柑橘(Citrus)是全球第一大类水果,富含维生素、多糖、有机酸、蛋白质、膳食纤维以及抗氧化物等成分,深受广大消费者喜爱[1-4]。2019年世界柑橘产量为1.4 亿t[5]。在中国,2021 年柑橘产量达5 595.6万t,年产值超2000亿元,是中国农业的重要支柱产业[6]。近年来,随着柑橘栽培面积的不断扩大以及栽培生态环境变化等因素的影响,柑橘黑点病(Citrus melanose)的发生和流行等问题越来越突出,在中国的柑橘主产区,包括广西、湖南、湖北、浙江、江西、福建、云南等地普遍发生,发病严重的地区病果率达100%,影响柑橘鲜果外观和商品价格,严重制约中国柑橘产业的健康发展[7-9]。
柑橘黑点病也称柑橘砂皮病,由间座壳菌属Diaporthe 引起,其病原菌也会引起柑橘树脂病或柑橘褐色蒂腐病。其中柑橘间座壳菌Diaporthe citri为优势种,无性态为柑橘拟茎点霉Phomopsis citri[10]。柑橘间座壳菌可侵染所有柑橘栽培品种,其中柠檬和葡萄柚易感病[11],目前尚未发现对其完全免疫的柑橘品种。近年来,已有多个柑橘品种以及柑橘间座壳菌的基因组信息被成功测序和注释[12-17],为进一步分析柑橘间座壳菌的侵染机制以及挖掘柑橘抗病基因提供了重要参考。针对柑橘黑点病发生流行的严重性以及其对柑橘产业健康发展的影响,笔者就国内外近年来柑橘黑点病的危害症状与分布、病原种类、遗传多样性、生物学特性、侵染过程、致病机制、流行规律以及防治措施等方面的研究进展进行综述,并对未来柑橘黑点病的研究方向及防控策略进行展望。
1 柑橘黑点病的症状与分布
1.1 症状
柑橘黑点病的症状可能因地理位置、寄主品种、发生季节、生理因素和感染严重程度而异[18]。柑橘果实发病后,果面散生黑色至红褐色小点(图1-A),或黑点连成片,果实表皮细胞木栓化并开裂,形成坚硬的黑色裂纹区(图1-B),与锈螨危害后引起果皮光滑的斑纹明显不同[19]。储藏期病果的果蒂及果肉等部位出现褐色腐烂[20]。柑橘新叶发病初期呈水浸状褐色斑点,周围呈半透明黄色晕圈,后期病叶表皮破裂并形成褐色或黑色坚硬的小粒点突起(图1-C)。柑橘新梢发病后在表面形成黄褐色或黑褐色的粒点突起(图1-D),柑橘主干发病后常引起流胶或干枯[21],并且病原菌在枯死枝条上产生大量的分生孢子或少量的子囊孢子[11]。
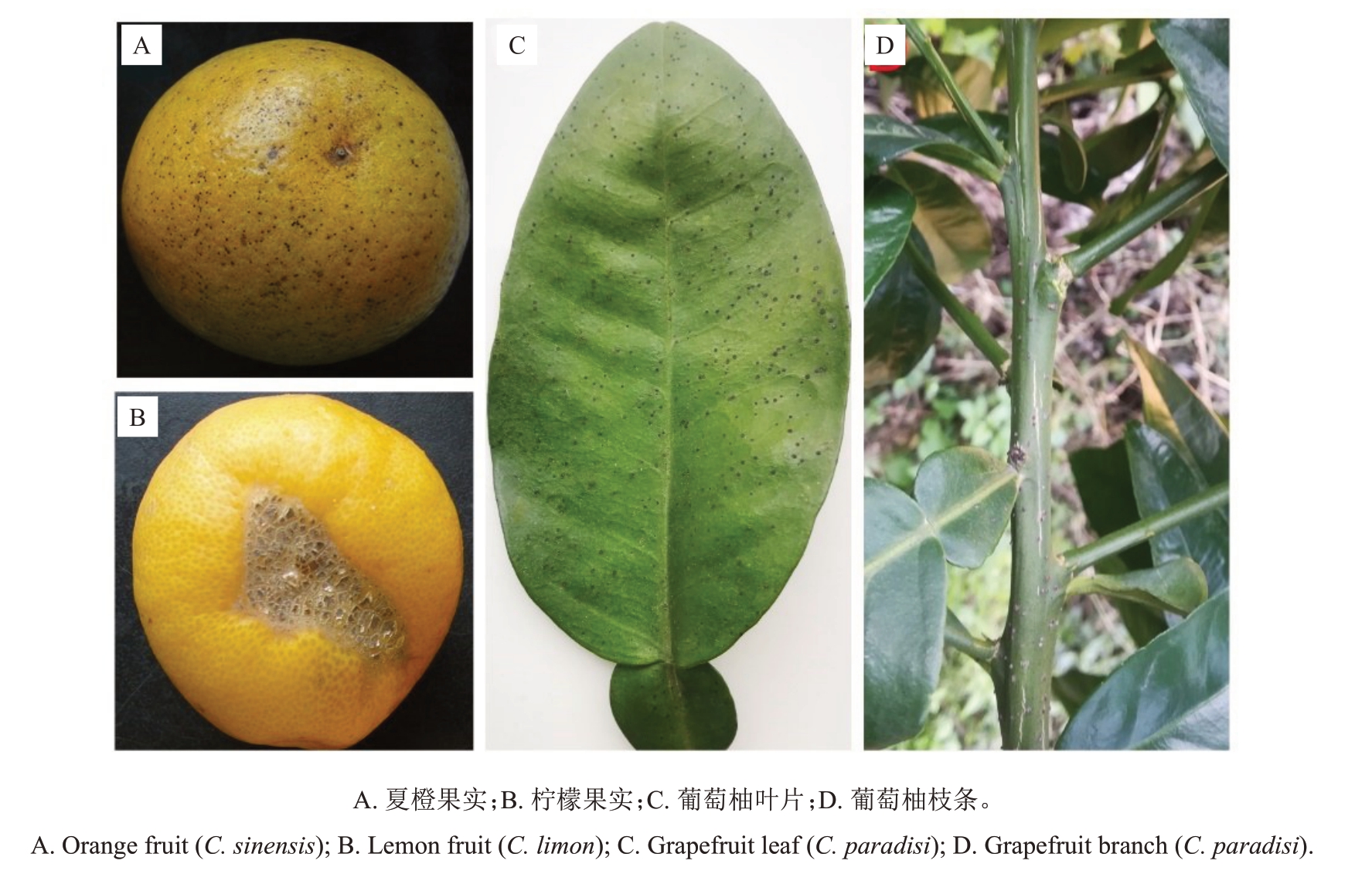
图1 柑橘黑点病菌侵染不同柑橘属植物组织的典型发病症状
Fig.1 The typical symptoms of citrus melanose pathogen infection on various tissues of different citrus plants
1.2 分布
柑橘黑点病在世界各地柑橘产区均有发生[18],包括中国、菲律宾、日本、韩国、泰国、缅甸、柬埔寨、斐济、毛里求斯、美国、墨西哥、海地、古巴、多米尼加、巴拿马、波多黎各、委内瑞拉、特立尼达和多巴哥、巴西、塞浦路斯、葡萄牙(亚速尔群岛)、新西兰、纽埃、萨摩亚、汤加、库克群岛、科特迪瓦和津巴布韦等国家[22-25]。在中国,柑橘黑点病在多个柑橘产区,包括广西、湖南、湖北、浙江、江西、福建、云南、贵州、重庆、广东、四川以及上海等地均普遍发生[7,9,11]。
2 病原种类、遗传多样性及生物学特性
2.1 病原种类
间座壳菌属(Diaporthe)真菌具有丰富的物种多样性,包含植物病原菌、内生菌和腐生菌[26]。寄主专化性不强,同一种间座壳菌可寄生在多种寄主植物上,或在同一种寄主植物上也常被多种间座壳菌复合寄生[26-30]。截至目前,寄生在柑橘属植物的间座壳菌属真菌数量达33 种[22],包含内生菌和致病菌[18,22,31]。在中国,柑橘间座壳菌(Diaporthe citris)是引起柑橘黑点病的重要病原菌,致病力强,可以侵染所有栽培柑橘,包括宽皮柑橘(Citrus reticulata)、甜橙(C.sinensis)、柚子(C.grandis)、柠檬(C.limon)和葡萄柚(C.paradisi)等。柑橘间座壳菌(D.citris)也是引起国外柑橘黑点病的优势种[30]。此外,在中国柑橘产区还分布D. citriasiana 和D. citrichinensis 菌株,他们能引起柑橘果实的蒂腐病[30]。其中,D.citrichinensis与柑橘间座壳菌之间的基因组平均核苷酸同一性(average nucleotide identity,ANI)达91%,说明这两个物种具有密切的亲缘关系[16],但D.citrichinensis的致病力较柑橘间座壳菌弱[22,30],造成他们致病力分化的原因还有待进一步研究。基于多基因位点包括核糖体内转录间隔区(rDNA internal transcribed spacer,ITS)、转录延伸因子1-α(translation elongation factor 1-alpha,TEF1-α)、β-微管蛋白(beta-tubulin,TUB)、组蛋白-H3(histone H3,HIS)、钙调蛋白(calmodulin,CAL)和交配型MAT1基因等序列进行的系统发育分析,为柑橘间座壳菌的准确、快速鉴定以及检测技术开发利用提供了重要参考[24,26,32-36]。
2.2 遗传多样性
目前,已对多个柑橘间座壳菌的菌株(包含MAT1-1 和MAT1-2 交配型菌株)以及近缘种的基因组信息进行测序和注释[16-17],柑橘间座壳菌的基因组大小为52.06~63.61 Mb,包含15 977~16 622 个蛋白编码基因,柑橘间座壳菌不同菌株的基因组平均核苷酸同一性达99%[16],这些基因组信息为进一步分析柑橘间座壳菌的产孢调控、致病力分化以及群体遗传进化等相关的分子机制奠定了重要基础。Xiong 等[11]研究表明,来源于中国南方5 个省份的339 个柑橘间座壳菌株群体中,具有不同交配型(MAT1-1-1和MAT1-2-1)和不同多位点基因型(multilocus genotypes,MLG)的菌株经常从相同的病叶和病果分离出来,说明柑橘间座壳菌的有性繁殖和基因重组在自然条件下频繁发生,因此他们具有较高的遗传多样性;同时,他们的遗传分化与其地理隔离(geographic separation)密切相关,而与他的寄主类别和寄主的不同组织相关性较差[11]。总之,柑橘间座壳菌是一种异宗配合(heterothallism)真菌,在自然环境条件下有性繁殖频繁发生,意味着他的有性孢子——子囊孢子在病害侵染循环中可能发挥着极其重要的作用[11,37],今后应重点关注柑橘间座壳菌子囊孢子的形成规律及其侵染过程,有利于对柑橘黑点病的有效防控。
2.3 生物学特性
柑橘间座壳菌的菌丝生长和产孢过程与其代谢物变化密切相关,其中氧化脂类代谢物是柑橘间座壳菌产孢的关键代谢物[38]。此外,菌丝生长、菌落形态、产孢与营养条件、温度、光照、pH 等因素密切相关。柑橘间座壳菌在PDA 培养基上生长的菌落边缘白色,气生菌丝蓬松,菌落背面呈淡黄色(图2-A~B);而在MEA 和OA 培养基上生长的菌落正面呈白色,扁平,培养后期菌落背面呈黄色[39]。柑橘间座壳菌生长的最适温度为26~30 ℃,最适pH 为6~9,在光暗交替条件下有利于菌丝生长[40]。

图2 柑橘间座壳菌的菌落和分生孢子形态特征
Fig.2 Morphological characteristics of colonies and conidia of D.citri
柑橘间座壳菌在PDA培养基上仅形成α型分生孢子[30],但来源于印度柠檬上的柑橘间座壳菌在相似条件下生长,不仅可以产生α型分生孢子,还可以产生β 型分生孢子[23],这可能与不同地域来源的菌株相关。将柑橘间座壳菌接种至无菌的柑橘枝条上,置于水琼脂平板上26 ℃培养30 d,可见许多黄色分生孢子液滴形成(图2-C),α型分生孢子含有1~2个油滴,呈椭圆形(图2-D);β型分生孢子呈直线或弯钩状(图2-E)。据报道,柑橘间座壳菌的分生孢子能在含柚子成分的培养液中萌发,最适温度为29.2 ℃[41],但温度低于17 ℃或高于35 ℃时,其不能成功侵染寄主[42]。
3 侵染过程及致病机制
柑橘间座壳菌的分生孢子与寄主叶片接触后,萌发形成的芽管直接穿透柑橘叶片的角质层,并且菌丝在相邻表皮细胞的侧壁之间向下延伸至叶片的栅栏薄壁组织中,并分枝生长[10]。柑橘间座壳菌分泌的果胶酶(pectinase)降解薄壁细胞的细胞壁,瓦解细胞后从破裂的叶片角质层中渗出黏性胶状物质,变硬后形成粗糙的黑色或棕色突起[10,42]。此外,在柑橘果实成熟期或贮藏期,其分泌的果胶酶对促进果实腐烂症状的形成也具有明显作用[10,43-44]。Gai等[16]测序和分析了柑橘间座壳菌的基因组信息,预测其含有1231~1287 个PHI(pathogen-host interaction)基因,具有1837~1885 个分泌蛋白以及1600 多个碳水化合物活性酶(carbohydrate-active enzymes,CAZymes),包括糖苷水解酶(glycoside hydrolases)、糖基转移酶(glycosyl transferases)、碳水化合物酯酶(carbohydrate esterases)、多糖裂解酶(polysaccharide lyases)等,他们可能与柑橘间座壳菌的致病性相关,但这些蛋白的功能有待进一步分析和验证。此外,有关柑橘间座壳菌是否形成特殊的侵染结构、形成哪些果胶酶种类以及如何调控果胶酶合成的分子调控机制等科学问题仍有待进一步研究。
Li 等[45]研究表明,柑橘间座壳菌侵染柑橘叶片后,叶际微生物组的群落均匀度显著降低,但对其具有拮抗活性的泛菌Pantoea asv90和甲基杆菌Methylobacterium asv41 的群落增加,这可能与柑橘植物的免疫反应相关。早期,有学者基于显微观察和高效液相色谱分析检测的方法,证明了柑橘叶片在受到柑橘间座壳菌侵染后会激活植物防御反应,包括诱导植物保卫素——6,7-二甲氧基香豆素(6,7-dimethoxy coumarin)的形成等,限制柑橘间座壳菌在寄主细胞的进一步侵染和扩展[46-48]。Li等[49]采用RNASeq 方法分析了柑橘间座壳菌侵染柑橘叶片3 d 后和14 d后的转录组数据,发现与柑橘叶片细胞壁生物发生(cell wall biogenesis)相关的基因在侵染3 d后被大量诱导表达,而参与胼胝质沉淀反应相关的基因、果胶甲基酯酶(pectin methylesterase,PME)基因以及香豆素及其衍生物合成的关键酶——阿魏酰辅酶A 6'-羟化酶1(Feruloyl-CoA 6'-Hydroxylase1)和东莨菪素8-羟化酶(scopoletin 8-hydroxylase)基因等在侵染14 d后被大量诱导表达,进一步从分子水平上证明了柑橘叶片被柑橘间座壳菌侵染后,激活了柑橘的防御反应。然而,有关柑橘如何利用其抗性蛋白或者其他受体蛋白识别柑橘间座壳菌的侵染,进而抑制病原菌的进一步扩展,以及柑橘间座壳菌如何逃避寄主的免疫反应的分子互作机制等问题仍有待进一步研究。
4 柑橘黑点病发生规律及防治措施
4.1 发生规律
柑橘间座壳菌的寄主范围仅限于柑橘属植物[22]。柑橘黑点病的发生流行与侵染源的数量、气候条件、柑橘品种、树龄以及果园栽培管理措施等密切相关[9,42,50-51]。柑橘枯死枝条是柑橘间座壳菌越冬和繁殖的重要场所[42,51],也是田间柑橘黑点病发生的重要侵染源[9]。枯死枝条上形成分生孢子器或分生孢子的数量与柑橘黑点病发生的严重程度、湿度、温度和树枝大小等相关[19,52]。有意思的是,尚未枯死的感病枝条不形成任何分生孢子器或分生孢子[52]。因此,感病枯死枝条在柑橘黑点病菌产孢方面起主要作用[11]。然而,有关调控柑橘间座壳菌无性孢子和子囊孢子发育成熟的分子调控通路有待进一步明确。
柑橘间座壳菌的分生孢子随雨水传播,具有从上至下和传播距离较短等特点,而子囊孢子通过自身的弹射力从子囊孔口释放,并通过气流传播扩散,具有传播距离较远的特点[9]。他们能否成功侵染寄主与柑橘的感病期、环境条件(温度、湿度)等密切相关。柑橘的嫩叶、嫩枝以及谢花后12周内的幼果均处于易感病期,也是预防和防控柑橘黑点病发生的关键时期。柑橘新叶完全展开后或者谢花后12 周以上的幼果对柑橘间座壳菌的抗性逐渐增强。人工接种试验结果表明,在25 ℃条件下柑橘间座壳菌分生孢子成功侵染柑橘需要10~12 h的湿润条件[42],柑橘叶片黑点病的发生潜育期为4~7 d[9]。在自然条件下,当平均气温大于22 ℃、叶片维持湿度超过80 h(每周)时,柑橘黑点病发病率将明显增加[53]。此外,柑橘果实生长期的平均温度为20 ℃,该时期的降雨量与柑橘黑点病的发生密切相关[9]。在不同的国家或地区,由于气候条件以及柑橘品种的差异,发病的严重程度或高峰期也存在差异,但与柑橘的易感物候期密切相关[21,52,54]。
4.2 防治方法
4.2.1 化学防治 施用杀菌剂是当前防治柑橘黑点病的主要方法。铜制剂和代森锰锌等保护性杀菌剂对柑橘黑点病具有较好的预防和保护作用[8,55-59],但铜制剂的保护作用容易因雨水冲刷丧失[22],同时高温(大于35 ℃)条件下使用铜制剂容易产生药害[42,60]。用100 μg·mL-1的二氧化硅和200 μg·mL-1的季铵化合物(季铵盐)的复合物替换铜制剂使用可降低对植物的毒性[61]。此外,在中国柑橘黑点病发病严重的果园中,代森锰锌的使用量(4 g·L-1)已明显大于推荐的使用量(1.34 g·L-1)[62],长期大量使用杀菌剂也容易造成环境污染。0.1 g·L-1的醚菌酯(kresoxim-methyl)和1 g·L-1的代森锰锌混合使用防治效果与2.66 g·L-1的代森锰锌的防效相当[62]。代森锰锌与矿物油(绿颖)或乙氧基改性聚三硅氧烷(GE公司)混合使用也可以提高对柑橘黑点病的防效[9]。此外,由恶唑烷二酮和代森锰锌复配而成的杀菌剂对柑橘黑点病的防效超过73%,在生产上具有推广应用前景[63]。
具有治疗性的甲氧基丙烯酸酯类(strobilurin)对柑橘黑点病具有较好的防效,但该类药剂如吡唑醚菌酯(pyraclostrobin)、嘧菌酯(azoxystrobin)等易产生抗药性,在1 年内该类型药剂的使用次数不能超过2 次[42]。苯醚菌酯(E-2-[2-(2,5-dimethyl-phenoxy)-phenylmethyl]-3-methoxyacrylic acid methylester)是中国自主研发的苯醌外部抑制剂(quinone outside inhibitor,QoI)类杀菌剂,当前中国的柑橘间座壳菌种群对其仍然敏感,可用于柑橘黑点病的防治[64]。同时,0.1 μg·mL-1 的醚菌酯和肟菌酯(trifloxystrobin)能完全抑制柑橘间座壳菌分生孢子的萌发[62]。此外,肟菌酯(trifloxystrobin)和吡唑醚菌酯(pyraclostrobine)分别与铜制剂混合使用均可提高对柑橘黑点病的防治效果[65-66]。然而,有关抑制柑橘间座壳菌分生孢子器形成的杀菌剂的报道较少[52],仅苯并咪唑类的苯菌灵(benomyl)可以抑制柑橘间座壳菌在枯死枝条上的产孢,但对柑橘果实和叶片黑点病的防治效果较差[67]。
4.2.2 生物防治 利用拮抗微生物防治植物病害可以减少因过度使用农药造成的环境污染等问题,并且一些拮抗微生物还可促进植物生长及增强植物抗性[22]。研究表明,唐菖蒲伯克霍尔德菌(Burkholderia gladioli)、恶臭假单胞菌(Pseudomonas pudia)和荧光假单胞菌(P.fluorescens)对柑橘间座壳菌具有拮抗活性[68],如抑制分生孢子萌发,引起病原菌致病力明显降低[69]。枯草芽孢杆菌(Bacillus subtilis)[70]、贝氏芽孢杆菌(B.velezensis)[71]、淀粉芽孢杆菌(B. amyloliquefaciens)[72]等对柑橘间座壳菌的菌丝生长或分生孢子萌发均有较强的抑制作用,部分拮抗菌株已应用于柑橘黑点病的田间防治,防效达74%[73]。此外,硫杆菌(Thiobacillus species)产生的生物硫(bio-sulfur)能显著抑制柑橘黑点病的发生[74]。棘孢木霉(Trichoderma asperellum)和类棘孢木霉(T.asperelloides)生防菌不仅能抑制柑橘间座壳菌的生长,还可以分泌漆酶(laccase)降解柑橘枯枝,减少感染枯枝上侵染源的形成[75]。然而,这些具有生防潜力的菌株在防控柑橘黑点病的商业化应用方面还有待进一步研究和推广。
4.2.3 农业防治及诱导植物抗性 加强柑橘果园的栽培管理,合理密植与修剪枝条,降低果园湿度,增施有机肥和磷钾肥,提高寄主的抗病性,并且及时防治害虫等措施可显著降低柑橘黑点病的发生率[21,76]。同时,部分抗性诱导化合物(Oxycom、Serenade、ReZist、Aliette、Nutriphite、Actigard 和Benlate)的使用,可显著提高柑橘的抗病性[77]。不过,目前抗性诱导剂在防治柑橘黑点病方面的商业化应用还较少。
5 展 望
中国是柑橘生产大国,柑橘产业在提高农民收入、实现乡村振兴以及促进农业发展等方面具有重要作用,但柑橘黑点病的流行和危害严重影响了柑橘鲜销和出口创汇,制约着柑橘产业的健康发展。近年来,针对柑橘黑点病的病原检测、种类鉴定、基因组信息、遗传多样性、生物学特性、侵染循环、致病机制、发生规律和防控措施等方面取得了一些进展。然而,以下几个问题仍有待进一步深入的探究:(1)柑橘间座壳菌是否形成特殊的侵染结构以便顺利穿透具有蜡质层的柑橘叶片和果皮。(2)柑橘间座壳菌分泌的果胶酶是其重要的致病因子,所形成的果胶酶种类、编码基因以及功能仍有待明确。同时,柑橘间座壳菌的基因组分析预测结果显示,其含有大量假定的致病基因、分泌蛋白以及碳水化物活性酶,他们是否参与其致病过程仍有待进一步探析。(3)柑橘间座壳菌仅在枯死的柑橘枝条上形成繁殖体,而在未枯死的枝条上不产孢,其无性孢子和子囊孢子发育调控的分子机制有待研究。(4)柑橘叶片受到柑橘间座壳菌侵染后,会增加叶片拮抗微生物的群落[43],寄主是如何识别柑橘间座壳菌的分子信号来调节自身免疫的,以及柑橘间座壳菌如何逃逸植物的防御反应的分子互作关系仍有待明确。
总之,深入认识柑橘间座壳菌的侵染结构、致病因子、产孢调控分子机制以及寄主识别柑橘间座壳菌的分子信号通路等方面的内容,将有助于为柑橘的抗病育种提供资源,也可为柑橘黑点病防治药剂的研发和应用提供新的靶标。同时,柑橘间座壳菌的种群丰富,是异宗配合真菌,有性繁殖频繁,应加强监测其对代森锰锌等农药的敏感性,科学合理混配农药,结合生防制剂以及植物抗性诱导物的使用,延缓其抗药性的形成,共同提高柑橘黑点病的综合防控能力。
[1] 李勋兰,洪林,杨蕾,王武,韩国辉,农江飞,谭平.11 个柑橘品种果实营养成分分析与品质综合评价[J].食品科学,2020,41(8):228-233.LI Xunlan,HONG Lin,YANG Lei,WANG Wu,HAN Guohui,NONG Jiangfei,TAN Ping.Analysis of nutritional components and comprehensive quality evaluation of citrus fruit from eleven varieties[J].Food Science,2020,41(8):228-233.
[2] ZOU Z,XI W P,HU Y,NIE C,ZHOU Z Q.Antioxidant activity of citrus fruits[J].Food Chemistry,2016,196:885-896.
[3] 邓秀新.世界柑橘品种改良的进展[J].园艺学报,2005,32(6):1140-1146.DENG Xiuxin.Advances in worldwide Citrus breeding[J].Acta Horticulturae Sinica,2005,32(6):1140-1146.
[4] 丁晓波,张华,刘世尧,廖益均,周志钦.柑橘果品营养学研究现状[J].园艺学报,2012,39(9):1687-1702.DING Xiaobo,ZHANG Hua,LIU Shiyao,LIAO Yijun,ZHOU Zhiqin. Current status of the study in Citrus nutriology[J].Acta Horticulturae Sinica,2012,39(9):1687-1702.
[5] FAO.Citrus fruit statistical compendium 2020[M].Rome,2021.
[6] 国家统计局.中国统计年鉴[M].北京:中国统计出版社,2022.National Bureau of Statistics of China. China Statistical Yearbook[M].Beijing:China Statistics Press,2022.
[7] 何永林,黄晓琴,黎起秦,陆温,林纬,袁高庆.柑橘砂皮病菌鉴定及室内药剂筛选试验[J].广东农业科学,2019,46(11):92-97.HE Yonglin,HUANG Xiaoqin,LI Qiqin,LU Wen,LIN Wei,YUAN Gaoqing. Pathogenic identification of citrus melanose and indoor screening of fungicides[J]. Guangdong Agricultural Sciences,2019,46(11):92-97.
[8] 陈国庆,姜丽英,徐法三,李红叶.防治柑橘黑点病药剂的离体和田间筛选[J].浙江大学学报(农业与生命科学版),2010,36(4):440-444.CHEN Guoqing,JIANG Liying,XU Fasan,LI Hongye. In vitro and in vivo screening of fungicides for controlling citrus melanose caused by Diaporthe citri[J].Journal of Zhejiang University(Agriculture and Life Sciences),2010,36(4):440-444.
[9] 姜丽英,徐法三,黄振东,黄峰,陈国庆,李红叶.柑橘黑点病的发病规律和防治[J].浙江农业学报,2012,24(4):647-653.JIANG Liying,XU Fasan,HUANG Zhendong,HUANG Feng,CHEN Guoqing,LI Hongye. Occurrence and control of citrus melanose caused by Diaporthe citri[J]. Acta Agriculturae Zhejiangensis,2012,24(4):647-653.
[10] BACH W J,WOLF F A.The isolation of the fungus that causes citrus melanose and the pathological anatomy of the host[J].Journal of Agricultural Research,1928,37(4):243-252.
[11] XIONG T,ZENG Y T,WANG W,LI P D,GAI Y P,JIAO C,ZHU Z R,XU J P,LI H Y.Abundant genetic diversity and extensive differentiation among geographic populations of the citrus pathogen Diaporthe citri in southern China[J]. Journal of Fungi,2021,7(9):749.
[12] LIU H,WANG X,LIU S J,HUANG Y,GUO Y X,XIE W Z,LIU H,TAHIR UL QAMAR M,XU Q,CHEN L L.Citrus pangenome to breeding database (CPBD):A comprehensive genome database for citrus breeding[J]. Molecular Plant,2022,15(10):1503-1505.
[13] WU G A,PROCHNIK S,JENKINS J,SALSE J,HELLSTEN U,MURAT F,PERRIER X,RUIZ M,SCALABRIN S,TEROL J,TAKITA M A,LABADIE K,POULAIN J,COULOUX A,JABBARI K,CATTONARO F,DEL FABBRO C,PINOSIO S,ZUCCOLO A,CHAPMAN J,GRIMWOOD J,TADEO F R,ESTORNELL L H,MUÑOZ-SANZ J V,IBANEZ V,HERREROORTEGA A,ALEZA P,PÉREZ-PÉREZ J,RAMÓN D,BRUNEL D,LURO F,CHEN C X,FARMERIE W G,DESANY B,KODIRA C,MOHIUDDIN M,HARKINS T,FREDRIKSON K,BURNS P,LOMSADZE A,BORODOVSKY M,REFORGIATO G,FREITAS-ASTÚA J,QUETIER F,NAVARRO L,ROOSE M,WINCKER P,SCHMUTZ J,MORGANTE M,MACHADO M A,TALON M,JAILLON O,OLLITRAULT P,GMITTER F,ROKHSAR D. Sequencing of diverse mandarin,pummelo and orange genomes reveals complex history of admixture during citrus domestication[J]. Nature Biotechnology,2014,32:656-662.
[14] LI Q,QI J J,QIN X J,DOU W F,LEI T G,HU A H,JIA R R,JIANG G J,ZOU X P,LONG Q,XU L Z,PENG A H,YAO L X,CHEN S C,HE Y R.CitGVD:A comprehensive database of citrus genomic variations[J].Horticulture Research,2020,7:12.
[15] WU G A,TEROL J,IBANEZ V,LÓPEZ-GARCÍA A,PÉREZROMÁN E,BORREDÁ C,DOMINGO C,TADEO F R,CARBONELL-CABALLERO J,ALONSO R,CURK F,DU D L,OLLITRAULT P,ROOSE M L,DOPAZO J,GMITTER F G,ROKHSAR D S,TALON M.Genomics of the origin and evolution of Citrus[J].Nature,2018,554:311-316.
[16] GAI Y P,XIONG T,XIAO X E,LI P D,ZENG Y T,LI L,RIELY B K,LI H Y. The genome sequence of the citrus melanose pathogen Diaporthe citri and two citrus-related Diaporthe species[J].Phytopathology,2021,111(5):779-783.
[17] LIU X Y,CHAISIRI C,LIN Y,YIN W X,LUO C X.Whole-genome sequence of Diaporthe citri isolate NFHF-8-4,the causal agent of Citrus melanose[J]. Molecular Plant-Microbe Interactions,2021,34(7):845-847.
[18] UDAYANGA D,CASTLEBURY L A,ROSSMAN A Y,HYDE K D. Species limits in Diaporthe:Molecular re-assessment of D.citri,D.cytosporella,D.foeniculina and D.rudis[J].Persoonia,2014,32:83-101.
[19] MONDAL S N,VICENT A,REIS R F,TIMMER L W. Saprophytic colonization of citrus twigs by Diaporthe citri and factors affecting pycnidial production and conidial survival[J]. Plant Disease,2007,91(4):387-392.
[20] 周娜,胡军华,姚廷山,王雪莲,王娟,彭凤格,洪棋斌,江东.柑橘种质抗柑橘蒂腐病菌扩展能力的评价[J]. 园艺学报,2015,42(10):1889-1898.ZHOU Na,HU Junhua,YAO Tingshan,WANG Xuelian,WANG Juan,PENG Fengge,HONG Qibin,JIANG Dong.Evaluation of anti-expansion capacity of different Citrus germplasm against Diaporthe citri[J]. Acta Horticulturae Sinica,2015,42(10):1889-1898.
[21] 郭鄂平,陆学忠,杨树国,王娅.十堰市柑橘树脂病的发生特点与综合防治[J].中国南方果树,2013,42(2):94-95.GUO E’ping,LU Xuezhong,YANG Shuguo,WANG Ya.Occurrence characteristics and comprehensive prevention and control of citrus melanose in Shiyan City[J].South China Fruits,2013,42(2):94-95.
[22] CHAISIRI C,LIU X Y,LIN Y,LUO C X. Diaporthe citri:A fungal pathogen causing melanose disease[J]. Plants,2022,11(12):1600.
[23] MAHADEVAKUMAR S,YADAV V,TEJASWINI G S,SANDEEP S N,JANARDHANA G R. First report of Phomopsis citri associated with Dieback of Citrus lemon in India[J]. Plant Disease,2014,98(9):1281.
[24] CHAISIRI C,LIU X Y,LIN Y,LI J B,XIONG B,LUO C X.Phylogenetic analysis and development of molecular tool for detection of Diaporthe citri causing melanose disease of Citrus[J].Plants,2020,9(3):329.
[25] AGUILERA-COGLEY V,VICENT A. Etiology and distribution of foliar fungal diseases of citrus in Panama[J]. Tropical Plant Pathology,2019,44(6):519-532.
[26] GOMES R R,GLIENKE C,VIDEIRA S I R,LOMBARD L,GROENEWALD J Z,CROUS P W.Diaporthe:A genus of endophytic,saprobic and plant pathogenic fungi[J].Persoonia,2013,31:1-41.
[27] 王先洪,姜佳琦,洪霓,王国平.贵州梨芽枯间座壳菌属Diaporthe 的种类[J].菌物学报,2022,41(8):1151-1164.WANG Xianhong,JIANG Jiaqi,HONG Ni,WANG Guoping.Diaporthe species causing pear bud witherings in Guizhou,Southwest China[J].Mycosystema,2022,41(8):1151-1164.
[28] ZHANG Q M,YU C L,LI G F,WANG C X.First report of Diaporthe eres causing twig canker on Zizyphus jujuba (jujube) in China[J].Plant Disease,2018,102(7):1458.
[29] 柴思睿.中国江西省柑橘黑点病菌种群结构研究[D].武汉:华中农业大学,2018.Chai Sirui.Characterization of the populations of Diaporthe species on citrus in Jiangxi province,China[D]. Wuhan:Huazhong Agricultural University,2018.
[30] HUANG F,HOU X,DEWDNEY M M,FU Y S,CHEN G Q,HYDE K D,LI H Y. Diaporthe species occurring on citrus in China[J].Fungal Diversity,2013,61(1):237-250.
[31] CUI M J,WEI X,XIA P L,YI J P,YU Z H,DENG J X,LI Q L.Diaporthe taoicola and D.siamensis,two new records on Citrus sinensis in China[J].Mycobiology,2021,49(3):267-274.
[32] UDAYANGA D,LIU X Z,CROUS P W,MCKENZIE E H C,CHUKEATIROTE E,HYDE K D. A multi-locus phylogenetic evaluation of Diaporthe (Phomopsis) [J]. Fungal Diversity,2012,56(1):157-171.
[33] FARR D F,CASTLEBURY L A,ROSSMAN A Y. Morphological and molecular characterization of Phomopsis vaccinii and additional isolates of Phomopsis from blueberry and cranberry in the eastern United States[J].Mycologia,2002,94(3):494-504.
[34] FARR D F,CASTLEBURY L A,ROSSMAN A Y,PUTNAM M L.A new species of Phomopsis causing twig dieback of Vaccinium vitis-idaea (lingonberry)[J]. Mycological Research,2002,106(6):745-752.
[35] 赖宝春,姚锦爱.蜜柚间座壳黑点病菌(Diaporthe citri)LAMP可视化检测技术的建立[J].福建农业学报,2022,37(11):1470-1475.LAI Baochun,YAO Jin’ai. Establishment of a LAMP assay for rapid detecting Diaporthe citri on pomelo[J]. Fujian Journal of Agricultural Sciences,2022,37(11):1470-1475.
[36] 曾雅婷,熊桃,李红叶.柑橘黑点病菌(Diaporthe citri)快速分子检测技术[J].浙江农业学报,2022,34(7):1457-1465.ZENG Yating,XIONG Tao,LI Hongye. Rapid molecular detection of Diaporthe citri,the pathogen of citrus melanose[J].Acta Agriculturae Zhejiangensis,2022,34(7):1457-1465.
[37] 熊桃.柑橘间座壳菌的群体遗传结构和有性生殖研究[D].杭州:浙江大学,2021.XIONG Tao. Population genetic structure and sexual reproduction of Diaporthe citri[D]. Hangzhou:Zhejiang University,2021.
[38] 蒲占湑,朱莉,杜丹超,胡秀荣,鹿连明,黄振东.基于超高效液相色谱-串联质谱技术的柑橘黑点病菌(Diaporthe citri)发育关联代谢物分析[J].微生物学报,2023,63(6):2472-2487.PU Zhanxu,ZHU Li,DU Danchao,HU Xiurong,LU Lianming,HUANG Zhendong.Development-associated metabolites of Diaporthe citri:A metabolomics analysis based on UPLC-MS/MS[J].Acta Microbiologica Sinica,2023,63(6):2472-2487.
[39] VLADIMIRO G,CROUS PEDRO W. Species of Diaporthe on camellia and citrus in the Azores Islands[J]. Phytopathologia Mediterranea,2018,57(2):307-319.
[40] 方天露.柑橘砂皮病菌的分离鉴定及生物学特性研究[D].长沙:湖南农业大学,2017.FANG Tianlu.Isolation,identification and biological characteristics of Diaporthe citri[D].Changsha:Hunan Agricultural University,2017.
[41] HONG S J,YUN S C.Effects of dryness,moisture interruption,and temperature on germination of Diaporthe citri pycnidiospores on Yuzu[J]. Research in Plant Disease,2018,24(2):132-137.
[42] GOPAL K,LAKSHMI L M,SARADA G,NAGALAKSHMI T,SANKAR T G,GOPI V,RAMANA K T V. Citrus melanose(Diaporthe citri Wolf):A review[J].International Journal of Current Microbiology and Applied Sciences,2014,3(4):113-124.
[43] BAHGAT M. The action of Phomopsis californica in producing a stem-end decay of citrus fruits[J]. Hilgardia,1928,3(6):153-181.
[44] PRUSKY D,ALKAN N,MENGISTE T,FLUHR R. Quiescent and necrotrophic lifestyle choice during postharvest disease development[J].Annual Review of Phytopathology,2013,51:155-176.
[45] LI P D,ZHU Z R,ZHANG Y Z,XU J P,WANG H K,WANG Z Y,LI H Y. The phyllosphere microbiome shifts toward combating melanose pathogen[J].Microbiome,2022,10(1):56.
[46] ARIMOTO Y,HOMMA Y,OHSAWA T.Studies on citrus melanose and citrus stem-end rot by Diaporthe citri (Faw.) Wolf.Part 5. Identification of a phytoalexin in melanose spot[J]. Japanese Journal of Phytopathology,1986,52(4):620-625.
[47] ARIMOTO Y,HOMMA Y,MISATO T. Studies on citrus melanose and citrus stem-end rot by Diaporthe citri (Faw.) Wolf.Part 4.Antifungal substance in melanose spot[J]. Japanese Journal of Phytopathology,1986,52(1):39-46.
[48] ARIMOTO Y,HOMMA Y,MISATO T. Studies on citrus melanose and citrus stem-end rot by Diaporthe citri(Faw.)wolf.Part 3. Mode of reaction in citrus fruit and leaf against infection of D. citri[J]. Japanese Journal of Phytopathology,1982,48(5):559-569.
[49] LI P D,XIAO X E,WANG J R,NIU F,HUANG J N,XIE B Y,YE L,ZHANG C F,WANG D L,WU Q,ZHENG X L,GAI Y P,LI H Y,JIAO C.Transcriptional insights of citrus defense response against Diaporthe citri[J]. BMC Plant Biology,2023,23(1):614.
[50] DAVIS R M,WILHITE H S. Relationships between melanose incidence and dead wood in Texas grapefruit[J]. Journal of the Rio Grande Valley Horticultural Society,1983,36:41-49.
[51] KIM K H,KIM G H,SON K I,KOH Y J. Outbreaks of Yuzu dieback in Goheung area:Possible causes deduced from weather extremes[J].The Plant Pathology Journal,2015,31(3):290-298.
[52] MONDAL S N,AGOSTINI J P,ZHANG L,TIMMER L W.Factors affecting pycnidium production of Diaporthe citri on detached citrus twigs[J].Plant Disease,2004,88(4):379-382.
[53] AGOSTINI J P,BUSHONG P M,BHATIA A,TIMMER L W.Influence of environmental factors on severity of citrus scab and melanose[J].Plant Disease,2003,87(9):1102-1106.
[54] 蒋飞,张喜喜,肖小娥,李红叶,祝增荣.上海柑橘黑点病田间流行与降雨关系研究[J].植物保护,2022,48(2):139-144.JIANG Fei,ZHANG Xixi,XIAO Xiaoe,LI Hongye,ZHU Zengrong. Relationship between citrus melanose and precipitation in Shanghai[J].Plant Protection,2022,48(2):139-144.
[55] IDREES M,NAZ S,EHETISHAM-UL-HAQ M,MEHBOOB S,KAMRAN M,ALI S,IQBAL M.Protectant and curative efficacy of different fungicides against citrus melanose caused by Phomopsis citri under in vivo conditions[J]. International Journal of Biosciences,2019,15(2):194-199.
[56] ANWAR U,MUBEEN M,IFTIKHAR Y,ZESHAN M A,SHAKEEL Q,SAJID A,UMER M,ABBAS A. Efficacy of different fungicides against citrus melanose disease in Sargodha,Pakistan[J]. Pakistan Journal of Phytopathology,2021,33(1):67-74.
[57] 刘欣,王明爽,梅秀凤,姜丽英,韩国兴,李红叶.柑橘黑点病菌种群对代森锰锌的敏感性评价及其替代药剂的筛选[J].植物保护学报,2018,45(2):373-381.LIU Xin,WANG Mingshuang,MEI Xiufeng,JIANG Liying,HAN Guoxing,LI Hongye. Sensitivity evaluation of Diaporthe citri populations to mancozeb and screening of alternative fungicides for citrus melanose control[J]. Journal of Plant Protection,2018,45(2):373-381.
[58] INUMA T. Decreasing the frequency of conrol of citrus melanose by using the fungicides dithianon for Satsuma Mandarin cultivation[J].Annual Report of the Kansai Plant Protection Society,2014,56:85-87.
[59] 李伟龙,周晓肖,郭巧慧,李辞海,李红叶.烯肟·丙森锌等杀菌剂对柑橘黑点病的防治效果比较[J]. 中国果树,2020(5):98-102.LI Weilong,ZHOU Xiaoxiao,GUO Qiaohui,LI Cihai,LI Hongye. Comparison of the prevention and control effects of fungicides such as oxime and prosenzinc on citrus melanose[J].China Fruits,2020(5):98-102.
[60] GRAHAM J H,DEWDNEY M M,MYERS M E. Streptomycin and copper formulations for control of citrus canker on grapefruit[J]. Proceedings of the Florida State Horticultural Society,2010,123:92-99.
[61] YOUNG M,OZCAN A,RAJASEKARAN P,KUMRAH P,MYERS M E,JOHNSON E,GRAHAM J H,SANTRA S.Fixed-quat:An attractive nonmetal alternative to copper biocides against plant pathogens[J]. Journal of Agricultural and Food Chemistry,2018,66(50):13056-13064.
[62] LIU X Y,CHAISIRI C,LIN Y,FU Y P,YIN W X,ZHU F X,LI J B,XIONG B,WU H,XU A,LUO C X. Effective management of citrus melanose based on combination of ecofriendly chemicals[J].Plant Disease,2023,107(4):1172-1176.
[63] 程小梅,龚碧涯,彭亚军,孔佑涵,肖伏莲,李先信.矿物油-杀菌剂联用防控柑橘砂皮病试验[J].中国果树,2020(3):84-86.CHENG Xiaomei,GONG Biya,PENG Yajun,KONG Youhan,XIAO Fulian,LI Xianxin.Test on prevention and control of citrus melanose by combination of mineral oil and fungicide[J].China Fruits,2020(3):84-86.
[64] 侯欣,陈国庆,王兴红,朱丽,李红叶.3 种柑橘病原真菌对苯醚菌酯和苯醚甲环唑敏感基线研究[J].浙江大学学报(农业与生命科学版),2013,39(1):62-68.HOU Xin,CHEN Guoqing,WANG Xinghong,ZHU Li,LI Hongye. Baseline sensitivities of three fungal pathogens of citrus to strobilurin fungicide and difenoconazole[J]. Journal of Zhejiang University (Agriculture and Life Sciences),2013,39(1):62-68.
[65] MUHAMMAD I,SUMERA N,SAIRA M,MUHAMMAD I. In vitro management of citrus melanose caused by Phomopsis citri through commercially available fungicides[J].International Journal of Biosciences,2019,14(6):179-183.
[66] 赵霞,席亚东,夏丽娟.4 种吡唑醚菌酯复配剂对柑橘砂皮病的田间防效[J].现代农药,2022,21(2):65-68.ZHAO Xia,XI Yadong,XIA Lijuan. Control effects of 4 types of pyraclostrobin mixtures on citrus melanose[J]. Modern Agrochemicals,2022,21(2):65-68.
[67] WHITESIDE J O. Sites of action of fungicides in the control of citrus melanose[J].Phytopathology,1977,67(8):1067-1072.
[68] KO Y J,KANG S Y,JEUN Y C.Suppression of citrus melanose on the leaves treated with rhizobacterial strains after inoculation with Diaporthe citri[J]. Research in Plant Disease,2012,18(4):331-337.
[69] KO Y J,KIM J S,KIM K D,JEUN Y C.Microscopical observation of inhibition-behaviors against Diaporthe citri by pre-treated with Pseudomonas putida strain THJ609-3 on the leaves of citrus plants[J]. Journal of Microbiology,2014,52(10):879-883.
[70] NNAM M H,SHIN J H,CHOI J P,HONG S I,KIM Y G,KIM H T. Identification of rhizo-bacterium inhibiting Diaporthe citri causing citrus melanose[J].The Korean Journal of Pesticide Science,2009,13(4):332-335.
[71] LEE D R,MAUNG C E H,CHOI T G,KIM K Y. Large scale cultivation of Bacillus velezensis CE 100 and effect of its culture on control of Citrus melanose caused by Diaporthe citri[J].Korean Journal of Soil Science and Fertilizer,2021,54(3):297-310.
[72] LEE D R,CHAW E H M,AJUNA H,KIM K Y.Effect of largescale cultivation of Bacillus amlyoliquefaciens Y1 using fertilizer based medium for control of citrus melanose causing Diaporthe citri[J]. Korean Journal of Soil Science and Fertilizer,2019,52(2):84-92.
[73] 李审微,洪艳云,李新文,何可佳,戴良英,卢晓鹏,宋娜,易图永. 枯草芽孢杆菌M-23 对柑橘砂皮病防效及柑橘叶际细菌群落多样性的影响[J]. 南方农业学报,2020,51(7):1699-1705.LI Shenwei,HONG Yanyun,LI Xinwen,HE Kejia,DAI Liangying,LU Xiaopeng,SONG Na,YI Tuyong. Effects of Bacillus subtilis M-23 on Diaporthe citri and diversity of bacterial community in the citrus phyllosphere[J]. Journal of Southern Agriculture,2020,51(7):1699-1705.
[74] SHIN Y H,KO E J,KIM S J,HYUN H N,JEUN Y C.Suppression of melanose caused by Diaporthe citri on citrus leaves pretreated with bio-sulfur[J].The Plant Pathology Journal,2019,35(5):417-424.
[75] 刘常利.柑橘黑点病生防菌筛选、鉴定和制剂开发[D].杭州:浙江大学,2021.LIU Changli.Biocontrol fungi screening,identification formulation develpoment for citrus melanose[D]. Hangzhou:Zhejiang University,2021.
formulation develpoment for citrus melanose[D]. Hangzhou:Zhejiang University,2021.
[76] 曾蓉,陆金萍,张学英,叶正文,戴富明.农业和化学措施对柑橘树脂病和炭疽病的协同控制作用[J].上海农业学报,2010,26(4):26-30.ZENG Rong,LU Jinping,ZHANG Xueying,YE Zhengwen,DAI Fuming. Synergistic effects of agronomic and chemical measures on controlling citrus melanosis and anthracnose[J].Acta Agriculturae Shanghai,2010,26(4):26-30.
[77] AGOSTINI J P,BUSHONG P M,TIMMER L W. Greenhouse evaluation of products that induce host resistance for control of scab,melanose,and Alternaria brown spot of citrus[J]. Plant Disease,2003,87(1):69-74.