猕猴桃(Actinidia spp.)原产于中国,在“大食物观”背景下是中国水果食物的重要组成部分,因其独特的风味及富含维生素C、氨基酸、有机酸、可溶性糖和矿物质等营养物质而成为备受大众喜爱的水果之一[1-2]。据不完全统计,中国已经累计选育猕猴桃新品种近400个,栽培总面积20万hm2,年产量超过250万t(布瑞克农业大数据,2024)。中国虽是猕猴桃生产大国,却非生产强国,主要表现为价格低廉、高品质果率低等[3]。金艳是由中国科学院武汉植物园猕猴桃学科组人员利用毛花猕猴桃和中华猕猴桃进行种间杂交选育而成,为江西地区的主栽品种之一[4]。生产上常用氯吡脲(CPPU)进行幼果期膨大处理,但因使用不当出现畸形果率高[5]、果皮色泽暗淡[6]、生理成熟期提前[7]、库存损耗高等问题[8-9],从而导致采后猕猴桃果实综合品质下降。
植物生长调节剂是一类由人工合成或天然提取,具有和植物体内激素相似生长发育调节作用的有机化合物的统称。在果树上常用的有CPPU[10-11]、6-苄氨基腺嘌呤(6-BA)[12-14]、赤霉素(GA3)[15-18]、吲哚乙酸(IAA)[15,19-20]、褪黑素(MT)[21-22]、萘乙酸(NAA)[23-25]、色氨酸(Trp)[26-27]和三十烷醇(Tri)[28]等,具有增强植物抗性及调控果实大小、色素积累水平、糖酸组分含量和成熟衰老进程等多种生理作用。笔者在本研究中以金艳猕猴桃为材料,开展不同植物生长调剂及质量浓度对果实生长发育和果实品质的影响研究,以期为绿色高效植物生长调节的开发利用提供理论基础,从而促进猕猴桃产业的健康发展。
1 材料和方法
1.1 试验材料与处理
以种植于宜春市奉新县博士猕猴桃基地(E 114°45', N 28°34')的8 年生金艳猕猴桃为试验材料,于2023 年5 月底(授粉后25~30 d),根据文献[11,13,16,19,22-23,26,28]报道分别采用不同质量浓度的植物生长调节剂[MT、CPPU、Tri、GA3、IAA、NAA、6-BA和Trp(北京索莱宝)]进行喷施处理,具体处理质量浓度如表1 所示,以清水处理为对照。每种植物生长调节剂的每个质量浓度分别处理3株(计3个生物学重复),每株猕猴桃树的留果量为800~900个。
表1 不同植物生长调节剂处理对金艳猕猴桃果实外观指标的影响
Table 1 Effects of different plant growth regulators treatments on fruit appearance indexes of Jinyan kiwifruit
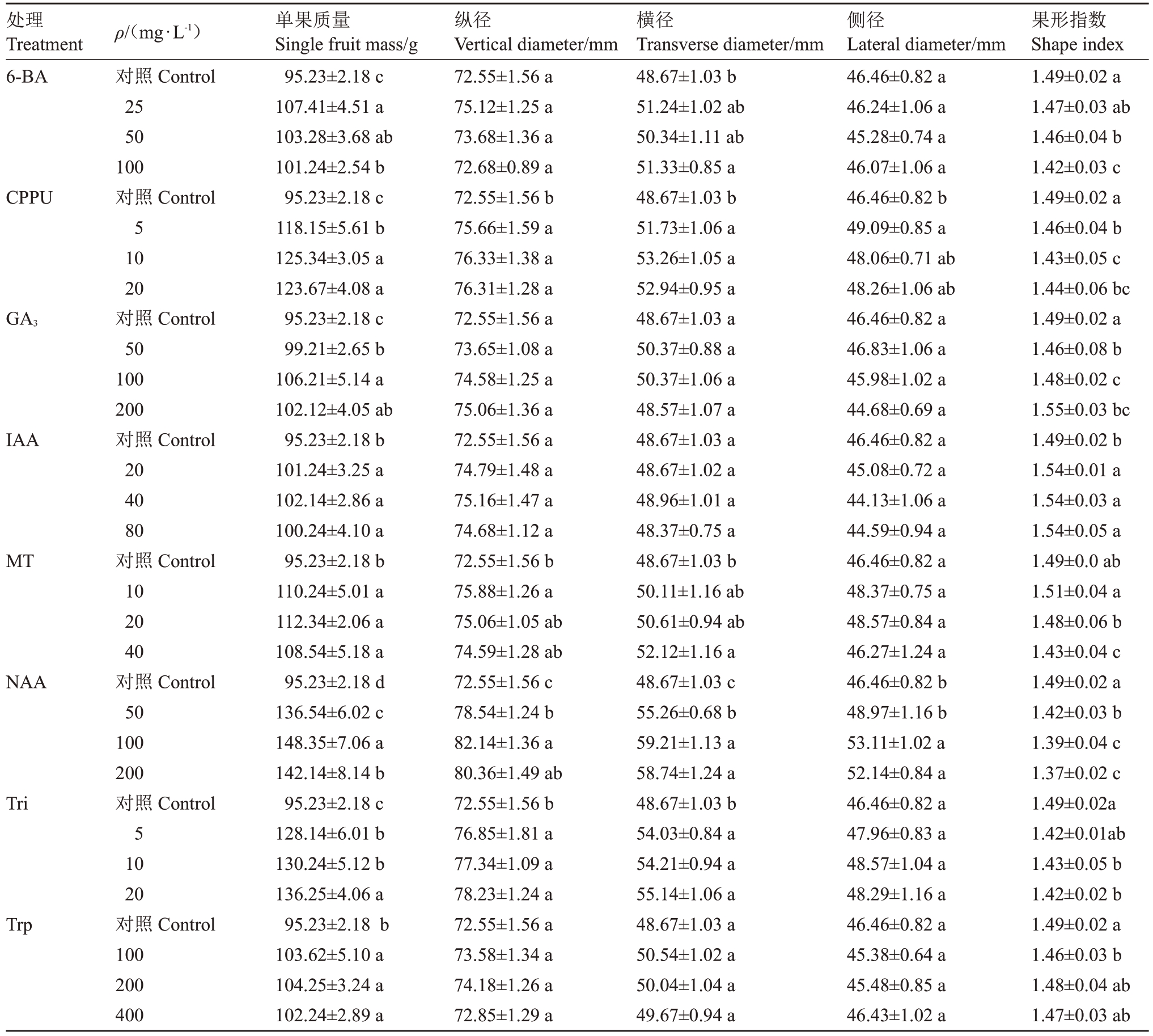
注:同一列中不同字母表示不同处理与对照相比差异显著(p<0.05)。下同。
Note:Within the same column, different letters indicate different treatments and are significantly different compared to control (p<0.05).The same below.
处理Treatment 6-BA果形指数Shape index 1.49±0.02 a 1.47±0.03 ab 1.46±0.04 b 1.42±0.03 c 1.49±0.02 a 1.46±0.04 b 1.43±0.05 c 1.44±0.06 bc 1.49±0.02 a 1.46±0.08 b 1.48±0.02 c 1.55±0.03 bc 1.49±0.02 b 1.54±0.01 a 1.54±0.03 a 1.54±0.05 a 1.49±0.0 ab 1.51±0.04 a 1.48±0.06 b 1.43±0.04 c 1.49±0.02 a 1.42±0.03 b 1.39±0.04 c 1.37±0.02 c 1.49±0.02a 1.42±0.01ab 1.43±0.05 b 1.42±0.02 b 1.49±0.02 a 1.46±0.03 b 1.48±0.04 ab 1.47±0.03 ab CPPU GA3 IAA MT NAA Tri Trp ρ/(mg·L-1)对照Control 25 50 100对照Control 5 10 20对照Control 50 100 200对照Control 20 40 80对照Control 10 20 40对照Control 50 100 200对照Control 5 10 20对照Control 100 200 400单果质量Single fruit mass/g 95.23±2.18 c 107.41±4.51 a 103.28±3.68 ab 101.24±2.54 b 95.23±2.18 c 118.15±5.61 b 125.34±3.05 a 123.67±4.08 a 95.23±2.18 c 99.21±2.65 b 106.21±5.14 a 102.12±4.05 ab 95.23±2.18 b 101.24±3.25 a 102.14±2.86 a 100.24±4.10 a 95.23±2.18 b 110.24±5.01 a 112.34±2.06 a 108.54±5.18 a 95.23±2.18 d 136.54±6.02 c 148.35±7.06 a 142.14±8.14 b 95.23±2.18 c 128.14±6.01 b 130.24±5.12 b 136.25±4.06 a 95.23±2.18 b 103.62±5.10 a 104.25±3.24 a 102.24±2.89 a纵径Vertical diameter/mm 72.55±1.56 a 75.12±1.25 a 73.68±1.36 a 72.68±0.89 a 72.55±1.56 b 75.66±1.59 a 76.33±1.38 a 76.31±1.28 a 72.55±1.56 a 73.65±1.08 a 74.58±1.25 a 75.06±1.36 a 72.55±1.56 a 74.79±1.48 a 75.16±1.47 a 74.68±1.12 a 72.55±1.56 b 75.88±1.26 a 75.06±1.05 ab 74.59±1.28 ab 72.55±1.56 c 78.54±1.24 b 82.14±1.36 a 80.36±1.49 ab 72.55±1.56 b 76.85±1.81 a 77.34±1.09 a 78.23±1.24 a 72.55±1.56 a 73.58±1.34 a 74.18±1.26 a 72.85±1.29 a横径Transverse diameter/mm 48.67±1.03 b 51.24±1.02 ab 50.34±1.11 ab 51.33±0.85 a 48.67±1.03 b 51.73±1.06 a 53.26±1.05 a 52.94±0.95 a 48.67±1.03 a 50.37±0.88 a 50.37±1.06 a 48.57±1.07 a 48.67±1.03 a 48.67±1.02 a 48.96±1.01 a 48.37±0.75 a 48.67±1.03 b 50.11±1.16 ab 50.61±0.94 ab 52.12±1.16 a 48.67±1.03 c 55.26±0.68 b 59.21±1.13 a 58.74±1.24 a 48.67±1.03 b 54.03±0.84 a 54.21±0.94 a 55.14±1.06 a 48.67±1.03 a 50.54±1.02 a 50.04±1.04 a 49.67±0.94 a侧径Lateral diameter/mm 46.46±0.82 a 46.24±1.06 a 45.28±0.74 a 46.07±1.06 a 46.46±0.82 b 49.09±0.85 a 48.06±0.71 ab 48.26±1.06 ab 46.46±0.82 a 46.83±1.06 a 45.98±1.02 a 44.68±0.69 a 46.46±0.82 a 45.08±0.72 a 44.13±1.06 a 44.59±0.94 a 46.46±0.82 a 48.37±0.75 a 48.57±0.84 a 46.27±1.24 a 46.46±0.82 b 48.97±1.16 b 53.11±1.02 a 52.14±0.84 a 46.46±0.82 a 47.96±0.83 a 48.57±1.04 a 48.29±1.16 a 46.46±0.82 a 45.38±0.64 a 45.48±0.85 a 46.43±1.02 a
1.2 取样方法
授粉140 d后,每2 d取20个果实进行可溶性固形物含量的测定,当果实的可溶性固形物含量大于6.5%时记为该处理的生理成熟期;在对应处理的生理成熟期,每株分别随机采取120 个健康的猕猴桃果实,采摘后进行单果质量、淀粉含量、横径、纵径和侧径指标的测定。常温放置至软熟状态(硬度小于1.2 kg·cm-2),其间每2 d随机抽取9个果实进行硬度指标测定。软熟后进行可溶性固形物含量和可滴定酸含量的测定,剩余果实液氮处理粉碎混匀后-80 ℃保存用于后续生理指标的测定。
1.3 指标测定与方法
单果质量用千分之一电子天平测量;果实横径、纵径和侧径用游标卡尺测量,果形指数计算方法:纵径/横径;可溶性固形物含量的测定用数显糖度计;可滴定酸含量的测定采用滴定法,具体步骤参考曹健康等[29]的报道;取果实赤道位置1.5 mm 左右的切片,65 ℃烘干至恒质量,以干质量/鲜质量的比值计为干物质含量;采用GY-4型果实硬度计测定果实硬度;软熟期的淀粉(Cat#BC0700)、总酚(Cat#BC1345)、类黄酮(Cat#BC1330)、葡萄糖(Cat#BC2500)、蔗糖(Cat#BC2460)和果糖(Cat#BC2450)含量的测定采用试剂盒法(北京索莱宝),步骤按说明书。奎宁酸、柠檬酸、苹果酸和抗坏血酸含量的测定采用高效液相色谱法进行,具体步骤参考周元等[30]的报道。
1.4 数据分析
使用Microsoft Excel 2021 进行统计处理及作图,使用SPSS17.0对数据进行差异显著性分析。
2 结果与分析
2.1 不同处理对果实外观指标的影响
授粉后25~30 d 不同处理对生理成熟期金艳猕猴桃果实外观指标的影响如表1 所示。结果表明,不同植物生长调节剂处理均能显著提高单果质量,NAA 处理对提高单果质量的效果最佳,与对照相比可将单果质量提高43.07%~55.78%。其次是Tri和CPPU 处理,可分别将单果质量提高34.56%~43.07%和24.07%~31.62%。此外,不同质量浓度处理效果差异明显,100 mg·L-1的NAA处理效果显著高于50 mg·L-1和200 mg·L-1,20 mg·L-1的Tri处理效果显著高于5 mg·L-1和10 mg·L-1,10 mg·L-1和20 mg·L-1的CPPU处理效果显著高于5 mg·L-1。不同质量浓度的CPPU、GA3、IAA、NAA、Tri及50 mg·L-1和100 mg·L-1的6-BA、40 mg·L-1的MT 和100 mg·L-1的Trp 处理均显著降低了果形指数。
2.2 不同处理对生理成熟期及淀粉含量的影响
由图1和图2可知,与对照相比CPPU处理将金艳猕猴桃果实的生理成熟期提前了18~20 d,NAA处理则将生理成熟期延迟了6~8 d,其他植物生长调节剂对生理成熟期无显著影响。CPPU处理对提高生理成熟期淀粉含量的效果最佳,与对照相比可将淀粉含量提高8.53%~17.32%,且5 mg·L-1处理效果显著高于10 mg·L-1和20 mg·L-1。其次是10 mg·L-1的MT与50 mg·L-1、100 mg·L-1的6-BA处理,可分别将淀粉含量提高8.82%和6.51%~7.60%。不同质量浓度的Tri、25 mg·L-1的6-BA、100 mg·L-1和200 mg·L-1的NAA 与100 mg·L-1的GA3处理则显著降低了生理成熟期的淀粉含量。
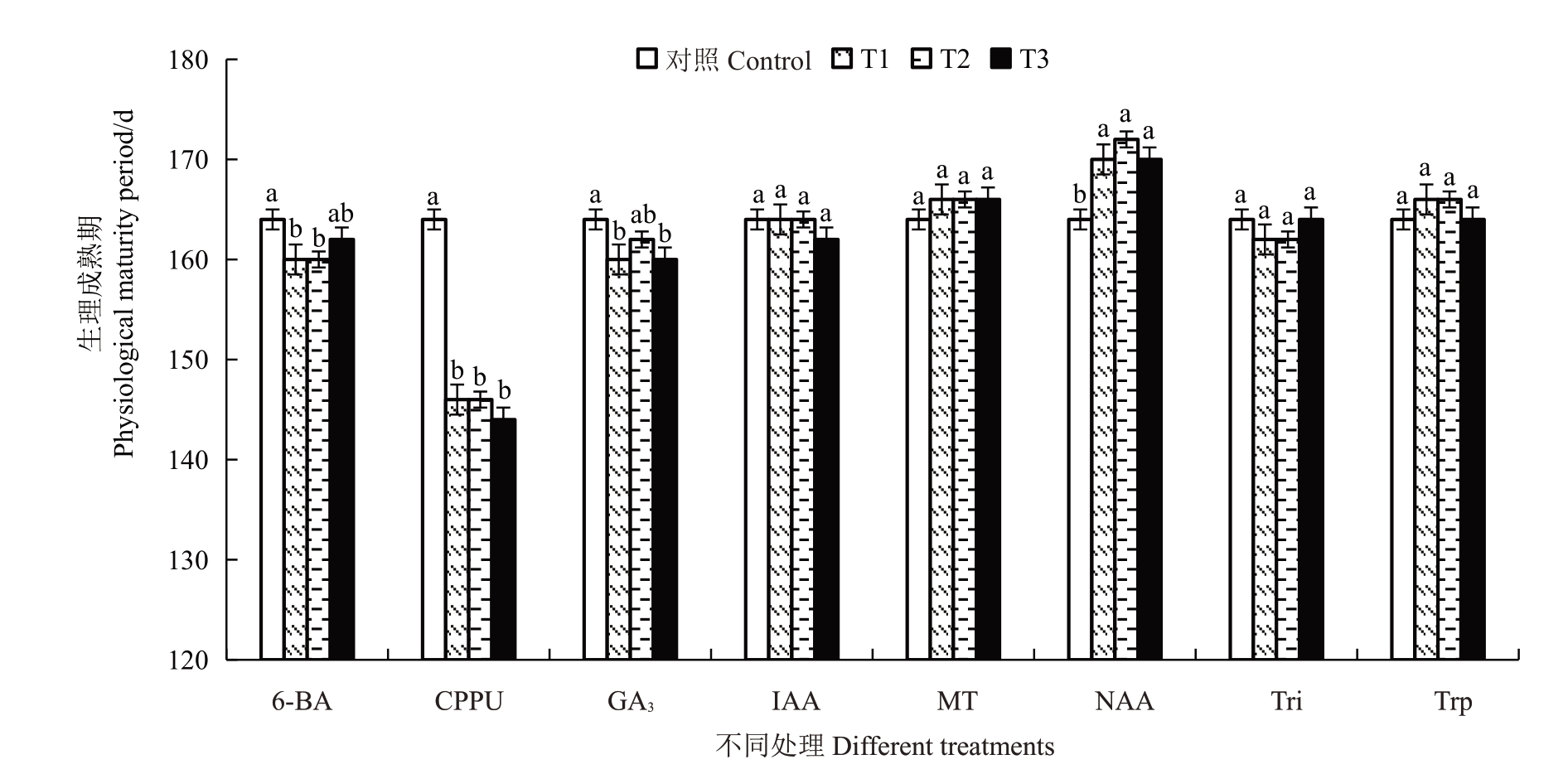
图1 不同植物生长调节剂处理对金艳猕猴桃果实生理成熟期的影响
Fig.1 Effects of different plant growth regulators treatments on the physiological maturity periods of Jinyan kiwifruit
T1、T2 和T3 分别代表不同植物生长调节剂从低到高的3 种处理质量浓度。下同。
T1,T2 and T3 respectively represent the three treatment concentrations of different plant growth regulators from low to high.The same below.
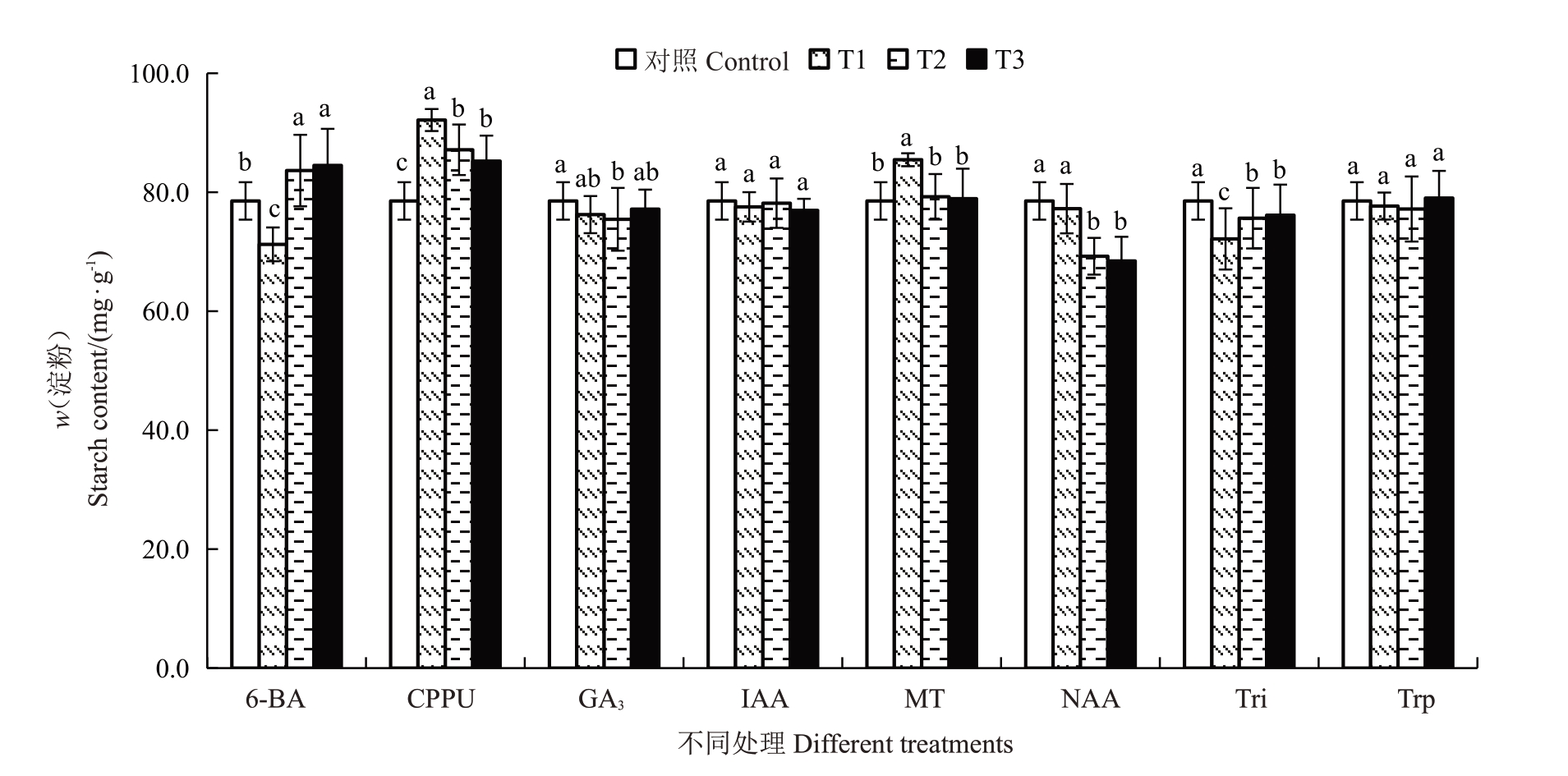
图2 不同植物生长调节剂处理对金艳猕猴桃果实生理成熟期淀粉含量的影响
Fig.2 Effects of different plant growth regulators treatments on the starch content in the physiological maturity periods of Jinyan kiwifruit
2.3 不同处理对硬度及软化速率的影响
不同处理对生理成熟期果实硬度的影响如图3 所示,结果表明,不同质量浓度的MT、Trp、NAA、5 mg·L-1的Tri、25 mg·L-1和50 mg·L-1的6-BA 处理均可以显著提高果实硬度。其中NAA 的处理效果最佳,与对照相比可将果实硬度提高15.30%~16.37%。其次是MT处理,可将果实硬度提高5.24%~10.89%,且不同质量浓度MT的处理效果差异显著,10 mg·L-1的处理效果显著高于20 mg·L-1和40 mg·L-1。

图3 不同植物生长调节剂处理对金艳猕猴桃果实生理成熟期硬度的影响
Fig.3 Effects of different plant growth regulators treatments on the fruit firmness in the physiological maturity periods of Jinyan kiwifruit
不同处理对猕猴桃采后果实常温条件下软化速率的影响由表2 可知。分析发现,不同质量浓度的NAA、200 mg·L-1的GA3和25 mg·L-1的6-BA处理均能显著抑制猕猴桃采后果实的软化速率。其中NAA处理效果最佳,与对照相比软化速率降低了10.96%~16.44%,软化周期延长了3~5 d。不同质量浓度的CPPU、200 mg·L-1的Trp、100 mg·L-1的GA3、40 mg·L-1的MT和20 mg·L-1的Tri则能显著提高采后果实的软化速率。CPPU处理的效果最显著,与对照相比软化速率提高了13.69%~26.03%,软化周期提前了4~6 d。
表2 不同植物生长调节处理对采后猕猴桃果实软化速率的影响
Table 2 Effects of different plant growth regulators treatments on the softening rate of postharvest Jinyan kiwifruit(kg·cm-2·d-1)
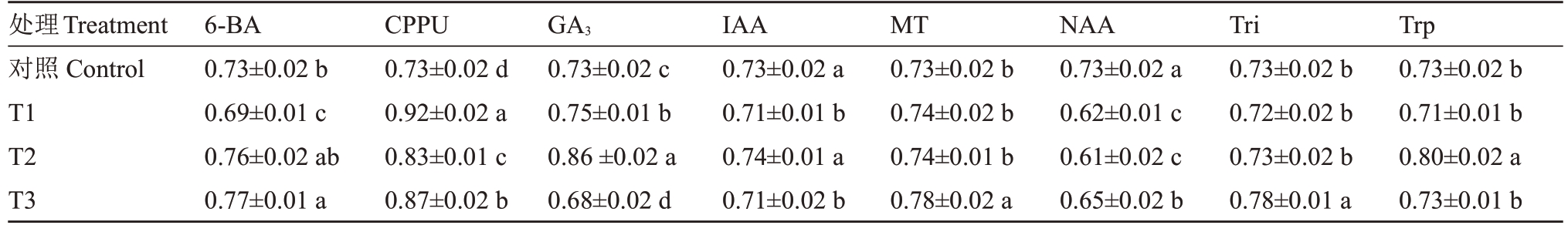
处理Treatment对照Control T1 T2 T3 6-BA 0.73±0.02 b 0.69±0.01 c 0.76±0.02 ab 0.77±0.01 a CPPU 0.73±0.02 d 0.92±0.02 a 0.83±0.01 c 0.87±0.02 b GA3 0.73±0.02 c 0.75±0.01 b 0.86±0.02 a 0.68±0.02 d IAA 0.73±0.02 a 0.71±0.01 b 0.74±0.01 a 0.71±0.02 b MT 0.73±0.02 b 0.74±0.02 b 0.74±0.01 b 0.78±0.02 a NAA 0.73±0.02 a 0.62±0.01 c 0.61±0.02 c 0.65±0.02 b Tri 0.73±0.02 b 0.72±0.02 b 0.73±0.02 b 0.78±0.01 a Trp 0.73±0.02 b 0.71±0.01 b 0.80±0.02 a 0.73±0.01 b
2.4 不同处理对软熟期果实品质的影响
2.4.1 不同处理对果实可溶性固形物含量的影响不同处理对果实软熟期可溶性固形物含量的影响如图4 所示。结果表明,不同质量浓度的CPPU 处理均能显著提高果实软熟期可溶性固形物含量,且20 mg·L-1和5 mg·L-1的处理效果显著高于10 mg·L-1,与对照相比将可溶性固形物含量提高了6.89%~15.04%。不同质量浓度的NAA和Tri处理则显著降低了果实软熟期可溶性固形物含量,与对照相比分别将可溶性固形物含量降低了4.21%~7.32%和3.70%~6.67%。其他植物生长调节剂处理则对果实软熟期可溶性固形物含量无显著影响。
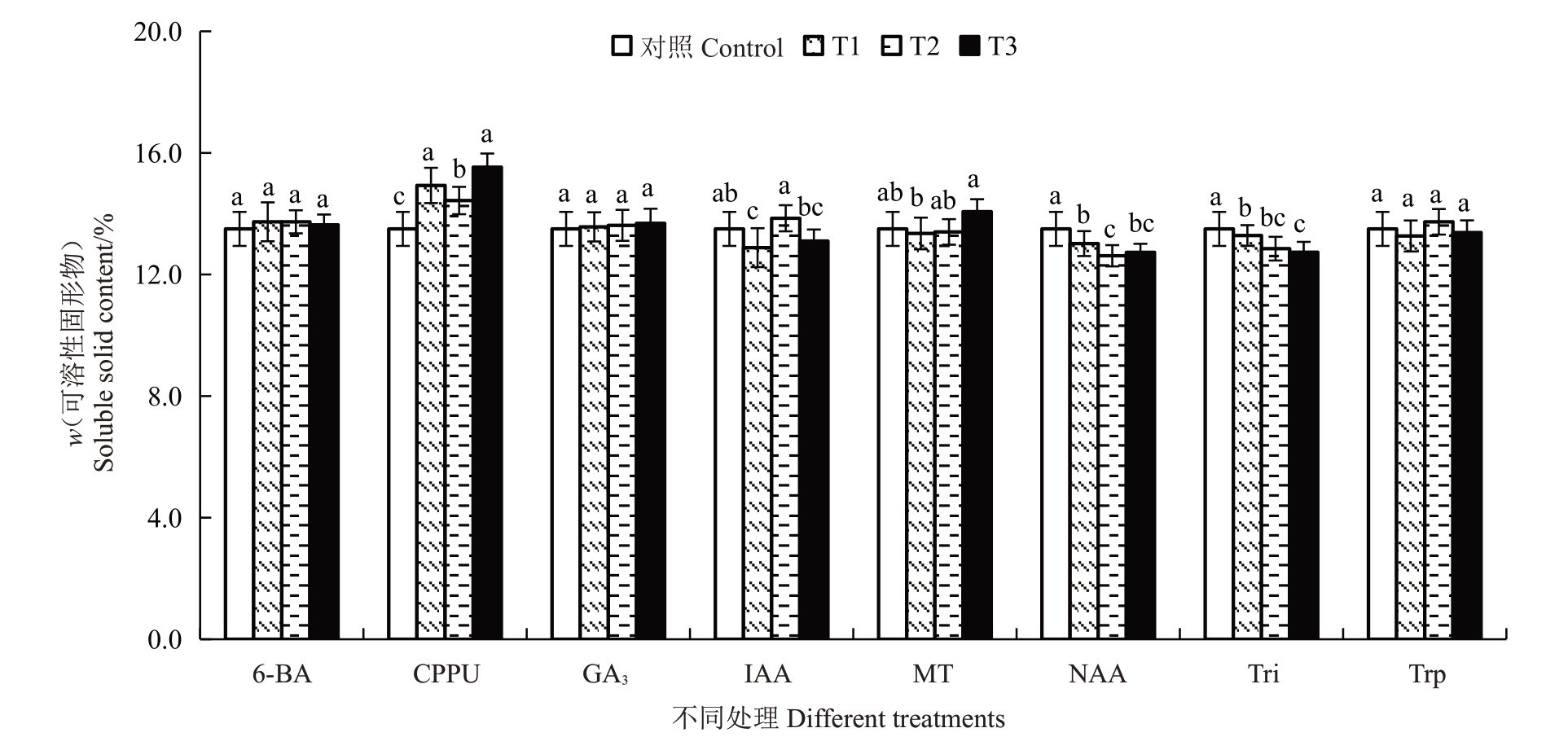
图4 不同植物生长调节剂处理对金艳猕猴桃果实软熟期可溶性固形物含量的影响
Fig.4 Effects of different plant growth regulators treatments on the soluble solids content in the softening periods of Jinyan kiwifruit
2.4.2 不同处理对果实可滴定酸含量的影响 软熟期金艳猕猴桃果实的可滴定酸含量介于1.16%~1.22%之间。不同处理对果实软熟期可滴定酸含量的影响如图5所示。结果表明,不同质量浓度的NAA、100 mg·L-1的6-BA、5 mg·L-1的CPPU、50 mg·L-1和100 mg·L-1的IAA、20 mg·L-1的Tri 及100 mg·L-1和400 mg·L-1的Trp处理均能显著提升金艳猕猴桃果实软熟期可滴定酸含量。50 mg·L-1的GA3和20 mg·L-1的MT 处理显著降低了果实软熟期可滴定酸含量。其他植物生长调节剂或质量浓度处理对果实软熟期可定酸含量则无显著影响。
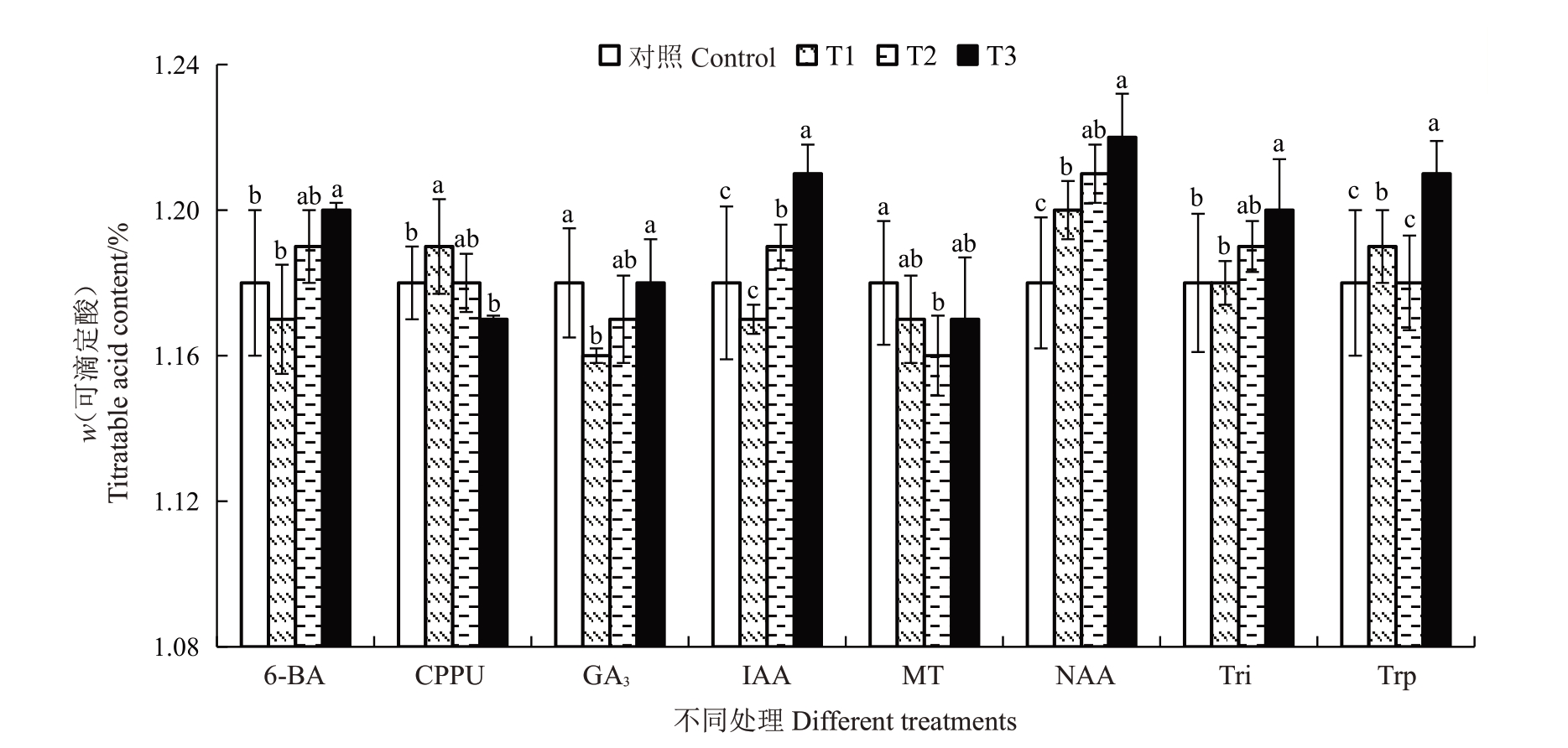
图5 不同植物生长调节剂处理对金艳猕猴桃果实软熟期可滴定酸含量的影响
Fig.5 Effects of different plant growth regulators treatments on the titratable acid content in the softening periods of Jinyan kiwifruit
2.4.3 不同处理对抗坏血酸含量的影响 不同处理对果实软熟期抗坏血酸含量的影响如图6所示。结果发现,不同质量浓度的CPPU、NAA 和Trp 处理均显著降低了软熟期金艳猕猴桃果实的抗坏血酸含量,但不同质量浓度之间的处理效果无显著差异。其中CPPU 的处理效果最为显著,与对照相比将果实软熟期抗坏血酸含量降低了10.53%~14.47%。其次是Trp和NAA处理,分别降低了9.21%~11.84%和6.24%~8.34%。不同质量浓度的MT和Tri处理则能显著提高果实软熟期抗坏血酸含量,分别提高了8.89%~10.53%和6.58%~9.21%。此外,80 mg·L-1的IAA处理也能显著提高果实软熟期抗坏血酸含量,其他植物生长调节剂或质量浓度则都对抗坏血酸的含量无显著影响。
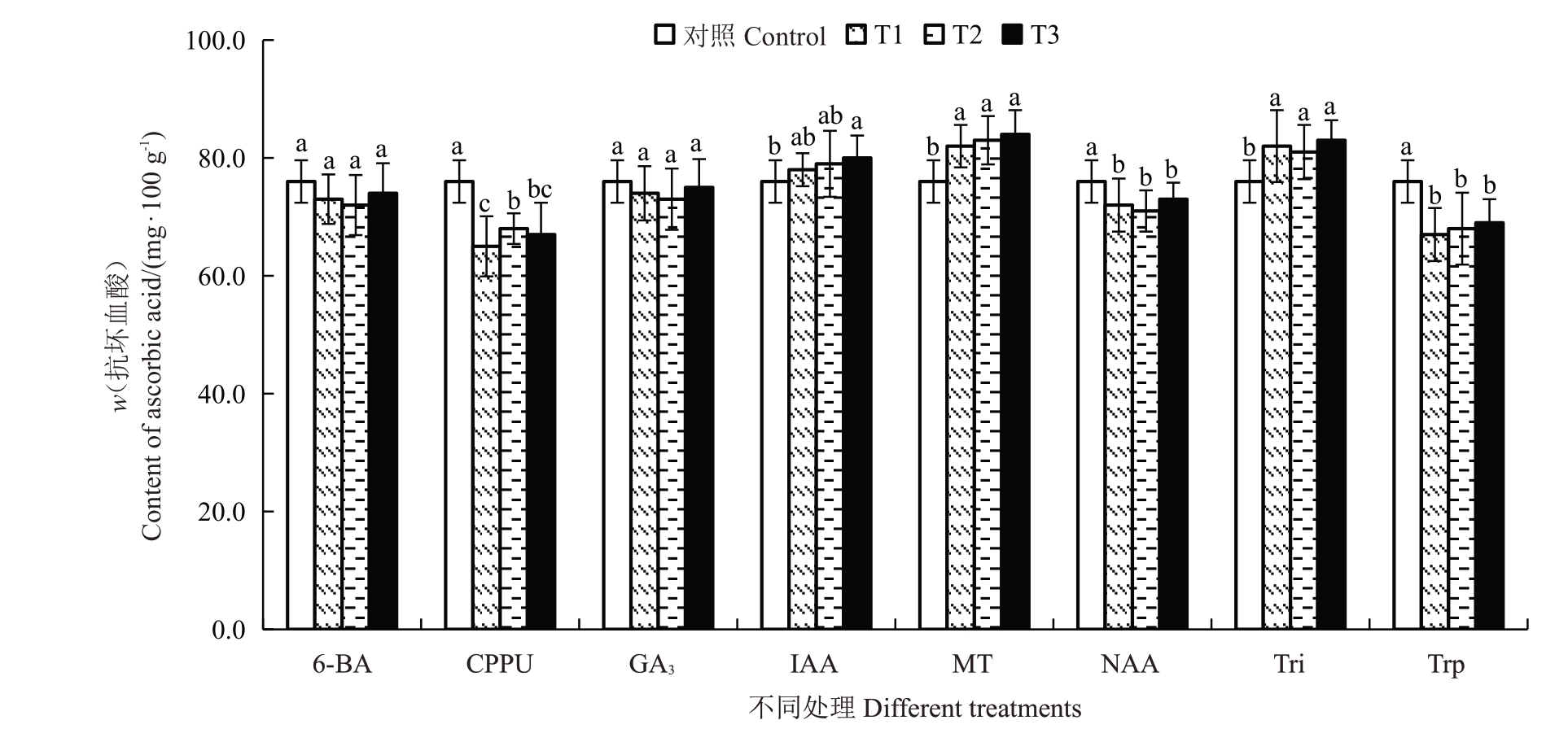
图6 不同植物生长调节剂处理对金艳猕猴桃果实软熟期抗坏血酸含量的影响
Fig.6 Effects of different plant growth regulators treatments on the ascorbic acid content in the softening periods of Jinyan kiwifruit
2.4.4 不同处理对可溶性糖含量的影响 不同处理对果实软熟期可溶性糖含量的影响如图7所示。结果表明,200 mg·L-1的GA3、200 mg·L-1的NAA、10 mg·L-1和20 mg·L-1的MT处理能显著提高果实软熟期果糖含量。其中效果最为显著的是10 mg·L-1的MT,与对照相比将果实软熟期果糖含量提高了17.43%,其次是200 mg·L-1的GA3、20 mg·L-1的MT和200 mg·L-1的NAA,分别将果实软熟期果糖含量提高了13.47%、11.82%和10.64%。其他植物生长调节剂或质量浓度对果实软熟期果糖含量无显著影响。不同质量浓度的CPPU、40 mg·L-1的MT、5 mg·L-1的Tri和400 mg·L-1的Trp 处理均能显著提高软熟期蔗糖含量。与对照相比,CPPU 处理可将蔗糖含量提高34.23%~41.23%,且不同质量浓度间的处理效果无显著差异。不同质量浓度的NAA、50 mg·L-1 和200 mg·L-1的GA3处理则显著降低了软熟期蔗糖含量,与对照相比,NAA 处理将软熟期蔗糖含量降低了15.81%~22.21%。不同质量浓度的MT、20 mg·L-1和40 mg·L-1 的IAA 处理可显著提高软熟期葡萄糖含量,20 mg·L-1 的IAA 处理效果最佳,其次是10 mg·L-1的MT,可分别将果实软熟期葡萄糖含量提高28.39%和26.59%。
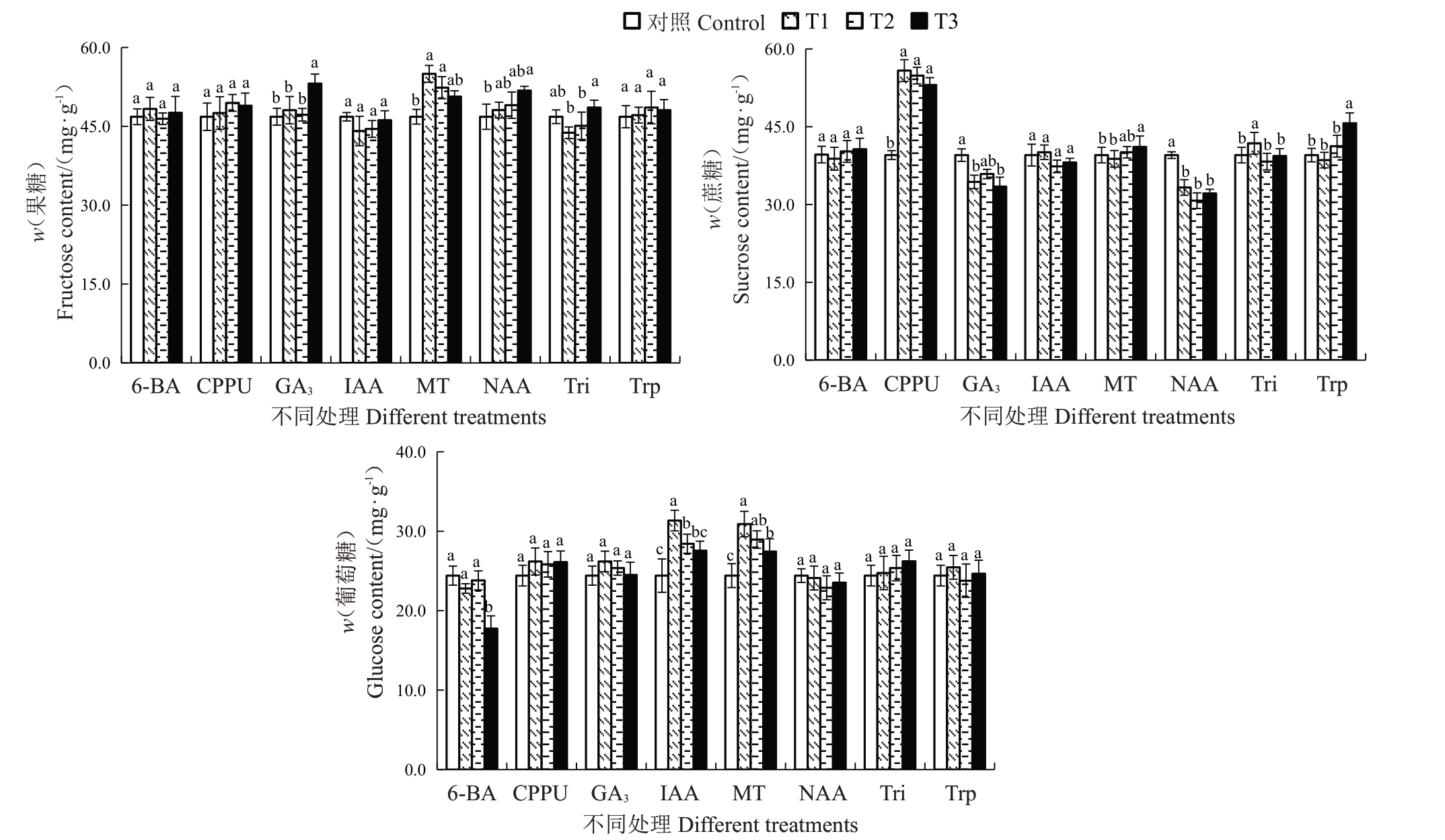
图7 不同植物生长调节剂处理对金艳猕猴桃果实软熟期可溶性糖含量的影响
Fig.7 Effects of different plant growth regulators treatments on the soluble sugar content in the softening periods of Jinyan kiwifruit
2.4.5 不同处理对有机酸含量的影响 不同处理对软熟期有机酸含量的影响如图8所示。结果表明,不同质量浓度的CPPU 和MT 处理均显著降低了软熟期果实柠檬酸、奎宁酸和苹果酸含量。与对照相比,柠檬酸含量分别降低了8.75%~10.63%和8.75%~15.01%,奎宁酸含量分别降低了10.77%~15.38%和9.23%~12.30%,苹果酸含量分别降低了14.29%~20.01%和23.81%~24.76%。不同质量浓度的Trp则显著提高了软熟期柠檬酸、奎宁酸和苹果酸含量,与对照相比柠檬酸、奎宁酸和苹果酸含量分别提高了7.51%~13.75%、9.23%~13.85%和21.90%~29.05%。此外,不同质量浓度的NAA处理显著提高了果实软熟期奎宁酸和苹果酸含量,100 mg·L-1的NAA处理显著提高了果实软熟期柠檬酸含量。
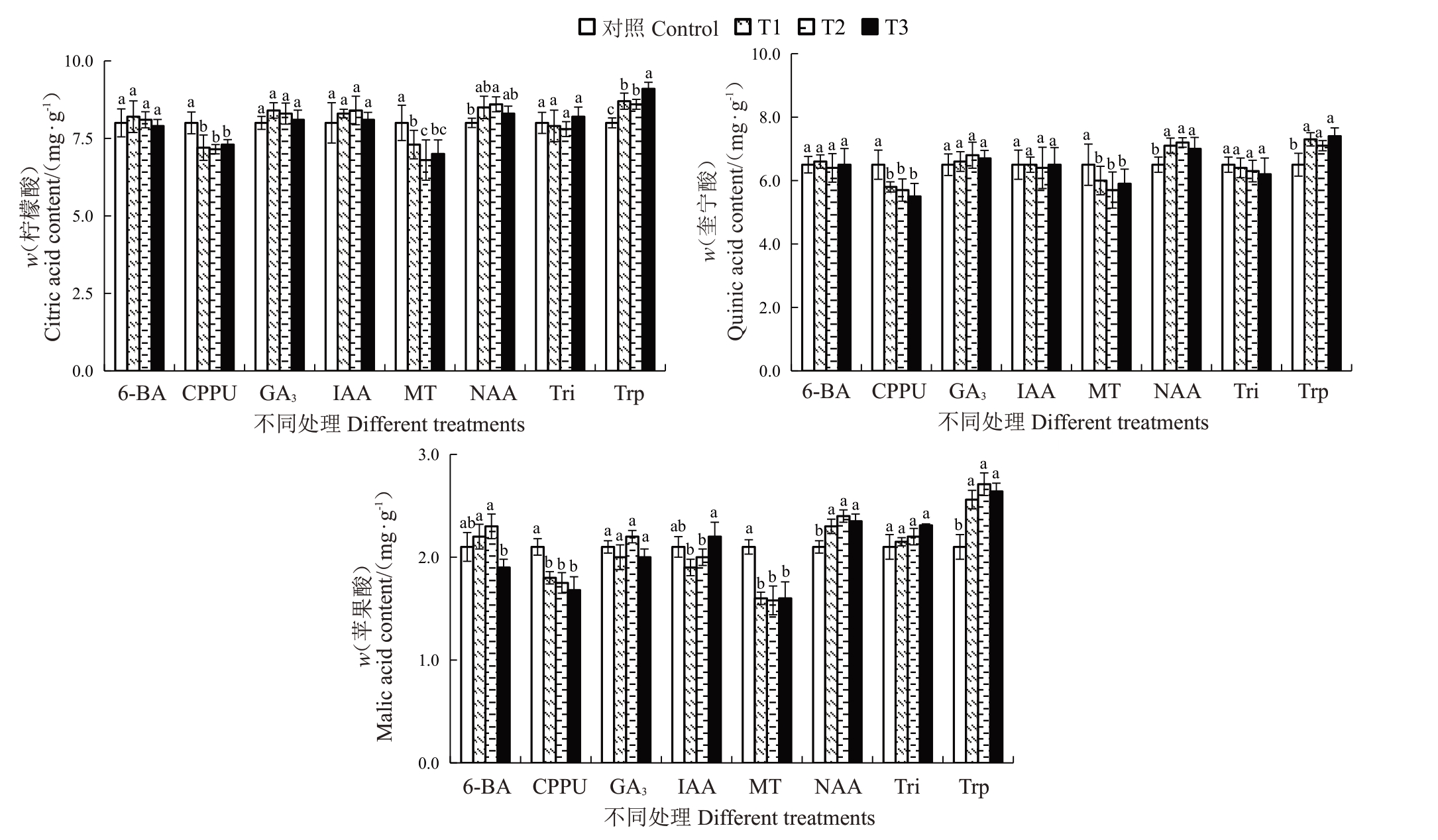
图8 不同植物生长调节剂处理对金艳猕猴桃果实软熟期有机酸含量的影响
Fig.8 Effects of different plant growth regulators treatments on the organic acid content in the softening periods of Jinyan kiwifruit
2.4.6 不同处理对总酚和总黄酮含量的影响 软熟期金艳猕猴桃果实总酚和总黄酮含量分别介于98~126 mg·100 g-1之间。不同处理对软熟期总酚和总黄酮含量的影响如图9、图10 所示。结果表明,CPPU和GA3处理均显著降低了软熟期果实总酚含量,与对照相比分别降低了7.14%~8.93%和6.25%~12.51%。不同质量浓度的NAA、MT、20 mg·L-1的Tri和80 mg·L-1的IAA处理则显著提高了果实软熟期总酚含量。不同质量浓度的CPPU 和MT 处理均显著提高了果实软熟期果实总黄酮含量,与对照相比分别提高了22.56%~28.75%和8.75%~15.00%,且不同CPPU质量浓度处理之间无显著差异,MT处理质量浓度越大总黄酮含量越高。不同质量浓度NAA、Trp、40 mg·L-1和80 mg·L-1的IAA处理则显著抑制了果实软熟期总黄酮的积累,与对照相比NAA和Trp 处理分别将果实软熟期总黄酮含量降低了10.00%~15.00%和8.75%~11.25%。其他植物生长调节剂或质量浓度则对果实软熟期总酚和总黄酮含量无显著影响。
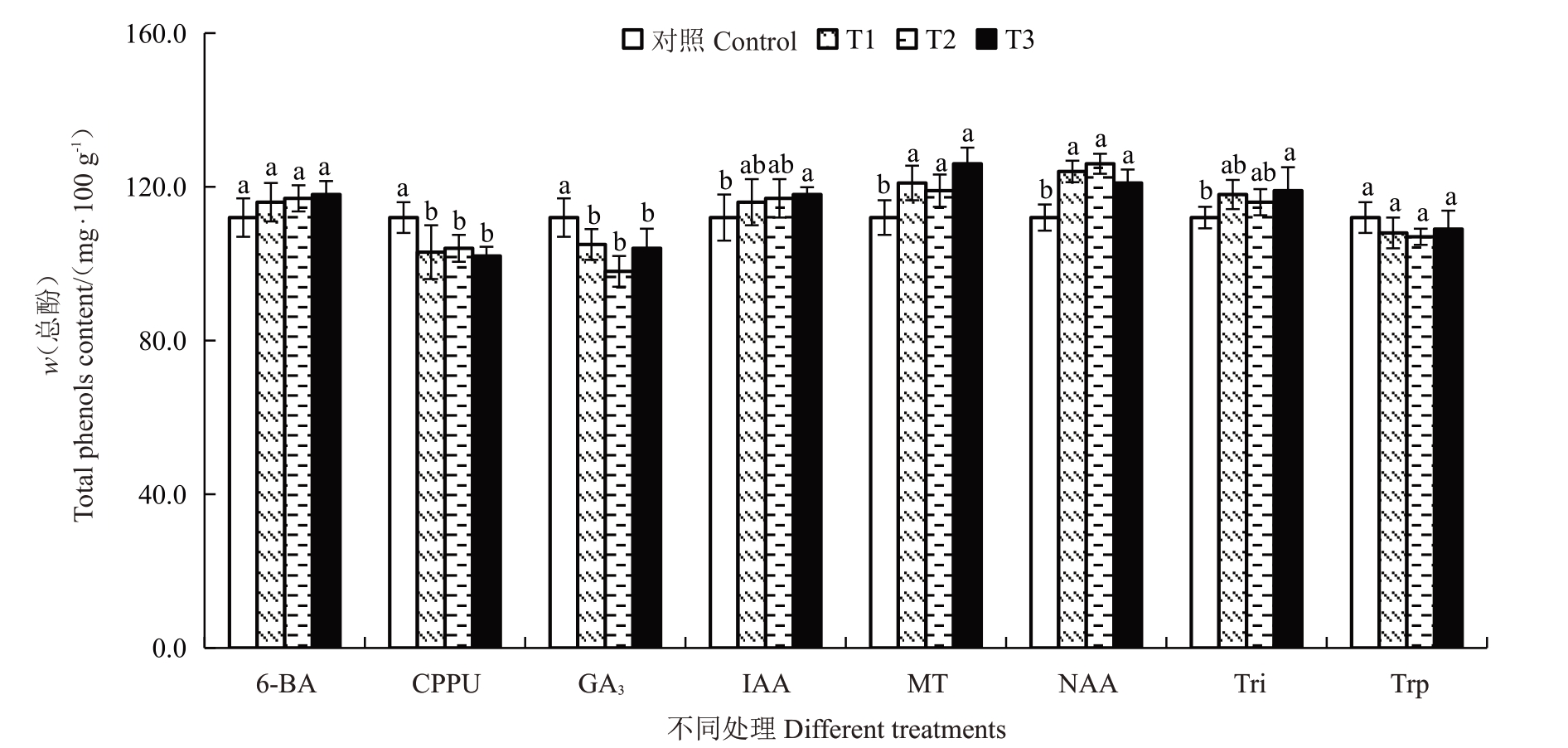
图9 不同植物生长调节剂处理对金艳猕猴桃果实软熟期总酚含量的影响
Fig.9 Effects of different plant growth regulators treatments on the total phenols content in the softening periods of Jinyan kiwifruit
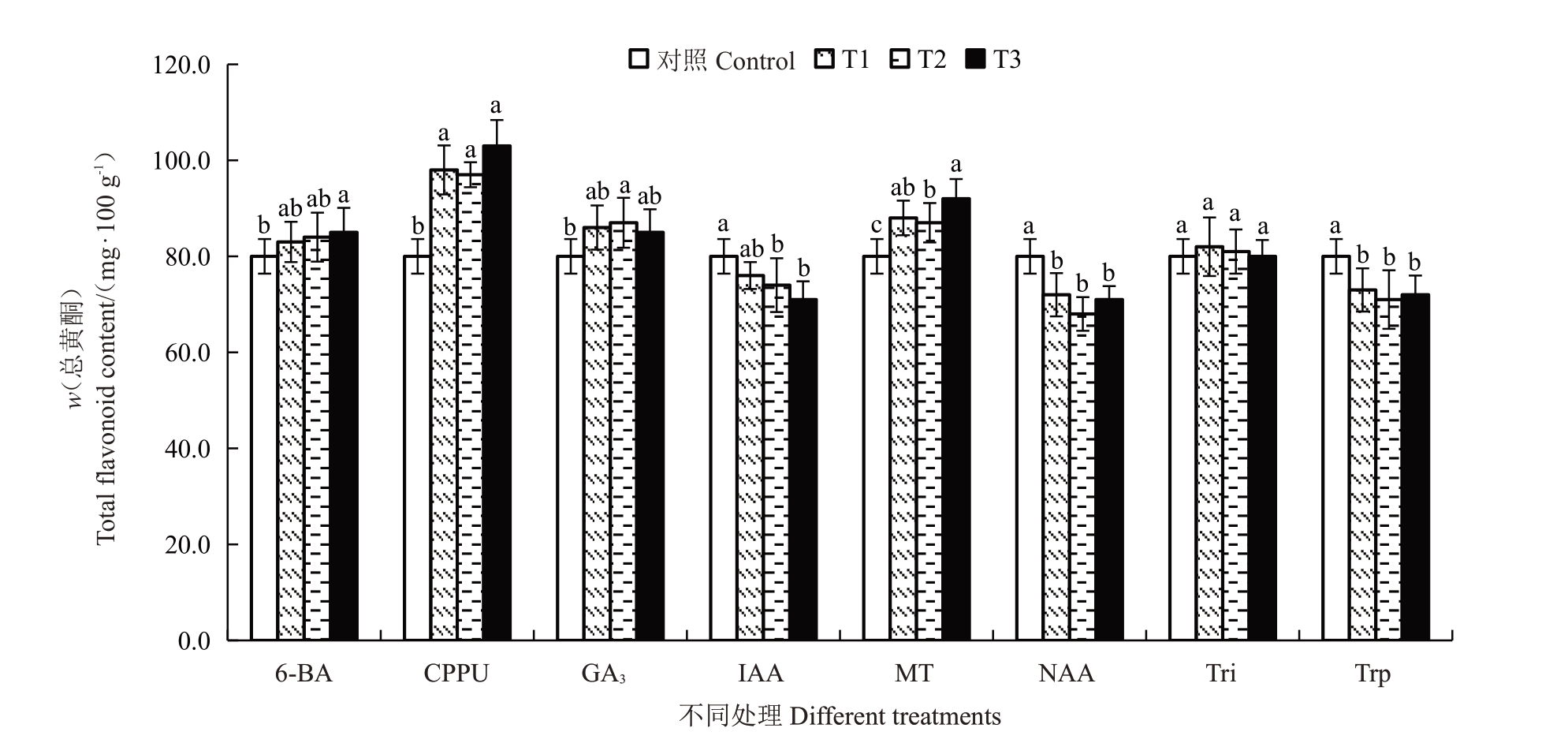
图10 不同植物生长调节剂处理对金艳猕猴桃果实软熟期总黄酮含量的影响
Fig.10 Effects of different plant growth regulators treatments on the total flavonoid content in the softening periods of Jinyan kiwifruit
3 讨 论
植物生长调节剂因具有促进植株生长和果实发育、提高产量和抗性等生理作用而被广泛应用于园艺植物的生产过程中[31]。本研究在果实外观指标、生理成熟期、果实硬度,以及淀粉、可溶性固形物、可溶性糖、有机酸、总酚和总黄酮含量等方面系统地分析了不同植物生长调节剂及质量浓度处理对金艳猕猴桃果实发育和采后品质指标的影响。果实外观指标是影响消费者喜好和商品价值的重要因素。研究表明,所有的处理均能显著提高生理成熟期金艳猕猴桃果实的单果质量,这与上述植物生长调节剂在金奉(原奉黄1 号)[9]、东红[7]、红阳[17]、秦美[6,8]和海沃德[6,18]等其他猕猴桃品种中的应用效果一致。然而除个别植物生长调节剂的处理质量浓度外,绝大部分的处理均显著降低了果形指数,这与李圆圆等[6]和伍梦婷等[9]报道的研究结果一致。
生理成熟期、果实硬度和淀粉含量是影响采后果实品质形成和贮藏性能的关键因素。本研究中发现,CPPU处理在提高淀粉含量的同时,也提前了金艳猕猴桃的生理成熟期并降低了果实硬度。这可能是生产上CPPU处理可以提高软熟期果实糖度但贮藏性能较差的原因之一。不同质量浓度的NAA 处理则可以将金艳猕猴桃的生理成熟期延后6~8 d,这在以往关于NAA 的应用研究中未见报道。结合CPPU 和NAA 这种相对立的结果,可为人工调控猕猴桃生理成熟期关键技术开发提供参考。
不同质量浓度的CPPU处理均能显著提高软熟期猕猴桃果实可溶性固形物含量,这与李圆圆[6]、熊浩等[7]和伍梦婷等[9]报道的研究结果一致。虽然不同植物生长调节剂或质量浓度对果实软熟期可滴定酸含量的影响差异显著,但可滴定酸含量均介于1.16%~1.22%之间,对果实风味品质形成的贡献相对较少。不同质量浓度的CPPU、NAA 和Trp 处理均显著降低了果实软熟期抗坏血酸含量。李圆圆[6]研究发现5 mg·L-1的CPPU 处理对秦美猕猴桃对抗坏血酸含量无显著影响,10 mg·L-1和20 mg·L-1的CPPU处理则显著降低抗坏血酸含量,3种质量浓度的CPPU 处理均能降低海沃德猕猴桃抗坏血酸含量,初步说明不同猕猴桃品种对CPPU 处理的响应程度不一致,这与本研究的结果基本一致。张春红等[23]研究发现50~200 mg·L-1的NAA 处理均能显著提高蓝莓果实的抗坏血酸含量。杜丽清等[26]也发现200 mg·L-1和600 mg·L-1的Trp 处理能显著提高菠萝果实的抗坏血酸含量,这与本研究的结果相反,这说明NAA 和Trp 处理对不同品种水果的效果有很大差别。
可溶性糖和有机酸作为猕猴桃最为关键的风味物质,其含量的多少直接影响了软熟期猕猴桃口感和风味。MT 处理能显著提高果实软熟期果糖、蔗糖、葡萄糖、总酚和总黄酮含量,但同时降低了柠檬酸、奎宁酸和苹果酸含量。贾润普等[21]和胡容平等[32]研究发现适宜浓度的MT处理能显著提高葡萄果实葡萄糖和果糖含量,李强等[33]也研究发现MT处理能显著提高葡萄果实总酚和总黄酮含量。这与本研究的结果一致。但MT处理能降低有机酸组分含量的研究尚未见报道。CPPU处理能显著提高软熟期猕猴桃果实蔗糖含量,但对葡萄糖和果糖含量无显著影响,同时能显著降低果实软熟期柠檬酸、奎宁酸和苹果酸含量。关于CPPU处理对猕猴桃果实具体糖酸组分的影响研究尚未见报道,该结果为解析CPPU处理影响猕猴桃果实品质的研究提供了一定的理论基础。不同质量浓度的NAA处理显著抑制果实软熟期蔗糖的积累与柠檬酸、奎宁酸和苹果酸的降解。王西成等[24]研究也发现50 mg·L-1、100 mg·L-1和200 mg·L-1的NAA 处理能显著抑制可溶性糖组分的积累和有机酸组分的降解,这与本研究的结果一致。综合表明,不同植物生长调节剂和质量浓度对金艳猕猴桃果实发育和采后品质指标含量具有不同的促进或抑制作用。
4 结 论
不同质量浓度的6-BA、CPPU、GA3、IAA、MT、NAA、Tri 和Trp 处理均能显著提高果实的单果质量,但降低了果形指数。不同植物生长调节剂或质量浓度对猕猴桃可溶性固形物、抗坏血酸、可溶性糖、有机酸、总酚和总黄酮含量等果实品质指标含量具有不同的促进或抑制作用。10 mg·L-1的CPPU处理促进淀粉和可溶性固形物积累的效果最佳,100 mg·L-1的NAA 处理提升果实外观和贮藏性能的效果最佳,MT 处理对提升果实风味品质的效果最佳,适宜质量浓度的CPPU、NAA和MT复合处理有望提高猕猴桃综合品质。
[1] WANG R C,SHU P,ZHANG C,ZHANG J L,CHEN Y,ZHANG Y X,DU K,XIE Y,LI M Z,MA T,ZHANG Y,LI Z G,GRIERSON D,PIRRELLO J,CHEN K S,BOUZAYEN M,ZHANG B,LIU M C.Integrative analyses of metabolome and genome-wide transcriptome reveal the regulatory network governing flavor formation in kiwifruit (Actinidia chinensis) [J].New Phytologist,2022,233(1):373-389.
[2] XIONG Y,YAN P,DU K,LI M Z,XIE Y,GAO P.Nutritional component analyses of kiwifruit in different development stages by metabolomic and transcriptomic approaches[J].Journal of the Science of Food and Agriculture,2020,100(6):2399-2409.
[3] 齐秀娟,郭丹丹,王然,钟云鹏,方金豹.我国猕猴桃产业发展现状及对策建议[J].果树学报,2020,37(5):754-763.QI Xiujuan,GUO Dandan,WANG Ran,ZHONG Yunpeng,FANG Jinbao.Development status and suggestions on Chinese kiwifruit industry[J].Journal of Fruit Science,2020,37(5):754-763.
[4] 胡新龙,金玲莉,王璠,王斯妤,吴庭观,李小飞,陈东元,吴美华.江西猕猴桃产业现状及“十四五”发展对策与建议[J].江西农业学报,2022,34(5):34-39.HU Xinlong,JIN Lingli,WANG Fan,WANG Siyu,WU Tingguan,LI Xiaofei,CHEN Dongyuan,WU Meihua.Development status and suggestions on kiwifruit industry in Jiangxi province during“the 14th Five-Year Plan”period[J].Acta Agriculturae Jiangxi,2022,34(5):34-39.
[5] WAN H L,KONG X B,LIU Y H,JIN F,HAN L X,XU M,LI X M,LI L,YANG J,LAI D N,NIE J Y.Residue analysis and effect of preharvest forchlorfenuron (CPPU) application on quality formation of kiwifruit[J].Postharvest Biology and Technology,2023,195:112144.
[6] 李圆圆.采前CPPU 处理对猕猴桃采后品质及细胞超微结构的影响机制研究[D].杨凌:西北农林科技大学,2018.LI Yuanyuan.Effect mechanism of preharvest CPPU treatment on postharvest quality and cell ultrastructure of kiwifruit[D].Yangling:Northwest A&F University,2018.
[7] 熊浩,郑浩,韩佳欣,苑馨予,李吉涛,钟彩虹,张琼.CPPU 处理对猕猴桃果实品质的影响[J].植物科学学报,2022,40(1):74-83.XIONG Hao,ZHENG Hao,HAN Jiaxin,YUAN Xinyu,LI Jitao,ZHONG Caihong,ZHANG Qiong.Effects of CPPU treatment on fruit quality in Actinidia[J].Plant Science Journal,2022,40(1):74-83.
[8] 方金豹,黄宏文,李绍华.CPPU 对猕猴桃果实发育过程中糖、酸含量变化的影响[J].果树学报,2002,19(4):235-239.FANG Jinbao,HUANG Hongwen,LI Shaohua.Influence of CPPU on kiwifruit sugar content and titratable acidity during fruit development[J].Journal of Fruit Science,2002,19(4):235-239.
[9] 伍梦婷,钟文奇,陶俊杰,陈双双,贾慧敏,徐小彪,黄春辉.氯苯脲(CPPU)浸果对‘奉黄1 号’猕猴桃品质的影响[J].中国南方果树,2023,52(4):100-107.WU Mengting,ZHONG Wenqi,TAO Junjie,CHEN Shuangshuang,JIA Huimin,XU Xiaobiao,HUANG Chunhui.Effect of chlorphenylurea (CPPU) on the quality of‘Fenghuang No.1’kiwifruit[J].South China Fruits,2023,52(4):100-107.
[10] 李国田,张雪丹,孙山,安淼.氯吡脲处理对‘泰山1 号’猕猴桃采后果实呼吸和品质的影响[J].北方园艺,2020(12):52-57.LI Guotian,ZHANG Xuedan,SUN Shan,AN Miao.Effects of CPPU on respiration intensity and fruit quality of‘Taishan No.1’kiwifruit during storage[J].Northern Horticulture,2020(12):52-57.
[11] WU L,LAN J B,XIANG X X,XIANG H Y,JIN Z,KHAN S,LIU Y Q.Transcriptome sequencing and endogenous phytohormone analysis reveal new insights in CPPU controlling fruit development in kiwifruit (Actinidia chinensis) [J].PLoS One,2020,15(10):e0240355.
[12] 张利英,刘雪霞,朱昌华,甘立军.KT、6-BA 对红颜草莓果实品质的影响[J].生物学杂志,2019,36(5):62-66.ZHANG Liying,LIU Xuexia,ZHU Changhua,GAN Lijun.Effects of KT and 6-BA on the fruit quality in‘Hongyan’strawberry[J].Journal of Biology,2019,36(5):62-66.
[13] 孙志伟.花期喷施GA3 和6-BA 对“小目手撕”菠萝采后果实品质的影响[D].昆明:云南农业大学,2023.SHUN Zhiwei.Effects of spraying GA3 and 6-BA at flowering stage on postharvest fruit quality of“small eye hand-torn”pineapple[D].Kunming:Yunnan Agricultural University,2023.
[14] ASÍN L,VILARDELL P,BONANY J,ALEGRE S.Effect of 6-BA,NAA and their mixtures on fruit thinning and fruit yield in‘Conference’and‘Blanquilla’pear cultivars[J].Acta Horticulturae,2010(884):379-382.
[15] 杨卫民,杜京旗,赵君.6-BA、IAA、GA3与ABA 对木枣果实成熟衰老的调控作用[J].生物技术通报,2016,32(1):88-91.YANG Weimin,DU Jingqi,ZHAO Jun.The regulation of 6-BA,IAA,GA3 and ABA on the ripening and senescence of Mu jujube’s fruits (Ziziphus jujuba L.)[J].Biotechnology Bulletin,2016,32(1):88-91.
[16] 蔡仲慧,李秀杰,王悦,李勃,谢兆森.赤霉素处理对阳光玫瑰葡萄果实生长动态及品质的影响[J].南方农业学报,2024,55(2):499-508.CAI Zhonghui,LI Xiujie,WANG Yue,LI Bo,XIE Zhaosen.Growth dynamics observation and quality analysis of grape berry under different gibberellin treatments[J].Journal of Southern Agriculture,2024,55(2):499-508.
[17] 杨海英.赤霉素对猕猴桃果实采后成熟的调控及其分子机制研究[D].烟台:鲁东大学,2023.YANG Haiying.Studies on regulation and molecular mechanism of gibberellin treatment on ripening of postharvest kiwifruit[D].Yantai:Ludong University,2023.
[18] 尹翠波,周庆阳.GA3和CPPU 对猕猴桃果实发育及品质的影响[J].福建果树,2007(4):5-9.YIN Cuibo,ZHOU Qingyang.Effects of GA3 and CPPU on fruit development and quality of kiwifruit[J].Fujian Fruits,2007(4):5-9.
[19] 钟晓红,石雪晖,马定渭,黄远飞,戴思慧.IAA 色氨酸处理对索非亚草莓营养生长和果实品质的调控[J].果树学报,2004,21(6):565-568.ZHONG Xiaohong,SHI Xuehui,MA Dingwei,HUANG Yuanfei,DAI Sihui.Study on the effects of tryptophan on the growth and fruit quality of Sophie strawberry cultivar[J].Journal of Fruit Science,2004,21(6):565-568.
[20] LIU N N.Effects of IAA and ABA on the immature peach fruit development process[J].Horticultural Plant Journal,2019,5(4):145-154.
[21] 贾润普,王玥,李勃,李蓥男,姚玉新.外源褪黑素处理对‘阳光玫瑰’葡萄果实品质的影响[J].植物生理学报,2022,58(10):2034-2044.JIA Runpu,WANG Yue,LI Bo,LI Yingnan,YAO Yuxin.Effects of exogenous melatonin treatment on quality of‘Shine Muscat’grape berries[J].Plant Physiology Journal,2022,58(10):2034-2044.
[22] 苏金龙.褪黑素对猕猴桃果实后熟衰老的调控及其生理机制研究[D].西安:西北大学,2021.SU Jinlong.Studies on regulation and physiological mechanism of melatonin treatment on ripening and senescence of kiwifruit[D].Xi’an:Northwest University,2021.
[23] 张春红,吴文龙.NAA 处理对蓝莓果实发育和品质性状的影响研究[J].中国南方果树,2021,50(4):147-152.ZHANG Chunhong,WU Wenlong.Effects of NAA on fruit development and quality traits of blueberry[J].South China Fruits,2021,50(4):147-152.
[24] 王西成,吴伟民,赵密珍,钱亚明,王壮伟.NAA 对葡萄果实中糖酸含量及相关基因表达的影响[J].园艺学报,2015,42(3):425-434.WANG Xicheng,WU Weimin,ZHAO Mizhen,QIAN Yaming,WANG Zhuangwei.Effect of NAA treatment on sugar acid content and related gene expression in grape berries[J].Acta Horticulturae Sinica,2015,42(3):425-434.
[25] 吴琼,吴文龙,李维林,张春红.NAA 处理对黑莓果实品质及细胞壁酶活性的影响[J].北方园艺,2021(10):15-21.WU Qiong,WU Wenlong,LI Weilin,ZHANG Chunhong.Effects of NAA treatment on fruit quality and cell wall degrading enzyme activities of blackberry fruits[J].Northern Horticulture,2021(10):15-21.
[26] 杜丽清,姚艳丽,孙光明,张秀梅.不同浓度IAA 色氨酸处理对菠萝果实品质发育的影响[J].热带作物学报,2014,35(3):433-437.DU Liqing,YAO Yanli,SUN Guangming,ZHANG Xiumei.Different concentrations of IAA and tryptophan effects on the quality development of pineapple[J].Chinese Journal of Tropical Crops,2014,35(3):433-437.
[27] WÓJCIK P,FILIPCZAK J,WÓJCIK M.Effects of prebloom sprays of tryptophan and zinc on calcium nutrition,yielding and fruit quality of‘Elstar’apple trees[J].Scientia Horticulturae,2019,246:212-216.
[28] 吴建辉,陈清香,覃振强,王泽清.0.1%三十烷醇对柑橘的生长调节作用[J].广东农业科学,2009,36(7):141-142.WU Jianhui,CHEN Qingxiang,QIN Zhenqiang,WANG Zeqing.Effect of growth regulator to citrus on applying 0.1% triacotanol[J].Guangdong Agricultural Sciences,2009,36(7):141-142.
[29] 曹健康,姜微波,赵玉梅.果蔬采后生理生化实验指导[M].北京:中国轻工业出版社,2007.CAO Jiankan,JIANG Weibo,ZHAO Yumei.Experiment guidance of postharvest physiology and biochemistry fruits and vegetables[M].Beijing:China Light Industry Press,2007.
[30] 周元,傅虹飞.猕猴桃中的有机酸高效液相色谱法分析[J].食品研究与开发,2013,34(19):85-87.ZHOU Yuan,FU Hongfei.Determination of organic acids in kiwifruit by reversed phase HPLC method[J].Food Research and Development,2013,34(19):85-87.
[31] 高佳缘.植物生长调节剂在果树生产上的应用[J].黑龙江农业科学,2019(2):163-164.GAO Jiayuan.Application of plant growth regulators in fruit tree production[J].Heilongjiang Agricultural Sciences,2019(2):163-164.
[32] 胡容平,范中菡,董义霞,钟莉莎,林立金,廖明安.褪黑素对葡萄果实品质的影响[J].北方园艺,2022(4):39-44.HU Rongping,FAN Zhonghan,DONG Yixia,ZHONG Lisha,LIN Lijin,LIAO Ming’an.Effects of melatonin on fruit quality of grape[J].Northern Horticulture,2022(4):39-44.
[33] 李强,史星雲,马宗桓,李春玲,赵连鑫,刘伟,张勤德.褪黑素对葡萄农艺性状及果实品质的影响[J].林业科技通讯,2021(12):32-36.LI Qiang,SHI Xingyun,MA Zonghuan,LI Chunling,ZHAO Lianxin,LIU Wei,ZHANG Qinde.Effects of melatonin on agronomic traits and fruit quality of grape[J].Forest Science and Technology,2021(12):32-36.