荔枝(Litchi chinensis Sonn.)是原产于中国的热带亚热带无患子科荔枝属常绿果树。2023 年中国荔枝的种植面积为50.2 万hm2,占世界荔枝种植面积的62.75%,产量为309.7 万t,占世界荔枝产量的77.43%,产值为290.2亿元[1]。荔枝产业在中国热带亚热带区域经济中发挥着重要的作用,但产业发展中存在一些技术难题,如荔枝开花问题一直备受关注。荔枝是雌雄同株异花植物,其单性花由两性花在减数分裂后通过花性器官选择性败育形成[2-3]。荔枝花穗是聚伞形圆锥状花序,具有花量大、雌雄花交替开放、雌花率低等特性,而该特性导致自然状态下花穗营养消耗大、坐果率低。在荔枝生产上,常需通过物理疏花或化学疏花降低雄花量,减少营养消耗,提高产量[4-5]。妃子笑是中国种植范围最广也是出口最多的荔枝品种[6],其果实焦核率高,肉质清香、蜜甜多汁,广受市场欢迎。但是,妃子笑由于花量过大,自然条件下坐果率低于0.5%[4],生产上多需采用乙烯利、烯效唑、多效唑单剂或复合试剂等对花穗进行处理[5,7-8],这些试剂也应用于控梢、保果等荔枝生长发育过程[9]。笔者团队在生产调研中发现,树体中过量的乙烯利累积容易导致老叶异常脱落,过量的烯效唑、多效唑容易对后期新梢的生长产生抑制作用。因此,筛选适用于荔枝花穗处理的新型植物生长调节剂,对荔枝产业的可持续健康发展具有重要意义。
生长素是植物生长发育必不可少的植物激素,它参与包括胚胎形成、种子发育、根系发育、幼苗生长、花发育、新器官形成等生物学过程[10]。外源生长素或抑制剂处理常应用于调节植物的开花结果。有研究表明,在花生的初花期喷施IAA可显著提高主茎高、花数量、果针长度以及果针数,而喷施生长素抑制剂TIBA 可显著增加侧枝长及分枝数,但显著降低主茎高、花数量以及果针数[11]。而在大豆中的有关研究表明,短日照处理后施加TIBA 可将每株花芽穗数由28.8 提高至51.7,并使子叶至第二节位的花芽数占比由19.4%提高至55.3%[12]。在黑豆中的有关研究表明,在始花期喷施TIBA 可显著提高第一分枝坐荚数和坐荚率[13]。青花椒上常出现“开黄花”现象,实际是雌蕊缺失、雄蕊发育异常引起的,而施加TIBA处理可显著改善该现象[14]。
胡香英等[8]对荔枝花穗进行NAA 处理,结果发现NAA显著抑制了荔枝的雌花率,并且降低了单穗产量。与之相反,若使用生长素抑制剂是否会抑制雄花而促进雌花发育从而有利于坐果?本研究中通过采用不同质量浓度不同类型的生长素抑制剂对妃子笑荔枝的花穗进行喷施处理,以清水为对照,分析各处理对荔枝雌花与雄花发育、坐果量以及果实品质的影响,以期为荔枝的丰产与提质增效提供技术参考。
1 材料和方法
1.1 供试材料与生长条件
试验地点位于海南省农业科学院热带果树研究所澄迈永发科研基地,土壤类型为玄武岩砖红壤土,经纬度为19°23′~20°01′N、109°45′~110°15′E,年平均温度26 ℃,总降雨量1124 mm。以荔枝品种妃子笑(Litchi chinensis Sonn.‘Feizixiao’)为试材,砧木品种为怀枝,树龄27 a(年),常规管理,生长发育良好。供试药剂为1-萘乙酸钠(Naphthylacetic acid,NAA)、4-苯氧基苯基硼酸(4-Phenoxyphenylboronic acid,PPBo)、三碘苯甲酸(2,3,5-Triiodobenzoic acid,TIBA)。
1.2 试验方法
1.2.1 试验处理 本试验共设计5种植物生长调节剂处理,选取长势、大小、物候期一致的植株和花穗,于2024 年2—5 月进行处理与调查统计。在花序轴停止伸长,且第1 批花蕾饱满待放时喷施不同植物生长调节剂溶液(具体处理见表1),以清水为对照。由于笔者课题组前期发表的文章[8]中,NAA 的处理质量浓度与效应已经比较明确,因此NAA处理只采用了一种质量浓度作为参照;而生长素抑制剂PPBo 与TIBA 依照NAA 的质量浓度范围采用了2个跨度较大的质量浓度进行试验,以初步探明该类生长调节剂对荔枝开花坐果的调控效应。试验以单株为小区,每个处理3次生物学重复,每株树在阴面与阳面各选取2个生长状态一致的花穗进行处理,并对花穗进行固定调查。处理时每个花穗套上38 cm×30 cm 自封袋后喷施约1 L 溶液,以花穗滴水为度。待花穗不滴水后取下自封袋,保持自然生长状态。
表1 不同生长调节剂的处理质量浓度
Table 1 The concentration of different plant growth regulators
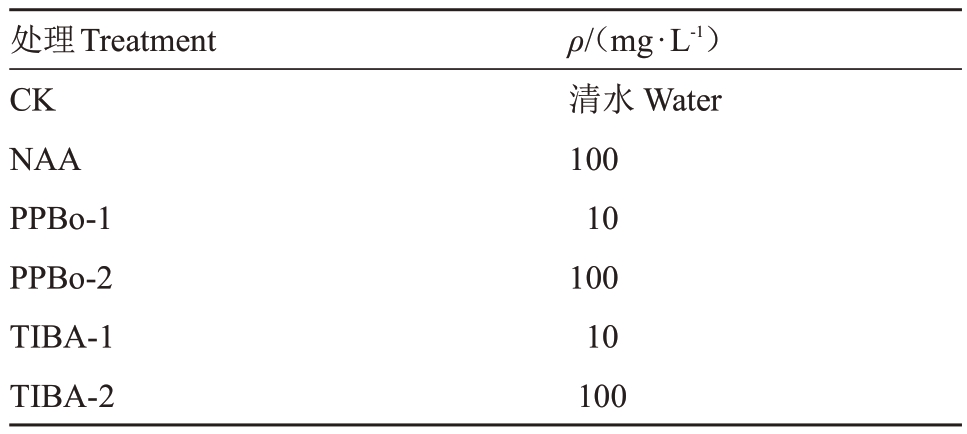
处理Treatment CK NAA PPBo-1 PPBo-2 TIBA-1 TIBA-2 ρ/(mg·L-1)清水Water 100 10 100 10 100
1.2.2 花穗处理与调查统计 对每个处理的花穗进行固定调查。在处理后持续关注花穗的开放动态,每隔1 d调查雌雄花的开花量,直至花期结束,对不同处理花穗的雄花与雌花开放数量、总花量、雌花率、雄花累计开放时间、雌花累计开放时间、雌花与雄花重叠开放时间,分别进行统计分析。
1.2.3 坐果量调查 雌花谢后持续观察花穗的坐果情况,调查雌花谢后1 周花穗的初坐果量以及成熟时的终坐果量。
1.2.4 果实品质分析 在果实成熟期,根据《荔枝、龙眼种质资源描述规范》[15],每个处理选取10 个单果测定果实品质相关指标,包括纵径、横径、单果质量、果皮质量、果皮厚度、种子质量、可食率、可溶性固形物含量。
1.3 数据分析
采用Excel 软件对数据进行统计分析,采用SPSS 26软件进行方差分析(多重比较采用Duncan’s test)以及相关性分析(采用Spearman correlation)。
2 结果与分析
2.1 生长素抑制剂对妃子笑雌雄花分化发育的效应
为了明确外源生长素抑制剂对妃子笑开花特性的影响,采用不同质量浓度的生长素合成抑制剂PPBo和生长素极性运输抑制剂TIBA对妃子笑的花穗进行处理,花量变化结果如表2 所示。NAA、PPBo 与TIBA 处理后花穗的雄花量均低于对照,其中,仅TIBA 处理与对照具有显著差异。在雌花量上,各处理与对照均无显著差异,但NAA 处理低于对照,生长素抑制剂TIBA-2处理高于对照。总花量上,除TIBA-1 处理显著低于对照,其余处理均与对照无显著差异。NAA处理后雌花率低于对照,而生长素抑制剂PPBo-2、TIBA-1、TIBA-2处理后雌花率均高于对照,其中TIBA-2的雌花率与对照达到显著差异水平,高达30.29%,是对照雌花率的2.9倍。以上结果表明,外源生长素处理可抑制雌花的发育,而生长素抑制剂处理可抑制雄花的发育,所以生长素在荔枝花芽性别分化发育过程中扮演着重要的角色。
表2 生长素抑制剂对荔枝花性别分化与发育的影响
Table 2 Effects of auxin inhibitors on floral sex differentiation and development of litchi

注:表中数据为(平均值±标准差),数据后的不同字母表示差异显著(p<0.05)。下同。
Note:The data is(mean±standard deviation),and different letters after the data indicate significant differences(p<0.05).The same below.
处理Treatment CK NAA PPBo-1 PPBo-2 TIBA-1 TIBA-2雌花率Ratio of female flowers/%10.61±4.88 bc 3.77±2.47 c 9.76±2.00 bc 12.32±2.37 bc 20.36±4.37 ab 30.29±3.89 a雄花量Male flowers number 1 661.17±244.89 a 1 549.83±206.56 a 1 082.67±196.49 ab 1 325.00±211.58 ab 788.17±41.84 b 909.33±137.54 b雌花量Female flowers number 189.67±84.78 ab 72.33±54.88 b 123.17±49.16 b 189.33±47.72 ab 189.17±44.82 ab 361.00±103.57 a总花量Total flowers number 1 850.83±161.72 a 1 622.17±259.94 ab 1 205.83±240.79 ab 1 514.33±246.97 ab 977.33±81.04 b 1 270.33±240.38 ab
对处理后花穗雌花与雄花的累计开放时间进一步统计分析,结果如表3 所示。不同处理后雄花累计开放时间与对照相比均没有显著差异;NAA和PPBo 处理后雌花累计开放时间有所减少,而TIBA处理后雌花累计开放时间有所延长但与对照没有明显差异;不同处理的雌雄花重叠开放时间与对照相比均没有显著差异,其中PPBo 处理的雌雄花重叠开放时间最短。
表3 生长素抑制剂对荔枝雄花与雌花累积开花时间的影响
Table 3 Effects of auxin inhibitors on the flowering period of males and females in litchi
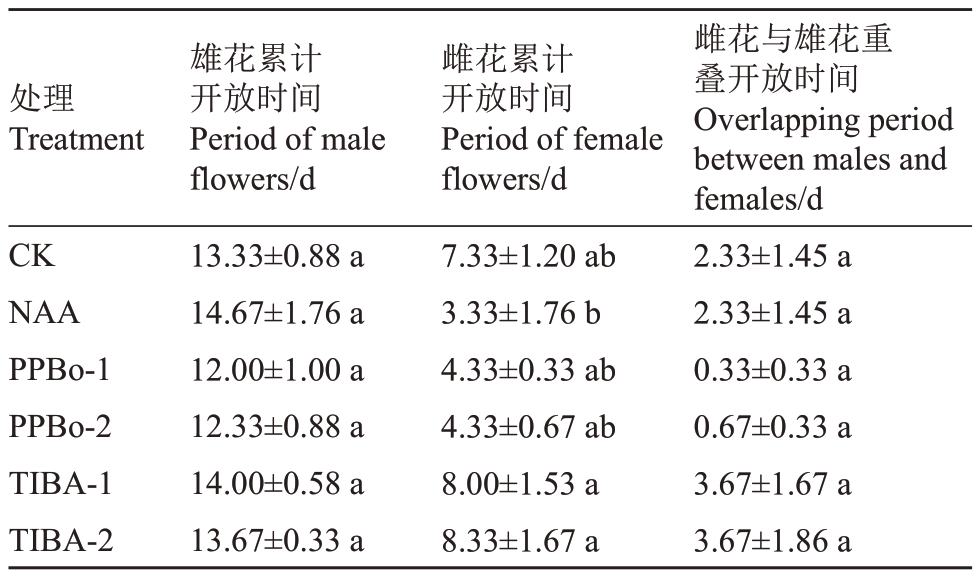
处理Treatment CK NAA PPBo-1 PPBo-2 TIBA-1 TIBA-2雄花累计开放时间Period of male flowers/d 13.33±0.88 a 14.67±1.76 a 12.00±1.00 a 12.33±0.88 a 14.00±0.58 a 13.67±0.33 a雌花累计开放时间Period of female flowers/d 7.33±1.20 ab 3.33±1.76 b 4.33±0.33 ab 4.33±0.67 ab 8.00±1.53 a 8.33±1.67 a雌花与雄花重叠开放时间Overlapping period between males and females/d 2.33±1.45 a 2.33±1.45 a 0.33±0.33 a 0.67±0.33 a 3.67±1.67 a 3.67±1.86 a
2.2 生长素抑制剂对妃子笑初坐果量与终坐果量的影响
为了分析不同处理对妃子笑荔枝坐果情况的影响,统计了雌花谢后1周的初坐果量以及7周后的最终坐果量,结果如表4所示。NAA处理的初坐果量低于对照,而PPBo与TIBA处理的初坐果量均高于对照,其中,TIBA-2 初坐果量高达59.17 个·穗-1,显著高于对照及其他处理;NAA 处理的终坐果量低于对照,TIBA-2 处理的终坐果量显著高于对照,其他生长素抑制剂处理的终坐果量高于对照但无显著差异。以上结果表明,生长素抑制剂处理可提高荔枝花穗的坐果能力,其中生长素极性运输抑制剂TIBA-2 的处理效果最佳,单穗终坐果量可达到15.00个·穗-1。
表4 生长素抑制剂对荔枝坐果量的影响
Table 4 Effects of auxin inhibitors on the amount of fruit set in litchi

处理Treatment CK NAA PPBo-1 PPBo-2 TIBA-1 TIBA-2初坐果量/(个·穗-1)Initial fruit set amount per panicle 15.00±5.77 b 2.50±2.50 b 28.00±9.57 b 26.50±12.51 b 23.50±6.26 b 59.17±15.97 a终坐果量/(个·穗-1)Final fruit set amount per panicle 4.50±1.89 b 0.67±0.67 b 5.83±2.60 ab 6.33±3.35 ab 8.33±3.24 ab 15.00±5.35 a
2.3 妃子笑花穗开花性状与坐果量的相关性分析
为了进一步探究妃子笑荔枝花穗各开花性状以及坐果量之间的关联性,对它们进行了相关性分析,结果如表5所示。数据表明,雄花量与总花量、雄花累积开放时间呈显著正相关,而与雌花率、初坐果量、终坐果量呈显著负相关;雌花量与雌花率、初坐果量、终坐果量呈显著正相关;总花量与雄花累积开放时间呈显著正相关,而与雌花率、初坐果量呈显著负相关;雌花率与雌花累积开放时间、初坐果量、终坐果量呈显著正相关;雄花累积开放时间与雌雄花重叠开放时间呈显著正相关,而与初坐果量呈显著负相关;雌花累积开放时间与雌雄花重叠开放时间呈显著正相关,相关系数达到0.74;初坐果量与终坐果量呈显著正相关,相关系数达到0.73。以上结果表明,与初坐果量最紧密相关的是雌花率,两者表现出极显著正相关,相关系数达到0.70。该结果表明花穗雌花率可作为筛选适合荔枝花穗处理的生长调节剂的重要指标。此外,初坐果量与雄花量、雌花量、总花量的相关性也极为显著,但与雌雄花开放时间及其重叠开放时间相关性较弱。终坐果量除了与初坐果量呈现极显著正相关外,与雄花量、雌花量、雌花率也具有显著的相关关系,但其相关性均弱于初坐果量与这些开花性状之间的相关性。
表5 荔枝各开花性状及坐果量间的相关性分析
Table 5 Correlation analysis of flowering characters and fruit setting quantity in litchi

注:*表示差异显著(*p<0.05,**p<0.01)。
Note:*indicates significant difference(*p<0.05,**p<0.01).
指标Index雌花量Female flowers number总花量Total flower number雌花率Ratio of female flowers雄花累积开放时间Period of Male flowers雌花累积开放时间Period of Female flowers雌花与雄花重叠开放时间Overlap period between males and females初坐果量Initial fruit set amount终坐果量Final fruit set amount雄花量Male flowers number雌花量Female flowers number总花量Total flower number雌花率Ratio of female flowers雄花累积开放时间Period of Male flowers雌花累积开放时间Period of Female flowers雌花与雄花重叠开放时间Overlap period between males and females初坐果量Initial fruit set amount终坐果量Final fruit set amount雄花量Male flowers number 1.00-0.14 1.00 0.95**0.09 1.00-0.58**0.80**-0.37*1.00 0.40*-0.14 0.32*-0.29 1.00-0.14 0.18-0.08 0.33*0.15 1.00 0.07-0.12 0.06-0.09 0.37*0.74**1.00-0.57**0.51**-0.43**0.70**-0.32*0.28-0.11 1.00 1.00-0.34*0.40*-0.24 0.51**0.16 0.31-0.05 0.73**
2.4 外源生长素抑制剂对妃子笑果实品质的影响
为了解不同生长素抑制剂对妃子笑荔枝果实品质的影响,分析了不同处理成熟果实的纵径、横径、单果质量、果皮质量、果皮厚度、种子质量、可食率以及可溶性固形物含量,结果如表6 所示。在果实纵径上,不同处理与对照均无显著差异;横径上,TIBA-2处理显著高于对照,其余处理与对照均无显著差异;单果质量上,PPBo-1和TIBA-2处理均显著高于对照,其余处理与对照均无显著差异;果皮质量上,NAA 处理显著低于对照,其余处理与对照均无显著差异;果皮厚度上,NAA、TIBA-1 和TIBA-2 处理均显著低于对照;种子质量与可溶性固形物TSS上,不同处理与对照均无明显差异;可食率上,NAA和TIBA-2 处理均显著高于对照,均超过80%,其余处理与对照均无显著差异。NAA 处理后果皮质量与果皮厚度均显著低于对照,可食率显著高于对照,其余品质性状与对照均无显著差异;PPBo-1处理具有显著提高单果质量的效果,其余品质性状与对照均无显著差异;TIBA 两处理均具有提高单果质量、降低果皮厚度、提高可食率的效果,尤其TIBA-2 的处理效果更为明显。
表6 生长素抑制剂对妃子笑果实品质的影响
Table 6 Effects of auxin inhibitors on fruit quality of Feizixiao
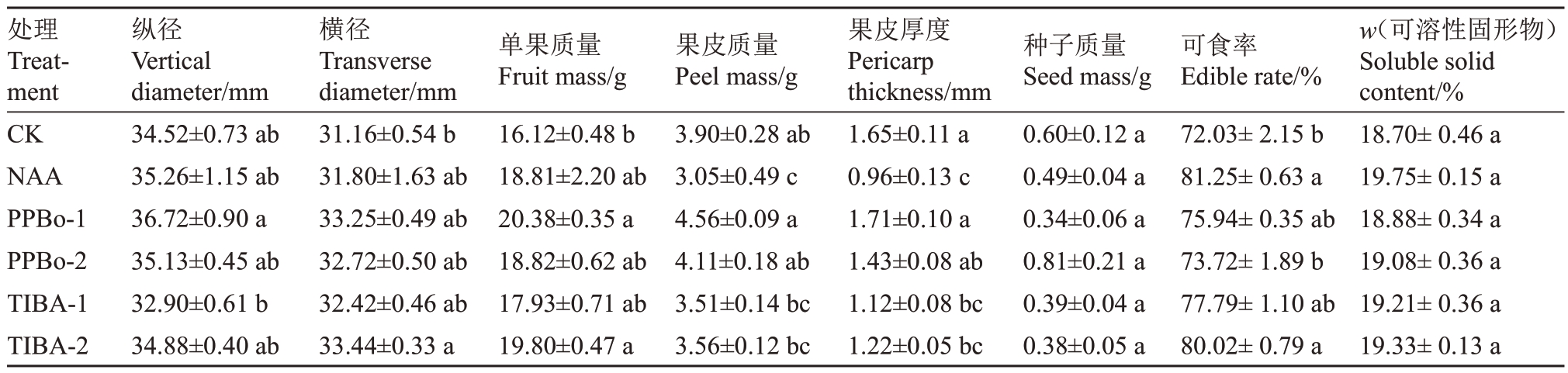
处理Treatment CK NAA PPBo-1 PPBo-2 TIBA-1 TIBA-2纵径Vertical diameter/mm 34.52±0.73 ab 35.26±1.15 ab 36.72±0.90 a 35.13±0.45 ab 32.90±0.61 b 34.88±0.40 ab横径Transverse diameter/mm 31.16±0.54 b 31.80±1.63 ab 33.25±0.49 ab 32.72±0.50 ab 32.42±0.46 ab 33.44±0.33 a单果质量Fruit mass/g 16.12±0.48 b 18.81±2.20 ab 20.38±0.35 a 18.82±0.62 ab 17.93±0.71 ab 19.80±0.47 a果皮质量Peel mass/g 3.90±0.28 ab 3.05±0.49 c 4.56±0.09 a 4.11±0.18 ab 3.51±0.14 bc 3.56±0.12 bc果皮厚度Pericarp thickness/mm 1.65±0.11 a 0.96±0.13 c 1.71±0.10 a 1.43±0.08 ab 1.12±0.08 bc 1.22±0.05 bc种子质量Seed mass/g 0.60±0.12 a 0.49±0.04 a 0.34±0.06 a 0.81±0.21 a 0.39±0.04 a 0.38±0.05 a可食率Edible rate/%72.03±2.15 b 81.25±0.63 a 75.94±0.35 ab 73.72±1.89 b 77.79±1.10 ab 80.02±0.79 a w(可溶性固形物)Soluble solid content/%18.70±0.46 a 19.75±0.15 a 18.88±0.34 a 19.08±0.36 a 19.21±0.36 a 19.33±0.13 a
3 讨 论
笔者基于前期的研究发现外源生长素处理荔枝花穗对雌花发育产生抑制作用,且生长素抑制剂在其他园艺作物中具有调节花果发育的作用[11-14],通过采用生长素合成抑制剂PPBo 与运输抑制剂TIBA处理妃子笑荔枝的花穗,并对处理后荔枝雄花与雌花开放量及开放时间、坐果量、果实品质等方面进行分析。结果表明,各自与对照相比,TIBA比PPBo处理更能抑制雄花发育进而提高雌花率;NAA处理的初坐果量低于对照,而PPBo和TIBA处理的初坐果量与终坐果量均高于对照,其中TIBA-2效果最佳,初坐果量达到59.17个·穗-1,终坐果量达到15.00个·穗-1,比对照多10.5 个·穗-1。相关性分析结果显示,初坐果量与雌花率呈显著正相关,而与雄花量呈显著负相关,终坐果量与初坐果量呈较显著正相关。果实品质分析结果表明TIBA 处理具有提高单果质量、降低果皮厚度、提高果实可食率的作用。不同处理花穗花量与坐果量的数据结果存在一定的误差,这主要是由于树体之间不可避免存在一些差异。但试验时笔者通过保持对照与不同处理的花穗均处于相同的状态并位于相同的树体方位,尽可能确保所有处理的内部与外部环境条件的一致性。因此,虽然测量值存在误差,不同处理与对照的统计学分析结果仍然具有较强的可靠性与说服力。
生长素作为植物重要的信号物质,在花原基的形成、花性器官的发育中发挥着重要的调节作用[16]。本研究中使用外源生长素NAA 处理荔枝花穗导致雌花量、雌花率均降低,而适宜浓度的生长素抑制剂PPBo 与TIBA 处理均可使雄花量降低、雌花率升高,表明过量的生长素可抑制荔枝雌花的发育,而雄花的发育需要适量的生长素。该结果与生长素抑制剂TIBA在玉米、青花椒、黄瓜上的处理效应一致,均表现为显著抑制雄蕊发育或降低雄花比例[14,17-18]。此外,对番木瓜施以生长素转运抑制剂N-(1-萘基)邻氨甲酰苯甲酸(NPA)处理可恢复雄花中的雌蕊发育[19]。本研究所使用的两种生长素抑制剂,PPBo 的作用原理是抑制生长素合成最后一步关键酶YUC 蛋白从而抑制生长素合成[20],而TIBA 的作用原理是竞争性结合IAA 的转运体PIN和ABC 等从而抑制IAA的运输[21-22]。通过比较不同浓度及不同类别生长素抑制剂的处理效果,可以发现生长素运输抑制剂TIBA 比合成抑制剂PPBo 处理的雄花量更低,雌花率更高,表明在荔枝雌雄花分化发育过程中,特定的生长素区域位置比含量更重要。不同浓度的PPBo或TIBA处理,雄花量都低于对照;低浓度的PPBo-1 处理表现出雌花量低于对照,高浓度的PPBo-2 处理与对照的雌花量没有显著差异,推测在花芽发育中保持内源适量的生长素对荔枝雌花发育也具有促进作用,而低浓度的生长素抑制剂处理打破了荔枝雌花发育所需的激素平衡。
相关性分析结果表明,初坐果量与花穗的雌花率相关系数最高,呈现显著的正相关关系,而这与TIBA-2处理雌花率最高,同时初坐果量也是最高的结果相符。终坐果量与初坐果量的相关系数最大,其次是与雌花率,均呈现显著正相关。在其他园艺作物上的研究也证明,生长素或生长素抑制剂的应用可显著提高作物的产量,例如花生[11]与黑豆[13]。因此,在荔枝生产上,采用适宜浓度的生长素抑制剂处理花穗,通过抑制雄花量、提高雌花率,可达到提高初坐果量的目的,再辅以一些保果措施防止落果,将可大幅度提高荔枝的产量。
本研究中,NAA处理后的荔枝果实除了果皮质量与果皮厚度外,其余的品质性状均与对照无显著差异,这与之前的研究结果较为相符[8]。PPBo与TIBA处理均有提高荔枝妃子笑单果质量的效应。此外,TIBA 还有降低果皮厚度从而提高可食率的作用,这可能与生长素对不同果实组织细胞的影响存在差异有关。在樱桃上,盛花期采用NAA 处理,可导致果实发育后期中外果皮细胞的大小显著高于对照,中果皮的细胞密度下降,最终果实单果质量显著增加[23]。荔枝果皮来源于子房壁,由外、中、内果皮三部分组成[24],生长素可能对荔枝果皮的细胞生长具有促进作用,而TIBA可能改变了生长素分布,从而降低果皮厚度与果皮质量;NAA外源处理也降低了果皮厚度与果皮质量,这可能是由于过量的生长素展示出的抑制作用。荔枝的果肉来源于假种皮,生长素对假种皮的发育是否存在影响仍需进一步的研究。
4 结 论
与清水对照相比,荔枝花穗上采用生长素NAA处理,雌花量与雌花率降低,平均单穗终坐果量降低至0.67个;而适宜浓度的生长素合成抑制剂PPBo与运输抑制剂TIBA处理,具有降低花穗雄花量,提高雌花率,并提高坐果量的作用,其中,100 mg·L-1的TIBA-2 处理效果最佳。此外,TIBA-2 处理还具有提高荔枝单果质量、降低果皮厚度、提高可食率的效应。
[1] 农业农村部南亚热带作物中心.中国荔枝、龙眼产业发展报告[J].中国热带农业,2024(3):5-7.South Subtropical Crops Center,Ministry of Agriculture and Rural Affairs.Report on the development of China’s litchi and longan industries[J].China Tropical Agriculture,2024(3):5-7.
[2] 肖华山,吕柳新,王湘平,王平.荔枝(Litchi chinensis Sonn.)花芽分化过程的细胞超微结构观察[J].福建师范大学学报(自然科学版),2002,18(2):57-60.XIAO Huashan,LÜ Liuxing,WANG Xiangping,WANG Ping.Observation on cell ultrastructure of flower bud differentiation in litchi (Litchi chinensis Sonn.)[J]. Journal of Fujian Teachers University(Natural Science),2002,18(2):57-60.
[3] 王平,郑伟,陈伟. 荔枝花性别分化过程的荧光显微观察[J].热带作物学报,2010,31(5):740-744.WANG Ping,ZHENG Wei,CHEN Wei. Fluorescence microscopic observation on flower sex differentiation in litchi (Litchi chinensis Sonn.)[J].Chinese Journal of Tropical Crops,2010,31(5):740-744.
[4] 胡福初,范鸿雁,何凡,华敏,王祥和.妃子笑荔枝高效花穗处理及保果壮果技术[J].中国热带农业,2014(3):65-67.HU Fuchu,FAN Hongyan,HE Fan,HUA Min,WANG Xianghe. High efficient panicle treatment and fruit retention technology on Feizixiao litchi[J]. China Tropical Agriculture,2014(3):65-67.
[5] 李冬波,朱建华,彭宏祥,黄凤珠,徐宁,陆贵锋,秦献泉,黎光旺. 花穗修剪与多效唑处理对妃子笑荔枝产量与效益的影响[J].南方农业学报,2011,42(2):182-184.LI Dongbo,ZHU Jianhua,PENG Hongxiang,HUANG Fengzhu,XU Ning,LU Guifeng,QIN Xianquan,LI Guangwang. Effects of pruning blossomed spikelets and MET treatment on the yield and economic benefits in litchi (Litchi chinensis Sonn. cv.Feizixiao) production[J]. Journal of Southern Agriculture,2011,42(2):182-184.
[6] 苏钻贤,杨胜男,陈厚彬,申济源.2020 年我国荔枝主产区的生产形势分析[J].南方农业学报,2020,51(7):1598-1605.SU Zuanxian,YANG Shengnan,CHEN Houbin,SHEN Jiyuan.Analysis of the production situation for litchi in main planting areas of China in 2020[J]. Journal of Southern Agriculture,2020,51(7):1598-1605.
[7] 严婷婷,王满青,董余思,杨明超,周文静,周瑞云,陈哲,胡福初,王祥和.复合植物生长调节剂对荔枝开花及坐果的影响[J].中国果树,2024(4):83-88.YAN Tingting,WANG Manqing,DONG Yusi,YANG Mingchao,ZHOU Wenjing,ZHOU Ruiyun,CHEN Zhe,HU Fuchu,WANG Xianghe. Effect of compound plant growth regulators on the flowering and fruit setting in litchi[J]. China Fruits,2024(4):83-88.
[8] 胡香英,胡福初,范鸿雁,王祥和,韩冰,林尤奋.5 种植物生长调节剂对妃子笑荔枝开花坐果调控效应的比较[J].西南农业学报,2016,29(4):915-919.HU Xiangying,HU Fuchu,FAN Hongyan,WANG Xianghe,HAN Bing,LIN Youfen. Effects of five plant growth regulators on blooming and fruit-setting of‘Feizixiao’litchi[J]. Southwest China Journal of Agricultural Sciences,2016,29(4):915-919.
[9] 海南省市场监督管理局. 农产品全产业链生产规范荔枝:DB46/T 595—2023[S].海南:中国标准出版社,2023.Hainan Market Supervision Administration.Technical specification for production of the whole industrial chain:Litchi:DB46/T 595—2023[S].Hainan:China Standards Press,2023.
[10] ZHAO Y D. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes[J].Annual Review of Plant Biology,2018,69:417-435.
[11] 彭琼.花生开花下针期生长素的极性运输及分布研究[D].长沙:湖南农业大学,2013.PENG Qiong. Studies on the polar transport and distribution of auxin at pegging stage of peanut (Arachis hypogaea L.)[D].Changsha:Hunan Agricultural University,2013.
[12] 张志良,颜季琼.2,3,5-三碘苯甲酸(TIBA)对大豆开花的影响[J].植物生理学通讯,1958(3):30-32.ZHANG Zhiliang,YAN Jiqiong. Effects of 2,3,5-triiodobenzoic acid(TIBA)on soybean flowering[J].Plant Physiology Communications,1958(3):30-32.
[13] 王昊文.三种植物生长调节剂对黑豆生长发育的调控效应研究[D].杨凌:西北农林科技大学,2018.WANG Haowen. Regulatory effects of three plant growth regulators on growth and development of black beans[D].Yangling:Northwest A&F University,2018.
[14] 王正江,张灿,王帅,赵敬坤,彭先容,杨裕然,李振轮.青花椒开黄花的生理变化及调控初步研究[J].植物生理学报,2023,59(2):315-323.WANG Zhengjiang,ZHANG Can,WANG Shuai,ZHAO Jingkun,PENG Xianrong,YANG Yuran,LI Zhenlun. Preliminary study on physiological changes and regulation of Zanthoxylum armatum with yellow flower[J].Plant Physiology Journal,2023,59(2):315-323.
[15] 中华人民共和国农业部.荔枝、龙眼种质资源描述规范:NY/T 1691—2009[S].北京:中国农业出版社,2009.Ministry of Agriculture of the People’s Republic of China. Descriptors standard for germplasm of litchi and longan:NY/T 1691—2009[S].Beijing:China Agriculture Press,2009.
[16] CUCINOTTA M,CAVALLERI A,CHANDLER J W,COLOMBO L. Auxin and flower development:A blossoming field[J].Cold Spring Harbor Perspectives in Biology,2021,13(2):a039974.
[17] MOORE R H. Several effects of maleic hydrazide on plants[J].Science,1950,112(2898):52-53.
[18] WITTWER S H,HILLYER I G.Chemical induction of male sterility in cucurbits[J].Science,1954,120(3126):893-894.
[19] 周平.基于高通量测序的番木瓜性别决定机制研究[D].福州:福建农林大学,2019.ZHOU Ping. The research of sex determination in papaya based on high-throughput sequencing[D]. Fuzhou:Fujian Agriculture and Forestry University,2019.
[20] KAKEI Y,YAMAZAKI C,SUZUKI M,NAKAMURA A,SATO A,ISHIDA Y,KIKUCHI R,HIGASHI S,KOKUDO Y,ISHII T,SOENO K,SHIMADA Y.Small-molecule auxin inhibitors that target YUCCA are powerful tools for studying auxin function[J].The Plant Journal,2015,84(4):827-837.
[21] THOMSON K S,HERTEL R,MÜLLER S,TAVARES J E.1-Nnaphthylphthalamic acid and 2,3,5-triiodobenzoic acid:In-vitro binding to particulate cell fractions and action on auxin transport in corn coleoptiles[J].Planta,1973,109(4):337-352.
[22] STRADER L C,BARTEL B. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid[J]. Molecular Plant,2011,4(3):477-486.
[23] 郑奇志.植物生长调节剂对上海地区甜樱桃坐果率及果实品质的影响[D].上海:上海交通大学,2019.ZHENG Qizhi. Effect of plant growth regulators on sweet cherry fruit setting and fruit quality in Shanghai[D]. Shanghai:Shanghai Jiao Tong University,2019.
[24] 李建国.荔枝学[M].北京:中国农业出版社,2008:224-227.LI Jianguo. The litchi[M]. Beijing:China Agriculture Press,2008:224-227.