可溶性糖是水果营养物质的重要组成部分,也是调控植物生长发育的重要信号分子[1]。在新鲜水果中,蔗糖(Suc)、果糖(Fru)和葡萄糖(Glu)是果实品质的主要决定因子,它们的含量和比例会对果实甜味产生重要影响[2]。在大多数植物中,蔗糖是源器官(叶)进行光合作用的主要产物,蔗糖合成后会通过韧皮部长距离运输转运至果实、根、茎尖等不同的库器官中维持其生长发育[3-4]。在库器官中,包括蔗糖在内的可溶性糖首先会被代谢以满足库器官对能量和碳源的需求,过量的可溶性糖可被转化为淀粉贮藏在质体中,或者经由液泡糖转运蛋白介导被转运至液泡中贮藏[3,5]。
库器官中蔗糖的积累水平主要取决于蔗糖合成和降解的速率。一方面,果实中的蔗糖可被转化酶(INV)水解成葡萄糖和果糖,也可在蔗糖合酶(SUSY)催化下分解为UDP-葡萄糖(UDPG)和果糖;另一方面,在成熟果实中,蔗糖磷酸合酶(SPS)可将果糖-6-磷酸(F6P)和UDPG 转化为蔗糖6-磷酸,蔗糖-6-磷酸进一步在蔗糖-6-磷酸磷酸酶(SPP)的催化下转化为蔗糖而积累[1]。在蔗糖代谢过程中,SPS、SUSY 和INV 被认为是负责蔗糖合成或降解的主要酶[1,6],其中,SPS被认为是负责蔗糖合成的限速酶[7]。在玉米和水稻中的研究表明,SPS基因对农作物生长速度和产量具有重要的促进作用[8-10]。对一些园艺作物,如香蕉[11]、柑橘[12]、甜瓜[13]等的研究发现,果实中蔗糖快速积累的阶段,SPS基因的表达也同步显著上调,暗示SPS 基因对果实中蔗糖合成和积累发挥重要作用。目前,猕猴桃果实中SPS 酶对蔗糖代谢功能的研究还处于起步阶段,调控蔗糖合成和积累的关键基因需要进一步挖掘。
毛花猕猴桃(Actinidia eriantha)是猕猴桃属中一个独特的种类,其果实风味浓郁,抗坏血酸(AsA)含量非常高[14-16],且具有较强的抗逆性,是猕猴桃育种和生产中极具应用潜力的重要种质资源。但是野生毛花猕猴桃果实普遍存在可溶性糖含量偏低、风味偏酸等缺点[16],限制了其在猕猴桃育种和生产中的有效利用。笔者课题组前期从野生毛花猕猴桃种质资源中选育出了一个可溶性糖含量高且综合性状优异的毛花猕猴桃新品种赣绿1 号[16],可作为果实糖代谢特征和高糖积累机制研究的理想材料。笔者在本研究中以高糖型的赣绿1 号为研究材料,以低糖型毛花猕猴桃品种赣绿6 号(前期编号:D6)作为对照,系统分析了成熟期果实品质指标、糖组分含量,发现蔗糖是导致不同毛花猕猴桃品种果实可溶性糖含量差异的主要糖类;进一步分析了蔗糖代谢相关酶活性和相关基因表达特征,发现SPS 是影响果实蔗糖积累的关键代谢酶,推测其编码基因AeSPS 是影响SPS 活性和果实蔗糖含量的关键调控基因。本研究为毛花猕猴桃果实甜味品质形成和调控机制提供了理论依据,亦为进一步解析猕猴桃蔗糖磷酸合酶基因的分子功能奠定了重要研究基础。
1 材料和方法
1.1 材料及处理
选择可溶性糖含量差异较大的高糖型毛花猕猴桃品种赣绿1 号和低糖型毛花猕猴桃赣绿6 号(对照)为试验材料,这两个品种均系从江西省南城县麻姑山野生毛花猕猴桃资源中选育而来,其中,赣绿1号为综合性状优异的毛花猕猴桃新品种,已获得农业农村部植物新品种权[16]。供试材料的嫁接苗种植于江西省奉新县农业农村局猕猴桃果园内。每个品种选择3 株长势一致的植株作为3 个生物学重复。2021 年,在果实可溶性固形物含量(SSC,w,后同)达到7.0%的生理成熟期,选择无病虫害和无机械损伤的果实采样,每株随机采集20个果实。果实采集后置于冰盒中带回实验室。首先测定鲜果的单果质量、果实纵径、果实横径,计算果形指数。之后将果实置于室温条件,在软熟期时,测定其果实SSC、干物质含量(DM);将剩余果实去除果皮、种子和果心部分,将果肉立即切碎并置于液氮中冷冻,冷冻样品置于-80 ℃冰箱中保存备用。
1.2 外观和内在品质指标测定
对生理成熟期的鲜果样品,使用电子天平测定单果质量;使用游标卡尺分别测定果实纵径、横径,以果实纵径与横径的比值作为果形指数。对软熟期鲜果样品,使用便携式糖度计(PLA-1;ATAGO)测定果实SSC;参考Jia 等[14]的方法,使用厚度约为2 mm的果实薄片测定果实DM。对软熟期果实冷冻样品,使用蒽酮比色法测定可溶性糖含量(SS)[17];使用氢氧化钠滴定法测定可滴定酸含量(TA)[17];使用2,6-二氯靛酚法测定抗坏血酸(AsA)含量[17];以可溶性糖含量与可滴定酸含量的比值作为样品糖酸比。果实甜度值计算参考姚改芳等[18]的方法,略有改动,计算公式为:甜度值=果糖含量×1.75+葡萄糖含量×0.70+蔗糖含量×1。
1.3 可溶性糖组分含量测定
采用高效液相色谱法(HPLC,LC-10A,Shimadzu)测定果实冻样葡萄糖、果糖和蔗糖等主要可溶性糖组分含量。为获得糖组分提取液,称取4 g冷冻果实样品在液氮中冷冻研磨后转移至10 mL 离心管,加入5.0 mL 80%乙醇,置于35 ℃水浴20 min,室温下10 000 r·min-1离心15 min,将上清液转至15 mL容量瓶中,重复提取3 次,将上清液合并,定容至15 mL。吸取1 mL提取液将其旋转蒸干,加入1 mL超纯水溶解干粉,使用过滤器(孔径0.45 μm)过滤溶液,获得的纯化提取液用于HPLC含量测定。测定糖组分含量时,使用Waters Spherisorb NH2柱(4.6 mm×250 mm,5.0 μm)进行样品分离,柱温为35 ℃,流动相为8.5:1.5 的乙腈:超纯水,进样量为20 μL,流速设置为1.0 mL·min-1,使用RID-10A检测器测量。
1.4 蔗糖代谢相关酶活性检测
使用索莱宝试剂盒分别检测冷冻果实样品的蔗糖磷酸合酶、蔗糖合酶、酸性转化酶(AINV)和中性转化酶(NINV)的活性,测定方法参照相应试剂盒的说明书。
1.5 基因表达分析
从毛花猕猴桃华特基因组数据库网站(https://kiwifruitgenome.org/organism/1)分别下载蔗糖磷酸合酶基因AeSPS(DTZ79_13g06220)、蔗糖合酶基因AeSUSY(DTZ79_12g00380)、酸性转化酶基因AeNINV(DTZ79_14g05920)和中性转化酶基因AeAINV(DTZ79_29g07640)的编码序列,以相关序列为模板设计基因定量引物(表1),使用实时荧光定量(qRT-PCR)法分析各基因相对表达量。
表1 用于qRT-PCR 分析的基因及相关引物序列
Table 1 Genes and the related primer sequences for qRT-PCR analysis
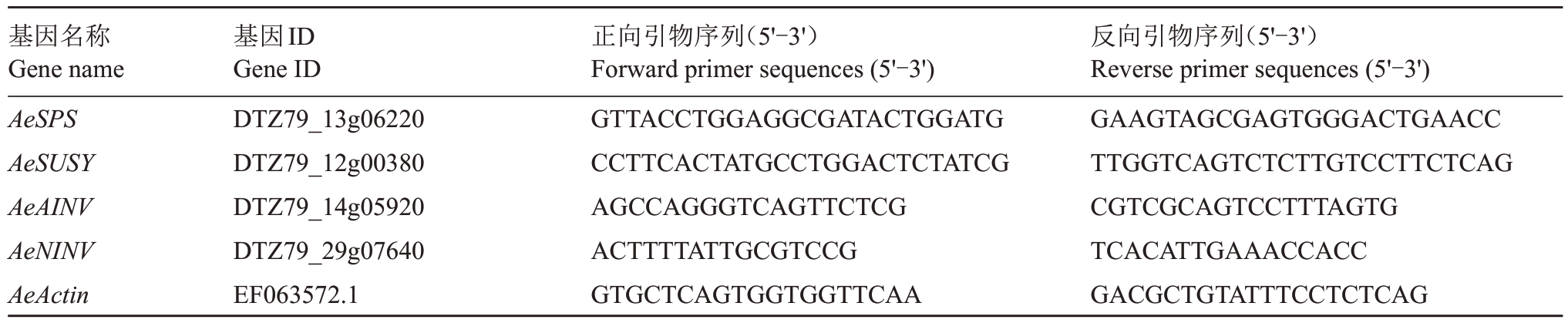
基因名称Gene name AeSPS AeSUSY AeAINV AeNINV AeActin基因ID Gene ID DTZ79_13g06220 DTZ79_12g00380 DTZ79_14g05920 DTZ79_29g07640 EF063572.1正向引物序列(5'-3')Forward primer sequences(5'-3')GTTACCTGGAGGCGATACTGGATG CCTTCACTATGCCTGGACTCTATCG AGCCAGGGTCAGTTCTCG ACTTTTATTGCGTCCG GTGCTCAGTGGTGGTTCAA反向引物序列(5'-3')Reverse primer sequences(5'-3')GAAGTAGCGAGTGGGACTGAACC TTGGTCAGTCTCTTGTCCTTCTCAG CGTCGCAGTCCTTTAGTG TCACATTGAAACCACC GACGCTGTATTTCCTCTCAG
使用聚合美试剂盒提取冷冻果实样品总RNA;使用Hifair Ⅲ1st Strand cDNA Synthesis SuperMix for qPCR(gDNA digester plus)试剂盒,去除样品中基因组DNA后对其进行反转录合成第一链cDNA;使用Hieff UNICON Universal Blue qPCR SYBR Green Master Mix 试剂盒对cDNA 样品开展qRTPCR 分析。以猕猴桃AeActin 基因(EF063572.1)作为qRT-PCR 反应的内参基因,以赣绿6 号果实样品为对照,参照Livak等[19]的方法计算各基因的相对表达量,每个反应设置3次生物学重复。
1.6 相关性分析
使用皮尔逊法(SPSS 软件,20.0 版,下同)分析果实品质指标[SS、SSC、甜度值(SV)、可溶性总糖含量(TS)、Glu含量、Fru含量、Suc含量]与蔗糖代谢相关酶(SPS、SUSY、AINV、NINV)活性相互之间的相关性,其中Glu 含量、Fru 含量、Suc 含量以鲜质量计;使用相同方法分析AeSPS、AeSUSY、AeAINV 和AeNINV 等基因相对表达量与蔗糖含量及其相应酶(SPS、SUSY、AINV和NINV)活性之间的相关性。
1.7 数据处理
利用SPSS 软件对不同品种之间相关数据分别在p<0.05 和p<0.01 水平进行独立样本T 检验分析,大多数结果以平均值±标准差的形式表示。
2 结果与分析
2.1 赣绿1号和赣绿6号猕猴桃果实生理成熟期外观品质指标
如图1-A、B所示,在果实生理成熟期,赣绿1号猕猴桃的果个明显大于赣绿6 号;两个品种果实基部的形状存在明显差异,赣绿6 号果实基部接近于圆锥形,而赣绿1 号果实基部接近于椭球形。对两个品种果实生理成熟期外观品质指标进行了测定,结果发现,赣绿1 号猕猴桃的果实纵径、果实横径、果形指数和单果质量均极显著高于赣绿6 号猕猴桃。赣绿1 号猕猴桃果实纵径为62.04 mm,赣绿6号果实纵径为40.55 mm(图1-C);赣绿1号果实横径为30.66 mm,赣绿6 号果实横径为24.24 mm(图1-D);赣绿1号的果形指数为2.05,赣绿6号的果形指数为1.68(图1-E)。此外,相比于赣绿6 号果实13.52 g 的平均单果质量,赣绿1 号平均单果质量为36.69 g,是前者的2.71倍(图1-F)。
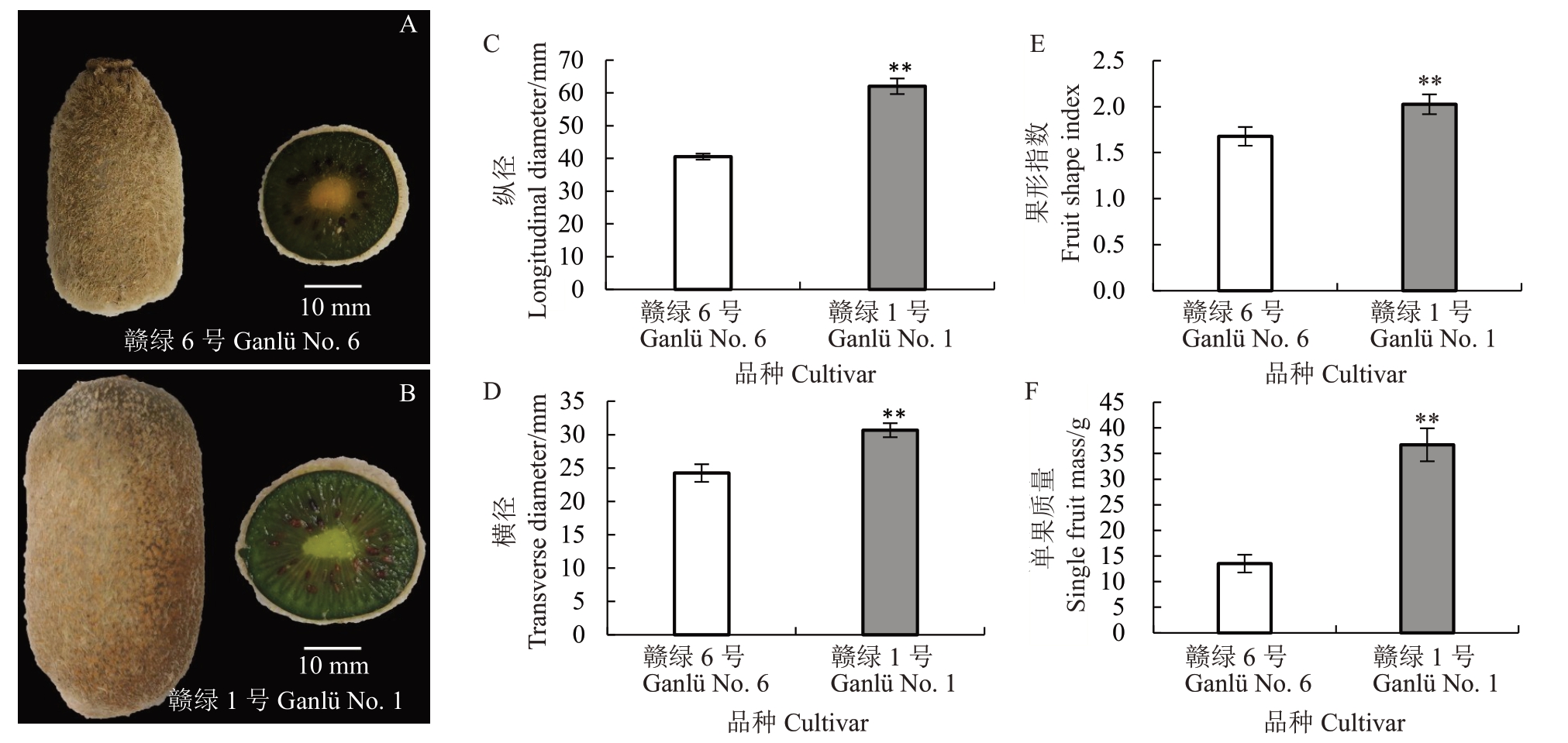
图1 赣绿1 号和赣绿6 号果实生理成熟期外观品质指标
Fig.1 Indicators of external quality in fruit of Ganlü No.1 and Ganlü No.6 at physiological maturity stage
“*”和“**”分别表示显著水平差异(p ≤0.05)和极显著水平差异(p ≤0.01)。下同。
“*”and“**”represent significant differences at p ≤0.05 and p ≤0.01 levels,respectively.The same below.
2.2 赣绿1号和赣绿6号猕猴桃果实软熟期内在品质指标
在果实软熟期,赣绿1号和赣绿6号果实干物质含量均为17%左右,两者之间并无显著差异(图2-A)。然而,赣绿1号果实的可溶性固形物含量和可溶性糖含量均极显著高于赣绿6 号,赣绿1 号果实的SSC为20.73%,赣绿6号的为16.27%(图2-B);赣绿1号果实的SS为17.56%,赣绿6号的为11.46%,前者为后者的1.53倍(图2-C)。相反,赣绿1号果实TA含量为0.80%,极显著低于赣绿6号的1.09%(图2-D)。与SS和TA数据相一致,赣绿1号果实糖酸比为22.04,极显著高于赣绿6号果实(图2-E),说明赣绿1号果实风味品质明显优于赣绿6号。此外,赣绿1号果实抗坏血酸含量也略微高于赣绿6号果实(图2-F)。
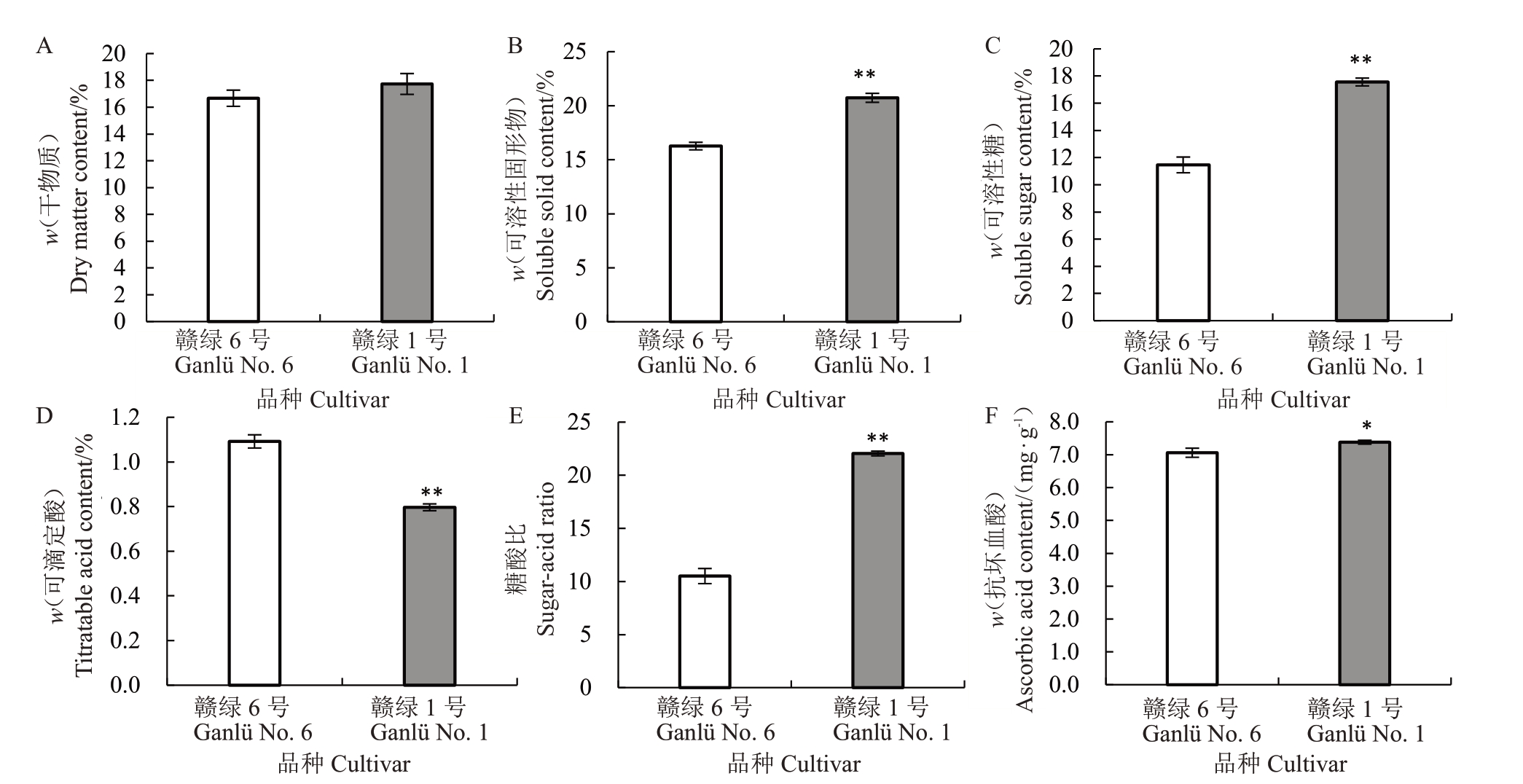
图2 赣绿1 号和赣绿6 号果实软熟期内在品质指标
Fig.2 Indicators of internal fruit quality of Ganlü No.1 and Ganlü No.6 at soft-ripe stage
2.3 赣绿1号和赣绿6号猕猴桃果实可溶性糖组分含量
对赣绿1 号和赣绿6 号猕猴桃果实软熟期葡萄糖、果糖和蔗糖含量进行了测定,结果发现,赣绿1号果实中葡萄糖含量为26.35 mg·g-1,略低于赣绿6号果实的27.67 mg·g-1,但两者之间无显著差异(图3-A)。赣绿1号果实果糖含量为28.99 mg·g-1,极显著高于赣绿6 号的20.66 mg·g-1(图3-B)。此外,赣绿1号果实蔗糖含量达28.83 mg·g-1,极显著高于赣绿6号果实的7.84 mg·g-1,其蔗糖含量是赣绿6号的3.29倍(图3-C)。赣绿1号果实中,3种可溶性总糖含量为81.17 mg·g-1,显著高于赣绿6号的56.18 mg·g-1(图3-D)。可见,蔗糖是两个毛花猕猴桃品种糖组分中含量差异最大的可溶性糖。此外,赣绿1 号果实的甜度值为95.00,极显著高于赣绿6号果实的甜度值(63.38)(图3-E)。
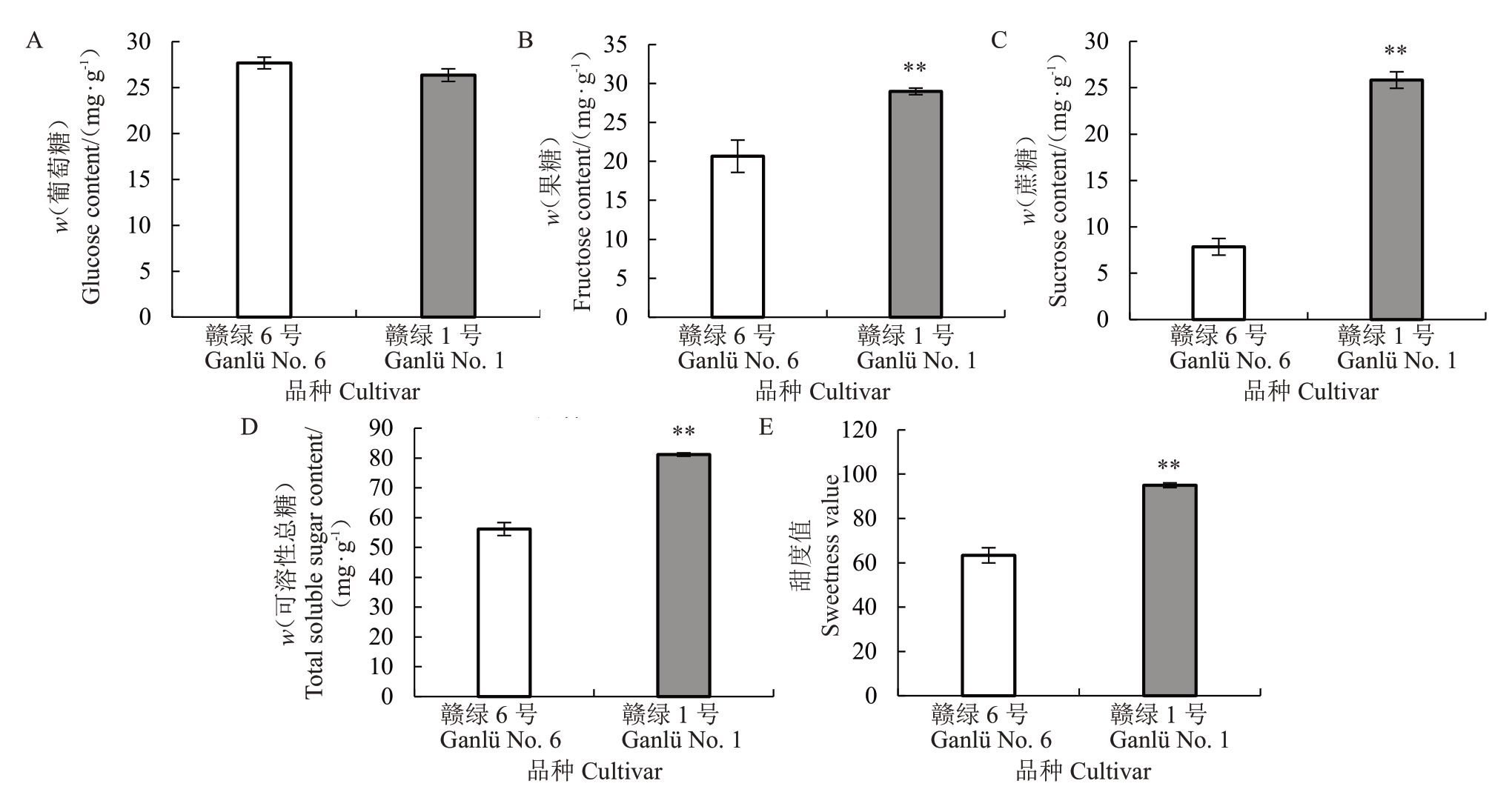
图3 赣绿1 号和赣绿6 号果实软熟期可溶性糖组分含量和甜度值
Fig.3 Contents of main sugar components in fruit of Ganlü No.1 and Ganlü No.6 at soft-ripe stage
2.4 蔗糖代谢相关酶活性
对赣绿1 号和赣绿6 号果实蔗糖代谢主要酶活性进行了测定,结果发现,负责蔗糖合成和降解的4个酶(SPS、SUSY、AINV、NINV)的活性在两个品种之间均存在显著差异。其中,赣绿1 号果实SPS 活性为631.34 U·g-1,极显著高于赣绿6 号果实的437.50 U·g-(1图4-A)。赣绿1 号果实SUSY 活性为807.56 U·g-1,极显著高于赣绿6 号的575.60 U·g-1(图4-B)。相反,在赣绿1 号果实中,负责催化蔗糖不可逆降解的两个酶的活性均显著低于赣绿6号果实。其中,赣绿1号果实AINV的活性为7.82 U·g-1,极显著低于赣绿6号的19.06 U·g-(1图4-C);而赣绿1 号果实NINV 活性为6.65 U·g-1,同样显著低于赣绿6 号果实的9.57 U·g-1(图4-D)。在两个品种中,负责蔗糖可逆或不可逆合成的两个酶(SPS 和SUSY)的活性大幅度高于负责蔗糖不可逆降解的两个酶(AINV和NINV)的活性,表明软熟期的果实中蔗糖的合成速率可能远大于其降解速率,蔗糖可能仍处于积累过程中。
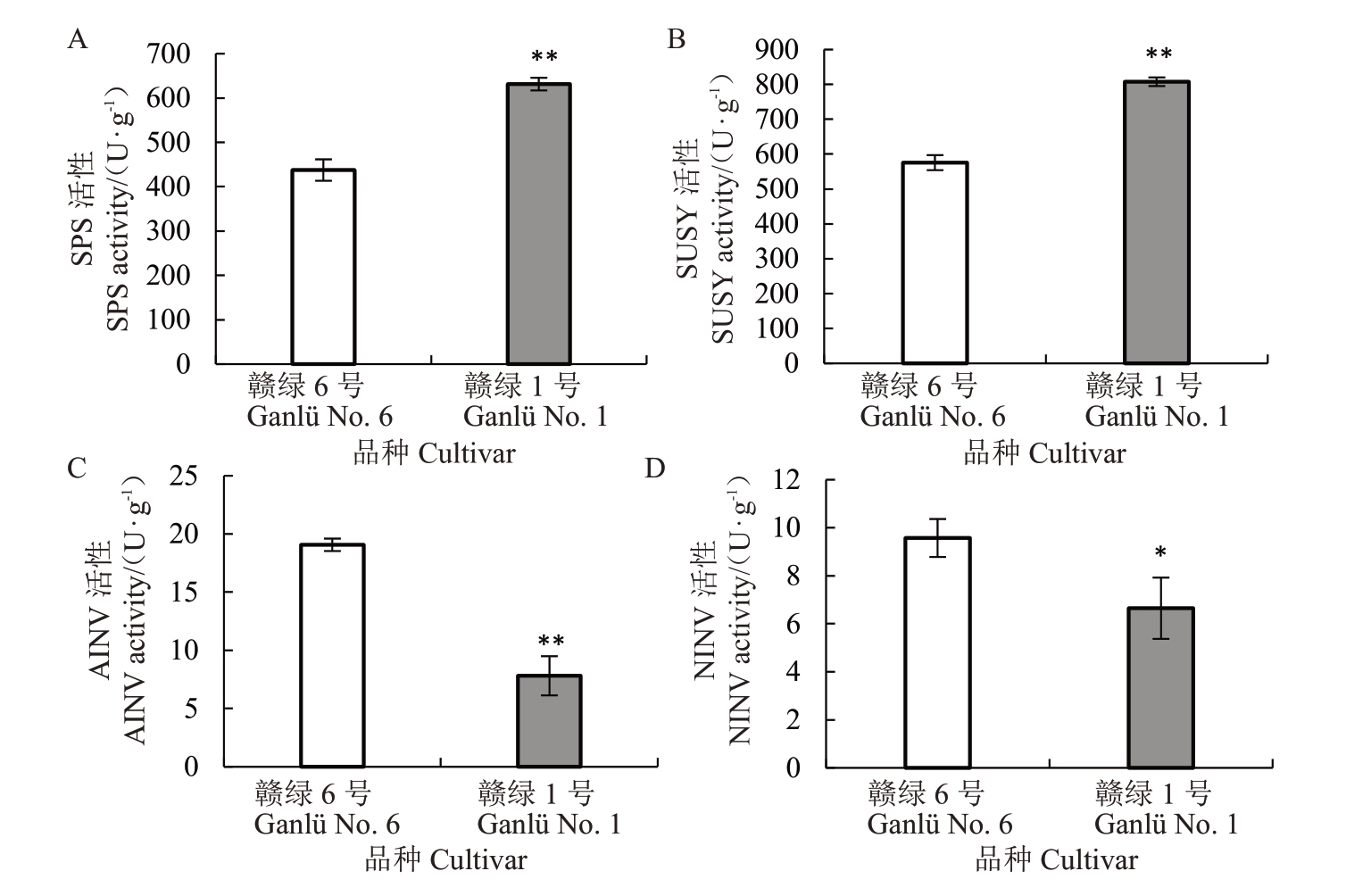
图4 赣绿1 号和赣绿6 号果实软熟期主要蔗糖代谢酶活性
Fig.4 Activities of main metabolic enzymes related to sucrose in fruit of Ganlü No.1 and Ganlü No.6 at soft-ripe stage
2.5 蔗糖代谢酶基因相对表达量
使用qRT-PCR 法对赣绿1 号和赣绿6 号果实SPS、SUSY、AINV 和NINV 等蔗糖代谢相关酶相应的编码基因AeSPS、AeSUSY、AeAINV和AeNINV相对表达量进行分析,结果发现,AeSPS 在赣绿1 号中的相对表达量是赣绿6号的4.81倍(图5-A),这与预期结果相一致。然而,赣绿1 号果实中AeSUSY 和AeAINV的相对表达量均低于赣绿6号(图5-B,C),但两个品种间并无显著差异。与预期结果相反,赣绿1号果实中AeNINV基因的相对表达量显著高于赣绿6号,这与两者的NINV 活性趋势相反(图4-D,图5-D)。通过比较可以发现,猕猴桃AeSPS基因的表达趋势与其编码的SPS活性趋势相一致(图4-A,图5-A)。
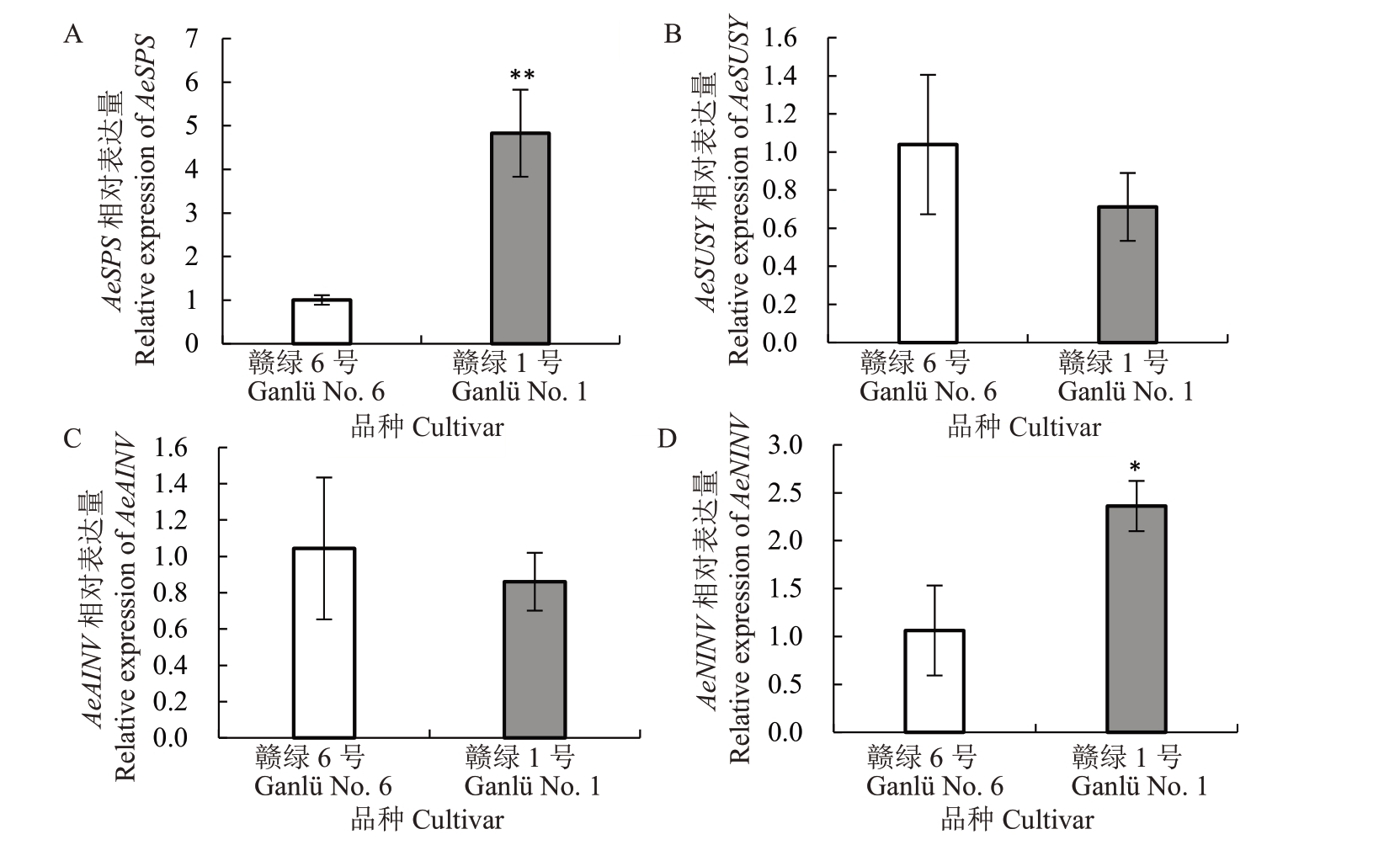
图5 赣绿1 号和赣绿6 号果实软熟期蔗糖代谢酶相关编码基因相对表达量
Fig.5 Relative expressions of genes encoding enzymes related to sucrose metabolism in fruit of Ganlü No.1 and Ganlü No.6 at soft-ripe stage
2.6 果实品质指标、糖组分含量、蔗糖代谢酶活性之间的相关性
对果实成熟期不同品质指标之间的相关性进行分析。结果(图6)表明,SS与SSC呈极显著正相关,它们之间的相关系数高达0.980,且SS 和SSC 与其他指标的相关系数也非常接近;SS与SV、TS、Fru含量、Suc含量均呈极显著正相关,而SS与Glu含量呈负相关但并无显著差异;SS与SPS活性、SUSY活性均呈显著正相关,而与AINV 活性、NINV 活性均呈显著负相关。
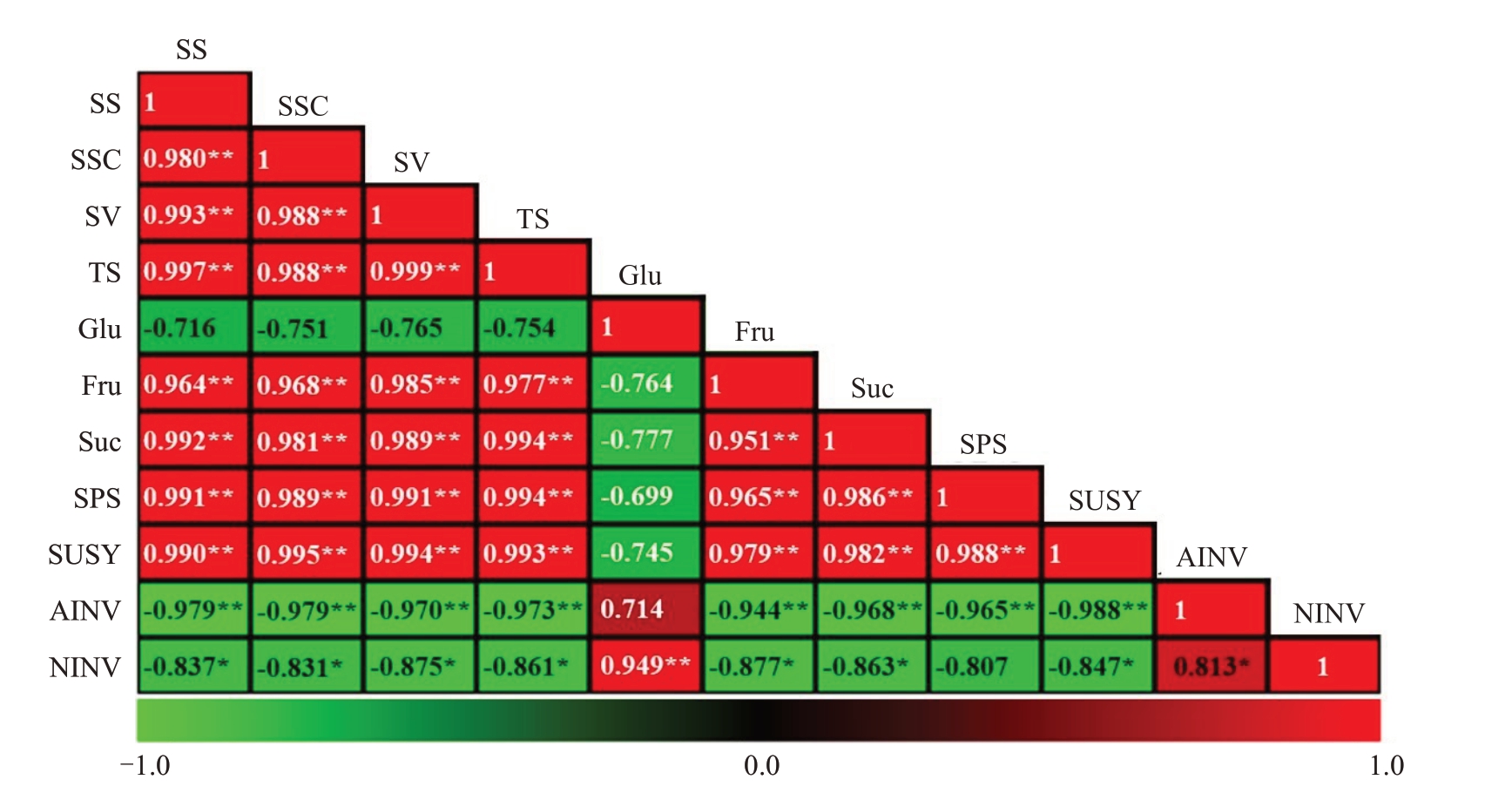
图6 猕猴桃果实品质指标与糖组分含量、蔗糖代谢酶活性的相关性
Fig.6 The correlation between quality indicators and sugar components contents or activities of metabolic enzymes related to sucrose
SS.可溶性糖含量;SSC.可溶性固形物含量;SV.甜度值;TS.可溶性总糖含量;Glu.葡萄糖含量;Fru.果糖含量;Suc.蔗糖含量;SPS.蔗糖磷酸合酶活性;SUSY.蔗糖合酶活性;AINV.酸性转化酶活性;NINV.中性转化酶活性。
SS.Soluble sugar content;SSC.Soluble solids content;SV.Sweetness value;TS.Total soluble sugar content;Glu.Glucose content;Fru.Fructose content;Suc.Sucrose content;SPS.Sucrose phosphate synthase activity;SUSY.Sucrose synthase activity;AINV.Acid invertase activity;NINV.Neutral invertase activity.
果实成熟期糖组分和总糖含量方面,Glu 含量与TS、Fru 含量、Suc 含量均呈负相关,但均无显著差异;TS、Fru 含量、Suc 含量三者相互之间均呈极显著正相关(图6)。糖代谢酶活性方面,SPS 活性与SUSY 活性呈极显著正相关;AINV 活性与NINV活性呈显著正相关;SPS 活性与AINV 活性呈极显著负相关,与NINV 活性呈负相关但无显著差异;SUSY 与AINV 活性、NINV 活性分别呈极显著负相关和显著负相关(图6)。
2.7 蔗糖代谢酶基因表达量与蔗糖含量和蔗糖代谢酶活性的相关性
对果实成熟期4个蔗糖代谢酶基因(AeSPS、Ae-SUSY、AeAINV、AeNINV)的相对表达量与果实蔗糖含量、主要蔗糖代谢酶(SPS、SUSY、AINV、NINV)活性之间的相关性进行了分析。结果表明,蔗糖磷酸合酶基因AeSPS相对表达量与果实Suc含量呈极显著正相关;AeSPS 相对表达量与SPS 活性、SUSY活性同样呈极显著正相关,但是与AINV 活性、NINV 活性之间分别呈极显著负相关、显著负相关(表2)。蔗糖合酶基因AeSUSY相对表达量与Suc含量呈无显著差异的负相关;与SPS 活性、SUSY 活性、AINV 活性之间均呈负相关,但均无显著差异;与NINV活性呈正相关但无显著差异(表2)。
表2 蔗糖代谢酶基因相对表达量与蔗糖含量与蔗糖代谢酶活性之间的相关性
Table 2 Correlations between sucrose content and the activity of sucrose-metabolizing enzymes or the relative expression levels of genes encoding sucrose-metabolizing enzymes
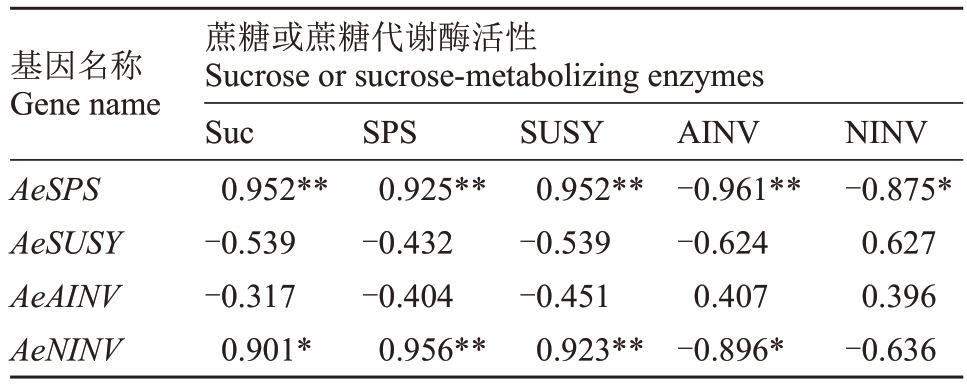
基因名称Gene name AeSPS AeSUSY AeAINV AeNINV蔗糖或蔗糖代谢酶活性Sucrose or sucrose-metabolizing enzymes Suc 0.952**-0.539-0.317 0.901*SPS 0.925**-0.432-0.404 0.956**SUSY 0.952**-0.539-0.451 0.923**AINV-0.961**-0.624 0.407-0.896*NINV-0.875*0.627 0.396-0.636
酸性转化酶基因AeAINV 相对表达量与Suc 含量呈负相关;与其编码酶AINV的活性呈正相关;与SPS 活性、SUSY 活性之间均呈负相关;与NINV 活性呈正相关。然而,上述各指标之间的相关性均无显著差异(表2)。与预期结果相反,蔗糖中性转化酶基因AeNINV 相对表达量与Suc 含量呈显著正相关,而其与其编码酶NINV 的活性之间却呈无显著差异的负相关。此外,AeNINV相对表达量与SPS活性、SUSY 活性之间均呈极显著正相关;与AINV 活性呈显著负相关(表2)。可见,猕猴桃果实中蔗糖代谢调控是一个非常复杂的过程,蔗糖的代谢过程可能受到多个基因和多个代谢酶共同影响。
3 讨 论
3.1 高可溶性糖含量显著提高了赣绿1号成熟期毛花猕猴桃果实品质
可溶性糖是调控水果风味尤其是甜味的重要物质,其含量会显著影响消费者对水果的偏好[14,20]。笔者在本研究中发现,赣绿1号猕猴桃果实成熟期可溶性糖含量是赣绿6号的1.53倍,而其果实中可滴定酸含量仅为赣绿6号的72.96%。相比于赣绿6号,赣绿1号可被视为高糖型猕猴桃果实。在成熟期,两者果实可溶性固形物含量趋势与可溶性糖含量趋势一致,而干物质含量并无显著差异。因此,赣绿1号果实中高可溶性糖含量很大程度上引起可溶性固形物含量的增加,这也解释了两个品种的SS和SSC之间存在极高的相关系数(0.980);而且,赣绿1号果实具备的高可溶性糖含量也显著提高了果实甜度值以及糖酸比,改善了果实风味品质,使其成为一个具有独特优势的毛花猕猴桃品种。
3.2 蔗糖含量对毛花猕猴桃果实成熟期可溶性糖含量具有重要影响
笔者在本研究中发现,在果实成熟期,赣绿1号果实中3种主要可溶性糖(葡萄糖、果糖、蔗糖)的总含量是赣绿6 号果实的1.44 倍,这种含量差异主要由两者蔗糖和果糖含量的差异引起,其中蔗糖含量差异尤为明显。蔗糖是引起毛花猕猴桃赣绿1号和赣绿6号果实成熟期可溶性糖含量存在显著差异的主要糖类。任金立[21]对两个薄皮甜瓜品种进行研究发现,在果实发育的不同时期,两个品种果实糖含量均表现出显著差异,而蔗糖含量的差异引起两个甜瓜品种间甜度产生显著差异,这一结果与本研究结果一致。姚改芳等[18]对不同栽培种梨果实的研究发现,成熟期梨果实糖分主要由果糖、葡萄糖、蔗糖和山梨醇组成,其中果糖含量最高且含量稳定,其余糖分含量存在较大差异。刘涵[22]对3个不同品种(系)猕猴桃果实可溶性糖进行了研究,结果发现,在果实成熟阶段,两个中华猕猴桃品种皖金和红阳的葡萄糖含量在可溶性糖中占比最高,两者均为葡萄糖优势型果实;而对萼猕猴桃果实中果糖含量占比最高,为果糖优势型果实。本研究中,赣绿1 号猕猴桃果实成熟期3 种可溶性糖含量差异较小,它们占可溶性总糖含量的比例分别为32.47%(葡萄糖)、35.71%(果糖)和31.82%(蔗糖);而赣绿6 号猕猴桃果实中葡萄糖占可溶性总糖含量的比例最高(49.26%),其次为果糖(36.78%)、蔗糖(13.96%);类似于上述皖金和红阳猕猴桃,赣绿6 号同样可被视为葡萄糖优势型果实。相似地,刘春宏等[23]对一个野生毛花猕猴桃的研究发现,在成熟阶段,野生毛花猕猴桃果实中葡萄糖积累最多,其次为果糖,蔗糖积累最少。对不同品种软枣猕猴桃进行的研究发现,蔗糖通常是果实中积累量最多的可溶性糖种类[24-25]。此外,蔗糖也是糯米糍荔枝[26]和10个不同品种杏[27]果实成熟阶段积累量最多的可溶性糖。而成熟阶段的苹果果实中,果糖占可溶性糖的比例超过55%[28]。可见,不同种类果树中,果实可溶性糖的积累可能存在较大差异;同一种类不同品种的果树,果实中不同可溶性糖的含量也可能存在明显差异。
3.3 蔗糖磷酸合酶及蔗糖磷酸合酶基因对猕猴桃果实成熟期蔗糖代谢具有显著调控作用
蔗糖是植物光合作用的主要产物,其对植物生长发育、逆境防御和果实品质提升等发挥重要作用。蔗糖及其代谢产物也是植物生长发育的能量来源;同时又能作为信号分子参与细胞代谢调控[1]。SPS 对蔗糖积累起重要作用,多数果树果实成熟过程中蔗糖积累水平与SPS活性呈正相关。本研究发现,在果实成熟期,赣绿1号果实SPS活性极显著高于赣绿6 号果实,且两个品种果实蔗糖含量与SPS活性呈极显著正相关。由此可推测,蔗糖磷酸合酶是调控毛花猕猴桃果实成熟期蔗糖代谢的关键酶。在甜瓜中,反义表达蔗糖磷酸合酶基因CmSPS1后,转基因植株成熟果实中蔗糖浓度和SPS 活性均降低,果实变得更小,说明SPS在调控甜瓜果实发育和果实品质形成中起重要作用[29]。笔者在本研究中发现,赣绿1号猕猴桃成熟果实中SPS编码基因AeSPS的相对表达量达是赣绿6号的4.81倍,AeSPS基因表达量与果实SPS 活性和蔗糖含量均呈极显著正相关,推测AeSPS 基因是调控毛花猕猴桃果实成熟期SPS活性的关键基因。
本研究发现,赣绿1号成熟果实中SUSY活性也显著高于赣绿6号,但其编码基因AeSUSY在两个品种中的相对表达量并无显著差异,且AeSUSY 基因相对表达量与果实蔗糖含量呈负相关,说明SUSY的活性调节机制可能比较复杂,AeSUSY基因并不是毛花猕猴桃果实蔗糖积累的关键调控基因。SUSY能够可逆催化蔗糖分解与合成,但通常认为在库器官中SUSY 主要起分解蔗糖的作用[30-31]。将一个番茄SUSY 编码基因反义表达后,转基因番茄果实SUSY 活性被显著抑制,蔗糖含量与对照相比并无显著差异,而番茄植株坐果率下降,推测SUSY主要参与番茄幼果中蔗糖输入调控[32]。笔者在本研究中还发现,赣绿1 号果实中,负责调控蔗糖降解的AINV和NINV的活性均显著低于赣绿6号果实,但它们的活性均大幅度低于两个品种中SPS和SUSY的活性,表明它们介导的蔗糖分解代谢对毛花猕猴桃果实成熟期蔗糖代谢的影响可能相对有限。此外,AINV和NINV的活性与各自的编码基因AeAINV和AeNINV的表达水平并不完全一致,暗示蔗糖转化酶活性不仅与其编码基因的表达量有关,亦受蛋白水调控[33],而相关作用机制需要进一步研究。
4 结 论
笔者在本研究中以高糖型(赣绿1号)和低糖型(赣绿6 号)两个毛花猕猴桃品种为研究对象,对成熟期果实品质指标进行了系统分析,首次发现蔗糖是引起毛花猕猴桃果实可溶性糖含量存在较大差异的主要糖类,推测蔗糖磷酸合酶及其编码基因AeSPS 是负责毛花猕猴桃果实成熟期蔗糖积累的关键代谢酶和关键调控基因。本研究结果为深入理解毛花猕猴桃果实蔗糖调控机制和果实风味品质的遗传改良提供了重要的理论依据。
[1] SADDHE A A,MANUKA R,PENNA S.Plant sugars:Homeostasis and transport under abiotic stress in plants[J].Physiologia Plantarum,2021,171(4):739-755.
[2] NIE X S,HONG C,WANG Q Y,LU M,AN H M.Sugar composition and transcriptome analysis in developing‘Fengtang’plum (Prunus salicina Lindl.) reveal candidate genes regulating sugar accumulation[J].Plant Physiology and Biochemistry,2023,202:107955.
[3] MEHDI F,GALANI S,WICKRAMASINGHE K P,ZHAO P F,LU X,LIN X Q,XU C H,LIU H B,LI X J,LIU X L.Current perspectives on the regulatory mechanisms of sucrose accumulation in sugarcane[J].Heliyon,2024,10(5):e27277.
[4] YOON J,CHO L H,TUN W,JEON J S,AN G.Sucrose signaling in higher plants[J].Plant Science,2021,302:110703.
[5] ZHU L C,LI B Y,WU L M,LI H X,WANG Z Y,WEI X Y,MA B Q,ZHANG Y F,MA F W,RUAN Y L,LI M J.MdERDL6-mediated glucose efflux to the cytosol promotes sugar accumulation in the vacuole through up-regulating TSTs in apple and tomato[J].Proceedings of the National Academy of Sciences of the United States of America,2021,118(1):e2022788118.
[6] QIAO K K,ZENG Q T,LV J Y,CHEN L L,HAO J X,WANG D,MA Q F,FAN S L.Exploring the role of GhN/AINV23:Implications for plant growth,development,and drought tolerance[J].Biology Direct,2024,19(1):22.
[7] ZHANG L H,ZHU L C,XU Y,LÜ L,LI X G,LI W H,LIU W D,MA F W,LI M J,HAN D G.Genome-wide identification and function analysis of the sucrose phosphate synthase MdSPS gene family in apple[J].Journal of Integrative Agriculture,2023,22(7):2080-2093.
[8] BHARALI A,BARUAH K K.Effects of integrated nutrient management on sucrose phosphate synthase enzyme activity and grain quality traits in rice[J].Physiology and Molecular Biology of Plants,2022,28(2):383-389.
[9] MA M Y,ZHU T,CHENG X Y,LI M Y,YUAN G L,LI C B,ZHANG A H,LU C M,FANG Y,ZHANG Y.Sucrose phosphate synthase 8 is required for the remobilization of carbon reserves in rice stems during grain filling[J].Journal of Experimental Botany,2024,75(1):137-151.
[10] ISHIMARU K,ONO K,KASHIWAGI T.Identification of a new gene controlling plant height in rice using the candidategene strategy[J].Planta,2004,218(3):388-395.
[11] DO NASCIMENTO J R,CORDENUNSI B R,LAJOLO F M,ALCOCER M J.Banana sucrose-phosphate synthase gene expression during fruit ripening[J].Planta,1997,203(3):283-288.
[12] KOMATSU A,MORIGUCHI T,KOYAMA K,OMURA M,AKIHAMA T.Analysis of sucrose synthase genes in citrus suggests different roles and phylogenetic relationships[J].Journal of Experimental Botany,2002,53(366):61-71.
[13] YU X Y,WANG X F,FAN J D,TIAN H M,ZHENG C C.Cloning and characterization of a sucrose phosphate synthase-encoding gene from muskmelon[J].Journal of the American Society for Horticultural Science,2007,132(4):557-562.
[14] JIA D F,XU Z Y,CHEN L,HUANG Q,HUANG C H,TAO J J,QU X Y,XU X B.Analysis of organic acid metabolism reveals citric acid and malic acid play major roles in determining acid quality during the development of kiwifruit (Actinidia eriantha)[J].Journal of the Science of Food and Agriculture,2023,103(12):6055-6069.
[15] JIA D F,GAO H,HE Y Q,LIAO G L,LIN L T,HUANG C H,XU X B.Kiwifruit Monodehydroascorbate reductase 3 gene negatively regulates the accumulation of ascorbic acid in fruit of transgenic tomato plants[J].International Journal of Molecular Sciences,2023,24(24):17182.
[16] 徐小彪,廖光联,黄春辉,贾东峰,钟敏,曲雪艳,刘青,高欢.甜香型毛花猕猴桃新品种赣绿1 号的选育[J].果树学报,2024,41(2):358-361.XU Xiaobiao,LIAO Guanglian,HUANG Chunhui,JIA Dongfeng,ZHONG Min,QU Xueyan,LIU Qing,GAO Huan.A novel sweet aromatic cultivar of Actinidia eriantha‘Ganlü No.1’[J].Journal of Fruit Science,2024,41(2):358-361.
[17] 曹建康,姜微波,赵玉梅.果蔬采后生理生化实验指导[M].北京:中国轻工业出版社,2007.CAO Jiankang,JIANG Weibo,ZHAO Yumei.Experiment guidance of postharvest physiology and biochemistry of fruits and vegetables[M].Beijing:China Light Industry Press,2007.
[18] 姚改芳,张绍铃,曹玉芬,刘军,吴俊,袁江,张虎平,肖长城.不同栽培种梨果实中可溶性糖组分及含量特征[J].中国农业科学,2010,43(20):4229-4237.YAO Gaifang,ZHANG Shaoling,CAO Yufen,LIU Jun,WU Jun,YUAN Jiang,ZHANG Huping,XIAO Changcheng.Characteristics of components and contents of soluble sugars in pear fruits from different species[J].Scientia Agricultura Sinica,2010,43(20):4229-4237.
[19] LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J].Methods,2001,25(4):402-408.
[20] ZHU L C,LI Y Z,WANG C C,WANG Z Q,CAO W J,SU J,PENG Y J,LI B Y,MA B Q,MA F W,RUAN Y L,LI M J.The SnRK2.3-AREB1-TST1/2 cascade activated by cytosolic glucose regulates sugar accumulation across tonoplasts in apple and tomato[J].Nature Plants,2023,9(6):951-964.
[21] 任金立.薄皮甜瓜品系DX108 与DX3-5 果实甜度差异分析及其响应基因的筛选[D].大庆:黑龙江八一农垦大学,2023.REN Jinli.Differential mechanism analysis and screening of responsive genes related to sweetness of fruits in two orient melon lines,DX108 and DX3-5[D].Daqing:Heilongjiang Bayi Agricultural University,2023.
[22] 刘涵.猕猴桃生理生化变化规律及调控猕猴桃果实大小发育的基因筛选[D].重庆:重庆三峡学院,2024.LIU Han.Physiological and biochemical changes and screening of genes regulating fruit size in kiwifruit[D].Chongqing:Chongqing Three Gorges University,2024.
[23] 刘春宏,邱国良,刘志斌,杨毅,庄启国,张茜.毛花猕猴桃果实发育过程中理化性质的变化研究[J].四川大学学报(自然科学版),2019,56(5):951-956.LIU Chunhong,QIU Guoliang,LIU Zhibin,YANG Yi,ZHUANG Qiguo,ZHANG Qian.Study on the changes of physicochemical properties of A.eriantha during the fruit development[J].Journal of Sichuan University (Natural Science Edition),2019,56(5):951-956.
[24] HE Y L,QIN H Y,WEN J L,CAO W Y,YAN Y P,SUN Y N,YUAN P Q,SUN B W,FAN S T,LU W P,LI C Y.Characterization of key compounds of organic acids and aroma volatiles in fruits of different Actinidia argute resources based on highperformance liquid chromatography (HPLC) and headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS)[J].Foods,2023,12(19):3615.
[25] 安娇.软枣猕猴桃果实发育过程中糖酸组分及其相关酶活性的变化[D].延吉:延边大学,2020.AN Jiao.Changes of sugar and acid components and related enzyme activities during fruit development of Actinidia arguta[D].Yanji:Yanbian University,2020.
[26] 王惠聪,黄辉白,黄旭明.荔枝果实的糖积累与相关酶活性[J].园艺学报,2003,30(1):1-5.WANG Huicong,HUANG Huibai,HUANG Xuming.Sugar accumulation and related enzyme activities in the litchi fruit of‘Nuomici’and‘Feizixiao’[J].Acta Horticulturae Sinica,2003,30(1):1-5.
[27] 陈美霞,陈学森,慈志娟,史作安.杏果实糖酸组成及其不同发育阶段的变化[J].园艺学报,2006,33(4):805-808.CHEN Meixia,CHEN Xuesen,CI Zhijuan,SHI Zuoan.Changes of sugar and acid constituents in apricot during fruit development[J].Acta Horticulturae Sinica,2006,33(4):805-808.
[28] TAO H X,SUN H Q,WANG Y F,SONG X N,GUO Y P.New insights on‘Gala’apple fruit development:Sugar and acid accumulation:A transcriptomic approach[J].Journal of Plant Growth Regulation,2020,39(2):680-702.
[29] TIAN H M,MA L Y,ZHAO C,HAO H,GONG B,YU X Y,WANG X F.Antisense repression of sucrose phosphate synthase in transgenic muskmelon alters plant growth and fruit development[J].Biochemical and Biophysical Research Communications,2010,393(3):365-370.
[30] GESSLER A.Sucrose synthase- an enzyme with a central role in the source-sink coordination and carbon flow in trees[J].New Phytologist,2021,229(1):8-10.
[31] KHANBO S,SOMYONG S,PHETCHAWANG P,WIROJSIRASAK W,UKOSKIT K,KLOMSA-ARD P,POOTAKHAM W,TANGPHATSORNRUANG S.A SNP variation in the Sucrose synthase (SoSUS) gene associated with sugar-related traits in sugarcane[J].PeerJ,2023,11:e16667.
[32] D’AOUST M A,YELLE S,NGUYEN-QUOC B.Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit[J].The Plant Cell,1999,11(12):2407-2418.
[33] 杨月,程远,阮美颖,王荣青,叶青静,姚祝平,周国治,万红建.蔗糖转化酶抑制蛋白研究进展[J].浙江农业科学,2023,64(5):1236-1241.YANG Yue,CHENG Yuan,RUAN Meiying,WANG Rongqing,YE Qingjing,YAO Zhuping,ZHOU Guozhi,WAN Hongjian.The research progress of invertase inhibitor[J].Journal of Zhejiang Agricultural Sciences,2023,64(5):1236-1241.