近年来,受全球气候变化和人类生产活动的影响,我国土壤盐渍化范围不断扩大,而苹果适宜的生长环境是弱酸性或中性土壤,土壤盐渍化使苹果的生长和果实产量及品质都面临着诸多挑战,影响我国苹果产业的健康发展,这已成为限制苹果产业发展的重要因素之一[1]。盐胁迫主要通过引起渗透胁迫、离子毒害和氧化胁迫等方式影响植物的生长和发育[2]。遭受盐害的植株生长减缓,叶绿体结构遭到破坏,叶尖、叶缘或叶脉变黄并逐渐萎蔫脱落,主根变短,须根减少,若植物长期受到高浓度盐胁迫,植物的根系结构也会被破坏,导致根系无法正常从外界吸收营养物质,造成植株发黄腐烂、停止生长甚至死亡[3]。此外,在盐胁迫下植物体内产生的氧化胁迫导致光合速率下降,影响植物蒸腾作用,从而影响植物水分和营养物质的运输[4]。同时盐胁迫还会使植株遭受渗透、水分和氧化胁迫,甚至会由于调节失衡而产生离子毒害,影响植株对其他营养物质的吸收,导致营养失衡[5]。受到盐胁迫的植物细胞内的Na+和Cl-积累过量,会造成活性氧的产生与清除之间的动态平衡失衡,引起脂质过氧化和脱脂作用,伤害膜蛋白和膜脂,从而破坏膜结构[6]。随着盐胁迫的加重,植物的生长量、干鲜质量、光合能力、碳水化合物含量和叶绿素含量等都呈现降低趋势。已有研究证实,提高植物抗氧化系统酶活性及提升抗氧化代谢水平是提高植物耐盐能力的途径之一[7]。植物受到盐胁迫时会产生和积累渗透调节物质保证机体渗透平衡和内部稳定,包括游离氨基酸、可溶性糖等。其中游离氨基酸脯氨酸是关键渗透调节物质之一,在植物响应逆境胁迫中起重要作用。研究表明,盐胁迫下植物体内脯氨酸含量有所增加,且外源施加脯氨酸能够改变植物的耐盐能力[8]。
苹果实生苗的耐盐性较弱,而砧木可以为苹果提供根系,砧木品质直接影响果树对养分的吸收及对逆境的适应能力,因此砧木是不同砧穗组合耐盐能力的主要决定因素[9]。在同一盐浓度下,当砧木的相对生长量与盐害指数成反比时,砧木的耐盐能力就越强,并且同一砧木嫁接不同品种后的耐盐能力基本一致[10]。因此在生产实践中,通常通过嫁接耐盐性较高的砧木来增强整株的耐盐性。有研究报道,柑橘不同砧木间以及嫁接接穗后树体养分吸收及抗逆性均存在较大差异[11]。过去,我国苹果生产常用由原产地自然条件下形成的乔化砧木,其对气候和土壤适应能力强,抗性强,寿命长,如山定子、海棠和富平楸子等[12-13]。但由于乔化栽培存在树体骨架大,成形慢,整形修剪技术复杂,操作难度大等生产局限,逐渐被矮化密植所替代[14]。近年来利用从国外引进的M9-T337、B9 和Pajam2 等矮化自根砧栽培模式在各苹果主产区逐步推广[15],该模式相较于过去国内常用的乔化砧、矮化中间砧栽培模式,具有矮化效果显著、苗木整齐度高、节省劳动力及早果丰产性好等显著优势[16],然而多数引进的矮化砧木源于气候相对较为温和、土壤肥沃的西欧平原,所以并不具备很强的耐逆境胁迫等特性,从国外引进的砧木在国内的适应能力有待考查[17]。有研究表明,国内外常用的矮化砧木M9 嫁接亲和力高、对土壤适应能力强,但抗旱、抗寒性较差,在我国许多苹果栽培地区容易抽条[18];砧木JM 系矮化效果明显,适应性广,但繁殖速度慢,对自然灾害抵抗力弱,且很难通过扦插繁殖[19-20];生产中常用的G 系矮化砧木早实丰产性好、抗重茬,但根系较浅,抗逆性较差,在再植条件下树势衰弱明显[21]。
笔者所在的苹果抗逆与品质改良创新团队前期得到了多株以富平楸子与R3(美国康奈尔大学和Geneva 试验站选育的矮化砧木,亲本为M27× Robusta 5,树体生长表现良好,适应性较强[22])为亲本的杂交1年生盆栽幼苗,经过干旱胁迫处理,从中筛选出抗旱和干旱敏感株系k15 和m2[23],分别建立外植体,并选择适宜的继代及生根培养基,成功扩繁出了两个砧木株系。为进一步探索其综合抗性,笔者以此砧木株系(k15和m2)、生产中应用广泛的砧木(M9-T337和M26)及其亲本(富平楸子)5种砧木资源为试验材料,对其耐盐性进行研究,分别测定了处理结束时的植株生长指标、生理指标、光合相关指标、Na+和K+含量、抗氧化酶活性及氨基酸含量,并利用隶属函数法对5种砧木资源耐盐能力进行综合评价,以期选育出适合我国土壤和环境的综合抗逆性较强的苹果矮化砧木。
1 材料和方法
1.1 材料
以苹果砧木富平楸子×R3 的杂交后代经抗性筛选出的砧木株系k15、m2、亲本富平楸子及生产上应用广泛的砧木M9-T337 和M26,5 种无性繁殖的组培苗为试验材料。
1.2 方法
本试验盐胁迫处理在西北农林科技大学苹果抗逆与品质改良创新团队土肥楼水培室内进行。将上述材料扩繁至足够数量后,移至生根培养基中生长40 d,经开盖炼苗后移栽到8 cm × 8 cm 的营养钵中,钵内装有(V 基质∶V 蛭石∶V 珍珠岩=4∶1∶1)的混合物,置于恒温光照培养箱中。待幼苗长出7~8片完全展开的真叶后,将其移至装有6.5 L 1/2浓度Hoagland营养液的水培盆中,每3 d换一次营养液。经过1周的预适应,选取大小一致且健康的植株进行水培盐处理。处理分为两组:(1)对照(CK),1/2 Hoagland营养液;(2)盐处理(ST),NaCl浓度为150 mmol·L-1。两组的pH 均用H3PO4调至为6.5±0.2。每3 d 更换1 次营养液,处理时间为15 d。处理结束时,分别取鲜样、冻样和干样,用于指标测定。
1.3 生长指标测定
株高的测定:处理结束时,对照组和处理组分别取5株植株,用直尺测定株高(从根茎连接处到植株顶芽的距离)。
叶片数的测定:准确查数并记录叶片数。
干鲜质量的测定:将植株分为根、茎、叶三部分,用清水洗净并擦干,用万分之一天平测定各部分鲜质量,放入烘箱105 ℃杀青30 min,然后转至65 ℃下烘干至恒质量,再用万分之一天平测定各部分干质量。
1.4 生理指标测定
根系构型:处理结束时,将植株根部洗净并擦干,利用WinRHIZO根系扫描仪分析植株根部构型。
相对电导率测定:取相同部位新鲜功能叶,用打孔器在叶片上打10 个圆片,注意避开叶脉,装入放有10 mL 纯净水的15 mL 离心管中,浸泡4 h,其间不断震荡混匀,利用电导率仪测量电导率,记为S1,而后沸水浴20 min,冷却至室温后震荡混匀,再次测量电导率,记为S2,测量纯净水的电导率,记为S0,计算叶片相对电导率,公式如下:相对电导率(REL)/%=(S1-S0)/(S2-S0)×100。
丙二醛(MDA)含量测定:按照生产厂家说明书(苏州科铭生物技术有限公司,江苏苏州),利用MDA检测试剂盒进行测定。
根系活力测定:处理结束时,取植株新鲜白根,按照生产厂家说明书(苏州科铭生物技术有限公司,江苏苏州)测定反映根系活力的脱氢酶(DHA)活性。
1.5 光合相关指标测定
光合参数测定:分别在处理的第0天、第3天、第6 天、第9 天和第12 天利用CIRAS-3 便携式光合系统仪(CIRAS,amesbury,MA,USA),测定同一位置功能叶片的净光合速率Pn、蒸腾速率Tr、气孔导度Gs和胞间CO2浓度Ci。
叶绿素含量测定:处理结束时,取新鲜叶片洗净擦干后,用剪刀将其剪成细丝状(避开叶脉),称取0.1 g 置于准备好的8 mL 80%丙酮中,暗处理24 h,其间不断震荡,使样品充分接触丙酮。待叶片组织完全脱色变白,吸取1 mL 于比色皿,利用UV-2600分光光度计,测定663 nm、645 nm和470 nm波长下的吸光值[24]。
叶绿素荧光参数测定:处理结束时,取新鲜功能叶,暗处理30 min,利用叶绿素荧光成像系统,测定荧光参数并拍摄荧光图片。
1.6 活性氧及抗氧化酶活性测定
活性氧积累:处理结束时,取新鲜叶片进行NBT、DAB 化学染色,观察H2O2和O2-积累情况,并利用H2O2和O2-检测试剂盒(苏州格锐思生物科技有限公司,江苏苏州)分别测定叶片组织中H2O2和O2-含量。
抗氧化酶活性测定:将处理结束时存好的叶片冻样研磨,称取0.1 g,利用相应试剂盒(苏州格锐思生物科技有限公司,江苏苏州)分别测定超氧化物歧化酶(SOD)、过氧化物酶(POD)和过氧化氢酶(CAT)活性。
1.7 Na+、K+含量测定
将处理结束时存好的根茎叶干样研磨过筛,称取0.1 g 于消解管中,加入硝酸,利用微波消解炉充分消化后,去离子水定容至50 mL,利用M410 火焰光度计测定Na+、K+含量。
1.8 氨基酸含量测定
将处理结束时的叶片冻样研磨,称取0.1 g,浸泡于1 mL 50%的乙醇中,4 ℃摇匀,离心机设置为12 000 r·min-1,时间10 min,过滤杂质,取上清液,用甲醇稀释,并保存在样品瓶中。使用高效液相色谱(HPLS-MS)系统测定氨基酸含量,色谱柱为Inertsil ODS-4 C18(4.6 mm × 250 mm,5 μm),流速设定为0.3 mL·min-1。洗脱溶剂体系为含有0.1%甲酸的超纯水(A)和乙腈(B)[6]。
1.9 隶属函数的综合分析
考虑到本试验的试验材料遗传背景存在差异,各项指标存在较大差异,故利用对照组和盐胁迫组各项指标的相对变化率进行隶属函数的计算,以评价其耐盐能力。
若该指标与耐盐能力呈正相关,该指标的隶属函数计算公式为:U(X)=(X-Xmin)/(Xmax-Xmin);若该指标与耐盐能力呈负相关,该指标的隶属函数计算公式为:U(X)=1-(X-Xmin)/(Xmax-Xmin)。式中,U(X)为隶属函数值,X 指某一指标的相对变化率([SST-SCK]/SCK×100);Xmax指某一指标相对变化率的最大值,Xmin指某一指标相对变化率的最小值。在笔者测定的指标中,与耐盐能力负相关的指标有相对电导率、MDA 含量、过氧化氢含量和Na+/K+,其余指标与耐盐能力均呈正相关。
1.10 数据分析
使用SPSS 26.0进行数据的统计分析,并使用单因素分析和Tukey的多重比较(p<0.05)进行差异显著性分析;利用Origin 2021绘图。
2 结果与分析
2.1 盐胁迫下植株表型及生长指标分析
由图1 可知,处理结束时,对照组无明显差异;盐处理组胁迫表型出现的时间存在一定差异,M26表型出现最早,第9 天时叶片便出现严重的萎蔫和坏死,其次是m2叶片在第10天出现褐斑,而后富平楸子、k15 和M9-T337 在处理的第12 天陆续出现盐胁迫表型,但处理结束时富平楸子和k15 只是上部叶片出现明显褐斑,而M9-T337叶片已出现大面积坏死,植株萎蔫。
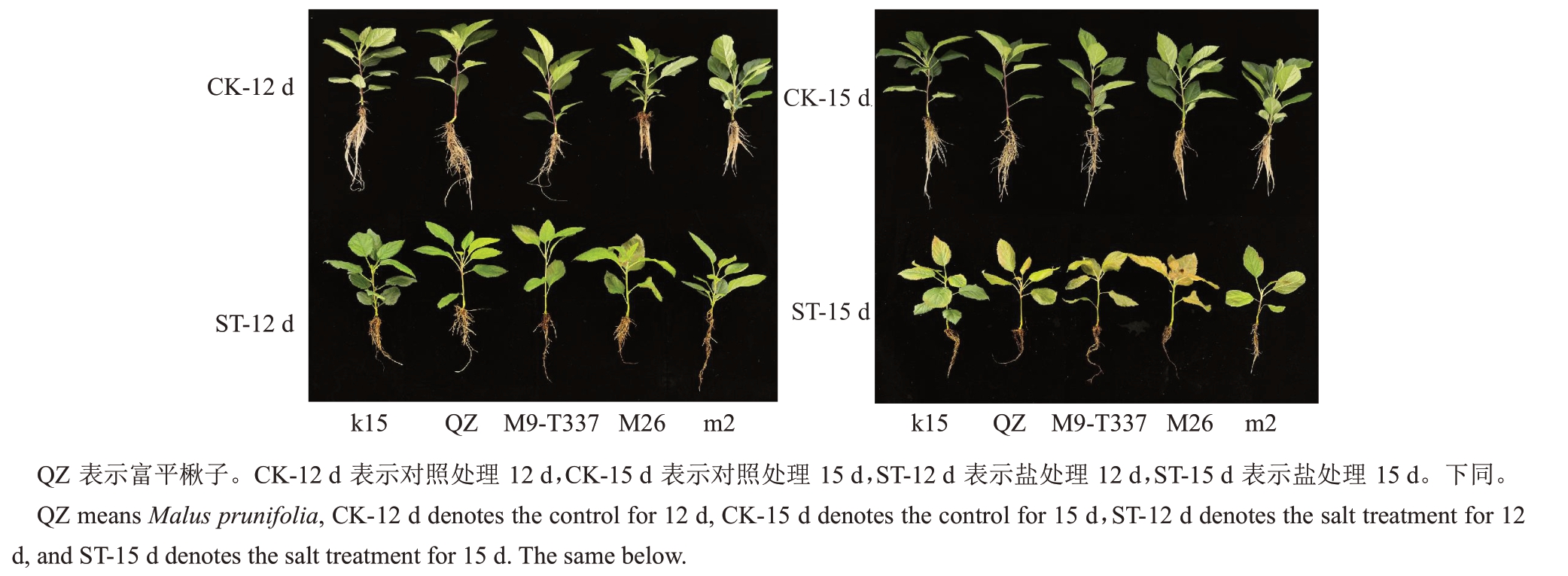
图1 盐胁迫下各株系表型图
Fig.1 Phenotypic of each strain under salt stress
从干鲜质量上看,与对照组相比,处理组各株系均明显降低,其中k15降幅最小,干鲜质量分别下降43.14%和22.87%;M26 降幅最大,分别显著降低71.11%和52.27%(图2-a~b)。
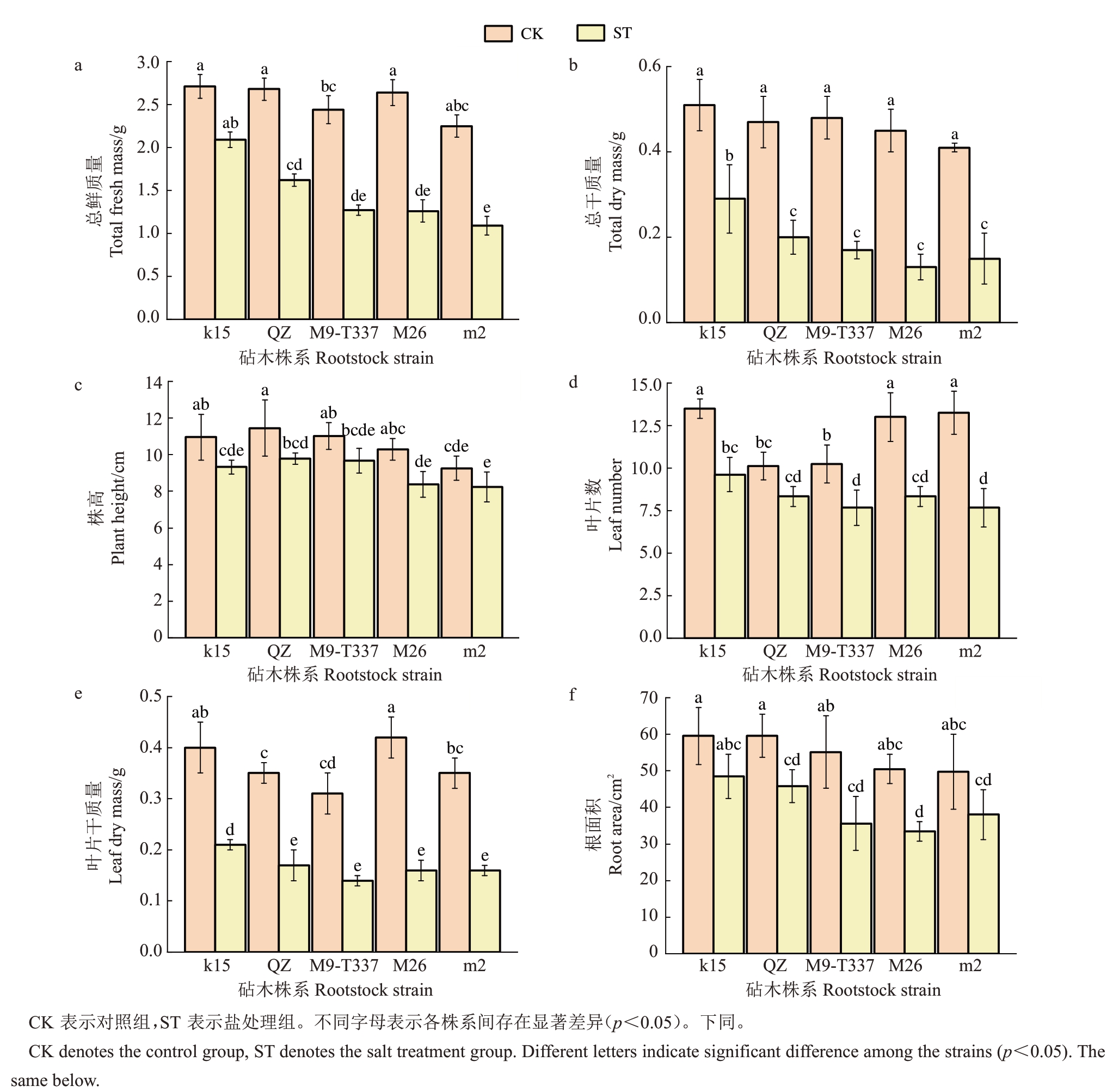
图2 盐胁迫下各株系生长量变化
Fig.2 Growth changes of each strain under salt stress
从株高、叶片数上看,与对照组相比,处理组各株系均有所下降(图2-c~d)。但值得注意的是,k15和m2相对来说节间较短(株高一定的情况下,叶片数相对较多),说明两个砧木株系自身存在一定的矮化现象。
从根部状态看,盐处理组根系均呈褐色,但k15根系明显更密,根面积下降幅度最小,仅下降18.6%,M9-T337根面积减少幅度最大,为31.6%(图2-f)。
2.2 盐胁迫下植株生理指标分析
通过比较相对电导率、MDA 含量以及根系活力,发现与对照组相比,盐处理组各株系相对电导率和MDA含量显著上升,根系活力显著降低。其中,相比于对照组,处理组k15 的相对电导率上升幅度最小,为42.6%,处理组M26 上升幅度最大,为55.1%(图3-a);盐处理后的MDA含量,富平楸子和k15 显著低于其他3 个株系,与对照组相比,分别显著增加了28.7%和27.9%,M9-T337 上升幅度最大,为54.3%(图3-b)。相比于对照组,处理组k15 根系活力下降幅度最小,其次为m2 和M9-T337,下降幅度分别为30.9%、33.3%和40.0%(图3-c)。
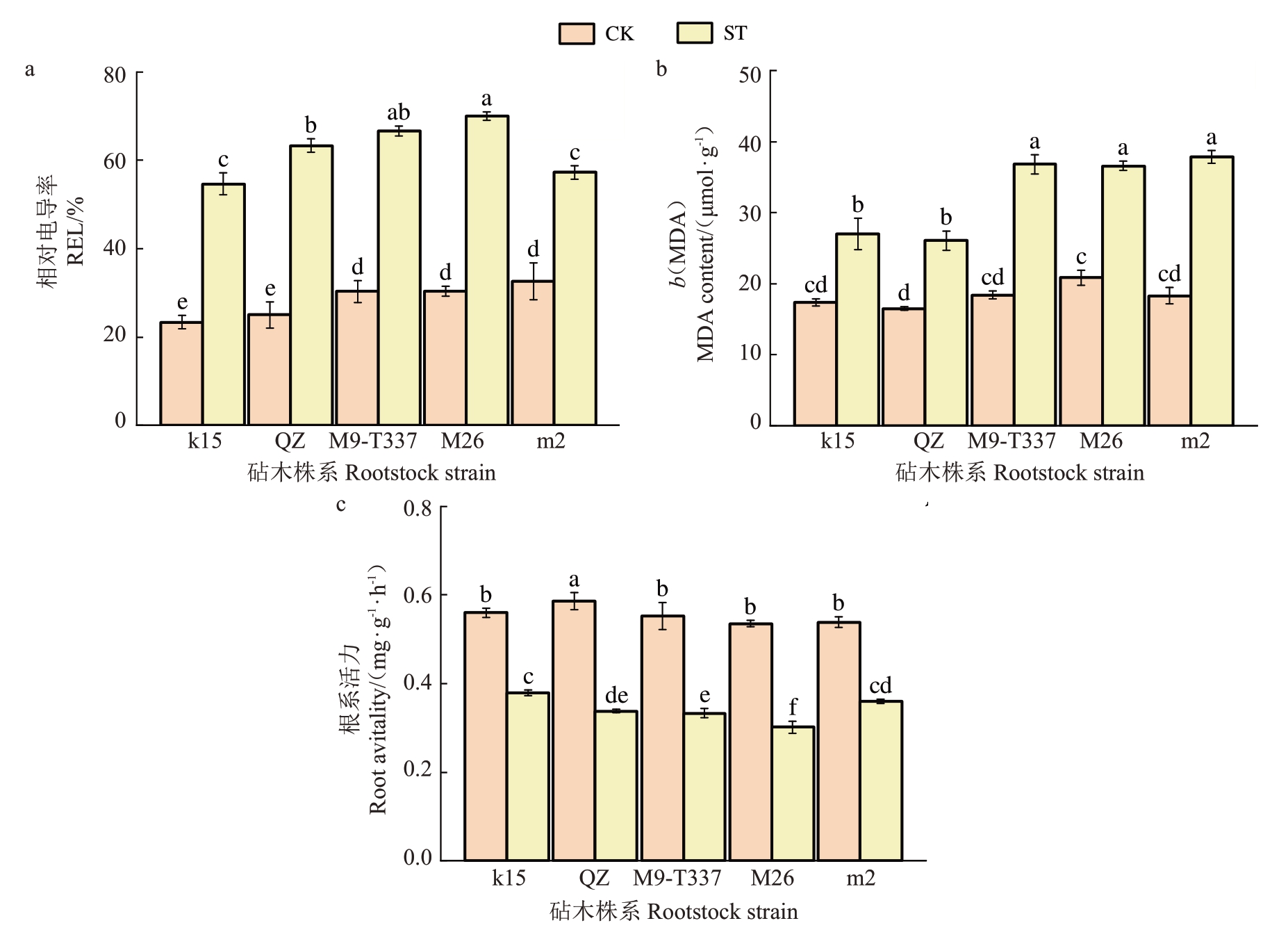
图3 盐胁迫对各株系相对电导率、MDA 含量及根系活力的影响
Fig.3 Effects of salt stress on relative electrical conductivity,MDA content and root vitality of each strain
2.3 盐胁迫下活性氧含量和抗氧化酶活性分析
为评价盐胁迫下各株系的抗氧化能力,对NBT和DAB 进行了染色,并测定了O2-和H2O2含量。从NBT染色图上来看,k15、富平楸子和M9-T337蓝色斑点明显少于M26 和m2(图4-a);从DAB 染色来看,k15 棕色斑点明显少于其他株系(图4-b);从O2-和H2O2含量上看,盐胁迫导致各株系均积累了一定量的O2-和H2O2,其中M26 积累量最多,富平楸子积累量最少(图4-c~d)。
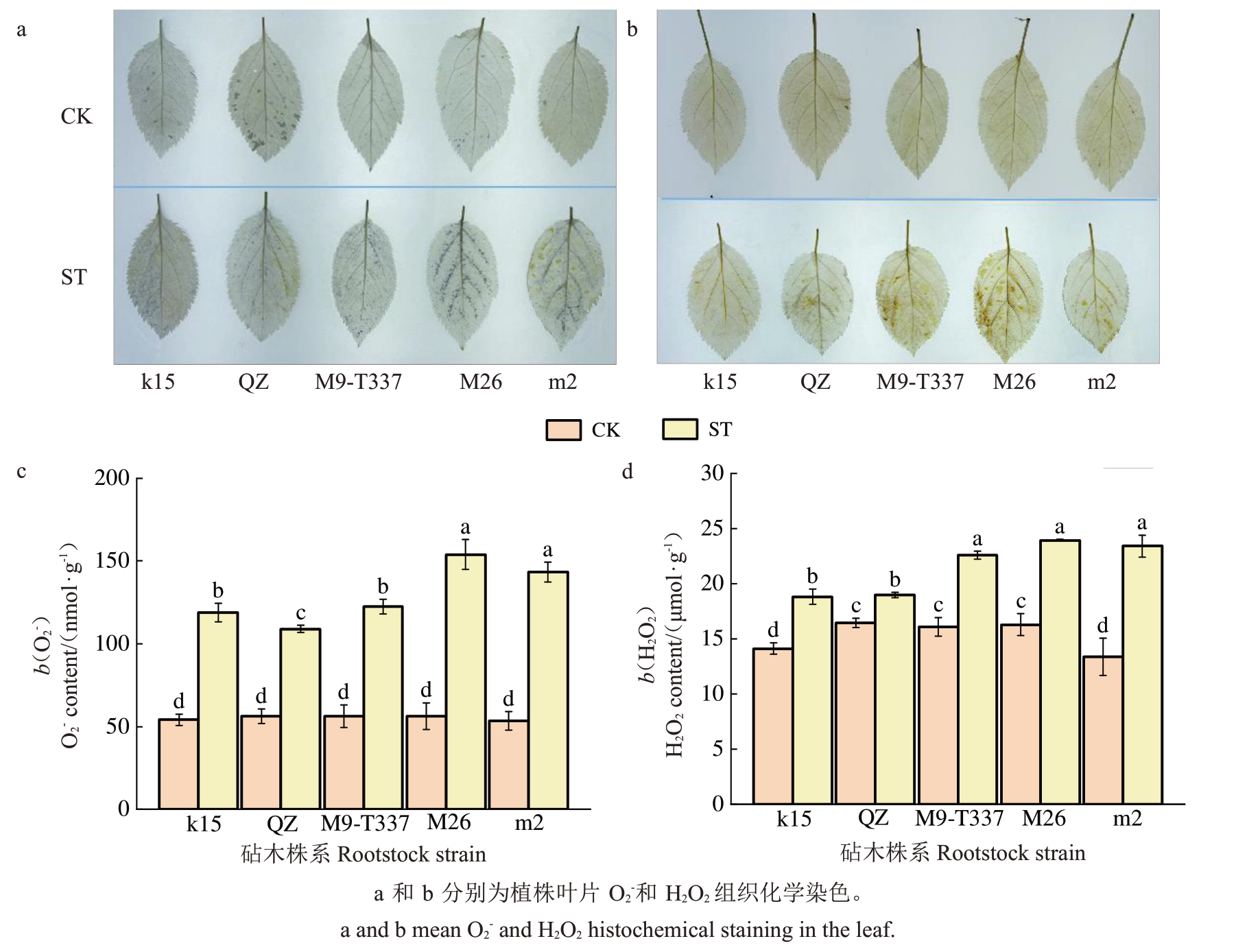
图4 盐胁迫对各株系活性氧积累的影响
Fig.4 Effects of salt stress on the accumulation of active oxygen in each strain
抗氧化相关酶活性结果显示,盐胁迫处理后各株系SOD、POD 和CAT 活性均显著上升。5 个株系的SOD 活性分别显著升高33.1%、23.0%、27.6%、12.2%和11.1%(图5-a);从POD 活性上看,盐胁迫下,k15 的POD 活性最高,m2 的POD 活性最低,与对照组相比,分别显著提高了42.9%和36.2%(图5-b);CAT 活性分别显著升高62.6%、60.5%、48.1%、43.2%和44.5%(图5-c)。由此可以推断,盐胁迫下各株系抗氧化相关酶活性均显著上升,其中k15 和富平楸子表现较为突出。
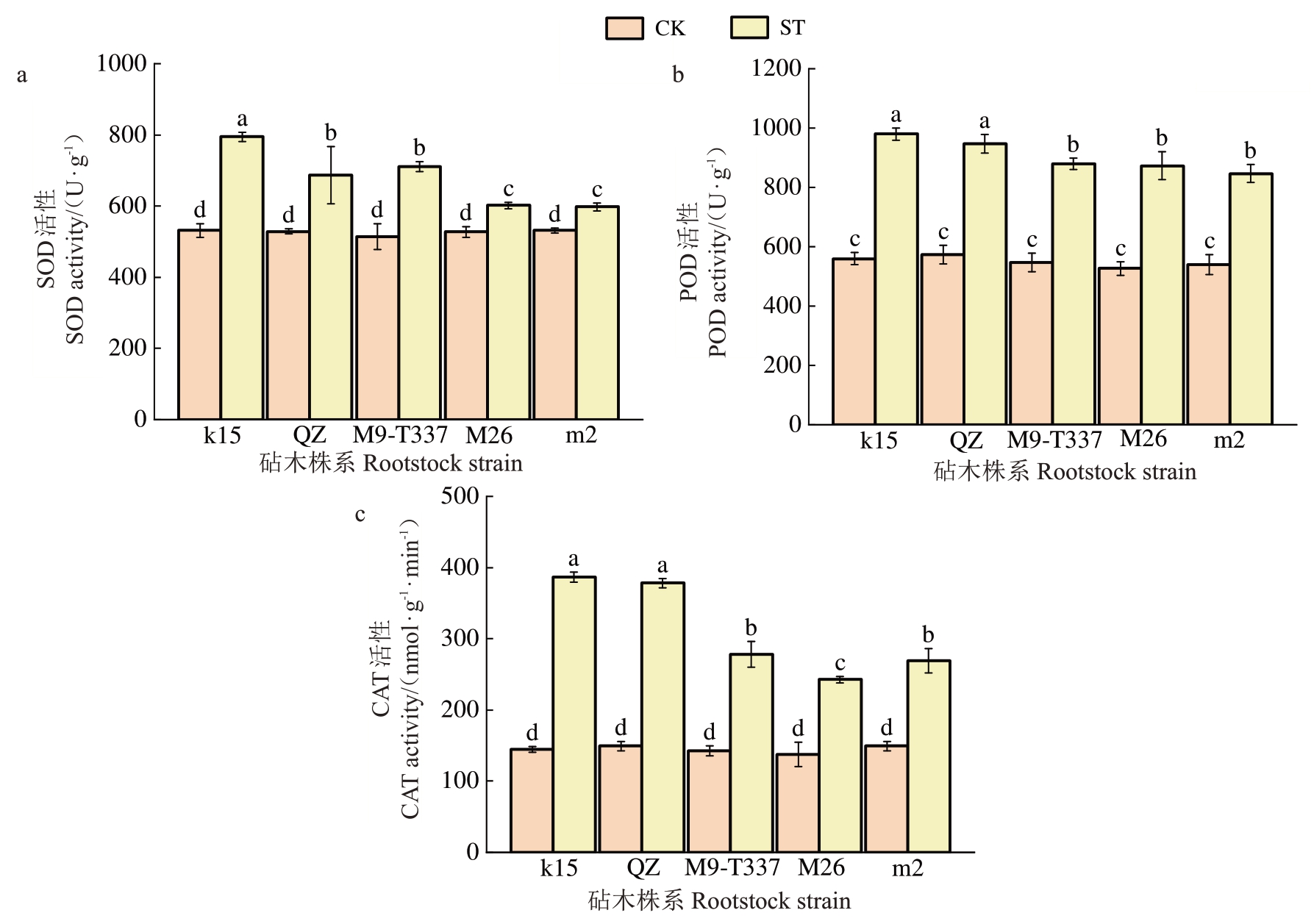
图5 盐胁迫对各株系抗氧化酶活性的影响
Fig.5 Effects of salt stress on antioxidant enzyme activities of each strain
2.4 盐胁迫下植株光合相关指标分析
在盐胁迫处理过程中,每3 d 测定1 次光合参数。结果表明,盐胁迫组第3天,各株系Pn、Tr、Gs和Ci均出现大幅度下降,随后下降幅度有所减缓(图6)。

图6 盐胁迫对各株系光合作用的影响
Fig.6 Effects of salt stress on photosynthesis of each strain
k15从第6天开始,光合参数Pn和Tr与其他株系出现分化,而Gs及Ci表现较为突出的是富平楸子,下降幅度相对较缓。结合两者在盐胁迫中的表现,相比于其他3个株系,k15和富平楸子表现出较强的光合能力,增强了一定的抗盐能力。
从总叶绿素含量上看,与对照组相比,盐胁迫组各株系均显著下降,k15、富平楸子、M9-T337、M26 和m2 下降幅度分别为32.4%、25.7%、36.4%、49.1%和47.3%,其中富平楸子降幅最小,M26 降幅最大,这与处理结束时M26 叶片大面积失绿相符(图7-b)。
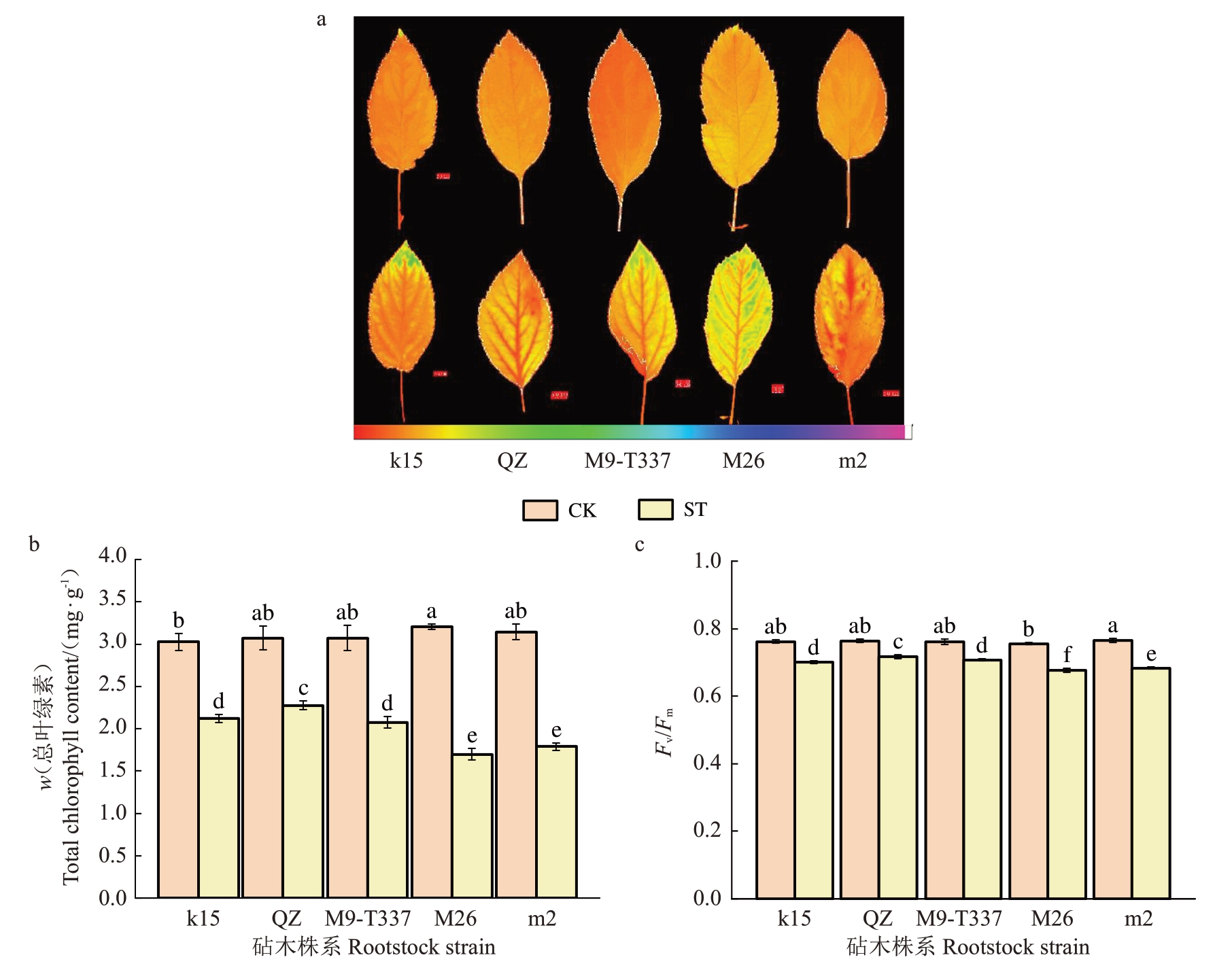
图7 盐胁迫对叶绿素含量及叶绿素荧光的影响
Fig.7 Effects of salt stress on chlorophyll content and chlorophyll fluorescence
叶绿素荧光图像清晰显示了各株系在盐胁迫后叶片的荧光变化情况(图7-a);同时从Fv/Fm值来看,对照组除M26 外,几乎无差异,而盐处理后k15、富平楸子和M9-T337 较其他2 个株系下降幅度更小,光系统损伤程度更小(图7-c)。
2.5 盐胁迫下植株离子稳态分析
水培盐胁迫会导致植物根部积累较多的Na+,根部被迫吸收过量Na+并运输到其他部位,使得植物细胞内Na+过量积累,产生离子毒害,同时影响K+及其他营养元素的吸收,打破细胞内部的离子稳定。本试验在处理结束时测定了各部位Na+、K+含量及Na+/K+。结果表明,对照组各株系根、茎、叶的Na+含量均较低,盐处理组均表现出显著升高,其中M26 涨幅最大,富平楸子、k15 涨幅相对较小(图8-a~c);K+则在盐处理后表现出不同程度的降低,其中M26 降幅最大,富平楸子和k15 变幅最小(图8-d~f);处理后各株系总Na+/K+显著升高,其中富平楸子与k15 涨幅相对较小,分别为95.49%和95.94%,M26涨幅最大,为97.78%(图8-g)。
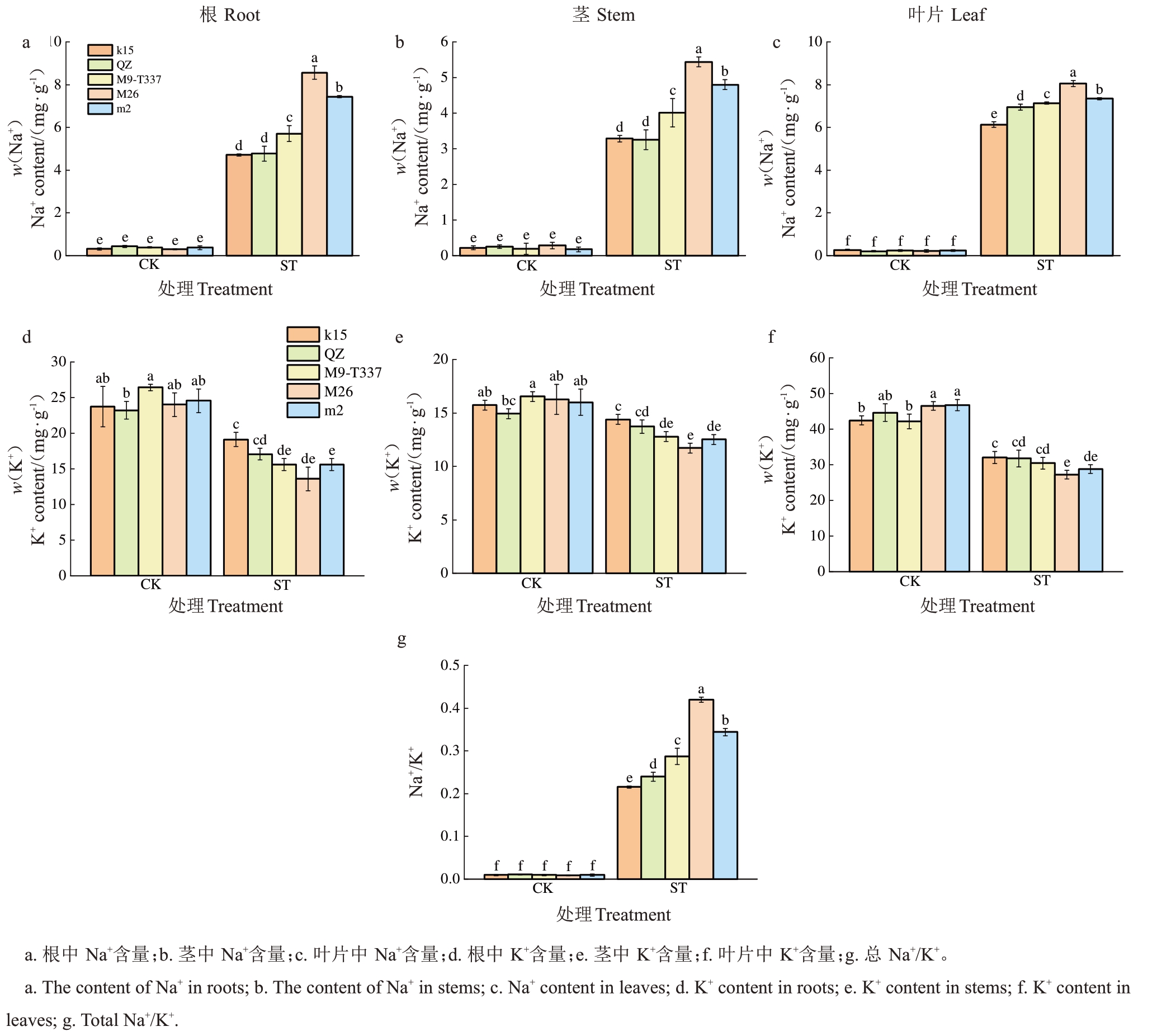
图8 盐胁迫对各株系离子稳态的影响
Fig.8 Effects of salt stress on ion homeostasis of each strain
2.6 盐胁迫下植株氨基酸含量分析
在植物响应盐胁迫时,氨基酸能够起到保护作用。其中脯氨酸为逆境胁迫的关键游离氨基酸,可参与渗透调节来保持机体渗透平衡。研究表明,植物遭受逆境胁迫时,脯氨酸含量有所增加,且在抗逆性较强的植株中增加量明显低于敏感型植株,而其他游离氨基酸浓度变化暂无明显固定规律[25]。为分析盐胁迫下各株系氨基酸变化情况,笔者在处理结束时对氨基酸含量进行了测定。结果(表1)表明,脯氨酸(Pro)、酪氨酸(Tyr)和苯丙氨酸(Phe)3 种氨基酸含量在处理后呈显著上升趋势,与对照组相比,5个株系(k15、富平楸子、M9-T337、M26和m2)脯氨酸含量分别提高了29.94%、20.27%、27.36%、35.72%和31.69%,其中M26 变化幅度最大,富平楸子变化幅度最小;酪氨酸含量分别提高了27.44%、18.79%、28.19%、32.39%和37.13%,其中m2 变化幅度最大,富平楸子变化幅度最小;苯丙氨酸含量分别提高了21.13%、32.53%、38.79%、50.94%和45.99%,其中M26 变化幅度最大,k15 变化幅度最小;而甘氨酸(Gly)、亮氨酸(Leu)和天冬氨酸(Asp)含量均显著下降,与对照组相比,5个株系甘氨酸含量分别降低了16.67%、7.14%、12.19%、19.69%和17.90%,其中富平楸子变化幅度最小,M26 降幅最大;亮氨酸含量分别降低了19.67%、21.40%、17.72%、30.71%和46.29%,其中M9-T337 变幅最小,m2 降幅最大;天冬氨酸含量分别降低了23.15%、22.75%、45.33%、57.05%和37.88%,其中富平楸子降幅最小,M26 降幅最大。综上所述,相比于对照组,盐胁迫组各株系氨基酸含量变化幅度最小的是富平楸子,最大的是M26和m2。
表1 盐胁迫对各株系氨基酸含量的影响
Table 1 Effects of salt stress on amino acids content in each strain(μg·g-1)
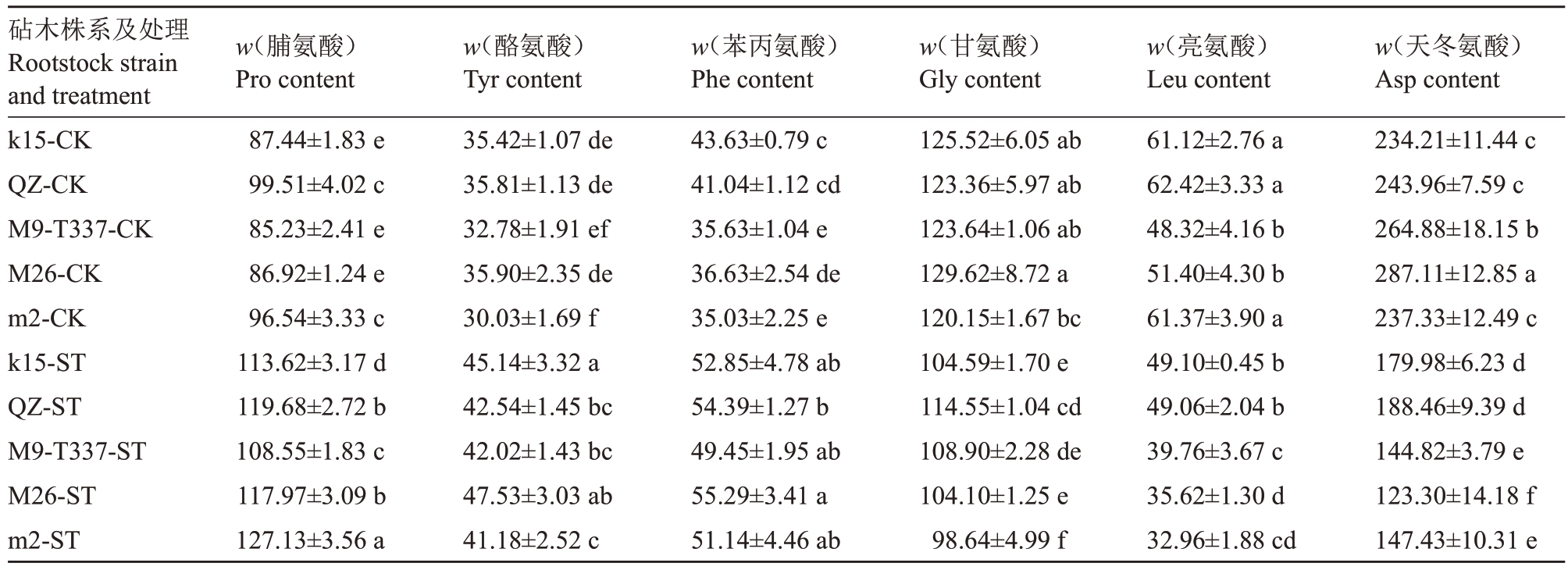
砧木株系及处理Rootstock strain and treatment k15-CK QZ-CK M9-T337-CK M26-CK m2-CK k15-ST QZ-ST M9-T337-ST M26-ST m2-ST w(脯氨酸)Pro content 87.44±1.83 e 99.51±4.02 c 85.23±2.41 e 86.92±1.24 e 96.54±3.33 c 113.62±3.17 d 119.68±2.72 b 108.55±1.83 c 117.97±3.09 b 127.13±3.56 a w(酪氨酸)Tyr content 35.42±1.07 de 35.81±1.13 de 32.78±1.91 ef 35.90±2.35 de 30.03±1.69 f 45.14±3.32 a 42.54±1.45 bc 42.02±1.43 bc 47.53±3.03 ab 41.18±2.52 c w(苯丙氨酸)Phe content 43.63±0.79 c 41.04±1.12 cd 35.63±1.04 e 36.63±2.54 de 35.03±2.25 e 52.85±4.78 ab 54.39±1.27 b 49.45±1.95 ab 55.29±3.41 a 51.14±4.46 ab w(甘氨酸)Gly content 125.52±6.05 ab 123.36±5.97 ab 123.64±1.06 ab 129.62±8.72 a 120.15±1.67 bc 104.59±1.70 e 114.55±1.04 cd 108.90±2.28 de 104.10±1.25 e 98.64±4.99 f w(亮氨酸)Leu content 61.12±2.76 a 62.42±3.33 a 48.32±4.16 b 51.40±4.30 b 61.37±3.90 a 49.10±0.45 b 49.06±2.04 b 39.76±3.67 c 35.62±1.30 d 32.96±1.88 cd w(天冬氨酸)Asp content 234.21±11.44 c 243.96±7.59 c 264.88±18.15 b 287.11±12.85 a 237.33±12.49 c 179.98±6.23 d 188.46±9.39 d 144.82±3.79 e 123.30±14.18 f 147.43±10.31 e
2.7 盐胁迫下各株系耐盐性综合评价
以各株系对照组与盐处理组相对变化率计算隶属函数值,以各株系隶属函数值的平均值为依据进行耐盐性综合评价,平均隶属函数值越大说明植株耐盐能力越强。通过分析相对电导率、MDA 含量、过氧化氢含量、SOD 活性、POD 活性、CAT 活性、净光合速率、叶绿素含量、Fv/Fm值、Na+/K+和氨基酸含量16 项指标,计算出了各株系的平均隶属函数值。结果(表2)表明,k15 的平均隶属函数值最大,表明盐胁迫下其各指标的相对变化程度最小,与其他种质资源相比耐盐能力最强。平均隶属函数值最小的是M26,表明其耐盐能力最弱。由此得出各株系耐盐能力依次为k15>富平楸子>M9-T337>m2>M26。
表2 隶属函数值
Table 2 Membership function value
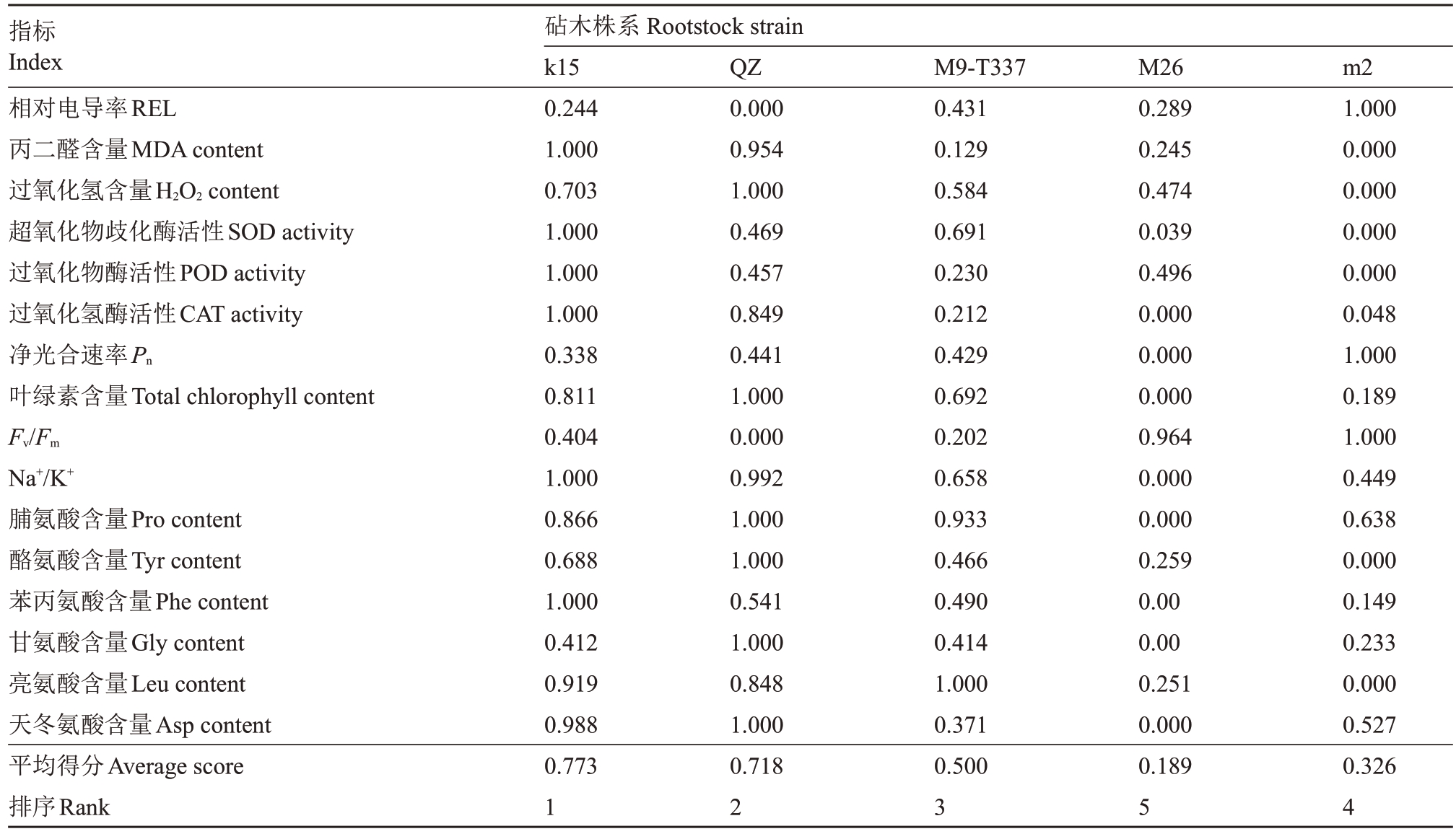
指标Index相对电导率REL丙二醛含量MDA content过氧化氢含量H2O2 content超氧化物歧化酶活性SOD activity过氧化物酶活性POD activity过氧化氢酶活性CAT activity净光合速率Pn叶绿素含量Total chlorophyll content Fv/Fm Na+/K+脯氨酸含量Pro content酪氨酸含量Tyr content苯丙氨酸含量Phe content甘氨酸含量Gly content亮氨酸含量Leu content天冬氨酸含量Asp content平均得分Average score排序Rank砧木株系Rootstock strain k15 0.244 1.000 0.703 1.000 1.000 1.000 0.338 0.811 0.404 1.000 0.866 0.688 1.000 0.412 0.919 0.988 0.773 1 QZ 0.000 0.954 1.000 0.469 0.457 0.849 0.441 1.000 0.000 0.992 1.000 1.000 0.541 1.000 0.848 1.000 0.718 2 M9-T337 0.431 0.129 0.584 0.691 0.230 0.212 0.429 0.692 0.202 0.658 0.933 0.466 0.490 0.414 1.000 0.371 0.500 3 M26 0.289 0.245 0.474 0.039 0.496 0.000 0.000 0.000 0.964 0.000 0.000 0.259 0.00 0.00 0.251 0.000 0.189 5 m2 1.000 0.000 0.000 0.000 0.000 0.048 1.000 0.189 1.000 0.449 0.638 0.000 0.149 0.233 0.000 0.527 0.326 4
3 讨 论
盐胁迫下植株生长受到抑制,根系发育迟缓,叶片失水、萎蔫甚至脱落[26]。本研究中,150 mmol·L-1盐胁迫处理15 d导致各个株系均出现不同程度的盐害表现。株高受到抑制,叶片出现褐斑并随着处理时间加长逐渐萎蔫脱落,根系生长迟缓并呈现褐色,新根变少[27]。盐胁迫下植物通过提高根系活力来适应环境变化,确保根系正常吸收水分及矿质营养,植株正常生长。研究表明,在低盐浓度下或短期中高盐浓度下,植物根系活力提高,且提高幅度与耐盐能力呈正相关,当盐浓度超过一定承受范围时,根系活力会大幅下降[28]。本研究中,由于150 mmol·L-1盐浓度超过苹果植株耐受能力,各株系根系活力均呈下降趋势。处理结束时,M26 盐害表现最为严重,其次为M9-T337和m2。相比之下,k15和富平楸子盐害表型出现较晚,且盐害程度相对较轻,与前人的研究结果相近[29]。
遭受盐胁迫的植株内部生理出现紊乱,抗性较强的株系则可以通过自身调节作用减轻损伤[30-31]。相对电导率和MDA含量能够反映生物膜的损伤程度,可作为植物耐逆境胁迫能力的关键生理指标[32]。MDA作为膜脂过氧化的产物,其含量反映了植物膜脂过氧化程度,盐胁迫下MDA过量积累,破坏生物膜结构和功能,导致膜通透性增强,电解质外渗,电导率增大[33-34]。植物耐受性越强,相对电导率和MDA含量越稳定。笔者测定了各株系相对电导率及MDA含量,结果表明,盐胁迫下各株系的相对电导率及MDA含量均有所上升,但k15和富平楸子的涨幅明显更小,侧面反映出两者较强的耐受性。
植物在盐胁迫下,细胞内会产生过量的活性氧,这些活性氧若没有及时清除便会引起氧化胁迫[35]。有研究证实,提高植物体内抗氧化系统酶活性及增强抗氧化代谢能力是提高植物抗盐能力的途径之一[36]。耐盐能力较强的植株可以通过启动抗氧化系统消除过量的活性氧,使活性氧与抗氧化酶保持动态平衡,以减轻盐害[37]。笔者通过NBT和DAB染色来分析活性氧含量,判断各株系O2-和H2O2积累情况,结果表明,k15、富平楸子和M9-T337 的O2-含量显著低于M26和m2;k15和富平楸子的H2O2含量显著低于其他株系。k15在盐胁迫下体内O2-和H2O2积累均较少,这可能与k15 自身较好的抗氧化调节能力有关。关于抗氧化酶活性变化与逆境胁迫的关系,有不同的研究结果。研究表明,植物抗氧化相关酶活性会随逆境胁迫加重而提高,并存在阈值[38];也有研究报道,保护酶活性会随胁迫程度增加而下降[39]。不同砧木逆境胁迫下的响应机制不同,需要的保护酶也不同,且变化规律差异较大[40]。本研究在盐胁迫处理15 d 后,5 个株系中POD、SOD 和CAT活性上升幅度最大的均为k15,由此可以推断,150 mmol·L-1盐胁迫下各植株都启动了保护酶系统,且保护酶活性与植株抗性呈正相关,k15表现出较强的耐盐能力。
光合作用是植物生长的必要条件,盐胁迫下光合能力强弱间接体现植株耐盐能力[41]。本试验的结果表明,盐胁迫组从第3 天开始,各株系Pn、Tr、Gs和Ci均出现大幅度下降,随着处理时间延长下降幅度有所减缓,这可能与植株建立了稳态耐受机制有关。研究表明,植物受到盐害时,短期内光合作用受气孔因素限制而降低;随着盐害时间变长,植物光合器官受到破坏,限制因素逐渐转为非气孔因素[42]。当植物净光合速率下降、胞间二氧化碳浓度升高时,光合作用受到非气孔因素的限制而下降[43]。由此可以推测,本研究第6 天开始光合能力下降可能是受到非气孔因素的影响,光合器官受到破坏。株系k15从第6天开始,净光合速率和蒸腾速率与其他株系出现分化,而从气孔导度和胞间CO2浓度上看,富平楸子较其他4 个株系表现最突出,下降幅度相对较缓。结合两者在盐胁迫中的表现,相比于其他3个株系,k15和富平楸子表现出较强的光合能力,增强了其抗盐能力。研究表明,盐胁迫下,小麦由于吸收不到充足的水分和矿质元素,叶绿素降解酶活性增强,叶绿素含量大幅降低,叶片失绿[44]。本研究在处理结束时测定了叶绿素含量及荧光参数。从总叶绿素含量上看,M26 下降幅度最大,这与处理结束时M26 叶片大面积失绿萎蔫的表型相符。叶绿素荧光参数可以作为分析植物受光抑制程度的重要指标[45]。通过分析Fv/Fm值,盐处理后k15、富平楸子、M9-T337 较M26 和m2 两个株系下降幅度小,表明其受光抑制程度较低。
植物根系处于高盐环境下,会引起细胞内离子浓度发生改变,植物会顺着电化学梯度从外界吸收大量的Na+,从而产生离子毒害[46]。当细胞内Na+浓度过高时会置换结合内膜上的Ca2+,导致细胞膜功能遭到破坏,同时细胞内K+等有机溶质外渗,导致植物生长代谢受到影响[47-49]。研究表明,碱蓬之所以具有较好的耐盐能力,是因为根系通过渗透调节将体内的盐分转运、区域化,降低细胞内盐浓度,同时增加液泡内Na+浓度,保持细胞正常吸水,调节离子平衡[50-51]。可见,耐盐能力强的植株根系能够通过类似排盐、拒盐机制维持内部Na+/K+稳定。由此推断,盐胁迫下,Na+/K+越稳定,植株耐盐能力越强。笔者通过分析各部位Na+、K+含量及Na+/K+,结果表明,富平楸子和k15 的Na+和K+含量以及Na+/K+变化幅度较小,侧面反映出其较强的耐盐能力。
植物体内氨基酸在逆境胁迫中发挥重要作用,如植物遭受逆境胁迫时会通过脯氨酸的积累来提高自身耐受性[52]。研究表明,不同物种间脯氨酸含量与植物抗逆性强弱的关系存在差异,如耐热性强的不结球白菜高温胁迫下积累更多的脯氨酸[53],番茄幼苗耐盐能力则与脯氨酸含量呈负相关[54]。本研究5 个株系中M26 的脯氨酸含量涨幅最大,富平楸子涨幅最小,可见脯氨酸含量与植株抗盐能力呈负相关,这与前人在苹果抗盐性评价中的结果存在差异[55],该研究认为抗盐性较强的砧木优系盐胁迫后脯氨酸含量增加幅度大,这可能与不同砧木自身的脯氨酸代谢方式有关,具体机制还有待研究。此外,本研究中酪氨酸(Tyr)和苯丙氨酸(Phe)含量在盐处理后呈现显著上升趋势,而甘氨酸(Gly)、亮氨酸(Leu)和天冬氨酸(Asp)含量均显著下降。植物响应逆境胁迫时氨基酸代谢机制较为复杂,单一组分变化无法作为抗盐性评价的绝对依据[56],需综合分析。
4 结 论
150 mmol·L-1的水培盐处理下5 个株系中k15表现出较强的耐盐能力,M26 耐盐性较差。盐胁迫下,各植株生长量下降,生长受到抑制,根面积减小,干鲜质量下降;相对电导率和MDA含量显著升高,根系活力显著降低;植物体内活性氧积累,抗氧化酶活性升高;叶绿素含量降低,光合作用明显受到抑制;Na+含量显著升高,K+含量下降,Na+/K+失衡;脯氨酸(Pro)、酪氨酸(Tyr)和苯丙氨酸(Phe)3 种氨基酸含量显著升高,甘氨酸(Gly)、亮氨酸(Leu)和天冬氨酸(Asp)含量显著降低。但由于各株系抗性存在差异,不同指标变化率有所不同。通过隶属函数综合分析,最终得出各株系耐盐能力顺序为:k15 >富平楸子>M9-T337 >m2 >M26。因此,苹果砧木富平楸子×R3 杂交后代k15 的耐盐能力较强,有望成为新的优质砧木资源。
[1] 郭全恩.干旱地区果树对土壤盐渍化胁迫的响应机制[D].杨凌:西北农林科技大学,2006.GUO Quan’en.The response mechanism of stress of soil salinization on apple tree in arid region[D].Yangling:Northwest A&F University,2006.
[2] MUNNS R,TESTER M. Mechanisms of salinity tolerance[J].Annual Review of Plant Biology,2008,59:651-681.
[3] 靳娟,鲁晓燕,王依. 果树耐盐性研究进展[J]. 园艺学报,2014,41(9):1761-1776.JIN Juan,LU Xiaoyan,WANG Yi. Advances in the studies on salt tolerance of fruit trees[J]. Acta Horticulturae Sinica,2014,41(9):1761-1776.
[4] HAMAMOTO S,HORIE T,HAUSER F,DEINLEIN U,SCHROEDER J I,UOZUMI N. HKT transporters mediate salt stress resistance in plants:From structure and function to the field[J].Current Opinion in Biotechnology,2015,32:113-120.
[5] SHAHID M A,BALAL R M,KHAN N,SIMÓN-GRAO S,ALFOSEA-SIMÓN M,CÁMARA-ZAPATA J M,MATTSON N S,GARCIA-SANCHEZ F. Rootstocks influence the salt tolerance of Kinnow mandarin trees by altering the antioxidant defense system,osmolyte concentration,and toxic ion accumulation[J].Scientia Horticulturae,2019,250:1-11.
[6] 霍柳青.自噬基因MdATG8i、MdATG10 和MdATG18α 在苹果响应盐和高温胁迫中的功能分析[D].杨凌:西北农林科技大学,2020.HUO Liuqing. Functional analysis of MdATG8i,MdATG10,MdATG18a in response to salt and heat stress in apple[D].Yangling:Northwest A&F University,2020.
[7] NISA Z U,MALLANO A I,YU Y,CHEN C,DUAN X B,AMANULLAH S,KOUSAR A,BALOCH A W,SUN X L,TABYS D,ZHU Y M. GsSNAP33,a novel Glycine soja SNAP25- type protein gene:Improvement of plant salt and drought tolerances in transgenic Arabidopsis thaliana[J]. Plant Physiology and Biochemistry,2017,119:9-20.
[8] SIDDIQUE A B,ISLAM M R,HOQUE M A,HASAN M M,RAHMAN M T,UDDIN M M. Mitigation of salt stress by foliar application of proline in rice[J].Universal Journal of Agricultural Research,2015,3(3):81-88.
[9] 杜中军,翟衡,罗新书,潘志勇,程述汉.苹果砧木耐盐性鉴定及其指标判定[J].果树学报,2002,19(1):4-7.DU Zhongjun,ZHAI Heng,LUO Xinshu,PAN Zhiyong,CHENG Shuhan. Salt-tolerance identification on apple rootstocks[J].Journal of Fruit Science,2002,19(1):4-7.
[10] 朱国芳.苹果不同砧穗组合在洛川地区的栽培适应性研究[D].杨凌:西北农林科技大学,2018.ZHU Guofang.Adaptation of different apple scion-stock combination in Luochuan,Shaanxi[D]. Yangling:Northwest A & F University,2018.
[11] 韩佳,周高峰,李峤虹,刘永忠,彭抒昂.缺镁、铁、硼胁迫对4个柑橘砧木生长及养分吸收的影响[J]. 园艺学报,2012,39(11):2105-2112.HAN Jia,ZHOU Gaofeng,LI Qiaohong,LIU Yongzhong,PENG Shu’ang. Effects of magnesium,iron,boron deficiency on the growth and nutrition absorption of four major citrus rootstocks[J].Acta Horticulturae Sinica,2012,39(11):2105-2112.
[12] 王米云. 苹果砧木的作用、特点、类型与选用[J]. 河北果树,2020(2):19-20.WANG Miyun.Functions,characteristics,types and selection of apple rootstock[J].Hebei Fruits,2020(2):19-20.
[13] 杨锋,王冬梅,闫忠业,黄金凤,刘志.M9 在苹果矮化砧木育种实践中的应用价值[J].北方果树,2024(1):1-6.YANG Feng,WANG Dongmei,YAN Zhongye,HUANG Jinfeng,LIU Zhi.Practiced value of M9 in breeding of apple dwarfing rootstocks[J].Northern Fruits,2024(1):1-6.
[14] 韩明玉.苹果矮砧集约高效栽培模式[J].果农之友,2009(9):12.HAN Mingyu. Intensive and efficient cultivation model of apple dwarf anvil[J].Fruit Growers’Friend,2009(9):12.
[15] 王小丫,张仲兴,高彦龙,董永娟,马小兰,王延秀.盐碱胁迫下苹果矮化砧木M9-T337 对外源柠檬酸(CA)的响应[J].果树学报,2024,41(2):252-265.WANG Xiaoya,ZHANG Zhongxing,GAO Yanlong,DONG Yongjuan,MA Xiaolan,WANG Yanxiu. Response of apple dwarfing rootstocks M9-T337 to exogenous citric acid (CA) under saline and alkaline stresses[J]. Journal of Fruit Science,2024,41(2):252-265.
[16] SIBOZA X I,KOTZE W P,COOK N C,STEYN W J. Evaluating more yield efficient dwarfing,semi-dwarfing and semi-vigorous rootstocks for the South African apple industry[J].Acta Horticulturae,2018(1228):161-166.
[17] MUSHTAQ R,PANDIT A,RAJA R H S,MIR M A,SHARMA M K,BHAT R,BABA J A. Performance of exotic apple varieties grafted on M9T337 clonal rootstock under high density plantation[J].Indian Journal of Horticulture,2019,76(3):530.
[18] 郝玉金,沙广利. 苹果营养系砧木选育进展[J]. 落叶果树,2018,50(1):3-7.HAO Yujin,SHA Guangli.Progress in selection and breeding of apple vegetative stock[J].Deciduous Fruits,2018,50(1):3-7.
[19] 张莹莹,李泓,赵文平,陈运娣.苹果矮化密植栽培现状及问题与建议[J].河北林业科技,2023(1):51-54.ZHANG Yingying,LI Hong,ZHAO Wenping,CHEN Yundi.Present situation,problems and suggestions of apple dwarfing and close planting cultivation[J]. Journal of Hebei Forestry Science and Technology,2023(1):51-54.
[20] 田利静,徐岭勇. 苹果矮化砧木筛选试验研究[J]. 新农业,2019(10):35-37.TIAN Lijing,XU Lingyong. Research on screening test of dwarfing rootstock for apple[J].New Agriculture,2019,(10):35-37.
[21] 王大江,BUS VINCENT G M,王昆,高源,赵继荣,刘立军,李连文,朴继成.美国苹果砧木育种历史、现状及其商业化砧木特性[J].中国果树,2018(6):107-110.WANG Dajiang,BUS VINCENT G M,WANG Kun,GAO Yuan,ZHAO Jirong,LIU Lijun,LI Lianwen,PIAO Jicheng.The history and present situation of apple rootstock breeding in America and its commercial rootstock characteristics[J]. China Fruits,2018(6):107-110.
[22] 赵德英.国内外常用苹果矮化砧木:G 系[J].果树实用技术与信息,2024(7):21-22.ZHAO Deying. Apple dwarfing rootstock commonly used at home and abroad:G series[J]. Fruit Tree Practical Technology and Information,2024(7):21-22.
[23] 徐硕.苹果砧木富平楸子与R3 杂交后代的抗旱性评价及抗旱机制初步分析[D].杨凌:西北农林科技大学,2024.XU Shuo. Evaluation and preliminary mechanism analysis on drought resistance of apple rootstock Malus prunifolia and R3 hybrid[D].Yangling:Northwest A&F University,2024.
[24] SUN X,WANG P,JIA X,HUO L Q,CHE R M,MA F W. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple[J]. Plant Biotechnology Journal,2018,16(2):545-557.
[25] 徐宇,肖化云,郑能建,张忠义,瞿玲露.植物组织中游离氨基酸在盐胁迫下响应的研究进展[J].环境科学与技术,2016,39(7):40-47.XU Yu,XIAO Huayun,ZHENG Nengjian,ZHANG Zhongyi,QU Linglu. Progress on responding of free amino acid in plants to salt stress[J]. Environmental Science &Technology,2016,39(7):40-47.
[26] CHEN K Q,SONG M R,GUO Y N,LIU L F,XUE H,DAI H Y,ZHANG Z H. MdMYB46 could enhance salt and osmotic stress tolerance in apple by directly activating stress-responsive signals[J]. Plant Biotechnology Journal,2019,17(12):2341-2355.
[27] YIN R,BAI T H,MA F W,WANG X J,LI Y H,YUE Z Y.Physiological responses and relative tolerance by Chinese apple rootstocks to NaCl stress[J]. Scientia Horticulturae,2010,126(2):247-252.
[28] BRANQUINHO C,BROWN D H,CATARINO F. The cellular location of Cu in lichens and its effects on membrane integrity and chlorophyll fluorescence[J]. Environmental and Experimental Botany,1997,38(2):165-179.
[29] 李春容.七种苹果砧木抗盐性和氮素利用效率的评价[D].杨凌:西北农林科技大学,2023.LI Chunrong.Evaluation of salt resistance and nitrogen use efficiency of seven apple rootstocks[D].Yangling:Northwest A&F University,2023.
[30] AN J P,ZHANG X W,XU R R,YOU C X,WANG X F,HAO Y J.Apple MdERF4 negatively regulates salt tolerance by inhibiting MdERF3 transcription[J]. Plant Science,2018,276:181-188.
[31] 李增裕,孙建设,孙宁.苹果耐盐性研究进展[J].河北农业大学学报,2003,26(增刊1):45-48.LI Zengyu,SUN Jianshe,SUN Ning.Progress of studies on salt tolerance of apple[J]. JJournal of Agricultural University of Hebei,2003,26(Suppl.1):45-48.
[32] FU M Y,LI C,MA F W. Physiological responses and tolerance to NaCl stress in different biotypes of Malus prunifolia[J]. Euphytica,2013,189(1):101-109.
[33] 朱润洁,闻蒙蒙,郎红珊,段好鑫,李玥,汤晓丽.红阳猕猴桃盐胁迫下生理生化响应及相关基因表达分析[J].果树学报,2024,41(11):2224-2234.ZHU Runjie,WEN Mengmeng,LANG Hongshan,DUAN Haoxin,LI Yue,TANG Xiaoli. Physiological and biochemical response and related gene expression of Hongyang kiwiftuit seedlings under salt stress[J].Journal of Fruit Science,2024,41(11):2224-2234.
[34] WOO S K,NAHM O,KWON H M. How salt regulates genes:Function of a Rel-like transcription factor TonEBP[J].Biochemical and Biophysical Research Communications,2000,278(2):269-271.
[35] JIANG Y W,HUANG B R. Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation[J]. Crop Science,2001,41(2):436-442.
[36] HU D G,MA Q J,SUN C H,SUN M H,YOU C X,HAO Y J.Overexpression of MdSOS2L1,a CIPK protein kinase,increases the antioxidant metabolites to enhance salt tolerance in apple and tomato[J].Physiologia Plantarum,2016,156(2):201-214.
[37] MILLER G,SUZUKI N,CIFTCI-YILMAZ S,MITTLER R.Reactive oxygen species homeostasis and signalling during drought and salinity stresses[J]. Plant,Cell & Environment,2010,33(4):453-467.
[38] 夏更寿,王加真.高盐胁迫对沟叶结缕草叶片抗氧化酶活性的影响[J].河北农业大学学报,2009,32(1):30-33.XIA Gengshou,WANG Jiazhen. Effects of NaCl on Zoysia matrella[J]. Journal of Agricultural University of Hebei,2009,32(1):30-33.
[39] 潘伟彬,邓恢.4 种草本水土保持植物的耐旱生理特性[J].华侨大学学报(自然科学版),2009,30(3):305-308.PAN Weibin,DENG Hui. Studies on drought-tolerant physiology of 4 herbage plants for soil and water conservation[J]. Journal of Huaqiao University (Natural Science Edition),2009,30(3):305-308.
[40] 焦灰敏.不同苹果砧木实生后代耐盐特性研究[D].阿拉尔:塔里木大学,2019.JIAO Huimin. Study on salt tolerance of different apple rootstocks in their offspring[D].Ala’er:Tarim University,2019.
[41] HUO L Q,GUO Z J,WANG P,ZHANG Z J,JIA X,SUN Y M,SUN X,GONG X Q,MA F W.MdATG8i functions positively in apple salt tolerance by maintaining photosynthetic ability and increasing the accumulation of arginine and polyamines[J]. Environmental and Experimental Botany,2020,172:103989.
[42] PARIHAR P,SINGH S,SINGH R,SINGH V P,PRASAD S M.Effect of salinity stress on plants and its tolerance strategies:A review[J]. Environmental Science and Pollution Research International,2015,22(6):4056-4075.
[43] 朱新广,张其德.NaCl 对光合作用影响的研究进展[J].植物学通报,1999,34(4):332-338.ZHU Xinguang,ZHANG Qide.Advances in the research on the effects of NaCl on photosynthesis[J]. Chinese Bulletin of Botany,1999,34(4):332-338.
[44] 王婵,侯格平,朱妍钰,马君君,王万鹏,李元昊,吴海燕,惠永芳,刘鹏伟,张文宇,马小乐.NaCl 胁迫对春小麦苗期叶绿素及保护性酶活性的影响[J].寒旱农业科学,2024(6):555-559.WANG Chan,HOU Geping,ZHU Yanyu,MA Junjun,WANG Wanpeng,LI Yuanhao,WU Haiyan,HUI Yongfang,LIU Pengwei,ZHANG Wenyu,MA Xiaole. Effects of NaCl stress on chlorophyll contents and protective enzyme activities of spring wheat at seedling stage[J]. Journal of Cold-Arid Agricultural Sciences,2024(6):555-559.
[45] STEFANOV M A,RASHKOV G D,APOSTOLOVA E L. Assessment of the photosynthetic apparatus functions by chlorophyll fluorescence and P700 absorbance in C3 and C4 plants under physiological conditions and under salt stress[J].International Journal of Molecular Sciences,2022,23(7):3768.
[46] ACOSTA-MOTOS J R,ORTUÑO M F,BERNAL-VICENTE A,DIAZ-VIVANCOS P,SANCHEZ-BLANCO M J,HERNANDEZ J A. Plant responses to salt stress:Adaptive mechanisms[J].Agronomy,2017,7(1):18.
[47] BATISTA-SILVA W,HEINEMANN B,RUGEN N,NUNES-NESI A,ARAÚJO W L,BRAUN H P,HILDEBRANDT T M.The role of amino acid metabolism during abiotic stress release[J].Plant,Cell&Environment,2019,42(5):1630-1644.
[48] HU L Y,ZHOU K,LIU Y,YANG S L,ZHANG J Y,GONG X Q,MA F W. Overexpression of MdMIPS1 enhances salt tolerance by improving osmosis,ion balance,and antioxidant activity in transgenic apple[J].Plant Science,2020,301:110654.
[49] HUANG Y H,CUI X,CEN H F,WANG K H,ZHANG Y W.Transcriptomic analysis reveals vacuolar Na+ (K+)/H+ antiporter gene contributing to growth,development,and defense in switchgrass (Panicum virgatum L.)[J]. BMC Plant Biology,2018,18(1):57.
[50] YU W C,WU W W,ZHANG N,WANG L P,WANG Y H,WANG B,LAN Q K,WANG Y. Research advances on molecular mechanism of salt tolerance in Suaeda[J]. Biology,2022,11(9):1273.
[51] 朱紫檀,洪晓松,张晓宁,申祺.盐地碱蓬耐盐碱响应机制研究进展[J].现代农业科技,2024(6):94-97.ZHU Zitan,HONG Xiaosong,ZHANG Xiaoning,SHEN Qi.Research progress on salt-alkali tolerance response mechanism of salt-alkali salt-alkali ponderum[J]. Modern Agricultural Science and Technology,2024(6):94-97.
[52] OLIVA M,GUY A,GALILI G,DOR E,SCHWEITZER R,AMIR R,HACHAM Y.Enhanced production of aromatic amino acids in tobacco plants leads to increased phenylpropanoid metabolites and tolerance to stresses[J]. Frontiers in Plant Science,2021,11:604349.
[53] 常开振.高温对不结球白菜幼苗脯氨酸代谢的影响[D].福州:福建农林大学,2023.CHANG Kaizhen. High temperature on non-heading Chinese cabbage seedlings effects of proline metabolism[D].Fuzhou:Fujian Agriculture and Forestry University,2023.
[54] 付珊,雷婷,金苇,李影,陈秀君,陈梦丽,陈亮.盐胁迫对番茄幼苗生长及生理指标的影响[J].湖北师范大学学报(自然科学版),2023,43(3):9-15.FU Shan,LEI Ting,JIN Wei,LI Ying,CHEN Xiujun,CHEN Mengli,CHEN Liang.Effects of salt stress on growth and physiological indexes of tomato seedlings[J]. Journal of Hubei Normal University(Natural Science),2023,43(3):9-15.
[55] 孙楠.苹果砧木优系31 的抗逆性研究[D].泰安:山东农业大学,2022.SUN Nan.Study on stress resistance of superior line 31 of apple rootstock[D].Tai’an:Shandong Agricultural University,2022.
[56] 潘凯,吴凤芝.枯萎病不同抗性黄瓜(Cucumis sativus L.)根系分泌物氨基酸组分与抗病的相关性[J]. 生态学报,2007,27(5):1945-1950.PAN Kai,WU Fengzhi. Correlation analysis of amino acids components in cucumber root exudates and Fusarium wilt resistance[J].Acta Ecologica Sinica,2007,27(5):1945-1950.