关于不同品种梨组培快繁体系的研究已有很多报道,但品种间差异较大。李昌珠等[1]发现欧洲梨Koporeka 继代培养28 d 后繁殖系数达5.60,生根培养14 d 后,生根率达88.65%,而Vila 连续培养60 d后只形成少量愈伤组织,无芽、根的分化;宋梅等[2]研究表明,与砀山酥梨相比,诱导库尔勒香梨茎尖分化更难,且繁殖系数较小;刘小芳等[3]发现库尔勒香梨的繁殖系数最高为1.30,生根诱导效果较差,仅有一株组培苗在1/2MS+1.00 mg·L-1 IBA+0.05 g·L-1活性炭生根培养基上诱导出一条根;其他品种如中梨1号在1/2MS+1.00 mg·L-1 IBA中生根率为44.80%[4],黄冠梨生根率最高为33.33%[5]。综上所述,不同梨品种间组培快繁效率的差异较大,有必要针对具体品种开展相关研究,建立适合某一品种的组培快繁体系。
玉露香梨是山西省农业科学院果树研究所通过库尔勒香梨×雪花梨杂交选育出的优新品种,该品种具有果皮薄、果肉细腻、口感香甜、石细胞少等优点,受到国内外市场的普遍认可[6-7],是农业农村部主推的中晚熟梨优良品种,且已在山西、河北等地进行大面积推广[8]。目前,玉露香梨研究主要集中在生理及栽培方面[7-9],尚未建立它的组培快繁体系。笔者旨在建立玉露香梨高效、稳定的组培快繁体系,为深入开展玉露香梨的分子生物学研究和培育脱毒苗提供理论和技术支撑。
1 材料和方法
1.1 试验材料
试验材料为实验室保存的继代30 d的玉露香梨组培苗。常规培养条件:温度(25±2)℃,光照度2000~3000 lx,光周期(昼/夜)=16 h/8 h。
1.2 试验方法
1.2.1 材料获得 盛花5~10 d 后,于河北农业大学标本园采集玉露香梨新梢外植体。将外植体去掉叶片,留下叶柄,用枝剪刀剪为1 cm单芽茎段,放于干净三角瓶中,流水冲洗30 min。在超净台上用0.1%HgCl2消毒6 min,75%乙醇消毒1 min,无菌水冲洗3次,无菌滤纸吸干水分后,接种于MS+1.00 mg·L-1 6-BA+0.10 mg·L-1 IBA+30.0 g·L-1蔗糖+6.0 g·L-1琼脂+2.0 g·L-1 聚乙烯醇(PVA)(pH值为5.8~6.0)培养基上,扩繁到一定数量后开展试验。
1.2.2 基本培养基对玉露香梨组培苗继代的影响以MS、1/2MS、1/4MS、NN69、WPM 为基本培养基,附加1.00 mg·L-1 6-BA、0.10 mg·L-1 NAA、30.0 g·L-1蔗糖、6.0 g·L-1琼脂、2.0 g·L-1PVA(pH值为5.8~6.0),共5 个处理开展试验,每个处理接6 瓶,每瓶接5 个1 cm单芽茎段,采用完全随机试验设计,3次重复,常规培养40 d后,调查统计繁殖系数及平均有效新梢数(繁殖系数=调查总株数/接种株数;有效新梢为继代苗中株高≥1.5 cm且可用于生根的嫩梢;平均有效新梢数=调查有效新梢数/接种株数;玻璃苗发生率/%=发生玻璃化的株数/接种株数×100;下同)。
1.2.3 植物生长调节剂对玉露香梨组培苗继代的影响 以MS+0.10 mg·L-1 NAA 为基本培养基,附加0.50、1.00、1.50 和2.00 mg·L-1 6-BA;以MS+1.00 mg·L-1 6-BA 为基本培养基,附加0.05、0.10、0.15 和0.20 mg·L-1 IBA 或NAA(pH 值为5.8~6.0),共11个处理用于试验。
1.2.4 基本培养基对玉露香梨组培苗生根的影响以MS、1/2MS、1/4MS、NN69、1/2NN69、1/4NN69 为基本培养基,附加2.00 mg·L-1 NAA、20.0 g·L-1蔗糖、6.0 g·L-1琼脂(pH 值为5.8~6.0),共6 个处理用于试验,每个处理接9 瓶,每瓶接5 个1 cm 单芽茎段,采用完全随机试验设计,3 次重复,常规培养40 d 后,调查统计生根率及平均生根数(生根率/%=生根株数/接种株数×100;平均生根数/条=主根发生总数/接种株数;茎尖枯死率/%=茎尖枯死的株数/接种株数×100;下同)。
1.2.5 植物生长调节剂对玉露香梨组培苗生根的影响 以1/2MS 为基本培养基,附加0.20、0.50、1.00、2.00、5.00 mg·L-1 IBA 或NAA(pH 值为5.8~6.0),共10个处理用于试验。
1.2.6 暗培养时间对玉露香梨组培苗生根的影响 以1.2.4、1.2.5 筛选到的生根培养基为基础,设置暗培养时间为0、5、10、15、20 d,共5 个处理用于试验。
1.2.7 活性炭对玉露香梨组培苗生根的影响 以1.2.4、1.2.5 筛选到的生根培养基为基础,设置活性炭质量浓度为0.0、0.5、1.0、2.0、4.0 g·L-1,共5个处理用于试验。
1.2.8 玉露香梨组培生根苗驯化移栽 选取生根诱导40 d 的玉露香梨组培苗132 棵,于温室强光(18 000~35 000 lx)闭瓶锻炼7 d 后再开瓶锻炼3 d,然后移栽到基质(草炭土、蛭石体积比为1∶2,121 ℃高压灭菌20 min 后晾凉备用)中。移栽后,喷施0.1%多菌灵预防病害,扣育苗塑料盖保温保湿。移栽7 d 后逐渐通风,10 d 后去掉塑料盖。调查移栽10、15、25、40、60 d 后玉露香梨组培苗的成活率(移栽成活率/%=成活株数/移栽株数×100),并观察记录幼苗生长状态。
1.3 数据分析
使用Excel 进行数据统计,利用DPS 软件进行数据分析,采用Duncan’s新复极差法进行差异显著性分析。
2 结果与分析
2.1 基本培养基对玉露香梨组培苗继代的影响
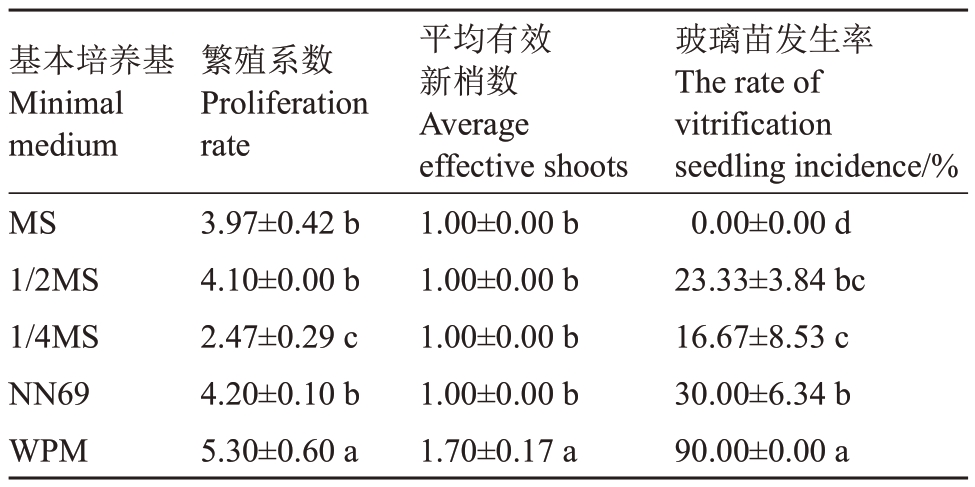
试验结果表明,WPM 处理的繁殖系数、平均有效新梢数及玻璃苗发生率均显著高于其他处理。MS、1/2MS、NN69 三者间的繁殖系数无显著差异,但均显著高于1/4MS 处理;MS、1/2MS、1/4MS、NN69 处理间的平均有效新梢数均无显著差异(表1)。除了MS 处理无玻璃苗出现外,1/2MS、1/4MS、NN69、WPM 处理组培苗均有不同程度的玻璃化现象。由图1可知,MS处理叶片大,叶色浓绿,相对更加健壮;1/2MS、1/4MS、NN69 处理叶片小而黄,轻微玻璃化;而WPM 处理叶片较大,叶色发黄,顶端卷曲,质脆,整体玻璃化严重。综合评价认为,适合玉露香梨组培苗继代增殖的基本培养基为MS培养基。
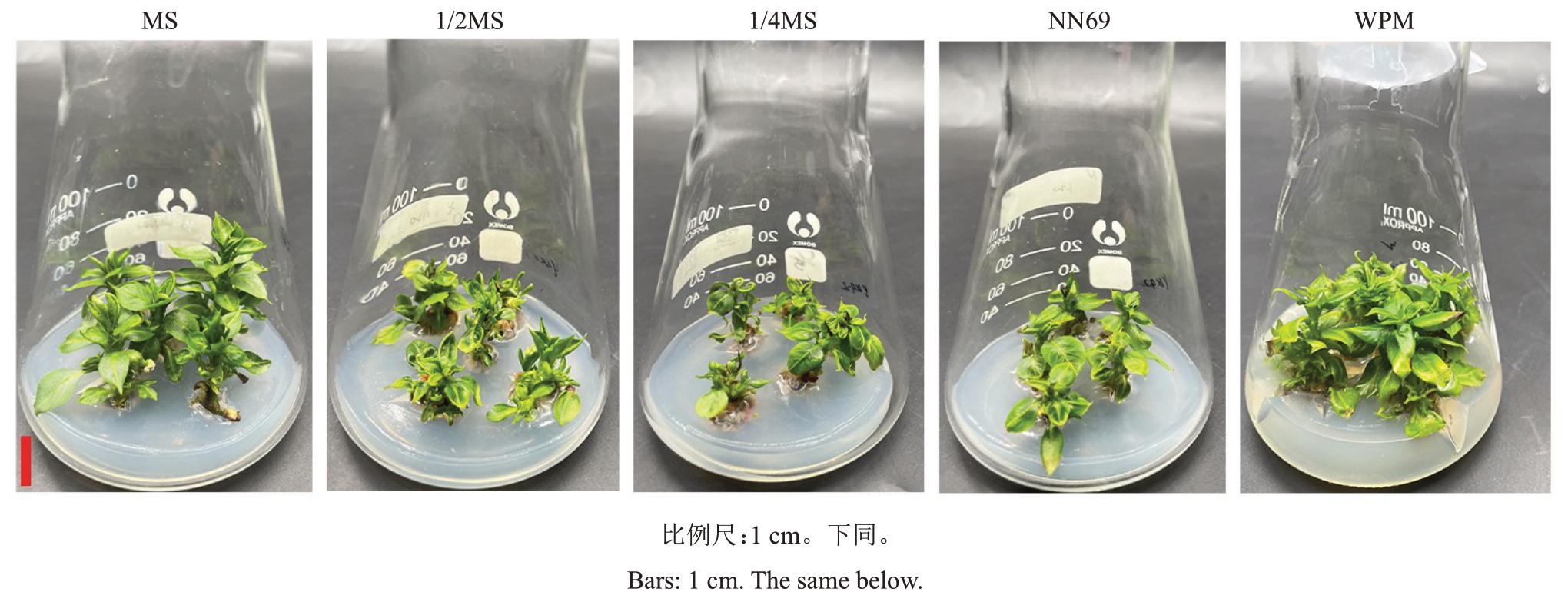
图1 基本培养基对玉露香梨组培苗生长的影响
Fig.1 Effects of minimal medium types on the growth of Yuluxiang pear in vitro
表1 基本培养基对玉露香梨组培苗继代增殖的影响
Table 1 Effects of minimal medium types on the proliferation of Yuluxiang pear in vitro
注:同一列中不同小写字母代表p<0.05 水平上的差异。下同。
Note:The different small letters in the same column indicated significant difference at p<0.05.The same below.
玻璃苗发生率The rate of vitrification seedling incidence/%0.00±0.00 d 23.33±3.84 bc 16.67±8.53 c 30.00±6.34 b 90.00±0.00 a基本培养基Minimal medium MS 1/2MS 1/4MS NN69 WPM繁殖系数Proliferation rate 3.97±0.42 b 4.10±0.00 b 2.47±0.29 c 4.20±0.10 b 5.30±0.60 a平均有效新梢数Average effective shoots 1.00±0.00 b 1.00±0.00 b 1.00±0.00 b 1.00±0.00 b 1.70±0.17 a
2.2 植物生长调节剂对玉露香梨组培苗继代的影响
当NAA质量浓度为0.10 mg·L-1时,1.00 mg·L-1 6-BA处理的繁殖系数显著高于低质量浓度(0.50 mg·L-1 6-BA)和较高质量浓度(1.50~2.00 mg·L-1 6-BA)处理,1.00 mg·L-1 6-BA处理与其他处理间的平均有效新梢数差异不显著(表2,图2)。总体而言,当NAA质量浓度为0.10 mg·L-1时,培养基中添加1.00 mg·L-1 6-BA 较合适,6-BA 质量浓度过高或过低均抑制玉露香梨继代增殖。
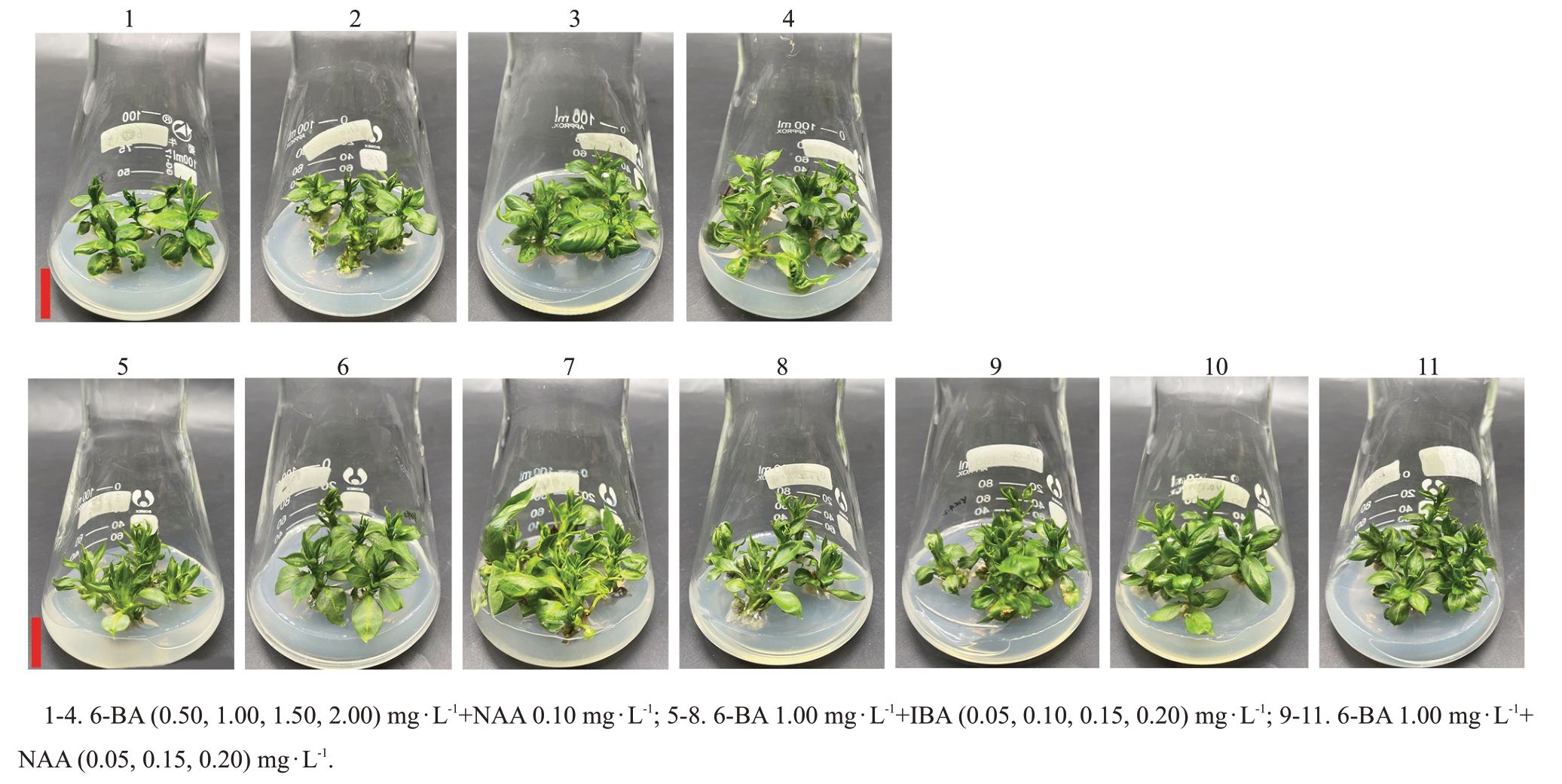
图2 植物生长调节剂对玉露香梨组培苗生长的影响
Fig.2 Effects of plant growth regulators on the growth of Yuluxiang pear in vitro
表2 植物生长调节剂对玉露香梨组培苗继代增殖的影响
Table 2 Effects of plant growth regulators on the proliferation of Yuluxiang pear in vitro
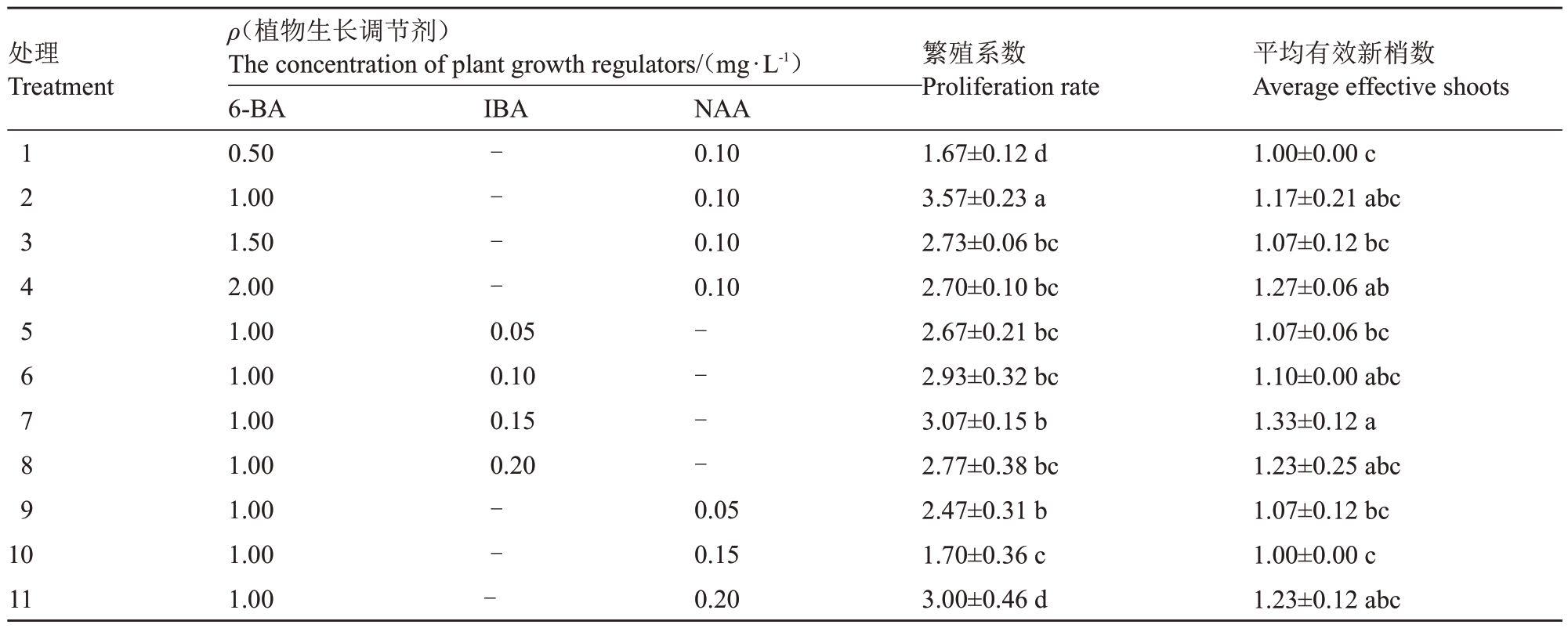
处理Treatment IBA繁殖系数Proliferation rate平均有效新梢数Average effective shoots 1 2 3 4 5 6 7 8 9----NAA 0.10 0.10 0.10 0.10 ρ(植物生长调节剂)The concentration of plant growth regulators/(mg·L-1)6-BA 0.50 1.00 1.50 2.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 0.05 0.10 0.15 0.20----10 11---0.05 0.15 0.20 1.67±0.12 d 3.57±0.23 a 2.73±0.06 bc 2.70±0.10 bc 2.67±0.21 bc 2.93±0.32 bc 3.07±0.15 b 2.77±0.38 bc 2.47±0.31 b 1.70±0.36 c 3.00±0.46 d 1.00±0.00 c 1.17±0.21 abc 1.07±0.12 bc 1.27±0.06 ab 1.07±0.06 bc 1.10±0.00 abc 1.33±0.12 a 1.23±0.25 abc 1.07±0.12 bc 1.00±0.00 c 1.23±0.12 abc
当6-BA质量浓度为1.00 mg·L-1时,不同质量浓度IBA 处理间繁殖系数差异不显著,0.15 mg·L-1 IBA 处理的平均有效新梢数显著高于0.05 mg·L-1 IBA 处理,但与0.10、0.20 mg·L-1 IBA 处理间差异不显著,说明0.10、0.15、0.20 mg·L-1IBA均适合玉露香梨继代增殖。当6-BA质量浓度为1.00 mg·L-1时,不同质量浓度NAA 处理间平均有效新梢数差异不显著,但0.10 mg·L-1 NAA 的繁殖系数显著高于0.05、0.15、0.20 mg·L-1 NAA 处理,表明0.10 mg·L-1 NAA适合玉露香继代增殖(表2,图2)。
整体看,1.00 mg·L-1 6-BA+0.10 mg·L-1 NAA 处理的繁殖系数显著高于其他处理,平均有效新梢数与其他处理间无显著差异,更适合玉露香梨组培苗继代扩繁。
2.3 基本培养基对玉露香梨组培苗生根的影响
MS、1/2MS 两处理间生根率、平均生根条数均无显著差异,但均显著高于其他处理,这两个处理的植株地上部叶片均较大、较为舒展、叶色油绿,长势好于其他处理(表3、图3)。在不同浓度NN69 处理下,随NN69浓度降低,生根率显著下降,1/4NN69处理的生根率、生根数均为0(表3);不同NN69处理下玉露香组培苗均出现叶小、卷曲、皱缩、发黄的情况,整体长势较差。除去1/2MS 与NN69 处理的茎尖枯死率为0 外,其他处理均有不同程度茎尖枯死情况出现。综合评价认为,适合玉露香梨组培苗生根的基本培养基为1/2MS(图3)。
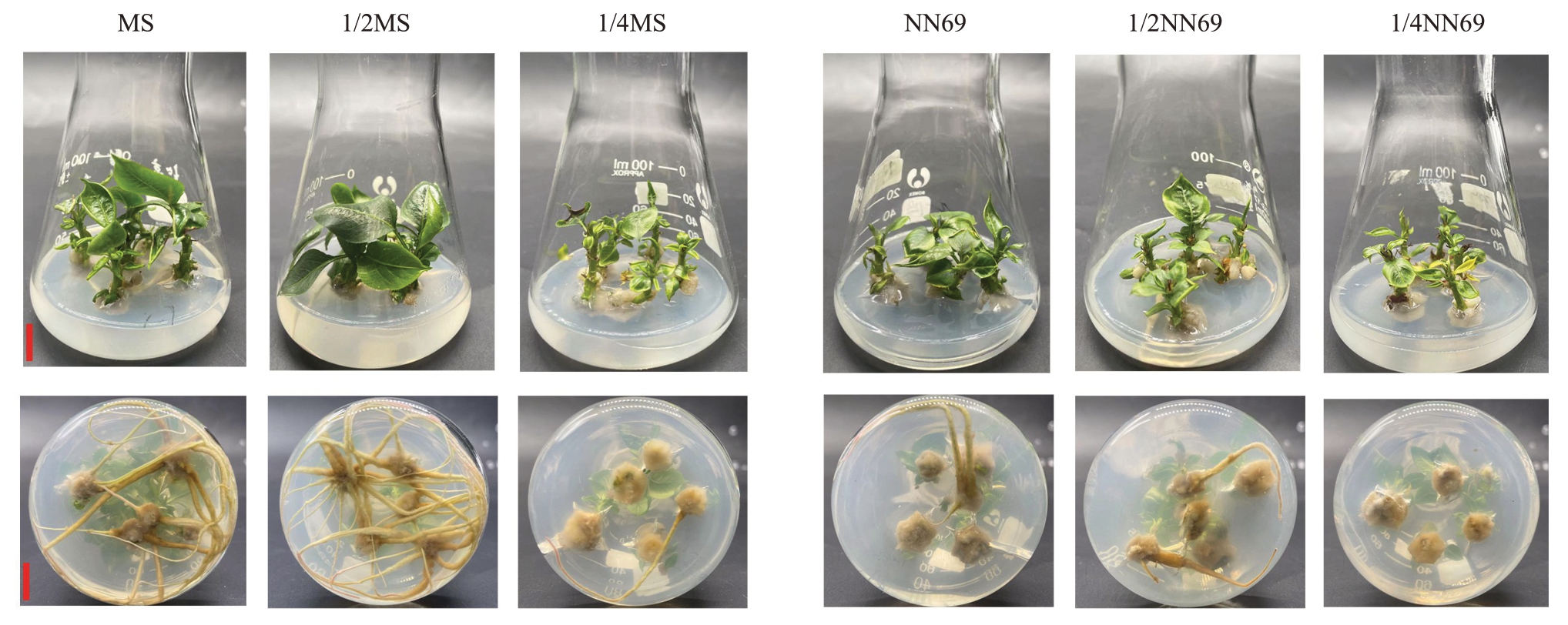
图3 基本培养基对玉露香梨组培苗生长的影响
Fig.3 Effects of minimal medium on the growth of Yuluxiang pear in vitro
表3 基本培养基对玉露香梨组培苗生根的影响
Table 3 Effects of different minimal medium on the rooting of Yuluxiang pear in vitro
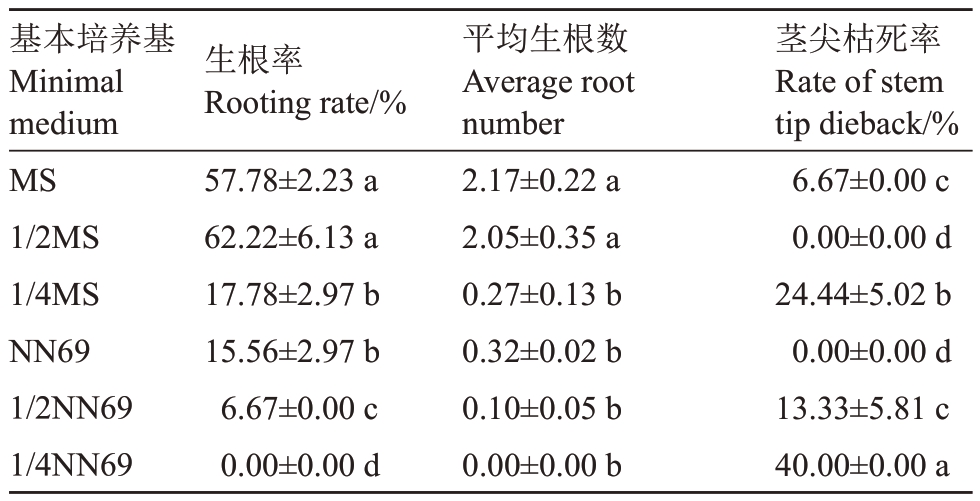
基本培养基Minimal medium MS 1/2MS 1/4MS NN69 1/2NN69 1/4NN69生根率Rooting rate/%57.78±2.23 a 62.22±6.13 a 17.78±2.97 b 15.56±2.97 b 6.67±0.00 c 0.00±0.00 d平均生根数Average root number 2.17±0.22 a 2.05±0.35 a 0.27±0.13 b 0.32±0.02 b 0.10±0.05 b 0.00±0.00 b茎尖枯死率Rate of stem tip dieback/%6.67±0.00 c 0.00±0.00 d 24.44±5.02 b 0.00±0.00 d 13.33±5.81 c 40.00±0.00 a
2.4 植物生长调节剂对玉露香梨组培苗生根的影响
在0.20~5.00 mg·L-1NAA范围内,随质量浓度增加,生根率显著提高;当NAA质量浓度为5.00 mg·L-1时,生根率达到最高,为70.00%;而除5.00 mg·L-1 IBA 处理外,其他质量浓度IBA 处理间的生根率差异均不显著。当NAA质量浓度为2.00 mg·L-1时,平均生根数为3.40,显著高于其他处理,此时生根率为60.00%(表4、图4)。以上结果表明,2.00 mg·L-1 NAA更适于玉露香梨组培苗生根。
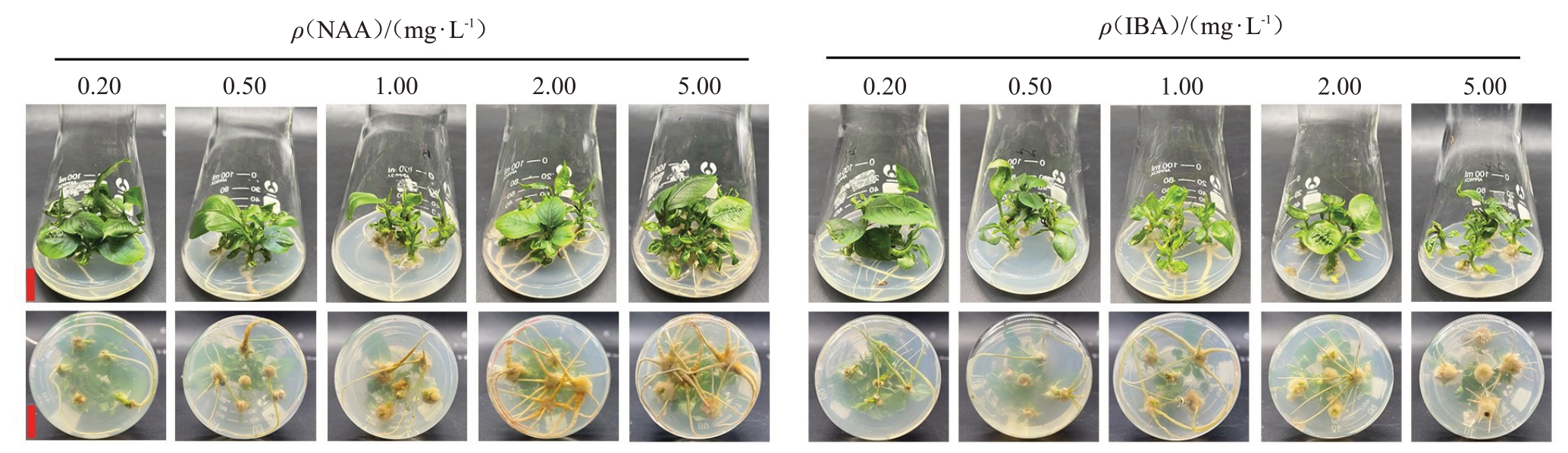
图4 植物生长调节剂对玉露香梨组培苗生长的影响
Fig.4 Effects of plant growth regulators on the growth of Yuluxiang pear in vitro
表4 植物生长调节剂对玉露香梨组培苗生根的影响
Table 4 Effects of plant growth regulators on the rooting of Yuluxiang pear in vitro
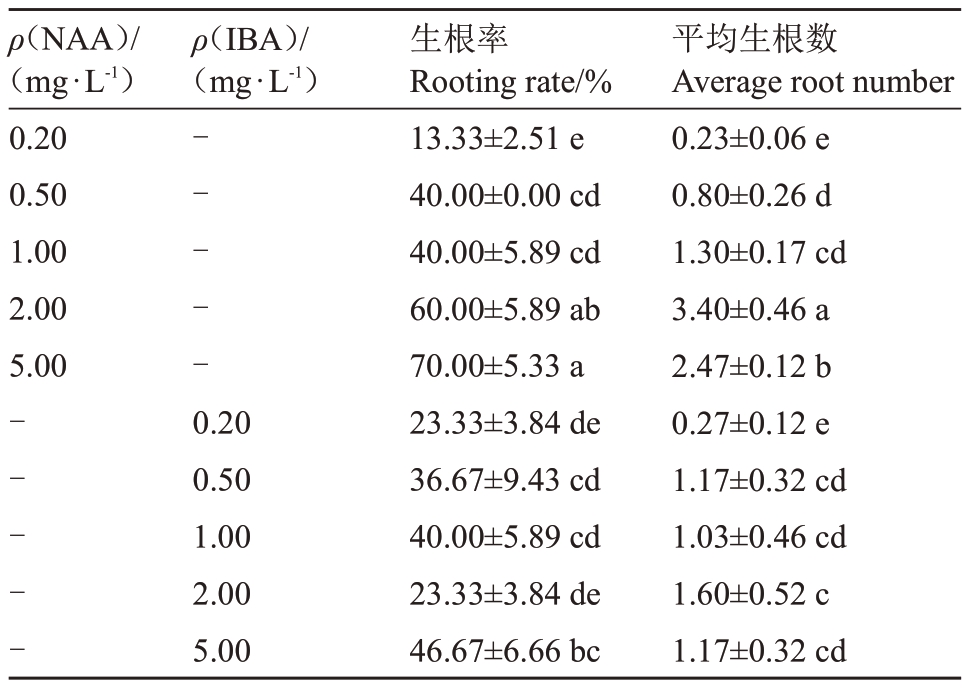
ρ(NAA)/(mg·L-1)0.20 0.50 1.00 2.00 5.00 ρ(IBA)/(mg·L-1)----------0.20 0.50 1.00 2.00 5.00生根率Rooting rate/%13.33±2.51 e 40.00±0.00 cd 40.00±5.89 cd 60.00±5.89 ab 70.00±5.33 a 23.33±3.84 de 36.67±9.43 cd 40.00±5.89 cd 23.33±3.84 de 46.67±6.66 bc平均生根数Average root number 0.23±0.06 e 0.80±0.26 d 1.30±0.17 cd 3.40±0.46 a 2.47±0.12 b 0.27±0.12 e 1.17±0.32 cd 1.03±0.46 cd 1.60±0.52 c 1.17±0.32 cd
2.5 暗培养时间对玉露香梨组培苗生根的影响
暗培养0、5、15 d 处理间生根率、平均生根数均无显著差异,但均显著高于暗培养10、20 d 处理。未经暗培养处理时,根系较粗壮;随暗培养时间增加,根系变细,茎尖枯死率显著增加,根部愈伤变大;暗培养20 d 后,茎尖全部枯死,愈伤达到最大(表5、图5)。以上结果表明,玉露香梨组培苗生根的培养条件为常规光培养,不适宜暗培养。
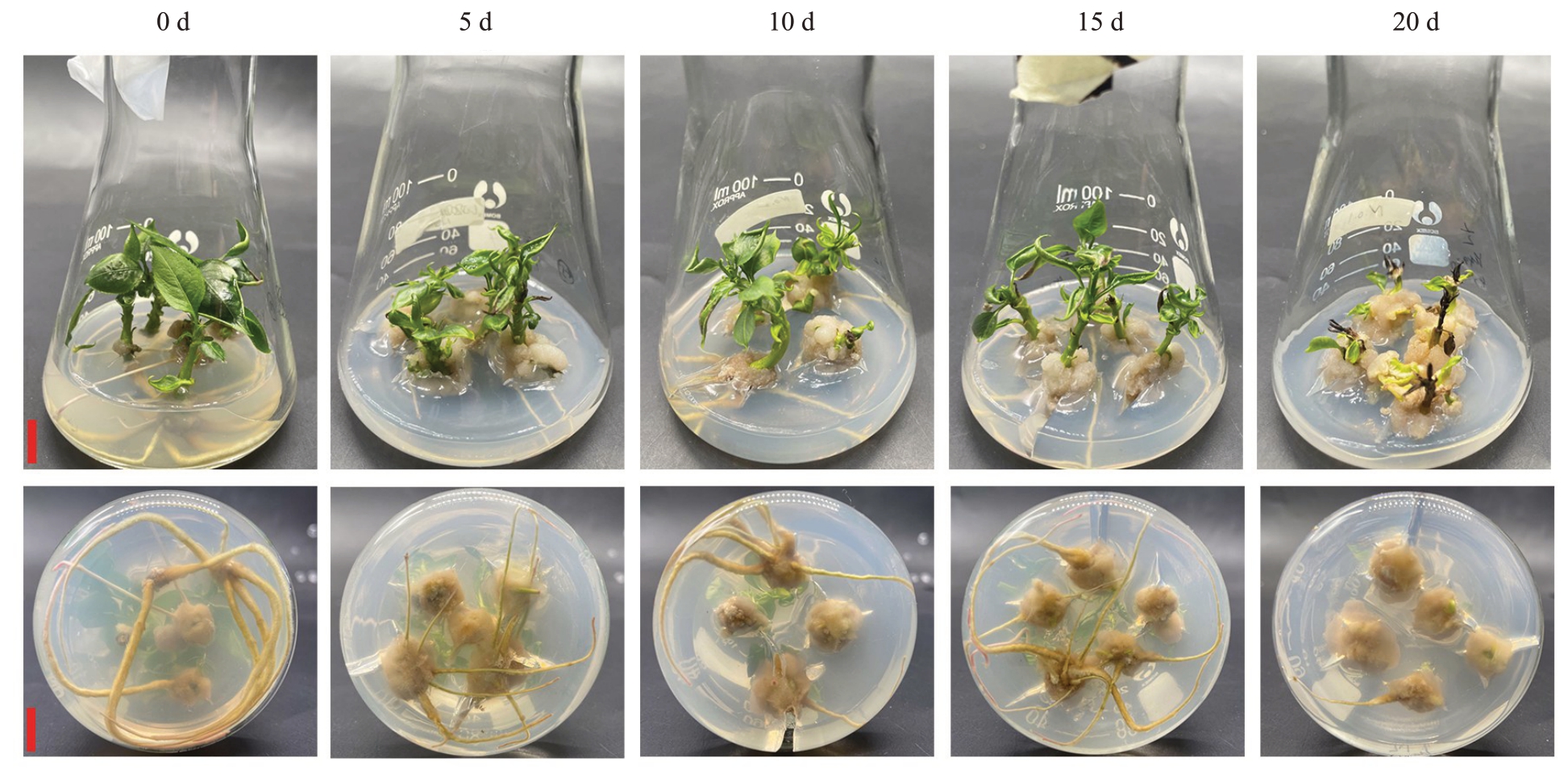
图5 暗培养时间对玉露香梨组培苗生长的影响
Fig.5 Effects of dark culture time on the growth of Yuluxiang pear in vitro
表5 暗培养时间对玉露香梨组培苗生根的影响
Table 5 Effects of darkness culture on the rooting of Yuluxiang pear in vitro
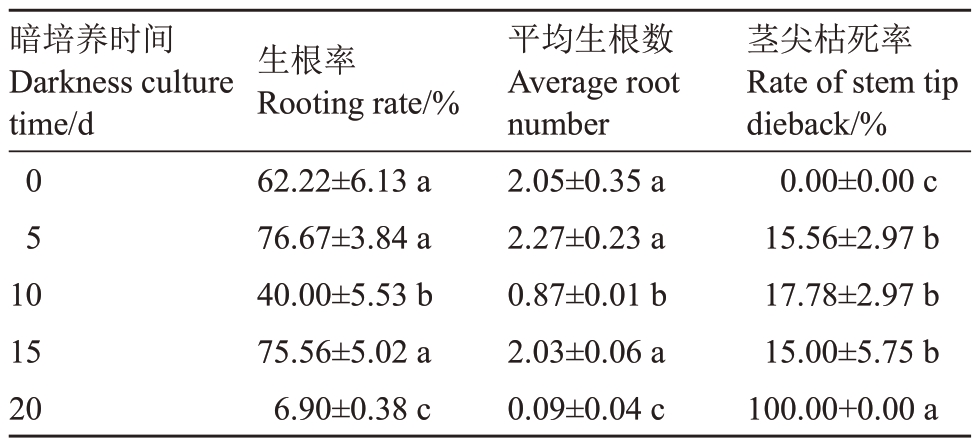
暗培养时间Darkness culture time/d 0 5 10 15 20生根率Rooting rate/%62.22±6.13 a 76.67±3.84 a 40.00±5.53 b 75.56±5.02 a 6.90±0.38 c平均生根数Average root number 2.05±0.35 a 2.27±0.23 a 0.87±0.01 b 2.03±0.06 a 0.09±0.04 c茎尖枯死率Rate of stem tip dieback/%0.00±0.00 c 15.56±2.97 b 17.78±2.97 b 15.00±5.75 b 100.00+0.00 a
2.6 活性炭对玉露香梨组培苗生根的影响
随活性炭质量浓度增加,玉露香梨组培苗生根率及生根数均显著降低或减少,根部愈伤也变小;当活性炭质量浓度等于或高于1.0 g·L-1时,生根率及生根数均为0;当活性炭质量浓度为4.0 g·L-1时,无愈伤产生(表6)。0.5 g·L-1活性炭可使叶片变绿,但质量浓度高于1.0 g·L-1时,叶片开始变黄,部分出现褐化现象(图6)。结果表明,活性炭对玉露香梨组培苗生根有显著抑制作用。
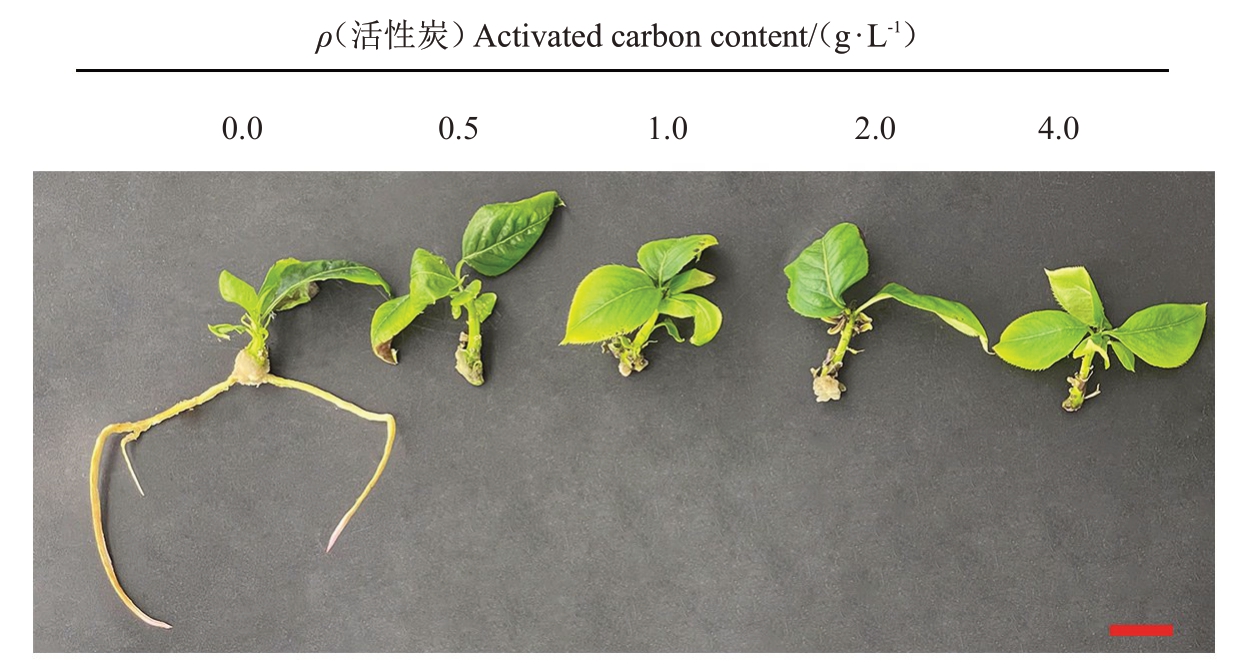
图6 不同活性炭质量浓度对玉露香梨组培苗生长的影响
Fig.6 Effects of different concentrations of activated carbon on the growth of Yuluxiang pear in vitro
表6 活性炭对玉露香梨组培苗生根的影响
Table 6 Effects of different concentrations of activated carbon on the rooting of Yuluxiang pear in vitro
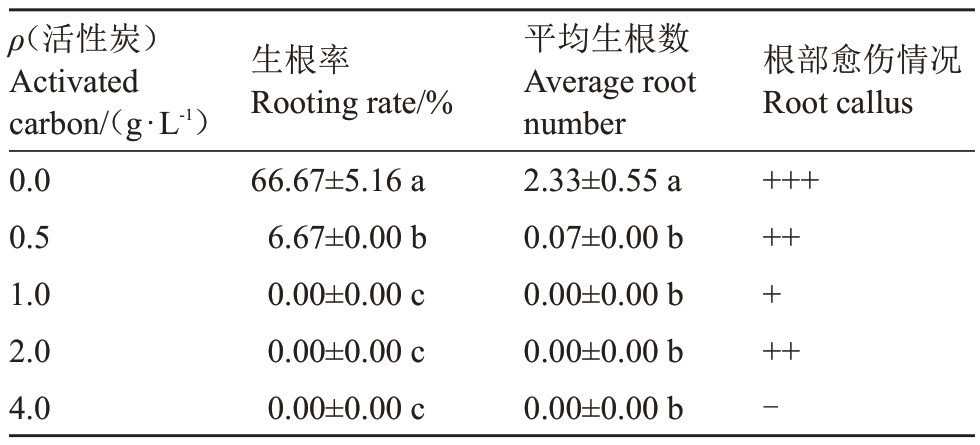
注:根部愈伤中标注“-”“+”分别表示无愈伤、有愈伤产生,且“+”越多,表示产生的愈伤越大。
Note:The marks“-”and“+”in root callus respectively indicated that there was no callus,and there was callus,and the more“+”indicated the bigger callus.
ρ(活性炭)Activated carbon/(g·L-1)0.0 0.5 1.0 2.0 4.0生根率Rooting rate/%66.67±5.16 a 6.67±0.00 b 0.00±0.00 c 0.00±0.00 c 0.00±0.00 c平均生根数Average root number 2.33±0.55 a 0.07±0.00 b 0.00±0.00 b 0.00±0.00 b 0.00±0.00 b根部愈伤情况Root callus++++++++-
2.7 玉露香梨组培生根苗驯化移栽
将生根诱导40 d 的玉露香梨组培苗,于温室驯化10 d 后移栽至营养钵中。观察发现,玉露香梨组培苗在移栽10 d 后开始有死亡现象出现,随着时间推移死亡增多,移栽60 d 后,死亡情况趋于稳定,成活率为32.57%,存活的植株生长良好(图7)。
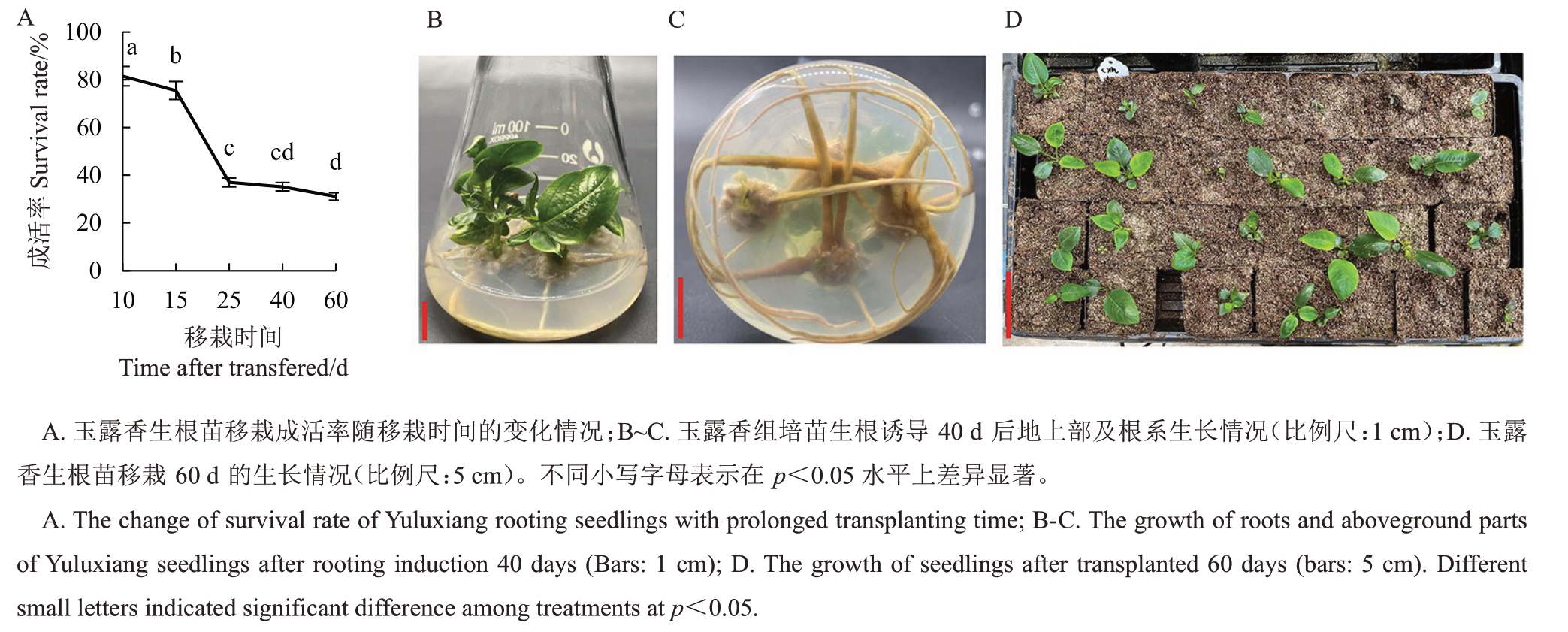
图7 玉露香梨移栽成活情况
Fig.7 The survival of Yuluxiang pear seedlings after transplanted
3 讨 论
3.1 基本培养基对玉露香梨组培苗生长的影响
基本培养基是继代增殖及生根的关键因素,梨常用基本培养基有MS、NN69、1/2MS、WPM 等[10-11];其中,WPM 有助于木本植物的生长和分化,在木本植物组织培养中较为常用[12]。本试验通过对比MS、1/2MS、1/4MS、NN69、WPM等5种基本培养基,发现使用WPM的繁殖系数显著高于其余4种培养基,但组培苗的玻璃化程度也显著增高。郭静等[13]报道,与MS相比,WPM培养基可显著降低苹果砧木G.11的玻璃化率,但在玉露香梨中得出了相反结果,具体原因有待进一步研究。
3.2 植物生长调节剂对玉露香梨组培苗继代和生根的影响
植物生长调节剂对组培苗继代增殖及生根有重要影响[14-16]。组培苗继代增殖中常用激素为6-BA、NAA、IBA、IAA,其中6-BA 常与NAA、IBA、IAA 配合使用。笔者通过对比6-BA 与NAA、IBA 不同组合下玉露香梨的继代增殖效果发现,当NAA 的质量浓度为0.10 mg·L-1时,添加1.00 mg·L-1的6-BA才会对继代增殖有显著促进作用,添加低质量浓度(0.50 mg·L-1)或较高质量浓度(1.50~2.00 mg·L-1)6-BA 时,繁殖系数均较低,不利于玉露香继代扩繁。杨冠宇等[17]研究也发现,6-BA质量浓度过高或过低均不适宜杜梨7-4株系进行扩繁,当6-BA质量浓度为0.30~1.50 mg·L-1时,杜梨7-4 株系组培苗生长正常,繁殖系数较高,均在3.90以上;当6-BA质量浓度降低到0.10~0.20 mg·L-1或提高到3.00 mg·L-1时,繁殖系数均降低。其他品种如西洋梨矮化砧木BA-29[18]、砀山酥梨[19]等也得出了相同的结论。笔者发现当6-BA 质量浓度为1.00 mg·L-1时,添加0.10~2.00 mg·L-1 IBA 或NAA 均能促进玉露香梨组培苗继代扩繁,但6-BA 与0.10 mg·L-1NAA 组合的扩繁效果要好于6-BA与不同质量浓度IBA处理的组合,因此适合玉露香梨继代扩繁的激素种类为6-BA 与NAA。前人报道适合津香蜜、巴梨、南果梨继代扩繁的激素也为6-BA与NAA[20-21]。
组培生根常用激素为NAA、IBA、IAA,使用其中一种或两种以上均可促进生根。笔者发现仅用NAA 或IBA 便可促进玉露香生根,IBA 处理生根率最高为46.67%,NAA 处理生根率可达70.00%。前人报道丰水梨、云南榅桲在仅添加NAA的生根培养基上也可生根,生根率分别为91.30%、62.50%[22-23]。
配合使用IBA、IAA 也可促进生根,如巴梨在1/2MS+1.00 mg·L-1 IAA+1.00 mg·L-1 IBA 生根培养基中生根率为56.70%,津香蜜在1/2MS+1.00 mg·L-1 IAA+3.00 mg·L-1 IBA中生根率为70.30%[20],杜梨在1/2MS+2.00 mg·L-1 IAA+0.50 mg·L-1 IBA 中生根率为86.70%[24]。NAA与IBA配合使用也有较好效果,如西洋梨矮化砧木BA-29 在1/2MS+0.50 mg·L-1 NAA+0.50 mg·L-1 IBA中生根率为81.56%[18]。
3.3 影响玉露香梨组培苗生根的其他因素
光照强弱对梨组培苗生根有重要影响,汤浩茹等[25]发现前期适当暗培养可促进早酥和身不知生根;田海青[26]将新梨7号暗培养7 d后转到光下常规培养,生根率达75.00%。而汤浩茹等[25]发现巴梨和考密斯等西洋梨在暗培养时,生根诱导效果较差;苗冉冉等[20]也发现暗培养对津香蜜和巴梨不定根诱导均无显著促进作用,且会使组培苗出现细弱、枯尖现象。本试验发现暗培养0~20 d对玉露香梨组培苗生根均无显著促进作用,且随暗培养时间增加,愈伤明显增大,茎尖枯死现象加重。
一般来说,适当质量浓度活性炭有助于组培苗生根,如现代月季叶片再生植株添加0.1%活性炭可形成完整根系[27];甜樱桃组培苗添加1.0 g·L-1活性炭生根率可达100.00%[28];黄金梨组培苗加入0.5 g·L-1活性炭后,生根率超过80.00%[29];秋子梨添加1.0 g·L-1活性炭后生根率由45.50%提高到90.00%,平均生根条数由2.24 提高到6.20,根系长度和植株表面积均显著增加[30-31]。但也有研究表明活性炭会抑制组培苗生根,如0.5 g·L-1和1.0 g·L-1活性炭显著抑制中矮1号组培苗生根[32]。本试验发现,活性炭对玉露香组培苗生根有显著抑制作用,当活性炭质量浓度为1.0~4.0 g·L-1时,生根率及生根数均为0。
4 结 论
适合玉露香梨组培苗继代增殖的培养基配方为MS+1.00 mg·L-1 6-BA+0.10 mg·L-1 NAA,繁殖系数为3.57,平均有效新梢数为1.17;适合玉露香梨组培苗生根的培养基配方为1/2MS+2.00 mg·L-1 NAA,生根率为60.00%,平均生根条数为3.40;暗培养和添加活性炭均不适于玉露香梨组培苗生根。
[1] 李昌珠,JIRI S,BLAZEK J. 不同基因型欧洲梨离体繁殖研究[J].果树学报,2002,19(4):227-230.LI Changzhu,JIRI S,BLAZEK J. Studies on in vitro propagation of common pear(Pyrus communis Linn.)with different genotypes[J].Journal of Fruit Science,2002,19(4):227-230.
[2] 宋梅,王淑娟,刘振江,刘红旗.香梨、砀山梨组织培养及脱毒快繁技术[J].新疆农业科学,2003,40(6):376-377.SONG Mei,WANG Shujuan,LIU Zhenjiang,LIU Hongqi. Tissue culture and de-poison high-speed breeding technique of Fragrant pear and Dangshan pear[J]. Xinjiang Agricultural Sciences,2003,40(6):376-377.
[3] 刘小芳,冯建荣,梁晓桐,吕文娟,李文慧,樊新民.库尔勒香梨组织培养的研究[J].山东农业科学,2016,48(5):9-13.LIU Xiaofang,FENG Jianrong,LIANG Xiaotong,LÜ Wenjuan,LI Wenhui,FAN Xinmin. Research on tissue culture of Korla fragrant pear[J].Shandong Agricultural Sciences,2016,48(5):9-13.
[4] 段莹莹,田彩芳,宋宇琴,李六林,王旭.‘中梨一号’离体培养与快速繁殖[J].北方果树,2014(5):7-9.DUAN Yingying,TIAN Caifang,SONG Yuqin,LI Liulin,WANG Xu. In vitro culture and rapid propagation of‘Zhongli 1’pear[J].Northern Fruits,2014(5):7-9.
[5] 宗娟,朱立武,贾兵. 黄冠梨茎尖离体培养再生体系建立初探[J].广东农业科学,2010,37(2):51-53.ZONG Juan,ZHU Liwu,JIA Bing. Primary study of regeneration system of pear cultivar‘Huangguan’stem apex by in vitro culture[J]. Guangdong Agricultural Sciences,2010,37(2):51-53.
[6] 郭黄萍,李晓梅,张建功.优质中熟红梨新品种‘玉露香’(暂定名)[J].山西果树,2001(1):3-4.GUO Huangping,LI Xiaomei,ZHANG Jiangong. A new variety of high-quality medium ripe red pear‘Yuluxiang’(tentative name)[J].Shanxi Fruits,2001(1):3-4.
[7] 谢鹏,蔚露,王红宁,林琭,牛自勉.不同产区玉露香梨果实品质特性综合分析[J].果树学报,2023,40(11):2371-2380.XIE Peng,YU Lu,WANG Hongning,LIN Lu,NIU Zimian.Comprehensive analysis on the fruit quality of Yuluxiang pear in different production areas[J]. Journal of Fruit Science,2023,40(11):2371-2380.
[8] 于宛婷,王文辉,张鑫楠,阎维巍,孙晓楠,贾晓辉.外源褪黑素对玉露香梨常温贮藏品质和生理特性的影响[J].果树学报,2023,40(8):1583-1591.YU Wanting,WANG Wenhui,ZHANG Xinnan,YAN Weiwei,SUN Xiaonan,JIA Xiaohui. Effects of exogenous melatonin on fruit quality and physiological characteristics during room temperature storage in Yuluxiang pear[J]. Journal of Fruit Science,2023,40(8):1583-1591.
[9] 贾晓辉,王文辉,姜云斌,王志华,杜艳民,佟伟.采收成熟度对‘玉露香’梨果实品质和耐贮性的影响[J].果树学报,2016,33(5):594-603.JIA Xiaohui,WANG Wenhui,JIANG Yunbin,WANG Zhihua,DU Yanmin,TONG Wei. Effects of harvest maturity on fruit quality and storage life of‘Yuluxiang’pears[J].Journal of Fruit Science,2016,33(5):594-603.
[10] 叶宇,欧春青,王斐,张艳杰,马力,杨冠宇,李佳纯,姜淑苓.梨组织培养研究进展及其在育种中的应用[J]. 中国果树,2022(5):1-7.YE Yu,OU Chunqing,WANG Fei,ZHANG Yanjie,MA Li,YANG Guanyu,LI Jiachun,JIANG Shuling. Research progresses of tissue culture in pear and its application in breeding[J].China Fruits,2022(5):1-7.
[11] 王海燕.农杆菌介导rolB 基因转化杜梨的研究[D].保定:河北农业大学,2014.WANG Haiyan.The rolB gene transformation via agrobacterium tumefaciens mediated in pear rootstock‘Pyrus betulifolia Bge.’[D].Baoding:Hebei Agricultural University,2014.
[12] 王蒂,陈劲枫.植物组织培养[M].2 版.北京:中国农业出版社,2013.WANG Di,CHEN Jinfeng. Plant tissue culture[M]. 2nd ed. Beijing:China Agriculture Press,2013.
[13] 郭静,柴慈江,史燕山,骆建霞,江文.苹果砧木G.11 试管苗增殖与生根培养研究[J].天津农学院学报,2019,26(4):38-42.GUO Jing,CHAI Cijiang,SHI Yanshan,LUO Jianxia,JIANG Wen.Micro shoot proliferation and rooting of apple rootstock G.11[J].Journal of Tianjin Agricultural University,2019,26(4):38-42.
[14] 闫帅,徐锴,袁继存,程存刚,张少瑜,赵德英.梨组织培养及遗传转化研究进展[J].中国果树,2017(增刊1):72-77.YAN Shuai,XU Kai,YUAN Jicun,CHENG Cungang,ZHANG Shaoyu,ZHAO Deying. Review of pear regeneration and genetic transformation system[J]. China Fruits,2017(Suppl. 1):72-77.
[15] JIN W M,WANG Y H,WANG H.Adventitious shoot regeneration from leaves of apple rootstock‘Pingyitiancha’(Malus hupehensis var. pinyiensis) and genetic fidelity of regenerated plantlets using SSR markers[J]. Canadian Journal of Plant Science,2014,94(8):1345-1354.
[16] SAINI S,SHARMA I,KAUR N,PATI P K. Auxin:A master regulator in plant root development[J].Plant Cell Reports,2013,32(6):741-757.
[17] 杨冠宇,王斐,张艳杰,马力,李佳纯,欧春青,姜淑苓.杜梨砧木组培扩繁技术研究[J].中国果树,2022(6):59-63.YANG Guanyu,WANG Fei,ZHANG Yanjie,MA Li,LI Jiachun,OU Chunqing,JIANG Shuling. Study on tissue culture propagation technology of Pyrus betulifolia rootstocks[J]. China Fruits,2022(6):59-63.
[18] 王苏珂,杨健,王龙,周厚成,李秀根.西洋梨矮化砧木BA-29的组培快繁技术研究[J].果树学报,2010,27(6):1002-1005.WANG Suke,YANG Jian,WANG Long,ZHOU Houcheng,LI Xiugen. Studies on tissue culture and rapid propagation of dwarf rootstock BA-29 of European pear[J].Journal of Fruit Science,2010,27(6):1002-1005.
[19] 金青,蔡永萍.‘砀山酥梨’的组织培养和脱毒快速繁殖[J].植物生理学通讯,2006,42(5):900.JIN Qing,CAI Yongping. Tissue culture and rapid propagation of Pyrus bretschneideri Rehd.[J].Plant Physiology Communications,2006,42(5):900.
[20] 苗冉冉,乔月莲,吴沅洙,王莉,师校欣,杜国强.‘津香蜜’和‘巴梨’组织培养快速繁殖[J]. 分子植物育种,2019,17(7):2297-2302.MIAO Ranran,QIAO Yuelian,WU Yuanzhu,WANG Li,SHI Xiaoxin,DU Guoqiang. Rapid propagation of‘Jinxiangmi’and‘Bartlett’pear by tissue culture[J]. Molecular Plant Breeding,2019,17(7):2297-2302.
[21] 陈丽静,于春叶,李浩戈,张丽,钟鸣,马慧.南果梨茎尖离体快繁技术研究[J].中国农学通报,2011,27(31):168-173.CHEN Lijing,YU Chunye,LI Haoge,ZHANG Li,ZHONG Ming,MA Hui. Rapid propagation of Pyrus ussuriensis Maxin.by shoot tip culture[J]. Chinese Agricultural Science Bulletin,2011,27(31):168-173.
[22] 侯修胜. 丰水梨试管快速繁殖技术研究[J]. 河北林业科技,2003(1):1-2.HOU Xiusheng. Study on the tube rapid propagation techniques of‘Hosui’pear[J].Journal of Hebei Forestry Science and Technology,2003(1):1-2.
[23] 徐凌飞,李致慧,贾东峰,李慧.梨矮化砧木云南榅桲离体快繁研究[J].北方园艺,2012(5):113-115.XU Lingfei,LI Zhihui,JIA Dongfeng,LI Hui. Study on in vitro propagation of Yunnan quince as a dwarf rootstock of pear[J].Northern Horticulture,2012(5):113-115.
[24] 闫帅,张少瑜,徐锴,袁继存,李晓光,周江涛,程存刚,赵德英.杜梨组培生根过程中多胺、内源激素及相关氧化酶活性的变化[J].果树学报,2019,36(3):318-326.YAN Shuai,ZHANG Shaoyu,XU Kai,YUAN Jicun,LI Xiaoguang,ZHOU Jiangtao,CHENG Cungang,ZHAO Deying. Dynamic changes in polyamines,endogenous hormones and oxidase activities during rooting of in vitro plantlets of Pyrus betulifolia Bunge[J].Journal of Fruit Science,2019,36(3):318-326.
[25] 汤浩茹,刘翠琼,罗娅,王小蓉.培养基和培养条件对4 个梨基因型试管苗生根的影响[J]. 果树学报,2006,23(2):283-286.TANG Haoru,LIU Cuiqiong,LUO Ya,WANG Xiaorong. Effects of media and cultural conditions on the rooting ability in vitro of 4 genotypes of Pyrus spp.[J]. Journal of Fruit Science,2006,23(2):283-286.
[26] 田海青. 新梨7 号梨组培快繁体系建立及微嫁接技术的研究[D].保定:河北农业大学,2014.TIAN Haiqing.Study on establishment of rapid propagation system and micrografting of the pear cultivar Xinli Pear 7[D].Baoding:Hebei Agricultural University,2014.
[27] 任桂芳,王建红,冯慧,李毅,李燕,施雪波. 现代月季(Rosa hybrida)叶片植株再生体系的建立[J].园艺学报,2004,31(4):533-536.REN Guifang,WANG Jianhong,FENG Hui,LI Yi,LI Yan,SHI Xuebo.Establishment of plant regeneration from leaves explants of Rosa hybrida[J].Acta Horticulturae Sinica,2004,31(4):533-536.
[28] 韩文璞,袁明莲.活性炭在甜樱桃组织培养中的应用[J].落叶果树,2001,33(3):7-8.HAN Wenpu,YUAN Minglian.Application of active carbon in tissue culture of sweet cherry[J]. Deciduous Fruits,2001,33(3):7-8.
[29] 王献革,及华,王利民.黄金梨的组织培养和快速繁殖[J].植物生理学通讯,2003,39(6):621.WANG Xiange,JI Hua,WANG Limin. Tissue culture and rapid propagation of Pyrus pyrifolia cv.Whangkumbe[J]. Plant Physiology Communications,2003,39(6):621.
[30] 王德芬,张梅,李鼎立,王然,马春晖,宋健坤.秋子梨叶片高效再生体系的构建[J].北方园艺,2016(4):97-101.WANG Defen,ZHANG Mei,LI Dingli,WANG Ran,MA Chunhui,SONG Jiankun.Establishment of high efficient regeneration system of Pyrus ussuriensis leaves[J]. Northern Horticulture,2016(4):97-101.
[31] 栾晓龙,史昊,许波,张倩男,刘莉.活性炭对秋子梨组培幼苗生根的影响[J].分子植物育种,2023,21(2):589-593.LUAN Xiaolong,SHI Hao,XU Bo,ZHANG Qiannan,LIU Li.Effect of activated carbon on rooting of tissue culture seedlings of Qiuzi pear(Pyrus ussuriensis Maxim.)[J]. Molecular Plant Breeding,2023,21(2):589-593.
[32] 王艺衡,冯静涵,于春亮,李涛,李金斗,赵健霄,张海霞,张玉星,马辉,许建锋.梨矮化砧木中矮1 号组培快繁技术研究[J].山东农业科学,2023,55(6):32-41.WANG Yiheng,FENG Jinghan,YU Chunliang,LI Tao,LI Jindou,ZHAO Jianxiao,ZHANG Haixia,ZHANG Yuxing,MA Hui,XU Jianfeng.Study on tissue culture and rapid propagation technology of pear dwarfing rootstock Zhong’ai 1[J]. Shandong Agricultural Sciences,2023,55(6):32-41.