桃(Prunus persica L.)作为全球广泛栽培的重要水果,深受消费者喜爱。中国地处东亚,地域辽阔,生态气候类型多样,是优质桃的适宜产区[1]。植物树(株)型是重要的农艺性状之一。依据树高与分枝角度两个主要因素相结合的方法可将桃树型分为普通型、直立型、帚型(包括柱型和盘龙型)、紧凑型、矮化型和垂枝型等[2]。
目前,桃生产上主栽品种以普通型为主,其树形开张,随着结果年限的延长,分枝角度变大,树冠内枝叶密集,中下部透光差,结果表面化,在一定程度上影响了果实品质的提升[3]。而柱型桃的显著特点是分枝数量少和分枝角度小,导致其树冠小,是研究桃分枝形成的理想材料[4]。分枝角度作为果树树型建成的关键构成要素,直接决定树冠大小、栽培密度、果实产量等[4]。分枝角度是指主干与着生枝条的角度,包括基角(crotch angle)、腰角(equilibrium angle)和梢角(geotropic angle)。植物分枝角度的形成受遗传因素、内源激素、环境因素等共同影响[5-6]。其中,植物激素介导遗传和环境信号,对分枝角度的形成发挥至关重要的作用。
油菜素内酯(brassinosteroids,BRs)是一类在植物生长和发育过程中具有重要作用的甾体类激素,活性最高的油菜素内酯(brassinolide,BL)以菜油甾醇为前体物质经DWF、CPD、BR6OX、D2 等酶催化合成[7]。BRs首次从植物花粉中分离和鉴定,在植物生长发育进程中发挥重要调控作用,广泛参与调节植物种子萌发、外界环境胁迫、植物株高、分枝数量、分枝角度等众多农艺性状[8-10]。在植物分枝形成过程中,BR 信号通路相关基因突变体Osdwarf4-1[11]、CPD[12]、m107[13]、d61[14]等均对植物分枝角度产生影响。外施BR 合成抑制剂丙环唑可导致毛竹(Phyllostachys edulis)节间长度增长、植株变高、叶夹角显著增大[15]。过表达BR 合成基因DWF4 可显著促进水稻分蘖数增加[11-12],而BR合成或信号转导基因突变体则呈现分蘖数显著减少等表型[9,16]。此外,BR还可协同多激素途径(独脚金内酯、细胞分裂素、生长素、茉莉酸等)来调控植物分枝形成[9,17-18]。目前,BR 合成相关基因在植物分枝中的研究主要集中在模式植物和农作物,如拟南芥(Arabidopsis thaliana)[19]、水稻(Oryza sativa L.)[20]和小麦(Triticum aestivum L.)[21]等,如水稻LEAF AND TILLER ANGLE INCREASED CONTROLLER 基因编码CCCH型锌指蛋白并调节BR信号转导[20]。
笔者在本研究中以普通型桃大久保和柱型桃洒红龙柱为试验材料,分析两种树型桃1 年生枝条分枝角度和内源BR 含量的差异;利用两种树型桃茎尖转录组数据筛选差异表达BR 合成关键基因PpD2,对PpD2 的组织特异性表达进行分析;克隆PpD2基因并对其功能进行验证,为后续通过分子育种手段培育桃新种质提供理论支撑。
1 材料和方法
1.1 试验材料
1年生普通型桃大久保和柱型桃洒红龙柱定植于河南农业大学毛庄科教园区。其中,大久保采集部位为腋芽;洒红龙柱采集部位包括腋芽、茎尖、嫩叶、1 年生枝条上部和中部、1 年生枝条分枝连接处韧皮部上部和下部,所有样品采集标记后均用液氮速冻,并带回实验室于-80 ℃冰箱保存以备后续试验使用。
用于基因功能验证的哥伦比亚野生型拟南芥(Arabidopsis thaliana ecotype Columbia)为本实验室保存。
1.2 大久保和洒红龙柱1年生枝条分枝角度测定
为明确普通型桃大久保和洒红龙柱分枝角度是否存在差异,利用SC-K1原位活体植物分枝角自动测量仪系统(杭州万深)对长势一致的1年生大久保和洒红龙柱枝条基角角度进行统计,每处理选取2株,每株随机选取3个枝条测定其分枝角度,3次重复。
1.3 大久保和洒红龙柱腋芽内源BR含量测定
内源BR 含量按照Xia 等[18]描述的方法进行测定(南京瑞源科技有限公司,中国南京)。采集大久保和洒红龙柱腋芽并用液氮研磨成粉末,用1 mL冰冻的乙腈重新悬浮并在4°C 下过夜孵育。收集上清液,并加入0.3 g MCX@BBII和3 mL ddH2O一起涡旋5 min。在4 ℃下10 000 g离心1 min后,添加5 mL含有0.5%甲酸的90%丙酮溶液重悬沉淀后高速离心1 min。弃上清液,向含有沉淀物的离心管中加入1.2 mL 90%丙酮和20~50 mg 乙酸铵,并涡旋1 min后离心3 min。氮气干燥后重新溶解在100 μL 45%乙腈中,用于BR含量测定分析。
1.4 转录组数据分析
普通型桃大久保和柱型桃洒红龙柱茎尖转录组数据参考谭彬等[22]的报道。根据转录组数据,使用TB tools 软件对BR 合成基因在两个品种中茎尖的表达量进行热图分析。
1.5 PpD2组织特异性分析
柱型桃洒红龙柱不同组织样品包括腋芽、茎尖、嫩叶、1 年生枝条上部、1 年生枝条中部、1 年生枝条分枝连接处韧皮部上部和1年生枝条分枝连接处韧皮部下部。RNA提取采用柱式植物总RNA提取试剂盒(Spin Column Plant total RNA Purification Kit)(上海生物工程股份有限公司),详细操作步骤参考试剂盒说明书。使用Nanodrop 2000 分光光度计和琼脂糖凝胶电泳对提取的总RNA的浓度、纯度及完整性进行检测。用反转录试剂盒合成cDNA(TaKa-Ra PrimeScript TM RT reagent Kit)。依据参考基因组中的PpD2编码序列,利用Primer Premier 5.0软件设计定量引物,以桃PpGAPDH为内参基因,反转录获得的cDNA 为模板,用SYBR Select Master Mix(Applied Biosystems,Mardrid,CA,USA)荧光定量试剂盒进行定量分析,每个样品设置3 个生物学重复,并用2-ΔΔCt法计算基因PpD2 在不同组织中的相对表达量。qRT-PCR 反应体系为:cDNA 1 μL,SYBR 10 μL,上下游引物各1 μL,ddH2O 补足至20 μL。qRT-PCR 反应程序为:95 ℃预变性2 min;95 ℃变性30 s,60 ℃退火30 s,72 ℃延伸30 s,共42个循环。引物序列详见表1。
表1 PpD2 基因qRT-PCR 分析、克隆及功能验证所用引物
Table 1 Primers of PpD2 used for qRT-PCR analysis,gene cloning and function verification.
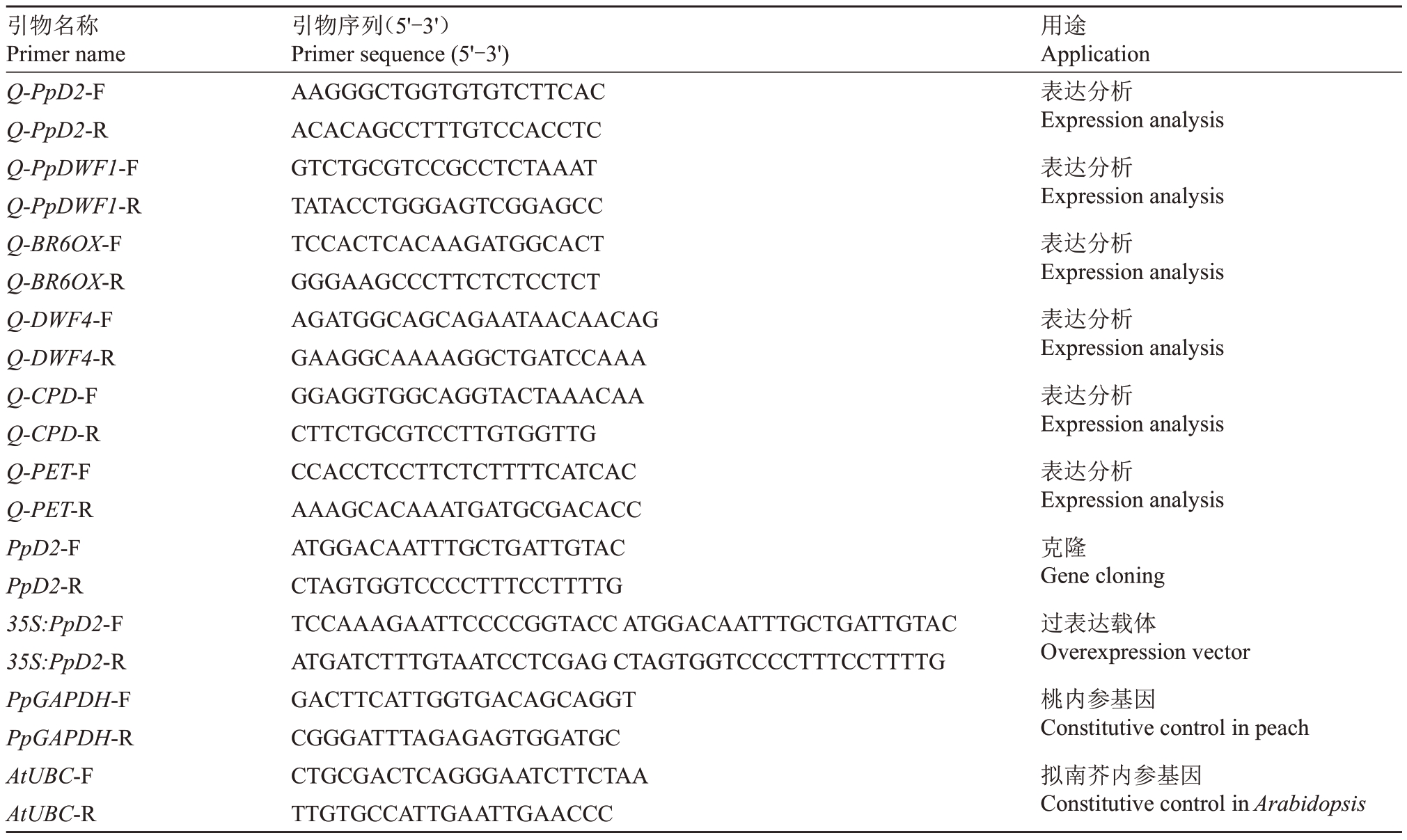
引物名称Primer name Q-PpD2-F Q-PpD2-R Q-PpDWF1-F Q-PpDWF1-R Q-BR6OX-F Q-BR6OX-R Q-DWF4-F Q-DWF4-R Q-CPD-F Q-CPD-R Q-PET-F Q-PET-R PpD2-F PpD2-R 35S:PpD2-F 35S:PpD2-R PpGAPDH-F PpGAPDH-R AtUBC-F AtUBC-R引物序列(5'-3')Primer sequence(5'-3')AAGGGCTGGTGTGTCTTCAC ACACAGCCTTTGTCCACCTC GTCTGCGTCCGCCTCTAAAT TATACCTGGGAGTCGGAGCC TCCACTCACAAGATGGCACT GGGAAGCCCTTCTCTCCTCT AGATGGCAGCAGAATAACAACAG GAAGGCAAAAGGCTGATCCAAA GGAGGTGGCAGGTACTAAACAA CTTCTGCGTCCTTGTGGTTG CCACCTCCTTCTCTTTTCATCAC AAAGCACAAATGATGCGACACC ATGGACAATTTGCTGATTGTAC CTAGTGGTCCCCTTTCCTTTTG TCCAAAGAATTCCCCGGTACC ATGGACAATTTGCTGATTGTAC ATGATCTTTGTAATCCTCGAG CTAGTGGTCCCCTTTCCTTTTG GACTTCATTGGTGACAGCAGGT CGGGATTTAGAGAGTGGATGC CTGCGACTCAGGGAATCTTCTAA TTGTGCCATTGAATTGAACCC用途Application表达分析Expression analysis表达分析Expression analysis表达分析Expression analysis表达分析Expression analysis表达分析Expression analysis表达分析Expression analysis克隆Gene cloning过表达载体Overexpression vector桃内参基因Constitutive control in peach拟南芥内参基因Constitutive control in Arabidopsis
1.6 PpD2基因克隆
依据桃基因组中PpD2 的CDS(coding sequence,编码序列)序列,利用软件Primer Premier 5.0设计特异性PCR引物。洒红龙柱叶片总RNA提取及反转录参照1.5。以获得的cDNA 为模板进行PCR 扩增。PCR 扩增体系为:cDNA 2 μL,Prime-STAR Max Premix(2×)25 μL,上下游引物各2 μL,ddH2O补足至50 μL。PCR扩增程序为:98 ℃3 min;98 ℃10 s,58 ℃10 s,72 ℃40 s,34个循环;72 ℃延伸3 min,4 ℃保存。
PCR 扩增产物经胶回收试剂盒(北京庄盟国际生物科技公司)纯化后与连接pClone007-T 并转化DH5α 大肠杆菌感受态。载体连接体系为:总体系为10 μL,其pEASY®-Blunt Ⅴector 1 μL,Insert DNA 30~50 ng,ddH2O 补足至10 μL。待单克隆长出后进行菌液PCR 验证阳性并送至上海生物科技股份有限公司进行测序验证。
1.7 35S:PpD2过表达载体构建
用软件Primer Premier 5.0 设计含同源臂的PpD2 同源重组引物(表1)。以构建成功的pClone007:PpD2为模板进行PCR扩增。PCR扩增体系和程序参照1.6。用限制性内切酶XhoⅠ和XbaⅠ双酶切空载质粒pSAK-277。酶切反应体系为:pSAK-277 载体10 μL,Cutsmart Buffer 5 μL,XbaⅠ1 μL,XhoⅠ1 μL,ddH2O补足至50 μL。
PCR 反应产物和酶切产物用胶回收试剂盒纯化(北京庄盟国际生物科技公司),并于-20 ℃保存备用。用同源重组酶(南京,诺唯赞)进行重组反应。重组反应体系、转化及阳性鉴定参照1.6。将测序成功的35:PpD2过表达质粒置于-20 ℃保存。
1.8 稳定转化拟南芥及鉴定分析
将测序正确的35S:PpD2 过表达载体转化GⅤ3101 农杆菌菌株,转化后的农杆菌接种于YEB液体培养基中(50 mg·L-1壮观霉素和利福平),并在28 ℃、200 r·min-1条件下培养至菌液浓度为OD600=0.7~0.8。12 000 r·min-1离心弃上清液,沉淀用重悬液(2.2 g·L-1 MS盐、50 g·L-1蔗糖、500 μL·L-1表面活性剂Silwet L-77)重悬(OD600=0.7~0.8)后用蘸花法稳定转化拟南芥。果荚成熟后收获种子。在超净工作台中将晾干并4 ℃春化后的T0代种子用次氯酸钠(6.25%)消毒3~4次,用灭菌的蒸馏水清洗5~6次后将种子均匀铺在MS 固体培养基(50 mg·L-1硫酸卡那霉素)培养。在16 h光照/8 h黑暗下培养1周左右移栽绿苗。移栽后3 周取拟南芥叶片用DNA 提取试剂盒提取基因组DNA,并以DNA 为模板进行PCR 扩增,对T0 植株进行阳性鉴定。以拟南芥AtUBC 为内参基因,以鉴定为阳性的拟南芥植株叶片提取RNA 反转录得到的cDNA 为模板对转基因株系进行PpD2 相对表达量分析。PCR 扩增体系和程序参照1.6,拟南芥叶片RNA提取、反转录和qRTPCR方法见1.5。引物序列详见表1。
选取PCR检测呈阳性且PpD2相对表达量高的3个株系T2代转基因拟南芥进行表型观察和统计。T2代转基因拟南芥和野生型拟南芥WT移栽3周后,用游标卡尺统计其莲座叶的长度和宽度。待拟南芥抽薹2周后,利用SC-K1原位活体植物分枝角自动测量仪系统(杭州万深)分别对长势一致的转基因株系和野生型拟南芥分枝角度(主薹上抽生的前两个枝条基角角度)进行统计,每株系统计2株,每株拟南芥选取主薹上抽生的前2个枝条测定分枝角度,3次重复。
1.9 数据统计与分析
SPSS 17.0 软件用于试验数据统计及差异显著性分析,Adobe Illustrator(AI)用于作图。
2 结果与分析
2.1 普通型桃大久保分枝角度显著大于柱型桃洒红龙柱
对1年生普通型桃大久保和柱型桃洒红龙柱1年生枝条分枝角度进行统计。结果表明,大久保1年生枝条分枝角度显著大于洒红龙柱(图1-A)。大久保和洒红龙柱腋芽内源BR含量测定结果显示,大久保腋芽内源BR含量显著高于洒红龙柱(图1-B),这与两种树型桃1年生枝条分枝角度差异一致。推测两种树型桃腋芽内源BR含量的差异可能导致两种树型桃1年生枝条分枝角度的差异。
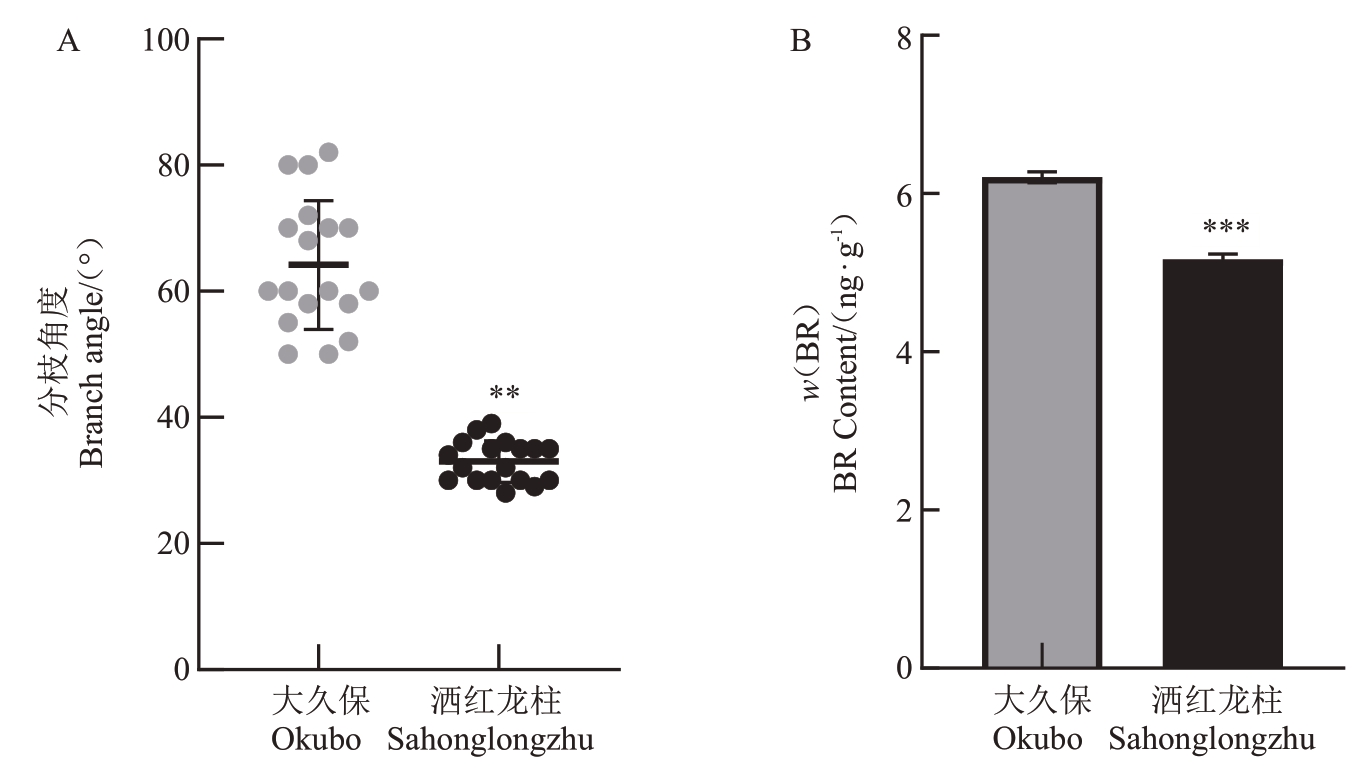
图1 普通型桃大久保和柱型桃洒红龙柱的分枝角度和内源BR 含量差异分析
Fig.1 Branch angle and endogeneous BR content analysis of standard peach Okubo and pillar peach Sahonglongzhu
A.两种树型桃1 年生枝条分枝角度;B.两种树型桃内源BR 含量。*表示差异显著(p<0.05),**表示差异极显著(p<0.01),***表示差异极显著(p<0.001)。下同。
A.Branch angles of Okubo and Sahonglongzhu;B.The endogenous BR content in Okubo was significantly higher than in that of Sahonglongzhu.* indicates significant difference (p<0.05),** indicates extremely significant difference (p<0.01), *** indicates extremely significant difference(p<0.001).The same below.
2.2 BR合成相关基因在两种树型桃中的表达分析
对普通型桃大久保和柱型桃洒红龙柱茎尖转录组数据中BR 合成相关基因表达趋势进行分析,结果发现,BR 合成相关基因中仅PpDET 在柱型桃洒红龙柱中表达量高于大久保;而PpDWF1、PpBR6OX、PpDWF4、PpCPD 和PpD2 表达量在柱型桃洒红龙柱中均低于普通型桃大久保,其中PpD2在洒红龙柱中的表达量显著低于大久保(图2-A),这与BR含量在两种树型桃腋芽中的差异一致。进一步的qRT-PCR结果发现BR合成相关基因在两种树型中的表达趋势和转录组数据一致,且PpD2 在两个树型中的表达量差异极显著(图2-B)。上述结果表明BR 合成相关基因PpD2 可能与桃分枝角度形成相关。
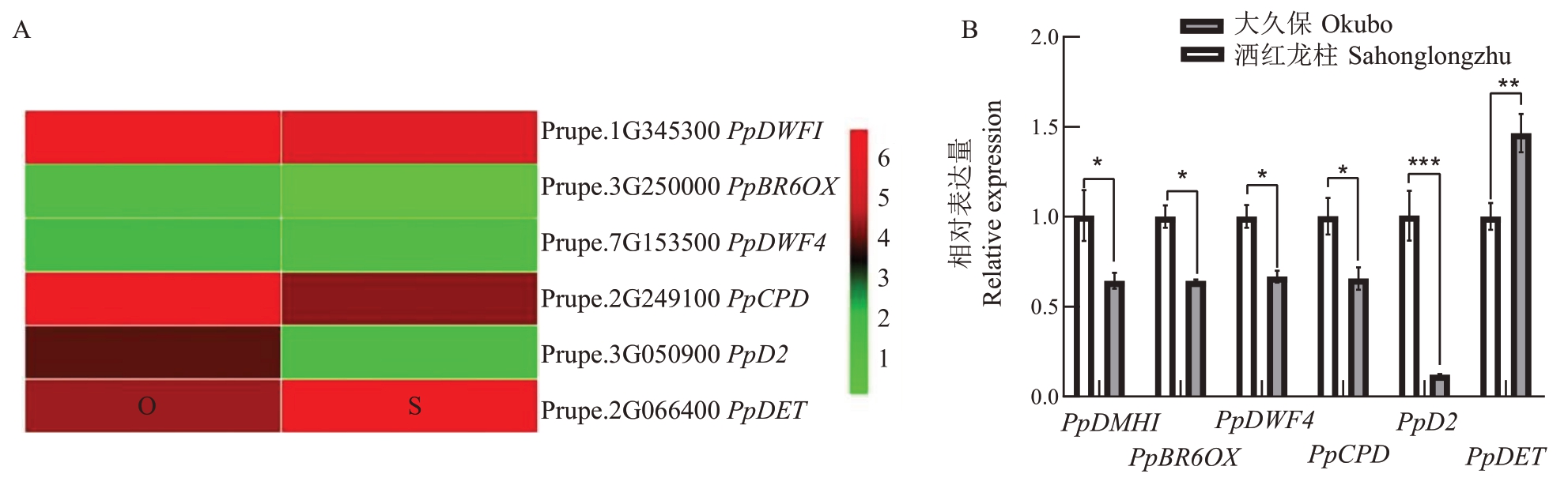
图2 BR 合成基因在普通型桃大久保和柱型桃洒红龙柱中的表达分析
Fig.2 The expression profile of BR biosynthesis-related genes in standard peach Okubo and pillar peach Sahonglongzhu
A.BR 合成基因在两种树型桃转录组分析;B.BR 合成基因在两种树型桃qRT-PCR 分析。
A.Transcriptome analysis of BR synthesis genes in two tree peach types;B.Analysis of BR synthesis genes by qRT-PCR in two tree peach types.
2.3 PpD2 在两种树型桃1 年生枝条分枝连接处韧皮部上部和下部的表达量存在显著差异
为进一步明确PpD2 的表达模式,采集洒红龙柱茎尖、嫩叶、1年生枝条上部、1年生枝条中部、1年生枝条分枝连接处韧皮部上部和1年生枝条分枝连接处韧皮部下部用于qRT-PCR 分析。结果显示,PpD2 在1 年生枝条分枝连接处韧皮部下部的表达量最高,在茎尖和嫩叶中的表达量相对较高,在1年生枝条上、中部表达量次之,而在1年生枝条分枝连接处韧皮部上部的表达量最低(图3)。分枝连接处是植物分枝角度形成的关键部位,而PpD2基因在1年生枝条分枝连接处韧皮部上部的表达量极显著低于在分枝连接处韧皮部下部,进一步表明PpD2可能与桃分枝角度的形成有关。
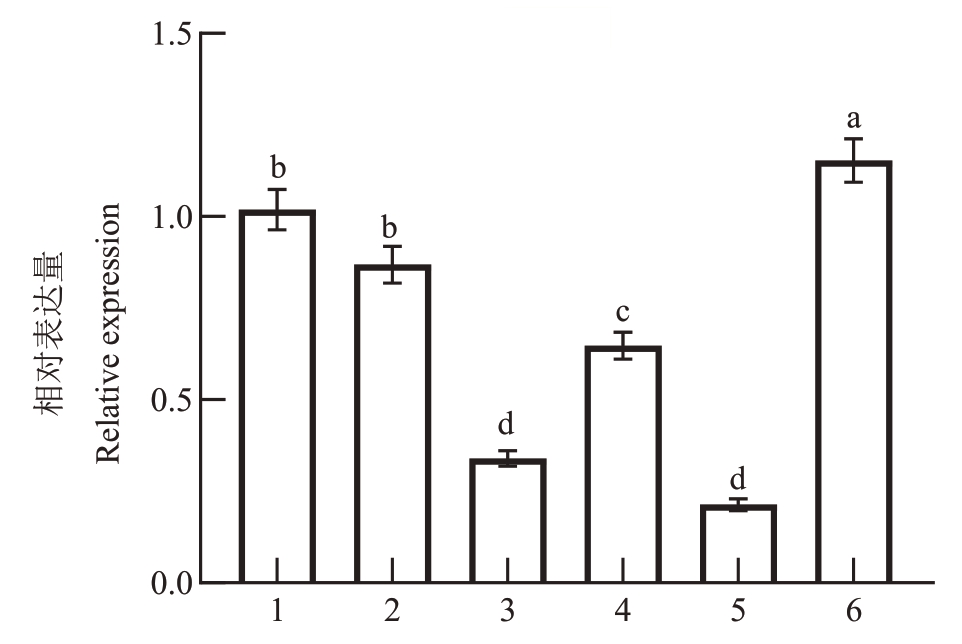
图3 PpD2 在柱型桃洒红龙柱中的组织特异性表达分析
Fig.3 The expression level of PpD2 in different tissues of pillar peach Sahonglongzhu
1.茎尖;2.嫩叶;3.1 年生枝条上部;4.1 年生枝条中部;5.1 年生枝条分枝连接处韧皮部上部;6.1 年生枝条分枝连接处韧皮部下部。不同小写字母表示差异显著(p<0.05)。下同。
1. Shoot tips; 2. Represented Young leaves; 3. Indicated upper part of annual branch;4.Represented middle part of annual branch;5.Represented upper part of the phloem at branch junction;6.Represented lower part of phloem at branch junction. Different small letters represent significant different at p<0.05.The same below.
2.4 PpD2转基因拟南芥株系表型观察与分析
为明确PpD2 功能,将构建好的35S:PpD2 过表达载体稳定转化拟南芥。PCR 结果显示水和野生型拟南芥WT中均未扩增出PpD2条带,而在转基因株系L1~L7 中均能扩增出目标条带,表明转基因株系L1~L7为阳性植株(图4-A)。qRT-PCR结果表明PpD2 基因在转基因拟南芥株系L1~L7 中的表达量均显著高于野生型拟南芥WT(图4-B),且转基因株系L1、L3 和L4 中PpD2 的表达量最高(图4-B),故选L1、L3和L4株系做进一步研究。
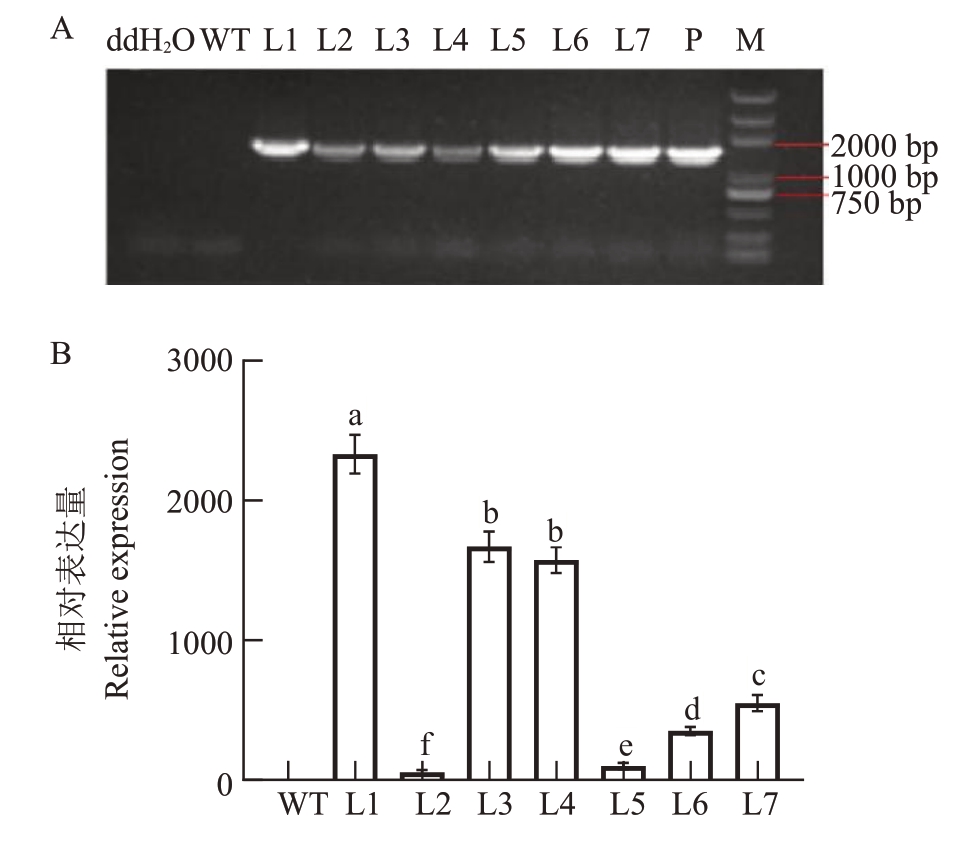
图4 PpD2 转基因拟南芥阳性鉴定
Fig.4 Identification of PpD2 transgenic lines
A.转基因拟南芥PCR 鉴定;B.转基因拟南芥定量鉴定;WT.野生型拟南芥;L1~L7.PpD2 转基因拟南芥株系;P.35:PpD2 质粒。
A.Ⅴerify through PCR; B.Ⅴerify via qRT-PCR;WT. Represent wild type Arabidopsis; L1-L7. Indicated PpD2 overexpression transgenic lines.P.Represented 35:PpD2 plasmid.
转基因拟南芥株系L1、L3、L4和野生型拟南芥WT移栽到温室中3周后,分别统计其莲座叶叶长和叶宽。结果表明,与野生型拟南芥WT相比,转基因株系L1、L3 和L4 莲座叶叶长显著增加(图5-A),WT 的莲座叶叶长平均值为4.3 cm,而转基因株系L1、L3 和L4 莲座叶叶长的平均值分别为6.4、6.1 和6.5 cm(图5-B)。同时转基因株系L1、L3和L4莲座叶叶宽也明显大于野生型拟南芥WT(图5-C)。以上结果表明PpD2对拟南芥莲座叶的生长具有一定调控作用。
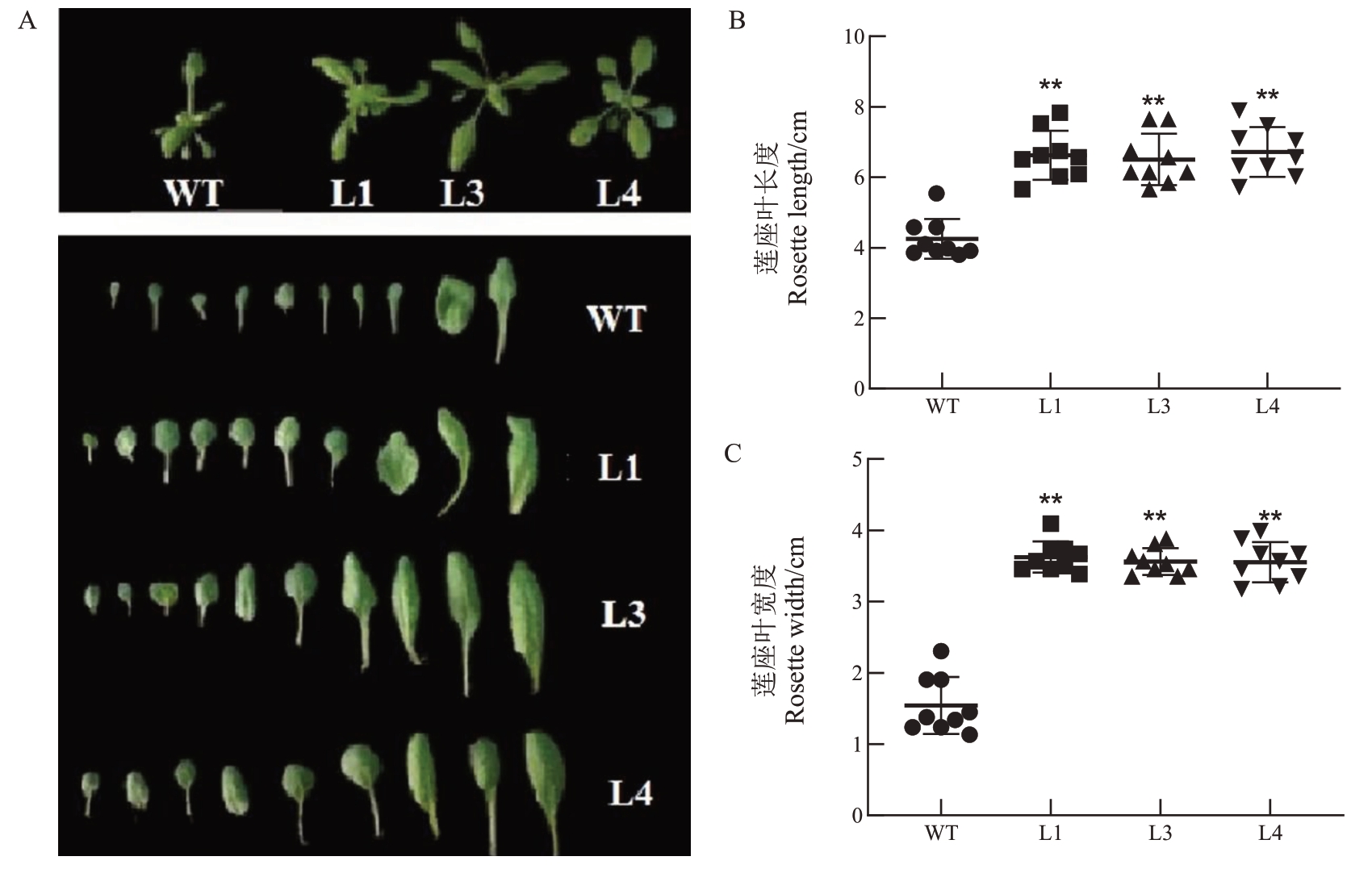
图5 PpD2 转基因拟南芥叶片长宽显著增加
Fig.5 The rosette width and length of PpD2 transgenic lines were obviously increased compared with WT
A.转基因拟南芥叶片表型图;B.转基因拟南芥株系L1、L3 和L4 的叶片长度显著大于野生型拟南芥WT;C.转基因拟南芥L1、L3 和L4的叶片宽度显著大于野生型拟南芥。
A.The phenotype of transgenic lines overexpression of PpD2.B.The rosette length of L1,L3 and L4 was significantly longer than wild type Arabidopsis WT.C.The rosette width of L1,L3 and L4 was obviously increased compared with WT.
当转基因拟南芥株系L1、L3、L4和野生型拟南芥WT 抽薹2 周后,分别统计主薹上着生的前两个分枝的基角。结果显示转基因株系L1、L3 和L4 的分枝角度显著大于野生型拟南芥(图6-A)。野生型拟南芥WT主薹上分枝的分枝角度平均值为48.5°,而转基因拟南芥株系L1、L3、L4 主薹上分枝的分枝角度的平均值分别为89.7°、86.5°和82.4°(图6-B)。以上结果表明过表达PpD2能显著增加转基因拟南芥枝条分枝角度。
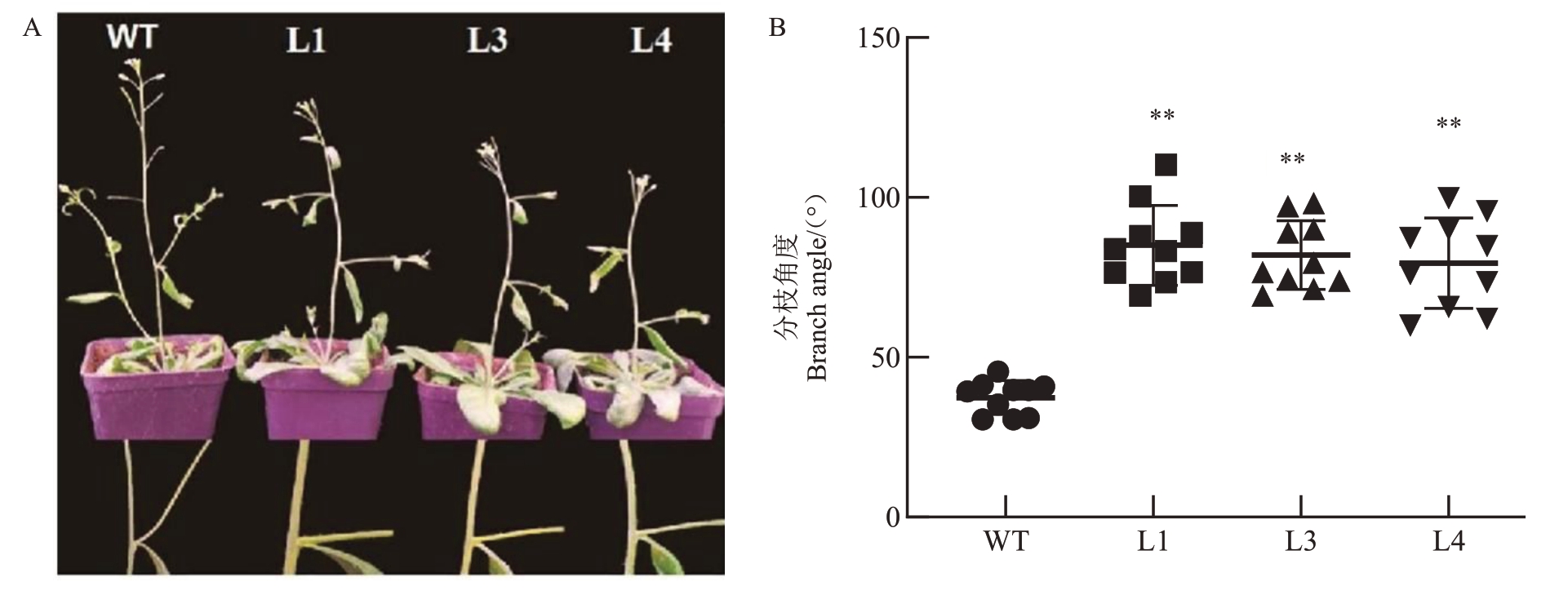
图6 PpD2 转基因拟南芥分枝角度显著增加
Fig.6 The branch angle of PpD2 transgenic lines was significantly increased compared with WT
A~B.野生型拟南芥WT 和转基因株系角度统计分析。
A~B.Branch angle analysis of wild type and transgenic Arabidopsis lines L1,L3,L4.
3 讨 论
分枝角度是影响果树树型的一个重要农艺性状,直接决定植物的栽培密度及产量[6]。普通型桃分枝角度大且分枝数量多,而柱型桃因分枝角度小且二级分枝数量少受到育种工作者的青睐[4]。
植物激素在调节分枝中起关键作用[23]。BR 是继生长素(IAA)、细胞分裂素(CK)、赤霉素(GA)、脱落酸(ABA)和乙烯之后发现的第六种植物激素[24],在植物生长发育进程中发挥重要的调控作用,BR 可影响植物叶片光合作用、调节植物根生长发育、促进细胞分裂与伸长、调控植物分枝等[10,25]。水稻BR合成基因dwarf2突变呈现分蘖角度显著减小等表型,而外施BR 能显著恢复dwarf2 突变体表型[26];miR6288b-3p 通过靶定并剪切的方式抑制PpTCP4 的表达,进而解除PpTCP4 对PpD2 的转录抑制,增加内源BR含量进而促进桃发枝[27];TaSPL8结合并激活BR 合成基因启动子TaD2 活性并调控小麦叶角,进而影响其种植密度和产量[21]。本研究中对分枝角度大的普通型桃大久保和分枝角度小的柱型桃洒红龙柱腋芽激素测定显示,大久保BR 含量显著高于洒红龙柱,且两种树型桃茎尖转录组和qRT-PCR 分析发现,BR 合成基因PpD2 在大久保中的表达量显著高于洒红龙柱,组织特异性表达结果表明PpD2在分枝连接处韧皮部上/下的表达量存在显著差异,而分枝连接处的弯曲程度直接决定分枝角度[28]。上述结果表明PpD2可能通过影响内源BR含量进而参与桃分枝角度的形成。
本研究过表达桃BR 合成基因PpD2 可显著增加拟南芥转基因株系分枝角度,这与草本植物水稻、拟南芥、番茄中的研究结果相似。BR合成相关基因OsBR6ox、ebisu dwarf(d2)突变直接影响水稻分蘖数量及角度[29-31];过表达BR 合成基因DWF4 显著促进拟南芥分枝形成[32];水稻BR 相关基因GSK3、GSK4、OsBZR4突变体直接影响水稻株高、叶夹角和水稻产量[33];OsPUB9、GSK2 和OsOFP8 形成一个调控网络,通过BR信号通路介导基因表达、叶片倾角和粒大小[34];番茄BR合成基因突变体dwf呈现发枝增多等表型,并且BR可通过影响赤霉素、细胞分裂素、独脚金内酯和糖等多条通路来调控番茄发枝[18,35];同时研究表明BR可能通过调节细胞质膜上的ATP酶活性,并影响植物细胞细胞壁的可塑性,进而调控植物分枝的形成[17];BR可促进叶夹角处细胞的分裂与伸长并导致叶夹角的变化[28]。本研究获得的PpD2 拟南芥过表达转基因株系中PpD2 的表达量显著高于野生型拟南芥WT,且分枝角度也明显大于野生型,表明PpD2 是导致普通型桃大久保分枝角度大的关键基因,为桃树型的遗传改良提供了理论依据。
4 结 论
普通型桃大久保1年生枝条分枝角度显著大于柱型桃洒红龙柱且腋芽内源BR含量显著高于洒红龙柱;PpD2在大久保茎尖中的表达量显著高于洒红龙柱,且在1 年生枝条分枝连接处韧皮部下部的表达量显著高于分枝连接处韧皮部上部;过表达PpD2能显著增加转基因拟南芥株系分枝角度,表明PpD2在分枝角度形成中发挥重要作用。
[1] 王志强,牛良,崔国朝,鲁振华,曾文芳.我国桃栽培模式现状与发展建议[J].果农之友,2015(9):3-4.WANG Zhiqiang,NIU Liang,CUI Guozhao,LU Zhenhua,ZENG Wenfang. Present situation and development suggestion of peach cultivation model in China[J]. Fruit Growers’Friend,2015(9):3-4.
[2] 谭彬,程钧,郑先波,王志强,冯建灿.桃树型及其调控关键基因研究进展[J].果树学报,2020,37(4):599-605.TAN Bin,CHENG Jun,ZHENG Xianbo,WANG Zhiqiang,FENG Jiancan. Progress on tree architecture and key genes of its regulation in peach[J]. Journal of Fruit Science,2020,37(4):599-605.
[3] 李天豪,张杰,高红珠,魏鹏程,郑先波,连晓东,王小贝,张海朋,程钧,王伟,谭彬,冯建灿.桃热激转录因子PpHSF18 基因的克隆及功能分析[J].果树学报,2023,40(5):852-860.LI Tianhao,ZHANG Jie,GAO Hongzhu,WEI Pengcheng,ZHENG Xianbo,LIAN Xiaodong,WANG Xiaobei,ZHANG Haipeng,CHENG Jun,WANG Wei,TAN Bin,FENG Jiancan.Cloning and functional analysis of heat shock transcription factor PpHSF18 in peach (Prunus persica)[J]. Journal of Fruit Science,2023,40(5):852-860.
[4] BASSI D,DIMA A,SCORZA R.Tree structure and pruning response of six peach growth forms[J]. Journal of the American Society for Horticultural Science,1994,119(3):378-382.
[5] KOTOⅤA A,KOTOⅤA L M,ROMANOⅤG A. Signaling network regulating plant branching:Recent advances and new challenges[J].Plant Science,2021,307:110880.
[6] WANG W G,GAO H B,LIANG Y,LI J Y,WANG Y H.Molecular basis underlying rice tiller angle:Current progress and future perspectives[J].Molecular Plant,2022,15(1):125-137.
[7] DIⅤI U K,KRISHNA P.Brassinosteroid:A biotechnological target for enhancing crop yield and stress tolerance[J]. New Biotechnology,2009,26(3/4):131-136.
[8] ZEBOSI B,ⅤOLLBRECHT E,BEST N B. Brassinosteroid biosynthesis and signaling:Conserved and diversified functions of core genes across multiple plant species[J]. Plant Communications,2024,5(9):100982.
[9] YIN W C,DONG N N,LI X C,YANG Y Z,LU Z F,ZHOU W B,QIAN Q,CHU C C,TONG H N. Understanding brassinosteroid-centric phytohormone interactions for crop improvement[J]. Journal of Integrative Plant Biology,2025,67(3):563-581.
[10] CHEN R Z,DENG Y W,DING Y L,GUO J X,QIU J,WANG B,WANG C S,XIE Y Y,ZHANG Z H,CHEN J X,CHEN L T,CHU C C,HE G C,HE Z H,HUANG X H,XING Y Z,YANG S H,XIE D X,LIU Y G,LI J Y. Rice functional genomics:Decades’efforts and roads ahead[J]. Science China Life Sciences,2022,65(1):33-92.
[11] SAKAMOTO T,MORINAKA Y,OHNISHI T,SUNOHARA H,FUJIOKA S,UEGUCHI- TANAKA M,MIZUTANI M,SAKATA K,TAKATSUTO S,YOSHIDA S,TANAKA H,KITANO H,MATSUOKA M. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice[J].Nature Biotechnology,2006,24(1):105-109.
[12] CHEN L S,XIONG G S,CUI X,YAN M X,XU T,QIAN Q,XUE Y B,LI J Y,WANG Y H. OsGRAS19 may be a novel component involved in the brassinosteroid signaling pathway in rice[J].Molecular Plant,2013,6(3):988-991.
[13] TANABE S,ASHIKARI M,FUJIOKA S,TAKATSUTO S,YOSHIDA S,YANO M,YOSHIMURA A,KITANO H,MATSUOKA M,FUJISAWA Y,KATO H,IWASAKI Y. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant,dwarf11,with reduced seed length[J].The Plant Cell,2005,17(3):776-790.
[14] YAMAMURO C,IHARA Y,WU X,NOGUCHI T,FUJIOKA S,TAKATSUTO S,ASHIKARI M,KITANO H,MATSUOKA M. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint[J].The Plant Cell,2000,12(9):1591-1605.
[15] ZHANG Z,YANG X L,CHENG L,GUO Z J,WANG H Y,WU W H,SHIN K,ZHU J Y,ZHENG X L,BIAN J H,LI Y C,GU L F,ZHU Q,WANG Z Y,WANG W F. Physiological and transcriptomic analyses of brassinosteroid function in moso bamboo(Phyllostachys edulis)seedlings[J].Planta,2020,252(2):27.
[16] FANG Z M,JI Y Y,HU J,GUO R K,SUN S Y,WANG X L.Strigolactones and brassinosteroids antagonistically regulate the stability of the D53-OsBZR1 complex to determine FC1 expression in rice tillering[J].Molecular Plant,2020,13(4):586-597.
[17] TIAN H Y,LⅤB S,DING T T,BAI M Y,DING Z J.Auxin-BR interaction regulates plant growth and development[J]. Frontiers in Plant Science,2018,8:2256.
[18] XIA X J,DONG H,YIN Y L,SONG X W,GU X H,SANG K Q,ZHOU J,SHI K,ZHOU Y H,FOYER C H,YU J Q. Brassinosteroid signaling integrates multiple pathways to release apical dominance in tomato[J]. Proceedings of the National Academy of Sciences of the United States of America,2021,118(11):e2004384118.
[19] GUO Z X,FUJIOKA S,BLANCAFLOR E B,MIAO S,GOU X P,LI J.TCP1 modulates brassinosteroid biosynthesis by regulating the expression of the key biosynthetic gene DWARF4 in Arabidopsis thaliana[J].The Plant Cell,2012,22(4):1161-1173.
[20] WANG L,XU Y Y,ZHANG C,MA Q B,JOO S H,KIM S K,XU Z H,CHONG K. OsLIC,a novel CCCH-type zinc finger protein with transcription activation,mediates rice architecture via brassinosteroids signaling[J].PLoS One,2008,3(10):e3521.
[21] LIU K Y,CAO J,YU K H,LIU X Y,GAO Y J,CHEN Q,ZHANG W J,PENG H R,DU J K,XIN M M,HU Z R,GUO W L,ROSSI Ⅴ,NI Z F,SUN Q X,YAO Y Y.Wheat TaSPL8 modulates leaf angle through auxin and brassinosteroid signaling[J].Plant Physiology,2019,181(1):179-194.
[22] 谭彬,杨丽萍,陈立川,冯玫侨,连晓东,魏鹏程,程钧,王小贝,冯建灿.桃PIN 蛋白基因家族鉴定及其在不同树型桃中的表达分析[J].农业生物技术学报,2020,28(10):1747-1760.TAN Bin,YANG Liping,CHEN Lichuan,FENG Meiqiao,LIAN Xiaodong,WEI Pengcheng,CHENG Jun,WANG Xiaobei,FENG Jiancan. Identification and expression analysis of PIN gene family in different tree architectures of peach (Prunus persica)[J]. Journal of Agricultural Biotechnology,2020,28(10):1747-1760.
[23] ONGARO Ⅴ,LEYSER O. Hormonal control of shoot branching[J].Journal of Experimental Botany,2008,59(1):67-74.
[24] SASSE J M. Recent progress in brassinosteroid research[J].Physiologia Plantarum,1997,100(3):696-701.
[25] 陈晨,陈虹,倪铭,张子晗,喻方圆.油菜素内酯调控植物生长发育的研究进展[J].林业科学,2022,58(7):144-155.CHEN Chen,CHEN Hong,NI Ming,ZHANG Zihan,YU Fangyuan. Research progress of brassinolide in regulating plant growth and development[J]. Scientia Silvae Sinicae,2022,58(7):144-155.
[26] HONG Z,UEGUCHI-TANAKA M,FUJIOKA S,TAKATSUTO S,YOSHIDA S,HASEGAWA Y,ASHIKARI M,KITANO H,MATSUOKA M. The rice brassinosteroid-deficient dwarf2 mutant,defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1,is rescued by the endogenously accumulated alternative bioactive brassinosteroid,dolichosterone[J]. The Plant Cell,2005,17(8):2243-2254.
[27] WANG X B,YAN L X,LI T H,ZHANG J,ZHANG Y J,ZHANG J J,LIAN X D,ZHANG H P,ZHENG X B,HOU N,CHENG J,WANG W,ZHANG L L,YE X,LI J D,FENG J C,TAN B. The lncRNA1-miR6288b-3p-PpTCP4-PpD2 module regulates peach branch number by affecting brassinosteroid biosynthesis[J].New Phytologist,2024,243(3):1050-1064.
[28] ZHANG L Y,BAI M Y,WU J X,ZHU J Y,WANG H,ZHANG Z G,WANG W F,SUN Y,ZHAO J,SUN X H,YANG H J,XU Y Y,KIM S H,FUJIOKA S,LIN W H,CHONG K,LU T G,WANG Z Y. Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis[J]. The Plant Cell,2010,21(12):3767-3780.
[29] MORI M,NOMURA T,OOKA H,ISHIZAKA M,YOKOTA T,SUGIMOTO K,OKABE K,KAJIWARA H,SATOH K,YAMAMOTO K,HIROCHIKA H,KIKUCHI S. Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis[J].Plant Physiology,2002,130(3):1152-1161.
[30] HONG Z,UEGUCHI-TANAKA M,UMEMURA K,UOZU S,FUJIOKA S,TAKATSUTO S,YOSHIDA S,ASHIKARI M,KITANO H,MATSUOKA M.A rice brassinosteroid-deficient mutant,ebisu dwarf (d2),is caused by a loss of function of a new member of cytochrome P450[J]. The Plant Cell,2003,15(12):2900-2910.
[31] DONG H J,ZHAO H,XIE W B,HAN Z M,LI G W,YAO W,BAI X F,HU Y,GUO Z L,LU K,YANG L,XING Y Z.A novel tiller angle gene,TAC3 together with TAC1 and D2 largely determine the natural variation of tiller angle in rice cultivars[J].PLoS Genetics,2016,12(11):e1006412.
[32] LI Q F,YU J W,LU J,FEI H Y,LUO M,CAO B W,HUANG L C,ZHANG C Q,LIU Q Q. Seed-specific expression of OsDWF4,a rate-limiting gene involved in brassinosteroids biosynthesis,improves both grain yield and quality in rice[J]. Journal of Agricultural and Food Chemistry,2018,66(15):3759-3772.
[33] LIU D P,YU Z K,ZHANG G X,YIN W C,LI L L,NIU M,MENG W J,ZHANG X X,DONG N N,LIU J H,YANG Y Z,WANG S M,CHU C C,TONG H N.Diversification of plant agronomic traits by genome editing of brassinosteroid signaling family genes in rice[J]. Plant Physiology,2021,187(4):2563-2576.
[34] XIE Y H,FAN Z P,LIANG X Y,TENG K C,HUANG Z J,HUANG M Y,ZHAO H,XU K Z,LI J X. OsPUB9 modulates leaf angle and grain size through the brassinosteroid signaling pathway in rice[J].The Plant Journal,2025,121(3):e17230.
[35] DONG H,WANG J C,SONG X W,HU C Y,ZHU C G,SUN T,ZHOU Z W,HU Z J,XIA X J,ZHOU J,SHI K,ZHOU Y H,FOYER C H,YU J Q.HY5 functions as a systemic signal by integrating BRC1-dependent hormone signaling in tomato bud outgrowth[J]. Proceedings of the National Academy of Sciences of the United States of America,2023,120(16):e2301879120.