猕猴桃作为中国重要的水果,在全国多地均有种植。多年来,众多学者针对猕猴桃的种植条件、抗病虫害能力和营养成分等方面进行了研究,旨在促进猕猴桃产业发展[1-3]。红阳猕猴桃(Actinidia chinensis‘Hongyang’)为中华猕猴桃变种,由四川苍溪县野生中华猕猴桃中心培育,果心带有红色放射状条纹,果实大且果形整齐,口感丰富,富含糖分和维生素C等营养物质,深受消费者喜爱,并且它的抗逆性强、果实耐贮藏,是中国重要的经济树种[1,4]。
果实香气是品质的重要指标,由酯、醛、酮、醇、萜烯类等多种挥发性香气物质综合构成。这些香气物质的组成以及含量会随着果实的成熟而改变,使得果实在不同阶段释放出不同的气味,因此,香气可侧面反映水果成熟度,用作评价果实适宜采收期的标准之一[5]。此外,水果的特征香气还可以刺激消费者的嗅觉,引起消费者食欲和购买欲,对水果产业发展具有重要意义[6]。
套袋在水果栽培中被广泛应用,能通过物理防护有效避免果实因雨水、鸟类、昆虫以及阳光照射不均等外界因素导致的落果、水果畸形以及食用口感差等问题,从而提高商品果率[7]。但套袋也会阻碍果实发育过程中接受光照,对果实的内在品质产生影响,这一影响因品种而异。对番石榴套袋,提高了果实质量以及抗坏血酸含量[7];对枇杷套袋能够显著提高果实质量和可溶性糖含量,降低可滴定酸含量[8];但对西南冷凉高地红富士苹果套袋则会降低果实品质[9]。就猕猴桃而言,有研究表明套袋显著降低海沃德猕猴桃果实中蔗糖以及叶绿素含量,但单果质量、可溶性固形物含量及干物质含量均无明显变化[10];而用适宜颜色的果袋对红阳猕猴桃套袋却能提高果实质量、干物质含量、可溶性糖含量、ASA含量和固酸比,降低可滴定酸含量[11]。
遮光对果实香气也具有显著影响。在果实的各个发育时期进行去光处理均会显著抑制玫瑰香葡萄中萜类物质的合成,但在果实成熟阶段进行遮光处理则会促进京香玉葡萄中醛、醇和酮的积累[12-13]。在硬核期前对白凤水蜜桃进行套袋处理能显著提高果皮中的香气含量,对果肉中的香气含量无影响[14]。利用橙色袋在果实缓慢增长期对玉露水蜜桃进行套袋遮光能显著提升果实中的醛类含量[15]。对红富士苹果进行套袋则减少了果实中香气物质种类和含量[16]。目前探究遮光对猕猴桃香气的影响文献较少,仅集中在探讨采后贮藏方式对香气的影响方面。0.5 μL·L-1的1-甲基环丙烯(1-MCP)被证明是改善猕猴桃布鲁诺采后品质并保持在贮藏期间香气发展的最适浓度[17];低温贮藏后使用脱落酸(ABA)处理增加了采后猕猴桃香气成分,并提高了相关合成酶醇酰基转移酶(AAT)、支链氨基酸转氨酶(BCAT)和过氧化氢裂解酶(HPL)活性[18];Han等[17]、Günther 等[18]和Huan 等[19]对华特猕猴桃进行冷藏后乙烯处理,发现相比未处理猕猴桃具有更浓郁的果香。此外,关于猕猴桃香气合成关键基因已有研究,Zhang 等[20]通过转录组分析鉴定出3 个酯类合成相关基因AdFAD1、AdALDH2 和AdAT17;张琳等[21]研究表明,乙烯抑制剂和外源乙烯处理下猕猴桃果实中的基因AcAT1、AcAT2、AcAT17表达量变化与AAT活性变化呈正相关;董婧等[22]对比6 种猕猴桃品种发现基因AcAT16 表达量与丁酸乙酯含量呈正相关。关于遮光影响猕猴桃香气合成的机制仍然未知。基于此,笔者以红阳猕猴桃为材料,将其从幼果到果实成熟分为六个时段利用套袋分别进行遮光处理,使用顶空-固相微萃取/气相色谱-质谱联用技术探究不同时段去光对红阳猕猴桃果实香气的影响,并对全时段遮光和全时段未遮光果实开展转录组测序分析,以期筛选出受光调控的香气形成关键基因,为探究遮光影响猕猴桃果实香气的分子机制奠定基础,同时找到猕猴桃套袋的适宜时段,为进一步优化猕猴桃栽培措施、生产高品质猕猴桃提供理论依据。
1 材料和方法
1.1 材料
笔者以红阳猕猴桃为材料,采自于广西植物研究所猕猴桃种质资源圃(东经110°17,北纬25°4)。试验树为5~6年生中华砧红阳猕猴桃,株行距为3 m×3 m,雌雄比例为8∶1,棚架栽培[23]。
1.2 试验处理
对猕猴桃果实使用黑色双层果袋进行遮光处理,遮光度95%。用花后天数(days after anthesis,简称DAA)标记处理的时间,选取6 株生长状态一致的果树,每组处理时分别在6株果树上各选取20颗位置、大小和发育相对一致的果实进行套袋,每2株作为一个生物学重复。在整个发育过程暴露于阳光下的果实为对照(CK),从花后20 d 开始到果实成熟,每20 d遮光为一个处理组,具体处理见表1。
表1 试验分组
Table 1 Experimental grouping
分组Group T1 T2 T3 T4 T5 T6 T7 CK遮光时段Shading period时段ⅠPeriod Ⅰ:20~40DAA时段ⅡPeriod Ⅱ:40~60DAA时段ⅢPeriod Ⅲ:60~80DAA时段ⅣPeriod Ⅳ:80~100DAA时段ⅥPeriod Ⅵ:100~120DAA时段ⅦPeriod Ⅶ:120~140DAA一直遮光Constant shading一直未遮光Without shading
果实可溶性固形物含量达到7%后采摘。采摘下来的果实在室温放置直到后熟完成,检测可溶性固形物含量达到20%,即进行香气成分的提取和检测[24]。
1.3 香气测定
香气测定方法参考刘翠霞等[25]。采用顶空固相萃取(HS-SPME)和气相色谱-质谱联用(GC-MS)检测果实香气成分。每个处理随机取5~10个果实,去皮后液氮速冻,加入10%的氯化钙,用磨样机预冷后打磨成粉末状,每次称取5 g,3 次重复,加入10 μL 41 mg·L-1的3-辛醇溶液作为内标,于20 mL 顶空瓶中进行萃取。萃取结束后,将萃取头插入气相色谱的进样口进行解析。萃取头采用30 μm PDMS/DVB SPME萃取头(Supelco公司,美国)。采用气质联用仪对芳香物质进行分析鉴定。气相色谱型号为7890A,质谱仪型号为5975C(安捷伦公司,美国)。定性分析通过AMDS 解卷积软件。解卷积后检索标准化合物的质谱图(NIST08)、广西落叶果树团队已经分析过的标准品的保留时间以及与相关文献报道进行保留指数等信息比对。对挥发物的定量分析采用3-辛醇作为内标物进行半定量分析。
1.4 转录组分析
测序样品为全时段遮光(T7)和全时段未遮光(CK)果实。取用于香气测定的T7和对照果实样品液氮速冻后使用磨样机快速研磨成粉末,装入离心管放至-80 ℃冰箱保存。委托武汉迈维代谢生物科技股份有限公司完成样品RNA提取和转录组测序,3 次重复。基于表达定量结果使用DESeq2 软件进行样品组间的差异表达分析,筛选阈值为:|log2Fold Change|≥1、FDR<0.05,将筛选出的基因在基因本体GO(Gene Ontology,GO)以及京都基因与基因组百科全书(Kyoto encyclopedia of genes and genomes,KEGG)数据库进行比对,获得不同样品差异表达基因的功能及相关代谢通路信息。根据GO和KEGG 注释结果,对差异基因进行功能分类与富集分析。
1.5 数据处理
采用Excel 2016 对数据进行处理,采用SPSS 20.0进行显著性分析以及主成分分析并计算综合得分,计算方法参考张文霖[26]的报道,使用Duncan 法对数据进行多重比较,采用Origin2021作图。
2 结果与分析
2.1 不同时段遮光处理下香气种类及含量分析
在红阳猕猴桃的处理组和对照组中,共检测到38种香气物质,包括15种酯类、11种醛类、8种萜烯类、3种醇类、1种其他类。
红阳猕猴桃果实在不同处理下的香气种类介于19~37 种(图1-A),其中T6 和T7 果实香气种类较多,分别为37和32种;T2和T5果实香气种类最少,均为19 种;T1、T3 和T4 的果实香气种类与对照相近,分别为24种、25种和22种。酯类物质种类在每个处理组中较稳定,均在14~15 种。醛类物质种类在各处理组中的变化较大,介于3~11 种之间,其中T6 红阳猕猴桃果实中醛类物质种类最多,为11 种,T7 其次,为8 种,T5 果实中醛类物质种类最少,为3种。萜烯类物质种类在各处理组中变化也较大,介于0~8 种之间,T7 红阳猕猴桃果实中萜烯类物质种类最多,为8种,T6其次,为7种,T4果实中无萜烯类物质,T2、T5和对照,仅为1种。各处理组中醇类物质种类较少,在0~3种之间。

图1 不同处理组红阳猕猴桃果实香气总含量及各成分占比
Fig.1 Aroma content of Hongyang kiwifruit fruits in different treatment groups and the proportion of each constituent
A.不同处理组果实香气种类;B.不同处理组果实香气总含量;C.不同处理组果实各类香气含量;D.不同处理组果实各类香气占比。不同小写字母表示差异显著(p<0.05)。下同。
A.Aroma components of fruit in different treatment groups; B.Total aroma content of fruits in different treatment groups; C.Content of various types of aroma in fruits in different treatment groups;D.Percentage of various types of aroma in fruits in different treatment groups.Different small letters show significant difference at 0.05 level.The same below.
在各时段遮光均抑制了红阳猕猴桃果实总挥发性香气的积累。其中,T4 果实中香气含量最高,T2和T5 其次。除T4 外,其他处理组香气物质总含量均显著低于对照,T2 和T5 约为对照香气物质总含量的76%和66%,而全遮光处理的T7 只有对照的13%(图1-B)。酯类物质为各处理组中红阳猕猴桃果实中的主要香气成分,在各处理组中含量均占各组香气物质总含量的90%以上;醛类物质次之,介于1%~7%之间;萜烯类和醇类物质含量在各处理组总含量中的占比均在2%以下(图1-C~D)。
各个时期遮光对各类香气物质的影响程度具有差别。对不同处理组中的酯类、醛类、萜烯类和醇类含量进行比较发现:T4红阳猕猴桃果实中酯类含量与对照无显著差异,T1、T2、T3、T5、T6 和T7 果实中的酯类含量显著低于对照,分别约占对照果实中酯类物质含量的24%、77%、41%、66%、30%和13%(图2-A);T4和T6果实中醛类物质含量与对照无显著差异,T1、T2、T3和T7中红阳猕猴桃醛类含量显著低于对照,分别约占对照果实中醛类物质含量的53%、64%、50%和49%(图2-B);T1和T2中萜烯类含量与对照无显著差异,T3、T5、T6和T7中萜烯类含量显著高于对照,相比对照果实中萜烯类物质含量提高59%、63%、147%和202%,此外,T4果实中无萜烯类物质累积(图2-C);仅T6红阳猕猴桃果实的醇类物质含量显著高于对照,相比于对照果实中的醇类物质含量提高136%,T2和T5果实中无醇类物质累积,其他处理组果实中醇类物质含量显著低于对照(图2-D)。
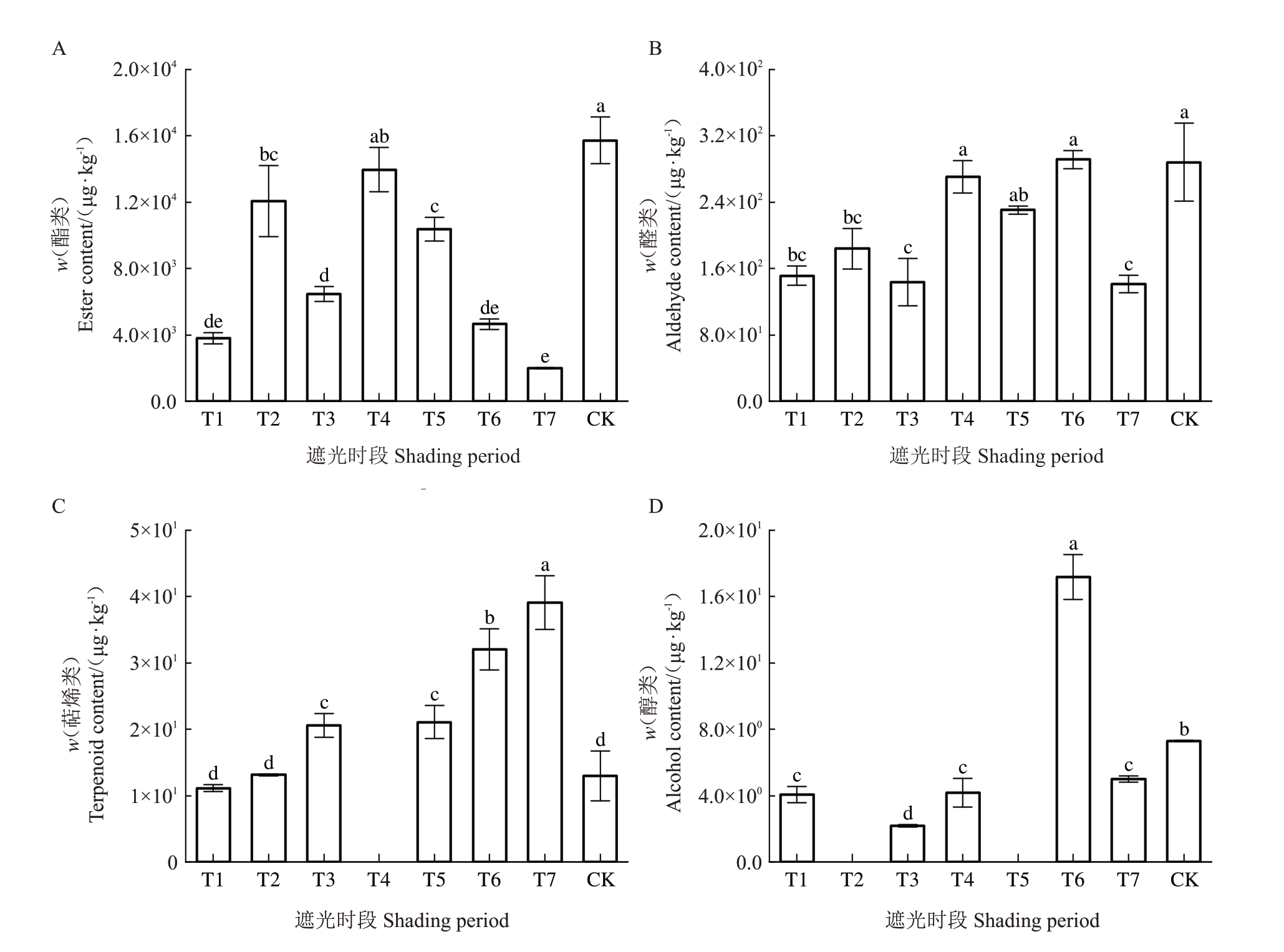
图2 不同处理组红阳猕猴桃果实各类别香气物质含量
Fig.2 Content of various types of aroma substances in Hongyang kiwifruit fruits of different treatment groups
A.不同处理组果实酯类含量;B.不同处理组果实醛类含量;C.不同处理组果实萜烯类含量;D.不同处理组果实醇类含量。
A.Ester content of fruits in different treatment groups;B.Aldehyde content of fruits in different treatment groups;C.Terpenoid content of fruits in different treatment groups;D.Alcohol content of fruits in different treatment groups.
2.2 不同时段遮光处理下红阳猕猴桃果实香气组分分析
在检测到的香气成分中,各处理组中红阳猕猴桃果实都含有的成分有15 种,分别是丁酸乙酯、丁酸甲酯、苯甲酸甲酯、丁酸丁酯、己酸乙酯、己酸甲酯、乙酸乙酯、苯甲酸乙酯、反式巴豆酸甲酯、甲酸丙酯、辛酸乙酯、戊酸乙酯、(E)-2-己烯醛、己醛和戊醛(表2)。
表2 不同处理组中红阳猕猴桃果实香气比较
Table 2 Comparison of fruit aroma of Hongyang kiwifruit in different treatment groups (μg·kg-1)
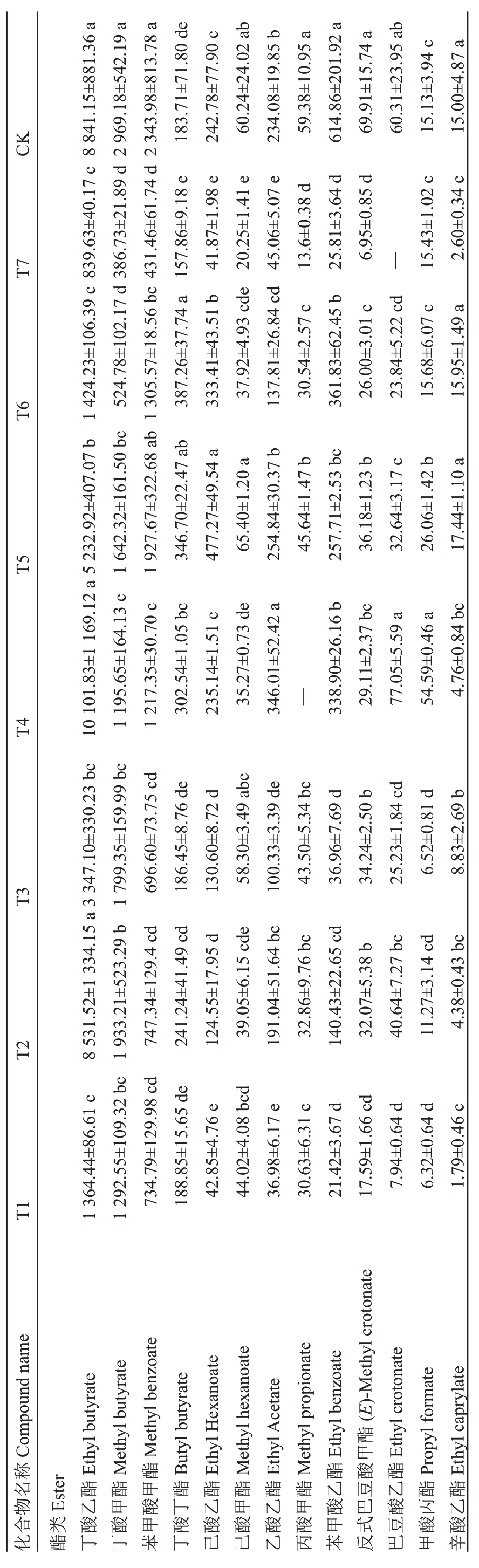
CK 8 841.15±881.36a 2 969.18±542.19a 2 343.98±813.78a 183.71±71.80de 242.78±77.90c 60.24±24.02 ab 234.08±19.85b 59.38±10.95 a 614.86±201.92 a 69.91±15.74 a 60.31±23.95 ab 15.13±3.94c 15.00±4.87a T7 839.63±40.17c 386.73±21.89d 41.87±1.98e 20.25±1.41e 431.46±61.74d 45.06±5.07e 157.86±9.18 e 13.6±0.38 d 25.81±3.64d 6.95±0.85 d—15.43±1.02c 2.60±0.34 c T6 1 424.23±106.39c 1 305.57±18.56 bc 387.26±37.74a 524.78±102.17 d 333.41±43.51b 37.92±4.93cde 137.81±26.84cd 30.54±2.57c 361.83±62.45b 26.00±3.01c 23.84±5.22cd 15.68±6.07c 15.95±1.49a T5 5 232.92±407.07b 1 927.67±322.68ab 477.27±49.54a 65.40±1.20a 254.84±30.37b 346.70±22.47ab 45.64±1.47b 257.71±2.53 bc 36.18±1.23b 32.64±3.17c 26.06±1.42b 17.44±1.10a 4.76±0.84 bc 302.54±1.05 bc 35.27±0.73de 10101.83±1 169.12 a 1 195.65±164.13c 1 642.32±161.50bc 235.14±1.51 c 346.01±52.42a—338.90±26.16b 29.11±2.37bc 77.05±5.59a 54.59±0.46a T4 1 217.35±30.70 c 58.30±3.49abc 43.50±5.34bc 36.96±7.69d 34.24±2.50b 25.23±1.84cd 6.52±0.81 d 8.83±2.69 b 696.60±73.75cd 186.45±8.76 de 130.60±8.72 d 100.33±3.39 de T3 3 347.10±330.23bc 1 799.35±159.99bc 4.38±0.43 bc T2 8 531.52±1 334.15 a 1 933.21±523.29b 747.34±129.4cd 241.24±41.49cd 124.55±17.95d 39.05±6.15cde 191.04±51.64bc 32.86±9.76bc 140.43±22.65cd 32.07±5.38b 40.64±7.27bc 11.27±3.14cd 1 364.44±86.61 c 1 292.55±109.32bc 734.79±129.98 cd 188.85±15.65de 42.85±4.76e 44.02±4.08bcd 36.98±6.17e 30.63±6.31c 21.42±3.67d 17.59±1.66cd 7.94±0.64 d 6.32±0.64 d 1.79±0.46 c T1 Compoundname Methylbenzoate Ethyl benzoate(E)-Methylcrotonate酯甲Ethyl crotonate称Ethyl butyrate Methylbutyrate酯Butyl butyrate Ethyl Hexanoate Methylhexanoate Ethyl Acetate Methylpropionate酯酸酯Propylformate Ethyl caprylate名酯酯甲酯酯酯酯酯乙豆乙酯酯物Ester乙甲酸丁乙甲乙甲酸巴酸丙乙合类酸酸甲酸酸酸酸酸甲式豆酸酸化酯丁丁苯丁己己乙丙苯反巴甲辛
表2 (续) Table 2 (Continued) (μg· kg-1)
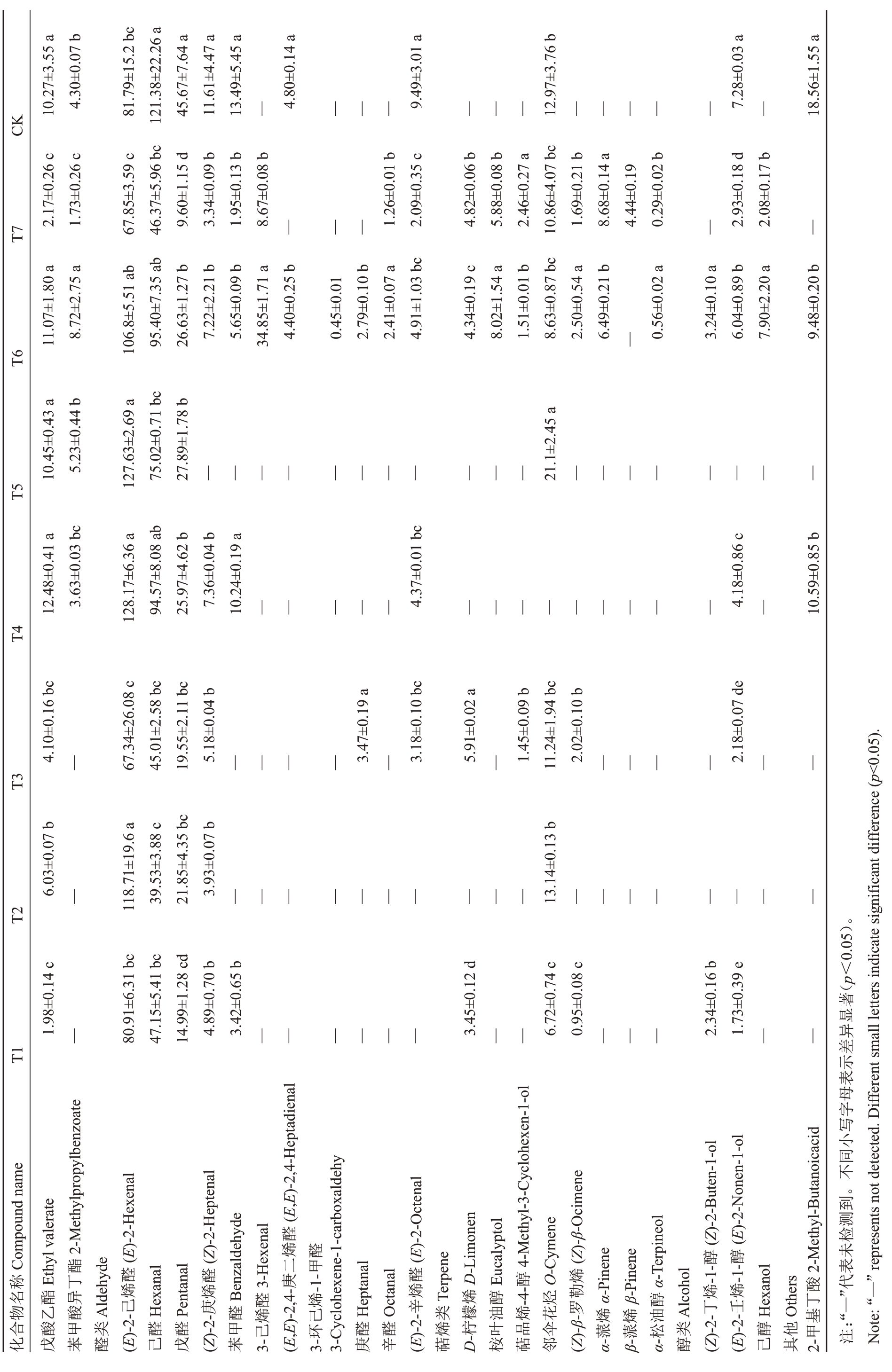
4.30±0.07 b CK 10.27±3.55a 81.79±15.2bc 121.38±22.26a 45.67±7.64a 11.61±4.47a 13.49±5.45a—4.80±0.14 a——9.49±3.01 a——12.97±3.76b——7.28±0.03 a—18.56±1.55a T7 2.17±0.26 c 1.73±0.26 c 67.85±3.59c 46.37±5.96bc 9.60±1.15 d 3.34±0.09 b 1.95±0.13 b 8.67±0.08 b——1.26±0.01 b 2.09±0.35 c 4.82±0.06 b 5.88±0.08 b 2.46±0.27 a 10.86±4.07bc 1.69±0.21 b 8.68±0.14 a 4.44±0.19 0.29±0.02 b—2.93±0.18 d 2.08±0.17 b—8.72±2.75 a 11.07±1.80a 106.8±5.51ab 5.65±0.09 b 26.63±1.27b 95.40±7.35ab 7.22±2.21 b 4.40±0.25 b 0.45±0.01 2.79±0.10 b 2.41±0.07 a 4.91±1.03 bc 4.34±0.19 c 8.02±1.54 a 1.51±0.01 b 8.63±0.87 bc 2.50±0.54 a 6.49±0.21 b 3.24±0.10 a 6.04±0.89 b 7.90±2.20 a 9.48±0.20 b T6 34.85±1.71a—0.56±0.02 a T5 10.45±0.43a 5.23±0.44 b 127.63±2.69 a 75.02±0.71bc 27.89±1.78b——21.1±2.45 a——12.48±0.41a 3.63±0.03 bc 94.57±8.08ab 25.97±4.62b 7.36±0.04 b 10.24±0.19a——4.37±0.01 bc——4.18±0.86 c—10.59±0.85b T4 128.17±6.36 a 4.10±0.16 bc 5.18±0.04 b 5.91±0.02 a 2.02±0.10 b T3—67.34±26.08 c 45.01±2.58bc 19.55±2.11bc——3.47±0.19 a—3.18±0.10 bc—1.45±0.09 b 11.24±1.94bc——2.18±0.07 de——6.03±0.07 b 3.93±0.07 b T2—118.71±19.6 a 39.53±3.88c 21.85±4.35bc——13.14±0.13b——1.98±0.14 c 4.89±0.70 b 3.42±0.65 b 0.95±0.08 c 1.73±0.39 e T1—80.91±6.31bc 47.15±5.41bc 14.99±1.28cd——3.45±0.12 d——6.72±0.74 c——2.34±0.16 b——Compoundname 2-Methylpropylbenzoate(E,E)-2,4-Heptadienal Ethyl valerate醛烯醛(Z)-2-Buten-1-ol(E)-2-Nonen-1-ol酯(E)-2-Hexenal(Z)-2-Heptenal二(E)-2-Octenal 4-Methyl-3-Cyclohexen-1-ol(Z)-β-Ocimene 2-Methyl-Butanoicacid称丁醛醛3-Hexenal醛D-Limonen Eucalyptol O-Cymene烯α-Terpineol-1-醇-1-醇名酯异烯烯Benzaldehyde-1-甲烯Terpene烯醇-4-醇烃勒α-Pinene β-Pinene烯烯酸物乙酸Aldehyde Hexanal Pentanal醛醛烯Heptanal Octanal类檬油烯花醇Alcohol Hexanol Others丁合酸甲类醛醛甲烯己醛醛烯叶品伞烯烯油类醇他基化戊苯醛(E)-2-己己戊(Z)-2-庚苯3-己(E,E)-2,4-庚3-环3-Cyclohexene-1-carboxaldehy庚辛(E)-2-辛萜D-柠桉萜邻(Z)-β-罗α-蒎β-蒎α-松醇(Z)-2-丁(E)-2-壬己其2-甲著(p<0.05)。显异差示表母字写小同不。到测检未表:“—”代注Note:“—”represents notdetected.Different smalllettersindicate significantdifference(p<0.05).
酯类物质作为猕猴桃果实的主要芳香物质,种类最多,含量也最丰富。由表2可以看出丁酸乙酯、丁酸甲酯、丁酸丁酯、己酸乙酯、乙酸乙酯、苯甲酸甲酯、苯甲酸乙酯是红阳猕猴桃果实中酯类香气物质的主要部分,其中丁酸乙酯、丁酸甲酯、己酸乙酯可以产生典型的猕猴桃香气,主要呈现果香和甜香,苯甲酸甲酯、苯甲酸乙酯可呈现花香[27]。(Z)-2-己烯醛、己醛、戊醛是醛类香气物质的主要部分,多呈现青草香[28]。邻伞花烃是主要萜烯类物质,仅在T4 中无积累。此外,萜烯类中的D-柠檬烯、桉叶油醇、萜品烯-4-醇、(Z)-β-罗勒烯、α-蒎烯、β-蒎烯、α-松油醇可分别在除T4外的处理组中检测到。
对不同处理组中红阳猕猴桃果实香气成分进行聚类分析发现,不同时段遮光处理对果实的影响可分为4类,其中T1、T2、T3和T5的果实为一类,T4和对照果实为一类,T6 和T7 分别为一类。说明T4中红阳猕猴桃的香气物质的组成和含量与对照更为接近(图3)。此外,酯类香气成分乙酸乙酯、己酸乙酯、苯甲酸乙酯、苯甲酸甲酯、丁酸甲酯、丁酸乙酯在T2、T5 和T4 中含量较高,说明时段Ⅱ、时段Ⅳ和时段Ⅴ遮光果实果香较时段Ⅰ、时段Ⅲ、时段Ⅵ以及全时段遮光果实浓郁。α-蒎烯、桉叶油醇、D-柠檬烯、α-松油醇、(Z)-β-罗勒烯、萜品烯-4-醇、庚醛、(Z)-2-丁烯-1-醇、1-己醇在T2、T4、T5 和对照中无累积,但在T6 果实中均有发现,说明时段Ⅵ遮光促进了上述成分的积累(图3)。
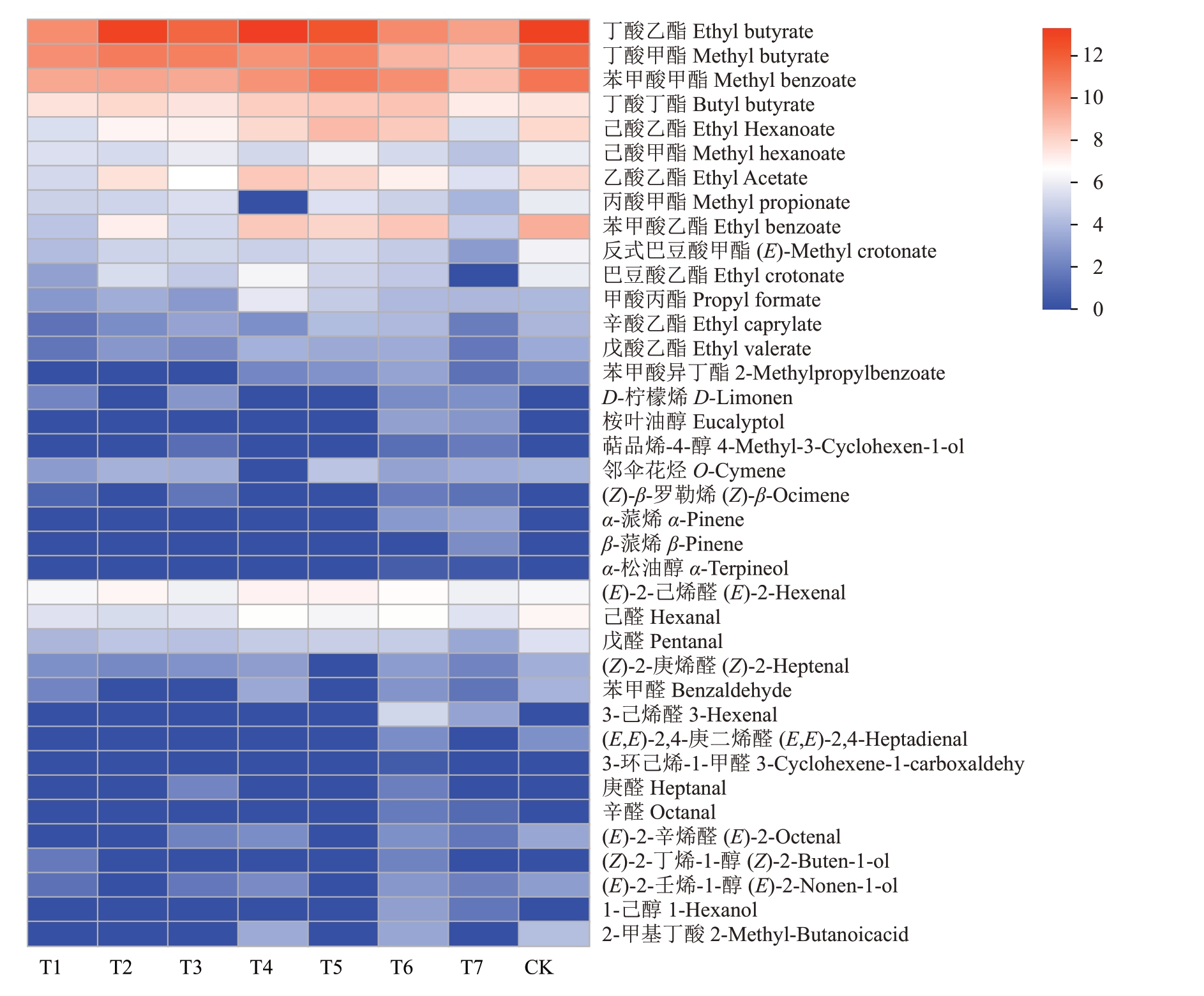
图3 不同处理组红阳猕猴桃果实香气物质聚类热图
Fig.3 Heat map of aroma clustering of Hongyang kiwifruits in different treatment groups
2.3 不同时段遮光处理下红阳猕猴桃果实香气的主成分分析
利用SPSS 软件对红阳猕猴桃果实香气物质进行主成分分析,以特征值大于1 提取主成分。结果显示提取了5 个主成分,第1 主成分和第2 主成分累积解释了总方差的90.147%,说明这两个主成分可以很好地反映样品中的大部分数据(表3)。利用主成分1和主成分2的特征值和贡献率分别计算各组的综合得分,并进行排名。结果显示除对照外,T6 综合得分最高,可能是因为T6 中醛类和醇类含量较其他组高。其次为T4、T5 和T2。结合香气总含量综合分析,T4、T2 和T5 的果实香气要优于其他处理(图4)。
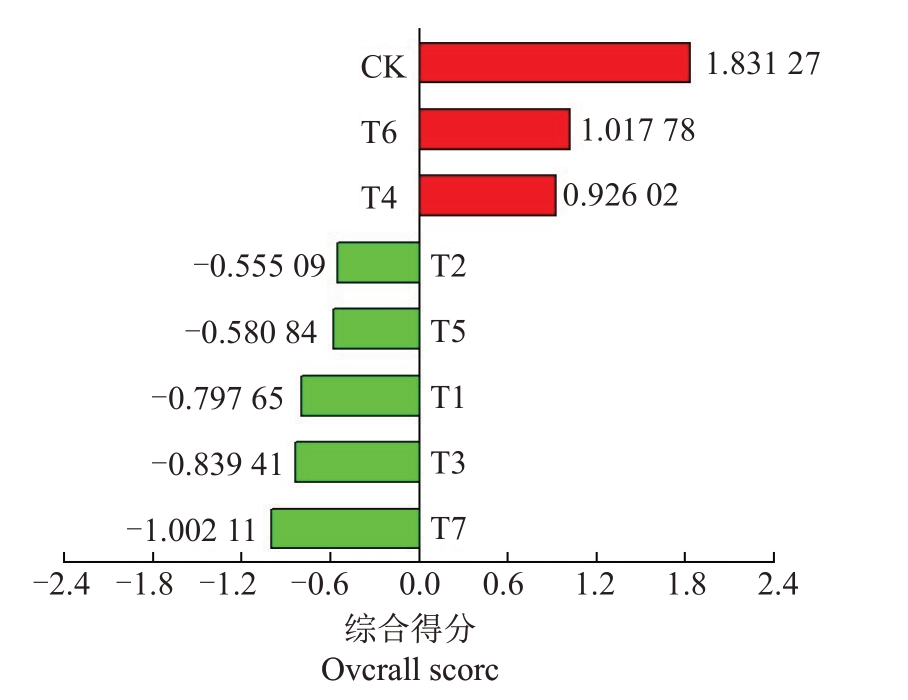
图4 不同处理组红阳猕猴桃果实香气综合得分
Fig.4 Overall score of Hongyang kiwifruits in different treatment groups
表3 5 个主成分特征向量及贡献率
Table 3 Five principal component eigenvectors and contribution rate
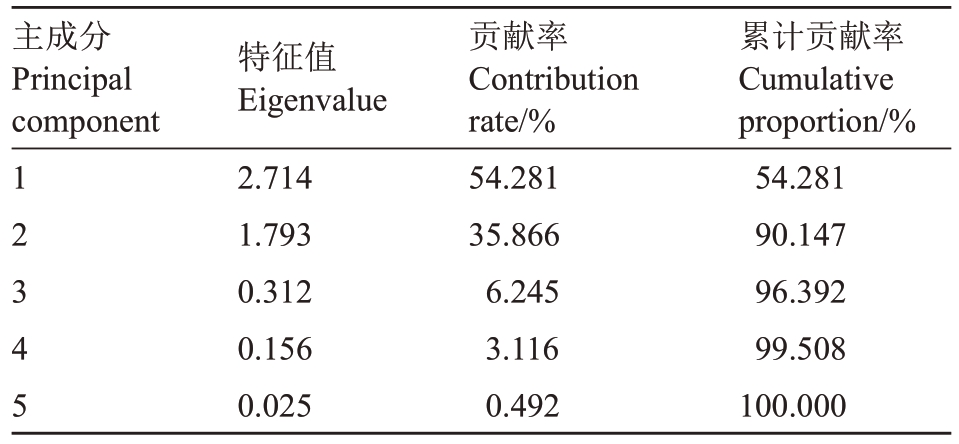
主成分Principal component 1 2 3 4 5特征值Eigenvalue 2.714 1.793 0.312 0.156 0.025贡献率Contribution rate/%54.281 35.866 6.245 3.116 0.492累计贡献率Cumulative proportion/%54.281 90.147 96.392 99.508 100.000
2.4 全时段遮光(T7)和全时段未遮光(CK)果实挥发性香气物质相关基因的表达
2.4.1 转录组数据整体概况 对T7 和对照果实进行转录组学分析,各样品Clean Data均达到6.74 GB以上,Q20碱基含量最小值为97.58%,Q30碱基含量最小值为93.35%,GC含量最小值为49.63%,符合质量要求。PCA 分析显示,主成分1 和主成分2 共解释了样品的52.41%,各组的样品都较高程度地聚集在一起,说明他们具有较好的重复性,组间差异显著(图5)。
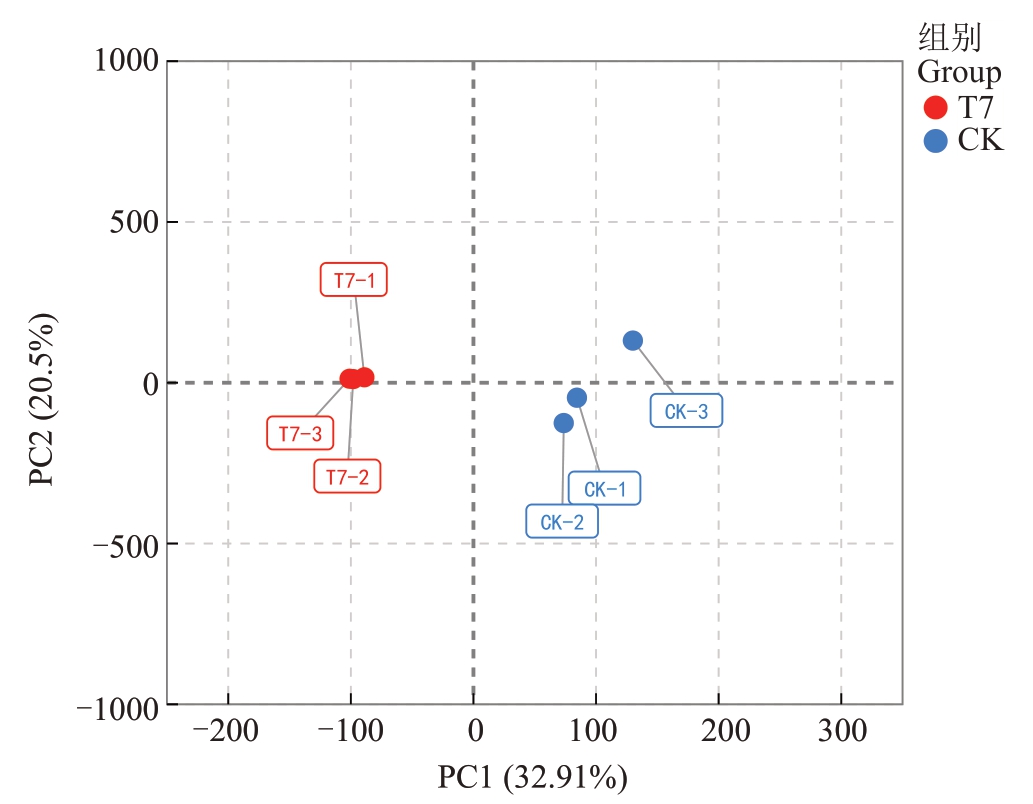
图5 样品PCA 图
Fig.5 Sample PCA plot
DEGs 分析结果显示,T7 和对照之间共有1248个差异表达基因,其中,T7 相对对照下调基因788个,上调基因460个(图6)。

图6 差异基因火山图
Fig.6 Differential gene volcano map
2.4.2 差异表达基因GO和KEGG富集分析 基于GO数据库,分析差异表达基因在生物过程(biological process)、细胞组成(cellular component)和分子功能(molecular function)的富集情况。这些差异基因注释在40 个GO 条目中,在生物过程主要注释到了细胞脂质代谢过程(cellular process)、代谢过程(metabolic process)、对刺激的响应(response to stimulus)和生物调控(biological regulation)等;在分子功能上,主要富集的条目是结合(binding)、催化活性(catalytic activity)、转录调控活性(transcription regulator activity)和转运载体活性(transporter activity)等;在细胞组成中富集的基因条目为细胞整体结构(cellular anatomical entity)和蛋白复合体(proteincontaining complex)(图7)。
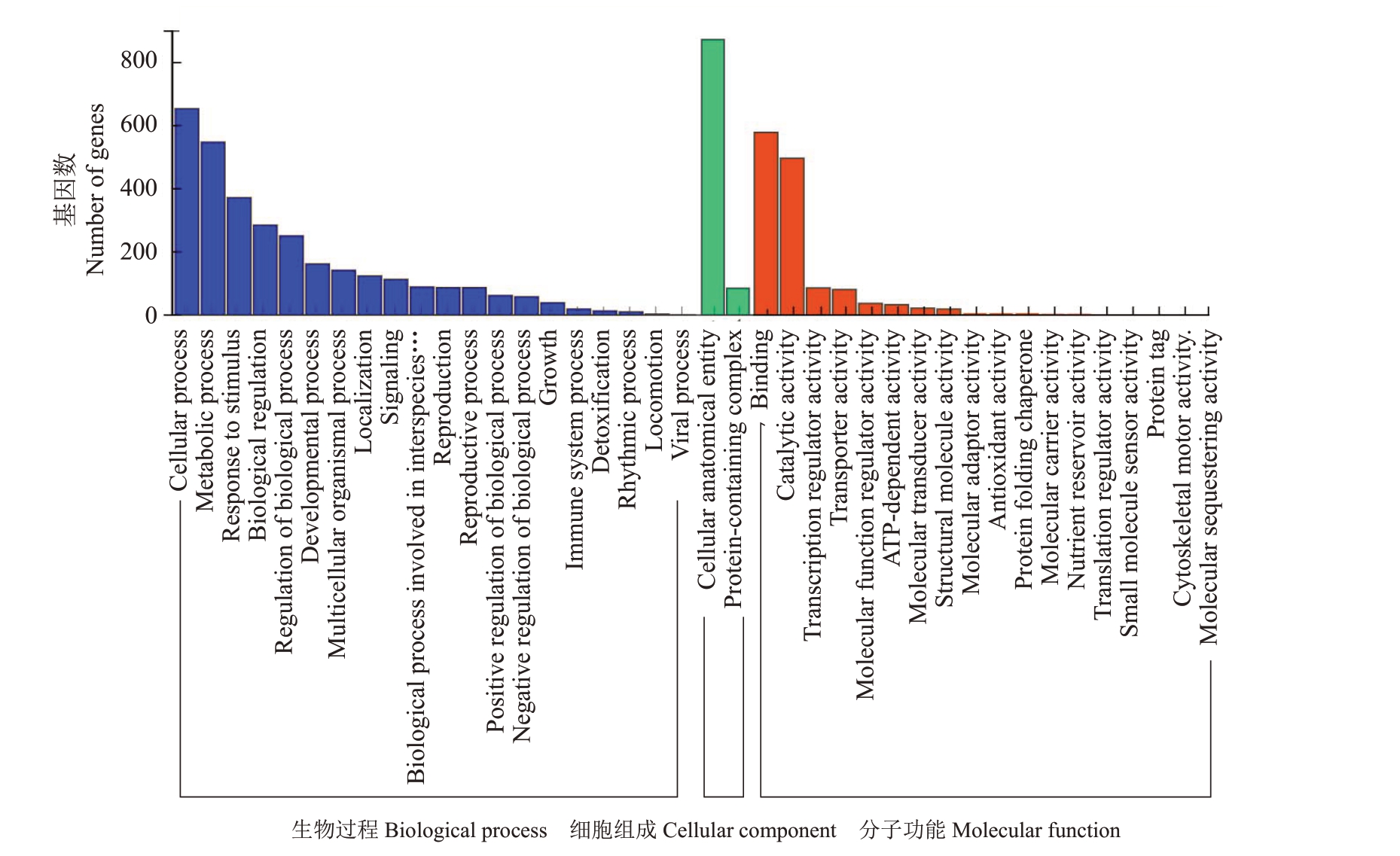
图7 GO 分类柱状图
Fig.7 GO Classification barplot
基于KEGG 数据库将T7 和对照之间的DEGs进行KEGG 富集分析,差异基因注释在130 个KEGG条目中,柱状图显示前50个富集最显著的条目。结果表明,大部分的差异基因富集在代谢中,其中,差异基因主要富集的条目是代谢途径(metabolic pathways)、次级代谢物的生物合成(biosynthesis of secondary metabolites)、内质网中的蛋白质加工(protein processing in endoplasmic)和氨基酸的生物合成(biosynthesis of amino acid)(图8)。以上结果说明遮光引发了红阳猕猴桃果实显著的生理变化。
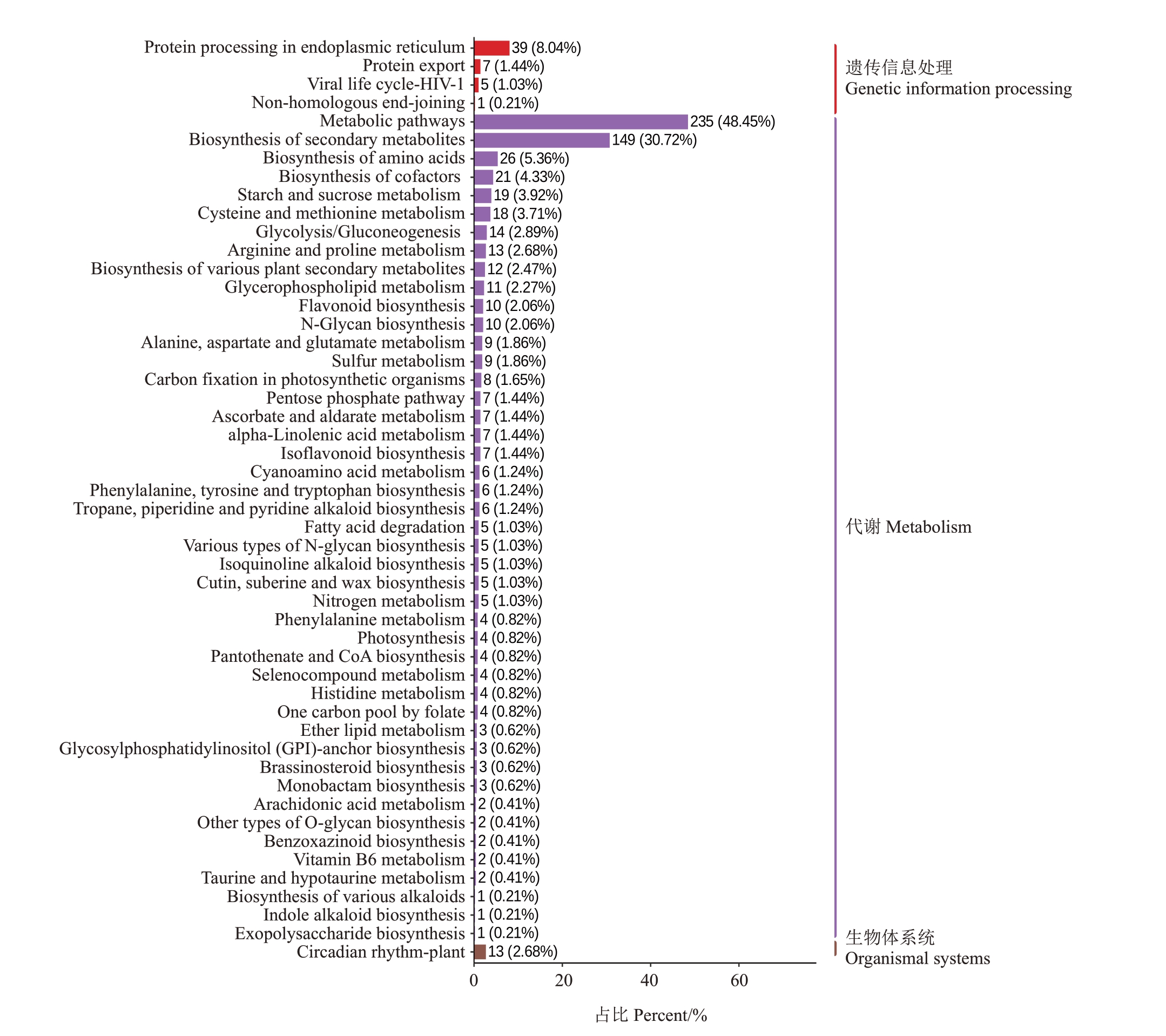
图8 KEGG 富集柱状图
Fig.8 KEGG enrichment barplot
2.4.3 受光照影响的香气合成基因分析 果实的挥发性香气物质合成途径主要为脂肪酸代谢途径、氨基酸代谢途径和萜类代谢途径[29]。对这3个代谢途径上的DEGs 进行分析,共筛选出15 个与香气合成相关的基因(图9)。其中包括2 个乙醇脱氢酶(ADH),1 个脂氧合酶(LOX),1 个羟基肉桂酰基转移酶(HCT),3个苯丙氨酸解氨酶(PAL),1个天冬氨酸转氨酶(GOT),1 个丙酮酸转氨酶(AGXT),1 个焦磷酸脱羧酶(MVD),1个2-C-甲基-d-赤藓糖醇-4-磷酸胞苷酰转移酶(IspD),1 个萜烯合酶(TPS),3个醛脱氢酶(ALDH)。遮光处理降低了T7 果实中ADH1、AGXT、GOT1 和IspD 基因的表达量,升高了MVD、ALDH和2个PAL基因的表达量,这可能是T7果实香气含量显著低于对照的原因。
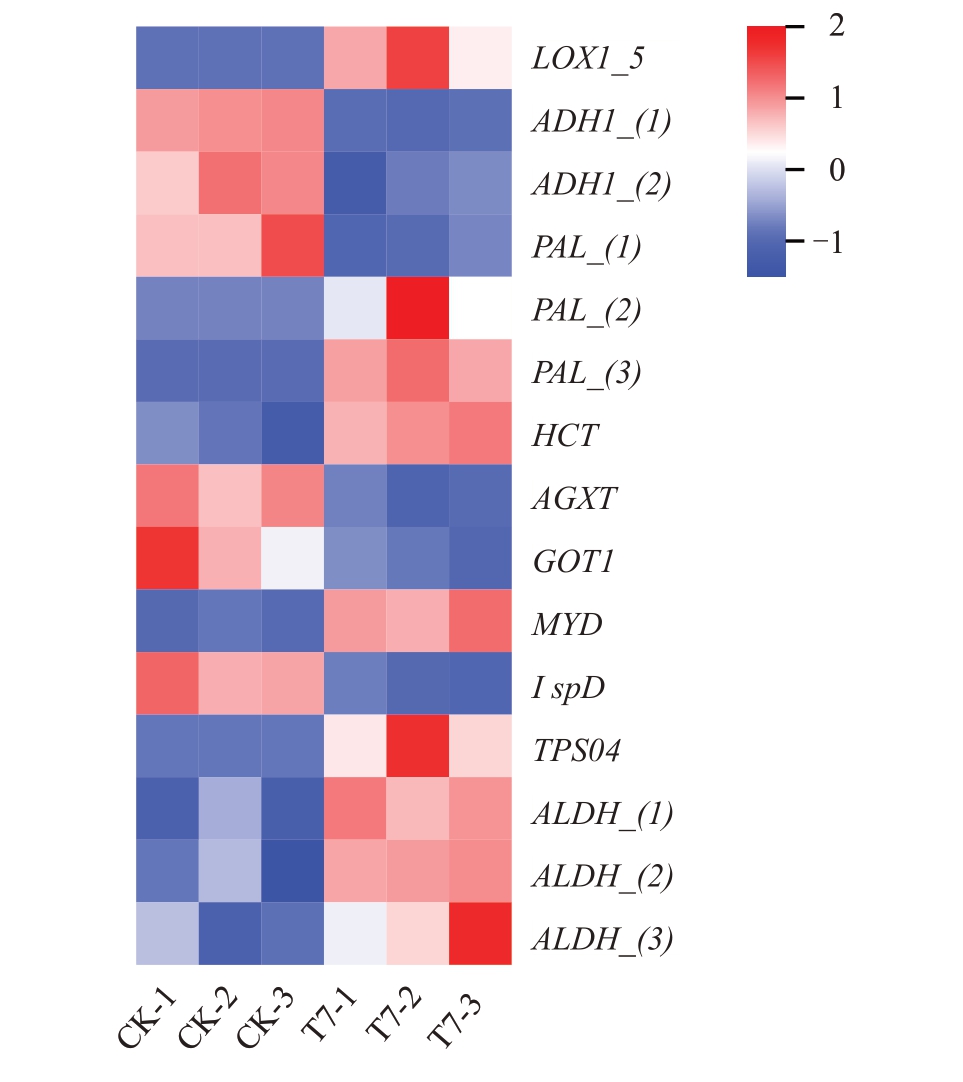
图9 香气合成途径相关基因热图
Fig.9 Heat map of genes involved in the aroma synthesis pathway
3 讨 论
酯类、醛类和醇类在猕猴桃果实香气中多有研究。其中酯类是猕猴桃特征香气的重要组分,为猕猴桃提供浓郁的果香和甜香,醛类和醇类则为猕猴桃富集青草香气[23-24]。本试验对对照中的红阳果实进行检测发现,其酯类含量占比超过90%,丁酸乙酯、丁酸甲酯在红阳果实酯类成分中的占比最大;醛类含量位居第二,(E)-2-己烯醛和己醛在醛类成分中占比最高,与前人的研究结果一致[25,30]。赵玉[27]等检测了翠香、徐香、秦美和华优猕猴桃的香气成分,将己醇鉴定为猕猴桃关键香气组分之一,但在红阳中己醇含量较低,未达到阈值,可能是因为品种差异。
将遮光处理组的果实香气与对照进行对比,结果显示除时段Ⅳ外,其他各时段的遮光处理均会导致果实酯类含量下降,其中T2 和T5 果实中酯类含量较其他组高,分别是对照的77%和66%,T1、T3和T6组中含量分别为对照的24%、41%和30%,表明时段Ⅰ、时段Ⅲ和时段Ⅵ为酯类合成的重要时期。在T4、T5 和T6 中,果实的醛类物质与对照无显著差异,而在T1、T2和T3中,醛类含量显著低于对照,说明时段Ⅰ~Ⅲ是醛类合成关键时期,已有研究也证明了醛类香气物质在红阳猕猴桃果实发育前期含量最高,而后逐渐降低[31]。上述结果说明了红阳猕猴桃中酯类和醛类的合成时期不完全相同,与前人在金艳猕猴桃中得出的结论类似[32]。此外,持续遮光显著降低了己醛的含量,但对(E)-2-己烯醛的含量无明显影响,与Liu等[33]、王继源等[34]的结果一致。
萜烯类多呈现花果香[35]。对照中只有邻伞花烃一种萜类,而王逍遥[29]研究发现桉叶油醇是红阳猕猴桃的主要萜类物质,可能是地域不同导致的。萜烯类的合成途径有甲羟戊酸途径(MVA)和2-C-甲基-D-赤藻糖醇-4-磷酸途径(MEP),其中,单萜通过MEP 途径产生[36]。在除对照和T4 外的其他组中分别检测到包含邻伞花烃在内的多种萜类物质,且均为单萜,说明红阳猕猴桃的萜烯类化合物合成途径为MEP途径,并且各类物质在果实发育的不同时段产生,依赖避光环境进行积累。T4果实中萜烯类物质的累积被完全抑制,而在其他时段遮光对果实内萜烯类物质含量无影响或显著提高,说明时段Ⅳ是萜烯类合成的关键时期。
在脂肪酸代谢途径中,前体物质经脂氧合酶(LOX)和乙醇脱氢酶(ADH)等酶的催化,转化为醛类和醇类,再转化成酯[37]。张曾等[31]认为AcLox3、AcLox4 和AcLox6 是红阳猕猴桃成熟过程中调控醛类合成的相关基因,AcLox1和AcLox5是调控酯类香气成分合成的相关基因。本研究转录组分析仅发现LOX1_5 基因(novel.3064)在遮光处理下表达量升高,认为其受遮光诱导,在猕猴桃酯类香气形成中起负调控作用。ALDH 能够将醛类催化为酸,从而进入倍半萜合成途径[38]。在T7 中2 个ADH 基因(Achv4p10g015598,Achv4p11g016521)表达被遮光抑制,而3 个ALDH 基因(Achv4p11g0116573,Achv4p06g008766,Achv4p04g006368)高表达,可能是T7果实醇类、醛类和酯类香气含量降低的原因。
在氨基酸代谢途径中,氨基酸可经过转氨酶催化形成酮酸,再由ADH催化形成各类香气成分,也可在PAL催化下形成醛,再向醇和酯转化[37]。在转录组分析结果中,AGXT 基因(Achv4p28g044216)和COT1 基因(Achv4p03g003648),以及PAL 基因(Achv4p24g037630)在T7 中被抑制表达,而PAL 基因(Achv4p28g04395,Achv4p26g040956)在T7 中表达量上调。推测PAL 基因(Achv4p28g043957,Achv4p26g040956)对香气合成起负调控作用,同时遮光 降 低 AGXT (Achv4p28g044216) 、COT1(Achv4p03g003648)和PAL基因(Achv4p24g037630)表达量,抑制T7果实醇类、醛类和酯类香气合成。
红阳猕猴桃萜烯类合成途径主要是MEP途径,IspD、MVD 和TPS 是该过程中的关键酶[39-40]。转录组分析结果显示,相比于对照,MVD 和TPS 基因在T7 中表达上调,IspD 基因表达下调。而T7 果实中萜烯类含量显著高于对照,推测MVD 基因(Achv4p22g034538)和TPS基因(Achv4p15g023672)是萜烯类合成的关键基因。有研究表明,TPSs基因表达受光照影响,如拟南芥中的TPS 合成基因AtTPS03、AtTPS06 和AtTPS29 受光诱导[41]。但在本试验中,TPS基因表达在遮光条件下上调,可能是由物种不同所致。
前人研究发现红阳猕猴桃套袋适宜开始在落花后30~40 d,过早套袋,容易伤害猕猴桃幼果,影响果实正常发育;而过晚套袋,猕猴桃果皮容易变得粗糙,达不到套袋的目的[42]。根据试验结果综合分析,对果实香气影响最小的遮光时段为时段Ⅳ,其次是时段Ⅴ和时段Ⅱ以及时段Ⅲ。结合已有研究,建议从时段Ⅰ后期(30~40 DAA)开始对猕猴桃套袋,时期Ⅴ结束(120 DAA左右)时取袋。试验结果显示,时段Ⅵ(120~140 DAA)遮光显著降低果实中酯类物质和总香气含量,已有研究也表明猕猴桃海沃德和红阳中酯类物质会在果实成熟后期大量累积[21,31],因此建议保持该时期果实充分的光照条件。
4 结 论
红阳猕猴桃果实香气含量被遮光处理显著影响,其中,T4、T5、T2果实香气受遮光影响最小,说明在40~60 DAA(时段Ⅱ)和80~120 DAA(时段Ⅳ和时段Ⅴ)为适宜遮光时段。遮光抑制了ADH、AGXT和COT1 基因的表达,提高了MVD 和ALDH 基因的表达量,是遮光果实中萜烯类香气物质含量升高和酯类、醛类、醇类含量下降的可能原因。此外,从试验结果来看,时段Ⅰ、Ⅲ、Ⅵ和时段Ⅳ分别是酯类和萜烯类香气物质合成的重要阶段,时段Ⅰ至时段Ⅲ为醛类物质合成的重要阶段。此结果可为猕猴桃精细化管理提供有益参考。
[1] 冯立团,张志强,贺浩浩,黄瑞,李宏武.陕西秦岭以北猕猴桃抗冻性调查及栽培建议[J].北方果树,2024(3):56-60.FENG Lituan,ZHANG Zhiqiang,HE Haohao,HUANG Rui,LI Hongwu.Investigation and cultivation suggestions on the frost resistance of kiwifruit north of Qinling Mountains in Shaanxi province[J].Northern Fruits,2024(3):56-60.
[2] 赵增玲,向阳,张卓,吴健笔,刘瑞玲,易图永.湘西地区猕猴桃溃疡病防治药剂筛选及田间防效评价[J].植物检疫,2024,38(5):47-51.ZHAO Zengling,XIANG Yang,ZHANG Zhuo,WU Jianbi,LIU Ruiling,YI Tuyong.Screening and field control effectiveness evaluation of fungicides for control of kiwifruit canker in Xiangxi[J].Plant Quarantine,2024,38(5):47-51.
[3] 王涛,张计育,王刚,贾展慧,潘德林,郭忠仁.猕猴桃细菌性溃疡病研究进展[J].中国农学通报,2020,36(3):123-128.WANG Tao,ZHANG Jiyu,WANG Gang,JIA Zhanhui,PAN Delin,GUO Zhongren.Advances in kiwifruit bacterial canker[J].Chinese Agricultural Science Bulletin,2020,36(3):123-128.
[4] 肖春,李玉琼,耿礼祥,张荣全,龙幔,罗惠引,张辉.红阳猕猴桃的特点及栽培技术要点[J].南方农业,2020,14(12):41-42.XIAO Chun,LI Yuqiong,GENG Lixiang,ZHANG Rongquan,LONG Man,LUO Huiyin,ZHANG Hui.Characteristics and cultivation techniques of Hongyang kiwifruit[J].South China Agriculture,2020,14(12):41-42.
[5] 陈成,王依,杨勇,阎永齐.采收成熟度对‘金艳’猕猴桃果实品质及香气成分的影响[J].中国农学通报,2020,36(31):28-36.CHEN Cheng,WANG Yi,YANG Yong,YAN Yongqi.Effects of maturity stage on fruit quality and aroma components of‘Jinyan’kiwifruit[J].Chinese Agricultural Science Bulletin,2020,36(31):28-36.
[6] 王成忠,任慧贤.食品风味化学进展[J].中国调味品,2011,36(5):8-11.WANG Chengzhong,REN Huixian.Food flavor chemical progress[J].China Condiment,2011,36(5):8-11.
[7] SRIVASTAVA K K,SONI S K,KUMAR D,DWIVEDI S K.Effect of different bagging materials on guava fruit physiology and its quality attributes[J].Plant Physiology Reports,2023,28(2):238-246.
[8] ZHI C,ALI M M,ZHANG J Y,SHI M,MA S F,CHEN F X.Effect of paper and aluminum bagging on fruit quality of loquat(Eriobotrya japonica Lindl.)[J].Plants,2021,10(12):2704.
[9] 王志琦.套袋及保护膜剂处理对富士苹果品质的影响[D].杨凌:西北农林科技大学,2023.WANG Zhiqi.Effects of bagged and Protective film treatment on quality of‘Fuji’apple[D].Yangling:Northwest A& F University,2023.
[10] 陈成,王依,宋思言,杨勇,万春雁,阎永齐.套袋对海沃德猕猴桃果实品质及叶绿素代谢的影响[J].西北农林科技大学学报(自然科学版),2022,50(7):138-146.CHEN Cheng,WANG Yi,SONG Siyan,YANG Yong,WAN Chunyan,YAN Yongqi.Effect of bagging on fruit quality and chlorophyll metabolism of Hayward kiwifruit[J].Journal of Northwest A&F University(Natural Science Edition),2022,50(7):138-146.
[11] 王斯妤,陈东元,王璠,吴庭观,曾明,邱家洪,刘伟.套袋处理对红阳猕猴桃果实品质及贮藏性的影响[J].江西农业学报,2020,32(6):41-46.WANG Siyu,CHEN Dongyuan,WANG Fan,WU Tingguan,ZENG Ming,QIU Jiahong,LIU Wei.Effects of different bagging treatments on quality and storage properties of‘Hongyang’kiwifruit[J].Acta Agriculturae Jiangxi,2020,32(6):41-46.
[12] ZHANG E P,CHAI F M,ZHANG H H,LI S H,LIANG Z C,FAN P G.Effects of sunlight exclusion on the profiles of monoterpene biosynthesis and accumulation in grape exocarp and mesocarp[J].Food Chemistry,2017,237:379-389.
[13] ZHANG H H,FAN P G,LIU C X,WU B H,LI S H,LIANG Z C.Sunlight exclusion from Muscat grape alters volatile profiles during berry development[J].Food Chemistry,2014,164:242-250.
[14] JIA H J,ARAKI A,OKAMOTO G.Influence of fruit bagging on aroma volatiles and skin coloration of‘Hakuho’peach(Prunus persica Batsch)[J].Postharvest Biology and Technology,2005,35(1):61-68.
[15] SHEN J Y,WU L,LIU H R,ZHANG B,YIN X R,GE Y Q,CHEN K S.Bagging treatment influences production of C6 aldehydes and biosynthesis-related gene expression in peach fruit skin[J].Molecules,2014,19(9):13461-13472.
[16] WANG G P,CHEN R,HAN X P,XUE X M.Effects and mechanism analysis of non-bagging and bagging cultivation on the growth and content change of specific substances of Fuji apple fruit[J].Plants,2023,12(18):3309.
[17] HAN X Y,WANG X Y,SHEN C,MO Y W,TIAN R G,MAO L C,LUO Z S,YANG H Y.Exogenous ABA promotes aroma biosynthesis of postharvest kiwifruit after low-temperature storage[J].Planta,2022,255(4):82.
[18] GÜNTHER C S,MARSH K B,WINZ R A,HARKER R F,WOHLERS M W,WHITE A,GODDARD M R.The impact of cold storage and ethylene on volatile ester production and aroma perception in‘Hort16A’kiwifruit[J].Food Chemistry,2015,169:5-12.
[19] HUAN C,ZHANG J,JIA Y,LI S E,JIANG T J,SHEN S L,ZHENG X L.Effect of 1-methylcyclopropene treatment on quality,volatile production and ethanol metabolism in kiwifruit during storage at room temperature[J].Scientia Horticulturae,2020,265:109266.
[20] ZHANG A D,ZHANG Q Y,LI J Z,GONG H S,FAN X G,YANG Y Q,LIU X F,YIN X R.Transcriptome co-expression network analysis identifies key genes and regulators of ripening kiwifruit ester biosynthesis[J].BMC Plant Biology,2020,20(1):103.
[21] 张琳.乙烯对1-MCP 处理的猕猴桃香气与醇酰基转移酶活性及基因表达的影响[D].西安:陕西师范大学,2013.ZHANG Lin.Effect of ethylene on aroma and alcohol acyltransferase activity and gene expression in 1-MCP-treated kiwifruit[D].Xi’an:Shaanxi Normal University,2013.
[22] 董婧,刘永胜,唐维.中华猕猴桃(Actinidia chinensis Planch.)果实香气成分及相关基因表达[J].应用与环境生物学报,2018,24(2):307-314.DONG Jing,LIU Yongsheng,TANG Wei.Volatile components and their corresponding synthetic gene expression profile in the fruits of Actinidia chinensis[J].Chinese Journal of Applied and Environmental Biology,2018,24(2):307-314.
[23] 李洁维,莫权辉,王发明,龚弘娟,叶开玉,蒋桥生.“红阳”猕猴桃高效栽培技术[J].农村新技术,2023(3):59-60.LI Jiewei,MO Quanhui,WANG Faming,GONG Hongjuan,YE Kaiyu,JIANG Qiaosheng.High efficiency cultivation technology of“Hongyang”kiwifruit[J].Nongcun Xin Jishu,2023(3):59-60.
[24] 蒋芯,颜丽菊,张海燕.22 个猕猴桃品种的果实品质比较研究[J].中国南方果树,2023,52(4):114-117.JIANG Xin,YAN Liju,ZHANG Haiyan.Comparative study of fruit quality of 22 kiwifruit cultivars[J].South China Fruits,2023,52(4):114-117.
[25] 刘翠霞,李洁维,高建有,莫权辉,王发明,叶开玉,刘平平,齐贝贝,龚弘娟.广西不同地区红阳猕猴桃果实香气分析[J].果树学报,2023,40(10):2170-2182.LIU Cuixia,LI Jiewei,GAO Jianyou,MO Quanhui,WANG Faming,YE Kaiyu,LIU Pingping,QI Beibei,GONG Hongjuan.Aromatic constituents analysis of Hongyang kiwifruits from different regions in Guangxi[J].Journal of Fruit Science,2023,40(10):2170-2182.
[26] 张文霖.主成分分析在SPSS 中的操作应用[J].市场研究,2005(12):31-34.ZHANG Wenlin.Operational application of principal component analysis in SPSS[J].Marketing Research,2005(12):31-34.
[27] 赵玉,詹萍,王鹏,田洪磊.猕猴桃中关键香气组分分析[J].食品科学,2021,42(16):118-124.ZHAO Yu,ZHAN Ping,WANG Peng,TIAN Honglei.Analysis of key aroma compounds in kiwifruits[J].Food Science,2021,42(16):118-124.
[28] FRANK D,O’RIORDAN P,VARELIS P,ZABARAS D,WATKINS P,CECCATO C,WIJESUNDERA C.Deconstruction and recreation of‘Hayward’volatile flavour using a trained sensory panel,olfactometry and a kiwifruit model matrix[J].Acta Horticulturae,2007(753):107-119.
[29] 王逍遥.特定低温诱导红阳猕猴桃萜类香气释放及调控机制初探[D].武汉:华中农业大学,2023.WANG Xiaoyao.Preliminary study of specific low temperatureinduced terpene aroma release and transcriptional regulation in-Hongyang kiwifruit[D].Wuhan:Huazhong Agricultural University,2023.
[30] 朱先波,潘亮,彭家清,吴伟,肖涛,任小林.“武当1 号”猕猴桃芳香物质的研究[J].北方园艺,2015(22):16-21.ZHU Xianbo,PAN Liang,PENG Jiaqing,WU Wei,XIAO Tao,REN Xiaolin.Study on aroma components of postharvests‘Wudang-1’kiwifruit[J].Northern Horticulture,2015(22):16-21.
[31] 张曾,曾维才,唐维,刘永胜.红阳猕猴桃成熟过程中脂氧合酶基因的表达与香气成分变化关系的研究[J].四川大学学报(自然科学版),2017,54(4):857-862.ZHANG Zeng,ZENG Weicai,TANG Wei,LIU Yongsheng.The relationship between the expression of lipoxygenase and flavor components at different Brix levels of Hongyang kiwifruit[J].Journal of Sichuan University (Natural Science Edition),2017,54(4):857-862.
[32] 谭皓.猕猴桃发育过程中香气成分变化规律研究[D].乌鲁木齐:新疆农业大学,2006.TAN Hao.Study on change regularities of aroma components of kiwifruit during fruit development[D].Urumqi:Xinjiang Agricultural University,2006.
[33] LIU Q,LI Y Q,LIAO G L,XU X B,JIA D F,ZHONG M,WANG H L,YE B.Transcriptome and Metabolome reveal AsA regulatory network between metabolites and genes after fruit shading by bagging in kiwifruit (Actinidia eriantha)[J].Scientia Horticulturae,2022,302:111184.
[34] 王继源,冯娇,侯旭东,陶建敏.不同果袋对‘阳光玫瑰’葡萄香气组分及合成相关基因表达的影响[J].果树学报,2017,34(1):1-11.WANG Jiyuan,FENG Jiao,HOU Xudong,TAO Jianmin.Effects of bagging treatments with different materials on aroma components and their biosynthetic gene expression in‘Shine Muscat’grape berry[J].Journal of Fruit Science,2017,34(1):1-11.
[35] LIU M Y,JI H L,JIANG Q Q,LIU T Y,CAO H,ZHANG Z W.Effects of full shading of clusters from véraison to ripeness on fruit quality and volatile compounds in Cabernet Sauvignon grapes[J].Food Chemistry,2024,21:101232.
[36] LICHTENTHALER H K,ROHMER M,SCHWENDER J.Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants[J].Physiologia Plantarum,1997,101(3):643-652.
[37] FEUSSNER I,WASTERNACK C.The lipoxygenase pathway[J].Annual Review of Plant Biology,2002,53(1):275-297.
[38] 张乐,张亚红,乔振羽,王亚楠,陈璐,周娟,黄嘉俊.不同昼夜温差对赤霞珠葡萄果实香气的影响及转录组分析[J].核农学报,2023,37(4):865-878.ZHANG Le,ZHANG Yahong,QIAO Zhenyu,WANG Yanan,CHEN Lu,ZHOU Juan,HUANG Jiajun.Effect of diurnal amplitude on fruit aroma of Cabernet Sauvignon and transcriptome analysis[J].Journal of Nuclear Agricultural Sciences,2023,37(4):865-878.
[39] MARSHALL B,AMRITKAR K,WOLFE M,KAÇAR B,LANDICK R.Evolutionary flexibility and rigidity in the bacterial methylerythritol phosphate (MEP) pathway[J].Frontiers in Microbiology,2023,14:1286626.
[40] 高雪倩,贾云彭,李昕悦,岳跃冲,范燕萍,玉云祎.建兰不同花器官花香代谢差异的转录组分析[J/OL].分子植物育种,2024:1- 11.(2024- 04- 23).https://kns.cnki.net/kcms/detail/46.1068.S.20240423.1334.007.html.GAO Xueqian,JIA Yunpeng,LI Xinyue,YUE Yuechong,FAN Yanping,YU Yunyi.Transcriptome analysis and gene mining reveal floral scent metabolic pathways in cymbidium ensifolium[J/OL].Molecular Plant Breeding,2024:1-11.(2024-04-23).https://kns.cnki.net/kcms/detail/46.1068.S.20240423.1334.007.html.
[41] MICHAEL R,RANJAN A,KUMAR R S,PATHAK P K,TRIVEDI P K.Light-regulated expression of terpene synthase gene,AtTPS03,is controlled by the bZIP transcription factor,HY5 in Arabidopsis thaliana[J].Biochemical and Biophysical Research Communications,2020,529(2):437-443.
[42] 汪洋,郑金成,周晓峰,李雪,贾淑娟,李俊德.猕猴桃果实套袋技术[J].落叶果树,2024,56(1):86-87.WANG Yang,ZHENG Jincheng,ZHOU Xiaofeng,LI Xue,JIA Shujuan,LI Junde.Kiwifruit fruit bagging technology[J].Deciduous Fruits,2024,56(1):86-87.