德阳柿(Diospyros deyangensis)原产于四川省德阳市,是柿科(Ebenaceae)柿属(Diospyros)多年生木本植物,属于雌雄异株落叶果树树种,又称红花野毛柿。前人通过形态学观察、分子标记、染色体倍性分析等手段发现德阳柿为四倍体,属于柿属植物新种[1-2]。木本果树植物的生命周期分为童期、成年期和衰老期,其中,童期指从种子萌发至具备正常开花能力的时期。木本植物的童期普遍较长,一般为3~8 a(年)[3],云杉甚至需要20~25 a 才能进入成年期[4]。然而,德阳柿作为栽培柿(Diospyros kaki)的近缘种,在播种数月后即可开花[5],是研究柿属植物短童期花发育机制的优良试材。研究短童期调控的分子机制对促进柿早花结实、提高育种效率具有重要的理论意义和实践价值。
通过对模式植物拟南芥的研究,发现了调控植物开花的重要整合因子SUPPRESSOR OF OVEREXPRESSION OF CO 1(SOC1)[6]。SOC1 隶属于MADS-box基因家族,它可以整合来自光周期、春化作用、自主途径等多个开花调控途径的信号,随后将信号传递给下游的花分生组织基因进而促进植物成花转化[6-7]。目前已从多种植物,如甜橙(Citrus sinensis)、银白杨(Populus trichocarpa)、苹果(Malus domestica)、欧洲葡萄(Vitis vinifera)中克隆出SOC1同源基因[8-11]。SOC1 调控开花的机制也被广泛研究[12]。前人研究发现,B3 结构域转录因子REM16通过结合SOC1和FT(FLOWERING LOCUS T)的启动子促进拟南芥开花[13];BjuWRKY71-1 通过调节SOC1 的表达促进芥菜开花[14];应激诱导型启动子rd29A 通过促进AtSOC1 的过表达可以将干旱胁迫下菊花的开花时间提前[15]。以上研究表明SOC1 作为转录因子受到其他蛋白或核酸的调控后调节植物的开花时间。此外,SOC1 也可以与其他蛋白质形成二聚体或高阶复合物靶向调控开花基因进而影响植物开花节奏[16]。SOC1 和AGL6 亚家族同源物DAL1相互作用介导松树营养生长到生殖生长的转变[4];TaSOC1 通过与MADS-box 开花调节因子TaVRT2 竞争结合TaVRN1,形成TaSOC1-TaVRN1模块,整合光周期和春化信号,调控小麦开花[17];BrSOC1b 通过与BrAGL9a、BrAGL9b、BrAGL2 和BrAGL8 蛋白相互作用,共同参与调节白菜的开花[18];拟南芥中SOC1与AGL16形成蛋白质复合物,共同作用于开花靶标基因[19]。SOC1 在调控植物开花时间的过程中起到关键的作用,然而至今还未有德阳柿SOC1基因相关的报道,SOC1基因在德阳柿短童期中的作用也尚不清楚。
笔者在本研究中基于前期获得的德阳柿基因组数据,分离DdSOC1 基因并分析其序列特征,探究DdSOC1 基因在不同组织器官中的表达模式;通过酵母双杂交(Y2H)筛库得到DdSOC1的互作蛋白并进行了Y2H、双分子荧光互补(BiFC)的互作验证;分析1年生、2年生德阳柿实生苗蕾期叶片中互作蛋白基因的表达量,以期探究DdSOC1 基因在德阳柿短童期中的功能,为研究德阳柿短童期分子调控网络提供理论依据。
1 材料和方法
1.1 试验材料
植物材料为种植于陕西杨凌西北农林科技大学国家柿种质资源圃(34°17′42.80″N,108°04′8.21″E)的1 年生、2 年生德阳柿(D.deyangensis)实生苗;本氏烟(Nicotiana tabacum)。用于实时荧光定量的试验材料取自1年生、2年生德阳柿蕾期实生苗叶片。
菌株:富平尖柿的酵母双杂交文库,Y2H Gold酵母菌株以及转入pGBKT7 空载、阳性对照(pGBKT7-53+pGADT7-T)、阴性对照(pGBKT7-Lam+pGADT7-T)的酵母菌株。
载体:Y2H载体pGBKT7(BD)、pGADT7(AD);BiFC载体pSPYNE(NE)、pSPYCE(CE)。
1.2 DdSOC1基因的克隆和序列分析
使用天根生化科技有限公司多糖多酚试剂盒(DP441)提取德阳柿叶片RNA,使用艾科瑞生物工程有限公司Evo M-MLV 反转录试剂盒(AG11728)将所提RNA 反转录合成模板cDNA。根据基因组数据查询基因的序列,利用SnapGene Viewer 2.4.3软件进行引物设计(表1)。采用艾科瑞高保真试剂盒(AG12202)扩增CDS 序列。DNA 片段连接到pMD19-T 载体后转化至大肠杆菌DH5α 中,经过菌落PCR和测序,最终得到阳性克隆。
表1 SOC1 克隆相关引物
Table 1 Related primers for cloning SOC1
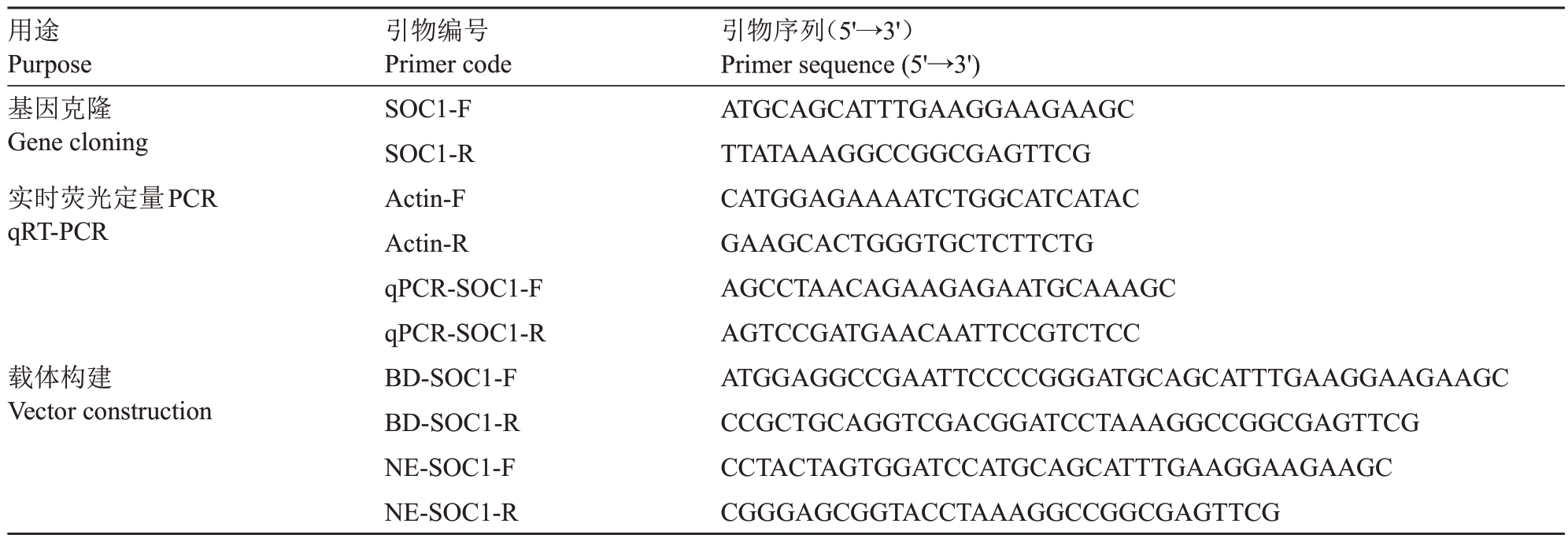
注:NE.pSPYNE;BD.pGBKT7。下划线碱基为不同载体同源臂序列。
Note:NE.pSPYNE;BD.pGBKT7.Underlined bases are homologous arms sequences of different vectors.
用途Purpose基因克隆Gene cloning实时荧光定量PCR qRT-PCR载体构建Vector construction引物序列(5'→3')Primer sequence(5'→3')ATGCAGCATTTGAAGGAAGAAGC TTATAAAGGCCGGCGAGTTCG CATGGAGAAAATCTGGCATCATAC GAAGCACTGGGTGCTCTTCTG AGCCTAACAGAAGAGAATGCAAAGC AGTCCGATGAACAATTCCGTCTCC ATGGAGGCCGAATTCCCCGGGATGCAGCATTTGAAGGAAGAAGC CCGCTGCAGGTCGACGGATCCTAAAGGCCGGCGAGTTCG CCTACTAGTGGATCCATGCAGCATTTGAAGGAAGAAGC CGGGAGCGGTACCTAAAGGCCGGCGAGTTCG引物编号Primer code SOC1-F SOC1-R Actin-F Actin-R qPCR-SOC1-F qPCR-SOC1-R BD-SOC1-F BD-SOC1-R NE-SOC1-F NE-SOC1-R
使用ORF Finder(https://www.ncbi.nlm.nih.gov/orffinder/)在线分析开放阅读框,使用Expasy 中的ProtParam(https://www.expasy.org/)工具预测编码蛋白的等电点和分子质量,在NCBI 上进行SOC1-like蛋白搜索,下载相似性较高的蛋白质序列,利用MEGA 7.0软件,选择邻接法将相关物种的SOC1序列构建系统进化树(bootstrap设为1000次)。
1.3 DdSOC1基因的表达特征
采用qRT-PCR 分析DdSOC1 基因的表达特征,引物见表1。将cDNA 质量浓度稀释至200 ng·μL-1后作为模板,用SYBR Green Pro Taq HS 预混型qPCR试剂盒(含Rox)(AG11718)进行定量实验。
取一年生开花德阳柿实生苗茎、叶、芽三个部位,在苗期、蕾期以及花期测定DdSOC1表达水平。
1.4 DdSOC1自毒自激活验证及互作蛋白筛选
1.4.1 DdSOC1 自激活验证 以DdSOC1 的连T 载体质粒为模板,设计pGBKT7(简称BD)引物(表1)并扩增,用SmaⅠ和BamHⅠ对BD 进行双酶切,同源重组后,通过菌液PCR和测序比对获得重组载体pGBKT7-DdSOC1(BD-SOC1)。依照酵母感受态制备及转化试剂盒(PT1183,源叶生物),获得BDSOC1阳性酵母菌株。
在SD/-Trp 培养基上分别点BD-SOC1 和BD 空载的酵母菌液,28 ℃倒置培养2~3 d 后观察酵母生长情况,若BD-SOC1 在SD/-Trp 平板上正常生长则表明其对酵母菌株无毒性。
在SD/-Trp/-Ade/-His+X-α-Gal(TDO/X)上分别点上阳性对照(pGBKT7-53+pGADT7-T)、阴性对照(pGBKT7-Lam+pGADT7-T)、pGBKT7 空载(BDempty)、BD-SOC1,观察酵母的生长情况。若BDSOC1的菌液在三缺板上不长斑则说明不存在自激活,可直接进行后续的筛库试验,若在三缺板上长斑并变蓝,则需筛选合适的抑制剂浓度抑制其自激活。
1.4.2 DdSOC1酵母文库筛选 将柿的核次级文库质粒转入BD-SOC1的酵母感受态细胞中,经过转化后,将菌液涂布至SD/-Trp/-Leu/-Ade/-His(QDO)培养基上,观察单菌落生长情况。挑取单菌落,稀释后点至SD/-Trp/-Leu/-Ade/-His/X-α-Gal/AbA(QDO/X/A)上,观察是否有变蓝的菌落。
菌落PCR及测序:将蓝色单菌落挑至离心管中加入20 μL 裂解液(Lysis Buffer for Microorganism to Direct PCR,9164,宝日医),热变性后低速离心,取上清液作为模板进行菌落PCR。选择电泳条带大于500 bp 且单一的菌落PCR 产物送至上海生工生物工程有限公司测序(表2)。
表2 酵母测序引物
Table 2 Primers of yeast sequencing
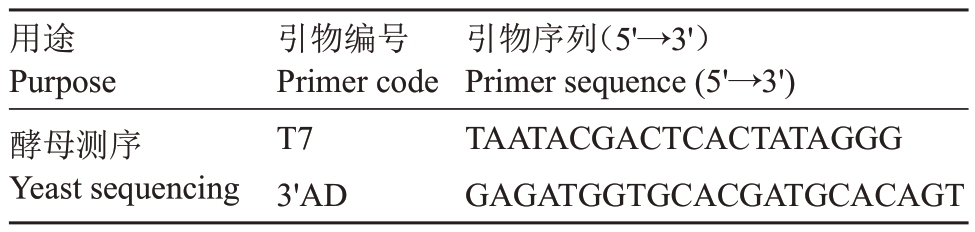
用途Purpose酵母测序Yeast sequencing引物编号Primer code T7 3'AD引物序列(5'→3')Primer sequence(5'→3')TAATACGACTCACTATAGGG GAGATGGTGCACGATGCACAGT
1.4.3 互作蛋白克隆 将筛库测序得到的序列在NCBI 的BLAST 数据库中进行比对,根据功能注释筛选互作蛋白,在德阳柿基因组中找出对应的CDS序列,并设计扩增引物(表3)。
表3 互作蛋白扩增引物
Table 3 Primers for interacting protein CDS amplification

用途Purpose基因克隆Gene cloning引物编号Primer code JOINTLESS-F JOINTLESS-R AGL14-F AGL14-R GL2-F GL2-R NBS-F NBS-R UBC7-F UBC7-R NOVEIN-F NOVEIN-R MIOX-F MIOX-R引物序列(5'→3')Primer sequence(5'→3')ATGAAGGATATACTTGGAAAGTACTC GTTATAAGGCAGCCCGAG ATGGTGAGAGGGAAGACCCAG GTTCTCCGGCGGCAGT ATGAGCTCTGGAAGCAGAGCA TCGCTTCGTTTTTCTTCTTGAC ATGTACGGAACAAAATCTCACCAG CTTGTGTTTGAGCAGATATTCG ATGTCGACGCCGGCGAGG GTCAGCTGTCCAGCTCTG ATGGAGGAAGAGCAACACCCG GGGAGCGATCCTCAGAGG ATGACCATTCTCGTCGAGCAG CCATCGGAGCTTGGCC
1.5 酵母双杂交(Y2H)
设计互作蛋白pGADT7(AD)的引物(表4)并扩增,用SmaⅠ和BamHⅠ对AD 载体进行双酶切,同源重组后,对菌落PCR、提质粒及测序。
表4 互作蛋白AD 载体引物
Table 4 Primers of interaction protein AD vector

注:AD.pGADT7。下划线碱基为不同载体同源臂序列。
Note:AD.pGADT7.Underlined bases are homologous arms sequences of different vectors.
用途Purpose载体构建Vector construction引物编号Primer code AD-JOINTLESS-F AD-JOINTLESS-R AD-AGL14-F AD-AGL14-R AD-GL2-F AD-GL2-R AD-NBS-F AD-NBS-R AD-UBC7-F AD-UBC7-R AD-NOVEIN-F AD-NOVEIN-R AD-MIOX-F AD-MIOX-R引物序列(5'→3')Primer sequence(5'→3')CAGTGAATTCCACCCGGGATGAAGGATATACTTGGAAAGTACTC CTCGAGCTCGATGGATCCGTTATAAGGCAGCCCGAG CAGTGAATTCCACCCGGGATGGTGAGAGGGAAGACCCAG CTCGAGCTCGATGGATCCGTTCTCCGGCGGCAGT CAGTGAATTCCACCCGGGATGAGCTCTGGAAGCAGAGCA CTCGAGCTCGATGGATCCTCGCTTCGTTTTTCTTCTTGAC CAGTGAATTCCACCCGGGATGTACGGAACAAAATCTCACCAG CTCGAGCTCGATGGATCCCTTGTGTTTGAGCAGATATTCG CAGTGAATTCCACCCGGGATGTCGACGCCGGCGAGG CTCGAGCTCGATGGATCCGTCAGCTGTCCAGCTCTG CAGTGAATTCCACCCGGGATGGAGGAAGAGCAACACCCG CTCGAGCTCGATGGATCCGGGAGCGATCCTCAGAGG CAGTGAATTCCACCCGGGATGACCATTCTCGTCGAGCAG CTCGAGCTCGATGGATCCCCATCGGAGCTTGGCC
参照1.4.2方法,将空载(AD-empty)质粒及互作蛋白的AD 质粒分别转入BD-SOC1 酵母感受态细胞,处理后的菌液涂布至DDO(SD/-Trp/-Leu)平板上。培养至长出单菌落后,挑取单菌落点至DDO、QDO/X/A板上,观察酵母生长及变色情况。设置阳性和阴性对照。
1.6 双分子荧光互补(BiFC)
设计DdSOC1 的pSPYNE(NE)以及互作蛋白pSPYCE(CE)的引物(表1,表5)并扩增,同源重组后测序检测,并提取重组质粒备用。
表5 互作蛋白CE 载体引物
Table 5 Primers of interaction protein CE vector
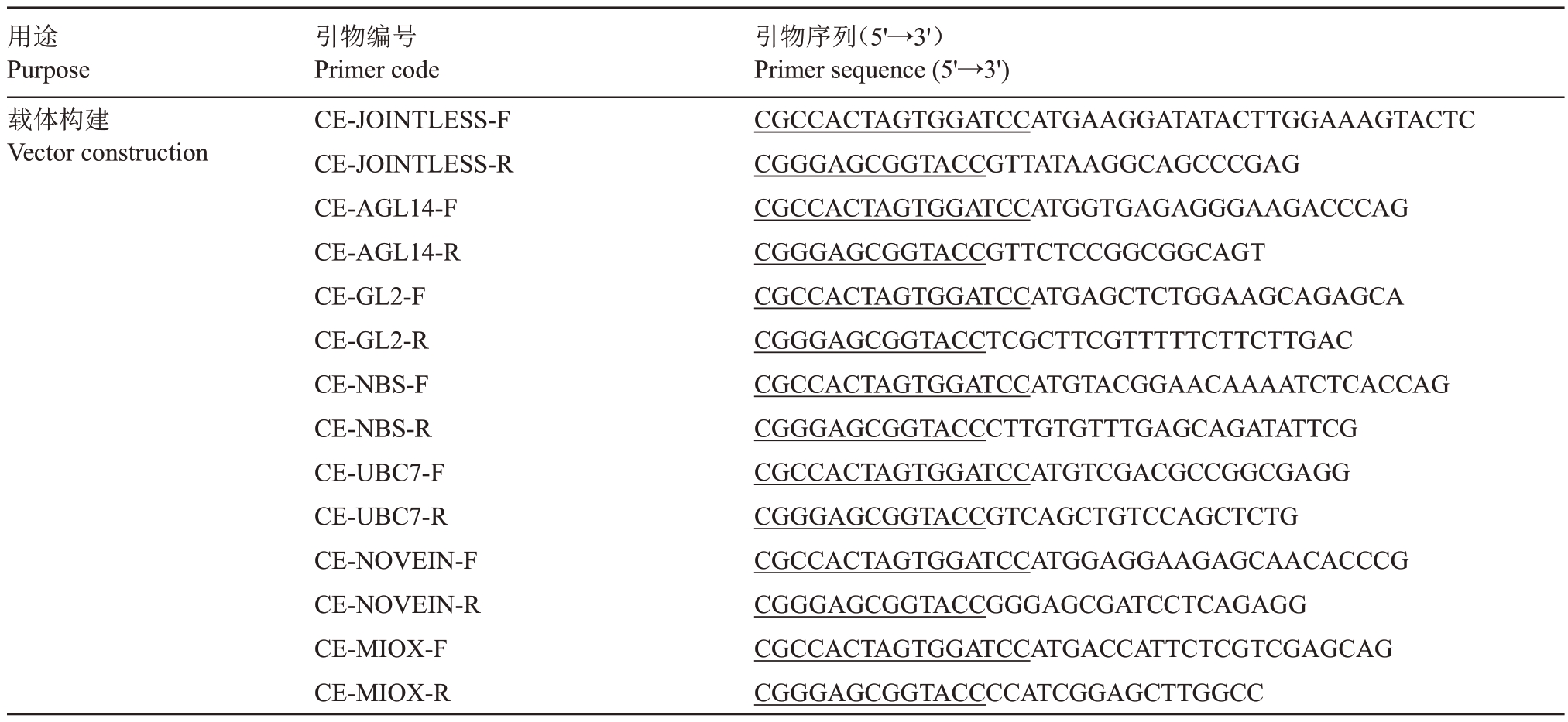
注:CE.pSPYCE。下划线碱基为不同载体同源臂序列。
Note:CE.pSPYCE.Underlined bases are homologous arms sequences of different vectors.
用途Purpose载体构建Vector construction引物编号Primer code CE-JOINTLESS-F CE-JOINTLESS-R CE-AGL14-F CE-AGL14-R CE-GL2-F CE-GL2-R CE-NBS-F CE-NBS-R CE-UBC7-F CE-UBC7-R CE-NOVEIN-F CE-NOVEIN-R CE-MIOX-F CE-MIOX-R引物序列(5'→3')Primer sequence(5'→3')CGCCACTAGTGGATCCATGAAGGATATACTTGGAAAGTACTC CGGGAGCGGTACCGTTATAAGGCAGCCCGAG CGCCACTAGTGGATCCATGGTGAGAGGGAAGACCCAG CGGGAGCGGTACCGTTCTCCGGCGGCAGT CGCCACTAGTGGATCCATGAGCTCTGGAAGCAGAGCA CGGGAGCGGTACCTCGCTTCGTTTTTCTTCTTGAC CGCCACTAGTGGATCCATGTACGGAACAAAATCTCACCAG CGGGAGCGGTACCCTTGTGTTTGAGCAGATATTCG CGCCACTAGTGGATCCATGTCGACGCCGGCGAGG CGGGAGCGGTACCGTCAGCTGTCCAGCTCTG CGCCACTAGTGGATCCATGGAGGAAGAGCAACACCCG CGGGAGCGGTACCGGGAGCGATCCTCAGAGG CGCCACTAGTGGATCCATGACCATTCTCGTCGAGCAG CGGGAGCGGTACCCCATCGGAGCTTGGCC
参照农杆菌感受态说明书将重组质粒转化至农杆菌感受态GV3101 中,利用菌落PCR 鉴定阳性菌株,在双抗LB 液体培养基中培养。离心后弃上清液,用MES溶液重悬菌液,OD600值调整至0.8后加入乙酰丁香酮,室温黑暗静置。将CE和NE侵染液等比例混合,将农杆菌侵染液注射进烟草叶背,暗培养48 h。使用激光共聚焦显微镜(TCS SP8 SR,Leica,德国)观察YFP荧光。
1.7 互作基因表达分析
参照1.3 的方法,设计互作蛋白的定量引物(表6),取蕾期1 年生、2 年生德阳柿(D.deyangensis)实生苗顶芽附近3~4枚叶(幼叶)及枝条基部的成年叶分析互作基因表达量。
表6 互作蛋白定量引物
Table 6 Quantitative primers for interacting proteins

用途Purpose实时荧光定量PCR qRT-PCR引物编号Primer code qPCR-JOINTLESS-F qPCR-JOINTLESS-R qPCR-AGL14-F qPCR-AGL14-R qPCR-GL2-F qPCR-GL2-R qPCR-NBS-F qPCR-NBS-R qPCR-UBC7-F qPCR-UBC7-R qPCR-NOVEIN-F qPCR-NOVEIN-R qPCR-MIOX-F qPCR-MIOX-R引物序列(5'→3')Primer sequence(5'→3')TCACACAAAGGGCGAACGAATC TCTGTTTCAGTTGCTGGTTCTCTTC GGCGAAGATGACGAAGAAGATAGAG AACAGAGCACGATCCGAGACC GGAAGCAGAGCAGCGTTGTG CGAAGCGGACGAGAATGAAGTG GTGGACAACGAGAAGCGAATGAG GTGATAGTGAGGACGCAGCAATC CATCATGCTCTGGAACGCTGTC GGTGGTTTATTTGGGTAATCTTCTGTG CCAAAGACTCCATTAAGGGTGCTAC TTAACAGGCAAGGCAGGCAATG GCAGAAGCCATTCGTAAGGACTAC TGTCACCAACAACAGCCCATTG
1.8 数据统计和分析
所有处理均包括3 次重复,数据均表示为平均值±标准误差,统计分析和作图使用GraphPad Prism 8软件完成。
2 结果与分析
2.1 DdSOC1基因的克隆和序列分析
以德阳柿叶的cDNA 为模板扩增得到DdSOC1基因,经测序验证扩增得到的序列与德阳柿基因组序列一致。该基因CDS 全长381 bp,编码126 个氨基酸;蛋白质的分子质量为14.56 ku、等电点为5.62。
利用MEGA 7.0 软件对德阳柿及其他物种的SOC1 序列进行系统发育树分析,结果(图1)显示,DdSOC1 和DlSOC1 关系最近,形成一个小的分支,与中华猕猴桃、毛花猕猴桃、茶SOC1蛋白序列一致性较高;与银白杨、欧洲葡萄、苹果、桃、杧果等SOC1 蛋白的亲缘关系较远;与水稻、小麦SOC1 蛋白亲缘关系最远。
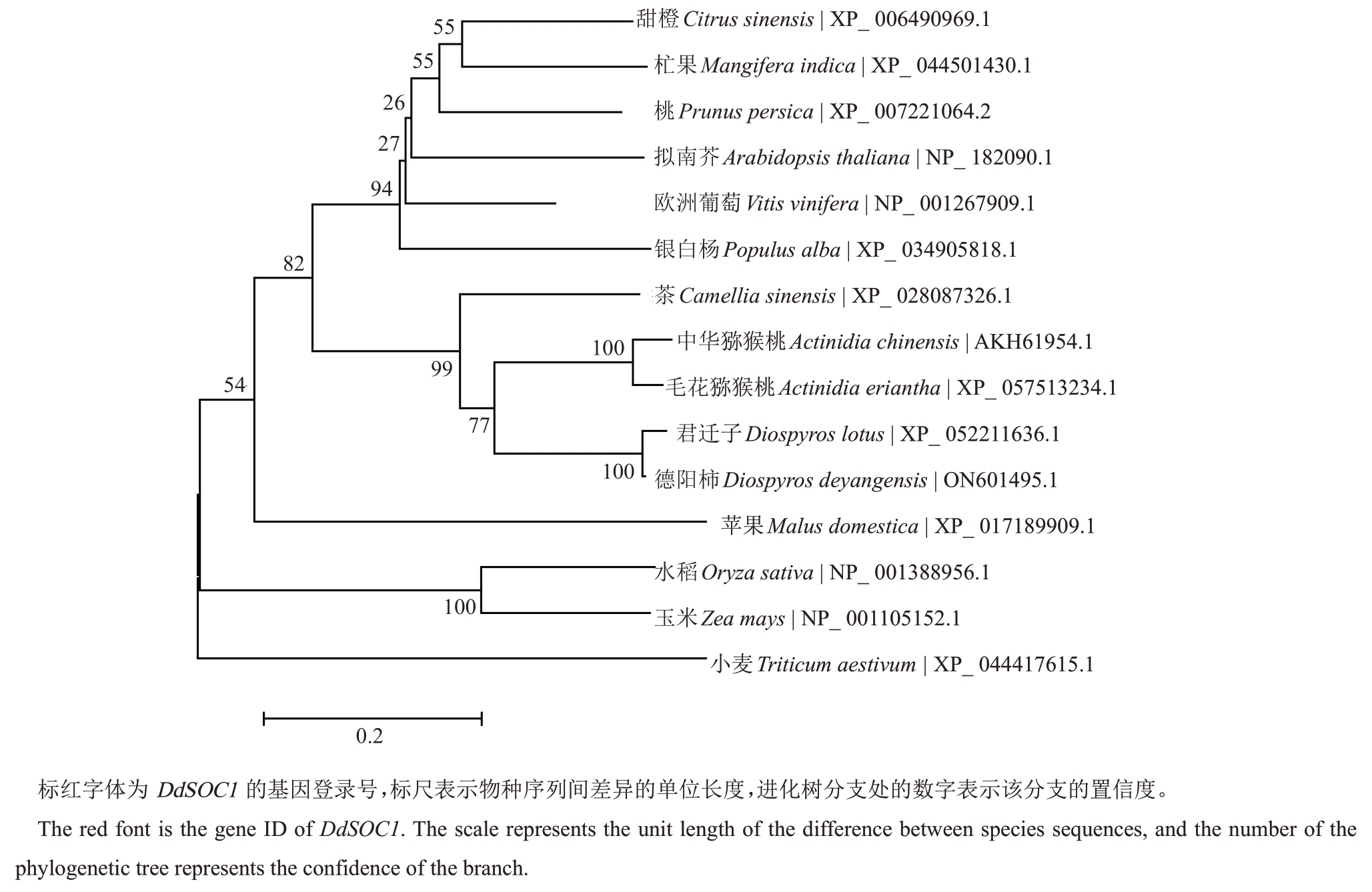
图1 德阳柿SOC1 与其他物种SOC1 序列的系统发育树分析
Fig.1 Phylogenetic tree of D.deyangensis and other species SOC1 sequences
2.2 DdSOC1基因的表达特征
1年生开花德阳柿不同器官(茎、叶、芽)在不同时期SOC1 的定量结果(图2)显示,茎中SOC1 表达量在蕾期最低,而在花期达到最高;叶中SOC1表达量随着开花进程逐渐升高;芽中SOC1 的表达量在幼苗期最高,而在花期最低。
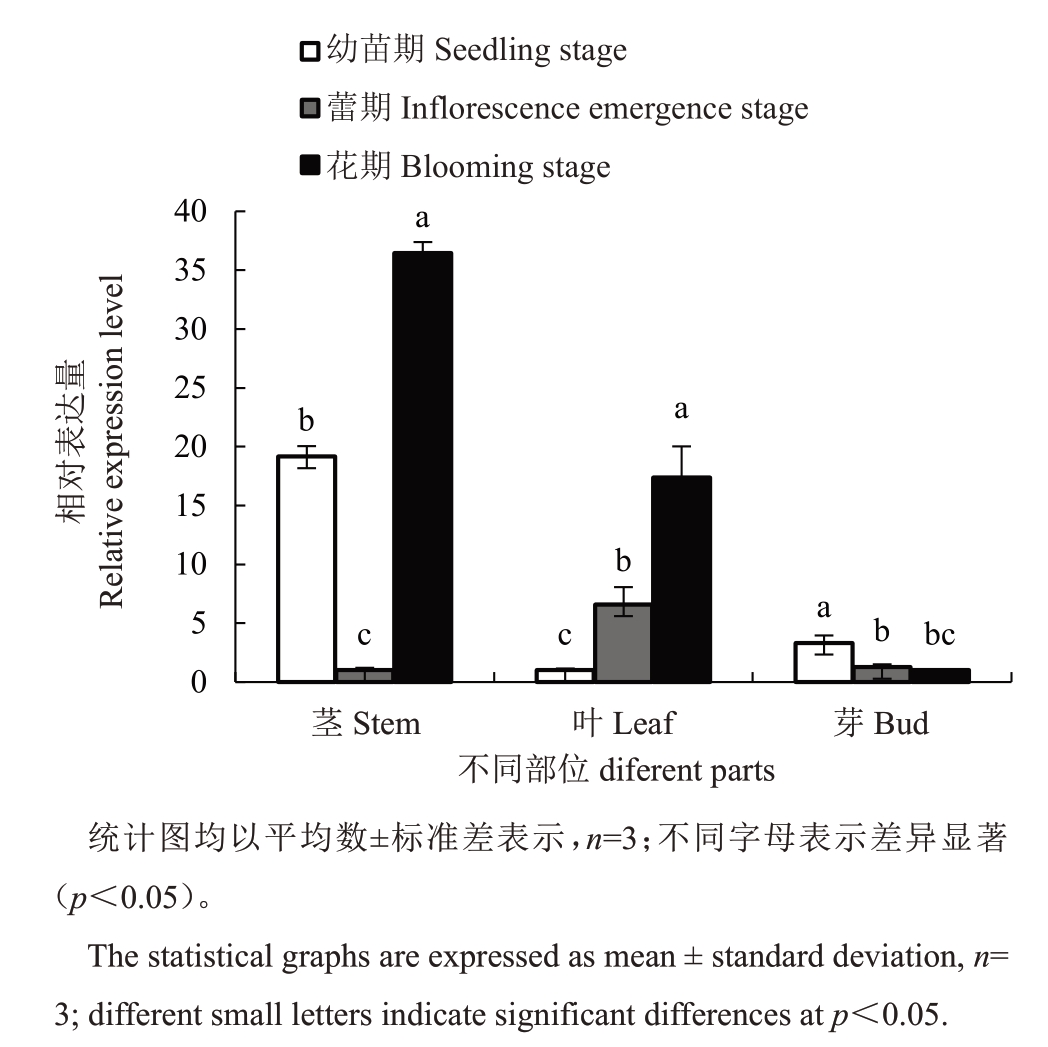
图2 在幼苗期、蕾期、花期时德阳柿茎叶芽中SOC1 表达量
Fig.2 The expression of SOC1 in stem and,leaf and buds of D.deyangensis at the seedling stage,inflorescence emergence and blooming stage
2.3 DdSOC1自毒自激活验证及酵母文库筛选
分别在SD/-Trp和SD/-Trp/Ade/His+X-α-gal培养基上对DdSOC1 毒性及自激活进行检测,结果如图3 所示,在SD/-Trp 培养基上,BD-SOC1 长出菌落,说明BD-SOC1 对酵母菌生长无影响;在SD/-Trp/-Ade/-His + X-α-gal 培养基上,只有阳性对照(Po)长出菌斑,阴性对照(Ne)、空白对照(BD-empty)、BD-SOC1 均未长出菌斑,说明DdSOC1 没有自激活现象。
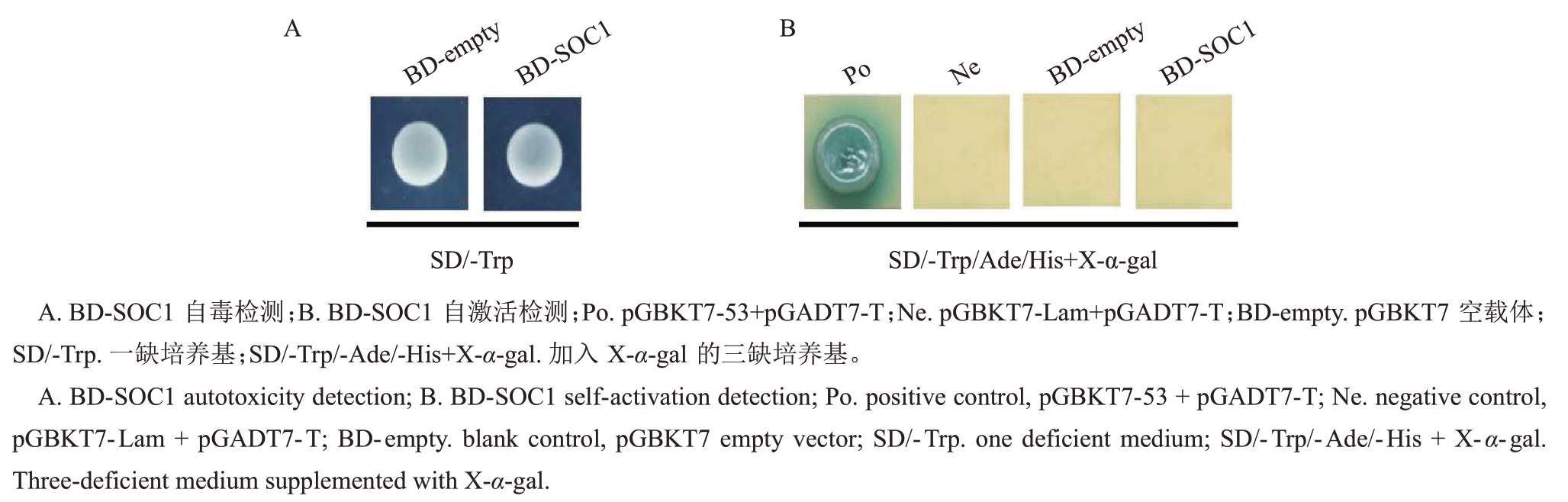
图3 DdSOC1 自毒自激活检测
Fig.3 Test of toxicity and self-activation of DdSOC1
酵母筛库结果显示,BD-SOC1 质粒与酵母文库质粒共转入的酵母菌在QDO板上长出白色单菌落,其在QDO/X/A 板上均变蓝。对QDO/X/A 上变蓝单菌落进行菌落PCR 检测,选择大于500 bp 的条带测序;利用NCBI BLAST 以及德阳柿基因组注释对比后从中筛选出7 条候选互作蛋白(AGL14、JOINTLESS、NOVEIN、GL2、UBC7、NBS、MIOX)。
2.4 酵母双杂交(Y2H)
酵母试验结果显示,9 组酵母组合在DDO 培养基上均长出白色菌落,表明9 组质粒均成功转入酵母菌株;在QDO及QDO/X/A培养基上,阴性和空白对照均无菌斑,阳性对照以及7 个酵母组合均能正常生长,且可转变为蓝色(图4)。因此,德阳柿SOC1蛋白与AGL14、JOINTLESS、NOVEIN、GL2、UBC7、NBS、MIOX均可发生互作。
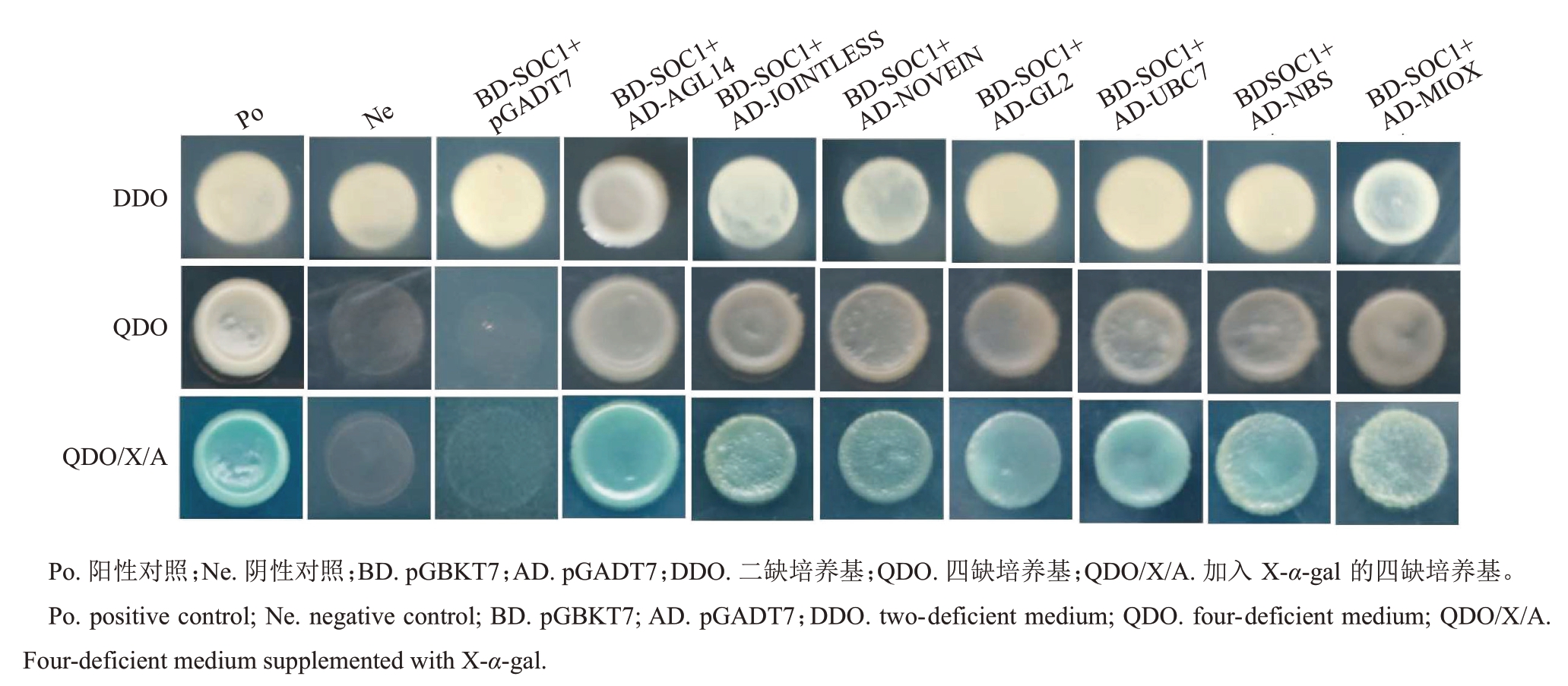
图4 DdSOC1 与互作蛋白的酵母双杂交试验
Fig.4 Interaction between DdSOC1 and related proteins in yeast two-hybrid assays
2.5 双分子荧光互补(BiFC)
为验证DdSOC1 与7 个候选互作蛋白在植物体内的互作关系,将DdSOC1 和候选互作蛋白分别构建BiFC 载体pSPYNE(NE)和pSPYCE(CE),并在烟草细胞中共注射后观察YFP荧光信号。结果(图5)显示,7 个组合中,JOINTLESS-cYFP+SOC1-nYFP在烟草细胞的细胞核、膜中发出较强烈的黄色荧光;AGL14-cYFP+Dd-SOC1-nYFP 组合在细胞核中发出黄色荧光;其他组合在细胞膜上显示黄色荧光。以上情况表明DdSOC1与7个候选互作蛋白在植物体内均存在互作关系。
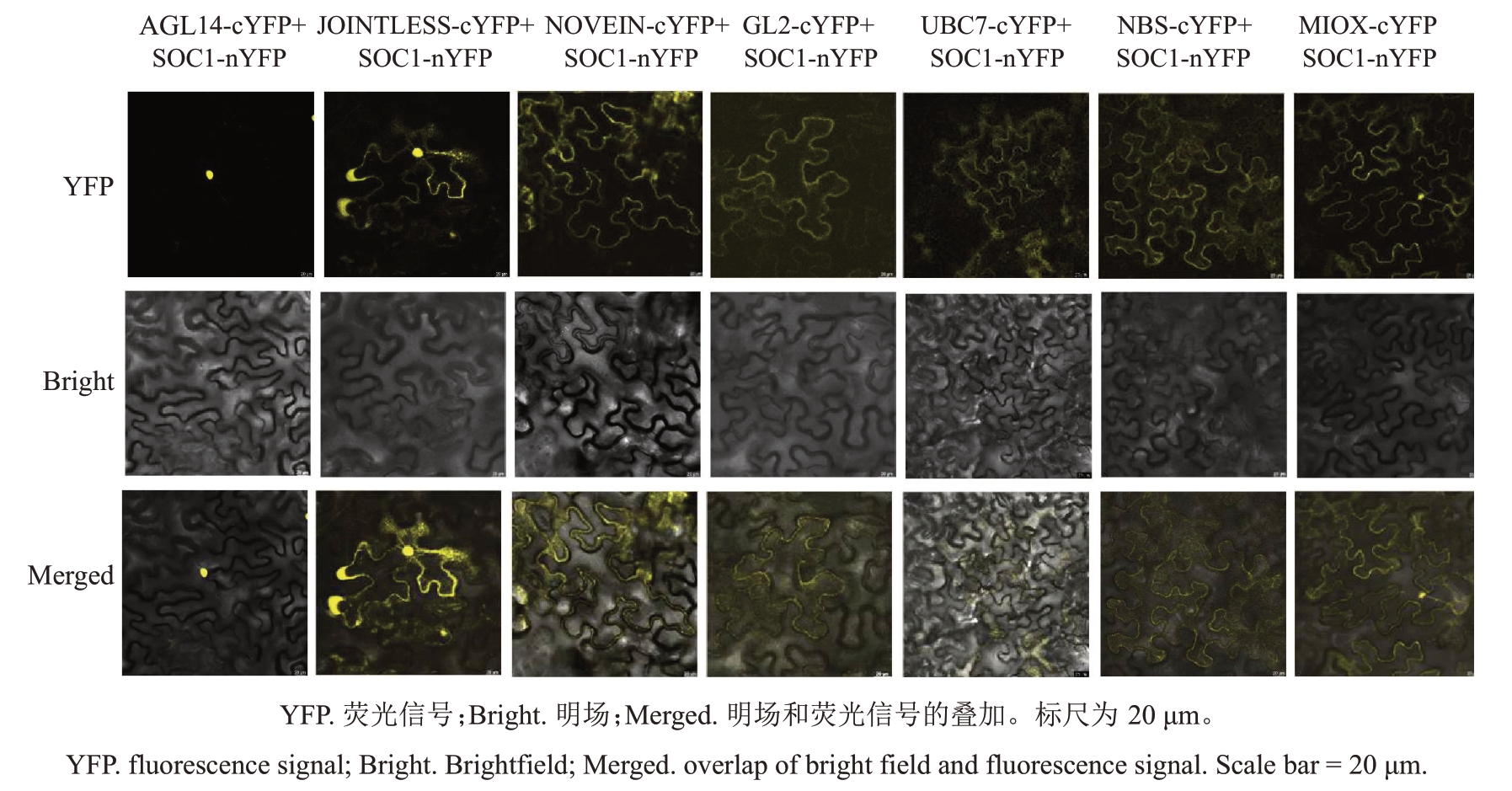
图5 DdSOC1 与互作蛋白的双分子荧光互补试验
Fig.5 Interaction between DdSOC1 and related proteins by bimolecular fluorescence complementation assays
2.6 DdSOC1 及互作蛋白基因在德阳柿中的表达情况
探究了蕾期1年生、2年生不同性状(已开花、未开花)德阳柿实生苗幼叶(顶芽附近叶片)、成叶(远离顶芽的成年叶)中DdSOC1 及互作蛋白基因的表达情况。试验结果(图6)显示,与未开花实生苗相比,1 年生已开花德阳柿实生苗的幼叶中SOC1、AGL14、NOVEIN、GL2、UBC7、NBS 的表达量较成叶中高,而MIOX 在幼叶的表达量则低于成叶;2 年生已开花德阳柿实生苗中,幼叶中SOC1、NOVEIN 表达量较成叶低,而其他基因(GL2、UBC7、NBS、AGL14、JOINTLESS)的表达量在幼叶中高于成叶;2年生未开花实生苗中,幼叶中SOC1 表达量略低于成叶,其他基因(GL2、AGL14、NOVEIN、JOINTLESS、UBC7、NBS、MIOX)在幼叶中的表达量均高于成叶。
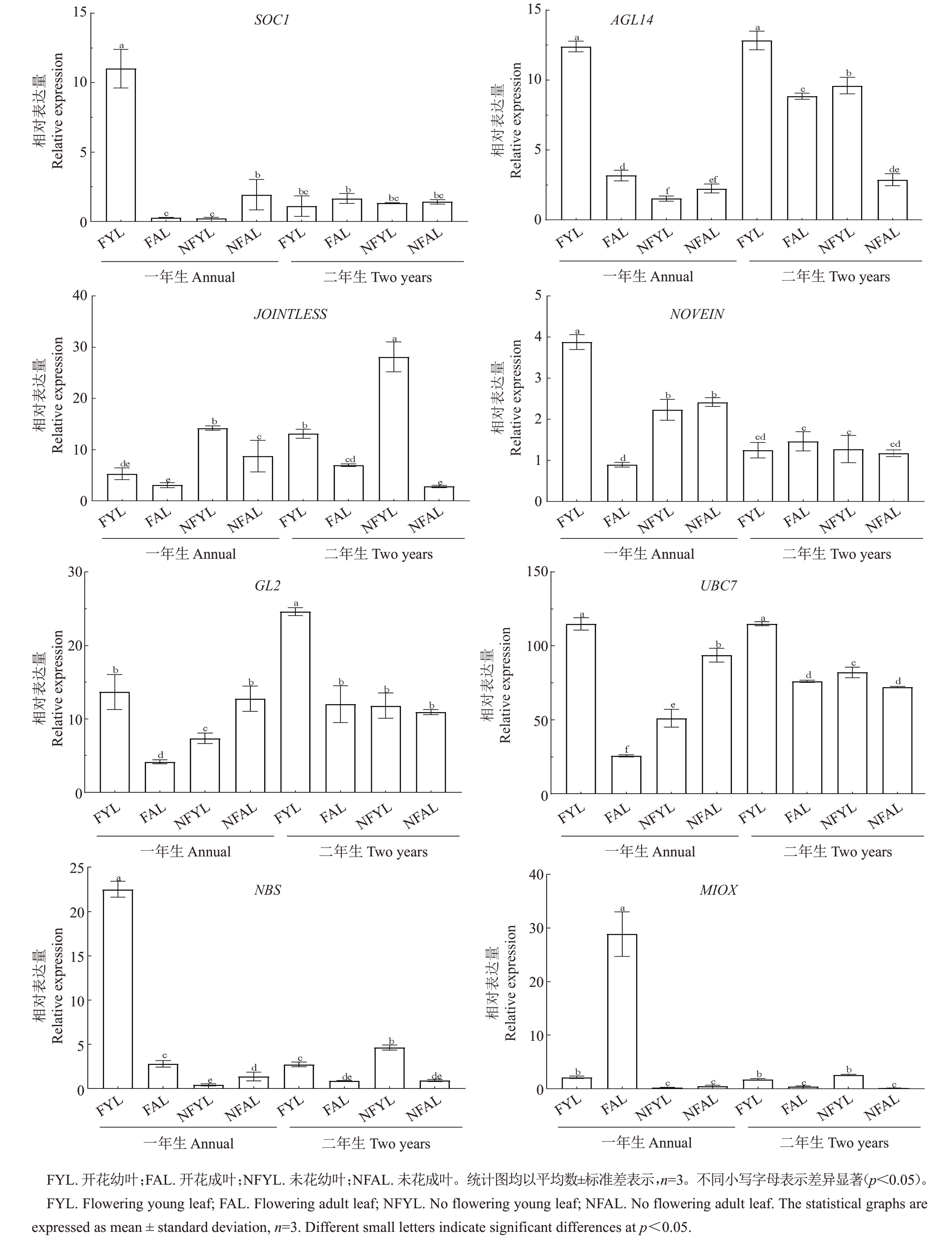
图6 DdSOC1 及互作蛋白基因在德阳柿中的表达量测定
Fig.6 Determination of the expression of DdSOC1 and its interacting proteins genes in D.deyangensis
3 讨 论
本研究中笔者克隆出了DdSOC1 基因,预测了DdSOC1蛋白的等电点及分子质量等理化性质。进化树分析表明,DdSOC1 蛋白与拟南芥AtSOC1、水稻OsSOC1 亲缘关系较远,与欧洲葡萄VvSOC1、茶CsSOC1、中华猕猴桃AcSOC1 等亲缘关系较近,与同属君迁子DlSOC1相似性最高,这表明SOC1在木本植物上进化较为保守。DdSOC1的表达特征结果显示,在整个成花过程中,叶、芽中DdSOC1 表达量分别是逐渐升高和逐渐降低,茎中DdSOC1 表达量先降低后升高;幼苗期DdSOC1在芽中表达量最高,蕾期在叶中表达量最高,花期在茎中表达量最高。以上结果表明,DdSOC1 整合了来自叶片的开花信号,以实现营养生长向生殖生长过渡的阶段,而不是直接调控花器官的发育,这与欧洲葡萄(V. vinifera)、竹子(Bambusa oldhamii)、白梨(Pyrus bretschneideri)、荔枝(Litchi chinensis)的研究结果较为相似[20-23]。
在前人已探明的SOC1调控植物开花时间的网络中,CO、FT、FLC、SVP、DELLA蛋白、AGL24蛋白及LFY 是重要的调控因子。其中,SOC1 与AGL24在植物茎顶端分生组织中相互作用形成复合物,然后结合在LFY启动子上激活其表达从而完成开花诱导[24-27]。明晰DdSOC1 的成花调控网络,对探究DdSOC1 调控德阳柿短童期分子机制具有重要意义。本研究基于富平尖柿cDNA 核次级文库对DdSOC1 进行互作蛋白的筛选,共筛选得到7 个互作蛋白。Y2H 和BiFC 试验结果均验证了DdSOC1与7个互作蛋白之间存在互作关系。此外,GL2-cYFP + SOC1-nYFP、NOVEIN-cYFP + SOC1-nYFP、JOINTLESS-cYFP+SOC1-nYFP三个组合荧光强度较高,表明DdSOC1 与这3 个互作蛋白(GL2、NOVEIN、JOINTLESS)互作关系较紧密。后续可以通过Co-IP或Pull-down蛋白试验进行进一步验证。
为了进一步探究DdSOC1及其互作蛋白在德阳柿短童期中的作用,测定了DdSOC1 基因在1 年生和2年生德阳柿实生苗叶片中的表达量。德阳柿短童期现象在德阳柿种内也存在差异,即存在开花和未花两种性状,因此本研究中选取蕾期的叶片作为试验材料。在1 年生德阳柿中,已开花实生苗幼叶SOC1 表达量比成叶高,而在未开花实生苗中幼叶SOC1表达量比成叶低,这表明SOC1对德阳柿开花有着积极影响。同样地,AGL14、JOINTLESS、NOVEIN、GL2、UBC7、NBS也表现出与SOC1相似的表达规律,即在1 年生已开花的德阳柿的幼叶中高表达,这说明以上6 个互作蛋白可能对德阳柿短童期具有正调控作用;而MIOX在1年生开花德阳柿实生苗幼叶中低表达,这说明其对德阳柿开花可能有阻遏作用。2年生实生苗,SOC1表达量在不同性状的实生苗中差异不大,而AGL14、JOINTLESS、GL2、UBC7 在开花实生苗中的表达量高于未开花实生苗。此外,前人研究发现,AGL14 通过促进LFY 和AP1 的表达使拟南芥出现早花表型[28],AGL14 也参与拟南芥花衰老方面的调节[29];JOINTLESS 为典型的MADS-box 基因,与花序分生组织发育有着密切关系,如在番茄中正调控番茄花序的分支和数量[30-31];GL2 作为GL2-zip 家族转录因子通过与ZIM结构域蛋白CsJAZ1 相互作用,进而调控延迟黄瓜雄花的开花时间[32];MIOX 参与了晚熟橙花和叶片的发育过程[33];转录组学分析表明,NBS在早开花紫苜蓿中高表达[34],而目前并未有关于NOVEIN、UBC7 参与植物成花调控的报道。综上,本研究中筛选得到7个互作蛋白,AGL14、JOINTLESS、GL2、NBS 可能正调控德阳柿短童期性状,MIOX 可能有延迟开花的作用,而NOVEIN、UBC7可能不参与德阳柿的成花调控。
4 结 论
本研究从柿中克隆分离出DdSOC1 基因,其序列在木本植物中进化较为保守;DdSOC1 可能整合了来自叶片的开花信号,以此实现营养生长向生殖生长的过渡;筛选得到了DdSOC1的7个互作蛋白;AGL14、JOINTLESS、GL2、NBS 可能正调控促进德阳柿开花,MIOX 可能有延迟开花的作用,而NOVEIN、UBC7可能不参与德阳柿成花调控。
[1] ZHANG Y F,YANG Y,GUO J,HU C Q,ZHU R S.Taxonomic status of Deyangshi based on chromosome number and SRAP markers[J].Scientia Horticulturae,2016,207:57-64.
[2] 胡杰.部分柿属种形态学研究与染色体倍性鉴定[D].杨凌:西北农林科技大学,2022.HU Jie. Morphological identification and chromosomal ploidy identification of some persimmon species[D]. Yangling:Northwest A&F University,2022.
[3] 楚乐乐,刘海强,盛星星,郑玮璇,龚赞,胡春根,张金智.果树成花转变途径与调控研究进展[J]. 植物科学学报,2022,40(2):281-290.CHU Lele,LIU Haiqiang,SHENG Xingxing,ZHENG Weixuan,GONG Zan,HU Chungen,ZHANG Jinzhi. Research progress on the pathways and regulation of flowering transformation in fruit trees[J].Plant Science Journal,2022,40(2):281-290.
[4] MA J J,CHEN X,SONG Y T,ZHANG G F,ZHOU X Q,QUE S P,MAO F,PERVAIZ T,LIN J X,LI Y,LI W,WU H X,NIU S H. MADS-box transcription factors MADS11 and DAL1 interact to mediate the vegetative-to-reproductive transition in pine[J].Plant Physiology,2021,187(1):247-262.
[5] 张平贤,张青林,徐莉清,郭大勇,罗正荣.部分柿属植物的早花现象观察[J].落叶果树,2017,49(3):24-26.ZHANG Pingxian,ZHANG Qinglin,XU Liqing,GUO Dayong,LUO Zhengrong. Observation on early flowering of some persimmon plants[J].Deciduous Fruits,2017,49(3):24-26.
[6] PARCY F. Flowering:A time for integration[J].The International Journal of Developmental Biology,2005,49(5/6):585-593.
[7] SIMPSON G G,DEAN C. Arabidopsis,the Rosetta stone of flowering time?[J].Science,2002,296(5566):285-289.
[8] 孙晓茜,戴洪义,张玉刚.柱型苹果MADS-box 家族的2 个同源基因克隆与生物信息学分析[J].华北农学报,2012,27(2):50-54.SUN Xiaoqian,DAI Hongyi,ZHANG Yugang.Cloning and bioinformatic analysis of 2 homologous genes of MADS-box in columnar apple[J].Acta Agriculturae Boreali-Sinica,2012,27(2):50-54.
[9] 苏文龙,刘程,苏哲,岳彩云,王晓霞,樊金会.毛白杨形成层中4 种MADS-box 基因的克隆和序列分析[J]. 分子植物育种,2015,13(3):653-657.SU Wenlong,LIU Cheng,SU Zhe,YUE Caiyun,WANG Xiaoxia,FAN Jinhui. Cloning and sequence analysis of four MADS-box genes from cambium of Populus tomentosa[J]. Molecular Plant Breeding,2015,13(3):653-657.
[10] 黄晓婧,张珺,夏惠,邓群仙,王进,吕秀兰,梁东.葡萄MADSbox 转录因子家族全基因组鉴定及表达分析[J]. 园艺学报,2019,46(10):1882-1896.HUANG Xiaojing,ZHANG Jun,XIA Hui,DENG Qunxian,WANG Jin,LÜ Xiulan,LIANG Dong.Genome-wide identification and expression analysis of the MADS-box gene family in Vitis vinifera[J]. Acta Horticulturae Sinica,2019,46(10):1882-1896.
[11] 贺新兴,杨杰,高钱辉,佟晓楠,张晓媛,李兴涛.脐橙SOC1 基因的克隆及其在脐橙花发育过程中的表达分析[J].分子植物育种,2022,20(15):4948-4957.HE Xinxing,YANG Jie,GAO Qianhui,TONG Xiaonan,ZHANG Xiaoyuan,LI Xingtao.Cloning and expression analysis of SOC1 gene during the development of navel orange flower[J].Molecular Plant Breeding,2022,20(15):4948-4957.
[12] MAPLE R,ZHU P,HEPWORTH J,WANG J W,DEAN C.Flowering time:From physiology,through genetics to mechanism[J].Plant Physiology,2024,195(1):190-212.
[13] YU Y C,QIAO L F,CHEN J C,RONG Y H,ZHAO Y H,CUI X K,XU J P,HOU X M,DONG C H.Arabidopsis REM16 acts as a B3 domain transcription factor to promote flowering time via directly binding to the promoters of SOC1 and FT[J]. The Plant Journal,2020,103(4):1386-1398.
[14] DENG Q L,WANG Y D,FENG J J,WEI D Y,WANG Z M,TANG Q L.Brassica juncea BjuWRKY71-1 accelerates flowering by regulating the expression of SOC1[J]. Chinese Journal of Biotechnology,2024,40(4):1017-1028.
[15] JIN Z P,YU X,PEI Y X. Ectopic expression of AtSOC1 gene driven by the inducible promoter rd29A,causes early flowering in Chrysanthemum[J]. Scientia Horticulturae,2020,261:109051.
[16] 齐联联,宿强,张珂.SOC1 调控植物开花时间的分子机制[J].草业科学,2022,39(1):149-160.QI Lianlian,SU Qiang,ZHANG Ke. Molecular mechanism of flowering time regulate by SOC1[J]. Pratacultural Science,2022,39(1):149-160.
[17] LUO X M,LIU B Y,XIE L,WANG K,XU D A,TIAN X L,XIE L N,LI L L,YE X G,HE Z H,XIA X C,YAN L L,CAO S H. The TaSOC1-TaVRN1 module integrates photoperiod and vernalization signals to regulate wheat flowering[J]. Plant Biotechnology Journal,2024,22(3):635-649.
[18] LI X,SHEN C W,CHEN R X,SUN B,LI D H,GUO X L,WU C H,KHAN N,CHEN B H,YUAN J P. Function of BrSOC1b gene in flowering regulation of Chinese cabbage and its protein interaction[J].Planta,2023,258(1):21.
[19] DONG X,ZHANG L P,TANG Y H,YU D M,CHENG F,DONG Y X,JIANG X D,QIAN F M,GUO Z H,HU J Y.Arabidopsis AGAMOUS- LIKE16 and SUPPRESSOR OF CONSTANS1 regulate the genome-wide expression and flowering time[J].Plant Physiology,2023,192(1):154-169.
[20] 刘丹,孙欣,慕茜,吴伟民,章镇,房经贵.葡萄花芽发育相关基因在不同节位芽中的表达分析[J].中国农业科学,2015,48(10):2007-2016.LIU Dan,SUN Xin,MU Qian,WU Weimin,ZHANG Zhen,FANG Jinggui. Analysis of expression levels of floral genes in the buds on different branch nodes of grapevine[J]. Scientia Agricultura Sinica,2015,48(10):2007-2016.
[21] HOU D,LI L,MA T F,PEI J L,ZHAO Z Y,LU M Z,WU A M,LIN X C. The SOC1-like gene BoMADS50 is associated with the flowering of Bambusa oldhamii[J]. Horticulture Research,2021,8(1):133.
[22] LIU Z,WU X P,CHENG M Y,XIE Z H,XIONG C L,ZHANG S L,WU J Y,WANG P. Identification and functional characterization of SOC1-like genes in Pyrus bretschneideri[J].Genomics,2020,112(2):1622-1632.
[23] SHI Y Y,ZHANG S W,GUI Q L,QING H W,LI M,YI C X,GUO H Q,CHEN H B,XU J Z,DING F.The SOC1 gene plays an important role in regulating litchi flowering time[J]. Genomics,2024,116(2):110804.
[24] LEE J,LEE I. Regulation and function of SOC1,a flowering pathway integrator[J].Journal of Experimental Botany,2010,61(9):2247-2254.
[25] LIU C,CHEN H Y,ER H L,SOO H M,KUMAR P P,HAN J H,LIOU Y C,YU H.Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis[J]. Development,2008,135(8):1481-1491.
[26] KHAN M R,KHAN I U,ALI G M. MPF2-like MADS-box genes affecting expression of SOC1 and MAF1 are recruited to control flowering time[J]. Molecular Biotechnology,2013,54(1):25-36.
[27] 蒋炜,周雯文,李朝闯,闫凯,王宇,王志敏,宋明,汤青林.青花菜开花促进因子AGL19 与整合子AGL24 和SOC1 的互作研究[J].园艺学报,2017,44(10):1905-1913.JIANG Wei,ZHOU Wenwen,LI Zhaochuang,YAN Kai,WANG Yu,WANG Zhimin,SONG Ming,TANG Qinglin.Interactions of flowering promoting factor AGL19 with integrator factors AGL24 and SOC1 in Brassica oleracea var. italica[J].Acta Horticulturae Sinica,2017,44(10):1905-1913.
[28] PÉREZ- RUIZ R V,GARCÍA- PONCE B,MARSCHMARTÍNEZ N,UGARTECHEA-CHIRINO Y,VILLAJUANABONEQUI M,DE FOLTER S,AZPEITIA E,DÁVILAVELDERRAIN J,CRUZ-SÁNCHEZ D,GARAY-ARROYO A,DE LA PAZ SÁNCHEZ M,ESTÉVEZ-PALMAS J M,ÁLVAREZ-BUYLLA E R.XAANTAL2(AGL14)is an important component of the complex gene regulatory network that underlies Arabidopsis shoot apical meristem transitions[J]. Molecular Plant,2015,8(5):796-813.
[29] CHEN W H,LIN P T,HSU W H,HSU H F,LI Y C,TSAO C W,HSU M C,MAO W T,YANG C H. Regulatory network for forever young flower-like genes in regulating Arabidopsis flower senescence and abscission[J]. Communications Biology,2022,5(1):662.
[30] HUERGA-FERNÁNDEZ S,DETRY N,ORMAN-LIGEZA B,BOUCHÉ F,HANIKENNE M,PÉRILLEUX C. JOINTLESS maintains inflorescence meristem identity in tomato[J]. Plant &Cell Physiology,2024,65(7):1197-1211.
[31] 王翔,尹钧.番茄花柄离区发育基因JOINTLESS 及互作蛋白基因的功能研究[J].园艺学报,2011,38(4):701-708.WANG Xiang,YIN Jun. Functional studies of JOINTLESS and its interacting MADS-domain proteins in tomato[J].Acta Horticulturae Sinica,2011,38(4):701-708.
[32] CAI Y L,BARTHOLOMEW E S,DONG M M,ZHAI X L,YIN S,ZHANG Y Q,FENG Z X,WU L C,LIU W,SHAN N,ZHANG X,REN H Z,LIU X W. The HD-ZIP IV transcription factor GL2-LIKE regulates male flowering time and fertility in cucumber[J]. Journal of Experimental Botany,2020,71(18):5425-5437.
[33] ALÓS E,REY F,GIL J V,RODRIGO M J,ZACARIAS L.Ascorbic acid content and transcriptional profiling of genes involved in its metabolism during development of petals,leaves,and fruits of orange (Citrus sinensis cv. Valencia Late) [J].Plants,2021,10(12):2590.
[34] MA D M,LIU B,GE L Q,WENG Y Y,CAO X H,LIU F,MAO P S,MA X Q. Identification and characterization of regulatory pathways involved in early flowering in the new leaves of alfalfa (Medicago sativa L.) by transcriptome analysis[J]. BMC Plant Biology,2021,21(1):8.