猕猴桃褐斑病是由多主棒孢菌(Corynespora cassiicola)侵染引起的一种叶部病害[1-2],主要在果实膨大期至品质形成期危害叶片,发病初期在叶片上出现褐色小圆斑,中期典型症状表现为具有同心轮纹的褐色坏死斑,后期病斑扩展合并,最终导致叶片大面积坏死及早落,影响果实品质及产量,致使减产15%~50%[3-4]。近年来,随着高感品种红阳猕猴桃种植面积的扩增,猕猴桃褐斑病逐渐成为危害四川地区猕猴桃最为严重的真菌性病害,且局部区域暴发成灾,严重制约了猕猴桃产业的发展[5-6]。目前生产上对猕猴桃褐斑病的防治以化学防治为主,但未见登记用于防治该病害的化学药剂,同时代森锰锌、多菌灵、百菌清等广谱性杀菌剂被广泛使用,药剂防治的针对性不强,防治效果参差不齐,现生产上发现用于防治褐斑病的复配药剂如戊唑醇·肟菌酯、氯氟醚菌唑·吡唑醚菌酯及苯醚甲环唑·嘧菌酯等防治效果有逐年减弱的趋势[7-8]。
针对多主棒孢菌在其他寄主上引起的病害,前人已有一些相关的药剂防治研究。Vawdrey 等[9]通过人工接种多主棒孢菌后进行药剂处理,发现百菌清及吡唑醚菌酯对病情抑制较其他药剂效果明显。祁之秋等[10]检测出苯醚甲环唑、咪鲜胺、代森锰锌及嘧霉胺对黄瓜上多主棒孢病菌菌丝生长具有强烈抑制作用,而烯肟菌酯、福美双、代森锰锌、烯酰吗啉、百菌清和多菌灵则对孢子萌发抑制作用更好。番华彩等[11]对来自香蕉的多主棒孢菌进行了7种杀菌剂的室内毒力测定,其中丙环唑对病原菌抑制效果较好,其次为多抗霉素和苯醚甲环唑·丙环唑。Vishwakarma等[12]对来自大豆的多主棒孢菌的室内毒力测定表明,当杀菌剂质量浓度为50µg·mL-1时,咪鲜胺·戊唑醇、嘧菌酯·戊唑醇·咪鲜胺、吡唑醚菌酯·氯氟醚菌唑·氟唑菌酰胺、多菌灵·代森锰锌和己唑醇可完全抑制病菌菌丝生长。崔丽红等[13]通过田间药效测定筛选出40%苯醚甲环唑·咪鲜胺水乳剂对猕猴桃褐斑病防治效果较好。目前,国内外对于多主棒孢菌的药剂毒力相关研究多集中在黄瓜及大豆等寄主上,且现有的药剂筛选工作多停留在单独的室内毒力测定或田间药效方面,不具有系统性。关于猕猴桃上多主棒孢菌的药剂毒力测定及药剂筛选鲜见报道,生产上亟待筛选出高效低毒的化学药剂,针对猕猴桃褐斑病做到高效防治[14-15]。
笔者在本研究中拟对啶酰菌胺、吡唑醚菌酯、肟菌酯、戊唑醇等9 种原药进行菌丝生长及孢子萌发的室内毒力测定,对测定的毒力进行评价并结合生产用药选择合适的复配药剂,然后进行复配药剂室内菌丝生长的毒力测定及田间药效试验,旨在系统地筛选出防治猕猴桃褐斑病的高效药剂。
1 材料和方法
1.1 材料
1.1.1 供试菌株 从猕猴桃褐斑病叶片上以单孢分离法得到多主棒孢菌(C.cassiicola)[16],病样采集于四川省成都市邛崃市固驿镇(E 103°59′83″,N 30°36′91″),病菌使用两种方法保存备用,分别为斜面培养基4 ℃临时保存及甘油-80 ℃长期保存。
1.1.2 供试药剂 原药:95%氟硅唑(Flusilazole)、95%嘧菌酯(Azoxystrobin)、97%戊唑醇(Tebuconazole)、95%肟菌酯(Trifloxystrobin)、98%啶酰菌胺(Boscalid)、98%多菌灵(Carbendazim)、98%恶唑菌酮(Famoxadone)、95%苯醚甲环唑(Difenoconazole)、95%吡唑醚菌酯(Pyraclostrobin),以上原药均由四川国光农化股份有限公司提供。复配药剂见表1。
表1 试验药剂及来源
Table 1 Test fungicide and its source

药剂Fungicides 3 3 2 2 5 5 g g··L L--11 苯D i醚fen甲oc环on唑a·z o嘧le菌·A酯zo悬xy浮str剂obin SC 18.7%丙环唑·嘧菌酯悬浮剂18.7%Propiconazole·Azoxystrobin SC 4 4 0 0 0 0 g g··L L--11 氯C l氟ofi醚ur菌fen唑a·z o吡le唑·P醚yra菌cl酯ost悬ro浮bin剂SC 42.4%氟唑菌酰胺·吡唑醚菌酯悬浮剂42.4%Fluzoxammide·Pyraclostrobin SC 43%氟吡菌酰胺·肟菌酯悬浮剂43%Fluriramide·Trifloxystrobin SC 75%戊唑醇·肟菌酯水分散粒剂75%Tebuconazole·Trifloxystrobin WG 40%苯醚甲环唑·肟菌酯悬浮剂40%Difenoconazole·Trifloxystrobin SC来源Source先正达集团中国Syngenta Group China先正达集团中国Syngenta Group China巴斯夫(中国)有限公司BASF Co.,Ltd.(China)巴斯夫(中国)有限公司BASF Co.,Ltd.(China)拜耳作物科学(中国)有限公司Bayer Crop Science(China)Co.,Ltd.拜耳作物科学(中国)有限公司Bayer Crop Science(China)Co.,Ltd.成都科利隆生化有限公司Chengdu Kelilong Co.,Ltd.
1.1.3 培养基 马铃薯葡萄糖琼脂(PDA)培养基:马铃薯200 g,葡萄糖20 g,琼脂粉15 g,蒸馏水1000 mL。
清水琼脂(WA)培养基:琼脂粉15 g,蒸馏水1000 mL。
1.1.4 试验条件 田间试验猕猴桃的选择应满足同一栽培模式、管理措施、品种及树龄的条件;试验区域位于都江堰市胥家镇猕猴桃种植区,露天栽培模式的5年生红阳品种园(E 103°71′98″,N 31°02′37″)。
1.2 方法
1.2.1 不同原药对多主棒孢菌的菌丝抑制效果 采用菌丝生长速率法[17-18]测定9种原药对病菌的室内毒力,分别设置0.1、1.0、10.0、25.0、50.0、100.0µg·mL-1 6个原药质量浓度梯度,将原药用丙酮预溶,随后用0.01%的吐温80 稀释到所需要的不同质量浓度;含药培养基的制作为每个培养皿加入1 mL 稀释后的药剂与9 mL 的PDA 培养液(相当于在原有的梯度质量浓度下再次稀释10倍),在培养基冷却至45 ℃左右混匀制成(最终培养基中丙酮的含量不得超过0.1%)。对照平板以1 mL 0.01%吐温80 加9 mL PDA培养基混合配制而成。将活化7 d的菌落沿外缘用5 mm 打孔器切取菌饼放置于含药平板中央,放入25 ℃恒温培养箱遮光培养7 d 后,用十字交叉法测量菌落直径,计算抑菌率及EC50值,每个药剂质量浓度设置3次重复。
菌丝生长抑制率/%=[(对照菌落直径-处理菌落直径)/(对照菌落直径-菌饼直径)]×100。
1.2.2 不同原药对多主棒孢菌孢子萌发的抑制试验 将多主棒孢菌分生孢子用无菌水从平板上洗脱下来,浓度调至1×106个孢子·mL-1。将30µL 孢子悬浮液与30µL系列质量浓度的药剂溶液混合而成药剂处理液加入凹玻片中,最终药剂处理液质量浓度设置0.1、1.0、10.0、25.0、50.0、100.0 µg·mL-1 6 个梯度,对照处理液为30 µL 孢子悬浮液与30 µL 0.01%吐温水混合而成,每个处理设置3 次重复,将凹玻片置于25 ℃培养箱中培养6 h,分别统计孢子萌发率,计算孢子萌发抑制率及EC50值[17]。
孢子萌发率/%=(孢子萌发数⁄调查的孢子总数)×100。
孢子萌发相对抑制率/%=([对照孢子萌发率-处理孢子萌发率)⁄对照孢子萌发率]×100。
1.2.3 复配药剂对多主棒孢菌的菌丝抑制试验 结合生产上用于防治猕猴桃褐斑病的化学药剂与原药试验效果,选择不同复配药剂进行室内毒力测定,将不同复配药剂按照有效成分含量用无菌水稀释设置1.0、10.0、20.0、50.0、100.0µg·mL-1 5 个质量浓度梯度。含药培养基的制作参考1.2.1,对照培养基用1 mL 无菌水与9 mL PDA培养基混合配制而成。将活化7 d的菌落沿外缘用5 mm打孔器切取菌饼放置在含药平板中央,每个处理设置3次重复,25 ℃黑暗培养7 d 后,用十字交叉法测量菌落直径,计算抑菌率。
1.2.4 复配药剂对猕猴桃褐斑病的田间防治试验将筛选出的复配药剂应用于田间防治试验,在猕猴桃褐斑病的病害防治关键期间分3 次进行施药,施药时间为2020年7月11日、7月18日和7月25日,田间各复配药剂施药质量浓度均设置为100µg·mL-1。采取随机区组设计:以3 株果树为1 个小区,设置3次重复小区。对照组同期喷洒清水处理。于药前、第1 次药后7 d 和第3 次药后7 d 调查猕猴桃褐斑病的严重度,每株树分东南西北中5 个方位各固定调查10 枚叶片,病害严重度分级标准见表2。其中病情指数计算公式为:病情指数=∑(各级病叶数×病级数值)(/病叶总数×9)×100。防治效果计算公式为:防治效果/%=[1-(CK0×PT1)(/CK1×PT0)]×100(CK0对照组药前病情指数,PT1处理组药后病情指数,CK1对照组药后病情指数,PT0处理组药前病情指数)[19]。
表2 猕猴桃褐斑病严重度分级标准
Table 2 The classification standard for severity of brown leaf spot on kiwifruit
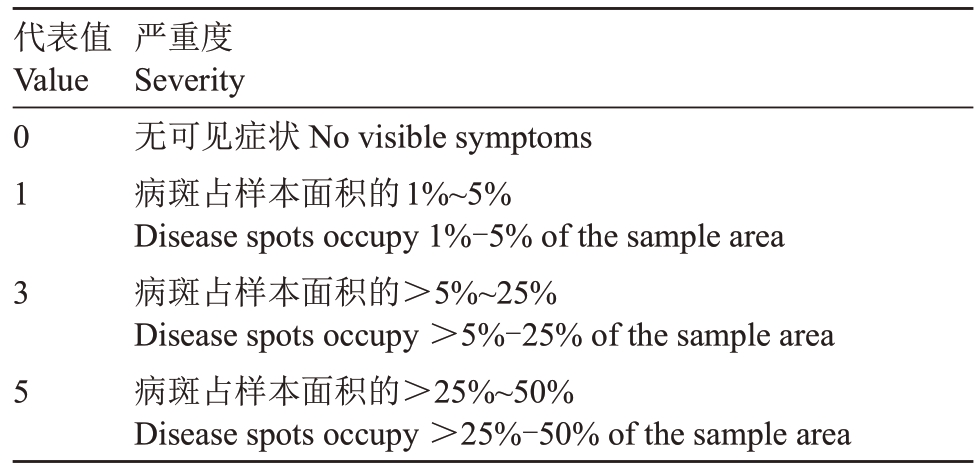
代表值Value 0 1 3 5严重度Severity无可见症状No visible symptoms病斑占样本面积的1%~5%Disease spots occupy 1%-5%of the sample area病斑占样本面积的>5%~25%Disease spots occupy >5%-25%of the sample area病斑占样本面积的>25%~50%Disease spots occupy >25%-50%of the sample area
1.2.5 田间大区应用试验 在四川省绵阳安州、都江堰、雅安芦山等猕猴桃种植区域进行药剂方案的推广应用,在种植区内选择典型果园进行药剂方案的施行。药剂方案选择的药剂为试验中筛选出的高效复配药剂,基于四川省地方标准DB51《猕猴桃褐斑病综合防控技术规程》,确定药剂施用时间及施药次数:在6月初褐斑病初发期开始施药,露天栽培条件下间隔7~10 d施药,连续施药4次,采果前20 d停药。在推广用药的果园设立对照组,不对其进行药剂防治,只进行正常的肥水管理。
1.3 数据分析
使用IBM SPSS Statistics 27软件对数据进行分析,以药剂质量浓度的对数值为横坐标,抑制率对应的概率值为纵坐标作图,得到毒力回归方程及有效抑制中浓度(EC50)值。使用Duncan 新复极差法比较各试验处理之间的差异显著性。
2 结果与分析
2.1 不同原药对多主棒孢菌的菌丝抑制效果
不同原药对多主棒孢菌菌丝的室内毒力试验表明,戊唑醇、吡唑醚菌酯、苯醚甲环唑对菌丝生长的毒力较高,EC50值依次为10.81、11.69、12.48µg·mL-1,多菌灵及啶酰菌胺对菌丝生长的毒力较低,EC50值分别为35.19、41.85µg·mL-1,抑制效果可见图1,具体毒力数据见表3。

图1 不同药剂在不同质量浓度梯度下对多主棒孢菌的抑制效果
Fig.1 The inhibitory effects of different fungicides at different concentration gradients on the C.cassiicola
表3 不同药剂对多主棒孢菌菌丝生长的毒力
Table 3 Toxicity of different fungicides to mycelia growth of C.cassiicola
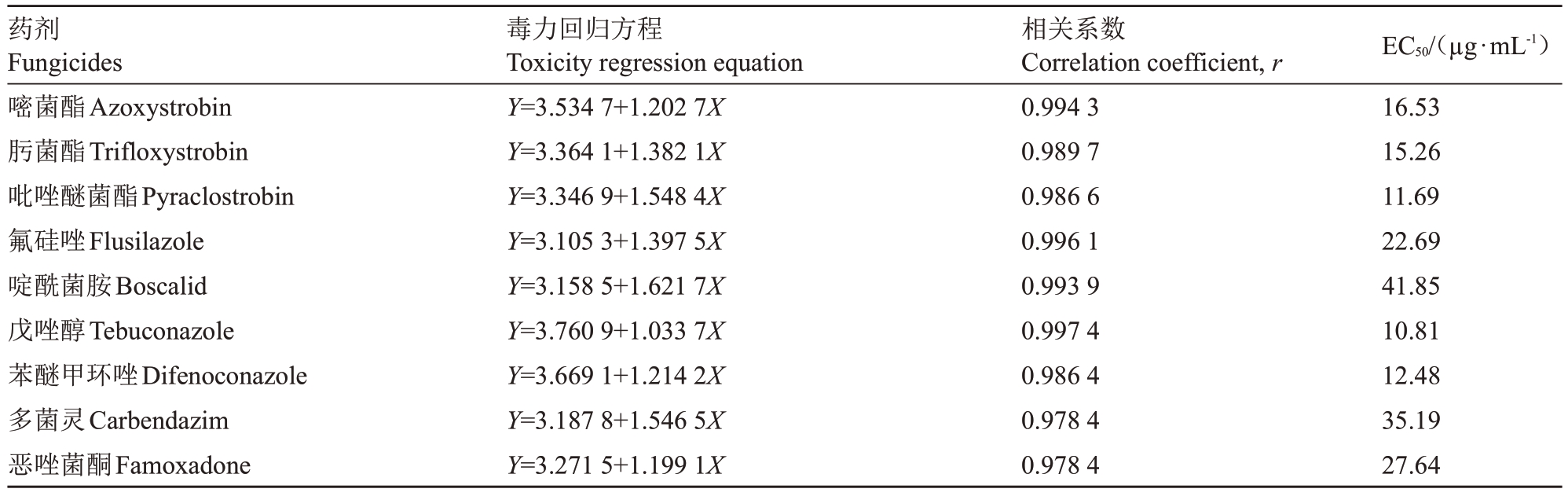
药剂Fungicides嘧菌酯Azoxystrobin肟菌酯Trifloxystrobin吡唑醚菌酯Pyraclostrobin氟硅唑Flusilazole啶酰菌胺Boscalid戊唑醇Tebuconazole苯醚甲环唑Difenoconazole多菌灵Carbendazim恶唑菌酮Famoxadone毒力回归方程Toxicity regression equation Y=3.534 7+1.202 7X Y=3.364 1+1.382 1X Y=3.346 9+1.548 4X Y=3.105 3+1.397 5X Y=3.158 5+1.621 7X Y=3.760 9+1.033 7X Y=3.669 1+1.214 2X Y=3.187 8+1.546 5X Y=3.271 5+1.199 1X相关系数Correlation coefficient,r 0.994 3 0.989 7 0.986 6 0.996 1 0.993 9 0.997 4 0.986 4 0.978 4 0.978 4 EC50/(µg·mL-1)16.53 15.26 11.69 22.69 41.85 10.81 12.48 35.19 27.64
2.2 不同原药对多主棒孢菌孢子萌发的抑制效果
通过不同原药对病菌孢子萌发的室内毒力试验表明:戊唑醇与肟菌酯的毒力较高,EC50值分别为8.30、8.55µg·mL-1;苯醚甲环唑、嘧菌酯、吡唑醚菌酯毒力相对次之,EC50 值分别为12.69、14.60、15.89 µg·mL-1,多菌灵与啶酰菌胺的毒力较低,EC50值分别为33.65、40.35 µg·mL-1,具体结果见表4。
表4 不同药剂对多主棒孢菌孢子萌发的毒力
Table 4 Toxicity of different fungicides to conidia germination of C.cassiicola
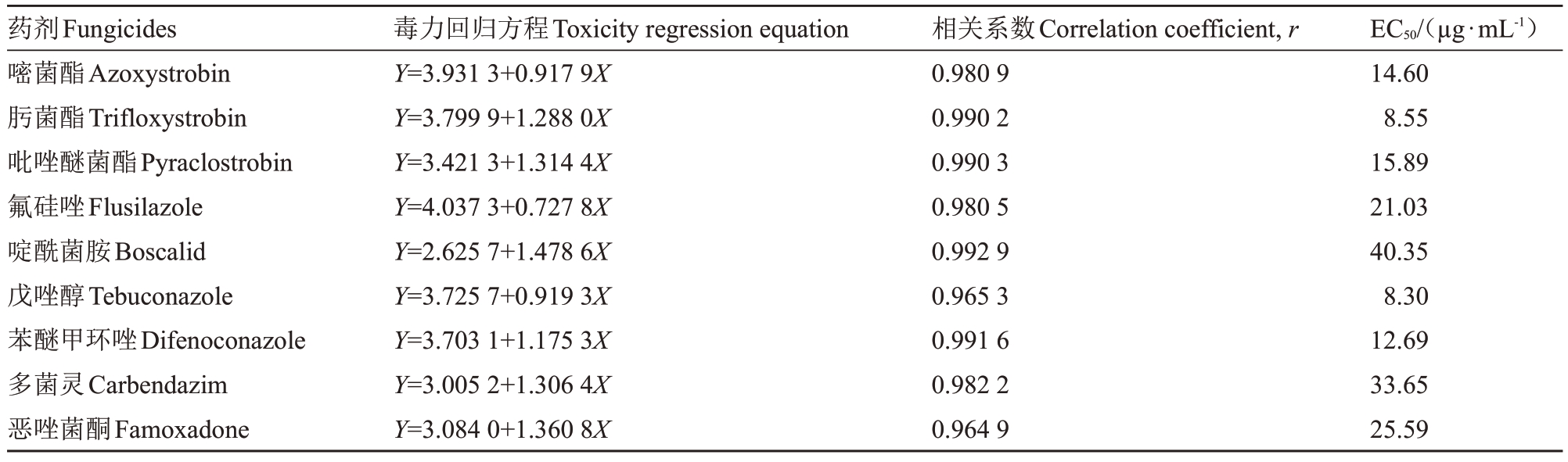
药剂Fungicides嘧菌酯Azoxystrobin肟菌酯Trifloxystrobin吡唑醚菌酯Pyraclostrobin氟硅唑Flusilazole啶酰菌胺Boscalid戊唑醇Tebuconazole苯醚甲环唑Difenoconazole多菌灵Carbendazim恶唑菌酮Famoxadone毒力回归方程Toxicity regression equation Y=3.931 3+0.917 9X Y=3.799 9+1.288 0X Y=3.421 3+1.314 4X Y=4.037 3+0.727 8X Y=2.625 7+1.478 6X Y=3.725 7+0.919 3X Y=3.703 1+1.175 3X Y=3.005 2+1.306 4X Y=3.084 0+1.360 8X相关系数Correlation coefficient,r 0.980 9 0.990 2 0.990 3 0.980 5 0.992 9 0.965 3 0.991 6 0.982 2 0.964 9 7月11日EC50/(µg·mL-1)14.60 8.55 15.89 21.03 40.35 8.30 12.69 33.65 25.59
2.3 不同复配药剂对多主棒孢菌菌丝生长的抑制效果
根据2.1 与2.2 的试验结果,以及田间生产用药调查情况,筛选出室内毒力抑制效果较好的原药种类,选择含有这些原药的商品复配药剂作为试验药剂,具体复配药剂详细信息见表1。从表5 可知,在药剂质量浓度为1µg·mL-1时,7 种复配药剂中以氟唑菌酰胺·吡唑醚菌酯对病菌的菌丝生长抑制效果最好,但仅达63.45%,其他均低于50%。在药剂质量浓度为10µg·mL-1时,氟唑菌酰胺·吡唑醚菌酯对病菌菌丝的抑制效果仍是最佳,抑制率可达到88.59%;苯醚甲环唑·肟菌酯与氟吡菌酰胺·肟菌酯抑制效果次之,抑制率分别达到77.30%与73.08%;戊唑醇·肟菌酯抑制效果最差,抑制率仅为39.44%。在药剂质量浓度为20µg·mL-1时,7种药剂对菌丝生长抑制效果在0.05水平上彼此之间均表现出差异,氟唑菌酰胺·吡唑醚菌酯抑制效果最佳,能100%完全抑制菌丝生长;氟吡菌酰胺·肟菌酯抑制效果次之,抑制率能达到85.85%;戊唑醇·肟菌酯抑制效果最差,抑制率为46.93%。在药剂质量浓度为50µg·mL-1时,氟唑菌酰胺·吡唑醚菌酯能100%抑制菌丝生长外,氟吡菌酰胺·肟菌酯与丙环唑·嘧菌酯抑制效果能分别达到96.08%与91.46%;另外苯醚甲环唑·肟菌酯的抑制效果达到了89.55%;戊唑醇·肟菌酯抑制效果仍是最差,抑制率为51.92%。在药剂质量浓度为100µg·mL-1时,氟唑菌酰胺·吡唑醚菌酯与氟吡菌酰胺·肟菌酯对菌丝生长抑制率均达到100%;丙环唑·嘧菌酯和苯醚甲环唑·肟菌酯的抑制率分别达到了99.46%与95.04%;氯氟醚菌唑·吡唑醚菌酯和戊唑醇·肟菌酯抑制效果最差,抑制率分别为60.03%与57.18%。
表5 不同复配药剂对多主棒孢菌菌丝生长抑制率
Table 5 Inhibition rate of different fungicides on mycelial growth of C.cassiicola%
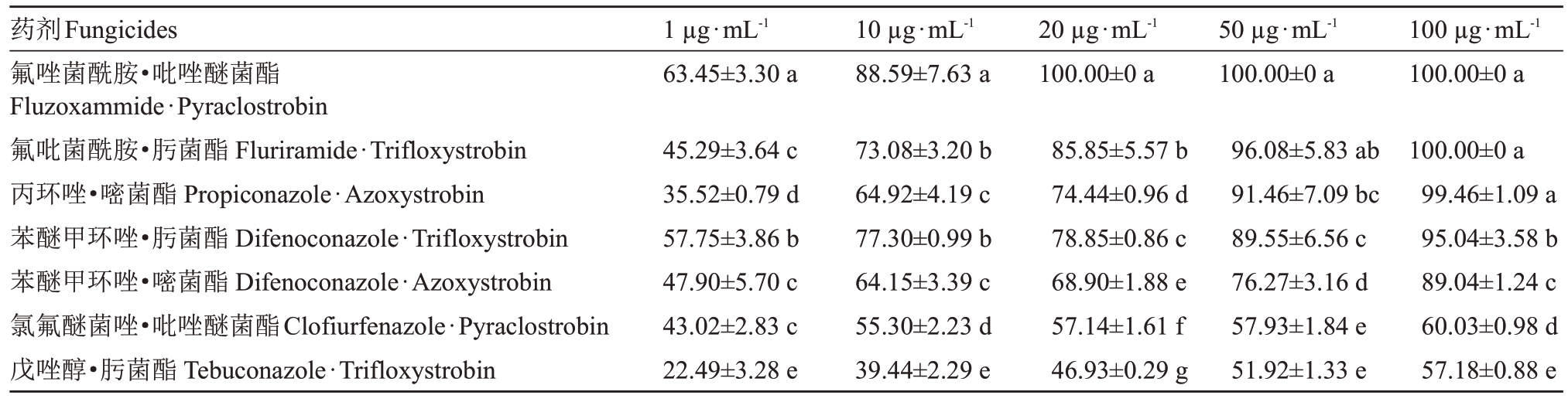
注:表中供试药剂的有效成分、剂型等信息请见表1。表中数据为平均数±标准差,同列数据后不同小写字母表示菌丝生长抑制率在0.05 水平具有显著差异。
Note:Details of the tested fungicides are shown in Table 1.Date are mean±SD.Different small letters after the data in the same column indicated that the inhibition rate of mycelia growth had significant difference at the level of 0.05.
50µg·mL-1 100.00±0 a药剂Fungicides氟唑菌酰胺·吡唑醚菌酯Fluzoxammide·Pyraclostrobin氟吡菌酰胺·肟菌酯Fluriramide·Trifloxystrobin丙环唑·嘧菌酯Propiconazole·Azoxystrobin苯醚甲环唑·肟菌酯Difenoconazole·Trifloxystrobin苯醚甲环唑·嘧菌酯Difenoconazole·Azoxystrobin氯氟醚菌唑·吡唑醚菌酯Clofiurfenazole·Pyraclostrobin戊唑醇·肟菌酯Tebuconazole·Trifloxystrobin 1µg·mL-1 63.45±3.30 a 10µg·mL-1 88.59±7.63 a 20µg·mL-1 100.00±0 a 100µg·mL-1 100.00±0 a 100.00±0 a 99.46±1.09 a 95.04±3.58 b 89.04±1.24 c 60.03±0.98 d 57.18±0.88 e 45.29±3.64 c 35.52±0.79 d 57.75±3.86 b 47.90±5.70 c 43.02±2.83 c 22.49±3.28 e 73.08±3.20 b 64.92±4.19 c 77.30±0.99 b 64.15±3.39 c 55.30±2.23 d 39.44±2.29 e 85.85±5.57 b 74.44±0.96 d 78.85±0.86 c 68.90±1.88 e 57.14±1.61 f 46.93±0.29 g 96.08±5.83 ab 91.46±7.09 bc 89.55±6.56 c 76.27±3.16 d 57.93±1.84 e 51.92±1.33 e 7月11日July 11th 42.4%氟唑菌酰胺·吡唑醚菌酯悬浮剂 42.4% Fluzoxammide·Pyraclostro0.7225 43%氟吡菌酰胺·肟菌酯悬浮剂 43% Fluriramide·Trifloxystrobin SC0.8325 75%戊唑醇·肟菌酯水分散粒剂 75% Tebuconazole·Trifloxystrobin WG0.935 40%苯醚甲环唑·肟菌酯悬浮剂 40% Difenoconazole·Trifloxystrobin SC0.5375
2.4 复配药剂对猕猴桃褐斑病的田间防治效果
根据2.3 中7 种复配药剂对病菌菌丝生长抑制效果及田间用药情况,选择氟唑菌酰胺·吡唑醚菌酯、氟吡菌酰胺·肟菌酯、苯醚甲环唑·肟菌酯、苯醚甲环唑·嘧菌酯、氯氟醚菌唑·吡唑醚菌酯及戊唑醇·肟菌酯6 种药剂进行田间防治试验,并在施药后持续调查至9 月。由图2 可知,连续3 次施用氟唑菌酰胺·吡唑醚菌酯的效果最好,至9 月5 日褐斑病病情指数只有17.13;施用苯醚甲环唑·肟菌酯、氟吡菌酰胺·肟菌酯、苯醚甲环唑·嘧菌酯的效果次之,至9 月5 日病情指数均小于30,距末次施药后20 d(8 月15 日)病情仍处于相对较低水平,可持续至果实完全采收,保证了果品的安全。氯氟醚菌唑·吡唑醚菌酯与戊唑醇·肟菌酯处理的试验区病情指数最高,到9 月5 日,病情指数分别达到61.58与52.98。由此可知:氟唑菌酰胺·吡唑醚菌酯有效地抑制了病情的发展;苯醚甲环唑·肟菌酯、氟吡菌酰胺·肟菌酯与苯醚甲环唑·嘧菌酯虽抑制了病情进一步发展,但是抑制效果不及氟唑菌酰胺·吡唑醚菌酯;氯氟醚菌唑·吡唑醚菌酯与戊唑醇·肟菌酯的病情指数最高,对病情发展的控制效果不佳。

图2 不同复配药剂防治下猕猴桃褐斑病的病情指数动态变化
Fig.2 The disease index of different fungicides on kiwifruit brown spot
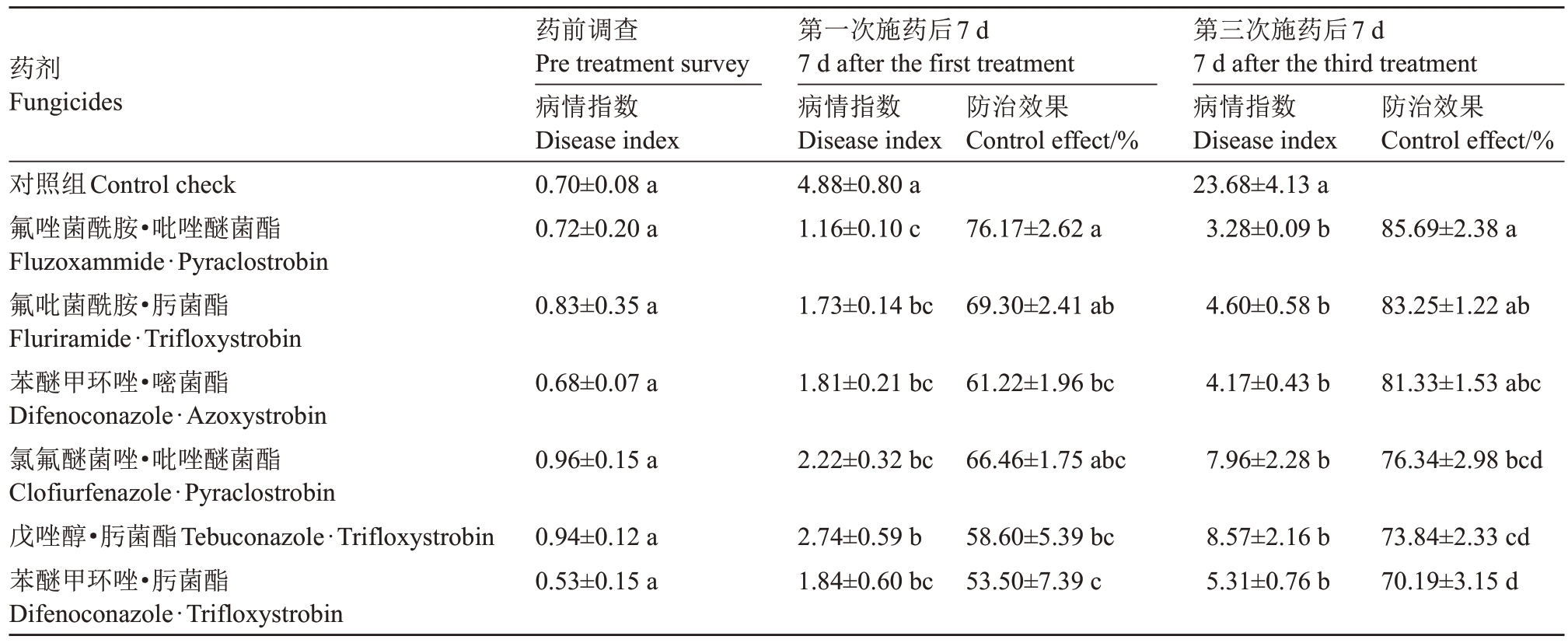
根据各处理猕猴桃褐斑病病情指数计算田间各复配药剂的防治效果,本次试验以首次施药后7 d(7月18 日)及末次施药后7 d(8 月1 日)的病情指数为代表,得出各复配药剂的防效见表6。
表6 不同复配药剂对猕猴桃褐斑病的防治效果
Table 6 The control effect of different fungicides on kiwifruit brown spot
注:表中供试药剂的有效成分、剂型等信息请见表1。表中数据为平均数±标准差,同列数据后不同小写字母表示病情指数及防效在0.05 水平上具有显著性的差异。
Note:Details of the tested fungicides are shown in Table 1.Date are mean±SD.Different lowercase letters after the data in the same column indicated that the disease index and control effect had significant difference at the level of 0.05.
药剂Fungicides药前调查Pre treatment survey病情指数Disease index 0.70±0.08 a 0.72±0.20 a第一次施药后7 d 7 d after the first treatment7 d after the third treatment病情指数防治效果病情指数防治效果Disease indexControl effect/%Disease indexControl effect/%4.88±0.80 a 1.16±0.10 c对照组Control check氟唑菌酰胺·吡唑醚菌酯Fluzoxammide·Pyraclostrobin氟吡菌酰胺·肟菌酯Fluriramide·Trifloxystrobin苯醚甲环唑·嘧菌酯Difenoconazole·Azoxystrobin氯氟醚菌唑·吡唑醚菌酯Clofiurfenazole·Pyraclostrobin戊唑醇·肟菌酯Tebuconazole·Trifloxystrobin苯醚甲环唑·肟菌酯Difenoconazole·Trifloxystrobin 76.17±2.62 a第三次施药后7 d 23.68±4.13 a 3.28±0.09 b 85.69±2.38 a 0.83±0.35 a 1.73±0.14 bc 69.30±2.41 ab 4.60±0.58 b 83.25±1.22 ab 0.68±0.07 a 1.81±0.21 bc 61.22±1.96 bc 4.17±0.43 b 81.33±1.53 abc 0.96±0.15 a 2.22±0.32 bc 66.46±1.75 abc 7.96±2.28 b 76.34±2.98 bcd 0.94±0.12 a 0.53±0.15 a 2.74±0.59 b 1.84±0.60 bc 58.60±5.39 bc 53.50±7.39 c 8.57±2.16 b 5.31±0.76 b 73.84±2.33 cd 70.19±3.15 d
在第1次施药7 d后,各复配药剂对褐斑病防治效果不一,其中氟唑菌酰胺·吡唑醚菌酯的防治效果达到了76.17%,氟吡菌酰胺·肟菌酯、苯醚甲环唑·嘧菌酯和氯氟醚菌唑·吡唑醚菌酯防治效果均在60%以上,戊唑醇·肟菌酯及苯醚甲环唑·肟菌酯防效较低分别只有58.60%和53.50%。在第3 次施药后7 d,氟唑菌酰胺·吡唑醚菌酯的防治效果达到了85.69%,氟吡菌酰胺·肟菌酯和苯醚甲环唑·嘧菌酯的防治效果分别达到83.25%和81.33%,其他3 种复配药剂的防效均未超过80%,其中苯醚甲环唑·肟菌酯防治效果最低只有70.19%。根据第1 次试验后7 d 与第3 次施药后7 d 的防治效果,氟唑菌酰胺·吡唑醚菌酯效果最好,氟吡菌酰胺·肟菌酯次之。
2.5 田间大区防治效果
根据药剂筛选结果在四川省都江堰等不同种植区域推广氟唑菌酰胺·吡唑醚菌酯与氟吡菌酰胺·肟菌酯两种药剂,在6月初褐斑病初发期开始施药,露天栽培条件下间隔7~10 d施药,连续施药4次(两种药剂交替使用,各施药2 次)。在7 月下旬至8 月上旬对不同种植区域进行褐斑病的调查。结果(表7)显示,2019—2021 年应用此套施药方案的种植区果园对猕猴桃褐斑病的防效均超过了80.00%,2019年雅安芦山、都江堰等5 个种植区果园对猕猴桃褐斑病的防治效果在81.45%~89.25%,处理组的发病率基本控制在50%以内,其中德阳绵竹与雅安芦山的发病率分别只有8.86%和12.40%;2020年绵阳安州、雅安芦山与都江堰种植区果园对病害的防治效果分别达到86.79%、86.61%和84.87%;2021 年绵阳安州与都江堰果园对病害的防治效果分别为81.03%和81.16%。根据2019—2021 年不同种植区病害的病情指数与发病率可知,该病害有逐年加重的趋势,但该套施药方案对病害的防治效果依旧能保持在80.00%以上。
表7 防治方案推广下四川省不同种植区对猕猴桃褐斑病防效
Table 7 The control effect on this disease of different planting areas in Sichuan province by the prevention scheme

调查日期Data地点Areas防效Control effect/%2019-07-26 2019-07-30 2019-08-10 2019-08-10 2019-08-13 2020-08-03 2020-08-07 2020-08-12 2021-08-13 2021-08-10芦山县Lushan county绵竹市Mianzhu city安州区Anzhou district邛崃市Qionglai city都江堰市Dujiangyan city安州区Anzhou district芦山县Lushan county都江堰市Dujiangyan city安州区Anzhou district都江堰市Dujiangyan city病情指数Disease index处理Treatment 1.64 1.11 6.06 9.23 2.62 7.41 13.36 10.31 14.22 9.62对照Control 15.26 9.96 32.67 77.72 19.97 56.11 99.76 68.15 74.96 51.06发病率Incidence rate/%处理Treatment 12.40 8.86 29.14 50.27 23.60 46.00 75.33 75.33 89.00 79.00对照Control 77.87 58.67 95.71 100.00 69.43 100.00 100.00 100.00 100.00 98.22 89.25 88.86 81.45 88.12 86.88 86.79 86.61 84.87 81.03 81.16
3 讨 论
对于由多主棒孢菌引起的植物病害,化学药剂防治是目前为止最有效的防治措施,化学防治药剂多属于琥珀酸脱氢酶抑制剂(Succinate dehydrogenase inhibitors,SDHIs)、甲氧基丙烯酸酯类(Strobilurin,QoIs)及甾醇合成抑制剂(Sterolbiosynthesis inhibitors,SBIs)三类杀菌剂。SDHIs 是市场上近些年销售额年复合增长率最高的杀菌剂,主要作用于病菌的线粒体呼吸链复合体Ⅱ,阻断能量代谢、抑制病菌生长直至死亡[20-21],SDHIs中啶酰菌胺,氟啶胺、吡唑萘菌胺、氟唑菌酰胺被先后报道对多主棒孢菌的菌丝生长有较强的抑制作用,其中氟啶胺及吡唑萘菌胺对多主棒孢菌孢子的萌发同样具有较强的抑制作用。QoIs作为全球市场份额最大的杀菌剂,近些年一直表现出较强的市场竞争力[22],其主要作用于病原菌线粒体呼吸链复合体Ⅲ,阻止电子传递从而抑制真菌生长,QoIs中嘧菌酯、吡唑醚菌酯、肟菌酯对多主棒孢菌菌丝生长抑制作用明显,并且吡唑醚菌酯与烯肟菌酯对病菌孢子萌发抑制作用较强[23-24]。作为农作物病害化学防治的主导药剂之一,SBIs杀菌剂主要抑制病菌麦角甾醇的自身合成,破坏病菌细胞膜结构从而起到杀菌作用,其中苯醚甲环唑、咪鲜胺、丙硫菌唑、氟硅唑、丙环唑与氟醚菌唑对多主棒孢菌菌丝的生长抑制作用明显,但尚未发现对病菌孢子萌发具有较强抑制作用的此类杀菌剂[25-27]。
本研究从杀菌剂原药出发对多主棒孢菌菌丝生长及孢子萌发进行室内毒力测定,根据毒力结果选择氟唑菌酰胺·吡唑醚菌酯在内的7 种复配药剂进行菌丝生长抑制试验,最后通过6 种复配药剂田间药效测定筛选出氟唑菌酰胺·吡唑醚菌酯与氟吡菌酰胺·肟菌酯2种高效杀菌剂。本试验中戊唑醇、吡唑醚菌酯及苯醚甲环唑原药对多主棒孢菌菌丝生长的抑制效果最强,戊唑醇与肟菌酯原药对该病菌孢子萌发的抑制效果最强,前人研究中对该病菌菌丝生长有较强抑制作用的啶酰菌胺在本试验中对菌丝生长及孢子萌发均表现出最差的抑制效果,此结果原因可能是来源于不同寄主的多主棒孢菌遗传背景及施药环境不同,导致对同一药剂的敏感性有所差异。戊唑醇原药对病菌的菌丝生长及孢子萌发均表现出较强的抑制效果,肟菌酯原药对病菌孢子萌发抑制效果较强,但复配药剂戊唑醇·肟菌酯在室内毒力测定及田间药效相较于其他复配药剂表现最差,这可能与复配药剂的剂型种类有关,除戊唑醇·肟菌酯为水分散粒剂(WG)外,其余6种复配药剂均为悬浮剂(SC),WG 的悬浮率及药效均低于或差于SC[28-29]。这说明单一的室内毒力或田间药效均不能反映药剂对病菌的具体作用效果,需要将二者结合分析,这也是本试验较前人相关研究的差异之处。本试验中对杀菌剂原药分别进行了菌丝生长及抑制孢子萌发毒力测定,综合对比原药毒力进行后续筛选;由于采集的病菌孢子仅能满足前期原药的毒力测定,不能长期保存,且该病菌在人工培养时不易产孢,故在后续复配药剂毒力测定中,仅测定了对菌丝生长的抑制效果,未测定对孢子萌发的抑制作用,此为本试验的不足之处,待室内诱导产孢技术成熟,可补充此部分试验。
通过复配药剂的室内毒力及田间药效筛选出氟唑菌酰胺·吡唑醚菌酯和氟吡菌酰胺·肟菌酯2种复配药剂均为SDHIs与QoIs两类杀菌剂组合而成,此2类杀菌剂作用机制均为高效的抑制线粒体呼吸链方式,并且作用位点不同,使得此种复配药剂具有高效、低抗药性风险的应用前景。根据2019—2021年田间推广应用结果,即从6 月初该病害初发阶段开始间隔7~10 d使用氟唑菌酰胺·吡唑醚菌酯或氟吡菌酰胺·肟菌酯2种药剂共4次,对该病害的防治效果均能超过80.00%,能有效防治猕猴桃褐斑病。后续应扩大对近些年生产上较为主流的复配药剂的药效测定,以便为猕猴桃褐斑病的高效防治及抗药性治理提供更多药剂选择。
4 结 论
通过原药及复配药剂一系列的毒力筛选及药效测定,戊唑醇、吡唑醚菌酯及苯醚甲环唑原药对多主棒孢菌菌丝生长的抑制效果明显,戊唑醇与肟菌酯原药对病菌孢子萌发有较强抑制效果;复配药剂42.4%氟唑菌酰胺·吡唑醚菌酯悬浮剂与43%氟吡菌酰胺·肟菌酯悬浮剂在室内毒力、田间药效、累年药剂防治结果中效果明显,推荐此2 种药剂用于猕猴桃褐斑病的防治。
[1] YUAN G Q,XIE Y L,TAN D C,LI Q Q,LIN W.First report of leaf spot caused by Corynespora cassiicola on kiwifruit (Actinidia chinensis)in China[J].Plant Disease,2014,98(11):1586.
[2] CUI Y L,GONG G S,YU X M,XU J,WEN X W,ZHANG M,CHEN H B,ZHENG X J,ZHOU Y,CHANG X L.First report of brown leaf spot on kiwifruit caused by Corynespora cassiicola in Sichuan,China[J].Plant Disease,2015,99(5):725.
[3] 龚国淑,李庆,张敏,崔永亮.猕猴桃病虫害原色图谱与防治技术[M].北京:科学出版社,2020:21-23.GONG Guoshu,LI Qing,ZHANG Min,CUI Yongliang.Kiwifruit diseases and insect pests original color map and control technology[M].Beijing:Science Press,2020:21-23.
[4] 邓蕾,潘慧,李文艺,赵昆松,钟彩虹,李黎.湖北省猕猴桃周年病害病原菌鉴定分析[J].中南农业科技,2023(4):25-30.DENG Lei,PAN Hui,LI Wenyi,ZHAO Kunsong,ZHONG Caihong,LI Li.Identification and analysis of annual disease pathogens of kiwifruit in Hubei Province[J].South-Central Agricultural Science and Technology,2023(4):25-30.
[5] 黄秀兰,崔永亮,徐菁,朱宇航,陈华保,常小丽,杨继芝,龚国淑.猕猴桃种质材料对褐斑病抗性评价[J].植物病理学报,2018,48(5):711-715.HUANG Xiulan,CUI Yongliang,XU Jing,ZHU Yuhang,CHEN Huabao,CHANG Xiaoli,YANG Jizhi,GONG Guoshu.Resistance evaluation of kiwifruit germplasm materials to brown leaf spot caused by Corynespora cassiicola[J].Acta Phytopathologica Sinica,2018,48(5):711-715.
[6] XU J,GONG G S,CUI Y L,ZHU Y H,WANG J,YAO K K,CHEN W,WU C P,YANG R,YANG X D,LI P,ZHAO H N,ZHONG S,LUO Y,LI Y,LIAO W F.Comparison and correlation of Corynespora cassiicola populations from kiwifruit and other hosts based on morphology,phylogeny,and pathogenicity[J].Plant Disease,2023,107(7):1979-1992.
[7] 苏文文,李苇洁,李良良,吴迪,韩振成,王加国,任春光.猕猴桃褐斑病的发生及防治[J].农技服务,2020,37(5):84-85.SU Wenwen,LI Weijie,LI Liangliang,WU Di,HAN Zhencheng,WANG Jiaguo,REN Chunguang.Occurrence and prevention of kiwifruit brown spot[J].Agricultural Technology Service,2020,37(5):84-85.
[8] 杨恩兰,王林,苟铁丞,龙彪,李荣.3 种杀菌剂对猕猴桃褐斑病的防治效果[J].农技服务,2021,38(1):74-76.YANG Enlan,WANG Lin,GOU Tiecheng,LONG Biao,LI Rong.Control effect of three fungicides on kiwifruit brown spot[J].Agricultural Technology Service,2021,38(1):74-76.
[9] VAWDREY L L,GRICE K R E,WESTERHUIS D.Field and laboratory evaluations of fungicides for the control of brown spot (Corynespora cassiicola) and black spot (Asperisporium caricae)of papaya in far north Queensland,Australia[J].Australasian Plant Pathology,2008,37(6):552-558.
[10] 祁之秋,纪明山,陆田,王英姿,李兴海,魏松红.黄瓜褐斑病防治药剂的离体活性筛选[J].植物保护,2009,35(2):140-143.QI Zhiqiu,JI Mingshan,LU Tian,WANG Yingzi,LI Xinghai,WEI Songhong. In vitro screening of effective fungicides against Corynespora cassiicola[J].Plant Protection,2009,35(2):140-143.
[11] 番华彩,郭志祥,白亭亭,徐胜涛,尹可锁,杨佩文,郑泗军,李迅东,曾莉.香蕉棒孢霉叶斑病菌防治药剂室内毒力测定[J].中国南方果树,2018,47(2):96-97.FAN Huacai,GUO Zhixiang,BAI Tingting,XU Shengtao,YIN Kesuo,YANG Peiwen,ZHENG Sijun,LI Xundong,ZENG Li.Laboratory determination of virulence of control fungicides for corynespora banana leaf spot pathogen[J].South China Fruits,2018,47(2):96-97.
[12] VISHWAKARMA V K,SAXENA M,SAXENA D R.Evaluation of strobilurin and triazole fungicides against target leaf spot caused by (Corynespora cassiicola) in vitro condition[J].International Journal of Current Microbiology and Applied Sciences,2020,9(12):1895-1902.
[13] 崔丽红,宋金秋,黄蔚.六种药剂对湘西地区猕猴桃褐斑病的防治效果[J].黑龙江农业科学,2023(2):51-54.CUI Lihong,SONG Jinqiu,HUANG Wei.Control effects of six fungicides on kiwifruit brown spot in western Hunan[J].Heilongjiang Agricultural Sciences,2023(2):51-54.
[14] 崔永亮.猕猴桃褐斑病的研究[D].雅安:四川农业大学,2015.CUI Yongliang.Study of kiwifruit brown leaf spot[D].Ya’an:Sichuan Agricultural University,2015.
[15] 张凯东,强遥,刘冰,李邦明,赵尚高,蒋军喜.猕猴桃叶斑病病菌生物学特性及室内药剂筛选[J].江苏农业科学,2021,49(18):106-110.ZHANG Kaidong,QIANG Yao,LIU Bing,LI Bangming,ZHAO Shanggao,JIANG Junxi.Biological characteristics of kiwi leaf spot pathogen and screening of indoor pesticides[J].Jiangsu Agricultural Sciences,2021,49(18):106-110.
[16] 龚国淑,徐琴,张敏,杨继芝,陈华保,申世安,唐太飞.一种简便的病原真菌单孢分离方法研究[J].玉米科学,2010,18(1):126-127.GONG Guoshu,XU Qin,ZHANG Min,YANG Jizhi,CHEN Huabao,SHEN Shian,TANG Taifei.A simple method for single fungal spore isolation[J].Journal of Maize Sciences,2010,18(1):126-127.
[17] 唐爽爽,刘志恒,余朝阁,赵廷昌.9 种杀菌剂对西瓜炭疽病菌的室内毒力测定及配比试验[J].植物保护,2014,40(6):171-175.TANG Shuangshuang,LIU Zhiheng,YU Zhaoge,ZHAO Tingchang.Toxicity determination and proportioning tests of nine fungicides to Colletotrichum orbiculare[J].Plant Protection,2014,40(6):171-175.
[18] 朱发娣.黄瓜多主棒孢菌(Corynespora cassiicola)对啶酰菌胺的抗性及其机理研究[D].北京:中国农业科学院,2018.ZHU Fadi.Resistance and its mechanism of Corynespora cassiicola from cucumber to boscalid[D].Beijing:Chinese Academy of Agricultural Sciences,2018.
[19] 王晓坤,郭贝贝,高杨杨,慕卫,刘峰.六种三唑类杀菌剂对番茄叶霉病菌的毒力及其安全性和田间防效评价[J].植物保护学报,2017,44(4):671-678.WANG Xiaokun,GUO Beibei,GAO Yangyang,MU Wei,LIU Feng.The toxicity of six triazole fungicides to Cladosporium fulvum and their safety and field efficacy in the control of tomato leaf mold[J].Journal of Plant Protection,2017,44(4):671-678.
[20] 仇是胜,柏亚罗.琥珀酸脱氢酶抑制剂类杀菌剂的研发进展(Ⅰ)[J].现代农药,2014,13(6):1-7.QIU Shisheng,BAI Yaluo.Progress on research and development of succinate dehydrogenase inhibitor fungicides (Ⅰ)[J].Modern Agrochemicals,2014,13(6):1-7.
[21] 魏阁,高梦琪,朱晓磊,杨光富.靶向琥珀酸脱氢酶的酰胺类杀菌剂的研究进展[J].农药学学报,2019,21(5/6):673-680 WEI Ge,GAO Mengqi,ZHU Xiaolei,YANG Guangfu.Research progress on carboxamide fungicides targeting succinate dehydrogenase[J].Chinese Journal of Pesticide Science,2019,21(5/6):673-680
[22] 严明,柏亚罗.甲氧基丙烯酸酯类等四大类杀菌剂市场概况及前景展望[J].现代农药,2016,15(6):1-8.YAN Ming,BAI Yaluo.Market overview and prospect outlook on four fungicide sectors including methoxyacrylates[J].Modern Agrochemicals,2016,15(6):1-8.
[23] 思彬彬,杨卓.甲氧基丙烯酸酯类杀菌剂作用机理研究进展[J].世界农药,2007,29(6):5-9.SI Binbin,YANG Zhuo.Studies on mechanism and resistance to strobilurin fungicides[J].World Pesticides,2007,29(6):5-9.
[24] 徐丽慧,高士刚,曾蓉,戴富明.黄瓜靶斑病菌致病性鉴定及药剂筛选[J].上海农业学报,2016,32(4):116-121.XU Lihui,GAO Shigang,ZENG Rong,DAI Fuming.Pathogenicity identification and fungicide screening of Corynespora cassiicola in cucumber[J].Acta Agriculturae Shanghai,2016,32(4):116-121.
[25] TERAMOTO A,MEYER M C,SUASSUNA N D,DA CUNHA M G. In vitro sensitivity of Corynespora cassiicola isolated from soybean to fungicides and field chemical control of target spot[J].Summa Phytopathologica,2017,43(4):281-289.
[26] 禾丽菲,李晓旭,朱佳美,慕卫,刘峰.不同杀菌剂对黄瓜靶斑病菌的毒力作用特性比较[J].农药学学报,2018,20(1):25-32.HE Lifei,LI Xiaoxu,ZHU Jiamei,MU Wei,LIU Feng.Comparison of toxicity properties of different types of fungicides against Corynespora cassiicola on cucumber[J].Chinese Journal of Pesticide Science,2018,20(1):25-32.
[27] 叶滔,马志强,毕秋艳,牛芳胜,韩秀英,张小风,王文桥,张利辉.植物病原真菌对甾醇生物合成抑制剂类(SBIs)杀菌剂的抗药性研究进展[J].农药学学报,2012,14(1):1-16.YE Tao,MA Zhiqiang,BI Qiuyan,NIU Fangsheng,HAN Xiuying,ZHANG Xiaofeng,WANG Wenqiao,ZHANG Lihui.Research advances on the resistance of plant pathogenic fungi to SBIs fungicides[J].Chinese Journal of Pesticide Science,2012,14(1):1-16.
[28] 华乃震.农药剂型的进展和动向(中)[J].农药,2008,47(3):157-160.HUA Naizhen.Advance and trend of pesticide formulation development(Middle)[J].Agrochemicals,2008,47(3):157-160.
[29] 王彦华,王鸣华,张久双.农药剂型发展概况[J].农药,2007,46(5):300-304.WANG Yanhua,WANG Minghua,ZHANG Jiushuang.Present situation on pesticide formulations[J].Agrochemicals,2007,46(5):300-304.