猕猴桃起源于中国,是世界上重要水果之一,具有重要的营养价值和经济价值。根据“Marketable Gross Production”指标,猕猴桃是世界上继柑橘、苹果、鲜食葡萄、桃子/油桃、梨之后第六重要水果[1]。2019年世界猕猴桃总收获面积约26.88万hm2,总产量约434.80 万t,其中中国收获面积约18.26 万hm2,总产量约219.67万t[2-4]。联合国粮食及农业组织数据显示,2022年中国的猕猴桃收获面积达19.91万hm2,产量上涨至238.03万t,面积和产量均居世界第一位且保持上涨趋势,猕猴桃产业对中国的经济发展和乡村振兴具有重要意义。然而,2022年中国猕猴桃单产约为12.00 t·hm-2,低于世界平均水平,远低于新西兰的41.10 t·hm-2,表明中国猕猴桃产业发展仍面临严峻问题且具有巨大的发展潜力[2]。猕猴桃褐斑病会引起猕猴桃的早期落叶,严重威胁猕猴桃的产量和质量,该病害在四川猕猴桃产区为仅次于溃疡病的第二大病害[5]。褐斑病危害严重果园的病叶率可达100%,进而导致枝条干枯和果实萎蔫脱落,产量损失可达50%,严重制约当地猕猴桃产业的发展[6-8]。掌握江西省猕猴桃褐斑病的发生情况,筛选高效药剂,对提高猕猴桃的产量和品质具有重要意义。
猕猴桃褐斑病是中国四川、江西等地非常严重的病害,但猕猴桃褐斑病在国内外研究报道甚少,中国于1988年在江西九江首次报道了猕猴桃褐斑病,描述为灰褐色病斑,病斑外围深褐色,中间褐色,发病严重时叶片脱落引发光杆[9],其症状报道与现在主流褐斑病症状报道一致。虽然1988 年福建地区也报道了褐斑病的发生,但对症状的描述为叶背面长出黑色煤污状霉层,其症状报道与现在猕猴桃黑霉病症状一致,应归为猕猴桃黑霉病而非褐斑病[10]。1990 年湖北省报道褐斑病的发生,症状与刘国池等描述一致[11]。2001年浙江报道了褐斑病的发生,症状为叶缘焦枯,严重时枯萎脱落,病害后期还会危害枝干,其症状报道与目前褐斑病的症状描述出入较大[12]。2013 年陕西地区报道了褐斑病的发生,但报道的危害对象为猕猴桃果实,应纳入果实软腐病或褐腐病的范畴[13]。猕猴桃褐斑病于2014 年在广西猕猴桃种植地区从病原的形态鉴定、分子鉴定、科赫氏法则验证等角度被首次系统报道[14]。此后,在四川[15]、贵州[16]、江西[17]、湖南[18]等地被相继系统报道,且田间叶片发病图片一致,表现为叶片散生褐色圆形病斑,病斑中央灰白或浅褐色,病斑呈轮纹或呈靶点状。至此,猕猴桃褐斑病的病状得到较为统一的共识。虽然猕猴桃褐斑病的病状得到较为统一的共识,但不同学者对褐斑病病原的鉴定存在差异。刘国池等[9]、吴德义等[11]分别将江西和湖北的猕猴桃褐斑病病原鉴定为叶点霉属真菌(Phyllosticta sp.);Yuan等[14]、Cui等[15]、秦双林等[19]、苏文文等[20]分别将广西、四川、江西、贵州的褐斑病病原鉴定为多主棒孢(Corynespora cassiicola);邹玉萍等[18]将湖南的猕猴桃褐斑病病原鉴定为多主棒孢和叶点霉属真菌;冉飞等[16]和Li 等[21]将贵州的褐斑病病原鉴定为细极链格孢(Alternaria tenuissima);Chen等[8]将贵州的褐斑病病原鉴定为禾谷镰刀菌(Fusarium graminearum);Li等[22]将山东的褐斑病病原鉴定为藤仓镰刀菌(F.fujikuroi)。目前,多主棒孢、细极链格孢、叶点霉属真菌、禾谷镰刀菌和藤仓镰刀菌等均被报道是褐斑病的病原,表现为不同地区或者同一地区不同团队的鉴定结果各有不同,但目前近半数报道认为多主棒孢为褐斑病病原。
多主棒孢在自然界中广泛存在,寄主范围广泛,可侵染380个属内的530种植物,甚至在偶然情况下可侵染人体的暴露组织[23-25]。多主棒孢病原菌主要在病残体或土壤中越冬,亦可在其他寄主上越冬,第二年越冬菌源产生分生孢子侵入猕猴桃叶片,并不断产生孢子进行再侵染和传播。目前,市场上登记的对猕猴桃褐斑病有效的药剂仅有苯醚甲环唑、己唑醇、甲基硫菌灵、唑醚·代森联、苯甲·丙环唑、氟菌·肟菌酯、氟酰羟·苯甲唑、唑醚·氟酰胺、唑醚·喹啉铜、小檗碱,可供选择的范围十分有限,易引发抗药性的发生。对褐斑病高效药剂的筛选是当前基层面临的紧迫问题。关于猕猴桃褐斑病的病原目前争论不断,不同地区或者同一地区不同团队之间鉴定结果存在较大差异,对病原的鉴定依然是各猕猴桃褐斑病流行地区的紧迫任务,且对褐斑病的高效药剂选择十分有限,需要针对当地猕猴桃褐斑病的病原种类进行更多新的高效药剂筛选。笔者拟通过系统的调查,掌握江西省猕猴桃褐斑病的发生情况,并对褐斑病流行区的病原进行分离鉴定,针对鉴定的病原,对其进行室内高效药剂筛选,以期为褐斑病的防控提供新的依据。
1 材料和方法
1.1 猕猴桃褐斑病发生情况调查
笔者所在课题组团队于2023 年8 月,分别前往江西省的赣东、赣西、赣南和赣北调查猕猴桃褐斑病发生情况,一共对21个果园进行了调查(表1)。其中每个区县至少调查5个果园,每果园至少调查2块园地,实地调查采用平行线取样法,于调查园地每隔数行随机选取1行,总计选取5行进行调查,每行至少随机调查5株树,每株树至少随机调查10片叶子,记录叶片的褐斑病发病等级。猕猴桃褐斑病叶片发病等级标准:0级,无病斑;1级,病斑面积≤5%;3级,病斑面积>5%~25%;5级,病斑面积>25%~50%;7级,病斑面积>50%~75%;9级,病斑面积>75%。病情指数计算公式:病情指数(disease index,DI)=Σ(各级病叶数×发病等级)/(调查叶片总数×9)×100。
表1 调查果园的信息
Table 1 The information of investigated orchard

区域Region奉新县Fengxin county武宁县Wuning county玉山县Yushan county寻乌县Xunwu county调查地点Investigated site赤岸果园Chi’an orchard金果家庭农场-新果园Jinguo family farm-new orchard金果家庭农场-老果园Jinguo family farm-old orchard新西蓝生态农业有限公司Xinxilan Ecological Agriculture Co.,Ltd石溪桃园春风果园Shixi Taoyuanchunfeng orchard兰田村果园Orchard in Lantian village平尧村寿新家庭农场Shouxin family farm in Pingyao village欢乐湾小镇果园Orchard in Happy Bay town夏柳村果园Orchard in Xialiu village界牌村果园Orchard in Jiepai village凤口村果园Orchard in Fengkou village六都乡果园Orchard in Liudu township祝村村果园Orchard in Zhucun village漏底果园Orchard in Loudi桥村村果园Orchard in Qiaocun village樟树镇果园Orchard in Zhangshu township李进坑果园Orchard in Lijinkeng东团村果园Orchard in Dongtuan village岗背村果园Orchard in Gangbei village杨梅村果园Orchard in Yangmei village长布村果园Orchard in Changbu village经度Longitude/(°)115.285 973 115.303 010 115.284 780 115.285 581 114.912 854 115.077 981 115.044 535 115.080 333 115.053 734 115.416 929 115.129 878 118.341 403 118.331 771 118.228 086 118.286 803 117.968 571 115.713 309 115.648 160 115.613 148 115.686 208 115.612 682纬度Latitude/(°)28.686 667 28.670 799 28.686 813 28.715 989 28.749 633 28.768 782 29.243 912 29.259 097 29.199 398 29.205 991 29.229 921 28.607 542 28.825 995 28.806 619 28.792 479 28.769 063 24.978 222 25.005 335 24.911 120 24.951 273 24.952 205
1.2 猕猴桃褐斑病病原分离和回接致病性鉴定
采用组织分离法对褐斑病病原进行分离,将采集的病叶用清水清洗并擦拭干净,再使用75%乙醇擦拭消毒。随后将病叶置于超净台中进行无菌操作(超净台中各器械均已进行消毒或灭菌处理),使用刀片将病健交界处按1 cm×1 cm 大小切下,依次在无菌水中清洗1 min,75%乙醇中浸泡45 s,无菌水清洗1 min,于无菌滤纸上晾干后置于PDA 培养基(索莱宝)中培养。对分离出的菌株挑取边缘菌丝进行纯化培养,观察菌落形态,并进行回接试验。挑取7 mm 直径菌饼接种于猕猴桃叶片(品种:金果),于保湿盒中25 ℃黑暗培养5 d 后观察叶片发病情况,对发病叶片采用组织分离法进行分离,观察分离得到的病原物是否与接种体一致。
1.3 病原菌的分子鉴定
将病原菌株于PDA 培养基25 ℃黑暗培养7 d后,使用枪头蘸取少量菌丝于2×Phanta Max Master Mix(诺唯赞)中进行ITS序列扩增(ITS1:TCCGTAGGTGAACCTGCGG/ITS4:TCCTCCGCTTATTGATATGC),PCR扩增体系:2×Phanta Max Master Mix 12.5 μL,上游引物ITS1 1 μL,下游引物ITS4 1 μL,ddH2O 10.5 μL,DNA模板为菌丝体少许。PCR反应程序为:95 ℃预变性5 min,95 ℃变性15 s,56 ℃退火15 s,72 ℃延伸1 min,32个循环,72 ℃终延伸5 min。采用1%琼脂糖凝胶电泳(电压125 V)观察扩增结果,将特异条带送测序,测序公司为生工生物(上海)股份有限公司。测序结果于NCBI网站进行序列比对(https://www.ncbi.nlm.nih.gov/),随后采用MEGA 7.0软件邻接法(neighbor-joining method)进行系统进化树构建,确定病原菌的亲缘关系。用于进化树构建的菌株(NCBI 登录号)为:KBLS-1-1(PP504495.1)、C.cassiicola isolate KC14(MH605273.1)、C.cassiicola isolate KC9(MH605272.1),C. cassiicola isolate ACC10(KP748298.1)、C. citricola isolate CBS169.77(FJ852594.1)、C. encephalarti culture CBS:145555(MK876383.1)、C.lignicola strain MFLUCC 16-1301(MN860549.1)、C.mengsongensis strain HJAUPC2000(OQ060574.1)、C.nabanheensis strain HJAUP C2048(OQ060577.1)、C.pseudocassiicola culture CPC:31708(MH327794.1)、C.smithii strain L133(KY984299.1)、C.submersa strain MFLUCC 16-1101(MN860548.1)、C.torulosa strain CBS 136419(MH866095.1)、C.thailandica culture CBS:145089(MK047455.1)、C. yunnanensis strain HJAUP C2132(OQ060579.1)、Didymella segeticola isolate MHT6(OP627529.1)、Didymella sp. strain PB- 96(MK334016.1)、F. graminearum strain TS- 152(MG832572.1)、F. graminearum strain PB-60(MK333980.1)、Diaporthe ambigua isolate UT15BD(MF139900.1)、D. ambigua isolate UT19BD(MF139899.1)、Colletotrichum fructicola strain MHT02(KY752036.1)、C. fructicola strain F11MHTFF04(KC012512.1)、Alternaria alternata strain KHF-5(MN173818.1)、A. alternata isolate SYP414(OR901852.1)、Verticillium dahliae strain Vdp83(LC070674.1)。
1.4 病原菌室内高效药剂筛选
采用菌丝生长速率法对9种杀菌剂的室内抑菌效果进行研究,使用的药剂分别为:药剂1:24%腈苯唑悬浮剂(美国陶氏益农公司);药剂2:8%宁南霉素水剂(德强生物股份有限公司);药剂3:30%丙硫菌唑可分散油悬浮剂(安徽久易农业股份有限公司);药剂4:40%环丙唑醇悬浮剂(盐城利民农化有限公司);药剂5:22.5%啶氧菌酯悬浮剂(美国杜邦公司);药剂6:6%嘧啶核苷类抗菌素水剂(陕西麦可罗生物科技有限公司);药剂7:50%克菌丹可湿性粉剂(安道麦马克西姆有限公司);药剂8:40%腈菌唑可湿性粉剂(美国陶氏益农公司);药剂9:80%三乙膦酸铝可湿性粉剂(利民化学有限责任公司)。根据厂家推荐使用剂量,按照有效成分计算,将药剂1~6及药剂8按照有效成分终质量浓度梯度10、30、90、270、810 μg·mL-1加入PDA 培养基中;药剂7 按照有效成分终质量浓度梯度30、90、270、810、2430 μg·mL-1加入PDA培养基中;药剂9按照有效成分终质量浓度梯度270、810、2430、7290、21 870 μg·mL-1加入PDA 培养基中。将各药剂按照浓度梯度倒好平板后,挑取菌饼放入平板中间,25 ℃黑暗培养7 d后采用十字交叉法测量各处理菌落直径。抑菌率/%=(对照组菌落直径-处理组菌落直径)/(对照组菌落直径-菌饼直径)×100。
1.5 数据分析
应用Excel 软件对数据进行整理和计算;应用SAS 8.0软件采用Duncan’s新复极差法进行多重比较(显著性水平p≤0.05)。
2 结果与分析
2.1 江西省猕猴桃褐斑病发生情况
于8月份前往江西省赣东、赣西、赣南、赣北4个片区调查猕猴桃褐斑病发生情况,一共对21个果园进行了调查。其中,猕猴桃褐斑病在赣北武宁县和赣西奉新县大面积暴发,褐斑病发病率达100%,病情指数分别为31.94 和37.16(表2)。武宁县和奉新县的11 个果园中有5 个果园发病等级中值(median disease rating,MDR)在5或以上,猕猴桃叶片呈现典型靶点状褐色病斑,即半数果园褐斑病属于严重发生,且导致严重的落叶现象(图1)。而玉山县和寻乌县病害较轻,病情指数分别为0.24和3.09,显著低于武宁县和奉新县,且发病中值均为0,表明病害的严重程度较低。此外,寻乌县由于纬度较低,夏季太阳强烈,猕猴桃面临较大的晒伤风险。

图1 猕猴桃褐斑病田间症状
Fig.1 Disease symptoms of kiwifruit brown leaf spot in orchard
表2 江西省猕猴桃褐斑病发生情况
Table 2 Occurrence of kiwifruit brown leaf spot in Jiangxi province

注:同列数据后不同小写字母表示差异显著(p≤0.05)。
Note:The different small letters after the same column indicate significant difference at p≤0.05.
调查地点Investigated site赤岸果园Chi’an orchard金果家庭农场-新果园Jinguo family farm-new orchard金果家庭农场-老果园Jinguo family farm-old orchard新西蓝生态农业有限公司Xinxilan Ecological Agriculture Co.,Ltd石溪桃园春风果园Shixi Taoyuanchunfeng orchard兰田村果园Orchard in Lantian village平尧村寿新家庭农场Shouxin family farm in Pingyao village欢乐湾小镇果园Orchard in Happy Bay town夏柳村果园Orchard in Xialiu village界牌村果园Orchard in Jiepai village凤口村果园Orchard in Fengkou village六都乡果园Orchard in Liudu township祝村村果园Orchard in Zhucun village漏底果园Orchard in Loudi桥村村果园Orchard in Qiaocun village樟树镇果园Orchard in Zhangshu township李进坑果园Orchard in Lijinkeng东团村果园Orchard in Dongtuan village岗背村果园Orchard in Gangbei village杨梅村果园Orchard in Yangmei village长布村果园Orchard in Changbu village调查点病情指数DI of investigated sites 74.89 69.20 37.40 25.07 2.62 13.79 9.61 18.00 69.00 2.67 60.44 0.60 0.00 0.00 0.31 0.31 0.00 6.67 3.62 0.27 4.89调查点发病等级中值MDR of investigated sites 775000017050000000000各县病情指数平均值Average DI of county 37.16±12.02 a 31.94±13.67 a 0.24±0.11 b 3.09±1.30 b
2.2 褐斑病的病原分离与鉴定
通过组织分离法对褐斑病严重危害地区武宁县和奉新县的猕猴桃病叶进行分离,从病叶组织分离出不同形态分离物共4 种类型(图2),分别记为KBLS-1、KBLS-2、KBLS-3 和KBLS-4,每种类型菌挑选2 个菌株(分别记为:KBLS-1-1、KBLS-1-2;KBLS-2-1、KBLS-2-2;KBLS-3-1、KBLS-3-2;KBLS-4-1、KBLS-4-2)进行科赫氏法则验证。分别将各菌株的菌饼接种猕猴桃叶片,接种示意图如图3,结果表明,仅KBLS-1 类型菌株接种猕猴桃叶片后产生典型侵染症状,其余3 个类型菌株均未产生典型侵染症状,仅在接种部位表面附着少许菌丝,表明仅KBLS-1 类型可能为褐斑病的病原。将KBLS-1 于PDA培养基上进行培养观察,菌落初期白色,培养7 d后菌落灰白色,菌落中菌丝浓密呈绒毛状,菌落中央隆起。分生孢子棍棒状,浅棕色,具有1至多个假隔膜(图4)。进一步对接种病叶进行再分离,结果表明,再分离得到的菌株与分离株KBLS-1 类型菌落形态一致,孢子形态一致,证明KBLS-1类型菌株为猕猴桃褐斑病的致病菌(图3、图4)。综合以上结果,将病原菌初步鉴定为多主棒孢(C.cassiicola)。进一步采用ITS序列进行分子鉴定,通过NCBI网站下载已公布的棒孢属下的12个种的ITS序列以及猕猴桃上5个常见病原真菌ITS序列,以亲缘关系较远的大丽轮枝菌(Verticillium dahliae)ITS序列为外群,构建了邻接法系统发育树。结果表明,KBLS-1与棒孢属下的多主棒孢亲缘关系紧密(图5)。以上结果表明,猕猴桃褐斑病病原为多主棒孢。
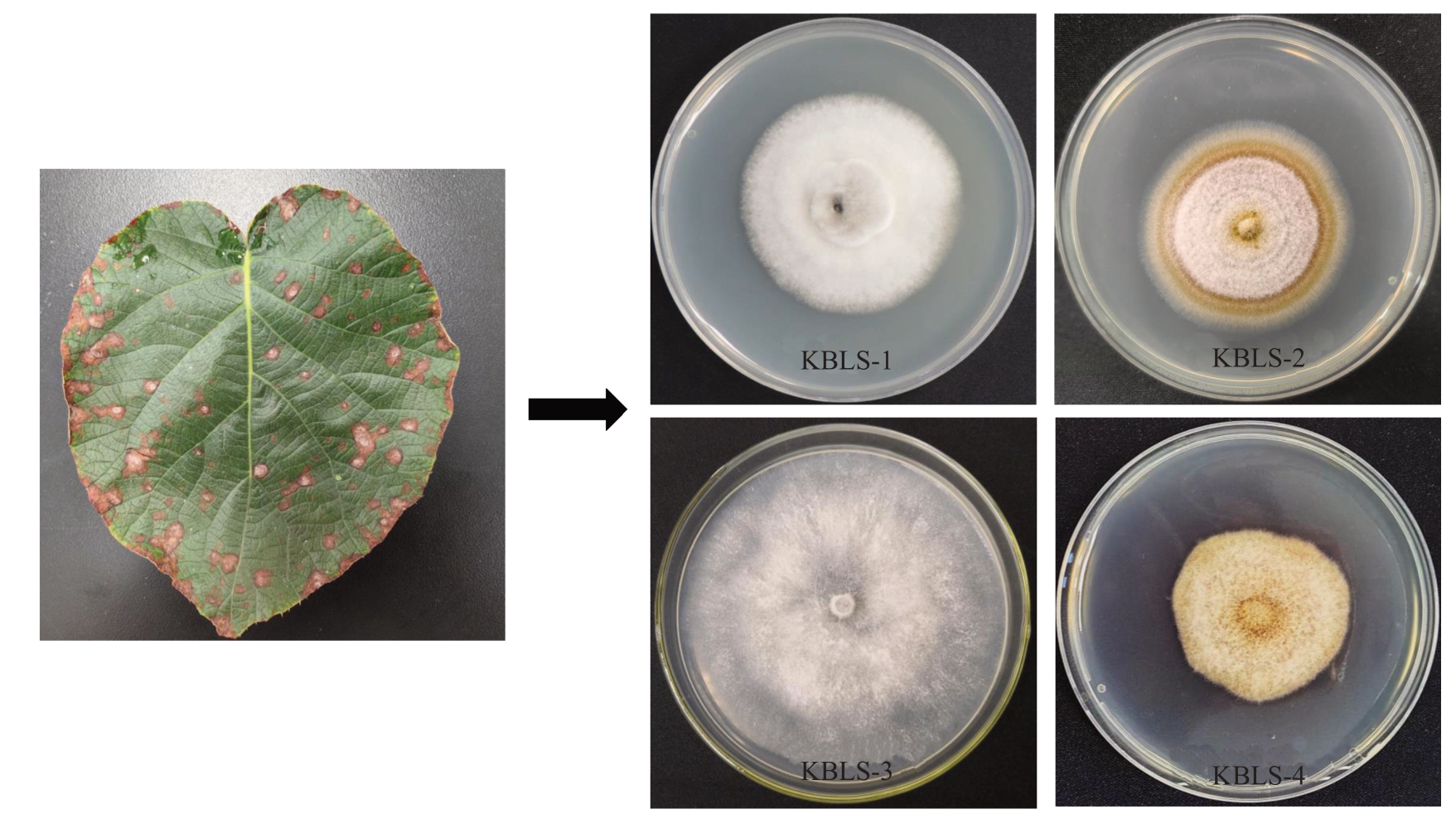
图2 猕猴桃褐斑病病原分离情况
Fig.2 Isolates from kiwifruit brown leaf spot diseased leaves

图3 科赫氏法则回接验证与接种后病原菌再分离
Fig.3 Verification of pathogen by Koch’s postulates and re-isolating of pathogen
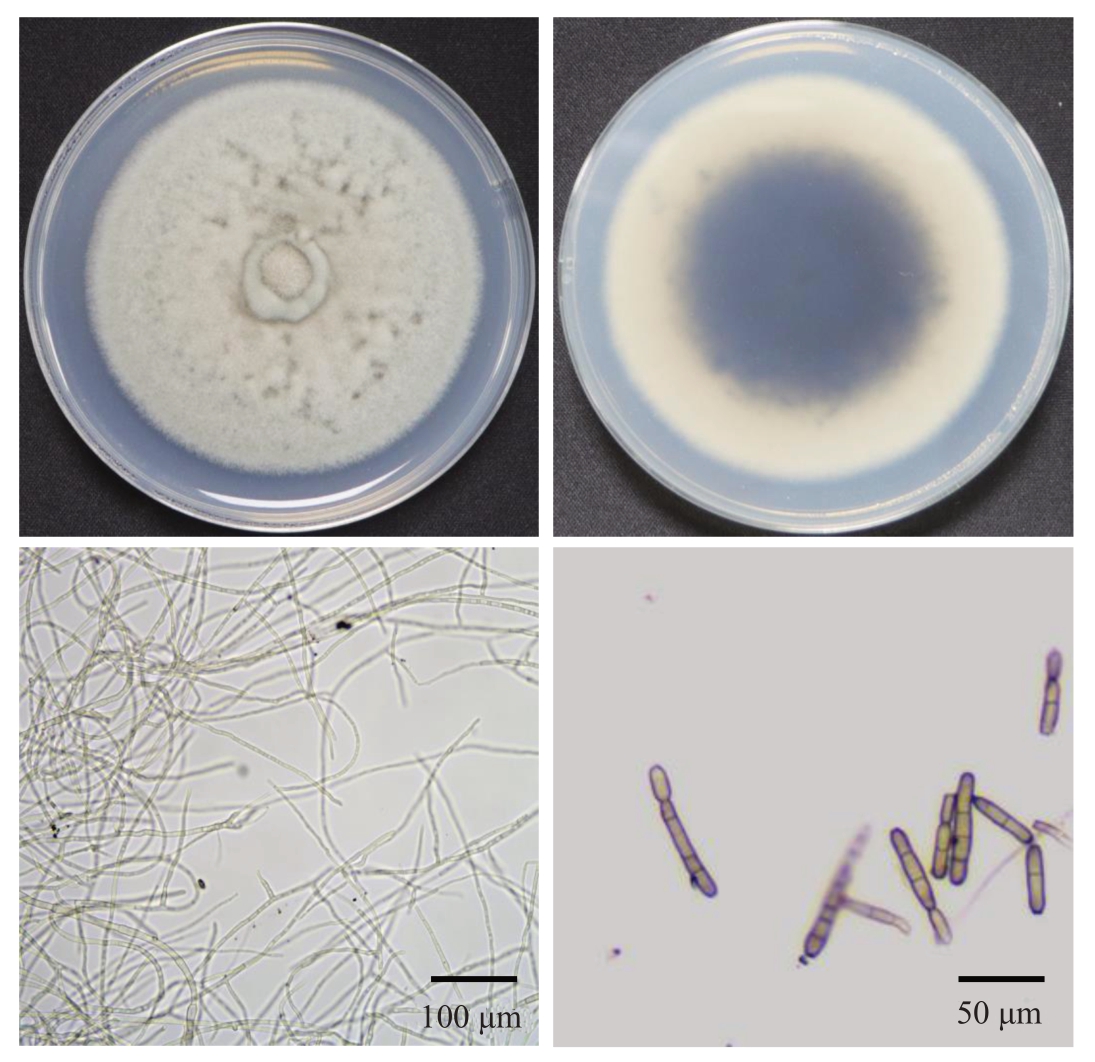
图4 猕猴桃褐斑病叶片分离物形态学观察
Fig.4 Morphology observation of isolates from kiwifruit brown leaf spot diseased leaves

图5 KBLS-1 菌株ITS 序列进化树构建
Fig.5 Phylogeny analysis of ITS sequences of KBLS-1
2.3 多主棒孢室内高效药剂筛选
猕猴桃褐斑病一旦进入暴发期,现有药剂均难以起效,故而筛选新的高效药剂是猕猴桃褐斑病防控的一项紧急任务。笔者对9 个未在猕猴桃褐斑病上进行过研究的药剂进行了室内毒力测定(图6)。得到9 种药剂的EC50,其中腈苯唑的EC50 为86.12 μg·mL-1,宁南霉素的EC50为106.07 μg·mL-1,丙硫菌唑的EC50为17.36 μg·mL-1,环丙唑醇的EC50为6.97 μg·mL-1,啶氧菌酯无明显抑菌效果,嘧啶核苷类抗菌素的EC50为304.46 μg·mL-1,克菌丹的EC50为5.30 μg·mL-1,腈菌唑的EC50为2.50 μg·mL-1,三乙膦酸铝的EC50为509.62 μg·mL-1(表3)。以上结果表明,丙硫菌唑、环丙唑醇、克菌丹和腈菌唑对猕猴桃褐斑病菌具有极强抑制作用,可作为防治褐斑病菌的潜在高效药剂。

图6 多主棒孢室内高效药剂筛选
Fig.6 Laboratory screening of highly efficient fungicides against C.cassiicola
表3 9 种药剂对多主棒孢的敏感性
Table 3 Inhibition effect of 9 fungicides against C.cassiicola

药剂名称Fungicide name腈苯唑Fenbuconazole宁南霉素Ningnanmycin丙硫菌唑Prothioconazole环丙唑醇Cyproconazole啶氧菌酯Pico xystrobin嘧啶核苷类抗菌素Pyrimidine nucleoside antibiotics克菌丹Captan腈菌唑Myclobutanil三乙膦酸铝Fosetyl aluminum毒力回归方程Toxicity regression equation y=4.619 3+0.196 7 x y=2.895 7+1.038 9 x y=4.796 6+0.164 1 x y=4.507 7+0.584 0 x y=2.011 9+0.063 2 x y=1.575 9+1.378 7 x y=4.925 6+0.102 7 x y=4.795 9+0.512 4 x y=2.055 4+1.087 7 x相关系数Correlation coefficient 0.910 8 0.988 8 0.989 4 0.970 8 0.891 5 0.991 7 0.952 4 0.924 4 0.942 2 EC50/(μg·mL-1)86.12 106.07 17.36 6.97 2.98E+59 304.46 5.30 2.50 509.62
3 讨 论
3.1 猕猴桃褐斑病流行情况
猕猴桃褐斑病是四川[7,26]、贵州[8]、江西[17]等地广泛发生的真菌性叶部病害,且为四川地区最为严重的真菌性病害,极大地制约当地猕猴桃产业的健康发展。对四川地区的调查中,猕猴桃褐斑病在红阳种植园的发病率可超过99%,病情指数约50,属于严重大范围发生[7]。对湖南地区的调查中,猕猴桃褐斑病在调查园区普遍发生,果园的发生率超过94%,病情指数约26[18]。江西地区猕猴桃褐斑病最早于1988年在九江地区发现,后于2013年在江西主产区奉新县山口猕猴桃种植基地发现并持续严重发生[9,19]。然而,尚不清楚其在奉新其他果园的发生情况以及其在全省的发生情况。笔者通过对赣东、赣西、赣南和赣北4 个方位的调查,结果表明褐斑病主要在赣西和赣北发生,赣东和赣南发生较轻。其中,猕猴桃褐斑病在赣北的武宁县和赣西奉新县大面积暴发,褐斑病发病率达100%,病情指数分别为31.94和37.16。
3.2 猕猴桃褐斑病病原鉴定
猕猴桃褐斑病的早期研究结果不一,表现为其病状在国内无统一认识,该病害病状于2014年通过系统的病原学研究后被最终确定为:叶片散生褐色圆形病斑,病斑呈现外围深褐色、中央灰白或浅褐色的靶点状,有时具有轮纹[14]。但即使病状统一后,猕猴桃褐斑病病原于不同地区乃至同一地区不同团队间的鉴定结果仍然存在差异。目前,禾谷镰刀菌[8]、叶点霉属真菌[9]、多主棒孢[14]、细极链格孢[21]和藤仓镰刀菌[22]等均被报道是褐斑病的病原。据此,猕猴桃褐斑病病原可能并不唯一,并存在复合侵染现象。因此,笔者系统调查了江西省猕猴桃褐斑病发生情况,并对褐斑病发生严重地区奉新县和武宁县的褐斑病病原进行了分离鉴定,一共分离得到了4种类型菌株(经过ITS 测序分别属于Corynespora、Didymella、Pestalotiopsis 和Alternaria),经过科赫氏法则验证及后续分子鉴定,其中致病菌为多主棒孢(C. cassiicola)。表明江西省猕猴桃褐斑病主要发生地区的病原为多主棒孢。
3.3 猕猴桃褐斑病高效药剂筛选
猕猴桃褐斑病作为国内新兴的暴发性病害,严重制约流行区域的果实品质。近十年来,针对猕猴桃褐斑病的药剂防治,已有不少报道对市场上大量不同药剂进行了筛选,得到了一些对褐斑病具有良好防效的药剂,如吡唑醚菌酯[27]、苯醚甲环唑[28]、小檗碱[29]、氨基寡糖素[30]、戊唑醇[20,31]、嘧菌酯[32]等。总体来说,对褐斑病的药剂选择十分有限,易引发抗药性的发生,因此,对褐斑病高效药剂的筛选是当前基层面临的紧迫问题。笔者针对9个未在猕猴桃褐斑病上进行过研究的药剂(腈苯唑、宁南霉素、丙硫菌唑、环丙唑醇、啶氧菌酯、嘧啶核苷类抗菌素、克菌丹、腈菌唑和三乙膦酸铝),采用生长速率法进行了室内毒力测定,结果表明,丙硫菌唑、环丙唑醇、克菌丹和腈菌唑对猕猴桃褐斑病菌具有极强抑制作用,可作为防治褐斑病菌的潜在高效药剂。
4 结 论
江西省猕猴桃褐斑病在赣北和赣西大范围严重发生,在赣东和赣南发生较轻。对江西省猕猴桃褐斑病严重发生地区的叶片进行病原分离,一共得到4 种不同类型分离物,通过科赫氏法则验证及病原鉴定确定最终病原为多主棒孢(C. cassiicola)。通过对9种未在该病害中研究过的药剂的室内毒力测定,发现丙硫菌唑、环丙唑醇、克菌丹和腈菌唑对猕猴桃褐斑病菌具有极强抑制作用,可作为防治褐斑病菌的新的潜在高效药剂。
[1] GUROO I,WANI S A,WANI S M,AHMAD M,MIR S A,MASOODI F A.A review of production and processing of kiwifruit[J].Journal of Food Processing Technology,2017,8(10):1000699.
Technology,2017,8(10):1000699.
[2] 钟彩虹,黄文俊,李大卫,张琼,李黎.世界猕猴桃产业发展及鲜果贸易动态分析[J].中国果树,2021(7):101-108.ZHONG Caihong,HUANG Wenjun,LI Dawei,ZHANG Qiong,LI Li. Development of world kiwifruit industry and dynamic analysis of fresh fruit trade[J].China Fruits,2021(7):101-108.
[3] 胡新龙,金玲莉,王璠,王斯妤,吴庭观,李小飞,陈东元,吴美华.江西猕猴桃产业现状及“十四五”发展对策与建议[J].江西农业学报,2022,34(5):34-39.HU Xinlong,JIN Lingli,WANG Fan,WANG Siyu,WU Tingguan,LI Xiaofei,CHEN Dongyuan,WU Meihua.Development status and suggestions on kiwifruit industry in Jiangxi Province during“the 14th five-year plan”period[J].Acta Agriculturae Jiangxi,2022,34(5):34-39.
[4] 钟曼茜,翟舒嘉,刘伟,睢国祥,段玉权,林琼,陶鑫凉.我国即食猕猴桃产业发展现状、问题与对策[J].中国果树,2023(2):122-127.ZHONG Manxi,ZHAI Shujia,LIU Wei,SUI Guoxiang,DUAN Yuquan,LIN Qiong,TAO Xinliang. The development status,problems and countermeasures of instant kiwifruit industry in China[J].China Fruits,2023(2):122-127.
[5] 龚国淑,李庆,张敏,崔永亮.猕猴桃病虫害原色图谱与防治技术[M].北京:科学出版社,2020.GONG Guoshu,LI Qing,ZHANG Min,CUI Yongliang. Primary color map and control techniques of kiwifruit diseases and pests[M].Beijing:Science Press,2020.
[6] 吴世权,高群,牛淑英.猕猴桃早期落叶病发生原因及防治对策[J].中国果树,2009(5):73.WU Shiquan,GAO Qun,NIU Shuying. The pathogen and prevention strategies of early leaf fall disease in kiwifruit[J]. China Fruits,2009(5):73.
[7] 崔永亮.猴桃褐斑病的研究[D].雅安:四川农业大学,2015.CUI Yongliang. Study of kiwifruit brown leaf spot[D].Yaan:Sichuan Agricultural University,2015.
[8] CHEN J,RAN F,SHI J Q,CHEN T T,ZHAO Z B,ZHANG Z Z,HE L N,LI W Z,WANG B C,CHEN X T,WANG W Z,LONG Y H.Identification of the causal agent of brown leaf spot on kiwifruit and its sensitivity to different active ingredients of biological fungicides[J].Pathogens,2022,11(6):673.
[9] 刘国池,熊致奥,董同琴,冯弋良.人工栽培猕猴桃褐斑病调查初报[J].江西植保,1988,11(1):22-23.LIU Guochi,XIONG Zhiao,DONG Tongqin,FENG Yiliang.Preliminary investigation of cultivatied kiwifruit[J]. Jiangxi Plant Protection,1988,11(1):22-23.
[10] 王清涛.中华猕猴桃褐斑病初探[J].福建农业科技,1988,19(3):8.WANG Qingtao. Preliminary study on brown leaf spot disease of Actinidia chinensis[J]. Fujian Agricrltural Science and Technology,1988,19(3):8.
[11] 吴德义,陈振旺.猕猴桃褐斑病初步研究[J].湖北农业科学,1990,29(2):31-32.WU Deyi,CHEN Zhenwang. Preliminary research on kiwifruit brown leaf spot[J].Hubei Agricultural Sciences,1990,29(2):31-32.
[12] 吴浙东.几种杀菌剂对猕猴桃褐斑病的药效试验[J].西南园艺,2001(3):12.WU Zhedong. Research on the efficacy of several fungicides against kiwifruit brown leaf spot[J]. Southwest Horticulture,2001(3):12.
[13] 赵金梅,高贵田,谷留杰,孙翔宇,薛敏,耿鹏飞,雷玉山.中华猕猴桃褐斑病病原鉴定及抑菌药剂筛选[J].中国农业科学,2013,46(23):4916-4925.ZHAO Jinmei,GAO Guitian,GU Liujie,SUN Xiangyu,XUE Min,GENG Pengfei,LEI Yushan.Identification and pharmaceutical screening of brown spot disease on Actinidia chinensis[J].Scientia Agricultura Sinica,2013,46(23):4916-4925.
[14] YUAN G Q,XIE Y L,TAN D C,LI Q Q,LIN W. First report of leaf spot caused by Corynespora cassiicola on kiwifruit (Actinidia chinensis) in China[J]. Plant Disease,2014,98(11):1586.
[15] CUI Y,GONG G,YU X,XU J,WEN X,ZHANG M,CHEN H,ZHENG X,ZHOU Y,CHANG X. First report of brown leaf spot on kiwifruit caused by Corynespora cassiicola in Sichuan,China[J].Plant Disease,2015,99(5):725.
[16] 冉飞,张荣全,袁腾,马继玲,尹显慧,李文志,樊荣,吴小毛,龙友华.‘红阳’猕猴桃褐斑病病原菌分离鉴定及防治药剂毒力测定[J].中国果树,2021(6):27-32.RAN Fei,ZHANG Rongquan,YUAN Teng,MA Jiling,YIN Xianhui,LI Wenzhi,FAN Rong,WU Xiaomao,LONG Youhua.Isolation and identification of the pathogen of‘Hongyang’kiwifruit brown spot and toxicity of fungicides to the pathogen[J].China Fruits,2021(6):27-32.
[17] 张凯东.猕猴桃棒孢叶斑病菌生物学特性研究及室内药剂筛选[D].南昌:江西农业大学,2021.ZHANG Kaidong. Biological characteristics of Corynespora cassiicola and screening of fungicides in laboratory[D]. Nanchang:Jiangxi Agricultural University,2021.
[18] 邹玉萍,王仁才,崔丽红,王琰.猕猴桃褐斑病病情调查及病原菌鉴定[J].湖南林业科技,2022,49(4):1-8.ZOU Yuping,WANG Rencai,CUI Lihong,WANG Yan.Investigation on the occurrence of kiwifruit brown spot and identification of pathogenic bacteria[J]. Hunan Forestry Science  Technology,2022,49(4):1-8.
Technology,2022,49(4):1-8.
[19] 秦双林,王园秀,蒋军喜,崔朝宇,欧阳慧,黄婷.江西奉新县猕猴桃叶斑病病原菌鉴定[J]. 江西农业大学学报,2016,38(3):488-491.QIN Shuanglin,WANG Yuanxiu,JIANG Junxi,CUI Chaoyu,OUYANG Hui,HUANG Ting. Identification of pathogenic fungus causing kiwifruit leaf spot in Fengxin County of Jiangxi Province[J].Acta Agriculturae Universitatis Jiangxiensis,2016,38(3):488-491.
[20] 苏文文,任春光,潘丽珊,吴迪,韩振诚,王加国,李良良,李苇洁.红阳猕猴桃褐斑病病原菌鉴定与室内药剂筛选[J].中国南方果树,2022,51(4):119-124.SU Wenwen,REN Chunguang,PAN Lishan,WU Di,HAN Zhencheng,WANG Jiaguo,LI Liangliang,LI Weijie. Pathogen identification and fungicide screening for brown leaf spot on Hongyang kiwifruit[J]. South China Fruits,2022,51(4):119-124.
[21] LI L,PAN H,DENG L,WANG Z P,LI D W,ZHANG Q,CHEN M Y,ZHONG C H.First report of Alternaria tenuissima causing brown spot disease of kiwifruit foliage in China[J].Plant Disease,2019,103(3):582.
[22] LI H H,TANG W,LIU K,ZHANG L,TANG X F,MIAO M,LIU Y S. First report of Fusarium fujikuroi causing brown leaf spot on kiwifruit[J].Plant Disease,2020,104(5):1560.
[23] DIXON L J,SCHLUB R L,PERNEZNY K,DATNOFF L E.Host specialization and phylogenetic diversity of Corynespora cassiicola[J].Phytopathology,2009,99(9):1015-1027.
[24] SUMABAT L G,KEMERAIT R C J,BREWER M T. Phylogenetic diversity and host specialization of Corynespora cassiicola responsible for emerging target spot disease of cotton and other crops in the southeastern United States[J]. Phytopathology,2018,108(7):892-901.
[25] FENG Y H,ZENG Q,QIU Y,LI D M,SHI D M.Successful application of photodynamic therapy for skin infection caused by Corynespora cassiicola in an immunosuppressed patient and literature review[J]. Photodiagnosis and Photodynamic Therapy,2023,41:103279.
[26] ZHU Y H,YAO K K,MA M M,CUI Y L,XU J,CHEN W,YANG R,WU C P,GONG G S. Occurrence regionalization of kiwifruit brown spot in Sichuan[J].Journal of Fungi,2023,9(9):899.
[27] 白伟,姜军侠,朱岁层,李建明.60%吡唑醚菌酯·代森联水分散粒剂防治猕猴桃褐斑病田间药效试验[J].农药科学与管理,2019,40(2):54-57.BAI Wei,JIANG Junxia,ZHU Suiceng,LI Jianming. Field efficacy trials of pyraclostrobin + metiram 60% WG against kiwifruit brown blotch[J]. Pesticide Science and Administration,2019,40(2):54-57.
[28] 胡妍月,孙杨,郭明程,涂贵庆,赵尚高,黄水金.8 种药剂对猕猴桃灰霉病和褐斑病的防治效果评价[J].湖南农业科学,2021(4):82-85.HU Yanyue,SUN Yang,GUO Mingcheng,TU Guiqing,ZHAO Shanggao,HUANG Shuijin.Evaluation on the control effects of eight fungicides on kiwifruit gray mold disease and brown spot disease[J].Hunan Agricultural Sciences,2021(4):82-85.
[29] 刘欣. 0.5%小檗碱水剂防治猕猴桃褐斑病田间药效试验[J].现代农业,2020(1):26-27.LIU Xin. Field efficacy trial of 0.5% berberine aqueous solution for controlling kiwifruit brown leaf spot disease[J]. Modern Agriculture,2020(1):26-27.
[30] 潘丽珊,任春光,苏文文,韩振诚,李苇洁.不同抗病诱导剂对猕猴桃褐斑病田间防效及果实品质的影响[J].经济林研究,2023,41(1):154-164.PAN Lishan,REN Chunguang,SU Wenwen,HAN Zhencheng,LI Weijie. The effect of different disease-resistant inducers on the field control of kiwi brown spot disease and their effects on fruit quality[J]. Non-wood Forest Research,2023,41(1):154-164.
[31] 杨恩兰,王林,苟铁丞,龙彪,李荣.3 种杀菌剂对猕猴桃褐斑病的防治效果[J].农技服务,2021,38(1):74-76.YANG Enlan,WANG Lin,GOU Tiecheng,LONG Biao,LI Rong. The control effect of three fungicides on kiwifruit brown leaf spot disease[J]. Agricultural Technology Service,2021,38(1):74-76.
[32] 吴晓枝.猕猴桃褐斑病化学防治试验[J].现代园艺,2018(15):45-46.WU Xiaozhi. Chemical control experiment of kiwifruit brown leaf spot disease[J].Xiandai Horticulture,2018(15):45-46.