苹果(Malus pumila Mill.)为蔷薇科(Rosaceae)苹果属(Malus)落叶乔木,因其果实具有营养价值高、耐贮性好和供应周期长等特点,已成为农民增收致富的支柱产业之一[1]。然而,苹果早期落叶病在国内苹果主产区广泛发生,给苹果产业高质量发展带来了挑战[2-3]。该病害发生后常导致苹果树提早大量落叶,严重削弱树势,发生严重情况下造成当年或翌年果实品质及产量下降[4]。相关研究表明,苹果早期落叶病种类主要包括苹果褐斑病、斑点落叶病、轮斑病、圆斑病、灰斑病和炭疽叶枯病等,并且不同区域的优势病害种类不同,其中苹果褐斑病和苹果斑点落叶病较为常见[5-7]。链格孢菌(Alternaria spp.)是引起多种苹果早期落叶病的重要病原之一[8]。Harteveld 等[9]在澳大利亚发现引起苹果链格孢叶斑病和果斑病的主要病原为A. longipes、A. arborescens、A. alternata/A. tenuissima 和A. tenuissima/A. mali,且同一物种内分离株在致病性和毒力方面表现出显著变异和交叉致病性;Toome-Heller 等[10]首次在新西兰发现苹果链格孢复合种(A. arborescens)可引起苹果叶斑病且出现褐斑病症状。何劲等[11]研究发现,贵州地区苹果早期落叶病种类主要有苹果轮斑病和斑点落叶病,其病原分别为A. mali 和A. alternata,其中,由交链格孢(A. alternata)引起的苹果斑点落叶病是苹果生产中危害严重的病害之一,在全球苹果主产区均有发生[12]。然而,也有相关研究发现,苹果斑点落叶病和苹果轮斑病可由A. mali 的不同毒力菌株引起[13-15],其中A. mali 弱毒菌株引起的苹果轮斑病主要危害叶片,也可危害果实且病斑较大,该病害流行时植株发病率可达100%[16],而A. mali 强毒菌株主要危害苹果叶片和嫩枝,可引起苹果斑点落叶病,发生后影响花芽形成和果实正常生长[17],导致叶部出现褐色病斑和树势衰弱,病害流行时引起70%苹果树叶片早期脱落[18]。
邵旭平等[19]将引起甘肃省苹果斑点落叶病的病原鉴定为A. mali 的强毒菌株,苹果轮斑病的病原鉴定为A. mali 的弱毒菌株,并发现A. mali 强弱毒菌株间具有交叉保护作用,可有效降低病害的发生。同时,苹果斑点落叶病的发生和蔓延与环境因素密切相关,特别是温度和湿度可以通过影响苹果斑点落叶病菌(A. mali)孢子的萌发来调控病菌的生长,在30 ℃和100%相对湿度下,孢子萌发率最高,而在极端温湿度条件下,萌发率显著下降[20]。此外,另有研究发现在9 种不同温度(4~36 ℃)和8种保湿时间(2~48 h)的组合条件下,A. mali 均能侵染苹果幼苗,且随着保湿持续时间的延长,病害发生的程度显著加剧[21]。但是,目前有关A. mali 强弱毒菌株在不同环境因素下的侵染规律、发病条件及苹果品种对其抗性方面缺乏全面系统的研究。鉴于此,笔者以课题组前期分离鉴定的A. mali 强弱毒菌株作为供试菌株,采用离体叶片接种测定不同环境因子(温度、相对湿度和光照)和不同苹果品种对A. mali 强弱毒菌株潜育期及致病力强弱的影响,以期为苹果早期落叶病的防控提供理论支撑。
1 材料和方法
1.1 材料
1.1.1 供试菌株及其孢子悬浮液制备 供试A.mali 强弱毒菌株均保存于甘肃农业大学植物保护学院植物病毒学与分子生物学实验室。参考Harimoto等[22]的方法配制浓度为1×105个·mL-1的A. mali强弱毒菌株孢子悬浮液,备用。
1.1.2 供试苹果品种 供试苹果品种分别为新红星、富士和金冠。选择长势和粗细一致的健康苹果枝条作为室内离体接种试材,均采集自兰州市七里河区苹果种植基地,树龄为18~21 a(年)。
1.2 方法
1.2.1 不同温度对Alternaria mali 强弱毒菌株致病活性的影响 将采集的长势和粗细一致的新红星健康苹果枝条置于装有无菌水的无菌三角瓶内,每瓶5 枝,采用喷施接种法将A. mali 强弱毒菌株孢子悬浮液(1×105个·mL-1)分别接种于供试枝条叶片的正面和反面,并以接种等体积无菌水作为对照。随后,将各处理和对照分别置于塑料罩内以保持湿度(相对湿度90%),并分别置于15、20、25、30 和35 ℃的人工气候箱(16 h 光照/8 h 黑暗)内培养,并待接种后每隔培养6 h 观察和记录潜育期。同时,待接种7 d 后,统计叶片病情指数。试验过程中不同温度处理下,每个处理和对照均重复3次。
参照崔琳霞等[23]和王程亮等[24]分级标准进行分级。具体分级如下:0 级,叶片上未观察到斑点;1级,斑点覆盖面积占叶片总面积比例小于10%;3级,斑点覆盖面积占叶片总面积比例为11%~25%;5级,斑点覆盖面积占叶片总面积比例为26%~40%;7级,斑点覆盖面积占叶片总面积比例为41%~65%;9级,斑点覆盖面积占叶片总面积比例大于66%。

1.2.2 不同相对湿度对Alternaria mali 强弱毒菌株致病活性的影响 将按上述接种方法处理后的枝条分别置于干燥器中,利用不同浓度的H2SO4调整并设置相对湿度分别为60%、70%、80%、90%和100%。然后,置于温度为25 ℃和光照条件为16 h光照/8 h 黑暗的培养箱中培养,并待接种后每隔培养6 h 观察和记录潜育期。待接种7 d 后,统计叶片病情指数。试验每个处理和对照均设置3个重复。
1.2.3 不同光照条件对Alternaria mali 强弱毒菌株致病活性的影响 将经上述接种处理后的枝条分别置于不同光照[持续光照(24 h·d-1)、持续黑暗(24 h·d-1)、光照与黑暗交替(12 h 光照/12 h 黑暗)、紫外照射3 h 和光照处理21 h]、温度为25 ℃和相对湿度为90%条件下培养,并待接种后每隔培养6 h观察和记录潜育期。待接种7 d后,统计和计算叶片的病情指数。试验每个处理和对照均设置3个重复。
1.2.4 不同品种对Alternaria mali 强弱毒菌株致病活性的影响 将采集的长势和粗细一致的健康新红星、富士及金冠3 个品种枝条分别置于装有无菌水的无菌三角瓶内,每瓶5枝。然后,采用喷施接种法将A. mali强弱毒菌株孢子悬浮液(1×105个·mL-1)分别接种于不同品种供试枝条叶片的正反面,并以接种等体积无菌水作为对照,每个处理和对照均设置3 次重复。然后,将其放置在温度为25 ℃、相对湿度为90%、光照条件为16 h 光照/8 h 黑暗的环境中培养。待接种后每隔培养6 h观察和记录潜育期,并待接种7 d 后,统计叶片病情指数。同时,参照王昆等[25]抗病性评价标准对不同品种进行抗病性评价。病情指数(DI)≤5,高抗;5<DI≤10,中抗;10<DI≤30,抗病;30<DI≤50,感病;DI>50,高感。
2 结果与分析
2.1 不同温度对Alternaria mali强弱毒菌株致病活性的影响
不同温度对A. mali强弱毒菌株潜育期和病情指数均具有不同程度的影响。随着温度升高,A. mali强弱毒菌株潜育期呈先降低(15~30 ℃)后升高(30~35 ℃)的变化趋势(图1-A),而病情指数表现出先升高后降低的变化趋势(图1-B)。在不同温度条件下,A. mali弱毒菌株的叶片潜育期始终较A. mali强毒菌株长,当温度条件为30 ℃时,A. mali强弱毒菌株的潜育期均最短,分别为54 h和72 h;在温度为20~30 ℃范围时,A. mali强弱毒菌株接种叶片后,发病程度较为严重,其中接种强弱毒菌株后,A. mali强毒菌株在温度为25 ℃时致病活性最强,病情指数为23.06,而弱毒菌株在30 ℃时致病活性最强,病情指数为17.53。然而,当温度为15 ℃时,A. mali强弱毒菌株潜育期均大于100 h,并在此温度下接种A. mali强弱毒菌株后,接种A. mali强毒菌株的叶片发病严重程度显著低于接种弱毒菌株的叶片,病情指数分别为5.86和8.42。
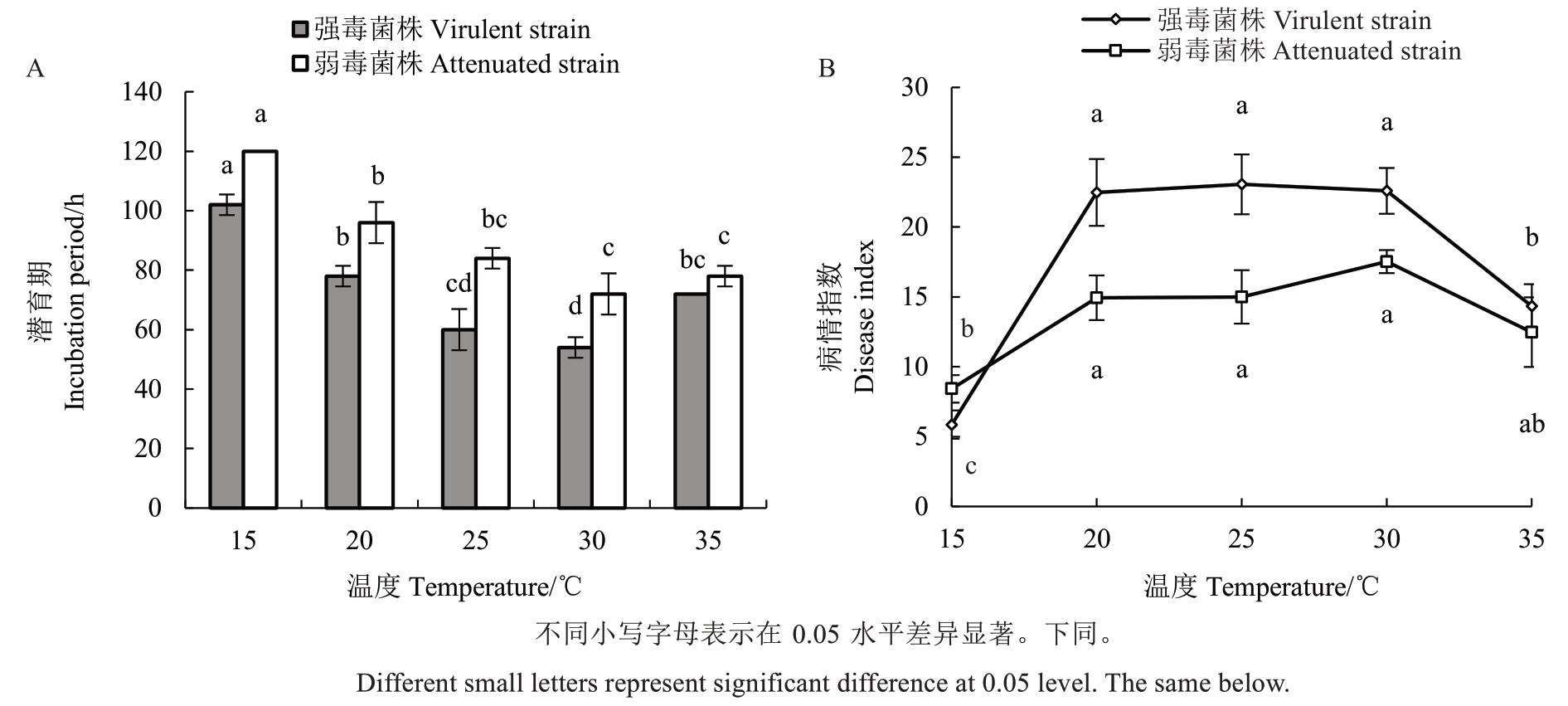
图1 不同温度对A. mali 强弱毒菌株潜育期(A)和致病活性(B)的影响
Fig. 1 Effects of different temperatures on incubation periods (A) and pathogenic activity (B) of virulent and attenuated strains of A. mali
2.2 不同相对湿度对Alternaria mali强弱毒菌株致病活性的影响
由图2-A可知,与A. mali弱毒菌株相比,A. mali强毒菌株在叶片上的潜育期整体较短,并且随着相对湿度(60%~100%)的增加,A. mali 强毒菌株在叶片上的潜育期呈逐渐变短到趋于稳定的趋势,而弱毒菌株呈先趋于稳定后逐渐变短的趋势。当相对湿度为60%时,A. mali强弱毒菌株的潜育期最长,均为120 h,而当相对湿度达到100%时,潜育期均达到最短,为72 h。由图2-B 可知,在不同相对湿度条件下培养7 d后,发现接种A. mali弱毒菌株的叶片发病程度低于A. mali强毒菌株,并且在60%的相对湿度条件下,接种强弱毒菌株后的叶片病情指数均最低,分别为10.88 和9.42,而在90%的相对湿度条件下,接种A. mali强弱毒菌株后的叶片病情指数均最高,分别为19.01和12.50。
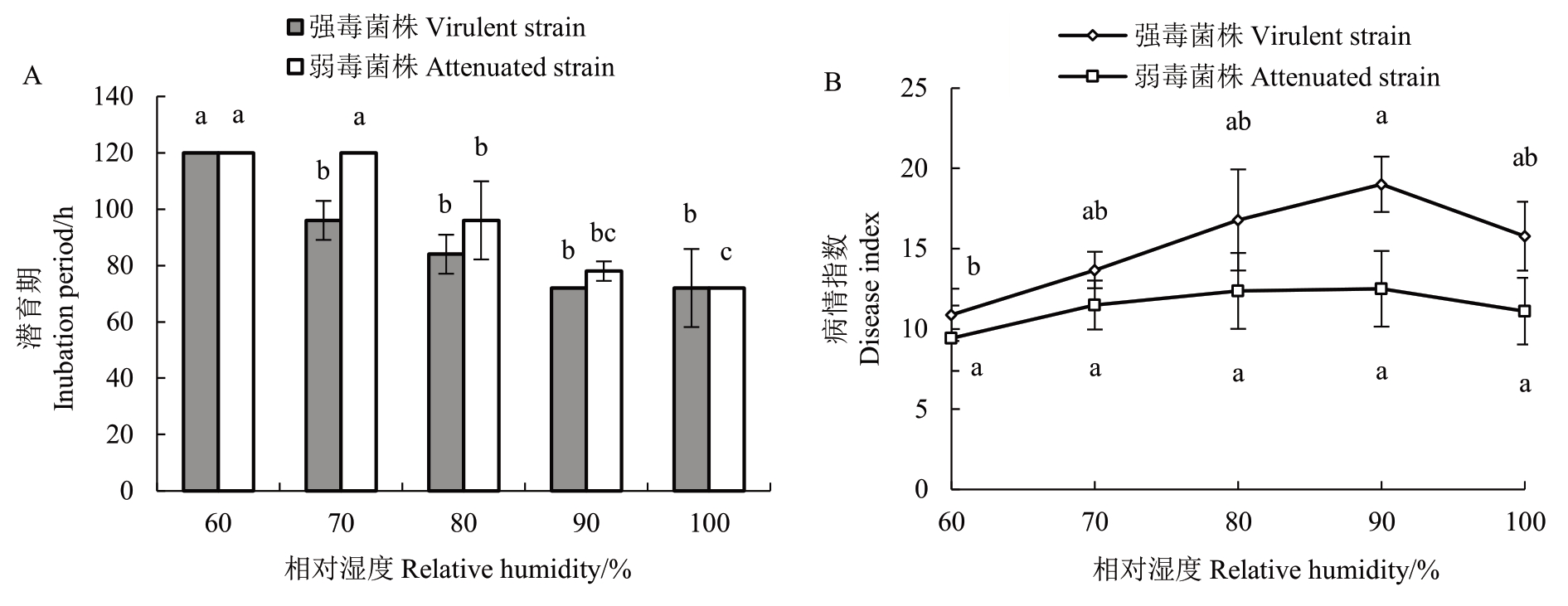
图2 不同相对湿度对A. mali 强弱毒菌株潜育期(A)和致病活性(B)的影响
Fig. 2 Effects of different relative humidity on incubation periods (A) and pathogenic activity (B) of virulent and attenuated strains of A. mali
2.3 不同光照条件对Alternaria mali强弱毒菌株致病活性的影响
在持续光照、持续黑暗、光暗交替、紫外照射+持续光照条件下,A. mali强毒菌株的潜育期均较A. mali弱毒菌株短,并且在光暗交替、紫外照射和持续光照条件下,强弱毒菌株潜育期较短,其中强毒菌株在光暗交替、紫外照射+持续光照条件下潜育期均为60 h,而弱毒菌株在此条件下均为72 h(图3-A)。在不同光照条件下培养7 d 后,除紫外照射+持续光照条件外,在其他不同光照处理下,A. mali强毒菌株接种叶片后,叶片的病情指数均高于弱毒菌株,其中A.mali 强毒菌株在光暗交替条件下病情指数最高,为22.59,但是在紫外照射+持续光照条件,A. mali弱毒菌株侵染叶片后病情指数最高,为21.24。然而,A.mali 强弱毒菌株均在持续黑暗条件下,接种叶片后病情指数最低,分别为13.58和13.33(图3-B)。
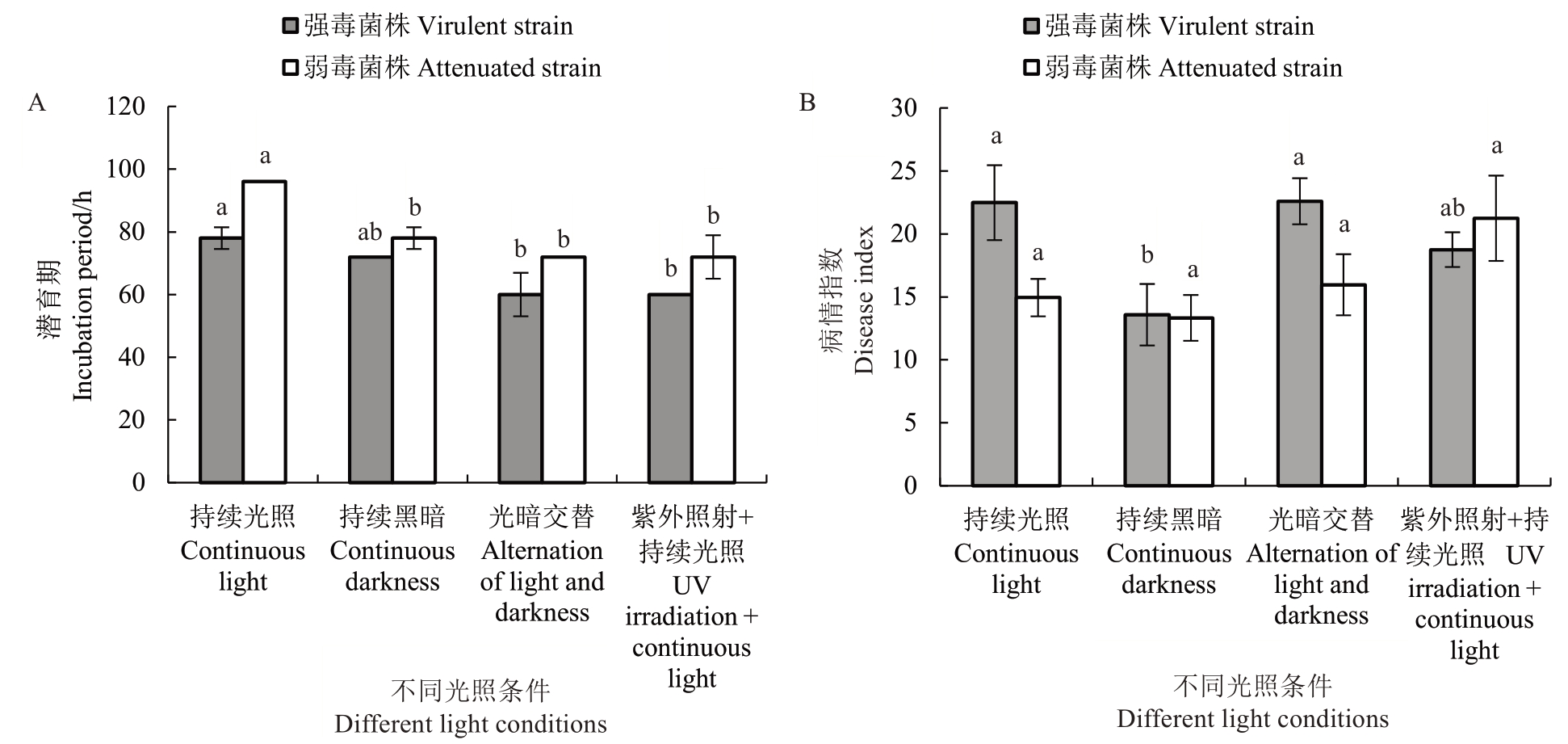
图3 不同光照条件对A. mali 强弱毒菌株潜育期(A)和致病活性(B)的影响
Fig. 3 Effects of different light intensity on incubation periods (A) and pathogenic activity (B) of virulent and attenuated strains of A. mali
2.4 不同品种对Alternaria mali强弱毒菌株致病活性的影响
不同苹果品种叶片接种A. mali强弱毒菌株后,A. mali强弱毒菌株在新红星和金冠品种上的潜育期均显著低于富士品种,并且强弱毒菌株均在金冠品种上潜育期最短且相同(48 h)(图4-A)。待接种A. mali强弱毒菌株7 d后,A. mali强弱毒菌株对富士品种的致病力整体低于新红星和金冠品种,其中在富士品种上的病情指数分别为14.13 和8.30。与A. mali强弱毒菌株对新红星的致病力相比,其对金冠的致病力较强,病情指数最高,分别为16.82 和22.09(图4-B)。不同品种抗病性评价结果表明,富士品种对A. mali弱毒菌株表现为中抗,而对A. mali强毒菌株表现为抗病,但是新红星和金冠对A. mali强弱毒菌株均表现为抗病。
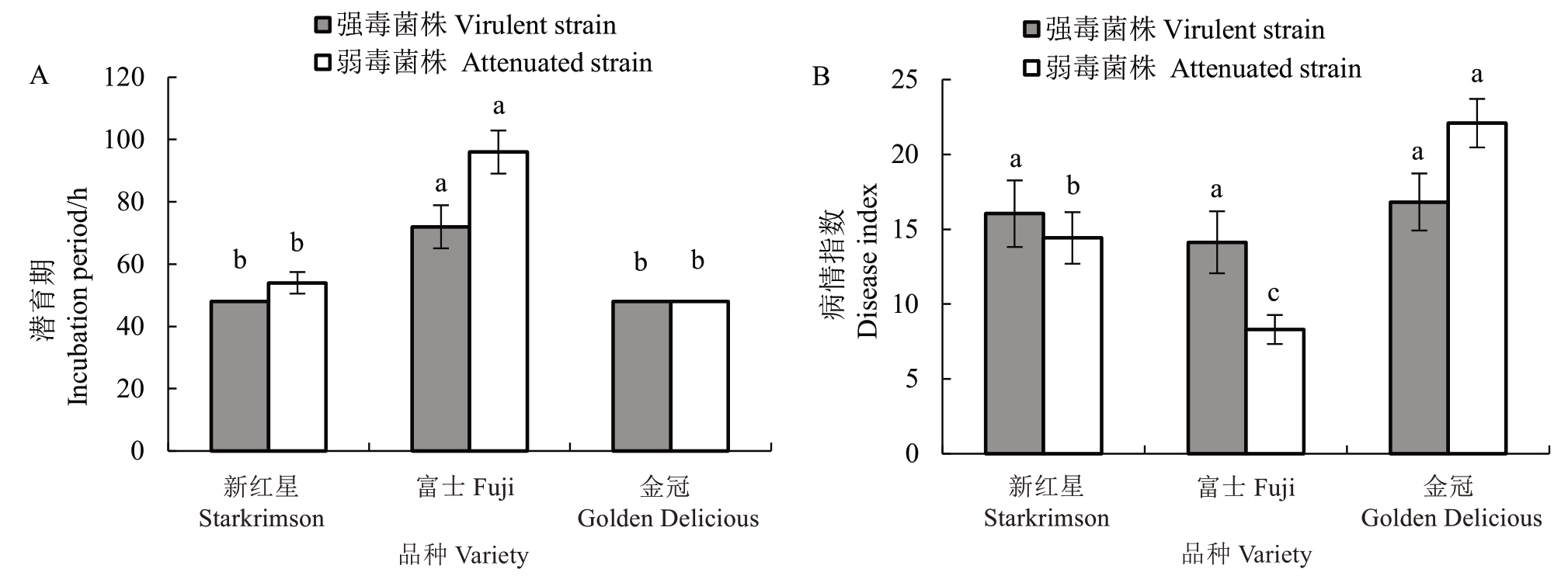
图4 不同苹果品种对A. mali 强弱毒菌株潜育期(A)和致病活性(B)的影响
Fig. 4 Effects of different apple varieties on incubation periods (A) and pathogenic activity (B) of virulent and attenuated strains of A. mali
3 讨 论
笔者通过测定不同环境(温度、相对湿度和光照)对A. mali强弱毒菌株潜育期和致病活性的影响,发现在不同的温度、相对湿度和光照条件下,A. mali强毒菌株的潜育期整体上较A. mali 弱毒菌株短,并且强毒菌株接种后的苹果叶片发病程度整体上高于弱毒菌株。吴桂本等[26]研究表明,胶东地区A. alternata f. sp. mali 菌株A1 致病性明显弱于A2。研究表明,A. mali 强毒菌株接种印度苹果品种叶片后,在温度为25~30 ℃时,其潜育期最短为48 h,病叶率最高为90%[27]。本研究表明,A. mali 强弱毒菌株接种新红星苹果品种叶片后,在温度为30 ℃时潜育期最短,且在温度为20 ~30 ℃范围时,叶片发病程度较为严重,尤其温度为25 ℃和30 ℃。然而,在温度为25 ℃、相对湿度为90%条件下,A. mali 强毒菌株接种苹果叶片后,其潜育期最短且病情指数最高,这一结果与胡同乐等[28]关于苹果斑点落叶病在降雨后,相对湿度为90%且持续10 h 以上会导致病原菌大量侵染的研究结果相吻合。此外,与持续光照相比,在12 h 光照与12 h 黑暗交替、3 h 紫外线照射和21 h 持续光照条件下,A. mali 强弱毒菌株在叶片上的潜育期均显著缩短,病情指数均达到最高峰,初步发现紫外线照射可增强A. mali弱毒菌株的致病性,进而促进了病斑的形成速度。薛军等[29]研究表明,苹果斑点落叶病的发病条件与田间光照时长及紫外线辐射强烈相关,光照时长及紫外线辐射可促使病原菌在叶片上的潜育期缩短,从而促进病害的发生,与本试验室内条件下的研究结果一致。
吕松等[30]发现在新疆的野生苹果和引进的西洋苹果上苹果斑点落叶病的发病率较高,而在国产苹果和野生品种中,发病率则显著降低。本试验结果表明,A. mali 强弱毒菌株在金冠品种上的潜育期最短且致病性最强,与徐秉良[27]研究发现致病力较强的A. mali 菌株(兰州1 号)对金冠品种致病活性较强的结果一致。另外,红星、印度和金冠等苹果品种在接种A. mali后,其中红星和印度均为高感品种,金冠为中抗品种[27],而本试验初步发现富士对A. mali 强弱毒菌株分别表现为抗病和中抗,新红星和金冠对强弱毒菌株均表现为抗病。因此,本试验明确了不同环境因子和苹果品种对A. mali 强弱毒菌株致病活性具有显著影响,而有关不同环境因子和苹果品种对A. mali 强弱毒菌株潜育期和致病性影响的机制还有待进一步深入研究。
4 结 论
不同温度、相对湿度、光照条件及苹果品种对A. mali 强弱毒菌株潜育期和致病性具有不同程度的影响,当温度为25~30 ℃、相对湿度为90%~100%、光照条件为光暗交替、紫外照射+持续光照时,A. mali 强弱毒菌株在新红星品种上的潜育期较短,致病性较强。富士苹果对A. mali强弱毒菌株均具有较强抗病性,分别表现为抗病和中抗。
[1] CHAO X F,SUN G Y,ZHAO H K,LI M,HE D J. Identification of apple tree leaf diseases based on deep learning models[J].Symmetry,2020,12(7):1065.
[2] 寿园园,李春敏,赵永波,陈东玫,张新忠. 苹果抗褐斑病离体鉴定的方法[J]. 果树学报,2009,26(6):912-914.SHOU Yuanyuan,LI Chunmin,ZHAO Yongbo,CHEN Dongmei,ZHANG Xinzhong. In vitro evaluation of resistance to Marssonina mali in apple[J]. Journal of Fruit Science,2009,26(6):912-914.
[3] 程守云,周吉生,周中磊,张耀峰. 苹果树早期落叶病综合防治技术[J]. 山西果树,2019(5):80-82.CHENG Shouyun,ZHOU Jisheng,ZHOU Zhonglei,ZHANG Yaofeng. Prevention and treatment technology of apple leaf defoliation diseases[J]. Shanxi Fruits,2019(5):80-82.
[4] 杨文渊,谢红江,陈善波,江国良,陈栋,涂美艳,李靖,孙淑霞.川西高原苹果早期落叶病调查及防治研究[J]. 安徽农业科学,2011,39(31):19181-19182.YANG Wenyuan,XIE Hongjiang,CHEN Shanbo,JIANG Guoliang,CHEN Dong,TU Meiyan,LI Jing,SUN Shuxia. Occurrence regularity and control of apple early defoliation disease in West Sichuan Plateau[J]. Journal of Anhui Agricultural Sciences,2011,39(31):19181-19182.
[5] 赵华,黄丽丽,谢芳芹,康振生. 苹果盘二孢的分离培养研究[J].菌物学报,2009,28(4):490-495.ZHAO Hua,HUANG Lili,XIE Fangqin,KANG Zhensheng.Culture study of Marssonina coronaria from diseased apple leaves[J]. Mycosystema,2009,28(4):490-495.
[6] 邓振山,白重炎,李军,李东旭. 陕北苹果常见真菌病害病原菌的分离鉴定研究[J]. 延安大学学报(自然科学版),2006,25(4):65-70.DENG Zhenshan,BAI Chongyan,LI Jun,LI Dongxu. Isolation and identification of apple common fungal pathogenes in northern Shaanxi[J]. Journal of Yanan University (Natural Science Edition),2006,25(4):65-70.
[7] 张书辉,王连起,曲向远. 苹果斑点落叶病的发生原因与防治方法[J]. 中国果树,2007(1):62-63.ZHANG Shuhui,WANG Lianqi,QU Xiangyuan. Causes and prevention of apple leaf spot disease[J]. China Fruits,2007(1):62-63.
[8] LIU B Y,LI Z W,DU J F,ZHANG W,CHE X Z,ZHANG Z R,CHEN P,WANG Y Z,LI Y,WANG S L,DING X H. Loop-mediated isothermal amplification (LAMP) for the rapid and sensitive detection of Alternaria alternata (Fr.) Keissl in apple Alternaria blotch disease with Aapg-1 encoding the endopolygalacturonase[J]. Pathogens,2022,11(11):1221.
[9] HARTEVELD D O C,AKINSANMI O A,DRENTH A. Pathogenic variation of Alternaria species associated with leaf blotch and fruit spot of apple in Australia[J]. European Journal of Plant Pathology,2014,139(4):789-799.
[10] TOOME-HELLER M,BASKARATHEVAN J,BURNIP G,ALEXANDER B. First report of apple leaf blotch caused by Alternaria arborescens complex in New Zealand[J]. New Zealand Journal of Crop and Horticultural Science,2018,46(4):354-359.
[11] 何劲,雷帮星,宋贞富,乔光,宋莎,康冀川,文晓鹏. DEB-2 菌株对贵州苹果早期落叶病病原的毒力效应[J]. 西南农业学报,2014,27(2):652-657.HE Jin,LEI Bangxing,SONG Zhenfu,QIAO Guang,SONG Sha,KANG Jichuan,WEN Xiaopeng. Toxicity of endophyte DEB-2 to pathogens of Guizhou apple early defoliation disease[J].Southwest China Journal of Agricultural Sciences,2014,27(2):652-657.
[12] 胡清玉,胡同乐,王亚南,王树桐,曹克强. 中国苹果病害发生与分布现状调查[J]. 植物保护,2016,42(1):175-179.HU Qingyu,HU Tongle,WANG Yanan,WANG Shutong,CAO Keqiang. Survey on the occurrence and distribution of apple diseases in China[J]. Plant Protection,2016,42(1):175-179.
[13] 胡同乐,曹克强,王树桐,甄文超. 生长季苹果斑点落叶病流行主导因素的确定[J]. 植物病理学报,2005,35(4):374-377.HU Tongle,CAO Keqiang,WANG Shutong,ZHEN Wenchao.Study on the main factor for the epidemic of Alternaria blotch on apple[J]. Acta Phytopathologica Sinica,2005,35(4):374-377.
[14] 陆敏,吴明勤,王金友. 苹果斑点落叶病菌形态及侵染行为的超微结构研究[J]. 植物保护,1994,20(5):16-18.LU Min,WU Mingqin,WANG Jinyou. Ultrastructural study on morphology and infection behavior of Alternaria mali[J]. Plant Protection,1994,20(5):16-18.
[15] 张彩霞,陈莹,李壮,康国栋,张利义,周宗山,丛佩华. 苹果斑点落叶病致病菌的鉴定及生物学特性研究[J]. 生物技术,2011,21(4):58-61.ZHANG Caixia,CHEN Ying,LI Zhuang,KANG Guodong,ZHANG Liyi,ZHOU Zongshan,CONG Peihua. Identification and biological characteristics of a pathogenic strain causing Alternaria blotch of apples[J]. Biotechnology,2011,21(4):58-61.
[16] 郭小侠,陈川,唐周怀,石晓红,石勇强. 苹果早期落叶病的发生规律及生物防治[J]. 陕西农业科学,2004,50(1):62-64.GUO Xiaoxia,CHEN Chuan,TANG Zhouhuai,SHI Xiaohong,SHI Yongqiang. Occurrence and biological control of early leaf litter disease in apple[J]. Shaanxi Journal of Agricultural Sciences,2004,50(1):62-64.
[17] FONTAINE K,FOURRIER-JEANDEL C,ARMITAGE A D,BOUTIGNY A L,CRÉPET M,CAFFIER V,GNIDE D C,SHILLER J,LE CAM B,GIRAUD M,IOOS R,AGUAYO J.Identification and pathogenicity of Alternaria species associated with leaf blotch disease and premature defoliation in French apple orchards[J]. PeerJ,2021,9:e12496.
[18] LI Y,ZHANG L Y,ZHANG Z,CONG P H,CHENG Z M. A simple sequence repeat marker linked to the susceptibility of apple to Alternaria blotch caused by Alternaria alternata apple pathotype[J]. Journal of the American Society for Horticultural Science,2011,136(2):109-115.
[19] 邵旭平,钟小刚,薛应钰,刘佳,张树武,王卫雄,徐秉良. 甘肃省苹果叶斑病病原菌鉴定及交叉保护作用研究[J]. 甘肃农业大学学报,2014,49(3):78-84.SHAO Xuping,ZHONG Xiaogang,XUE Yingyu,LIU Jia,ZHANG Shuwu,WANG Weixiong,XU Bingliang. Identification and its cross protection on pathogens of apple leaf spot in Gansu Province[J]. Journal of Gansu Agricultural University,2014,49(3):78-84.
[20] AALUM K,WANI I A,SINGH S D. Influence of temperature and relative humidity on the sorulation and growth of the fungus(Alternaria mali),causal agent of Alternaria leaf spot/blotch of apple (Malus domestica Borkh.)[J]. International Journal of Advance Research in Science and Engineering,2018,7(4):312-321.
[21] FILAJDIĆ N,SUTTON T B. Influence of temperature and wetness duration on infection of apple leaves and virulence of different isolates of Alternaria mali[J]. Phytopathology,1992,82(11):1279-1283.
[22] HARIMOTO Y,TANAKA T,KODAMA M,YAMAMOTO M,OTANI H,TSUGE T. Multiple copies of AMT2 are prerequisite for the apple pathotype of Alternaria alternata to produce enough AM-toxin for expressing pathogenicity[J]. Journal of General Plant Pathology,2008,74(3):222-229.
[23] 崔琳霞,武艳芍. 3%多抗霉素水剂防治苹果斑点落叶病田间药效试验[J]. 中国农学通报,2008,24(12):394-395.CUI Linxia,WU Yanshao. Control effects of 3% polyoxin AS on apple spot leaf drop in field[J]. Chinese Agricultural Science Bulletin,2008,24(12):394-395.
[24] 王程亮,张潞生,高微微,张文,戚元勇,杨庆军. 芽孢杆菌TS-01 对苹果斑点落叶病菌的拮抗作用及防病效果[J]. 植物保护学报,2008,35(2):183-184.WANG Chengliang,ZHANG Lusheng,GAO Weiwei,ZHANG Wen,QI Yuanyong,YANG Qingjun. Antagonism and control effect produced by Bacillus strain TS-01 against Alternaria mali[J].Acta Phytophylacica Sinica,2008,35(2):183-184.
[25] 王昆,龚欣,刘立军,王大江,高源. 苹果地方品种资源苹果斑点落叶病抗性调查与评价[J]. 中国果树,2015(5):81-84.WANG Kun,GONG Xin,LIU Lijun,WANG Dajiang,GAO Yuan. Investigation and evaluation of resistance to apple spot deciduous disease of apple local variety resources[J]. China Fruits,2015(5):81-84.
[26] 吴桂本,王英姿,王培松,王继秋,宫本义. 苹果斑点落叶病菌的分化及生物学研究[J]. 中国果树,1999(4):11-15.WU Guiben,WANG Yingzi,WANG Peisong,WANG Jiqiu,GONG Benyi. Study on the differentiation and biology of Alternaria mali[J]. China Fruits,1999(4):11-15.
[27] 徐秉良. 苹果链格孢菌侵染条件及致病性研究[J]. 甘肃农业大学学报,1997,32(1):43-47.XU Bingliang. The infection factors and pathogenicity of Alternaria mall Roberts[J]. Journal of Gansu Agricultural University,1997,32(1):43-47.
[28] 胡同乐,王树桐,宋萍,张凤巧,甄文超,曹克强. 苹果斑点落叶病菌大量侵染的决定性天气条件初探[J]. 河北农业大学学报,2006,29(1):63-66.HU Tongle,WANG Shutong,SONG Ping,ZHANG Fengqiao,ZHEN Wenchao,CAO Keqiang. Primary study on the crucial weather conditions of mass infection of Alternaria mali on apple[J]. Journal of Agricultural University of Hebei,2006,29(1):63-66.
[29] 薛军,孙继玲,王孝友. 苹果斑点落叶病发生规律的研究[J].落叶果树,2006,38(1):36-37.XUE Jun,SUN Jiling,WANG Xiaoyou. Study on the regularity of apple leaf spot disease[J]. Deciduous Fruits,2006,38(1):36-37.
[30] 吕松,王忆,张新忠,李天红,韩振海. 苹果斑点落叶病抗病性种质评价及遗传分析[J]. 中国农业大学学报,2012,17(4):68-74.LÜ Song,WANG Yi,ZHANG Xinzhong,LI Tianhong,HAN Zhenhai. Evaluation of resistance of Malus germplasms to apple Alternaria blotch (Alternaria mali) and analysis of inheritance for resistance[J]. Journal of China Agricultural University,2012,17(4):68-74.