梨黑斑病(Pear black spot)是由链格孢(Alternaria spp.)侵染梨叶片、果实和新梢等组织引起的重要病害之一[1],该病害在东南亚地区均有发生,严重影响梨的产量和品质,给梨产业造成了极大的经济损失[2-4]。研究报道,梨黑斑病组织中可分离出9种Alternaria spp.[5-6],Alternaria gaisen 和Alternaria alternata(Fr.)Keissler(简称A. alternata)被认为是梨黑斑病的主要病原菌,而引起河北省鸭梨黑斑病的主要病原菌为A.alternata[2]。
通常A. alternata 以分生孢子和菌丝体在寄主病残体上越冬,翌年春季产生的分生孢子借风雨传播至梨的不同组织上,遇合适的温、湿度萌发芽管,芽管顶端膨大形成附着胞,下方生长侵入丝,侵入气孔、皮孔或伤口,吸取梨组织中的养料和水分,完成初侵染,然后以发病植株为中心引起再侵染。树体密闭、树势衰弱、地势低洼、偏施氮肥、土壤贫瘠、害虫猖獗等不利因素均可加重梨黑斑病的发生和流行[4]。
当前,针对梨黑斑病的防治仍以化学药剂为主,但化学药剂种类的盲目选择和过量使用加速了A.alternata 抗性的产生[7],造成了环境污染,影响了人体健康[8-9]。果园生草技术可改善果园小气候,改良果园土壤,增强树势,恶化病原菌的生长环境,降低病虫害的发生,目前已广泛应用于生产中。梨园生草技术应用中,园内留存的植物种类繁杂多样,而A.alternata 具有广泛的寄主植物[10],留存于梨园中的植物种类中或存在A. alternata 的良好寄主。理论上,寄主植物可助力A. alternata 的增殖,而来自寄主植物的A. alternata 亦将成为梨黑斑病传播和流行的一个潜在侵染源头。目前,关于梨园中存在的A.alternata 寄主植物种类的研究尚无报道,而显症寄主植物中的A. alternata 感染梨叶片显症的研究亦罕见报道。
笔者拟利用保存的A. alternata 室内接种健康的梨叶片以活化A.alternata,对分离自显症梨叶片中的A.alternata 进行鉴定和检测,选择梨园中常见的植物种类为接种对象进行A. alternata 的室内接种试验,分析和筛选A. alternata 的寄主植物种类,然后将分离自显症寄主植物中的A.alternata再接种健康的梨叶片进行致病性验证,研究结果为后期深入了解梨园中黑斑病的发生、传播和流行提供理论支撑。
1 材料和方法
1.1 材料
供试梨品种为鸭梨(Pyrus bretschneideri)。
供试筛选的寄主植物种类:河北省梨园中常见的25 种植物(白萝卜、打碗花、海棠、红苋菜、狗尾草、白茅草、龙葵、葎草、花生、马唐草、苹果、牛筋草、铁笕菜、樱桃、小飞蓬、圆叶牵牛、月季、枣、裂叶牵牛、葡萄、苘麻、苦荬菜、甜椒、猪秧秧、萝藦),详见表1。
表1 供试植物种类
Table 1 Plant species in the experiment

序号No.1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25名称Name白萝卜White radish打碗花Japanese bindweed海棠Chinese flowering crab apple红苋菜Red Amaranth狗尾草Green bristlegrass白茅草Cogongrass龙葵Black nightshade葎草Japan hop花生Peanut马唐草Crabgrass苹果Apple牛筋草Goosegrass铁苋菜Three-seeded copperleaf樱桃Cherry小飞蓬Canadian fleabane圆叶牵牛Common morning glory月季Chinese rose枣Chinese jujube裂叶牵牛Ivyleaf morning glory葡萄Grape苘麻Piemarker苦荬菜Herb of denticulate Ixeis甜椒Bell pepper猪秧秧False cleavers萝藦Rough potato拉丁名Latin name Raphanus sativus(L.)Calystegia hederacea Wall.Malus spectabilis Amaranthus mangostanus(L.)Setaria viridis(L.)Beauv.Imperata cylindrica(L.)Beauv.Solanum nigrum(L.)Humulus scandens(Lour.)Merr.Arachis hypogaea(L.)Digitaria sanguinalis(L.)Scop.Malus pumila Mill.Eleusine indica(L.)Gaertn.Acalypha australis(L.)Prunus pseudocerasus(Lindl.)G.Don Conyza canadensis(L.)Cronq.Pharbitis purpurea(L.)Voigt Rosa chinensis Jacq.Ziziphus jujuba Mill.Pharbitis nil(L.)Choisy Vitis vinifera(L.)Abutilon theophrasti Medicus Ixeris polycephala Cass Capsicum annuum var.grossum Sendt.Galium spurium(L.)Metaplexis japonica(Thunb.)Makino接种方法Method of inoculation活体接种In-vivo inoculation活体接种In-vivo inoculation离体接种Vitro inoculation活体接种In-vivo inoculation活体接种In-vivo inoculation活体接种In-vivo inoculation活体接种In-vivo inoculation活体接种In-vivo inoculation离体接种Vitro inoculation活体接种In-vivo inoculation离体接种Vitro inoculation活体接种In-vivo inoculation活体接种In-vivo inoculation离体接种Vitro inoculation活体接种In-vivo inoculation离体接种Vitro inoculation离体接种Vitro inoculation离体接种Vitro inoculation离体接种Vitro inoculation离体接种Vitro inoculation活体接种In-vivo inoculation活体接种In-vivo inoculation活体接种In-vivo inoculation活体接种In-vivo inoculation离体接种Vitro inoculation
供试A. alternata 菌株:Alternaria alternata(Fr.)Keissler-SGS,保存于河北省农林科学院石家庄果树研究所。A.alternata菌株鉴定与检测的引物由生工生物工程(上海)股份有限公司合成,序列见表2。PDA琼脂培养基制备方法:去皮马铃薯200 g切成小块,加水煮烂(煮沸20~30 min),八层纱布过滤,收集滤液并加入15~20 g 琼脂粉,继续加热搅拌至琼脂粉完全溶解,加入葡萄糖20 g 搅拌均匀至溶解,稍冷却后补足水(40 ℃)至1000 mL,121 ℃,20 min灭菌。
表2 试验用PCR 引物
Table 2 The primers used in PCR
基因名称Gene name ITS基因长度Length of fragment/bp 570 HB引物名称Primer name ITS1-F ITS4-R HB-F HB-R引物序列(5'-3')Primer sequences(5'-3')TCCGTAGGTGAACCTGCGG TCCTCCGCTTATTGATATGC TCACCCTTGTCTTTTGCGTA ACCTTTGCTGATAGAGAGTG 398
1.2 方法
1.2.1 A.alternata孢悬液的制备 使用无菌解剖刀从保存于4 ℃的PDA 琼脂培养基边缘切取长有A.alternata 白色菌丝的琼脂块(5 mm×5 mm),接种于新的PDA琼脂培养基上,在(26±1)℃下孵育4 d,活化A.alternata。吸取2 mL 无菌水冲洗PDA 琼脂培养基表面的A.alternata,用无菌涂布器刮取生长于PDA 琼脂培养基上的A. alternata,用八层纱布(灭菌烘干)过滤去除菌丝,制备用于接种的A.alternata孢子粗悬液。
1.2.2 A. alternata 的巢氏PCR 鉴定 吸取1.2.1 制备的A.alternata 孢悬液800 μL,12 000 r·min-1离心5 min,选用真菌基因组DNA 提取试剂盒D2300(北京索莱宝科技有限公司)提取A. alternata 的总DNA,使用巢式PCR鉴定A.alternata[11]。
ITS 序列PCR 扩增体系(25 μL):10 μL PCR mix,上下游引物(100 nmol·μL-1)各1 μL,2 μL 模板(上述A. alternata 孢悬液的总DNA),补足dd H2O(11 μL)至25 μL。反应程序:94 ℃预变性4 min;95 ℃变性30 s,58 ℃退火30 s,72 ℃延伸1 min,35个循环;72 ℃终延伸10 min;4 ℃保存,获得ITS 序列的PCR产物。
HB序列(巢氏基因序列)PCR扩增体系(25 μL):11 μL PCR mix,上下游引物(100 nmol·μL-1)各1 μL,0.05 μL 模板(上述ITS 基因序列的PCR 产物),补足dd H2O(12.5 μL)至25 μL。反应程序:94 ℃预变性4 min;95 ℃变性30 s,55.8 ℃退火30 s,72 ℃延伸30 s,35个循环;72 ℃终延伸10 min;4 ℃保存,获得HB序列的PCR产物。
1.2.3 A.alternata接种梨叶片和分离 用血球计数板记录A.alternata 孢悬液中的孢子数量,无菌水稀释孢悬液至106cfu·L-1。吸取10 μL稀释的孢悬液接种于健康梨叶片表面,用灭菌的棉花包裹叶柄,平铺于无菌的培养皿中,用10 μL 无菌水处理的健康梨叶片为对照,选取3 个梨叶片为3 次生物学重复,所有叶片均置于光照培养箱,设置光照∶黑暗=16 h(28 ℃)∶8 h(25 ℃),相对湿度(80±5)%,8 d后观察梨叶片症状,验证活化的A.alternata 对梨叶片是否具有致病力。
用无菌水冲洗接种A.alternata孢悬液的梨叶片和无菌水处理的对照组梨叶片,用灭菌的剪刀分别剪取接种显症梨叶片和对照梨叶片各10 mg,用植物基因组提取试剂盒DP360[天根生化科技(北京)有限公司]提取叶片中的总DNA,以A. alternata 的ITS 序列为目标基因进行巢氏PCR 反应(参照1.2.2),检测梨叶片中的A.alternata。
用无菌水冲洗接种A.alternata显症的梨叶片的正反面,再用95%的酒精冲洗无菌水冲洗过的显症梨叶片的正反面;将酒精冲洗消毒的显症梨叶片继续浸泡于0.3%的NaClO 溶液中2 min;用无菌水再次冲洗浸泡NaClO溶液的显症梨叶片的正反面,去除梨叶片表面残留的次氯酸钠溶液。在超净台上,用灭菌的剪刀剪取显症梨叶片病健交界处1 cm2的叶组织接种于PDA琼脂培养基表面,置于(26±1)℃下黑暗培养72 h,切取PDA琼脂培养基边缘的分离的白色菌丝块(5 mm×5 mm)接种于新的PDA 琼脂培养基4 d,制备孢悬液(参照1.2.1),以A.alternata的ITS 序列为目标基因进行巢氏PCR 反应,鉴定分离的微生物(参照1.2.2)。
1.2.4 A.alternata寄主植物的筛选 选择梨园中常见的25种植物(采集的植物需远离梨园10 km以上)叶片为A.alternata 接种对象,室内难以栽培的试验植物种类采用叶片离体接种,其他植物种类(室内便于种植的试验植物种类)进行活体接种(表1)。每种植物选取3片健康完整的叶片作为3次生物学重复,吸取上述1.2.3 制备的A.alternata 孢悬液10 μL接种于待测植物的叶片表面,10 μL 无菌水处理的植物叶片为对照组,所有试验的叶片均置于光照培养箱中,设置光照∶黑暗=16 h(28 ℃)∶8 h(25 ℃),相对湿度(80±5)%,14 d后观察显症情况,采用方格纸法测定叶片病斑面积并进行显著性分析。显症的植物叶片用于微生物的分离(参照1.2.3),以A. alternata 的ITS 序列进行巢氏PCR 反应,鉴定分离的微生物(参照1.2.2)。
1.2.5 分离自显症寄主植物的A. alternata 再接种梨叶片 将1.2.4中显症植物中分离并验证的A.alternata制备孢悬液,用无菌水稀释至106 cfu·L-1,10 μL接种于健康梨叶片表面的一个部位,10 μL 无菌水处理的健康梨叶片为对照,每个叶片接种3个部位,置于光照培养箱中,设置光照∶黑暗=16 h(28 ℃)∶8 h(25 ℃),相对湿度(80±5)%,10 d 观察显症,设置3 次试验重复。A.alternata 的ITS 序列为目标基因进行巢氏PCR 检测梨叶片中的A.alternata(参照1.2.2)。
1.3 数据统计与分析
利用Microsoft Excel 进行数据汇总和处理,采用SPSS 软件(IBM,Armonk,New York,USA)进行Student t检验。
2 结果与分析
2.1 A.alternata的活化
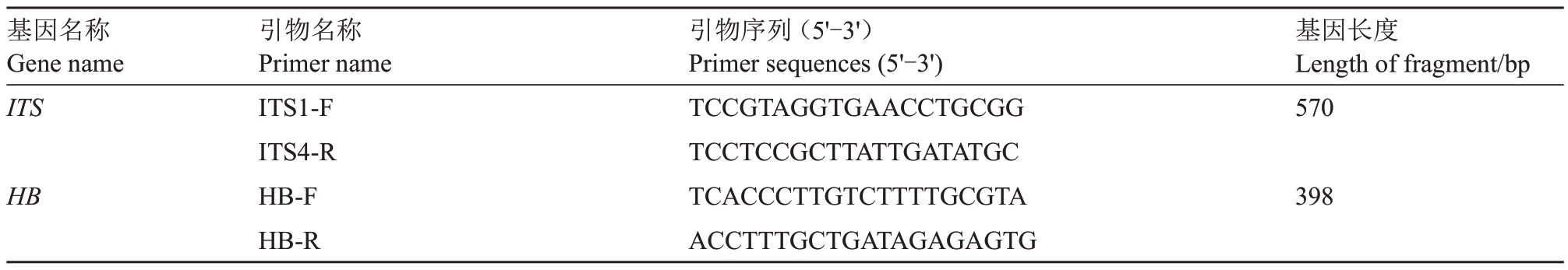
PDA 琼脂培养基活化4 d 的A.alternata 中部呈棕色,边缘有白色的菌丝(图1-A)。选用A.alternata 的ITS 为目标基因进行巢氏PCR 反应,琼脂糖凝胶电泳出现与预期大小一致的阳性亮带(570 bp 和398 bp)(图1-B)。健康梨叶片接种活化的A.alternata 孢悬液后8 d,3 个接种的健康梨叶片均能出现明显的黑色病斑,而无菌水处理的3 个健康梨叶片无明显症状(图1-C)。将分离自显症梨叶片中的微生物接种于PDA 琼脂培养基上4 d,生长的微生物菌落表型与A. alternata 相似,表现为中部呈棕色、边缘为白色的菌丝(图1-D)。利用A. alternata 的ITS 序列进行巢氏PCR 反应,琼脂糖凝胶电泳仍出现与预期大小一致的阳性亮带(570 bp 和398 bp)(图1-E)。利用A. alternata 的ITS 序列进行巢氏PCR 反应检测3 个显症的梨叶片,琼脂糖凝胶电泳再次出现与预期大小一致的阳性亮带(570 bp 和398 bp)(图1-F)。表明储存于4 ℃的A.alternata活化后接种于健康的梨叶片仍具有致病力,从显症的梨叶片中可再分离获得A.alternata。
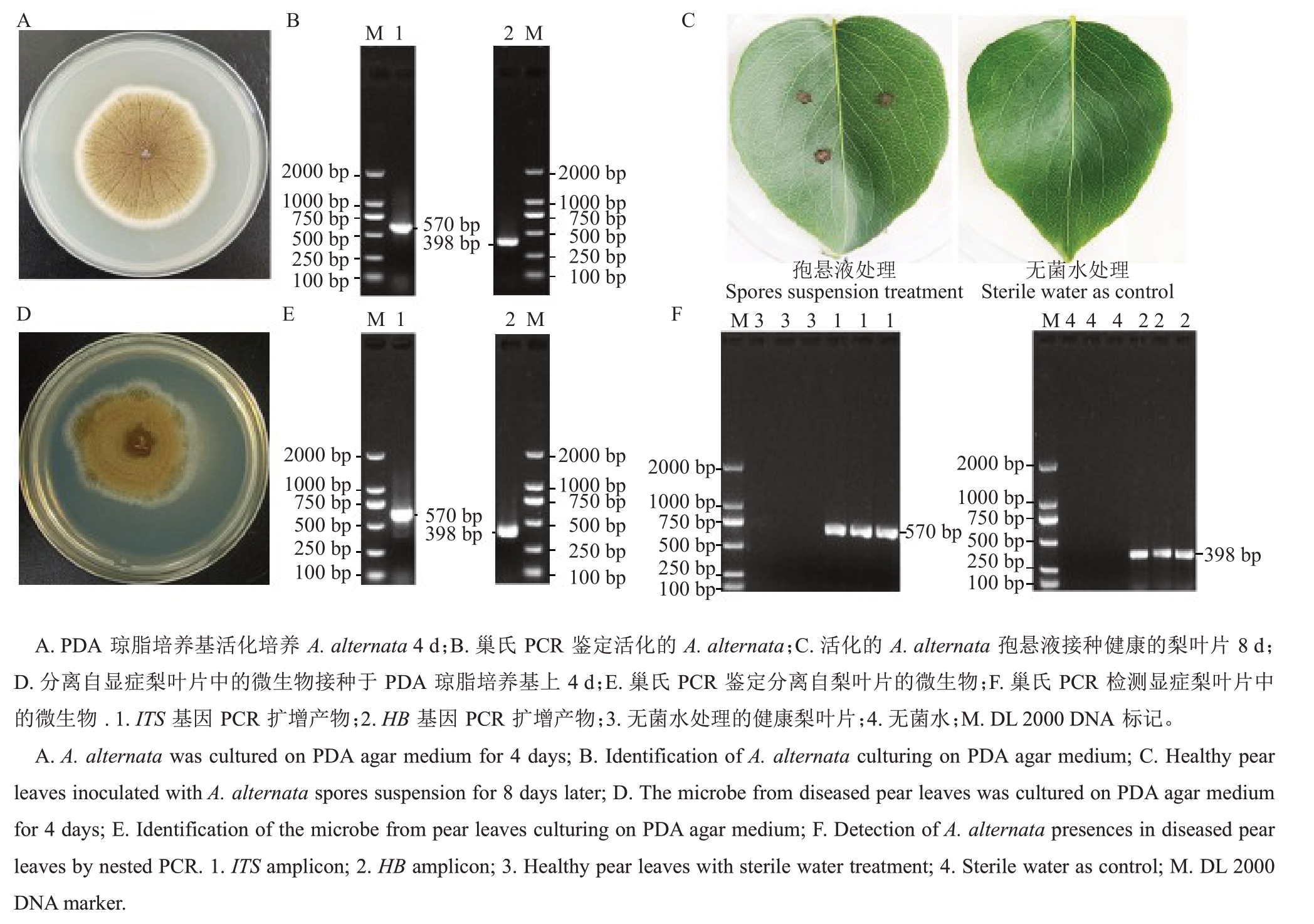
图1 A.alternata 侵染梨叶片的验证
Fig.1 Validation experiment of A.alternata pathogenicity on pear leaves
2.2 A.alternata寄主植物的分析与筛选
将上述分离自显症梨叶片中的A. alternata 制备孢悬液,分别接种于25 种植物叶片14 d,测定25种不同植物叶片的病斑面积,并进行显著性分析,结果表明,6种植物(苹果、海棠、樱桃、月季、花生、枣)叶片上的病斑面积显著大于其无菌水处理的对照叶片(p<0.01),而接种A. alternata 的其他19 种植物(白萝卜、打碗花、红苋菜、狗尾草、白茅草、龙葵、葎草、马唐草、牛筋草、铁苋菜、小飞蓬、圆叶牵牛、裂叶牵牛、葡萄、苘麻、苦荬菜、甜椒、猪秧秧、萝藦)和其无菌水处理的叶片病斑面积差异不显著(图2-A),与观察到的症状相同(图2-B)。表明苹果、海棠、樱桃、月季、花生、枣是A.alternata的寄主植物。
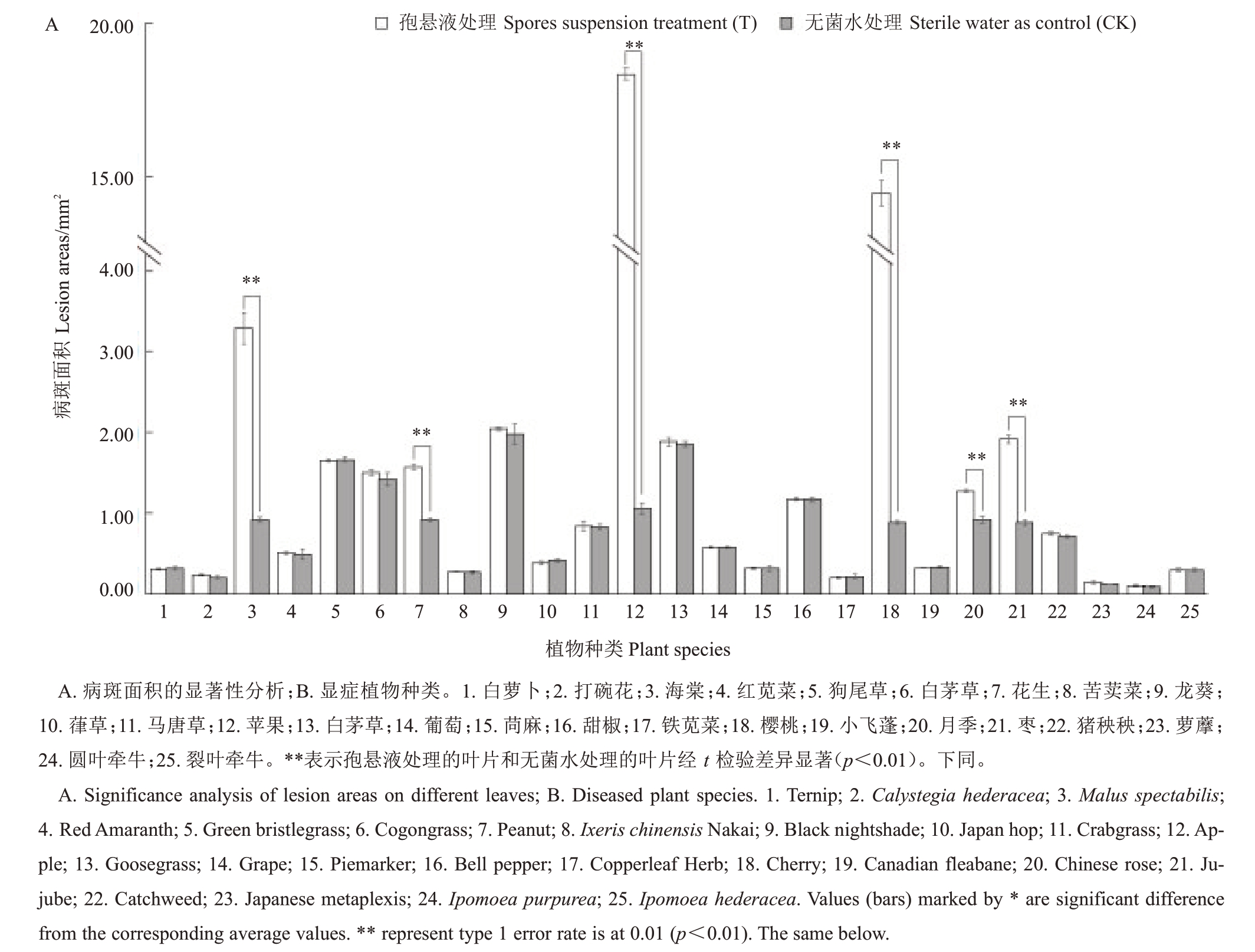
图2 A.alternata 寄主植物分析
Fig.2 Analysis on plant hosts of A.alternata
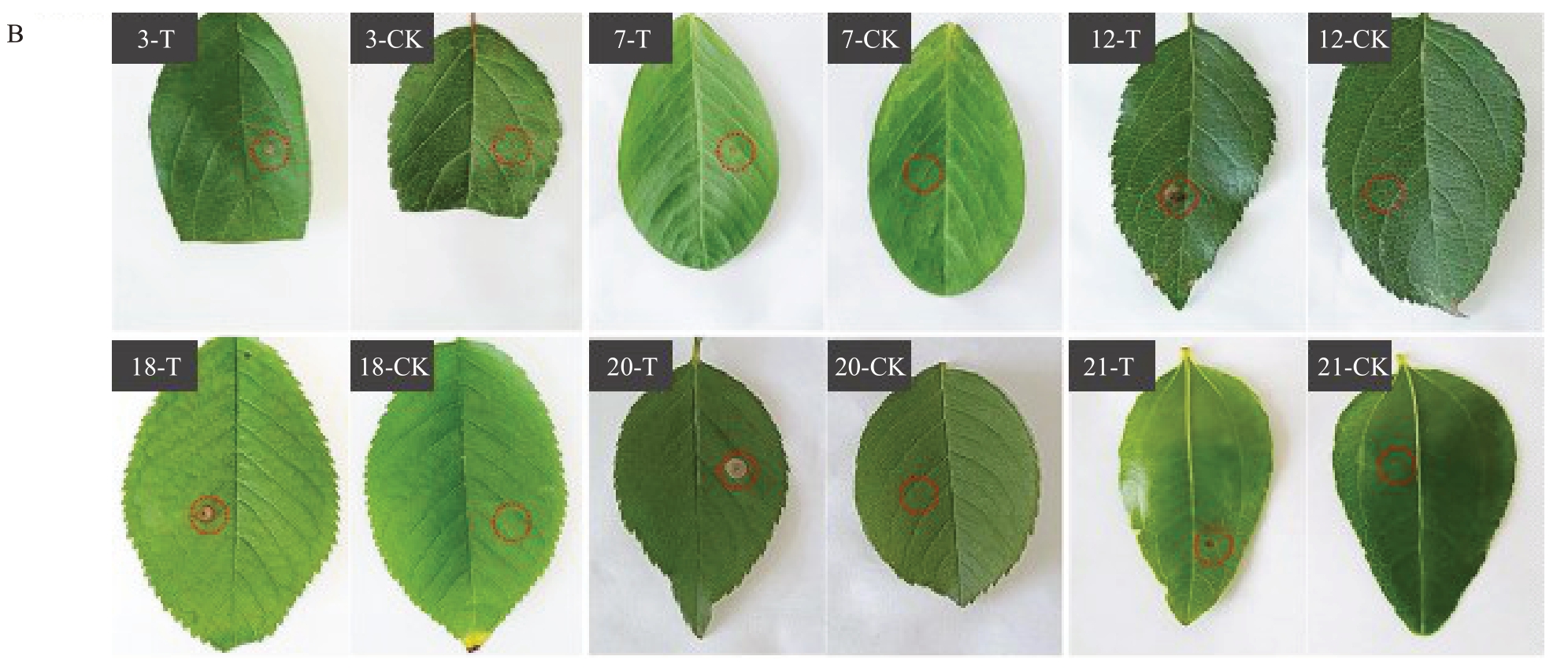
图2 (续) Fig.2 (Continued)
2.3 显症寄主植物中A.alternata的再分离
将显症的6 种寄主植物(苹果、海棠、樱桃、月季、花生、枣)叶片中分离的6 个微生物在PDA 琼脂培养基上培养4 d,结果显示,6个微生物菌落的表型与A.alternata 相同,表现为中部呈棕色、边缘为白色的菌丝(图3),表明在感染A.alternata 显症的寄主植物叶片中,再分离获得的微生物或为A. alternata。

图3 显症寄主植物中微生物的分离
Fig.3 Microbe isolation from diseased plant hosts respectively
2.4 自寄主植物分离的A.alternata 对梨叶片的致病性
分离自上述6 种显症寄主植物叶片中的6 个微生物菌株按A. alternata 孢悬液制备方法制备孢子悬浮液,分别接种于健康的梨叶片10 d,结果显示,自显症寄主植物中分离获得的微生物菌株可再感染梨叶片,而无菌水处理的健康梨叶片症状不明显(图4-A)。以A.alternata 的ITS 基因序列进行巢氏PCR 检测显症的梨叶片,琼脂糖凝胶电泳出现与预期大小一致的阳性亮带(570 bp 和398 bp),而无菌水处理的梨叶片无阳性条带(图4-B 和图4-C),表明分离自6 种寄主植物中的6 株微生物均为A. alternata,这些A. alternata 均可感染梨叶片引发症状。

图4 分离自寄主植物中的A.alternata 对梨叶片致病性
Fig.4 Pathogenicity of A.alternata isolation from plant host on pear leaves
3 讨 论
A.alternate 以分生孢子和菌丝体在梨病叶、病果、病残枝中越冬,翌年春季产生的分生孢子借风雨传播至梨的不同组织中完成初侵染,然后以发病植株为中心引起再侵染。目前,梨黑斑病以化学防治为主,而化学药剂长期和广泛使用加速了病原菌的抗药性。梨黑斑病防控常用的化学药剂为14α-脱甲基反应抑制剂(14α-demethylation inhibitors,DMIs),如苯醚甲环唑、烯唑醇等羊毛甾醇,但DMIs杀菌剂作用位点具有专一性,多种植物病原菌均有对DMIs杀菌剂产生田间抗药性的报道[7]。此外,化学药剂使用不当会造成环境污染并危害人体健康[8-9]。为减轻梨园病虫害发生,以生态调控为目标的果园种草技术广泛应用于生产中。在果园种草技术应用中植物种类的留存较为盲目,但A.alternate寄主广泛[10],可侵染海南番木瓜[12]、杧果[13]、深州蜜桃[14]、哈密瓜[15]等植物,而关于梨园中存在A.alternate 寄主植物的研究罕有报道。
在实际梨园环境中,验证感染A.alternate 显症的寄主植物是梨黑斑病发生潜在侵染源头的田间试验较难开展。笔者忽略了风雨传播A.alternate的因素,选用梨园中常见的植物为接种对象,利用室内接种的方法分析接种A.alternate 显症的植物种类,验证了显症寄主植物中的A.alternate对梨叶片的致病能力。通常室内接种A.alternate的方法有菌丝块接贴法[16]和孢子悬浮液喷雾接种法[9]。菌丝块贴接法需固定菌丝块的位置,而孢子悬浮液喷雾接种法对喷雾机要求较高,需保证液滴在接种部位均匀展布。为便于定点观察显症,笔者采用制备的A.alternate孢悬液定点接种植物。试验在分离自显症寄主中的A. alternate 再接种梨叶片验证致病性的过程中,若选用的接种对象为梨老叶片,接种后孵育时间较长(如23 d),且会出现黄化及干枯,影响后期症状的观察,故而宜选用幼嫩的梨叶为接种对象以优化实验方案。
关于A. alternata 的鉴定,分子生物学方法(RAPD[17]、AFLP[18]、SSH[19]、SCAR[20])具有快速、便捷的优势,已广泛应用于试验中,但这些鉴定方法均不能准确区分A.alternata至“种”的水平,因此传统的柯赫氏法则仍是植物病原菌鉴定的常用方法。此外,李云飞等[11]以梨黑斑病菌的ITS 序列为目标基因,设计巢氏PCR 引物,在梨组织中成功检测并鉴定出A. alternate,并把检测精准度提高到pg 水平[11]。尽管ITS 基因不是鉴别A.alternata 的最优基因,笔者在本研究中为避免干扰均采用无菌操作,且所用的A.alternate 菌株经分离和纯化后再进行ITS基因的巢氏PCR鉴定,以确保试验结果的科学性和客观性。
梨园中生态环境极其复杂,农事操作、昆虫啃食、风雨冰雹等极易造成留存的植物叶片上产生机械伤口,而机械伤口可助力病原菌侵入寄主[21-22],增加园内菌原基数,为梨黑斑病的发生、传播和流行提供了大量的侵染源头。因此,实际生产中应铲除梨园内部和周围的A.alternate 寄主植物,利用减少A.alternate 潜在侵染源头的方式降低梨黑斑病的发生概率。
4 结 论
梨园中存在黑斑病菌的寄主植物,显症寄主植物中的A. alternate 具有感染梨叶片致病的能力,A.alternate寄主植物或为梨黑斑病发生的一个侵染源头。
[1] 杨晓平,胡红菊,王友平,田瑞,陈启亮,张靖国.梨黑斑病病原菌的生物学特性及其致病性观察[J]. 华中农业大学学报,2009,28(6):680-684.YANG Xiaoping,HU Hongju,WANG Youping,TIAN Rui,CHEN Qiliang,ZHANG Jingguo. Biological characteristics and pathogenicity of pear black spot by Alternaria alternata (Fr.)Keissl[J].Journal of Huazhong Agricultural University,2009,28(6):680-684.
[2] 张志铭,宋福,孙淑贞,赵文胜.河北鸭梨黑斑病病原菌的鉴定[J].植物检疫,2003,17(4):212-214.ZHANG Zhiming,SONG Fu,SUN Shuzhen,ZHAO Wensheng.The identification of pathogen causing Ya pears black spot[J].Plant Quarantine,2003,17(4):212-214.
[3] TERAKAMI S,ADACHI Y,IKETANI H,SATO Y,SAWAMURA Y,TAKADA N,NISHITANI C,YAMAMOTO T. Genetic mapping of genes for susceptibility to black spot disease in Japanese pears[J].Genome,2007,50(8):735-741.
[4] 杨晓平,陈启亮,张靖国,范净,何秀娟,胡红菊.梨黑斑病及抗病育种研究进展[J].果树学报,2017,34(10):1340-1348.YANG Xiaoping,CHEN Qiliang,ZHANG Jingguo,FAN Jing,HE Xiujuan,HU Hongju. Research progress of pear black spot and breeding for disease resistance[J]. Journal of Fruit Science,2017,34(10):1340-1348.
[5] ROBERTS R G. Alternaria yaliinficiens sp. nov. on Ya Li pear fruit:From interception to identification[J].Plant Disease,2005,89(2):134-145.
[6] ROBERTS R G.Two new species of Alternaria from pear fruit[J].Mycotaxon,2007,100:159-167.
[7] 杨晓平.梨黑斑病菌Alternaria sp.鉴定及砂梨抗黑斑病分子机制初探[D].武汉:武汉大学,2015.YANG Xiaoping. Identification of pear black spot Alternaria sp.and study on molecular mechanism of sand pear disease resistance to pear black spot[D].Wuhan:Wuhan University,2015.
[8] KNIGHT S C,ANTHONY V M,BRADY A M,GREENLAND A J,HEANEY S P,MURRAY D C,POWELL K A,SCHULZ M A,SPINKS C A,WORTHINGTON P A,YOULE D. Rationale and perspectives on the development of fungicides[J].Annual Review of Phytopathology,1997,35:349-372.
[9] NGUYEN V N,NGUYEN D M C,SEO D J,PARK R D,JUNG W J. Antimycotic activities of Cinnamon-derived compounds against Rhizoctonia solani in vitro[J]. BioControl,2009,54(5):697-707.
[10] ROTEM J. The genus Alternaria:Biology,epidemiology and pathogenicity[M].Minnesota,USA:Amer Phytopathological Society,1994.
[11] 李云飞,陈雪娇,张爱芳,杨雪,陈雨,姚剑.砀山梨黑斑病分子检测技术研究[J].中国农学通报,2016,32(4):150-154.LI Yunfei,CHEN Xuejiao,ZHANG Aifang,YANG Xue,CHEN Yu,YAO Jian.Molecular detection of Alternaria alternata causing black spot of Dangshan pear[J]. Chinese Agricultural Science Bulletin,2016,32(4):150-154.
[12] 刘秀娟,杨业铜,黄圣明.海南省番木瓜果实潜伏侵染真菌种类及其分布状况的研究[J]. 植物病理学报,1994,24(4):313-317.LIU Xiujuan,YANG Yetong,HUANG Shengming. The species and distribution of latent fungi of papaya fruit in Hainan province[J].Acta Phytopathologica Sinica,1994,24(4):313-317.
[13] PRUSKY D.Assessment of latent infections as a basis for control of postharvest disease of mango[J]. Plant Disease,1983,67(7):816.
[14] 张志铭,王江柱,李玉琴,赵志芬,曹健美.深州蜜桃黑斑病(Alternaria alternata)的研究Ⅰ. 发生情况、症状和病原鉴定[J].河北农业大学学报,1995,18(4):49-52.ZHANG Zhiming,WANG Jiangzhu,LI Yuqin,ZHAO Zhifen,CAO Jianmei.The study on occurrence,symptoms and identification on pathogen of Shenzhou peach black spot(Alternaria alternata) Ⅰ[J]. Journal of Hebei Agricultural University,1995,18(4):49-52.
[15] 张辉,李学文,张唯一,冯宏鹰.新疆哈密瓜果实潜伏侵染真菌种类研究[J].新疆农业科学,2000,37(3):127-130.ZHANG Hui,LI Xuewen,ZHANG Weiyi,FENG Hongying.Studes on the species of latent fungi of Xingjiang Hami melon fruit[J].Xinjiang Agricultural Sciences,2000,37(3):127-130.
[16] 王文青,李扬,向均,洪霓,王国平.我国梨产区引起黑斑病的链格孢种类鉴定与致病性研究[J]. 果树学报,2020,37(12):1922-1933.WANG Wenqing,LI Yang,XIANG Jun,HONG Ni,WANG Guoping. Identification and pathogenicity of Alternaria species causing black spot in pear producing regions in China[J]. Journal of Fruit Science,2020,37(12):1922-1933.
[17] ROBERTS R G,REYMOND S T,ANDERSEN B. RAPD fragment pattern analysis and morphological segregation of smallspored Alternaria species and species groups[J]. Mycological Research,2000,104(2):151-160.
[18] SOMMA S,POSE G,PARDO A,MULÈ G,PINTO V F,MORETTI A,LOGRIECO A F.AFLP variability,toxin production,and pathogenicity of Alternaria species from Argentinean tomato fruits and puree[J]. International Journal of Food Microbiology,2011,145(2/3):414-419.
[19] ROBERTS R G,BISCHOFF J F,REYMOND S T. Differential gene expression in Alternaria gaisen exposed to dark and light[J].Mycological Progress,2012,11(2):373-382.
[20] STEWART J E,ANDREW M,BAO X D,CHILVERS M I,CARRIS L M,PEEVER T L. Development of sequence characterized amplified genomic regions (SCAR) for fungal systematics:Proof of principle using Alternaria,Ascochyta and Tilletia[J].Mycologia,2013,105(4):1077-1086.
[21] FERRANTE P,FIORILLO E,MARCELLETTI S,MAROCCHI F,MASTROLEO M,SIMEONI S,SCORTICHINI M. The importance of the main colonization and penetration sites of Pseudomonas syringae pv. actinidiae and prevailing weather conditions in the development of epidemics in yellow kiwifruit,recently observed in central Italy[J]. Journal of Plant Pathology,2012,94:455-461.
[22] WU J P,DIAO Y,GU Y C,HU Z L. Infection pathways of soft rot pathogens on Amorphophallus konjac[J]. African Journal of Microbiology Research,2010,4(14):1495-1499.
[23] 刘勇,张雅雯,南志标,段廷玉.天然草地管理措施对植物病害的影响研究进展[J].生态学报,2016,36(14):4211-4220.LIU Yong,ZHANG Yawen,NAN Zhibiao,DUAN Tingyu.Progress of research into the effects of native grassland management practices on plant disease[J]. Acta Ecologica Sinica,2016,36(14):4211-4220.