桃[Prunus persica(L.)Batsch]为蔷薇科(Rosaceae)李属(Prunus)桃亚属(Persica)植物,原产于中国[1],有众多野生资源、地方品种及育成品种[2-3]。据统计,2021年中国桃种植面积和产量为82.50万hm2、1 601.65 万t,分别占全球比重的54.83%、64.08%。在品种结构上,近年中国培育出“果个大、产量高、耐贮运、风味浓郁”等类型多样的优良品种,基本满足了消费者对桃果品的多样性需求。根据着红色部位,可分为果皮红色与果肉红色,二者均因积累花色苷而呈现不同程度的红色[4]。花色苷是植物中广泛存在的一类水溶性黄酮类色素,在不同pH、温度等条件下,呈现出不同的颜色[5]。另外,花色苷还具有清除体内自由基、预防心血管硬化、抗肿瘤等保健功效[6]。以此特点,红肉桃被众多消费者所青睐,亦被越来越多的育种家作为重点选育对象。
花色苷合成通路上结构基因或调控转录因子的变异直接决定了花色苷的积累。例如,PpMYB10.1启动子上5243 bp 转座子插入导致桃果皮不能合成花色苷[7];PpBL启动子上6688 bp转座子是形成红肉桃的关键[8]。另外,转录因子PpNAC1、PpSPL1、PpHYH和PpHY5可直接激活或作用于PpMYB10.1,进而影响下游花色苷合成途径结构基因的表达,促进或抑制花色苷的合成[9-11]。环境因素如光照、温度、激素等对花色苷合成同样有较大影响。本文基于前人的报道,对桃果皮和果肉红色形成的遗传变异、变异前后作用机制差异以及环境因素影响的分子调控机制等进行了总结,为利用和开发分子标记进行桃亲本选配和杂种后代的早期选择提供帮助。
1 桃果实颜色多样性及与花色苷含量的关系
桃亚属在中国分布有6个种,包括普通桃[P.persica(L.)Batsch]、甘肃桃(P.kansuensis Rehd.)、山桃[P. davidiana(Carr.)Franch]、陕甘山桃(P. potanini Rehd.)、光核桃(P. mira Koehne)和新疆桃[P. ferganensis(Kost.et Kiab)Kov.et Kost][1]。其中,普通桃在中国被广泛栽培,在果皮颜色和果肉颜色多样性及遗传变异方面存在较大差异[12]。在《桃种质资源描述规范和数据标准》一书中,王力荣等[13]将果肉颜色分为红肉、白肉、黄肉,根据花色苷含量分为多、中、少、无(图1-A);将果皮底色分为乳白、绿、乳黄、黄等,根据着色面积分为多、中、少、无(图1-B)。徐子媛[15]调查了73份桃种质资源品质性状,发现果皮底色为乳白色的占比最高,为45.2%,果皮不同程度红色的有66 份,占90.4%,仅7 份材料果皮不着红色;果肉红色的种质资源有12份,占16.4%。这也从侧面反映了中国桃种质资源多样性的丰富程度。

图1 桃果肉和果皮颜色类型
Fig.1 Peach flesh and skin color types
为探究果实红色与花色苷含量的关系,查阅了前人对桃果实花色苷含量测定的相关文献。白肉桃果皮和果肉花色苷分布范围在0.65~37.21 mg·100 g-1和0.07~25.20 mg·100 g-1,黄肉桃为0.61~32.33 mg·100 g-1和0.17~18.59 mg · 100 g-1,而红肉桃为1.04~113.11 mg·100 g-1和0.73~129.06 mg·100 g-1,值得注意的是红肉桃中果皮和果肉总酚含量是白肉桃和黄肉桃的2~3 倍[16-17]。丁体玉等[18]根据桃果肉着色面积和花色苷含量的动态变化,将红肉桃分为两类:(1)“成熟积累型”在果实成熟期果肉花色苷含量达到最大值(170~320 mg·kg-1);(2)“发育中期积累型”在盛花后70~80 d 果肉花色苷含量达到峰值(150~800 mg·kg-1),随着果实成熟花色苷含量逐渐下降。章秋平等[19]比较了12 份不同果肉颜色品种中花色苷含量,红肉桃品种花色苷平均含量显著高于白肉和黄肉品种,红肉桃中果肉花色苷含量1.249~19.503 mg·g-1,白肉桃中0.393~2.264 mg·g-1,黄肉桃中0.552~1.465 mg·g-1。而红肉桃品种间花色苷含量也有很大差异,有的红肉品种花色苷含量甚至显著低于白肉品种,这可能是由于红肉桃果肉中存在大量的多酚物质从而干扰了花色苷含量的测定,而且花色苷含量与栽培环境、试验取样等也存在一定关系。
2 桃果实主要花色苷组分及合成途径
花色苷是类黄酮物质中含量和分布最为广泛的一类色素物质,普遍存在于植物的花、果实、叶片等组织中[20],对植物器官的色泽、风味和香气等都有一定影响[21]。常见的花色苷有矢车菊色素(cyanindin,Cy)、天竺葵色素(pelargonidin,Pg)、飞燕草色素(delphinidin,Dp)、芍药色素(peonidin,Pn)、牵牛色素(petunidin,Pt)和锦葵色素(malvidin,Mv)6 种[22]。花色苷糖基化的糖分子也具有多样性,多数为葡萄糖(glucose),少数为半乳糖、木糖、鼠李糖和阿拉伯糖,以及由这些单糖所构成的二糖或者多糖[5]。不同色素结合糖基而形成不同的颜色,矢车菊色素和天竺葵色素呈红色,飞燕草色素及甲基化的衍生物牵牛色素和锦葵色素呈现蓝紫色。
花色苷在植物体内的合成主要由一系列结构基因(CHS、CHI、F3H、DFR、ANS、UFGT等)所调控,这些结构基因通过编码不同功能的酶来参与花色苷的合成,同时这些结构基因受上游转录因子(PpMYB10.1、PpbHLH、PpWD40 及PpBL 等)的转录调控。苯丙氨酸是花色苷生物合成的直接前体物质,由苯丙氨酸到花色苷大致有3 个阶段。桃果实中花色苷的生物合成途径已基本明晰[23-24],如图2所示。第一阶段:苯丙氨酸在苯丙氨酸解氨酶(PAL)催化作用下,形成肉桂酸;肉桂酸经肉桂酸-4-羟化酶(C4H)和4-香豆酰-CoA 连接酶(4CL)形成4-香豆酰-CoA;再经查尔酮合成酶(CHS)催化合成黄色的查尔酮。第二阶段:在查尔酮异构酶(CHI)以查尔酮为底物,异构化合成黄烷酮;在黄烷酮-3-羟化酶(F3H)催化下形成二氢山柰酚。第三阶段:二氢山柰酚经二氢黄酮醇-4-还原酶(DFR)合成无色花色苷;无色花色苷由花青素苷合成酶(ANS)催化形成彩色的花色苷;最后通过类黄酮-3-O-糖基转移酶(UFGT)的作用,游离的有色花色苷形成能够稳定存在于植物中的花色苷,使植物呈现出鲜艳的颜色;花色苷在细胞质中合成后,经谷胱甘肽-S-转移酶(GST)将其转移运输到液泡中,是花色苷显色的关键代谢途径;未修饰的花色苷由无色花色苷还原酶(LAR)或者花色苷还原酶(ANR)的作用产生。
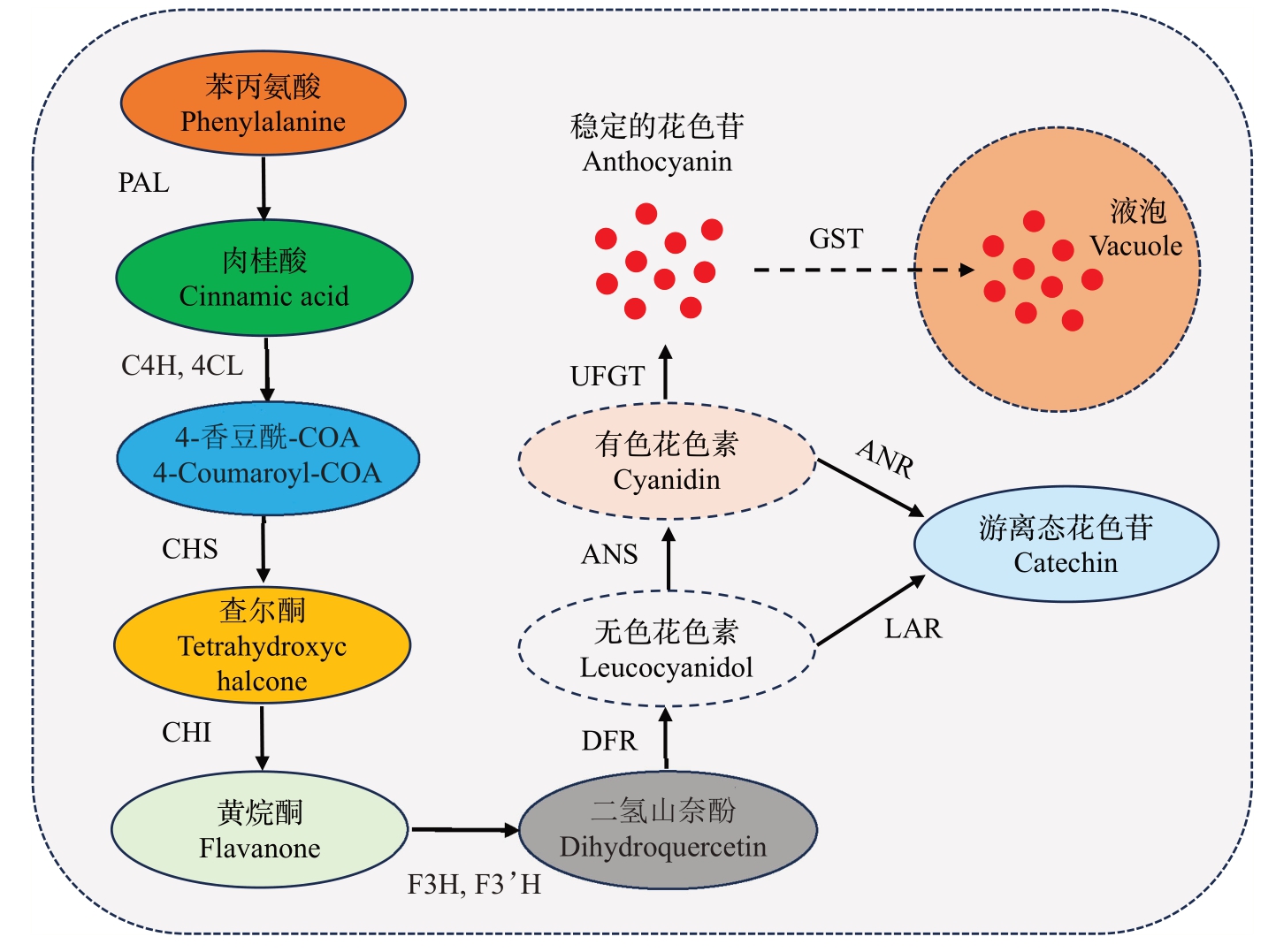
图2 桃果实花色苷生物合成途径
Fig.2 Anthocyanin biosynthesis pathway in peach fruit
3 桃果实红色形成的遗传变异及调控机制
3.1 桃果皮红色形成的遗传变异及调控机制
桃果皮颜色由底色和盖色(或红晕)共同决定,在果实成熟过程中,果皮底色由绿色转为白色或黄色。在此阶段,因不同品种基因型差异,果皮底色上有不同类型(斑点、条纹、红晕)且深浅不同的红色沉积[12]。研究表明,果皮红晕由多个基因控制[25-27],但同时也取决于环境因素[28]。2003 年,Beckman 等[29]将整个果面红色性状描述为“全红”,基因型为FR,果面无或极少红色性状被描述为“高亮”,基因型为frfr。2004年,Dirlewanger等[30]发现,位于基因组第3 连锁群上的3 个MYB10 基因,PpMYB10.1(ppa026640m) ,PpMYB10.2 (ppa016711m) 和PpMYB10.3(ppa021385m)与颜色性状的Anther color(Ag)标记关联。2005 年,Beckman 等[31]进一步将果皮颜色描述为红与非红,红色对非红为显性(H/h)。
对桃果皮花色苷合成通路研究发现,PpMYB10.1 和PpMYB10.3 可调控花色苷合成通路上结构基因的表达,促进花色苷积累,进而使果皮呈红色[23,32]。Tuan 等[7]分析非红品种Mochizuki 中PpMYB10基因簇3个基因(PpMYB10.1、PpMYB10.2、PpMYB10.3)的表达,表明桃果皮花色苷含量与PpMYB10.1 表达量密切相关,进一步分析其序列发现PpMYB10.1 启动子-1173 nt 处存在5243 bp 的转座子插入,导致PpMYB10.1 丧失功能,下游结构基因不能被激活转录,使得Mochizuki 果皮不能合成积累花色苷(图3-A)。对63 份桃野生资源、地方品种和育成品种进行PpMYB10.1 启动子上变异鉴定,发现育成品种表型和基因型完全吻合,而部分地方品种果皮颜色表型与基因型不匹配,猜测可能存在其他调控机制或变异,还有待继续研究[33]。而PpMYB10.1启动子上483 bp的缺失会增强其驱动活性,有利于PpMYB10.1 对下游结构基因的转录调控[18]。Zhao等[9]研究光照对桃果皮着色分子机制发现,在光照条件下,光响应基因PpHYH 与伴侣蛋白PpBBX 互作形成异源二聚体激活PpMYB10.1,促进果皮着红色;然而在黑暗条件下,光信号抑制因子PpCOP1 在细胞核大量积累,引起PpHYH 蛋白降解,导致花色苷积累受阻(图3-B)。
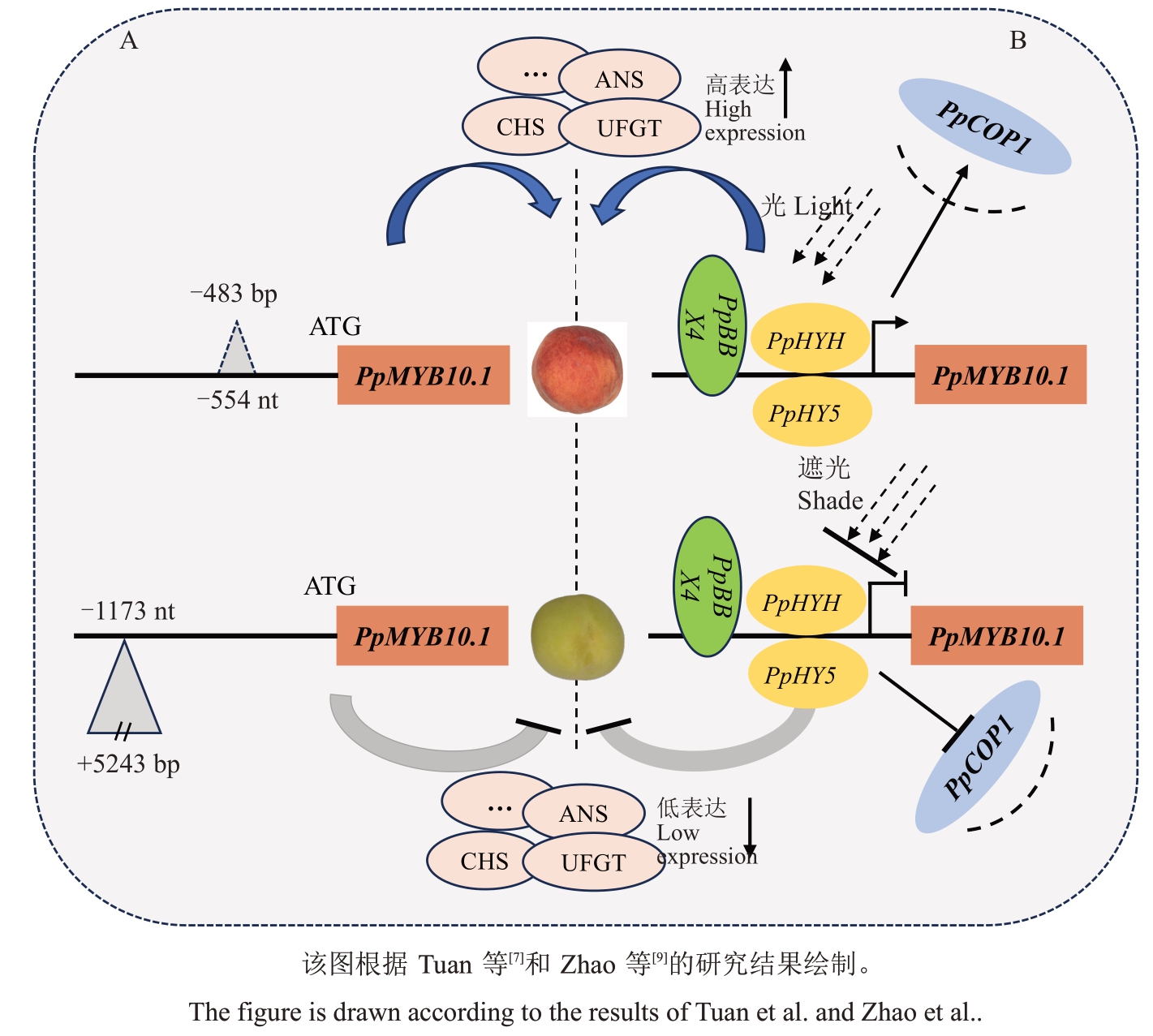
图3 桃果皮花色苷合成调控模型
Fig.3 Regulation model of anthocyanin synthesis in peach skin
另外,表观遗传修饰也能影响桃果实花色苷的合成。Zhu 等[34]研究表明,当贮藏温度升高到16 ℃时,随着DNA去甲基化程度增加,白肉桃果肉中会积累大量花色苷;Cheng 等[35]研究发现,桃果实花色苷合成与PpUGT78A1和PpUGT78A2糖基化有关。
3.2 桃果肉红色形成的遗传变异及调控机制
研究表明,桃红肉性状存在两种类型,由多个基因或者QTL 位点调控。第一种类型以加拿大红肉品种Harrow Blood 为代表,从硬核前期就开始大量积累花色苷,且叶片背面叶脉变红[36]。Werner 等[37]分析了Harrow Blood×Rutgers Red Leaf 2n的F2后代中果肉颜色分离情况,发现红肉桃和普通桃的比例符合1∶3,提出红肉性状是由一个隐形基因bf控制;Gil 等[38]进一步将bf 基因定位到第4 连锁群的顶端,但没有候选基因被报道。章秋平[39]研究了2个杂交群体组合,发现后代果肉均为红色,但红色深浅不同,推断红肉性状并非单一的隐性性状,而是受多个基因控制的数量性状。第二种是血桃类型,果实进入成熟期果肉才开始积累花色苷而变红,且叶脉不变红。Shen 等[40]对中国血桃品种五月鲜进行研究,提出这类血桃是由一对显性基因DBF控制的,利用SSR 标记将此位点定位到第5 连锁群的顶端。周晖[41]在大红袍×曙光杂交群体中鉴定发现,大红袍血桃性状为显性遗传,与Harrow Blood 的隐性红肉性状不同。
Zhou 等[11]在5 号染色体上发现一个NAC 家族转录因子,BLOOD(BL)与红肉性状密切相关,PpBL与PpNAC1 形成二聚体促进PpMYB10.1 表达,进而促进果肉花色苷合成;同时鉴定到PpSPL1蛋白可以抑制PpBL-PpNAC1复合体活性,导致花色苷含量降低。Miyuki 等[8]研究了日本红肉品种Tenshin-suimitsuto的PpBL基因结构,发现该品种的PpBL启动子上存在6688 bp 的转座子插入是果肉红色的关键变异,同时PpBL 表达与果肉红色深浅不同有关。Wang等[42]对该转座子blood-TE进一步分析,发现白肉桃中PpWRKY70 抑制了PpBL 的转录活性;而红肉桃中存在blood-TE 的插入,导致PpWRKY70 的抑制作用减弱,导致PpBL高表达,进而形成红肉表型(图4-A)。
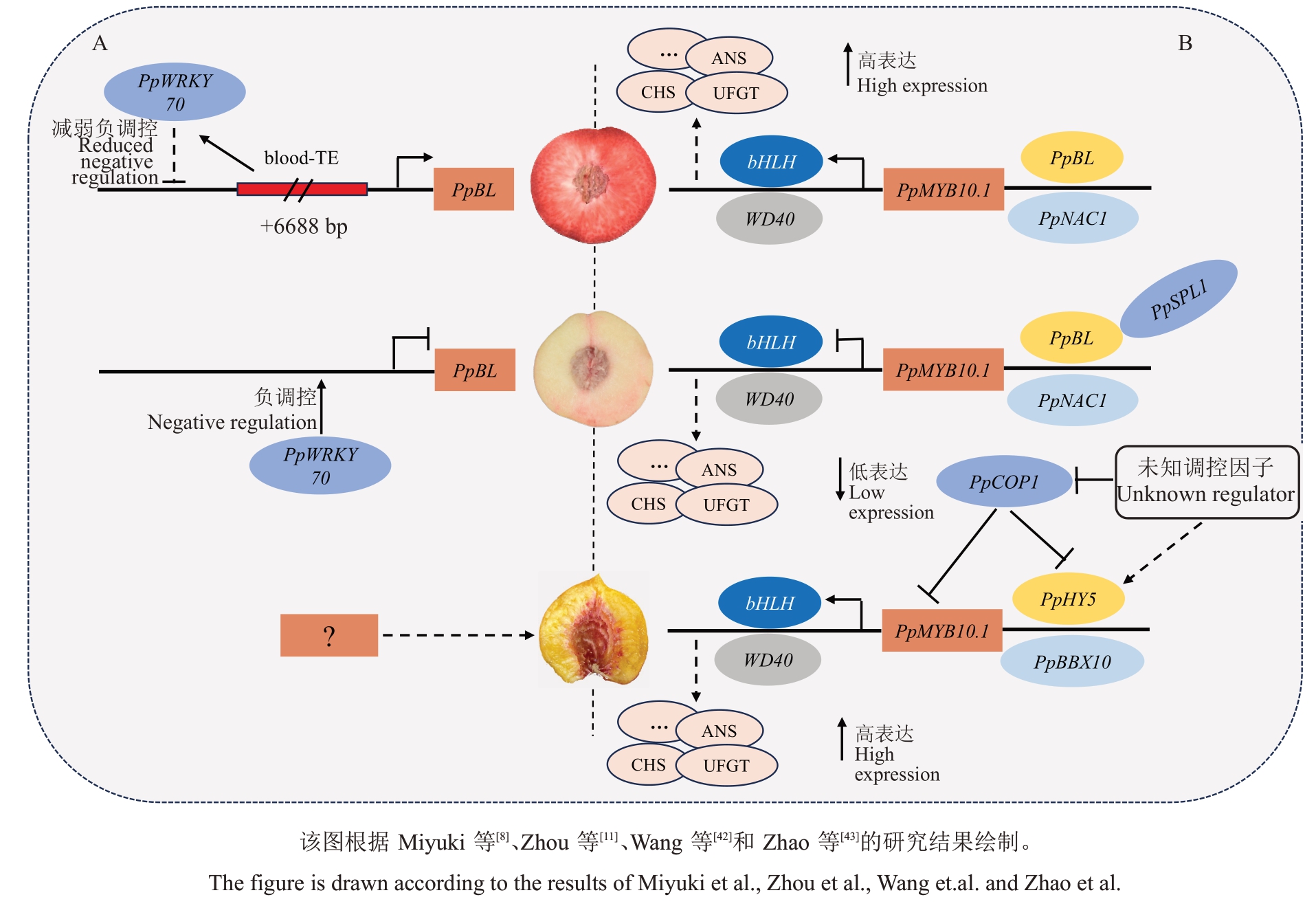
图4 桃果肉花色苷合成调控模型
Fig.4 Regulation model of anthocyanin synthesis in peach flesh
另外,还有一种红肉类型是近核处周围果肉呈红色(Cs)且伴有苦味,但目前尚未有关键遗传变异的报道。Zhao 等[43]通过比较转录组挖掘到调控桃近核处红色性状的光响应基因PpHY5,在PpBBX10的协同下可促进PpMYB10.1的转录激活。然而,靠近果核处果肉始终处于黑暗条件下,PpCOP1 与PpHY5 以及PpMYB10.1 的相互作用并未导致靠近果核处果肉花色苷积累受阻,PpHY5 和PpMYB10.1表达在近核处形成过程中反而上调,猜测可能是上游存在未知的调节因子参与对PpCOP1功能的调节以及对PpHY5的转录调控(图4-B)。
4 桃果实红色形成的影响因素及调控机制
花色苷含量还受外界环境影响其合成[44-45],比如光照、温度、激素、糖以及矿质元素等[46]。
4.1 光照
外部环境中尤其是光照对桃果实着色影响最大。光敏色素感应到光信号后,提高了花色苷合成途径相关酶的活性,促进果实着色[47]。丁云龙等[48]对不同树体部位桃果实着色差异研究,发现上部果实比中部、下部以及内膛着色更深,其光照条件差异是主要因素。何平等[49]研究套袋对桃果实着色的影响,表明套袋果实果皮花色苷含量显著下降,去袋后受光诱导花色苷迅速积累,且相关基因表达量迅速上升。不同光质对桃果实着色的研究表明,紫外光、蓝光明显增加果皮花色苷含量[50-51],有利于着色。Zhao 等[10]发现光形态转录因子PpHY5 响应UVA 和UVB共同调节PpMYB10.1转录表达,下游花色苷合成结构基因表达量升高,促进桃果皮着色,UVA 和UVB 同时处理着色更明显。然而光照影响桃果肉花色苷合成的报道较少。Rumainum 等[52]通过套袋对桃果皮和果肉花色苷积累的影响试验表明,桃果肉花色苷积累不完全依赖于光照,即在黑暗条件下,果肉花色苷可以正常合成。综上,光照可以提高相关酶活性或基因表达量,促进花色苷合成,而这种作用只是在桃果皮上。
4.2 温度
温度对果实花色苷合成也具有重要影响。昼夜温差大和夜间温度低的地区果实着色更好,可能是由于低温减弱了呼吸速率,促进糖分积累,从而有利于花色苷积累[53]。Zhou 等[54]研究表明,高温和遮光处理显著降低了桃红色叶片中花色苷合成基因的表达,导致花色苷积累减少。研究者提出,高温条件下花色苷合成速率减慢,降解加快,其稳定性差,降低了花色苷的积累,又称“高温褪色”反应[55]。在苹果[56]、葡萄[57]研究中表明,适当低温处理可使花色苷合成相关基因高表达,促进花色苷的积累。Yin等[58]研究表明,高海拔地区不同光质和低温有利于葡萄果皮花色苷合成。
4.3 激素
外源激素处理有利于果实着色。Zhang 等[59]研究表明,乙烯通过抑制花色苷合成上游转录因子的活性,抑制桃果皮花色苷合成,而1-甲基环丙烯(1-MCP)有着与乙烯相反的作用。果实采后用不同激素处理研究表明,茉莉酸酯类、苯丙氨酸、L-谷氨酸和油菜素内酯均可以增加桃果皮花色苷含量,促进果实着色[60-62]。另外,在苹果、梨、葡萄中,激素促进或抑制花色苷合成的研究较为深入。例如,在苹果中乙烯和茉莉酸通过MdERF1B-MdMYC2基因模块协同正调控苹果花色苷合成[63];Li等[64]鉴定发现,乙烯和生长素响应因子之间相互作用来调节苹果果皮花青素积累。在梨中,乙烯单独或者与茉莉酸共同作用,抑制梨果皮花色苷形成[65-66];乙烯通过PpERF9-PpTPL1 共抑制复合体介导的组蛋白去乙酰化效应抑制PpRAP2.4和PpMYB114的表达,从而抑制梨果皮花色苷合成的分子机制[67]。孙玉帅等[68]对葡萄外施ABA 和乙烯的研究表明,ABA 能通过VlMybA1 直接调控着色,也可间接通过乙烯促进VlMybA2的表达来调控葡萄着色。
4.4 糖、矿质元素
Wang等[69]试验表明,葡萄糖、蔗糖、果糖及山梨醇均能诱导桃果肉中花色苷的积累,且PpDFR 及PpUFGT 表达量显著升高。Zhou 等[70]用热空气和UV-C 处理桃果实发现,两种处理通过调节蔗糖、苹果酸和柠檬酸含量增强花色苷积累,同时上调了相关酶活性和基因表达。外源蔗糖处理显著增加了桃果皮花色苷含量,促进着色[71]。Maatallah 等[72]研究N-P-K 施肥对桃果实品质的影响,认为N 和K 在增加产量和品质的同时,增加了花色苷含量进而改善桃果实颜色。
5 展 望
桃基因组数据公布[73]和测序技术的进步,直接加速了对目标性状候选基因的定位,实现了对表型性状的精准鉴定,为桃分子辅助育种和果树遗传改良奠定了良好的基础[74]。分子标记辅助育种主要有两种方法:(1)在控制目的性状的基因序列上开发分子标记,直接完成表型鉴定;(2)首先鉴定亲本基因型,在目标性状位点两侧开发分子标记,进而完成表型鉴定[75]。例如,桃果皮红色与纯色性状,直接取决于PpMYB10.1启动子上的等位变异,开发出分子标记MYB10.1- 1/MYB10.1- 1(全红)、MYB10.1- 1/MYB10.1-2(半红)以及MYB10.1-2/MYB10.1-2(纯色),在苗期即可对果实颜色进行鉴定[7]。另外中国特有的一些地方品种抗逆性强,且果皮纯色(绿色、浅黄、白色),分子标记MYB10.1-2/MYB10.1-2 并不能100%预测[33]。因此,找到新目的基因、新等位变异对目的基因的精准鉴定,并开发出标记被育种家所利用,是目前桃树上快速从杂交后代中筛选符合育种目标优株的主要手段。
根据桃果肉颜色分为白肉、黄肉和红肉。红肉桃富含花色苷等抗氧化成分,有益于人体健康,但大多红肉桃品种风味偏酸且早熟,阻碍了红肉桃的育种进程和新品种推广。由于桃尚未形成成熟的遗传转化体系,基因编辑技术尚不能被高效利用,所以分子标记快速鉴定表型成为当前育种中最直接有效的改良性状的手段。例如,选择高糖低酸且综合性状优良的桃品种作为亲本,与红肉桃品种杂交获得果肉性状分离的群体,同时利用红肉性状分子标记和酸味性状分子标记可快速筛选到低酸且红肉的桃优株,加快了育种进程。中国早熟且风味甜的红肉蟠桃金陵血蟠品种[76]的选育,也标志着中国桃育种工作在颜色多样性方面迈向新的台阶。另外,桃近核处果肉红色且伴有苦味受多个基因协同调控,不受光环境诱导,存在的遗传机制尚不清楚,可利用中国丰富的种质资源优势,发掘候选基因以及可能存在的遗传变异,解析果肉近核处泛红的分子机制,开发分子标记为桃育种服务。
[1] 汪祖华,庄恩及.中国果树志-桃卷[M].北京:中国林业出版社,2001.WANG Zuhua,ZHUANG Enji. Chinese fruit tree:Peach[M].Beijing:China Forestry Publishing House,2001.
[2] CAO K,LI Y,DENG C H,GARDINER S E,ZHU G R,FANG W C,CHEN C W,WANG X W,WANG L R.Comparative population genomics identified genomic regions and candidate genes associated with fruit domestication traits in peach[J].Plant Biotechnology Journal,2019,17(10):1954-1970.
[3] YU Y,FU J,XU Y G,ZHANG J W,REN F,ZHAO H W,TIAN S L,GUO W,TU X L,ZHAO J,JIANG D W,ZHAO J B,WU W Y,WANG G C,MA R C,JIANG Q,WEI J H,XIE H.Genome re-sequencing reveals the evolutionary history of peach fruit edibility[J].Nature Communications,2018,9(1):5404.
[4] ZHOU H J,YU Z F,YE Z W. Key proteins associated to coloured compounds of peach peel using iTRAQ proteomic techniques during development and postharvest[J].Scientia Horticulturae,2018,239:123-132.
[5] 孙建霞,张燕,胡小松,吴继红,廖小军.花色苷的结构稳定性与降解机制研究进展[J]. 中国农业科学,2009,42(3):996-1008.SUN Jianxia,ZHANG Yan,HU Xiaosong,WU Jihong,LIAO Xiaojun. Structural stability and degradation mechanisms of anthocyanins[J]. Scientia Agricultura Sinica,2009,42(3):996-1008.
[6] WANG J,MAZZA G.Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFNgamma-activated RAW 264.7 macrophages[J]. Journal of Agricultural and Food Chemistry,2002,50(4):850-857.
[7] TUAN P A,BAI S L,YAEGAKI H,TAMURA T,HIHARA S,MORIGUCHI T,ODA K.The crucial role of PpMYB10.1 in anthocyanin accumulation in peach and relationships between its allelic type and skin color phenotype[J]. BMC Plant Biology,2015,15:280.
[8] HARA-KITAGAWA M,UNOKI Y,HIHARA S,ODA K.Development of simple PCR-based DNA marker for the red-fleshed trait of a blood peach‘Tenshin-suimitsuto’[J].Molecular Breeding,2019,40(1):5.
[9] ZHAO L,SUN J L,CAI Y M,YANG Q R,ZHANG Y Q,OGUTU C O,LIU J J,ZHAO Y,WANG F R,HE H P,ZHENG B B,HAN Y P. PpHYH is responsible for light-induced anthocyanin accumulation in fruit peel of Prunus persica[J]. Tree Physiology,2022,42(8):1662-1677.
[10] ZHAO Y,MIN T,CHEN M J,WANG H X,ZHU C Q,JIN R,ALLAN A C,KUI L W,XU C J. The photomorphogenic transcription factor PpHY5 regulates anthocyanin accumulation in response to UVA and UVB irradiation[J]. Frontiers in Plant Science,2021,11:603178.
[11] ZHOU H,KUI L W,WANG H L,GU C,DARE A P,ESPLEY R V,HE H P,ALLAN A C,HAN Y P. Molecular genetics of blood-fleshed peach reveals activation of anthocyanin biosynthesis by NAC transcription factors[J]. Plant Journal,2015,82(1):105-121.
[12] 王力荣,朱更瑞,方伟超.中国桃遗传资源[M].北京:中国农业出版社,2012.WANG Lirong,ZHU Gengrui,FANG Weichao. Peach genetic resource in China[M].Beijing:China Agriculture Press,2012.
[13] 王力荣,朱更瑞.桃种质资源描述规范和数据标准[M].北京:中国农业出版社,2005.WANG Lirong,ZHU Gengrui.Descriptors and data standard for peach(Prunus persica L.)[M].Beijing:China Agriculture Press,2005.
[14] 王蛟,曹珂,王玲玲,王力荣.PpMYB10.1 启动子483 bp 缺失与红肉桃果肉颜色形成关系的研究[J].植物遗传资源学报,2023,24(3):758-766.WANG Jiao,CAO Ke,WANG Lingling,WANG Lirong. Deciphering the genetic effect of a 483 bp deletion in the PpMYB10.1 promoter to determine intensities of the red-colored flesh peach[J]. Journal of Plant Genetic Resources,2023,24(3):758-766.
[15] 徐子媛.73 份桃种质资源果实品质评价研究[D].南京:南京农业大学,2021.XU Ziyuan. Fruit quality evaluation of 73 peach germplasm resources[D].Nanjing:Nanjing Agricultural University,2021.
[16] 沈志军,马瑞娟,俞明亮,许建兰,蔡志翔,倪林箭,颜少宾.桃三种肉色类型果实抗氧化因子的比较评价[J].中国农业科学,2012,45(11):2232-2241.SHEN Zhijun,MA Ruijuan,YU Mingliang,XU Jianlan,CAI Zhixiang,NI Linjian,YAN Shaobin. Evaluation of antioxidant factors in peach with three types of flesh color[J].Scientia Agricultura Sinica,2012,45(11):2232-2241.
[17] 王莉,殷益明,庞钰洁,沈玉丽,贾惠娟.早熟红肉桃新品种庚村阳桃的选育[J].果树学报,2022,39(6):1121-1124.WANG Li,YIN Yiming,PANG Yujie,SHEN Yuli,JIA Huijuan.Gengcunyangtao,a new early ripening red-flesh peach cultivar[J].Journal of Fruit Science,2022,39(6):1121-1124.
[18] 丁体玉,曹珂,方伟超,朱更瑞,陈昌文,王新卫,王力荣.红肉桃两类花色素苷积累模式与相关基因表达差异[J].中国农业科学,2017,50(13):2553-2563.DING Tiyu,CAO Ke,FANG Weichao,ZHU Gengrui,CHEN Changwen,WANG Xinwei,WANG Lirong. The difference of anthocyanin accumulation pattern and related gene expression in two kinds of red flesh peach[J]. Scientia Agricultura Sinica,2017,50(13):2553-2563.
[19] 章秋平,李疆,王力荣,朱更瑞,方伟超,曹珂,陈昌文,冯义彬.红肉桃果实发育过程中色素和糖酸含量的变化[J].果树学报,2008,25(3):312-315.ZHANG Qiuping,LI Jiang,WANG Lirong,ZHU Gengrui,FANG Weichao,CAO Ke,CHEN Changwen,FENG Yibin.Study on the changes of contents of pigments,sugar and acid of Blood-flesh peach cultivar during fruit development[J]. Journal of Fruit Science,2008,25(3):312-315.
[20] VIZZOTTO M,CISNEROS- ZEVALLOS L,BYRNE D H,RAMMING D W,OKIE W R.Large variation found in the phytochemical and antioxidant activity of peach and plum germplasm[J]. Journal of the American Society for Horticultural Science,2007,132(3):334-340.
[21] KAYESH E,SHANGGUAN L F,KORIR N K,SUN X,BILKISH N,ZHANG Y P,HAN J,SONG C N,CHENG Z M,FANG J G.Fruit skin color and the role of anthocyanin[J].Acta Physiologiae Plantarum,2013,35(10):2879-2890.
[22] SAKATA K,SAITO N,HONDA T.Ab initio study of molecular structures and excited states in anthocyanidins[J]. Tetrahedron,2006,62(15):3721-3731.
[23] RAVAGLIA D,ESPLEY R V,HENRY-KIRK R A,ANDREOTTI C,ZIOSI V,HELLENS R P,COSTA G,ALLAN A C.Transcriptional regulation of flavonoid biosynthesis in nectarine(Prunus persica)by a set of R2R3 MYB transcription factors[J].BMC Plant Biology,2013,13:68.
[24] CAO K,DING T Y,MAO D M,ZHU G R,FANG W C,CHEN C W,WANG X W,WANG L R.Transcriptome analysis reveals novel genes involved in anthocyanin biosynthesis in the flesh of peach[J].Plant Physiology and Biochemistry,2018,123:94-102.
[25] FRETT T J,REIGHARD G L,OKIE W R,GASIC K. Mapping quantitative trait loci associated with blush in peach[Prunus persica (L.) Batsch][J]. Tree Genetics & Genomes,2014,10(2):367-381.
[26] EDUARDO I,PACHECO I,CHIETERA G,BASSI D,POZZI C,VECCHIETTI A,ROSSINI L. QTL analysis of fruit quality traits in two peach intraspecific populations and importance of maturity date pleiotropic effect[J]. Tree Genetics & Genomes,2011,7(2):323-335.
[27] CANTÍN C M,CRISOSTO C H,OGUNDIWIN E A,GRADZIEL T,TORRENTS J,MORENO M A,GOGORCENA Y. Chilling injury susceptibility in an intra- specific peach[Prunus persica (L.) Batsch] progeny[J]. Postharvest Biology and Technology,2010,58(2):79-87.
[28] LAYNE D R,JIANG Z W,RUSHING J W.Tree fruit reflective film improves red skin coloration and advances maturity in peach[J].HortTechnology,2001,11(2):234-242.
[29] BECKMAN T G,SHERMAN W B. Probable qualitative inheritance of full red skin color in peach[J]. HortScience,2003,38(6):1184-1185.
[30] DIRLEWANGER E,GRAZIANO E,JOOBEUR T,GARRIGACALDERÉ F,COSSON P,HOWAD W,ARÚS P. Comparative mapping and marker-assisted selection in Rosaceae fruit crops[J]. Proceedings of the National Academy of Sciences of the United States of America,2004,101(26):9891-9896.
[31] BECKMAN T G,ALCAZAR J R,SHERMAN W B,WERNER D J. Evidence for qualitative suppression of red skin color in peach[J].HortScience,2005,40(3):523-524.
[32] RAHIM M A,BUSATTO N,TRAINOTTI L. Regulation of anthocyanin biosynthesis in peach fruits[J]. Planta,2014,240(5):913-929.
[33] GUO T F,WANG J,LU X X,WU J L,WANG L R.The development of molecular markers for peach skin blush and their application in peach breeding practice[J]. Horticulturae,2023,9(8):887.
[34] ZHU Y C,ZHANG B,ALLAN A C,KUI L W,ZHAO Y,WANG K,CHEN K S,XU C J.DNA demethylation is involved in the regulation of temperature-dependent anthocyanin accumulation in peach[J].Plant Journal,2020,102(5):965-976.
[35] CHENG J,WEI G C,ZHOU H,GU C,VIMOLMANGKANG S,LIAO L,HAN Y P. Unraveling the mechanism underlying the glycosylation and methylation of anthocyanins in peach[J].Plant Physiology,2014,166(2):1044-1058.
[36] CHAPARRO J X,WERNER D J,WHETTEN R W,O’MALLEY D M. Inheritance,genetic interaction,and biochemical characterization of anthocyanin phenotypes in peach[J]. Journal of Heredity,1995,86(1):32-38.
[37] WERNER D J,CRELLER M A,CHAPARRO J X. Inheritance of the blood-flesh trait in peach[J]. HortScience,1998,33(7):1243-1246.
[38] GIL M I,TOMÁS-BARBERÁN F A,HESS-PIERCE B,KADER A A. Antioxidant capacities,phenolic compounds,carotenoids,and vitamin C contents of nectarine,peach,and plum cultivars from California[J]. Journal of Agricultural and Food Chemistry,2002,50(17):4976-4982.
[39] 章秋平.红肉桃果实发育期色素变化规律研究及其遗传趋向分析与初步定位[D].乌鲁木齐:新疆农业大学,2008.ZHANG Qiuping. Study on content changes of pigments during fruit development in blood-flesh peach and its heredity and preliminary QTLs analysis[D]. Urumqi:Xinjiang Agricultural University,2008.
[40] SHEN Z J,CONFOLENT C,LAMBERT P,POËSSEL J L,QUILOT-TURION B,YU M L,MA R J,PASCAL T.Characterization and genetic mapping of a new blood-flesh trait controlled by the single dominant locus DBF in peach[J]. Tree Genetics&Genomes,2013,9(6):1435-1446.
[41] 周晖.桃花青苷着色及原花青素合成的调控机制研究[D].北京:中国科学院大学,2015.ZHOU Hui. Mechanisms underlying the regulation of anthocyanin coloration and proanthocyanidin synthesis in peach[D].Beijing:University of Chinese Academy of Sciences,2015.
[42] WANG J,CAO K,LI Y,WU J L,LI W Q,WANG Q,ZHU G R,FANG W C,CHEN C W,WANG X W,DONG W X,LIU W S,WANG L R. Genome variation and LTR-RT analyses of an ancient peach Landrace reveal mechanism of blood-flesh fruit color formation and fruit maturity date advancement[J]. Horticulture Research,2023,11(1):uhad265.
[43] ZHAO L,ZHANG Y Q,SUN J L,YANG Q R,CAI Y M,ZHAO C P,WANG F R,HE H P,HAN Y P.PpHY5 is involved in anthocyanin coloration in the peach flesh surrounding the stone[J].Plant Journal,2023,114(4):951-964.
[44] TIAN Y Y,WANG H Y,SUN P,FAN Y G,QIAO M M,ZHANG L X,ZHANG Z Q. Response of leaf color and the expression of photoreceptor genes of Camellia sinensis cv.Huangjinya to different light quality conditions[J]. Scientia Horticulturae,2019,251:225-232.
[45] LADO J,ALÓS E,MANZI M,CRONJE P J R,GÓMEZ-CADENAS A,RODRIGO M J,ZACARÍAS L. Light regulation of carotenoid biosynthesis in the peel of mandarin and sweet orange fruits[J].Frontiers in Plant Science,2019,10:1288.
[46] 周丹蓉,方智振,叶新福,潘少霖,廖汝玉,姜翠翠,王小安.李果中花色素苷研究进展[J].东南园艺,2015,3(3):43-46.ZHOU Danrong,FANG Zhizhen,YE Xinfu,PAN Shaolin,LIAO Ruyu,JIANG Cuicui,WANG Xiaoan. Research progress of anthocyanin in plum[J].Southeast Horticulture,2015,3(3):43-46.
[47] GALVÃO V C,FANKHAUSER C. Sensing the light environment in plants:Photoreceptors and early signaling steps[J]. Current Opinion in Neurobiology,2015,34:46-53.
[48] 丁云龙,张斌斌,严娟,马瑞娟,姜卫兵.桃树体不同部位果实着色差异及其与环境因子的关系研究[J]. 西北植物学报,2019,39(4):660-668.DING Yunlong,ZHANG Binbin,YAN Juan,MA Ruijuan,JIANG Weibing.Study on difference of peach fruit coloring and relationship with environmental factors in different tree canopy position[J].Acta Botanica Boreali-Occidentalia Sinica,2019,39(4):660-668.
[49] 何平,李林光,王海波,常源升.套袋对‘秋雪’桃果实品质及花青素合成相关基因表达的影响[J]. 植物生理学报,2018,54(2):273-281.HE Ping,LI Linguang,WANG Haibo,CHANG Yuansheng. Effect of bagging on fruit quality and anthocyanin synthesis-related gene expression of‘Qiuxue’peach[J].Plant Physiology Journal,2018,54(2):273-281.
[50] 池铭,孙丽娟,马立杰,赵婧,周宏胜,凌军,罗淑芬,李国锋,李鹏霞,张映曈.不同光质处理对采后桃果皮色泽及花色苷代谢的影响[J].食品科学,2023,44(3):209-217.CHI Ming,SUN Lijuan,MA Lijie,ZHAO Jing,ZHOU Hongsheng,LING Jun,LUO Shufen,LI Guofeng,LI Pengxia,ZHANG Yingtong. Effects of different light qualities on color development and anthocyanin metabolism of peach skin during postharvest storage[J].Food Science,2023,44(3):209-217.
[51] 郑晓翠,刘凤之,王海波,王孝娣.不同光质补光对设施内桃果实品质及叶片质量的影响[J].西北植物学报,2023,43(6):979-987.ZHENG Xiaocui,LIU Fengzhi,WANG Haibo,WANG Xiaodi.Effects of different supplemental light on fruit and leaf quality of peach in the facility[J]. Acta Botanica Boreali-Occidentalia Sinica,2023,43(6):979-987.
[52] RUMAINUM I M,WORARAD K,YAMAKI Y,YAMANE K.Effects of developmental stages,light,and an auxin polar transport inhibitor on the skin and flesh pigmentation of red-fleshed peach fruit[J].The Horticulture Journal,2016,85(2):141-147.
[53] 俞明亮.苹果花青苷色素的形成[J].北方果树,1992(4):34-36.YU Mingliang. Formation of apple anthocyanin[J]. Northern Fruits,1992(4):34-36.
[54] ZHOU Y,GUO D,LI J,CHENG J,ZHOU H,GU C,GARDINER S,HAN Y P. Coordinated regulation of anthocyanin biosynthesis through photorespiration and temperature in peach(Prunus persica f.atropurpurea)[J].Tree Genetics&Genomes,2013,9(1):265-278.
[55] MORI K,SUGAYA S,GEMMA H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition[J].Scientia Horticulturae,2005,105(3):319-330.
[56] BAN Y,HONDA C,HATSUYAMA Y,IGARASHI M,BESSHO H,MORIGUCHI T. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin[J].Plant&Cell Physiology,2007,48(7):958-970.
[57] YAMANE T,JEONG S T,GOTO-YAMAMOTO N,KOSHITA Y,KOBAYASHI S. Effects of temperature on anthocyanin biosynthesis in grape berry skins[J]. American Journal of Enology and Viticulture,2006,57(1):54-59.
[58] YIN H N,WANG Z X,WANG L,CAO J H,WANG J K,XI Z M. Effects of mesoclimate and microclimate variations mediated by high altitude and row orientation on sucrose metabolism and anthocyanin synthesis in grape berries[J]. Horticultural Plant Journal,2024,10(3):713-731.
[59] ZHANG Y T,LING J,ZHOU H S,TIAN M Y,HUANG W,LUO S F,HU H L,LI P X. 1-Methylcyclopropene counteracts ethylene inhibition of anthocyanin accumulation in peach skin after harvest[J]. Postharvest Biology and Technology,2022,183:111737.
[60] 马立杰,邵小达,赵晟,潘泓,赵婧,张映曈,凌军,李鹏霞,黄雯,周宏胜.采后苯丙氨酸处理对‘湖景蜜露’桃果皮色泽的影响[J].食品与发酵工业,2023,49(18):195-201.MA Lijie,SHAO Xiaoda,ZHAO Sheng,PAN Hong,ZHAO Jing,ZHANG Yingtong,LING Jun,LI Pengxia,HUANG Wen,ZHOU Hongsheng. Effects of postharvest phenylalanine treatment on peel coloration of‘Hujingmilu’peach[J]. Food and Fermentation Industries,2023,49(18):195-201.
[61] 尚娟娥.不同品种桃(Prunus persica L.)果实发育过程中品质变化和花色苷代谢及外源物质调控研究[D].福州:福建农林大学,2023.SHANG Juan’e. Changes in fruit quality,anthocyanin metabolism and regulation of exogenous substances during fruit development of different varieties of peach (Prunus persica L.)[D].Fuzhou:Fujian Agriculture and Forestry University,2023.
[62] 何平,李林光,王海波,常源升.茉莉酸酯类对秋甜桃果实着色及品质的影响[J].分子植物育种,2019,17(7):2371-2378.HE Ping,LI Linguang,WANG Haibo,CHANG Yuansheng. Effects of jasmonates on coloration and quality of Qiutian peach[J].Molecular Plant Breeding,2019,17(7):2371-2378.
[63] WANG S,LI L X,FANG Y,LI D,MAO Z L,ZHU Z H,CHEN X S,FENG S Q. MdERF1B-MdMYC2 module integrates ethylene and jasmonic acid to regulate the biosynthesis of anthocyanin in apple[J].Horticulture Research,2022,9:uhac142.
[64] LI H L,LIU Z Y,WANG X N,HAN Y P,YOU C X,AN J P.E3 ubiquitin ligases SINA4 and SINA11 regulate anthocyanin biosynthesis by targeting the IAA29-ARF5-1-ERF3 module in apple[J].Plant,Cell&Environment,2023,46(12):3902-3918.
[65] NI J B,PREMATHILAKE A T,GAO Y H,YU W J,TAO R Y,TENG Y W,BAI S L.Ethylene-activated PpERF105 induces the expression of the repressor-type R2R3-MYB gene PpMYB140 to inhibit anthocyanin biosynthesis in red pear fruit[J]. Plant Journal,2021,105(1):167-181.
[66] NI J B,ZHAO Y,TAO R Y,YIN L,GAO L,STRID Å,QIAN M J,LI J C,LI Y J,SHEN J Q,TENG Y W,BAI S L.Ethylene mediates the branching of the jasmonate-induced flavonoid biosynthesis pathway by suppressing anthocyanin biosynthesis in red Chinese pear fruits[J].Plant Biotechnology Journal,2020,18(5):1223-1240.
[67] NI J B,WANG S M,YU W J,LIAO Y F,PAN C,ZHANG M M,TAO R Y,WEI J,GAO Y H,WANG D S,BAI S L,TENG Y W. The ethylene-responsive transcription factor PpERF9 represses PpRAP2.4 and PpMYB114 via histone deacetylation to inhibit anthocyanin biosynthesis in pear[J]. The Plant Cell,2023,35(6):2271-2292.
[68] 孙玉帅,王菲,管雪强,郗慧茹,姚玉新.ABA 和乙烯互作调控葡萄VlMybA1 和VlMybA2 表达并促进果皮着色[J].园艺学报,2023,50(11):2323-2336.SUN Yushuai,WANG Fei,GUAN Xueqiang,CHI Huiru,YAO Yuxin.ABA and ethylene enhance the expression VlMybA1 and VlMybA2 and promote pigmentation in the berry skin via their interaction[J]. Acta Horticulturae Sinica,2023,50(11):2323-2336.
[69] WANG J,CAO K,WANG L R,DONG W X,ZHANG X,LIU W S. Two MYB and three bHLH family genes participate in anthocyanin accumulation in the flesh of peach fruit treated with glucose,sucrose,sorbitol,and fructose in vitro[J]. Plants,2022,11(4):507.
[70] ZHOU D D,LI R,ZHANG H,CHEN S X,TU K. Hot air and UV-C treatments promote anthocyanin accumulation in peach fruit through their regulations of sugars and organic acids[J].Food Chemistry,2020,309:125726.
[71] 田梦瑶,周宏胜,唐婷婷,张映曈,凌军,罗淑芬,李鹏霞.外源蔗糖处理对采后桃果皮色泽形成的影响[J].食品科学,2022,43(1):177-183.TIAN Mengyao,ZHOU Hongsheng,TANG Tingting,ZHANG Yingtong,LING Jun,LUO Shufen,LI Pengxia.Effect of exogenous sucrose treatment on the peel coloration in postharvest peaches[J].Food Science,2022,43(1):177-183.
[72] MAATALLAH S,GUIZANI M,ELLOUMI O,MONTEVECCHI G,ANTONELLI A,GHRAB M,DABBOU S. Yield and biochemical fruit quality of irrigated peach cultivars subjected to conventional farmer’s fertilization practices in warm production area[J]. Journal of Food Composition and Analysis,2024,129:106121.
[73] INITIATIVE I P G,VERDE I,ABBOTT A G,SCALABRIN S,JUNG S,SHU S Q,MARRONI F,ZHEBENTYAYEVA T,DETTORI M T,GRIMWOOD J,CATTONARO F,ZUCCOLO A,ROSSINI L,JENKINS J,VENDRAMIN E,MEISEL L A,DECROOCQ V,SOSINSKI B,PROCHNIK S,MITROS T,POLICRITI A,CIPRIANI G,DONDINI L,FICKLIN S,GOODSTEIN D M,XUAN P F,DEL FABBRO C,ARAMINI V,COPETTI D,GONZALEZ S,HORNER D S,FALCHI R,LUCAS S,MICA E,MALDONADO J,LAZZARI B,BIELENBERG D,PIRONA R,MICULAN M,BARAKAT A,TESTOLIN R,STELLA A,TARTARINI S,TONUTTI P,ARÚS P,ORELLANAA,WELLS C,MAIN D,VIZZOTTO G,SILVA H,SALAMINI F,SCHMUTZ J,MORGANTE M,ROKHSAR D S. The high-quality draft genome of peach (Prunus persica)identifies unique patterns of genetic diversity,domestication and genome evolution[J].Nature Genetics,2013,45(5):487-494.
[74] FRESNEDO-RAMÍREZ J,FRETT T J,SANDEFUR P J,SALGADO-ROJAS A,CLARK J R,GASIC K,PEACE C P,ANDERSON N,HARTMANN T P,BYRNE D H,BINK M C A M,VAN DE WEG E,CRISOSTO C H,GRADZIEL T M. QTL mapping and breeding value estimation through pedigree-based analysis of fruit size and weight in four diverse peach breeding programs[J].Tree Genetics&Genomes,2016,12(2):25.
[75] 孟君仁,曾文芳,邓丽,潘磊,鲁振华,崔国朝,王志强,牛良.桃若干重要性状的KASP 分子标记开发与应用[J].中国农业科学.2021,54(15):3295-3307.MENG Junren,ZENG Wenfang,DENG Li,PAN Lei,LU Zhenhua,CUI Guochao,WANG Zhiqiang,NIU Liang. Development and application of KASP molecular markers of some important traits for peach[J]. Scientia Agricultura Sinica,2021,54(15):3295-3307.
[76] 许建兰,马瑞娟,张斌斌,张妤艳,张春华,郭磊,沈志军,俞明亮.早熟红肉蟠桃新品种‘金陵血蟠’[J].园艺学报,2021,48(1):193-194.XU Jianlan,MA Ruijuan,ZHANG Binbin,ZHANG Yuyan,ZHANG Chunhua,GUO Lei,SHEN Zhijun,YU Mingliang. A new early ripening red-flesh flat peach cultivar‘Jinling xuepan’[J].Acta Horticulturae Sinica,2021,48(1):193-194.