草莓(Fragaria×ananassa)属于蔷薇科草莓属多年生草本植物。在世界上有着广泛的栽植面积。其风味独特,营养丰富,果实内含有高水平的维生素C、叶酸和酚类成分[1-2],且果实具有抗氧化等功效[3-4],深受广大消费者喜爱。盐胁迫是自然界最主要的非生物胁迫之一,是影响生态环境和农业生产的全球性问题。由于草莓对盐敏感[5],盐渍化的土壤严重影响了草莓植株发育和果实品质,限制了草莓的栽培推广和生产发展[6]。
在盐胁迫下,根系对土壤盐胁迫感受最为迅速和明显,也是受危害最直接的部位[7]。呼吸代谢是一切生命活动的基础,为植物的生命活动提供能量和生物合成的原料[8-9]。盐胁迫会抑制根系呼吸速率,从而影响生理代谢活动,导致根系吸收和运输等主要功能紊乱[10]。盐渍化对草莓的根系呼吸影响尤为显著,但目前关于盐胁迫条件下草莓根系呼吸速率以及相关酶活性的研究鲜有报道。
生物光子辐射(biophoton emission)是自然界普遍存在的一种生物发光现象,其辐射强度仅为0~103 hv·s-1·cm-2,波长范围为180~800 nm[11-12],因为极为微弱的发光强度又被称为超微弱发光(ultra weak luminescence,UWL)。UWL 普遍存在于生命体中,包括各个器官、组织和细胞都会自发性地向外辐射光子[13],其涉及植物体内许多主要的生物功能,如细胞分裂、能量代谢、信息传递[14]。尽管UWL 与生物体生理反应以及生化过程有着紧密联系,并且呼吸作用为植株生长最关键的生理活动之一,但目前对于园艺植物的相关研究主要集中于叶片、果实、种子等器官,对于最先受到土壤盐害影响的根系呼吸作用与UWL之间的相关性仍知之甚少。
丛枝菌根真菌(arbuscular mycorrhizal fungi,AMF)是自然界普遍存在的一种根系共生真菌,具有天然、无污染等优势,因具有提升植株抗性的特点,已有AMF 应用在果蔬种植方面的报道。草莓根系呼吸速率是逆境条件下重要的生理指标,那么草莓根系在盐胁迫下呼吸相关指标如何变化?AMF如何缓解盐胁迫对草莓根系的影响?笔者在本研究中以草莓根系为材料,在前期已进行AMF对草莓植株抗逆性影响研究的基础上,探究盐胁迫下接种AMF后草莓根系呼吸速率和相关酶活性的变化特性,以UWL与根系呼吸指标的相关性变化为主要切入点,研究在盐胁迫环境下AMF提高草莓耐盐性的机制。
1 材料和方法
1.1 试验材料
试验选取生长健壮、长势相似的红颜草莓苗为材料,草莓苗苗龄3个月,平均茎粗3 mm。
1.2 试验设计
试验为双因素随机区组设计,包含AMF和盐胁迫两个因素。采用温室内盆栽控制的方法,将草莓苗移栽到含有基质的花盆中,栽培基质为泥炭、蛭石、珍珠岩体积比1∶1∶1的混合物,接种菌剂经高温干热灭菌处理后作为不接种AMF 的对照。将草莓苗分为4组处理,包括只接种AMF组、接种AMF后进行盐胁迫组、对照组和只进行盐胁迫组,分别记为FF、FY、CK和YY,每组分别在5个采样时期进行相关指标测量,每个处理组在每个测量时期内设置3盆草莓作为重复。
1.3 AMF处理
AMF 选取与草莓根系亲和性好的摩西球囊霉(Glomus mosseae)[15]为供试菌种,以含有孢子、菌丝及侵染根段的根土混合物作为接种菌剂。将AMF接种于草莓苗根系,接种时先在花盆中装略低于1/3高度的栽培基质,将30 g 菌剂均匀撒下后再将草莓苗栽在菌剂表面,使其与根系充分接触,继续加入适量的栽培基质覆盖住根系并浇足定根水。待草莓与AMF建立共生关系后进行盐胁迫处理。
1.4 盐胁迫处理
盐胁迫处理采用内蒙古地区盐碱土壤中含量较高的NaCl和Na2SO4复合盐,将两种盐配置为摩尔比1∶1、浓度90 mmol·L-1的盐溶液,处理时按照每盆600 mL浇灌。为使盐溶液充分浸润土壤,需将流出的盐溶液反复回浇直到不再流出,非盐胁迫组浇灌等量蒸馏水作为对照。试验期间对草莓苗正常浇水与田间管理。从盐胁迫的第1 天开始,每2 d 进行1次指标测量,处理时长为9 d(采样时间表示为D1、D3、D5、D7、D9,共5次)。
1.5 试验指标及测定方法
1.5.1 菌根侵染率 将接菌草莓根系取出后用毛刷轻柔刷去表面附着的土壤,冲洗洁净后剪成1 cm长根段备用。根系AMF染色使用台盼蓝染色剂,染色及侵染率测定参考王思雨等[16]的方法。
1.5.2 根系呼吸速率测定 选用液相Oxy-Lab氧电极(英国HANSATECH 公司)用于测量根系呼吸速率,参考毛志泉等[17]的方法并进行改良。取直径基本一致的健康根系,迅速称取0.05 g,切成2 mm 左右根段放入反应杯,加盖启动测量程序。
1.5.3 根系呼吸相关酶活性测定 选用索莱宝公司试剂盒测定草莓根系呼吸相关酶活性,包括6-磷酸葡萄糖脱氢酶(G-6-PDH)、磷酸果糖激酶(PFK)、NAD-苹果酸脱氢酶(NAD-MDH)活性。
1.5.4 UWL强度测定 选用BPCL-GP15型超微弱发光分析仪(北京建新力拓科技有限公司)测量UWL强度,参考孙聪等[18]的方法并适当调整。仪器开机后调控高压950 V 预热30 min,设定采样时间5 s。各处理叶片选择直径10 mm打孔器取样测定,根系剪取0.1 g用于测量。
1.6 数据处理与方法
数据处理使用Microsoft Excel(2021),图形设计使用Origin 2022 软件(Origin Lab,Northampton,MA,USA)。采用SPSS 26(IBM SPSS STATISTICS,USA)进行统计学和相关性分析,以p<0.05为差异有统计学意义。
2 结果与分析
2.1 草莓菌根侵染率
菌根侵染率可以作为AMF 与草莓植株的共生情况是否良好的参考指标,由表1可知FF、FY组草莓接种后AMF定殖情况良好,菌根侵染率均超过60%;未接菌的CK、YY组侵染率为0,对后续试验无影响。
表1 不同处理组草莓根系侵染率
Table 1 Root infestation rate of strawberry in different treatment groups
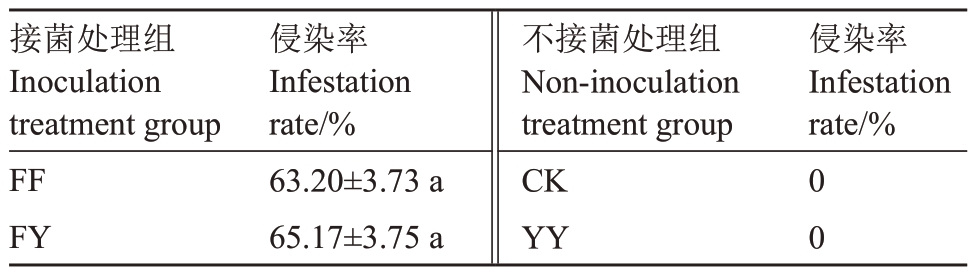
接菌处理组Inoculation treatment group FF FY侵染率Infestation rate/%侵染率Infestation rate/%63.20±3.73 a 65.17±3.75 a不接菌处理组Non-inoculation treatment group CK YY 0 0
2.2 盐胁迫下草莓根系呼吸速率的变化
根系呼吸速率测定结果如图1所示。随着胁迫时间的延长,测定期内草莓根系呼吸速率总体呈下降趋势,受到盐胁迫的FY、YY组呼吸速率下降趋势明显强于未受到盐胁迫组,并且随着盐胁迫时间的增加,呼吸速率持续下降。FF组草莓根系呼吸速率显著高于其他处理组,且呼吸速率随时间变化幅度最小;CK组呼吸速率高于盐胁迫处理的两组,维持波动稳定。在盐胁迫处理的情况下,接菌的FY 组比未接菌YY组呼吸速率更高,且变化幅度更小,由此推断AMF可以缓解被盐抑制的呼吸速率。
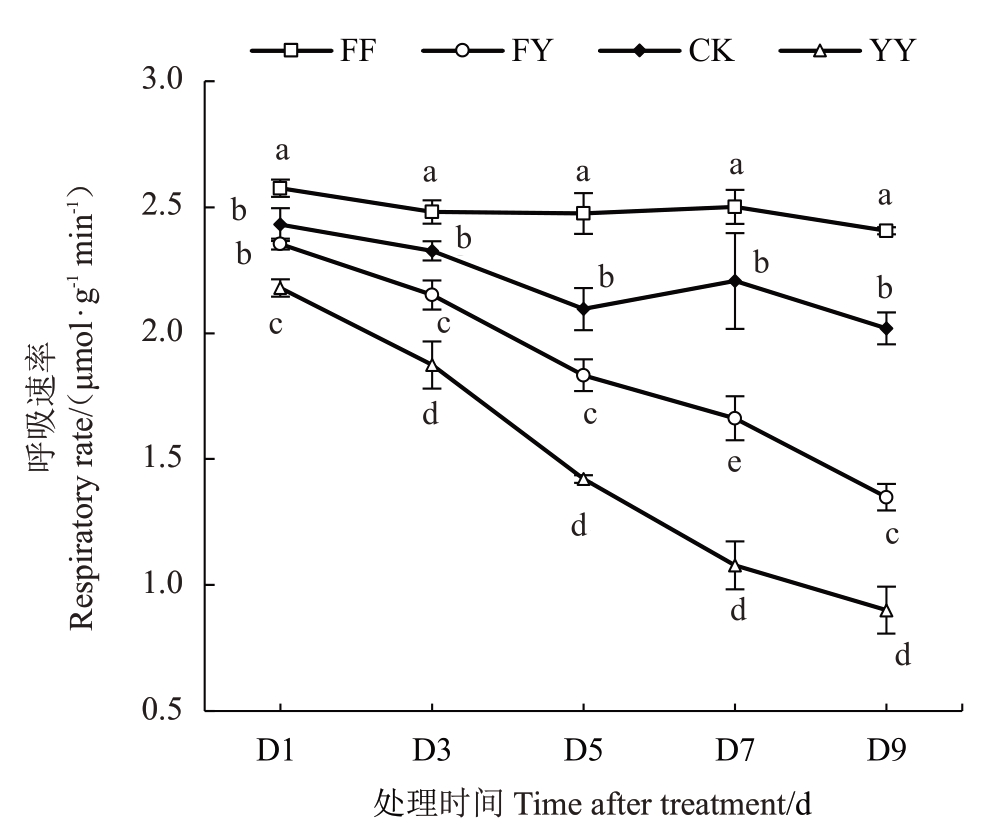
图1 盐胁迫对草莓根系呼吸速率的影响
Fig.1 Effect of salt stress on the respiration rate of strawberry roots
2.3 盐胁迫下草莓根系呼吸相关酶活性的变化
2.3.1 6-磷酸葡萄糖脱氢酶活性 G-6-PDH活性的高低在一定程度上反映生物体的生物合成能力和抗氧化能力。通过在340 nm波长下测定NADPH生成的速率,可以评估G-6-PDH的催化活性。草莓根系酶活性测定结果如图2-A 所示,在测定时期内G-6-PDH活性随时间延长呈下降趋势。除D5时期外FF组酶活性均明显高于其他3组;CK组呈现波动下降趋势;FY 组酶活性在D1、D3 时与CK 无显著差异;YY组酶活性最低,均与同期其他3组达到差异显著水平。由此推断AMF 可以提高盐胁迫条件下草莓根系G-6-PDH活性。
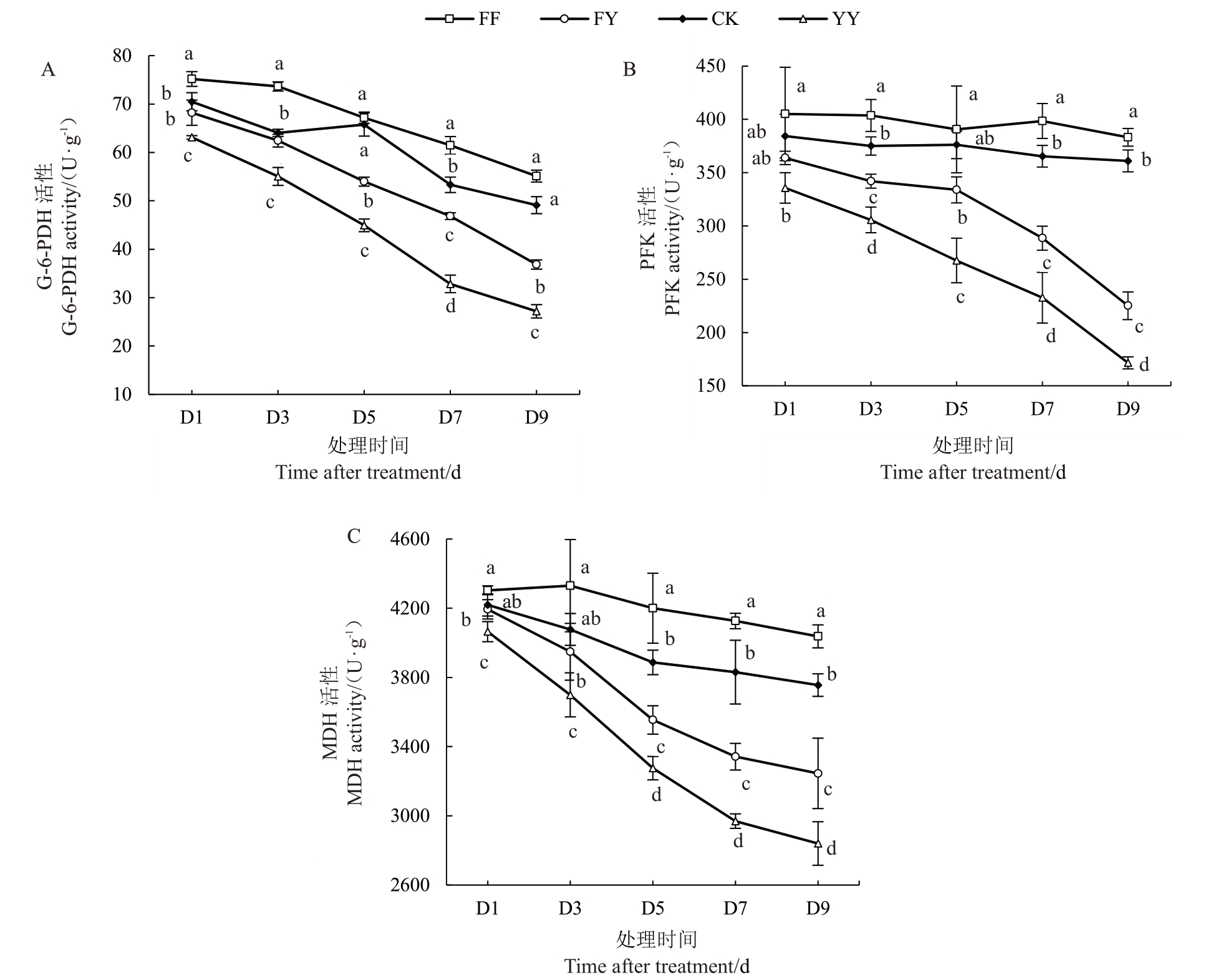
图2 盐胁迫下草莓根系呼吸相关酶活性的变化
Fig.2 Changes in respiration-related enzyme activities in strawberry roots under salt stress
2.3.2 磷酸果糖激酶活性 PFK活性测定结果如图2-B所示。通过在340 nm波长下测定NADH浓度的下降速率,可以有效评估PFK 活性。在测定时期内,盐胁迫处理使PFK 活性下降,未受盐胁迫的FF和CK组酶活性保持稳定。FF组酶活性均高于其他3组,对照CK组变化与FF组大致相同,都呈现波动稳定趋势。在D1 时期FY 和YY 组分别为同期CK组的94.67%和87.34%,随测定时期延长PFK活性均呈降低趋势,FY组酶活性整体高于YY组。通过测定数据推断AMF 可以提高盐胁迫条件下草莓根系磷酸果糖激酶活性。
2.3.3 NAD-苹果酸脱氢酶活性 苹果酸脱氢酶(MDH)广泛分布于动物、植物、微生物和培养细胞中。图2-C 表明草莓根系NAD-苹果酸脱氢酶(NAD-MDH)活性随着处理时间延长总体呈下降趋势。FF组酶活性在D5~D9中显著高于CK组,FY组酶活性在胁迫时期内显著高于YY 组,FF 组酶活性整体较CK组变化波动更稳定,YY组酶活性下降比例比FY组更大。由此推断AMF可以提高盐胁迫条件下草莓根系NAD-MDH 活性,缓解盐胁迫对根系呼吸的抑制。
2.4 盐胁迫下草莓UWL强度的变化
2.4.1 草莓根系UWL 强度 试验结果如图3-A 所示,随着时间的延长,未经盐胁迫处理两组UWL 强度略微波动下降,盐胁迫处理下的两组根系UWL强度均呈现明显下降趋势。FF组整体UWL强度高于CK 且下降幅度较小,推测接种AMF 可以提高草莓根系UWL 强度,且能使UWL 强度维持在较为稳定状态。胁迫结束时FY、YY 组UWL 强度分别为774.00、619.67,比胁迫第1 天降低了14.3% 、27.6%。由以上数据分析可以得出,盐胁迫会导致草莓根系UWL强度下降,接种AMF可以缓解UWL强度下降趋势,减少盐胁迫对草莓根系造成的影响。
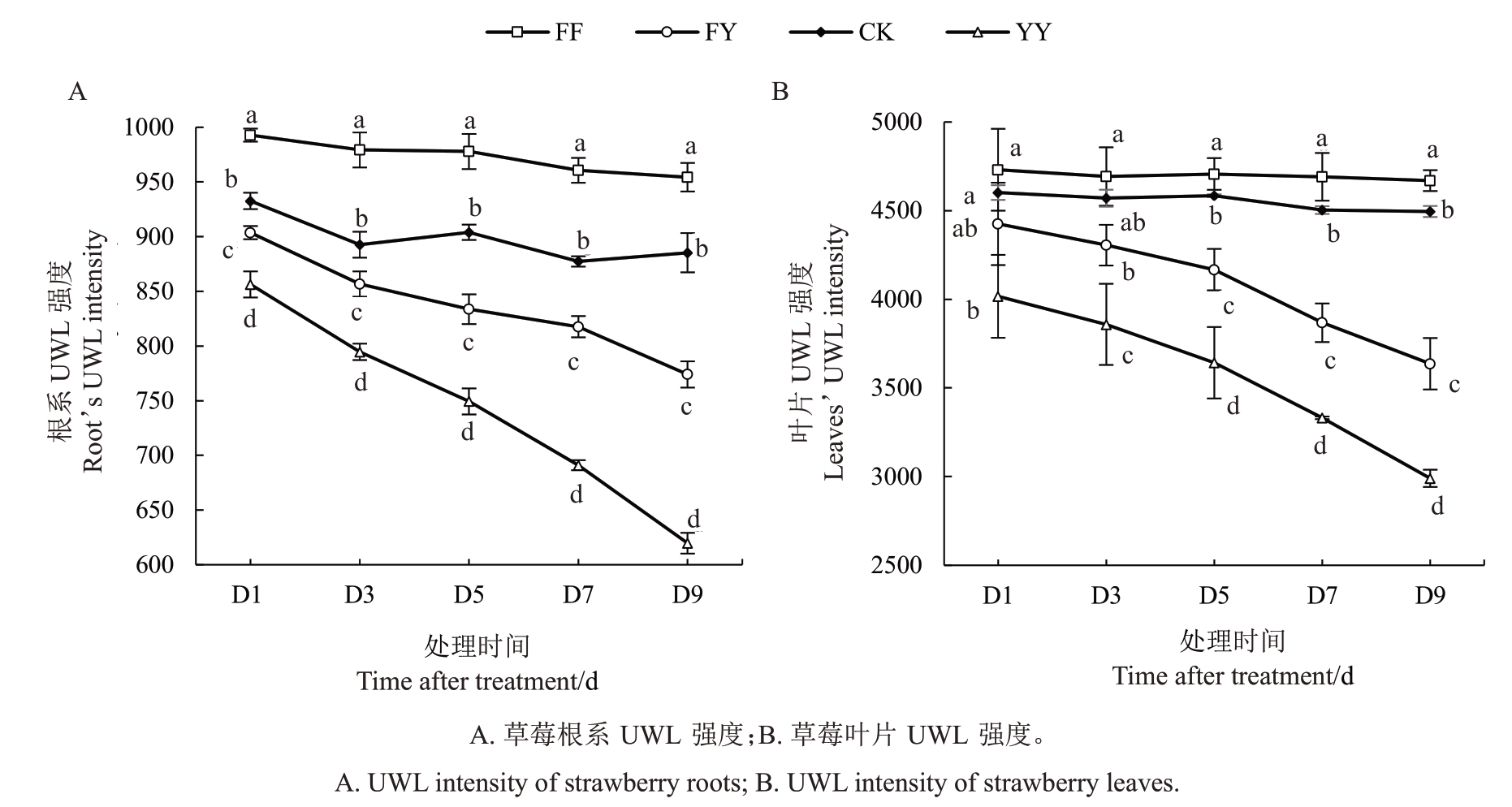
图3 盐胁迫下草莓根系和叶片UWL 强度的变化Fig.3 Changes in UWL intensity of strawberry roots and leaves under salt stress
2.4.2 草莓叶片UWL 强度 草莓叶片UWL 强度试验结果如图3-B 所示,在处理时间内,FF 组叶片UWL 强度呈现略微增强趋势而CK 组则略微减弱,整体上FF 组UWL 强度高于CK 组,从而推断AMF可以提高草莓叶片UWL强度。盐胁迫下是否接种AMF对叶片UWL强弱有显著影响。盐胁迫导致草莓叶片UWL 强度呈现随时间延长而下降的趋势,FY 与YY 组在9 d 内UWL 强度分别下降了17.82%、25.55%。D1 为两组UWL 强度差距最小的测试时间,FY组强度为4 425.22,YY组为4 016.56,接种AMF 后根系发光强度增强了10.17%;D9 为两组UWL 强度差距最大的测试时间,FY 组强度为3 636.50,YY组为2 990.17,FY相比于YY强度增强了21.62%。以上表明盐胁迫会导致草莓叶片UWL强度减弱,接种AMF可以缓解这种减弱现象。
2.5 盐胁迫下草莓根系呼吸及相关酶活性与UWL强度的关系
在盐胁迫下,草莓根系呼吸速率、三种根系呼吸相关酶活性、根系UWL 强度以及叶片UWL 强度均随着试验时间的延长而下降;接种了AMF 的FY 组虽呈现测量指标下降趋势但速率低于未接菌的YY组。经相关性分析,纯接菌组FF与对照组CK(图4-A、B)的根系UWL 强度与根系呼吸速率、G-6-PDH活性、PFK 活性、NAD-MDH 活性呈正相关,叶片UWL强度与根系UWL强度呈正相关。盐胁迫下的FY(图4-C)组、YY(图4-D)组叶片UWL强度与根系UWL 强度、根系呼吸速率、G-6-PDH 活性、PFK 活性、NAD-MDH 活性均呈极显著正相关;根系UWL强度与根系呼吸速率、G-6-PDH 活性、PFK 活性、NAD-MDH 活性也呈极显著正相关。分析表明,草莓在盐胁迫下根系UWL 强度与根系呼吸相关酶活性密切相关,因酶活性降低导致草莓根系呼吸速率降低,从而导致根系UWL 强度降低,随着胁迫时间的延长,根系受到盐胁迫伤害加重,叶片也表现出盐害特征,UWL强度也随之降低。

图4 盐胁迫下草莓根系呼吸速率、相关酶活性和UWL 强度的相关性
Fig.4 Correlation of respiration rate,related enzyme activities and UWL intensity in strawberry roots under salt stress
3 讨 论
笔者在本研究中旨在探讨AMF 在盐胁迫下对草莓根系呼吸和UWL 强度的影响。结果显示,盐胁迫显著抑制了草莓根系的呼吸速率和相关酶活性,且随着时间的推移,根系和叶片的UWL强度逐渐下降。这一发现与陈晓晶等[9]、孙聪等[18]的研究一致。在无盐胁迫条件下,接种AMF的草莓植株表现出更高的根系呼吸速率、酶活性和UWL强度,这表明AMF能够增强植物的代谢活动,促进植物健康生长。在盐胁迫条件下,接种AMF显著减缓了草莓根系呼吸速率和酶活性的下降幅度,并且减缓了UWL强度的下降。这一发现与之前的研究一致。谭英等[19]研究得出接种AMF可以增强盐胁迫下紫花苜蓿抗氧化酶活性。吴艳芬等[20]发现接种AMF可促进大豆的呼吸代谢。本研究中的数据进一步支持了AMF在盐胁迫条件下可对植物发挥保护作用的观点。
呼吸作用是植物维持正常生长的重要一环,根系的呼吸作用可以释放能量满足植物各种生理活动需要,同时呼吸作用的中间产物为植物体其他组织器官的形成提供重要的碳骨架[21]。G-6-PDH是磷酸戊糖途径的关键酶,其还原生成的NADPH 在生物合成和维持细胞内还原态中起着至关重要的作用。PFK 主要负责将果糖-6-磷酸和ATP 转化为果糖-1,6-二磷酸和ADP,是糖酵解途径中的重要调控酶。MDH 在细胞的多种生理功能中发挥着至关重要的作用,在线粒体内为三羧酸循环的关键酶,在胞质中则催化草酰乙酸还原生成苹果酸。以上三种酶在草莓根系呼吸过程中起重要作用,因此试验选取以上三种关键酶作为检测指标。草莓根系对盐分变化较为敏感,试验中随着盐胁迫时间延长,根系呼吸相关的G-6-PDH、PFK、NAD-MDH 活性下降,导致草莓根系呼吸速率降低,而接种AMF可以缓解三种呼吸相关酶活性和呼吸速率的下降趋势。
植物的UWL 强度被认为来自体内的核酸代谢、呼吸代谢等各种氧化还原过程,可以作为植物抗逆性的参考指标[22]。笔者在本研究中发现,未受到盐胁迫的草莓根系、叶片UWL强度保持波动稳定,接种AMF 后发光强度有明显提升;受到盐胁迫时,随着时间延长叶片和根系UWL 强度逐渐下降,接种AMF 同样可以缓解这种下降趋势。结果表明接种AMF可以缓解草莓在盐胁迫下造成的伤害。
根据试验数据的相似变化趋势,考虑到草莓地上部叶片的UWL强度与地下部根系的UWL强度之间可能存在一定相关关系,同时探讨根系UWL强度与呼吸作用及酶活性之间的关系。通过相关性分析,发现在盐胁迫环境下,草莓叶片UWL 强度与草莓根系UWL强度、根系呼吸强度及几种关键酶活性(G-6-PDH、PFK、NAD-MDH)之间存在显著的正相关关系。这表明UWL 强度可以作为衡量盐浓度下草莓根系受胁迫程度的一个有效指标。根系是首先受到盐胁迫影响的部位,直接受到盐离子浓度变化的影响,导致呼吸作用和酶活性的变化。由于植物生长在土壤中,直接检测根系的生理生化指标具有一定难度。笔者发现,在试验设置的盐浓度条件下,通过测量草莓叶片的UWL强度,可以间接评估根系的呼吸作用强度和相关酶活性,以此了解植物受胁迫程度。这种方法不仅提高了检测的便捷性,还提供了一种非侵入性的手段来监测植物健康状态和盐胁迫反应的思路。综合上述结果,通过相关性分析,发现在4组处理中草莓叶片生长与根系呼吸均存在正相关性,进一步支持了UWL强度作为植物生理状态指标的潜力,为实际农业生产中的胁迫监测提供了理论依据和实践指导。
4 结 论
盐胁迫显著抑制了草莓根系的呼吸相关酶活性,从而导致根系呼吸速率下降,根系和叶片UWL强度随时间的推移而下降。AMF 有效减小了呼吸速率和酶活性的下降幅度,并抑制了UWL强度的下降。草莓叶片UWL 强度与根系UWL 强度、根系呼吸强度及关键酶活性(G-6-PDH、PFK、NAD-MDH)之间存在正相关关系,通过测量草莓叶片的UWL强度,可以提供在重度盐胁迫情况下根系呼吸强度和相关酶活性的评估指标。
[1] PROTEGGENTE A R,PANNALA A S,PAGANGA G,VAN BUREN L,WAGNER E,WISEMAN S,VAN DE PUT F,DACOMBE C,RICE-EVANS C A.The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition[J]. Free Radical Research,2002,36(2):217-233.
[2] GIAMPIERI F,TULIPANI S,ALVAREZ- SUAREZ J M,QUILES J L,MEZZETTI B,BATTINO M. The strawberry:Composition,nutritional quality,and impact on human health[J].Nutrition,2012,28(1):9-19.
[3] TULIPANI S,ROMANDINI S,BUSCO F,BOMPADRE S,MEZZETTI B,BATTINO M. Ascorbate,not urate,modulates the plasma antioxidant capacity after strawberry intake[J]. Food Chemistry,2009,117(1):181-188.
[4] WANG S Y,LIN H S. Antioxidant activity in fruits and leaves of blackberry,raspberry,and strawberry varies with cultivar and developmental stage[J].Journal of Agricultural and Food Chemistry,2000,48(2):140-146.
[5] 吴雯雯,安玉艳,汪良驹.5-氨基乙酰丙酸缓解‘红颜’草莓盐胁迫伤害的时间效应研究[J]. 园艺学报,2017,44(6):1038-1048.WU Wenwen,AN Yuyan,WANG Liangju. Study on time effects of exogenous 5-aminolevulinic acid treatment on alleviating salinity injury in‘Benihoppe’strawberry[J].Acta Horticulturae Sinica,2017,44(6):1038-1048.
[6] ONDRAŠEK G,ROMIĆ D,ROMIĆ M,DURALIJA B,MUSTAĆ I. Strawberry growth and fruit yield in a saline environment[J].Agriculturae Conspectus Scientificus,2006,71(4):155-158.
[7] MCCORMACK M L,DICKIE I A,EISSENSTAT D M,FAHEY T J,FERNANDEZ C W,GUO D L,HELMISAARI H S,HOBBIE E A,IVERSEN C M,JACKSON R B,LEPPÄLAMMI-KUJANSUU J,NORBY R J,PHILLIPS R P,PREGITZER K S,PRITCHARD S G,REWALD B,ZADWORNY M. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes[J].New Phytologist,2015,207(3):505-518.
[8] 马怀宇,刘国成,吕德国,秦嗣军.‘寒富’苹果花芽呼吸代谢途径对低温胁迫的响应特征[J].果树学报,2012,29(3):317-321.MA Huaiyu,LIU Guocheng,LÜ Deguo,QIN Sijun.Responsive characteristics of respiratory metabolism pathway activity in‘Hanfu’apple flower buds under cold stress[J]. Journal of Fruit Science,2012,29(3):317-321.
[9] 陈晓晶,徐忠山,赵宝平,米俊珍,严威凯,刘景辉.盐胁迫对燕麦根系呼吸代谢、抗氧化酶活性及产量的影响[J].生态学杂志,2021,40(9):2773-2782.CHEN Xiaojing,XU Zhongshan,ZHAO Baoping,MI Junzhen,YAN Weikai,LIU Jinghui. Effects of salt stress on root respiratory metabolism,antioxidant enzyme activities,and yield of oats[J].Chinese Journal of Ecology,2021,40(9):2773-2782.
[10] COSTA J H,JOLIVET Y,HASENFRATZ-SAUDER M P,ORELLANO E G,DA GUIA S L M,DIZENGREMEL P,DE MELO D F.Alternative oxidase regulation in roots of Vigna unguiculata cultivars differing in drought/salt tolerance[J]. Journal of Plant Physiology,2007,164(6):718-727.
[11] POPP F A,LI K H,MEI W P,GALLE M,NEUROHR R.Physical aspects of biophotons[J].Experientia,1988,44(7):576-585.
[12] 郭金丽,刘欢,梁爽,朱冠宇,白杨,李连国.活性氧调控下草莓果实衰老过程中活性氧与超微弱发光的关系[J].果树学报,2017,34(3):363-369.GUO Jinli,LIU Huan,LIANG Shuang,ZHU Guanyu,BAI Yang,LI Lianguo.Relationship between reactive oxygen species and ultraweak luminescence in strawberry fruit during senescence under various reactive oxygen regulation treatments[J].Journal of Fruit Science,2017,34(3):363-369.
[13] PRASAD A,GOURIPEDDI P,DEVIREDDY H R N,OVSII A,RACHAKONDA D P,WIJK R V,POSPÍŠIL P. Spectral distribution of ultra-weak photon emission as a response to wounding in plants:An in vivo study[J].Biology,2020,9(6):139.
[14] 程海鹏,王君晖,池浩超,朱睦元.豌豆种子萌发过程中超微弱发光的研究[J].浙江大学学报(理学版),2001,28(6):682-685.CHENG Haipeng,WANG Junhui,CHI Haochao,ZHU Muyuan.Study on ultraweak luminescence of Pisum sativum seeds at the stage of germination[J]. Journal of Zhejiang University (Sciences Edition),2001,28(6):682-685.
[15] 雷晓光.丛枝菌根真菌(AMF)与草莓共生效应研究[D].呼和浩特:内蒙古农业大学,2017.LEI Xiaoguang. Symbiotic effect of arbuscular mycorrhizal fungi(AMF)on strawberry[D].Hohhot:Inner Mongolia Agricultural University,2017.
[16] 王思雨,魏涵,陈科宇,董强,纪宝明,张静. 丛枝菌根真菌(AMF)孢子、菌丝密度及侵染率定量测定方法[J]. Bio-101,2021:e2104253.WANG Siyu,WEI Han,CHEN Keyu,DONG Qiang,JI Baoming,ZHANG Jing. Practical methods for arbuscular mycorrhizal fungal spore density,hyphal density and colonization rate of AMF[J].Bio-101,2021:e2104253.
[17] 毛志泉,王丽琴,沈向,束怀瑞,邹岩梅.有机物料对平邑甜茶实生苗根系呼吸强度的影响[J].植物营养与肥料学报,2004,10(2):171-175.MAO Zhiquan,WANG Liqin,SHEN Xiang,SHU Huairui,ZOU Yanmei. Effect of organic materials on respiration intensity of annual Malus hupehensis Rehd.root system[J].Plant Nutrition and Fertilizing Science,2004,10(2):171-175.
[18] 孙聪,郭金丽.盐胁迫下欧李叶片叶绿素代谢与超微弱发光的关系[J].果树学报,2023,40(7):1411-1420.SUN Cong,GUO Jinli. The relationship between chlorophyll metabolism and ultraweak luminescence of leaves under salt stress in Cerasus humilis[J]. Journal of Fruit Science,2023,40(7):1411-1420.
[19] 谭英,尹豪.盐胁迫下根施AMF 和褪黑素对紫花苜蓿生长、光合特征以及抗氧化系统的影响[J].草业学报,2024,33(6):64-75.TAN Ying,YIN Hao.Effects of root application of an arbuscular mycorrhizal fungus and melatonin on the growth,photosynthetic characteristics,and antioxidant system of Medicago sativa under salt stresss[J].Acta Prataculturae Sinica,2024,33(6):64-75.
[20] 吴艳芬,刘秋鸣,刘卫欢,蒙爱萍,陈振翔,刘灵.AMF 与根瘤菌对间作大豆光合与呼吸代谢的影响[J].广西师范大学学报(自然科学版),2022,40(2):231-241.WU Yanfen,LIU Qiuming,LIU Weihuan,MENG Aiping,CHEN Zhenxiang,LIU Ling.Effects of inoculation of AMF and Rhizobium on photosynthetic and respiratory metabolism and growth of intercropping Glycine max[J].Journal of Guangxi Normal University(Natural Science Edition),2022,40(2):231-241.
[21] 高相彬,赵凤霞,沈向,胡艳丽,郝云红,杨树泉,苏立涛,毛志泉.肉桂酸对平邑甜茶幼苗根系呼吸速率及相关酶活性的影响[J].中国农业科学,2009,42(12):4308-4314.GAO Xiangbin,ZHAO Fengxia,SHEN Xiang,HU Yanli,HAO Yunhong,YANG Shuquan,SU Litao,MAO Zhiquan. Effects of cinnamon acid on respiratory rate and its related enzymes activity in roots of seedlings of Malus hupehensis Rehd.[J]. Scientia Agricultura Sinica,2009,42(12):4308-4314.
[22] 习岗.植物超弱发光及其在农业上的应用[J].物理,1994,23(9):548-552.XI Gang. Ultra-weak luminescence in plants and its application to agriculture[J].Physics,1994,23(9):548-552.