阳光玫瑰是日本选育出的中晚熟葡萄品种,因其无核(人工处理后)、皮薄、甜度高和香气浓郁等优异品质以及较强的环境适应性,近年来广受消费者和种植者喜爱[1]。随着农业栽培技术和设施的不断优化,当前阳光玫瑰已成为南方地区发展的热门品种之一[2]。然而随着全球气候变暖,中国南方夏季极端高温的日数、频率和强度全区域呈现明显增长趋势,极端高温天气已成为南方夏季的一种“新常态”[3-4]。夏季持续高温会导致葡萄叶片卷枯、果实出现日灼,严重影响果实的产量和品质[5],而且南方地区葡萄普遍采用的避雨栽培模式也会进一步加重夏季高温逆境的发生。高温热害已成为南方地区阳光玫瑰健康生产最主要的限制因素。
葡萄架形可通过改变葡萄叶幕形状,改变叶片群体的受光面积及园内光照度、温度、湿度、通风性等微环境因子,也可调控葡萄营养生长和生殖生长的平衡,影响树体长势,最终影响葡萄果实产量及品质[6]。选用合理的架形有利于减少园内高温郁积,提高树体抗逆性,增强葡萄对高温的适应力。因此,观测不同架形阳光玫瑰葡萄在自然高温天气下的生长表现,评价不同架形阳光玫瑰葡萄耐热性的差异,对保证夏季阳光玫瑰葡萄正常生长和果实发育具有重要意义。
由于受地形和气候等多方面的影响,南方地区葡萄栽培多采用V 形架和飞鸟形架[7]。近年来,随着葡萄现代化种植的发展,H 形平棚架因修剪技术简单、标准化生产程度高以及架面下活动空间大,在南方平地果园和葡萄观光园采用的也越来越多[8]。在植物对高温的生理响应中,光合作用是最敏感的生理过程[9]。高温会导致气孔关闭、叶绿素分解、光合作用的相关酶钝化或变性,使叶片光合作用减弱[10]。高温还会破坏光系统Ⅱ(PSⅡ),使PSⅡ捕光天线色素分解、反应中心受损、放氧复合体失活以及受体侧光合电子传递受阻,从而造成植物光能捕获减少、热耗散增加,最终导致叶片光化学效率和光能利用率降低[9]。与PSⅡ相比,PSI在高温下比较稳定[11]。另外,植物叶片组织结构在高温胁迫下的变化也十分明显,正常情况下植物叶肉组织排列紧密,而高温胁迫会导致叶片组织发生紊乱,在葡萄[12]、甜樱桃[13]、虎耳草[14]等植物中均发现叶肉组织在高温后出现膨大、细胞排列疏松等现象。热激蛋白(Heat Shock Protein,HSP)是植物受高温刺激后大量合成的一类蛋白质,大部分HSP的功能是作为分子伴侣参与蛋白质的运输、折叠、组装和定位,阻止高温胁迫下一些蛋白质的错误折叠,帮助变性蛋白质复性或降解,从而减少高温下细胞内受损蛋白的积累,维护细胞正常功能,减少高温伤害[15]。HSP根据分子质量大小可分为HSP100s、HSP90s、HSP70s、HSP60s 和分子质量为15~50 kDa 的小分子热激蛋白[16-17]。热激转录因子(Heat Shock Factor,HSF)位于信号传导途径下游,当接收到外界传来的热激信号时,就会与热激蛋白基因的启动子结合,从而促进HSPS基因的表达,增加植物的耐热性[18-19]。HSFS根据低聚物结构域的特点可以分为三大类,即HSFA、HSFB 和HSFC,在高温处理的葡萄及拟南芥中发现,HSFA2 可与一个肌醇半乳糖苷合成相关基因GLOS1(Galactinol Synthesis)启动子结合,共同上调表达参与耐热性调节[20-21]。
目前,关于不同架形葡萄耐热性差异的研究较少。笔者在本研究中以阳光玫瑰为试验材料,在夏季田间高温环境下,观测V形架、飞鸟形架和H形架3种架形葡萄叶片的组织结构、光合特性、叶绿素荧光特性和抗逆基因的表达量,综合分析不同架形葡萄高温胁迫应答差异,以期为葡萄耐热机制及抗热栽培研究提供理论依据。
1 材料和方法
1.1 试验材料与处理
试验于2022 年6—8 月在四川省成都市双流区太平镇前进村5组“一米阳光”葡萄园内(N 30°27’,E 104°13’)进行,年均气温16.5 ℃,年均降水量895.6 mm,年均日照时数1 032.9 h。试验材料为阳光玫瑰葡萄,树龄为5 a(年),南北行向种植,设置V形架(V-shaped,Vs)、飞鸟形架(Flying Bird-shaped,Fs)和H 形架(H-shaped,Vs)3 种架形处理。V 形架株行距2 m×3 m,叶幕分布高度为距地面1.0~1.9 m,叶幕开张角度约为45°;飞鸟形架株行距2 m×3 m,叶幕分布高度为距地面1.5 ~1.8 m,叶幕开张角度约为55°,新梢下垂长度约60 cm;H 形架株行距4 m×6 m,叶幕水平分布于距地面1.8 m 高度。3 种架形均采用避雨栽培模式,避雨棚为连栋钢架拱棚,单棚跨6 m,脊高4.3 m,肩高2.5 m,南北向种植,同侧新梢间距约为20 cm,新梢主梢保留12枚叶片左右,生长期内修剪及肥水管理等技术基本一致。
成都地区每年会出现连续极端高温天气,将日最高气温>38 ℃出现的第1天定义为田间高温胁迫第1天。2022年8月2日,根据天气预报试验基地日最高气温不超过38 ℃,因此将8 月2 日定义为高温发生前,并开展相关指标的调查收集。到2022 年8月8 日,试验基地露地日最高气温超过38 ℃,达到了39.2 ℃,且根据天气预报此后将出现连续高温,因此将8月8日定义为自然高温胁迫第1天,而后每间隔2~3 d开展一次相关调查,直至高温结束。具体试验开展时间为自然高温发生前6 d(8月2日)及胁迫发生后的3、6、9、15 d(8 月10 日、13 日、16 日、22日),用B6 代表自然高温发生前6 d,用A3、A6、A9、A15分别代表高温胁迫发生后的3、6、9、15 d。如图1所示为2022年8月试验区实际露地日均温和日最高温的变化情况,8月8日之前日最高温低于38 ℃,在8月8—24日每天至少有1 h的温度超过38 ℃,并且最高温达到42 ℃。
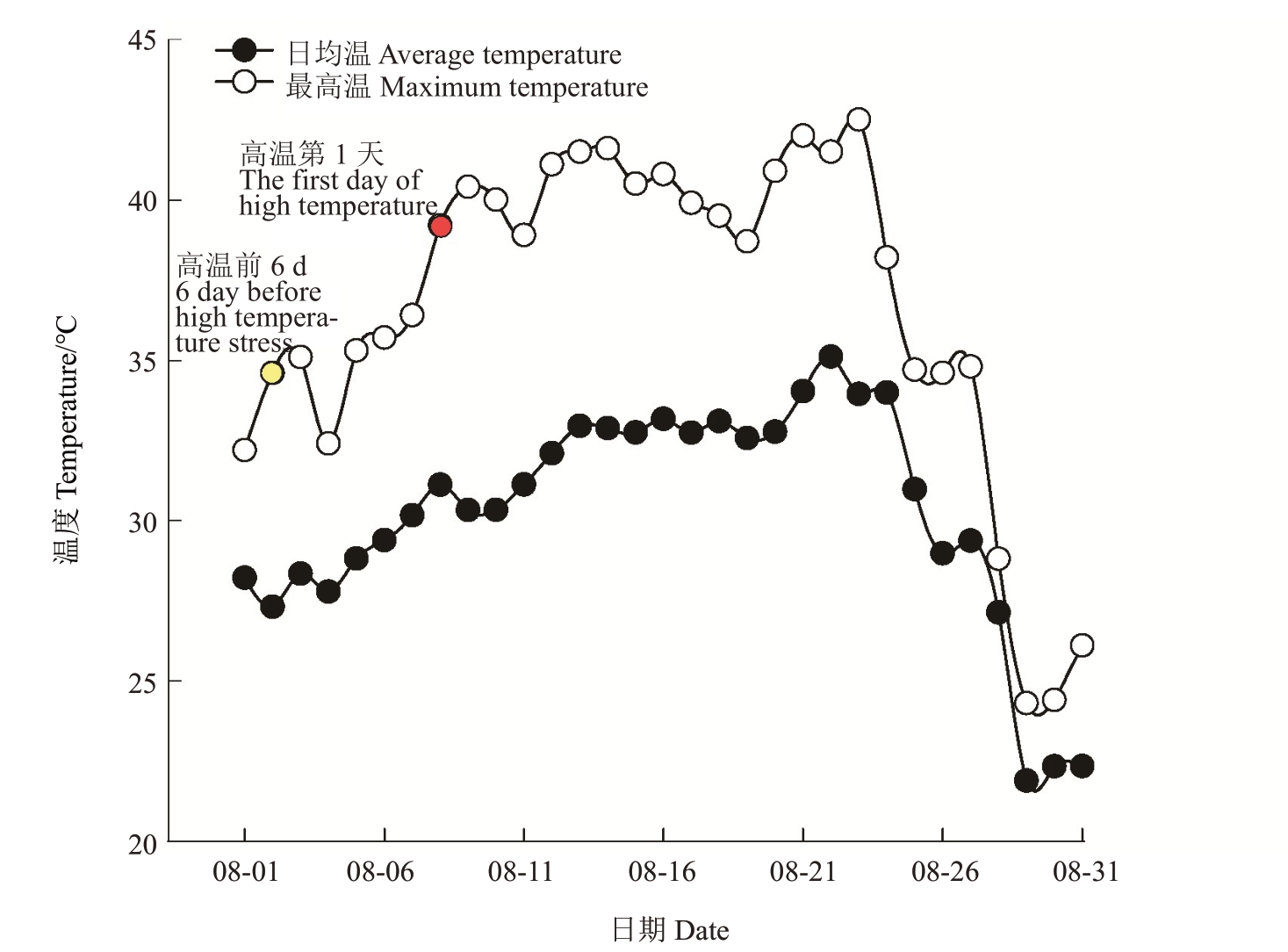
图1 2022 年8 月露地气温
Fig.1 The temperature in open fields in August 2022
在试验开始前每处理选取10 株生长一致的葡萄植株挂牌标记确定为样株,于B6、A3、A6、A9、A15日采集葡萄植株第6~8节位叶片进行光合色素含量测定,每处理每次采集10枚叶片,其中第6、7、8节位每次分别采摘3、4、3枚叶片;再于当日13:00—14:00每处理采集新展开幼叶10枚用于抗逆基因的表达分析。同时,试验开始前每处理在样株中选取第7 节位叶片6 枚进行标记,其中3 枚用于B6、A3、A6、A9、A15日连续观测葡萄叶片光合参数日变化,另外3枚用于连续观测叶绿素荧光参数日变化。另外,于B6和A15日分别采集第7节位叶片5枚,用于制作石蜡切片观测叶片解剖结构。所有分析测试所用的叶片均选择行间内同一朝向叶片。
1.2 测定项目与方法
1.2.1 高温期间温、湿度监测 于2022年6—8月成都夏季高温期间,在果穗附近叶幕处悬挂温、湿度记录仪(ZDR-U1W1S-T2,杭州泽大仪器有限公司),每处理3台,每1 h记录1次叶幕温、湿度,直至试验结束。统计6—8 月的日均温、月均温、月最高温、月最低温、极温差(每个月的最高温与最低温之差);计算>40 ℃及>45 ℃的高温时长,将其与该月份的总时长相比得到该月的高温比例;统计6—8月的日均湿度、月均湿度、月最高湿度、月最低湿度,并检索湿度记录仪60%~80%及>80%的湿度时长,与该月总时长相比得到该湿度时长比例。
1.2.2 高温前后叶片组织结构观测 石蜡切片参照范志霞等[22]的方法略作调整制作。以叶主脉中部为中心剪取0.5 cm×1.0 cm的小块鲜样,立即用FAA固定液(百奥莱博)固定24 h以上,再取固定好的叶片经不同浓度乙醇脱水、二甲苯透明、浸蜡、包埋处理后,制成厚度为10 μm的连续石蜡切片,后经脱蜡、番红-固绿染色后,使用荧光显微镜(BX41,Olympus)测微尺测量栅栏组织、海绵组织和叶片厚度,计算叶片栅海比。每1个样片观察10个视野,取平均值。
1.2.3 光合色素含量测定 测定方法参照李合生[23]和张洁[7]的方法,略有改动。取0.1 g 叶片,置于10 mL 80%丙酮内,在室温下避光浸提24 h,用紫外-可见分光光度计UV-1800(岛津,日本)测定663、645和470 nm波长下的吸光值,计算叶绿素a(Chl a)、叶绿素b(Chl b)、类胡萝卜素(Car)含量,同时计算总光合色素含量=Chl a+Chl b+Car。
1.2.4 光合作用测定 在08:00—18:00 期间,每隔2 h 用便携式光合仪LI-6400(LI-COR,美国)测定叶片的净光合速率(Pn)、气孔导度(Gs)、蒸腾速率(Tr)和胞间二氧化碳浓度(Ci),取日平均值。设定参数为:流速500 μmol·s-1,相对湿度60%,CO2浓度400 μmol·mol-1,温度25 ℃,光照度1500 μmol·m-2·s-1。测定当天晴朗无风,每种架形测定3 枚叶片,3 次重复。
1.2.5 叶绿素荧光参数测定 用便携式调制叶绿素荧光仪PAM2500(Walz,德国)测定叶绿素荧光参数,测定时间同1.2.4 中所述光合参数的测定时间。测定前将叶片置于暗适应夹中适应30 min,依次测定叶片初始荧光(Fo)、最大荧光(Fm)、最大光化学效率(Fv/Fm)、潜在光化学效率(Fv/Fo)、实际光化学效率(ΦPSII)、光化学猝灭系数(qP)、非光化学猝灭系数(NPQ)与电子传递速率(ETR)。每种架形测定3 枚叶片。
1.2.6 抗逆基因表达分析 利用RNA prep Pure 多糖多酚植物总RNA 提取试剂盒(DP441,天根,中国)提取葡萄叶片总RNA,采用1%琼脂糖凝胶电泳检测RNA 完整度,并利用核酸蛋白检测仪检测RNA 浓度及纯度。以提取的RNA 为模板,参照Prime ScriptTMRT reagent Kit with gDNA Eraser(Perfect Real Time)试剂盒(TaKaRa,日本)操作说明反转录合成第一链cDNA。采用TB Green Premix Ex TagTMⅡ(Tli RNaseH Plus)试剂盒(TaKaRa,日本)和CFX96 Real-Time System荧光定量PCR仪(Bio-Rad,美国)进行实时荧光定量PCR(qRT-PCR)。反应体系共10 μL,其中含有TB Green Premix Ex Taq Ⅱ(Tli RNaseH Plus)(2×)5 μL,cDNA模板1 μL,10 μmol·L-1上游和下游引物各0.5 μL,ddH2O 3 μL。反应程序:95 ℃预变性30 s后,运行40个循环的95 ℃变性5 s、56.8 ℃退火30 s;然后按照以下梯度采集溶解曲线:95 ℃保持10 s,降温到65 ℃后开始以0.5 ℃每步升温,并维持5 s采集荧光信号,反应至95 ℃结束。设3次生物学重复。
葡萄目的抗逆基因为热激蛋白HSP基因(VvHSP17.9、VvHSP22、VvHSP70、VvHSP90、VvHSP100、VvHSP101)、热激转录因子HSF 基因(VvHSFA1、VvHSFA2、VvHSFB1)和GLOS1,内参基因为VvGAPDH,使用Primer 5.0设计荧光定量PCR引物(表1),引物由北京擎科生物科技股份有限公司合成。采用2-△△CT法[24]计算基因的相对表达量,将高温前V形架各目的基因的相对表达量定义为1,计算其他处理中基因的表达倍数。
表1 葡萄目的基因实时荧光定量PCR 引物序列
Table 1 The sequence of primers for PCR analysis of target genes in grape

目的基因Target gene VvHsp17.9 VvHsp22 VvHsp70 VvHsp90 VvHsp100 VvHsp101 VvHsfA1 VvHsfA2 VvHsfB1 VvGOLS1 VvGAPDH登录号Accession number引用文献Reference[25]XM_034819224.1 XM_010650267.3 XM_010659145.3[26]NM_001280893.1[26]XM_010650040.3[26]XM_002279078.5上游引物(5'-3')Forward primer sequence(5'-3')AACTTCCCCACCCTCCTCT TGCTGCTATTGCTTGTGTTCT ATGCTACGGCTGCTGAGTTT ATATGGCTTCGCAACCCCAA AAGGGCATCATGGTGTTC GGACATGATGAAGGTGGGCA GTCTTCGGCAATCTCCTC GGAGGGTCGAATGCAGAACA TGCGAGGAGCTGATAGCG CCGAGAACCAGACCCAGTTC TTCCGTGTTCCTACTGTTG上游引物(3'-5')Reverse primer sequence(3'-5')CGTCAAGGAGTACCCCAATTC GCTTGGGGTTCTCTTCCTCA GCTTGCCATCTGAATCCCCT TCTAAGCAAGGGAGCAAGGC TGTCCCTCAAGTCGTCAAG TCCTGACACTCCCGCATCTA GCTACTCCACGATACCACC CAGTCTGAAGGCGTTGCAAC TCACCAACCAACCCACCAT TGCATGTTGTCCTCCTTCCC CCTCTGACTCCTCCTTGAT [27]
1.3 数据处理
使用Microsoft Excel 2010 对数据进行初步处理,采用SPSS 19.0 软件进行方差分析,用邓肯氏法进行差异显著性检验,显著水平为α=0.05。使用https://www.chiplot.online/网站绘制相关性分析热图,使用SigmaPlot 14.0绘制折线图及柱形图。
2 结果与分析
2.1 夏季不同架形葡萄叶幕温湿度比较
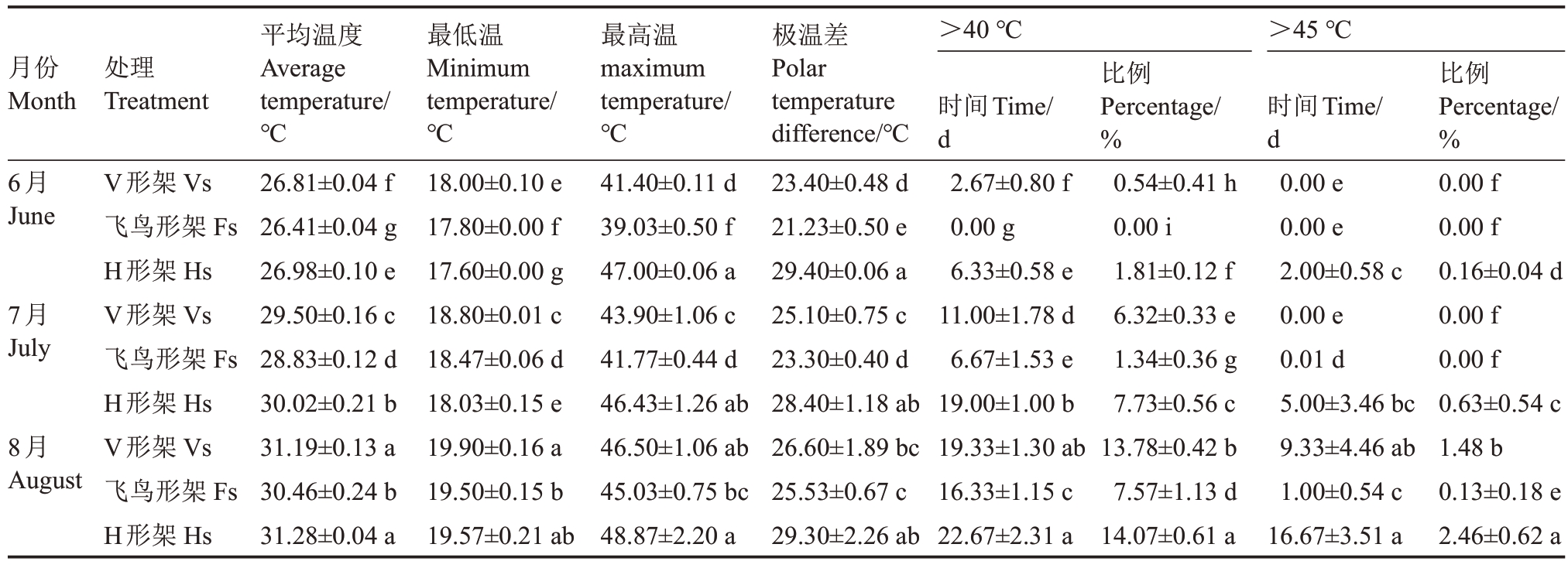
2.1.1 叶幕温度 图2反映了夏季(6—8月)不同架形葡萄叶幕每日温度范围及平均温度。如图2 所示,夏季(6—8月)飞鸟形架和V形架葡萄叶幕极限温度总体少于H 形架。表2 表明,6—8 月飞鸟形架叶幕极温差为21.23~25.53 ℃,V 形架为23.40~26.60 ℃,H 形架为28.40~29.40 ℃。6 月V 形架、飞鸟形架和H 形架叶幕最高温分别是41.40、39.03、47.00 ℃,V形架叶幕≥40 ℃的天数有2.67 d,H形架叶幕≥40 ℃的天数有6.33 d,其中有2 d最高温突破45 ℃;7月V形架、飞鸟形架和H形架叶幕最高温分别是43.90、41.77、46.43 ℃,≥40 ℃的天数V 形架有11 d,飞鸟形架仅6.67 d,而H形架有19 d,其中有5 d最高温突破45 ℃;8 月不同架形葡萄叶幕温度均突破45 ℃,V 形架、飞鸟形架和H 形架叶幕最高温分别是46.50、45.03、48.87 ℃,≥40 ℃的天数V 形架、飞鸟形架、H形架分别有19.33、16.33、22.67 d,其中≥45 ℃的天数分别有9.33、1.00、16.67 d。8 月份飞鸟形架叶幕40 ℃以上的高温比例较V 形架降低了45.07%,较H形架降低了46.20%。就日平均温度来看,飞鸟形架在高温天气出现的时候降温效果较显著,V形架次之,H形架叶幕温度最高。
表2 架形对夏季叶幕最低温、最高温及高于40 ℃和45 ℃比例的影响
Table 2 Effects of trellis systems on the minimum temperature,maximum temperature and the frequency of temperature
higher than 40 ℃and 45 ℃of the grape canopy during the high-temperature months
注:同列数据后不同小写字母表示差异显著(p<0.05)。下同。
Note:Different small letters in each column indicate significant difference(p<0.05).The same below.
处理Treatment>40 ℃>45 ℃平均温度Average temperature/℃26.81±0.04 f 26.41±0.04 g 26.98±0.10 e 29.50±0.16 c 28.83±0.12 d 30.02±0.21 b 31.19±0.13 a 30.46±0.24 b 31.28±0.04 a最低温Minimum temperature/℃18.00±0.10 e 17.80±0.00 f 17.60±0.00 g 18.80±0.01 c 18.47±0.06 d 18.03±0.15 e 19.90±0.16 a 19.50±0.15 b 19.57±0.21 ab最高温maximum temperature/℃41.40±0.11 d 39.03±0.50 f 47.00±0.06 a 43.90±1.06 c 41.77±0.44 d 46.43±1.26 ab 46.50±1.06 ab 45.03±0.75 bc 48.87±2.20 a极温差Polar temperature difference/℃23.40±0.48 d 21.23±0.50 e 29.40±0.06 a 25.10±0.75 c 23.30±0.40 d 28.40±1.18 ab 26.60±1.89 bc 25.53±0.67 c 29.30±2.26 ab月份Month 6月June 7月July 8月August比例Percentage/%0.00 f 0.00 f 0.16±0.04 d 0.00 f 0.00 f 0.63±0.54 c 1.48 b 0.13±0.18 e 2.46±0.62 a V形架Vs飞鸟形架Fs H形架Hs V形架Vs飞鸟形架Fs H形架Hs V形架Vs飞鸟形架Fs H形架Hs时间Time/d 2.67±0.80 f 0.00 g 6.33±0.58 e 11.00±1.78 d 6.67±1.53 e 19.00±1.00 b 19.33±1.30 ab 16.33±1.15 c 22.67±2.31 a比例ercentage/0.54±0.41 h 0.00 i 1.81±0.12 f 6.32±0.33 e P%1.34±0.36 g 7.73±0.56 c 13.78±0.42 b 7.57±1.13 d 14.07±0.61 a时间Time/d 0.00 e 0.00 e 2.00±0.58 0.00 e 0.01 d c 5.00±3.46 bc 9.33±4.46 ab 1.00±0.54 c 16.67±3.51 a
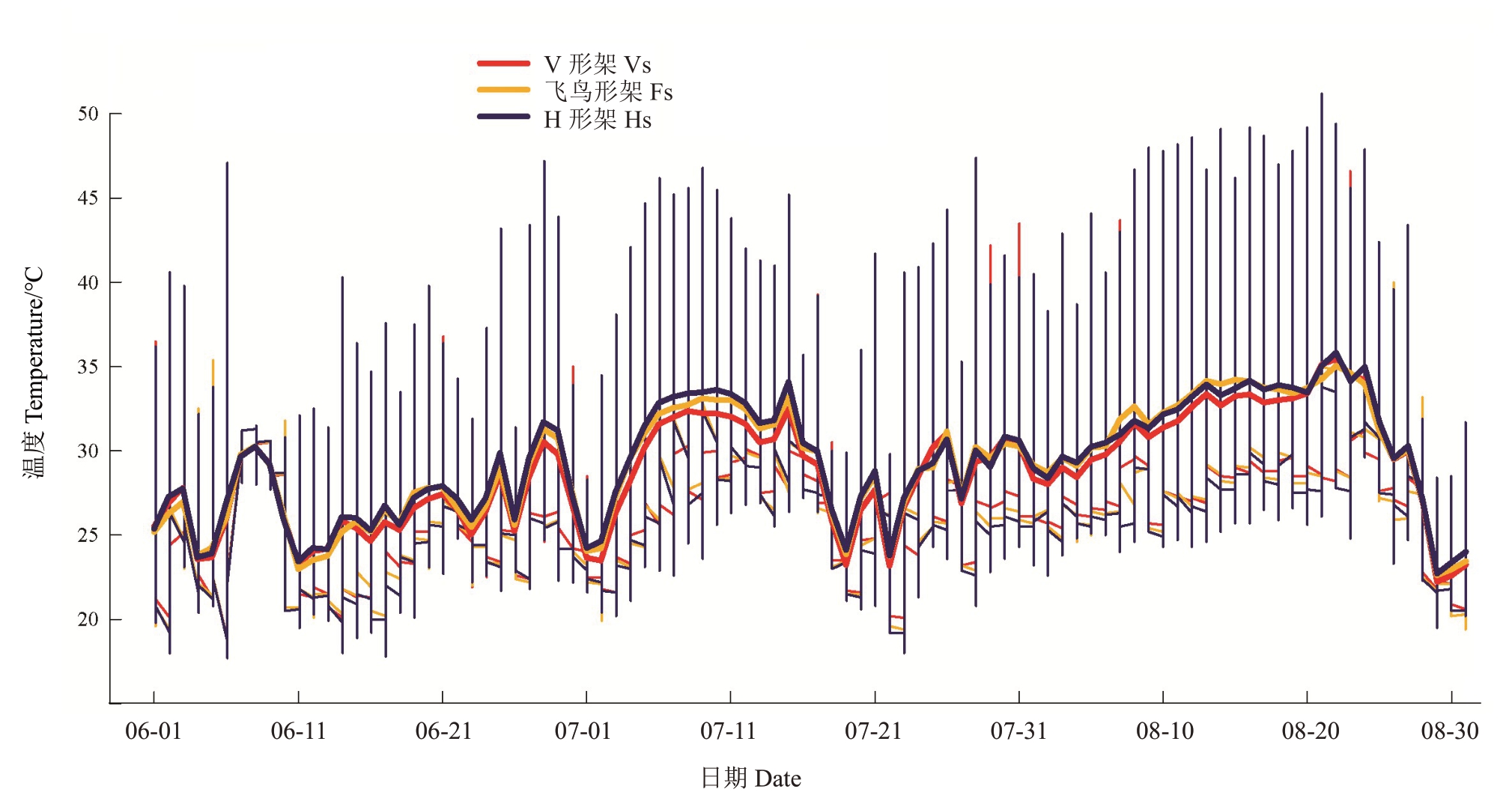
图2 架形对葡萄叶幕温度的影响
Fig.2 Effects of trellis systems on the canopy temperature of grapes
细竖线代表每日的温度范围,竖线最高点和最低点分别反映当日的最高温度值和最低温度值,水平粗实线代表每日的平均温度值。
The thin vertical line represents the daily temperature range,the highest and lowest points of the vertical line reflect the highest and lowest temperature of the day,respectively;the horizontal thick solid line represents the average temperature of the day.
2.1.2 叶幕湿度 如图3 所示,6—8 月,随温度升高,各架形叶幕湿度逐渐降低。飞鸟形架叶幕湿度最高,V 形架次之,H 形架最低,但波动范围相反,H形架叶幕湿度波动大。表3 表明,6—8 月飞鸟形架叶幕湿度60%~80%所占比例为20.39%~26.37%,比V形架平均降低4.37%,比H形架平均增高17.32%,而6—8 月飞鸟形架叶幕湿度>80%所占比例为38.82%~48.91%,比V 形架平均增高8.89%,比H 形架平均增高4.53%,即H 形架叶幕湿度较低,V 形架叶幕适宜叶片果实发育的湿度比例(60%~80%)较高,飞鸟形架叶幕高湿比例较高。
表3 架形对夏季叶幕最低湿度、最高湿度及60%~80%湿度比例的影响
Table 3 Effects of trellis systems on the minimum humidity,maximum humidity and the ratio of humidity between 60%and
80%of the grape canopy during the high-temperature months
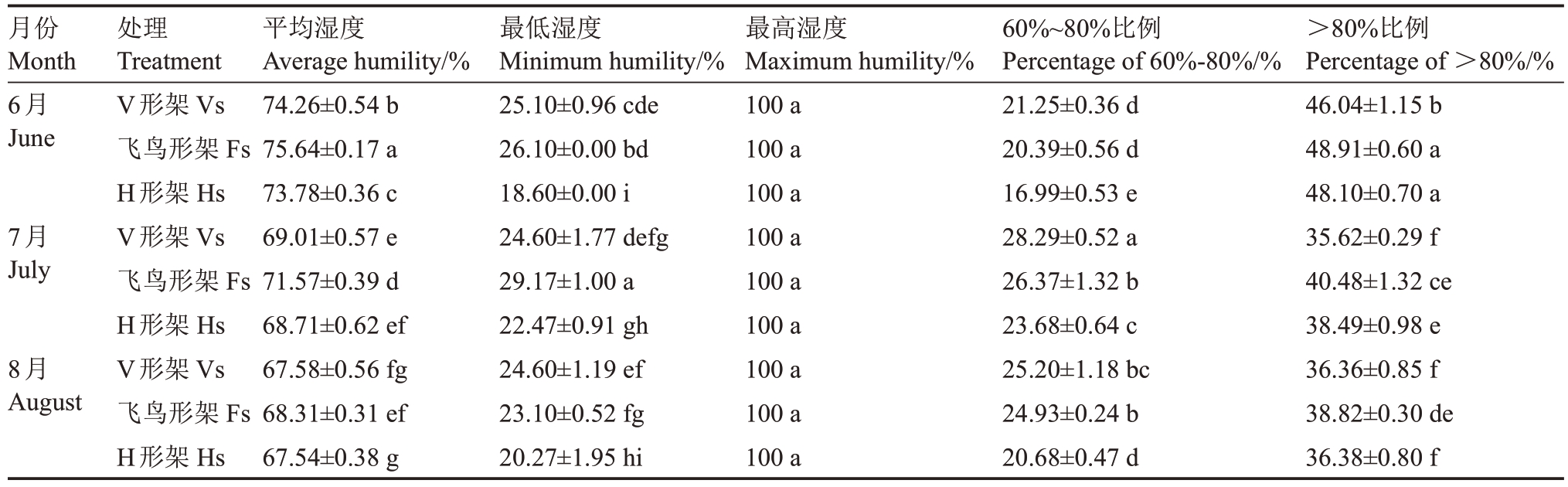
月份Month 6月June 7月July 8月August处理Treatment V形架Vs飞鸟形架Fs H形架Hs V形架Vs飞鸟形架Fs H形架Hs V形架Vs飞鸟形架Fs H形架Hs平均湿度Average humility/%74.26±0.54 b 75.64±0.17 a 73.78±0.36 c 69.01±0.57 e 71.57±0.39 d 68.71±0.62 ef 67.58±0.56 fg 68.31±0.31 ef 67.54±0.38 g最低湿度Minimum humility/%25.10±0.96 cde 26.10±0.00 bd 18.60±0.00 i 24.60±1.77 defg 29.17±1.00 a 22.47±0.91 gh 24.60±1.19 ef 23.10±0.52 fg 20.27±1.95 hi最高湿度Maximum humility/%100 a 100 a 100 a 100 a 100 a 100 a 100 a 100 a 100 a 60%~80%比例Percentage of 60%-80%/%21.25±0.36 d 20.39±0.56 d 16.99±0.53 e 28.29±0.52 a 26.37±1.32 b 23.68±0.64 c 25.20±1.18 bc 24.93±0.24 b 20.68±0.47 d>80%比例Percentage of >80%/%46.04±1.15 b 48.91±0.60 a 48.10±0.70 a 35.62±0.29 f 40.48±1.32 ce 38.49±0.98 e 36.36±0.85 f 38.82±0.30 de 36.38±0.80 f
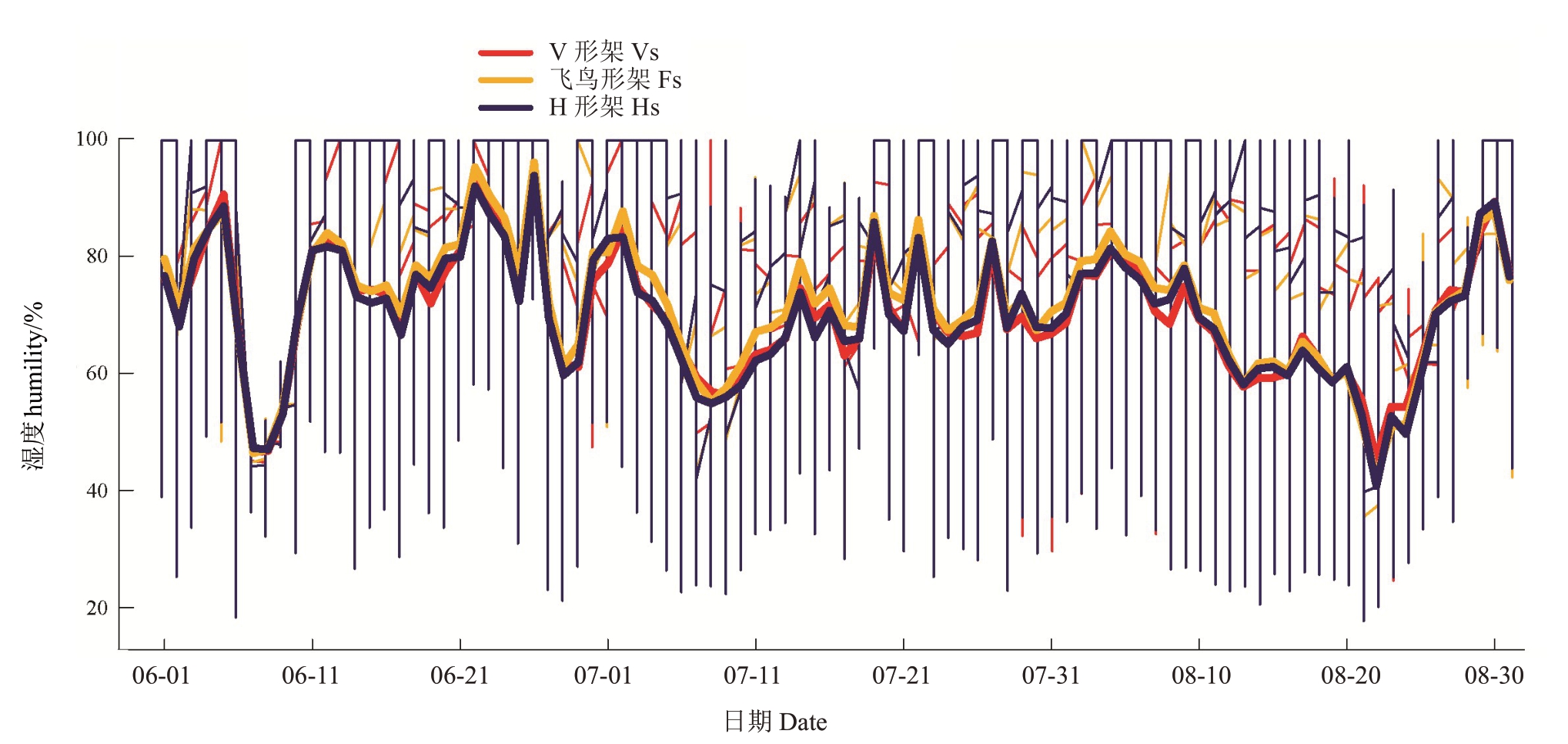
图3 架形对葡萄叶幕湿度的影响
Fig.3 Effects of trellis systems on the canopy humidity
细竖线代表每日的湿度范围,竖线最高点和最低点分别反映当日的最高湿度值和最低湿度值,水平粗实线代表每日的平均湿度值。
The thin vertical line represents the daily humility range,the highest and lowest points of the vertical line reflect the highest and lowest humility of the day,respectively;the horizontal thick solid line represents the average humility of the day.
2.2 高温对葡萄叶片解剖结构的影响
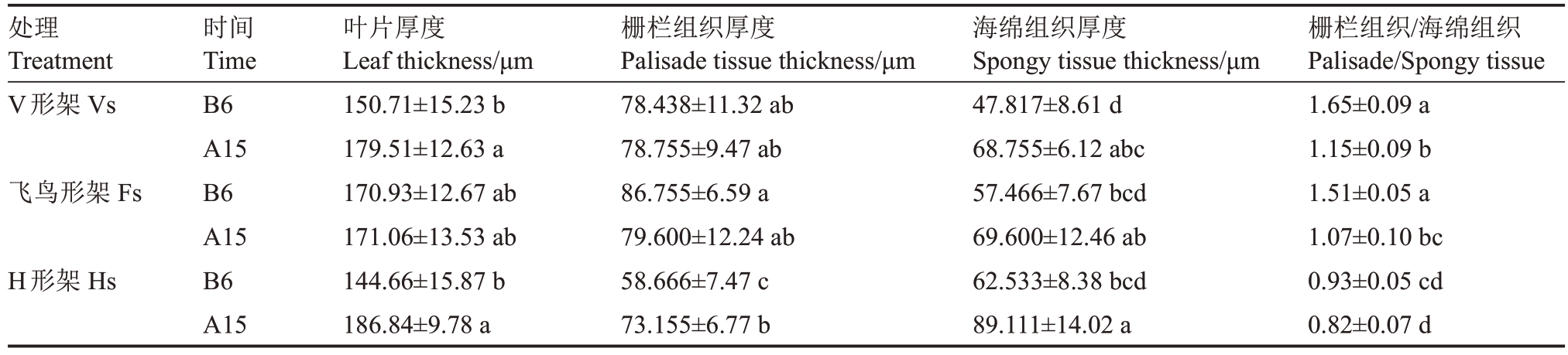
高温发生前及胁迫发生后均可明显区分葡萄叶片上下表皮、栅栏组织和海绵组织(图4)。高温发生前,3种架形葡萄叶片栅栏组织细胞呈长柱形,排列整齐、紧密,海绵组织细胞呈不规则形,排列紧凑;在高温胁迫发生15 d后,3种架形葡萄叶片厚度增加,栅栏组织细胞形状和排列变得不规则,海绵组织空隙增多增大,排列疏松。表4表明,高温发生前,3种架形葡萄叶片厚度及海绵组织厚度无显著差异,但飞鸟形架和V形架葡萄叶片栅栏组织厚度显著高于H形架;高温胁迫发生后,V形架和H形架葡萄叶片厚度显著增加,V形架增加了19.11%,H形架增加了29.16%,而飞鸟形架叶厚无显著变化,其中H形架栅栏组织厚度和海绵组织厚度在高温后显著增加24.70%、42.50%,V 形架海绵组织厚度显著增加43.79%;V形架和飞鸟形架的栅/海比在高温后显著降低,分别降低了30.30%、29.14%,而H 形架降低11.83%,但与高温前无显著差异。
表4 高温前后葡萄叶片解剖结构的变化
Table 4 Changes in the anatomical structure of grape leaves after high-temperature treatment
注:B 代表高温发生前的天数,A 代表高温胁迫发生后的天数。
Note:B represents the days before heat stress;A represents the days after heat stress.
处理Treatment V形架Vs飞鸟形架Fs H形架Hs栅栏组织/海绵组织Palisade/Spongy tissue 1.65±0.09 a 1.15±0.09 b 1.51±0.05 a 1.07±0.10 bc 0.93±0.05 cd 0.82±0.07 d时间Time B6 A15 B6 A15 B6 A15叶片厚度Leaf thickness/μm 150.71±15.23 b 179.51±12.63 a 170.93±12.67 ab 171.06±13.53 ab 144.66±15.87 b 186.84±9.78 a栅栏组织厚度Palisade tissue thickness/μm 78.438±11.32 ab 78.755±9.47 ab 86.755±6.59 a 79.600±12.24 ab 58.666±7.47 c 73.155±6.77 b海绵组织厚度Spongy tissue thickness/μm 47.817±8.61 d 68.755±6.12 abc 57.466±7.67 bcd 69.600±12.46 ab 62.533±8.38 bcd 89.111±14.02 a
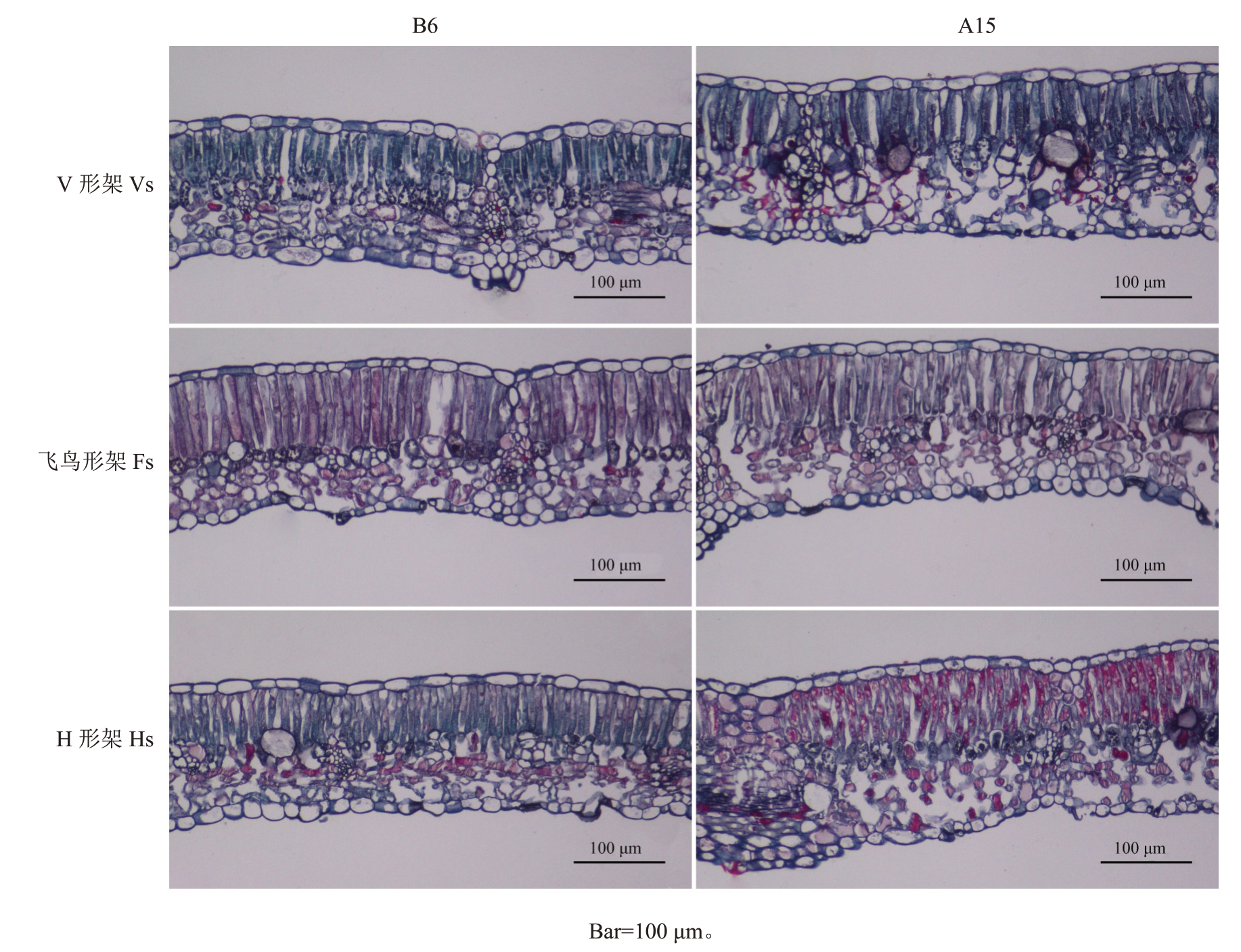
图4 高温胁迫对葡萄叶片解剖结构的影响
Fig.4 Effects of high-temperature stress on the anatomical structure of grape leaves
2.3 高温对葡萄叶片光合色素含量的影响
如图5所示,高温发生前3种架形葡萄叶片Chl a、Chl b、Car总体无显著差异,且在高温胁迫期间均呈先上升后下降的变化趋势,但达到最大值的时间不同,V形架、飞鸟形架Chl a和Chl b含量在胁迫6 d时升至最高值,而H 形架在胁迫3 d 时达到最大值,3种架形的Car 含量均在胁迫3 d 时达到最大值。整个试验期间,飞鸟形架叶绿素及类胡萝卜素含量最高,V形架次之,H形架最低,其中飞鸟形架Chl a和Car含量与V形架无显著差异,仅Chl b在胁迫6 d和胁迫9 d时显著高于V形架,飞鸟形架各光合色素含量总体显著高于H 形架,而V 形架和H 形架各光合色素含量在胁迫中期有显著差异,到胁迫15 d时则无显著差异。就总光合色素而言,到胁迫6 d 时,3种架形差异最明显,此时飞鸟形架总光合色素含量较V 形架显著提高6.74%,较H 形架显著提高49.87%,而后各架形之间的差异逐渐缩小。
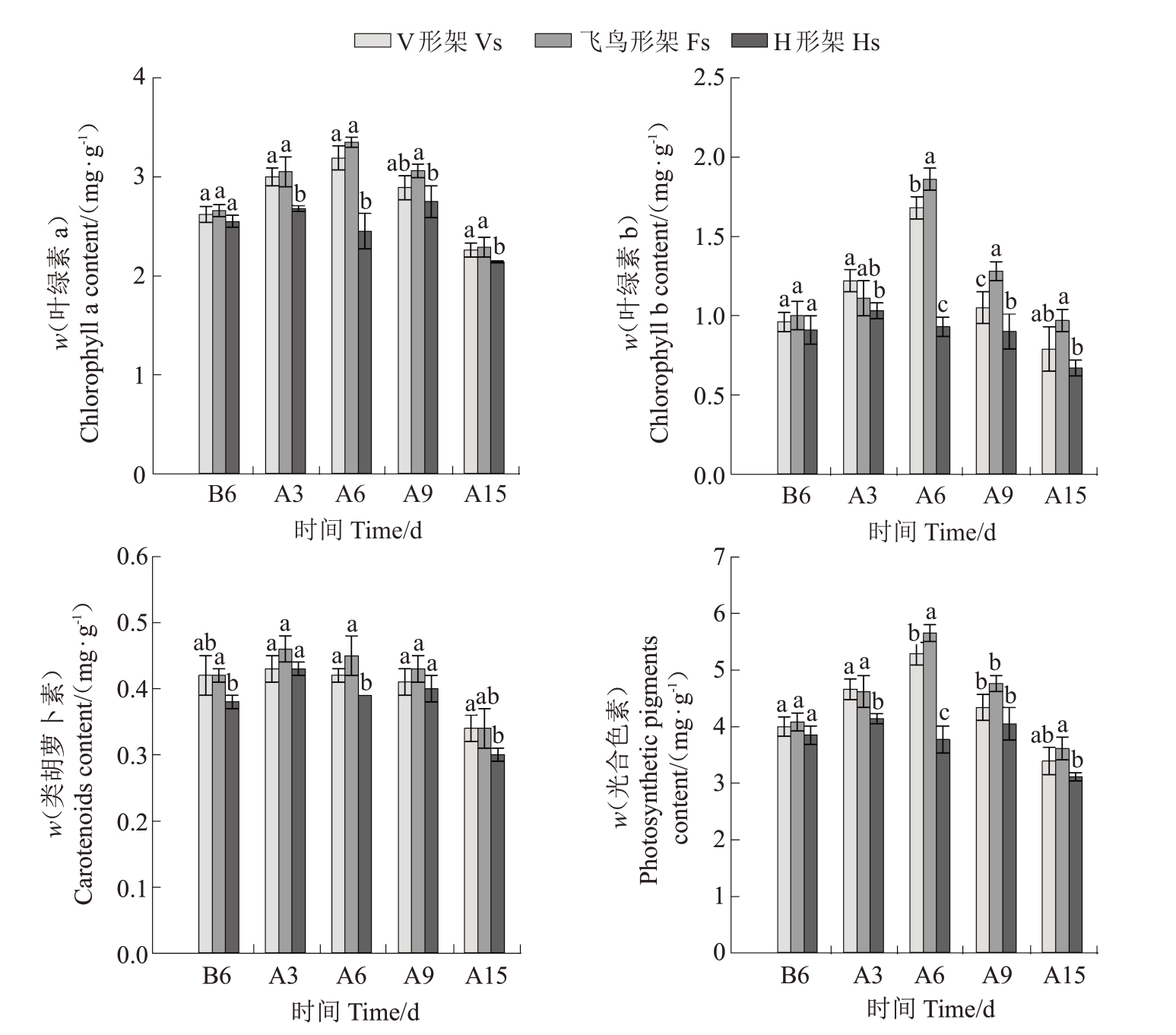
图5 高温胁迫对葡萄叶片光合色素含量的影响
Fig.5 Effects of high-temperature stress on the photosynthetic pigment content of grape leaves
2.4 高温对葡萄叶片光合参数的影响
如图6所示,3种架形葡萄叶片日均Pn、日均Gs、日均Tr和日均Ci变化规律一致,随高温胁迫时间的延长先上升后下降,除V 形架Pn、Tr和Ci在胁迫6 d时达到最大值,其余均在胁迫3 d时达到峰值。整个试验期间,Pn总体表现为飞鸟形架>V 形架>H 形架,Gs、Tr和Ci则表现相反,为H形架>V形架>飞鸟形架,其中3 种架形之间Pn的差异随胁迫时间增加而逐渐增大,到胁迫15 d时,飞鸟形架(Pn)较V形架显著增加14.21%,较H形架显著增加76.22%;3种架形的Gs、Tr和Ci在胁迫3d 和胁迫6 d 时差异较明显,胁迫6 d 时,H 形架的Gs、Tr和Ci分别比V 形架提高21.46%、38.38%、1.56%,比飞鸟形架提高49.04%、81.18%、11.16%,而后差异逐渐缩小,到胁迫15 d时,除H形架的Ci显著高于V形架和飞鸟形架外,其余无显著差异。
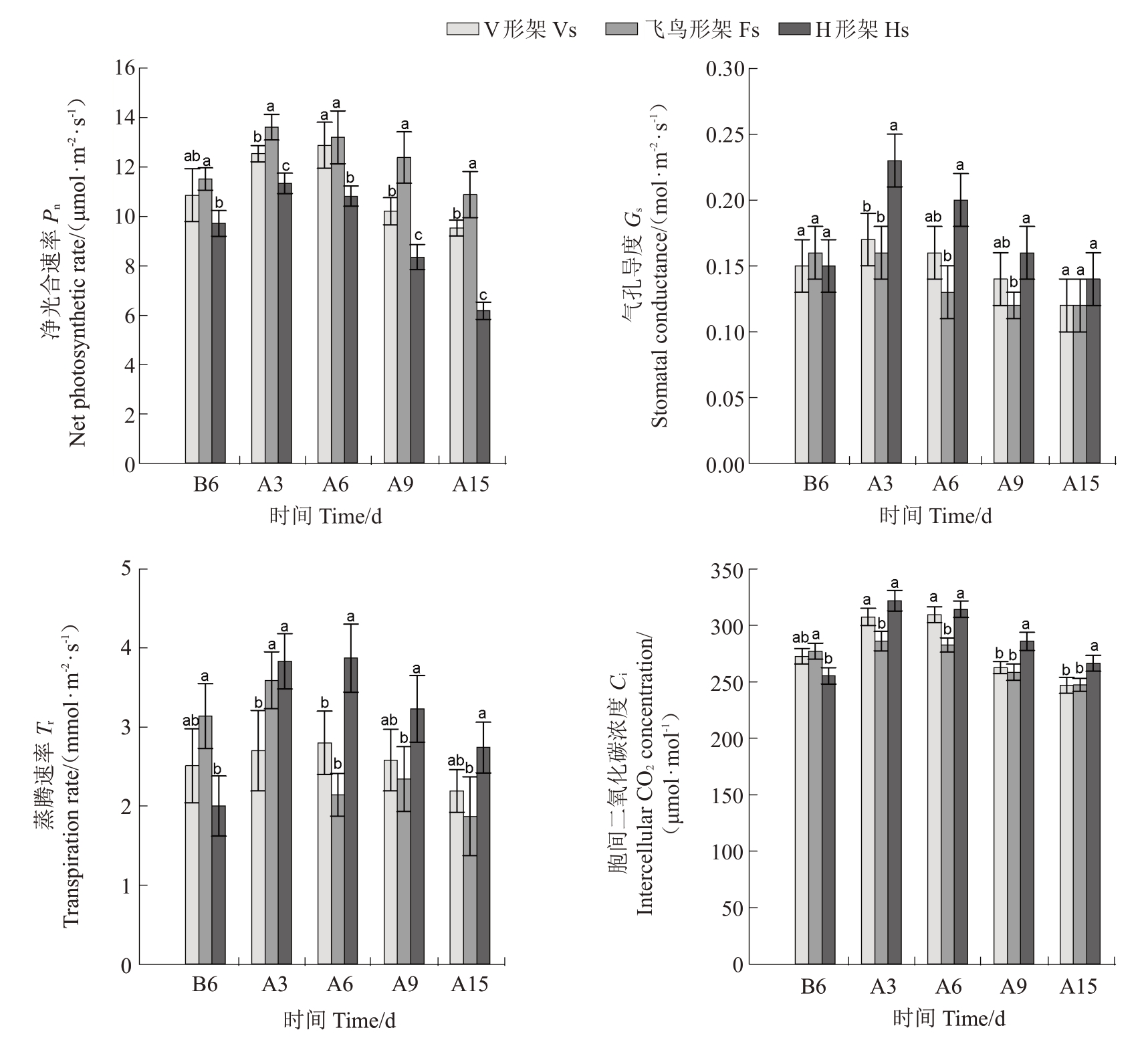
图6 高温胁迫对葡萄叶片光合参数的影响
Fig.6 Effects of high-temperature stress on photosynthetic parameters of grape leaves
2.5 高温对葡萄叶片叶绿素荧光参数的影响
如图7 所示,高温前,3 种架形各荧光参数值总体无显著差异;在高温胁迫期间,3种架形葡萄叶片各荧光参数变化规律大致相同。初始荧光(Fo)和非光化学猝灭系数(NPQ)呈逐渐上升趋势,到胁迫15 d时,V 形架、飞鸟形架和H 形架的Fo 分别上升了66.37% 、39.80% 、101.25% ,NPQ 分别上升了67.02%、55.16%、83.86%;最大荧光(Fm)和光化学猝灭系数(qP)呈先上升后下降趋势,Fm和qP 分别在胁迫3 d和胁迫6 d时达到最大值,而后快速降低,与高温前相比,胁迫15 d 后,V 形架、飞鸟形架和H 形架的Fm分别下降了1.51%、14.16%、8.06%,qP 分别下降了34.16%、32.72%、55.82%;最大光化学效率(Fv/Fm)、潜在光化学效率(Fv/Fo)和实际光化学效率(ΦPSII)在胁迫前期值较平稳,Fv/Fm和Fv/Fo在胁迫6 d时快速降低,ΦPSII在胁迫9 d时快速降低,到胁迫15 d时,飞鸟形架的Fv/Fm(0.76)、Fv/Fo(3.33)和ΦPSII(0.37)最高,H 形架的Fv/Fm(0.62)、Fv/Fo(1.78)和ΦPSII(0.19)最低;电子传递速率(ETR)在高温前至胁迫6 d呈小幅降低再小幅上升的趋势,胁迫6 d后呈急剧下降趋势,与高温前相比,胁迫15 d后,V形架、飞鸟形架和H 形架分别降低42.04%、33.86%、65.92%。在高温胁迫期间H 形架的各荧光参数变化幅度较大,导致H形架的Fo和NPQ明显高于V形架和飞鸟形架,其余荧光参数则明显低于V 形架和飞鸟形架;V 形架除了NPQ 明显高于飞鸟形架外,其余荧光参数两者无显著差异。
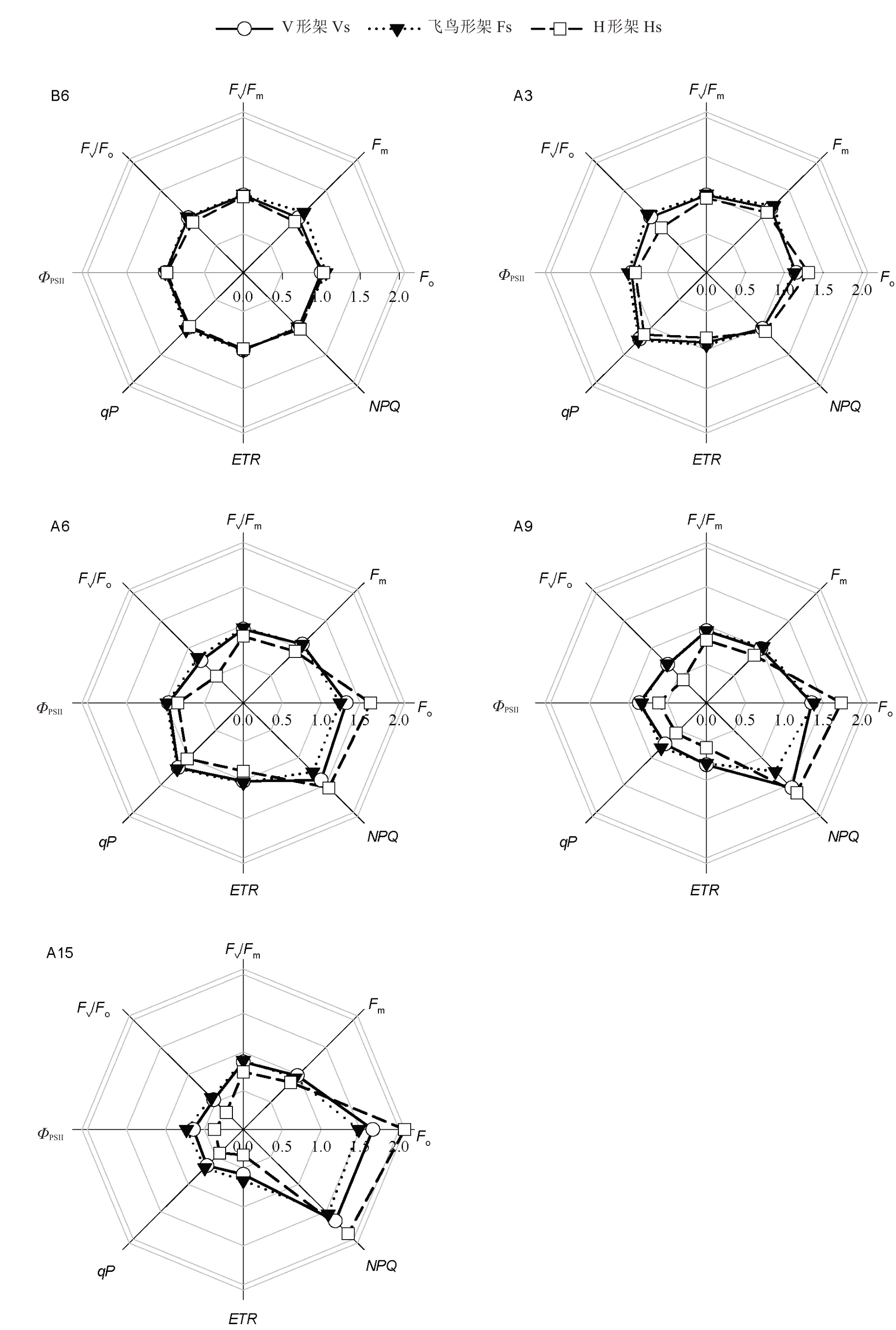
图7 高温胁迫对葡萄叶片叶绿素荧光参数的影响
Fig.7 Effects of high-temperature stress on the chlorophyll fluorescence parameters of grape leaves
2.6 高温对葡萄叶片抗逆基因表达的影响
2.6.1 热激蛋白HSP基因的表达分析 如图8,3种架形葡萄叶片的VvHSP17.9 和VvHSP90 相对表达量呈上升趋势,其中VvHSP17.9在高温胁迫6 d至胁迫15 d 时表达量明显上调,此时飞鸟形架VvHSP17.9 的表达量较高,H 形架次之,V 形架较低;胁迫期间V 形架和H 形架VvHSP90 表达量仅略微上升,而飞鸟形架在胁迫15 d 时显著上调了VvHSP90的表达量。3 种架形的VvHSP22、VvHSP70、VvHSP101表达量变化趋势较为一致,呈先上升后下降,VvHSP70、VvHSP101 表达量在胁迫3~6 d 较高,而VvHSP22 表达量在胁迫9~15 d 时较高;在大部分采样时间点,飞鸟形架VvHSP22、VvHSP70 和VvHSP101 的表达量最高,其中V 形架VvHSP22 和VvHSP101 表达量高于H 形架,而VvHSP70 表达量低于H 形架。飞鸟形架和H 形架的VvHSP100 先上调后下降,分别在胁迫3 d 和胁迫6 d 时达到峰值,而后表达量急剧下调,V 形架的VvHSP100 在胁迫9 d 后显著上调,并在胁迫15 d时达到最高值,总体而言V形架VvHSP100 表达量高于飞鸟形架,而H 形架表达量最低。

图8 高温胁迫对葡萄叶片热激蛋白基因表达的影响
Fig.8 Effects of high-temperature stress on the expression of HSP genes in grape leaves
2.6.2 热激转录因子HSF 基因及GLOS1 的表达分析 进一步对葡萄叶片中3个重要的热激转录因子基因(VvHSFA1、VvHSFA2、VvHSFB1)和GLOS1 进行表达分析,结果如图9。随高温胁迫时间的延长,3种架形葡萄的VvHSFA1 和GLOS1 相对表达量总体呈上升趋势,而VvHSFA2、VvHSFB1 呈先上升后降低趋势,VvHSFB1在胁迫3 d至胁迫9 d时表达量较高,而VvHSFA2 在胁迫6 d 至胁迫15 d 时表达量较高。总体而言,飞鸟形架对上述基因表达量上调的促进作用最显著,V形架次之,H形架上述基因表达量相对较低,且H形架GLOS1在整个胁迫期间表达量均较低。
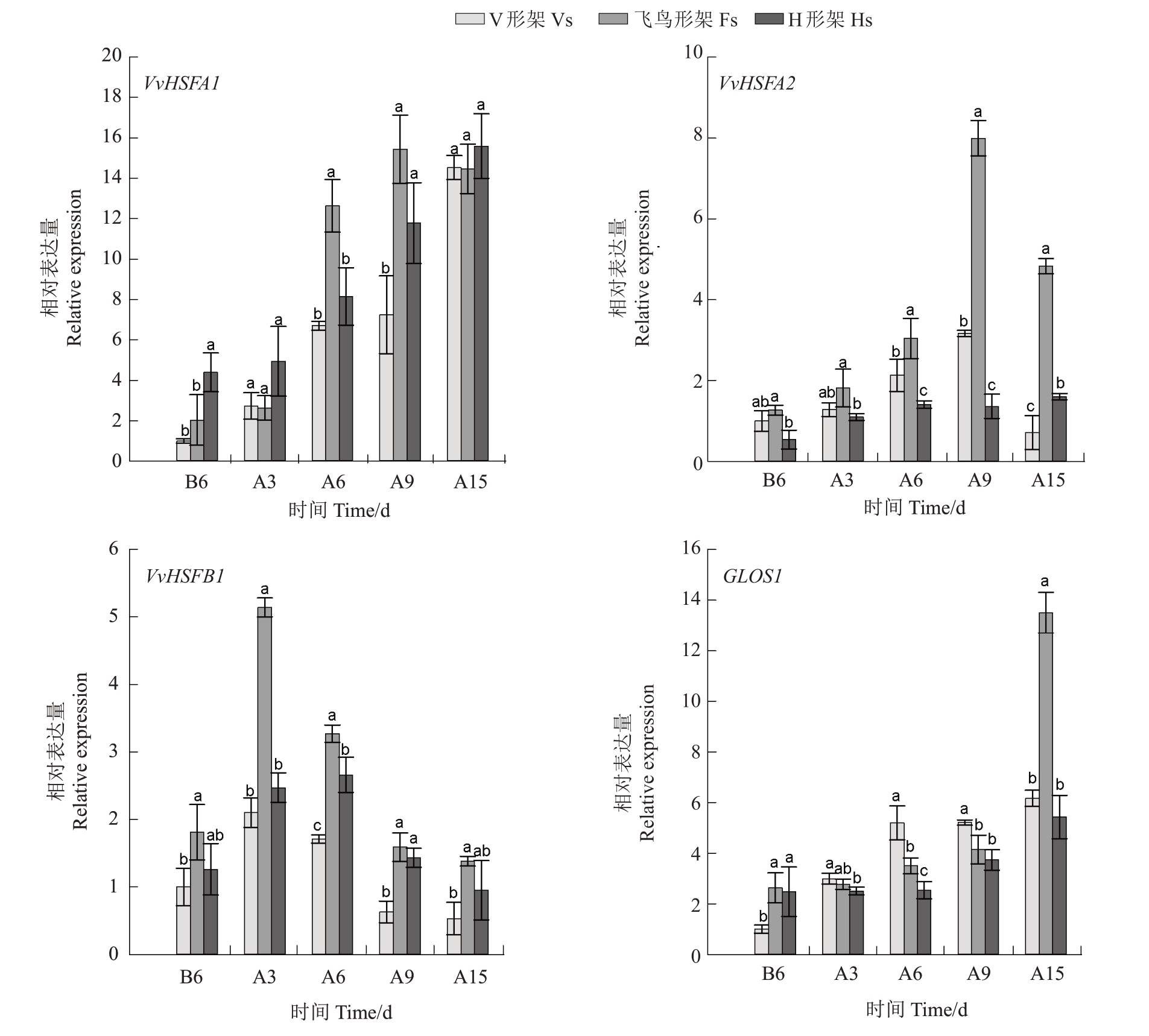
图9 高温胁迫对葡萄叶片热激转录因子基因及GLOS1 表达的影响
Fig.9 Effects of high-temperature stress on the expression of HSF genes and GLOS1 in grape leaves
2.7 光合色素、光合荧光参数与抗逆基因间的相关性分析
选取3 种架形葡萄夏季高温发生前6 d 及胁迫发生后3、6、9、15 d的共15组数据,进行叶片光合色素、光合荧光参数与抗逆基因表达量间的相关性分析。如图10 所示,在光合色素、光合参数和叶绿素荧光参数之间,Pn 与3 种光合色素(Chl a、Chl b、Car)、6种叶绿素荧光参数(Fm、Fv/Fm、Fv/Fo、ΦPSII、qP、ETR)之间均呈显著或极显著正相关,但与Fo、NPQ之间呈显著负相关。在10 个抗逆基因中,VvHSP17.9、VvHSP90、VvHSFA1、GOLS1 4个抗逆基因与光合色素、光合参数和叶绿素荧光参数(除与Fo、NPQ 之间呈极显著正相关)之间呈负相关,特别是与Gs、Fv/Fm、Fv/Fo、ΦPSII、ETR之间呈显著或极显著负相关;而VvHSP70、VvHSP101,VvHSFB1 3 个抗逆基因与光合色素、光合参数和叶绿素荧光参数(除与Fo、NPQ 之间呈负相关)之间呈正相关,特别是与Chl a、Chl b、Car、Pn、Gs、Ci、Fm、qP 之间呈显著或极显著正相关。VvHSP22、VvHSP100、VvHSFA2 与光合色素、光合参数和叶绿素荧光参数之间的相关总体不显著。
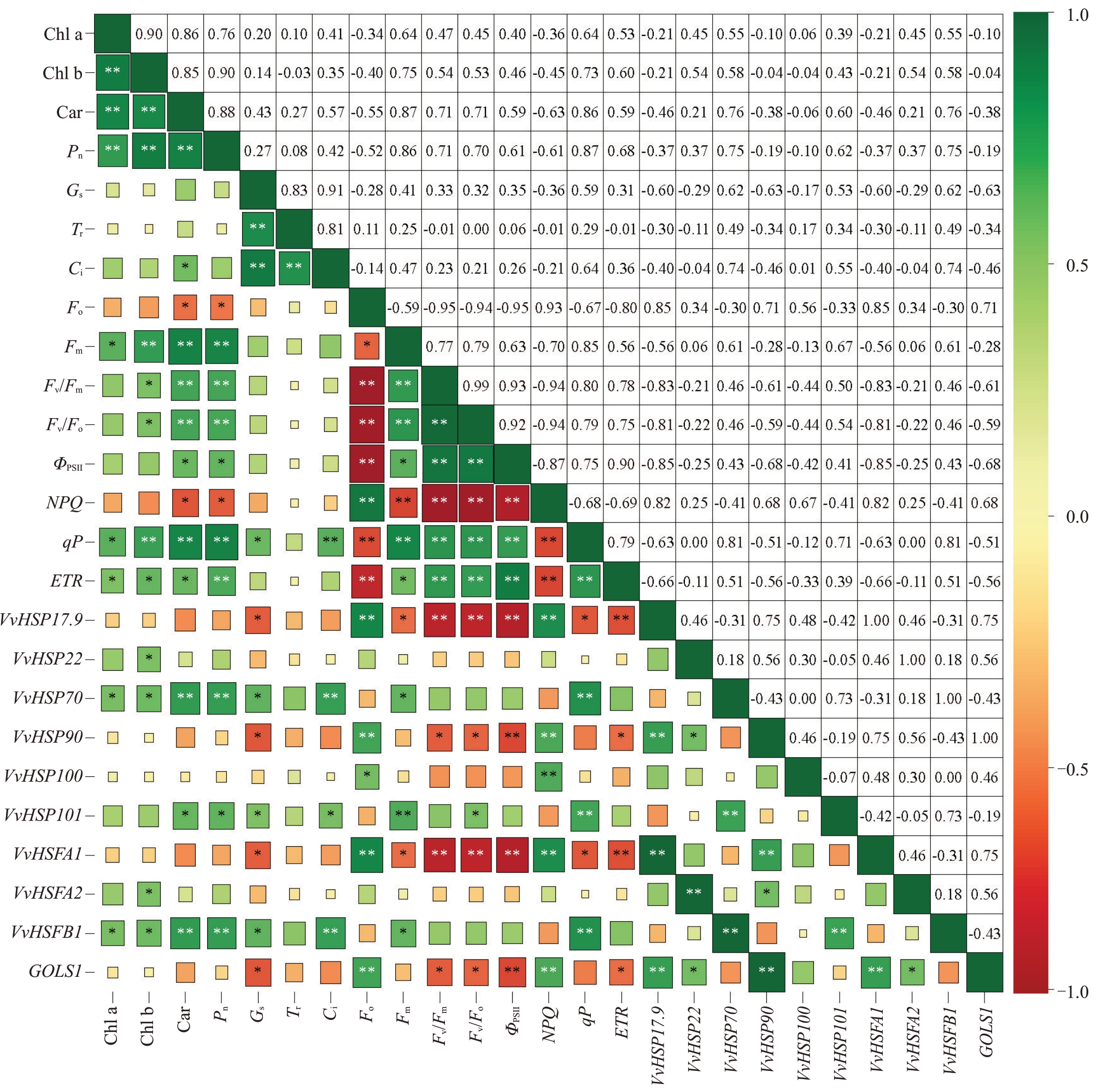
图10 光合色素、光合荧光参数与抗逆基因间的相关性分析
Fig.10 Correlation analysis between photosynthetic pigments,photosynthetic fluorescence parameters and resistance genes
“*”和“**”分别表示在p<0.05 和p<0.01 水平的相关性显著和极显著。
“*”and“**”indicate significant and highly significant correlations at the p<0.05 and p<0.01 levels.
3 讨 论
前人研究发现,在葡萄成熟期V 形架降低温度提高湿度效果优于H形架[6],研究表明,在夏季高温期间,飞鸟形架和V 形架叶幕极限温度出现的次数和程度明显低于H形架,飞鸟形架叶幕湿度较高,V形架次之,H 形架最低,与前人研究结果相似,表明高温天气下飞鸟形架和V形架叶幕的降温保湿效果较好,可避免高温干旱双重胁迫的发生,其中飞鸟形架优势更为显著。实际生产中,H 形架葡萄由于结果部位较高且叶幕呈水平分布,其园内通风效果优于飞鸟形架及V 形架,而本研究中H 形架葡萄叶幕温度最高,其原因可能是H 形架葡萄叶幕与棚顶距离较近,接受的光照度高,导致叶幕温度升高较快;另外H 形架叶幕透光性较强,致使悬挂在新梢中部的温湿度计易受阳光直射,最终导致H 形架叶幕所测定的温度较高。飞鸟形架葡萄枝条开张角度较大且主干较高,果园内通风透气性优于V形架葡萄,因此飞鸟形架叶幕温度最低。
叶片的栅栏组织不仅可以使叶片含有较多叶绿素,还可以保护叶肉细胞免受灼伤[9,12]。已有研究表明,植物叶片栅栏组织厚度与耐热性呈正相关,海绵组织厚度与耐热性呈负相关[28]。试验研究中,高温前飞鸟形架和V形架叶片栅栏组织厚度显著高于H形架,与前人在京蜜葡萄上的研究结果一致[29],说明飞鸟形架及V形架葡萄耐热性高于H形架葡萄。高温胁迫可改变植物叶肉细胞结构,使栅栏组织和海绵组织厚度增加,且耐热性差的品种其变化幅度较大[12]。在本研究中,高温胁迫15 d后,飞鸟形架叶片栅栏组织及海绵组织厚度变化不明显,V 形架叶片海绵组织显著增加,H 形架叶片栅栏组织及海绵组织厚度明显增加,进一步说明H 形架葡萄耐热性弱于飞鸟形架和V 形架葡萄,其叶片受高温伤害较严重。
高温胁迫会抑制光合色素合成,同时胁迫产生的活性氧会加速叶绿素降解,导致叶片光合色素含量降低,光合速率降低[26]。试验研究发现,3 种架形葡萄叶片的Chl a、Chl b、Car、Chl 含量及Pn、Gs、Tr、Ci随高温胁迫时间的延长呈先上升后降低的趋势,这与前人在多个葡萄品种[26,30]及灰岩皱报春[31]中的研究结果一致,表明短时间的田间高温会使葡萄产生一定的抵抗来应对高温胁迫,具体表现为叶片气孔开度增大以促进蒸腾散热、降低叶温,同时叶肉细胞内CO2含量与光合色素含量增加,使叶片光合速率升高。但随着高温胁迫时间的延长,为避免叶片水分散失过多,叶片气孔大量关闭,叶肉细胞内Ci降低,同时叶绿素含量减少,导致Pn大幅降低[30];另外高温下叶片衰老加速也将导致Pn降低[32]。高温胁迫下,引起植物Pn下降的原因主要分为气孔限制和非气孔限制2种,其中气孔限制因素强调叶片Gs降低,导致CO2供给不足从而限制叶片光合作用,具体表现为Gs、Ci、Pn同时降低;而非气孔限制因素偏重高温破坏光合机构、抑制光合作用关键酶活性,进而对光合作用产生影响,非气孔限制因素引起的下降表现为Gs 下降的同时Ci 升高[30,33]。本研究结果表明,胁迫后期3 种架形葡萄叶片Pn降低的同时Gs、Tr、Ci均显著下降,说明Pn降低主要由气孔限制因素导致,类似的研究结果在南丰蜜橘[9]和白桦[34]中也得到过证实。值得注意的是,笔者在本研究中通过相关性分析发现,Pn与光合色素含量、光化学效率之间呈显著正相关,而与Gs、Tr、Ci之间无显著相关性,说明光合色素含量降低及光合机构受损等的非气孔限制因素也导致了葡萄Pn降低,但具体机制有待进一步研究。前人研究表明,夏季飞鸟形架[7]、V 形架[29]葡萄叶片叶绿素含量、光合速率高于水平棚架。类似地,在本研究中,飞鸟形架葡萄叶片Chl、Car含量及Pn最高、V形架次之,H形架最低,且飞鸟形架葡萄叶片Chl a、Chl b 达到最大值后下降时间晚于V 形架和H 形架,表明飞鸟形架葡萄耐热能力较强,在受到高温胁迫时可维持较高水平的光合色素含量,使植株具有较强的光合能力。
叶绿素荧光参数中Fo可以反映PSⅡ反应中心状态,Fo增加则表明PSⅡ反应中心失活[30]。在本研究中,随着高温胁迫时间增加,3种架形葡萄叶片Fo逐渐上升,说明高温下葡萄PSⅡ反应中心逐渐失活。Fm与光合机构中天线色素含量成正比[35];Fv/Fm和Fv/Fo是鉴定植物光合机构是否受高温伤害的重要指标,可衡量反应中心的光化学效率[30];ΦPSII反映叶片在当前生长环境中的实际光能转换效率,qP反映PSⅡ天线色素吸收的光能用于光合作用的份额,NPQ反映植物耗散过剩光能为热的能力,也就是光保护能力,ETR 反映实际光照度下光合电子链电子传递速率[36]。在本研究中,胁迫前期3 种架形葡萄叶片Fm增大,表明天线色素含量增加,这与本文中胁迫前期3种架形葡萄叶片叶绿素含量增加的研究结果一致,此时叶片NPQ增加,而Fv/Fm、Fv/Fo、ΦPSII、ETR 保持稳定,表明胁迫前期3 种架形葡萄叶片以热耗散方式耗散多余光能来保护光合机构,维持叶片光能转换效率的稳定,此时叶片PSⅡ反应中心失活或可逆,这与王虹[37]在红叶桃中的研究结果一致。胁迫6 d 后,3 种架形葡萄叶片NPQ 继续升高,但Fm、Fv/Fm、Fv/Fo、ΦPSII、qP、ETR陆续降低,说明长时间自然高温胁迫下葡萄叶片吸收的过量光能不能以热能形式完全耗散,过度光能造成光合机构中天线色素含量降低,光合电子传递速率减缓,此时3种架形葡萄叶片的光合机构已经受到高温伤害,吸收的光能用于光合作用的份额减少,叶片光能转换效率降低,这与窦飞飞等[30]、张睿佳等[38]的研究结果一致。就供试的3 种葡萄架形而言,在相同高温胁迫下,飞鸟形架和V 形架葡萄叶片的Fm、Fv/Fm、Fv/Fo、ΦPSII、qP和ETR较H形架较高,Fo、NPQ较低,与H形架差异显著,而飞鸟形架和V形架差异不明显,表明飞鸟形架和V 形架葡萄叶片在高温下PSⅡ反应中心较为稳定,耐热性较强。
通过对3 种架形葡萄叶片的6 个HSP、3 个HSF和GLOS1共10个基因检测,结果显示高温胁迫显著上调了这些基因的表达,说明VvHSP 和VvHSF、GLOS1 基因可以在短期内迅速响应以提高葡萄抗热能力,这与前人在牡丹[39]、葡萄[26-27]上的研究结果一致。HSP70基因在转基因植物中过表达可促进植株耐热性提高[40],HSP101蛋白在高温下能迅速诱导启动圆锥南芥的光化学修复,使圆锥南芥在高温胁迫中仍具有较高的光化学效率[41]。笔者在本研究中通过相关性分析发现,VvHSP70、VvHSP101,VvHSFB1 3 个抗逆基因与Chl a、Chl b、Car、Pn、Gs、Ci、Fm、qP 之间呈显著正相关,也表明VvHSP70、VvHSP101,VvHSFB1 3个抗逆基因与葡萄耐热性呈正相关,可增强高温下葡萄光合系统的稳定性。VvHSP17.9、VvHSP90、VvHSFA1、GOLS1 4个抗逆基因与Fv/Fm、Fv/Fo、ΦPSII、ETR之间呈显著负相关,主要原因是胁迫后期上述4 个基因的表达量仍在持续增加,但葡萄PSⅡ反应受损程度逐渐加深,说明葡萄耐热性受多种基因共同调控,VvHSP17.9、VvHSP90、VvHSFA1、GOLS1 4 个抗逆基因并不起主导作用。此外,笔者在本研究中还发现VvHSP22、VvHSP100、VvHSFA2 与光合色素、光合参数和叶绿素荧光参数之间的相关性总体不显著,但与VvHSP90、GOLS1之间存在显著正相关,VvHSP22、VvHSP100、VvHSFA2 可能通过其他抗逆基因间接调控葡萄的耐热性。总体而言,高温胁迫下飞鸟形架葡萄对上述除VvHSP100 外的9 个基因表达量的上调促进作用最显著,其中V 形架葡萄VvHSP22、VvHSP101 和VvHSFA1、VvHSFA2、VvHSFB1、GLOS1 基因表达量明显高于H 形架,而VvHSP17.9 和VvHSP70 表达量明显低于H 形架,表明飞鸟形架葡萄抗高温胁迫能力较强,更能适应高温环境,H 形架葡萄耐热性较差。
4 结 论
通过叶片组织解剖结构、光合荧光参数及热激基因表达量的观测,夏季连续自然高温(>38 ℃)胁迫下,V形架、飞鸟形架和H形架阳光玫瑰葡萄叶片厚度增加,PSⅡ反应中心受损,光合色素含量和光合速率随高温胁迫时间延长先上升后降低,气孔闭合是引起葡萄光合速率最终下降的主要因素。飞鸟形架阳光玫瑰葡萄叶幕温度较低、叶肉组织紧实、光合色素含量高、热激基因(VvHSP、VvHSP、GLOS1)表达量更高、PSⅡ反应中心较为稳定是其耐热性较强的主要原因。
[1] 宋献策,王世平,顾巧英,蔡红玲,张伟达,曹伟婷.阳光玫瑰葡萄在上海的引种表现及优质栽培技术[J].中外葡萄与葡萄酒,2015(4):48-51.SONG Xiance,WANG Shiping,GU Qiaoying,CAIHongling,ZHANG Weida,CAO Weiting. Introduction performance and high-quality cultivation techniques of Shine Muscat grape in Shanghai[J].Sino-Overseas Grapevine&Wine,2015(4):48-51.
[2] 王荣,雷舒敏,杜肇轩,岳领齐,张雪枫,杨国顺,王美军,谭君,许延帅.南方六个地区不同成熟期‘阳光玫瑰’葡萄果实品质评价[J/OL].分子植物育种,2024:1-15(2024-05-31)[2024-07-11].https://kns.cnki.net/kcms/detail/46.1068.S.20240530.1707.006.html.WANG Rong,LEIShumin,DU Zhaoxuan,YUE Lingqi,ZHANG Xuefeng,YANG Guoshun,WANG Meijun,TAN Jun,XU Yanshuai.‘Shine Muscat’grape fruit quality evaluation in different mature periods of six areas in the South of China[J/OL].Molecular Plant Breeding,2024:1-15(2024-05-31)[2024-07-11].https://kns.cnki.net/kcms/detail/46.1068.S.20240530.1707.006.html.
[3] PAPALEXIOU S M,AGHAKOUCHAK A,TRENBERTH K E,FOUFOULA-GEORGIOU E. Global,regional,and megacity trends in the highest temperature of the year:Diagnostics and evidence for accelerating trends[J].Earth’s Future,2018,6(1):71-79.
[4] 贾子康.中国南方夏季极端高温和地表气温的多时间尺度变化及预报研究[D].兰州:兰州大学,2023.JIA Zikang. Multi-timescale variations and forecasts of summer extreme high-temperature and surface air temperature in Southern China[D].Lanzhou:Lanzhou University,2023.
[5] 江莉,牛先前,王鹏博,陈婷,刘鑫铭,谢倩,陈清西,雷龑.开窗对设施大棚葡萄植株光合特性和果实品质的影响[J].西北农业学报,2023,32(12):1933-1942.JIANG Li,NIU Xianqian,WANG Pengbo,CHEN Ting,LIU Xinming,XIE Qian,CHEN Qingxi,LEIYan. Effects of windows opening on photosynthetic characteristics and fruit quality of grape plants in greenhouses[J].Acta Agriculturae Boreali-occidentalis Sinica,2023,32(12):1933-1942.
[6] 郭伟琛.不同葡萄品种果实质地评价及‘阳光玫瑰’两种不同架型比较研究[D].杨凌:西北农林科技大学,2023.GUO Weichen.Evaluation of fruit texture of different grape varieties and comparative study on two different trellis system of‘Shine-Muscat’[D]. Yangling:Northwest A & F University,2023.
[7] 张洁.棚架不同叶幕类型对葡萄冠层结构、光截获及光合的数字化模拟研究[D].石河子:石河子大学,2020.ZHANG Jie. Impacts of canopy shapes on canopy structure,light interception and photosynthetic capacities for pergola trellis grapevines based on digitizing and modelling[D]. Shihezi:Shihezi University,2020.
[8] 郑婷,吴江,刘凡启,许瀛之,李生保,房经贵.葡萄种植架式及其应用[J].中外葡萄与葡萄酒,2021(2):40-45.ZHENG Ting,WU Jiang,LIU Fanqi,XU Yingzhi,LIShengbao,FANG Jinggui. Introduction and application of training system on grapevine[J].Sino-Overseas Grapevine&Wine,2021(2):40-45.
[9] 徐超,杨再强,王雨亭,刘布春,杨惠栋,汤雨晴,胡新龙,胡钟东. 南丰蜜橘对高温热害的生理响应及耐热性评价模型构建[J].果树学报,2023,40(12):2638-2651.XU Chao,YANG Zaiqiang,WANG Yuting,LIU Buchun,YANG Huidong,TANG Yuqing,HU Xinlong,HU Zhongdong.Physiological response to high temperature and heat tolerance evaluation of different lines in Nanfeng tangerine[J]. Journal of Fruit Science,2023,40(12):2638-2651.
[10] GU L H. Comment on“Climate and management contributions to recent trends in U. S. agricultural yields”[J]. Science,2003,300(5625):1505.
[11] HAVAUX M,TARDY F. Temperature-dependent adjustment of the thermal stability of photosystem II.In vivo:Possible involvement of xanthophyll-cycle pigments[J]. Planta,1996,198(3):324-333.
[12] 查倩,奚晓军,和雅妮,蒋爱丽.田间高温对不同葡萄叶片组织结构的影响[J].中国农学通报,2019,35(13):74-77.ZHA Qian,XIXiaojun,HE Yani,JIANG Aili.High temperature in field:Effect on the leaf tissue structure of grape varieties[J].Chinese Agricultural Science Bulletin,2019,35(13):74-77.
[13] 孟祥丽.五种不同甜樱桃砧木旱涝和高温胁迫适应性评价[D].金华:浙江师范大学,2011.MENG Xiangli. Comprehensive evaluation of the tolerance of sweet cherry drafted on five different rootstocks under drought,waterlogging and high-temperature stress by subordinate function values analysis[D]. Jinhua:Zhejiang Normal University,2011.
[14] 贺安娜,林文强,姚奕,谭晓利.不同温度处理对虎耳草叶片气体交换及叶肉结构的影响[J].植物研究,2012,32(4):410-414.HE Anna,LIN Wenqiang,YAO Yi,TAN Xiaoli. Gas exchange and leaf structure of Saxifraga stolonifera Curt. under different temperatures[J]. Bulletin of Botanical Research,2012,32(4):410-414.
[15] XU Y,ZHAN C Y,HUANG B R.Heat shock proteins in association with heat tolerance in grasses[J]. International Journal of Proteomics,2011:529648.
[16] 王涛,田雪瑶,谢寅峰,张往祥.植物耐热性研究进展[J].云南农业大学学报(自然科学),2013,28(5):719-726.WANG Tao,TIAN Xueyao,XIE Yinfeng,ZHANG Wangxiang.Research advance on heat-stress tolerance in plants[J]. Journal of Yunnan Agricultural University (Natural Science),2013,28(5):719-726.
[17] HELM K W,LAFAYETTE P R,NAGAO R T,KEY J L,VIERLING E. Localization of small heat shock proteins to the higher plant endomembrane system[J]. Molecular and Cellular Biology,1993,13(1):238-247.
[18] SULEMAN P,REDHA A,AFZAL M,AL-HASAN R.Temperature-induced changes of malondialdehyde,heat-shock proteins in relation to chlorophyll fluorescence and photosynthesis in Conocarpus lancifolius(Engl.)[J].Acta Physiologiae Plantarum,2013,35(4):1223-1231.
[19] 刘冬峰.砂梨对高温胁迫的响应及耐热机理研究[D].杭州:浙江大学,2014.LIU Dongfeng. Studies on the pesponse of sand pear to hightemperature and heat-tolerance mechanism[D]. Hangzhou:Zhejiang University,2014.
[20] OGAWA D,YAMAGUCHIK,NISHIUCHIT. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth[J].Journal of Experimental Botany,2007,58(12):3373-3383.
[21] PILLET J,EGERT A,PIERIP,LECOURIEUX F,KAPPEL C,CHARON J,GOMÈS E,KELLER F,DELROT S,LECOURIEUX D. VvGOLS1 and VvHsfA2 are involved in the heat stress responses in grapevine berries[J]. Plant & Cell Physiology,2012,53(10):1776-1792.
[22] 范志霞,陈越悦,付荷玲.成都地区10 种园林灌木叶片结构与抗旱性关系研究[J].植物科学学报,2019,37(1):70-78.FAN Zhixia,CHEN Yueyue,FU Heling.Study on drought resistance and leaf structure in 10 species of garden shrubs in Chengdu[J].Plant Science Journal,2019,37(1):70-78.
[23] 李合生.植物生理生化实验原理和技术[M].北京:高等教育出版社,2000.LIHesheng. Principles and techniques of plant physiological biochemical experiment[M]. Beijing:Higher Education Press,2000.
[24] LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔcT Method[J].Methods,2001,25(4):402-408.
[25] 杨娅倩.外源亚精胺对高温胁迫下葡萄幼苗生理生化特性的影响[D].泰安:山东农业大学,2020.YANG Yaqian. Effects of exogenous spermidine on physiological and biochemical characteristics of grape seedlings under high temperature stress[D]. Taian:Shandong Agricultural University,2020.
[26] 肖芳. 高温胁迫对苗期红提葡萄生理及基因表达特性的影响[D].南京:南京信息工程大学,2018.XIAO Fang. Effects of high temperature stress on physiological and gene expression characteristics of grapevine (Vitis vinifera L. Hongti) during seedling stage[D]. Nanjing:Nanjing University of Information Science&Technology,2018.
[27] 查倩,奚晓军,蒋爱丽,田益华.高温胁迫对葡萄高温相关基因和蛋白表达的影响[J].中国农业科学,2017,50(9):1674-1683.ZHA Qian,XIXiaojun,JIANG Aili,TIAN Yihua. Influence of heat stress on the expression of related genes and proteins in grapevines[J]. Scientia Agricultura Sinica,2017,50(9):1674-1683.
[28] 申惠翡,赵冰,徐静静.15 个杜鹃花品种叶片解剖结构与植株耐热性的关系[J].应用生态学报,2016,27(12):3895-3904.SHEN Huifei,ZHAO Bing,XU Jingjing. Relationship between leaf anatomical structure and heat resistance of 15 Rhododendron cultivars[J]. Chinese Journal of Applied Ecology,2016,27(12):3895-3904.
[29] 史祥宾,刘凤之,程存刚,王孝娣,王宝亮,郑晓翠,王海波.不同叶幕形对设施葡萄叶幕微环境、叶片质量及果实品质的影响[J].应用生态学报,2015,26(12):3730-3736.SHIXiangbin,LIU Fengzhi,CHENG Cungang,WANG Xiaodi,WANG Baoliang,ZHENG Xiaocui,WANG Haibo. Effects of canopy shapes of grape on canopy microenvironment,leaf and fruit quality in greenhouse[J].Chinese Journal of Applied Ecology,2015,26(12):3730-3736.
[30] 窦飞飞,张利鹏,王永康,于坤,刘怀锋.高温胁迫对不同葡萄品种光合作用和基因表达的影响[J].果树学报,2021,38(6):871-883.DOU Feifei,ZHANG Lipeng,WANG Yongkang,YU Kun,LIU Huaifeng. Effects of high temperature stress on photosynthesis and gene expression of different grape cultivars[J]. Journal of Fruit Science,2021,38(6):871-883.
[31] 张路,张启翔.高温胁迫对灰岩皱叶报春生理指标的影响[J].西南农业学报,2011,24(5):1728-1732.ZHANG Lu,ZHANG Qixiang. Effects of high temperature stress on physiological indicators of Primula forrestii[J]. Southwest China Journal of Agricultural Sciences,2011,24(5):1728-1732.
[32] 雷虎,江晓东,张建取.高温高湿环境下调亏灌溉对番茄叶片光合和衰老特性的影响[J].中国瓜菜,2023,36(3):58-63.LEIHu,JIANG Xiaodong,ZHANG Jianqu.Effects of regulated deficit irrigation on photosynthetic and senescence characteristics of tomato leaves under high temperature and high relative humidity environment in summer[J]. China Cucurbits and Vegetables,2023,36(3):58-63.
[33] 黄显雅,陈格,黄永才,桂杰,黄诚梅,鞠莹,盛静文,蒋萍,杨柳.持续高温条件对4 个百香果品种开花坐果习性及光合的影响[J/OL]. 果树学报,2024:1-13(2024-05-16)[2024-07-11].https://doi.org/10.13925/j.cnki.gsxb.20240144.HUANG Xianya,CHEN Ge,HUANG Yongcai,GUI Jie,HUANG Chengmei,JU Ying,SHENG Jingwen,JIANG Ping,YANG Liu. Effects of continuous high-temperature on flowering and fruit-setting habits and photosynthesis of four passion fruit varieties[J/OL]. Journal of Fruit Science,2024:1-13(2024-05- 16) [2024- 07- 11]. https://doi.org/10.13925/j.cnki.gsxb.20240144.
[34] 胡荣云.沈阳地区高温干旱和强光胁迫条件下白桦光合生理研究[D].沈阳:沈阳农业大学,2019.HU Rongyun. Photosynthetic physiology of Betula platyphylla under high temperature,drought and high light stress in Shenyang area[D]. Shenyang:Shenyang Agricultural University,2019.
[35] CHEN L S,LIP M,CHENG L L. Effects of high temperature coupled with high light on the balance between photooxidation and photoprotection in the Sun-exposed peel of apple[J].Planta,2008,228(5):745-756.
[36] 曾宝珍,成永娟,车莉莉,杨娟博,卢世雄,梁国平,吴志国,赵毅,毛娟.纳米零价铁对武威产区黑比诺葡萄新梢和叶片生长及光合特性的影响[J].果树学报,2024,41(3):481-493.ZENG Baozhen,CHENG Yongjuan,CHE Lili,YANG Juanbo,LU Shixiong,LIANG Guoping,WU Zhiguo,ZHAO Yi,MAO Juan. Effects of nano zero-valent iron on the growth and photosynthetic characteristics of the new shoots and leaves of Pinot Noir in Wuwei production area[J]. Journal of Fruit Science,2024,41(3):481-493.
[37] 王虹.夏秋季节干旱胁迫对红叶桃光合特性及相关生理指标的影响[D].南京:南京农业大学,2008.WANG Hong. Study on the photosynthesis characteristics and relative physiological index of red-leaf peach under drought stress in summer and autumn[D]. Nanjing:Nanjing Agricultural University,2008.
[38] 张睿佳,李瑛,虞秀明,娄玉穗,许文平,张才喜,赵丽萍,王世平.高温胁迫与外源油菜素内酯对‘巨峰’葡萄叶片光合生理和果实品质的影响[J].果树学报,2015,32(4):590-596.ZHANG Ruijia,LIYing,YU Xiuming,LOU Yusui,XU Wenping,ZHANG Caixi,ZHAO Liping,WANG Shiping. Effects of heat stress and exogenous brassinolide on photosynthesis of leaves and berry quality of‘Kyoho’grapevine[J]. Journal of Fruit Science,2015,32(4):590-596.
[39] 郝力慧,董彬,朱绍华,马进.牡丹响应高温胁迫的转录组分析及PsHSP 基因表达[J].浙江农林大学学报,2021,38(4):802-811.HAO Lihui,DONG Bin,ZHU Shaohua,MA Jin.Transcriptome analysis and PsHSP gene expression of Paeonia suffruticosa in response to high temperature stress[J]. Journal of Zhejiang A&F University,2021,38(4):802-811.
[40] SAKUMA Y,MARUYAMA K,QIN F,OSAKABE Y,SHINOZAKIK,YAMAGUCHI-SHINOZAKIK. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression[J]. Proceedings of the National Academy of Sciences of the United States of America,2006,103(49):18822-18827.
[41] 唐婷,郑国伟,李唯奇.高山植物圆锥南芥的光合系统耐热性及其修复机制[J].植物分类与资源学报,2015,37(1):46-54.TANG Ting,ZHENG Guowei,LIWeiqi. The thermotolerance and repair mechanism of photosystem in alpine plant Arabis paniculata(Cruciferae)[J].Plant Diversity,2015,37(1):46-54.