葡萄(Vitis vinifera L.)作为重要的经济水果作物,果实中富含花青素、黄酮醇及白藜芦醇等多种生物活性成分有利于人体健康[1-2]。在葡萄的生长发育过程中,大多数幼果在开花后9~10 d发生严重的脱落[3]。坐果过程是决定葡萄产量和品质的关键阶段,是由内部特定基因的调控和植物激素水平的变化共同驱动的[4]。细胞分裂素处理可以显著提高坐果率和产量[5-7]。氯吡苯脲(CPPU)是一种细胞分裂素类的植物生长调节剂,已被证实能够通过提高果实中碳水化合物水平来促进鳄梨坐果[8],此外CPPU还可以通过降低呼吸代谢和维持较高的能量电荷水平来促进葡萄果实坐果[9]。CPPU 处理还可以促进甜瓜坐果和提高产量[10]。然而,关于CPPU 促进葡萄果实坐果的分子机制尚不清楚。因此,深入研究CPPU 在葡萄坐果中的作用机制,对提升葡萄果实的产量和品质具有重要的生产实践意义。
CKX 是一种不可逆降解细胞分裂素为腺嘌呤/腺苷的黄素酶,是细胞分裂素信号转导中降解分支的关键酶[11-12]。CKX酶具有N端FAD和C端细胞分裂素结合位点(CK-binding)两个保守结构域[13]。CKX 基因家族成员不仅可以调控对非生物胁迫的响应[14]、根的形成[15]和果实发育[16],还可以调控植物细胞分裂素水平进而影响产量[17]。在水稻中,沉默OsCKX11可以提高旗叶中的细胞分裂素水平,增加分蘖数和穗粒数从而提高产量[18]。同样,沉默OsCKX2可以增加水稻花序分生组织中细胞分裂素含量,提高盐胁迫条件下的产量[19]。油菜中突变CKX3CKX5 使细胞分裂素浓度增加,花和胚珠增加,产量提高[20]。然而VlCKX5基因在葡萄中的功能尚不清楚,还需进一步研究。
VlAGL6a也称为VlMADS3,是MADS-box基因家族中的成员之一[21]。MADS-box 基因分为Ⅰ型和Ⅱ型,Ⅰ型MADS-box 基因被分为3 个不同的进化群:Mα、Mβ 和Mγ,Ⅱ型MADS-box 基因包括动物和酵母中的MEF2-like 基因以及植物中特有的MIKC 型基因[22-23]。MIKC 型基因又分为MIKCC型和MIKC*型,植物中大多数MIKC 型基因为MIKCC型,包括12 个亚家族SVP、FLC、TM3、AP1/FUL、SEP、AGL6、AG、AGL12、ANR1、AP3/PI、AGL15、BS[24-25]。MADS-box在植物发育中发挥重要作用,在番茄中异源过表达VvMADS45 基因会使花、果和种子变大,而沉默其同源基因SlAGL104 会使花、果实和种子变小[26]。在拟南芥中AGL6 可以抑制FLC/MAF基因转录调控开花时间[27]。FveSEP3在草莓花发育中起主要作用,FveSEP3 可以抑制单性果实生长和促进正常授粉的果实成熟[28]。HvMADS1 直接调控细胞分裂素降解酶HvCKX3基因进而保持细胞分裂素的稳态,在高温下维持无分支的穗结构[29]。
笔者课题组前期研究发现植物生长调节剂CPPU处理降低了葡萄内源细胞分裂素含量,显著提高葡萄坐果率[30],但其潜在的分子机制尚不清楚。本研究在巨峰葡萄基因组中克隆VlCKX5 基因及其启动子,通过生物信息学分析、表达特异性分析、GUS组织化学染色、亚细胞定位、酵母单杂交和双荧光素酶分析等方法,对VlCKX5 的功能以及转录调控模式进行初步探索,为后续进一步研究VlCKX5 调控细胞分裂素水平进而影响葡萄坐果的分子机制奠定基础。
1 材料和方法
1.1 材料处理
植物材料取自中国河南省洛阳市偃师葡萄种植区种植的10 年生巨峰葡萄(Vitis vinifera L.×Vitis labrusca L.)。在盛花后5 d,使用10 mg·L-1的CPPU溶液(含0.03%Silwet-L77 表面活性剂)浸蘸葡萄幼果10 s,蒸馏水(含0.03% Silwet-L77 表面活性剂)处理作为对照,具体处理参考Sun 等[31]的方法。在CPPU 处理和蒸馏水处理后1、2、4和8 d选择长势一致的整串葡萄幼果进行采样。在盛花后13 d 时,采集自然发育的根(1 年生)、茎(1 年生)、叶(1 年生枝条从基部数起的第4至6枚叶)、花序(1年生枝上)、卷须(1年生枝上)和幼果(1年生枝上),用于基因的组织特异性表达分析。本氏烟草(Nicotiana benthamiana)在25 ℃培养室中生长,光照16 h/黑暗8 h。
1.2 序列分析
使用在线网站Ensembl Plants(https://plants.ensembl.org/index.html)查询VlCKX5(Vitvi13g01614)的编码序列(CDS)和蛋白质序列。使用在线网站Expasy(https://web.expasy.org/protparam/)查询VlCKX5蛋白序列的各种物理和化学参数。使用在线网站Interpro(https://www.ebi.ac.uk/interpro/)查询VlCKX5 的保守结构域。在NCBI(https://www.ncbi.nlm.nih.gov/)数据库中下载VlCKX5 的同源氨基酸序列,使用MEGA11 软件对VlCKX5 及其同源氨基酸序列进行比对,生成系统发育树。利用GeneDoc 2.7对VlCKX5同源物进行氨基酸序列比对。
1.3 RNA提取与VlCKX5克隆
使用RNA提取试剂盒(诺唯赞,南京)提取巨峰葡萄的RNA。使用反转录试剂盒(雅礼,江苏)获得葡萄的全长cDNA,并以此为模板通过PCR 扩增VlCKX5 的编码序列(CDS),扩增引物为pSAK277-VlCKX5-F:TAGTGGATCCAAAGAATTCCATGTTGAGGGGCTTCTGTCTTTGG,pSAK277-VlCKX5-R:CGAGAAGCTTTTTGAATTCGATCACAAGAAGGGTGTCGCCTTTC。使用胶回收试剂盒(康为世纪,北京)回收目标片段。将连接产物转化入大肠杆菌感受态(Trans5α),使用质粒提取试剂盒(聚合美,北京)提取pSAK277-VlCKX5 质粒,并送至苏州金唯智生物科技有限公司进行测序得到pSAK277-VlCKX5序列。测序结果正确后将质粒转至农杆菌感受态(GV3101)中。
1.4 实时荧光定量(RT-qPCR)
使用反转录试剂盒(雅礼,江苏)进行反转录获取片段cDNA,经内参基因Ubiqutin1(GenBank:CA808925)检测后在-20 ℃保存备用。采用2-ΔΔCT法计算基因的相对表达量,并使用Excel 2016 和Graphpad Prism 8 软件对所得数据进行分析。构建载体所用引物序列为VlCKX5-F:GCATTCGTTTCATAGCAAGC,VlCKX5-R:AATGCCCGTCAACAGAAAGT;VlAGL6a- F:ACTTTCTGTGCTTTGTGATGCT,VlAGL6a-R:TGATACCGCTCTAGGGTTTTG。
1.5 启动子的克隆与GUS组织化学染色
使用DNA提取试剂盒(雅礼,江苏)提取葡萄果实的DNA。使用添加同源臂的引物以DNA为模板使用高保真酶Primer STAR Max Premix(TaKaRa,大连)克隆VlCKX5 的5′端上游1566 bp 序列,连接GUS 载体,获得pC0390-GUS-proVlCKX5 质粒。将其质粒转到GV3101 中,获得农杆菌菌液。使用PlantCARE 网站(https://bioinformatics.psb.ugent.be/webtools/plantcare/html/)预测VlCKX5启动子的顺式作用元件。使用真空渗透方法进行烟草叶片的瞬时转化,GUS组织化学染色试验的具体方法参考GUS染色试剂盒(酷来搏,北京)的说明书。使用生长素(IBA)(100 μmol·L-1)、水杨酸(SA)(100 μmol·L-1)、脱落酸(ABA)(100 μmol·L-1)、氯吡苯脲(CPPU)(40 μmol·L-1)和茉莉酸甲酯(MeJA)(100 μmol·L-1)溶液喷施浸染pC0390-GUS-proVlCKX5菌液后的烟草叶片,喷施叶片的正反面,用湿润的脱脂棉包裹住叶柄,放入托盘中正常光照培养24 h。以pC0390-GUS载体为阴性对照,带有35S启动子的GUS载体作为阳性对照。将培养好的烟草叶片放进一次性培养皿中,加入GUS 染色剂,使叶片完全浸入,放在37 ℃培养箱中过夜培养,其间使用封口膜将容器封闭避免GUS染色液挥发,使用70%乙醇脱色2~3次,至绿色完全脱去,肉眼或显微镜下观察到白色背景上的蓝色小点即为GUS 表达位点。观察GUS 染色情况并拍照记录。构建载体所用引物序列为GUSproVlCKX5-F:TGGGCCCGGCGCGCCAAGCTTGGGAGCCACCTTGAGCATCTC,GUS-proVlCKX5-R:GGTGGACTCCTCTTAGAATTCCATGGGTCTAGGGAAAGGAGCAG。
1.6 靶向VlCKX5的转录因子调控预测
通过CIS-BP 数据库(https://cisbp.ccbr.utoronto.ca/)、JASPAR 数据库(https://jaspar.elixir.no/)和PlantTFDB 数据库(http://planttfdb.gao-lab.org)得到葡萄转录因子信息,并使用TB-tools 的GTF/GFF3 Sequences Extractor 插件提取VlCKX5 启动子上游2000 bp 序列,根据PlantTFDB、CIS-BP 和JASPAR数据库公布的转录因子结合位点信息,使用Find Individual Motif Occurences(FIMO)在线网站(https://meme-suite.org/meme/tools/fimo)在VlCKX5 启动子上进行TFBS预测,依据10-5截取阈值作为筛选预测结果。通过GENIE3 预测转录因子与VlCKX5 之间可能存在的共表达调控关系,依据weigh>0.1 截取阈值作为筛选预测结果。根据GENIE3预测得到的共表达调控关系,通过Gephi0.10软件将得到的预测信息进行可视化,使用Fruchterman Reingold 布局,绘制共表达调控网络图。
1.7 亚细胞定位
去除VlAGL6a(Vitvi15g00776)编码区的终止密码子后,将其插入到101LYFP 载体中,形成101LYFP-VlAGL6a 重组质粒。将重组质粒转化到GV3101 中,将核标记物(VirD2NLS-mCherry)与转化后的菌液混合,转化到烟叶中瞬时表达。25 ℃下暗处理2 d,正常培养1 d 后,用激光共聚焦显微镜(奥林巴斯,日本)观察荧光信号。空白101LYFP作为对照。构建载体所用引物序列为101LYFPVlAGL6a- F:ATGGGATCTACTAGTGAATTCATGGGGAGAGGAAGAGTGGAGC,101LYFP- VlAGL-6a-R:GGGGGTACCGTCGACGGATCCAAGAACCCACCCTTGGATGAAG。
1.8 酵母单杂交(Y1H)
克隆VlCKX5 启动子中VlAGL6a 的结合片段P并插入pAbAi 载体中,构建重组饵料质粒pAbAiproVlCKX5/P。将重组诱饵质粒pAbAi- proVlCKX5/P用BstbⅠ线性化,采用PEG/LiAc转化方法将其整合到酵母菌株Y1H Gold 中。转化后的酵母菌株在SD/-Ura 缺陷型培养基上培养3~5 d,将培养基放置于30 ℃培养箱中。通过加入不同浓度的金担子素A(AbA)的SD/-Ura 培养基筛选抗性浓度。随后,将重组猎物质粒pGADT7-VlAGL6a转化到阳性酵母菌株(包含诱饵基因组)中,并涂布在SD/-Leu 缺陷型培养基上,在30 ℃培养箱中倒置培养3~5 d,以pGADT7 空载体转化到阳性酵母菌株(包含诱饵基因组)中作为对照。在SD/-Leu/AbA 培养基上培养共转化酵母细胞,检测VlCKX5 与VlAGL6a的相互作用。构建载体所用引物序列为pAbAiproVlCKX5/P-F:TTGAATTCGAGCTCGGTACCCCATGACCGAGTCCTCGTTATTTTG,pAbAi-proVlCKX5/P- R:TACAGAGCACATGCCTCGAGGAGCAACACCTAATCCTCCTCTC;pGADT7-VlAGL6a-F:GCCATGGAGGCCAGTGAATTCATGGGGAGAGGAAGAGTGGAGC,pGADT7-VlAGL6a-R:CAGCTCGAGCTCGATGGATCCTCAAAGAACCCACCCTTGGATGAAG。
1.9 双荧光素酶测定
从巨峰葡萄DNA中PCR扩增出VlCKX5的ATG起始密码子上游1566 bp 的DNA 序列,并插到pGreenII0800-LUC 载体中。所生成的质粒ProVlCKX5-LUC 作为报告子。将VlAGL6a 的CDS 编码区插入载体pSAK277 中,生成作为效应子的35SVlAGL6a。未插入启动子的载体pGreenII0800-LUC和未插入VlAGL6a的载体pSAK277作为阴性对照。将效应子和报告子分别导入农杆菌GV3101中,报告质粒与效应质粒以1∶9混合共转化到本氏烟草叶片中[10]。48 h 后使用双荧光素酶报告基因检测系统(Promega,美国)测定每个样品的荧光素酶活性。构建载体所用引物序列为LUC-VlCKX5-F:GTCGACGGTATCGATAAGCTTGGGAGCCACCTTGAGCATCTC,LUC-VlCKX5-R:CGCTCTAGAACTAGTGGATCCCATGGGTCTAGGGAAAGGAGCAG,pSAK277-VlAGL6a-F:TAGTGGATCCAAAGAATTCCATGGGGAGAGGAAGAGTGGAGC,pSAK277-Vl-AGL6a-R:CGAGAAGCTTTTTGAATTCGATCAAAGAACCCACCCTTGGATGAAG。
2 结果与分析
2.1 VlCKX5克隆及序列分析
为进一步对VlCKX5 的功能进行探究,克隆到VlCKX5(Vitvi13g01614)。VlCKX5 的CDS 长度为1641 bp,编码546个氨基酸,分子质量为61.516 62 kDa,等电点为8.36,不稳定系数和脂肪系数分别为36.64和94.27,蛋白质稳定。VlCKX5 具有CKXs 特征结构域-甲酚甲基羟化酶(PCMH)型黄素腺嘌呤二核苷酸(FAD)结合域和细胞分裂素结合位点(CKbinding)(图1-A)。系统发育树显示,VlCKX5 与中华猕猴桃的同源关系最近(图1-B)。VlCKX5 与AcCKX(中华猕猴桃)、OsCKX4(水稻)和AtCKX6(拟南芥)蛋白的多序列比对结果显示VlCKX5含有FAD和Cytokin-bind的核心保守结构域(图1-C)。

图1 VlCKX5 的序列分析
Fig.1 Sequence analysis of VlCKX5
2.2 VlCKX5基因表达模式分析
通过RT-qPCR 检测巨峰根、茎、叶、花序、幼果、卷须中VlCKX5 的表达量,结果显示VlCKX5 基因在葡萄根和叶中相对表达量最高,其次是在花序中,在茎和卷须中的相对表达量最低(图2-A)。检测CPPU 处理后不同时期VlCKX5 的表达量,VlCKX5在CPPU处理后表达受到显著抑制,在1、2、4、8 d的表达量显著降低(图2-B)。
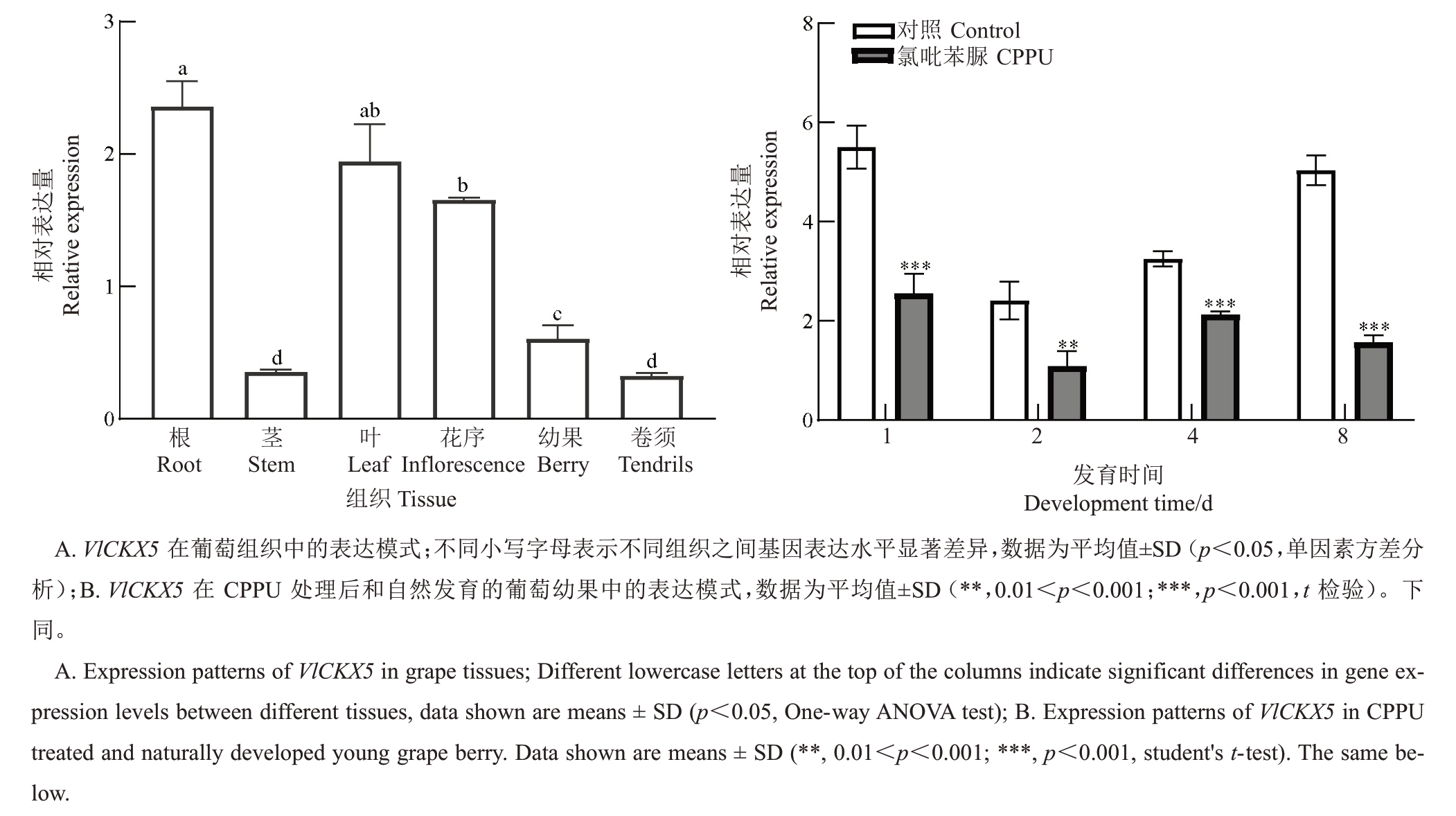
图2 VlCKX5 的表达量分析
Fig.2 Expression analysis of VlCKX5
2.3 VlCKX5启动子克隆及分析
对VlCKX5 启动子序列的顺式作用元件进行预测,结果显示VlCKX5 启动子区域除了启动子和增强子区域中常见的顺式作用元件CAAT-box和核心启动子元件TATA-box 外,还包括光、分生组织表达和激素的响应元件(表1)。在VlCKX5 的启动子区域发现生长素、水杨酸和脱落酸的植物激素响应元件(图3-A)。克隆VlCKX5的5′端上游1566 bp序列并进行GUS染色试验,与阴性对照相比,Mock处理的烟草叶片明显呈蓝色,ProVlCKX5 显著增强了GUS 基因的活性。此外,VlCKX5 启动子对IBA、SA、ABA 和CPPU 均表现出激素应答信号,其中IBA、SA、ABA 和CPPU 处理与Mock 处理的叶片颜色相比,表现出更深的蓝色,MeJA 处理与Mock 处理的叶片颜色相比没有明显的差别,对VlCKX5 启动子激活效果不明显(图3-B)。
表1 VlCKX5 启动子中顺式作用元件与数量
Table 1 The cis-acting elements and number in VlCKX5 promoter
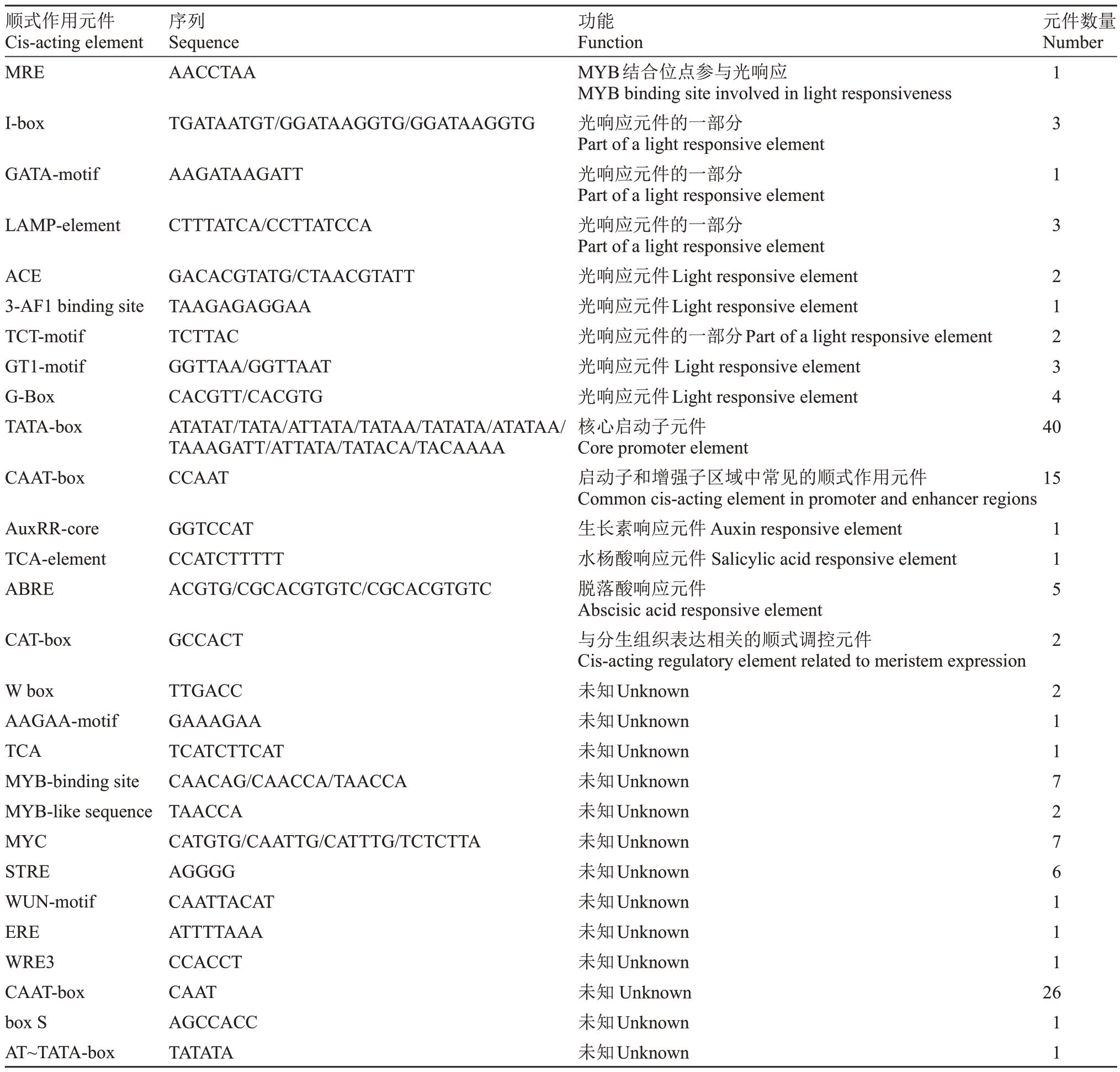
顺式作用元件Cis-acting element MRE序列Sequence AACCTAA元件数量Number I-box TGATAATGT/GGATAAGGTG/GGATAAGGTG GATA-motif LAMP-element AAGATAAGATT CTTTATCA/CCTTATCCA ACE 3-AF1 binding site TCT-motif GT1-motif G-Box TATA-box 1 3 1 3 2 1 2 3 4 40 CAAT-box GACACGTATG/CTAACGTATT TAAGAGAGGAA TCTTAC GGTTAA/GGTTAAT CACGTT/CACGTG ATATAT/TATA/ATTATA/TATAA/TATATA/ATATAA/TAAAGATT/ATTATA/TATACA/TACAAAA CCAAT 15 AuxRR-core TCA-element ABRE GGTCCAT CCATCTTTTT ACGTG/CGCACGTGTC/CGCACGTGTC CAT-box GCCACT W box AAGAA-motif TCA MYB-binding site MYB-like sequence MYC STRE WUN-motif ERE WRE3 CAAT-box box S AT~TATA-box TTGACC GAAAGAA TCATCTTCAT CAACAG/CAACCA/TAACCA TAACCA CATGTG/CAATTG/CATTTG/TCTCTTA AGGGG CAATTACAT ATTTTAAA CCACCT CAAT AGCCACC TATATA功能Function MYB结合位点参与光响应MYB binding site involved in light responsiveness光响应元件的一部分Part of a light responsive element光响应元件的一部分Part of a light responsive element光响应元件的一部分Part of a light responsive element光响应元件Light responsive element光响应元件Light responsive element光响应元件的一部分Part of a light responsive element光响应元件Light responsive element光响应元件Light responsive element核心启动子元件Core promoter element启动子和增强子区域中常见的顺式作用元件Common cis-acting element in promoter and enhancer regions生长素响应元件Auxin responsive element水杨酸响应元件Salicylic acid responsive element脱落酸响应元件Abscisic acid responsive element与分生组织表达相关的顺式调控元件Cis-acting regulatory element related to meristem expression未知Unknown未知Unknown未知Unknown未知Unknown未知Unknown未知Unknown未知Unknown未知Unknown未知Unknown未知Unknown未知Unknown未知Unknown未知Unknown 1 1 5 2 2 1 1 7 2 7 6 1 1 1 26 1 1
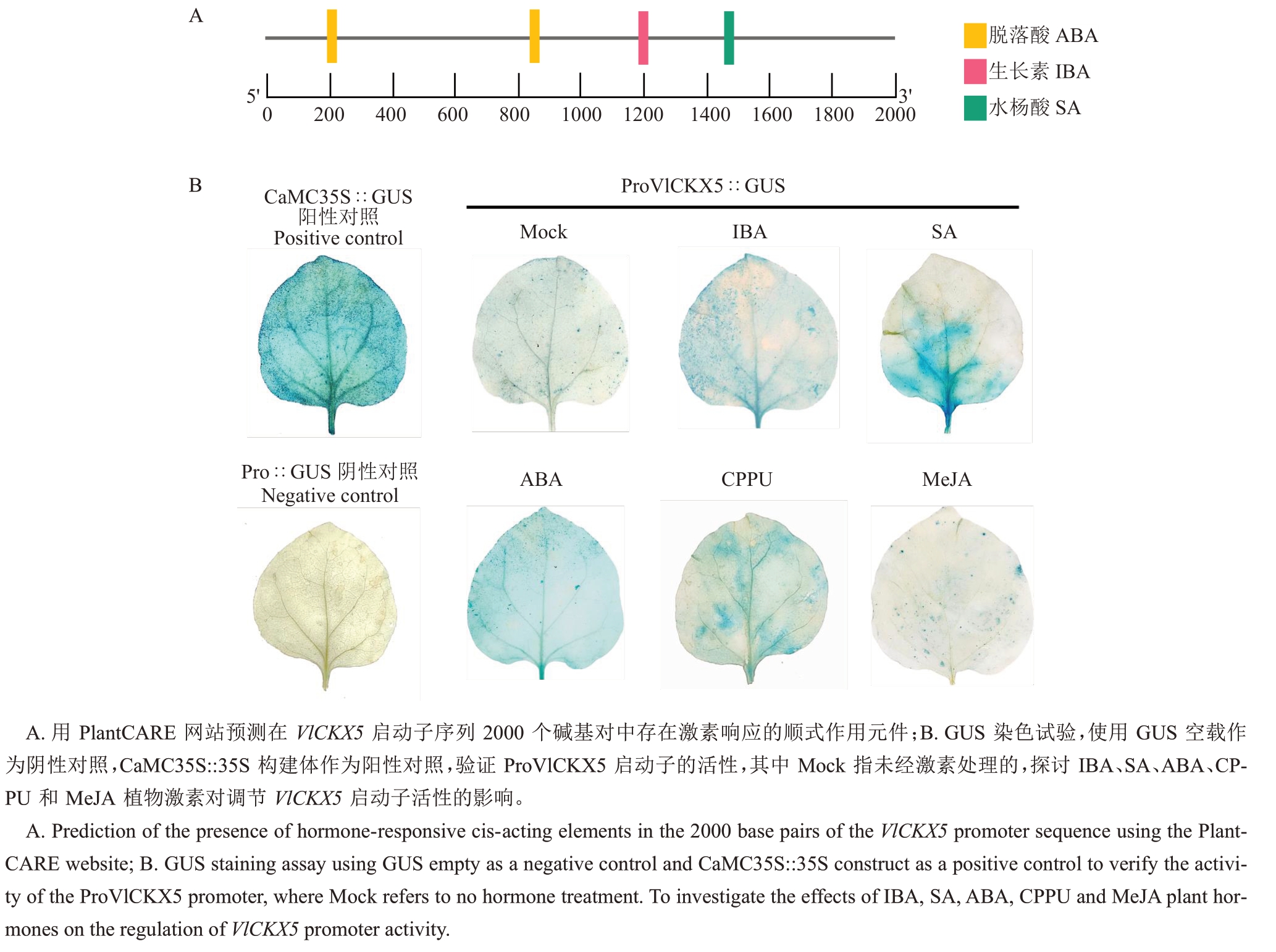
图3 VlCKX5 启动子活性分析
Fig.3 Analysis of VlCKX5 promoter activity
2.4 靶向调控VlCKX5的转录因子预测及分析
预测了可能靶向VlCKX5 的转录因子,在PlantTFDB、CIS-BP和JASPAR数据库中发现了共同靶向VlCKX5 的5 个转录因子(图4-A)。通过Gephi0.10 软件对这5 个转录因子以及VlCKX5 之间的共表达网络关系进行可视化,结果可以看出VlCKX5和这5个转录因子之间都存在靶向关系(图4-B)。对转录因子进行功能注释发现这5 个转录因子属于BPC、DOF、MADS和FLC家族,结合VlCKX5的表达量分析和转录因子热图(图2-B,图4-C),挑选出与VlCKX5表达趋势同为下调的AGL6a作为关键转录因子。
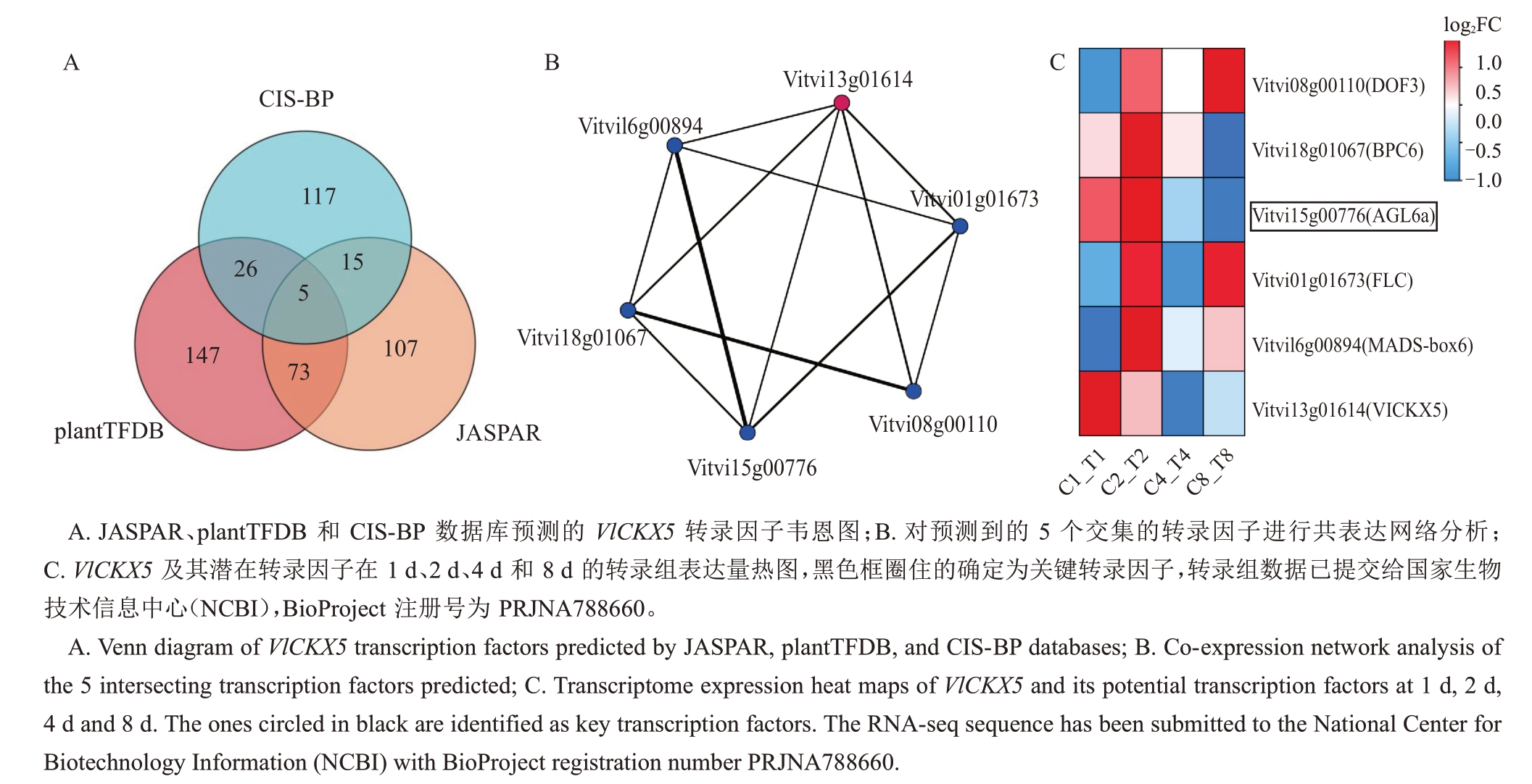
图4 VlCKX5 转录因子的预测
Fig.4 Prediction of VlCKX5 transcription factor
2.5 VlAGL6a的亚细胞定位及RT-qPCR
亚细胞定位试验结果显示,101LYFP-VlAGL6a标记的荧光与mcherry标记的荧光在细胞核内共定位(图5-A)。RT-qPCR结果显示,在巨峰葡萄的根、茎、叶、花序、幼果和卷须组织的表达模式中,VlAGL6a在花序中相对表达量最高,其次是在果实和卷须中,在根、茎和叶中的相对表达量最低(图5-B)。CPPU 处理后,VlAGL6a 在1、2、4 和8 d 的表达量显著降低(图5-C)。

图5 VlAGL6a 的亚细胞定位和表达量分析
Fig.5 Subcellular localization and expression analysis of VlAGL6a
2.6 VlAGL6a与VlCKX5相互作用
在VlCKX5启动子区域预测出VlAGL6a的结合位点,标记结合位点片段为P(图6-A)。利用酵母单杂交技术,将P片段插入pAbAi载体并作为诱饵导入Y1HGold酵母菌株中。并将重组猎物质粒pGADT7-VlAGL6a转化到阳性酵母菌株(包含诱饵基因)中,以验证VlCKX5与VlAGL6a的相互作用。结果显示只有同时含有pGADT7-VlAGL6a 和pAbAi-proVlCKX5/P 的酵母菌落才可以在浓度为400 ng·mL-1的AbA 筛选培养基上正常生长,而pGADT7-VlAGL6a 和pAbAi-VlCKX5/P 的酵母菌落400 ng·mL-1的AbA筛选培养基上不能正常生长(图6-B)。
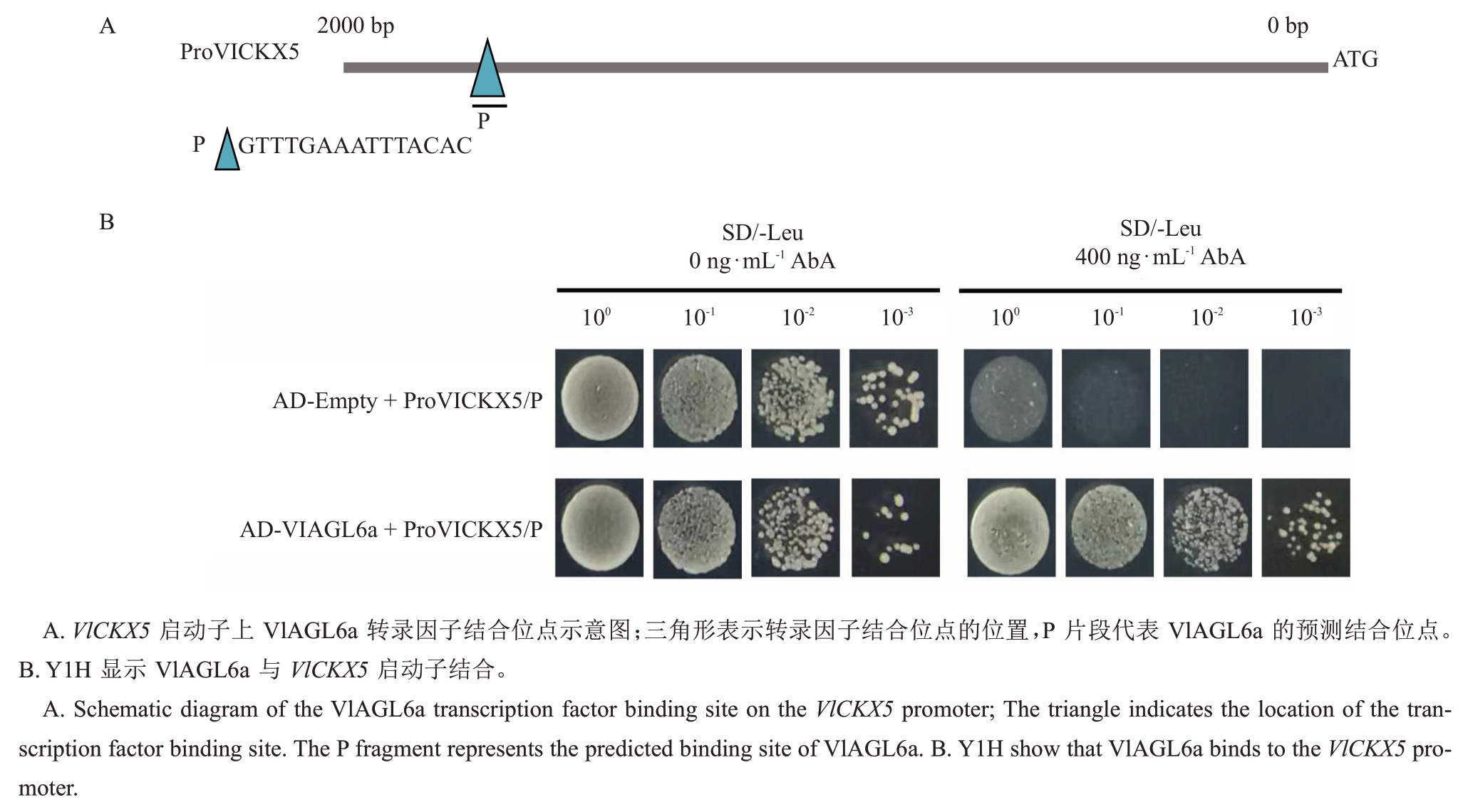
图6 酵母单杂交试验
Fig.6 Y1H assay
为了进一步研究VlAGL6a在调控VlCKX5表达中的功能,进行了双荧光素酶试验。构建了双荧光素酶载体(图7-A)。设计了一个实验组:ProVlCKX5-LUC+35S-VlAGL6a,以及一个空白对照组:LUC0800+pSAK277。在烟草叶片中注入等量菌液瞬时表达,用LUC/REN 比值计算荧光素酶的相对活性。结果显示,与对照组相比,35S-VlAGL6a 试验组的LUC/REN 比率显著增加(图7-B)。酵母单杂交和双荧光素酶试验证明了VlAGL6a 可以与VlCKX5启动子内P段基序特异性结合,且VlAGL6a作为VlCKX5的正调控转录因子,促进了VlCKX5基因的表达。

图7 双荧光素酶试验
Fig.7 Dual luciferase assay
3 讨 论
细胞分裂素是影响植物生长发育的一类重要激素,可以促进细胞分裂、调控根生长、延缓叶片衰老和调节作物产量等[32],CKXs可以通过切割不饱和的类异戊二烯侧链促进生物活性细胞分裂素的不可逆降解。笔者在本研究中对VlCKX5进行生物信息学分析的结果显示,VlCKX5具有黄素腺嘌呤二核苷酸(FAD)结合域和细胞分裂素结合位点(CK-binding),这与谷子[33]、白菜[34]、鸽豆[35]等CKX基因的研究结果相同,完全符合CKX 基因的结构特征。VlCKX5 基因的特异性表达分析显示,VlCKX5 在巨峰葡萄的根、茎、叶、花序、果实和卷须中均能表达,其中VlCKX5 基因在根和叶中相对表达量较高,在花序中的表达量次之,在茎和卷须中的表达量最低。VlCKX5 的表达具有组织特异性,这说明VlCKX5 可能在葡萄的营养生长和生殖生长过程中都发挥着重要作用。与此一致的是,在水稻中,OsCKX 基因家族成员也表现出组织特异性的表达模式,其中OsCKX4 在根部表达显著,OsCKX9 在叶片和腋芽中表达量较高,而OsCKX5 则在所有检测的组织中均表现出高水平的表达,ckx4和ckx9双突变体会影响水稻的分蘖数和穗长[36]。在本试验中,经CPPU处理后,VlCKX5的表达量在1 d、2 d、4 d和8 d 时均显著降低,说明CPPU 处理抑制了VlCKX5基因的表达,有研究发现CPPU可以抑制CKX的活性,是提高作物生产力的农用制剂[37]。CKX 基因对调控植物产量发挥重要功能[38-39]。在大麦中沉默HvCKX1 基因可降低细胞分裂素氧化酶/脱氢酶水平,提高植株产量[40]。在水稻中,降低OsCKX2的表达量会提高细胞分裂素在花序分生组织中积累从而提高籽粒产量[41]。在笔者课题组前期的研究中发现CPPU处理降低了葡萄内源细胞分裂素含量并促进葡萄坐果[30],在拟南芥中过表达葡萄VlCKX4基因可促进拟南芥坐果使角果数增多[42]。因此推测VlCKX5 基因可能通过提高细胞分裂素水平促进葡萄坐果,从而提高葡萄产量。
在基因表达的过程中,启动子是决定基因是否被转录以及转录效率的关键因素之一。本研究中对VlCKX5启动子序列的顺式作用元件进行预测,结果显示VlCKX5 启动子区域的顺式作用元件包含多种与生长素、水杨酸和脱落酸相关的响应元件。GUS染色试验结果显示,IBA、SA、ABA 和CPPU 激素的处理都增强了VlCKX5 启动子的活性,IBA、SA、ABA 和CPPU 激素可能串扰调控VlCKX5 的表达,影响葡萄的生长发育。CKX 可能是赤霉素/细胞分裂素串扰的一个重要环节[43]。BjuCKX 基因具有生长素、脱落酸、茉莉酸甲酯、乙烯和水杨酸的激素响应元件,可以不同程度应答激素[44]。细胞分裂素与脱落酸密切串扰,细胞分裂素水平及其信号转导的调节影响脱落酸依赖和脱落酸不依赖的途径,使植物适应不利条件[45]。综上所述,VlCKX5 可能受IBA、SA、ABA和CPPU激素的串扰影响参与到多种激素通路中发挥作用。
MADS-box基因编码高度保守的DNA结合转录因子,可调控花和果实发育过程[46]。有研究发现MADS-box 基因的表达模式与指定功能之间存在密切的相关性[47],而在本研究中VlAGL6a 在葡萄花序中的相对表达量最高,其次是在果实和卷须中,在根、茎和叶组织中表达量极低,VlAGL6a可能在葡萄的生殖生长中发挥重要作用。与此一致的是,Ac-MADS1 启动子在番茄和拟南芥的花器官和果实发育中的高水平表达可能促进花发育和果实成熟[48],PeMADS5 基因在花中高水平表达促进了竹子开花[49],TaAGL6 在花器官开始分化时的穗中高表达,过表达TaAGL6 会导致小麦小穗数和穗粒数增多[50]。酵母单杂交和双荧光素酶分析结果显示MADS-box 转录因子VlAGL6a 可以靶向VlCKX5 促进其表达。有研究表明MADS 家族的FUL2 和MBP20可以抑制CKX5/6/8基因的表达,从而促进花分生组织中的细胞分裂素信号转导,促进植物向生殖生长过渡,进而抑制番茄花序分枝[51]。TaMADSGS 蛋白会抑制TaCKX 基因的表达使小麦籽粒发育早期的细胞分裂素含量维持在正常水平,促使籽粒正常发育[52]。因此推测VlAGL6a 可以靶向VlCKX5通过调控细胞分裂素水平影响葡萄坐果。
4 结 论
VlCKX5 基因编码546 个氨基酸,具有FAD 和CK-bind的典型CKXs家族保守结构域。VlCKX5具有组织表达特异性,在根和叶中高表达,其次是花序中。CPPU 处理后VlCKX5 的表达量显著降低。外源CPPU 处理可以激活VlCKX5 启动子活性。转录因子VlAGL6a 定位在细胞核中,在花序中高表达。CPPU 处理后VlAGL6a 的相对表达量显著下调,与VlCKX5 的表达趋势一致。进一步研究证明,VlAGL6a 可以与VlCKX5 相互作用并促进VlCKX5 的表达,因此,VlAGL6a通过靶向VlCKX5调控细胞分裂素水平而影响葡萄坐果,为CPPU 激素调控葡萄坐果提供了理论依据。
[1] SABRA A,NETTICADAN T,WIJEKOON C. Grape bioactive molecules,and the potential health benefits in reducing the risk of heart diseases[J].Food Chemistry:X,2021,12:100149.
[2] ZHOU D D,LI J H,XIONG R G,SAIMAITI A,HUANG S Y,WU S X,YANG Z J,SHANG A,ZHAO C N,GAN R Y,LI H B. Bioactive compounds,health benefits and food applications of grape[J].Foods,2022,11(18):2755.
[3] BÖTTCHER C,DAVIES C. Hormonal control of grape berry development and ripening[M]. Sharjah:The Bentham Science Publishers Ltd.,2012:194-217.
[4] RIMPIKA,JAIN S,RATHOD M,BANJARE R,NIDHI N,SOOD A,SHILPA,SHARMA R.Physiological aspects of flowering,fruit setting,fruit development and fruit drop,regulation and their manipulation:A review[J].International Journal of Environment and Climate Change,2023,13(12):205-224.
[5] CROSBY K E,AUNG L H,BUSS G R. Influence of 6-benzylaminopurine on fruit-set and seed development in two soybean,Glycine max(L.)Merr.genotypes[J].Plant Physiology,1981,68(5):985-988.
[6] CLIFFORD P E. Control of reproductive sink yield in mung beans[J].Zeitschrift Für Pflanzenphysiologie,1981,102(2):173-181.
[7] ZUÑIGA- MAYO V M,BAÑOS- BAYARDO C R,DÍAZRAMÍREZ D,MARSCH-MARTÍNEZ N,DE FOLTER S. Conserved and novel responses to cytokinin treatments during flower and fruit development in Brassica napus and Arabidopsis thaliana[J].Scientific Reports,2018,8(1):6836.
[8] MOSTAFA L Y,MOSTAFA Y S,EL-BERRY I M.Effect of NAA and CPPU on fruit drop,yield and quality of avocado trees[J].Egyptian Journal of Horticulture,2020,47(2):137-147.
[9] YU Y H,LI X F,YANG S D,BIAN L,YU K K,MENG X X,LIU H N,PEI M S,WEI T L,GUO D L.CPPU-induced changes in energy status and respiration metabolism of grape young berry development in relation to berry setting[J]. Scientia Horticulturae,2021,283:110084.
[10] LIU Y,LI Y,GUO H X,LV B S,FENG J,WANG H H,ZHANG Z H,CHAI S. Gibberellin biosynthesis is required for CPPU- induced parthenocarpy in melon[J]. Horticulture Research,2023,10(6):uhad084.
[11] WANG C N,WANG H,ZHU H,JI W K,HOU Y L,MENG Y Y,WEN J Q,MYSORE K S,LI X S,LIN H. Genome-wide identification and characterization of cytokinin oxidase/dehydrogenase family genes in Medicago truncatula[J]. Journal of Plant Physiology,2021,256:153308.
[12] 王泽琛,肖荣,欧阳乐军,李莉梅,梁楚炎,潘璟茵,刘智超.基于CRISPR/Cas9 的拟南芥CKX3 基因编辑载体构建及转化研究[J].植物研究,2021,41(6):1015-1022.WANG Zechen,XIAO Rong,OUYANG Lejun,LI Limei,LIANG Chuyan,PAN Jingyin,LIU Zhichao. Construction and transformation of Arabidopsis CKX3 gene editing vector based on CRISPR/Cas9[J]. Bulletin of Botanical Research,2021,41(6):1015-1022.
[13] LIU Y,WANG X,WANG X F,GAO W S,YOU C X.Identification and functional characterization of apple MdCKX5.2 in root development and abiotic stress tolerance[J].Horticulturae,2022,8(1):62.
[14] LI S X,AN Y R,HAILATI S,ZHANG J,CAO Y M,LIU Y S,GENG J C,HU T M,YANG P Z. Overexpression of the cytokinin oxidase/dehydrogenase (CKX) from Medicago sativa enhanced salt stress tolerance of Arabidopsis[J]. Journal of Plant Biology,2019,62(5):374-386.
[15] TANG D,LI Y J,ZHAI L M,LI W,KUMAR R,YER H,DUAN H,CHENG B P,DENG Z N,LI Y. Root predominant overexpression of iaaM and CKX genes promotes root initiation and biomass production in citrus[J]. Plant Cell,Tissue and Organ Culture,2023,155(1):103-115.
[16] ZHAO X,ZHAO Z X,CHENG S S,WANG L H,LUO Z,AI C F,LIU Z G,LIU P,WANG L L,WANG J R,LIU M Z,LI Y,LIU M J. ZjWRKY23 and ZjWRKY40 promote fruit size enlargement by targeting and downregulating Cytokinin oxidase/dehydrogenase 5 expression in Chinese jujube[J].Journal of Agricultural and Food Chemistry,2023,71(46):18046-18058.
[17] ZENG J Y,YAN X Y,BAI W Q,ZHANG M,CHEN Y,LI X B,HOU L,ZHAO J,DING X Y,LIU R C,WANG F L,REN H,ZHANG J Y,DING B,LIU H R,XIAO Y H,PEI Y.Carpel-specific down-regulation of GhCKXs in cotton significantly enhances seed and fiber yield[J]. Journal of Experimental Botany,2022,73(19):6758-6772.
[18] ZHANG W,PENG K X,CUI F B,WANG D L,ZHAO J Z,ZHANG Y J,YU N N,WANG Y Y,ZENG D L,WANG Y H,CHENG Z K,ZHANG K W. Cytokinin oxidase/dehydrogenase OsCKX11 coordinates source and sink relationship in rice by simultaneous regulation of leaf senescence and grain number[J].Plant Biotechnology Journal,2021,19(2):335-350.
[19] JOSHI R,SAHOO K K,TRIPATHI A K,KUMAR R,GUPTA B K,PAREEK A,SINGLA-PAREEK S L.Knockdown of an inflorescence meristem-specific cytokinin oxidase- OsCKX2 in rice reduces yield penalty under salinity stress condition[J].Plant,Cell&Environment,2018,41(5):936-946.
[20] SCHWARZ I,SCHEIRLINCK M T,OTTO E,BARTRINA I,SCHMIDT R C,SCHMÜLLING T. Cytokinin regulates the activity of the inflorescence meristem and components of seed yield in oilseed rape[J]. Journal of Experimental Botany,2020,71(22):7146-7159.
[21] CHENG Y Z,SUN Y D,PEI M S,LIU H N,WEI T L,GUO D L.Transcription factor VviAGL6a regulates fruit ripening by directly activating grape VviJMJ21[J]. Scientia Horticulturae,2024,336:113396.
[22] ZHANG L M,ZHAO J,FENG C F,LIU M J,WANG J R,HU Y F.Genome-wide identification,characterization of the MADSbox gene family in Chinese jujube and their involvement in flower development[J].Scientific Reports,2017,7(1):1025.
[23] PARENICOVÁ L,DE FOLTER S,KIEFFER M,HORNER D S,FAVALLI C,BUSSCHER J,COOK H E,INGRAM R M,KATER M M,DAVIES B,ANGENENT G C,COLOMBO L.Molecular and phylogenetic analyses of the complete MADSbox transcription factor family in Arabidopsis:New openings to the MADS world[J].The Plant Cell,2003,15(7):1538-1551.
[24] 付学森,刘紫璇,王玲,龙雨青,曾娟,周日宝,刘湘丹.灰毡毛忍冬MADS-box 基因家族鉴定与CMB1 基因克隆[J].湖南中医药大学学报,2024,44(3):383-394.FU Xuesen,LIU Zixuan,WANG Ling,LONG Yuqing,ZENG Juan,ZHOU Ribao,LIU Xiangdan.Identification of the MADSbox gene family and cloning of the CMB1 gene in Lonicera macranthoides[J]. Journal of Hunan University of Chinese Medicine,2024,44(3):383-394.
[25] SCHILLING S,KENNEDY A,PAN S R,JERMIIN L S,MELZER R. Genome-wide analysis of MIKC-type MADS-box genes in wheat:pervasive duplications,functional conservation and putative neofunctionalization[J]. New Phytologist,2020,225(1):511-529.
[26] SUN X M,ZHANG S L,LI X M,ZHANG X M,WANG X H,WANG L,LI Z,WANG X P.A MADS-box transcription factor from grapevine,VvMADS45,influences seed development[J].Plant Cell,Tissue and Organ Culture,2020,141(1):105-118.
[27] YOO S K,WU X L,LEE J S,AHN J H.AGAMOUS-LIKE 6 is a floral promoter that negatively regulates the FLC/MAF clade genes and positively regulates FT in Arabidopsis[J]. The Plant Journal,2011,65(1):62-76.
[28] PI M T,HU S Q,CHENG L C,ZHONG R H,CAI Z Y,LIU Z C,YAO J L,KANG C Y. The MADS-box gene FveSEP3 plays essential roles in flower organogenesis and fruit development in woodland strawberry[J].Horticulture Research,2021,8(1):247.
[29] LI G,KUIJER H N J,YANG X J,LIU H R,SHEN C Q,SHI J,BETTS N,TUCKER M R,LIANG W Q,WAUGH R,BURTON R A,ZHANG D B. MADS1 maintains barley spike morphology at high ambient temperatures[J]. Nature Plants,2021,7(8):1093-1107.
[30] WANG L L,SHI Q F,JING P W,WANG R X,ZHANG H M,LIU Y T,LI C Y,SHI T Z,ZHANG L X,YU Y H. VlMYB4 and VlCDF3 co-targeted the VlLOG11 promoter to regulate fruit setting in grape (Vitis vinifera L.)[J]. Plant Cell Reports,2024,43(8):194.
[31] SUN Y D,YUE Y H,LI X F,LI S Q,SHI Q F,YU Y H.Transcription factor VviWOX13C regulates fruit set by directly activating VviEXPA37/38/39 in grape (Vitis vinifera L.)[J]. Plant Cell Reports,2023,43(1):19.
[32] LI S M,ZHENG H X,ZHANG X S,SUI N. Cytokinins as central regulators during plant growth and stress response[J]. Plant Cell Reports,2021,40(2):271-282.
[33] WANG Y G,LIU H H,XIN Q G. Genome-wide analysis and identification of cytokinin oxidase/dehydrogenase (CKX) gene family in foxtail millet (Setaria italica)[J]. The Crop Journal,2014,2(4):244-254.
[34] LIU Z N,LV Y X,ZHANG M,LIU Y P,KONG L J,ZOU M H,LU G,CAO J S,YU X L. Identification,expression,and comparative genomic analysis of the IPT and CKX gene families in Chinese cabbage (Brassica rapa ssp. pekinensis)[J]. BMC Genomics,2013,14:594.
[35] SHARMA S,ARPITA K,NIRGUDE M,SRIVASTAVA H,KUMAR K,SREEVATHSA R,BHATTACHARYA R,GAIKWAD K. Genomic insights into cytokinin oxidase/dehydrogenase(CKX)gene family,identification,phylogeny and synteny analysis for its possible role in regulating seed number in Pigeonpea[Cajanus cajan(L.)Millsp.][J].International Journal of Biological Macromolecules,2024,277:134194.
[36] RONG C Y,LIU Y X,CHANG Z Y,LIU Z Y,DING Y F,DING C Q. Cytokinin oxidase/dehydrogenase family genes exhibit functional divergence and overlap in rice growth and development,especially in control of tillering[J]. Journal of Experimental Botany,2022,73(11):3552-3568.
[37] KHABLAK S H,SPIVAK S I,PASTUKHOVA N L,YEMETS A I,BLUME Y B. Cytokinin oxidase/dehydrogenase as an important target for increasing plant productivity[J]. Cytology and Genetics,2024,58(2):115-125.
[38] CHEN L,ZHAO J Q,SONG J C,JAMESON P E.Cytokinin dehydrogenase:A genetic target for yield improvement in wheat[J].Plant Biotechnology Journal,2020,18(3):614-630.
[39] SHARMA A,PRAKASH S,CHATTOPADHYAY D. Killing two birds with a single stone-genetic manipulation of cytokinin oxidase/dehydrogenase (CKX) genes for enhancing crop productivity and amelioration of drought stress response[J]. Frontiers in Genetics,2022,13:941595.
[40] ZALEWSKI W,GALUSZKA P,GASPARIS S,ORCZYK W,NADOLSKA-ORCZYK A. Silencing of the HvCKX1 gene decreases the cytokinin oxidase/dehydrogenase level in barley and leads to higher plant productivity[J]. Journal of Experimental Botany,2010,61(6):1839-1851.
[41] ASHIKARI M,SAKAKIBARA H,LIN S Y,YAMAMOTO T,TAKASHI T,NISHIMURA A,ANGELES E R,QIAN Q,KITANO H,MATSUOKA M. Cytokinin oxidase regulates rice grain production[J].Science,2005,309(5735):741-745.
[42] SHI Q F,LI X F,YANG S D,ZHAO X C,YUE Y H,YANG Y J,YU Y H.Dynamic temporal transcriptome analysis reveals grape VlMYB59-VlCKX4 regulatory module controls fruit set[J].Horticulture Research,2024,11(9):uhae183.
[43] TODOROVA D,VASEVA I,MALBECK J,TRÁVNÍČKOVÁ A,MACHÁČKOVÁ I,KARANOV E. Cytokinin oxidase/dehydrogenase activity as a tool in gibberellic acid/cytokinin cross talk[J].Biologia Plantarum,2007,51(3):579-583.
[44] LI M Y,ZHOU J,GONG L,ZHANG R,WANG Y,WANG C,DU X M,LUO Y,ZHANG Y,WANG X R,TANG H R.Identification and expression analysis of CKX gene family in Brassica juncea var.tumida and their functional analysis in stem development[J].Horticulturae,2022,8(8):705.
[45] HA S,VANKOVA R,YAMAGUCHI-SHINOZAKI K,SHINOZAKI K,TRAN L S P. Cytokinins:Metabolism and function in plant adaptation to environmental stresses[J]. Trends in Plant Science,2012,17(3):172-179.
[46] GRIMPLET J,MARTÍNEZ-ZAPATER J M,CARMONA M J.Structural and functional annotation of the MADS-box transcription factor family in grapevine[J].BMC Genomics,2016,17:80.
[47] ALVAREZ-BUYLLA E R,LILJEGREN S J,PELAZ S,GOLD S E,BURGEFF C,DITTA G S,VERGARA-SILVA F,YANOFSKY M F. MADS-box gene evolution beyond flowers:Expression in pollen,endosperm,guard cells,roots and trichomes[J].Plant Journal,2000,24(4):457-466.
[48] MOYLE R L,KOIA J H,VREBALOV J,GIOVANNONI J,BOTELLA J R. The pineapple AcMADS1 promoter confers high level expression in tomato and Arabidopsis flowering and fruiting tissues,but AcMADS1 does not complement the tomato LeMADS-RIN (rin) mutant[J]. Plant Molecular Biology,2014,86(4/5):395-407.
[49] ZHANG Y T,TANG D Q,LIN X C,DING M Q,TONG Z K.Genome-wide identification of MADS-box family genes in moso bamboo (Phyllostachys edulis) and a functional analysis of PeMADS5 in flowering[J]. BMC Plant Biology,2018,18(1):176.
[50] KONG X C,WANG F,GENG S F,GUAN J T,TAO S,JIA M L,SUN G L,WANG Z Y,WANG K,YE X G,MA J,LIU D C,WEI Y M,ZHENG Y L,FU X D,MAO L,LAN X J,LI A L.The wheat AGL6-like MADS-box gene is a master regulator for floral organ identity and a target for spikelet meristem development manipulation[J].Plant Biotechnology Journal,2022,20(1):75-88.
[51] JIANG X B,LUBINI G,HERNANDES- LOPES J,RIJNSBURGER K,VELTKAMP V,DE MAAGD R A,ANGENENT G C,BEMER M. FRUITFULL-like genes regulate flowering time and inflorescence architecture in tomato[J].The Plant Cell,2022,34(3):1002-1019.
[52] ZHANG J N,ZHANG Z H,ZHANG R J,YANG C F,ZHANG X B,CHANG S Y,CHEN Q,ROSSI V,ZHAO L,XIAO J,XIN M M,DU J K,GUO W L,HU Z R,LIU J,PENG H R,NI Z F,SUN Q X,YAO Y Y. Type I MADS-box transcription factor TaMADS-GS regulates grain size by stabilizing cytokinin signalling during endosperm cellularization in wheat[J]. Plant Biotechnology Journal,2024,22(1):200-215.