猕猴桃(Actinidia Lindl.)富含维生素C,被称为“水果之王”,深受大众的喜爱。随着猕猴桃产业的不断发展,根据联合国粮食及农业组织(FAO)最新数据(http://www.fao.org/faostat/en/),2022 年中国收获面积近20 万hm2,占全球的70%;年产量238 万t,占全球的52%,种植面积和产量稳居世界第一。由丁香假单胞菌猕猴桃致病变种(Pseudomonas syringae pv. actinidiae)侵染导致的猕猴桃溃疡病,具有潜伏期长、传播迅速、发生面积广、预防和防治困难等特点,已成为猕猴桃产业的毁灭性病害。猕猴桃溃疡病可危害猕猴桃树干、叶片、花器和根系[1];其中,病菌可危害花苞、花瓣、花梗,直接导致花蕾感病后不能张开,随后变褐枯死并脱落,是导致园区当年产量损失的直接因素;此外,病菌侵染雄花导致的花粉带菌,是溃疡病远距离快速传播的最主要途径,严重影响猕猴桃的产量[2-3]。目前化学防治仍是猕猴桃溃疡病的主要防治方法[1],但是过量施用化学农药造成了病原菌抗药性增强和生态环境污染,不利于猕猴桃产业持续健康的发展[2]。相比于化学防治,生物防治具有安全高效的优点,近年来逐渐成为猕猴桃溃疡病防控的重要研究领域[3]。植物中的一些微生物与寄主植物经长期的进化,发展出一种互利共生关系。寄主植物能够为微生物提供空间和营养,微生物在促进植物养分吸收、生长发育、对不良环境的抵抗等方面发挥相应的功能[4-5]。植物表面或内部存在的大量微生物作为植物的防线,可以通过竞争生态位[6-7]、产生抑菌物质[8-9]和诱导植物的抗性[10-11]等多种作用机制抑制病原菌的生长,减轻病害对寄主的影响。
通过高通量测序技术对猕猴桃花进行微生物组成和动态表征,可进行潜在生防有益菌的筛选。Lee等[12]发现苹果花际细菌中伯克霍尔德菌属(Burkholderaceae)显著富集,该菌对多种植物病原菌均表现出较强的拮抗效应[13-14]。Fridman等[15]发现不动杆菌属(Acinetobacter)在扁桃(Amygdalus communis)、葡萄柚(Citrus paradisi)和光烟草(Nicotiana glauca)的花蜜中显著富集,该属细菌兼具病菌拮抗及植物促生作用。此外,当受到病原菌侵染时,有益菌会通过特有的分泌物或代谢产物在病原菌与植物的互作体系中发挥作用。Kong等[16]发现相较于感染欧文氏杆菌(Erwinia amylovora)后的苹果花,健康花中成团泛菌(Pantoea agglomerans)和P.allii显著富集,成团泛菌分泌的关键抗真菌化合物—Herbicolin A通过直接结合和破坏含有麦角甾醇的脂筏而发挥抑菌作用。
此前,对猕猴桃植株的微生物组研究主要集中在地下生态位,譬如根及根际土[17-19]。溃疡病的发病症状主要集中在地上部分,而花作为溃疡病重要的感病部位,目前对猕猴桃花际微生物还未见系统研究。因此,笔者拟针对不同溃疡病发病程度下东红猕猴桃品种花际样品的细菌和真菌的群落结构进行研究,探究猕猴桃花际微生物群落对溃疡病菌侵染的影响,以期为猕猴桃溃疡病的生物防治提供新思路。
1 材料和方法
1.1 试验材料和采样地点
在湖北省十堰市丹江口市习家店镇猕猴桃试验园区进行采样。采集品种为中国科学院武汉植物园选育的红肉猕猴桃品种东红。根据花的发病症状,设置3个不同感病等级,其中F1对应健康状态(溃疡病菌检测为阴性);F2对应中等发病程度(花瓣和萼片变成褐色,但能正常开放);F3对应严重发病程度(花瓣和萼片变成褐色,且不能开放)。
于2022年4—5月东红猕猴桃病情高发期,对不同发病程度的植株样本的花进行取样。将园区分成3 个样地,每块样地中随机选取同一发病等级的猕猴桃植株3 株,共计9 株。针对健康/中等/严重发病程度样本,分别在每块样地的3份样品中随机选择1份,将3块样地中选择到的3份样品混样作为1个生物学重复,其余2 个生物学重复以此类推。最终获得不同发病等级的生物学重复样本共计9个。将上述采取的样品装入无菌袋中,并迅速放入-80 ℃超低温冰箱进行低温冷冻。
1.2 DNA提取和测序
称取2 g 的花样品,经液氮冷冻后进行研磨,放入2 mL 离心管中。由北京百迈客生物科技有限公司进行微生物DNA 提取,并对细菌16s RNA(V3+V4区)和真菌ITS1 RNA微生物进行扩增,扩增引物如表1,使用Illumina novaseq 6000进行测序。
表1 扩增引物序列
Table 1 Amplified primer sequence

类型Type细菌Bacteria扩增区域Amplification region 16S V3+V4真菌Fungi ITS1扩增引物Amplification Primer 338F 806R ITS1F ITS2引物序列Primer sequence 5'-ACTCCTACGGGAGGCAGCA-3 5'-GGACTACHVGGGTWTCTAAT-3'5'-CTTGGTCATTTAGAGGAAGTAA-3 5'-GCTGCGTTCTTCATCGATGC-3'
1.3 序列数据处理与统计分析
利用QIIME2(versoin 2020.6)中DADA2 方法将测序数据中的低质量序列剔除,低质量序列包括平均质量分数较低以及长度较短的序列,得到有效序列。利用Uparse 软件对不同样本中有效序列进行聚类,序列相似性超过97%的聚类成为OTUs。
(1)群落组成分析:在QIIME 1.91 中,使用BLAST算法分别将细菌、真菌代表性序列与SILVA参考数据库(versoin 12.8)[20]及UNITE 数据库(versoin 7.0)进行比对[21],通过物种注释得到不同分类水平的微生物丰度数据。利用QIIME 软件生成不同分类水平上的物种丰度表,对不同发病程度的猕猴桃组织丰度大于1%的门和属进行统计,对比分析不同发病程度的微生物门属的变化。
(2)微生物组多样性分析:对不同发病程度的多样性香农指数(Shannon index)和丰富度Chao1指数进行统计,评估序列文库的α 多样性,使用QIIME(versoin 2020.6)(beta_diversity.py scripts)计算β 多样性指数。采用主坐标分析法(PCoA)计算并可视化Bray-Curtis 距离矩阵,分析不同发病程度样本之间的相似性或差异性。
(3)微生物组间差异Lefse 分析:首先使用非参数Kruskal-Wallis 秩对不同发病程度样本中丰度差异显著的物种进行表征,然后采用线性回归分析(LDA)来评估每个组分(物种)丰度对差异贡献的大小。
使用IBM SPSS Statics 对不同样本中的数据进行单因素方差分析,分析结果用平均值和标准误表示,并采用Duncan氏新复极差法对不同样本之间的差异性是否显著进行检验。当p<0.05 时认为不同样本呈显著差异。
2 结果与分析
2.1 测序结果
质控后共获得584 580 条细菌16SrRNA 和520 169个真菌ITS1高质量片段。单一样本细菌的序列数在61 141~69 967个之间,平均为64 953个序列。单一样本真菌的序列数在48 990~60 651 个之间,平均为57 796个。对上述序列进行聚类,共检测到3410 个细菌OTUs 和12 986 个真菌OTUs。在不同发病程度花样本中检测到37个共同细菌OTUs和383 个共同真菌OTU(s图1)。在健康、中度发病和重度发病样本中,细菌独有的OTUs 分别占37.5%、27.0%和18.6%,真菌独有的OTUs 分别占18.3%、25.6%和19.4%。对测序深度进行分析,当测序序列在30 000个以后,曲线的变化幅度趋于平缓,说明对更多的序列进行检测只能产生较少的OTUs,表明测序深度合理,可对花中的大部分物种进行表征(图2)。
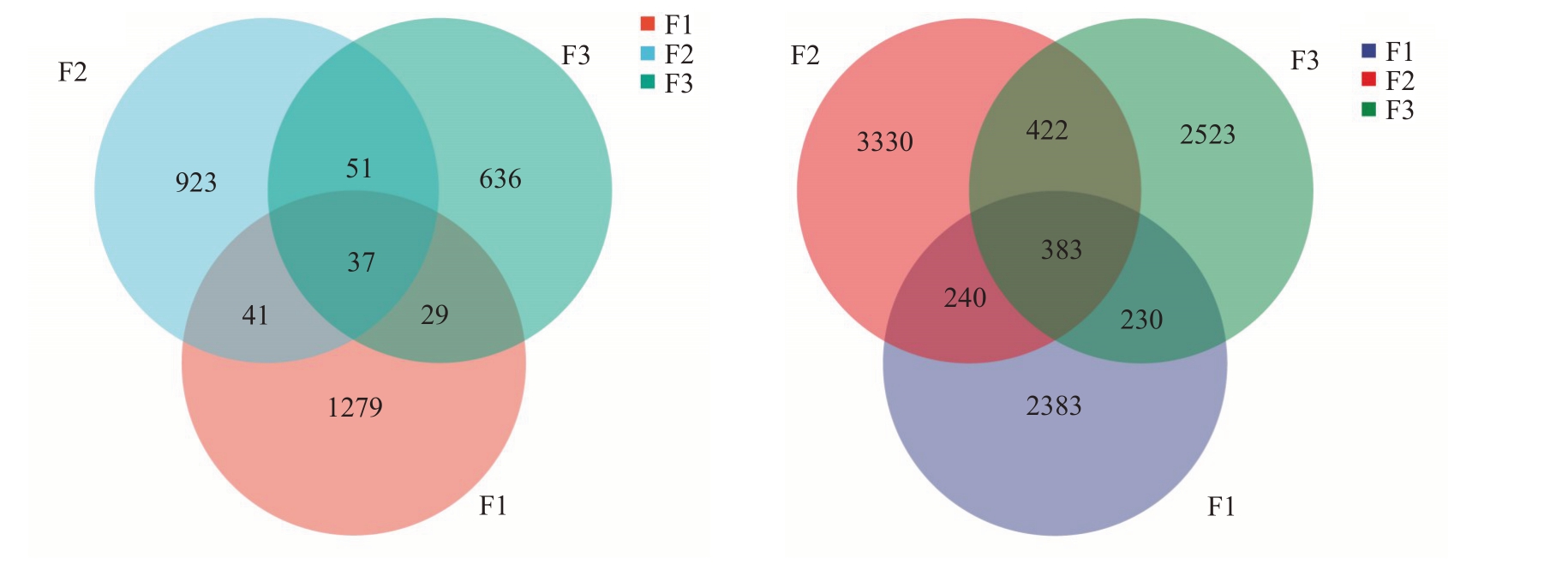
图1 不同发病程度花组织中OUTs 分布
Fig.1 Venn diagram illustrating OUTs distribution in flower samples with different disease degrees
A.细菌;B.真菌。
A.Bacteria;B.Fungi.
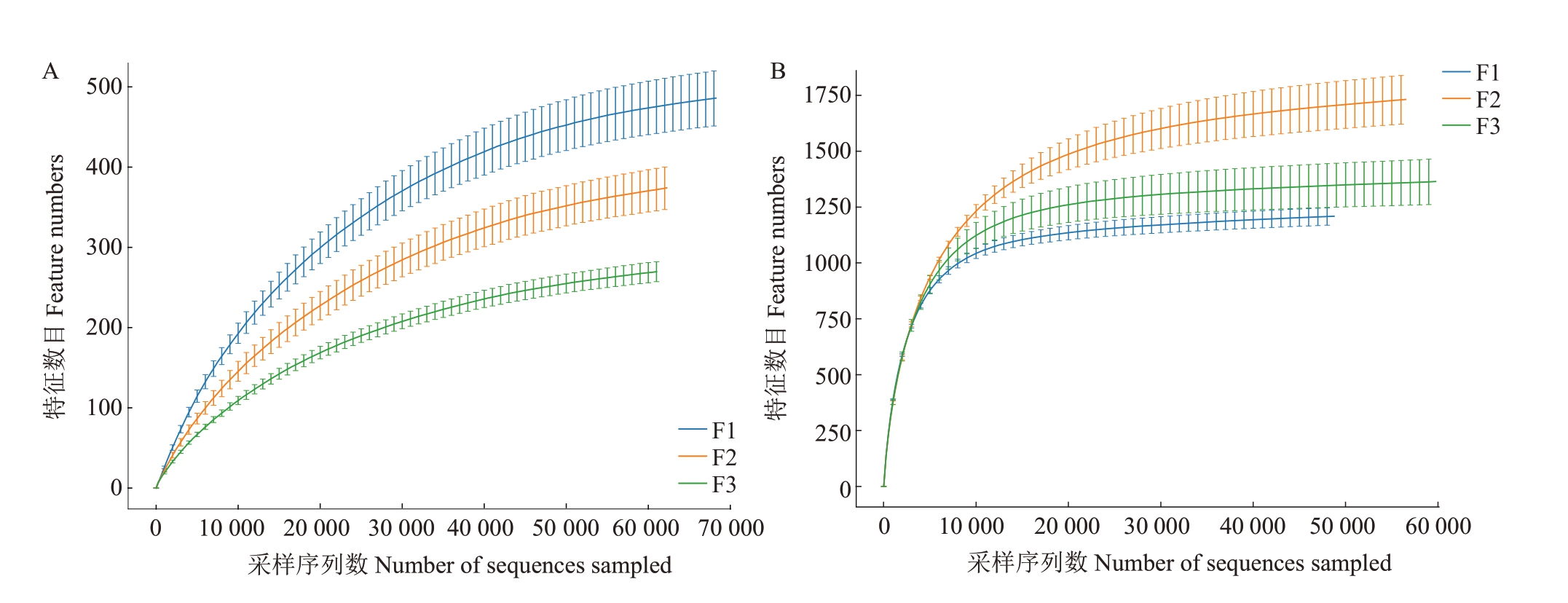
图2 不同溃疡病发病程度下花组织稀释性曲线
Fig.2 The dilutive curves of flower with different severity of kiwifruit canker disease
A.细菌;B.真菌。
A.Bacteria;B.Fungi.
2.2 不同发病程度猕猴桃花样本微生物多样性和群落结构
为分析不同溃疡病发病程度花细菌和真菌微生物群落的差异,对不同发病程度花样本中细菌和真菌的α多样性指数进行分析(表2)。α多样性分析结果表明,随着感病程度的增加,细菌OTUs数量和丰富度指数下降;健康样本中细菌的丰富度指数与感病程度样本呈显著差异(p<0.05);真菌的OTUs 数量和丰富度指数先增加后减少。其中,中等发病程度样本与其他样本中的真菌丰富度指数呈显著差异(p<0.05)。
表2 不同发病程度花组织α 多样性指数
Table 2 Alpha diversity index of flower with different disease degrees
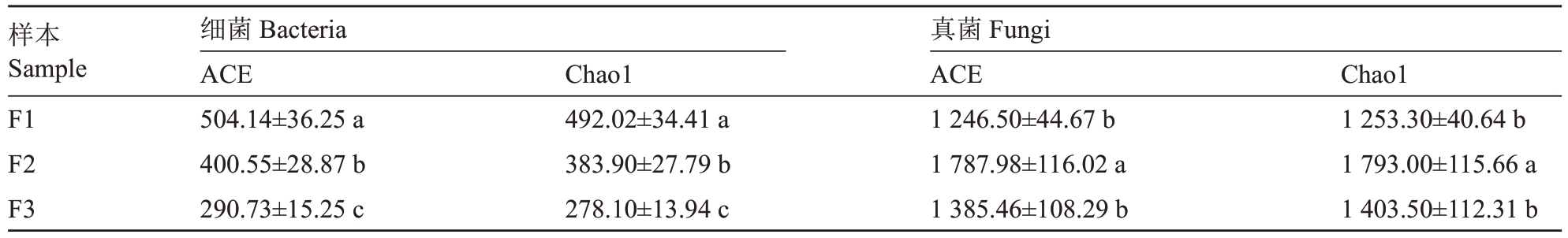
注:表中数据均为平均值±SE。同列不同字母表示在0.05 水平差异显著。
Note:The data in the table are average±SE.Different small letters in the same column indicate significant difference at 0.05 level.
样本Sample细菌Bacteria ACE Chao1 Chao1真菌Fungi ACE F1 F2 F3 504.14±36.25 a 400.55±28.87 b 290.73±15.25 c 1 253.30±40.64 b 1 793.00±115.66 a 1 403.50±112.31 b 492.02±34.41 a 383.90±27.79 b 278.10±13.94 c 1 246.50±44.67 b 1 787.98±116.02 a 1 385.46±108.29 b
为了更具体地描述不同发病程度对猕猴桃花部细菌和真菌群落结构的影响,进行了主坐标分析(PCoA)。在细菌群落结构中,第1主成分的累积方差贡献率为95.11%,第2 主成分的累积方差贡献率为3.01%。在真菌群落结构中,第1主成分的累积方差贡献率为28.52%,第2 主成分的累积方差贡献率为14.93%。不同发病程度样本之间的细菌群落呈极显著差异(p<0.001)(图3-A),健康样本与中等发病或严重感病程度样本之间的真菌群落均呈显著差异(p<0.05)(图3-B)。由此说明,溃疡病病菌的入侵显著改变了猕猴桃花细菌和真菌微生物的群落结构。
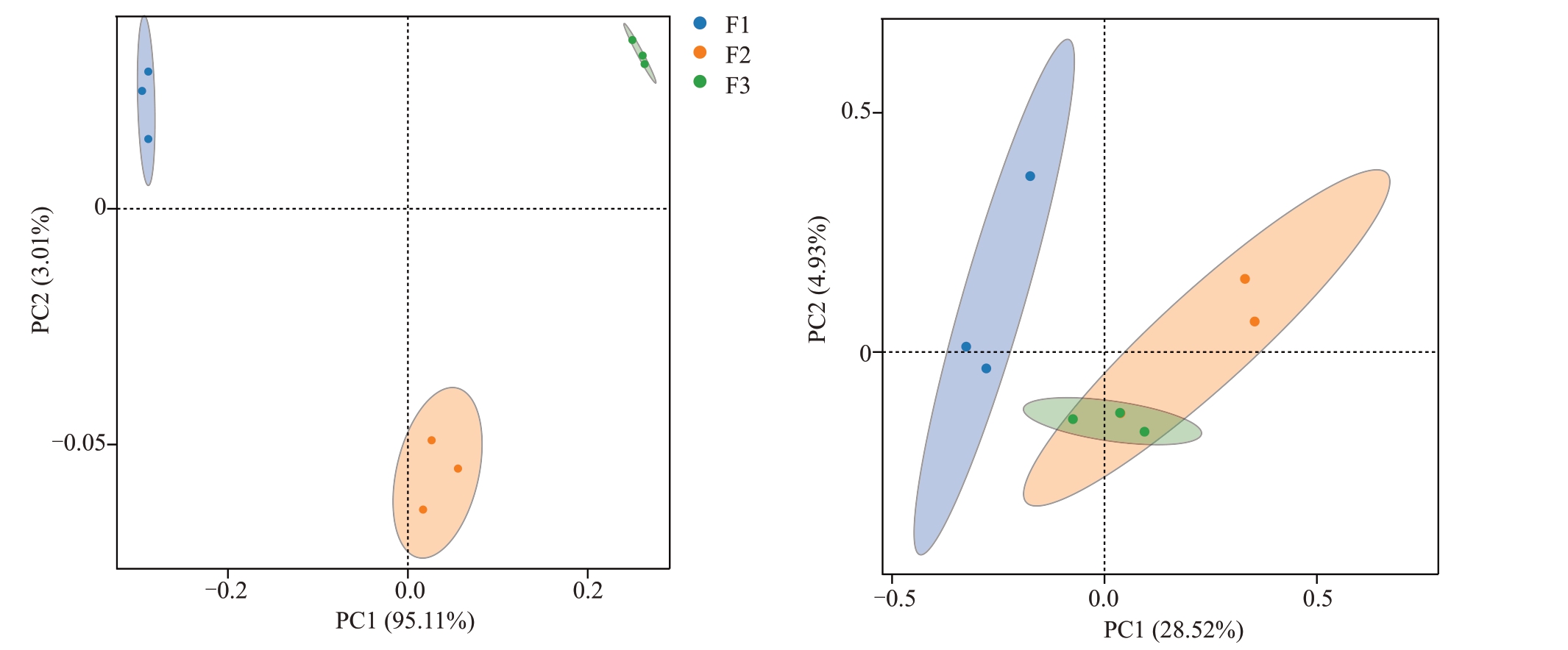
图3 不同发病程度对花组织微生物β 多样性的影响
Fig.3 The effect of different disease degrees on the beta diversity of bacterial and fungal communities in kiwifruit flower
A.细菌;B.真菌。A.Bacteria;B.Fungi.
2.3 猕猴桃溃疡病对花际微生物群落组成的影响
在猕猴桃不同发病程度样本中,共鉴定到细菌的27 个门、55 个纲、134 个目和210 个科,以及真菌的16个门、52个纲、118个目和252个科。在细菌群落中,与健康花相比,中等发病猕猴桃花的细菌各分类学水平数量有一定程度的下降;严重发病程度样本中各分类水平数量进一步下降。在真菌群落中,与健康花相比,中等感病程度花的真菌各分类水平数量有一定程度的上升;而在严重感病程度花样本中的各分类水平数量有一定程度的下降;但整体数量仍高于健康样本中的数量(表3)。
表3 不同发病程度花组织的物种数量
Table 3 The species number of flower with different disease degrees
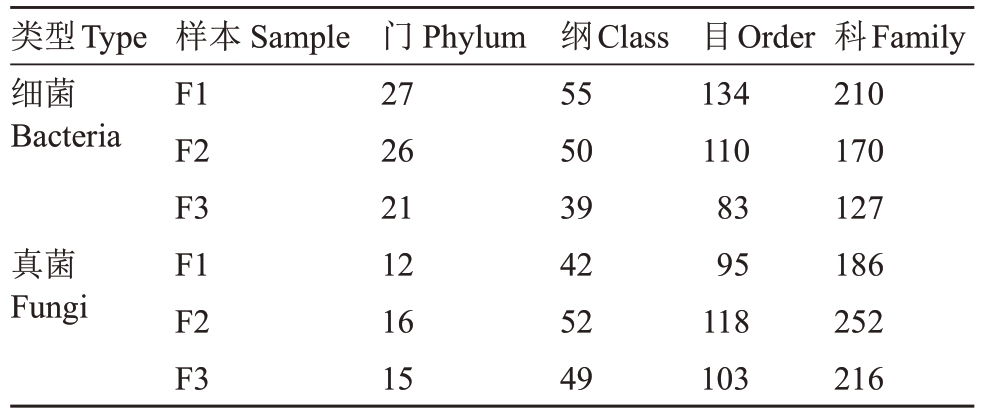
类型Type细菌Bacteria真菌Fungi样本Sample F1 F2 F3 F1 F2 F3门Phylum 27 26 21 12 16 15纲Class 55 50 39 42 52 49目Order 134 110 83 95 118 103科Family 210 170 127 186 252 216
不同发病程度花样本中的微生物组成不同。不同发病程度下细菌门水平上的群落组成及相对丰度如图4-A所示,变形菌门所有样本中相对丰度最高,蓝细菌门、厚壁菌门、拟杆菌门、放线菌门次之。其中,变形菌门的相对丰度变化随感病程度的变化最显著,在中等、严重发病程度样本中,变形菌门相对丰度分别为健康样本的22.74 倍、40.50 倍;蓝细菌门、厚壁菌门、拟杆菌门在健康样本中相对丰度较高,与细菌的丰度指数变化趋势相同。
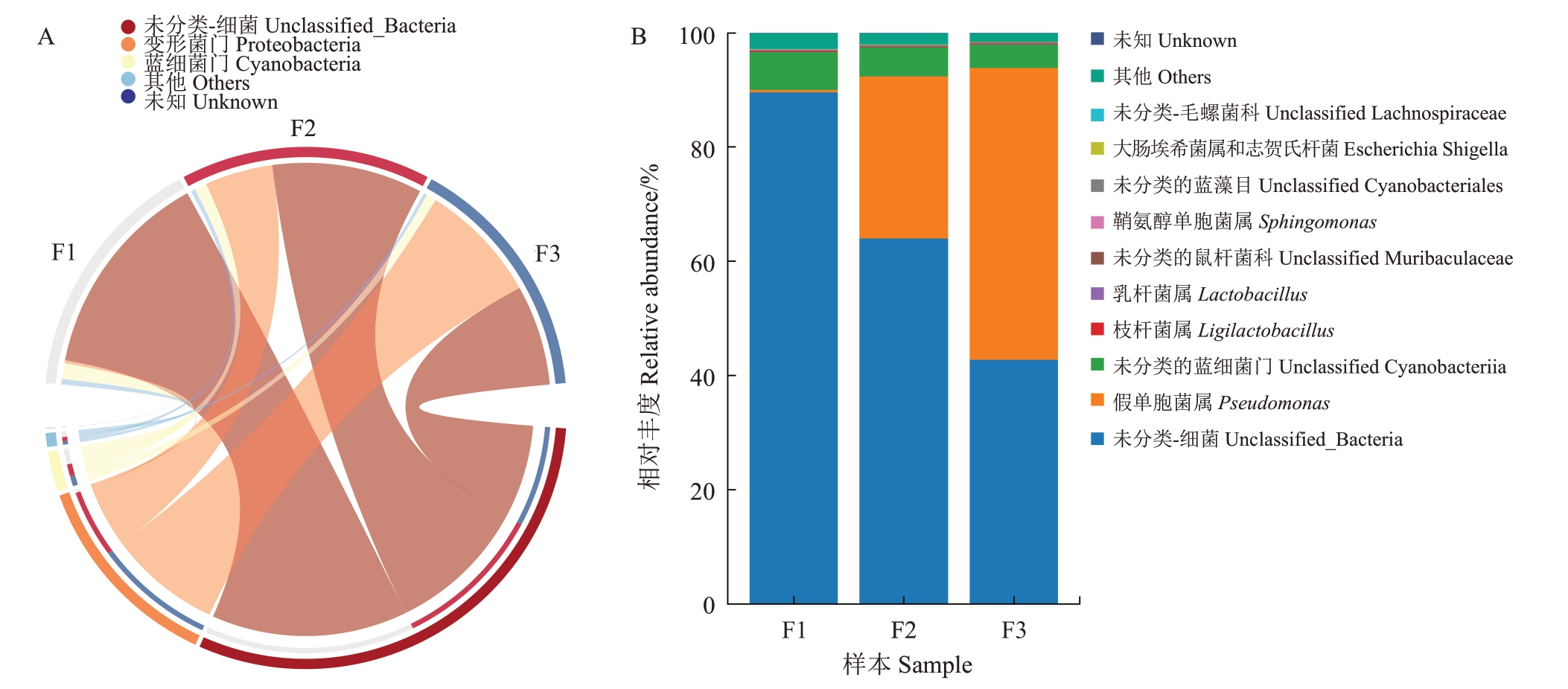
图4 不同发病程度对猕猴桃花组织细菌相对丰度的影响
Fig.4 The influence of different disease degrees on the relative abundance of bacteria in kiwifruit flower
A.门水平;B.属水平。A.Phylum level;B.Genus level.
在属水平上,不同发病程度样本中的假单胞菌属、蓝细菌属、Ligilactobacillus、乳杆菌属、鞘氨醇单胞属相对丰度存在差异(图4-B),随着感病程度的增加,假单胞菌属的相对丰度逐渐提升,在中等、严重发病程度样本中的相对丰度分别为健康样本的70.75倍、127.60倍。而蓝细菌属的相对丰度逐渐下降,在严重发病程度样本中的相对丰度相较于健康样本中下降了2.47%。对不同发病程度样本中丰度变化较为明显的假单胞菌属进行差异性分析,结果显示,假单胞菌属在严重发病程度样本与感病水平较低样本(健康和中等发病水平)中的相对丰度呈显著差异(p<0.05)(图5-A)。
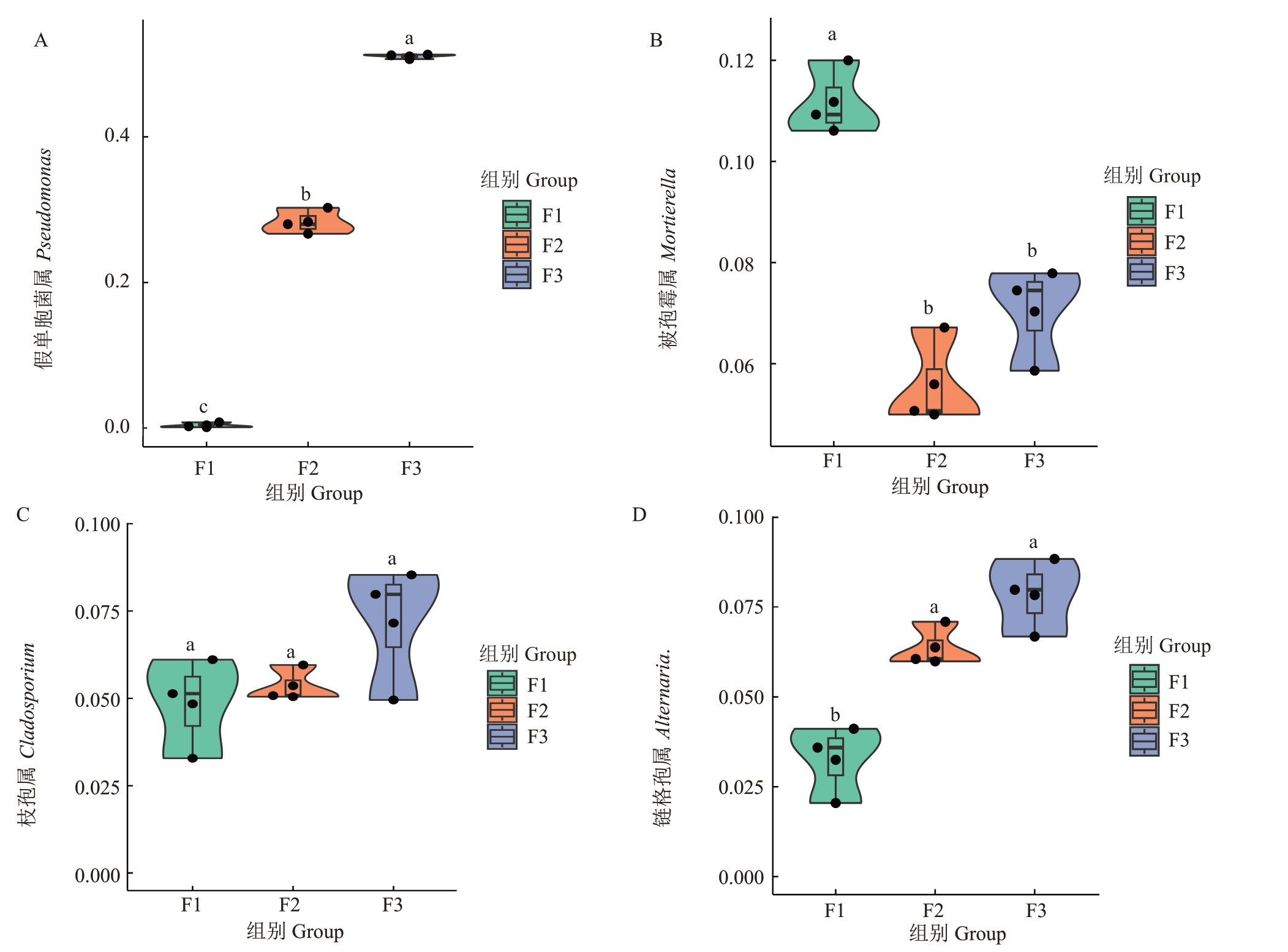
图5 不同发病程度花组织核心菌群差异分析
Fig.5 Difference analysis of main bacterial and fungal groups in flower with different disease degrees
不同小写字母表示差异显著(p<0.05)。
Different small letters indicate significant difference at p<0.05.
在真菌群落门水平上(图6-A),健康样本中子囊菌门和担子菌门的相对丰度低于感病样本;在不同发病样本中,子囊菌门和担子菌门有小幅度的下降;而被孢菌门和罗兹菌门在健康样本中的相对丰度高于感病花组织的相对丰度,在不同发病样本,被孢菌门和罗兹菌门的相对丰度有小幅度的提升。
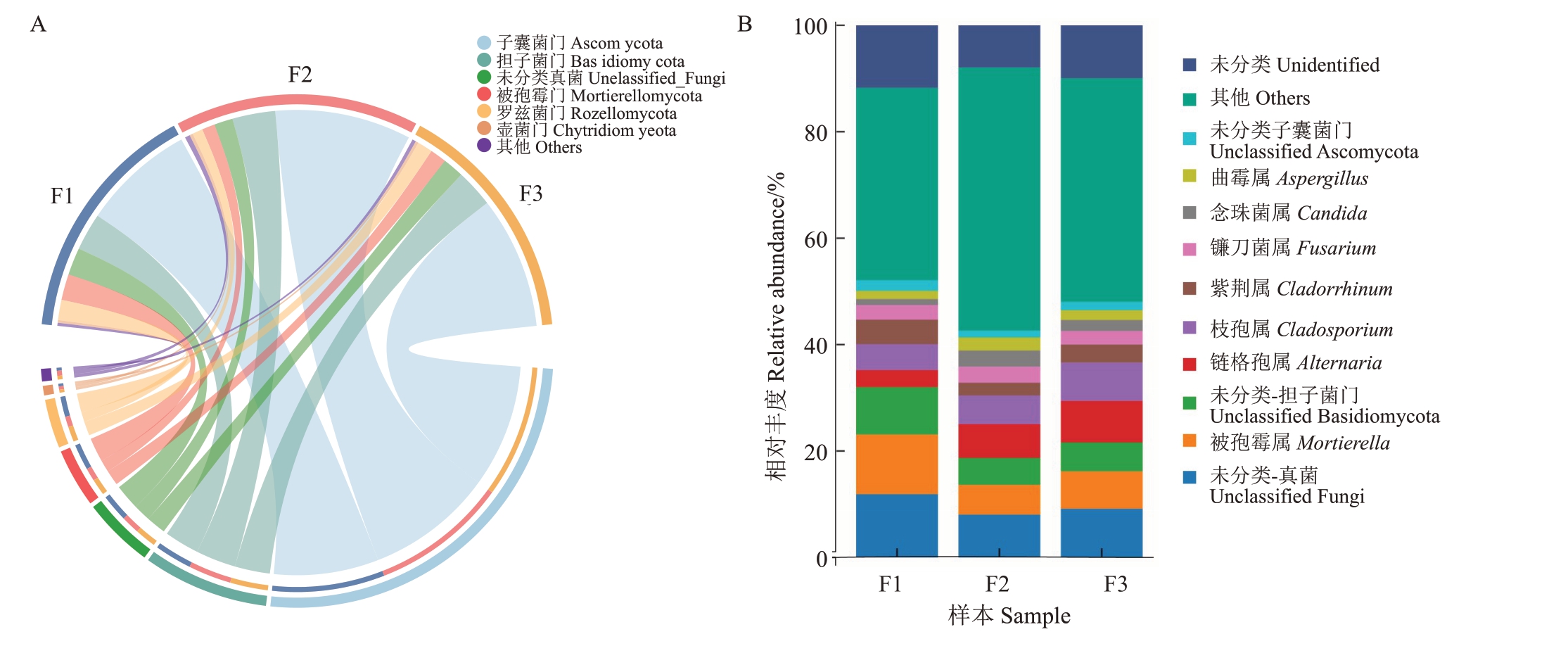
图6 不同发病程度对猕猴桃花组织真菌相对丰度的影响
Fig.6 The influence of different disease degrees on the relative abundance of fungi in kiwifruit flower
A.门水平;B.属水平。A.Phylum level;B.Genus level.
在属水平上(图6-B),被孢霉属在健康样本中的相对丰度高于感病样本;而枝孢属、链格孢属、枝孢属在严重发病程度样本中相对丰度较高,链格孢属和枝孢属相对丰度相较于健康样本中提高了4.62%和2.42%。曲霉属和维希尼克氏酵母在中等发病程度样本中的相对丰度较高。对不同样本中丰度变化较为明显的被孢霉属、链格孢属和枝孢属进行差异性分析,结果显示,健康样本中被孢霉属和链格孢属的相对丰度与感病样本呈显著差异(图5-B、D);而不同发病程度的枝孢属差异不显著(图5-C)。
2.4 不同发病程度花际微生物差异物种分析
利用LefSe(LDA Effect Size)对不同发病程度花微生物群落的差异物种进行分析,结果如图7-A所示,健康样本中的差异物种为黄色土源菌、Pedobacter、蓝细菌和丰祐菌属;随着感病程度的增加,中等发病程度中无差异物种;假单胞菌属为严重发病程度样本中的主要差异物种。
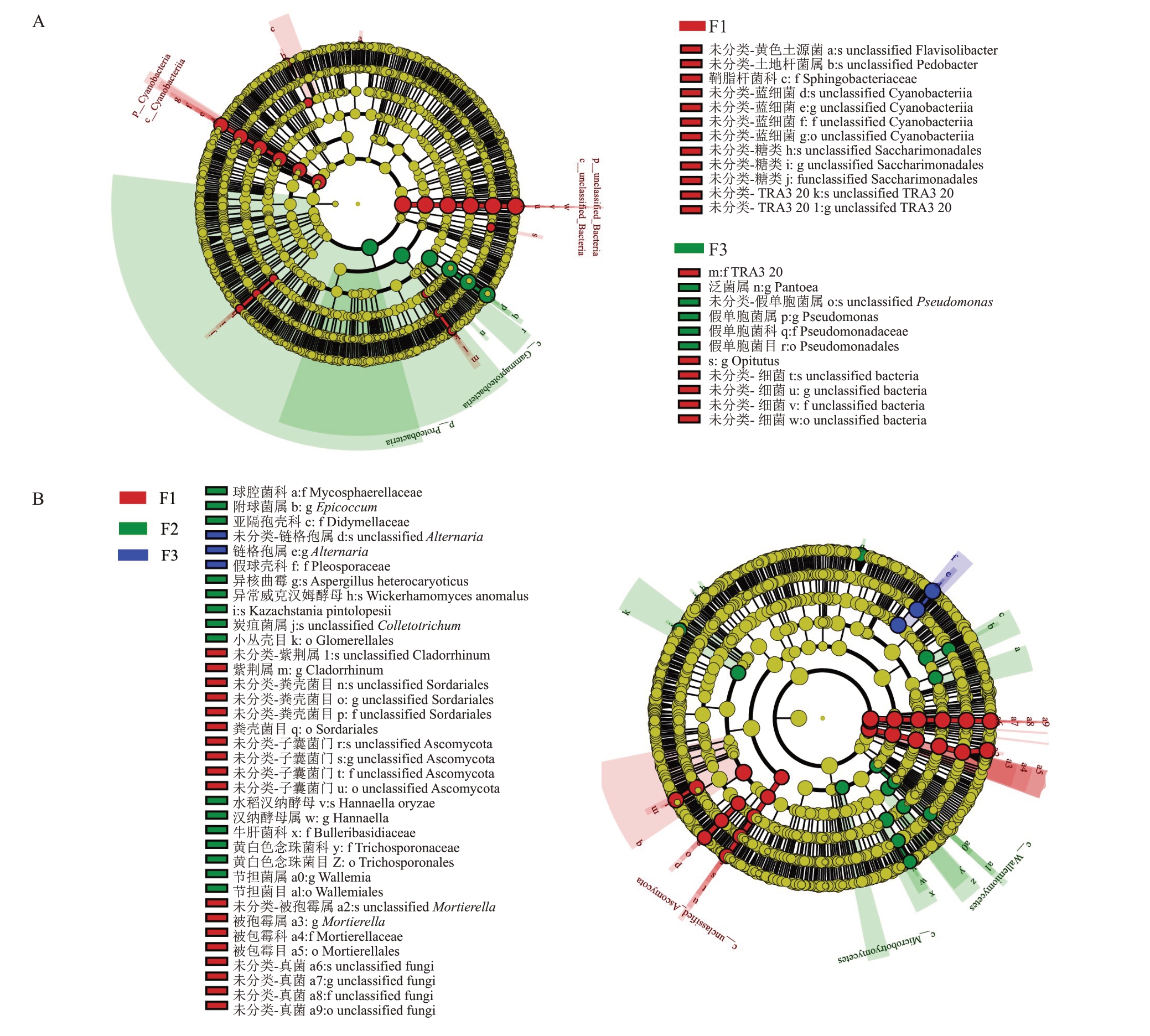
图7 不同发病程度的猕猴桃花组织差异物种LeFSe 分析
Fig.7 LeFSe analysis of differential species in kiwifruit flower of different disease degrees
A.细菌;B.真菌。
A.Bacteria;B.Fungi.
对真菌群落差异贡献较大的物种进行分析,如图7-B 所示,健康样本中的差异物种为粪壳菌目、子囊菌门和被孢霉属。随着感病程度的增加,中等发病程度中的差异物种为球腔菌属、附球菌属、亚隔孢壳科;链格孢属为严重发病程度样本中的差异物种。
3 讨 论
大量研究表明,外部病原物入侵作为主要的选择压力影响植物微生物群落的构建[22-23]。目前,花部微生物对病原菌侵入的响应机制的研究较少,可参照叶际微生物应对病原菌侵入的响应结果进行分析。笔者使用高通量测序技术对不同发病程度的猕猴桃花中的微生物群落结构进行表征,研究结果与柑橘黑点病菌侵入叶际时叶际微生物群落变化规律一致,整体微生物群落的均匀程度显著降低[24]。
前期研究表明,植物组织中微生物门水平的相对丰度存在差异,会导致寄主对病原菌的抗性不同[25]。本研究结果表明,不同发病程度猕猴桃花样本中的微生物门水平的相对丰度存在显著差异。厚壁菌门可以促进氮素循环帮助寄主吸收营养,还可产生一些代谢产物抑制病原菌的生长[26]。本研究结果表明,随感病程度增加,花样本中厚壁菌门的相对丰度下降;推测厚壁菌门的含量显著下降可能与猕猴桃寄主感染溃疡病后抗病能力下降有关,在青枯病病菌侵染番茄和黑胫病病菌侵染烟草过程中观察到相同的现象[27-28]。被孢菌门可以分解纤维素和木质素,是碳循环的重要参与者,前期研究中发现健康样本中被孢菌门的相对丰度最高,可能是因为被孢菌门可提高碳源或有机磷的含量,丰富的碳源可以招募一些如放线菌[29-30]等益生菌。笔者在本研究中同样发现,在猕猴桃花的健康样本中被孢菌门的相对丰度较高,可能是猕猴桃树对溃疡病抗性较强的原因之一。
变形菌门中多为病原菌,含量变化可能与发病程度增加直接相关,其中假单胞菌属中包含导致猕猴桃溃疡病的重要病原菌——丁香假单胞菌猕猴桃致病变种。笔者在本研究中发现,猕猴桃花样本中的假单胞菌含量在不同发病程度中存在显著差异,随着感病程度的增加逐渐上升。除了一些致病菌外,该属中多个荧光假单胞菌被报道为植物的有益促生菌,可以通过产生抗生素以及与病原菌争夺营养元素来抑制病原菌的生长[31-33]。无论作为病原菌或者益生菌,假单胞菌属均与猕猴桃溃疡病的发生存在相关性。
真菌中的链格孢属包含大量病原菌,其中链格孢菌也是猕猴桃软腐病的病原菌[34],可以侵染猕猴桃的叶片及花。在本研究中,随着病害程度增加,猕猴桃花中的链格孢属含量显著上升;推测该属侵染后留下的伤口可能有助于溃疡病病菌对猕猴桃植株的侵染,此外该菌可能与溃疡病病菌协同侵染,加重病害的发生[35]。被孢霉属可以产生花生四烯酸(arachidonic acid,ARA),ARA作为一种不饱和脂肪酸,可以诱导多种植物对病原菌产生防御反应[36]。本研究中被孢霉属在健康样本中显著富集,可能是因为被孢霉属促进养分的吸收,增强了树势,从而提高了对溃疡病菌的抵抗能力。季也蒙毕赤酵母可在植物的伤口处快速定殖,对伤口形成起保护作用,阻碍病原菌侵染,同时还可提高寄主对病原菌抗性相关的酶活性,从而提高病原菌抗性[37]。笔者在本研究中同样发现,季也蒙毕赤酵母在健康样本中的含量较高,可能抑制了溃疡病病菌在伤口的附着,从而降低溃疡病的发生。
4 结 论
笔者通过高通量测序技术研究了不同溃疡病发病程度下猕猴桃花部的微生物群落,发现溃疡病病菌的入侵改变了猕猴桃花部的微生物群落结构,降低了细菌的多样性,提高了真菌的丰富度。被孢霉属等有益菌在健康样本中富集,假单胞菌属在严重发病程度样本中富集。与健康的样本相比,细菌中变形菌门及真菌中子囊菌门和担子菌门的相对丰度与发病程度呈正相关;被孢菌门、厚壁菌门和蓝细菌门相对丰度与发病程度呈负相关。猕猴桃感染溃疡病后期花可能通过招募有益微生物来抵抗溃疡病病菌的侵染,增强植株抗病性。本研究结果有助于从微生态的角度探明猕猴桃溃疡病的发病机制,此外,研究中发现的一些潜在的益生菌株可为猕猴桃溃疡病的生物防治提供一定的研究方向。
[1] 杜贞娜,程斐,郭怀宇,臧威,孙剑秋,郭天荣.猕猴桃溃疡病病原菌的研究进展[J].绍兴文理学院学报,2022,42(2):50-55.DU Zhenna,CHENG Fei,GUO Huaiyu,ZANG Wei,SUN Jianqiu,GUO Tianrong.Research progress on pathogen of kiwifruit canker[J].Journal of Shaoxing University,2022,42(2):50-55.
[2] 韩明丽,张志友,陈丽萍,钱伟红,李艳冬,赵根.猕猴桃溃疡病发生的影响因素及其防治方法[J].湖南农业科学,2013(21):77-80.HAN Mingli,ZHANG Zhiyou,CHEN Liping,QIAN Weihong,LI Yandong,ZHAO Gen.Influencing factors and control method for bacterial canker disease of kiwifruit[J].Hunan Agricultural Sciences,2013(21):77-80.
[3] 田野,李丽丽,杜春梅,李黎,田立娟,申健,斯克里普琴科N V,刘德江.猕猴桃溃疡病的研究进展[J].江苏农业科学,2023,51(15):8-15.TIAN Ye,LI Lili,DU Chunmei,LI Li,TIAN Lijuan,SHEN Jian,SCRIPCENCO N V,LIU Dejiang.Research progress of kiwifruit canker disease[J].Jiangsu Agricultural Sciences,2023,51(15):8-15.
[4] 游雨欣,戴德江,罗金燕,朱洁,李斌.猕猴桃溃疡病防治策略的研究现状与展望[J].浙江农业科学,2022,63(6):1322-1328.YOU Yuxin,DAI Dejiang,LUO Jinyan,ZHU Jie,LI Bin.Research status and prospect of control strategies for kiwifruit canker[J].Journal of Zhejiang Agricultural Sciences,2022,63(6):1322-1328.
[5] 李黎,钟彩虹,李大卫,张胜菊,黄宏文.猕猴桃细菌性溃疡病的研究进展[J].华中农业大学学报,2013,32(5):124-133.LI Li,ZHONG Caihong,LI Dawei,ZHANG Shengju,HUANG Hongwen.Research progress on bacterial canker disease of kiwifruit[J].Journal of Huazhong Agricultural University,2013,32(5):124-133.
[6] SHAFI J,TIAN H,JI M S.Bacillus species as versatile weapons for plant pathogens:A review[J].Biotechnology & Biotechnological Equipment,2017,31(3):446-459.
[7] GE A H,LIANG Z H,XIAO J L,ZHANG Y,ZENG Q,XIONG C,HAN L L,WANG J T,ZHANG L M.Microbial assembly and association network in watermelon rhizosphere after soil fumigation for Fusarium wilt control[J].Agriculture,Ecosystems&Environment,2021,312:107336.
[8] KWAK M J,KONG H G,CHOI K,KWON S K,SONG J Y,LEE J,LEE P A,CHOI S Y,SEO M,LEE H J,JUNG E J,PARK H,ROY N,KIM H,LEE M M,RUBIN E M,LEE S W,KIM J F.Rhizosphere microbiome structure alters to enable wilt resistance in tomato[J].Nature Biotechnology,2018,36(11):1100-1109.
[9] MAZURIER S,CORBERAND T,LEMANCEAU P,RAAIJMAKERS J M.Phenazine antibiotics produced by fluorescent pseudomonads contribute to natural soil suppressiveness to Fusarium wilt[J].The ISME Journal,2009,3(8):977-991.
[10] 佐长赓,王静怡,牛新湘,刘萍,管力慧,党文芳,杨红梅,楚敏,王宁,林青,王有武,娄恺,史应武.内生菌与根际细菌对棉花的促生与诱导抗病作用[J].西南农业学报,2022,35(4):757-763.ZUO Changgeng,WANG Jingyi,NIU Xinxiang,LIU Ping,GUAN Lihui,DANG Wenfang,YANG Hongmei,CHU Min,WANG Ning,LIN Qing,WANG Youwu,LOU Kai,SHI Yingwu.Effects of endophytes and rhizosphere bacteria on cotton growth promotion and disease resistance induction[J].Southwest China Journal of Agricultural Sciences,2022,35(4):757-763.
[11] 农倩,张雯龙,蓝桃菊,苏琴,陈艳露,张艳,覃丽萍,谢玲.一株抗香蕉枯萎病DSE 菌株的筛选鉴定及抗病机理初探[J].热带作物学报,2017,38(3):559-564.NONG Qian,ZHANG Wenlong,LAN Taoju,SU Qin,CHEN Yanlu,ZHANG Yan,QIN Liping,XIE Ling.Screening and identification of dark septate endophyte strain L-14 and its mechanism of banana Fusarium wilt disease resistance[J].Chinese Journal of Tropical Crops,2017,38(3):559-564.
[12] LEE H J,KIM S H,KIM D R,CHO G,KWAK Y S.Dynamics of bacterial communities by apple tissue:Implications for apple health[J].Journal of Microbiology and Biotechnology,2023,33(9):1141-1148.
[13] 王瑜.伯克霍尔德氏菌CR7 防治小麦赤霉病的作用机制初探[D].杨凌:西北农林科技大学,2023.WANG Yu.A preliminary study on the mechanism of Burkholderia CR7 in the control of Fusarium head blight[D].Yangling:Northwest A&F University,2023.
[14] 于静,宋新颖,李莹,何康,郭志青,许曼琳,张霞,迟玉成.伯克氏菌对群结腐霉引起的花生果腐病的生防潜力[J].花生学报,2023,52(2):52-60.YU Jing,SONG Xinying,LI Ying,HE Kang,GUO Zhiqing,XU Manlin,ZHANG Xia,CHI Yucheng.Biocontrol potentiality of endophytic Burkholderia cepacia against peanut pod rot caused by Pythium myriotylum[J].Journal of Peanut Science,2023,52(2):52-60.
[15] FRIDMAN S,IZHAKI I,GERCHMAN Y,HALPERN M.Bacterial communities in floral nectar[J].Environmental Microbiology Reports,2012,4(1):97-104.
[16] KONG H G,HAM H,LEE M H,PARK D S,LEE Y H.Microbial community dysbiosis and functional gene content changes in apple flowers due to fire blight[J].Plant Pathology Journal,2021,37(4):404-412.
[17] 欧光敏,郑良豹,梁红,周玲艳.猕猴桃林下套种大豆土壤微生物群落结构分析[J].广东农业科学,2024,51(1):63-72.OU Guangmin,ZHENG Liangbao,LIANG Hong,ZHOU Lingyan.Analysis of interplanting soybean under kiwifruit forest on soil microbial community structure[J].Guangdong Agricultural Sciences,2024,51(1):63-72.
[18] 朱海云,马瑜,柯杨,李勃.不同种植年限猕猴桃园土壤微生物功能多样性研究[J].微生物学杂志,2019,39(5):64-72.ZHU Haiyun,MA Yu,KE Yang,LI Bo.Functional diversities of soil microbial community in kiwifruit orchards of different planting years[J].Journal of Microbiology,2019,39(5):64-72.
[19] 吴文能,李勇,雷霁卿,王瑞.高通量测序技术对猕猴桃叶斑病微生物多样性研究[J].北方园艺,2020(20):21-26.WU Wenneng,LI Yong,LEI Jiqing,WANG Rui.Microbial diversity of kiwifruit leaf spot disease by high-throughput sequencing[J].Northern Horticulture,2020(20):21-26.
[20] QUAST C,PRUESSE E,YILMAZ P,GERKEN J,SCHWEER T,YARZA P,PEPLIES J,GLÖCKNER F O.The SILVA ribosomal RNA gene database project:Improved data processing and web-based tools[J].Nucleic Acids Research,2013,41(Database issue):D590-D596.
[21] KÕLJALG U,LARSSON K H,ABARENKOV K,NILSSON R H,ALEXANDER I J,EBERHARDT U,ERLAND S,HØILAND K,KJØLLER R,LARSSON E,PENNANEN T,SEN R,TAYLOR A F S,TEDERSOO L,VRÅLSTAD T,URSING B M.UNITE:A database providing web-based methods for the molecular identification of ectomycorrhizal fungi[J].New Phytologist,2005,166(3):1063-1068.
[22] CHAPELLE E,MENDES R,BAKKER P A H M,RAAIJMAKERS J M.Fungal invasion of the rhizosphere microbiome[J].The ISME Journal,2016,10(1):265-268.
[23] FERNÁNDEZ-GONZÁLEZ A J,CARDONI M,CABANÁS C G L,VALVERDE- CORREDOR A,VILLADAS P J,FERNÁNDEZ-LÓPEZ M,MERCADO-BLANCO J.Linking belowground microbial network changes to different tolerance level towards Verticillium wilt of olive[J].Microbiome,2020,8(1):11.
[24] DEYETT E,ROLSHAUSEN P E.Temporal dynamics of the sap microbiome of grapevine under high pierce’s disease pressure[J].Frontiers in Plant Science,2019,10:1246.
[25] XI H,SHEN J L,QU Z,YANG D Y,LIU S M,NIE X H,ZHU L F.Effects of long-term cotton continuous cropping on soil microbiome[J].Scientific Reports,2019,9:18297.
[26] RÄDECKER N,POGOREUTZ C,VOOLSTRA C R,WIEDENMANN J,WILD C.Nitrogen cycling in corals:The key to understanding holobiont functioning?[J].Trends in Microbiology,2015,23(8):490-497.
[27] LEE S M,KONG H G,SONG G C,RYU C M.Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease[J].The ISME Journal,2021,15(1):330-347.
[28] 吴寿明,高正锋,白茂军,潘首慧,范成平,董延鑫,杨索,王莹,陈汶,杨小龙,岑浩,田玉琴,昝建朋,吴海,吕芬.烟草黑胫病不同发病程度与根际微生物间的响应关系[J].中国土壤与肥料,2023(7):223-231.WU Shouming,GAO Zhengfeng,BAI Maojun,PAN Shouhui,FAN Chengping,DONG Yanxin,YANG Suo,WANG Ying,CHEN Wen,YANG Xiaolong,CEN Hao,TIAN Yuqin,ZAN Jianpeng,WU Hai,LÜ Fen.Response between different incidence of tobacco black shank disease and rhizosphere microorganisms[J].Soil and Fertilizer Sciences in China,2023(7):223-231.
[29] KOECHLI C,CAMPBELL A N,PEPE-RANNEY C,BUCKLEY D H.Assessing fungal contributions to cellulose degradation in soil by using high-throughput stable isotope probing[J].Soil Biology and Biochemistry,2019,130:150-158.
[30] 乔乔,王淮,姚日生,朱慧霞,邓胜松.长孢被孢霉PFY 降解木质素的初步研究[J].化工进展,2012,31(增刊1):80-85.QIAO Qiao,WANG Huai,YAO Risheng,ZHU Huixia,DENG Shengsong.Degradation of lignin by Mortierella elongata PFY[J].Chemical Industry and Engineering Progress,2012,31(Suppl.1):80-85.
[31] 代鹏,陈海琴,顾震南,张灏,陈永泉,陈卫.高山被孢霉生产多不饱和脂肪酸发酵条件的研究进展[J].食品工业科技,2014,35(5):354-359.DAI Peng,CHEN Haiqin,GU Zhennan,ZHANG Hao,CHEN Yongquan,CHEN Wei.Research progress in fermentation condition for polyunsaturated fatty acids by Mortierella alpina[J].Science and Technology of Food Industry,2014,35(5):354-359.
[32] 汪心玉,芦钰,邱艳红,张海军,张力群,李健强,王红阳,罗来鑫,徐秀兰.荧光假单胞菌2P24 防控瓜类果斑病机制初探[J].中国生物防治学报,2023,39(3):575-584.WANG Xinyu,LU Yu,QIU Yanhong,ZHANG Haijun,ZHANG Liqun,LI Jianqiang,WANG Hongyang,LUO Laixin,XU Xiulan.Preliminary study on the control mechanism of Pseudomonas fluorescens 2P24 on bacterial fruit blotch[J].Chinese Journal of Biological Control,2023,39(3):575-584.
[33] 施河丽,谭军,谭绍安,彭五星,尹忠春,祁高富,向必坤.荧光假单胞菌缓解植烟土壤酸化效果及对烟草青枯病的防治作用[J].烟草科技,2023,56(2):19-25.SHI Heli,TAN Jun,TAN Shaoan,PENG Wuxing,YIN Zhongchun,QI Gaofu,XIANG Bikun.Effects of Pseudomonas fluore-scens on alleviating soil acidification and controlling tobacco bacterial wilt[J].Tobacco Science & Technology,2023,56(2):19-25.
[34] LI L,PAN H,CHEN M Y,ZHANG S J,ZHONG C H.Isolation and identification of pathogenic fungi causing postharvest fruit rot of kiwifruit(Actinidia chinensis)in China[J].Journal of Phytopathology,2017,165(11/12):782-790.
[35] 冯中红,孙广宇.链格孢属及相关属分类研究新进展[J].菌物研究,2020,18(4):294-303.FENG Zhonghong,SUN Guangyu.Advances in the classification of Alternaria and related genera[J].Journal of Fungal Research,2020,18(4):294-303.
[36] EROSHIN V K,DEDYUKHINA E G.Effect of lipids from Mortierella hygrophila on plant resistance to phytopathogens[J].World Journal of Microbiology and Biotechnology,2002,18(2):165-167.
[37] YAN Y,ZHANG X Y,ZHENG X F,APALIYA M T,YANG Q Y,ZHAO L N,GU X Y,ZHANG H Y.Control of postharvest blue mold decay in pears by Meyerozyma guilliermondii and it’s effects on the protein expression profile of pears[J].Postharvest Biology and Technology,2018,136:124-131.