柑橘是芸香科柑橘属的重要经济果树,中国柑橘产量居全球首位[1]。在果树生产中,通常利用嫁接繁殖具有优良性状的果树品种。偶然情况下,嫁接口的砧木细胞和接穗细胞会共同发育成不定芽并生长为一个新的枝条,形成嫁接嵌合体[2]。Schmidt[3]“原套原体”学说提出植物茎尖分生组织由原套和原体两部分组成,由外向内,分别为L1 层、L2 层、L3层。通常,L1 层发育成表皮细胞,L2 层发育成生殖细胞,L3层形成器官内部组织[2]。当某个细胞层或三层细胞的一个扇区发生突变,就形成植物嵌合体[4]。
人工合成的周缘嵌合体,在植物组织发生规律研究中具有重要作用。Chen等[5]通过离体嫁接培养获得了榨菜(Brassica juncea)和紫甘蓝(Brassica oleracea)种间嵌合体,研究表明该嵌合体含有两个嫁接供体的染色体,且具有两个供体的宝贵性状。Zhang等[6]在温州蜜柑(Citrus unshiu)作为中间砧高接罗伯逊脐橙(C.sinensis)的树上发现了嫁接嵌合体早红脐橙,其果实汁胞主要来源于L1供体温州蜜柑,而果皮、果形和叶片香气等性状来源于L2 供体罗伯逊脐橙。Zhang 等[7]在湖北省秭归县的一个果园进行芽变调查时发现嫁接嵌合体红肉桃叶橙(C.sinensis+C.unshiu),研究发现其果肉性状主要来源于L1 层,花粉、种子和果皮香气特征来源于L2/L3层。Yasuda等[8]对Meiwa金柑的突变体Yubeni进行流式细胞术分析,发现Yubeni是一个由二倍体和四倍体细胞构成的周缘嵌合体,其L1 层细胞为二倍体,L2和L3层为四倍体。Nukaya等[9]发现Meiwa金柑周缘嵌合体叶片、花瓣、花丝、花柱、子房、果皮着色层是二四混倍体,表明这些部分由L1和L2/L3共同构成;而叶中脉、种子和果实白皮层均是纯合四倍体,表明这些组织只来源于L2/L3。
由于存在组织异质性,周缘嵌合体的配子或珠心胚由茎尖分生组织中的哪一层细胞发育而来,对其实生后代表型有着决定性作用[4]。Schmülling等[10]研究表明在烟草嵌合体中,L2产生雄性和雌性配子。Zonneveld等[11]利用流式细胞术对玉簪(Hosta Tratt.)嵌合体12 个组织和器官进行起源分析,发现95%的配子来源于L2 层,表明L2 细胞层是配子形成的主要细胞层。柑橘类植物还具有多胚现象[12],多胚柑橘实生后代往往由珠心细胞发育而来并表现出母本的性状。因此,柑橘周缘嵌合体实生后代表型特征还需要考虑配子或珠心胚来源于哪个供体[13]。
柑橘四倍体有着重要的育种价值[14]。与二倍体砧木相比,柑橘四倍体砧木对盐胁迫[15-16]、重金属毒害[17-18]、干旱胁迫[19]以及低温胁迫[20]等非生物胁迫的耐受力更强[21]。柑橘四倍体往往表现出大果、少核的表型特征[22-23],比如,金柑四倍体具有少核、厚果皮、含糖量高等特点,具有良好的市场潜力[8-9]。最重要的是,柑橘四倍体可以作为倍性杂交亲本创制多倍体砧木和三倍体接穗品种[24]。研究表明,利用多胚柑橘品种珠心细胞存在自然加倍的特点,通过实生播种已从多个柑橘品种发掘出四倍体种质[12]。但四倍体发生频率在品种间存在差异,且同一品种的发生频率因生长环境不同而存在显著差异[25]。在高纬度、高海拔、低温条件下,柑橘四倍体发生频率显著提高[25]。目前,通过实生播种结合倍性分析,已报道很多四倍体柑橘种质。谢善鹏等[26]通过实生播种在常山胡柚、温岭高橙、新会橙、橘血橙杂种、衢州香橙和酸橙中获得四倍体;周锐等[12]通过实生播种在红江橙、贡柑、年橘、新会柑、滑皮金柑中获得四倍体;Ren等[14]通过秋水仙素原位活体诱导在秋辉橘、黄陵庙蜜橘、琥珀甜橙和HB 柚等单胚性品种中获得四倍体;Aleza等[27]利用秋水仙素诱导结合茎尖微嫁接在克里曼丁橘中获得四倍体。但柑橘周缘嵌合体实生多倍体后代发掘鲜有报道。
红肉胡柚(C.unshiu+C.aurantium,“OCC”)是笔者课题组前期报道的周缘嵌合体,经SSR(simple sequence repeat)分子标记证明红肉胡柚叶片中含有两个供体的细胞核、叶绿体和线粒体基因组,表明红肉胡柚是常山胡柚(C.aurantium,“C”)和温州蜜柑(C.unshiu,“O”)嫁接形成的周缘嵌合体,其L1层来源于温州蜜柑,L2/L3 层细胞来源于常山胡柚[2],其果肉颜色、质地和风味与温州蜜柑相似,果实大小、种子数量、果皮着色层和白皮层等性状与常山胡柚相似[2,28]。配子通常由L2 层细胞发育而来,但仍有研究发现某些周缘嵌合体实生后代在形态学上表现出异于L2 供体的特征,并在表观遗传等方面受到L1 供体的影响,因此,挖掘周缘嵌合体的四倍体实生后代,有可能获得具备L1 供体性状特征的新种质。Yu 等[4]在研究榨菜(Brassica juncea)(L1 供体)和紫甘蓝(Brassica oleracea)(L2 供体)合成的嵌合体时发现,其实生后代表现出叶色更绿、蜡质显著减少等区别于L2供体的表型特征,同时伴随着siRNA和基因组甲基化改变。Marcotrigiano 等[29]在研究6组普通烟草(Nicotiana tabacum)和光烟草(Nicotiana glauca)的周缘嵌合体时也发现,部分实生后代表现出与L1供体表型一致的情况。目前,柑橘周缘嵌合体实生后代研究和多倍体发掘鲜有报道。因此,笔者在本研究中以柑橘周缘嵌合体红肉胡柚四倍体实生后代为材料,通过SSR分子标记分析其遗传规律,对实生后代四倍体单株的性状进行评价,为柑橘周缘嵌合体实生后代用于育种奠定基础。
1 材料和方法
1.1 试验材料
2022年10月,在浙江农林大学实验基地,采集红肉胡柚花后6 个月的果实,剥出种子后在1 mol·L-1 NaOH 溶液中摇晃浸泡5 min,洗净种子后,使用0.2 mol·L-1 次氯酸钠溶液对种子进行消毒,将处理好的种子用MT(Murashge-Tucker)培养基(4.43 g MT+30 g 蔗糖+7 g 琼脂粉)进行无菌接种。待种子萌发并长至8 cm 株高时,将小苗移栽入V 腐殖土:V 基质=1∶1的栽培土壤。
1.2 种子单多胚鉴定
收获红肉胡柚成熟果实,剥出种子后随机挑选100粒种子,参考张斯淇[30]的方法统计每粒种子胚数并在体视显微镜下观察和拍照。
1.3 倍性鉴定
以成年态二倍体红肉胡柚叶片为对照,剪取实生后代植株0.5 cm2 嫩叶,参照解凯东等[31]的方法进行样品制备,并采用流式细胞仪(Cy-Flow®Ploidy Analyser,Sysmex,Germany)检测植株倍性。
1.4 SSR分子标记鉴定四倍体实生后代
采用Simgen Plant DNA Kit试剂盒,提取红肉胡柚及其实生后代、嫁接供体温州蜜柑和常山胡柚的叶片DNA。SSR 分子标记的反应体系为10 μL:无菌水2 μL,正反向引物各1 μL,DNA 模版1 μL,2×Accurate Taq Master Mix 5 μL。使用T100TM Thermal Cycler PCR 仪(Biorad,America)进行PCR 扩增,PCR 扩增程序为:95 ℃预变性3 min,95 ℃变性15 s,61 ℃退火15 s,72 ℃延伸30 s,35 个循环后72 ℃延伸5 min,4 ℃保存。扩增产物用12%PAGE胶先于90 V、400 mA电压下电泳30 min,再于200 V、400 mA电压下电泳90 min,后进行银染7 min,显影5 min,结束后拍照观察。利用分布于柑橘9 条染色体的28 对SSR 引物分析红肉胡柚实生四倍体后代的遗传组成,SSR引物序列源自相关参考文献,由杭州有康生物技术有限公司合成。表1 列出了其中3对引物的序列。
表1 部分SSR 引物序列
Table 1 Partial SSR primers sequences
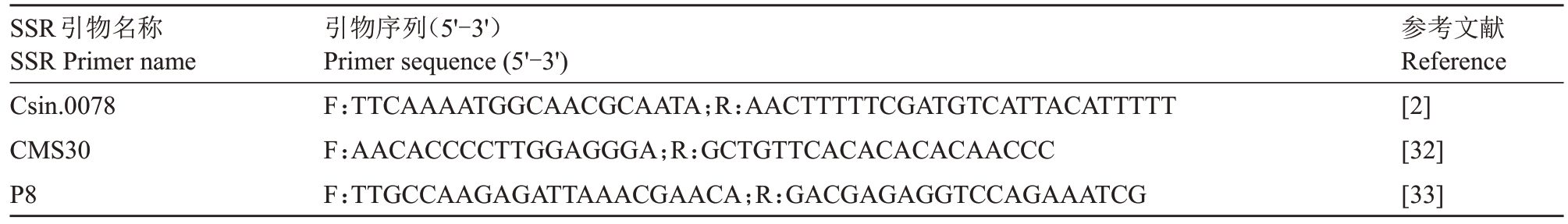
SSR引物名称SSR Primer name Csin.0078 CMS30 P8引物序列(5'-3')Primer sequence(5'-3')F:TTCAAAATGGCAACGCAATA;R:AACTTTTTCGATGTCATTACATTTTT F:AACACCCCTTGGAGGGA;R:GCTGTTCACACACACAACCC F:TTGCCAAGAGATTAAACGAACA;R:GACGAGAGGTCCAGAAATCG参考文献Reference[2][32][33]
1.5 叶片形态学观察
以红肉胡柚二倍体实生后代植株作为对照,测定同一生长发育时期的四倍体植株形态指标,包括叶长、叶宽、节间距、叶片厚度。节间距用直尺测量,叶长、叶宽、叶片厚度用游标卡尺测量,取平均值。
1.6 叶片透射电镜样品制备与观察
取红肉胡柚实生四倍体和二倍体后代植株的叶片,在叶中脉附近剪取0.5 cm2的正方形,样品放置于2.5%戊二醛溶液中固定,使用真空泵抽除样品中的空气,4 ℃条件下固定24 h。采用Chen 等[5]的方法,对样品进行固定、脱水、渗透、包埋,将处理好的部分样品在LKB 11800 PYRAMITOME型半薄切片机中进行切片,获得厚度为2 μm 的半薄切片,使用次甲基蓝对切片进行染色,染色完成后在光学显微镜下观察切片并拍照。其余样品通过半薄切片观察定位,使用LEICA EM UC7型超薄切片机切片,得到厚度为70~90 nm 的超薄切片,经过柠檬酸铅溶液、醋酸双氧铀50%乙醇饱和溶液分别染色5 min后,在Hitachi H-7650型透射电镜中观察。
1.7 叶片扫描电镜样品制备与观察
取红肉胡柚实生四倍体和二倍体后代植株的叶片,双面刀片将叶片切成1.0 cm2的正方形,采用Chen 等[5]的方法处理和制备样品,样品经过干燥和镀膜后,在Hitachi SU-8010 型扫描电镜中观察并拍照。
1.8 叶片叶绿素和总类胡萝卜素含量测定
称取剪碎的红肉胡柚实生四倍体和二倍体后代植株的新鲜叶片各0.1 g,置于棕色试管,在每个试管中加入5 mL 95%乙醇,置于暗处24 h,将提取液倒入光径1 cm 的比色杯内,以95%乙醇作为对照,使用分光光度计在波长665、649、470 nm 下测定吸光度。试验设置3个生物学重复。参照张泽群[34]的方法计算红肉胡柚实生四倍体和二倍体后代植株叶片的叶绿素和总类胡萝卜素含量。
1.9 数据分析
使用ImageJ 2.14.0版本软件进行叶片解剖结构和气孔特征参数测量。试验数据均采用IBM SPSS 25.0 中的独立样本T 检验进行统计分析。采用Excel 2010和Photoshop CS6软件制图。
2 结果与分析
2.1 红肉胡柚种子单多胚鉴定
拍摄红肉胡柚及其两个供体的果实(图1-A),随机挑选100 粒红肉胡柚种子统计胚数。结果显示,红肉胡柚属于单多胚类型,其中,单胚种子(图1-B)有42粒,占比为42.0%,播种于培养基,每粒种子获得1 株小苗(图1-D);多胚种子(图1-C)有58 粒,占比为58.0%,胚数量为每粒种子2~4 个,播种于培养基,每粒种子可获得2~4株小苗(图1-E)。
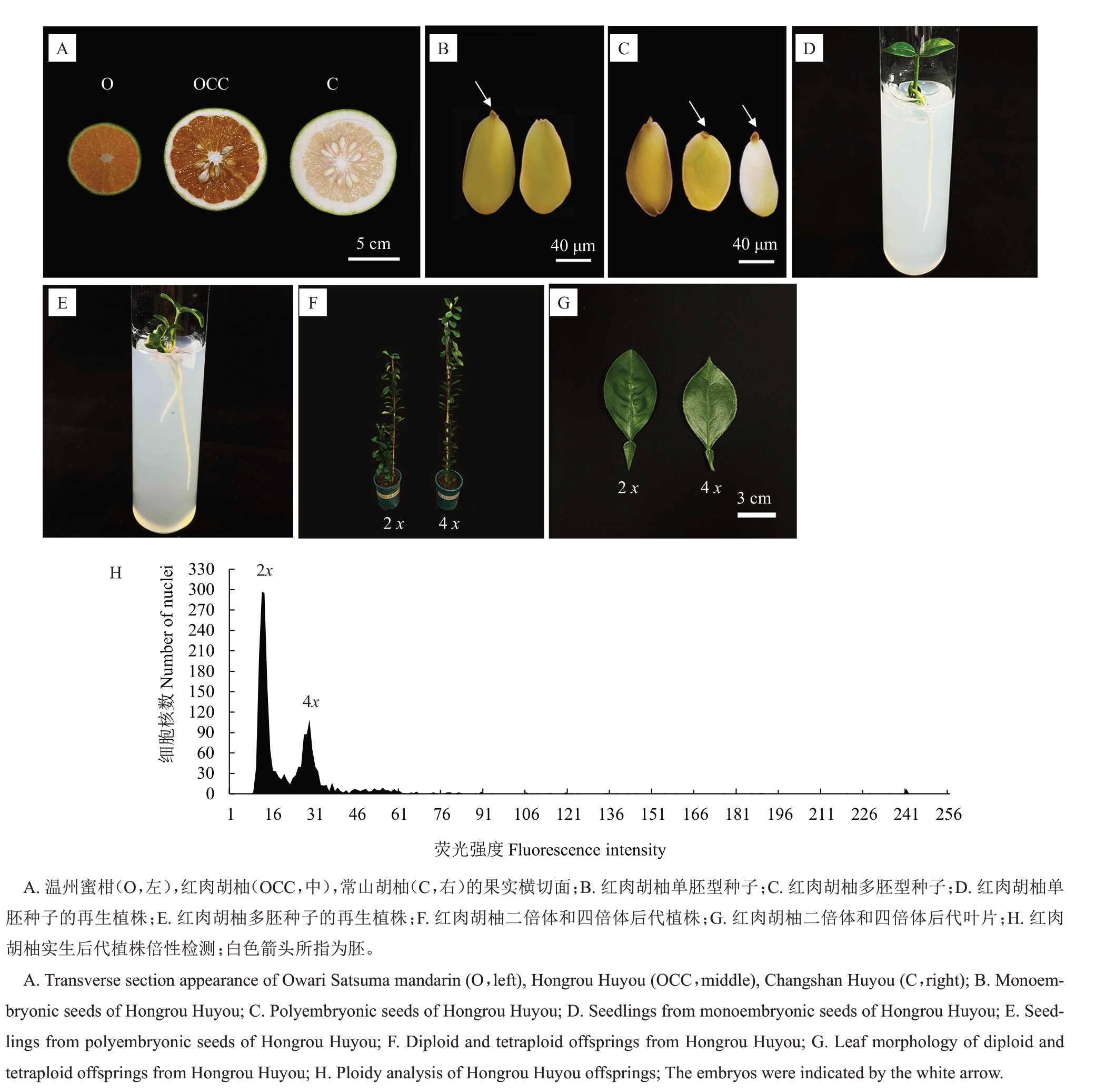
图1 红肉胡柚果实形态、种子表型及植株、实生后代植株倍性检测及叶片形态
Fig.1 Fruit morphology,seed phenotype and plant,ploidy analysis and leaf morphology of Hongrou Huyou offsprings
2.2 红肉胡柚后代植株倍性鉴定
移栽成活的120 株红肉胡柚实生后代单株,参照解凯东等[31]的方法,以成年态红肉胡柚二倍体叶片作为对照,采用流式细胞仪对其进行倍性鉴定。结果表明,120株实生后代中,二倍体119株,四倍体1 株。图1-H 显示成年态红肉胡柚二倍体与四倍体后代叶片等比例混合进样,四倍体后代的荧光强度对应的横坐标数值为二倍体的两倍。
2.3 红肉胡柚实生四倍体后代SSR分子标记鉴定
利用分布于柑橘9条染色体的28对SSR引物分析红肉胡柚实生四倍体后代的遗传组成,表明四倍体单株与红肉胡柚L2供体常山胡柚带型一致,即红肉胡柚四倍体后代具有常山胡柚双二倍体的带型特征。图2显示其中3对引物的扩增图谱。
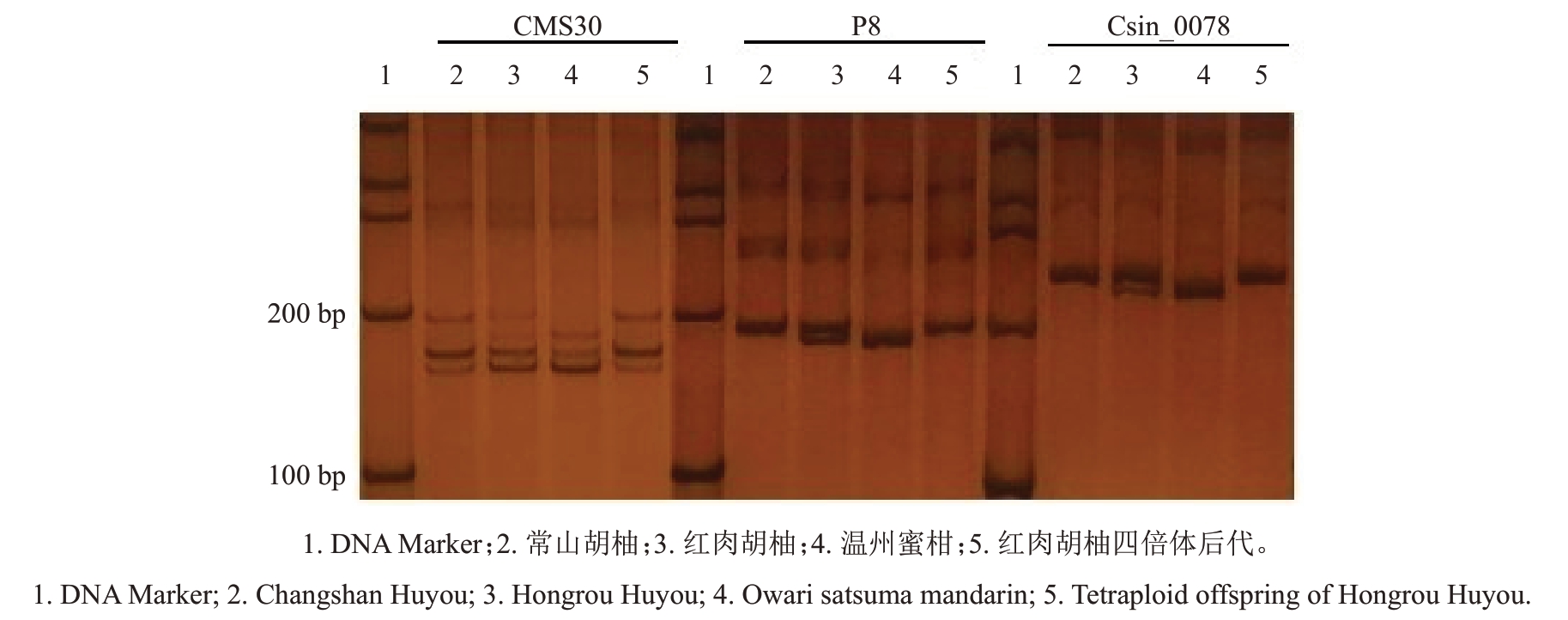
图2 部分实生后代四倍体植株SSR 分子鉴定
Fig.2 SSR analysis of the tetraploid plant in partial and its donor plants
2.4 实生四倍体和二倍体后代叶片形态比较
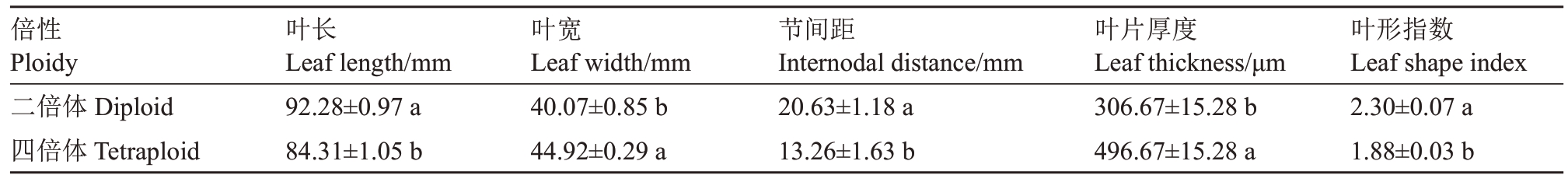
观察结果表明,四倍体叶片相较于二倍体更宽、更厚(图1-F、G)。红肉胡柚四倍体植株的叶宽和叶厚显著大于二倍体,叶长、节间距和叶形指数显著小于二倍体(表2)。
表2 红肉胡柚二倍体与四倍体后代叶片形态比较
Table 2 Comparison of leaf morphology between diploid and tetraploid offsprings of Hongrou Huyou
注:不同小写字母表示差异显著(p<0.05)。下同。
Note:Different small letters indicate significant differences at p<0.05 level.The same below.
倍性Ploidy二倍体Diploid四倍体Tetraploid叶形指数Leaf shape index 2.30±0.07 a 1.88±0.03 b叶长Leaf length/mm 92.28±0.97 a 84.31±1.05 b叶宽Leaf width/mm 40.07±0.85 b 44.92±0.29 a节间距Internodal distance/mm 20.63±1.18 a 13.26±1.63 b叶片厚度Leaf thickness/μm 306.67±15.28 b 496.67±15.28 a
2.5 实生四倍体和二倍体后代叶片解剖学观察
红肉胡柚二倍体和四倍体后代叶片的半薄切片显示,四倍体薄壁细胞面积大于二倍体,且四倍体上表皮、下表皮、栅栏组织、海绵组织的厚度显著大于二倍体,分别增加了47.1%、34.2%、77.0%和64.6%(图3-A~D,表3)。红肉胡柚二倍体和四倍体后代叶片的超薄切片显示,四倍体叶片的细胞增大,胞内脂肪球数量显著高于二倍体,平均密度为40.0个·100 μm-2;淀粉粒数量较二倍体显著减少,平均密度为8.67个·100 μm-2(图3-E~H,表3)。

图3 红肉胡柚二倍体与四倍体后代植株叶片解剖结构、透射电镜结构、叶片气孔表型比较
Fig.3 Comparison of anatomical structures,transmission electron microscopies and stomatal phenotypes of leaves between diploid and tetraploid offsprings from Hongrou Huyou
表3 红肉胡柚二倍体与四倍体后代植株叶片解剖结构参数比较
Table 3 Comparison of leaf anatomical parameters between diploid and tetraploid offsprings of Hongrou Huyou
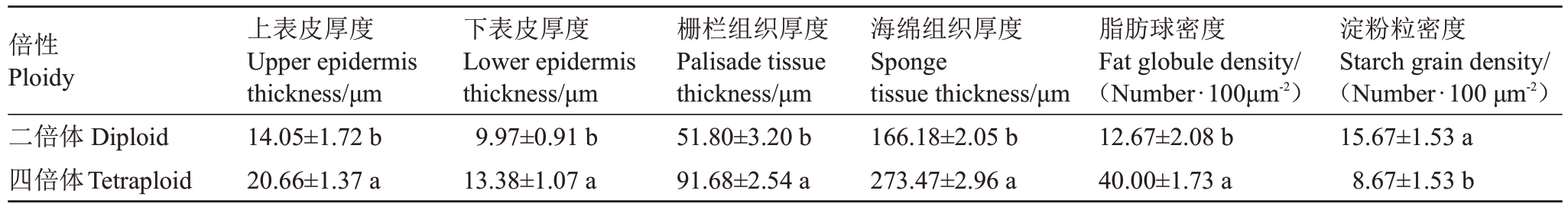
倍性Ploidy二倍体Diploid四倍体Tetraploid上表皮厚度Upper epidermis thickness/μm 14.05±1.72 b 20.66±1.37 a下表皮厚度Lower epidermis thickness/μm 9.97±0.91 b 13.38±1.07 a栅栏组织厚度Palisade tissue thickness/μm 51.80±3.20 b 91.68±2.54 a海绵组织厚度Sponge tissue thickness/μm 166.18±2.05 b 273.47±2.96 a脂肪球密度Fat globule density/(Number·100μm-2)12.67±2.08 b 40.00±1.73 a淀粉粒密度Starch grain density/(Number·100 μm-2)15.67±1.53 a 8.67±1.53 b
2.6 实生四倍体和二倍体后代叶片气孔形态学观察
在扫描电镜下观察红肉胡柚实生二倍体与四倍体后代叶片的气孔,结果表明,二倍体和四倍体叶片下表皮的气孔形状大多数为椭圆形。四倍体的气孔和保卫细胞均显著大于二倍体,四倍体气孔的平均长度为6.76 μm,平均宽度为4.34 μm,四倍体保卫细胞的平均长度为25.07 μm,平均宽度为18.64 μm。四倍体气孔的平均密度为269.80个·mm-2,相较于二倍体显著减少(图3-I~L,表4)。
表4 红肉胡柚二倍体与四倍体后代植株叶片气孔表型比较
Table 4 Comparison of stomatal phenotypes in leaves between diploid and tetraploid offsprings of Hongrou Huyou

倍性Ploidy二倍体Diploid四倍体Tetraploid气孔长Stoma length/μm 5.51±0.69 b 6.76±1.03 a气孔宽Stoma width/μm 3.17±0.52 b 4.34±0.80 a保卫细胞长Guard cell length/μm 17.73±0.99 b 25.07±1.17 a保卫细胞宽Guard cell width/μm 13.66±1.59 b 18.64±2.32 a气孔密度Stomatal density/(No.·mm-2)458.30±28.05 a 269.80±19.03 b
2.7 实生四倍体和二倍体后代光合色素含量比较
对叶片叶绿素和类胡萝卜素含量进行测定,结果表明,红肉胡柚四倍体叶片中的叶绿素含量和总类胡萝卜素含量均显著高于红肉胡柚二倍体。四倍体叶片中的叶绿素a 含量(w,后同)为1.75 mg·g-1,叶绿素b含量为0.75 mg·g-1,叶绿素总含量为2.49 mg·g-1,总类胡萝卜素含量为0.34 mg·g-1,相较于二倍体分别高17.4%、23.0%、19.1%、6.3%(表5)。四倍体植株的叶色相较于二倍体植株更浓绿。
表5 红肉胡柚二倍体与四倍体后代植株叶绿素和类胡萝卜素含量比较
Table 5 Comparison of chlorophyll and carotenoid contents in diploid and tetraploid progeny plants of Hongrou Huyou(mg·g-1)
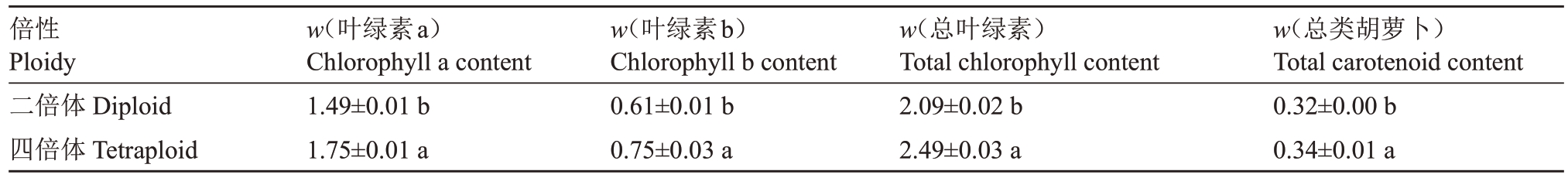
倍性Ploidy二倍体Diploid四倍体Tetraploid w(叶绿素a)Chlorophyll a content 1.49±0.01 b 1.75±0.01 a w(叶绿素b)Chlorophyll b content 0.61±0.01 b 0.75±0.03 a w(总叶绿素)Total chlorophyll content 2.09±0.02 b 2.49±0.03 a w(总类胡萝卜)Total carotenoid content 0.32±0.00 b 0.34±0.01 a
3 讨 论
研究周缘嵌合体实生后代的遗传规律,对嵌合体后代的应用具有重要意义。在双子叶植物中,配子通常由茎尖分生组织L2 层细胞发育而来[29]。徐远涛[13]利用开发的InDel标记,对红肉桃叶橙嵌合体实生后代遗传组成进行研究,结果表明红肉桃叶橙外种皮、子叶、胚以及实生苗的遗传组成与L2 供体桃叶橙一致。Goffreda等[35]在研究野生番茄(Lycopersicon pennellii)和栽培番茄品种(Lycopersicon esculentum)种间嵌合体时,通过细胞层标记基因和颜色标记分析嵌合体自交后代,发现后代植株只有一种基因型,且与L2供体基因型一致。笔者在本研究中利用位于柑橘9 条染色体的28 对SSR 引物对红肉胡柚实生四倍体后代进行分子标记鉴定,结果显示四倍体单株与红肉胡柚L2 供体常山胡柚带型一致,与徐远涛[13]的研究结果是一致的。
柑橘实生四倍体发生途径主要有珠心细胞自然加倍产生同源四倍体,或通过染色体未减数2n雌配子与2n 花粉自然授粉产生异源/同源四倍体,以及合子自然加倍产生四倍体[36]。红肉胡柚及其嫁接供体品种均为二倍体,生长栽培环境附近不存在多倍体柑橘品种,红肉胡柚是单多胚并存的柑橘品种,其实生子代可能存在杂交子代和珠心苗两种情况。因此,笔者在本研究中获得的1 株四倍体后代植株可能是通过自身未减数的2n花粉与2n雌配子自交途径形成的,也可能由红肉胡柚珠心细胞自然加倍而成。然而,曹丽雯[37]在研究榨菜(Brassica juncea)和紫甘蓝(Brassica oleracea)合成的周缘嵌合体时,发现与L2层供体榨菜相比,部分自交后代植株叶形发生变异,表现出趋向于L1 供体紫甘蓝的叶形特征,而且自交后代植株在siRNA 数量和表达量以及CHH甲基化等表观遗传方面发生显著改变,体现出周缘嵌合体层间细胞互作。虽然分子标记分析表明笔者在本研究中发掘的四倍体单株只具备L2 供体常山胡柚的SSR带型特征,但L1供体温州蜜柑对红肉胡柚实生四倍体后代是否存在表观遗传等方面的影响,还需要进一步研究。
植物四倍体较二倍体会有明显的形态差异,通常表现为叶片变宽、变厚及叶形指数变小,同时抗逆性更强[16]。在本研究中,红肉胡柚四倍体的叶宽及上下表皮、栅栏组织和海绵组织厚度较二倍体显著增大,四倍体叶片叶绿素含量和类胡萝卜素含量显著高于二倍体,且四倍体脂肪球数量丰富,淀粉粒数量较二倍体显著降低,这些特点有利于净光合速率、电子传递量子效率和光化学猝灭系数的提高,有助于叶片进行光合作用[22]。在本研究中,四倍体气孔密度显著减小,气孔和保卫细胞大小较二倍体显著增大;Ren等[14]对秋水仙素诱导获得的秋辉橘、黄陵庙蜜橘、琥珀甜橙、HB 柚四倍体与其二倍体进行形态差异比较,表明气孔密度随着植物倍性增加而显著减小。
4 结 论
笔者在本研究中从柑橘周缘嵌合体红肉胡柚实生后代中发掘到1 株四倍体,分子标记鉴定其遗传组成与红肉胡柚L2 供体常山胡柚一致。对该四倍体单株进行了形态学、解剖学、色素含量等的评价,为未来培育三倍体无核新品种提供了四倍体新种质。
[1] 梁武军,解凯东,谢宗周,徐强,伊华林,郭文武.三倍体葡萄柚实生后代多倍体的发掘与SSR 遗传鉴定[J].果树学报,2015,32(1):13-18.LIANG Wujun,XIE Kaidong,XIE Zongzhou,XU Qiang,YI Hualin,GUO Wenwu.Exploitation of polyploids from open-pollinated triploid grapefruit progenies and their genetic identifica-tion by SSR molecular markers[J]. Journal of Fruit Science,2015,32(1):13-18.
[2] ZHANG M,ZHANG Z Q,WU Q,KE F Z,XU J G,ZHAO S Q,WANG G,ZHANG C. Chimerism evaluation of‘Hongrou Huyou’,a grafted chimera between Citrus changshan-huyou and Citrus unshiu[J]. Horticultural Science and Technology,2020,38(1):107-117.
[3] SCHMIDT A. Histologische studien an phanerogamen vegetations-punkten[J].Bot Arch,1924(8):345-404.
[4] YU N N,CAO L W,YUAN L,ZHI X,CHEN Y Q,GAN S S,CHEN L P. Maintenance of grafting-induced epigenetic variations in the asexual progeny of Brassica oleracea and B. juncea chimera[J].The Plant Journal,2018,96(1):22-38.
[5] CHEN L P,GE Y M,ZHU X Y.Artificial synthesis of interspecific chimeras between tuber mustard(Brassica juncea)and cabbage (Brassica oleracea) and cytological analysis[J]. Plant Cell Reports,2006,25(9):907-913.
[6] ZHANG M,DENG X X,QIN C P,CHEN C L,ZHANG H Y,LIU Q,HU Z Y,GUO L L,SONG W H,TAN Y,LIAO S C.Characterization of a new natural periclinal navel-satsuma chimera of citrus:‘Zaohong’navel orange[J].Journal of the American Society for Horticultural Science,2007,132(3):374-380.
[7] ZHANG M,XIE Z Z,DENG X X,LIAO S C,SONG W H,TAN Y. Characteristics of‘Hongrou Taoye’,a grafted chimera in sweet orange and satsuma mandarin[J]. Horticultural Science and Technology,2015,33(3):390-395.
[8] YASUDA K,KUNITAKE H,NAKAGAWA S,KUROGI H,YAHATA M,HIRATA R,YOSHIKURA Y,KAWAKAMI I,SUGIMOTO Y.The confirmation of ploidy periclinal Chimera and its morphological characteristics in meiwa kumquat‘Yubeni’[J].Horticultural Research(Japan),2008,7(2):165-171.
[9] NUKAYA T,SUDO M,YAHATA M,OHTA T,TOMINAGA A,MUKAI H,YASUDA K,KUNITAKE H.The confirmation of a ploidy periclinal chimera of the meiwa kumquat (Fortunella crassifolia Swingle) induced by colchicine treatment to nucellar embryos and its morphological characteristics[J]. Agronomy,2019,9(9):562.
[10] SCHMÜLLING T,SCHELL J.Transgenic tobacco plants regenerated from leaf disks can be periclinal chimeras[J]. Plant Molecular Biology,1993,21(4):705-708.
[11] ZONNEVELD B J M. Nuclear DNA content of ploidy chimeras of Hosta Tratt.(Hostaceae)demonstrate three apical layers in all organs,but not in the adventitious root[J].Plant Systematics and Evolution,2007,269(1):29-38.
[12] 周锐,解凯东,王伟,彭珺,谢善鹏,胡益波,伍小萌,郭文武.依据多倍体形态特征快速高效发掘柑橘四倍体[J].园艺学报,2020,47(12):2451-2458.ZHOU Rui,XIE Kaidong,WANG Wei,PENG Jun,XIE Shanpeng,HU Yibo,WU Xiaomeng,GUO Wenwu.Efficient identification of tetraploid plants from seedling populations of apomictic citrus genotypes based on morphological characteristics[J].Acta Horticulturae Sinica,2020,47(12):2451-2458.
[13] 徐远涛.柑橘无融合生殖和嫁接嵌合的分子基础研究[D].武汉:华中农业大学,2019.XU Yuantao. Molecular basis of apomixis and grafting chimerism in citrus[D]. Wuhan:Huazhong Agricultural University,2019.
[14] REN J,LU X,DUAN Y Y,XIAO G A,XIE K D,WU X M,GUO W W. In vivo tetraploid induction of mono-embryonic citrus genotypes by colchicine treatment[J].Scientia Horticulturae,2024,338:113701.
[15] RUIZ M,QUIÑONES A,MARTÍNEZ-ALCÁNTARA B,ALEZA P,MORILLON R,NAVARRO L,PRIMO- MILLO E,MARTÍNEZ-CUENCA M R. Effects of salinity on diploid (2x)and doubled diploid (4x) Citrus macrophylla genotypes[J]. Scientia Horticulturae,2016,207:33-40.
[16] WEI T L,WANG Y,LIU J H.Comparative transcriptome analysis reveals synergistic and disparate defense pathways in the leaves and roots of trifoliate orange(Poncirus trifoliata)autotetraploids with enhanced salt tolerance[J]. Horticulture Research,2020,7(1):88.
[17] RUIZ M,QUIÑONES A,MARTÍNEZ-ALCÁNTARA B,ALEZA P,MORILLON R,NAVARRO L,PRIMO- MILLO E,MARTÍNEZ-CUENCA M R. Tetraploidy enhances boron-excess tolerance in carrizo citrange(Citrus sinensis L.Osb.×Poncirus trifoliata L. Raf.)[J]. Frontiers in Plant Science,2016,7:701.
[18] BALAL R M,SHAHID M A,VINCENT C,ZOTARELLI L,LIU G D,MATTSON N S,RATHINASABAPATHI B,MARTÍNEZ-NICOLAS J J,GARCIA-SANCHEZ F. Kinnow mandarin plants grafted on tetraploid rootstocks are more tolerant to Cr-toxicity than those grafted on its diploids one[J].Environmental and Experimental Botany,2017,140:8-18.
[19] ALLARIO T,BRUMOS J,COLMENERO-FLORES J M,IGLESIAS D J,PINA J A,NAVARRO L,TALON M,OLLITRAULT P,MORILLON R. Tetraploid Rangpur lime rootstock increases drought tolerance via enhanced constitutive root abscisic acid production[J].Plant,Cell&Environment,2013,36(4):856-868.
[20] OUSTRIC J,MORILLON R,LURO F,HERBETTE S,LOURKISTI R,GIANNETTINI J,BERTI L,SANTINI J.Tetraploid Carrizo citrange rootstock (Citrus sinensis Osb. × Poncirus trifoliata L. Raf.) enhances natural chilling stress tolerance of common clementine (Citrus clementina Hort. ex Tan.)[J].Journal of Plant Physiology,2017,214:108-115.
[21] DAHRO B,LI C L,LIU J H. Overlapping responses to multiple abiotic stresses in citrus:From mechanism understanding to genetic improvement[J].Horticulture Advances,2023,1(1):4.
[22] XUE H,ZHANG B,TIAN J R,CHEN M M,ZHANG Y Y,ZHANG Z H,MA Y. Comparison of the morphology,growth and development of diploid and autotetraploid‘Hanfu’apple trees[J].Scientia Horticulturae,2017,225:277-285.
[23] SUDO M,YASUDA K,YAHATA M,SATO M,TOMINAGA A,MUKAI H,MA G,KATO M,KUNITAKE H. Morphological characteristics,fruit qualities and evaluation of reproductive functions in autotetraploid satsuma mandarin (Citrus unshiu Marcow.)[J].Agronomy,2021,11(12):2441.
[24] XIE K D,YUAN D Y,WANG W,XIA Q M,WU X M,CHEN C W,CHEN C L,GROSSER J W,GUO W W. Citrus triploid recovery based on 2x × 4x crosses via an optimized embryo rescue approach[J].Scientia Horticulturae,2019,252:104-109.
[25] ALEZA P,FROELICHER Y,SCHWARZ S,AGUSTÍ M,HERNÁNDEZ M,JUÁREZ J,LURO F,MORILLON R,NAVARRO L,OLLITRAULT P. Tetraploidization events by chromosome doubling of nucellar cells are frequent in apomictic citrus and are dependent on genotype and environment[J]. Annals of Botany,2011,108(1):37-50.
[26] 谢善鹏,解凯东,夏强明,周锐,张成磊,郑浩,伍小萌,郭文武.柑橘6 个地方品种资源四倍体高效发掘及分子鉴定[J].果树学报,2022,39(1):1-9.XIE Shanpeng,XIE Kaidong,XIA Qiangming,ZHOU Rui,ZHANG Chenglei,ZHENG Hao,WU Xiaomeng,GUO Wenwu. Efficient exploration and SSR identification of 53 doubled diploid seedlings from six local citrus cultivars and germplasm resources[J].Journal of Fruit Science,2022,39(1):1-9.
[27] ALEZA P,JUÁREZ J,OLLITRAULT P,NAVARRO L.Production of tetraploid plants of non apomictic citrus genotypes[J].Plant Cell Reports,2009,28(12):1837-1846.
[28] ZHANG M,JING L Y,WU Q,ZHU K J,KE F Z,XU J G,ZHAO S Q,WANG G,ZHANG C. Metabolite profile comparison of a graft chimera‘Hongrou Huyou’(Citrus changshanhuyou + Citrus unshiu) and its two donor plants[J]. BMC Plant Biology,2019,19(1):582.
[29] MARCOTRIGIANO M,BERNATZKY R. Arrangement of cell layers in the shoot apical meristems of periclinal chimeras influences cell fate[J].The Plant Journal,1995,7(2):193-202.
[30] 张斯淇.柑橘无融合生殖的遗传分析和相关基因挖掘[D].武汉:华中农业大学,2017.ZHANG Siqi.Genetic analysis of citrus apomixis and its related genes discovery[D].Wuhan:Huazhong Agricultural University,2017.
[31] 解凯东,王惠芹,王晓培,梁武军,谢宗周,伊华林,邓秀新,GROSSER J W,郭文武.单胚性二倍体为母本与异源四倍体杂交大规模创制柑橘三倍体[J].中国农业科学,2013,46(21):4550-4557.XIE Kaidong,WANG Huiqin,WANG Xiaopei,LIANG Wujun,XIE Zongzhou,YI Hualin,DENG Xiuxin,GROSSER J W,GUO Wenwu. Extensive Citrus triploid breeding by crossing monoembryonic diploid females with allotetraploid male parents[J].Scientia Agricultura Sinica,2013,46(21):4550-4557.
[32] 郭雁君,曾继武,胡亚平,郭丽英,蒋惠,周希琴,吉前华.基于SSR 标记的肇庆地区柑橘品种分类地位研究[J].中国农学通报,2014,30(4):137-143.GUO Yanjun,ZENG Jiwu,HU Yaping,GUO Liying,JIANG Hui,ZHOU Xiqin,JI Qianhua. Classification of Zhaoqing local citrus germplasm resources based on simple sequence repeat molecular marker analysis[J]. Chinese Agricultural Science Bulletin,2014,30(4):137-143.
[33] 李益,马先锋,唐浩,李娜,江东,龙桂友,李大志,牛英,韩瑞玺,邓子牛.柑橘品种鉴定的SSR 标记开发和指纹图谱库构建[J].中国农业科学,2018,51(15):149-159.LI Yi,MA Xianfeng,TANG Hao,LI Na,JIANG Dong,LONG Guiyou,LI Dazhi,NIU Ying,HAN Ruixi,DENG Ziniu. SSR markers screening for identification of Citrus cultivar and construction of DNA fingerprinting library[J]. Scientia Agricultura Sinica,2018,51(15):149-159.
[34] 张泽群.‘红肉胡柚’起源及特征分析[D].杭州:浙江农林大学,2019.ZHANG Zequn. Analysis on the origin and characteristics of‘Hongrou Huyou’[D]. Hangzhou:Zhejiang A & F University,2019.
[35] GOFFREDA J C,SZYMKOWIAK E J,SUSSEX I M,MUTSCHLER M A.Chimeric tomato plants show that aphid resistance and triacylglucose production are epidermal autonomous characters[J].The Plant Cell,1990,2(7):643-649.
[36] 彭滢,李晓妍,肖璇.柑橘多胚性砧木枳橙同源四倍体的发掘与SSR 鉴定[J].分子植物育种,2020,18(4):1211-1215.PENG Ying,LI Xiaoyan,XIAO Xuan. Excavation and SSR identification of autotetraploids in citrus polyembryonic rootstock‘Citrange’[J]. Molecular Plant Breeding,2020,18(4):1211-1215.
[37] 曹丽雯.榨菜与紫甘蓝嵌合体后代变异性状发生的分子机理研究[D].杭州:浙江大学,2018.CAO Liwen.Studies on the molecular mechanism of phenotypic variations in the progenies of chimeras produced by in vitro grafting between Brassica juncea and B. oleracea[D]. Hangzhou:Zhejiang University,2018.