葡萄(Vitis vinifera L.)作为世界范围内广泛种植的水果之一,具有极大的经济效益[1-2]。随着气候变化和环境条件对葡萄生产的不良影响逐渐加剧,设施栽培技术应运而生[3]。中国作为鲜食葡萄主产国,通过设施栽培技术如利用避雨棚、日光温室等结构,显著降低霜霉病等病害发生率并有效延长了货架期[4-5]。然而,覆盖材料对光谱的选择性吸收导致设施内的光环境异化(如蓝紫光衰减>40%),造成光合效率下降、花芽分化受抑制及次生代谢紊乱等连锁反应[6],成为设施葡萄优质生产的核心瓶颈。
光环境调控是突破设施栽培光限制的关键途径。研究表明,光质直接关系到植株生长、光合效率、果实品质及产量[7]。光照不足会导致树体瘦弱、叶片黄化、花芽分化不良、落花落果,甚至引发病害,从而导致品质下降[8-9]。近年来,随着设施园艺快速发展,LED补光技术可通过光谱定制精准调控果树生理响应,成为提高光能利用效率、促进植物生长和提升产量的有效手段,在果树生产中被广泛应用[10-11]。不同光质波长不同,影响植物生长的不同发育阶段[12]。红光(600~700 nm)能促进植物茎和叶片的伸长、增加叶片数、叶面积及叶片厚度[13-14],蓝光(400~500 nm)则能显著改善果实的品质,如提高草莓果实糖度和花青素含量等[15-16]。然而,光质互作效应存在显著的品种依赖性,且关于补光对花芽分化分子机制的调控路径尚不明晰。
阳光玫瑰(V.labrusca×V.vinifera‘Shine Muscat’)作为高附加值鲜食葡萄品种,在设施栽培中普遍存在新梢徒长、坐果率波动及香气物质积累不足等问题,且该品种对光照条件较为敏感,光照不足或光质不合理会导致植株生长受限,光合能力下降,进而影响果实品质和产量[17]。不同光质对葡萄的生长发育影响也存在差异,如何合理选择和应用补光措施以优化阳光玫瑰葡萄的生长发育和果实品质,仍需进一步研究。本研究旨在通过不同补光处理,探讨光质对阳光玫瑰葡萄叶片、茎干和果实品质等生长指标,以及光合特性、花芽分化等生理特性方面的影响,为优化葡萄避雨栽培中的补光措施提供一定的理论依据与实践指导。
1 材料和方法
1.1 材料
试验在山东省泰安市金牛山基地(116°99′ E,36°13′N)进行,供试材料为5 年生避雨栽培的阳光玫瑰葡萄,采用“T”形水平棚架栽培,主干高度1.2 m,株行距为3 m×2 m,南北走向,除补光处理外,其余管理条件一致。试验采用红光(RL),白光(WL),红光∶蓝光=2∶1(RBL 2∶1)LED灯补光,遮阳网进行遮光处理(ST),设施不额外补光为对照(CK),试验所用LED 补光灯的功率均为36 W,白光为全光谱,波长350~750 nm,红光波长640 nm,在树体上方30 cm处安装灯管进行补光处理,各处理间以遮光膜相隔。每组处理10 株葡萄,每组设3 次重复。阳光玫瑰葡萄补光从芽萌发(2023-04-11)开始,至第一批果实成熟(2023-09-07)结束,每日补光12 h(8:00—20:00)。
1.2 方法
1.2.1 生长指标测定 处理两个月后,于6 月15 日进行叶片大小(叶面积)、茎基部粗度和节间长度等指标的测定,随机取样,每个处理采集20 个枝条。叶面积计算采用几何近似法,计算公式:叶面积=长轴×短轴×π/4。
1.2.2 叶绿素荧光诱导动力学曲线(OJIP)及荧光参数测定 在8 月下旬,使用快速荧光测定仪(汉莎,Handy-PEA)对各处理的阳光玫瑰葡萄叶片进行叶绿素荧光诱导动力学曲线及相关参数的测定。选择粗度相同的枝条上第3枚叶片进行测定,每个处理测定10枚叶片,每个样本重复测定5次。测定前,将叶片在黑暗环境中静置20 min,之后用3000 μmol·m-²·s-1的饱和光进行1 s的荧光诱导。采用JIP-test方法对OJIP荧光诱导曲线进行分析,并对相关荧光数据进行标准化处理,参考李冠宇等[18]的方法计算可变荧光(Vt)、相对可变荧光(V)和相对可变荧光的差值(ΔVt),同时,利用P-test对OJIP曲线进行分析,获得单位反应中心吸收的能量(ABS/RC)、被反应中心捕获的能量(TRo/RC)、光化学性能指数(PI abs)及t=0时最大光化学效率(φPo)等其他荧光参数。
1.2.3 叶片光合参数测定 于果实膨大后期,选择晴朗无风的天气08:00—18:00 之间,选取阳光玫瑰葡萄枝条中部的成熟叶片,使用便携式光合测定仪(汉莎,CIRAS-3)测定光合参数。光照度设定为500 μmol·m-2·s-1,设置6 个时间梯度(08:00、10:00、12:00、14:00、16:00、18:00),分别测定各处理叶片的净光合速率(Pn)、气孔导度(Gs)、胞间CO2浓度(Ci)和蒸腾速率(Tr)。
1.2.4 果实大小和品质测定 随机选取20个果穗,分别从果穗上部、中部和下部随机选取1个果粒,用游标卡尺测量果粒的最大纵径、横径。使用数显式糖酸测定仪(ATAGO PAL-BXIACID2)测定葡萄果实中的总可溶性固形物(TSS)和总酸(TA)含量。具体方法:将样品果实榨汁后,取适量果汁滴入数显式糖酸测定仪中,读取仪器显示的可溶性固形物含量(%),稀释50 倍后测定有机酸含量(%)。每个处理样品重复测定3次,取平均值。
1.2.5 成花相关基因的表达分析和第2年花芽数量统计 在第2 年春季新梢萌发时,观察并统计花芽数量。成花率定义为每个处理中冬芽萌发出花序的总数占萌发芽的数量的比率。在花序主轴分化完成后,选取葡萄第5 节的成熟花芽,立即置于液氮中,并带回实验室备用。提取RNA使用RNA提取试剂盒(诺唯赞,RC411-01),利用Nanodrop 2000 检测RNA 浓度和纯度,琼脂糖凝胶电泳检测RNA 的完整性。反转录后进行qRT-PCR 实验。通过Genebank 和Phytozome 网站查询葡萄候选基因序列,并使用Primer 5.0 软件设计特异性引物(表1)。选择VvActin1 Gank参考序列(XP_008654957.1)作为内参基因,采用2-ΔΔCt 法计算基因的相对表达量。qRT-PCR 使用南京诺唯赞生物科技有限公司的ChamQ Universal SYBR qPCR Master Mix试剂盒,反应体系为:one step RT-qPCR Master Mix 10 μL,上、下游引物(10 μmol·L-1)各0.4 μL,cDNA(200 ng·μL-1)0.6 μL,加水补充至20 μL。反应程序为:预变性95℃30 s;循环反应95 ℃10 s,60 ℃30 s,共40次循环。所有样品进行3次重复。
表1 实时荧光定量PCR 引物序列
Table 1 Primer sequences for the quantification of transcripts by real-time PCR
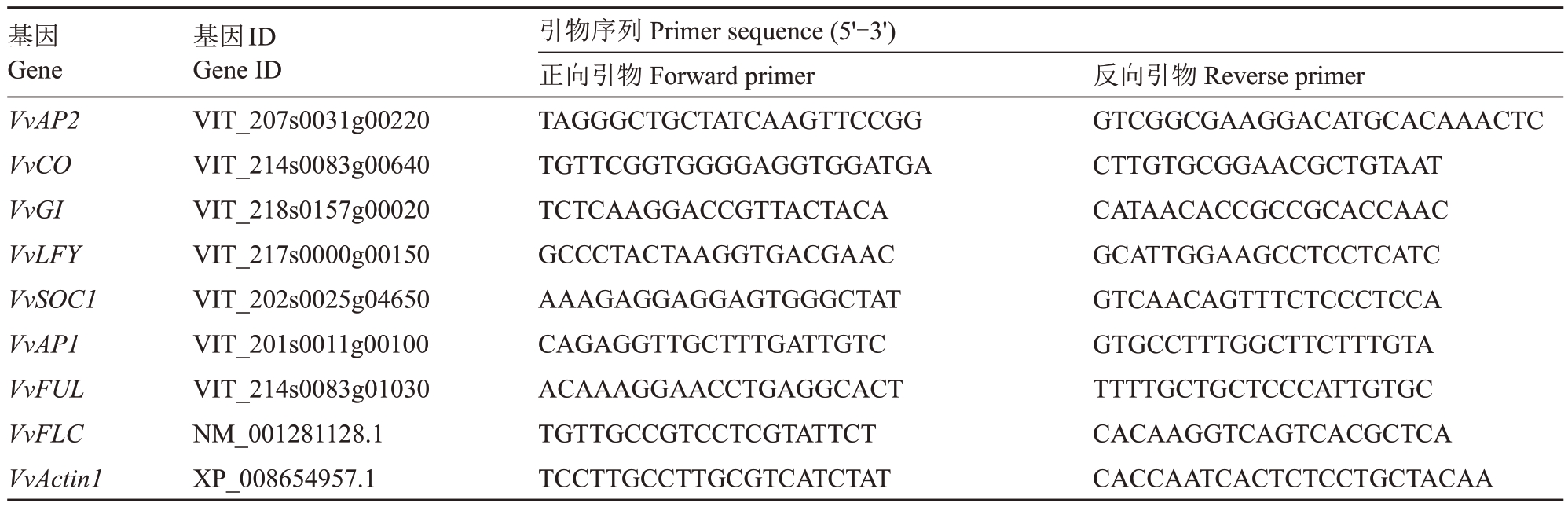
基因Gene VvAP2 VvCO VvGI VvLFY VvSOC1 VvAP1 VvFUL VvFLC VvActin1基因ID Gene ID VIT_207s0031g00220 VIT_214s0083g00640 VIT_218s0157g00020 VIT_217s0000g00150 VIT_202s0025g04650 VIT_201s0011g00100 VIT_214s0083g01030 NM_001281128.1 XP_008654957.1引物序列Primer sequence(5'-3')正向引物Forward primer TAGGGCTGCTATCAAGTTCCGG TGTTCGGTGGGGAGGTGGATGA TCTCAAGGACCGTTACTACA GCCCTACTAAGGTGACGAAC AAAGAGGAGGAGTGGGCTAT CAGAGGTTGCTTTGATTGTC ACAAAGGAACCTGAGGCACT TGTTGCCGTCCTCGTATTCT TCCTTGCCTTGCGTCATCTAT反向引物Reverse primer GTCGGCGAAGGACATGCACAAACTC CTTGTGCGGAACGCTGTAAT CATAACACCGCCGCACCAAC GCATTGGAAGCCTCCTCATC GTCAACAGTTTCTCCCTCCA GTGCCTTTGGCTTCTTTGTA TTTTGCTGCTCCCATTGTGC CACAAGGTCAGTCACGCTCA CACCAATCACTCTCCTGCTACAA
1.3 数据分析
利用Excel 2010软件对数据进行整理,数据为平均值±标准差,利用SPSS 25.0 进行方差分析(One-way ANOVA),p<0.05 为差异显著,采用Graphpad Prism 8.0进行可视化作图。
2 结果与分析
2.1 不同处理对叶面积、茎粗和节间长度的影响
由图1 可以看出,处理60 d 后,与对照相比,红蓝光2∶1 和红光处理的叶片长度增加,分别达到21.11 cm和20.05 cm;而遮光处理的叶片长度减少,仅为18.40 cm。叶片宽度方面,CK和红光处理最大,分别为30.25 cm 和27.20 cm;遮光处理的叶片宽度最小,仅为22.10 cm。在叶面积方面,CK处理的叶面积最大,为474.50 cm²;其次是红光和红蓝光2∶1 处理,分别为450.39 cm²和405.02 cm²;遮光处理的叶面积最小,仅为323.54 cm²。

图1 不同处理对叶面积、茎粗和节间长度的影响
Fig.1 Effects of different treatments on area of leaves,thickness of stem,and length of internodes
葡萄新梢粗度和长度是衡量生长状况的重要指标[19]。如图1所示,补光处理和CK处理的茎基部粗度显著高于遮光处理(p<0.05)。新梢长度统计结果显示,与CK处理相比,红蓝光2∶1、白光和红光处理的新梢长度分别增加了12.48%、6.24%和3.73%,而遮光处理降低了13.11%。对茎1~8节的节间长度进行统计发现,1~3 节的节间长度在各处理之间无显著差异;而在第4~7节,红蓝光2∶1、白光和CK处理的节间长度显著大于红光和遮光处理(p<0.05);第8节的节间长度以红蓝光2∶1和CK处理的最长,白光和红光处理次之,遮光处理的节间长度最短。以上结果说明合理的补光措施能有效促进葡萄叶片和新梢的生长。
2.2 不同处理对叶片OJIP的影响
最大光化学量子产量(Fv/Fm)是反映PSⅡ活性中心光能转化效率的重要参数[18],将各处理阳光玫瑰葡萄叶绿素荧光参数进行标准化,并绘制OJIP曲线,发现各处理荧光强度均在短时间内达到峰值(P点),随后趋于平缓(图2-A)。对荧光值归一化后的OJIP 曲线显示,各处理均在2 ms 和30 ms 处产生了两个拐点,即J点和I点,趋势无显著变化(图2-B)。
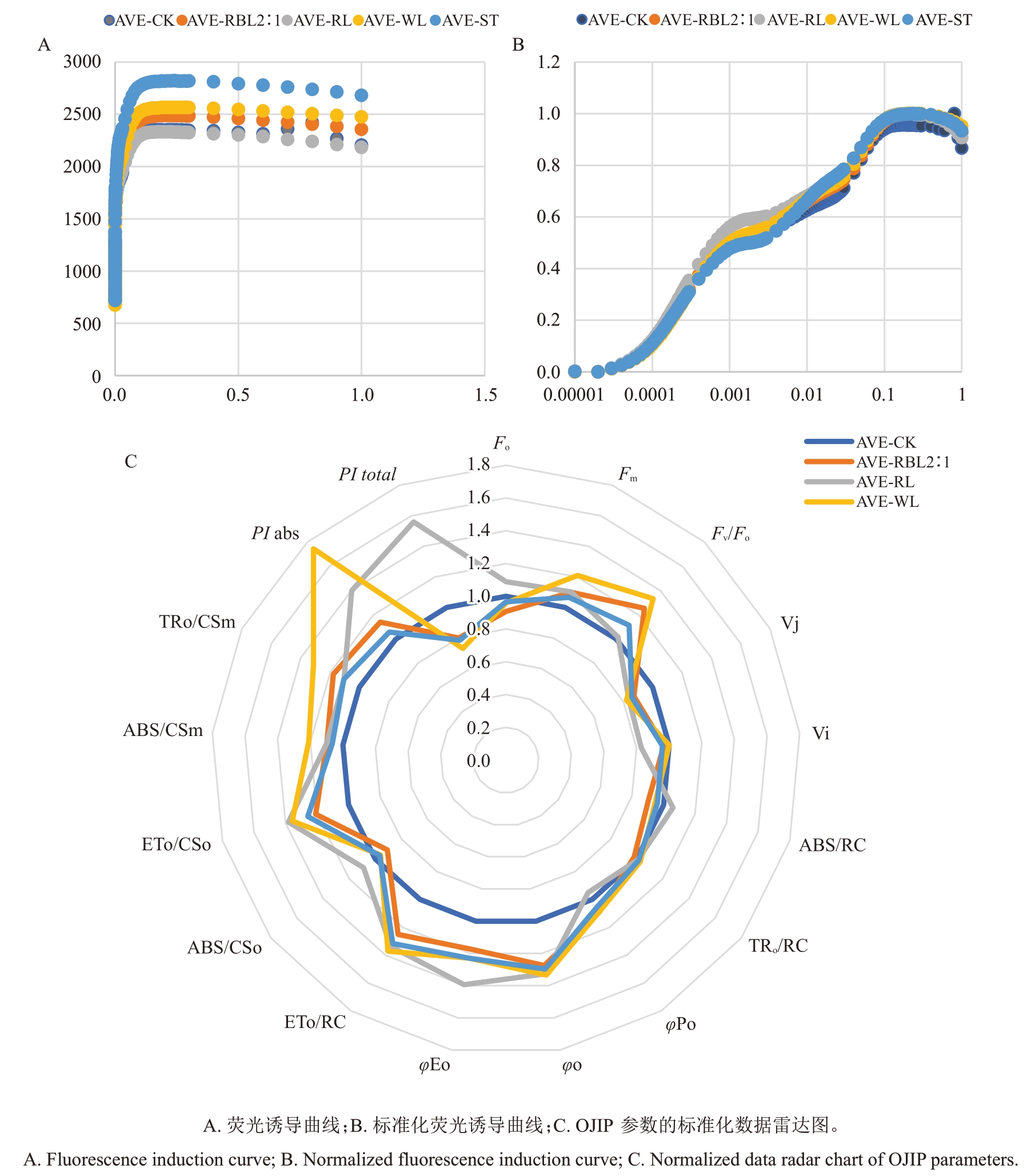
图2 不同处理对叶片快速叶绿素荧光诱导动力学曲线(OJIP)的影响
Fig.2 Effects of different treatments on the rapid chlorophyll fluorescence induction kinetics curve(OJIP)of leaves
与对照相比,红蓝光2∶1 和白光组叶片PSⅡ最大光化学效率(Fv/Fm)显著升高(p<0.05),红蓝光2∶1和红光处理的PI abs显著高于CK(p<0.05)(表2),最大光化学效率(φPo)红蓝光2∶1 处理高于CK 处理,白光和红蓝光2∶1 处理的单位叶面积捕获的光能(TRO/CSm)和单位叶面积吸收的光能(ABS/CSm)显著高于CK和其他处理(p<0.05),而单位反应中心吸收的能量(ABS/RC)则相反(图2-C)。
表2 不同处理对阳光玫瑰葡萄Fo、Fm、Fv、Fv/Fo、Fv/Fm及PI abs 的影响
Table 2 Effects of different treatments on Fo,Fm,Fv,Fv/Fo,Fv/Fm,and PI abs of Shine Muscat grape

注:不同小写字母表示同列数据在p<0.05 水平显著差异。
Note:Different small letters indicate significant differences in the same column data at the p<0.05 level.
处理Treatment对照CK红蓝光2∶1 RBL 2:1红光RL白光WL遮光ST Fo Fm Fv 698.333±37.018 a 693.667±55.609 ab 3 068.667±71.122 a 2527±135.072 b 2 370.333±106.327 ab 1 833.333±84.500 b Fv/Fo 3.406±0.327 ab 2.502±0.123 c Fv/Fm 0.702±0.065 b 0.755±0.010 a PI abs 0.852±0.111 b 1.283±0.075 a 1.254±0.130 a 1.217±0.366 ab 0.954 7±0.134 ab 658±16.093 b 571.667±36.088 c 689±18.037 a 2197±366.504 bc 2557±206.369 b 1 931.500±306.785 c 1539±351.574 c 1858±368.664 b 2 576.125±290.168 a 2.332±0.475 c 3.090±0.292 b 3.708±0.339 a 0.685 6±0.017 c 0.760±0.011 a 0.734±0.005 ab
2.3 不同处理对叶片光合特性的影响
由图3 可以看出,各处理的净光合速率日变化均呈现“升-降-再升-再降”的双峰趋势。其中,红蓝光2∶1 处理大部分时间的净光合速率保持最高水平。各处理第一个高峰均出现在10:00,随后CK、红光、白光和遮光处理的第二个高峰出现在14:00,而红蓝光2∶1处理的第二个高峰延迟至16:00,随后迅速下降。
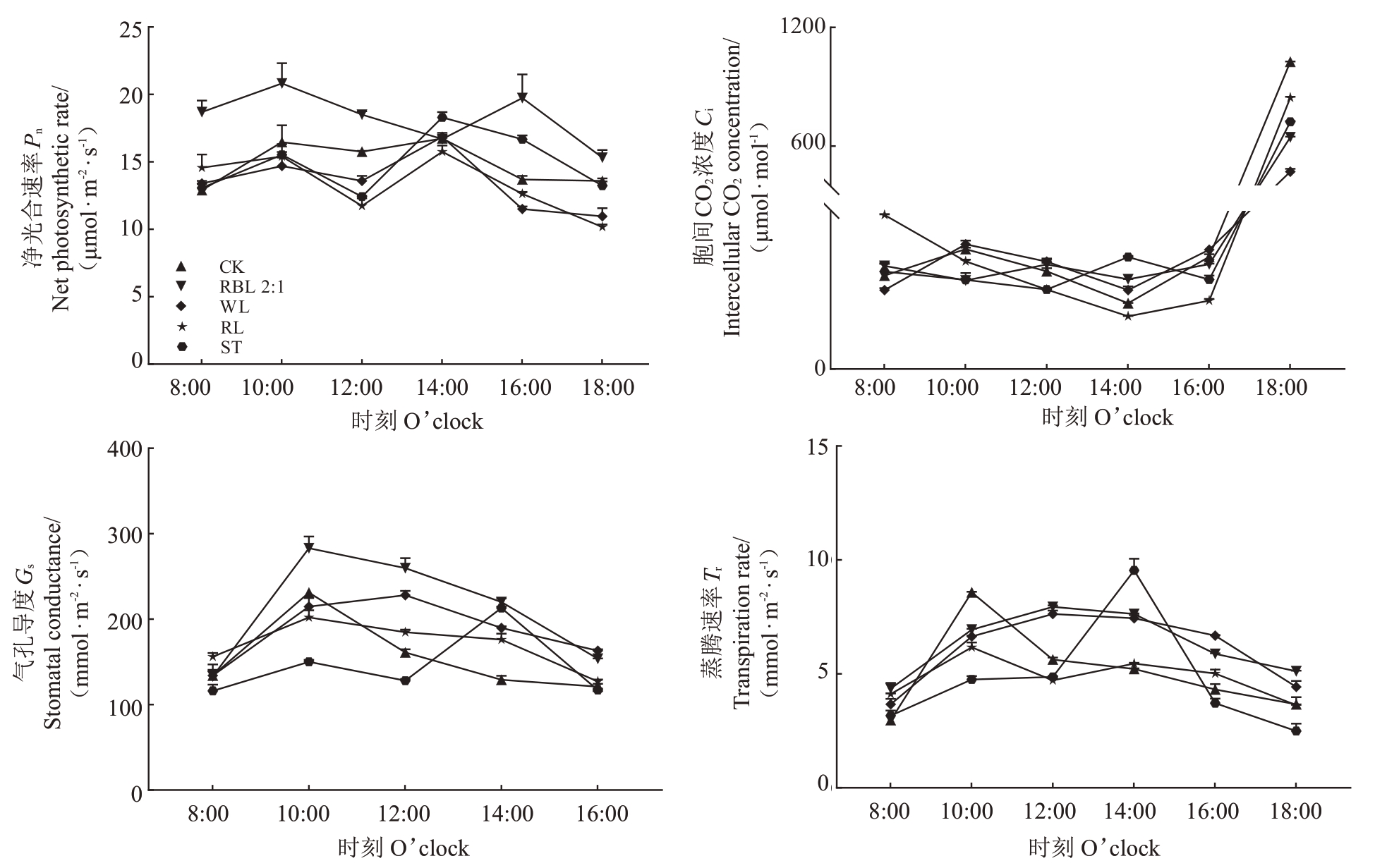
图3 不同处理对叶片光合特性的影响
Fig.3 Effects of different treatments on the photosynthetic characteristics of leaves
在胞间CO2浓度方面,CK和白光处理整体出现“升-降-再升”的变化趋势,而除遮光处理外,CK和补光处理的胞间CO2浓度在12:00—14:00 之间呈下降趋势,随后,在16:00后呈上升趋势。红蓝光2∶1处理在8:00—12:00 之内胞间CO2浓度低于CK 组,而白光和红光组在此时间区间内高于CK,表明该时间段内红蓝光2∶1处理的光合效率较高。16:00—18:00,各处理的胞间CO2浓度明显上升,其中CK 处理在18:00 浓度最高,红光处理次之,白光和红蓝光2∶1处理相对较低。
气孔导度(Gs)是影响光合碳同化速率的重要因素。本研究中补光处理的气孔导度均呈现先升后降的趋势,红蓝光2∶1 处理气孔导度整体显著高于白光和红光处理(p<0.05);而CK 和遮光处理的气孔导度则分别在10:00和14:00达到高峰。
蒸腾速率的日变化趋势与气孔导度一致,补光处理同样呈现“先升后降”的趋势,CK 和遮光处理的高峰时间分别为10:00 和14:00。综上所述,从光合特性来看,红蓝光2∶1处理表现最佳,白光处理次之。
2.4 不同处理对果实生长的影响
由图4 可以看出,随着果实的生长,红蓝光2∶1处理的果实生长速度最快,表现为成熟期(9月3日)果实纵径、横径及单果质量均为最大,特别是单果质量,显著高于其他处理(p<0.05)。白光、CK和遮光这3个处理的单果质量无显著差异。红光处理显著抑制果实的生长(p<0.05),主要表现是红光处理抑制果实纵径的伸长。果形指数的统计结果显示,各处理对果实发育期的果形指数影响较大,而对果实成熟期的影响相对较小。果实成熟期果形指数在1.29~1.42之间,果实呈椭圆形或长椭圆形[20],红蓝光2∶1 和红光组的果形指数均高于对照组和遮光组,这表明补光处理对果实形状具有一定的改善效果。
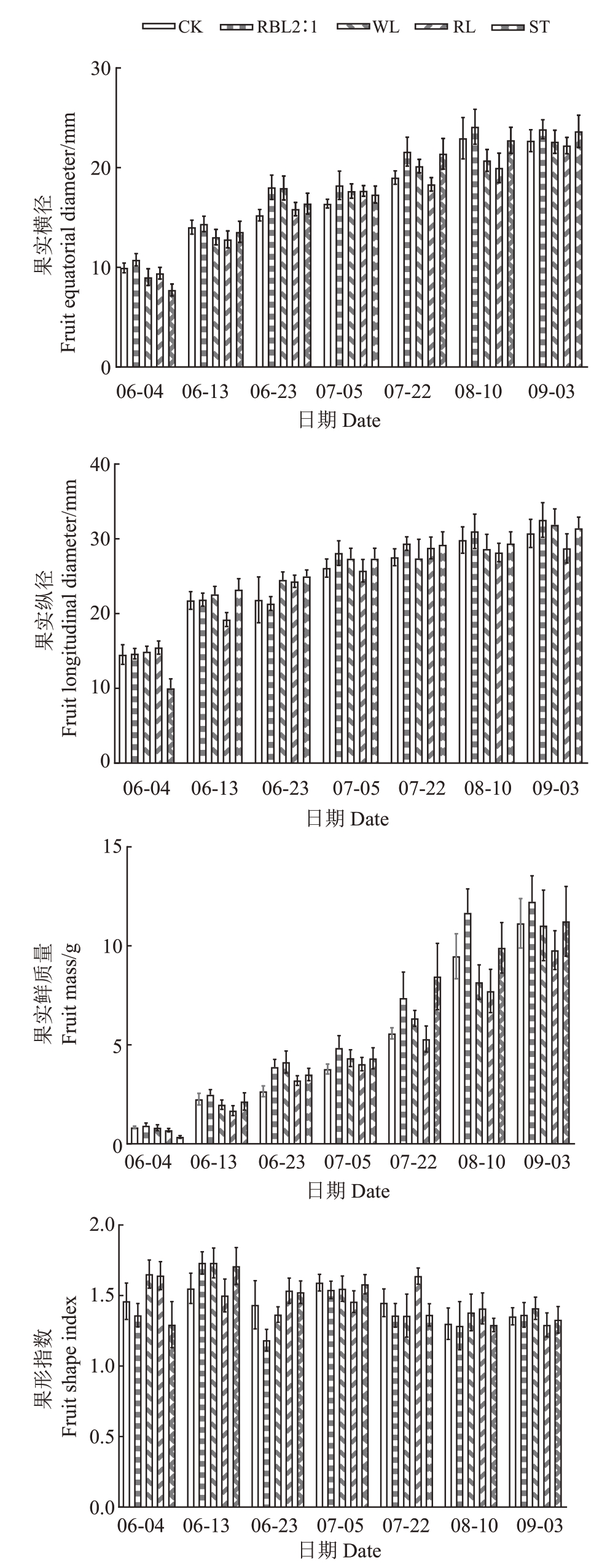
图4 不同处理对果实生长的影响
Fig.4 Effects of different treatments on fruit growth
2.5 不同处理对果实品质的影响
由图5 可以看出,随着果实的发育,不同处理下,阳光玫瑰葡萄果实的TSS 含量逐渐增加。特别是在果实成熟期,红蓝光2∶1 和白光处理的TSS 含量(w,后同)显著高于其他处理(p<0.05),分别为19.67%和19.63%;红光处理的TSS 含量最低,为16.90%,表明红光处理在一定程度上抑制了TSS 的积累。随着果实的成熟,各处理的TA含量呈下降趋势。到果实成熟期,CK、红蓝光2∶1 和白光处理的TA 含量无显著差异,均为0.60%,而红光和遮光处理的TA 含量显著高于其他处理(p<0.05),分别为0.86%和0.76%。各处理的固酸比也随着果实成熟显著增大(p<0.05),在果实成熟期,红蓝光2∶1和白光处理在果实成熟期的固酸比显著高于其他处理(p<0.05),达到了最高值,表明这两个处理改善了果实的甜酸平衡。

图5 不同处理对果实品质的影响
Fig.5 Effects of different treatments on fruit quality
2.6 不同处理对葡萄第2年花芽分化的影响
光质对花芽分化具有显著影响。由图6-A可以看出,第2 年花芽的萌发时间及萌发数量有较大差异。补光处理(红蓝光2∶1、红光和白光)花芽萌发明显较早,而CK和遮光处理萌发较晚。第1节位的花芽萌发率分别为红蓝光2∶1(100%)=白光(100%)>CK(92%)>红光(88%)>遮光(72%);在第2 节位,花芽萌发率分别为红光(100%)>红蓝光2∶1(96%)>白光(92%)>CK(88%)>遮光(76%)(图6-B)。综合来看,白光和红蓝光补光处理的花芽萌发率显著高于CK 和遮光处理,说明其不仅可以提早花芽萌发时间,还能提高花芽萌发率。

图6 不同处理对冬芽萌发(A)及第1、2 节位花芽率(B)的影响
Fig.6 Effects of different treatments on winter bud germination(A)and flower bud rates at the first and second nodes(B)
2.7 不同处理对葡萄成花基因的影响
本研究参考王海波等[17]的研究,在花序主轴分化完成后,对不同处理条件下花芽中9 个成花相关基因的表达水平进行了分析(图7)。结果显示,VvGI基因和VvSOC1基因在白光和红蓝光2∶1处理显著高于CK、红光和遮光处理(p<0.05);VvLFY 基因和VvFLC基因的表达模式相近,表达量排序依次为CK>RL>RBL 2∶1>ST>WL;VvFUL 基因与VvAP1 基因表达模式相近,除红光处理的表达量略高于CK,红蓝光2:1 和白光处理表达量比CK 低。VvCO 基因表达量在白光和红光处理组显著高于CK,红蓝光2∶1 和遮光处理的表达则低于CK。而VvSOC1 基因与VvFLC 基因的表达模式完全相反,说明这两个基因在该阶段的花芽分化过程中可能具有相反的功能。此外,本研究在此阶段未检测到成花关键基因VvFT的表达。
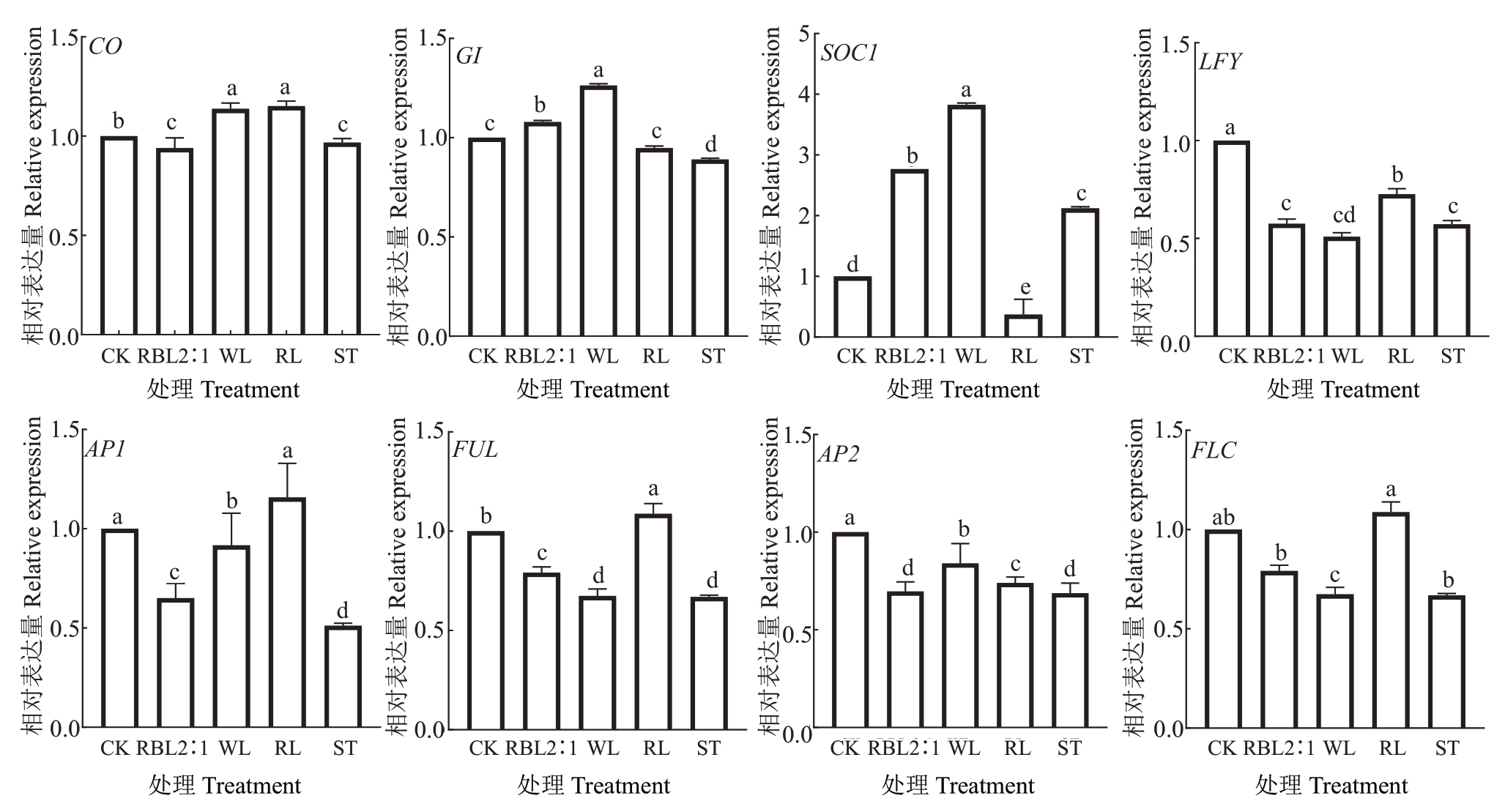
图7 不同处理对葡萄成花基因的影响
Fig.7 Effects of different treatments on grape flowering genes
3 讨 论
补光是解决葡萄避雨栽培光照不足的有效方法。研究表明,红光能够有效促进植物株高、叶片数、叶面积以及叶片厚度等生长指标的增加[21]。笔者在本研究中发现,相较于遮光组,红光和红蓝光2∶1补光处理显著增大了叶片的长度和叶面积(p<0.05),这与王琦等[22]研究中红蓝比7∶3的补光处理显著增加株高和叶面积的结果相一致。同时,笔者在本研究中还发现红蓝光2∶1处理和白光处理整体增加了新梢的节间的长度,表明合理的光质组合不仅对叶片生长有促进作用,也对新梢的发育具有积极影响,尤其是红蓝光的比例可能是一个重要的调控因素。
遮光显著影响葡萄纵横径和单果质量[23]。黄秋凤等[24]对巨峰葡萄进行夜间补光,发现其果粒质量、纵径、横径及果实可溶性固形物含量均有所提升。笔者在本研究中发现红蓝光2∶1处理显著提高了葡萄的可溶性糖含量(p<0.05),导致TSS/TA 比显著增大(p<0.05)。前人在桃中研究发现,蓝光可显著提高果实质量、可溶性糖含量等指标[25],在火龙果中,红蓝光(4∶1)可显著提高单株产量[26];在南高丛越橘上研究发现,红蓝光(3∶1)能够显著提高果实可溶性糖和可溶性固形物含量[27];在巨峰葡萄中也观察到了类似的结果,红光和蓝光处理显著提高了葡萄果实中的糖浓度[28]。此外,RBL2∶1处理表现出较高的糖积累水平和TSS/TA 比率,表明该光质组合在促进葡萄可溶性糖积累方面具有最优效果,这与时晓芳等[29]的红光显著促进果实的可溶性固形物和糖分的积累,蓝光则可降低可滴定酸含量的研究结果相符。此现象可能与红蓝光组合处理对葡萄光合作用效率的提升密切相关,因为红蓝光组合能够较大限度地提高叶片的光能利用效率,从而促进了糖分的积累。
叶片是植物光合作用的主要器官。刘文海等[30]通过采取不同遮阴方式研究桃树的耐弱光性,发现随着光照度的降低,叶片的光补偿点、光饱和点、CO2补偿点、CO2饱和点以及羧化效率均下降,光呼吸速率也逐渐降低。王欣欣等[31]的研究中补充红光可提高巨峰葡萄叶片的净光合速率,并促进新梢和叶片的生长。笔者在本研究中发现,RBL2∶1 处理对叶片Fv/Fm、PI abs 及Pn和Gs等光合参数有显著的提升作用。值得注意的是,RBL2∶1处理PI abs值较CK 提升50.59%,表明该光质比通过促进QA 向QB电子传递[17],提升光能向化学能转化效率。这与樱桃红蓝光比例6∶1的补光处理显著提高樱桃叶片光合速率的结果相一致[9],进一步支持了红蓝光组合能够提升植物光合作用效率的观点。相较于单一补光处理,红蓝光组合在促进光合作用和光能利用方面表现出明显优势。
此外,笔者在本研究中还发现补光处理对第2年冬芽的花芽分化具有积极影响。在生理层面,红蓝光2∶1 处理显著提高了花芽萌发率。在分子层面,多个光周期相关基因(如VvGI 和VvSOC1)的表达有显著变化。这与刘鑫等[32]在红地球葡萄中的研究相符,表明适宜的光质不仅有助于当年果实的生长,还能对翌年的花芽分化产生正向影响,为提高下一年的产量打下基础。因此,补光处理对葡萄的生长发育具有长远的正向影响,合理的补光能够有效提升产量和品质。
4 结 论
与CK组相比,RBL 2∶1补光提高了阳光玫瑰葡萄新梢生长速率,增大了叶片面积,且提高了叶片PI abs 值、Fv/Fm值及光利用效率;同时,果实单果质量和糖酸比均有所提升;此外,花芽萌发率得到显著提升,VvGI、VvSOC1等光响应基因的表达水平显著高于CK。这为补光技术在避雨栽培条件下的应用提供了较好的理论基础。
[1] 祁栋灵,周庆阳,刘三军,李靖.利用叶形结构数值分析葡萄种质亲缘关系的研究[J].中国南方果树,2005,34(3):64-66.QI Dongling,ZHOU Qingyang,LIU Sanjun,LI Jing. Study on the foliar structure data and the relationship of grapes[J]. South China Fruits,2005,34(3):64-66.
[2] 刘柯含,张芮,高彦婷,张红娟,温健,薛莲,李青青.中国葡萄产业现状分析及其发展对策[J].中国果树,2024(7):132-138.LIU Kehan,ZHANG Rui,GAO Yanting,ZHANG Hongjuan,WEN Jian,XUE Lian,LI Qingqing. Present situation analysis and development countermeasures of grape industry in China[J].China Fruits,2024(7):132-138.
[3] 李勃.山东省葡萄产业发展现状、问题与对策[J].落叶果树,2024,56(5):1-5.LI Bo. The current situation,problems and countermeasures of grape industry development in Shandong Province[J]. Deciduous Fruits,2024,56(5):1-5.
[4] ZHANG J X,LI W P,ZHANG P,ZHANG X H,WANG J F,WANG L J,CHEN K Q,FANG Y L,ZHANG K K. Effect of supplementary light with different wavelengths on anthocyanin composition,sugar accumulation and volatile compound profiles of grapes[J].Foods,2023,12(22):4165.
[5] YIN Y G,LI M M,JIA N,SUN Y,HAN B,LIU C J,LIU S Y,ZHAO S J,GUO Z J. Effects of trellis system and berry thinning intensity on vine performance and quality composition of two table grape cultivars under protected cultivation in northern China[J].Scientia Horticulturae,2022,299:111045.
[6] 王世平,李勃.中国设施葡萄发展概况[J].落叶果树,2019,51(1):1-5.WANG Shiping,LI Bo.Development of protected grape cultivation in China[J].Deciduous Fruits,2019,51(1):1-5.
[7] 邢阿宝,崔海峰,俞晓平,张雅芬,叶子弘.光质及光周期对植物生长发育的影响[J].北方园艺,2018(3):163-172.XING Abao,CUI Haifeng,YU Xiaoping,ZHANG Yafen,YE Zihong. Effects of different lights qualities and photoperiods on plant growth and development[J]. Northern Horticulture,2018(3):163-172.
[8] 郑晓翠,王海波,王宝亮,王孝娣,魏长存,刘万春,何锦兴,刘凤之.补光对设施葡萄果实品质及叶片质量的影响[J].中国果树,2013(2):31-33.ZHENG Xiaocui,WANG Haibo,WANG Baoliang,WANG Xiaodi,WEI Changcun,LIU Wanchun,HE Jinxing,LIU Fengzhi.Effects of supplementary light on fruit quality and leaf quality of facility-grown grapes[J].China Fruits,2013(2):31-33.
[9] 李都岳,陈翔,华爱君,李永丽,吴延军.设施栽培不同光质补光对两个中国樱桃品种生长发育及品质的影响[J].中国南方果树,2024,53(3):251-255.LI Duyue,CHEN Xiang,HUAAijun,LI Yongli,WU Yanjun.Effects of different quality supplementary light on growth and quality of two varieties of Chinese cherry in protected cultivation[J].South China Fruits,2024,53(3):251-255.
[10] 齐志国.LED 补光对设施葡萄植株生长及果实品质的影响[D].沈阳:沈阳农业大学,2023.QI Zhiguo. Effects of LED supplementary light on plant growth and fruit quality of facility grape[D]. Shenyang:Shenyang Agricultural University,2023.
[11] 朱静娴. 人工补光对植物生长发育的影响[J]. 作物研究,2012,26(1):74-78.ZHU Jingxian. Influence of artificial supplement of light on plant growth and development[J]. Crop Research,2012,26(1):74-78.
[12] 杨其长,张成波.植物工厂概论[M].北京:中国农业科学技术出版社,2005.YANG Qichang,ZHANG Chengbo.An introduction to plant factory[M]. Beijing:China Agriculmual Science and Technology Press,2005.
[13] KIGEL J,COSGROVE D J. Photoinhibition of stem elongation by blue and red light:Effects on hydraulic and cell wall properties[J].Plant Physiology,1991,95(4):1049-1056.
[14] KIM S J,HAHN E J,HEO J W,PAEK K Y.Effects of LEDs on net photosynthetic rate,growth and leaf stomata of chrysanthemum plantlets in vitro[J].Scientia Horticulturae,2004,101(1/2):143-151.
[15] XU F,CAO S F,SHI L Y,CHEN W,SU X G,YANG Z F.Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit[J]. Journal of Agricultural and Food Chemistry,2014,62(20):4778-4783.
[16] ZHANG Y T,JIANG L Y,LI Y L,CHEN Q,YE Y T,ZHANG Y,LUO Y,SUN B,WANG X R,TANG H R. Effect of red and blue light on anthocyanin accumulation and differential gene expression in strawberry (Fragaria × ananassa)[J]. Molecules,2018,23(4):820.
[17] 王海波,王孝娣,赵君全,史祥宾,王宝亮,郑晓翠,刘凤之.设施促早栽培下耐弱光能力不同的葡萄品种冬芽的花芽分化[J].园艺学报,2016,43(4):633-642.WANG Haibo,WANG Xiaodi,ZHAO Junquan,SHI Xiangbin,WANG Baoliang,ZHENG Xiaocui,LIU Fengzhi. Studies on the flower bud differentiation of grape cultivars with different tolerant ability of low light in greenhouse[J].Acta Horticulturae Sinica,2016,43(4):633-642.
[18] 李冠宇,马闯,田淑芬,王超霞,王荣.硅酸钠对碱性盐胁迫下阳光玫瑰葡萄叶片光系统Ⅱ(PSⅡ)的影响[J]. 果树学报,2024,41(7):1359-1367.LI Guanyu,MA Chuang,TIAN Shufen,WANG Chaoxia,WANG Rong.Effect of Na2SiO3 on leaf photosystem Ⅱ(PS Ⅱ)under alkaline salt stress in Shine Muscat grape[J]. Journal of Fruit Science,2024,41(7):1359-1367.
[19] 李双海.福建省高海拔山区葡萄优质高效栽培技术研究[D].福州:福建农林大学,2022.LI Shuanghai. Research on high-quality and high-efficiency cultivation techniques of grapes in high-altitude mountainous areas of Fujian province[D]. Fuzhou:Fujian Agriculture and Forestry University,2022.
[20] 刘崇怀,沈育杰,陈俊.葡萄种质资源描述规范和数据标准[M].北京:中国农业出版社,2006.LIU Chonghuai,SHEN Yujie,CHEN Jun. Descriptors and data standard for grape (Vitis L.) [M]. Beijing:China Agriculture Press,2006.
[21] FAN X X,XU Z G,LIU X Y,TANG C M,WANG L W,HAN X L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light[J].Scientia Horticulturae,2013,153:50-55.
[22] 王琦,谭占明,程云霞,杨帆,邱宇杰,付予笑,唐瑾,杨桂臻,马全会.不同比例红蓝光夜间补光对番茄生长生理的影响[J].山东农业科学,2024,56(9):58-66.WANG Qi,TAN Zhanming,CHENG Yunxia,YANG Fan,QIU Yujie,FU Yuxiao,TANG Jin,YANG Guizhen,MA Quanhui.Effects of supplementing red and blue light at different ratios at night on growth and physiology of tomato[J].Shandong Agricultural Sciences,2024,56(9):58-66.
[23] 马宗桓,姜雪峰,毛娟,卢世雄,何红红,陈佰鸿.不同光照强度对‘马瑟兰’葡萄果实发育及着色的影响[J].中外葡萄与葡萄酒,2019(5):47-50.MA Zonghuan,JIANG Xuefeng,MAO Juan,LU Shixiong,HE Honghong,CHEN Baihong. Effects of different light intensity on berries development and coloration of‘Marselan’grapevine[J].Sino-Overseas Grapevine&Wine,2019(5):47-50.
[24] 黄秋凤,谢蜀豫,曹慕明,陈立,李敏,覃锦声,李玮,余欢,阙名锦,陈国品.夜间补光对巨峰葡萄春果叶片营养及果实品质的影响[J].南方农业学报,2019,50(4):781-787.HUANG Qiufeng,XIE Shuyu,CAO Muming,CHEN Li,LI Min,QIN Jinsheng,LI Wei,YU Huan,QUE Mingjin,CHEN Guopin. Effects of supplementary illumination at night on leaf nutrition and fruit quality for spring fruit of Kyoho grape[J].Journal of Southern Agriculture,2019,50(4):781-787.
[25] 郑晓翠,刘凤之,王海波,王孝娣.不同光质补光对设施内桃果实品质及叶片质量的影响[J].西北植物学报,2023,43(6):979-987.ZHENG Xiaocui,LIU Fengzhi,WANG Haibo,WANG Xiaodi.Effects of different supplemental light on fruit and leaf quality of peach in the facility[J]. Acta Botanica Boreali-Occidentalia Sinica,2023,43(6):979-987.
[26] 尤小婷,陈士伟,李栋宇,张曼其,姚雷业,张正贺,刘伟清,徐杨玉.不同光质LED 补光对火龙果植株生长发育的影响[J].热带农业科学,2021,41(4):7-10.YOU Xiaoting,CHEN Shiwei,LI Dongyu,ZHANG Manqi,YAO Leiye,ZHANG Zhenghe,LIU Weiqing,XU Yangyu. Effects of LEDs with different spectra on the growth and development of pitaya[J]. Chinese Journal of Tropical Agriculture,2021,41(4):7-10.
[27] 王佳淇,何莹钰,韦晓桐,李永强,杨莉,陈文荣,廖芳蕾,郭卫东.LED 补光组合对大棚越橘生长发育的影响[J].园艺学报,2020,47(6):1183-1193.WANG Jiaqi,HE Yingyu,WEI Xiaotong,LI Yongqiang,YANG Li,CHEN Wenrong,LIAO Fanglei,GUO Weidong. Effects of LED supplemental light on the growth and development of blueberry in greenhouse[J]. Acta Horticulturae Sinica,2020,47(6):1183-1193.
[28] KOYAMA K,IKEDA H,POUDEL P R,GOTO-YAMAMOTO N. Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape[J]. Phytochemistry,2012,78:54-64.
[29] 时晓芳,林玲,黄秋秘,白先进,白扬,黄桂媛,韩佳宇,李洪艳,曹雄军,郭荣荣.阳光玫瑰葡萄冬果品质及糖代谢响应光质机理[J].南方农业学报,2024,55(8):2286-2294.SHI Xiaofang,LIN Ling,HUANG Qiumi,BAI Xianjin,BAI Yang,HUANG Guiyuan,HAN Jiayu,LI Hongyan,CAO Xiongjun,GUO Rongrong. Response mechanism of Shine Muscat grape winter berries quality and sugar metabolism to light quality[J].Journal of Southern Agriculture,2024,55(8):2286-2294.
[30] 刘文海,高东升,束怀瑞.不同光强处理对设施桃树光合及荧光特性的影响[J].中国农业科学,2006,39(10):2069-2075.LIU Wenhai,GAO Dongsheng,SHU Huairui. Effects of different photon flux density on the characteristics of photosynthesis and chlorophyll fluorescence of peach trees in protected culture[J].Scientia Agricultura Sinica,2006,39(10):2069-2075.
[31] 王欣欣,赵文东,郭修武,满丽婷,高圣华,赵海亮.不同光质对延迟栽培‘巨峰’葡萄新梢生长及生理特性的影响[J].北方果树,2009(3):3-5.WANG Xinxin,ZHAO Wendong,GUO Xiuwu,MAN Liting,GAO Shenghua,ZHAO Hailiang. Effects of supplemental lighting with different light quality on the shoot growth and physiology of‘Kyoho’grape growing in greenhouse for delay[J].Northern Fruits,2009(3):3-5.
[32] 刘鑫,张亚红,袁苗,刘帅,葛静,周娟.红蓝光对设施‘红地球’葡萄花芽分化的影响[J].西南农业学报,2023,36(3):637-646.LIU Xin,ZHANG Yahong,YUAN Miao,LIU Shuai,GE Jing,ZHOU Juan.Effect of red and blue light on flower bud differentiation of‘Red Globe’grape in protected culture[J]. Southwest China Journal of Agricultural Sciences,2023,36(3):637-646.