苹果(Malus domestica Borkh.)为蔷薇科苹果属落叶乔木,因果实味道甜美、营养丰富而深受消费者喜爱[1]。疏花疏果是调整苹果树体负荷、克服大小年结果、保证产量、提高品质和市场竞争力的一项必要技术[2]。传统人工疏花疏果的精准度高,但费时费力费钱,与目前的用工难、用工贵的现状相悖,尤其不适用于规模化果园和大型合作社。化学疏花疏果是当前发达国家广泛应用的一项省力化花果管理措施[3-4],因其突出的省人工、低成本优势已成为研究的重点和热点[5]。
硫代硫酸铵(Ammonium thiosulfate,ATS)是一种化学疏花剂[6-7]。笔者在前期研究了ATS在红富士上的应用浓度和喷施时期,并从营养和激素角度初步探讨了其疏花机制[8-9],但是更深一步的机制尚未涉及。有研究表明,代谢产物关乎植物生命周期的全过程[10]。葡萄遮光处理时,赖氨酸、异亮氨酸的生物合成途径受到抑制,可导致花朵脱落[11];大豆花荚脱落与黄酮类、氨基酸及其衍生物、酚酸及萜类等次生代谢物的生物合成有关[12]。
利用传统分析方法检测植物次生代谢产物较为困难,但代谢组学的发展实现了对复杂代谢物的分析和完整代谢网络的构建[13],并在种子萌发、开花、果实发育等方面[14-16]出现诸多成功案例,使得运用代谢组分析ATS 疏花后引起的次生代谢物差异成为可能。为此,笔者在前期研究基础上,以威海金苹果为试材,通过广泛靶向代谢组学技术分析ATS 处理后幼果代谢产物种类和组分含量差异,旨在筛选造成幼果脱落的关键代谢物,进一步丰富疏花机制,为花朵疏除剂的应用推广提供理论支撑。
1 材料和方法
1.1 试验地概况
试验地点为山东省果树研究所泰东实验基地(117°10′47″ E,36°11′44″ N),园内年平均气温13.2 ℃,年日照数2 627.1 h,年降水量679 mm,无霜期195 d,属温带大陆性半湿润季风气候区。园地平坦,土质为砂壤土,肥力中等。供试材料为5年生威海金苹果(Malus domestica‘Harlikar’),基砧CG11,南北行向,株行距1 m×3.5 m,采用行间生草、树盘覆盖、设立支架、高纺锤树形、水肥一体化等技术,管理水平较高且一致,树体生长健壮。
1.2 试验设计
2023年4月2日进行疏花试验,在中心花90%开放时对全树均匀喷施疏花剂ATS,浓度为2.0 g·L-1,以清水为对照。采用背负式电动喷雾器喷施,时间段为上午8:00—10:00,喷洒的顺序是先上后下、先内后外,喷头与花朵的距离为10~15 cm,重点喷洒花柱,直至花柱湿润轻微滴水为止。
本试验采用单株小区设计,9次重复,处理之间选取1~2 株树作为隔离株,选取干径、树势、花量相当的树为试验树,挂牌标记。处理后10 d 采样,从试验树上取处理及对照的边果(图1),每株树采20个,3株树共计60个边果混成一个生物学样本,9株树共计3 个生物学重复,摘去果梗和花萼后,用铝箔纸包裹,在液氮罐中冷冻,送到实验室,用-80 ℃冰箱保存。

图1 ATS 处理10 d 及对照的边果样品
Fig.1 ATS processed 10 day and control edge result samples
1.3 代谢物提取
将ATS 处理组(TR)和对照组(CK)各3 个样本送至武汉迈特维尔生物科技有限公司,利用广靶代谢组技术进行代谢物的定性定量分析。将样本置于冻干机真空冷冻干燥后研磨成粉末,称样50 mg 加入1200 μL-20 ℃预冷的70%甲醇水内标提取液;30 min 1 次涡旋,每次30 s,共6 次;之后离心(转速12 000 r·min-1,3 min),抽上清液过0.22 μm 的微孔滤膜,将滤液储存于进样瓶,即为待测样品。
1.4 数据采集
利用超高效液相色谱-串联质谱系统(Ultra Performance Liquid Chromatography-Tandem mass spectrometry,UPLC-MS 系统)分析检测代谢物。液相条件主要包括:色谱柱—Agilent SB-C18 1.8 μm,2.1 mm×100 mm;流动相—A相为超纯水(加入0.1%的甲酸),B相为乙腈(加入0.1%的甲酸);洗脱梯度—0.00 min 时B 相的比率为5%,9.00 min 后,B 相的比率呈直线上升,直至95%,10.00~11.10 min 时,B 相的比率下降到5%,再经过5%的均衡,直至14 min;流速0.35 mL·min-1;柱温40 ℃;进样量2 μL。
1.5 数据统计分析
利用软件Analyst1.6.3 处理质谱数据;根据|Log2(fold change)|≥2 与p-value<0.05 筛选差异积累代谢物(Differential Expressed Metabolites,DEMs)。采用R 软件(Base Package)3.5.0 对样本进行主成分分析(Principal Component Analysis,PCA)与热图制作;使用SPSS19.0(SPSS,IBM Corporation,USA)进行数据分析。
2 结果与分析
2.1 代谢物定性定量分析
混样质控样本在正、负离子模式下,代谢物检测保留时间与离子检测的离子流强度较为统一,重叠性高,说明每个样本在不同时间段内的检测信号基本稳定,数据可靠且重复性高。
2.2 PCA分析和重复相关性评估
根据皮尔逊相关系数(Pearson’s Correlation Coefficient,PCC)来分析ATS处理组(TR)和对照组(CK)边果样品间的变异度。如图2-A所示,处理组和对照组之间相关系数均大于0.96,表明处理和对照这两组样品在生物学特性上高度相关;主成分1(PC1)、主成分2(PC2)和主成分3(PC3)的累计方差贡献率分别为35.48%、18.26%和16.29%,3 个主成分能够很好地代表本试验代谢组数据的整体结构(图2-B)。为了比较ATS处理组和对照组之间的代谢物差异,使用OPLS-DA 得分三点图(图2-C),并采用响应排序法进行200次验证(图2-D),结果表明ATS 处理组和对照组之间区分效果明显,数据结果较好。Q2>0.5表示模型可靠稳定,能根据ⅤIP值筛选代谢差异物。
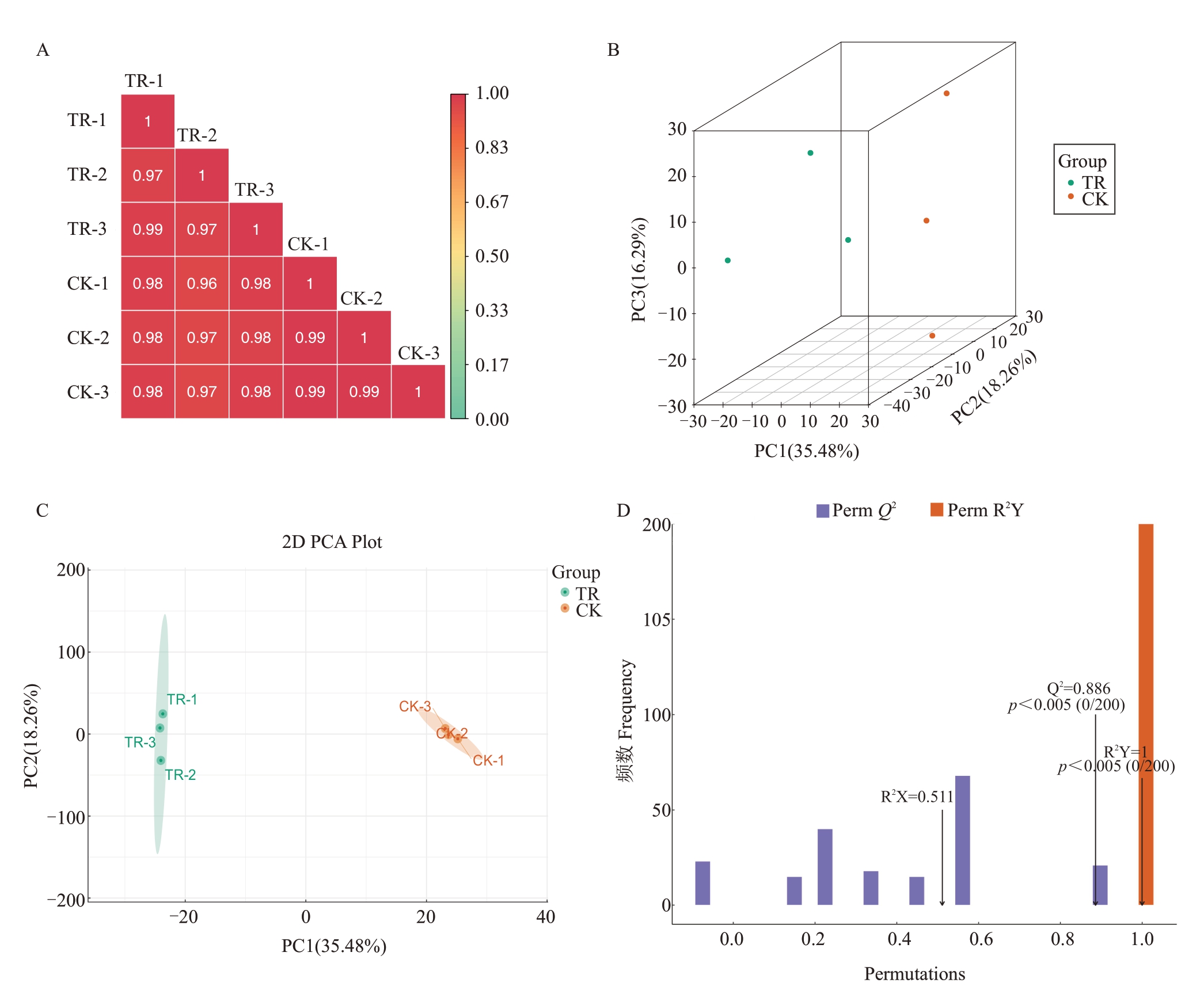
图2 代谢物质控和PCA 分析
Fig.2 Metabolic material control and PCA analysis
A.样品间重复相关性评估;B.OPLS-DA 得分图;C.主成分分析图;D.OPLS-DA 验证图。TR.ATS 处理组;CK.对照组。下同。
A. Sample repetitive correlation assessment; B. OPLS-DA score chart; C. Principal component analysis;D. OPLS-DA validation plot. TR.ATStreated group;CK.Control group.The same below.
2.3 OPLS-DA S-plot差异代谢物的筛选
对苹果果实进行广泛靶向代谢组技术分析,通过单变量统计(Fold Change≥1和Fold Change≤1)和多元统计分析(OPLS-DA 结果的ⅤIP>1)相结合的方法,对差异代谢物进行挖掘。采用偏最小二乘判别分析对果实样品进行差异区分,筛选差异代谢物,并依据差异代谢物的FC、ⅤIP 和P 值绘制火山图。如图3 所示,共检测到1946 种代谢物,其中有1861种代谢物差异不显著,差异显著的85 种代谢物中,有63种代谢物下调,22种代谢物上调。
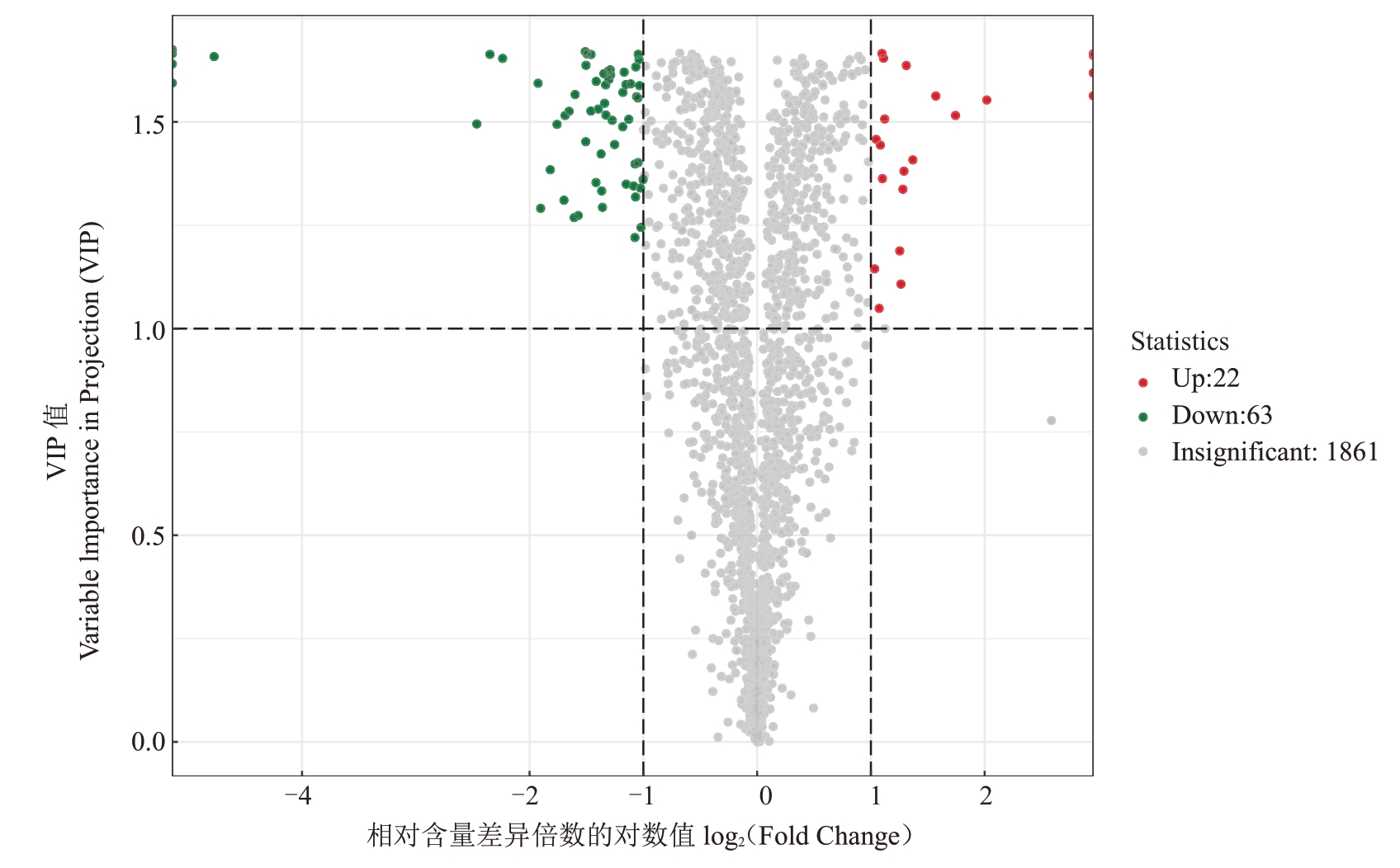
图3 差异代谢物筛选火山图
Fig.3 Differential metabolites screening volcano map
2.4 差异代谢物的鉴定分析
对显著差异代谢物进行多元统计分析(表1),包括酚酸类16种、核苷酸及其衍生物3种、氨基酸及其衍生物1种、黄酮类物质34种、木脂素和香豆素8种、鞣质6种、生物碱3种、萜类3种、有机酸2种、脂质4种和其他类5种。其中,黄酮类物质占比最高,达到40%,酚酸类代谢物、木脂素和香豆素代谢物以及鞣质代谢物也占比较大,分别为18.82%、9.41%和7.06%。在黄酮类化合物中,淫羊藿糖苷、苜蓿素-7-O-葡萄糖苷、山柰酚-3-O-(6''-对香豆酰)半乳糖苷、山柰酚-3-芥子酰双葡萄糖苷-7-葡萄糖苷、3,5,7,3',5'-五羟基-4'-甲氧基黄酮-3-O-葡萄糖苷等糖苷类代谢物表达量显著上调。酚酸类含量较高的差异显著代谢物有邻苯二甲酸、2-苯乙基-1-O-β-D-葡萄糖、双没食子酸、芥子醛和去鼠李糖基异洋丁香酚苷。
表1 不同处理组间果实差异代谢物数目统计
Table 1 Statistics of fruit differential metabolites number between different treatment groups
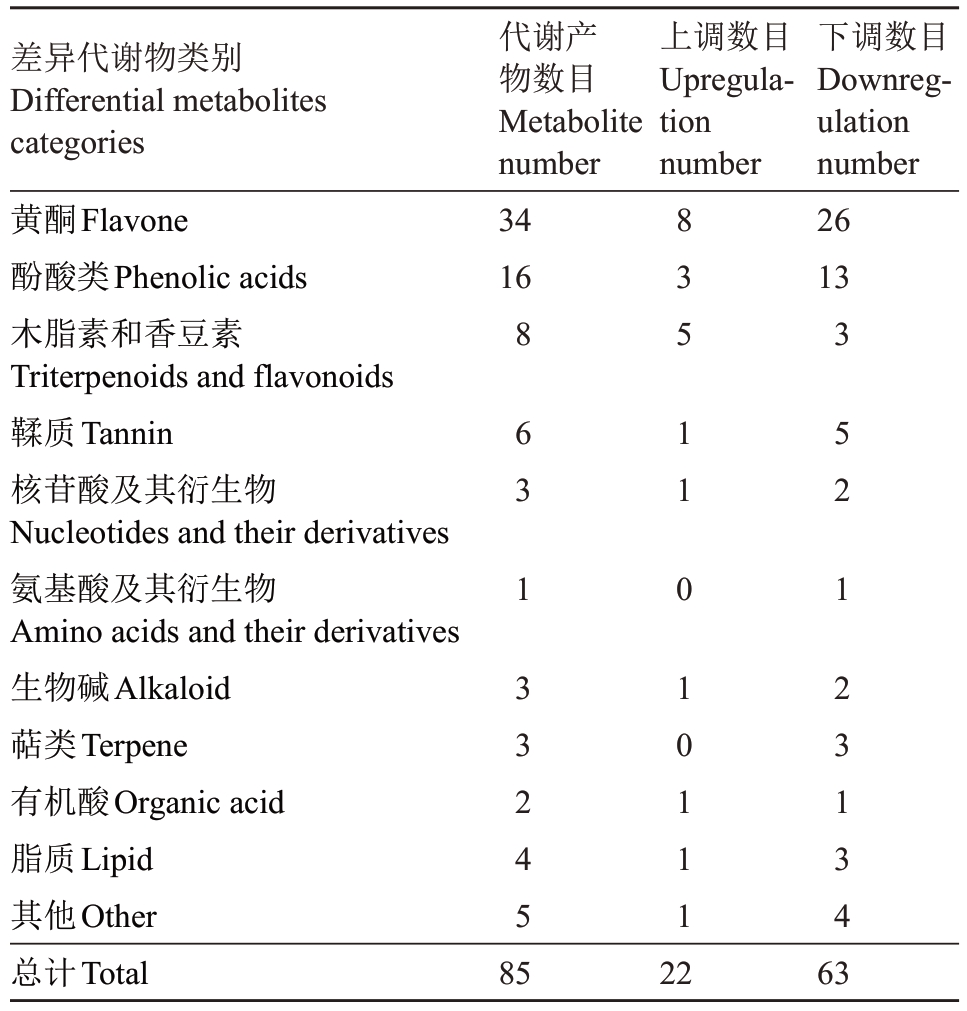
差异代谢物类别Differential metabolites categories黄酮Flavone酚酸类Phenolic acids木脂素和香豆素Triterpenoids and flavonoids鞣质Tannin核苷酸及其衍生物Nucleotides and their derivatives氨基酸及其衍生物Amino acids and their derivatives生物碱Alkaloid萜类Terpene有机酸Organic acid脂质Lipid其他Other总计Total代谢产物数目Metabolite number 34 16上调数目Upregulation number下调数目Downregulation number 26 13 8 6 3 1 3 3 2 4 5 8 3 5 1 1 0 1 0 1 1 1 3 5 2 1 2 3 1 3 4 85 22 63
2.5 差异代谢物聚类分析
为直观展示两组样本间的代谢物差异,对85种显著差异代谢物表达量进行层次聚类分析(图4)。TR 和CK 两组代谢物在表达丰度上差异明显。在CK 组中,红花素(Carthamidin)、8,9-环氧匹马拉18-酸甲酯(Methyl 8,9-epoxypimaran-18-oate)、3,4,5-三咖啡酰奎宁酸(3,4,5-Tricaffeoylquinic acid)、槲皮素-3-O-芹糖基(1→2)半乳糖苷[Quercetin-3-O-apiosyl(1→2)galactoside]、3-羟基根皮素(3-Hydroxyphloretin)、二氢咖啡酰腐胺(Dihydrocaffeoylputrescine)、槲皮素-3-O-洋槐糖苷(Quercetin-3-O-robinobioside)等代谢物的含量较高,溶血磷脂酰乙醇胺(LysoPE)、愈创木酚基甘油-β-愈创木基醚葡萄糖苷(Guaiacylglycerol-β-Guaiacyl Ether glucoside)、甘肃黄芩素I(Rehderianin I)和山柰酚-3-芥子酰双葡萄糖苷-7-葡萄糖苷(kaempferol-3-sinapoyldiglucoside-7-glucoside)等代谢物含量较低;在TR 组中含量较高的差异代谢物有芥子醛(Sinapinaldehyde)、淫羊藿糖苷A(Epimedoside A)、左旋肉碱(L-Carnitine)和愈创木酚基甘油-β-愈创木基醚葡萄糖苷(Guaiacylglycerol-β-Guaiacyl Ether glucoside),含量较低的差异代谢物有山柰酚-7-O-葡萄糖醛酸苷(Kaempferol-7-O-glucuronide)、槟榔鞣质C1(Arecatannin C1)、槲皮素-3-O-新橘皮糖苷(Quercetin-3-O-neohesperidoside)、4-甲基苯甲醛(4-Methylbenzaldehyde)、1-O-亚油酰基-(碳二十四烯酸)[1-O-Linoleoyl-(carbon eicosapentaenoic acid)]、蜡梅苷(Meratin)等。以上结果表明,TR和CK组幼果中代谢物存在明显差异,ATS处理影响了果实内部的生理代谢。
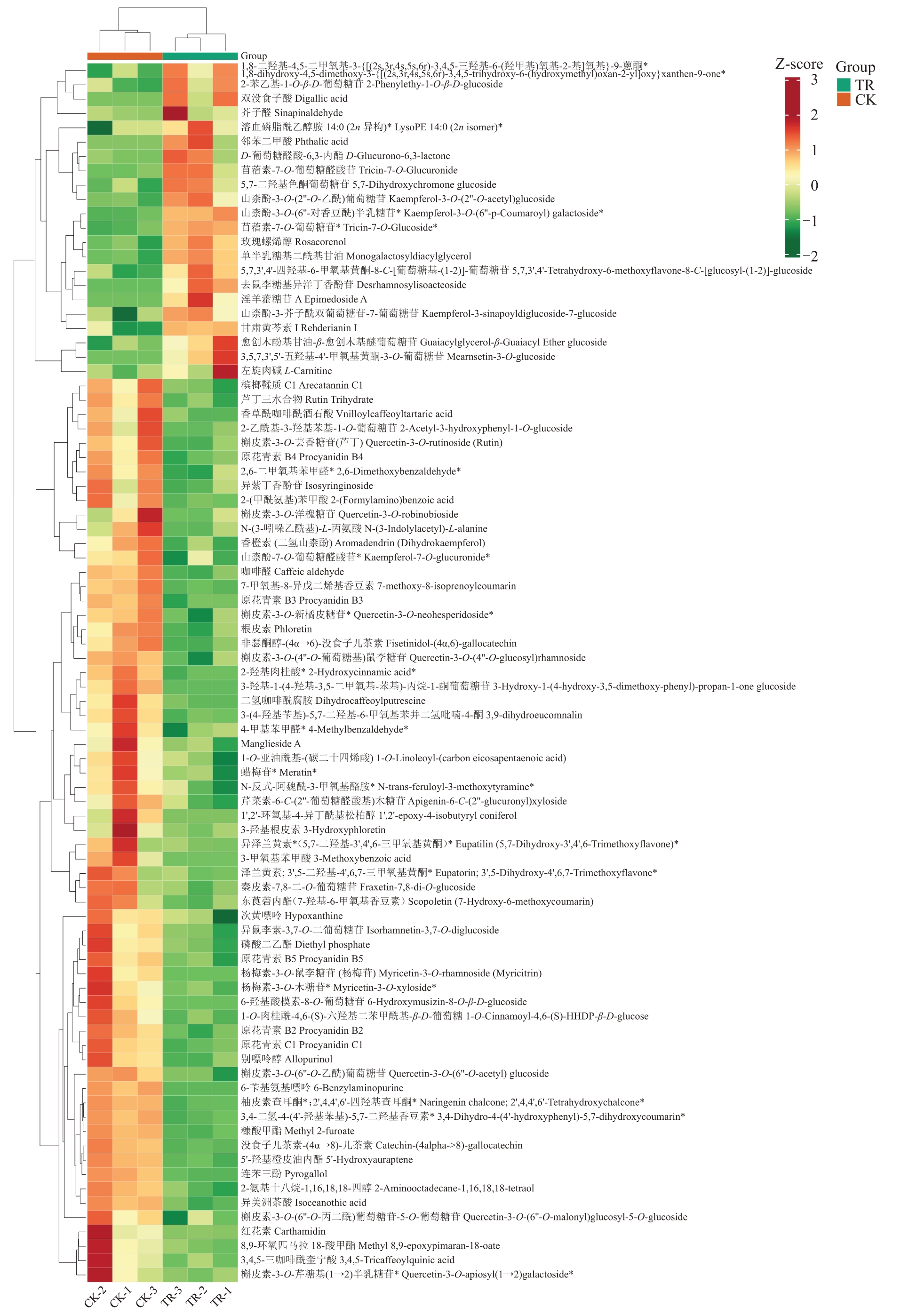
图4 85 种差异代谢物的聚类分析
Fig.4 Cluster analysis of the 85 differential metabolites
2.6 差异代谢物含量Log2FC分析
为明确关键代谢物质,进一步筛选出前20种差异代谢物(图5)。相比于对照组,处理组的差异代谢物质显著下调的有:黄酮类包括芹菜素-6-C-(2''-葡萄糖醛酸基)木糖苷[Apigenin-6-C-(2''-glucuronyl)xyloside]等,鞣质包括槟榔鞣质C1(Arecatannin C1),酮类化合物包括6-羟基酸模素-8-O-葡萄糖苷(6-Hydroxymusizin-8-O-β-D-glucoside),萜类包括8,9-环氧匹马拉18-酸甲酯(Methyl 8,9-epoxypimaran-18-oate)等,木脂素和香豆素包括7-甲氧基-8-异戊二烯基香豆素(7-methoxy-8-isoprenoylcoumarin)等,酚酸类包括2-(甲酰氨基)苯甲酸[2-(Formylamino)benzoic acid]等,有机酸类包括磷酸二乙酯(Diethyl phosphate)。显著上调的代谢物主要有:萜类包括玫瑰螺烯醇(rosacorenol),黄酮类包括苜蓿素-7-O-葡萄糖醛酸苷(Tricin-7-O-Glucoside),山柰酚-3-O-(2''-O-乙酰)葡萄糖苷[Kaempferol-3-O-(2''-O-acetyl)glucoside]。
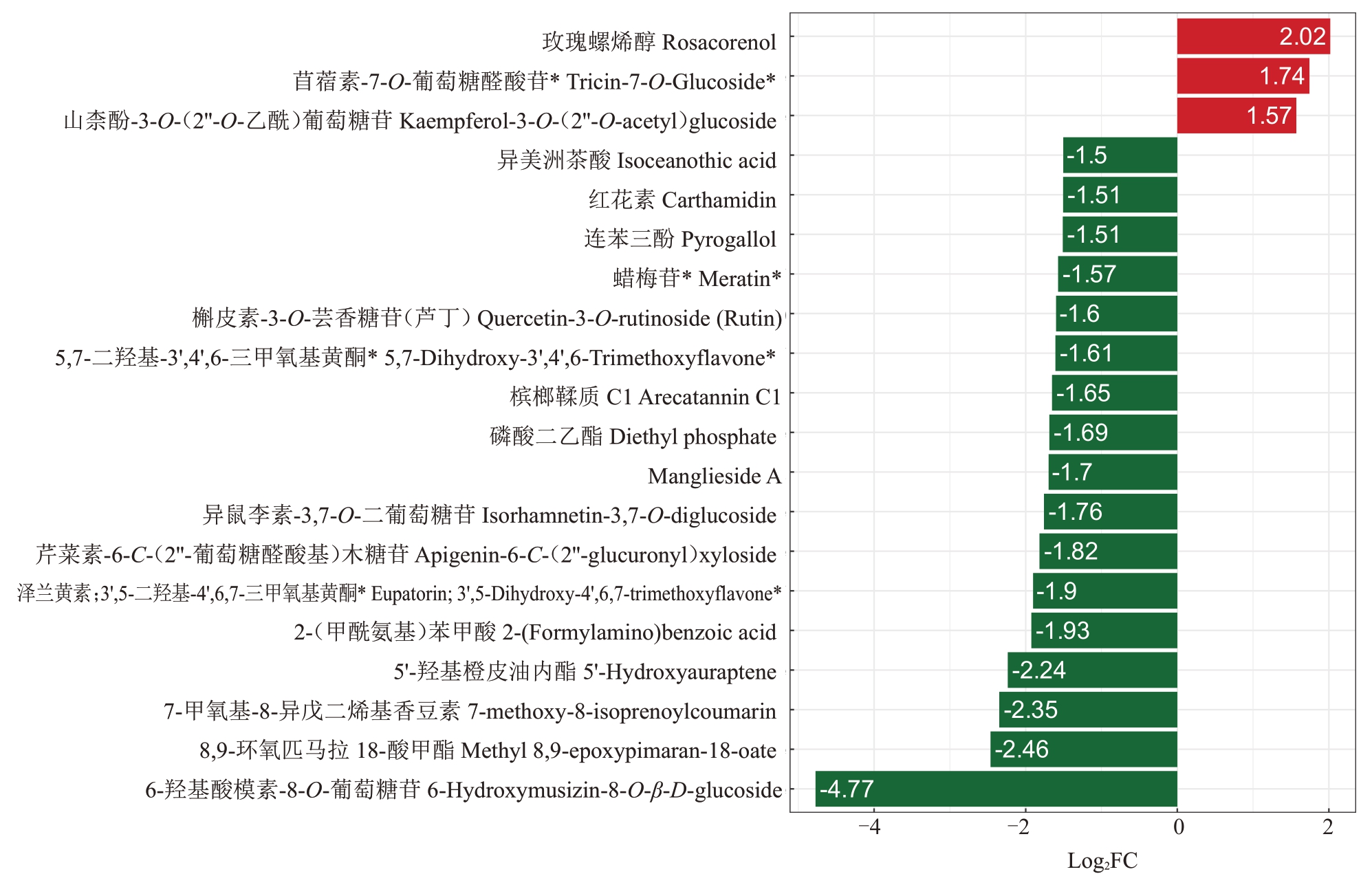
图5 差异代谢物条形图
Fig.5 Bar chart of differential metabolites
2.7 差异代谢物相关性分析
为进一步了解ATS影响幼果内代谢物之间的相互调节关系,利用Pearson分析方法对ⅤIP值最大的前50 个差异代谢物进行相关性分析(图6)。在TR组中,玫瑰螺烯醇(rosacorenol)是萜类中含量较高的代谢物,与其呈正相关的代谢物有去鼠李糖基异洋丁香酚苷(Desrhamnosyl isoacteoside)、淫羊藿糖苷A(Epimedoside A)、邻苯二甲酸(Phthalic acid)和山柰酚-3-O-(2''-O-乙酰)葡萄糖苷[Kaempferol-3-O-(2''-O-acetyl)glucoside]。在CK组中,黄酮类中苜蓿素-7-O-葡萄糖醛酸苷(Tricin-7-O-Glucoside)和山柰酚-3-O-(2''-O-乙酰)葡萄糖苷[Kaempferol-3-O-(2''-O-acetyl)glucoside]含量相对较高,与苜蓿素-7-O-葡萄糖醛酸苷(Tricin-7-O-Glucoside)呈正相关的代谢物有山柰酚-3-O-(6''-对香豆酰)半乳糖苷[Kaempferol-3-O-(6''-p-Coumaroyl)galactoside]和去鼠李糖基异洋丁香酚苷(Desrhamnosyl isoacteoside),与山柰酚-3-O-(2''-O-乙酰)葡萄糖苷[Kaempferol-3-O-(2''-O-acetyl)glucoside]呈正相关的代谢物有淫羊藿糖苷A(Epimedoside A)和邻苯二甲酸(Phthalic acid)。

图6 VIP 值排名前50 的差异代谢物含量的相关性分析
Fig.6 Correlation analysis of differential metabolites content in the top 50 of VIP values
2.8 差异代谢物KEGG 代谢通路功能注释及富集分析
对85 个差异代谢物进行KEGG 代谢通路注释和富集分析。从差异代谢物的KEGG 富集分析图(图7)中可以看出,差异代谢产物涉及多种代谢途径。其中占比最大的是次生代谢生物合成途径,其次是黄酮和黄酮醇生物合成、类黄酮生物合成及苯丙类生物合成等途径。此外,还有氨基酸代谢相关的通路,如苯丙氨酸代谢和色氨酸代谢;碳水化合物代谢相关的通路,如抗坏血酸和醛酸盐代谢;能量代谢相关的通路,如嘌呤代谢和核苷酸代谢。这些通路均与果实能量供应和物质转化密切相关,直接影响果实生长发育和品质形成。
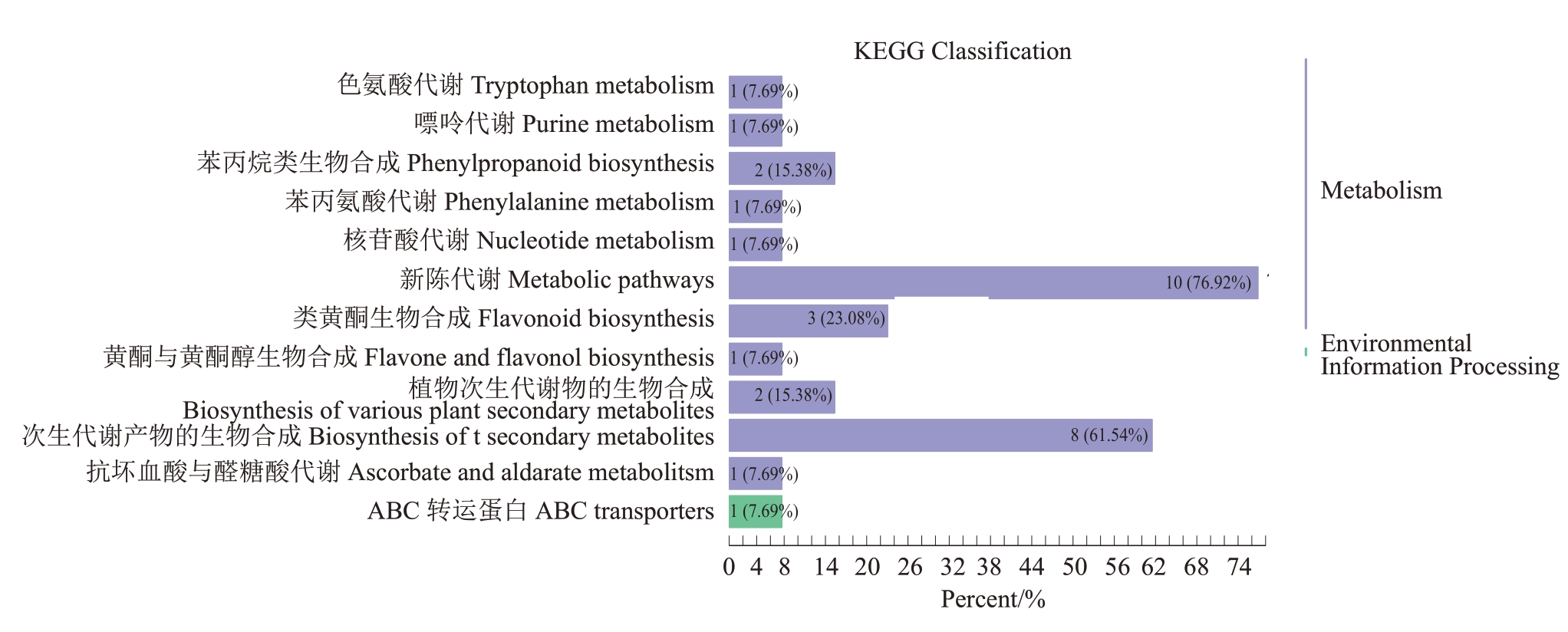
图7 差异代谢物KEGG 代谢通路
Fig.7 KEGG metabolic pathway of differential metabolites
由差异代谢物的KEGG富集分析图(图8)可以看出,显著富集的3 条代谢通路为次生代谢生物合成、类黄酮生物合成和苯丙烷类生物合成,在次生代谢生物合成中差异代谢物主要富集于糖酵解/糖异生(Glycolysis/Gluconeogenesis),其中差异代谢物反式-2-羟基肉桂酸酯(trans-2-Hydroxycinnamate)、6-羟基阿魏酰-CoA(6-Hydroxyferuloyl-CoA)下调;抗坏血酸和醛酸盐代谢途径中D-葡萄糖醛酸-6,3-内酯(D-Glucurono-6,3-lactone)上调。
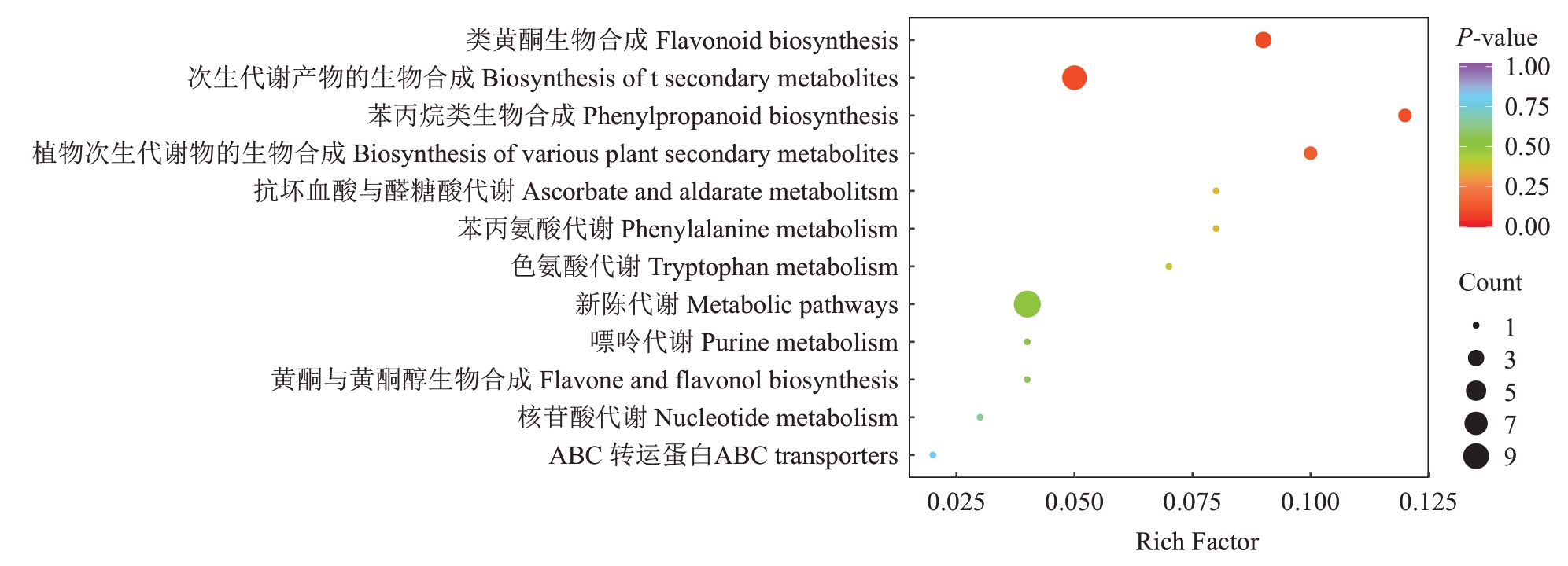
图8 差异代谢物KEGG 富集图
Fig.8 KEGG enrichment of differential metabolites
3 讨 论
次生代谢在植物生长发育过程中发挥重要作用,其中,在果树落花落果研究中,碳水化合物、激素、类黄酮等代谢起重要作用[17-18]。有研究表明,水分胁迫使根中1-氨基环丙烷-1-羧酸(ACC)积累,之后向上转移到枝条中并氧化形成乙烯,而乙烯的合成增加会促进花果的脱落[19]。
碳水化合物与果实生长发育密切相关。糖类既是能量物质,又是信号物质,在果实幼果脱落中占据重要地位。龙眼上研究表明,饥饿胁迫处理下果实蔗糖、葡萄糖和果糖含量降低,可溶性糖含量明显下降,引发果实大量脱落[20];荔枝、柑橘上存在同样现象[21-22]。笔者前期在苹果上的研究也表明,ATS疏花剂处理使幼果可溶性糖含量显著降低,可能是幼果脱落的一个主要原因[9]。本试验代谢组数据显示,次生代谢生物合成中差异代谢物富集于糖酵解/糖异生,其中差异代谢物反式-2-羟基肉桂酸酯与6-羟基阿魏酰-CoA下调,会造成葡萄糖生成受阻[23],进一步证实ATS疏花作用与幼果碳水化合物下降有关。
类黄酮是植物次生代谢的主要成分,能够对植物的生长发育及繁殖等生理活动产生重要影响[24-26]。糖类物质作为类黄酮的合成前体和信号物质与其息息相关[27]。黄酮、黄酮醇、花色素以及黄烷酮在糖基转移酶催化下形成各种糖苷衍生物[28],包括UDP-葡萄糖苷、UDP-鼠李糖苷、UDP-戊糖苷和UDP-半乳糖苷等。本试验中,ATS 处理的黄酮糖苷类物质如山柰酚-7-O-葡萄糖醛酸苷、芹菜素-6-C-(2''-葡萄糖醛酸基)木糖苷和槲皮素-3-O-芸香糖苷(芦丁)含量显著低于对照,推测其与ATS疏花导致边果脱落有关。但Farolfi等[29]研究发现油橄榄疏花处理对类黄酮类次生代谢物含量影响较小,对花青苷、环烯醚萜与LMW酚类物质含量影响较大,与本研究结果不一致;同时Stefanelli等[30]研究认为喷施过量氮肥会造成幼果多酚组分含量下降;分析可能与ATS为氮肥类疏花剂有关。
此外,本研究还发现,ATS处理后差异代谢物中下调的种类远多于上调的,尤其是黄酮醇类化合物中槲皮素、山柰酚等次生代谢物含量显著下调,而类黄酮物质作为天然生长素运输的调控因子,可影响生长素的转运和积累[31-32]。ATS 处理是否通过类黄酮次生代谢产物含量降低影响生长素运输进而导致幼果脱落,需要更多后续研究。
4 结 论
代谢组共检测到1946种代谢物,差异显著代谢物85种,包括34种黄酮、16种酚酸类、8种木脂素和香豆素等。显著富集的差异代谢物通路主要为次生代谢生物合成和类黄酮生物合成途径,筛选出9 种主要代谢物,分别为红花素、蜡梅苷、槲皮素-3-O-芸香糖苷、5,7-二羟基-3',4',6-三甲氧基黄酮、异鼠李素-3,7-O-二葡萄糖苷、3',5-二羟基-4',6,7-三甲氧基黄酮、芹菜素-6-C-(2''-葡萄糖醛酸基)木糖苷、苜蓿素-7-O-葡萄糖醛酸苷和山柰酚-3-O-(2''-O-乙酰)葡萄糖苷。从代谢组角度补充了ATS疏花机制。
[1] WANG N,QU C Z,JIANG S H,CHEN Z J,XU H F,FANG H C,SU M Y,ZHANG J,WANG Y C,LIU W J,ZHANG Z Y,LU N L,CHEN X S. The proanthocyanidin-specific transcription factor MdMYBPA1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples[J].The Plant Journal,2018,96(1):39-55.
[2] 薛晓敏,韩雪平,聂佩显,董放,王金政.苯嗪草酮疏果剂对苹果边果营养与激素含量的影响[J]. 农业工程学报,2021,37(7):206-211.XUE Xiaomin,HAN Xueping,NIE Peixian,DONG Fang,WANG Jinzheng. Effects of fruit thinning agent“metamitron”on nutrition and hormone content of apple lateral fruits[J].Transactions of the Chinese Society of Agricultural Engineering,2021,37(7):206-211.
[3] GONZALEZ L,BONANY J,ALEGRE S,ÀⅤILA G,CARBÓ J,TORRES E,RECASENS I,MARTIN B,ASIN L.Brevis thinning efficacy at different fruit size and fluorescence on‘Gala’and‘Fuji’apples[J].Scientia Horticulturae,2019,256:108526.
[4] GABARDO G C,KRETZCHMAR A A,PETRI J L,DE MARTIN M S,SEZERINO A A,BLATTMANN E.Influence of postflowering chemical thinning on development and fruit quality of‘Fuji Suprema’and‘Maxigala’apple trees[J]. Journal of Experimental Agriculture International,2019,32(4):1-13.
[5] 薛晓敏,路超,聂佩显,王翠玲,王金政.果树化学疏花疏果研究进展[J].江西农业学报,2012,24(2):52-57.XUE Xiaomin,LU Chao,NIE Peixian,WANG Cuiling,WANG Jinzheng.Research advances in chemical thinning of flowers and fruits in fruit tree[J].ActaAgriculturae Jiangxi,2012,24(2):52-57.
[6] BOUND S A. Optimising crop load and fruit quality of‘Packham’s Triumph’pear with ammonium thiosulfate,ethephon and 6-benzyladenine[J].Scientia Horticulturae,2015,192:187-196.
[7] FALLAHI E,KHODDAMZADEH A A,FALLAHI B,MAHDA-ⅤI S.Branched secondary alcohol ethoxylate,ammonium thiosulfate,calcium polysulfides-thiosulfate,and fish oil effects on blossom thinning,fruit set and quality of peaches and nectarines[J].American Journal of Plant Sciences,2020,11(12):1918-1933.
[8] 孟海凤,张春香,韩雪平,薛晓敏.硫代硫酸铵对红富士苹果的疏花效应[J].果树学报,2023,40(1):60-66.MENG Haifeng,ZHANG Chunxiang,HAN Xueping,XUE Xiaomin. Flower thinning effect of ammonium thiosulfate in Red Fuji apple[J].Journal of Fruit Science,2023,40(1):60-66.
[9] 孟海凤,黄剑,聂佩显,曹琪,李瑞洁,薛晓敏.硫代硫酸铵对苹果边果营养及激素含量的影响[J].北方园艺,2024(4):8-13.MENG Haifeng,HUANG Jian,NIE Peixian,CAO Qi,LI Ruijie,XUE Xiaomin.Effects of flower thinning agent ammonium thoisulfate on nutrition and hormone content of apple lateral fruit[J].Northern Horticulture,2024(4):8-13.
[10] CAO Y Q,YANG K,LIU W,FENG G Y,PENG Y,LI Z.Adaptive responses of common and hybrid bermudagrasses to shade stress associated with changes in morphology,photosynthesis,and secondary metabolites[J]. Frontiers in Plant Science,2022,13:817105.
[11] DOMINGOS S,FINO J,CARDOSO Ⅴ,SÁNCHEZ C,RAMALHO J C,LARCHER R,PAULO O S,OLIⅤEIRA C M,GOULAO L F.Shared and divergent pathways for flower abscission are triggered by gibberellic acid and carbon starvation in seedless Vitis vinifera L.[J].BMC Plant Biology,2016,16:38.
[12] SUN H X,HE D X,WANG N,YAO X D,XIE F T. Transcriptome and metabolome jointly revealed the regulation and pathway of flower and pod abscission caused by shading in soybean(Glycine max L.)[J].Agronomy,2024,14(1):106.
[13] 王梦迪,雍旭红,印敏,王奇志.代谢组学技术在植物次生代谢调控研究中的应用[J].植物科学学报,2023,41(2):269-278.WANG Mengdi,YONG Xuhong,YIN Min,WANG Qizhi.Application of metabonomics in regulation study of plant secondary metabolites[J].Plant Science Journal,2023,41(2):269-278.
[14] 肖金玲,沈蒙,葛云飞,康子悦,王娟,全志刚,王维浩,刁静静,曹龙奎.萌发绿豆中多酚类物质动态变化规律及其抗氧化活性的研究[J].中国粮油学报,2020,35(7):28-35.XIAO Jinling,SHEN Meng,GE Yunfei,KANG Ziyue,WANG Juan,QUAN Zhigang,WANG Weihao,DIAO Jingjing,CAO Longkui. Dynamic changes and antioxidant activity of polyphenols in germinated mung bean[J]. Journal of the Chinese Cereals and Oils Association,2020,35(7):28-35.
[15] 潘媛,赵晓,陈大霞.灰毡毛忍冬花不同发育阶段的转录组学与代谢组学研究[J].中国中药杂志,2021,46(11):2798-2805.PAN Yuan,ZHAO Xiao,CHEN Daxia. Different development phase of transcription proteomics and metabolomics of flower of Lonicera macranthoides[J]. China Journal of Chinese Materia Medica,2021,46(11):2798-2805.
[16] LIU J G,LIU Y Q,JIA M,KANG X D,WANG S M,SUN H,LIU M,WANG A Q,STRAPPE P,ZHOU Z K.Association of enriched metabolites profile with the corresponding volatile characteristics induced by rice yellowing process[J]. Food Chemistry,2021,349:129173.
[17] 曹小汉,毛惠敏,任莉萍.代谢组学技术在植物次生代谢产物研究中的应用[J].农业与技术,2022,42(11):1-3.CAO Xiaohan,MAO Huimin,REN Liping.Application of metabolomics in the study of plant secondary metabolites[J].Agriculture and Technology,2022,42(11):1-3.
[18] TAN B,TAN X,LIU C,ZENG Y,LI Y H.Effects of lead stress on rice (Oryza sativa L.) growth and metabolism in the rhizosphere microenvironment:The role of eicosanoid compounds[J].Plant Growth Regulation,2022,96(3):483-495.
[19] TAYLOR J E,WHITELAW C A. Signals in abscission[J]. New Phytologist,2001,151(2):323-340.
[20] 杨子琴,李茂,章笑赟,余意,王惠聪,黄旭明.饥饿胁迫对龙眼果实脱落及糖代谢的影响[J].果树学报,2011,28(3):428-432.YANG Ziqin,LI Mao,ZHANG Xiaoyun,YU Yi,WANG Huicong,HUANG Xuming. Effects of starvation stress on fruit abscission and sugar metabolism in Longan[J].Journal of Fruit Science,2011,28(3):428-432.
[21] KUANG J F,WU J Y,ZHONG H Y,LI C Q,CHEN J Y,LU W J,LI J G.Carbohydrate stress affecting fruitlet abscission and expression of genes related to auxin signal transduction pathway in litchi[J]. International Journal of Molecular Sciences,2012,13(12):16084-16103.
[22] IGLESIAS D J,TADEO F R,PRIMO-MILLO E,TALON M.Carbohydrate and ethylene levels related to fruitlet drop through abscission zone A in citrus[J].Trees,2006,20(3):348-355.
[23] PAYYAⅤULA R S,TSCHAPLINSKI T J,JAWDY S S,SYKES R W,TUSKAN G A,KALLURI U C. Metabolic profiling reveals altered sugar and secondary metabolism in response to UGPase overexpression in Populus[J]. BMC Plant Biology,2014,14(1):265.
[24] GUO Y,WANG T L,FU F F,EL-KASSABY Y A,WANG G B.Temporospatial flavonoids metabolism variation in Ginkgo biloba leaves[J].Frontiers in Genetics,2020,11:589326.
[25] SINGH P,ARIF Y,BAJGUZ A,HAYAT S.The role of quercetin in plants[J].Plant Physiology and Biochemistry,2021,166:10-19.
[26] DEBEAUJON I,NESI N,PEREZ P,DEⅤIC M,GRANDJEAN O,CABOCHE M,LEPINIEC L.Proanthocyanidin-accumulating cells in Arabidopsis testa:Regulation of differentiation and role in seed development[J].The Plant Cell,2003,15(11):2514-2531.
[27] SMEEKENS S. Sugar-induced signal transduction in plants[J].Annual Review of Plant Physiology and Plant Molecular Biology,2000,51:49-81.
[28] 赵莹,刘津,王长松,赵广荣.微生物合成黄酮类研究进展[J].中国生物工程杂志,2014,34(4):110-117.ZHAO Ying,LIU Jin,WANG Changsong,ZHAO Guangrong.Advances on flavonoids production of engineered microorganisms[J].China Biotechnology,2014,34(4):110-117.
[29] FAROLFI C,TOMBESI S,LUCINI L,CAPRI E,GARCÍAPÉREZ P. Influence of fruit load and water deficit on olive fruit phenolic profiling and yield[J].International Journal of Plant Biology,2024,15(3):895-913.
[30] STEFANELLI D,GOODWIN I,JONES R. Minimal nitrogen and water use in horticulture:Effects on quality and content of selected nutrients[J]. Food Research International,2010,43(7):1833-1843.
[31] PEER W A,MURPHY A S. Flavonoids and auxin transport:Modulators or regulators?[J]. Trends in Plant Science,2007,12(12):556-563.
[32] THOMPSON E P,WILKINS C,DEMIDCHIK Ⅴ,DAⅤIES J M,GLOⅤER B J. An Arabidopsis flavonoid transporter is required for anther dehiscence and pollen development[J]. Journal of Experimental Botany,2010,61(2):439-451.