桃富含维生素C、抗氧化剂以及膳食纤维等营养元素,是一种兼具营养价值和经济价值的水果[1],深受国人喜爱。鲜食桃消费量的上升促进了其种植面积的持续扩大[2]。然而,由于不合理的农艺措施,如过量施用化肥、农药以及不合理的控产措施等,导致桃树寿命普遍较短,一般10 a(年)左右便需要更新种植。在土地资源紧缺的背景下,农民往往在老桃园中种植新桃苗。然而,新种的树往往生长迟缓、植株矮小、抗性降低甚至死亡,称之为桃树再植障碍[3]。
目前,针对桃树再植障碍已有的调控措施主要包括深翻客土、土壤灭菌以及选用抗性砧木等。深翻客土常被看作是一种克服果树再植障碍的有效措施[4],但此法费时费工,在生产上不易大面积应用。土壤灭菌主要采用化学药剂或物理方法(蒸汽、太阳能等),然而针对土传病害的化学药剂存在使用量偏大、防治效果不佳、药剂高残留等问题[5],且化学药剂也会杀灭土壤中有益微生物,破坏土壤微生态环境[6]。选用抗性砧木可能缓解再植桃园中桃树生长过程中的某些问题,如提高桃树对线虫的抗性等[7],然而,桃树再植障碍是多种原因引起的,而绝大多数砧木的抗病性只针对单一问题起作用,加上对引起桃树再植障碍的原因还没有完全了解,因此很难筛选出符合要求的抗性砧木。鉴于上述各种方式的缺陷,目前缓解桃树再植障碍的合理措施依然短缺。
土壤深翻是农业生产中重要的耕作方式,可以促进土壤养分循环、调节养分利用效果、提高作物产量和品质[8-9]。Hu 等[10]研究发现深翻可以降低深层土壤的紧实度、提高土壤的pH、促进土壤微生物的均一性等。老桃园经过深翻后若再种植一年水稻,土壤性质可能因为淹水产生还原条件等因素而发生改变。水稻种植生产过程中,淹水为土壤提供了厌氧环境,能够改善因长期作物种植导致的土壤劣化。Wang等[5]研究表明,利用淹水能够提高长期连作土壤的pH,降低土壤电导率(electrical conductivity,EC)值,对土壤酸化和次生盐渍化具有明显的改良作用。另外,有研究表明,利用淹水创造厌氧环境可以改善土壤结构[11-12]。然而,土壤经过深翻后,底层土壤因未经过耕作,养分贫瘠,其中土壤中磷的有效性是影响作物生长的限制性因素[13]。尽管为促进桃树生长而施用大量磷肥,但是仅仅只有13%的磷被当季作物吸收[14]。Peng 等[15]分析指出,被人们忽略的自然溶磷作用对土壤有效磷的影响巨大。在自然界中影响磷素有效性的因素包括磷素浸出量低,磷对土壤矿物的亲和力强,以及解磷微生物组成和丰度等[16]。土壤中解磷微生物能够分泌磷酸酶,促进土壤磷素的释放。研究表明,phoD是编码磷酸酶的重要基因,是指示土壤中解磷微生物组成和丰度的主要参考基因[17-19]。土壤深翻结合种植水稻会改变土壤性质、微生物群落组成,然而,如何改变土壤解磷微生物以及对黄桃品质的影响还有待于进一步解析。
笔者利用深翻结合种植水稻的方式改良种植10 a 以上的黄桃园土壤,研究不同改良方式对黄桃再植土壤理化性质与土壤中磷代谢相关的微生物组成的影响,并进一步分析解磷微生物多样性、丰度和组成与黄桃产量和品质的关系,以期为后期改良黄桃再植土壤提供参考,同时为黄桃产业的可持续发展提供技术支撑。
1 材料和方法
1.1 试验地概况
试验地位于上海市奉贤区青村镇吴房村黄桃种植区内(30°58′N,121°20′E),海拔7 m,年降水量和年平均温度分别为1200 mm 和16 ℃。吴房村种植黄桃的历史悠久。所选试验地于2006 年开始种植黄桃,2016年出现老化症状:桃树生长势减弱,流胶严重,产量降低等。
1.2 试验设计
于2017年选取地势平坦的老桃园(其初始理化性质为pH 6.78;EC 值496 μS·cm-1;碱解氮含量(w,后同)110.5 mg·kg-1;速效钾含量783.5 mg·kg-1;有效磷含量116.9 mg·kg-1;有机质含量2.48%),选取20棵老桃树挖掉,不进行再植改良,闲置1 a 作为对照(CK)。选取40棵桃树挖掉,将土壤进行深度为50 cm的深翻,并进行土地平整,其中20 棵桃树的面积经过深翻后闲置1 a(DT),另外20棵桃树种植1 a水稻进行淹水处理(DT+RP)。于2018 年种植新桃苗。农业管理措施主要依据当地果农的管理习惯。桃树品种为锦绣黄桃,平均每666.7 m2种植40 棵。在生长第四年,为控制变量,对每棵桃树进行疏果(每棵桃树留120个果实)。
1.3 样品采集
黄桃样品于2022 年10 月采集。分别于3 种不同处理中采集土壤。在黄桃树滴水线左右50 cm处,采集深度为40 cm,采集同一处理3 棵桃树土壤混合作为1个土样,因此每个处理采集6个重复。土壤采集后置于冰上运回实验室,一部分保存于-80 ℃冰箱中,用于分子生物化学分析;一部分于室温下自然风干,用于测定土壤理化性质。
1.4 土壤理化性质和果实品质测定
土壤pH 以土水质量比1∶2.5 混合后测定[20];电导率(EC)以土水质量比1∶5 混合后测定;土壤有机质含量采用重铬酸钾容量法测定[21];土壤碱解氮含量利用碱解扩散法进行测定[22];土壤有效磷含量采用0.5 mol·L-1 NaHCO3浸提-钼锑抗比色法测定[23];土壤速效钾含量采用乙酸铵浸提-火焰光度法测定[24]。平均单果质量:果实成熟后,在每株桃树上随机摘取黄桃果实5个,同一处理的3株桃树共摘取15 个果实,称质量并计算每个重复的平均单果质量;黄桃666.7 m2产量采用单果质量×留果数×每666.7 m2株数进行换算;可溶性固形物含量采用手持糖量计测定;可滴定酸含量采用滴定法测定。
1.5 土壤总DNA提取与高通量测序
土壤总DNA 提取采用Power Max Soil DNA Isolation Kit 试剂盒(MOBIO,USA),每个样品称取0.25 g 土壤,按照试剂盒说明步骤进行。提取的土壤总DNA 分别经过1%琼脂糖凝胶电泳和Nano Drop测定DNA完整性、纯度和浓度。土壤解磷微生物的扩增引物为phoD-F733(TGG GAY GAT CAY GAR GT)/phoD-R1083(CTG SGC SAK SAC RTT CCA)[13]。扩增引物连接接头A、B 和样品识别序列。扩增体系为5 μL 10×Pyrobest缓冲液,4 μL dNTPs(2.5 mmol·L-1),上下游引物各2 μL(10 μmol·L-1),0.75 U Pyrobest DNA聚合酶和30 ng模板DNA。使用ABI GeneAmp® 9700 型PCR 仪进行扩增,扩增程序为:95 ℃预变性5 min,27 个循环包括95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸30 s,最后72 ℃延伸10 min[25-26]。全部PCR产物经2%琼脂糖凝胶电泳检测,并利用AxyPrepDNA 凝胶回收试剂盒(AXYGEN 公司)切胶回收。制备Amplicon 文库后,应用Illumina MiSeq PE250平台测序。
1.6 荧光定量PCR测定解磷微生物绝对丰度
解磷微生物绝对丰度采用SYBR Green法测定,反应在罗氏LightCycler 480 ⅡPCR(Roche Diagnostics,Indianapolis,IN,USA)仪器上进行。反应体系为FastFire qPCR PreMix 10 μL,10 nmol·L-1上下游引物,ROX Reference Dye 0.4 μL,1 μL DNA,补加ddH2O至终体积为20 μL。以含有phoD基因的重组pGEMR-T载体为标准质粒,质粒制备和后续方法参照文献[27]。
1.7 数据处理和分析
通过Illumina Miseq平台测序所得的原始数据经过QIIME(v1.8.0)软件进行质量控制。根据文献[28]所示具体过程为:1)过滤reads 尾部质量值20 以下的碱基,设置50 bp 的窗口,如果窗口内的平均质量值低于20,从窗口开始截去后端碱基,过滤质控后50 bp 以下的reads,去除含N 碱基的reads;2)根据PE reads 之间的overlap 关系,将成对reads 拼接(merge)成一条序列,最小overlap 长度为10 bp,最大错配率不超过20%;3)根据序列首尾两端的barcode 和引物区分样品,并调整序列方向,barcode 允许的错配数为0,最大引物错配数为2;去除引物序列,并根据GenBank 数据库检测并去除嵌合体,获得高质量序列。利用CROP 软件将核苷酸相似度大于97%的序列作为一个分类操作单元(ASⅤ)[29],利用GenBank 数据库对物种进行注释,并去除所有处理中只有一条序列的ASⅤ,将所有样品序列进行抽平。利用Mothur 软件(Ⅴ1.31.2)计算样品α 多样性。
利用Excel 2020、SPSS 24 和R 语言等进行数据分析。利用单因素方差分析(ANOⅤA,Tukey’s test)分析不同改良方式解磷微生物α 多样性、丰度、不同分类水平下解磷微生物相对丰度以及功能变化的差异。基于weighted Fast UniFrac 距离矩阵,运用非度量多维标度(non-metric multidimensional sealing,NMDS)对不同改良方式解磷细菌β多样性进行分析。利用偏最小二乘路径对土壤理化性质,解磷微生物丰度、组成和多样性以及黄桃产量、品质等指标的相关性进行分析。该分析利用R 语言中“plspm”程序包,模型的拟合度利用GOF(goodness-of-fit)进行评估,当GOF>0.7 可认为拟合度较好[30]。
2 结果与分析
2.1 不同改良方式对再植黄桃土壤理化性质的影响
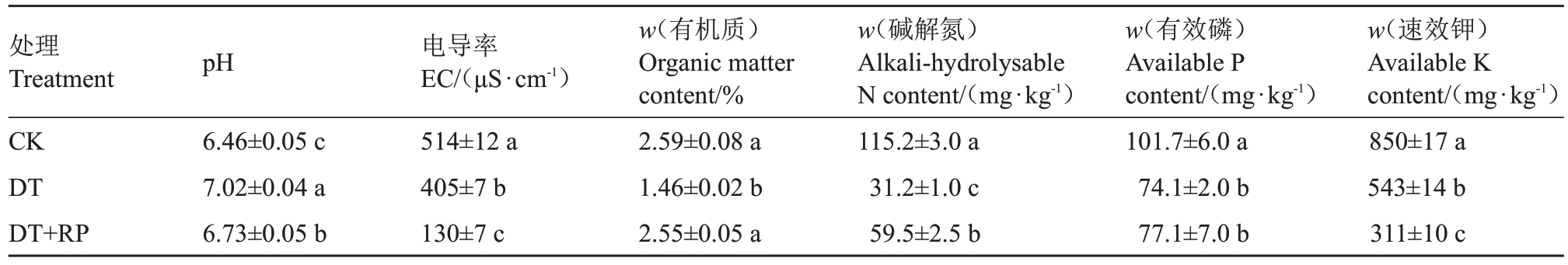
不同改良方式改变了再植黄桃土壤的理化性质。如表1所示,再植桃园深翻(DT)和深翻结合种植水稻(DT+RP)两种改良方式均显著提高了土壤pH,DT处理使土壤pH提高了8.7%,而DT+PR处理使土壤pH提高了4.2%。两种改良方式均显著降低了土壤的EC 值,DT 和DT+PR 处理分别降低了21.2%和74.7%。对土壤养分而言,两种改良方式均降低了土壤的有机质和有效养分含量。
表1 不同改良方式对土壤理化性质的影响
Table 1 The influence of different treatments on soil physical and chemical properties
注:数据后不同小写字母表示差异显著(p<0.05)。下同。
Note:Ⅴalues followed by different small letters indicate significant difference(p<0.05).The same below.
处理Treatment CK DT DT+RP w(速效钾)Available K content/(mg·kg-1)850±17 a 543±14 b 311±10 c pH 电导率EC/(μS·cm-1)6.46±0.05 c 7.02±0.04 a 6.73±0.05 b 514±12 a 405±7 b 130±7 c w(有机质)Organic matter content/%2.59±0.08 a 1.46±0.02 b 2.55±0.05 a w(碱解氮)Alkali-hydrolysable N content/(mg·kg-1)115.2±3.0 a 31.2±1.0 c 59.5±2.5 b w(有效磷)Available P content/(mg·kg-1)101.7±6.0 a 74.1±2.0 b 77.1±7.0 b
2.2 不同改良方式对解磷微生物丰度的影响
不同改良方式改变了再植桃园土壤解磷微生物的丰度(图1)。DT 处理对土壤中解磷微生物丰度没有显著影响,而DT+PR处理显著降低了土壤中解磷微生物丰度,比CK 处理降低了51.8%(图1-A)。相关性分析表明,土壤中解磷微生物丰度与土壤的EC值和有效磷含量呈显著正相关,而与土壤中有机质含量呈显著负相关(图1-B)。
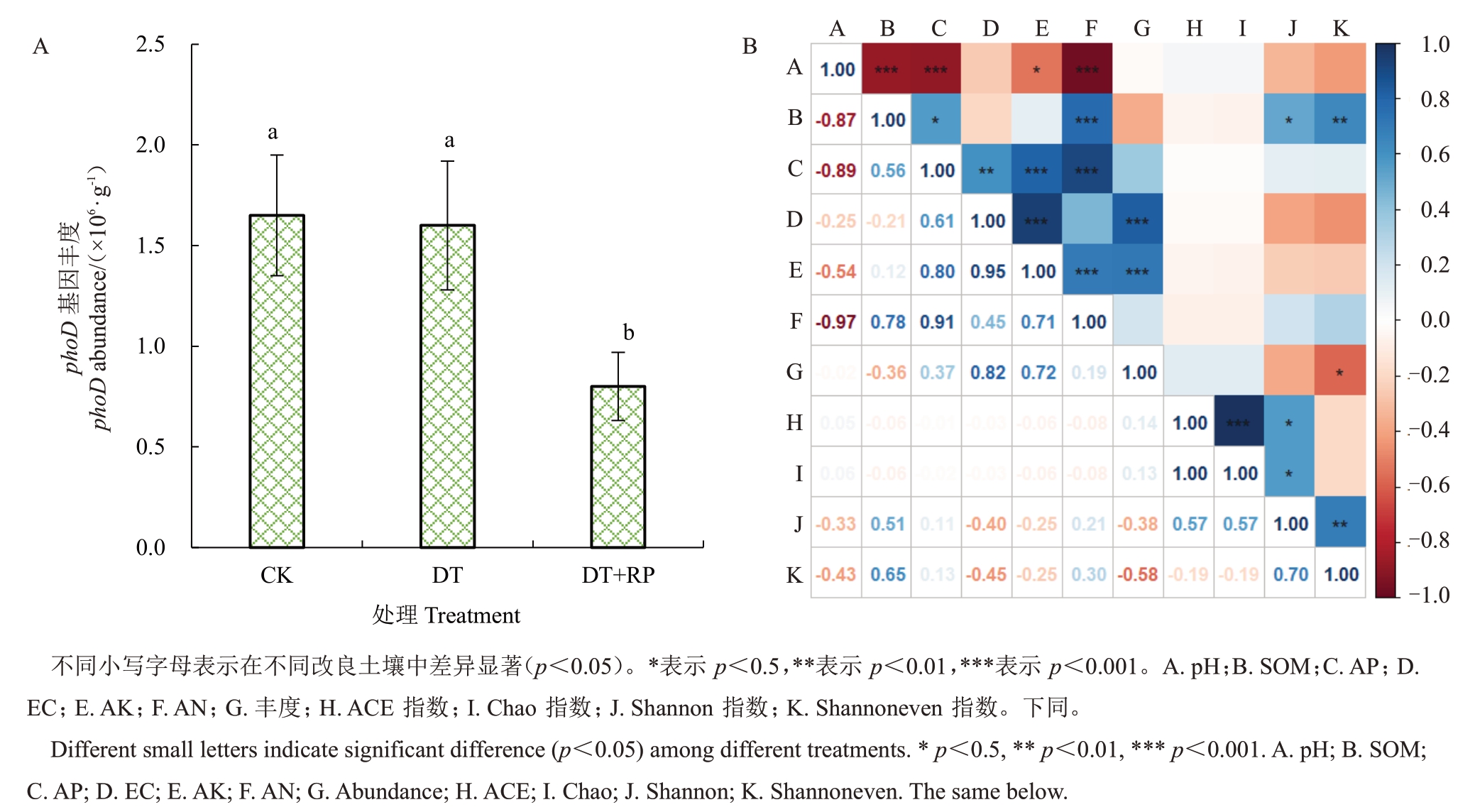
图1 不同改良措施对土壤解磷微生物丰度(A)的影响及解磷微生物丰度与土壤理化性质的关系(B)
Fig.1 The influence of different treatments on soil phosphorus microbial abundance(A)and correlation of soil properties and phosphorus microbial abundance(B)
2.3 不同改良方式对再植桃园土壤解磷微生物多样性的影响
不同改良方式对再植桃园土壤微生物多样性的影响见表2。由表2可知,不同改良方式对土壤中解磷微生物丰富度指数(ACE 和Chao)和Sobs 的影响不显著(p>0.05),但显著改变了解磷微生物的均匀性指数和多样性指数。与CK 相比,不同改良方式对Shannoneven 均匀度指数和Shannon 多样性指数影响不显著,而与DT 相比,DT+RP 显著提高了Shannoneven 均匀度指数和Shannon 多样性指数。与CK 相比,DT 处理使土壤Shannoneven 指数降低了1.96%,而DT+PR 处理使Shannoneven 指数提高了1.44%;同样,DT处理使土壤Shannon指数降低了1.68%,而DT+PR处理使Shannon指数提高了1.68%。
表2 不同改良方式对黄桃土壤解磷微生物多样性的影响
Table 2 The influence of different treatments on phosphorus-dissolving microbial diversity
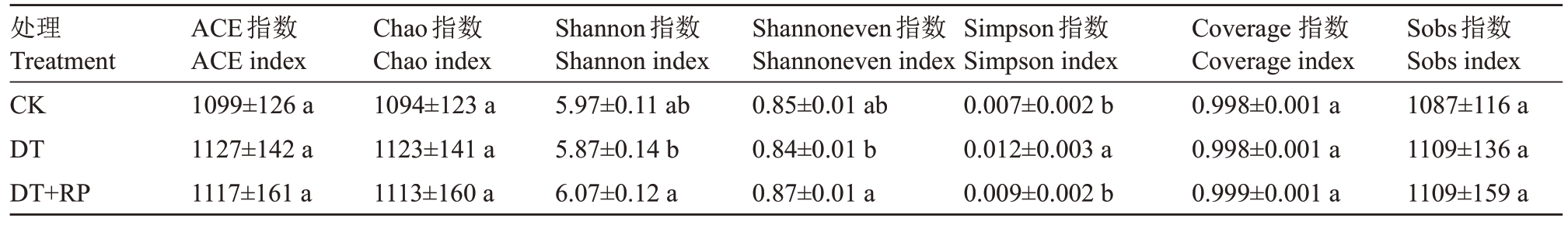
处理Treatment CK DT DT+RP ACE指数ACE index 1099±126 a 1127±142 a 1117±161 a Chao指数Chao index 1094±123 a 1123±141 a 1113±160 a Shannon指数Shannon index 5.97±0.11 ab 5.87±0.14 b 6.07±0.12 a Shannoneven指数Shannoneven index 0.85±0.01 ab 0.84±0.01 b 0.87±0.01 a Simpson指数Simpson index 0.007±0.002 b 0.012±0.003 a 0.009±0.002 b Coverage 指数Coverage index 0.998±0.001 a 0.998±0.001 a 0.999±0.001 a Sobs指数Sobs index 1087±116 a 1109±136 a 1109±159 a
2.4 不同改良方式对再植黄桃土壤解磷微生物群落组成的影响
不同改良方式对解磷微生物β 多样性的影响见图2,其中第一排序轴解释了解磷微生物48.27%群落组成变异,而第二排序轴解释了27.33%群落组成变异。不同改良方式改变了黄桃土壤解磷微生物群落组成,其中DT+RP处理解磷微生物主要在第一排序轴上显著差异,而DT处理解磷微生物组成与CK在第二排序轴上显著差异,说明DT+PR处理对解磷微生物群落组成的影响大于DT处理。
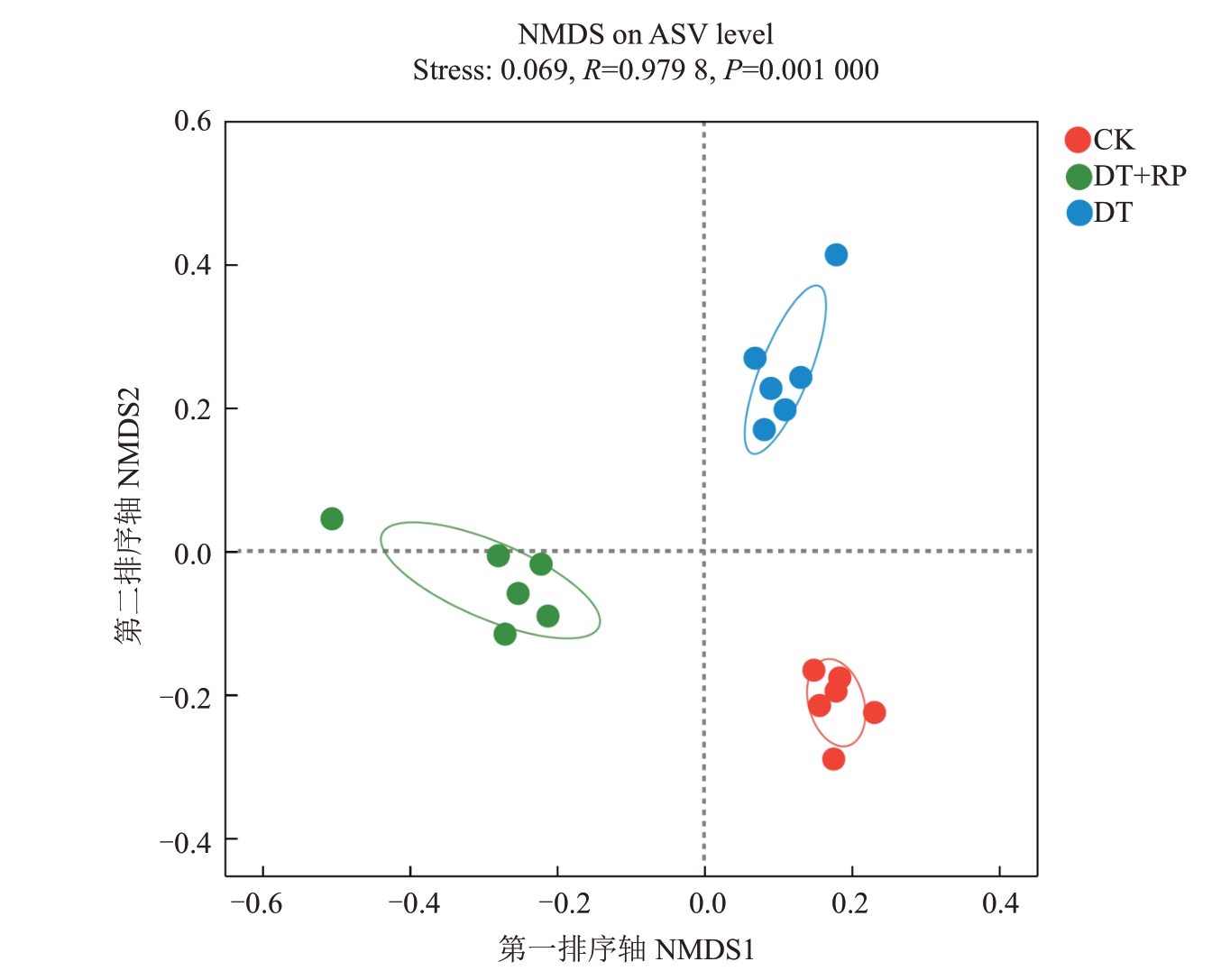
图2 不同改良措施对土壤解磷微生物β 多样性的影响
Fig.2 The influence of different treatments on soil phosphorus-dissolving microbial β-diversity
不同改良方式改变了再植桃园土壤中解磷微生物的群落组成。在属水平上,与CK相比,DT+PR改良方式提高了Bradyrhizobium、Cupriavidus、Rhizobacter、Sulfuricaulis 和Methylibium 的相对丰度,而降低了Marichromatium、Mycobacterium、Mesorhizobium、Nitrobacter、Polaromonas 和Thioalkalivibrio 的相对丰度。与CK 相比,DT 改良方式提高了Nocardioides、Cupriavidus、Mycobacterium、Rhizobacter、Starkeya、Methylibium、Actinomad、Polaromonas 和Sulfuricystis的相对丰度,降低了Marichromatium、Mesorhizobium、Nitrobacter、Thioalkalivibrio和Burkholderia的相对丰度(图3)。
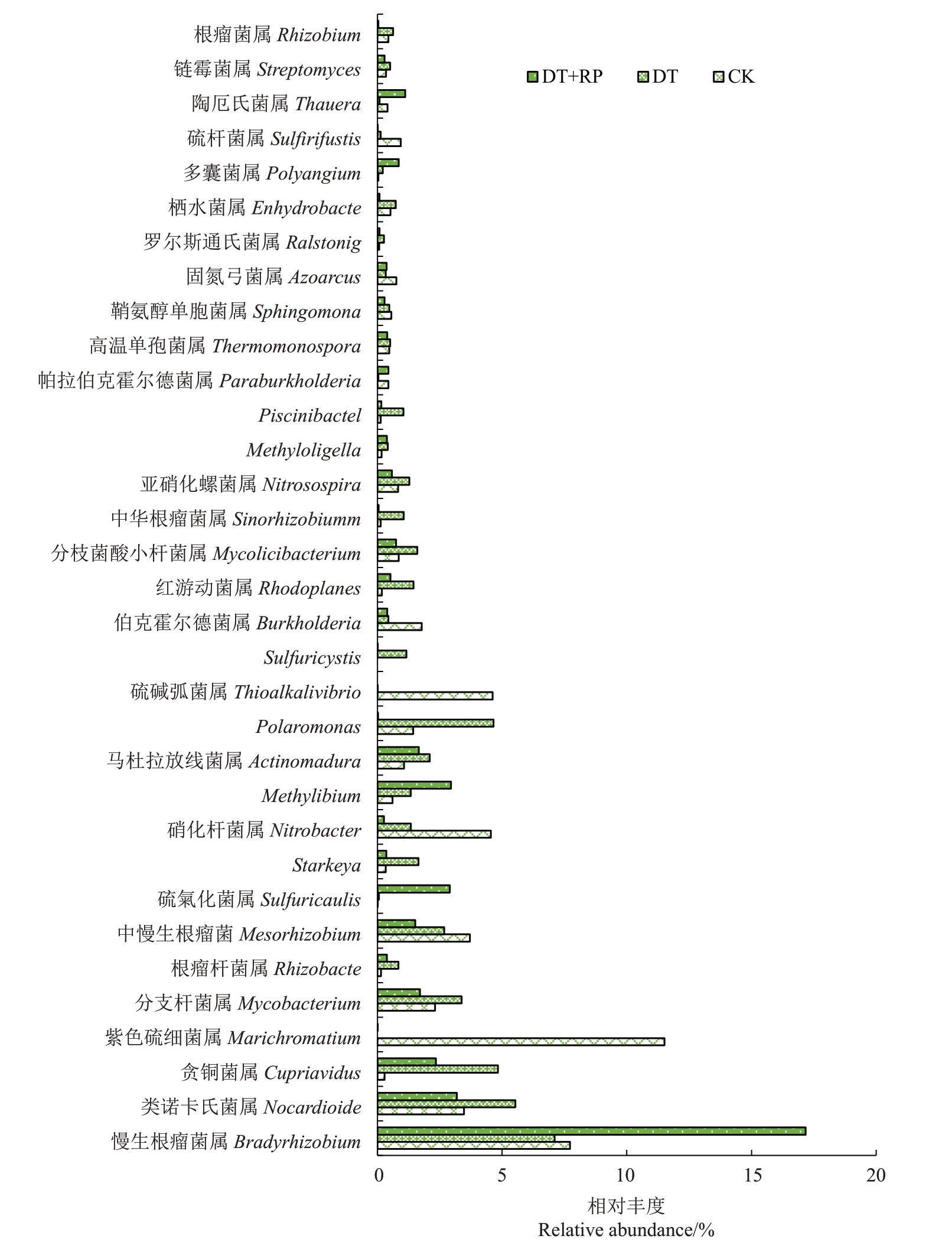
图3 不同改良措施对土壤解磷微生物属组成的影响(属水平)
Fig.3 The influence of different treatments on soil phosphorus-dissolving microbial composition(genus level)
2.5 不同改良方式对黄桃产量和品质的影响
不同改良方式改变了黄桃果实产量和果实品质(图4)。不同改良方式提高了黄桃产量,DT和DT+PR处理使黄桃产量比CK分别提高了7.1%和13.8%(图4-A)。对果实可溶性固形物含量而言,DT处理影响最大,比老桃园果实显著提高18.8%,而DT+PR处理仅显著提高了5.4%(图4-B)。不同改良方式都显著降低了黄桃果实的可滴定酸含量,DT 和DT+PR处理果实可滴定酸含量比CK分别降低了21.5%和27.9%(图4-C)。
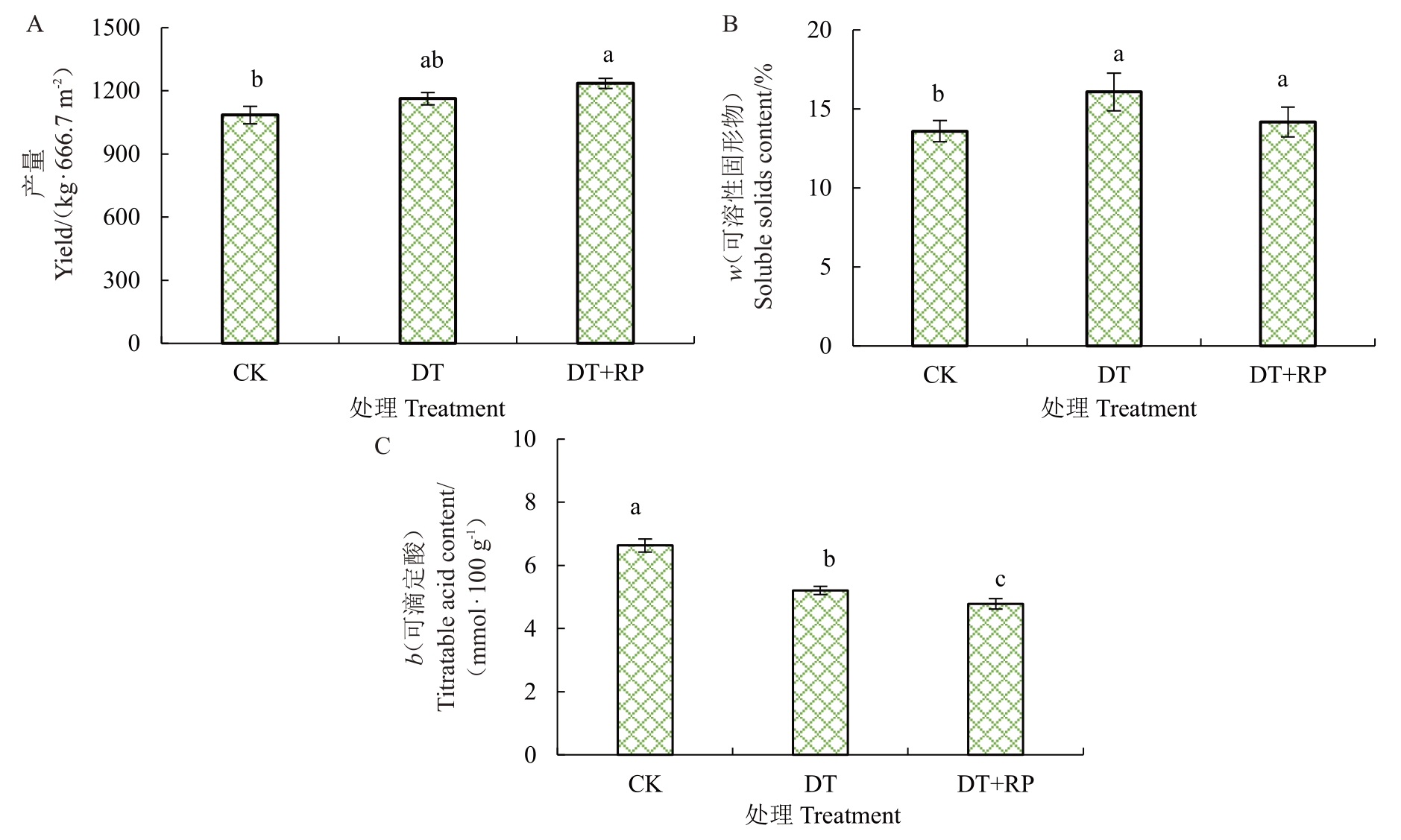
图4 不同改良措施对黄桃产量(A)、可溶性固形物(B)和可滴定酸含量(C)的影响
Fig.4 The influence of different treatments on yellow peach yield(A),soluble solids content(B)and titratable acid content(C)
2.6 土壤理化性质、解磷微生物结构以及黄桃产量和品质的相关性分析
通过偏最小二乘路径分析影响黄桃产量和品质的因素发现(图5),不同改良方式通过改变土壤理化性质直接改变黄桃的产量和品质(R=-0.855,p<0.05),也可以间接通过影响土壤中解磷微生物的丰度和组成改变黄桃产量和品质。土壤理化性质可以直接改变土壤中解磷微生物丰度(R=1.30,p<0.05)和群落组成(R =-0.890,p<0.05),土壤解磷微生物丰度(R =-0.614,p<0.05)和群落组成(R=0.953,p<0.05)又分别显著影响黄桃的产量和可溶性固形物含量。
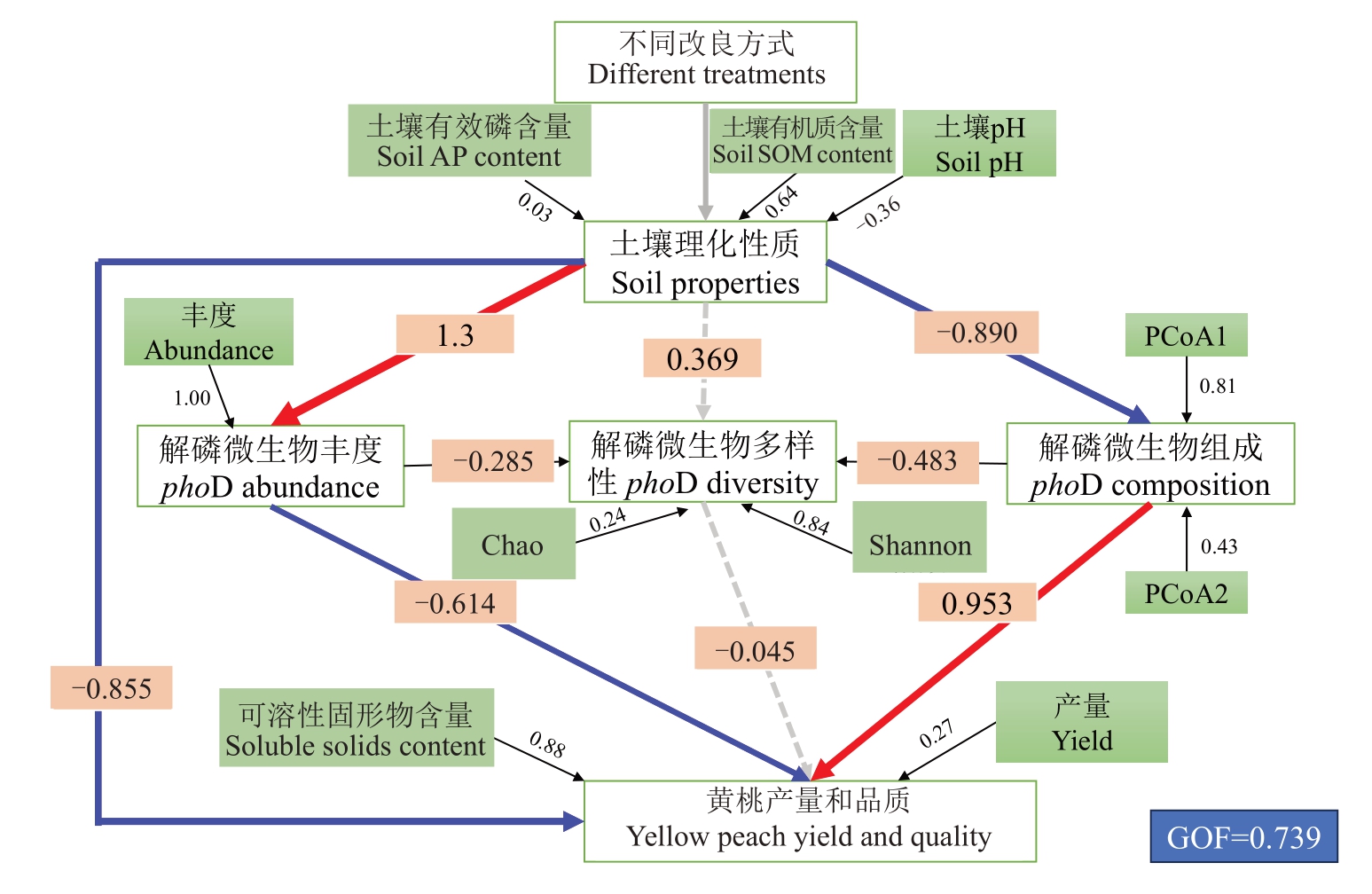
图5 PLS-PM 分析不同改良措施下影响黄桃产量和品质的因素
Fig.5 PLS-PM describing the biotic and abiotic factors that affect yellow peach yield and quality
3 讨 论
本研究结果表明,不同改良方式显著提高了再植桃园土壤pH,其中深翻处理使土壤pH 提高了8.7%,而深翻结合种植1 a水稻处理仅使土壤pH 提高了4.2%。前人研究表明,土壤深翻有助于土壤pH 上升[31]。在本研究中,新种桃苗前经过50 cm 的深翻,使原先因施肥等原因酸化的土壤得到改良。而DT+PR处理比DT处理土壤pH低,可能是因为淹水处理促进了枯枝、落叶的腐解,加速了有机酸等物质的释放,进而降低了土壤的pH[32]。对于土壤EC值而言,不同改良措施显著降低了土壤EC值,其中DT 处理降低到405 μS·cm-1,而DT+PR 处理降低到130 μS·cm-1。这可能是因为深翻使土壤盐渍化土壤进入下层,从而使上层土壤的EC值降低;而DT+PR处理可能使盐分溶解到水中,使土壤的EC 值进一步降低。根据土壤次生盐渍化程度标准[33],深翻处理使黄桃园土壤次生盐渍化程度降低,但还处于中盐度水平;而深翻结合种植水稻处理使土壤次生盐渍化水平显著降低,达到低盐度水平。本研究表明,通过深翻和种植水稻等措施能够降低老桃园土壤的酸化和盐渍化程度,有利于桃园的更新换代。然而,不同桃园改良措施,包括深翻和种植水稻,均降低了土壤中养分含量,包括土壤中有效磷含量,这可能是因为深翻导致深层养分含量低的土壤上移,使耕层土壤养分降低。因此,深翻改良老桃园应配合有机物料等的施用,以促进有机质等养分的积累。
在本研究中,笔者发现不同改良措施没有显著改变再植桃园解磷微生物的丰度指数(Chao和ACE指数)(p>0.05),但显著改变了解磷微生物的多样性指数(Shannon和Simpson指数),其中深翻结合种植水稻比单独深翻显著提高了土壤中解磷微生物多样性,该研究结果说明不同改良方式通过改变解磷微生物的均匀度而改变其多样性。笔者的研究也表明,深翻结合种植水稻比单独深翻显著改变了土壤解磷微生物的Shannoneven 均匀度指数,说明不同改良方式通过改变不同种类解磷微生物的相对丰度,从而使解磷微生物的多样性发生改变。
不同改良方式改变了解磷微生物的群落组成,其中深翻结合种植水稻提高了Bradyrhizobium,Cupriavidus 的相对丰度。Bradyrhizobium 除了与大豆共生为植物提供氮素外,还具有显著的溶磷作用[34]。在本研究中,淹水改良再植桃园土壤显著提高了慢生根瘤菌属丰度,说明淹水有助于土壤中根瘤菌属的生长,促进土壤中磷元素的活化,提高植物可利用养分含量。最近研究同样表明,根瘤菌属的微生物含有与磷代谢相关的基因,并在土壤有机磷和磷活化方面发挥重要作用[35]。在本研究中,DT+PR处理使Cupriavidus的相对丰度显著提高,可能促进了土壤中磷的代谢。该研究结果表明不同改良方式改变了土壤中与磷代谢相关微生物的群落组成,可能对黄桃生长、产量和品质产生影响。
土壤微生物丰度、群落组成以及多样性等对土壤功能改善具有重要意义[5]。在本研究中,不同土壤改良方式提高了黄桃产量和可溶性固形物含量,显著降低了可滴定酸含量,使黄桃品质得到改善。利用偏最小二乘路径分析表明,不同改良方式可以直接改变土壤理化性质从而对黄桃产量和品质产生影响,也可以间接通过影响土壤中解磷微生物丰度和组成对其产生影响。Fan等[36]研究表明,微生物群落结构改变可以改变植物生长状态,可能是因为微生物群落结构的改变对土壤元素循环产生影响,如与磷元素相关微生物的增多可以促进土壤磷素的释放以被植物利用。
4 结 论
相较于不改良处理,深翻结合种植水稻使土壤pH显著提高了4.2%,EC值显著降低了74.7%,但降低了土壤养分的含量;深翻结合种植水稻虽然使土壤解磷微生物绝对丰度显著降低了51.8%,但提高了解磷微生物多样性(Shannon指数)。不同改良方式可以通过直接改变土壤理化性质影响黄桃的产量和品质,也可以间接通过影响土壤中解磷微生物的丰度和组成改变黄桃产量和品质。
[1] 刘燕德,吴明明,孙旭东,朱丹宁,李轶凡,张智诚.黄桃表面缺陷和可溶性固形物光谱同时在线检测[J]. 农业工程学报,2016,32(6):289-295.LIU Yande,WU Mingming,SUN Xudong,ZHU Danning,LI Yifan,ZHANG Zhicheng. Simultaneous detection of surface deficiency and soluble solids content for Amygdalus persica by online visible-near infrared transmittance spectroscopy[J].Transactions of the Chinese Society of Agricultural Engineering,2016,32(6):289-295.
[2] 叶正文,苏明申,杜纪红,周慧娟,吴钰良,庄恩及.晚熟鲜食黄桃新品种‘锦花’的选育[J].果树学报,2012,29(5):952-953.YE Zhengwen,SU Mingshen,DU Jihong,ZHOU Huijuan,WU Yuliang,ZHUANG Enji.A new late ripening yellow peach cultivar:‘Jinhua’[J].Journal of Fruit Science,2012,29(5):952-953.
[3] 范洁群,王伟民,吴淑杭,褚长彬,周德平,宋卫国.生物质炭对老桃园再植障碍的土壤调理机制初探[J]. 上海农业学报,2017,33(2):48-51.FAN Jiequn,WANG Weimin,WU Shuhang,CHU Changbin,ZHOU Deping,SONG Weiguo.Preliminary study of biochar on the soil conditioning mechanism of replant problem in old peach orchard[J].Acta Agriculturae Shanghai,2017,33(2):48-51.
[4] 王海燕,盛月凡,李前进,王玫,潘凤兵,陈学森,沈向,尹承苗,毛志泉.葱、芥菜和小麦轮作对老龄苹果园土壤环境的影响[J].园艺学报,2019,46(11):2224-2238.WANG Haiyan,SHENG Yuefan,LI Qianjin,WANG Mei,PAN Fengbing,CHEN Xuesen,SHEN Xiang,YIN Chengmiao,MAO Zhiquan. Effects of Allium fistulosum,Brassica juncea and Triticum aestivum rotation on soil environment of old apple orchard[J].Acta Horticulturae Sinica,2019,46(11):2224-2238.
[5] WANG Q F,CHU C B,ZHAO Z,WU S H,ZHOU D P. Preflooding soil used in monocropping increased strawberry biomass and altered bacterial community composition[J]. Soil Science and Plant Nutrition,2021,67(6):643-652.
[6] SENNETT L,BURTON D L,GOYER C,ZEBARTH B J.Influence of chemical fumigation and biofumigation on soil nitrogen cycling processes and nitrifier and denitrifier abundance[J]. Soil Biology and Biochemistry,2021,162:108421.
[7] 王力荣,王新卫,朱更瑞,方伟超,陈昌文,曹珂,李勇,吴金龙,王玲玲,牛棚.桃抗再植病障碍砧木中桃抗砧1 号的选育[J].果树学报,2023,40(8):1766-1770.WANG Lirong,WANG Xinwei,ZHU Gengrui,FANG Weichao,CHEN Changwen,CAO Ke,LI Yong,WU Jinlong,WANG Lingling,NIU Peng. A replantation disease resistant new peach rootstock cultivar Zhong Tao Kang Zhen No. 1[J]. Journal of Fruit Science,2023,40(8):1766-1770.
[8] XU Y Z,NIE L X,BURESH R J,HUANG J L,CUI K H,XU B,GONG W H,PENG S B.Agronomic performance of late-season rice under different tillage,straw,and nitrogen management[J].Field Crops Research,2010,115(1):79-84.
[9] YIN H J,ZHAO W Q,LI T,CHENG X Y,LIU Q. Balancing straw returning and chemical fertilizers in China:Role of straw nutrient resources[J]. Renewable and Sustainable Energy Reviews,2018,81:2695-2702.
[10] HU R W,ZHENG B F,LIU Y J,PENG S G,GONG J,LI J H,QIN T,LIANG J S,XIONG K L,SHAO L J,ZHENG Z Y,YI Z X,ZHOU Q M,LI J.Deep tillage enhances the spatial homogenization of bacterial communities by reducing deep soil compaction[J].Soil and Tillage Research,2024,239:106062.
[11] HUANG X Q,LIU L L,WEN T,ZHANG J B,WANG F H,CAI Z C. Changes in the soil microbial community after reductive soil disinfestation and cucumber seedling cultivation[J].Applied Microbiology and Biotechnology,2016,100(12):5581-5593.
[12] MENG T Z,WEI Q,YANG Y J,CAI Z C. The influences of soil sulfate content on the transformations of nitrate and sulfate during the reductive soil disinfestation (RSD) process[J]. Science of the Total Environment,2022,818:151766.
[13] XU L,LI X Z,LI C N,KOU Y P,LI J B,YAO M J,ZHANG B C,WANG L X,XU H W,YOU C M,LI H,LIU S N,ZHANG L,LIU Y,TAN B,XU Z F. Disentangling the relative importance of precipitation,biocrust succession,and shrub cover in mediating soil phoD-harbouring communities and organic phosphorus mineralisation[J]. Soil Biology and Biochemistry,2023,186:109165.
[14] HUO W G,PENG Y,MAIMAITIAILI B,BATCHELOR W D,FENG G. Phosphorus fertilizer recommendation based on minimum soil surplus for cotton growing in salt-affected soils[J].Field Crops Research,2023,291:108799.
[15] PENG Y,HUO W G,FENG G. Maximising cotton phosphorus utilisation for zero surplus and high yields:A review of innovative P management strategies[J]. Field Crops Research,2024,313:109429.
[16] ELSER J J. Phosphorus:A limiting nutrient for humanity?[J].Current Opinion in Biotechnology,2012,23(6):833-838.
[17] WAN W J,HE D L,LI X,XING Y H,LIU S,YE L P,YANG Y Y.Linking rare and abundant phoD-harboring bacteria with ecosystem multifunctionality in subtropical forests:From community diversity to environmental adaptation[J]. Science of the Total Environment,2021,796:148943.
[18] WEI X M,HU Y J,RAZAⅤI B S,ZHOU J,SHEN J L,NANNIPIERI P,WU J S,GE T D. Rare taxa of alkaline phosphomonoesterase-harboring microorganisms mediate soil phosphorus mineralization[J]. Soil Biology and Biochemistry,2019,131:62-70.
[19] XU L,CAO H L,LI C N,WANG C H,HE N P,HU S Y,YAO M J,WANG C T,WANG J M,ZHOU S G,LI X Z.The importance of rare versus abundant phoD-harboring subcommunities in driving soil alkaline phosphatase activity and available P content in Chinese steppe ecosystems[J].Soil Biology and Biochemistry,2022,164:108491.
[20] LI X Y,DENG Y,LI Q,LU C Y,WANG J J,ZHANG H W,ZHU J G,ZHOU J Z,HE Z L. Shifts of functional gene representation in wheat rhizosphere microbial communities under elevated ozone[J].The ISME Journal,2013,7(3):660-671.
[21] STRICKLAND T C,SOLLINS P. Improved method for separating light- and heavy-fraction organic material from soil[J]. Soil Science Society of America Journal,1987,51(5):1390-1393.
[22] ZHANG X X,ZHANG R J,GAO J S,WANG X C,FAN F L,MA X T,YIN H Q,ZHANG C W,FENG K,DENG Y. Thirtyone years of rice-rice-green manure rotations shape the rhizosphere microbial community and enrich beneficial bacteria[J].Soil Biology and Biochemistry,2017,104:208-217.
[23] OLSEN S R,COLE C Ⅴ,WATANABE F S,DEAN L A.Estimation of available phosphorus in soils by extraction with sodium bicarbonate[J]. United States Department of Agriculture,1954,939:19.
[24] SPARK D L,PAGE A L,HELMKE P A,LOEPPERT R H.Lithium,sodium,potassium,rubidium,and cesium[M]. SSSA-ASA,1973:791-796.
[25] ZHOU J,JIANG X,ZHOU B K,ZHAO B S,MA M C,GUAN D W,LI J,CHEN S F,CAO F M,SHEN D L,QIN J. Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in NorthEast China[J].Soil Biology and Biochemistry,2016,95:135-143.
[26] ZHOU J,GUAN D W,ZHOU B K,ZHAO B S,MA M C,QIN J,JIANG X,CHEN S F,CAO F M,SHEN D L,LI J.Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in NorthEast China[J]. Soil Biology and Biochemistry,2015,90:42-51.
[27] LI H,WENG B S,HUANG F Y,SU J Q,YANG X R.pH regulates ammonia-oxidizing bacteria and archaea in paddy soils in Southern China[J]. Applied Microbiology and Biotechnology,2015,99(14):6113-6123.
[28] BOKULICH N A,SUBRAMANIAN S,FAITH J J,GEⅤERS D,GORDON J I,KNIGHT R,MILLS D A,CAPORASO J G.Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing[J].Nature Methods,2013,10(1):57-59.
[29] EDGAR R C. UPARSE:Highly accurate OTU sequences from microbial amplicon reads[J]. Nature Methods,2013,10(10):996-998.
[30] ZHOU G P,FAN K K,GAO S J,CHANG D N,LI G L,LIANG T,LIANG H,CAO W D. Green manuring relocates microbiomes in driving the soil functionality of nitrogen cycling to obtain preferable grain yields in thirty years[J].Science China Life Sciences,2024,67:596-610.
[31] 张凯歌,兰挚谦,付玉芳,王晓卓,张雪艳.柠条堆肥与耕作深度对连作黄瓜土壤细菌群落组成与代谢功能的影响[J].植物营养与肥料学报,2022,28(3):460-469.ZHANG Kaige,LAN Zhiqian,FU Yufang,WANG Xiaozhuo,ZHANG Xueyan. Effects of Caragana compost and tillage depth on bacterial community composition and metabolic functions in continuous cucumber soils[J]. Journal of Plant Nutrition and Fertilizers,2022,28(3):460-469.
[32] LIU X,LIU H,ZHANG Y,CHEN G,LI Z,ZHANG M. Straw return drives soil microbial community assemblage to change metabolic processes for soil quality amendment in a rice-wheat rotation system[J]. Soil Biology and Biochemistry,2023,185:12.
[33] 西安市市场监督管理局.设施栽培土壤次生盐渍化改良技术规范:DB6101/T 195—2022[S].北京:中国标准出版社,2022.Xi’an Administration For Market Regulation. Technical specifications for secondary salinization improvement of protected cultivation soil:DB6101/T 195—2022[S]. Beijing:Standards Press of China,2022.
[34] 马鸣超,刘丽,姜昕,关大伟,李俊.胶质类芽孢杆菌与慢生大豆根瘤菌复合接种效果评价[J].中国农业科学,2015,48(18):3600-3611.MA Mingchao,LIU Li,JIANG Xin,GUAN Dawei,LI Jun.Evaluation of the effect of co-inoculant of Paenibacillus mucilaginosus and Bradyrhizobium japonicum in application[J]. Scientia Agricultura Sinica,2015,48(18):3600-3611.
[35] CHEN Q Q,ZHAO Q,XIE B X,LU X,GUO Q,LIU G X,ZHOU M,TIAN J H,LU W G,CHEN K,TIAN J,LIANG C Y.Soybean (Glycine max) rhizosphere organic phosphorus recycling relies on acid phosphatase activity and specific phosphorus-mineralizing-related bacteria in phosphate deficient acidic soils[J]. Journal of Integrative Agriculture,2024,23(5):1685-1702.
[36] FAN K K,DELGADO-BAQUERIZO M,GUO X S,WANG D Z,ZHU Y G,CHU H Y. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment[J].The ISME Journal,2021,15(2):550-561.