果树设施栽培通过人为调控生长环境,提前或延长鲜果的供应时间,为反季节水果的供应创造了条件,提高了土地利用率与农业经济效益,是现代化设施农业不可缺少的组成部分。草莓(Fragaria×ananassa Duch.)是设施栽培中重要的物种之一,具有生长周期短、总采摘期长、甜酸可口、营养丰富等优点,是老百姓非常喜爱的一种水果。草莓的生长期和上市时间主要集中在9 月至翌年5 月,即深秋、冬季与翌年早春时段,此时设施内的环境条件远达不到其在露天环境下正常生长所需的温度和光照条件,果实容易出现风味淡、色泽不鲜艳、含糖量大幅减少、畸形率高等问题[1-2],急需通过各种有效的辅助技术措施调控设施中的环境因子。其中,光质的调控是最易操作的辅助栽培技术之一,其对叶片的光合性能改善、同化物合成与运转、果实糖分积累均具有重要意义。
UV-B(280~320 nm)是太阳辐射中具有高能量的不可见光,对植物生长发育各方面如叶片厚度、面积大小、形态、节间长短、植物高矮和生物量积累等都有影响。研究表明,高强度的UV-B 辐射会引起叶片海绵组织和薄壁细胞结构紊乱,积累自由基,破坏细胞[3-4],降低气孔导度和CO2的同化率[5],改变叶绿体中叶绿素a/b的比例,损害叶绿体的光能捕获和电子转移能力[6],削弱PSⅡ反应中心的活性并破坏电子传递系统,还能影响光合相关基因的表达,捕光叶绿素a/b 结合蛋白基因Cab、ATP 合酶基因等[7-8],最终对光合作用产生负面效应。而适宜剂量的UV-B则对植物的生长有正向作用,可以改善叶肉光合组织的显微结构,增强叶片光能利用率和电子传递转化效率[9],快速提高糖转运蛋白基因的表达量,促进果实的糖分积累[10],还能改善果实色泽及提高花青素含量,综合提升园艺作物的果实品质[11]。在弱紫外光的设施环境中适量补充UV-B 能够改善桃树叶绿体的超微结构并提高光系统之间的电子传递效率,使PSⅡ的捕光色素复合体吸收光能的效果增强[9];青花菜在一定剂量的UV-B 辐射下叶绿素含量提高了36.8%[12];还有研究表明,温室补充UV-B 辐射提高了番茄产量、硬度和酚类物质含量[13];紫光辐射对设施草莓朱砂叶螨有较好的趋避作用[14],而国内外对UV-B影响草莓光合和品质的研究鲜有报道。
叶绿素分子通过PSⅡ和PSⅠ两个光系统吸收光能并传递电子的过程是光合作用的重要一步。激发态的叶绿素分子在能量转变时有三种方式:能量传递、发射荧光或磷光、放热,此三者此消彼长。因此可以通过检测叶绿素荧光的变化来反映出色素分子间的能量传递情况以及PSⅡ到PSⅠ的电子传递情况[15]。以往有关UV-B对叶片光合性能的研究中,主要集中在PSⅡ活性对UV-B 的响应,缺少对PSⅡ和PSⅠ之间电子传递过程的解析,特别是在低、中、高的不同梯度辐射下光系统之间的不同响应尚不十分明确。因此笔者在温室中设置不同梯度的UV-B补充剂量,研究其对草莓光系统性能和果实品质的影响,以期为设施果树光合能力的提高、环境调控技术的改进以及果实品质的改善提供理论依据。
1 材料和方法
1.1 材料
试验材料为本实验室育出的设施专用品种雪里香(Fragaria ×ananassa Duch. ‘Xuelixiang’)草莓。2022年9月中旬栽植于山东农业大学园艺实验站节能型日光温室内,10月上旬覆盖棚膜,11月20日左右第一序果成熟。栽培模式为立体化无土栽培,将基质袋放入栽培槽中吊挂,栽培槽宽20 cm,高15 cm,袋中的基质材料主要为椰糠。草莓苗采用三角形种植,株距15 cm,每行栽种60株,水肥一体化滴灌,其中水溶性肥为氮磷钾平衡肥,常规管理。处理前,选择长势一致的草莓植株,去除植株老叶及已有果实,1个花序周围保留5~7枚功能叶。
1.2 试验设计及处理方法
为尽可能消除低温的影响,选择在翌年3 月中旬进行试验。测定日光温室内与露天环境下日平均UV-B 强度,将内外的强度差值0.6 W·m-2记为1 Δt,据此在日光温室内设置4 个补充剂量:1/3 Δt、2/3 Δt、1 Δt和4/3 Δt,即补充的辐射强度分别为0.2、0.4、0.6、0.8 W·m-2,记为T1~T4,以温室内不进行任何处理的设施草莓为对照(CK),见表1。以上每个处理分3个小区,每小区有9株草莓。采用313 nm、40 W·s-1的UV-B 灯管(南京电子卡知科技有限公司)进行补光,通过UV-B 型单通道辐照计(北京师范大学光电仪器厂)检测并调节灯管的高度达到所需的辐射剂量,每天08:30—10:00定时处理1.5 h,处理时间及梯度均结合预试验和课题组前人研究而定。阴雨雪天暂停照射,处理25 d。选取中心展开叶外第3 枚功能叶进行本试验中所有叶片的采样与测定,选取红熟期果实进行果实品质测定。
表1 试验处理
Table 1 Experimental treatments
处理Treatment对照CK T1 T2 T3 T4补充剂量Supplementary dose/(W·m-2)0.0 0.2 0.4 0.6 0.8
1.3 测定指标及方法
1.3.1 环境指标监测 采用L92-LXWS 型空气温湿度记录仪(杭州路格科技有限公司)、3415F 型光量子计(Spectrum LightScout,美国)、UV-B 型单通道辐照计分别记录温度、湿度、光照度、UV-B强度。
1.3.2 叶片抗性指标测定 参考赵世杰等[16]的方法,分别采用茚三酮法、考马斯亮蓝G-250染色法和硫代巴比妥酸法测定脯氨酸、可溶性蛋白和丙二醛(MDA)含量;参考Zhang等[17]的方法,采用电导率仪(上海雷磁公司)测定相对电导率。均为3次重复。
1.3.3 叶绿素荧光相关参数测定 采用FMS-2 型便携式脉冲调制式荧光仪(Hansatech公司,英国)测定设施草莓叶片的叶绿素荧光参数,5 次重复。在天气晴朗的08:30—16:30,每隔2 h 进行一次测定。选择植物外围朝阳受光方向一致的第3 枚功能叶,使用暗适应夹暗处理20 min 后测定叶片的最大荧光(Fm)和最小荧光(F0),参照Demmig-Adams 等[18]计算最大光化学效率Fv/Fm=(Fm-F0)/Fm。测定光适应下的最大荧光(Fm')、最小荧光(F0')、稳态荧光(Fs),计算最大光化学效率Fv'/Fm'=(Fm'-F0')/Fm',PSⅡ光化学量子产量φPSⅡ=(Fm'-Fs)/Fm',光化学淬灭系数qP=(Fm'-Fs)/(Fm'-F0'),非光化学淬灭系数qN=(Fm-Fm')/(Fm-F0),ETR=φPSⅡ×PFD×0.84×0.5(PFD 为光子通量密度,0.5 代表光能在两个光系统分配系数,0.84代表假设叶片表面光能的平均吸收率)。
1.3.4 叶绿素荧光诱导动力学曲线测定 采用连续激发式荧光仪M-PEA(Hansatech 公司,英国)测定叶片快速叶绿素荧光诱导动力学曲线(OJIP 曲线),叶片均经过30 min 暗反应,5 次重复。对测定的OJIP曲线进行标准化,Vt=(Ft-F0)/(Fm-F0)。Vt(试验组)减去Vt(对照组)得到相对可变荧光的差异动力学ΔVt曲线,ΔVt=Vt-Vt(CK)。OJIP 曲线上特殊位点的荧光参数进行标准化处理:F0(初始荧光)、Fk(0.3 ms时荧光)、FJ (2 ms时荧光)、FI (30 ms时荧光)、Fm(最大荧光)。根据Strasser 方法对OJIP 曲线进行JIPtest 分析[15]。PSⅠ活性用820 nm 光吸收的最大值与最小值的差值/最小值来表示,即△I/Io。
1.3.5 叶绿体色素含量及光合参数的测定 对叶片进行95%乙醇浸提后采用分光光度计法测定叶绿素含量,3 次重复;采用CIRAS-3 型便携光合仪(PPSystemes,美国)测定光合参数,取样及测定时间为晴朗上午的09:00—11:00,设定光合有效辐射强度为1200 μmol·m-2·s-1,叶室温度为25 ℃。测定光合参数包括胞间CO2浓度(Ci )、气孔导度 (Gs)、蒸腾速率(Tr)和净光合速率(Pn),5次重复。
1.3.6 植物生长和果实品质指标测定 每个处理随机选取5株,分别测定地上部分和地下部分鲜质量,置于烘箱中105 ℃杀青20 min,80 ℃烘干至恒质量后测定干质量,并计算干鲜质量比值。使用万分之一天平称量单果质量,10次重复。采用手持折光仪(LYT-330)测定果实可溶性固形物含量(TSS),5次重复。采用蒽酮比色法测定果实可溶性糖含量[19],3 次重复。采用GMK-835N 型水果酸度计测定(G-WON,韩国)可滴定酸含量,5 次重复。将得到的可溶固形物含量和可滴定酸含量通过计算得到果实的固酸比。
1.4 数据处理与统计分析
采用 Microsoft Excel 2010 软件统计分析数据,采用Origin 2021 作图,采用SPSS 26.0 进行显著性方差分析。
2 结果与分析
2.1 日光温室内和露天的环境因子差异分析
为更好地调控温室内的环境因子,首先比较了温室内和露天的主要环境因子差异。一天之中温室内温度在29.8~37.2 ℃之间,露天温度在23.9~30.0 ℃,均呈单峰曲线变化,温室内平均温度比露天环境高6.13 ℃(图1-A)。相对湿度的趋势与温度相反,呈先降后升的变化趋势,维持在40%~60%之间,温室内外基本无差别(图1-B)。一天内温室中的光照度和UV-B 强度均低于露天,但两者的变化规律相似 ,均随着时间推移先升后降,且在11:30—12:30之间达到最大值。12:30之前,温室内光照度是露天的49.7%~75.3%,12:30 之后,室内光照度下降较快,仅为露天的36.2%~68.9%(图1-C)。温室内UV-B日平均强度为露天的51.84%,与露天差值在0.55~1.13 W·m-2范围内,中午前后的差值最大(图1-D)。由此可见,温室内的光环境质量劣于露天,这为笔者在温室中补充UV-B进行光质调控提供了数据支持。

图1 温室内和露天的温度、相对湿度、光照度、UV-B 强度比较
Fig. 1 Comparison of temperature, relative humidity, light intensity and UV-B intensity between greenhouse and open air
2.2 UV-B辐射对设施草莓叶片抗性的影响
如图2所示,分析了各梯度UV-B辐射下草莓叶片的抗性及伤害性指标,以初步判断剂量设定是否合理。随着UV-B 剂量的增加,脯氨酸含量(图2-A)和可溶性蛋白含量(图2-B)均呈增加趋势,T4 最高。丙二醛含量中(图2-C),UV-B 辐射剂量最高的T4 处理显著高于其他处理,而剂量相对较小的T1~T3处理与对照无显著差异;此外,对照与各处理之间的相对电导率也没有显著差异(图2-D)。由此可见,补充一定剂量UV-B 的草莓叶片提高了叶片的抗逆能力,并未发现其对叶片产生的可见伤害,可用于后续试验。
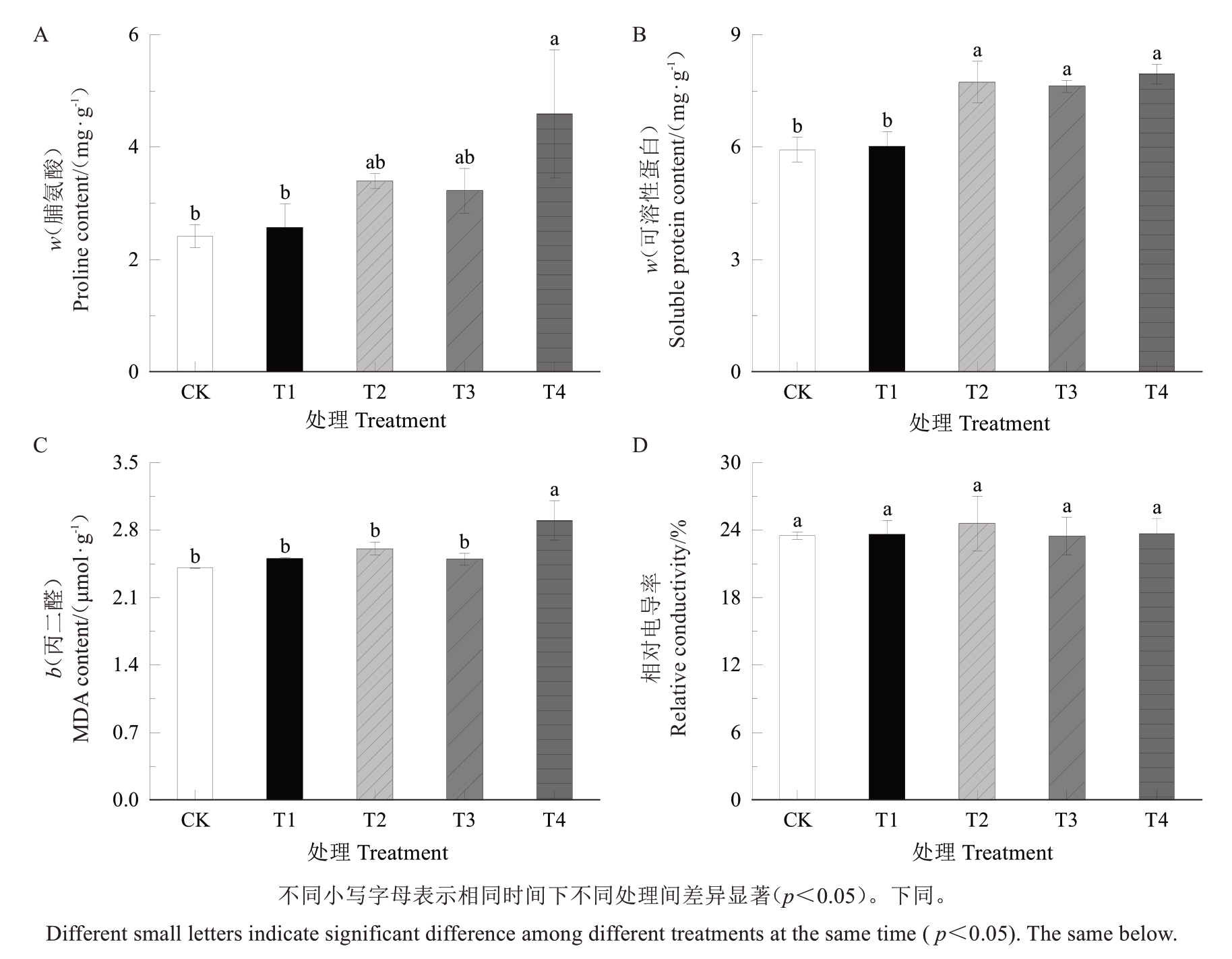
图2 UV-B 辐射对叶片抗性的影响
Fig. 2 Effects of UV-B radiation on the resistance in leaves
2.3 UV-B辐射对设施草莓叶片荧光参数的影响
2.3.1 UV-B 辐射下叶片的快速叶绿素荧光动力学曲线变化 OJIP 曲线的变化能够反映PSⅡ反应中心电子传递的情况(图3-A~E),ΔVt曲线可清晰直观地看出叶绿素荧光诱导曲线O、J、I、P 相(OJIP)的特征及变化情况(图3-a~e)。开始进行UV-B 处理时,处理组的Vt 值均低于对照,OJIP 曲线均为典型趋势,J 点、I 点明显(图3-A、a)。10:40 时T4 处理叶片的OJIP 曲线已显示出较大的升幅,尤其J 相的变化较为明显,ΔVJ和ΔVI>0,其后的时间内一直处于高值水平,其余处理尚低于对照(图3-B、b)。在12:30时,T1、T3、T4 处理叶片的OJIP 曲线均处于上升时段,并发生不同程度的变形(图3-C、c)。14:30时(图3-D、d),T1~T4 处理叶片的OJIP 曲线均高于对照,差异动力学OJIP 曲线显示,试验组的ΔVK>0(图3-c、d),T4 最高,T3 次之,T2 最低,可见T2 处理的OJIP曲线受影响较小。16:30 时T1、T2和T3处理均已降至对照之下,T4 处理虽然也在逐渐下降但仍明显高于对照(图3-E、e)。

图3 UV-B 辐射对叶片快速叶绿素荧光诱导动力学OJIP 曲线的影响
Fig. 3 Effects of UV-B radiation on fast chlorophyll fluorescence induction kinetics OJIP curve in leaves
2.3.2 UV-B 辐射对设施草莓叶片叶绿素荧光参数的影响 Fv'/Fm'、φPSⅡ分别反映了光下的最大光化学效率、PSⅡ有效光化学量子产量(图4-A~B),两者的日变化趋势均为先降后升,在UV-B 处理的时间段内(08:30—10:30),Fv'/Fm'、φPSⅡ均高于对照,10:30 之后,T2 处理的Fv'/Fm'仍然显著高于对照;T4则逐渐下降;qP表明了PSⅡ吸收的光能用于光化学反应电子传递的份额,qP 的日变化趋势为先升后降,在UV-B 处理时段内,T2~T4处理的qP 较对照提高了2.7%、7.4%、10.2%(图4-C),10:30 之后,T2 处理的qP 值升高,T4 处理则低于对照及其他处理,T1和T3 处理稍高于对照。qN 则反映了植物热耗散的能力,体现了植物在环境胁迫中的保护性协调能力。qN 在一天之中持续下降,UV-B 处理期间下降幅度较大,之后趋于平缓,14:30 之后又迅速下降。随着UV-B 辐射强度的提升,试验组qN 值均高于对照(图4-D),多数时间段为T2>T1>T3>T4>CK。ETR 日变化的总体趋势为先升后降(图4-E),各处理到达峰值的时间不同,T4 处理在10:30达到峰值,而对照和T2处理在12:30达到峰值,T1和T3处理均后推至14:30。UV-B 辐射剂量越高,ETR 值越小,T3、T4 处理多数时间段与对照及其他2 个处理呈显著差异。

图4 UV-B 辐射对叶片叶绿素荧光相关参数的影响
Fig. 4 Effects of UV-B radiation on chlorophyll fluorescence related parameters in leaves
2.3.3 UV-B 辐射对设施草莓PSⅡ、PSⅠ光系统活性的影响 Fv/Fm是指PSⅡ的潜在最大光合效率,常用来表征PSⅡ的活性。如图5 所示,Fv/Fm日变化浮动较小,随着时间在0.74~0.83 之间呈轻微“V”字形变化。在进行UV-B 处理时,Fv/Fm与补充剂量呈正相关,其中T4、T3 处理显著高于其他处理组(图5-A)。结束UV-B 处理后的时段,以T2 处理最高,且与对照呈显著差异。
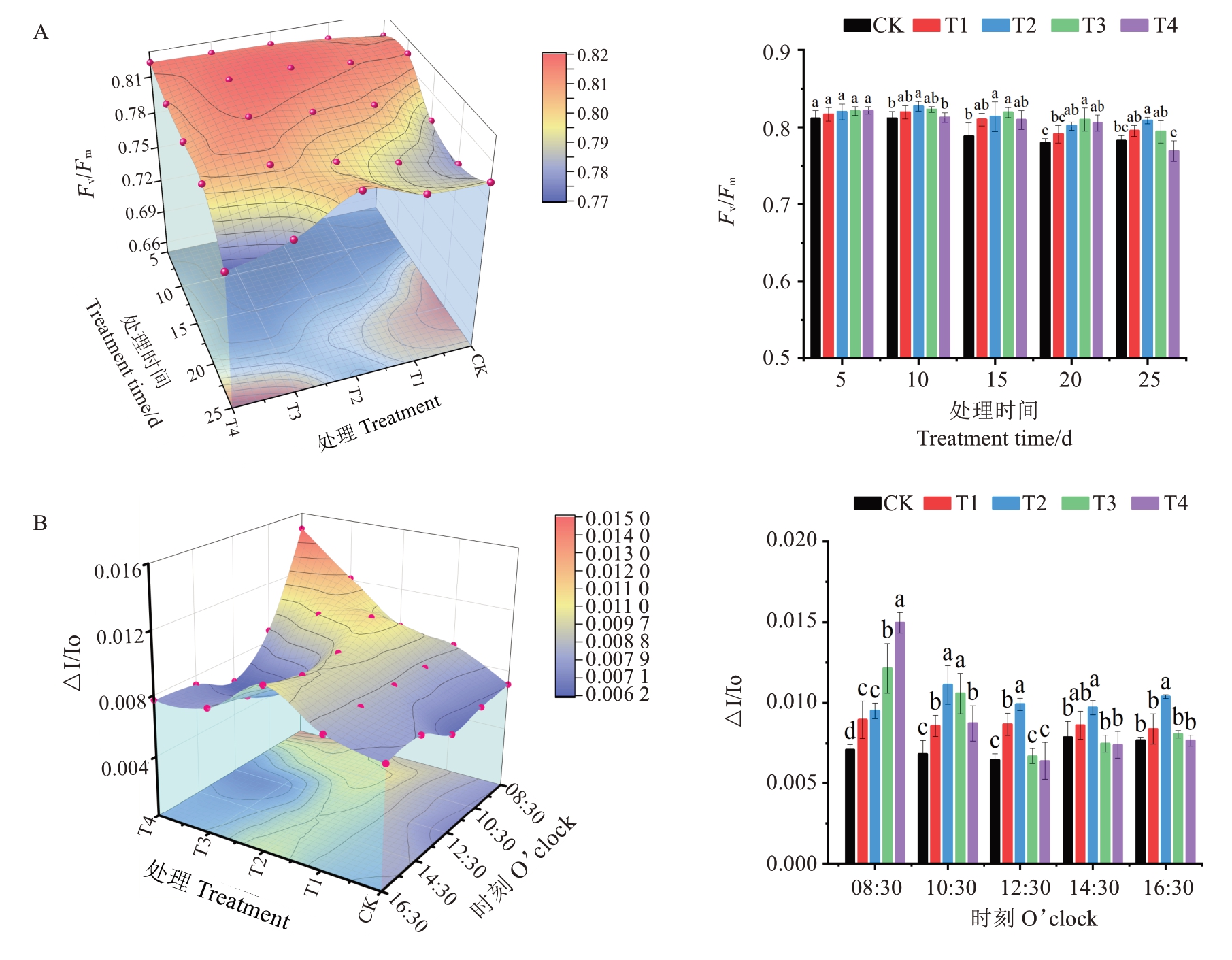
图5 UV-B 辐射对叶片PSⅡ、PSⅠ光系统活性的影响
Fig. 5 Effects of UV-B radiation on photosystem activity of PSⅡ and PSⅠ in leaves
PSⅠ活性的高低受UV-B 辐射影响相对较大,如图5-B 所示。处理伊始(08:30),△I/Io 随剂量的增加呈现上升趋势,然后均呈先升后降的单峰变化,以T2 处理最高,T4 处理和对照较低,T1 和T3 处理居中,且随着时间的推移,除T2 处理一直显著高于对照外,其余处理逐渐变得与对照无显著差异。对同一剂量处理的△I/Io 值而言,剂量较低的对照和T1 处理日变化不大,中高剂量的T2、T3 和T4 处理均在上午持续下降,然后缓慢回升,T2 处理能回升至原始水平,但T3和T4处理不能。
2.4 UV-B辐射对设施草莓叶片光合能力的影响
2.4.1 UV-B 辐射对叶片叶绿体色素含量的影响 叶绿素a、叶绿素b 和类胡萝卜素含量三者随着UV-B 辐射剂量的升高,均表现为先升后降的趋势,其中T2 处理的含量达到最高,T1 处理次之,T3、T4 处理与对照无显著差异。总叶绿素含量的变化趋势与叶绿素a、b 基本一致,T1、T2 处理相较于对照分别显著增加了10.44%、20.47%(图6)。
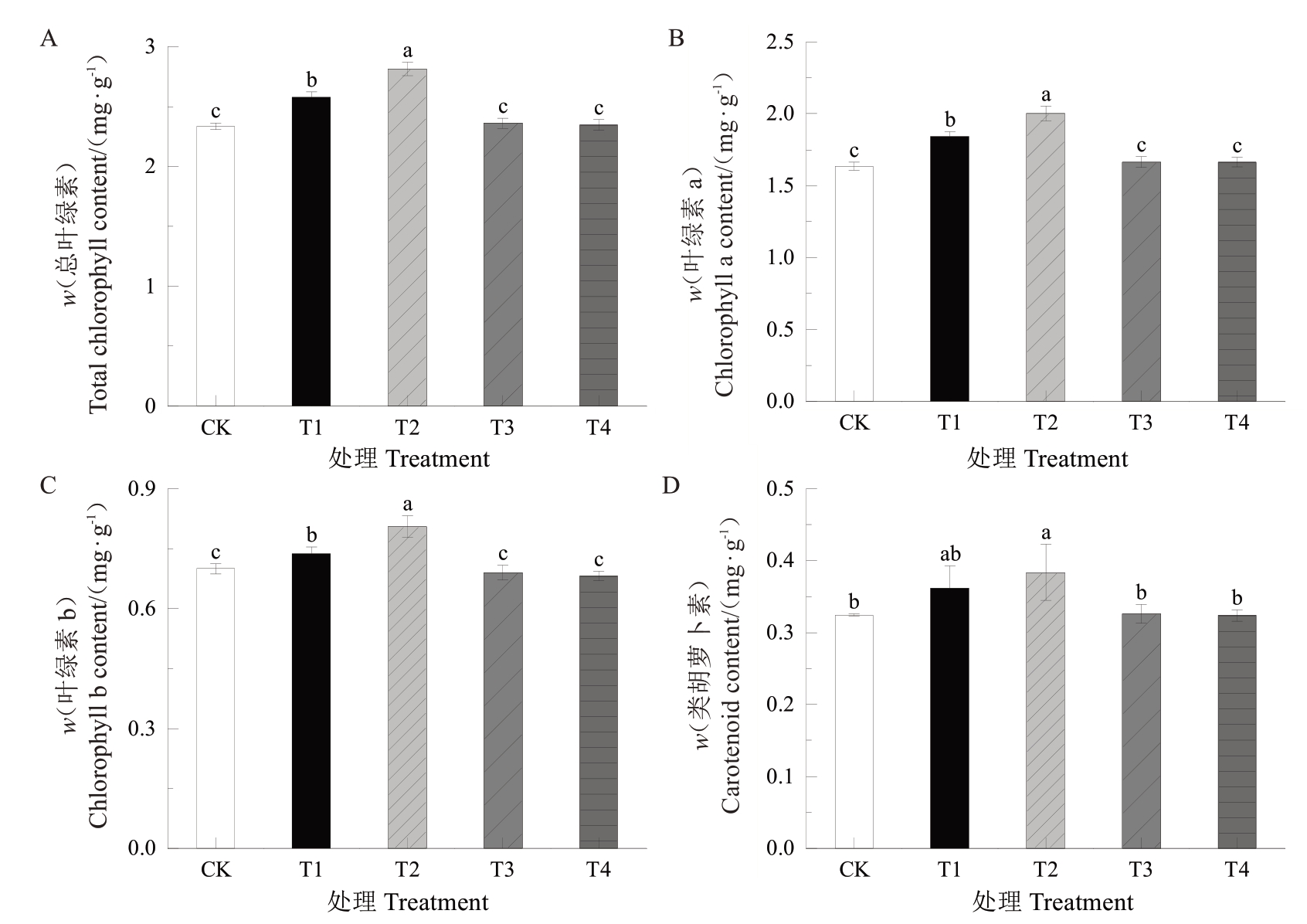
图6 UV-B 辐射对叶片叶绿体色素含量的影响
Fig. 6 Effects of UV-B radiation on chloroplast pigment contents in leaves
2.4.2 UV-B 辐射对叶片气体交换参数的影响 UVB 处理组草莓叶片Pn、Gs、Tr表现为随着梯度的升高呈先升后降的趋势,Ci变化趋势则相反。T1~T4 处理的Pn和Tr均高于对照,前者升高了6.8%~11.8%,后者比对照升高了11.3%~12.1%(图7-A,D);T1~T4 处理的Ci低于对照,其中T2处理最低(图7-B);T1~T3处理的Gs显著高于对照,T4 处理与对照无显著差异(图7-C)。
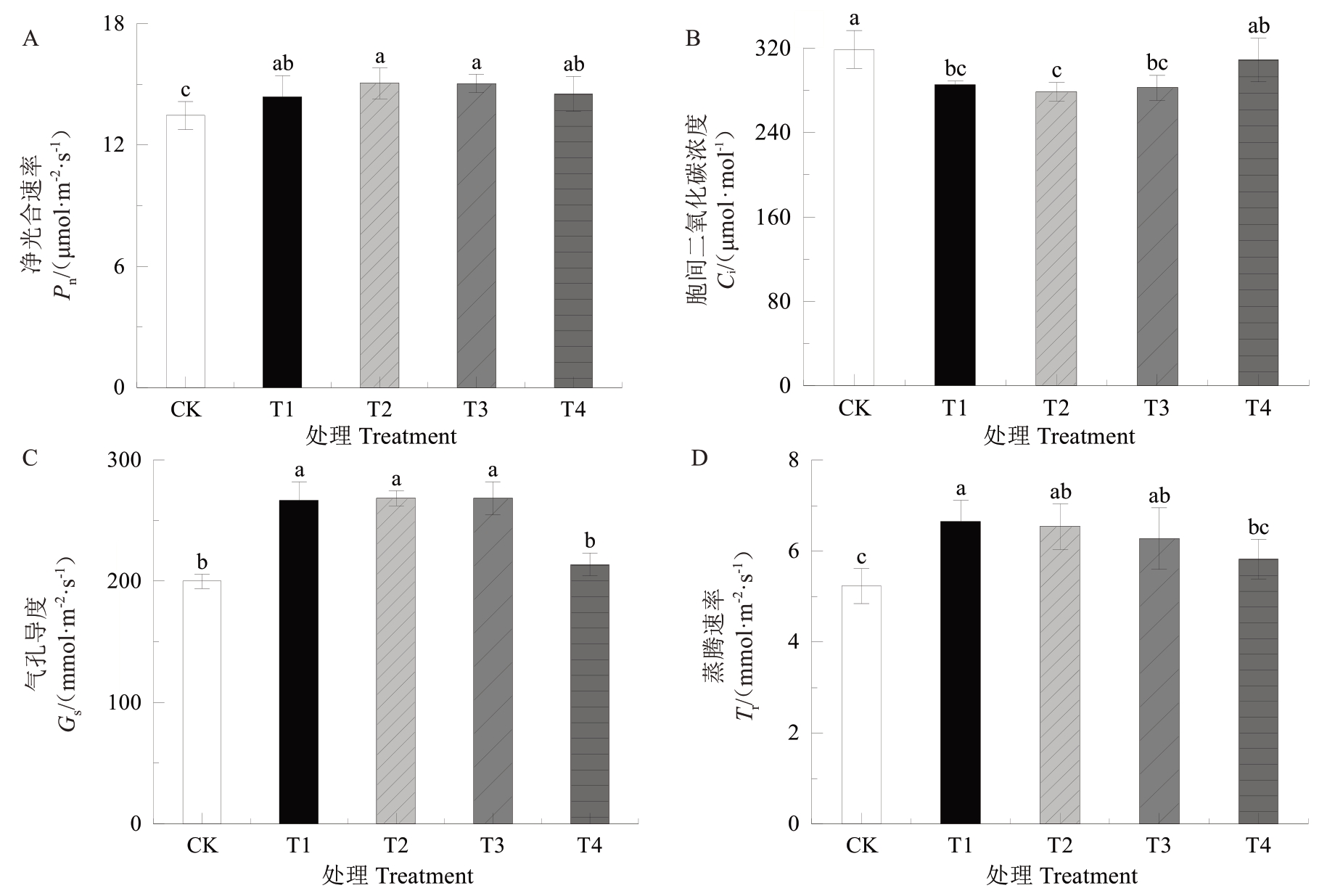
图7 UV-B 辐射对叶片光合相关参数的影响
Fig. 7 Effects of UV-B radiation on photosynthetic parameters in leaves
2.5 UV-B 辐射对设施草莓植株生长和果实品质的影响
2.5.1 UV-B 辐射对植株干鲜质量的影响 图8-A显示了不同处理下叶片的生长情况,T2 处理叶片最大,对照和其他处理区别不明显。地上部与地下部的干鲜质量如图8-B~C 所示,均随着剂量的增大表现为先升后降,其中T1、T2 处理的地上部鲜质量和干质量均显著高于对照,地上部鲜质量分别比对照显著增加了41.4%、46.3%。处理组的地上部干鲜质量比值均显著低于对照,处理间无显著差异,地下部的干鲜质量比与对照无显著差异(图8-D)。

图8 UV-B 辐射对植株干鲜质量的影响
Fig. 8 Effect of UV-B radiation on dry and fresh mass of strawberry plants
2.5.2 UV-B 辐射对果实品质的影响 如表2 所示,设施草莓经UV-B 处理后,单果质量及可溶性固形物含量和可溶性糖含量随着补充剂量的增加呈先升后降的趋势,且处理组均高于对照,其中T2 处理均为最高,分别较对照显著提高了24.9%、25.7%、64.9%。处理组的可滴定酸含量和糖酸比与对照均无显著差异。
表2 UV-B 辐射对设施草莓果实品质的影响
Table 2 Effects of UV-B radiation on fruit quality of protected strawberry
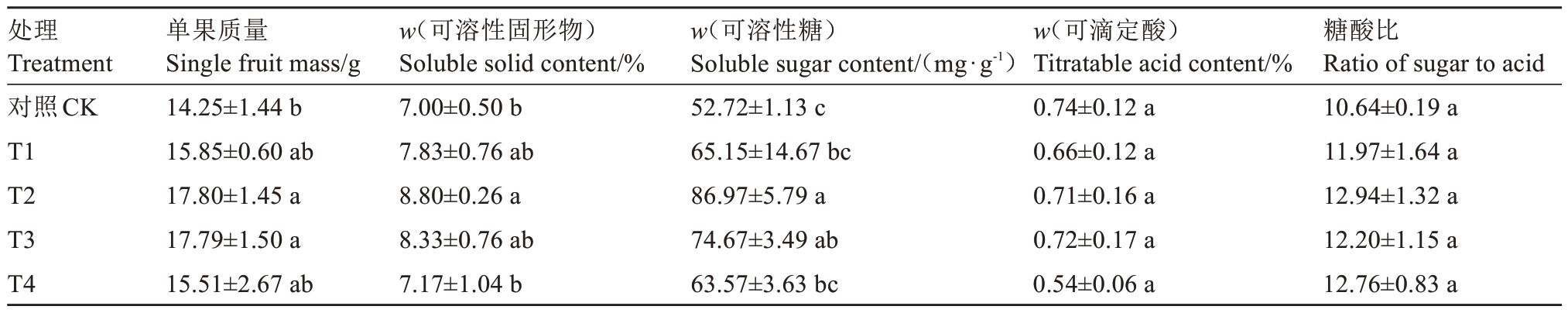
注:不同小写字母表示在0.05 水平差异显著。
Note:Different small letters indicate significant difference at 0.05 level.
处理Treatment对照CK T1 T2 T3 T4糖酸比Ratio of sugar to acid 10.64±0.19 a 11.97±1.64 a 12.94±1.32 a 12.20±1.15 a 12.76±0.83 a单果质量Single fruit mass/g 14.25±1.44 b 15.85±0.60 ab 17.80±1.45 a 17.79±1.50 a 15.51±2.67 ab w(可溶性固形物)Soluble solid content/%7.00±0.50 b 7.83±0.76 ab 8.80±0.26 a 8.33±0.76 ab 7.17±1.04 b w(可溶性糖)Soluble sugar content/(mg·g-1)52.72±1.13 c 65.15±14.67 bc 86.97±5.79 a 74.67±3.49 ab 63.57±3.63 bc w(可滴定酸)Titratable acid content/%0.74±0.12 a 0.66±0.12 a 0.71±0.16 a 0.72±0.17 a 0.54±0.06 a
2.6 UV-B 调控设施草莓叶片光系统性能和果实品质的生理机制
总结以上内容,得出如图9所示的工作模式图,UV-B 处理初始阶段(08:30),叶片PSⅡ和PSⅠ活性均上升;中午时段温室内温度持续升高至37 ℃左右,UV-B 梯度处理的叶片的PSⅠ光化学活性受到了不同程度的抑制;温度下降后,低、中剂量处理的叶片PS系统活性率先较快恢复,高剂量处理逐渐恢复。
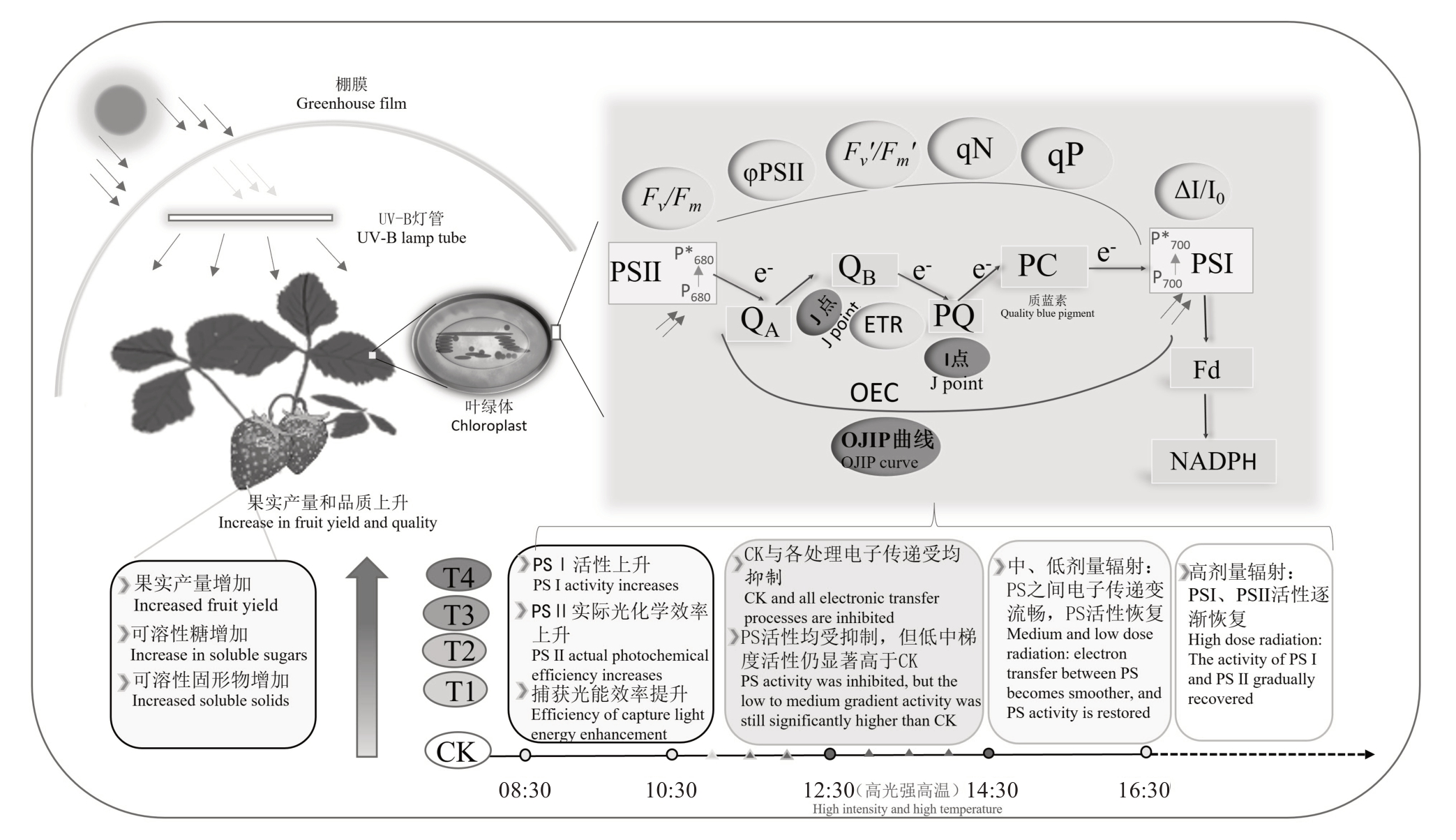
图9 UV-B 调控设施草莓叶片光系统性能和果实品质的模式图
Fig. 9 Model of UV-B regulating leaf photosystem performance and fruit quality in greenhouse strawberry
3 讨 论
3.1 适宜的UV-B辐射有利于提高设施草莓植株的抗性与果实品质
当植物处于逆境时,细胞膜的选择透性增大,电解质外渗,导致叶片的电导率上升;同时受自由基攻击的脂质产生过氧化反应,形成产物丙二醛;而脯氨酸和可溶性蛋白作为参与细胞渗透调节的物质,他们的积累可抵抗逆境,维持细胞膜结构,与抗氧化系统共同发挥保护作用[20]。在本试验中,经一个果实生长周期的UV-B 处理后,T1~T3 处理的脯氨酸和可溶性蛋白含量有所增加,电导率和丙二醛含量与对照差异不显著,说明低中UV-B 补充剂量下叶片的渗透调节物质足够保护植株免受胁迫;T4 处理的丙二醛、脯氨酸、可溶性蛋白含量显著增加,暗示植株需要产生大量渗透调节物质来增强自身抗性,抵御高强度的辐射[21]。本研究中低剂量处理组(T1、T2)地上部和地下部干鲜质量均增加,且叶绿素含量和净光合速率上升,验证了光合速率是叶片物质积累的主要影响因素。猜测以此为前提,促进了叶片和果实C 代谢和转运过程,而果实糖积累是碳代谢的重要一环,此外,还可能为适宜UV-B辐射提高了同化物的分配率从而影响相关的果实指标[19],最终T1、T2 处理果实的单果质量和可溶性糖含量升高,而可滴定酸含量和固酸比受UV-B 辐射影响较小。综上,低剂量的UV-B 补充对设施草莓的物质积累和内在果实品质有改善作用,为草莓的生产上进一步改善果实品质奠定了理论基础。不过果实品质相关指标在UV-B 辐射下具体的响应机制尚不完全明确,有待深入研究。
3.2 UV-B 可通过影响叶片PSⅡ、PSⅠ活性来调控光合性能
作物种类或品种不同,UV-B 辐射剂量不同,所处栽培环境不同,都会导致光系统和光合机构对UV-B 的响应方式不尽相同[22]。高丛蓝莓品种Brigitta和Bluegold暴露于UV-B下,前者光合性能受抑制,而后者光合效率提升[23]。在低温光胁迫的环境下,紫外线可加剧黄瓜叶片PSⅡ光抑制,却减轻了PSⅠ光抑制[24];增强大田草莓的UV-B 强度使草莓叶片细胞间隙增大并对光合速率产生了负面作用[25]。温室内的光照弱、UV-B 强度低[26],易存在枝条徒长、叶薄色浅、光合性能低等轻微胁迫现象,补充适宜剂量的UV-B 可以改善叶绿体超微结构并提高叶片的光合性能[9,27-28]。
原初反应中光能的捕获和光化学反应的电子传递由PSⅡ和PSⅠ共同承担,两者的活性及串联工作效率是影响光合速率的重要因素。借助快速叶绿素荧光诱导动力学曲线即可探究叶片PSⅡ反应中心开闭情况以及电子传递至PSⅠ转化为ATP 的光化学反应过程的变化情况[29]。在本试验中,UV-B 处理时,试验组的J 点和I 点较低,ΔJ 和ΔI<0,表明PSⅡ中QA的还原速率及其下游电子受体还原情况优于对照,UV-B 辐射提升了PSⅡ捕获光能的能力、QA向下传递电子的效率和PQ库接受电子的能力[30-31]。随着时间的推移,温室内温度持续升高,OJIP 曲线开始变化,T4、T1、T3处理渐次上升,12:30时三者均出现在零线之上,说明此时试验组叶片的QA-大量积累[32],PSⅡ供体侧和受体侧电子传递受到了不同程度的抑制[28]。14:30 时T2 也升至零线之上,但位于较低的位置,表明T2 处理受到的抑制较轻,意味着当存在高温胁迫时,UV-B 辐射强度越高,电子传递受抑制越厉害[33],这可能也是光合“午休”的主要原因之一。随着下午温度的下降,T1、T2 和T3 处理的曲线很快恢复,QA的还原速率相对于对照有所上升,且PSⅡ和PSⅠ活性受抑制后恢复能力较好,暗示低中UV-B 剂量处理下的PSⅡ光合机构对即时的外部环境有较强的恢复适应能力[34]。其可能原因在于PSⅡ反应中心修复循环(D1 蛋白周转)、脯氨酸等渗透调节物质含量上升、活性氧清除系统等光保护系统的持续运转等[29,35],而电子传递链并未受到实质性伤害,所以温度降至常温后会很快恢复[35]。16:30 时,T1~T3 处理的活性略高于对照,表示其光系统完全恢复,UV-B对叶片光合机构的调控实现了良性循环,并推测16:30 至翌日08:30(夜间)T4 处理也恢复正常,且其间各处理与对照的荧光参数差异较小[16]。进一步的试验结果表明,各UV-B 处理的ETR 值(即单位时间内进入光合作用反应中的电子数)均低于对照,T4、T3 处理的降幅大于T2、T1 的降幅,结合qP、qN的变化规律,可能是光合系统机智地将所吸收的过剩光能分配到了热耗散、发射荧光等其他去处,从而降低ETR,以此来减少电能向活跃化学能的转化,使电子传递链免受实质性损伤[36]。
综上,在本试验中,低、中剂量的UV-B处理提高了叶绿体色素含量和两个光系统的活性,使两者之间的电子传递更流畅,从而提高了叶片的光合能力,改善了果实品质。其中T2处理的PSⅡ活性(Fv/Fm)、PSⅠ活性(△I/Io)和糖酸比最高,表明此强度的UV-B 辐射最有利于光能的捕获、反应中心的开放和电子的传递以及果实内在品质的改善。
4 结 论
阈值范围内,补充适宜剂量的UV-B辐射能不同程度地提高设施草莓叶绿体色素含量和光系统工作效率及光合能力,增加植株干物质积累并优化分配,从而改善果实品质。在本试验条件下,补充0.4 W·m-2(T2)的剂量最为适宜,0.2 W·m-2(T1)和0.6 W·m-2(T3)次之,此三者的光系统活性和果实品质均优于对照。
[1] 高东升. 中国设施果树栽培的现状与发展趋势[J]. 落叶果树,2016,48(1):1-4.GAO Dongsheng. The current situation and development trend of facility fruit tree cultivation in China[J]. Deciduous Fruits,2016,48(1):1-4.
[2] FENG Y,CUI X,SHAN H,SHI Z S,LI F H,WANG H W,ZHU M,ZHONG X M. Effects of solar radiation on photosynthetic physiology of barren stalk differentiation in maize[J].Plant Science,2021,312:111046.
[3] CHEN M H,HUANG Y Y,LIU G,QIN F,YANG S,XU X. Effects of enhanced UV-B radiation on morphology,physiology,biomass,leaf anatomy and ultrastructure in male and female mulberry (Morus alba) saplings[J]. Environmental and Experimental Botany,2016,129:85-93.
[4] MA M,WANG P,YANG R Q,ZHOU T,GU Z X. UV-B mediates isoflavone accumulation and oxidative-antioxidant system responses in germinating soybean[J]. Food Chemistry,2019,275:628-636.
[5] RANJBARFORDOEI A,SAMSON R,VAN DAMME P. Photosynthesis performance in sweet almond [Prunus dulcis (Mill.)D. Webb] exposed to supplemental UV-B radiation[J]. Photosynthetica,2011,49(1):107-111.
[6] FENG L H,JIANG H,ZHANG Y B,ZHANG S. Sexual differences in defensive and protective mechanisms of Populus cathayana exposed to high UV-B radiation and low soil nutrient status[J]. Physiologia Plantarum,2014,151(4):434-445.
[7] ZAVAFER A,KOINUMA W,CHOW W S,CHEAH M H,MINO H. Mechanism of photodamage of the oxygen evolving Mn cluster of photosystem Ⅱ by excessive light energy[J]. Scientific Reports,2017,7(1):7604.
[8] MISHRA V,SRIVASTAVA G,PRASAD S M,ABRAHAM G.Growth,photosynthetic pigments and photosynthetic activity during seedling stage of cowpea (Vigna unguiculata) in response to UV-B and dimethoate[J]. Pesticide Biochemistry and Physiology,2008,92(1):30-37.
[9] WANG X X,FU X L,CHEN M,HUAN L,LIU W H,QI Y H,GAO Y G,XIAO W,CHEN X D,LI L,GAO D S. Ultraviolet B irradiation influences the fruit quality and sucrose metabolism of peach (Prunus persica L.)[J]. Environmental and Experimental Botany,2018,153:286-301.
[10] YANG J F,LI B B,SHI W J,GONG Z Z,CHEN L,HOU Z X.Transcriptional activation of anthocyanin biosynthesis in developing fruit of blueberries (Vaccinium corymbosum L.) by preharvest and postharvest UV irradiation[J]. Journal of Agricultural and Food Chemistry,2018,66(42):10931-10942.
[11] STRASSERF R J,SRIVASTAVA A,GOVINDJEE. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria[J]. Photochemistry and Photobiology,1995,61(1):32-42.
[12] MOREIRA-RODRÍGUEZ M,NAIR V,BENAVIDES J,CISNEROS-ZEVALLOS L,JACOBO-VELÁZQUEZ D A. UVA,UVB light,and methyl jasmonate,alone or combined,redirect the biosynthesis of glucosinolates,phenolics,carotenoids,and chlorophylls in broccoli sprouts[J]. International Journal of Molecular Sciences,2017,18(11):2330.
[13] 郭建军,王晓芳,孙玉楚,李景原. 人工增加UV-B 辐照改善温室番茄品质[J]. 中国瓜菜,2020,33(2):31-36.GUO Jianjun,WANG Xiaofang,SUN Yuchu,LI Jingyuan. Effect of UV-B on the tomato quality in greenhouse[J]. China Cucurbits and Vegetables,2020,33(2):31-36.
[14] 郑佳寅,曲文雅,陈向荣,吴晓霞,赵明,高红胜,周福才. 紫光对设施草莓朱砂叶螨种群的控制作用[J]. 扬州大学学报(农业与生命科学版),2024,45(3):140-146.ZHENG Jiayin,QU Wenya,CHEN Xiangrong,WU Xiaoxia,ZHAO Ming,GAO Hongsheng,ZHOU Fucai. Control effect of purple light on Tetranychus cinnabarinus population in greenhouse strawberry[J]. Journal of Yangzhou University (Agricultural and Life Science Edition),2024,45(3):140-146.
[15] 李晨,刘建廷,樊永信,赵雪惠,肖伟,陈修德,付喜玲,李玲,李冬梅. UV-B 对设施桃叶片光合功能及叶绿体超微结构的影响[J]. 植物学报,2022,57(4):434-443.LI Chen,LIU Jianting,FAN Yongxin,ZHAO Xuehui,XIAO Wei,CHEN Xiude,FU Xiling,LI Ling,LI Dongmei. Effects of UV-B on photosynthetic function and chloroplast ultrastructure of peach leaves grown in greenhouse[J]. Chinese Bulletin of Botany,2022,57(4):434-443.
[16] 赵世杰,苍晶. 植物生理学实验指导[M]. 北京:中国农业出版社,2016.ZHAO Shijie,CANG Jing. Guidelines for plant physiology experiments[M]. Beijing:China Agriculture Press,2016.
[17] ZHANG R,XU C,BAO Z L,XIAO R,CHEN X D,XIAO W,LI D M,FU X L,YANG C,LI L. Auxin alters sodium ion accumulation and nutrient accumulation by playing protective role in salinity challenged strawberry[J]. Plant Physiology and Biochemistry,2021,164:1-9.
[18] DEMMIG-ADAMS B,ADAMS W W. The role of xanthophyll cycle carotenoids in the protection of photosynthesis[J]. Trends in Plant Science,1996,1(1):21-26.
[19] 于妮娜,李冬梅,谭秋平,张海森,高东升. UV-B 辐射对设施桃结果枝同化物转运和分配的影响[J]. 应用与环境生物学报,2013,19(1):157-163.YU Nina,LI Dongmei,TAN Qiuping,ZHANG Haisen,GAO Dongsheng. Effect of UV-B radiation on assimilate translocation and distribution in fruiting shoot of protected peach[J]. Chinese Journal of Applied and Environmental Biology,2013,19(1):157-163.
[20] 王鑫月,张立业,张雪梅. 树莓茎对低温胁迫的生理响应[J].中国农学通报,2023,39(34):63-72.WANG Xinyue,ZHANG Liye,ZHANG Xuemei. Physiological response of Rubus idaeus stems to cryogenic stress[J]. Chinese Agricultural Science Bulletin,2023,39(34):63-72.
[21] 张琳,王修蘋,李祖然,何永美,李元. 植物响应UV-B 辐射的表观遗传调控和胁迫记忆研究进展[J]. 植物生理学报,2023,59(7):1195-1210.ZHANG Lin,WANG Xiupin,LI Zuran,HE Yongmei,LI Yuan.Research progress on the epigenetic regulation and stress memory of plant to UV-B radiation responses[J]. Plant Physiology Journal,2023,59(7):1195-1210.
[22] INOSTROZA-BLANCHETEAU C,ACEVEDO P,LOYOLA R,ARCE-JOHNSON P,ALBERDI M,REYES-DÍAZ M. Shortterm UV-B radiation affects photosynthetic performance and antioxidant gene expression in highbush blueberry leaves[J]. Plant Physiology and Biochemistry,2016,107:301-309.
[23] ZHANG Z S,JIN L Q,LI Y T,TIKKANEN M,LI Q M,AI X Z,GAO H Y. Ultraviolet-B radiation (UV-B) relieves chillinglight-induced PSⅠ photoinhibition and accelerates the recovery of CO2 assimilation in cucumber (Cucumis sativus L.) leaves[J].Scientific Reports,2016,6:34455.
[24] 张子山. 低温弱光胁迫下黄瓜叶片光系统Ⅰ与光系统Ⅱ的相互作用[D]. 泰安:山东农业大学,2013.ZHANG Zishan. Interaction between photosystem Ⅰ and photosystem Ⅱ under chilling-light stress in cucumber leaves[D]. Tai’an:Shandong Agricultural University,2013.
[25] VALKAMA E,KIVIMÄENPÄÄ M,HARTIKAINEN H,WULFF A. The combined effects of enhanced UV-B radiation and selenium on growth,chlorophyll fluorescence and ultrastructure in strawberry (Fragaria × ananassa) and barley (Hordeum vulgare) treated in the field[J]. Agricultural and Forest Meteorology,2003,120(1/2/3/4):267-278.
[26] KATZIN D,MARCELIS L F M,VAN HENTEN E J,VAN MOURIK S. Heating greenhouses by light:A novel concept for intensive greenhouse production[J]. Biosystems Engineering,2023,230:242-276.
[27] 褚润,陈年来. UV-B 辐射增强对芦苇叶绿素荧光参数的影响[J]. 环境科学学报,2018,38(8):3375-3382.CHU Run,CHEN Nianlai. Effects of enhanced UV-B radiation on chlorophyll fluorescence characteristics of Phragmites australis[J]. Acta Scientiae Circumstantiae,2018,38(8):3375-3382.
[28] LYCOSKOUFIS I,KAVGA A,KOUBOURIS G,KARAMOUSANTAS D. Ultraviolet radiation management in greenhouse to improve red lettuce quality and yield[J]. Agriculture,2022,12(10):1620.
[29] 李鹏民. 快速叶绿素荧光诱导动力学在植物逆境生理研究中的应用[D]. 泰安:山东农业大学,2007.LI Pengmin. Application of chlorophyll a fluorescence transient instudy of plant physiology under stress conditions[D]. Tai’an:Shandong Agricultural University,2007.
[30] 王伟,王岩,梁变变,赵天宏,田荣荣,武红艳. 初花期喷镧对UV-B 辐射增强下紫花苜蓿光合及荧光特性的影响[J]. 中国农业气象,2017,38(4):230-239.WANG Wei,WANG Yan,LIANG Bianbian,ZHAO Tianhong,TIAN Rongrong,WU Hongyan. Effects of spraying lanthanum in early flowering stage on photosynthetic characters and chlorophyll fluorescence parameters of Medicago sativa under enhanced UV-B radiation[J]. Chinese Journal of Agrometeorology,2017,38(4):230-239.
[31] PALMA C F F,CASTRO-ALVES V,MORALES L O,ROSENQVIST E,OTTOSEN C O,STRID Å. Spectral composition of light affects sensitivity to UV-B and photoinhibition in cucumber[J]. Frontiers in Plant Science,2021,11:610011.
[32] 徐超,王明田,杨再强,韩玮,郑盛华. 高温对温室草莓光合生理特性的影响及胁迫等级构建[J]. 应用生态学报,2021,32(1):231-240.XU Chao,WANG Mingtian,YANG Zaiqiang,HAN Wei,ZHENG Shenghua. Effects of high temperature on photosynthetic physiological characteristics of strawberry seedlings in greenhouse and construction of stress level[J]. Chinese Journal of Applied Ecology,2021,32(1):231-240.
[33] 祁虹,段留生,王树林,王燕,张谦,冯国艺,杜海英,梁青龙,林永增. 全生育期UV-B 辐射增强对棉花生长及光合作用的影响[J]. 中国生态农业学报,2017,25(5):708-719.QI Hong,DUAN Liusheng,WANG Shulin,WANG Yan,ZHANG Qian,FENG Guoyi,DU Haiying,LIANG Qinglong,LIN Yongzeng. Effect of enhanced UV-B radiation on cotton growth and photosynthesis[J]. Chinese Journal of Eco-Agriculture,2017,25(5):708-719.
[34] 李冬梅,李少旋,徐功勋,李晨,付喜玲,陈修德,张海森,高东升. 设施作物响应UV-B 辐射的研究进展[J]. 植物生理学报,2018,54(1):36-44.LI Dongmei,LI Shaoxuan,XU Gongxun,LI Chen,FU Xiling,CHEN Xiude,ZHANG Haisen,GAO Dongsheng. Research advances of plant response to UV-B radiation in greenhouse[J].Plant Physiology Journal,2018,54(1):36-44.
[35] YOSHIOKA-NISHIMURA M. Close relationships between the PSⅡ repair cycle and thylakoid membrane dynamics[J]. Plant &Cell Physiology,2016,57(6):1115-1122.
[36] 孟令曾,张教林,曹坤芳,许再富. 迁地保护的4 种龙脑香冠层叶光合速率和叶绿素荧光参数的日变化[J]. 植物生态学报,2005,29(6):976-984.MENG Lingzeng,ZHANG Jiaolin,CAO Kunfang,XU Zaifu.Diurnal changes of photosynthetic characteristics and chlorophyll fluorescence in canopy leaves of four diptocarp species under ex-situ conservation[J]. Acta Phytoecologica Sinica,2005,29(6):976-984.