西番莲(Passiflora caerulea L.)果实俗称百香果,是一种特色的热带、亚热带果品,在中国广西、福建、海南、广东、云南等地广泛栽培[1-3]。西番莲果实富含多种营养物质,如维生素、酚类、有机酸、矿物质等[1,4-5],具有抗氧化、降血压、降血脂和抗肿瘤等功效[6-7],且因风味独特、香气浓郁而深受消费者的喜爱[6]。然而,因西番莲果实为呼吸跃变型果实,采后呼吸强度高,后熟衰老速度快,果实极易发生腐烂、皱缩等衰败现象,进而影响品质和商业价值[1,4]。因此,通过采后处理减缓果实品质败坏对西番莲的保鲜流通具有重要意义。
呼吸代谢是果实重要的生理代谢活动,跃变型果实采后成熟衰老和品质变化尤其与呼吸代谢密切相关[8-10]。呼吸强度及糖酵解(EMP)、三羧酸(TCA)循环、磷酸戊糖途径(PPP)等不同呼吸代谢途径的水平均可影响果实采后品质[10-12]。另外,呼吸代谢途径的改变也与呼吸代谢相关酶酶活性、吡啶核苷酸含量等有关[9]。有研究报道,过氧化氢处理[9]、病原菌侵染[11]等能导致果实采后较低水平的PPP以及较高水平的EMP 和TCA 循环,从而造成果实品质劣变。相反地,三磷酸腺苷[8]、酸性电解水[10]、棓酸丙酯[13]等处理可保持果实采后较高水平的PPP以及较低水平的EMP和TCA循环,从而维持果实品质。
海藻寡糖是由海藻多糖降解得到的低聚合度活性物质[14-15],主要有琼胶寡糖、岩藻寡糖、褐藻寡糖及卡拉胶寡糖。其中,琼胶寡糖是由琼二糖为重复单位连接形成,包含新琼寡糖(Neoagaro-oligosaccharide,NAOS)和琼寡糖[16]。有研究发现,海藻寡糖具有安全性高、成本低、水溶性好、生物相容性高等优点,兼具提高植物抗氧化能力和抗逆性及促进生长调节等作用[14-16]。相关研究表明,褐藻寡糖处理能提高草莓果实的采后品质,延长其货架期[17]。另外,蓝炎阳等[18]报道,海藻寡糖处理可保持蜜柚可溶性固形物(TSS)、总糖、有机酸等物质的含量,提高果实硬度,延长其贮藏期。Hou等[19]研究报道,琼胶寡糖处理可推迟樱桃番茄果实的呼吸高峰,延缓维生素C 与可滴定酸(TA)含量的下降,从而减缓品质劣变。然而,目前海藻寡糖维持果实采后品质的作用机制尚不清楚,关于海藻寡糖应用于西番莲果实采后保鲜的研究报道也较少。因此,笔者在本研究中采用龙须菜降解所得的海藻寡糖——NAOS溶液对西番莲果实进行采后处理,探究NAOS 溶液处理减缓西番莲果实衰老的作用及其与呼吸代谢的关系,以期为利用海藻寡糖维持果实采后品质、延长保鲜期提供理论依据。
1 材料和方法
1.1 材料与处理
成熟度为约八成熟的钦蜜9 号西番莲果实,采摘于福建省热带作物科学研究所(福建漳州),采后运到实验室,将大小、色泽均一,无病害、无损伤的健康果实清洗后备用。
NAOS 溶液由自然资源部第三海洋研究所(福建厦门)提供,主要成分为深海火色杆菌Flammeovirga sp.OC4降解龙须菜所得的四糖和六糖的混合溶液,有效质量浓度为6 g·L-1。该溶液为淡褐色透明水溶液,有轻微发酵腥臭味,常温下理化性状稳定,加水稀释至处理所用质量浓度(180 mg·L-1)后近乎无色无味。
在预试验中,选用0(对照)、60、120、180、240 mg·L-1 NAOS 溶液浸泡处理西番莲果实15 min,晾干后包装于聚乙烯薄膜袋(袋两侧各有9个3 mm微孔)中,在(25±1)℃、相对湿度85%条下贮藏18 d,定期取样测定果实腐烂率。结果发现,在贮藏第18 天时,不同处理组的果实腐烂率分别为:32.38%(对照)>27.62%(120 mg·L-1)>26.67%(60 mg·L-1)>24.76%(240 mg·L-1)>17.14%(180 mg·L-1)。因180 mg·L-1 NAOS 处理最能有效减缓西番莲果实采后衰败,所以本研究中使用该质量浓度处理。
将挑选出的果实分为处理组和对照组,每组3次重复。(1)NAOS处理组:180 mg·L-1 NAOS溶液浸泡果实15 min;(2)对照组(Control):蒸馏水浸泡果实15 min。随后将两组的果实均取出晾干、装入打孔聚乙烯薄膜袋(15个/袋),置于(25±1)℃、相对湿度85%条件下贮藏18 d,贮藏期内定期(每3 d)随机取样测定相关指标;另外,在贮藏1 d时,还测定呼吸强度。
1.2 测定方法
1.2.1 腐烂率 以果实腐烂率评价其衰败程度。取一袋西番莲果实,参照Li 等[20]的方法评价果实采后腐烂率。
1.2.2 硬度 参照Hao 等[21]的方法测定果实硬度,结果以N表示。
1.2.3 失重率 参照Lin 等[22]的方法测定果实失重率,结果以百分比表示。
1.2.4 果皮水分含量 参照罗振宇等[23]的方法,采用直接干燥法测定西番莲果皮水分含量,结果以百分比表示。
1.2.5 果肉TSS 和TA 含量 从西番莲果实中取出果肉,经研磨后过滤。参照Lin等[22]的方法测定果实果肉的TSS和TA含量,结果以百分比表示。
1.2.6 呼吸强度 参照Zhang 等[8]和Liu 等[10]的方法测定西番莲果实呼吸强度,结果用(以CO2计)mg·kg-1·h-1表示。
1.2.7 呼吸代谢途径相关酶活性 参照Lin 等[11]和Sun等[12]的方法测定西番莲果皮呼吸代谢途径关键酶(包括PGI、SDH 及G-6-PDH+6-PGDH)的活性。此外,参照Lin等[11]和Sun等[12]的方法测定西番莲果皮CCO、AAO 和PPO 等呼吸末端氧化酶活性。上述结果均以U·mg-1表示。
1.2.8 NADK活性 参照Lin等[9,13]的方法测定西番莲果皮NADK活性,结果以U·mg-1表示。
1.2.9 吡啶核苷酸含量 参照Lin 等[11]和Sun 等[12]的方法测定西番莲果皮吡啶核苷酸(NAD、NADH、NADP和NADPH)含量,结果用μmol·g-1表示。
1.3 数据处理
本研究中测定的指标均进行3 次重复,结果以平均值±标准误表示。利用SPSS软件(版本号:26)的方差分析与Duncan 检验对所获得的数据进行统计及分析。在数据图中,用*、**分别表示在同一贮藏时间NAOS 处理组和对照组间具有显著(p<0.05)、极显著(p<0.01)的差异。
2 结果与分析
2.1 果实腐烂率
腐烂率的高低可反映果实采后品质的水平。由图1可知,对照果实腐烂率在贮藏0~3 d均为0,随后迅速升高。然而,NAOS处理组果实在贮藏0~9 d未出现腐烂,在贮藏9~18 d腐烂率逐渐上升。贮藏6~18 d 期间NAOS 处理组果实的腐烂率均低于对照组,并在9~18 d期间差异达到极显著(p<0.01)。在贮藏18 d 时,NAOS 处理组果实的腐烂率仅为对照组的52.93%。
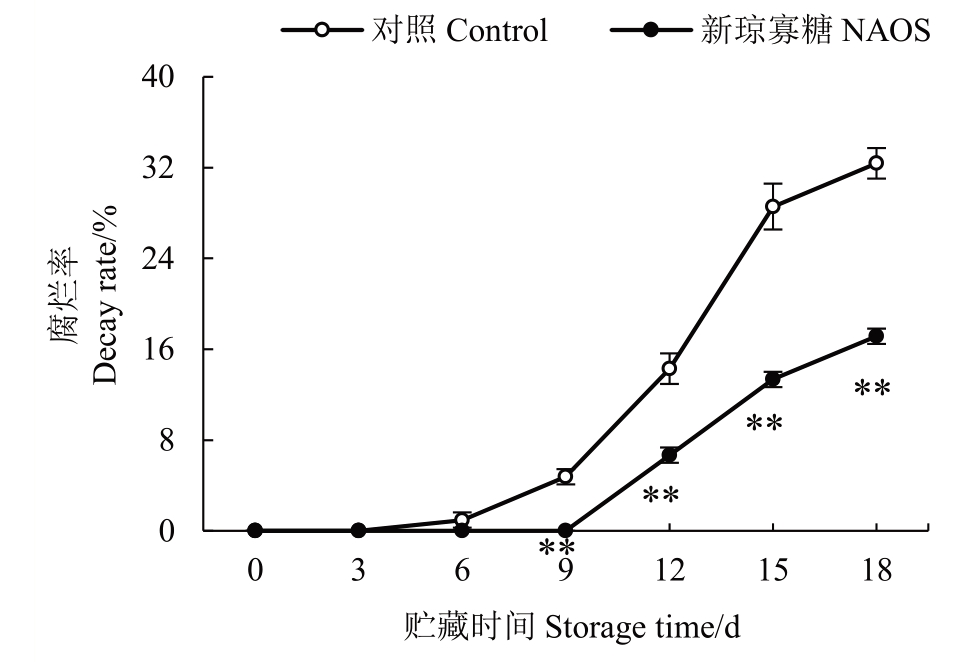
图1 NAOS 处理对西番莲果实腐烂率的影响
Fig.1 Effects of NAOS treatment on the decay rate of passion fruit
2.2 果实硬度和失重率
果实质地是反映跃变型果实后熟过程的重要指标。由图2-A 可知,对照组和NAOS 处理组果实的硬度从贮藏0 d 时的69.61 N 分别逐渐下降至贮藏18 d 时的19.68 N 和40.59 N。在贮藏6~18 d 期间,NAOS处理组果实的硬度高于对照组,且在贮藏12 d和18 d时差异为极显著(p<0.01)。
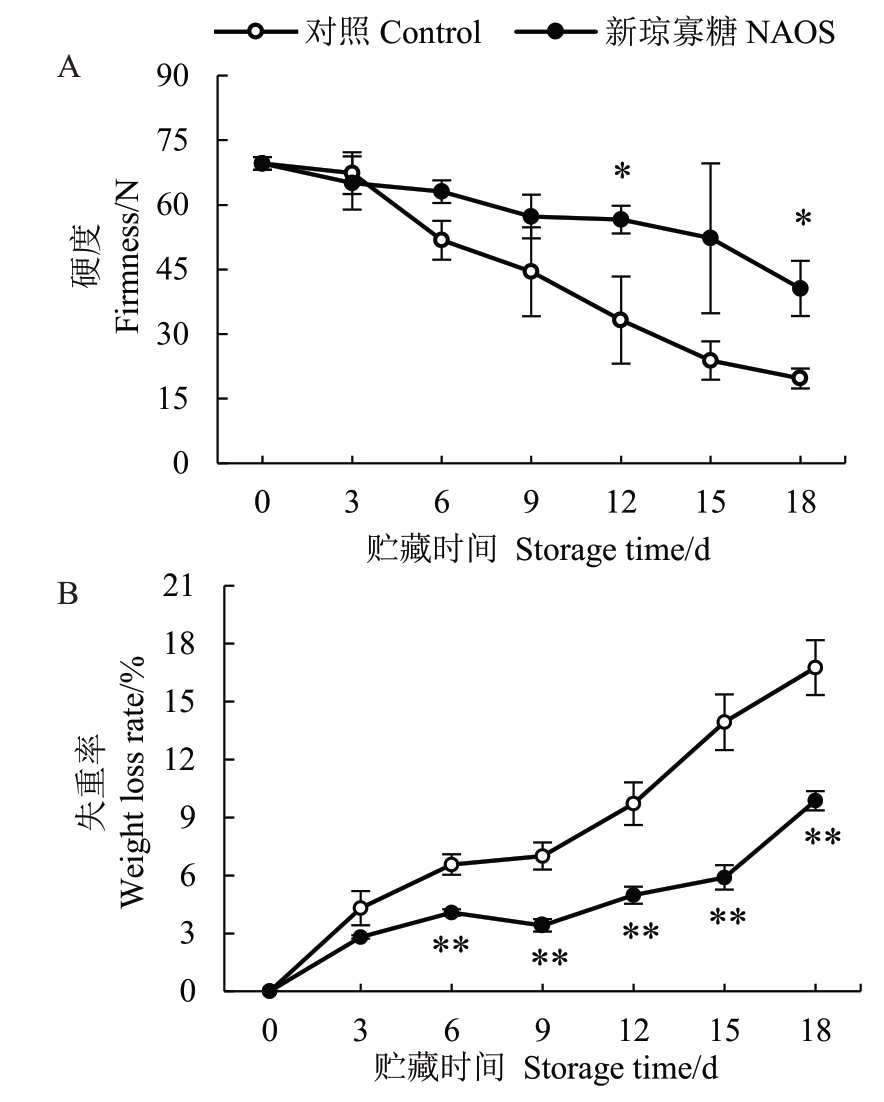
图2 NAOS 处理对西番莲果实硬度(A)和失重率(B)的影响
Fig.2 Effects of NAOS treatment on the firmness(A)and weight loss rate(B)of passion fruit
果实失重主要由蒸腾作用造成的水分流失以及呼吸消耗所导致[22]。由图2-B 可知,在贮藏期内西番莲果实失重率上升。在3~18 d 期间,NAOS 处理组果实的失重率均低于对照组,其中在贮藏6~18 d差异极显著(p<0.01),在18 d时处理组为对照组的58.86%。
2.3 果皮水分含量
皱缩是西番莲果实最显著的采后品质劣变特征之一,果皮失水是引起皱缩的主要原因,并加速果实耐贮性下降和衰败进程[23]。由图3 可知,对照组果实果皮水分含量在贮藏0~18 d 期间快速降低。NAOS处理组果实果皮水分含量在贮藏0~12 d期间变化较小,12~15 d 快速降低,15~18 d 无明显变化。在贮藏18 d时处理组与对照组的差异为极显著(p<0.01)。以上结果表明,在贮藏期间NAOS处理组果实可保持较高的果皮水分含量。
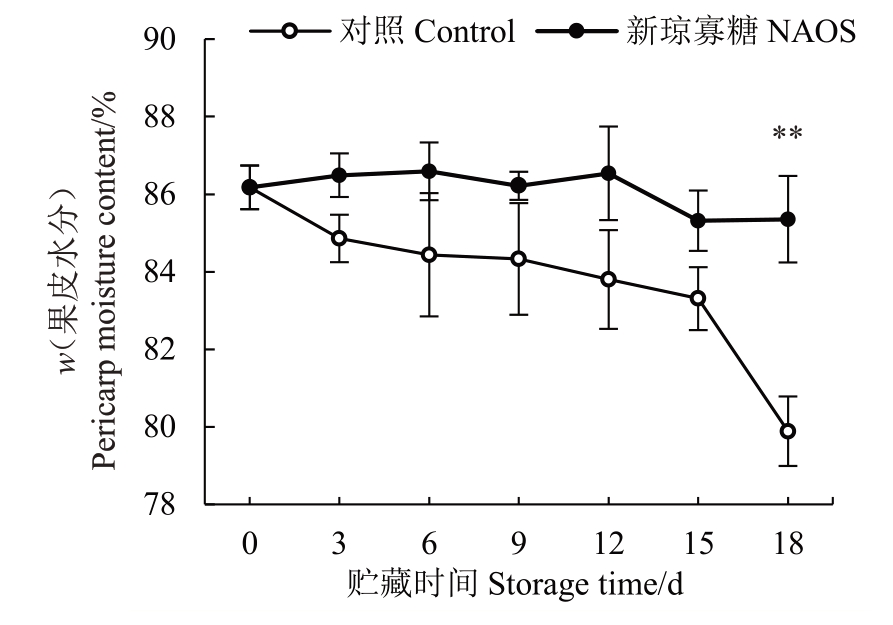
图3 NAOS 处理对西番莲果实果皮水分含量的影响
Fig.3 Effects of NAOS treatment on the moisture content of passion fruit pericarp
2.4 果肉TSS和TA含量
TSS 和TA 含量是影响果实食用品质的直接因素,其含量在采后的变化深受呼吸代谢等生理活动影响。由图4-A 可知,对照组西番莲果实TSS 含量在贮藏0~3 d 呈上升趋势而在贮藏3~18 d 下降。NAOS 处理组果实TSS 含量在贮藏0~6 d 与对照组相近,而在贮藏9~18 d 处理组果实TSS 含量虽缓慢下降但均显著(p<0.05)高于对照组。由图4-B 可知,对照组果实TA 含量在贮藏0~3 d 迅速降低,随后持续下降,从贮藏0 d的4.89%下降到贮藏18 d的1.26%。NAOS处理组果实TA含量在贮藏期内缓慢降低,在3~18 d 期间均高于对照组,并且在6~18 d差异达到极显著(p<0.01)水平。
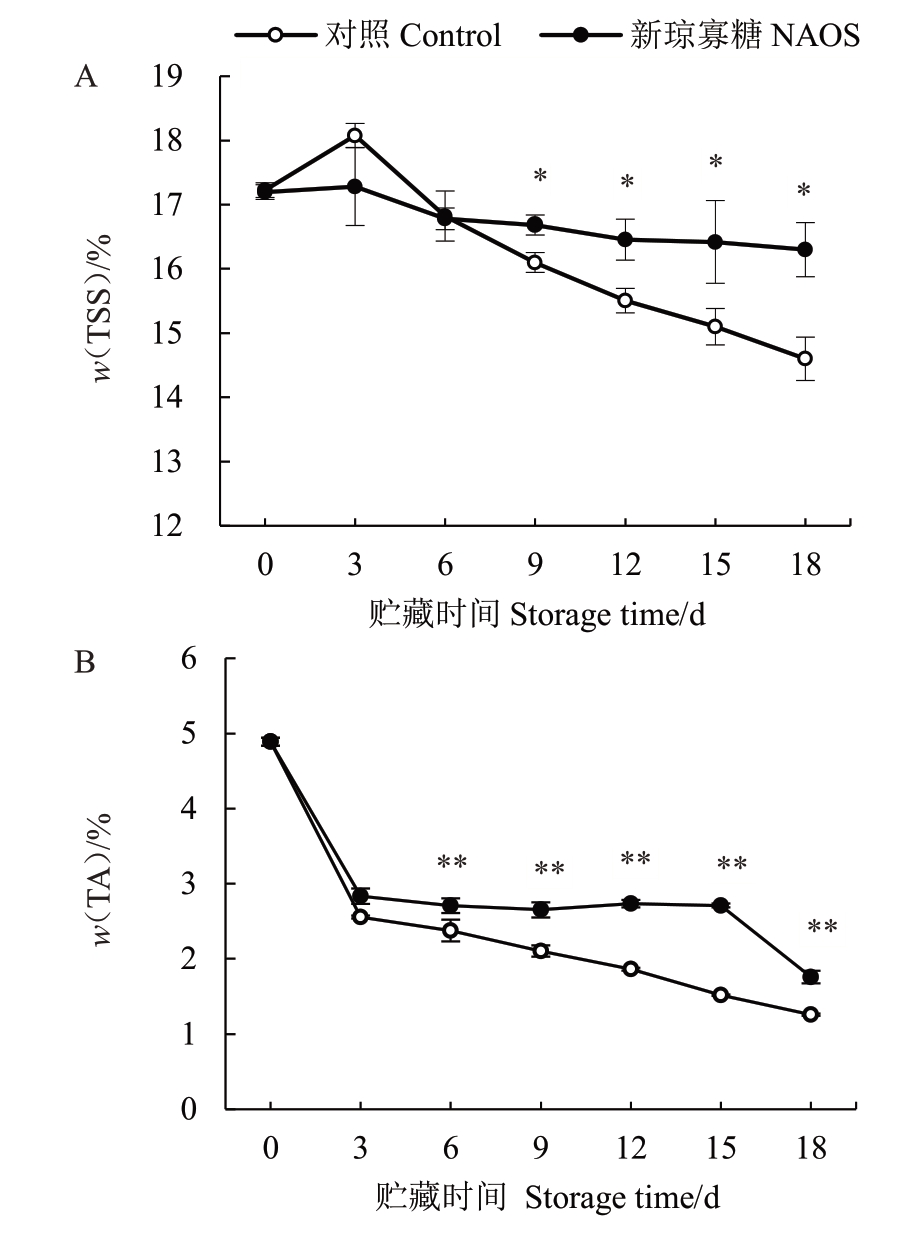
图4 NAOS 处理对西番莲果实果肉TSS(A)和TA(B)含量的影响
Fig.4 Effects of NAOS treatment on the contents of TSS(A)and TA(B)of passion fruit pulp
2.5 果实呼吸强度
呼吸是采后果实重要的生理代谢活动,高呼吸强度会导致底物消耗增多,促进果实衰败,缩短货架期[22]。由图5可知,对照组呼吸强度在贮藏0~1 d快速上升,并在1 d达到呼吸高峰(154.46 mg·kg-1·h-1),随后下降;而NAOS处理组果实呼吸强度在贮藏0~6 d上升,6 d时达到呼吸峰值(99.96 mg·kg-1·h-1),随后波动变化。NAOS 处理组果实呼吸强度在贮藏1 d时极显著(p<0.01)低于对照,在贮藏3 d 时显著(p<0.05)低于对照。
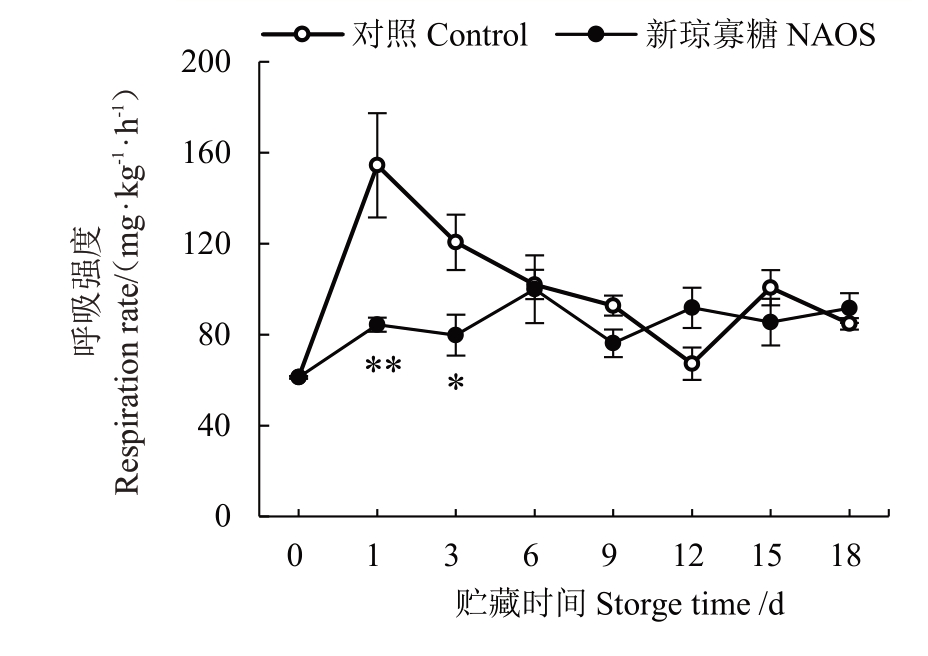
图5 NAOS 处理对西番莲果实呼吸强度的影响
Fig.5 Effects of NAOS treatment on the respiration rate of passion fruit
2.6 果皮呼吸代谢途径关键酶活性
PGI和SDH分别是EMP和TCA循环的关键酶,而G-6-PDH 与6-PGDH 是PPP 的关键酶,它们活性的高低可反映各呼吸代谢途径水平的变化[10-11]。由图6-A可知,西番莲果实PGI活性在贮藏0~3 d略有降低,3~12 d快速上升,随后迅速下降。与对照组相比,NAOS处理组果皮PGI活性维持在较低水平,并且在贮藏3、12、18 d 差异显著(p<0.05),在贮藏6~9 d和15 d差异为极显著(p<0.01)。
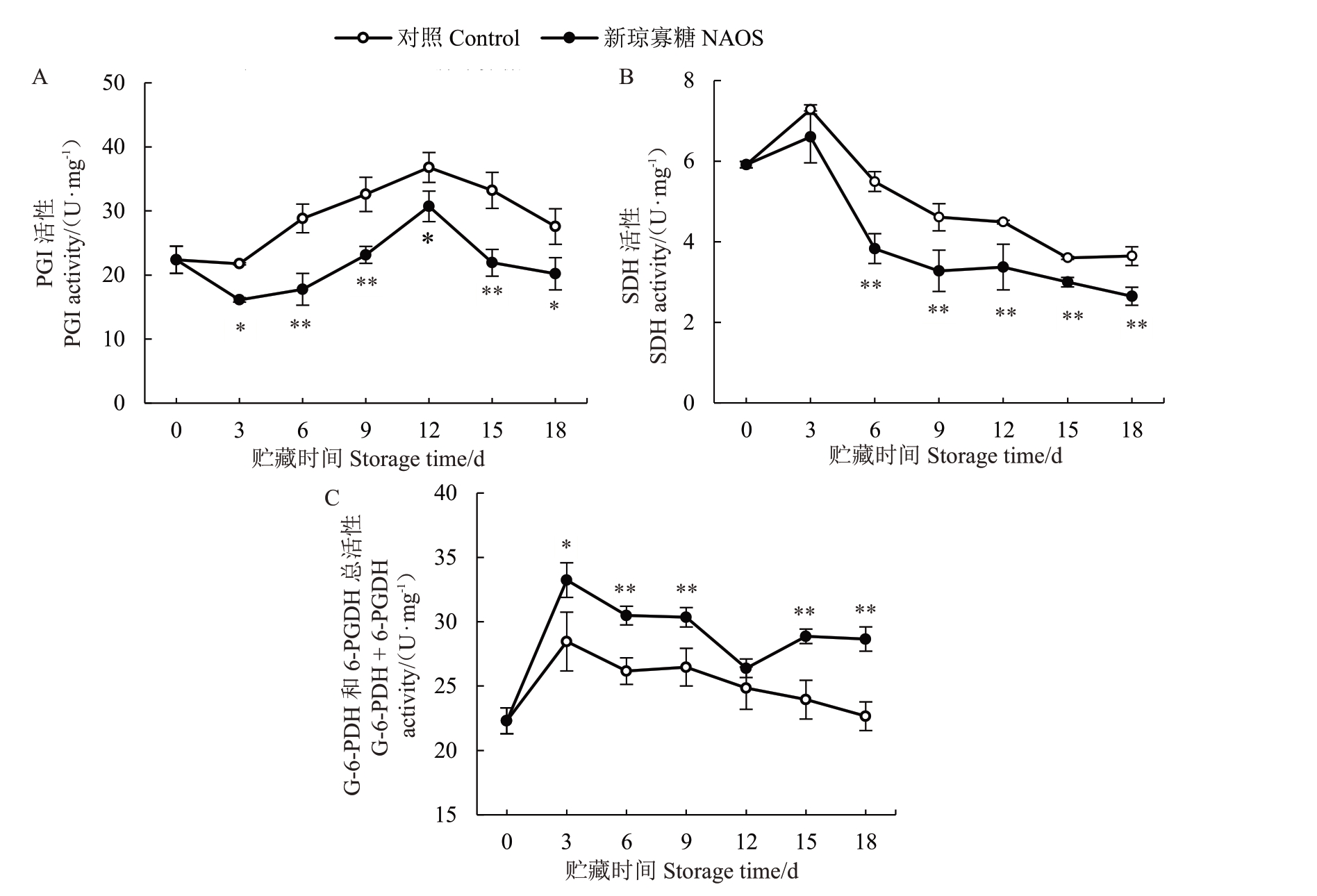
图6 NAOS 处理对西番莲果实果皮PGI(A)、SDH(B)和G-6-PDH+6-PGDH(C)活性的影响
Fig.6 Effects of NAOS treatment on activities of PGI(A),SDH(B)and G-6-PDH+6-PGDH(C)in pericarp of passion fruit
由图6-B 可知,对照组和NAOS 处理组果实的果皮SDH 活性均在贮藏0~3 d 升高,随后下降。在同一贮藏时间内NAOS 处理组的果皮SDH 活性均低于对照组,并在6~18 d达到极显著(p<0.01)差异水平。
如图6-C 所示,对照组果实果皮G-6-PDH 和6-PGDH总活性在贮藏0~3 d快速升高,3~18 d逐渐下降。NAOS处理组果皮G-6-PDH和6-PGDH总活性在贮藏0~3 d升高较快,3~18 d整体呈下降趋势。在同一贮藏时间内NAOS 处理组果皮G-6-PDH 和6-PGDH总活性均高于对照组,并且贮藏3 d时差异显著(p<0.05),6~18 d期间差异极显著(p<0.01)。
2.7 果皮NADK活性
NADK能催化NAD磷酸化生成NADP,它们分别参与不同的呼吸代谢途径[11]。由图7 可知,对照组果实果皮NADK 活性在贮藏0~3 d 降低,3~12 d呈现上升趋势,12~15 d下降后有所上升。NAOS处理组果皮NADK 活性在贮藏0~3 d较快上升,3~9 d下降,9~18 d上升。在相同贮藏时间,NAOS处理组果皮NADK 活性均高于对照组,并且在贮藏3~6 d和15~18 d 达到极显著(p<0.01)差异水平,在12 d时达到显著(p<0.05)差异水平。在贮藏18 d,NAOS处理组果皮NADK活性是对照组的1.60倍。
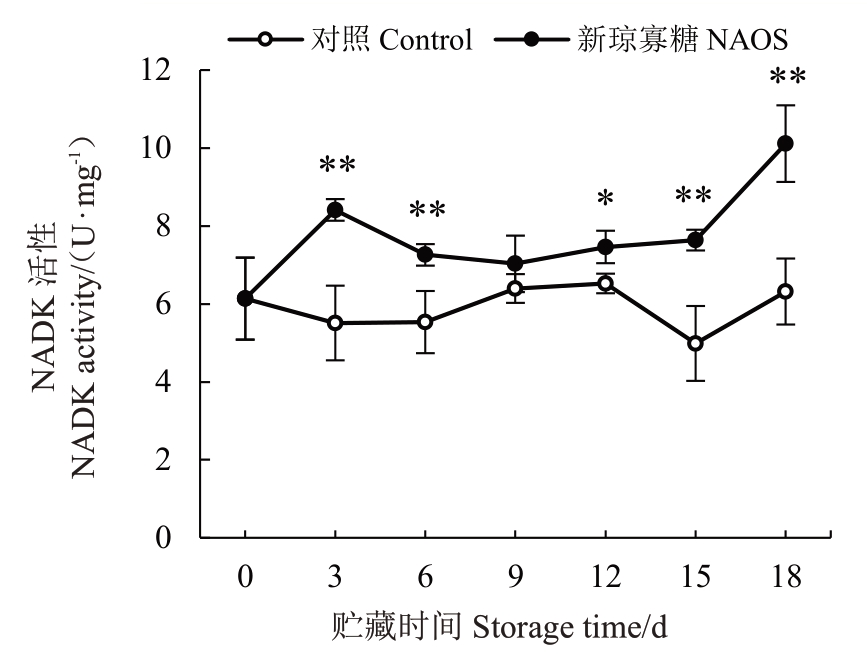
图7 NAOS 处理对西番莲果实果皮NADK 活性的影响
Fig.7 Effects of NAOS treatment on the NADK activity in pericarp of passion fruit
2.8 果皮吡啶核苷酸含量
NAD(H)与NADP(H)等吡啶核苷酸是参与调节呼吸代谢的重要辅助因子[10,13]。由图8-A可知,对照组果实果皮NAD 含量于贮藏0~9 d 快速升高,9~18 d整体略有下降;而NAOS处理组果皮NAD含量在贮藏0~3 d变化较小,3~9 d快速升高,随后快速降低。与对照组相比,在相同贮藏时间内NAOS 处理组果皮NAD 含量低于对照组,并在贮藏3~6 d 和15~18 d达到极显著(p<0.01)差异水平。
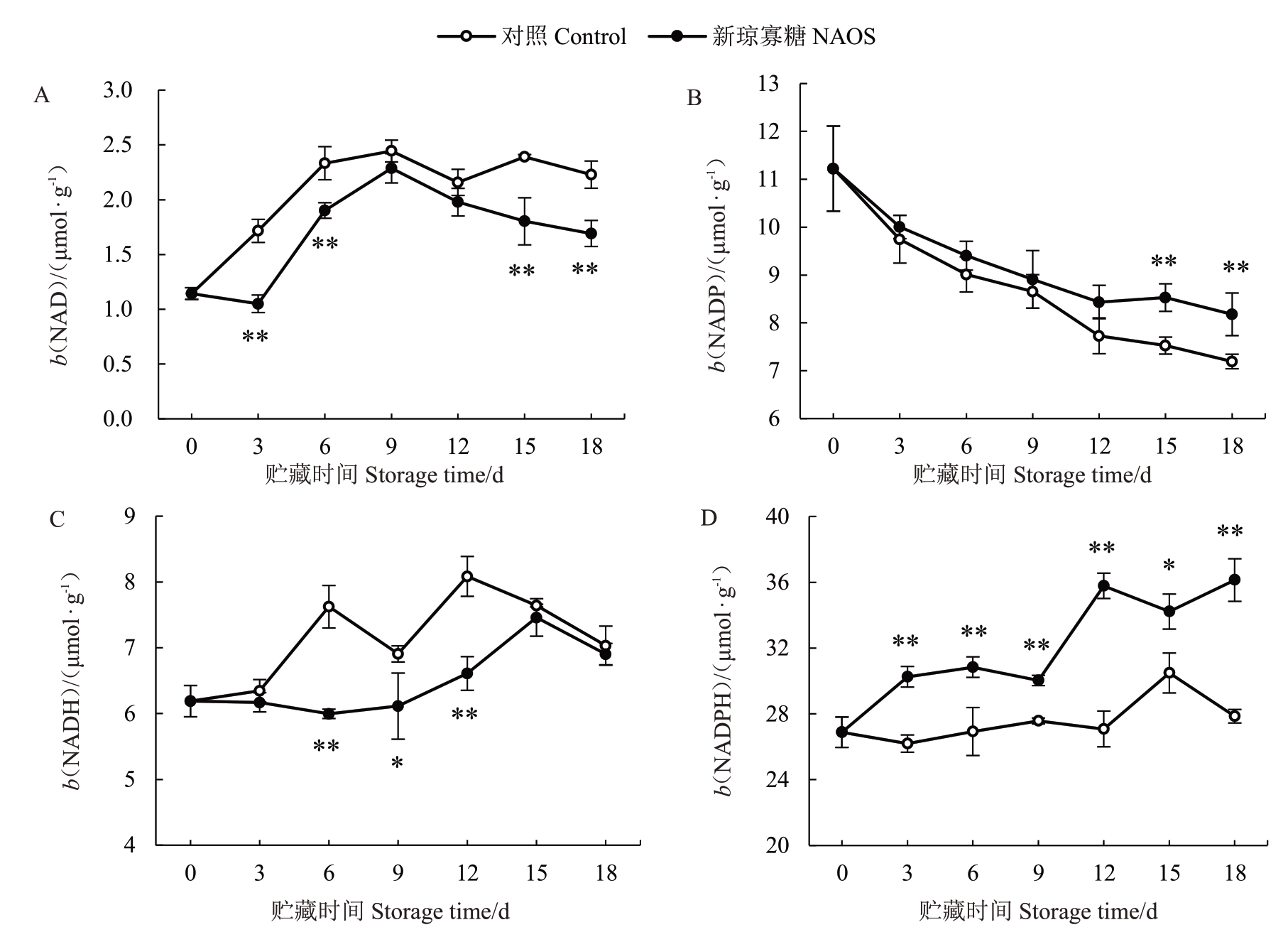
图8 NAOS 处理对西番莲果实果皮NAD(A)、NADP(B)、NADH(C)和NADPH(D)含量的影响
Fig.8 Effects of NAOS treatment on the contents of NAD(A),NADP(B),NADH(C)and NADPH(D)in pericarp of passion fruit
如图8-B 所示,对照组和NAOS 处理组果皮NADP 含量在贮藏期内不断降低,分别从0 d 时的11.22 μmol·g-1 下降至18 d 时的7.19 μmol·g-1 和8.18 μmol·g-1。与对照果实对比,在相同贮藏时间内NAOS 处理组果皮NADP 含量高于对照组,且在贮藏15~18 d达到极显著(p<0.01)差异水平。
由图8-C 可知,对照组果实的果皮NADH 含量于贮藏0~6 d 快速上升,6~9 d 下降,9~12 d 快速升高,随后逐渐降低。NAOS 处理组果皮NADH 含量在贮藏0~6 d略有下降,6~15 d快速上升,15~18 d快速下降。在3~18 d 期间NAOS 处理组果皮NADH含量低于对照组,且在贮藏6 d和12 d达到极显著差异(p<0.01)水平,在贮藏9 d 时达到显著差异(p<0.05)水平。
由图8-D可知,对照组果实果皮NADPH含量在贮藏0~3 d下降,3~15 d整体呈上升趋势,15~18 d快速降低。NAOS处理组果皮NADPH含量在贮藏0~6 d 快速上升,6~9 d 降低,在9~12 d 迅速升高,随后维持在较高水平。在贮藏3~12 d 和18 d 时,NAOS处理组果皮NADPH含量极显著(p<0.01)高于对照组,在15 d时显著(p<0.05)高于对照组。
2.9 果皮呼吸末端氧化酶活性
作为呼吸末端氧化酶,CCO 在电子传递链(ETC)细胞色素途径中起着关键作用,其活性可反映ETC 运转水平[10]。由图9-A 可知,两组果实果皮CCO 活性均在贮藏0~3 d 快速上升,在3 d 达到峰值(对照组为217.53 U·mg-1,NAOS 处理组为153.63 U·mg-1),随后不断降低。在整个贮藏期内NAOS处理组果实果皮CCO活性低于对照组,并在3 d 和9~15 d 达到极显著(p<0.01)差异水平,18 d时达到显著(p<0.05)差异水平。
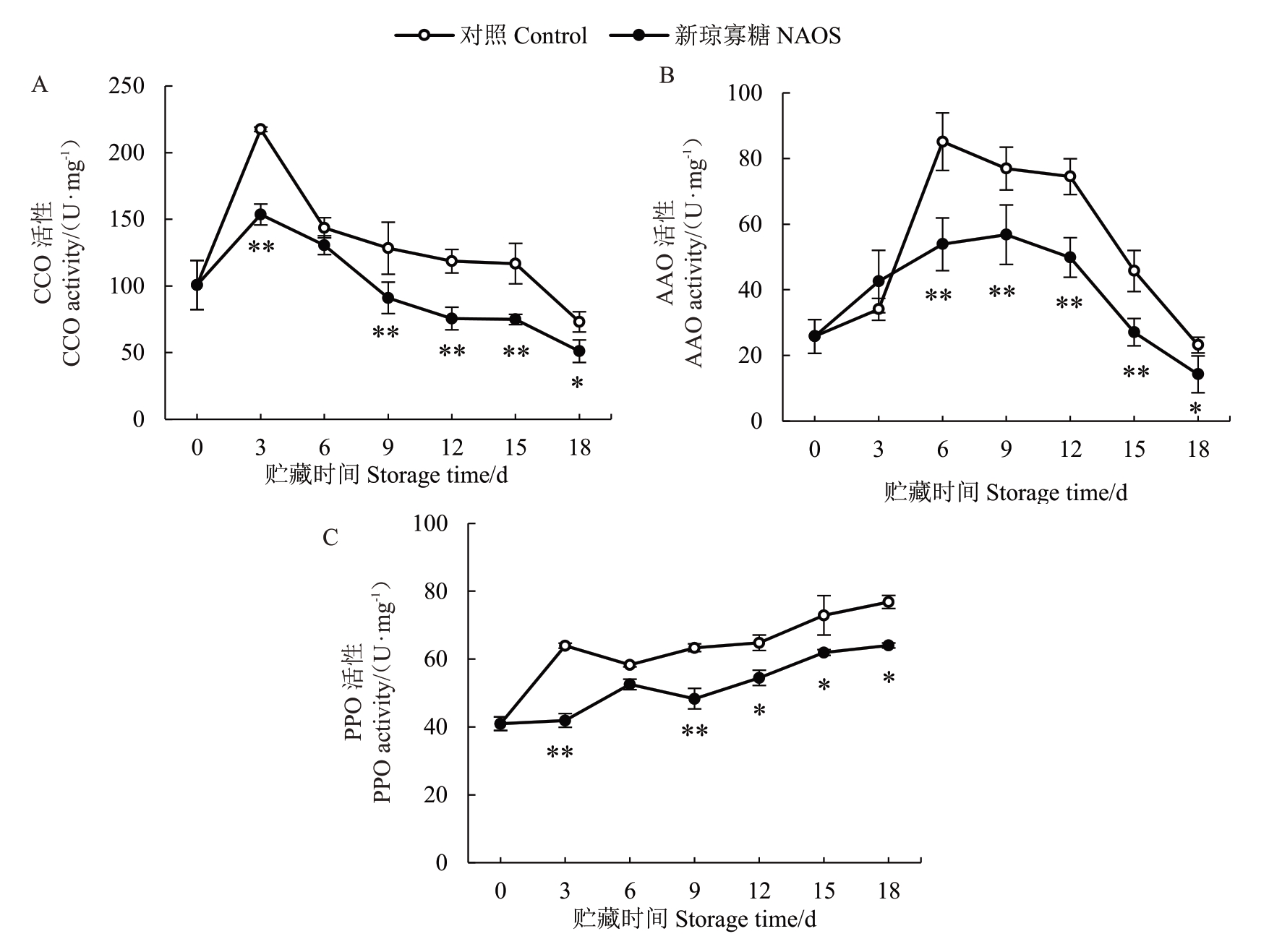
图9 NAOS 处理对西番莲果实果皮CCO(A)、AAO(B)和PPO(C)活性的影响
Fig.9 Effects of NAOS treatment on the activities of CCO(A),AAO(B)and PPO(C)in pericarp of passion fruit
如图9-B所示,对照组果皮AAO活性在贮藏0~6 d迅速上升,6~18 d快速下降。NAOS处理组果皮AAO 活性在贮藏0~9 d 逐渐升高,9~18 d 快速下降。在贮藏6~18 d 期间NAOS 处理组果皮AAO 活性均低于对照组,且在贮藏6~15 d 差异为极显著(p<0.01),18 d时差异为显著(p<0.05)。
如图9-C 所示,对照组和NAOS 处理组果实果皮PPO 活性在贮藏期间均呈现整体上升的趋势。在相同贮藏时间内NAOS处理组果皮PPO活性均低于对照组,并在贮藏3 d和9 d达到极显著(p<0.01)差异水平,在12~18 d达到显著(p<0.05)差异水平。
3 讨 论
呼吸作用是跃变型果实采后成熟衰老的标志之一,也是果实组织获取能量用于维持各种生理代谢的主要方式[24]。然而,呼吸代谢伴随着能量消耗和营养物质降解,高呼吸强度可促进果实成熟和衰老,加速果实品质变化[10,25]。笔者在本研究中发现,与对照组相比,NAOS 处理可延缓西番莲果实呼吸高峰,同时降低其峰值。NAOS 处理组果实还保持了较高的果实硬度、果皮水分含量、果肉TSS 和TA 含量,较低的失重率和腐烂率。笔者的试验结果证明,NAOS 处理可维持西番莲果实的采后品质、减缓果实失重和腐烂等衰老现象发生。推测原因是,NAOS 处理降低西番莲果实呼吸水平,一方面可能延缓果实后熟衰老,减少因组织结构、成分变化和代谢活动引起的水分流失,从而保持了果皮水分含量,维持果实硬度,减少皱缩;另一方面可能减少糖和有机酸等底物消耗,从而维持果肉TSS和TA等呈味物质的水平。类似地,Liu 等[10]研究表明,酸性电解水处理减缓龙眼果肉自溶发生、稳定果实品质与其保持较低的呼吸强度有关。Lin等[22]研究发现,壳聚糖处理可降低龙眼果实呼吸强度,保持较高的果肉营养物质含量,进而维持其品质。
EMP、TCA 循环与PPP 是主要的呼吸代谢途径,与果实采后品质有关[10-12]。其中,EMP是最基本的呼吸途径,它将葡萄糖氧化为丙酮酸,随后进入TCA循环,而TCA循环与能量供给有关。PPP不仅是植物中NADPH 的主要来源,也为许多合成反应提供中间产物,也与植物应对外界胁迫有关[26]。呼吸代谢途径关键酶可调节上述呼吸代谢途径及呼吸强度,进而影响果实采后品质[13,27-28]。在EMP 中,PGI 可将葡萄糖-6-磷酸转化成果糖-6-磷酸,PGI 活性高低可评价EMP 水平高低[29]。SDH 是TCA 循环的关键酶,可将琥珀酸脱氢转化成延胡索酸[9,30]。另外,G-6-PDH和6-PGDH是PPP的关键酶,其酶活性可评价PPP 水平[9-10]。此外,NAD(H)、NADP(H)等吡啶核苷酸也能调节呼吸代谢[9,13]。NAD在NADK的作用下被磷酸化成NADP[13]。NAD(H)含量与EMP、TCA循环水平有关,而NADP(H)含量将影响PPP 水平[13]。本研究发现,与对照组对比,NAOS 处理组具有延后的且较低的呼吸高峰及较低的腐烂率,同时也保持较低的PGI、SDH活性和NAD(H)含量,较高的G-6-PDH+6-PGDH、NADK活性和NADP(H)含量。因此,NAOS 处理降低西番莲果实的PGI、SDH活性而提升G-6-PDH+6-PGDH总活性,也提高果实的NADK 活性、NADP(H)含量而降低NAD(H)含量,提高了PPP 水平而削弱了EMP 与TCA循环水平,从而延缓果实呼吸高峰而减轻其采后品质变化,进而减缓腐烂发生。类似地,Lin 等[13]研究发现,棓酸丙酯处理减缓龙眼果肉发生自溶与其保持较高水平的PPP、较低水平的EMP和TCA循环有关。Li 等[29]研究表明,海藻糖处理提高苹果的G6PDH 活性而降低PGI、SDH 活性,进而提高PPP水平而降低EMP、TCA 循环水平,从而保持果实品质。
CCO、AAO 及PPO 是重要的呼吸末端氧化酶[12-13],它们影响着ETC,间接反映电子传递效率和呼吸代谢水平[10,31]。笔者在本研究中发现,与对照组相比,NAOS 处理组在贮藏0~18 d 保持较低CCO和PPO 活性,在贮藏6~18 d 保持较低AAO 活性。同时,NAOS 处理组具有较低的呼吸高峰与腐烂率。因此推测,NAOS 处理通过降低呼吸末端氧化酶水平而削弱电子传递效率及呼吸代谢,推移呼吸高峰,有利于减少能量与底物的消耗,进而稳定果实品质而延缓衰老发生。类似地,Lin 等[27]研究发现,较低活性的AAO 和CCO 是减缓龙眼发生果皮褐变的关键因素。Song等[32]研究报道,一氧化氮处理延缓桃果实冷害发生与其具有较低的CCO 活性有关。
4 结 论
NAOS处理降低了西番莲果实的PGI和SDH活性、NAD(H)含量,提高了NADK和G-6-PDH+6-PGDH 活性、NADP(H)含量,从而保持较低的EMP 和TCA 循环水平,并且减缓电子传递,提高PPP 水平,从而减少能量及营养物质的消耗。NAOS处理可调节西番莲果实采后呼吸代谢途径,抑制呼吸高峰,延缓后熟和衰老,从而减少水分散失和物质消耗,维持果实质量、质地、呈味物质和外观等品质特征,减缓果实衰老。本研究表明,NAOS 具有调控采后西番莲果实生理、延缓其品质败坏的作用,这为海藻寡糖在果蔬采后保鲜和生理调控方面的应用提供了理论依据。
[1] 林育钊,陈蕾伊,陈佳怡,蒋璇靓,郑金水,陈洪彬.ε-聚赖氨酸对西番莲果实采后病害与抗病物质代谢的影响[J].食品科学,2024,45(3):142-149.LIN Yuzhao,CHEN Leiyi,CHEN Jiayi,JIANG Xuanjing,ZHENG Jinshui,CHEN Hongbin. Effect of ε-poly-L-lysine on postharvest diseases and disease-resistant substance metabolism in passion fruits[J].Food Science,2024,45(3):142-149.
[2] 林育钊,陈蕾伊,陈洪彬,郑金水,蒋璇靓,杨菁美.4 种包装材料对西番莲果实贮藏效果的影响[J]. 食品与机械,2024,40(3):134-140.LIN Yuzhao,CHEN Leiyi,CHEN Hongbin,ZHENG Jinshui,JIANG Xuanjing,YANG Jingmei.Effects of four packaging materials on preservation of passion flower (Passiflora caerulea L.)fruit[J].Food&Machinery,2024,40(3):134-140.
[3] 郭欣,林育钊,曾玲珍,林静颖,余星星,林河通.不同浓度壳聚糖处理对采后西番莲果实耐贮性和贮藏品质的影响[J].热带作物学报,2020,41(8):1665-1673.GUO Xin,LIN Yuzhao,ZENG Lingzhen,LIN Jingying,YU Xingxing,LIN Hetong.Effects of different concentrations of chitosan treatment on storability and storage quality of passion fruit postharvest[J]. Chinese Journal of Tropical Crops,2020,41(8):1665-1673.
[4] 陈果,林育钊,郭欣,林河通.西番莲采后品质劣变及贮藏保鲜技术研究进展[J].亚热带植物科学,2020,49(4):323-328.CHEN Guo,LIN Yuzhao,GUO Xin,LIN Hetong. Research advances in quality deterioration and storage technologies of harvested passion fruit[J]. Subtropical Plant Science,2020,49(4):323-328.
[5] YOU M,DUAN X Y,LI X,LUO L J,ZHAO Y,PAN H H,GONG W L,YANG L R,XIANG Z,LI G F.Effect of 1-methylcyclopropene combined with chitosan-coated film on storage quality of passion fruit[J].Sustainable Chemistry and Pharmacy,2022,27:100679.
[6] WANG H L,CHEN H B,LIN Y,LI M L,LIU Q Q,LIN Y Z,JIANG X J,CHEN Y H. Insights into the isolation,identification,and biological characterization analysis of and novel control strategies for Diaporthe passiflorae in postharvest passion fruit[J].Journal of Fungi,2023,9(10):1034.
[7] DO CARMO SANTOS J T,PETRY F C,DE CASTRO TOBARUELA E,MERCADANTE A Z,GLORIA M B A,COSTA A M,LAJOLO F M,HASSIMOTTO N M A.Brazilian native passion fruit (Passiflora tenuifila Killip) is a rich source of proanthocyanidins,carotenoids,and dietary fiber[J]. Food Research International,2021,147:110521.
[8] ZHANG S,LIN H T,LIN Y F,LIN Y X,HUNG Y C,CHEN Y H,WANG H,SHI J. Energy status regulates disease development and respiratory metabolism of Lasiodiplodia theobromae(Pat.)Griff.&Maubl.-infected longan fruit[J].Food Chemistry,2017,231:238-246.
[9] LIN Y X,LIN H T,CHEN Y H,WANG H,LIN M S,RITENOUR M A,LIN Y F.The role of ROS-induced change of respiratory metabolism in pulp breakdown development of longan fruit during storage[J].Food Chemistry,2020,305:125439.
[10] LIU Q Q,XIE H L,CHEN Y H,LIN M S,HUNG Y C,WANG H,FAN Z Q,LIN Y F,LIN H T.Acidic electrolyzed oxidizing water delayed the breakdown occurrence in pulp of fresh longan by regulating the metabolisms of respiratory and energy[J].Postharvest Biology and Technology,2023,205:112531.
[11] LIN L J,LIN H T,CHEN Y,ZHANG H L,WANG X Q,LIN M S,CHEN Y H,WANG H,FAN Z Q,LIN Y F. Respiratory and energy metabolisms participate in the disease occurrence of fresh Chinese olive caused by Pestalotiopsis microspora[J].Postharvest Biology and Technology,2023,205:112514.
[12] SUN B L,KUANG X Y,LIN H T,LIN M S,CHEN Y Z,ZENG L Z,LIN Y F,CHEN Y H,WANG H,FAN Z Q. The role of respiratory metabolism in chilling injury development of Chinese olive fruit during cold storage[J]. Postharvest Biology and Technology,2023,205:112489.
[13] LIN Y X,LIN Y F,LIN M S,CHEN L,LI H,LIN H T. Propyl gallate postharvest treatment improves the storability of longans by regulating the metabolisms of respiratory and disease-resistance substances[J].Postharvest Biology and Technology,2023,206:112556.
[14] 余劲聪.海藻寡糖在农业领域的应用研究进展[J].南方农业学报,2016,47(6):921-927.YU Jincong. Research progress in application of seaweed oligosaccharides in agriculture[J]. Journal of Southern Agriculture,2016,47(6):921-927.
[15] LI L,JIANG J J,YAO Z,ZHU B W.Recent advances in the production,properties and applications of alginate oligosaccharides- a mini review[J]. World Journal of Microbiology & Biotechnology,2023,39(8):207.
[16] 万艳玲,温超,龚林锋,曾翰庭,王成鹏.新琼寡糖对多头切花月季‘爱丽丝’保鲜效果的影响[J]. 安徽农业科学,2023,51(17):154-157.WAN Yanling,WEN Chao,GONG Linfeng,ZENG Hanting,WANG Chengpeng. Effects of Neoagaro-oligosaccharides on preservation of spray cut rose‘Alice’[J].Journal of Anhui Agricultural Sciences,2023,51(17):154-157.
[17] BOSE S K,HOWLADER P,JIA X C,WANG W X,YIN H.Alginate oligosaccharide postharvest treatment preserve fruit quality and increase storage life via abscisic acid signaling in strawberry[J].Food Chemistry,2019,283:665-674.
[18] 蓝炎阳,高剑龙,曾润颖,王少峰.海藻寡糖对琯溪蜜柚保鲜品质的影响[J].福建农林大学学报(自然科学版),2013,42(5):480-484.LAN Yanyang,GAO Jianlong,ZENG Runying,WANG Shaofeng. Effects of algae- oligosaccharides treatment on storage quality of Guanxi honey pomelo fruit[J]. Journal of Fujian Agriculture and Forestry University(Natural Science Edition),2013,42(5):480-484.
[19] HOU Y P,GAO J L,GU L,WANG S F,ZENG R Y. Effects of agaro-oligosaccharide treatment on postharvest quality of cherry tomatoes during cold storage[J]. Journal of Food Processing and Preservation,2015,39(6):949-955.
[20] LI M L,LIN Q,CHEN Y Z,CHEN Y H,LIN M S,HUNG Y C,LIN H T. Acidic electrolyzed water treatment suppresses Phomopsis longanae Chi-induced the decreased storability and quality properties of fresh longans through modulating energy metabolism[J].Food Chemistry,2023,404:134572.
[21] HAO Y Q,CHEN F H,WU G B,GAO W Y.Impact of postharvest nitric oxide treatment on lignin biosynthesis-related genes in wax apple (Syzygium samarangense) fruit[J]. Journal of Agricultural and Food Chemistry,2016,64(45):8483-8490.
[22] LIN Y Z,LI N,LIN H T,LIN M S,CHEN Y H,WANG H,RITENOUR M A,LIN Y F.Effects of chitosan treatment on the storability and quality properties of longan fruit during storage[J].Food Chemistry,2020,306:125627.
[23] 罗振宇,陈晓婷,李雨函,汪莹,苏金强,杨静,兰嘉滢,倪辉,张珅,林河通.采后黄金百香果失水规律及其与品质劣变的关系[J].食品工业科技,2023,44(1):369-377.LUO Zhenyu,CHEN Xiaoting,LI Yuhan,WANG Ying,SU Jinqiang,YANG Jing,LAN Jiaying,NI Hui,ZHANG Shen,LIN Hetong. Postharvest water loss pattern of golden passion fruit and its relationship with quality deterioration[J]. Science and Technology of Food Industry,2023,44(1):369-377.
[24] HU W Y,ZHANG X Y,GODANA E A,GU X Y,ZHAO L N,ZHANG H Y. Yarrowia lipolytica reduces the disease incidence of Asparagus infected by Fusarium proliferatum by affecting respiratory metabolism and energy status[J]. Biological Control,2021,159:104625.
[25] TAN X L,FAN Z Q,ZENG Z X,SHAN W,KUANG J F,LU W J,SU X G,TAO N G,LAKSHMANAN P,CHEN J Y,ZHAO Y T.Exogenous melatonin maintains leaf quality of postharvest Chinese flowering cabbage by modulating respiratory metabolism and energy status[J]. Postharvest Biology and Technology,2021,177:111524.
[26] TAO S K,ZHU Y,PAN Y G,ZHANG Z K,HUANG L J. Enhancement of respiratory metabolism of the pentose phosphate pathway (PPP) strengthens the chilling tolerance of postharvest Papaya fruit stored at 1 ℃[J].Postharvest Biology and Technology,2022,191:111988.
[27] LIN Y F,CHEN Y Z,ZHENG Y,ZHANG H L,LIN M S,WANG H,FAN Z Q,CHEN Y H,LIN H T.Energy and respiratory metabolism participate in dicyclohexylcarbodiimide and disodium succinate-mediated the alteration of energy status modulating pericarp browning of fresh longan[J].Postharvest Biology and Technology,2024,213:112959.
[28] CHEN Y H,SUN J Z,LIN H T,LIN M S,LIN Y F,WANG H,HUNG Y C. Salicylic acid reduces the incidence of Phomopsis longanae Chi infection in harvested longan fruit by affecting the energy status and respiratory metabolism[J]. Postharvest Biology and Technology,2020,160:111035.
[29] LI C Y,SUN L,ZHU J,CHENG Y,HUANG R,FAN Y T,GUO M,GE Y H. Trehalose maintains the quality of Malus domestica by mediating sucrose and respiratory metabolism[J].Scientia Horticulturae,2022,295:110857.
[30] CHEN C Y,CAI N,WAN C P,KAI W B,CHEN J Y.Carvacrol delays Phomopsis stem-end rot development in pummelo fruit in relation to maintaining energy status and antioxidant system[J].Food Chemistry,2022,372:131239.
[31] WANG D,MA Q,LI D,LI W X,LI L,AALIM H,LUO Z S.Moderation of respiratory cascades and energy metabolism of fresh-cut pear fruit in response to high CO2 controlled atmosphere[J]. Postharvest Biology and Technology,2021,172:111379.
[32] SONG C C,ZHAO Y Y,LI A,QI S N,LIN Q,DUAN Y Q.Postharvest nitric oxide treatment induced the alternative oxidase pathway to enhance antioxidant capacity and chilling tolerance in peach fruit[J].Plant Physiology and Biochemistry,2021,167:113-122.