毛花猕猴桃(Actinidia eriantha)属于猕猴桃科(Actinidiaceae)猕猴桃属(Actinidia),是中国特有的野生宝贵资源,主要分布在中国长江以南的丘陵生态地形区域[1-2]。毛花猕猴桃不仅具有较强的生长势、抗逆性、抗病虫害能力,而且适应范围广,市场潜力大[3-5],被认为是继中华猕猴桃(A.chinensis)和美味猕猴桃(A.deliciosa)之后极具开发潜力的优良浆果种类[2]。
中国是猕猴桃生产大国,但猕猴桃产业各环节发展不均衡,果实品质不稳定、优果率低、贮藏保鲜技术不成熟等问题突出。其中贮藏保鲜效果差是导致中国猕猴桃国际竞争力弱的主要原因之一,也是制约猕猴桃健康可持续发展的关键因素[6]。猕猴桃采后具有典型的呼吸跃变和生理后熟特点,成熟后易软化腐烂、不耐贮藏[7]。在产业应用中,猕猴桃多采用冷藏来延缓果实后熟、维持果实品质及延长货架期。然而,猕猴桃冷藏保鲜需要消耗电力、设施、空间、管理等诸多资源,且消费者更倾向于购买完全成熟的猕猴桃[8]。留树后熟技术就是解决上述突出问题的新途径之一。留树后熟是通过延迟采收来贮藏保鲜的技术,留树果实仍然是生命体,在树上一般会经历生理成熟,随后完全成熟,口感达最佳,最后逐渐衰老的过程,具有提升果实品质,延长鲜果供应期和增收的作用[9-10]。因此,留树后熟不仅能节省资源,还能立即售卖和食用[8]。留树后熟已广泛应用于柑橘[11]、杧果[12]和葡萄柚[13]等果树作物上,在猕猴桃上尚未见相关研究报道。
前期研究发现,毛花猕猴桃果实生理成熟后可长期留树[14],但果实留树后熟期间的品质变化及品质最佳留树时间尚不清楚。据此,笔者在本研究中以毛花猕猴桃赣绿1 号为材料,通过测定其果实留树后熟过程中不同时期外在及内在品质的变化,并利用主成分分析探明赣绿1号果实品质最佳的留树后熟时期,为猕猴桃的留树后熟技术应用提供理论基础。
1 材料和方法
1.1 材料
供试材料样品来源于江西省奉新县农业农村局的猕猴桃园(28°70' N,115°38' E)。猕猴桃园栽培株行距为3 m×4 m,栽培架式为水平大棚架,单主干双主蔓多侧蔓整形。以毛花猕猴桃赣绿1 号为试验材料,单株小区,3 次生物学重复,在果实生理成熟期[可溶性固形物含量(w,后同)≥6.5%[15],2022-10-26]进行首次采样,之后每隔10 d 每株采集果样15 个,直至果实太皱缩不宜食用为止。分别于盛花后(DAFB)171、180、192、201、210、222 d 共6 个时期采集果样,各时期的气候特征如图1 所示。采回立即测定果实硬度、单果质量等外观指标,之后将果样除去果皮、种子和果心,用液氮速冻后,于-80 ℃保存备用。
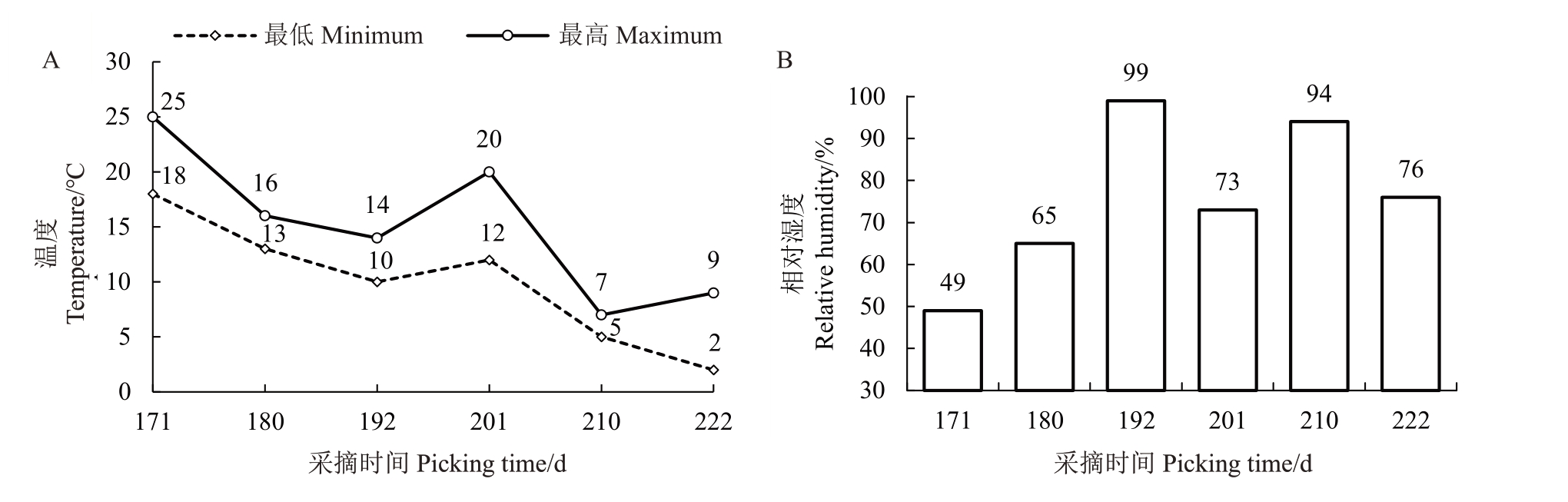
图1 赣绿1 号果实留树后熟期间的最低温度、最高温度(A)与相对湿度(B)
Fig.1 The minimum temperature,maximum temperature(A)and relative humidity(B)of Ganlü No.1(A.eriantha)fruit during on-vine ripening
1.2 指标测定与方法
随机选取10个果实,使用千分之一电子天平测量其单果质量;使用游标卡尺测量果实的横径、纵径和侧径,果形指数=纵径/横径;采用质构仪(型号为TA-XTplus)测量果实的果皮与果肉的硬度;采用色差仪(型号为CHROMA METER CR-400)测量果肉色差值;采用手持数显糖度计测定可溶性固形物含量;参照李合生[16]的方法测定叶绿素含量;采用蒽酮比色法测定可溶性糖含量,采用NaOH 中和滴定法测定果实可滴定酸含量[17];采用钼蓝比色法测定抗坏血酸含量(AsA)[18]。
1.3 数据处理
数据采用Microsoft Excel 2016软件进行初步的数据分析并制作相应柱形和折线图;利用IBM SPSS Statistics 25 软件进行差异显著性分析和主成分分析,差异性分析选择邓肯法和LSD 法,显著性水平为0.05。
2 结果与分析
2.1 果实外观和色泽的变化
分析果实外观与横切、纵切面在留树后熟过程中的变化,发现赣绿1号果实表面逐渐光滑,果面颜色褐色加深,盛花后222 d外果皮开始失水皱缩;盛花后171 d整个果肉为嫩绿色,盛花后180 d 靠近果心的果肉颜色开始从嫩绿色变为深绿色,并逐渐向外果皮扩散,直至盛花后222 d 整个果肉变为深绿色,且果肉逐渐变得透明;果心颜色逐渐变黄,从浅绿色逐渐变为淡黄色(图2-A)。
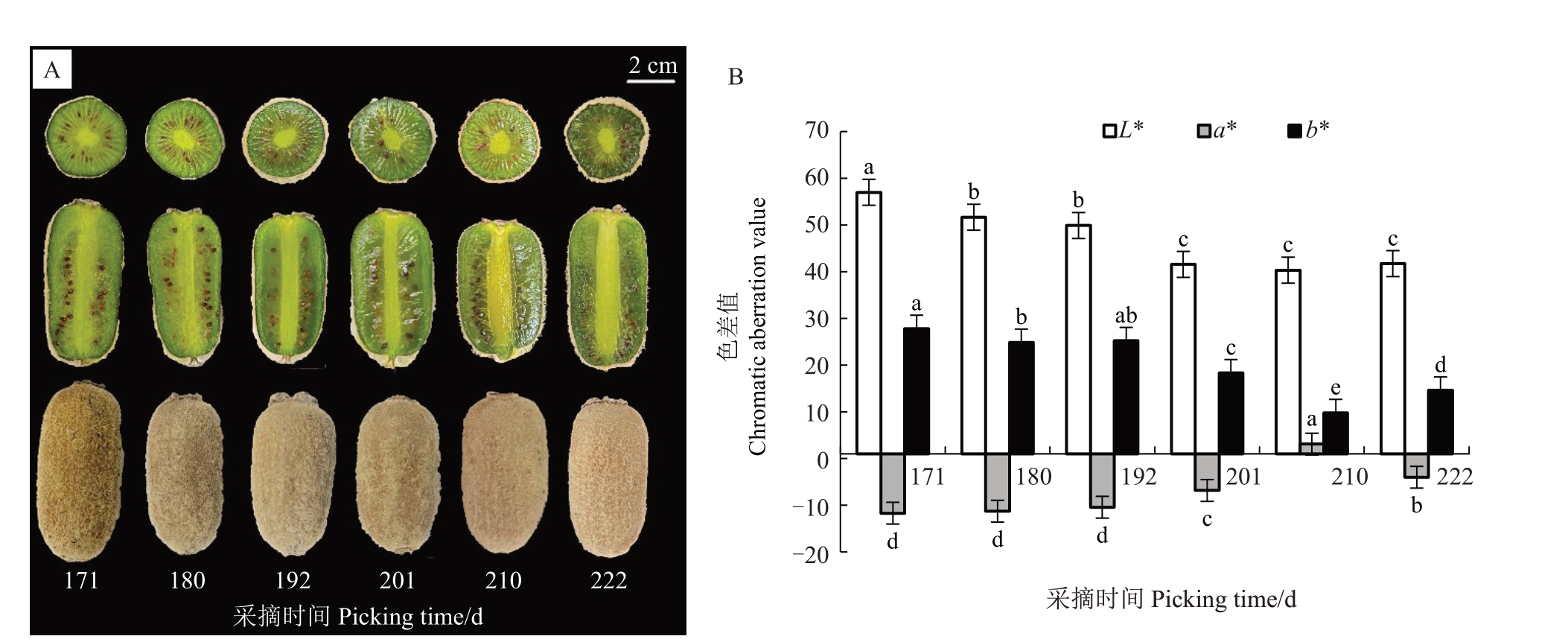
图2 赣绿1 号果实留树后熟期间外观和色泽变化
Fig.2 The appearance and color changes of Ganlü No.1(A.eriantha)fruit during on-vine ripening
A.果实横切面、纵切面与表面;B.果肉L*、a*、b*值变化。L*表示果肉亮度;a*、b*表示果肉色度组分,其绝对值越大则表示颜色越深。a*取正值为红色,负值为绿色;b*取正值为黄色,负值为蓝色[19]。同一指标不同时期间具有相同的小写字母表示差异在0.05 水平无显著差异。下同。
A.Transverse, longitudinal and surface cuts of the fruit; B.The L*, a*, b* values changes of the flesh.The L* represents the brightness of the flesh; a* and b* represent the chromaticity components of the flesh, the larger the absolute value, the darker the color.the positive value of a* indicates red, and the negative value indicates green; the positive value of b* indicates yellow, the positive value indicates blue[19].The same small letter for the same index in different time indicates that there is no significant difference at the 0.05 level.The same below.
在留树后熟过程中(图2-B),赣绿1号果肉的L*值、b*值整体都呈下降的趋势,L*值从最高值(盛花后171 d 的56.99)显著下降到最低值(盛花后210 d 的40.32),下降幅度达29.25%;b*值从最高值(盛花后171 d 的27.81)显著下降到最低值(盛花后210 d 的9.79),下降幅度达64.8%;a*值整体呈上升的趋势,从最低值(盛花后171 d的-11.7)显著上升到最高值(盛花后210 d 的3.09),而后下降至盛花后222 d的-4.00。L*、b*值下降,表明果实随着留树时间的增长,果肉亮度逐渐降低,果肉颜色逐渐加深、变黄;a*值上升,表明果肉红色加深。果实表型与色差值表现一致,果肉颜色从嫩绿变为偏黄深绿。
2.2 果实纵横径、硬度、单果质量及果形指数的变化
由表1可见,赣绿1号果实在留树后熟过程中,果形指数在盛花后171~210 d 呈下降的趋势,盛花后210 d 达最低值1.79。单果质量和硬度整体都呈下降的趋势,单果质量在盛花后171~180 d显著下降了23.72%;而盛花后180~222 d趋于平稳,无显著差异;果皮硬度在盛花后171 d达最高(573.24 g),盛花后222 d 显著下降到最低(162.45 g),下降幅度达71.66%;果肉硬度盛花后171 d最高(155.61 g),盛花后210 d 显著下降到最低,为14.76 g,下降幅度达90.51%。
表1 赣绿1 号果实留树后熟期间的果形指数、单果质量及硬度
Table 1 Shape index,single quality and hardness of Ganlü No.1(A.eriantha)fruit during on-vine ripening
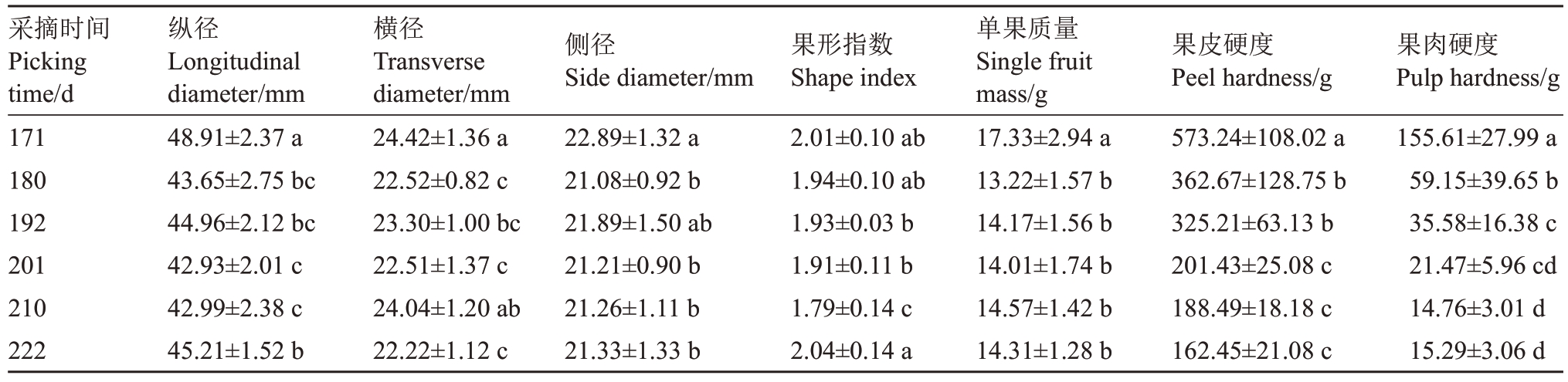
注:不同小写字母表示差异显著(p<0.05)。
Note:Different small letters indicate significant difference at p<0.05.
采摘时间Picking time/d 171 180 192 201 210 222纵径Longitudinal diameter/mm 48.91±2.37 a 43.65±2.75 bc 44.96±2.12 bc 42.93±2.01 c 42.99±2.38 c 45.21±1.52 b横径Transverse diameter/mm 24.42±1.36 a 22.52±0.82 c 23.30±1.00 bc 22.51±1.37 c 24.04±1.20 ab 22.22±1.12 c侧径Side diameter/mm 22.89±1.32 a 21.08±0.92 b 21.89±1.50 ab 21.21±0.90 b 21.26±1.11 b 21.33±1.33 b果形指数Shape index 2.01±0.10 ab 1.94±0.10 ab 1.93±0.03 b 1.91±0.11 b 1.79±0.14 c 2.04±0.14 a单果质量Single fruit mass/g 17.33±2.94 a 13.22±1.57 b 14.17±1.56 b 14.01±1.74 b 14.57±1.42 b 14.31±1.28 b果肉硬度Pulp hardness/g 155.61±27.99 a 59.15±39.65 b 35.58±16.38 c 21.47±5.96 cd 14.76±3.01 d 15.29±3.06 d果皮硬度Peel hardness/g 573.24±108.02 a 362.67±128.75 b 325.21±63.13 b 201.43±25.08 c 188.49±18.18 c 162.45±21.08 c
2.3 果实营养品质的变化
2.3.1 果实AsA 含量的变化 由图3 可见,赣绿1号果实在留树后熟过程中,AsA含量从盛花后171 d的947.89 mg·100 g-1上升至最高值(盛花后192 d的1 282.22 mg·100 g-1),上升了24.12%;盛花后201 d下降至905.17 mg·100 g-1,下降幅度达到29.41%;盛花后210 d 上升至1 028.95 mg·100 g-1,最后在盛花后222 d下降至最低值897.66 mg·100 g-1。果实AsA含量整体呈先上升后下降的趋势。
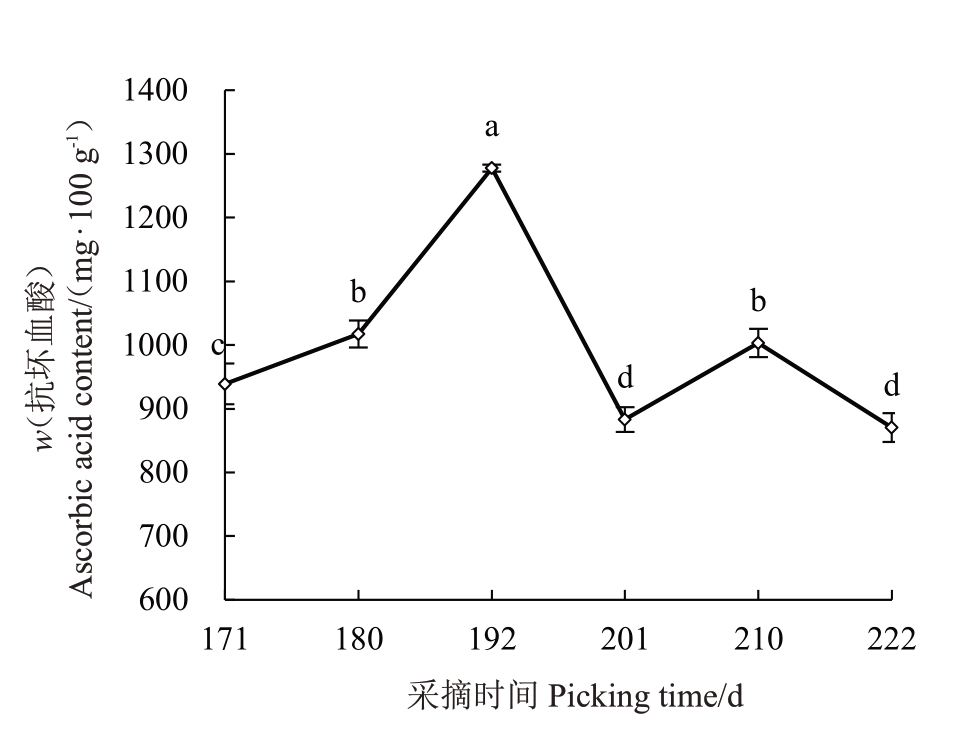
图3 赣绿1 号果实留树后熟期间的抗坏血酸含量变化
Fig.3 Ascorbic acid content changes of Ganlü No.1(A.eriantha)fruit during on-vine ripening
2.3.2 果实可滴定酸含量的变化 由图4 可见,赣绿1 号果实在留树后熟过程中,可滴定酸含量从盛花后171~180 d 上升缓慢且无显著差异,盛花后180~192 d 显著上升,于盛花后192 d 到达最高值1.26%;盛花后192~210 d缓慢下降至1.21%;盛花后210~222 d 急速下降至最低值1.02%,下降幅度达15.7%。可滴定酸含量先上升后下降,总体呈下降的趋势。
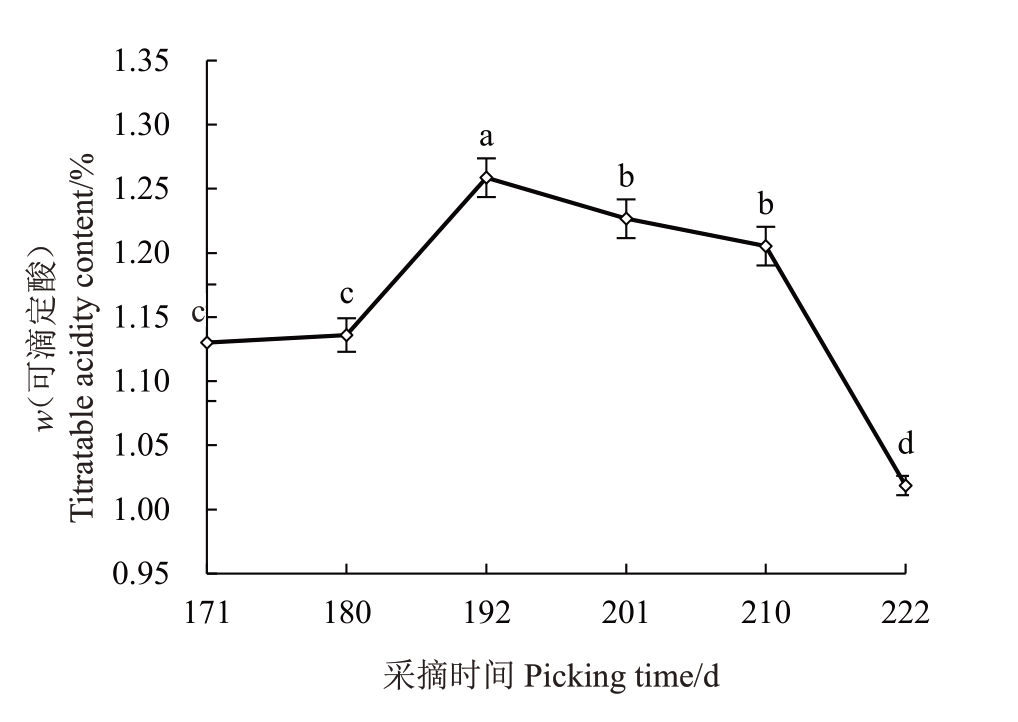
图4 赣绿1 号果实留树后熟期间的可滴定酸含量变化
Fig.4 Titratable acid content changes of Ganlü No.1(A.eriantha)fruit during on-vine ripening
2.3.3 果实可溶性固形物和可溶性糖含量的变化 由图5可见,赣绿1号果实在留树后熟过程中,可溶性固形物和可溶性糖含量变化趋势基本一致,盛花后171~210 d 呈上升的趋势并在盛花后210 d达到最高值,而后迅速下降。可溶性固形物含量从最低值(盛花后171 d 的7.46%)显著上升到最高值(盛花后210 d 的18.41%),上升幅度高达146.78%;果实可溶性糖含量从最低值(盛花后171 d 的6.42%)显著上升到最高值(盛花后210 d 的10.87%),上升幅度达40.94%。可溶性固形物和可溶性糖含量先上升后下降,总体呈上升的趋势。其中,盛花后192~222 d 的变化趋势表明可溶性糖以外的可溶性固形物迅速消耗。
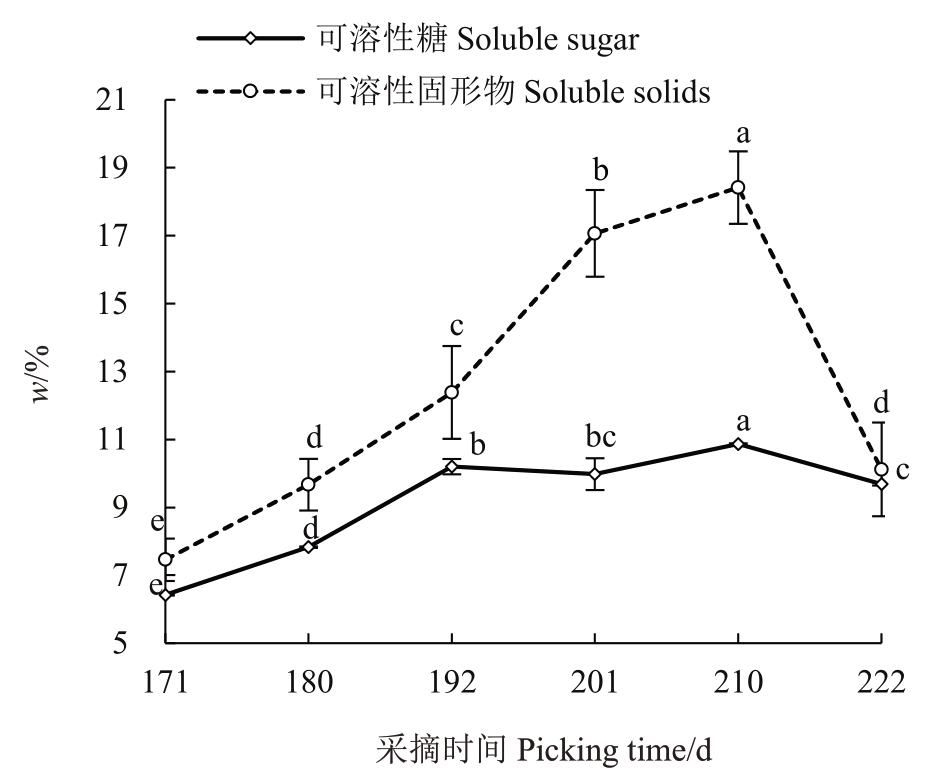
图5 赣绿1 号果实留树后熟期间的可溶性固形物、可溶性糖含量变化
Fig.5 Soluble solids and soluble sugar content changes of Ganlü No.1(A.eriantha)fruit during on-vine ripening
2.4 果肉叶绿素和类胡萝卜素含量的变化
由图6可见,赣绿1号果实在留树后熟过程中,叶绿素a、叶绿素b、类胡萝卜素含量的整体变化趋势基本一致,都是呈下降-上升-下降的趋势,总体呈现下降。盛花后180~201 d叶绿素a、叶绿素b、类胡萝卜素含量分别显著上升至2.73、1.68、1.62 mg·100 g-1,分别上升了52.51%、37.93%和44.64%;盛花后210 d后叶绿素a、叶绿素b、类胡萝卜素含量急剧分别下降至最低值(盛花后222 d的1.48、0.77、0.92 mg·100 g-1),分别下降了45.79%、61.5%、43.21%,下降速度为叶绿素b>叶绿素a>类胡萝卜素,果实转变为黄绿色。
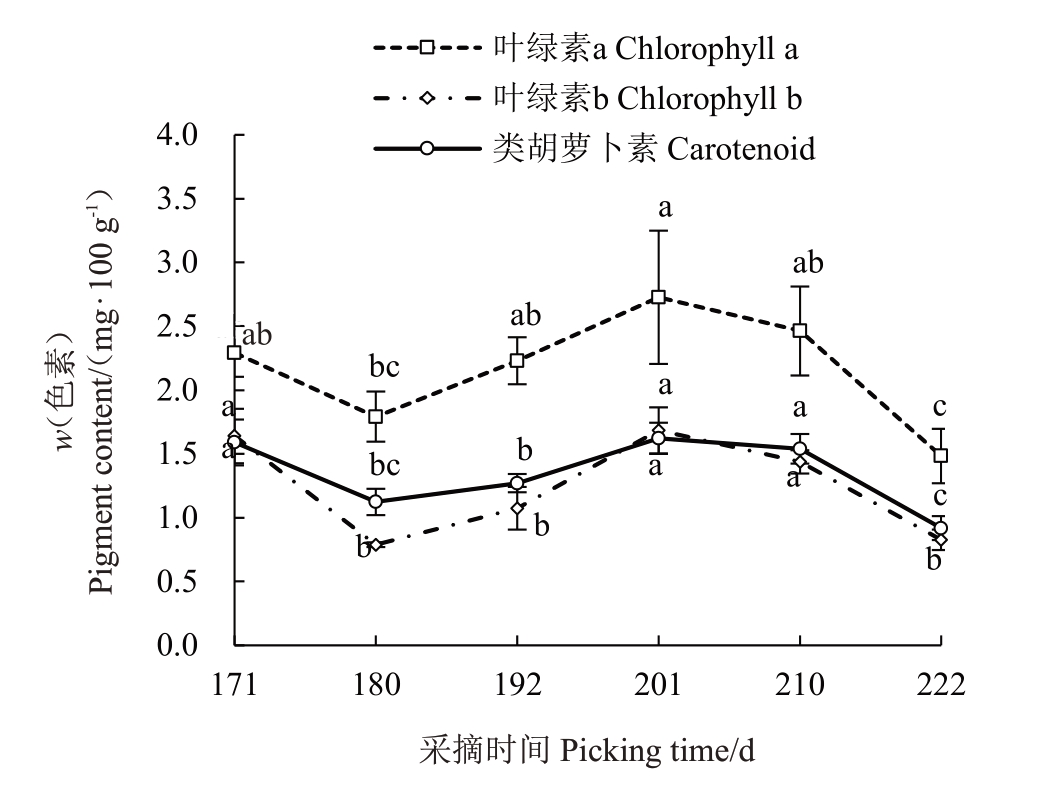
图6 赣绿1 号果实留树后熟期间的色素含量变化
Fig.6 Pigment content changes of Ganlü No.1(A.eriantha)fruit during on-vine ripening
2.5 赣绿1号果实留树后熟期间品质的综合评价
通过对6 个留树后熟时期的赣绿1 号毛花猕猴桃的6 项品质指标进行主成分分析(表2),发现前3个主成分的特征值大于1,累计方差贡献率达到98.143%。第一主成分的方差贡献率为50.52%,其中正向载荷的指标为可溶性糖含量(0.895)、可滴定酸含量(0.731)、可溶性固形物含量(0.935),负向载荷的指标为果肉硬度(-0.741),这些指标都拥有绝对值较高的载荷值;第二主成分的方差贡献率为27.59%,叶绿素+类胡萝卜素含量(0.766)有较大的正向载荷;第三主成分的方差贡献率为20.034%,抗坏血酸含量(0.898)对其产生主要的正向影响。根据(表2、表3)主成分的特征向量和载荷矩阵,Xi的系![]() (例:Y1中的X1的系
(例:Y1中的X1的系![]() ),可得到3 个主成分的得分函数表达式(Y1、Y2、Y3)。
),可得到3 个主成分的得分函数表达式(Y1、Y2、Y3)。
表2 不同留树后熟时期品质的主成分特征向量、特征值、贡献率和累计贡献率
Table 2 Principal component eigenvectors,eigenvalues,contribution rates and cumulative contribution rates of quality on different on-vine ripening time
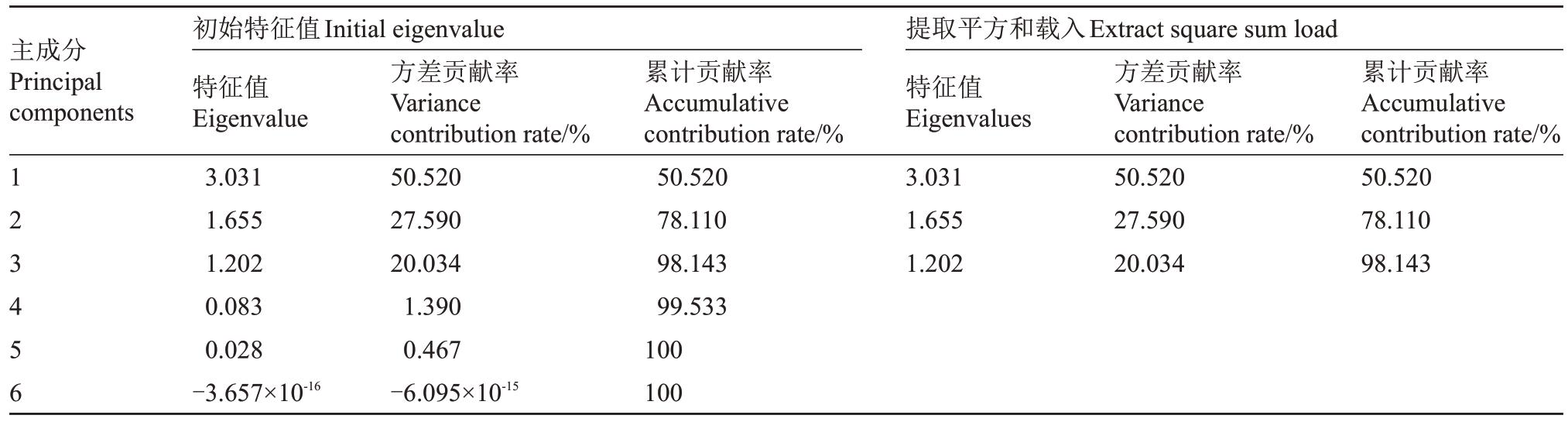
主成分Principal components 1 2 3 4 5 6初始特征值Initial eigenvalue特征值Eigenvalue 3.031 1.655 1.202 0.083 0.028-3.657×10-16方差贡献率Variance contribution rate/%50.520 27.590 20.034 1.390 0.467-6.095×10-15累计贡献率Accumulative contribution rate/%50.520 78.110 98.143 99.533 100 100提取平方和载入Extract square sum load特征值Eigenvalues 3.031 1.655 1.202方差贡献率Variance contribution rate/%50.520 27.590 20.034累计贡献率Accumulative contribution rate/%50.520 78.110 98.143
表3 主成分因子对应的载荷矩阵
Table 3 Load matrix corresponding to principal component factor
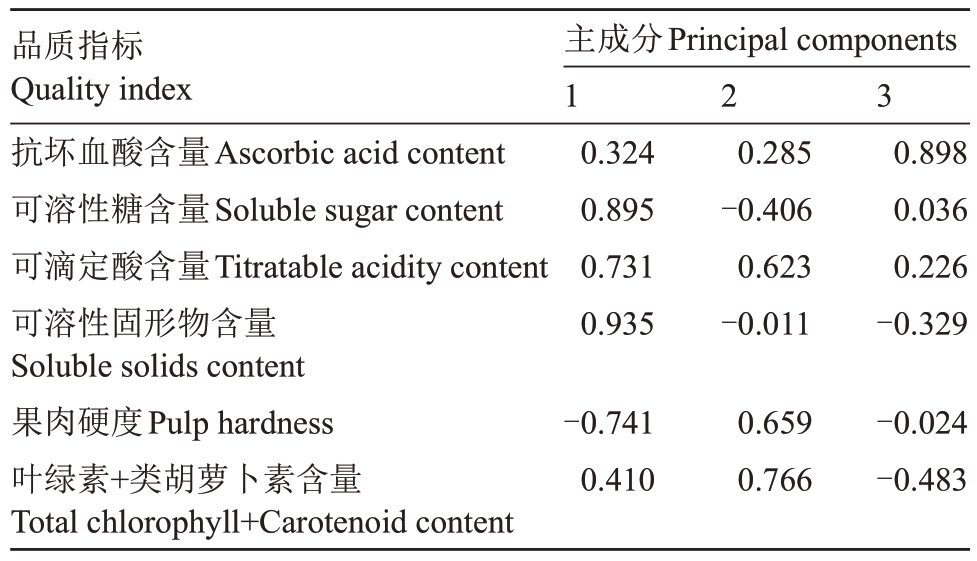
品质指标Quality index抗坏血酸含量Ascorbic acid content可溶性糖含量Soluble sugar content可滴定酸含量Titratable acidity content可溶性固形物含量Soluble solids content果肉硬度Pulp hardness叶绿素+类胡萝卜素含量Total chlorophyll+Carotenoid content 2 3主成分Principal components 1 0.324 0.895 0.731 0.935 0.285-0.406 0.623-0.011 0.898 0.036 0.226-0.329-0.741 0.410 0.659 0.766-0.024-0.483
Y1=0.186X1+0.514X2+0.420X3+0.537X4-0.426X5+0.235X6;
Y2=0.221X1-0.315X2+0.485X3-0.009X4+0.512X5+0.595X6;
Y3=0.820X1+0.033X2+0.206X3-0.300X4-0.022X5-0.441X6;
综合得分=0.505 2Y1+0.275 9Y2+0.200 34Y3。
式中,X1~X6分别对应标准化后的AsA、可溶性糖、可滴定酸、可溶性固形物、果肉硬度、叶绿素+类胡萝卜素含量等品质指标。各得分值与相应特征值的方差贡献率的乘积相加得出不同时期猕猴桃的综合得分,以此来评价不同留树后熟时期赣绿1 号毛花猕猴桃果实的综合品质。通过计算,得到综合得分和综合排名结果如表4所示,不同留树后熟时期的赣绿1 号毛花猕猴桃果实综合品质排名为:192 d>210 d>201 d>180 d>171 d>222 d,即留树21 d>39 d>30 d>9 d>0 d>50 d。
表4 不同留树后熟时期赣绿1 号果实的主成分得分及排名
Table 4 Principal component score and ranking of Ganlü No.1(A.eriantha)fruit on different on-vine ripening time
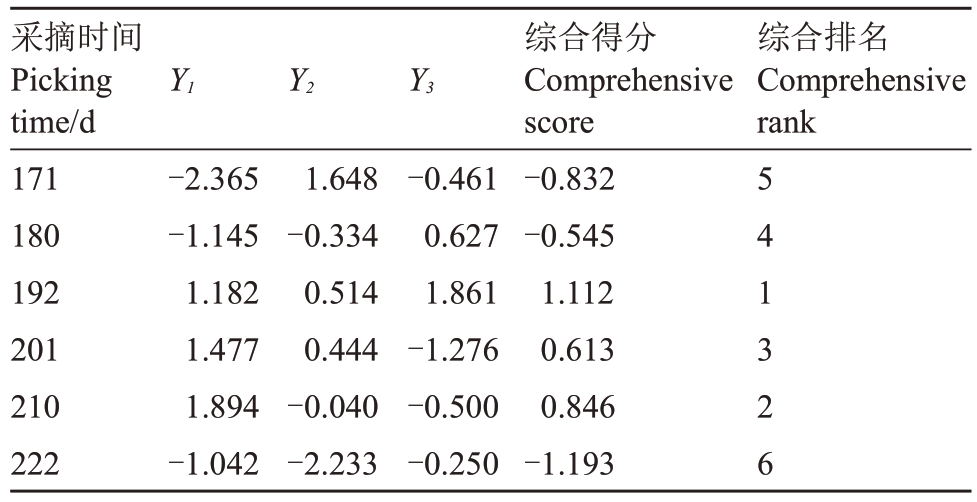
采摘时间Picking time/d 171 180 192 201 210 222 Y1 Y2 Y3综合排名Comprehensive rank-2.365-1.145 1.182 1.477 1.894-1.042 1.648-0.334 0.514 0.444-0.040-2.233-0.461 0.627 1.861-1.276-0.500-0.250综合得分Comprehensive score-0.832-0.545 1.112 0.613 0.846-1.193 5 4 1 3 2 6
3 讨 论
果实品质高低是决定赣绿1号留树后熟是否可行的重要因素。笔者在本研究中通过果实的外观及内在品质指标综合评价,发现与多项猕猴桃果实后熟研究结果的总体趋势一致,且果实颜色、质地变化相似。果实软化成熟是一个极其复杂的生理过程,受淀粉降解和细胞壁成分、结构变化等的影响[20-22]。然而果实硬度可以在一定程度上反映这种变化,因此成为猕猴桃成熟的典型指标[22-23]。有研究发现金艳、徐香、金魁猕猴桃在后熟过程中硬度不断下降[24-25],且Burdon 等[23]对海沃德的研究发现,在后熟前期果实硬度显著下降,后熟后期缓慢下降。本研究材料从盛花后171~180 d 硬度迅速下降,盛花后201~222 d平缓下降,与上述变化趋势基本一致。当成熟猕猴桃果实硬度达到可采收硬度标准时,果实开始发生成熟软化,内含物随之发生一系列变化[26]。后熟前期果实硬度的快速降低主要是与淀粉降解及细胞壁(主要为果胶)降解有关,消除了淀粉支撑和维持细胞膨压的作用[27-29]。后熟后期的果肉的软化则可能是细胞呼吸作用程度相对显著加强,果肉细胞蛋白质组成崩解,以及果肉细胞内含物(糖、TA、AsA、色素)逐渐被消耗殆尽后的结果[30-32],同时影响着其他品质指标。
AsA 也称为维生素C,是植物中含量最丰富的抗氧化剂之一[33]。有研究发现奉黄1号[34]、徐香[9]、海沃德[35]等猕猴桃的AsA和可滴定酸含量随采摘期的延后总体呈下降趋势,本研究与此研究结果一致。而海沃德[35]猕猴桃在0 ℃冷藏完成生理后熟的过程中,随着贮藏时间的延长,AsA和可滴定酸含量在20~100 d上升。本研究在盛花后171~210 d,AsA含量也上升,于盛花后192 d达到峰值1 282.22 mg·100 g-1。关于这个峰值,有研究发现叶绿体产生活性氧(ROS)时,AsA 在浓度为20 mmol·L-1或更高的水平。而AsA 在光保护中起着核心作用,包括光合作用和呼吸产生的ROS清除剂、紫黄质脱环氧化酶的辅因子和光系统Ⅱ电子供体[33],此时AsA 上升很可能是在为后熟期间呼吸高峰做准备。
果实的叶绿素中含有丰富的铁元素,有解毒、抗氧化、延缓衰老,美容养颜等功效[36]。类胡萝卜素是多种天然色素的总称,有维持视觉、增强免疫,保护皮肤等作用[37]。有研究发现奉黄1号不同采收期的总叶绿素、类胡萝卜素含量随采摘期的延后总体呈下降趋势,与本研究结果总体趋势一致[34]。而秦美[38]猕猴桃在20 ℃贮藏后熟过程中,各色素含量均迅速下降,下降幅度表现出:叶绿素b>叶绿素a>类胡萝卜素;在0 ℃贮藏过程中,L*和b*值逐渐减小,a*值逐渐增大,果实的亮度下降,果肉转变为黄绿色并趋于透明;本研究与秦美这两种贮藏温度下色素和色差研究结果基本一致。关于色素的下降,有研究表明猕猴桃后熟后期果肉细胞呼吸作用程度显著加强,蛋白质组成崩解,色素逐渐被消耗[38]。关于叶绿素含量的变化,有研究发现与叶绿体细胞壁和超微结构的变化直接相关,其降解过程中MDcase和Chlase酶可能发挥重要作用[39]。
可溶性固形物、可溶性糖含量是猕猴桃重要营养指标,其含量水平决定猕猴桃的食用口感[40]。有研究表明海沃德猕猴桃在冷藏条件下,随着采收期的延后猕猴桃果实可溶性固形物含量显著增加[35]。而张佳佳等[41]发现华特果实后熟前期呼吸作用加强,加速分解积累的有机物(淀粉),使得可溶性固形物和可溶性糖含量上升,果实口感风味增加;后熟后期可溶性固形物成为主要供能底物来源,使得果实可溶性固形物和可溶性糖含量下降。本研究表明,从盛花后171~210 d 果实可溶性固形物和可溶性糖含量上升,盛花后210 d 达到峰值10.87%后迅速下降,与华特整体趋势基本一致。淀粉降解还会受到乙烯的调控和低温的诱导[42],有研究发现β-淀粉酶基因(BAM3.2,BAM3L)、淀粉磷酸化酶基因(PHS2,PHS2.1)可能参与低温诱导的淀粉降解,AdDof3 和AcbHLH137 分别调控AdBAM3L 和AcBAM3 靶基因的表达,从而促进淀粉降解[43-45]。
因留树后熟时期不同,果实品质均有各自的特点,需综合性地进行评价与分析。目前主成分分析已被广泛应用在有关果实品质的数据分析和综合评价中[46]。通过主成分分析综合评价,果实品质综合得分排名前三(得分均大于0.6)的时期为:盛花后192、210、201 d,即留树21、39、30 d。有关贮藏研究发现,徐香挂树预贮7 d 的猕猴桃冷藏出库时AsA含量、可食状态下感官得分最高[9];秦美在采后20 ℃贮藏,仅能保鲜12 d 左右[38];华特采后20 ℃贮藏,约6 d 内完全软化,在完全软化后的6~12 d 保持营养物质相对稳定[41];而本研究赣绿1 号可以留树贮藏达39 d。与其他品种猕猴桃相比,赣绿1 号毛花猕猴桃生理成熟后不易落果,留树时间长,耐贮性较好。目前,猕猴桃采后贮藏催熟的相关设备不够齐全,贮藏、催熟技术不够规范[47]。留树后熟具有不占室内贮藏空间、省去贮藏环节、减少果实损伤和对第2年产量影响不大等优点[11]。在品质方面,留树预贮可以提高猕猴桃果实可溶性固形物含量,降低可滴定酸含量,提高淀粉降解速率,风味品质更佳[9]。在生产方面,市场需要即食的猕猴桃[47]。毛花猕猴桃挂树后熟期间,不同树体不同位置果实成熟存在差异,可自由挑选、随摘即食,有利于发展农家乐、旅游观光等产业,让人们品尝到更鲜嫩可口的猕猴桃,且节约贮藏成本[8]。但长时间留树贮藏,果实会在树上进入后熟阶段,导致采后贮藏时间大大缩短[7]。正常采后贮藏也可以通过冷藏、气调等方法减缓果实成熟、衰老,贮存更长时间。
4 结 论
赣绿1 号果实留树后熟期间,果实外观指标均呈现下降趋势,内在品质呈现先增后降趋势。各色素含量于盛花后210 d 后迅速下降,幅度表现出:叶绿素b>叶绿素a>类胡萝卜素,果肉颜色从嫩绿变为偏黄深绿。AsA 含量于盛花后192 d 达到峰值1 282.22 mg·100 g-1,可溶性糖含量于盛花后210 d达到峰值10.87%。通过主成分分析综合评价,果实留树21~39 d 果实综合品质最佳。本试验结果可为开创新的更环保的留树后熟猕猴桃贮藏手段提供理论依据。
[1] LIAO G L,XU X B,HUANG C H,ZHONG M,JIA D F.Resource evaluation and novel germplasm mining of Actinidia eriantha[J].Scientia Horticulturae,2021,282:110037.
[2] 黄宏文.猕猴桃属分类资源驯化栽培[M].北京:科学出版社,2013:2-78.HUANG Hongwen. Actinidia taxonomy germplasm domestication cultivation[M].Beijing:Science Press,2013:2-78.
[3] 邹梁峰.毛花猕猴桃雄株核心种质构建及遗传多样性分析[D].南昌:江西农业大学,2019.ZOU Liangfeng.Establishiment of the core collection of male germplasm resources of Actinidia eriantha and analysis of its genetic diversity[D].Nanchang:Jiangxi Agricultural University,2019.
[4] LIAO G L,XU Q,ALLAN A C,XU X B.L-Ascorbic acid metabolism and regulation in fruit crops[J].Plant Physiology,2023,192(3):1684-1695.
[5] 王海令,曹家乐,廖光联,黄春辉,贾东峰,曲雪艳,徐小彪.毛花猕猴桃AeAPX 基因家族鉴定与表达分析[J].果树学报,2022,39(12):2225-2240.WANG Hailing,CAO Jiale,LIAO Guanglian,HUANG Chunhui,JIA Dongfeng,QU Xueyan,XU Xiaobiao.Identification and expression analysis of AeAPX gene family in Actinidia eriantha[J].Journal of Fruit Science,2022,39(12):2225-2240.
[6] 袁云香.猕猴桃的储藏与保鲜技术[J].北方园艺,2011(6):168-170.YUAN Yunxiang.Technology of storage and fresh-keeping of kiwifruit[J].Northern Horticulture,2011(6):168-170.
[7] 王明召,阳廷密,张素英,门友均,唐明丽,易显荣,万保雄,娄兵海.‘红阳’猕猴桃不同时期采收果实品质及贮藏效果研究[J].中国果树,2018(4):31-33.WANG Mingzhao,YANG Tingmi,ZHANG Suying,MEN Youjun,TANG Mingli,YI Xianrong,WAN Baoxiong,LOU Binghai.Study on fruit quality and storage effect of‘Hongyang’kiwifruit in different harvest periods[J].China Fruits,2018(4):31-33.
[8] TILAHUN S,CHOI H R,PARK D S,LEE Y M,CHOI J H,BAEK M W,HYOK K,PARK S M,JEONG C S.Ripening quality of kiwifruit cultivars is affected by harvest time[J].Scientia Horticulturae,2020,261:108936.
[9] 屈魏,高萌,冉昪,李欢,舒雪瑶,饶景萍.挂树预贮对‘徐香’猕猴桃采后耐贮性和冷敏性的影响[J].食品科学,2020,41(23):197-204.QU Wei,GAO Meng,RAN Bian,LI Huan,SHU Xueyao,RAO Jingping.Effect of tree-hanging pre-storage on postharvest storability and cold sensitivity of‘Xuxiang’kiwifruits[J].Food Science,2020,41(23):197-204.
[10] 孙建城,王登亮,刘春荣,吴雪珍,吴群,程慧林.柑橘留树保鲜技术研究进展[J].中国果树,2021(7):1-6.SUN Jiancheng,WANG Dengliang,LIU Chunrong,WU Xuezhen,WU Qun,CHENG Huilin.Research progress of citrus ontree storage[J].China Fruits,2021(7):1-6.
[11] 陶爱群,易干军,石雪晖,姜小文.柑橘留树保鲜研究进展[J].广东农业科学,2012,39(24):45-49.TAO Aiqun,YI Ganjun,SHI Xuehui,JIANG Xiaowen.Overview of citrus storage on tree[J].Guangdong Agricultural Sciences,2012,39(24):45-49.
[12] KIENZLE S,CARLE R,SRUAMSIRI P,TOSTA C,NEIDHART S.Occurrence of alk (en) ylresorcinols in the fruits of two mango (Mangifera indica L.) cultivars during on-tree maturation and postharvest storage[J].Journal of Agricultural and Food Chemistry,2014,62(1):28-40.
[13] BURNS J K,ALBRIGO L G.Time of harvest and method of storage affect granulation in grapefruit[J].HortScience,1998,33(4):728-730.
[14] 徐小彪,廖光联,黄春辉,贾东峰,钟敏,曲雪艳,刘青,高欢.甜香型毛花猕猴桃新品种赣绿1 号的选育[J].果树学报,2024,41(2):358-361.XU Xiaobiao,LIAO Guanglian,HUANG Chunhui,JIA Dongfeng,ZHONG Min,QU Xueyan,LIU Qing,GAO Huan.A novel sweet aromatic cultivar of Actinidia eriantha‘Ganlü No.1’[J].Journal of Fruit Science,2024,41(2):358-361.
[15] LIAO G L,LI Z Y,HUANG C H,ZHONG M,TAO J J,QU X Y,CHEN L,XU X B.Genetic diversity of inner quality and SSR association analysis of wild kiwifruit(Actinidia eriantha)[J].Scientia Horticulturae,2019,248:241-247.
[16] 李合生.植物生理生化实验原理和技术[M].北京:高等教育出版社,2000:130-138.LI Hesheng.Principles and techniques of plant physiological biochemical experiment[M].Beijing:Higher Education Press,2000:130-138.
[17] 曹建康,姜微波,赵玉梅.果蔬采后生理生化实验指导[M].北京:中国轻工业出版社,2007:35-36.CAO Jiankang,JIANG Weibo,ZHAO Yumei.Postharvest physiological and chemical experiment guidance for fruits and vegetables[M].Beijing:China Light Industry Press,2007:35-36.
[18] 高俊凤.植物生理学实验指导[M].北京:高等教育出版社,2006:203-204.GAO Junfeng.Experimental guidance for plant physiology[M].Beijing:Higher Education Press,2006:203-204.
[19] 王利群,戴雄泽.色差计在辣椒果实色泽变化检测中的应用[J].辣椒杂志,2009,7(3):23-26.WANG Liqun,DAI Xiongze.Application of colorimeter for testing its color change during the development of hot pepper(Capsicum annuum L.) fruit[J].Journal of China Capsicum,2009,7(3):23-26.
[20] ZHANG B,CHEN K S,BOWEN J,ALLAN A,ESPLEY R,KARUNAIRETNAM S,FERGUSON I.Differential expression within the LOX gene family in ripening kiwifruit[J].Journal of Experimental Botany,2006,57(14):3825-3836.
[21] BRUMMELL D A,DAL CIN V,CRISOSTO C H,LABAVITCH J M.Cell wall metabolism during maturation,ripening and senescence of peach fruit[J].Journal of Experimental Botany,2004,55(405):2029-2039.
[22] WANG D D,YEATS T H,ULUISIK S,ROSE J K C,SEYMOUR G B.Fruit softening:Revisiting the role of pectin[J].Trends in Plant Science,2018,23(4):302-310.
[23] BURDON J,PIDAKALA P,MARTIN P,BILLING D.Softening of‘Hayward’kiwifruit on the vine and in storage:The effects of temperature[J].Scientia Horticulturae,2017,220:176-182.
[24] 杨丹,王琪凯,张晓琴.贮藏温度对采后‘金艳’猕猴桃品质和后熟的影响[J].北方园艺,2016(2):126-129.YANG Dan,WANG Qikai,ZHANG Xiaoqin.Effect of different storage temperatures on the quality and postharvest ripening of‘Jinyan’kiwifruit[J].Northern Horticulture,2016(2):126-129.
[25] 张计育,莫正海,黄胜男,刘永芝,郭忠仁.不同储藏温度对猕猴桃果实后熟过程中品质的影响[J].江苏农业科学,2013,41(11):295-297.ZHANG Jiyu,MO Zhenghai,HUANG Shengnan,LIU Yongzhi,GUO Zhongren.Effects of different storage temperatures on the quality of kiwifruit during ripening[J].Jiangsu Agricultural Sciences,2013,41(11):295-297.
[26] ITAI A,TANAHASHI T.Inhibition of sucrose loss during cold storage in Japanese pear (Pyrus pyrifolia Nakai) by 1-MCP[J].Postharvest Biology and Technology,2008,48(3):355-363.
[27] SCHRÖDER R,ATKINSON R.Kiwifruit cell walls:Towards an understanding of softening?[J].New Zealand Journal of Forestry Science,2006,36(1):112-129.
[28] ZHANG Q Y,GE J,LIU X C,WANG W Q,LIU X F,YIN X R.Consensus co-expression network analysis identifies AdZAT5 regulating pectin degradation in ripening kiwifruit[J].Journal of Advanced Research,2022,40:59-68.
[29] MOCHIZUKI T,KUROSAKI T.Histochemical changes of starch in kiwifruit (Actinidia chinensis Planch.) during fruit growth and storage[J].Nippon Shokuhin Kogyo Gakkaishi,1988,35(4):221-225.
[30] 陈金印,曾荣,李平.猕猴桃采后生理及贮藏技术研究进展[J].江西农业大学学报(自然科学版),2002,24(4):477-483.CHEN Jinyin,ZENG Rong,LI Ping.Advance of research on postharvest physiology of kiwifruit and its storage technology[J].Acta Agriculturae Universitis Jiangxiensis,2002,24(4):477-483.
[31] 顾子民.猕猴桃果实生长过程中种子发育及其激素含量的变化[D].杨凌:西北农林科技大学,2022.GUN Zimin.Changes of seed development and hormone content in kiwifruit during fruit growth[D].Yangling:Northwest A& F University,2022.
[32] 饶景萍,郭卫东,彭丽桃,任小林.猕猴桃后熟软化影响因素的研究[J].西北植物学报,1999,19(2):303-309.RAO Jingping,GUO Weidong,PENG Litao,REN Xiaolin.Study on the factors of kiwifruit ripening and softening[J].Acta Botanica Boreali-Occidentalia Sinica,1999,19(2):303-309.
[33] SMIRNOFF N.Ascorbate biosynthesis and function in photoprotection[J].Philosophical Transactions of the Royal Society of London.Series B,Biological Sciences,2000,355(1402):1455-1464.
[34] 陈双双,贺艳群,徐小彪,贾东峰,陶俊杰,梅奕阳,黄春辉.不同采收期对‘奉黄1 号’猕猴桃果实品质的影响[J].江西农业大学学报,2021,43(6):1259-1268.CHEN Shuangshuang,HE Yanqun,XU Xiaobiao,JIA Dongfeng,TAO Junjie,MEI Yiyang,HUANG Chunhui.Effect of different harvest time on fruit quality of‘Fenghuang No.1’kiwifruit[J].Acta Agriculturae Universitatis Jiangxiensis,2021,43(6):1259-1268.
[35] 吴彬彬,饶景萍,李百云,赖勤毅,张海燕.采收期对猕猴桃果实品质及其耐贮性的影响[J].西北植物学报,2008,28(4):4788-4792.WU Binbin,RAO Jingping,LI Baiyun,LAI Qinyi,ZHANG Haiyan.Effect of harvest date on fruit quality and storage duration of kiwifruit[J].Acta Botanica Boreali-Occidentalia Sinica,2008,28(4):4788-4792.
[36] 申申,段君禄,李宇,王承,傅佳佳,王鸿博.竹叶中叶绿素的提取工艺及其功能性应用研究[J].化工新型材料,2018,46(1):117-120.SHEN Shen,DUAN Junlu,LI Yu,WANG Cheng,FU Jiajia,WANG Hongbo.Research on the extraction and functional application of chlorophyll from bamboo leaves[J].New Chemical Materials,2018,46(1):117-120.
[37] 田清尹,岳远征,申慧敏,潘多,杨秀莲,王良桂.植物观赏器官中类胡萝卜素代谢调控的研究进展[J].生物技术通报,2022,38(12):35-46.TIAN Qingyin,YUE Yuanzheng,SHEN Huimin,PAN Duo,YANG Xiulian,WANG Lianggui.Research progress in the regulation of carotenoid metabolism in plant ornamental organs[J].Biotechnology Bulletin,2022,38(12):35-46.
[38] 张媛娥,雷生姣,夏辛珂,胡彪,陈钰亭.猕猴桃加工中叶绿素研究进展[J].食品科技,2021,46(2):44-50.ZHANG Yuane,LEI Shengjiao,XIA Xinke,HU Biao,CHEN Yuting.Research progress of chlorophyll in kiwifruit processing[J].Food Science and Technology,2021,46(2):44-50.
[39] 任亚梅.猕猴桃果实叶绿素代谢及生理特性研究[D].杨凌:西北农林科技大学,2009.REN Yamei.Study on chlorophyll metabolism and physiological characteristics of kiwifruit[D].Yangling:Northwest A & F University,2009.
[40] 刘科鹏,黄春辉,冷建华,陈葵,严玉平,辜青青,徐小彪.‘金魁’猕猴桃果实品质的主成分分析与综合评价[J].果树学报,2012,29(5):867-871.LIU Kepeng,HUANG Chunhui,LENG Jianhua,CHEN Kui,YAN Yuping,GU Qingqing,XU Xiaobiao.Principal component analysis and comprehensive evaluation of the fruit quality of‘Jinkui’kiwifruit[J].Journal of Fruit Science,2012,29(5):867-871.
[41] 张佳佳,郑小林,励建荣.毛花猕猴桃‘华特’果实采后生理和品质变化[J].食品科学,2011,32(8):309-312.ZHANG Jiajia,ZHENG Xiaolin,LI Jianrong.Physiological and quality changes in Actindia eriantha Benth‘Walter’fruit during storage at normal temperature[J].Food Science,2011,32(8):309-312.
[42] 冉欣雨,黄文俊,钟彩虹.猕猴桃果实淀粉代谢研究进展[J].果树学报,2024,41(2):325-337.RAN Xinyu,HUANG Wenjun,ZHONG Caihong.Advance in starch metabolism research of kiwifruit[J].Journal of Fruit Science,2024,41(2):325-337.
[43] ZHANG A D,WANG W Q,TONG Y,LI M J,GRIERSON D,FERGUSON I,CHEN K S,YIN X R.Transcriptome analysis identifies a zinc finger protein regulating starch degradation in kiwifruit[J].Plant Physiology,2018,178(2):850-863.
[44] 刘璐,王康,韩一璐,杨民杰,陈伟,曹士锋,施丽愉.猕猴桃AcbHLH137 功能鉴定及对淀粉降解基因AcBAM3 转录激活分析[J].核农学报,2022,36(3):544-553.LIU Lu,WANG Kang,HAN Yilu,YANG Minjie,CHEN Wei,CAO Shifeng,SHI Liyu.Functional identification of Acb-HLH137 and its transcriptional activation of starch degradation gene AcBAM3 in kiwifruit[J].Journal of Nuclear Agricultural Sciences,2022,36(3):544-553.
[45] 陈璐,高柱,毛积鹏,张小丽,卢玉鹏,林孟飞,公旭晨,王小玲.不同温度处理采后猕猴桃果实淀粉降解的转录组分析[J].江西农业大学学报,2023,45(3):591-604.CHEN Lu,GAO Zhu,MAO Jipeng,ZHANG Xiaoli,LU Yupeng,LIN Mengfei,GONG Xuchen,WANG Xiaoling.Transcriptome analysis of starch degradation in post-harvest kiwifruit treated at different temperatures[J].Acta Agriculturae Universitatis Jiangxiensis,2023,45(3):591-604.
[46] 胡光明,黎纯斌,杨斌,王周倩,申素云,李作洲,钟彩虹.宜昌市72 份野生中华猕猴桃果实性状多样性分析与综合评价[J].果树学报,2022,39(9):1540-1552.HU Guangming,LI Chunbin,YANG Bin,WANG Zhouqian,SHEN Suyun,LI Zuozhou,ZHONG Caihong.Analysis and comprehensive evaluation of fruit trait diversity of 72 Actinidia chinensis accessions in Yichang[J].Journal of Fruit Science,2022,39(9):1540-1552.
[47] 钟曼茜,翟舒嘉,刘伟,睢国祥,段玉权,林琼,陶鑫凉.我国即食猕猴桃产业发展现状、问题与对策[J].中国果树,2023(2):122-127.ZHONG Manxi,ZHAI Shujia,LIU Wei,SUI Guoxiang,DUAN Yuquan,LIN Qiong,TAO Xinliang.Current situation,problems and countermeasures of the development of ready-to-eat kiwifruit industry in China[J].China Fruits,2023(2):122-127.