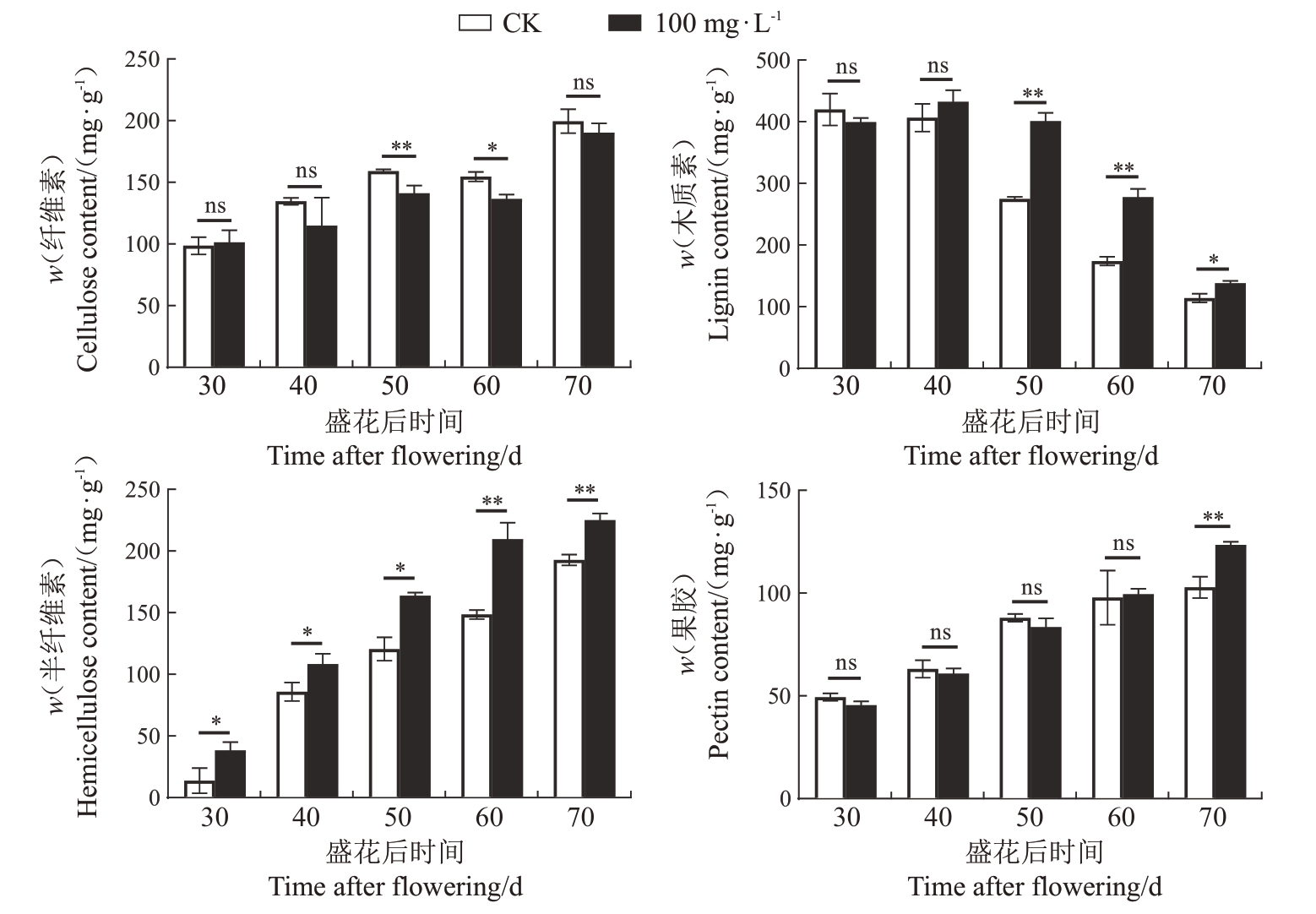
图6 100 mg·L-1 GA3处理对早蜜椪柑果皮纤维素、半纤维素、木质素、果胶含量的影响
Fig.6 Effects of 100 mg·L-1 GA3 treatment on cellulose,hemicellulose,lignin,and pectin content in the peel of Zaomi Ponkan
2.2.3 GA3对早蜜椪柑果皮结构的影响 为了探究GA3调控果皮增厚的细胞学原因,对果皮厚度差异形成的关键时期40 DAF的GA3处理和CK的果皮分别进行了细胞学观察。结果显示,单位面积(1 mm2)内CK和GA3处理的外果皮细胞数量分别为2086和2087 个,两者无显著差异;CK 和GA3处理的中果皮细胞数量分别为1651 和1670 个,同样无显著差异。统计果皮细胞层数发现,CK 果皮的细胞层数约为151.5,而GA3处理的果皮约为162.9,细胞层数显著增加7.5%。以上结果表明,GA3促进早蜜椪柑果皮增厚的细胞学原因是细胞层数的增加,而非细胞面积增大(图7)。

图7 CK 和100 mg·L-1 GA3处理后的果皮显微结构差异
Fig.7 Microstructural differences in the peel after treatments with CK and 100 mg·L-1 GA3
3 讨 论
裂果严重影响柑橘的产量和品质,导致其食用价值和商业价值显著下降。对农民而言,裂果意味着收入的减少和生产成本的上升,因此防控裂果对提高柑橘产业的整体效益至关重要。研究表明,赤霉素能够促进果皮增厚,从而有效减缓裂果的发生。赖剑锋等[21]通过赤霉素处理纽荷尔脐橙和阳光一号橘柚后,纽荷尔脐橙的裂果率由36.63%下降至4.52%,而阳光一号橘柚裂果率则从44.13%降至13.00%。代琳等[22]在明日见第二次生理落果期后喷施30 mg·L-1 GA3,发现其能够显著促进果皮增厚,从而降低裂果率。笔者在本研究中同样发现,分裂期喷施50、100、200 mg·L-1 GA3均能有效诱导早蜜椪柑果皮的增厚,进而显著抑制裂果的发生。尽管赤霉素单独使用可以预防裂果,但也有研究显示赤霉素和其他激素的协同使用[23],以及套袋、合理施肥、均衡供水等[24]措施同样对裂果表现较好的防控效果,这些措施或将可以进一步考虑作为裂果的防控手段。
虽然生产中外施GA3能有效防控柑橘裂果的发生,但同时也可能导致其果实发生“粗皮大果”的现象[25]。“粗皮大果”表现为果皮粗糙且增厚,果肉不化渣,可溶性固形物含量较低,从而显著降低果实的商品价值[26-27]。王智豪[28]发现50、100 mg·L-1 GA3的施用造成红美人杂柑成花少,且易形成粗皮大果,导致果实汁液、香味和化渣性程度均较低。Lu等[15]也发现,50 mg·L-1 GA3的应用同样引发了温州蜜柑的粗皮大果现象。在本研究中,早蜜椪柑分裂期应用外源GA3会在早期发育过程中引起果皮粗糙,但随着果实发育阶段的推进,果皮粗糙度逐渐下降,直至果实发育后期基本观察不到粗糙表型。因此,分裂期外施50、100、200 mg·L-1 GA3对早蜜椪柑果实外观品质的影响较小。
果皮细胞壁成分的差异也是造成裂果的重要因素[14,29]。温明霞等[30]研究发现,锦橙裂果果皮中的可溶性果胶含量高于正常果,而水溶性果胶含量呈现相反趋势。张雅馨[23]指出,甘平裂果的发生与纤维素含量无关,但与木质素含量呈显著正相关。笔者在本研究中发现,早蜜椪柑分裂期100 mg·L-1 GA3处理能够显著提高果皮中的木质素和半纤维素含量。这表明,GA3可能通过提高果皮中的木质素和半纤维素含量,从而赋予其更高的机械强度进而预防裂果[31]。
赤霉素通过促进细胞分裂和扩展来调节植物器官大小,如叶片扩张[32]、茎的伸长[33-34]、籽粒膨大[35]、根系生长[36]等。值得注意的是,果皮厚度的形成同样与细胞分裂和扩展具有密切关系[37-38]。邹河清等[19]发现,赤霉素喷施于红江橙后,果皮显著增厚,主要原因是促进了细胞的分裂。Renaudin 等[39]发现,200 mg·L-1 GA3处理番茄导致果皮显著增厚,并观测到中果皮细胞的扩大和拉长。Martí等[40]发现,干扰赤霉素响应的负调控基因SlDELLA 的表达,能够显著增加番茄果皮细胞长度。此外,细胞分裂素、生长素等其他激素也可通过促进细胞分裂或扩展来参与果皮的增厚过程[41-43]。笔者在本研究中发现,100 mg·L-1 GA3处理早蜜椪柑促进果皮增厚的关键时期是盛花后40 d。进一步分析发现,该时期外果皮和中果皮的细胞大小无显著差异,但细胞层数显著增加了7.5%,早蜜椪柑分裂期喷施GA3引起的果皮增厚源于细胞层数增加,而非细胞面积增大。
4 结 论
早蜜椪柑分裂期喷施外源GA3能有效促进果皮增厚,提高半纤维素和木质素含量,最终降低裂果率。分裂期100 mg·L-1 GA3处理对果皮增厚的效应在花后40 d 即显现,其细胞学原因主要是由于细胞层数增多造成的。适宜浓度的GA3通过改变果皮发育特征,有效预防裂果,但其分子机制仍需进一步研究。
[1] 齐乐,祁春节.世界柑橘产业现状及发展趋势[J].农业展望,2016,12(12):46-52.QI Le,QI Chunjie. Status quo and development trend of world’s citrus industry[J].Agricultural Outlook,2016,12(12):46-52.
[2] 彭际淼,杨水芝,龙桂友,杨雪华,石军,邓子牛,王岩,袁波.柑桔新品种早蜜椪柑的主要性状及栽培要点[J].中国南方果树,2012,41(4):56-58.PENG Jimiao,YANG Shuizhi,LONG Guiyou,YANG Xuehua,SHI Jun,DENG Ziniu,WANG Yan,YUAN Bo.Main characteristics and cultivation tips of the new citrus variety Zaomi Ponkan[J].South China Fruits,2012,41(4):56-58.
[3] 张文龙,万润楚,郑妮,陈焱,赖恒鑫,余歆,钱春,曹立.柑橘不育系新品种‘阳光1 号’[J].园艺学报,2024,51(9):2221-2222.ZHANG Wenlong,WAN Runchu,ZHENG Ni,CHEN Yan,LAI Hengxin,YU Xin,QIAN Chun,CAO Li.‘Yangguang 1’:A new monoembryonic sterile line of citrus[J]. Acta Horticulturae Sinica,2024,51(9):2221-2222.
[4] 王君秀,袁梦,李思静,袁高鹏,陈焱,彭良志,付行政,曹立,淳长品.不同柑桔品种的裂果程度及裂果成因研究[J].中国南方果树,2017,46(2):63-66.WANG Junxiu,YUAN Meng,LI Sijing,YUAN Gaopeng,CHEN Yan,PENG Liangzhi,FU Xingzheng,CAO Li,CHUN Changpin. Study on the degree and causes of fruit cracking in different citrus varieties[J]. South China Fruits,2017,46(2):63-66.
[5] 孙权,谢亮亮,于明华,马烈,陆奔宇.红美人、甘平柑橘在昆山市的引种表现及配套栽培技术[J].上海农业科技,2023(2):86-88.SUN Quan,XIE Liangliang,YU Minghua,MA Lie,LU Benyu.Performance of introducing‘Hongmeiren’and‘Ganping’citrus in Kunshan city and associated cultivation techniques[J].Shanghai Agricultural Science and Technology,2023(2):86-88.
[6] 周秋蓉.Ca+GA3处理对预防芦柑裂果的作用及其机理研究[D].福州:福建农林大学,2023.ZHOU Qiurong. The effect of Ca+GA3 treatment on preventing fruit cracking in Ponkan and its mechanism[D]. Fuzhou:Fujian Agriculture and Forestry University,2023.
[7] 马小焕.脐橙果皮内裂发生的解剖结构和矿质营养元素变化研究[D].重庆:西南大学,2011.MA Xiaohuan. Study on the changes of peel albedo microstructure and macroelement contents in peel of‘Navel’oranges during the development of peel pitting[D]. Chongqing:Southwest University,2011.
[8] 李会佳.番茄裂果性状的遗传分析及QTL 定位[D].哈尔滨:东北农业大学,2016.LI Huijia. Genetic analysis and QTL mapping in tomato cracking[D].Harbin:Northeast Agricultural University,2016.
[9] 仲钊江,吴震,周蓉,朱为民,杨学东,于筱薇,徐艳,高扬杨,蒋芳玲.番茄果胶裂解酶基因SlPL 参与调控裂果机制研究[J].园艺学报,2024,51(2):295-308.ZHONG Zhaojiang,WU Zhen,ZHOU Rong,ZHU Weimin,YANG Xuedong,YU Xiaowei,XU Yan,GAO Yangyang,JIANG Fangling. Study on the regulatory mechanism of SlPL gene affecting tomato fruit cracking[J]. Acta Horticulturae Sinica,2024,51(2):295-308.
[10] 吴建阳,何冰,陈妹,武爱龙,李伟才,魏永赞.果实裂果机理研究进展与展望[J].广东农业科学,2017,44(4):38-45.WU Jianyang,HE Bing,CHEN Mei,WU Ailong,LI Weicai,WEI Yongzan. Progress and prospects of mechanisms in fruit cracking[J]. Guangdong Agricultural Sciences,2017,44(4):38-45.
[11] 孙双双,杜晓云,王欢,王玉霞,张序,李延菊.甜樱桃裂果与果实主要性状的相关性研究[J].中国果树,2023(6):26-32.SUN Shuangshuang,DU Xiaoyun,WANG Huan,WANG Yuxia,ZHANG Xu,LI Yanju.Analysis of fruit characters and cracking ability of sweet cherry[J].China Fruits,2023(6):26-32.
[12] 廖南峤.西瓜果皮硬度及耐裂性基因挖掘与分子标记开发研究[D].杭州:浙江大学,2022.LIAO Nanqiao. Gene mining and molecular maker developing of rind hardness and cracking resistance in watermelon[D].Hangzhou:Zhejiang University,2022.
[13] 韦兰洁,陈依丽,李昌杰,黎俊辰,黄馨芸.火龙果裂果与果实主要性状的相关性分析[J].南方农业,2022,16(5):46-49.WEI Lanjie,CHEN Yili,LI Changjie,LI Junchen,HUANG Xinyun. Correlation analysis on the main character of pitaya fruits and its dehiscent fruit[J]. South China Agriculture,2022,16(5):46-49.
[14] LI J,CHEN J Z.Citrus fruit-cracking:Causes and occurrence[J].Horticultural Plant Journal,2017,3(6):255-260.
[15] LU X P,LI F F,XIONG J,CAO X J,MA X C,ZHANG Z M,CAO S Y,XIE S X. Transcriptome and metabolome analyses provide insights into the occurrence of peel roughing disorder on satsuma mandarin (Citrus unshiu Marc.) fruit[J]. Frontiers in Plant Science,2017,8:1907.
[16] CASTRO-CAMBA R,SÁNCHEZ C,ⅤIDAL N,ⅤIELBA J M.Plant development and crop yield:The role of gibberellins[J].Plants,2022,11(19):2650.
[17] JONA R,GOREN R,MARMORA M. Effect of gibberellin on cell-wall components of creasing peel in mature‘Ⅴalencia’orange[J].Scientia Horticulturae,1989,39(2):105-115.
[18] 李永杰,金国强,淳长品,朱潇婷,邱晓莹.柑橘果皮的发育特征及GA3的防裂效果[J].果树学报,2021,38(7):1092-1101.LI Yongjie,JIN Guoqiang,CHUN Changpin,ZHU Xiaoting,QIU Xiaoying. Developmental characteristics of citrus peel and the effect of gibberellic acid on fruit cracking[J].Journal of Fruit Science,2021,38(7):1092-1101.
[19] 邹河清,许建楷.红江橙的果皮结构与裂果的关系研究[J].华南农业大学学报,1995,16(1):90-96.ZOU Heqing,XU Jiankai. Study on the relationship between peel structure and fruit cracking in‘Hongjiang’oranges[J].Journal of South China Agricultural University,1995,16(1):90-96.
[20] 许园园,谭世水,张玲,段少伟,郭玲霞,周铁,李菲菲,韩健,李先信,王聪田,陈鹏.锦红冰糖橙大果芽变果实结构解剖、激素变化和转录组分析[J].果树学报,2024,41(4):611-624.XU Yuanyuan,TAN Shishui,ZHANG Ling,DUAN Shaowei,GUO Lingxia,ZHOU Tie,LI Feifei,HAN Jian,LI Xianxin,WANG Congtian,CHEN Peng. Anatomical structure,hormone change,and transcriptome analysis of the large-fruit mutant of Jinhong Bingtang Orange[J]. Journal of Fruit Science,2024,41(4):611-624.
[21] 赖剑锋,谌鹏飞,刘志文,梅磊,邹少丰,李昌,丁少华.3%赤霉酸+mgm 防治柑橘裂果的研究初报[J].江西农业学报,2023,35(5):30-34.LAI Jianfeng,CHEN Pengfei,LIU Zhiwen,MEI Lei,ZOU Shaofeng,LI Chang,DING Shaohua. Preliminary study on control of citrus fruit cracking by 3% gibberellic acid and mgm[J].Acta Agriculturae Jiangxi,2023,35(5):30-34.
[22] 代琳,张伦德,周志扬,陈泓臻,黄康,马晴晴,孙孝贤,熊博.外源赤霉素、氨基酸钙处理对‘明日见’柑橘裂果的影响[J].中国农学通报,2024,40(7):49-55.DAI Lin,ZHANG Lunde,ZHOU Zhiyang,CHEN Hongzhen,HUANG Kang,MA Qingqing,SUN Xiaoxian,XIONG Bo. Effects of exogenous gibberellin and calcium amino acid on fruit cracking of‘Asumi’citrus[J]. Chinese Agricultural Science Bulletin,2024,40(7):49-55.
[23] 张雅馨.外源激素与钙处理对‘甘平’杂柑裂果防控研究[D].武汉:华中农业大学,2023.ZHANG Yaxin. Study on prevention and control of‘Kanpei’citrus fruit cracking by exogenous hormone and calcium treatment[D].Wuhan:Huazhong Agricultural University,2023.
[24] 李娟,陈杰忠.柑橘裂果发生类型、过程及预防对策[J].广东农业科学,2011,38(10):32-33.LI Juan,CHEN Jiezhong. The style,process and control of cracking fruit in citrus[J]. Guangdong Agricultural Sciences,2011,38(10):32-33.
[25] KUBO T,HIRATSUKA S.Relationship between rind roughness and gibberellins in Satsuma mandarin fruit[J]. Journal of the Japanese Society for Horticultural Science,2000,69(6):718-723.
[26] 刘芳,刘永忠,彭抒昂.温州蜜柑粗皮大果形成过程的解剖学研究[J].果树学报,2012,29(5):789-793.LIU Fang,LIU Yongzhong,PENG Shu’ang. Anatomically study on the forming process of rough fruit of Satsuma[J]. Journal of Fruit Science,2012,29(5):789-793.
[27] 余歆,张晓楠,赵晓春.柑橘果皮的生物活性物质和重要园艺性状[J].园艺学报,2021,48(4):825-836.YU Xin,ZHANG Xiaonan,ZHAO Xiaochun. Bioactive compounds in citrus peel and peel-related important horticultural traits[J].Acta Horticulturae Sinica,2021,48(4):825-836.
[28] 王智豪.浙北地区‘红美人’杂柑不同结果母枝成花及果实品质研究[D].杭州:浙江大学,2023.WANG Zhihao. Study on flower bud formation and fruit quality of different female branches of‘Hongmeiren’citrus hybrid cultivated in northern Zhejiang province[D]. Hangzhou:Zhejiang University,2023.
[29] BROWNLEADER M D,JACKSON P,MOBASHERI A,PANTELIDES A T,SUMAR S,TREⅤAN M,DEY P M. Molecular aspects of cell wall modifications during fruit ripening[J]. Critical Reviews in Food Science and Nutrition,1999,39(2):149-164.
[30] 温明霞,石孝均.锦橙裂果的钙素营养生理及施钙效果研究[J].中国农业科学,2012,45(6):1127-1134.WEN Mingxia,SHI Xiaojun.Influence of calcium on fruit cracking of Jincheng orange and its physiological mechanism[J]. Scientia Agricultura Sinica,2012,45(6):1127-1134.
[31] 李茜玟,孙浩爽,庞学群,张昭其.荔枝裂果研究综述与展望[J].广东农业科学,2024,51(11):136-148.LI Xiwen,SUN Haoshuang,PANG Xuequn,ZHANG Zhaoqi.Research review and prospect of litchi fruit cracking[J]. Guangdong Agricultural Sciences,2024,51(11):136-148.
[32] JATHAR Ⅴ,SAINI K,CHAUHAN A,RANI R,ICHIHASHI Y,RANJAN A. Spatial control of cell division by GA-OsGRF7/8 module in a leaf explaining the leaf length variation between cultivated and wild rice[J].New Phytologist,2022,234(3):867-883.
[33] GAO X H,XIAO S L,YAO Q F,WANG Y J,FU X D.An updated GA signaling‘relief of repression’regulatory model[J].Molecular Plant,2011,4(4):601-606.
[34] 张玉博.赤霉素处理对海岛棉始果枝发育的影响及相关基因挖掘[D].阿拉尔:塔里木大学,2024.ZHANG Yubo. Effects of gibberellin treatment on the development of cotton branches and related gene mining[D]. Ala’er:Tarim University,2024.
[35] LÜ H Y,LI X,LI H,HU Y F,LIU H M,WEN S J,LI Y P,LIU Y H,HUANG H H,YU G W,HUANG Y B,ZHANG J J. Gibberellin induced transcription factor bZIP53 regulates CesA1 expression in maize kernels[J].PLoS One,2021,16(3):e0244591.
[36] ACHARD P,GUSTI A,CHEMINANT S,ALIOUA M,DHONDT S,COPPENS F,BEEMSTER G T S,GENSCHIK P.Gibberellin signaling controls cell proliferation rate in Arabidopsis[J].Current Biology,2009,19(14):1188-1193.
[37] ZHU Z G,LIANG H L,CHEN G P,LI F F,WANG Y S,LIAO C G,HU Z L. The bHLH transcription factor SlPRE2 regulates tomato fruit development and modulates plant response to gibberellin[J].Plant Cell Reports,2019,38(9):1053-1064.
[38] ZHANG S B,XU M,QIU Z K,WANG K T,DU Y C,GU L F,CUI X. Spatiotemporal transcriptome provides insights into early fruit development of tomato (Solanum lycopersicum)[J]. Scientific Reports,2016,6:23173.
[39] RENAUDIN J P,CHENICLET C,ROUYÈRE Ⅴ,CHEⅤALIER C,FRANGNE N. The cell pattern of tomato fruit pericarp is quantitatively and differentially regulated by the level of gibberellin in four cultivars[J]. Journal of Plant Growth Regulation,2023,42(9):5945-5958.
[40] MARTÍ C,ORZÁEZ D,ELLUL P,MORENO Ⅴ,CARBONELL J,GRANELL A.Silencing of DELLA induces facultative parthenocarpy in tomato fruits[J]. The Plant Journal,2007,52(5):865-876.
[41] GAN L J,SONG M Y,WANG X C,YANG N,LI H,LIU X X,LI Y. Cytokinins is involved in regulation of tomato pericarp thickness and fruit size[J]. Horticulture Research,2022,9:uhab041.
[42] LIU X,XU T,DONG X F,LIU Y D,LIU Z H,SHI Z H,WANG Y L,QI M F,LI T L.The role of gibberellins and auxin on the tomato cell layers in pericarp via the expression of ARFs regulated by miRNAs in fruit set[J]. Acta Physiologiae Plantarum,2016,38(3):77.
[43] SERRANI J C,FOS M,ATARÉS A,GARCÍA-MARTÍNEZ J L.Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv. Micro-Tom of tomato[J]. Journal of Plant Growth Regulation,2007,26(3):211-221.